Abstract
Objective:
This exploratory study seeks to identify distinct circulating immune signatures among patients having recurrent acute pancreatitis (RAP), chronic pancreatitis (CP), and pancreatic adenocarcinoma (PDAC).
Methods:
A retrospective analysis of human serum samples from collaborating institutions of the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) was performed. Samples came from the North American Pancreatitis Studies 2 (NAPS2) cohort and the Pancreatic Adenocarcinoma Gene Environment Risk Study (PAGER) and were analyzed using a 62-plex Luminex assay in a blinded fashion. Group and pairwise comparisons were performed to identify unique immune signature panels and to calculate diagnostic utility using area under the curve analysis.
Results:
A total of 179 patients’ samples were included: 41 controls, 40 CP, 78 PDAC and 20 RAP patients, of which 20 controls, 20 CP, and 58 PDAC patients had diabetes mellitus (DM). A unique immune signature panel could discriminate RAP, CP, and PDAC from controls with an AUC range from 0.77 – 0.86 (95% CI range: 0.64 – 0.94), RAP from CP, and CP from PDAC with an AUC of 0.77 (95% CI 0.64 – 0.90) and 0.76 (95% CI 0.67 – 0.86), respectively. Furthermore, an immune signature panel could also discriminate PDAC-DM from DM controls with an AUC of 0.96 (95% CI : 0.93–1.00)
Conclusion:
This study identifies unique immune analytes that may serve as novel diagnostic and predictive non-invasive biomarkers of RAP, CP, and PDAC. Further validation is warranted in prospective cohorts as developed by the CPDPC.
Keywords: Cytokines, Biomarkers, Pancreatitis, Pancreatic Cancer, Diabetes
INTRODUCTION
Chronic pancreatitis (CP) is a progressive chronic inflammatory disorder with subsequent irreversible fibrosis which affects an estimated 40 – 92/100,000 people in the United States.(1, 2) With disease progression, CP can lead to serious complications including maldigestion and malnutrition from exocrine pancreatic insufficiency (EPI), endocrine pancreatic insufficiency (i.e. diabetes mellitus, DM), chronic pain syndromes and pancreatic ductal adenocarcinoma (PDAC).(3–7) Currently, there are no sensitive and specific biomarker tests to detect CP or PDAC at early stages or predict progression to DM or PDAC among patients with CP.
PDAC is a lethal, recalcitrant cancer with an overall 5-year survival rate of 8.2%.(8) Approximately 68% of PDAC patients have DM at diagnosis, where 40% of these patients developed it within the last 3 years.(9) In these patients, the DM may function as an early marker for PDAC. Identifying these patients with new onset DM with a reliable diagnostic test may be a critical step in early PDAC diagnosis.
Immunological mechanisms that govern disease progression are of increasing interest.(10) The dynamic cellular and molecular interactions between the pancreas and the immune system in CP progression, however, remains unclear.(11) Correlating inflammatory cell-pancreas interactions with development of important clinical CP sequelae like fibrosis, DM, and PDAC may provide new diagnostic and therapeutic insights.(12) In both CP and PDAC, human studies and experimental animal modeling indicate that pancreatic stellate cells (PSCs) play an important pathophysiologic role.(13) In CP, increased expression and activity of inflammatory cytokines and macrophages appear to activate PSCs to form fibrosis indicating progression of CP. Pre-clinical animal studies demonstrate clear unique immune responses associated with acute (AP), recurrent acute (RAP), and CP as well as highlight potential immune targets to halt or reverse CP progression.(14–17) Furthermore, there have been some human studies looking at select cytokines in chronic pancreatitis and pancreatic cancer.(18–20) These studies suggest that distinct immune signaling signatures may function as biomarkers of CP and its sequelae of type 3c DM, PDAC, and PDAC-induced DM.
This exploratory study seeks to identify distinct immune signatures by performing in-depth immune profiling of 62 analytes from 4 distinct well-characterized clinical populations: non-disease controls, RAP, CP, and PDAC. In addition, since a subset of CP patients, PDAC patients, and controls have DM, the study aims to examine circulating immune signatures that might reflect interactions between pancreatic non-immune and immune cells underlying the pathogenesis of DM in these populations.
METHODS
Subject Samples
This was an IRB-approved, retrospective analysis of patient samples collected from collaborating institutions that are part of the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC), a cooperative agreement grant funded by the National Cancer Institute (NCI) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The participating sites, organizational structure of the CPDPC and its studies can be found at http://cpdpc.mdanderson.org. This study was approved by the CPDPC Steering Committee, and involved 3 member institutions: Stanford University (WGP, AH), University of Pittsburgh (REB, DY, DCW, KS), and the University of Texas MD Anderson Cancer Center (SA, WW, LL).
All samples were provided by the University of Pittsburgh in a blinded fashion to investigators at Stanford. Samples came from the North American Pancreatitis Studies (NAPS2) cohort and the Pancreatic Adenocarcinoma Gene Environment Risk (PAGER) Study. NAPS2 was a series of three consecutive cross-sectional studies that enrolled 1,195 patients with CP, 568 patients with RAP, and 1107 controls from 27 centers across the United States from 2000–2014. Detailed methodology for NAPS2 has been published previously.(21–23) All samples provided by the PAGER study consists of histologically-confirmed pancreatic adenocarcinoma from subjects undergoing care at the University of Pittsburgh Medical Center (UPMC) by any of the different medical and surgical disciplines involved in the care of malignant pancreatic diseases under an IRB-approved protocol. Samples were collected and frozen to −80°C within 4 hours and were only thawed once for testing. Non-diseased controls (i.e. patients without pancreatic disorders but possibly with other diseases that reflect the general population) were also enrolled at the University of Pittsburgh to support both the NAPS2 and PAGER studies. Biospecimens were collected prior to any treatment including chemotherapy or surgery and processed following the standard operating procedures of the Early Detection Research Network (EDRN). Patients with RAP had 2 or more episodes of documented acute pancreatitis without structural evidence of definite CP. Acute pancreatitis was diagnosed by meeting 2 of 3 clinical criteria: elevated lipase or amylase level greater than 3 times the upper limit of normal, abdominal pain consistent with pancreatic pain, or imaging evidence of acute pancreatitis. CP was defined by definitive changes on cross-sectional imaging, endoscopic ultrasound or histology. Diagnosis of DM was self-reported by controls, and in patients with CP was based on assessment by the enrolling physician. Patients with PDAC had biopsy-proven disease.
A total of 179 patients’ serum samples were included in this analysis (Table 1). From the NAPS2 and PAGER studies, samples were deliberately chosen to create a cohort that included non-disease controls (n = 41), and patients with CP (n=40), PDAC patients (n=78) and RAP patients (n=20). Within these groups DM was present in 20 controls, and 20 patients with CP, and 58 with PDAC. To minimize the impact of demographic variables on immune profiling results, only samples from Caucasians were selected. We tried to create a similar distribution of gender. Once these variables were set, samples were selected randomly from the available repository.
Table 1.
Patient characteristics by disease groups. Continuous variables are summarized by mean, standard deviation (SD), median, and interquartile range (IQR). Categorical variables are summarized by frequency and proportion. The p-values were from one-way ANOVA tests of the mean difference among the 4 groups for continuous variables, and from Fisher’s exact tests of the proportions among the 4 groups for categorical variables. Missing data were excluded from testing.
| Characteristic | Control (N=41) | RAP (N=20) | CP (N=40) | PDAC (N=78) | p-Value | |
|---|---|---|---|---|---|---|
| Age | N (n-Missing) | 41 (0) | 20 (0) | 40 (0) | 78 (0) | <0.0001 |
| Mean (SD) | 58.4 (10.9) | 52.5 (5.8) | 53.6 (9.4) | 68.4 (9.4) | ||
| Median (IQR) | 59 (49,65) | 53.5 (48,58) | 56 (47.5,60) | 68 (63,75) | ||
| Gender | Male | 22 (53.7) | 12 (60) | 24 (60) | 43 (55.1) | 0.9261 |
| Female | 19 (46.3) | 8 (40) | 16 (40) | 35 (44.9) | ||
| BMI | N (n-Missing) | 30 (11) | 20 (0) | 40 (0) | 0 (78) | 0.0509 |
| Mean (SD) | 29.2 (5.5) | 27.8 (4.9) | 25.8 (6.3) | |||
| Median (IQR) | 29.2 (26.4,31.6) | 27.8 (23.5,30.8) | 24.3 (21.8,27.9) | |||
| DM Present | Yes | 20 (48.8) | 0 (0) | 20 (50) | 58 (74.4) | 0.0052 |
| No | 21 (51.2) | 0 (0) | 20 (50) | 20 (25.6) | ||
| Missing | 0 (0) | 20 (100) | 0 (0) | 0 (0) | ||
| Current Smoking | Yes | 7 (17.1) | 6 (30) | 19 (47.5) | 14 (17.9) | 0.0037 |
| No | 34 (82.9) | 14 (70) | 21 (52.5) | 64 (82.1) | ||
| Ever Alcohol | Yes | 8 (19.5) | 17 (85) | 33 (82.5) | 48 (61.5) | 0.0228 |
| No | 5 (12.2) | 3 (15) | 6 (15) | 30 (38.5) | ||
| Missing | 28 (68.3) | 0 (0) | 1 (2.5) | 0 (0) |
A total of 100 microliters of serum from each subject was provided to investigators at Stanford in a blinded fashion. The results from the assay (see below) were then transmitted to investigators at MD Anderson. Investigators at the University of Pittsburgh then unblinded the samples to investigators at MD Anderson to complete the statistical analysis. Clinical and demographic variables including age, body mass index (BMI), gender, race, smoking history, alcohol history, DM status, and tumor location (for PDAC samples) were included.
Luminex Assay
The assay was performed in the Human Immune Monitoring Center at Stanford University (http://iti.stanford.edu/himc/protocols.html). Human 62-plex kits were purchased from Affymetrix and used according to the manufacturer’s recommendations along with the most updated technical report at our institution for processing and analyzing data from these kits.(24) In brief, 50 microliter samples were mixed with antibody-linked polystyrene beads on 96-well filter-bottom plates and incubated at room temperature for 2 hours followed by overnight incubation at 4°C. Room temperature incubation steps were performed on an orbital shaker at 500–600 rpm. Plates were vacuum filtered and washed twice, then incubated with biotinylated detection antibody for 2 hours at room temperature. Samples were then filtered and washed twice as above and resuspended in streptavidin-PE. After incubation for 40 minutes at room temperature, two additional vacuum washes were performed, and the samples resuspended in Reading Buffer. Each sample was run in duplicate to ensure technical reproducibility. Plates were read using a Luminex 200 instrument. Median fluorescence intensity, as an estimate of analyte concentration, was used to compare expression in each clinical group. The luminex assay includes the following 62 cytokines/chemokines/adhesion molecules: interleukins (IL; 1α, 1β, 1RΑ, 2, 4, 5, 6, 7, 8, 9, 10, 12p40, 12p70, 13, 15, 17A, 17F, 18, 21, 22, 27, 23, 31), BDNF, CD40L, EGF, ENA78, Eotaxin, FASL, FGFβ, GCSF, GMCSF, GROA, HGF, ICAM1, IFNα, IFNβ, IFNγ, IP10, LIF, Leptin, MCP1, MCP3, MCSF, MIG, MIP-1α, MIP-1β, PDGFβ, NGF, Rantes, Resistin, PAI1, SCF, SDF-1α, TGFα, TGFβ, TNFα, TNFβ, TRAIL, VCAM1, VEGF, VEGFD (Supplementary Table 1).
Statistical Analysis
The primary analysis of this study was to identify candidate biomarkers (proteins) that are differentially present between 4 disease groups: non-disease controls (subjects with no known pancreatic disease), RAP, CP, and PDAC. As a pilot study, a power calculation for sample size was not indicated and therefore not performed. Standard descriptive statistics were performed to summarize the clinical characteristics of patients by disease groups. Group differences were assessed using ANOVA test of means for continuous variables and Fisher’s Exact test for categorical variables. The overall difference in mean biomarker levels were compared among the four disease groups using one-way ANOVA. Log transformation was used to reduce the skewness of biomarkers and to make the modeling assumption plausible when necessary. Biomarkers with ANOVA p-values below the false discovery rate (FDR) cutoff of 5% were flagged as having statistically significantly different mean levels in the 4 disease group analysis; whereas FDR cutoff of 20% was used in the remaining analyses. The FDR cutoff was obtained by applying the Beta-Uniform Mixture (BUM) method to the ANOVA p-values.(25)
Next, all pairwise comparisons among the four groups (i.e. 6 possible pairs) were performed on each biomarker in the ‘short list’ identified from the aforementioned ANOVA analysis involving all four disease groups. Tukey adjustment was made to correct for family-wise error rate (FWER) in the multiple comparisons. The significant biomarkers identified in each pairwise comparison were further combined into a single biomarker score by a linear combination, which is determined by a logistic regression with the multiple biomarkers as covariates and the disease group indicator as the outcome. The ability of this combined biomarker score to discriminate disease groups was assessed by the ROC curve and area under the ROC curve (AUC).
Two 3-group comparisons were made to identify differentially expressed proteins based on clinical context. The first comparison focused on controls vs. RAP vs. CP to assess unique analytes to mark disease progression toward CP. The second comparison focused on controls vs. CP vs. PDAC to assess unique analytes to identify PDAC from patients with CP. A secondary analysis focusing on evaluating differences in candidate biomarkers based on DM status was performed to determine whether potential candidate biomarkers were a primary biomarker for DM rather than the disease group. All tests were two-sided and p-values that passed a priori clinically-defined FDR thresholds were considered as statistically significant. Statistical analysis was carried out using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.4 (R Foundation, Vienna, Austria).
RESULTS
Table 1 presents descriptive statistics of subject characteristics by disease groups including non-diseased controls. Despite fixing race and gender distribution, PDAC patients were older and had higher rates of DM. CP patients had lower BMI and higher tobacco use while patients with either RAP or CP had higher alcohol consumption rates. Twenty-four out of 62 candidate biomarkers were found to be differentially expressed between the four disease groups (Table 2). In comparing patients with PDAC, CP, and controls, 11 cytokines were differentially expressed: GM-CSF, Leptin, IL-31, Resistin, PDGFB, FASL, IFNβ, IL-17F, IL-27, TGFα, and TNFβ. In comparing patients with CP, RAP, and controls, 18 cytokines were differentially expressed: Resistin, Leptin, GM-CSF, IL-1β, IFNγ, IL-31, PDGFB, IL-27, TNFα, SCF, MIP1α, IFNβ, RANTES, IL-17F, IL-6, FASL, IP-10, and IL-13. Pairwise comparisons were performed on each of the 24 candidate biomarkers with Tukey adjustment for multiple comparisons. Detailed results of these comparisons are in Table 2 and Figure 1. A unique immune signature panel could discriminate each disease group (RAP, CP, PDAC) from controls with an AUC range from 0.77 – 0.86 (95% CI range: 0.64 – 0.94). Additionally, a unique immune signature panel could discriminate RAP from CP, and CP from PDAC with an AUC of 0.77 (95% CI 0.64 – 0.90) and 0.76 (95% CI 0.67 – 0.86), respectively. Further details of these comparisons are described.
Table 2.
Comparisons of candidate biomarkers among different disease groups. Listed are the biomarkers that were found to be significantly differently expressed. The AUC corresponds to the combined score formed by linear combination of the significant biomarkers.
| Comparison of interest | # Significantly differentially expressed biomarkers | AUC (95% CI) |
|---|---|---|
| Controls vs. RAP vs. CP vs. PDAC | 24 – RESISTIN, LEPTIN, GM-CSF, IL-1β, IFNγ, IL-31, PDGFB, IL-27, TNFα, SCF, MIP-1α, IFNβ, RANTES, IL-17F, IL-6, FASL, IP-10, IL-13, TNFβ, HGF, MIG, IL-12P40, TGFα, NGF | Not Significant |
| Controls vs. CP vs. PDAC | 11 – GM-CSF, LEPTIN, IL-31, RESISTIN, PDGFB, FASL, IFNβ, IL-17F, IL-27, TGFα, TNFβ | Not Significant |
| Controls vs. RAP vs. CP | 18 – RESISTIN, LEPTIN, GM-CSF, IL-1β, IFNγ, IL-31, PDGFB, IL-27, TNFα, SCF, MIP-1α, IFNβ, RANTES, IL-17F, IL-6, FASL, IP-10, IL-13 | Not Significant |
| RAP vs. Controls | 6 – IFN γ, IL-13, IL-1β, IL-27, IL-31, and FASL | 0.77 (0.64 – 0.90) |
| CP vs. Controls | 5 – GM-CSF, IFNβ, LEPTIN, PDGFB, and RESISTIN | 0.86 (0.77 – 0.94) |
| PDAC vs. Controls | 4 – GM-CSF, IL-31, LEPTIN, and FASL | 0.77 (0.68 – 0.85) |
| CP vs. RAP | 4 – RESISTIN, SCF, MIP-1α, and IL-17F | 0.77 (0.64 – 0.90) |
| PDAC vs. RAP | 5 – HGF, IL-12P40, MIG, SCF, and TNFα | 0.77 (0.66 – 0.89) |
| PDAC vs. CP | 3 – IFNβ, IL-17F and PDGFB | 0.76 (0.67 – 0.86) |
| Disease Subgroup Analysis | ||
| Controls with DM vs. no DM | 4 – IL-1α, SDF1A, RESISTIN, and VEGF | 0.85 (0.72 – 0.97) |
| DM Subgroup Analysis | ||
| DM Controls vs. CP with DM vs. PDAC with DM | 9 – RESISTIN, RANTES, GM-CSF, IL-31, IL-1α, PDGFB, SDF1A, FASL, and ICAM1 | Not Significant |
| CP with DM vs. DM Controls | 5 – GM-CSF, IL-1α, RANTES, RESISTIN, and SDF1A | 0.90 (0.81 – 0.99) |
| PDAC with DM vs. DM Controls | 6 – GM-CSF, IL-31, RANTES, RESISTIN, FASL, and ICAM1 | 0.96 (0.93 – 1.00) |
| PDAC with DM vs. CP with DM | 1 – PDGFB | 0.69 (0.56 – 0.82) |
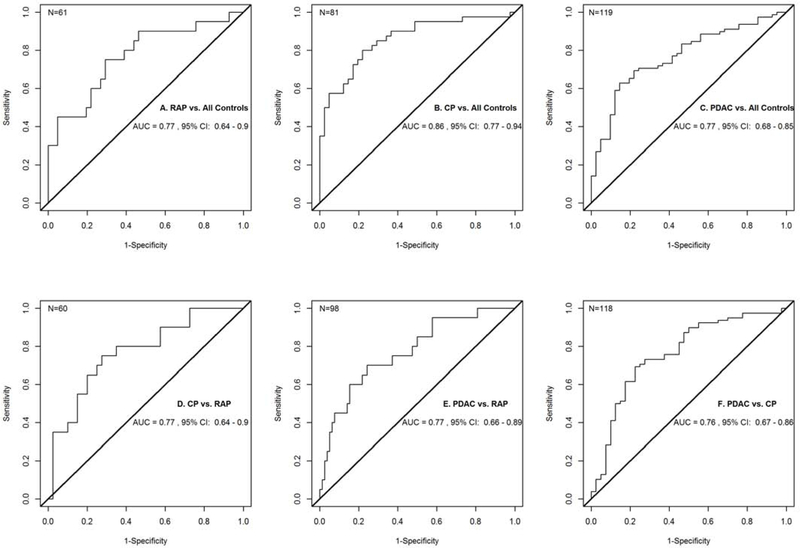
Figure 1.
Receiver Operator Characteristic Curves for A. RAP vs. Controls (IFNγ, IL-13, IL-1β, IL-27, IL-31, and FASL), B. CP vs. Controls (GM-CSF, IFNβ, Leptin, PDGFB, and Resistin), C. PDAC vs. Controls (GM-CSF, IL-31, Leptin, and FASL), D. CP vs. RAP (Resistin, SCF, MIP-1α, and IL-17F), E. PDAC vs. RAP (HGF, IL-12P40, MIG, SCF, and TNFα), and F. PDAC vs. CP (IFNβ, IL-17F, and PDGFB).
RAP vs. Controls
Six cytokines were differentially expressed between RAP and controls. These included IFNγ, IL-13, IL-1β, IL-27, IL-31, and FASL. All of these cytokines were relatively decreased in patients with RAP when compared with controls (Table 3). Together, the combined performance of these analytes differentiated RAP from controls with an AUC of 0.77 (95% CI: 0.64–0.90) (Figure 1A).
Table 3.
Detailed results on biomarkers that are significantly differentially expressed in the pairwise comparisons. The differential expression is quantified by mean change in the biomarkers.
| Comparison | Biomarker | Mean Fluorescence Intensity (MFI) Difference (A) - (B) (95% CI) | Tukey Adjusted p-Value |
|---|---|---|---|
| RAP (N = 20) (A) vs. Controls (N= 41) (B) | |||
| IFNγ | −0.39 (−0.72 – −0.05) | 0.016 | |
| IL-13 | −0.37 (−0.72 – −0.02) | 0.037 | |
| IL-1β | −0.36 (−0.68 – −0.04) | 0.022 | |
| IL-27 | −0.38 (−0.71 – −0.04) | 0.020 | |
| IL-31 | −0.37 (−0.69 – −0.06) | 0.011 | |
| FASL | −0.37 (−0.73 – −0.01) | 0.043 | |
| CP (N=40) (A) vs. Controls (N=41) (B) | |||
| GM-CSF | −0.78 (−1.35 – −0.22) | 0.003 | |
| IFNβ | 0.60 (0.00 – 1.20) | 0.048 | |
| Leptin | −1.36 (−2.38 – −0.34) | 0.004 | |
| PDGFB | −0.58 (−1.08 – −0.08) | 0.017 | |
| Resistin | 0.90 (0.22 – 1.59) | 0.004 | |
| PDAC (N=78) (A) vs. Controls (N=41) (B) | |||
| GM-CSF | −0.73 (−1.22 – −0.24) | 0.001 | |
| IL-31 | −0.29 (−0.51 – −0.07) | 0.004 | |
| Leptin | −0.96 (−1.85 – −0.08) | 0.027 | |
| FASL | −0.30 (−0.56 – −0.04) | 0.015 | |
| CP (N=40) (A) vs. RAP (N=20) (B) | |||
| Resistin | 1.05 (0.21 – 1.90) | 0.007 | |
| SCF | 0.38 (0.03 – 0.73) | 0.027 | |
| MIP-1α | 0.66 (0.03 – 1.30) | 0.035 | |
| IL-17F | 0.50 (0.01 – 0.99) | 0.045 | |
| PDAC (N=78) (A) vs. RAP (N=20) (B) | |||
| HGF | 0.45 (0.07 – 0.83) | 0.014 | |
| SCF | 0.36 (0.04 – 0.68) | 0.019 | |
| IL-12P40 | 0.49 (0.06 – 0.91) | 0.017 | |
| MIG | 0.54 (0.09 – 0.99) | 0.012 | |
| TNFα | 0.19 (0.01 – 0.38) | 0.032 | |
| PDAC (N=78) (A) vs CP (N=40) (B) | |||
| IFNβ | −0.54 (−1.06 – −0.02) | 0.040 | |
| IL-17F | −0.39 (−0.74 – −0.04) | 0.023 | |
| PDGFB | 0.46 (0.02 – 0.90) | 0.036 | |
| Disease Subgroup Analysis | |||
| Controls with DM (N=20) (A) vs. Controls without DM (N=21) (B) | |||
| IL-1α | −0.57 (−0.92 – −0.21) | 0.003 | |
| SDF1A | −0.46 (−0.75 – −0.16) | 0.003 | |
| Resistin | −0.82 (−1.36 – −0.27) | 0.004 | |
| VEGF | −0.47 (−0.81 – −0.12) | 0.009 | |
| DM Subgroup Analysis | |||
| CP with DM (N=20) (A) vs. DM Controls (N=20) (B) | |||
| GM-CSF | −0.91 (−1.63 – −0.17) | 0.012 | |
| Resistin | 1.39 (0.50 – 2.29) | 0.001 | |
| IL-1α | 0.56 (0.07 – 1.06) | 0.020 | |
| RANTES | 0.61 (0.16 – 1.06) | 0.005 | |
| SDF1A | 0.50 (0.05 – 0.94) | 0.027 | |
| PDAC with DM (N=20) (A) vs. DM Controls (N=20) (B) | |||
| GM-CSF | −0.83 (−1.43 – −0.22) | 0.004 | |
| IL-31 | −0.34 (−0.60 – −0.08) | 0.007 | |
| FASL | −0.33 (−0.63 – −0.04) | 0.022 | |
| RANTES | 0.50 (0.13 – 0.87) | 0.005 | |
| Resistin | 0.79 (0.06 – 1.53) | 0.031 | |
| ICAM1 | 0.70 (0.07 – 1.33) | 0.027 | |
| PDAC with DM (N=20) (A) vs. CP with DM (N=20) (B) | |||
| PDGFB | 0.57 (0.05 – 1.09) | 0.026 |
CP vs. Controls
Five cytokines were differentially expressed between CP and controls. These included GM-CSF, IFNβ, Leptin, PDGFB, and Resistin. GM-CSF, Leptin, and PDGFB were relatively decreased in patients with CP when compared with controls, while Resistin and IFNβ were relatively increased in CP patients when compared with controls (Table 3). Together, the combined performance of these analytes differentiated CP from controls with an AUC of 0.86 (95% CI: 0.77 – 0.94) (Figure 1B).
PDAC vs. Controls
Four cytokines were differentially expressed between PDAC and controls. These included GM-CSF, IL-31, Leptin, and FASL. All 4 of these analytes were relatively decreased in patients with PDAC when compared with controls (Table 3). Together, the combined performance of these analytes differentiated PDAC from controls with an AUC of 0.77 (95% CI: 0.68 – 0.85) (Figure 1C).
CP vs. RAP
Four cytokines were differentially expressed between CP and RAP. These included Resistin, SCF, MIP-1α, and IL-17F. All 4 of these analytes were relatively increased in patients with CP when compared with patients with RAP (Table 3). Together, the combined performance of these analytes differentiated CP from RAP with an AUC of 0.77 (95% CI: 0.64 – 0.90) (Figure 1D).
PDAC vs. RAP
Five cytokines were differentially expressed between PDAC and RAP. These included HGF, IL-12P40, MIG, SCF, and TNFα. All 5 cytokines were relatively increased in patients with PDAC compared to patients with RAP (Table 3). Together, the combined performance of these analytes differentiated PDAC from RAP with an AUC of 0.77 (95% CI: 0.66 – 0.89) (Figure 1E).
PDAC vs. CP
Three cytokines were differentially expressed between PDAC and CP. These included IFNβ, IL-17F, and PDGFB. IL-17F and IFNβ were relatively decreased in patients with PDAC when compared with patients with CP while PDGFB was relatively increased in PDAC patients compared to CP patients (Table 3). Together, the combined performance of these analytes differentiated PDAC from CP with an AUC of 0.76 (95% CI: 0.67 – 0.86) (Figure 1F).
DM Status within Same Disease Groups:
To evaluate whether DM influences the cytokine results, CP with DM (n=20) was compared to CP without DM (n=20), however no cytokines were differentially expressed. Similarly, PDAC with DM (n= 58) was compared to PDAC without DM (n=20) and no cytokines were differentially expressed. However, among controls with DM (n= 20) and no DM (n=21), 4 cytokines were differentially expressed: IL-1α, SDF1A, Resistin, and VEGF. All 4 cytokines were relatively decreased in controls with DM patients compared to no DM patients (Table 3). Together, the combined analytes diagnosed DM from no DM in non-diseased controls with an AUC of 0.85 (95% CI: 0.72 – 0.97).
DM Status between Different Disease Groups:
Differences in cytokine expression among those with DM were observed between CP and controls, PDAC and controls, and PDAC vs CP. An analysis of these groups was performed to see whether the differences persisted despite DM status. Nine candidate biomarkers were differentially expressed between the 3 DM groups (Table 2 and 3), of which 5 were similar to the comparison of the 3 groups overall (RESISTIN, GM-CSF, IL-31, PDGFB, and FASL).
CP with DM vs DM controls
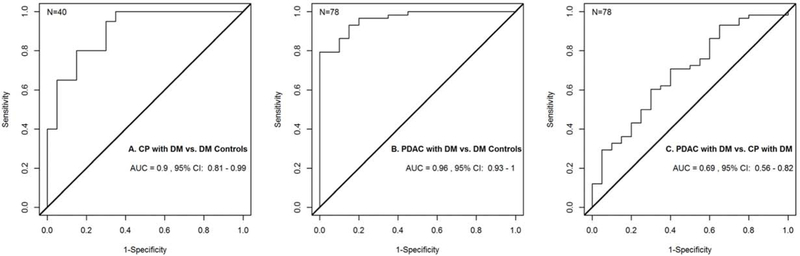
Five cytokines were differentially expressed between these 2 groups (Table 2). These included GM-CSF, IL-1α, RANTES, Resistin, and SDF1A. IL-1α, RANTES, Resistin, and SDF1A were relatively increased in CP with DM when compared with DM controls, while GM-CSF was relatively decreased in CP with DM compared to DM controls (Table 3). Together, the combined performance of these cytokines differentiated CP with DM from DM controls with an AUC of 0.90 (95% CI: 0.81 – 0.99) (Figure 2A).
Figure 2.
Receiver Operator Characteristic Curves for A. CP with DM vs. DM Controls (GM-CSF, IL-1α, RANTES, Resistin, and SDF1A), B. PDAC with DM vs DM Controls (GM-CSF, IL-31, RANTES, Resistin, FASL, and ICAM1), and C. PDAC with DM vs. CP with DM (PDGFB).
PDAC with DM vs. DM controls
Six cytokines were differentially expressed between these 2 groups (Table 2). These included GM-CSF, IL-31, RANTES, Resistin, FASL, and ICAM1. RANTES, Resistin and ICAM1 were relatively increased in PDAC with DM when compared with DM controls, while GM-CSF, IL-31 and FASL were relatively decreased in PDAC with DM when compared with DM controls (Table 3). Together, the combined performance of these cytokines differentiated PDAC with DM from DM controls with an AUC of 0.96 (95% CI: 0.93 – 1.00) (Figure 2B).
PDAC with DM vs. CP with DM
Only one cytokine, PDGFB, was differentially expressed between these 2 groups (Table 2). PDGFB was relatively increased in PDAC with DM when compared with CP with DM (Table 3). The performance of this cytokine differentiated PDAC with DM from CP with DM with an AUC of 0.69 (95% CI: 0.56 – 0.82) (Figure 2C).
Sensitivity Analysis
Our preliminary analysis (data not shown) identified one outlier among the controls. To assess whether this sample impacts the result, we conducted a sensitivity analysis by excluding this sample. This resulted in 27 candidate biomarkers being identified as having different means among the 4 disease groups. Twenty-two of the 24 biomarkers previously identified were still present. The 2 biomarkers no longer identified to be uniquely expressed in one of the 4 groups were IL-1β, and IL-6. Five additional biomarkers that were uniquely expressed in one of the 4 groups were identified in this sensitivity analysis: IL-18, ICAM-1, IL-1α, IFNα, and IL-4.
DISCUSSION
In this pilot study, 179 serum samples collected from rigorous multi-center protocols to ensure reliable, well-defined phenotypes was studied to identify a unique immune signature for each disease state. This was performed by in depth immunophenotyping using a relatively easy well-validated 62-multiplex assay. A panel of cytokines and chemokines were identified that were uniquely expressed to each of the 3 different disease groups. These observations have potential clinical utility in diagnosing early CP and PDAC particularly when not visible by conventional cross-sectional imaging and predicting disease progression among RAP and CP patients. In addition, they have potential to lead to novel hypotheses of mechanisms involved in disease etiology, progression, and prognosis.
Progression from RAP to CP
The current clinical paradigm for the evolution of CP involves repetitive inflammatory insults followed by fibrosis.(26) As this process is primarily governed by well-defined immunological mechanisms, it is rational to attempt to characterize and describe the progression of CP by the unique presence or absence of immune mediators. Interactions between pancreatic non-immune and immune cells mediated by the circulating cytokines and chemokines may also underlie the pathogenesis of CP complications. The presence of an immune signature (pattern of detectable cytokines) therefore, may have clinical utility in classifying disease state and predicting disease progression.
Earlier pre-clinical animal studies have demonstrated that AP and CP lead to distinct and opposing macrophage polarization and responses. Progression to CP is associated with up regulation of monocyte/macrophage chemoattractants and an increase in alternatively activated macrophages (M2, CD206hi and IL-10+). In these pancreata, infiltrating CD206 expressing (M2) macrophages localize in proximity to PSCs and interact via macrophage IL-4R-PSC Th2 (IL-4, IL-13) cytokine signaling suggesting a cross-talk between these cells that contribute to disease progression.(14) CP progression was associated with macrophage, Th2, and fibrosis associated cytokine profiles.
In this study, 6 cytokines were differentially expressed in patients with a history of RAP when compared to controls (IFNγ, IL-13, IL-1β, IL-27, IL-31, and FASL). These cytokines were relatively suppressed for patients with RAP. Many are mediators of inflammation suggesting that RAP may represent a heterogenous population of patients with acute inflammatory states that have not yet progressed to CP (suppressed Th2 or Th2 related cytokines: IL-13, IL-27, IL-31). Yet RAP might include patients on their way to CP, or with underlying subclinical CP, thus combination of immune profiles between the two states might be present. For example, IFNγ and FASL might be expected to increase in AP where significant acinar cell death is expected.
In patients with CP, 5 cytokines were differentially expressed when compared to controls (GM-CSF, IFNβ, Leptin, PDGFB, and Resistin). Interestingly, IFNβ was increasingly expressed in CP patients suggesting increase cytokine release by fibroblasts or PSCs or of cytokines known to activate fibroblasts or PSCs. PDGFB was decreased in CP compared to controls in this study, which differs from our previous observations that alternatively activated macrophages (M2) generate PDGFB in experimental CP and induces PSCs to up regulate extracellular matrix genes such as fibronectin and collagen 1A1.(16) However, our previous study assessed PDGFB expression within the pancreas and since macrophages by definition are not circulating cells, it is possible that PDGFB expression in the tissue do not correlate with those in circulation.
From RAP to CP, there were 4 cytokines (Resistin, SCF, MIP-1α, and IL-17F) that were increasingly expressed in CP patients compared to RAP patients. The increase in MIP-1α and IL-17F are consistent with our preclinical published (16) and unpublished (in press) results. These may represent a potential immune signature for CP.
Progression from CP to PDAC
Immunophenotyping may enable early detection of patients at risk for developing complications such as PDAC. Chronic inflammation has long been known to lead to neoplasia, and CP is also a known risk factor for PDAC development.(7) A small study reported higher levels of circulating TNFα, IL-6, and IL-1 in PDAC as compared to CP.(18) PDAC is associated with an extensive stromal reaction, presumably from PSC (or cancer associated fibroblasts) activation, along with an extensive presence of tumor-associated macrophages in an immunosuppressive environment. Thus, similar immune pathways investigated in CP might also be relevant in PDAC.
In this study, PDAC had 4 cytokines (GM-CSF, IL-31, Leptin, and FASL) that were decreased when compared to controls. Compared to RAP, 5 cytokines (HGF, IL-12P40, MIG, SCF, and TNFα) were increasingly expressed among PDAC patients. Compared to CP, PDAC had 3 candidate biomarkers (IFNβ, IL-17F, and PDGFB) that were differentially expressed in circulation. PDGFB was expressed at higher levels in PDAC patients which is a cytokine mediator that activates PSCs and serves as mitogenic cytokine for myofibroblasts. If validated, these cytokine signatures may help diagnose PDAC in CP patients.
DM immunophenotype in CP and PDAC
DM can be a long-term sequelae of CP. New onset DM is also risk factor for PDAC development and may be a paraneoplastic phenomenon for early PDAC.(9, 27–30) In this study, we evaluated for differences in immune profiling by DM status among controls, CP, and PDAC. Among controls, 4 cytokines were differentially expressed between controls with DM compared to non-DM controls (IL-1α, SDF1A, Resistin, VEGF) while none were identified among CP patients with and without DM and PDAC patients with and without DM. These results suggest that there are no identifiable analytes in our panel that could distinguish the DM contribution within the pancreatic disease groups. Alternatively, these results may fail to show a difference as a result of our sample size or due to limitations in our study where the DM diagnosis was self-reported
We identified 5 analytes that were differentially expressed between CP and controls with DM (GM-CSF, IL-1α, RANTES, Resistin, and SDF1A), and 6 analytes that were differentially expressed between PDAC and controls with DM (GM-CSF, IL-31, RANTES, Resistin, FASL, and ICAM1. RANTES, Resistin and ICAM1). The latter analysis is of particular interest given that distinguishing DM due to PDAC in the setting of new onset DM is a significant research focus of the CPDPC.(29) Our study suggests that this panel of 6 cytokines can differentiate these potential populations with an AUC of 0.96 (95% CI: 0.93 – 1.00). Finally, PDGFB was differentially expressed between CP and PDAC with DM. While there was some overlap in analytes when comparing groups that included both non-DM and the DM subgroups, the DM only analysis identified other analytes that were not observed in the analysis between control patients with and without DM. This suggests that some of these analytes are uniquely expressed due to an interaction of their pancreatic disease and DM status.
An attractive aspect about our approach is that it is non-invasive and consistent with current understandings of the role of the immune system in these chronic inflammatory states. Blood is an easily accessible biomarker with minimal harm to the patient. Serum cytokines as potential biomarkers in predicting development of severe AP(31)(30)(29)(28)(28)(25) or discriminating pancreatic cancer from benign pancreatic disease have been reported and showed promising leads.(18, 31–33) The approach used in this study is relatively more comprehensive and the samples assessed were derived from multiple centers and assayed in a blinded fashion.
There are clear limitations to this study. This is a relatively small pilot study that lacks independent external validation. Further, the small sample size per group makes internal cross-validation through bootstrapping or k-fold cross-validation approaches infeasible. There may be confounding variables that influence the current results including the observation that despite fixing for race, PDAC patients were older and had higher rates of DM. In addition, cytokine concentrations are estimated using median fluorescence intensity. Quantitative estimation for promising markers will require standard ELISA testing. An independent validation study using prospectively collected biospecimens within the CPDPC is planned. Clinical heterogeneity that exists within the groups such as number of RAP episodes, duration of CP, and stage of PDAC, and timing of sample collection to disease activity, may be critical in determining the immune signature, and this would not be adequately captured in this study. Our study also attempted to look at DM status and its impact on the RAP, CP, and PDAC immune signatures. There are 3 types of DM and it was not possible to specify which type existed in each sample likely lending to some heterogeneity of the results. Further, the presence of DM in controls was self-reported. Finally, by studying multiple analytes, levels of expression may seem contradictory between different clinical groups. This may reflect limitations of the accuracy of the groups chosen and the quality of the samples or perhaps reflect a novel observation that may challenge current paradigms associated with each disease group. These factors likely impacted our results in combination with using a small sample size.
This study, however, represents the first line of inquiry from a multi-center collaboration within the CPDPC consortium that is developing several prospective well-defined large patient cohorts in CP and PDAC. This study identifies promising cytokine biomarkers that can be further validated in these prospective cohorts, which will overcome many of the limitations in this study and opens up a new framework to classify pancreatic disease and predict disease progression. In conclusion, we demonstrate the feasibility and potential of developing unique immune signatures for RAP, CP, and PDAC to serve as diagnostic and predictive non-invasive biomarkers.
Supplementary Material
Acknowledgements:
Contributors to NAPS2 (Stuart Sherman, MD, Indiana University School of Medicine, Indianapolis IN; Samer Al-Kaade, MD, Saint Louis University School of Medicine, St. Louis MO; Timothy B. Gardner, MD, Dartmouth-Hitchcock Medical Center, Lebanon NH Michele D. Lewis, MD; Mayo Clinic, Jacksonville FL; Christopher E. Forsmark, MD; University of Florida College of Medicine, Gainesville FL; Joseph Romagnuolo MD MSc, Medical University of South Carolina, Charleston, SC; Darwin L. Conwell, MD, Peter A. Banks, MD; Brigham & Women’s Hospital, Boston, MA), Stanford Human Immune Monitoring Core, Stanford Diabetes Research Center.
Funding support:
Research reported in this publication was supported by National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers U01DK108306 (DCW, DY), DK061451 (DCW) and DK077906 (DY), and U01CA200466 (REB), P30 CA016672 (SA, LL), U01DK108300 (WGP, AH), U01CA21002002 (WGP), 1U01DK108320 (SJH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement:
DCW serves as a consultant for AbbVie, Regeneron, Ariel Precision Medicine, is a cofounder of Ariel Precision Medicine and may have equity. REB receives research support from Freenome and Immunovia. WGP serves as an advisory board member for Abbvie, Ariel Precision Medicine, Alnylam, Akcea Therapeutics, Interpace, and as a consultant to Recro Pharma. He receives research support from Kangan Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011;106(12):2192–9. [DOI] [PubMed] [Google Scholar]
- 2.Sellers ZM, MacIsaac D, Yu H, Dehghan M, Zhang KY, Bensen R, et al. Nationwide Trends in Acute and Chronic Pancreatitis Among Privately Insured Children and Non-Elderly Adults in the United States, 2007–2014. Gastroenterology. 2018;155(2):469–78 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez-Munoz JE. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency: when is it indicated, what is the goal and how to do it? Adv Med Sci. 2011;56(1):1–5. [DOI] [PubMed] [Google Scholar]
- 4.Rickels MR, Bellin M, Toledo FG, Robertson RP, Andersen DK, Chari ST, et al. Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatology. 2013;13(4):336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drewes AM, Bouwense SAW, Campbell CM, Ceyhan GO, Delhaye M, Demir IE, et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17(5):720–31. [DOI] [PubMed] [Google Scholar]
- 6.Andersen DK, Andren-Sandberg A, Duell EJ, Goggins M, Korc M, Petersen GM, et al. Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas. 2013;42(8):1227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112(9):1366–72. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas. 2013;42(2):198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16(2):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144(6):1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellin MD, Whitcomb DC, Abberbock J, Sherman S, Sandhu BS, Gardner TB, et al. Patient and Disease Characteristics Associated With the Presence of Diabetes Mellitus in Adults With Chronic Pancreatitis in the United States. Am J Gastroenterol. 2017;112(9):1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117(1):50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habtezion A, Kwan R, Akhtar E, Wanaski SP, Collins SD, Wong RJ, et al. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut. 2011;60(5):671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143(6):1670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X, Wang L, Elm MS, Gabazadeh D, Diorio GJ, Eagon PK, et al. Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats. Am J Pathol. 2005;166(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dima SO, Tanase C, Albulescu R, Herlea V, Chivu-Economescu M, Purnichescu-Purtan R, et al. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012;41(7):1001–7. [DOI] [PubMed] [Google Scholar]
- 19.Rasch S, Valantiene I, Mickevicius A, Beer S, Rosendahl J, Charnley RM, et al. Chronic pancreatitis: Do serum biomarkers provide an association with an inflammageing phenotype? Pancreatology. 2016;16(5):708–14. [DOI] [PubMed] [Google Scholar]
- 20.Padoan A, Plebani M, Basso D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int J Mol Sci. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, Anderson MA, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008;8(4–5):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox CM, Yadav D, Ye T, Gardner TB, Gelrud A, Sandhu BS, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol. 2015;13(3):552–60; quiz e28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox CM, Sandhu BS, Singh V, Gelrud A, Abberbock JN, Sherman S, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol. 2016;111(10):1488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes TH. General Utility Regression Model for Cytokine Assay, v. 7-July-2017. Technical Report of the Human Immune Monitoring Center, Institute for Immunity, Transplantation and Infection, Stanford University School of Medicine; 2017. [Google Scholar]
- 25.Pounds S, Morris SW. Estimating the Occurrence of False Positives and False Negatives in Microarray Studies by Approximating and Partitioning the Empirical Distribution of P-Values. Bioinformatics. 2003;19(10):1236–42. [DOI] [PubMed] [Google Scholar]
- 26.Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, et al. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal G, Rabe KG, Petersen GM, Chari ST. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology. 2012;12(2):156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitra A, Sharma A, Brand RE, Van Den Eeden SK, Fisher WE, Hart PA, et al. A Prospective Study to Establish a New-Onset Diabetes Cohort: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47(10):1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nature reviews Gastroenterology & hepatology. 2013;10(7):423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieminen A, Maksimow M, Mentula P, Kyhala L, Kylanpaa L, Puolakkainen P, et al. Circulating cytokines in predicting development of severe acute pancreatitis. Crit Care. 2014;18(3):R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dambrauskas Z, Giese N, Gulbinas A, Giese T, Berberat PO, Pundzius J, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16(15):1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw VE, Lane B, Jenkinson C, Cox T, Greenhalf W, Halloran CM, et al. Serum cytokine biomarker panels for discriminating pancreatic cancer from benign pancreatic disease. Mol Cancer. 2014;13:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.