Abstract
Two core features of depression include depressed mood (heightened distress) and anhedonia (reduced pleasure). Despite their centrality to depression, studies have not examined their contribution to treatment outcomes in a randomized clinical trial providing mainstream treatments like antidepressant medications (ADM) and cognitive therapy (CT). We used baseline distress and anhedonia derived from a factor analysis of the Mood and Anxiety Symptom Questionnaire to predict remission and recovery in 433 individuals with recurrent/chronic major depressive disorder. Patients were provided with only ADM or both ADM and CT. Overall, higher baseline distress and anhedonia predicted longer times to remission within one year and recovery within three years. When controlling for treatment condition, distress improved prediction of outcomes over and above anhedonia, while anhedonia did not improve prediction of outcomes over and above distress. Interactions with treatment condition demonstrated that individuals with higher distress and anhedonia benefited from receiving CT in addition to ADM, whereas there was no added benefit of CT for individuals with lower distress and anhedonia. Assessing distress and anhedonia prior to treatment may help select patients who will benefit most from CT in addition to ADM. For the treatments and outcome measures tested, utilizing distress to guide treatment planning may yield the greatest benefit.
Trial registration:
clinicaltrials.gov Identifier:
Keywords: Distress, Anhedonia, MASQ, Depression, Treatment outcome
The two main features of depression, reflected in its core symptoms, are depressed mood and anhedonia (American Psychiatric Association, 2013). Depressed mood involves feelings of sadness, hopelessness, and distress (increased negative emotions), while anhedonia involves a loss of interest or pleasure in usual activities (decreased positive emotions). Numerous psychometric and neurobiological studies have found that these features are separable and that both contribute to the expression of the disorder (Paulus et al., 2017; Watson, 2009). While most current treatments focus on lowering depressed patients’ distress, there has recently been a surge of interest in better understanding anhedonia and designing treatments to lift patients’ positive emotions (Craske et al., 2019; Geschwind, Arntz, Bannink, & Peeters, 2019).
Studies have found evidence for a relationship between both increased distress (Barlow et al., 2014; Mulder, 2002) and increased anhedonia (Downar et al., 2014; McMakin et al., 2012; Uher et al., 2012) at the start of treatment and smaller reductions in symptoms following treatment. Additionally, researchers have observed that higher neuroticism and lower extraversion, personality constructs related to distress and anhedonia, predict increased future symptoms of depression (Brown, 2007; Brown & Barlow, 2009; Naragon-Gainey et al., 2013). Most of these studies, however, did not compare distress and anhedonia as predictors of treatment outcome (Barlow et al., 2014; Downar et al., 2014; Mulder, 2002), and when both were included, they were among a variety of clinical characteristics studied (McMakin et al., 2012; Uher et al., 2012). Distress and anhedonia were examined using subsets of items from global depression scales instead of measures developed to assess these constructs with strong psychometric properties. Furthermore, distress and anhedonia were not examined in the context of mainstream treatments like standalone trials of antidepressant medications with or without psychotherapy, thereby limiting the applicability of the studies’ findings. These studies also examined treatment outcomes only up to six months, leaving open the question of whether distress and anhedonia predict longer-term treatment outcomes. Finally, some studies were naturalistic or open-label trials (Barlow et al., 2014; Downar et al., 2014; Mulder, 2002; Uher et al., 2012) open to the biases associated with these designs, like a lack of careful treatment monitoring or the inability to compare a treatment to another treatment or control group.
Understanding the role of distress and anhedonia in predicting treatment outcomes has both practical and theoretical implications. The most commonly utilized treatment for depression is antidepressant medication (Hollon et al., 2005). Cognitive therapy is an equally effective treatment, and is the most extensively tested form of psychotherapy for depression (Cuijpers et al., 2016; DeRubeis, Siegle, & Hollon, 2008). The relative impact of distress and anhedonia on outcomes for these mainstream treatments may clarify the utility of assessing one or both of these features prior to treatment to help with treatment planning. As depression is a recurrent disorder with high rates of relapse following treatment, understanding the role of these features in predicting long-term treatment outcomes is particularly crucial (DeRubeis et al., 2008). Furthermore, recent efforts to advance research on depression have focused on examining disrupted dimensions of functioning that may be targeted in treatment (Insel, 2014). Two of the dimensions under study, the Negative and Positive Valence Systems Domains, correspond to distress and anhedonia. Better understanding the relationships of distress and anhedonia to treatment outcomes may shed light on the benefits of studying these dimensions in relation to depression.
Additionally, while studies have found that combining medications with cognitive therapy tends to improve outcomes, it is more resource-intensive to administer therapy (Hollon et al., 1992; Keller et al., 2000; Kocsis et al., 2009). Some research has shown that combined therapy produces better outcomes only for those with more severe depression (Hollon et al., 2014; Thase et al., 1997). Understanding whether higher levels of distress and/or anhedonia predict better oucomes for combined therapy than for medications alone may clarify which of these features can be utilized to identify individuals who require additional resources to treat their symptoms.
The Present Study
To address these gaps, we used baseline measures of distress and anhedonia derived from the Mood and Anxiety Symptoms Questionnaire (MASQ; Watson & Clark, 1991) to predict treatment outcomes for depressed individuals in a randomized clinical trial. The MASQ, which assesses distress and anhedonia as distinguishable symptom dimensions, is particularly well suited to investigating these questions.
The clinical trial analyzed was appropriate for several reasons. First, treatment was offered for up to three years, allowing for the assessment of long-term treatment outcomes. Second, the trial included only participants with recurrent or chronic depression, thereby focusing on the patients for whom both short- and long-term treatment outcomes are particularly crucial (Bockting et al., 2015). Third, participants were treated with several classes of antidepressants using best clinical practice, and half of patients also received manualized cognitive therapy. Therefore, all participants were provided with gold standard, evidence-based treatment. Additionally, it was possible to examine outcomes for individuals given only antidepressant medications versus both medications and cognitive therapy. Importantly, the primary analyses for this trial found that individuals with more severe and non-chronic depression benefited from adding cognitive therapy to medication, whereas those with less severe and chronic depression did not (Hollon et al., 2014).
Method
Participants
Adults seeking treatment were recruited from clinics at the University of Pennsylvania (Philadelphia), Rush Medical Center (Chicago, Illinois), and Vanderbilt University (Nashville, Tennessee). All participants met criteria for recurrent (two or more episodes) or chronic (episode lasting more than two years) DSM-IV major depressive disorder (MDD) with a Hamilton Depression Rating Scale (HDRS) score of 14 or higher. All participants provided informed consent and the Institutional Review Boards at all sites approved the study.
Of the 452 participants who began the study, 10 did not begin treatment and were excluded from the longitudinal analyses. Of the 442 remaining participants, 6 did not have baseline MASQ scores and 3 had over 25% of MASQ items missing (see below for details) and were therefore excluded. The final sample included 433 participants: half in the antidepressant-only group (ADM; n = 216) and half in the combined group given both antidepressant medications and cognitive therapy (COM; n = 217). As factor analyses used only baseline data, we included all 443 participants with baseline MASQ scores. See Table 1 for demographic characteristics.
Table 1.
Demographic and Clinical Characteristics by Treatment Condition
| Demographic Characteristics | Antidepressant only group (n = 217) | Combined antidepressant and cognitive therapy group (n = 216) |
|---|---|---|
| Age | 43.32 (13.35) | 43.41 (12.69) |
| % Female | 60% | 56% |
| HDRS score | 22.32 (4.44) | 21.91 (3.96) |
| MASQ distress | 74.79 (19.42) | 77.23 (17.99) |
| MASQ anhedonia | 94.36 (11.66) | 95.65 (11.34) |
| MASQ somatic | 50.08 (16.80) | 48.42 (15.43) |
| Median time to remission (days) | 136 | 141 |
| Median time to recovery (days) | 332 | 254 |
Note. Results represent Mean (SD). MASQ = Mood and Anxiety Symptom Questionnaire. HDRS = Hamilton Depression Rating Scale.
Design
Participants were randomly assigned to one of the two groups described above. Randomization was stratified by sex, marital status, symptom severity, recurrence, chronicity, and comorbid Axis II disorders. In the acute phase, participants were treated until they met criteria for remission (4 consecutive weeks of minimal symptoms), and in the continuation phase, participants were treated until they met criteria for recovery (another 26 consecutive weeks without relapse). When participants relapsed during continuation treatment, they were required to meet remission criteria again before being eligible to meet criteria for recovery. Treatment was offered for a maximum of 42 months.
Pharmacotherapy used a principle-based algorithm that involved up to four classes of antidepressants and the use of any augmenting agents commonly used in clinical practice. The study goal was to provide personalized antidepressant therapy using best clinical practice. Cognitive therapy followed the procedures in the original treatment manual (Beck, Rush, Shaw, & Emery, 1979). Treatment was augmented when indicated only for participants with comorbid Axis II disorders (Beck, Freeman, & Davis, 2003). For a full study summary, see Hollon et al. (2014).
Measures
The Mood and Anxiety Symptom Questionnaire (MASQ).
The MASQ, completed at baseline, is a 90-item measure developed to differentiate among clusters of depression and anxiety symptoms. Items are rated on a 5-point scale ranging from “not at all” to “extremely” and describe symptoms that have been experienced over the last week.
MASQ scores were imputed at the item level for the three participants with less than 25% of responses missing (Schweizer et al., in prep). Scores were imputed using random forest-based imputation implemented in R with the package “missForest” (Stekhoven, 2013). This method generates a single imputed dataset, thereby circumventing the need to run analyses across multiple datasets, and has been found to outperform other imputation methods (Shah et al., 2014).
MASQ factor analyses.
The factor structure of the MASQ has been extensively debated. All models have found evidence for a three-factor solution consisting of distress symptoms (common to depression and anxiety), anhedonia symptoms (specific to depression), and somatic symptoms (specific to anxiety), but the items considered markers of these factors differ across studies. Following initial analyses (Watson et al., 1995a and Watson et al., 1995b), Watson et al. argued that the negatively worded items referencing loss of interest should be included in the depression-specific subscale, which consists of positively worded items describing high levels of positive emotions. Later studies found that these loss of interest items loaded primarily on the distress factor or were eliminated altogether (Bedford, 1997; Keogh & Reidy, 2000; see also Kendall et al., 2016). Additionally, while the original scale includes somatic symptoms relevant to both depression and anxiety (e.g., “felt nauseous”) in the distress subscale, subsequent factor analyses (Bedford, 1997; Keogh & Reidy, 2000; see also Watson et al., 1995b) supported the inclusion of these items on the somatic factor.
In our sample, the original distress and anhedonia subscales were highly correlated (r = .66; Watson & Clark, 1991), and there was a particularly strong relationship among distress and loss of interest items (r = .78). As our analyses centered on differences between the MASQ subscales, we wanted to ensure that these subscales had strong psychometric properties in our unique sample of treatment-seeking individuals with recurrent or chronic depression. We therefore ran exploratory and confirmatory factor analyses to arrive at three subscales accurately representing the relationship among items in our sample. These internally consistent subscales represented distress, anhedonia, and somatic symptoms (α = .92–.94), were less strongly inter-correlated than the original subscales (r’s = .10-.47) and were in line with the factors identified by Keogh and Reidy (2000) and Bedford (1997) rather than the original subscales (Watson & Clark, 1991). For details about these analyses and subscales, see Supplemental Item 1 and Supplemental Table 1.
The Hamilton Depression Rating Scale (HDRS).
The 17-item HDRS (Hamilton, 1960) was used to assess depression severity. Trained interviewers, blind to treatment condition, administered the measure at intake, biweekly through week 4, every 4 weeks through week 20 of the acute phase, and every 8 weeks thereafter until the conclusion of the study. Interrater reliability, established through re-ratings of recorded evaluations, was high (ICC = .96).
Treatment outcome variables.
Remission and recovery criteria have previously been described (Hollon et al., 2014) and were based on both HDRS scores and scores on the Longitudinal Interval Follow-up Evaluation (LIFE; Keller et al., 1987). Remission was defined as HDRS scores of 8 or less and LIFE ratings of 2 or less for 4 consecutive weeks; after 12 months, criteria were relaxed and partial remission was met with 4 weeks of HDRS scores of 12 or less and LIFE ratings of 3 or less. Relapse was defined as having HDRS scores of 16 or more or LIFE scores of 5 or more for 2 consecutive weeks. Recovery was achieved when participants met criteria for remission and then did not relapse for another 26 consecutive weeks. The first outcome measure, time to remission within the first year of the study, was chosen because remission criteria were relaxed after 12 months. The second outcome measure, time to recovery within three years, was chosen to represent treatment outcome across the duration of the study.
Data Analysis
To test overall treatment outcome, we first ran analyses controlling for treatment condition. We then tested differential treatment outcome by examining the interaction of scores with treatment condition. Given our primary interest in MASQ distress and anhedonia, we tested these subscales as predictors of outcomes separately and when controlling for one another. As we were interested in distress and anhedonia both as symptom dimensions and also as constructs separate from symptom severity, we present results with and without controls for baseline depression severity. To provide context for analyses focusing on distress and anhedonia, we also tested somatic symptoms as a predictor of outcomes.
Cox regression (survival) analyses and all follow-up analyses were performed in R 3.6.1 (R Core Team, 2016) using the ‘survival’ package (Therneau, 2015). Wald tests were employed to assess the statistical significance of individual predictors in regression models. We used ANOVAS comparing nested models with likelihood ratio tests to determine which variables significantly improved the prediction of outcomes. In line with norms for survival analyses, patients who dropped out before the end of the observation period were censored (Cox & Oakes, 1984). All scales were standardized before analysis such that the coefficients provided are standardized.
Results
Baseline Differences and Correlations
There were no differences in baseline MASQ variables or HDRS depending on study group, showing that randomization was successful (all t’s < 1.36, all p’s > .175; see Table 1). Distress was strongly related to anhedonia (r = .46, p < .001) and somatic symptoms (r = .47, p < .001), while anhedonia was weakly related to somatic symptoms (r = .10, p = .035). HDRS scores at intake were moderately related to the MASQ subscales (r’s = .24-.43, all p < .001). Time to remission and recovery were strongly related, but differentiable (r = .55, p < .001).
Predicting Treatment Outcomes
Overall treatment outcome.
Higher baseline distress predicted longer times to remission within one year and to recovery within three years, even when controlling for baseline depression severity (Table 2, top1). Model comparisons showed that depression severity did not significantly improve the prediction of times to remission or recovery (both X2 ‘s for distress < 2.07, both p’s for distress > .151). Higher baseline anhedonia also predicted longer times to remission within one year and recovery within three years, even when controlling for baseline depression severity. By contrast, these model comparisons showed that depression severity did significantly improve the prediction of time to remission (X2 for anhedonia = 4.81, p = .028), and trended towards improving the prediction of time to recovery (X2 for anhedonia = 2.96, p = .086).
Table 2.
MASQ Subscales Predicting Treatment Outcome
| Controlling for treatment condition | Time to remission within 1 year | Time to recovery within 3 years | ||||||
|---|---|---|---|---|---|---|---|---|
| β | p value | HR | NNT | β | p value | HR | NNT | |
| Only MASQ subscale | ||||||||
| Distress | −.33 | <.001 | 0.72 | 6.13 | −.33 | <.001 | 0.72 | 6.07 |
| Anhedonia | −.23 | <.001 | 0.79 | 8.68 | −.20 | <.001 | 0.82 | 10.09 |
| Somatic | −.20 | .002 | 0.82 | 10.12 | −.16 | .008 | 0.86 | 12.77 |
| Controlling for baseline HDRS | ||||||||
| Distress | −.30 | <.001 | 0.74 | 6.65 | −.32 | <.001 | 0.73 | 6.36 |
| Anhedonia | −.20 | <.001 | 0.81 | 10.00 | −.17 | .002 | 0.84 | 11.66 |
| Somatic | −.15 | .034 | 0.86 | 13.70 | −.11 | .086 | 0.89 | 17.78 |
| Controlling for other MASQ subscales | ||||||||
| Distress (cont. for anhedonia) | −.28 | <.001 | 0.75 | 7.15 | −.30 | <.001 | 0.73 | 6.16 |
| Anhedonia (cont. for distress) | −.09 | .163 | 0.91 | 21.68 | −.05 | .392 | 0.95 | 37.39 |
| Additional controls | ||||||||
| Distress (cont. for baseline HDRS, MASQ anhedonia, and MASQ somatic) | −.23 | .002 | 0.79 | 8.59 | −.29 | <.001 | 0.75 | 6.99 |
| Anhedonia (cont. for baseline HDRS, MASQ distress, and MASQ somatic) | −.10 | .149 | 0.91 | 20.46 | −.05 | .434 | 0.95 | 39.73 |
| Interacting with treatment | Time to remission within 1 year | Time to recovery within 3 years | ||||||
| Only MASQ subscale | β | p value | HR | NNTa | β | p value | HR | NNTa |
| Distress | .26 | .032 | 1.29 | −7.84 | .29 | .014 | 1.33 | −7.04 |
| Anhedonia | .24 | .036 | 1.27 | −8.32 | .30 | .007 | 1.34 | −6.79 |
| Somatic | .04 | .757 | 1.04 | −52.28 | .15 | .211 | 1.16 | −13.64 |
| Controlling for baseline HDRS | ||||||||
| Distress | .26 | .043 | 1.29 | −7.86 | .24 | .048 | 1.27 | −8.32 |
| Anhedonia | .24 | .041 | 1.27 | −8.37 | .27 | .017 | 1.31 | −7.36 |
| Somatic | −.01 | .969 | 0.99 | 369.37 | .06 | .671 | 1.06 | −36.15 |
| Controlling for other MASQ subscales | ||||||||
| Distress (cont. for anhedonia) | .19 | .160 | 1.21 | −10.49 | .19 | .153 | 1.21 | −10.46 |
| Anhedonia (cont. for distress) | .13 | .310 | 1.14 | −14.98 | .19 | .134 | 1.21 | −10.60 |
| Additional controls | ||||||||
| Distress (cont. for baseline HDRS, MASQ anhedonia, and MASQ somatic) | .21 | .185 | 1.23 | −9.72 | .13 | .405 | 1.13 | −15.94 |
| Anhedonia (cont. for baseline HDRS, MASQ distress, and MASQ somatic) | .14 | .313 | 1.15 | −14.54 | .20 | .120 | 1.23 | −9.83 |
Note. MASQ = Mood and Anxiety Symptom Questionnaire. HDRS = Hamilton Depression Rating Scale. β = Standardized regression coefficient; HR = Hazard ratio; NNT = Number needed to treat.
Higher baseline somatic symptoms predicted longer times to remission and recovery. Somatic symptoms continued to predict time to remission, and trended towards predicting time to recovery, when controlling for baseline depression severity. Model comparisons showed that depression severity trended towards improving the prediction of time to remission (X2 for somatic = 3.10, p = .078), but did not significantly improve the prediction of time to recovery (X2 for somatic = 2.40, p = .121).
When both distress and anhedonia were included in the same model so that they controlled for one another, distress continued to predict longer times to remission and recovery, but anhedonia did not. Model comparisons showed that adding distress as a predictor alongside anhedonia improved prediction of times to remission and recovery (both X2’s for distress > 16.47, both p’s for distress < .001), but adding anhedonia alongside distress did not (both X2’s for anhedonia < 1.93, both p’s for anhedonia > .165). Even when controlling for somatic symptoms, baseline depression severity, and anhedonia, distress continued to predict longer times to remission and recovery. By contrast, anhedonia did not continue to predict outcomes when controlling for somatic symptoms, baseline depression severity, and distress.
Differential treatment outcome2.
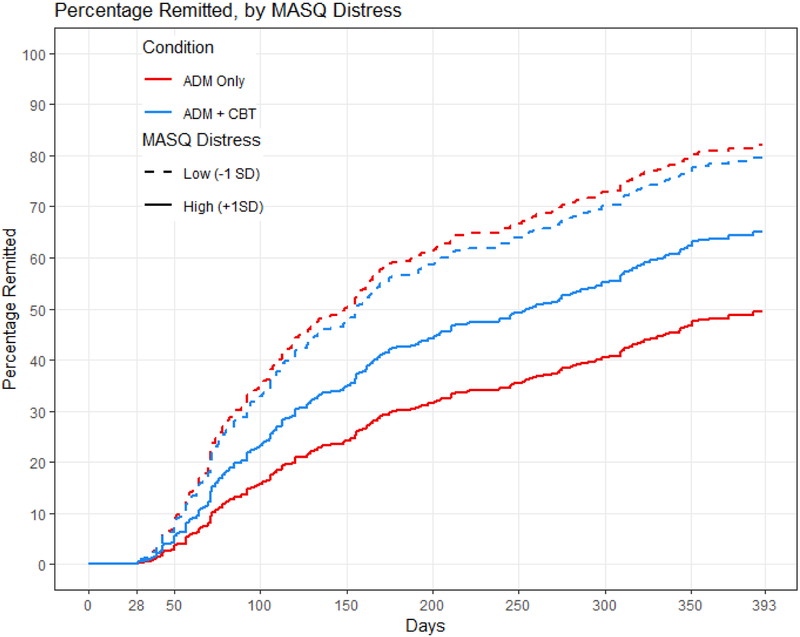
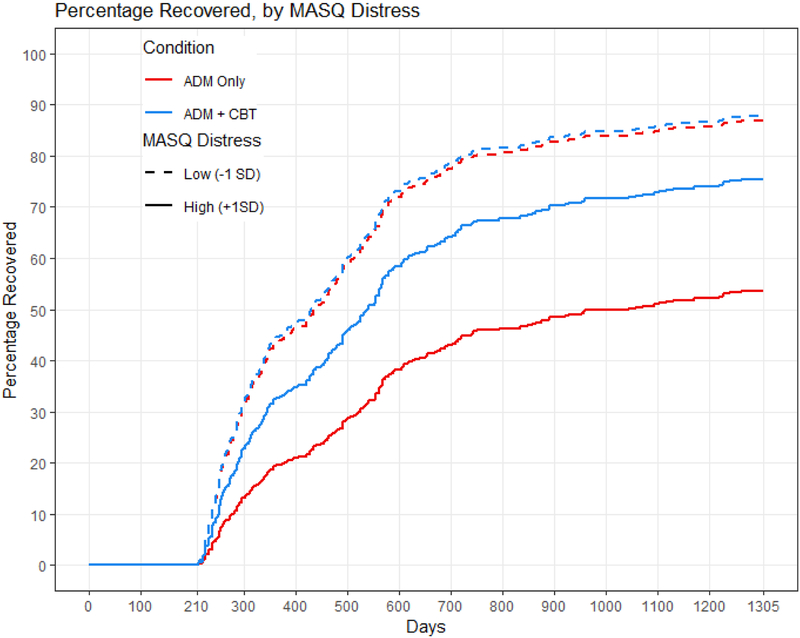
Distress interacted with treatment condition in its prediction of time to remission and recovery, even when controlling for the interaction of condition with baseline depression severity (Table 2, bottom). Model comparisons showed that adding the interaction of depression severity with treatment condition did not significantly improve the prediction of times to remission or recovery (both X2’s for distress < 1.80, both p’s for distress > .407). Treatment outcome was equivalent for individuals with low levels of distress regardless of condition. Individuals with high levels of distress, however, took longer to remit within one year (Figure 1) and to recover within three years (Figure 2) when receiving antidepressant medication only than when cognitive therapy was provided in addition to medication (combined treatment). Calculations of median survival time (Table 3, top) indicated that when receiving antidepressant medication only, individuals with high levels of distress (1 SD higher than the mean) remitted an average of 273 days later, and recovered an average of 609 days later, compared to individuals with low levels of distress (1 SD lower than the mean). When receiving cognitive therapy in addition to medication, individuals with high levels of distress remitted only an average of 100 days later, and recovered only an average of 110 days later, compared to individuals with low levels of distress.
Figure 1.
MASQ distress predicting time to remission within one year. The red lines represent the antidepressant medication (ADM) group, and the blue lines represent the combined group given both antidepressant medications and cognitive therapy. The solid lines represent individuals with MASQ distress scores 1 SD higher than the mean, and the dashed lines represent individuals with MASQ distress scores 1 SD lower than the mean.
Figure 2.
MASQ distress predicting time to recovery within three years. The red lines represent the antidepressant medication (ADM) group, and the blue lines represent the combined group given both antidepressant medications and cognitive therapy. The solid lines represent individuals with MASQ distress scores 1 SD higher than the mean, and the dashed lines represent individuals with MASQ distress scores 1 SD lower than the mean.
Table 3.
Median Survival Time by MASQ Subscale
| MASQ Distress | Time to remission (days) | Time to recovery (days) |
|---|---|---|
| Antidepressant medication group | ||
| Low MASQ distress | 121 | 225 |
| High MASQ distress | 394 | 834 |
| Difference in days | 273 | 609 |
| Combined group (antidepressant medication + cognitive therapy) | ||
| Low MASQ distress | 128 | 219 |
| High MASQ distress | 228 | 329 |
| Difference in days | 100 | 110 |
| MASQ Anhedonia | ||
| Antidepressant medication group | ||
| Low MASQ anhedonia | 128 | 255 |
| High MASQ anhedonia | 316 | 624 |
| Difference in days | 188 | 369 |
| Combined group (antidepressant medication + cognitive therapy) | ||
| Low MASQ anhedonia | 137 | 255 |
| High MASQ anhedonia | 193 | 295 |
| Difference in days | 56 | 40 |
Note. MASQ = Mood and Anxiety Symptom Questionnaire. Low MASQ distress = 1 SD below the mean; High MASQ distress = 1 SD above the mean; Low MASQ anhedonia = 1 SD below the mean; high MAS Anhedonia = 1 SD below the mean.
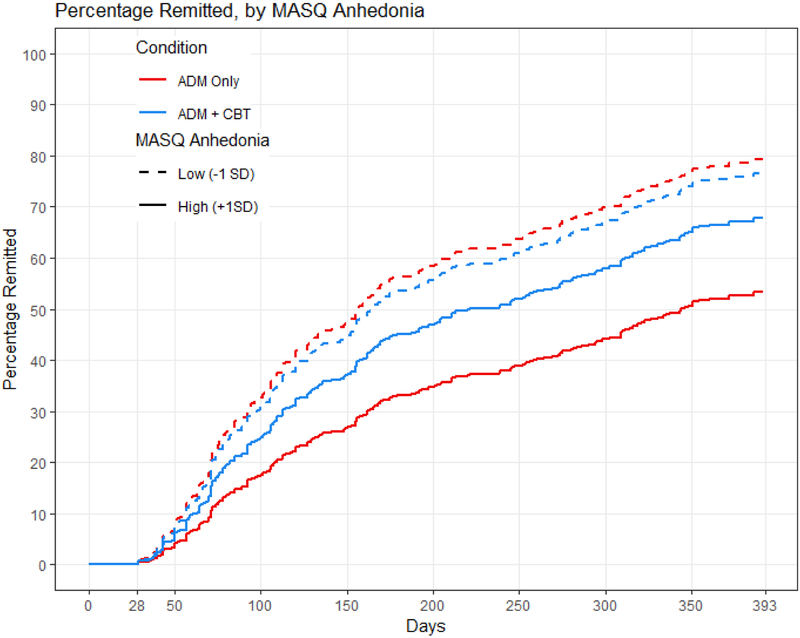
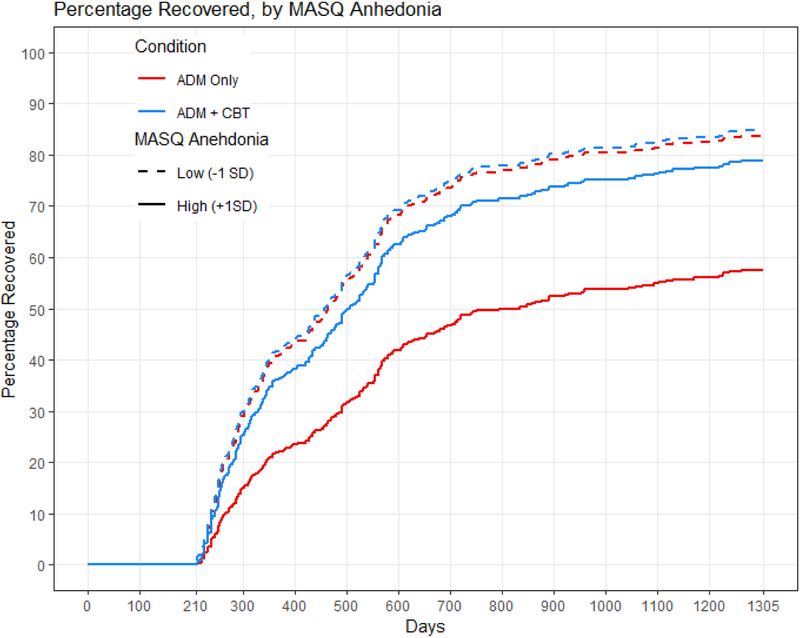
Anhedonia also interacted with treatment condition in its prediction of time to remission and recovery, even when controlling for the interaction of condition with baseline depression severity. Model comparisons showed that adding the interaction of depression severity with treatment condition improved the prediction of time to recovery (X2 for anhedonia = 7.32, p = .026) and trended towards improving the prediction of time to remission (X2 for anhedonia = 5.50, p = .064). Again, treatment outcome was equivalent for individuals with low levels of anhedonia regardless of condition. Individuals with high levels of anhedonia, however, took longer to remit within the first year (Figure 3) and to recover within three years (Figure 4) when receiving only antidepressant medication than when receiving combined treatment. Calculations of median survival time (Table 3, bottom) indicated that when receiving antidepressant medication only, individuals with high levels of anhedonia remitted an average of 188 days later, and recovered an average of 369 days later, compared to individuals with low levels of anhedonia. When receiving cognitive therapy in addition to medication, individuals with high levels of anhedonia remitted only an average of 56 days later, and recovered only an average of 40 days later, compared to individuals with low levels of anhedonia.
Figure 3.
MASQ anhedonia predicting time to remission within one year. The red lines represent the antidepressant medication (ADM) group, and the blue lines represent the combined group given both antidepressant medications and cognitive therapy. The solid lines represent individuals with MASQ anhedonia scores 1 SD higher than the mean, and the dashed lines represent individuals with MASQ anhedonia scores 1 SD lower than the mean.
Figure 4.
MASQ anhedonia predicting time to recovery within three years. The red lines represent the antidepressant medication (ADM) group, and the blue lines represent the combined group given both antidepressant medications and cognitive therapy. The solid lines represent individuals with MASQ anhedonia scores 1 SD higher than the mean, and the dashed lines represent individuals with MASQ anhedonia scores 1 SD lower than the mean.
Somatic symptoms did not interact with treatment condition to predict times to remission or recovery on their own or when controlling for baseline depression severity. Model comparisons showed that adding the interaction of depression severity with treatment condition did not significantly improve the prediction of times to remission or recovery (both X2’s for somatic < 4.52, both p’s for somatic > .104).
When both distress and anhedonia were included in the same model so that they controlled for one another, neither continued to interact with condition to predict times to remission or recovery, likely because the inclusion of these two interactions in one model decreased power. As we were still interested in comparing distress and anhedonia as predictors of differential treatment outcome, we ran model comparisons that should be interpreted with caution given this non-significant finding. The results of these comparisons were consistent with the results for overall treatment outcomes. While the interaction of distress with treatment condition significantly improved prediction of times to remission and recovery with anhedonia already in the model (both X2’s for distress > 18.09, both p’s for distress < .001), the interaction of anhedonia with treatment condition did not significantly improve prediction of times to remission and recovery with distress already in the model (both X2’s for anhedonia < 3.34, both p’s for anhedonia > .189)3.
Discussion
We used distress and anhedonia subscales derived from the MASQ to predict treatment outcomes in 433 individuals with recurrent or chronic depression. When examining overall treatment outcomes, higher baseline distress and anhedonia predicted longer times to remission within one year and recovery within three years over and above baseline depression severity. Interactions with treatment condition showed that individuals with higher distress and anhedonia remitted and recovered faster when receiving cognitive therapy in addition to antidepressant medications (combined treatment). By contrast, individuals with lower distress and anhedonia showed no differences in remission or recovery when cognitive therapy was provided alongside medications. These interactions remained significant when controlling for baseline depression severity. While both distress and anhedonia predicted overall and differential treatment outcome, distress improved the prediction of these outcomes over and above anhedonia, whereas anhedonia did not improve the prediction of these outcomes over and above distress. Finally, higher baseline somatic symptoms predicted time to remission and recovery when considering overall treatment outcomes, although they were a weaker predictor than distress and anhedonia and did not consistently predict outcomes over and above baseline depression severity. Unlike distress and anhedonia, somatic symptoms did not interact with treatment condition to predict remission or recovery.
Ours is the first study to test the interaction of anhedonia and distress with combined treatment versus medications alone. As previously described, some prior research has found that combined therapy results in better outcomes only for those with more severe depression (Hollon et al., 2014; Thase et al., 1997). We extend these findings by showing that both core features of depression predict which individuals benefit most from combined treatment. If replicated, these results suggest that assessing distress and anhedonia prior to treatment may help determine which patients will benefit most from adding cognitive therapy to antidepressant medications. Given the economic burden of treating depression (Greenberg et al., 2015), it is important to understand which features of the disorder can identify individuals for whom spending additional resources on treatment may lead to better outcomes.
Our results also demonstrated that while both distress and anhedonia are important predictors of treatment outcomes, distress was a relatively stronger predictor. Although these findings confirm evidence from some previous studies showing that negative emotional processes predict clinical outcomes more strongly than positive emotional processes (e.g., Conway et al., 2016; Klein et al., 2011; Quilty et al., 2008), other studies have found the opposite (e.g., Geschwind et al., 2011; Gorwood et al., 2015). Importantly, distress may have emerged as a stronger predictor than anhedonia because both the psychotherapy provided and the outcome measures utilized (HDRS and LIFE scores) focused on treating and assessing distress much more so than anhedonia. Our findings might not replicate if applied to newer treatments that focus more explicitly on improving anhedonia and that have shown promising early results (Craske et al., 2019; Dunn et al., 2019; Geschwind et al., 2019), or to outcome measures with a more balanced focus on positive and negative emotional processes. Although HDRS and LIFE scores currently represent the gold-standard approach for measuring depression treatment outcomes, assessments of daily functioning or wellbeing (Keyes, 2005) that focus less heavily on distress may have yielded different findings. Finally, while distress emerged as a relatively stronger predictor than anhedonia when examined as a moderator in this study, we did not show that distress was a stronger mediator of treatment outcome relative to anhedonia.
With these caveats in mind, our findings indicate that for the mainstream depression treatments assessed, prioritizing baseline measures of distress rather than anhedonia to guide treatment planning would yield the greatest benefit. In this context, anhedonia may be a less important predictor of treatment outcomes than previously argued, particularly when compared directly to distress. Additionally, studies addressing Positive Valence Systems processes in depression treatment must also take into account the role of Negative Valence Systems processes.
Our findings diverge from two former studies showing that anhedonia is a stronger predictor of outcomes than distress (McMakin et al., 2012; Uher et al., 2012). Unlike the present study, these studies were limited in that they considered a variety of depression features instead of focusing on anhedonia and distress, measured anhedonia and distress using subsets of items from depression scales not designed to assess these features, and focused only on shorter-term treatment outcomes.
We also found that somatic symptoms predicted overall treatment outcome, although less consistently than distress and anhedonia, and did not predict differential treatment outcome. The somatic symptoms factor is relatively specific to anxiety, and heightened somatic symptoms may indicate the presence of higher levels of comorbid anxiety. Our finding that somatic symptoms predicted overall treatment outcome is consistent with evidence showing that comorbid anxiety is associated with poorer treatment response (Fava, 2003). On the other hand, the comparatively weaker prediction of somatic symptoms relative to anhedonia and distress may be due to the provided treatments’ focus on alleviating symptoms of depression instead of anxiety. Along these lines, individuals with heightened anxiety may not have benefited more from the addition of psychotherapy to medications because while antidepressant medications can be effective for both depression and anxiety (Bandelow et al., 2012), the cognitive therapy provided targeted primarily depression-related cognitions and emotions.
Finally, our MASQ factor solution was in line with the subscales proposed by later studies (Bedford, 1997; Keogh & Reidy, 2000) rather than the original subscales (Watson & Clark, 1991). Our subscales differed from the original ones in two ways. First, loss of interest items loaded on the distress rather than the anhedonia subscale, indicating that they were more related to symptoms of general psychological distress than to the low positive affect symptoms specific to depression. Interestingly, factor analyses of the MASQ have consistently confirmed that the low positive affect items are stronger indicators of the anhedonia factor than the loss of interest items, which contain significant distress-related variance (Bedford, 1997; Kendall et al., 2016; Keogh & Reidy, 2000; Watson et al., 1995b). Second, the somatic factor included all somatic symptoms instead of only anxiety-specific ones. The broadening of the somatic factor to include some symptoms common to depression and anxiety has also been supported by previous factor analyses of the MASQ (Keogh & Reidy, 2000; Watson et al., 1995b). Therefore, careful examination of previous analyses support the factor structure confirmed in the present study and indicate that future use of the MASQ should prioritize this structure.
On the other hand, the particularly high loadings of loss of interest items on the distress factor in our study, as well as the inclusion of all somatic symptoms on the somatic factor instead of on both the somatic and distress factor, may be accounted for by characteristics of our sample. While previous studies have been conducted primarily in non-clinical samples, ours consisted of treatment-seeking individuals with recurrent or chronic depression. These individuals experienced more severe depression symptoms, and likely also more severe symptoms of anxiety and somatic distress (given high rates of comorbidity among these disorders; Maier & Falkai, 1999), than previous samples that may have influenced our findings.
The factor structure utilized in the present analyses therefore consisted of a negatively-worded distress subscale including items related to feelings of distress, pessimism, and lack of interest in enjoyable activities, and a positively-worded anhedonia subscale related to low levels of positive emotions. Anhedonia has been conceptualized as including both low positive emotions when experiencing rewarding stimuli, as well as deficits in the desire to pursue rewarding stimuli (Proceedings, 2011), constructs which have been differentiated from one another (Berridge & Kringelbach, 2008). As our measure included only the first aspect of anhedonia, our findings are limited to this definition of it. Our measure may also reflect broader negative and positive emotional processes rather than more specific behavioral or motivational processes associated with anhedonia and distress. The previous support for the factor structure utilized in the present study, however, raises questions about the definition of these constructs and the boundaries between them, particularly as measured by self-report.
This study should be considered alongside its limitations. First, as it is a secondary analysis of a randomized trial describing analyses that were planned following publication of the trial’s main outcomes, results should be considered exploratory and require replication. Second, the study did not include a therapy-only condition, as all patients received antidepressant medications. Additionally, there were no pill placebo or attention control groups. Therefore, we cannot determine whether patients with elevated distress or anhedonia benefitted from combined treatment specifically, or simply because they were exposed to cognitive therapy, greater therapeutic contact time, or any additional intervention. Third, as previously noted, the cognitive therapy provided and the outcome measures utilized emphasize distress more than anhedonia, and different interventions or measures may have produced different results. Despite these limitations, our study demonstrates that distress and anhedonia are robust predictors of remission and recovery, that individuals with high levels of distress and anhedonia benefit when receiving cognitive therapy alongside medications, and that distress is a relatively stronger predictor than anhedonia.
Supplementary Material
Highlights.
Elevated distress and anhedonia predicted treatment outcomes in depressed persons
Elevations predicted longer times to remission and recovery for up to 3 years
Elevations predicted greater benefit from cognitive therapy in addition to medications
Distress improved predictions of outcomes over and above anhedonia
Anhedonia did not improve predictions of outcomes over and above distress
Funding:
This work was supported by grants MH60713 and MH01697 (K02; Dr. Hollon) and MH60998 (Dr. DeRubeis) from the National Institute of Mental Health, as well as the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs (Dr. Khazanov).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Table 2 includes regression coefficients, hazard ratios, and Numbers Needed to Treat (NNT) to facilitate the interpretation of analyses. For example, higher baseline distress predicted longer times to remission with a standardized regression coefficient of −.33 (p < .001). Given a hazard ratio of .72, having high distress decreased the odds of remitting within 1 year by an average of 28%. An NNT of 6 indicates that for every 6 people with high distress, there was one fewer remission.
The Cox regression proportionality of hazards (PH) assumption was examined using the Schoenfeld residuals test for analyses involving treatment condition. All results aside from one indicated that the PH assumption was not violated (all X2 < .11, all p > .065). When examining somatic symptoms predicting time to remission, treatment condition emerged as potentially violating the PH assumption (X2 = .12, p = .042). Further testing with time-by-covariate interactions and plots of Schoenfeld residuals showed that the variables in this model did not change over time. Therefore, the original result of this analysis was retained in the paper.
We reran analyses using the original MASQ subscales (Watson & Clark, 1991). Results for overall treatment outcome were essentially unchanged. Results for differential treatment outcome were similar, although distress emerged as a less consistent predictor of time to remission and recovery than in the analyses presented. For additional details, see Supplemental Item 2 and Supplemental Table 2.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, (DSM-5): American Psychiatric Publishing. [Google Scholar]
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, … & WFSBP Task Force on Anxiety Disorders, OCD and PTSD. (2012). Guidelines for the pharmacological treatment of anxiety disorders, obsessive–compulsive disorder and posttraumatic stress disorder in primary care. International Journal of Psychiatry in Clinical Practice, 16(2), 77–84. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, & Ellard KK (2014). The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science, 2(3), 344–365. [Google Scholar]
- Beck AT, Freeman A, & Davis DD (2003). Cognitive Therapy of Personality Disorders (2nd ed.). New York, NY: Guilford Publications. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, & Emery G (1979). Cognitive Therapy of Depression. New York NY: Guilford press. [Google Scholar]
- Bedford A (1997). On Clark-Watson’s tripartite model of anxiety and depression. Psychological Reports, 80(1), 125–126. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Kringelbach ML (2008). Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology, 199(3), 457–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockting CL, Hollon SD, Jarrett RB, Kuyken W, & Dobson K (2015). A lifetime approach to major depressive disorder: The contributions of psychological interventions in preventing relapse and recurrence. Clinical Psychology Review, 41, 16–26. [DOI] [PubMed] [Google Scholar]
- Brown TA (2007). Temporal course and structural relationships among dimensions of temperament and DSM-IV anxiety and mood disorder constructs. Journal of Abnormal Psychology, 116(2), 313–328. [DOI] [PubMed] [Google Scholar]
- Brown TA, & Barlow DH (2009). A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: Implications for assessment and treatment. Psychological Assessment, 21(3), 256–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CC, Craske MG, Zinbarg RE, & Mineka S (2016). Pathological personality traits and the naturalistic course of internalizing disorders among high-risk young adults. Depression and Anxiety, 33(1), 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR; Oakes D (1984). Analysis of survival data. New York, NY: Chapman & Hall. [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, & Rosenfield D (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. Journal of Consulting and Clinical Psychology, 87(5), 457–471. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, & Huibers MJ (2016). How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry, 15(3), 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, & Hollon SD (2008). Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nature Reviews Neuroscience, 9(10), 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, … Giacobbe P (2014). Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biological Psychiatry, 76(3), 176–185. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Widnall E, Reed N, Owens C, Campbell J, & Kuyken W (2019). Bringing light into darkness: A multiple baseline mixed methods case series evaluation of Augmented Depression Therapy (ADepT). Behavior Research and Therapy, 120(103418). [DOI] [PubMed] [Google Scholar]
- Fava M (2003). Diagnosis and definition of treatment-resistant depression. Biological Psychiatry, 53(8), 649–659. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Nicolson NA, Peeters F, van Os J, Barge-Schaapveld D, & Wichers M (2011). Early improvement in positive rather than negative emotion predicts remission from depression after pharmacotherapy. European Neuropsychopharmacology, 21(3), 241–247. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Arntz A, Bannink F, & Peeters F (2019). Positive cognitive behavior therapy in the treatment of depression: A randomized order within-subject comparison with traditional cognitive behavior therapy. Behavior Research and Therapy, 116, 119–130. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Demyttenare K, Vaiva G, Corruble E, Llorca PM, Bayle F, & Courtet P (2015). An increase in joy after two weeks is more specific of later antidepressant response than a decrease in sadness. Journal of Affective Disorders, 185, 97–103. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, & Kessler RC (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). Journal of Clinical Psychiatry, 76(2), 155–162. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23(1), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Evans MD, Wiemer MJ, Garvey MJ, Grove WM, & Tuason VB (1992). Cognitive therapy and pharmacotherapy for depression. Singly and in combination. Archives of General Psychiatry, 49(10), 774–781. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Fawcett J, Amsterdam JD, Shelton RC, Zajecka J, … Gallop R (2014). Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: A randomized clinical trial. JAMA Psychiatry, 71(10), 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, & Rush AJ (2005). Psychotherapy and medication in the treatment of adult and geriatric depression: Which monotherapy or combined treatment? Journal of Clinical Psychiatry, 66(4), 455–468. [DOI] [PubMed] [Google Scholar]
- Insel TR (2014). The NIMH Research Domain Criteria (RDoC) Project: Precision medicine for psychiatry. American Journal of Psychiatry, 171(4), 395–397. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, & Andreasen NC (1987). The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry, 44(6), 540–548. [DOI] [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, … Zajecka J (2000). A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. New England Journal of Medicine, 342(20), 1462–1470. [DOI] [PubMed] [Google Scholar]
- Kendall AD, Zinbarg RE, Bobova L, Mineka S, Revelle W, Prenoveau JM, & Craske MG (2016). Measuring positive emotion with the Mood and Anxiety Symptom Questionnaire: Psychometric properties of the anhedonic depression scale. Assessment, 23(1), 86–95. [DOI] [PubMed] [Google Scholar]
- Keogh E, & Reidy J (2000). Exploring the factor structure of the Mood and Anxiety Symptom Questionnaire (MASQ). Journal of Personality Assessment, 74(1), 106–125. [DOI] [PubMed] [Google Scholar]
- Keyes CL (2005). Mental illness and/or mental health? Investigating axioms of the complete state model of health. Journal of Consulting and Clinical Psychology, 73(3), 539–548. [DOI] [PubMed] [Google Scholar]
- Klein DN, Kotov R, & Bufferd SJ (2011). Personality and depression: Explanatory models and review of the evidence. Annual Review of Clinical Psychology, 7, 269–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JH, Gelenberg AJ, Rothbaum BO, Klein DN, Trivedi MH, Manber R, … Investigators, R. (2009). Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: The REVAMP Trial. Archives of General Psychiatry, 66(11), 1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, & Falkai P (1999). The epidemiology of comorbidity between depression, anxiety disorders and somatic diseases. International Clinical Psychopharmacology, 14, S1–6. [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, … Brent DA (2012). Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. Journal of the American Academy of Child & Adolescent Psychiatry, 51(4), 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RT (2002). Personality pathology and treatment outcome in major depression: A review. American Journal of Psychiatry, 159(3), 359–371. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K, Gallagher MW, & Brown TA (2013). Stable “trait” variance of temperament as a predictor of the temporal course of depression and social phobia. Journal of Abnormal Psychology, 122(3), 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB, Craske MG, Bookheimer S, Taylor CT, Simmons AN, … Fan B (2017). Latent variable analysis of positive and negative valence processing focused on symptom and behavioral units of analysis in mood and anxiety disorders. Journal of Affective Disorders, 216, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilty LC, De Fruyt F, Rolland JP, Kennedy SH, Rouillon PF, & Bagby RM (2008). Dimensional personality traits and treatment outcome in patients with major depressive disorder. Journal of Affective Disorders, 108(3), 241–250. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2016). R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- Schweizer S, Cohen ZD, Hayes R, DeRubeis RJ, Crane C, Kuyken W, & Dalgleish T (in prep). Relapse prevention for antidepressant medication (ADM) responders with recurrent depression: Using the Personalized Advantage Index to decide between treatments.
- Shah AD, Bartlett JW, Carpenter J, Nicholas O, & Hemingway H (2014). Comparison of random forest and parametric imputation models for imputing missing data using MICE: A CALIBER study. American Journal of Epidemiology, 179(6), 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekhoven DJ (2013). missForest: Nonparametric Missing Value Imputation using Random Forest. R package version 1.4.
- Thase ME, Greenhouse JB, Frank E, Reynolds CF 3rd, Pilkonis PA, Hurley K, … Kupfer DJ (1997). Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Archives of General Psychiatry, 54(11), 1009–1015. [DOI] [PubMed] [Google Scholar]
- Therneau T (2015). A Package for Survival Analysis in R. version 2.38. Retrieved from https://CRAN.R-project.org/package=survival
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, … McGuffin P (2012). Depression symptom dimensions as predictors of antidepressant treatment outcome: Replicable evidence for interest-activity symptoms. Psychological Medicine, 42(5), 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D (2009). Differentiating the mood and anxiety disorders: A quadripartite model. Annual Review of Clinical Psychology, 5, 221–247. [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark AL (1991). The Mood and Anxiety Symptom Questionnaire (MASQ) Unpublished manuscript, University of Iowa, Iowa City. [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, & McCormick RA (1995a). Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology, 104(1), 15–25. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, & McCormick RA (1995b). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology, 104(1), 3–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.