Significance
The world’s agricultural landscapes have seen dramatic declines in their biodiversity. Because biodiversity responses to land-use changes can be delayed, understanding how current biodiversity is impacted by historical land-use changes is crucial to protect and restore agricultural biodiversity. By utilizing a long-term land-use record database, we show that historical land-use changes continue to shape current biodiversity. Our study expands our understanding of global biodiversity change by identifying land-use history effects to be as strong as the effects of current management and the amount of seminatural land cover, often assumed to be the major drivers of biodiversity change in agricultural landscapes. Accordingly, land-use legacy effects must be considered in management programs that aim to protect and restore biodiversity.
Keywords: agricultural ecosystems, biodiversity loss, functional diversity, grasslands, land-use changes
Abstract
Land-use change is a major driver of biodiversity loss worldwide. Although biodiversity often shows a delayed response to land-use change, previous studies have typically focused on a narrow range of current landscape factors and have largely ignored the role of land-use history in shaping plant and animal communities and their functional characteristics. Here, we used a unique database of 220,000 land-use records to investigate how 20-y of land-use changes have affected functional diversity across multiple trophic groups (primary producers, mutualists, herbivores, invertebrate predators, and vertebrate predators) in 75 grassland fields with a broad range of land-use histories. The effects of land-use history on multitrophic trait diversity were as strong as other drivers known to impact biodiversity, e.g., grassland management and current landscape composition. The diversity of animal mobility and resource-acquisition traits was lower in landscapes where much of the land had been historically converted from grassland to crop. In contrast, functional biodiversity was higher in landscapes containing old permanent grasslands, most likely because they offer a stable and high-quality habitat refuge for species with low mobility and specialized feeding niches. Our study shows that grassland-to-crop conversion has long-lasting impacts on the functional biodiversity of agricultural ecosystems. Accordingly, land-use legacy effects must be considered in conservation programs aiming to protect agricultural biodiversity. In particular, the retention of permanent grassland sanctuaries within intensive landscapes may offset ecological debts.
Habitat destruction caused by land-use change is a major driver of global biodiversity declines (1). While land-use changes often have time-delayed impacts on biodiversity (2, 3), we know very little about the relationship between land-use history and present-day biodiversity. The presence of remnant and alternative habitats in the landscape can buffer biodiversity loss through time, if organisms are able to persist in, and disperse to, these refuges (4). These metapopulation processes can delay or prevent species extinction for years or decades (4), meaning we may have underestimated the impacts of past land-use change on biodiversity. Understanding the long-term response of biodiversity to land-use change is crucial if we are to limit species extinctions and biodiversity decline by implementing sound and timely conservation and restoration efforts.
Species can differ significantly in their responses to land-use changes (5), making it hard to generalize biodiversity response as a whole, and to predict which species and taxa may suffer the most from habitat destruction in the long run. A solution to this problem is the use of species traits, as species sharing similar traits have been shown to respond consistently to land-use change (6, 7). Furthermore, in agricultural landscapes, trait diversity plays an important role in determining the capacity of ecosystems to cope with future environmental changes (8) and secure the provision of key ecosystem services. However, the long-term response of functional diversity to land-use changes is unknown as previous studies investigating the effects of land-use history have focused on species richness (3, 9), ignoring trait diversity. A general understanding of how the trait diversity of multiple trophic groups responds to land-use changes over time may therefore improve our capacity to manage agricultural biodiversity, and secure sustainable long-term agroecosystem functioning.
Here, we investigated how 20-y of past land-use changes affected the functional trait diversity of 7 taxonomic groups in grasslands (vascular plants, wild bees, hoverflies, grasshoppers, carabid beetles, spiders, and birds), belonging to 5 trophic groups (primary producers, pollinators, herbivores, invertebrate, and vertebrate predators) (see for details SI Appendix, Table S1). We used a unique database of 220,000 land-use records in an agricultural region of 430 km2 dominated by annual crop production in western France (11,000 fields recorded annually since 1994, ref. 10) to investigate the effect of multiple aspects of land-use history, operating at the field and landscape level, on functional diversity (SI Appendix, Fig. S1). Historically, the study region was a typical rural area composed of mixed crop-livestock systems. Fifty years ago, grassland was the dominant land use, covering 60% of the study area (10). These grasslands were either grazed or mown for livestock production. Since that time and up to the present day, shifts from livestock to annual crop production resulted in a strong decline in grassland cover and in 2014, grasslands covered only 12% of the area.
Within the study area, we sampled 75 grasslands with a wide range of land-use histories, while controlling for the effects of current landscape composition and the management of the sampled grassland field. Land-use history was assessed by the age of the sampled grassland field (“Field age”) and by 3 independent landscape metrics (SI Appendix, Fig. S1 and Table S2): 1) the time elapsed since the first grassland-to-crop conversion for all fields within a landscape of 1-km radius of the sampled grassland (“Time as cropland”). High value of time as cropland indicates that most grasslands in the landscape were converted into crops long ago; 2) the permanency of the grassland cover in the landscape (“Grassland permanency”). A high value of grassland permanency corresponds in our system to landscapes with old permanent grasslands; 3) the turnover of rotation from crop to grassland in the landscape (“Crop-grassland turnover”). A high value of crop-grassland turnover indicates that sown grasslands are maintained for several years. These 3 metrics complement each other to inform on the loss and the stability of grasslands, and the turnover from crops to grasslands in the landscape.
We focused on a core set of independent organismal traits that mediate species responses to land-use changes (SI Appendix, Fig. S2): body size, mobility (e.g., wing span) and resource-use traits (e.g., mandible strength or beak size). Such traits can directly determine a species’ ability to respond rapidly to land-use intensification, e.g., frequent disturbances and resource homogenization in agricultural landscape may select for species with smaller size, a generalist diet, shorter generation time, and higher dispersal abilities (6, 11). We summarized the functional diversity of the entire trophic chain using multitrophic and multitrait diversity metrics (5). We also investigated how land use affects different facets of functional trait diversity by compiling 3 multitrophic trait diversity indices for mobility, resource-acquisition, and body size traits separately. We controlled for confounding effects of the local species pool (the multitrophic density and species richness, following ref. 5) in our analyses to isolate net effects of land-use changes on functional diversity (see Methods). We hypothesized that landscapes in which grasslands were converted to crops long ago, i.e., landscapes with high values of time as cropland, would support low grassland multitrophic trait diversity by selecting generalist species and reducing the diversity of body size and mobility traits. We further hypothesized that increasing grassland permanency and increasing crop-grassland turnover could mitigate biodiversity loss by providing stable and high-quality habitat to low-mobility organisms and specialist-feeding species.
Results
All models accounted for the influence of multitrophic species richness and density on trait diversity (Fig. 1). Multitrophic trait diversity increased linearly with multitrophic species richness and decreased with multitrophic density (Fig. 2), with the exception of resource-acquisition trait diversity for which multitrophic density had no effect. We also found multitrophic species richness to be more strongly affected by land-use history (43% of explained variance) than by current land use (18% of explained variance) (SI Appendix, Figs. S3 and S4). On the contrary, multitrophic density was mostly driven by current land use (62 vs. 38% of variance explained by land-use history).
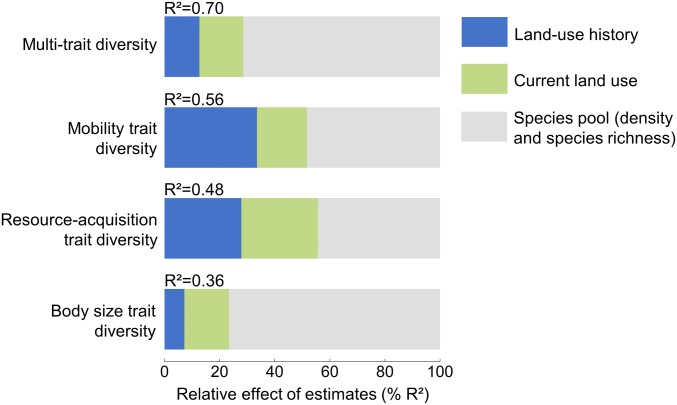
Fig. 1.
Importance of the drivers of multitrait diversity, mobility trait diversity, resource-acquisition trait diversity, and body size trait diversity. Relative effects (% R2), resulting from a model averaging procedure, were calculated for each group of predictors (i.e., land-use history, current land use, and the species pool). All predictors were scaled to interpret parameter estimates on a comparable scale. Note that for mobility, resource-acquisition, and body size trait diversity, we focused on animal traits and excluded plant traits from the analyses. Results were consistent considering spatial scales ranging from 500 to 1500 m radii surrounding the sampled grasslands (SI Appendix, Fig. S8).
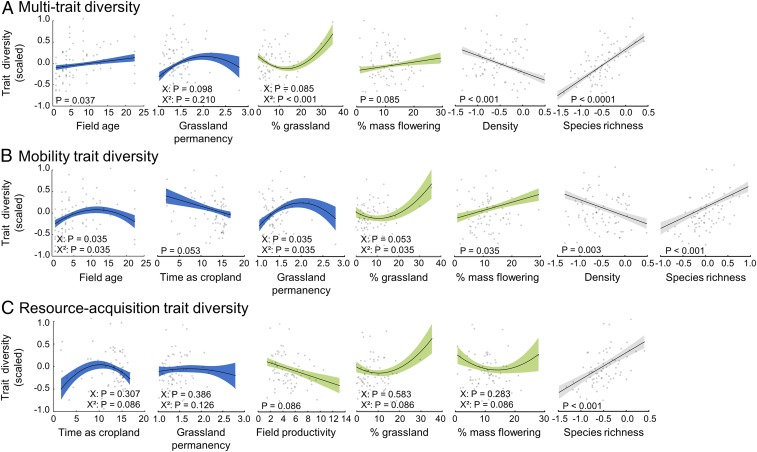
Fig. 2.
Effects of land-use history (field age, time as cropland, and grassland permanency), current land use (field productivity, % grassland, and % mass flowering), and of the species pool (multitrophic density and richness) on (A) multitrait diversity, (B) mobility trait diversity, and (C) resource-acquisition trait diversity. Lines show model fits and shaded areas correspond to the 95% confidence band. Model predictions were calculated using a model averaging procedure. Note that for mobility trait and resource-acquisition trait diversity, we focused on animal traits and excluded plant traits from the analyses. All predictors were scaled to interpret parameter estimates on a comparable scale. P values of the best selected models for each model parameter are given. P values were adjusted following (42) to control for false discovery rates (SI Appendix, Table S7). Gray dots (n = 75) correspond to observed data.
The best-selected model explained 70% of the variance in multitrait diversity (Fig. 1; see also SI Appendix, Table S3). Of this, current land use accounted for 16% of the explained variance (Fig. 1), where multitrait diversity was higher in landscapes with high grassland cover (Fig. 2). Land-use history explained 12% of multitrait diversity, through the effects of grassland field age (5% of the explained variance) and grassland permanency in the landscape (7% of the explained variance) (Fig. 2). These effects were positive: multitrait diversity was higher in fields that had been grasslands for a long time and were surrounded by other permanent grasslands (Fig. 2).
When considering each trait separately, we found that mobility, resource-acquisition, and body size trait diversity responded differently to past and current land use. Land-use history was the main driver of the diversity of mobility traits (34% of explained variance) (Fig. 1). Mobility trait diversity was lower in fields embedded in landscapes converted to cropland long ago (Fig. 2). In contrast, the diversity of mobility traits was higher in old grassland fields and in landscapes in which grassland cover was more permanent. Past and current land use accounted for an equal amount (28%) of explained variance for resource-acquisition trait diversity (Fig. 1). The diversity of resource-use strategies initially increased following grassland-to-cropland conversion in the landscape (during the first 10 y), but subsequently decreased (Fig. 2). Current land use could either mitigate this loss, as resource-acquisition trait diversity increased with present-day grassland cover, or accentuate it, as it decreased with grassland field productivity, a proxy of grassland management intensity, and the amount of mass flowering crops in the landscape. Our model explained less variation in body size diversity, although this did respond to current land use (16% of explained variance) (SI Appendix, Fig. S5).
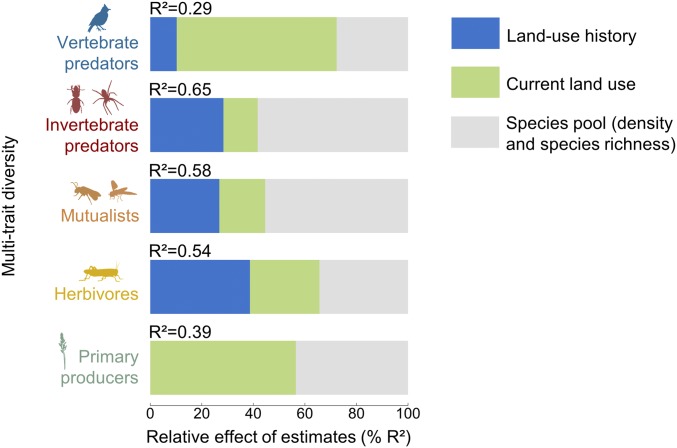
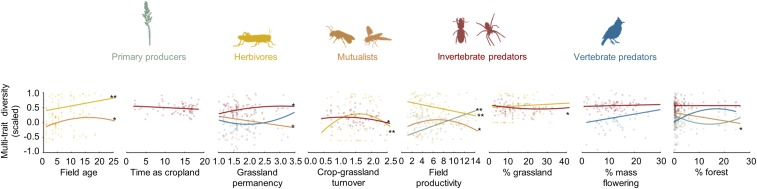
When considering each trophic group separately, plant trait diversity was only impacted by current grassland field productivity (27% of explained variance). For animals, the importance of land-use history decreased with increasing trophic level, while the importance of current landscape-scale predictors increased (Figs. 3 and 4, see also SI Appendix, Table S4 and Fig. S6). Herbivore (i.e., grasshoppers) and mutualist (i.e., wild bees and hoverflies) trait diversity was strongly affected by land-use history (39 and 27% of explained variance, respectively) and was higher in old grassland fields (Fig. 4). Land-use history accounted for 28% of the explained variance in invertebrate predator trait diversity, which increased in landscapes dominated by permanent grasslands. Vertebrate predator trait diversity was mainly driven by current landscape composition, and increased in landscapes with a high cover of mass flowering crops (16% of explained variance) and forest (46% of explained variance).
Fig. 3.
Importance of the drivers of multitrait diversity within the different trophic groups (primary producers, herbivores, mutualists, invertebrate predators, and vertebrate predators). Relative effects (% R2), resulting from the model averaging procedure, were calculated for each group of predictors (i.e., land-use history, current land use, and the species pool). All predictors were scaled to interpret parameter estimates on a comparable scale.
Fig. 4.
Effects of land-use history (field age, time as cropland, grassland permanency, and crop-grassland turnover), and current land use (field productivity, % grassland, % mass flowering, % forest) on the multitrait diversity of each trophic group. Lines show model fits for primary producers (green), herbivores (yellow), mutualists (orange), invertebrate predators (brown), and vertebrate predators (blue). Model predictions were calculated using the model averaging procedure. All predictors were scaled to interpret parameter estimates on a comparable scale. P values of the best selected models for each model parameter are given; *P < 0.05; **P < 0.01. P values were adjusted following (42) to control for false discovery rates (SI Appendix, Table S7). Dots (n = 75) correspond to observed data and colors indicate the trophic group.
Discussion
We investigated how land-use history affects the functional diversity of multiple trophic groups in grasslands. We found that historical land use continues to play an important role in shaping the present-day functional diversity across multiple trophic groups, and that it is of equal importance to well-established land-use drivers of biodiversity in agricultural landscapes, such as grassland management intensity (7) and current landscape composition (6). Ignoring the role of land-use legacies on functional diversity may therefore hinder our ability to predict how biodiversity responds to land-use changes and the functional consequences of biodiversity change on ecosystems.
Multitrait diversity was most strongly influenced by the species pool, i.e., the density of individuals and the taxonomic diversity observed within the sampled grasslands. There was a negative relationship between multitrait diversity and density. High population densities, together with low functional diversity, occur when a small number of functionally similar species benefit from land-use intensification in agricultural landscapes. These few species may benefit from the resources offered by particular crop monocultures and become hyper abundant, e.g., in pest outbreaks (12). However, by increasing functional diversity, we may limit dominance by a few species with potentially negative effects on crops, as suggested by the dilution effect hypothesis (13, 14). We also found that multitrait diversity strongly increased with taxonomic diversity (Fig. 2), highlighting low functional redundancy among species across all of the trophic groups studied. A low functional redundancy implies that species loss may be accompanied by strong declines in functional diversity and uncertain outcomes in agroecosystem functioning. While agricultural landscapes have the potential to host high trait diversity (15), the low functional redundancy observed in our study suggests that the functional biodiversity of agroecosystems is particularly vulnerable to land-use changes and species loss (16, 17).
Decomposing multitrait diversity into 3 independent indices (SI Appendix, Fig. S2) allowed us to investigate the mechanisms through which land-use history alters biodiversity in agricultural landscapes. The multitrophic diversity of mobility and resource-acquisition traits was lower in landscapes that were converted from grassland to cropland long ago (Fig. 2), highlighting that habitat destruction has not only immediate, but also long-lasting negative effects on the functional diversity of agroecosystems. The conversion of permanent grasslands into annual crops may have particularly strong effects on low-mobility organisms and species with narrow feeding niches. However, our results show that maintaining permanent grasslands in the surrounding landscape mitigates this loss (Fig. 2), probably by providing stable, heterogeneous, and resource-rich habitats in which many species can persist in and migrate between. While increasing the quantity of seminatural habitats in the landscape is often viewed as an important method to conserve biodiversity in agricultural landscapes (e.g., ref. 18), our results highlight that increasing the quality of these habitats—through the conservation of old permanent grasslands in the landscape—may be equally important. Finally, we observed that mobility trait diversity has decreased linearly with time since landscape conversion to cropland, while resource-acquisition trait diversity has shown a hump-shaped response: it increased during the first 10 y after grassland-to-crop conversion, but then decreased (Fig. 2). Spatial and temporal disturbances can create a concentration of transient species in remnant habitats, thus locally increasing resource-acquisition trait diversity (18). However, resource-acquisition trait diversity will decrease after some time due to the scarcity of habitats and resources in the landscape (9). Quantifying the duration of this effect may be essential to slow down species loss and delay extinction.
Resource-acquisition trait diversity was also strongly impacted by current land use. Locally, we found a strong negative effect of the grassland field productivity (Fig. 2). High grassland productivity is associated with high rates of fertilization, which decreases plant diversity, and in turn, the availability of feeding niches (19). This has been observed in both experiments and real-world landscapes, and leads to a global homogenization of species resource-use strategies at higher trophic levels (20). In addition, at the landscape scale, the proportion of mass flowering crops (ranging from 0 to 44% in the study area) negatively affected resource-acquisition trait diversity. Mass flowering crops offer short pulses of uniform and homogeneous resources for herbivores and mutualists that may select for particular resource-acquisition traits and therefore reduce their diversity (e.g., ref. 21). As the diversity of resource-acquisition traits drives overall resource utilization (22), the community level homogenization of resource-acquisition traits following land-use intensification could threaten the delivery of essential agroecosystem services such as nutrient recycling (23), pollination (24), and biological control (25).
We did not find clear response of body size to land-use changes (Fig. 1 and SI Appendix, Fig. S5), in contrast to other studies focusing on single trophic groups (e.g., ref. 26). Body size is an integrative trait related to many aspects of animal species physiology and ecology (e.g., metabolism, ref. 11; mobility and dispersal, or else stoichiometry, ref. 27) and may not have the same functional significance across multiple trophic groups (28). As a result, our synthetic index of multitrophic body size may not show a consistent response to land-use changes. This may explain why our analyses predicted less variation in body size trait diversity than they did for mobility or resource-acquisition traits.
We found that past and current land use had contrasting impacts on trait diversity of different trophic groups (Fig. 3). For instance, primary consumers (i.e., herbivores and mutualists) responded more strongly to local history (through the age of the sampled grasslands) while predators were more impacted by past and present-day landscape composition (Fig. 4 and SI Appendix, Table S4). Accordingly, delayed responses to land-use changes could create temporal mismatches between interacting trophic levels, if land-use changes disrupt interacting partners inconsistently. This may lead to the disruption of ecological interactions and further biodiversity loss (29). While plants are expected to experience time-delayed responses to habitat loss because of persistence in seed banks (30), plant trait diversity was not impacted by land-use history (Fig. 3). However, we found a strong effect of land-use history on plant species richness (SI Appendix, Fig. S6), suggesting that legacy effects of land use affect plant species independently of the traits investigated in this study. While the functional diversity of certain trophic groups showed divergent responses to land-use changes (Fig. 4), our study shows that there is a consistent pattern in the response of overall ecosystem trait diversity, thus suggesting that our integrated index of multitrait diversity provides a simple quantitative measure of the whole ecosystem biodiversity response.
Our results revealed that present-day functional biodiversity was impacted by land-use changes that happened up to 20 y ago, thus demonstrating that land-use actions have long-lasting impacts and that extinction debts (sensu 9) are commonplace in agricultural landscapes. In an era of global biodiversity change, our results emphasize the need to consider land-use legacies in conservation programs that aim to protect the biodiversity of agroecosystems and its associated ecosystem functions and services. In particular, our results emphasize the need to preserve permanent grasslands in agricultural landscapes, as they provide a shelter to low-mobility organisms and species with narrow feeding niches. While large-scale policy schemes encourage the retention of permanent grasslands, they have largely decreased in their cover over the last years (31). Our results call for immediate actions to conserve and restore permanent grasslands in order to preserve the functionality of agricultural landscapes and avoid future extinction debt.
Materials and Methods
Study Area.
The study was conducted in 2014 in the Long Term Socio-Ecological Research (LTSER) “Zone atelier Plaine & Val de Sèvre” (ZAPVS) located in western France (32). The LTSER covered ∼430 km2 of an intensively managed agricultural plain. Historically, it was a typical rural area characterized by the presence of mixed crops-livestock systems (dairy goats and cows). Grassland was the dominant land use 50 y ago covering about 60% of the area (32). Since that time, shifts from grazing livestock to feeding livestock, and shifts from livestock production to annual crop production have resulted in a strong decline in grassland cover. In 2014, grasslands covered about 12% of the area and included artificial grasslands (i.e., alfalfa with 3% of the area), temporary (sown with pure grasses or in mixtures with legume species and ≤5 y old), and permanent grasslands (>5 y old) managed by grazing, mowing, or abandoned. The remaining areas were covered by crops (66% of landscape area). Soils are mostly composed of karst, with calcareous rocks providing shallow calcareous soils with low water retention and pH > 7. Since 1994, land cover of the study area has been monitored on a yearly basis at the field scale, by using about 30 land-use types (11,000 fields approximately, see ref. 32 for methodological details on land cover monitoring), and has been stored in a Geographical Information System (GIS) database, running on QGIS v 2.14.
Grassland Selection and Land-Use Metrics.
We monitored 75 grassland fields within the study area. The grassland fields were selected among hay meadows of varying ages and vegetation types (pure legumes, pure or mixed grasses, legume and grass mixture, postcultural vegetation). The average age of the grassland field was 8 y-old (SD = 6.55). We calculated for each of the 75 grassland fields the current landscape composition, and landscape metrics linked to land-use history, within a 1 km-radius landscape (SI Appendix, Fig. S1). This scale was chosen to approximate the dispersal distance of different taxa (e.g., ref. 33). For landscape composition, we considered landscape elements known as favorable or resource-rich habitats for the different groups of taxa, i.e., the proportion of the landscape covered by grasslands, forests, and mass flowering crops (oilseed rape and sunflower). Landscape composition metrics varied between 0–35% for grassland, 0–32% for forests, and 0–44% for mass flowering crop covers. For land-use history metrics, we calculated for all fields in the 1 km-radius landscape surrounding the focal grasslands: (1) the time elapsed since the first grassland-to-crop conversion (SI Appendix, Fig. S1); (2) the time spent into current land use since the last tillage (hereafter called “Field age”). The age of crop-field was 1 y and the age of grassland field varied between 1 and 20 y (set to 20 y if it had not been plowed since 1994); and (3) the turnover from crop to grassland since the last conversion of the field into cropland. This metric is linked to the lifespan of grasslands sown in crop rotation and is higher when the average lifespan of sown grasslands in the landscape is longer. To account for the size of the field, all these metrics were weighted by the field area. We then averaged these 3 metrics at the landscape level (hereafter called “Time as cropland,” “Grassland permanency,” and “Crop-grassland turnover”) (SI Appendix, Fig. S1).
Species Richness and Density.
Seven taxonomic groups were sampled on the 75 grassland fields. The taxonomic groups were chosen for their association with key ecological functions: primary producers (vascular plants), pollinators (wild bees and hoverflies), herbivores involved in carbon and nitrogen cycling (grasshoppers), invertebrate predators that are natural enemies of pests (carabid beetles and spiders), and vertebrate predators (birds) of important cultural value (SI Appendix, Table S1). Sampling was performed throughout the growing season from April to August 2014 in each grassland field following standardized protocols (32). Details of sampling methods are provided in SI Appendix, Methods S1. Relative abundance per plant species was calculated as the sum of the species cover in the 10 quadrats divided by the total cover of all species. For animals, species density was estimated as the number of individuals captured (invertebrates) or recorded (birds) divided by sampling intensity (number of traps or point counts in the grassland). The number of species in each grassland field was determined in the laboratory for all invertebrate species.
Trait Measurements and Trait Diversity.
Functional trait data were collected for all taxonomic groups. Plant trait data came from a local database from the LTSER site (23). Bird morphological traits were compiled from the literature (34). For all other taxonomic groups, all measurements were performed using a stereo microscope (Leica Microsystems M50) equipped with an integrated high-definition microscope camera (Leica IC80 HD). Details of trait measurements are provided in SI Appendix, Methods S2. In total, we collected trait data for 178 species distributed across the different trophic groups (SI Appendix, Methods S2).
We calculated the community abundance-weighted variance (35) for each taxonomic group separately and each trait separately. For single traits, the variance is a measure of the trait dispersion within a given community weighted by the abundance of each individual species and is a measure of functional trait diversity of a given community (35). We also computed a multitrait index of functional diversity based on trait dispersions for each taxonomic group separately—the Functional Dispersion (FD) (36).
Multitrophic Trait Diversity Measurements.
We used methods developed to study ecosystem multifunctionality to calculate a multitrait diversity index considering all traits and taxonomic groups. This approach (averaging approach, see ref. 37) consists of calculating the average standardized values of multiple functional diversities. Our multitrait functional diversity index was thus calculated as the average standardized FD values across the taxonomic groups (similar to multidiversity indices following ref. 5). Using this index allows us to identify the environmental conditions, which maximize functional trait diversity across multiple taxonomic groups. Note that the averaged multitrait diversity index was highly correlated with the threshold-50 multitrait diversity index (37)—calculated as the percentage of community variance values that exceeds 50% of their maximum observed community variance (r = 0.87, see SI Appendix, Table S5). Similarly, we calculated an average index of multitrophic density—average standardized values of the total density for the different animal taxonomic groups (i.e., grasshoppers, wild bees, hoverflies, carabids, spiders and birds)—and an average index of multitrophic species richness, following ref. 5. In addition, to test if land-use changes have similar effects on different axes of trait variation, we also calculated averaged indices for mobility trait diversity, resource-acquisition trait diversity, and body size trait diversity values as the average standardized community variance values of these 3 types of traits, across the taxonomic groups. To calculate these trait indices, we excluded plant traits as the 3 selected plant traits are associated with different functions and other axes of variation. Finally, to test if the response of each trophic group to land-use changes is similar, we computed multitrait diversity, species richness, and density indices for 5 trophic groups separately: primary producers (vascular plants), mutualists (wild bees and hoverflies), herbivores (grasshoppers), invertebrate predators (carabids and spiders), and vertebrate predators.
Assessing the Effects of Past and Current Land Use.
We evaluated the effects of land-use changes on grassland trait diversity by using linear models. We ran separate analyses on: 1) the multitrait diversity; 2) the mobility, resource-acquisition, and body size trait diversity; 3) on the multitrait diversity of the different trophic groups separately. We included in our models the effects of the grassland field age and of the 3 landscape land-use history metrics as predictors of functional trait diversity. We also controlled for current landscape composition (% grassland, % forest, and % mass flowering crop areas). We used plant productivity as a proxy measure of grassland management, as it is related to fertilizer inputs. This was assessed by harvesting plant biomass each month (between February and August 2014) above a cutting height of 5 cm from the soil surface, within five 35 × 35 cm quadrats. Productivity was then calculated as the weight of dried-plant material (oven-dried at 60 °C for 72 h) product per square meter per day between the initial biomass measurement and peak biomass (end of May). We considered quadratic terms for grassland field age, landscape land-use history metrics, current grassland field productivity, and landscape composition to assess potential nonlinear effects of these variables. Further covarying factors accounted for the number of mowing events and soil depth were included. Our models also integrated the coordinates of the centroid of each sampled grassland (latitude and longitude) to correct for additional spatial effects not accounted for by the field and landscape predictors (38). Finally, as the local species pool (defined by both the density and the species richness) may affect functional trait diversity metrics (39), we included in the models the multitrophic density and multitrophic species richness indices to isolate the net effects of land-use changes on multitrophic trait diversity. We ran separate analyses on the multitrophic density and the species richness, and the results are presented in SI Appendix, Figs. S3 and Fig. S4.
We then performed a series of models for the multitrait diversity, mobility trait diversity, resource-acquisition trait diversity, and body size trait diversity; and for the multitrait diversity of each taxonomic group separately. We first used a backward stepwise regression procedure using the software JMP 11 (SAS Institute) to select, between all models, the best-fitting models with lower second-order Akaike Information Criterion (AICc) (Δ AICc < 2). Second, using the best selected models, we performed a model-averaging procedure based on AICc selection (delta AICc < 2) to determine parameter coefficients for the best final set of predictors of our response variables. This procedure was performed using the function dredge in the R package Multi-Model Inference (MuMIn) (40). Model residuals were inspected for constant variance and normality. We standardized all variables (z-scored: mean-centered and divided by the SD) to interpret parameter estimates on a comparable scale (41). Correlation among the predictors used was low (SI Appendix, Table S2) and did not induce multicollinearity issues in our analyses (SI Appendix, Table S6). The inclusion of many predictors in statistical models increases the chance of type I error (false positives). To account for this, we used a Benjamini and Hochberg procedure to control for false discovery rates and adjust P values (42). To evaluate the relative importance of the predictors as drivers of trait diversity, we expressed the importance of predictors as the percentage of variance they explain, based on the comparison between the absolute values of their standardized regression coefficients and the sum of all standardized regression coefficients from the predictors. This method is similar to a variance partitioning analysis because we previously transformed all predictors to z-scores (38, 43, 44). The following identifiable variance fractions were then examined: 1) land-use history, 2) current land use, and 3) the species pool. The data used in this paper are available in Figshare digital repository (45).
Data Availability.
The data that support the findings of this study are available through the Figshare repository, https://datadryad.org/stash (DOI: 10.6084/m9.figshare.11310086.v1).
Supplementary Material
Acknowledgments
We thank Franck Coudray, Hélène Deraison, Yoann Erreca, Thierry Fanjas-Mercère, Jean-Luc Gautier, Louis Gross, Nadine Guillon, Florian Mezerette, Sophie Pillaud, Alexis Saintilan, and Edoardo Tedesco for field assistance. The study was supported by the French government Initiatives d’Excellence—Initiatives Science/Innovation/Territoires/Économie (IDEX-ISITE) initiative 16-IDEX-0001 (CAP 20-25), the FarmLand research programme, an European Research Area Net (ERA-Net) BiodivERsA project funded by the French Agence Nationale de la Recherche (ANR-11-EBID-0004), and the Enhancing biodiversity-based ecosystem services to crops through optimized densities of green infrastructure in agricultural landscapes (ECODEAL) research programme, 2013–2014 BiodivERsA/Agriculture, Food Security & Climate Change (FACCE-JPI) joint call for research proposals, with the national funders Agence Nationale de la Recherche, Bundesministerium für Bildung und Forschung, Swedish Research Council for Environment (FORMAS) (2014-1783), Fonds zur Förderung der wissenschaftlichen Forschung, Ministerio de Economía y Competitividad, Netherlands Organisation for Scientific Research (NWO), and Projektträger im Deutschen Zentrum für Luft- und Raumfahrt (PT-DLR). G.L.P was supported by a region Poitou-Charentes- department Deux-Sèvres PhD grant. N.G. was supported by the AgreenSkills+ fellowship programme, which has received funding from the European Union’s Seventh Framework Programme under Grant Agreement FP7-609398 (AgreenSkills+ contract). Y.L.B.-P. was supported by the European Research Council (BIODESERT) and by a Marie Sklodowska-Curie Actions Individual Fellowship (MSCA-IF) within the European Program Horizon 2020 (Linking plant functional diversity to ecosystem multifunctionality in arid systems worldwide [DRYFUN] Project 656035). C.V. was supported by the European Research Council (ERC) Starting Grant Project “ecophysiological and biophysical constraints on domestication in crop plants”(Grant ERC-StG-2014-639706-CONSTRAINTS). We thank Eric Allan, Sandra Lavorel, Hervé Jactel, Colin Fontaine, and Pascal Carrère for their useful comments on the previous versions of the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Database deposition: The data that support the findings of this study are available through the Figshare repository, https://datadryad.org/stash (DOI: 10.6084/m9.figshare.11310086.v1).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910023117/-/DCSupplemental.
References
- 1.Newbold T., et al. , Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353, 288–291 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Tilman D., May R. M., Lehman C. L., Nowak M. A., Habitat destruction and the extinction debt. Nature 371, 65–66 (1994). [Google Scholar]
- 3.Krauss J., et al. , Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol. Lett. 13, 597–605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blitzer E. J., et al. , Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 146, 34–43 (2012). [Google Scholar]
- 5.Allan E., et al. , Interannual variation in land-use intensity enhances grassland multidiversity. Proc. Natl. Acad. Sci. U.S.A. 111, 308–313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gámez-Virués S., et al. , Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 6, 8568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn D. F., et al. , Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 12, 22–33 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Vogel A., et al. , Chapter three–Lost in trait space: Species-poor communities are inflexible in properties that drive ecosystem functioning. Adv. Ecol. Res. 61, 91–131 (2019). [Google Scholar]
- 9.Kuussaari M., et al. , Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 24, 564–571 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Bretagnolle V., et al. , Towards sustainable and multifunctional agriculture in farmland landscapes: Lessons from the integrative approach of a French LTSER platform. Sci. Total Environ. 627, 822–834 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B., Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004). [Google Scholar]
- 12.Hillebrand H., Bennett D. M., Cadotte M. W., Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 89, 1510–1520 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Bebber D. P., Holmes T., Gurr S. J., The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 23, 1398–1407 (2014). [Google Scholar]
- 14.Schmidt K. A., Ostfeld R. S., Biodiversity and the dilution effect in disease ecology. Ecology 82, 609–619 (2001). [Google Scholar]
- 15.Le Provost G., et al. , Trait-matching and mass effect determine the functional response of herbivore communities to land-use intensification. Funct. Ecol. 31, 1600–1611 (2017). [Google Scholar]
- 16.Laliberté E., et al. , Land-use intensification reduces functional redundancy and response diversity in plant communities. Ecol. Lett. 13, 76–86 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Perović D., et al. , Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J. Appl. Ecol. 52, 505–513 (2015). [Google Scholar]
- 18.Tscharntke T., et al. , Landscape moderation of biodiversity patterns and processes–Eight hypotheses. Biol. Rev. Camb. Philos. Soc. 87, 661–685 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Harpole W. S., et al. , Addition of multiple limiting resources reduces grassland diversity. Nature 537, 93–96 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Gossner M. M., et al. , Land-use intensification causes multitrophic homogenization of grassland communities. Nature 540, 266–269 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Kormann U., et al. , Local and landscape management drive trait-mediated biodiversity of nine taxa on small grassland fragments. Divers. Distrib. 21, 1204–1217 (2015). [Google Scholar]
- 22.Naeem S., et al. , Declining biodiversity can alter the performance of ecosystems. Nature 368, 734–737 (1994). [Google Scholar]
- 23.Deraison H., Badenhausser I., Loeuille N., Scherber C., Gross N., Functional trait diversity across trophic levels determines herbivore impact on plant community biomass. Ecol. Lett. 18, 1346–1355 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Holzschuh A., et al. , Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 19, 1228–1236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenop A., Woodcock B. A., Wilby A., Cook S. M., Pywell R. F., Functional diversity positively affects prey suppression by invertebrate predators: A meta-analysis. Ecology 99, 1771–1782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Palma A., et al. , Ecological traits affect the sensitivity of bees to land-use pressures in European agricultural landscapes. J. Appl. Ecol. 52, 1567–1577 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillebrand H., et al. , Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol. Lett. 12, 516–527 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Häussler J., et al. , The biggest losers: Habitat isolation deconstructs complex food webs from top to bottom. bioRxiv:10.1101/439190 (17 October 2018). [DOI] [PMC free article] [PubMed]
- 29.Valiente-Banuet A., et al. , Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307 (2015). [Google Scholar]
- 30.Purschke O., et al. , Interactive effects of landscape history and current management on dispersal trait diversity in grassland plant communities. J. Ecol. 102, 437–446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pe’er G., et al. , Agriculture policy. EU agricultural reform fails on biodiversity. Science 344, 1090–1092 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Bretagnolle V., et al. , Description of long-term monitoring of farmland biodiversity in a LTSER. Data Brief 19, 1310–1313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diekötter T., Kadoya T., Peter F., Wolters V., Jauker F., Oilseed rape crops distort plant–pollinator interactions. J. Appl. Ecol. 47, 209–214 (2010). [Google Scholar]
- 34.Cramp S., Handbook of the birds of Europe, the Middle East and North Africa (Oxford University Press, Oxford, UK, 1977–1994), Vol. 1–9. [Google Scholar]
- 35.Enquist B. J., et al. , Chapter nine-scaling from traits to ecosystems: Developing a general trait driver theory via integrating trait-based and metabolic scaling theories. Adv. Ecol. Res. 52, 249–318 (2015). [Google Scholar]
- 36.Laliberté E., Legendre P., A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Byrnes J. E., et al. , Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 5, 111–124 (2014). [Google Scholar]
- 38.Le Bagousse-Pinguet Y., et al. , Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl. Acad. Sci. U.S.A. 116, 8419–8424 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayfield M. M., et al. , What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Glob. Ecol. Biogeogr. 19, 423–431 (2010). [Google Scholar]
- 40.Bartoń K., MuMIn: Model Selection and Model Averaging Based on Information Criteria (AICc and alike) (R Package Version, 1–1, 2014). https://CRAN.R-project.org/package=MuMIn. Accessed 17 December 2019.
- 41.Schielzeth H., Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113 (2010). [Google Scholar]
- 42.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995). [Google Scholar]
- 43.Gross N., et al. , Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 1, 0132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sirami C., et al. , Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. U.S.A. 116, 16442–16447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Provost G., et al. , “Land-use history impacts functional diversity across multiple trophic groups.” Figshare. 10.6084/m9.figshare.11310086.v1. Deposited 3, December 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available through the Figshare repository, https://datadryad.org/stash (DOI: 10.6084/m9.figshare.11310086.v1).