Significance
Overfishing of tropical grouper- and snapper-spawning aggregations is a globally pervasive problem, but evidence is elusive that recovery can occur following stock collapse. We analyze a uniquely rich research and monitoring dataset from spawning aggregations in the Cayman Islands to reconstruct annual Nassau grouper spawner abundance across almost 2 decades of postcollapse adaptive-fisheries management. Using an integrated population model that leverages both mark–resight and video census techniques, we demonstrate that Nassau grouper have undergone a remarkable recovery during this time frame. Because of the implementation of these deliberate, science-based conservation strategies, Little Cayman is now home to the largest remaining identified Nassau grouper aggregation anywhere in the world.
Keywords: fish-spawning aggregation, Nassau grouper, integrated population model, mark–resight, tagging
Abstract
Many large-bodied marine fishes that form spawning aggregations, such as the Nassau grouper (Epinephelus striatus), have suffered regional overfishing due to exploitation during spawning. In response, marine resource managers in many locations have established marine protected areas or seasonal closures to recover these overfished stocks. The challenge in assessing management effectiveness lies largely in the development of accurate estimates to track stock size through time. For the past 15 y, the Cayman Islands government has taken a series of management actions aimed at recovering collapsed stocks of Nassau grouper. Importantly, the government also partnered with academic and nonprofit organizations to establish a research and monitoring program (Grouper Moon) aimed at documenting the impacts of conservation action. Here, we develop an integrated population model of 2 Cayman Nassau grouper stocks based on both diver-collected mark–resight observations and video censuses. Using both data types across multiple years, we fit parameters for a state–space model for population growth. We show that over the last 15 y the Nassau grouper population on Little Cayman has more than tripled in response to conservation efforts. Census data from Cayman Brac, while more sparse, show a similar pattern. These findings demonstrate that spatial and seasonal closures aimed at rebuilding aggregation-based fisheries can foster conservation success.
Reef fishes that form fish-spawning aggregations (FSAs), including many species of grouper, are at high risk of being overfished when fisheries target their FSAs (1–6). Heavy fishing pressure at FSAs can impact the targeted population in a number of ways, from decreasing mean length and skewing sex ratios for hermaphroditic species, to complete collapse and disappearance of the FSA (1, 7–11). Such overfishing is often masked by hyperstability, whereby catch-per-unit effort (CPUE) at the FSA remains high despite steep population declines (12–16); as a result, the onset and persistence of fisheries-induced population decline is poorly detected. The vulnerability of FSAs to fishing, coupled with poor catch-based indicators of overfishing, has resulted in pervasive overfishing of coral reef FSAs globally (4).
The Nassau grouper (Epinephelus striatus), once the most important reef fishery in the Caribbean, exemplifies the risks associated with FSA fishing (17). Following dramatic population declines throughout its range (Bermuda, Florida, and throughout the Caribbean), the Nassau grouper was listed as both “critically endangered” under the International Union for Conservation of Nature Red List process (18) and, more recently, “threatened” under the US Endangered Species Act (19). Nassau grouper form large FSAs at highly predictable times and locations throughout its range in the tropical Western Atlantic, Caribbean, and Gulf of Mexico (3, 9). Fish typically spend a week or more at FSAs, ultimately releasing gametes over the course of a few nights. Eggs are externally fertilized, with at least one female and multiple males participating in spawning events (20). The fertilized eggs hatch after ∼24 h (9) and remain as pelagic larvae for 35 to 45 d (21, 22). Larvae do not necessarily recruit to the locations in which they were spawned; population genetic studies show broad (but not complete) connectivity across the Caribbean (23, 24). Following recruitment, individuals take 5 to 7 y to reach reproductive maturity and may live 29 or more years (8). Because the total annual reproductive output for a stock happens over a short window of time and a single location, it is reasonable to expect high variability in recruitment (25, 26).
The spatiotemporal predictability of Nassau grouper FSAs makes them particularly susceptible to overfishing during the spawning season (2). Historically, some Nassau grouper FSAs may have hosted upwards of 100,000 individuals (27), attracting fish from as much as 260 km away (28, 29). Records from Cuba suggest that annual Nassau grouper catch, at its maximum in the 1960s, reached 1,800 t, although subsequent decades showed steady declines until collapse by the 1990s (30). While the Cuban example is unusually well documented, the fate of Cuban Nassau grouper populations has been repeated throughout the range of the species. Once FSAs are discovered, they are fished intensively; many such aggregations have ultimately been fished to the point where fish cease to aggregate (31–34). In a contemporary context, few of the known remaining FSAs host more than 1,000 individuals (2, 5, 35, 36).
Following regional collapses in Nassau grouper FSAs, many nations have sought to protect their remnant populations through a variety of management actions, including fishing moratoria, seasonal closures, or the establishment of marine protected areas at FSAs (8, 22, 37–41). To date, few published studies have quantitatively demonstrated the efficacy of such management strategies even after several decades of aggressive management (e.g., complete fishing moratoria). It is not clear if this lack of evidence stems from management ineffectiveness (e.g., enforcement/compliance failures), population processes, e.g., Allee effects (3, 42, 43), recruitment variability (44, 45), or an absence of data sufficient to document success.
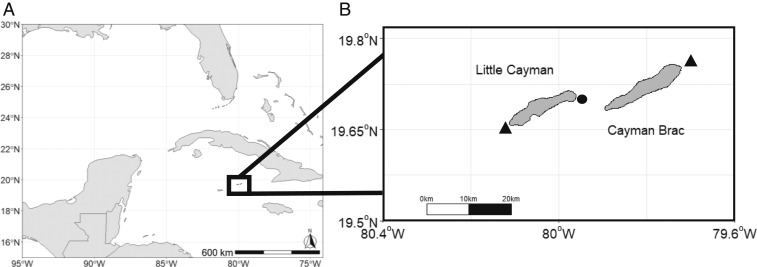
Like many Caribbean nations, the Cayman Islands (Fig. 1A) have a long history of Nassau grouper exploitation and a more recent history of overexploitation (see SI Appendix, Supplement A, Table S1 for a Cayman Island Nassau grouper fishery history). Following concerns of decreased catch voiced by Caymanian fishermen in the 1980s, the Cayman Islands Department of Environment (CI-DoE) began collecting catch data during the spawning season and carried out age and growth studies for the species in 1987 (8). These early efforts at monitoring fishing were abandoned by the late 1990s as catch across all 3 Cayman Islands had completely collapsed. In 2001, however, a Nassau grouper FSA was discovered by 2 local fishermen at the west end of Little Cayman, opposite the historical location at the east end of the island (Fig. 1B). At the time of discovery, the FSA consisted of ∼7,000 fish; in 2 y of fishing, the CI-DoE recorded at least 4,000 Nassau grouper caught (excluding undocumented take that was likely substantial) (8, 20). In 2003, the Cayman Islands Marine Conservation Board responded to this rapid decline by instituting no-take zones on all known (historical and active) Nassau grouper FSAs during the spawning season. Subsequently, in 2016, the Cayman Islands government enacted legislation aimed at recovering Nassau grouper (46). The 2016 regulations include a seasonal closure on Nassau grouper harvest from December through April inclusive (possession, sale, and take of Nassau grouper during this time is illegal); a 16- to 24-inch slot size limit; a bag limit of 5 Nassau grouper per vessel per day during the open season; and gear restrictions (no take of Nassau grouper via spear). Additionally, the Cayman Islands maintain a range of marine parks, some of which are no-take year-round; during this study, no-take zones covered ∼15% of the Cayman Islands shelf area (increasing to 45% in March of 2019).
Fig. 1.
Map showing location of the Cayman Islands in the greater Caribbean region (A) and close-up of the islands of Little Cayman and Cayman Brac (B). The close-up map shows the historical site on the east end of Little Cayman (circle) and active spawning sites on the west end of Little Cayman and the east end of Cayman Brac (triangles). The islands of Little Cayman and Cayman Brac are 10 km apart. The spawning site at Little Cayman is at a shelf break at 30 m, dropping off to >100 m, and the Cayman Brac site is at a gentle slope starting at 40 m, dropping to >100 m. In 2017, the human population of the Cayman Islands was 63,415, with around 200 on Little Cayman and just over 2,000 on Cayman Brac (85).
Since the discovery of the west-end Little Cayman FSA, Reef Environmental Education Foundation (REEF) and the CI-DoE have monitored Nassau grouper FSAs in the Cayman Islands through the collaborative Grouper Moon Project. The Grouper Moon Project aims to provide the Cayman Islands government with monitoring data necessary to effectively assess and manage Nassau grouper populations. While prior to fishery collapse, the CI-DoE collected CPUE data from aggregation-based fisheries (8), FSA closures precluded the continuation of these data. That, and because hyperstability tempers the value of such data for assessments, we developed and implemented a long-term (15+ year) diving-based census program to estimate fish abundance at FSAs in the Cayman Islands. For the purposes of this study, we assumed that the abundance of spawners at the FSAs on each island provide a direct proxy for the total spawning stock of Nassau grouper on that island. Thus, a time series of estimates of spawners on each island reflects the stock-level response of Nassau grouper to the abovementioned management efforts. We revisit and justify this assumption in Discussion.
Here, we provide evidence for the recovery of Little Cayman and Cayman Brac Nassau grouper populations over the last decade, based on data generated from both a mark–resight program and video census techniques (47, 48). Specifically, we report on an in situ mark–resight technique that uses external tags (Floy tags) deployed with pole spears and subsequent visual counts of marked and unmarked individuals by research divers (surveyors). Using these counts, we generate estimates of the number of spawning adults and, importantly, provide confidence intervals associated with these counts. Taking account of time, we develop an autoregressive state–space model of Nassau grouper populations on both islands and inform year-specific population estimates using mark–resighting observations. Additionally, we extend this state–space formulation into an integrated population model framework (49) by combining both mark–resighting observations and counts from video census conducted on both FSAs over the last decade. In doing so, we demonstrate how readily available observation technologies can be incorporated into a single robust population assessment that explicitly models the process of population recovery.
Methods
We carried out all mark–resight and video censuses at Nassau grouper FSAs on the west end of Little Cayman and the east end of Cayman Brac, in the Cayman Islands (Fig. 1B), during the winter spawning periods from 2005 through 2018. Research activities took place on the island of Cayman Brac less frequently than on Little Cayman (Table 1), as the conditions on Cayman Brac are variable and typically unsafe for diving. Conditions at the only known remaining Nassau grouper FSA on Grand Cayman (east end) precluded the research methods we outline below. However, divers made an in situ visual estimate of the number of aggregating fish in 2012, and in 2018, we estimated the size of the FSA using a remotely operated vehicle.
Table 1.
Number of fish tagged, the number of surveys conducted by research divers, the number of research divers (surveyors) participating, the average number of surveys conducted per dive per surveyor, and the number of video census counts completed each year on Little Cayman and Cayman Brac
| Year | Fish tagged, no. | Surveyors participating, no. | Surveys completed, no. | Surveys per dive per surveyor, average no. | Video counts, no. |
| Little Cayman | |||||
| 2005 | — | — | — | — | 10 |
| 2006 | — | — | — | — | 5 |
| 2008 | 36 | 4 | 42 | 6 | 8 |
| 2009 | 58 | 4 | 156 | 25 | 8 |
| 2010 | 57 | 7 | 128 | 6 | 7 |
| 2011 | 67 | 11 | 224 | 9 | — |
| 2012 | 103 | 6 | 771 | 18 | 9 |
| 2013 | 100 | 7 | 491 | 20 | 8 |
| 2014 | 42 | 8 | 430 | 12 | 5 |
| 2015 | 107 | 6 | 324 | 24 | 7 |
| 2016 | 93 | 12 | 452 | 15 | — |
| 2017 | 102 | 15 | 588 | 12 | 10 |
| 2018 | 118 | 13 | 793 | 24 | 8 |
| Cayman Brac | |||||
| 2008 | 15 | 2 | 5 | 3 | 15 |
| 2013 | — | — | — | — | 15 |
| 2017 | — | — | — | — | 20 |
| 2018 | 36 | 5 | 136 | 27 | 19 |
—, no data of that type were collected that year.
To tag Nassau grouper at the FSA sites, researchers on scuba deployed Floy FIM-96 Small Billfish tags using modified pole spears. Individuals were tagged opportunistically on either side of the body but always toward the back of the fish and immediately below the dorsal fin. The tag color was changed year-to-year as some tags remained on individuals for up to 3 y. In subsequent resight surveys (described below), we only recorded resightings of tags deployed in the same year to avoid issues with interannual tag loss. For the purposes of this study, we assume that tags stay on for a minimum of 8 d and thus our resightings were unaffected by short-term tag loss; we found no evidence to suggest tag shedding was occurring at the aggregation site. In all years, we concentrated tagging efforts over multiple days (usually 2 to 3) early in the spawning period. Typically, the FSA persists for at least 6 d after the full moon. We tagged a variable number of fish each year between 2008 and 2018 (Table 1), with the goal of tagging ∼100 fish per year on Little Cayman and 30 fish per year on Cayman Brac. We chose these tagging targets to balance the potential negative impacts of tagging with the need for consistent mark–resightings. In later years of the study when the populations were apparently larger, we increased our resightings survey effort (rather than tagging effort) in order to maintain or increase accuracy in population-size estimates (Table 1).
Following the completion of tagging, surveyors conducted resight surveys for 2 to 6 d each year following a set protocol (50). While diving on the aggregation site, surveyors pseudorandomly identified 50 consecutive individuals (where they could clearly and fully see the tagging area on each individual fish) and recorded a mark–resight only when the individual fish was tagged on the side closest to the surveyor. A survey consists of the count of tags (resights) seen from 50 fish. Surveyors typically achieved 5 to 20 surveys per dive (Table 1). In general, observers conducted surveys while swimming against the movement of fish in an effort to avoid double-counting individual fish within a count of 50. Because a fish could be tagged on either the right or left side, and because both sides of the same fish are not always observable, observers effectively counted 50 “sides” of fish and recorded the number of those sides that were tagged. Even if surveyors could see both sides of fish, they only counted the side closest to them, because the 2 sides of a single fish are nonindependent (fish were only tagged once). In our analysis, we excluded resighting data from any surveyor who completed 5 or fewer surveys during the entire study period (2008 to 2018).
We opportunistically collected video censuses (SI Appendix, Supplement B) of the FSAs on both islands when fish schooled into a loosely organized band along the shelf edge of the aggregation sites (20). To collect a video census, a diver started recording video at one end of the band and traversed the length of the band while keeping aggregating fish within the camera frame. When divers collected multiple video censuses within a given spawning season, the research team did preliminary counts of fish in the video to select the census that they judged to capture the most fish. Subsequently, multiple researchers and trained volunteers counted the number of fish captured during video census. The videos were typically enumerated by advancing the video slowly while continuously counting fish, advancing across paused frames and counting fish in still images, or some combination of these methods. The observers were not given a specific time interval to complete the counts in each video; rather, we instructed analysts to generate the most accurate count possible using methods that worked best for them and a time interval that would afford completion using that method. In any given video, issues of fish occlusion due to stacking, low light, and image resolution resulted in variable counts across observers. For these reasons, we collected at least 10 counts from each video in order to adequately capture count variability. The analytic framework outlined below explicitly models this observation error.
Basic State–Space Model.
We modeled the true number of fish at the aggregation site each year, , in log space using a state–space model, with the following state process:
| [1] |
where represents the population growth rate in year y (in log space). We treated yearly population growth, , terms as random effects drawn from a normal distribution with a mean (the model-estimated overall population mean growth rate in log space), and SD (process error). We use the following priors in the model: a uniform distribution with bounds −10 and 10 for ; a uniform distribution with bounds 0 and 10 on ; and for , an island-specific uniform distribution (0,1000 for Cayman Brac and 0,5000 for Little Cayman).
Informing Estimates from Binomial Resight Data.
Because surveyors made every effort to avoid sampling the same fish within a count of 50, sampling was effectively conducted without replacement. Typically, this data type would be modeled using the hypergeometric distribution, a special case of the binomial distribution applicable when sampling is without replacement. However, because individual sample sizes (the number of fish sides counted in survey i) were small relative to the total population size, , the hypergeometric distribution is approximated by the binomial distribution (51). We thus modeled the counts of sides of tagged individuals for survey in year , , using a binomial observation model:
| [2] |
where represents the proportion of sides of fish in the aggregating population that are tagged in year , and represents the number of fish sides counted in survey . The proportion of tagged sides of fish in the FSA is equal to:
| [3] |
where is the number of fish tagged at the aggregation site in year , and represents the model estimated population size from Eq. 1.
Incorporating Surveyor Effect.
Tag-detection rate may differ across surveyors. For instance, surveyors with more survey experience may detect tags with more accuracy than novices (i.e., they have fewer false-negative detections). Regardless of the specific mechanism, we can incorporate such surveyor random effects by altering the proportion of tagged sides of a fish in logit space. Eq. 2 becomes:
| [4] |
where represents the proportion of tagged fish sides detectable for surveyor a in year y, such that:
| [5] |
where represents the modified proportion of tagged sides of a fish in logit space, such that:
| [6] |
where is a surveyor-specific random deviate away from the proportion of tagged-fish sides (in logit space) for surveyor a, with a normal distribution for a prior with mean 0 and SD (which has a uniform prior with lower and upper bounds of 0 and 5, respectively).
Incorporating Video Census Data.
By separating the process and observation components of our model framework, we afford the flexibility to inform the process model by more than one class of observations, e.g., an integrated population model (49). We leveraged this flexibility in order to incorporate both mark–resighting observations and counts from video census into a single model. To do so, we treated video census counts as imprecise observations of a proportion of the true population size. In other words, we estimate , the proportion of the spawning population captured in the video. Thus, the video census observation process becomes:
| [7] |
where, is the count made by reviewer of video from year ; is the spawning population in year ; and is the SD in year . We used a uniform distribution with bounds 0 and 1 on the prior of the proportion, , and a uniform distribution with bounds 0 and 100 on the prior for .
Model Fitting.
We used the programming language R (52) and the package “R2jags” (53) to perform the Bayesian Monte Carlo Markov Chain sampling. To evaluate the need to include surveyor random effects on Little Cayman, we used the information criterion metric deviance information criterion (DIC). We compared the relative performance of different model parameterizations (parsimony) by calculating the difference between the DIC of each model and that of the model with the lowest DIC (∆DIC). Due to the limited amount of data from Cayman Brac for the tagging model, we did not attempt to fit the Cayman Brac model with random surveyor effects. To evaluate convergence, we calculated the Gelman–Rubin statistic for every parameter, along with the number of parameters with Gelman–Rubin statistics greater than 1.01, 1.05, and 1.10.
The model for Little Cayman with just tagging data ran from 2008 to 2018 (years when we conducted tagging studies). The Little Cayman model including video census data ran from 2005 to 2018 (the range of years during which we captured video census, except 2007, 2011, and 2016, when conditions and fish behavior precluded video capture). The model for Cayman Brac runs from 2008 to 2018 as both datasets cover this period, although the observations across years were sparser than on Little Cayman (2 y of mark–resight data, 4 y of video census). All data and code used to develop our results are available at https://github.com/WaterLynn.
Results
Across all years and both islands, we tagged 934 fish (Table 1). No tagged fish was ever resighted outside the island where it was tagged. Divers collected 4,540 resighting censuses in total, with the vast majority (4,399) from the Little Cayman aggregation site. Similarly, most year-specific video census data came from Little Cayman (11 vs. 4 y in Cayman Brac). The 2 estimates of FSA size on the east-end Grand Cayman site yielded similar estimates of abundance (500 or less fish) despite a 6-y interval between the observations. Below, we present findings from the mark–resight model on Little Cayman and Cayman Brac without the video census data included. Subsequently, we present results from the integrated population model that contains both mark–resight data and video census data. None of the final models have parameters with Gelman–Rubin statistics larger than 1.05, suggesting each achieved convergence.
Mark–Resight Model Results—Little Cayman: Basic Model and Model with Surveyor Effect.
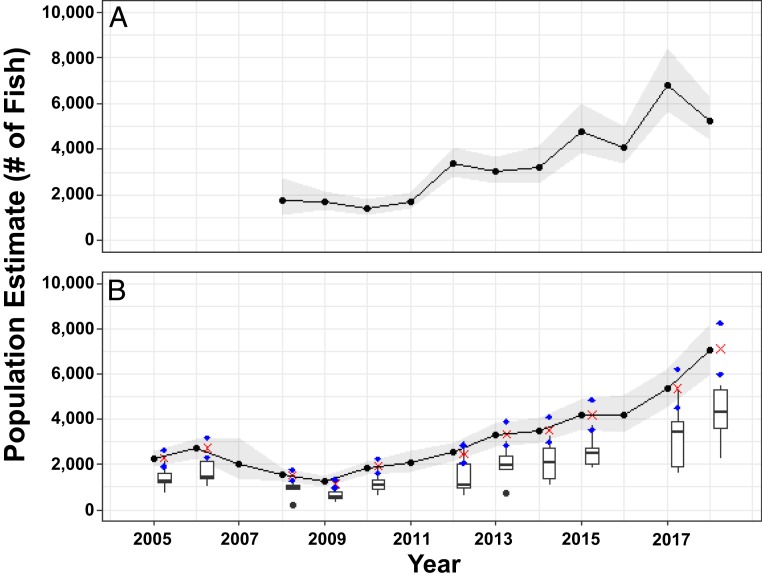
Both models show that the Little Cayman Nassau grouper FSA has dramatically increased since the start of the tagging program in 2008 (Table 2 and Fig. 2). The model with surveyor effect was ∼140 DIC units lower than the basic model, suggesting strong support for differences in tag-detection ability among surveyors (Table 2). For 2018, the size of the Nassau grouper population attending the FSA for Little Cayman Island is estimated to be 5,223 individuals, 95% Bayesian credible interval (CI) of 4,413 and 6,310, an increase from the estimated ∼2,000 fish in 2003.
Table 2.
Median posterior estimate of abundance and values for the 95% Bayesian CI from the 2 state–space models fit using only tagging data for Little Cayman: basic model and the model incorporating surveyor effect
| Year | Basic model DIC = 9,219.7 (∆DIC = 137.3) | Model incorporating surveyor effect DIC = 9,082.4 (∆DIC = 0) |
| 2008 | 1,902 (1,273, 3,031) | 1,732 (1,110, 2,761) |
| 2009 | 1,741 (1,479, 2,080) | 1,684 (1,326, 2,157) |
| 2010 | 1,425 (1,208, 1,695) | 1,416 (1,129, 1,790) |
| 2011 | 1,819 (1,607, 2,069) | 1,712 (1,383, 2,101) |
| 2012 | 3,170 (2,935, 3,426) | 3,387 (2,808, 4,066) |
| 2013 | 3,192 (2,905, 3,546) | 3,014 (2,524, 3,680) |
| 2014 | 3,784 (3,180, 4,542) | 2,107 (2,496, 4,120) |
| 2015 | 4,681 (4,086, 5,414) | 4,754 (3,861, 6,007) |
| 2016 | 3,542 (3,161, 3,972) | 4,074 (3,406, 5,023) |
| 2017 | 7,075 (6,201, 8,181) | 6,821 (5,636, 8,425) |
| 2018 | 4,847 (4,456, 5,280) | 5,223 (4,413, 6,310) |
DIC and ∆DIC values are given next to the model name.
Fig. 2.
Population estimates of Nassau grouper at the spawning aggregation on Little Cayman using the tagging data from 2008 to 2018 (A) and using the tagging and video census data for 2005 to 2018 (B). In both cases, the tagging model includes the surveyor effect. In both A and B, the gray shaded area represents the 95% Bayesian CI, and the black dots connected by the line are the median posterior estimates. In B, boxplots display counts from video censuses (jittered slightly along the x axis for ease of viewing). The red X denotes the median video census count divided by the median posterior estimate for the proportion of fish captured by the video census, and the blue diamonds are the median video census count divided by the 2.5 and 97.5% quantile of the posterior estimate for the proportion of fish captured by the video census.
To evaluate the assumption that the mean proportion of tagged fish remains constant across all surveys collected, we subset the data and compared model results from 1) all survey data, 2) surveys from dives on which more than 10 surveys were completed (i.e., dives where survey conditions were good), and 3) surveys from the day prior to, on, and after peak spawning (i.e., surveys conducted around the night we observed the most spawning). We found that posterior estimates of annual population size differed minimally (SI Appendix, Supplement C, Fig. S1), and thus present only results using all survey data.
Incorporating Video Census Data—Little Cayman and Cayman Brac.
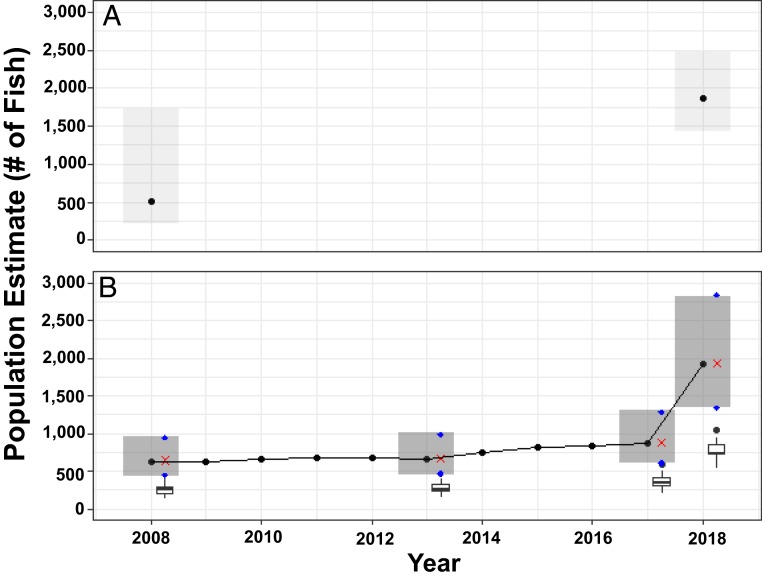
By incorporating video, we can extend our population estimates for Little Cayman back to 2005 (Fig. 2). For Little Cayman, the results show that the population decreases from around 2,267 fish in 2006 (95% Bayesian CI of 1,910 and 2,636) to 1,256 in 2009 (95% Bayesian CI of 1,043 and 1,484), at which point, it increased steadily through time to reach 7,070 fish in 2018 (95% Bayesian CI, 5,937 and 8,201). For Cayman Brac, the model results show minor increases in population size between 2008 and 2017 and then a large increase in the population size in 2018 to ∼1,900 fish (95% Bayesian CI, 1,346 and 2,820; Fig. 3).
Fig. 3.
Population estimates of Nassau grouper at the spawning aggregation on Cayman Brac using the tagging data from 2008 and 2018 (A) and using the tagging data and video counts for 2008 to 2018 (B). In both A and B, the black dots show the median posterior estimates, and the shaded boxes show the 95% Bayesian CI in years with observations (tag and/or video census counts). In B, boxplots (see Fig. 2 for detailed boxplot explanation) display counts from video censuses (jittered slightly along the x axis for ease of viewing).
Discussion
The targeting of reef fish FSAs has resulted in pervasive overfishing of aggregating species throughout the world’s oceans (14). At the same time, the importance of FSAs for population productivity and sustainability has crystalized within the fisheries-management community (3, 54). This recognition has precipitated recent efforts to establish place-based and seasonal protections for FSAs, particularly in the tropical western Atlantic. To date, however, clear evidence remains scarce that such management actions can succeed. In this study, we conclusively demonstrate that aggressive FSA protections in the Cayman Islands have resulted in the sustained recovery of an endangered reef fish that was previously on the brink of extirpation.
Nassau grouper populations have experienced dramatic declines over the last century due to overfishing on FSAs (2, 17, 33, 34, 39). Nonetheless, the species remains important culturally, economically, and ecologically. The anthropological importance of Nassau grouper stems from their role as a cultural symbol (29, 55) and as an economic tool (5, 39, 56), both as a commodity from fishing and for recreational divers/snorkelers to enjoy (57, 58). Nassau grouper may also provide important ecological service as a control on populations of mesopredators (59, 60) and as a potential predator of the invasive lionfish (61, 62). Given the ecological and economic value of the species, many governments throughout the Caribbean have made efforts to recover the species through management actions such as fishing bans (38), seasonal closures (7), or marine protected areas (46, 63). Understanding the population response of aggregating species to such management actions requires accurate estimates of population size across time. While previous studies have used length–frequency data to show Nassau grouper population responses to conservation, e.g., Heppell et al. (7), few studies have showed responses in terms of changes in population size. While it could be argued that this paucity of evidence for population growth following spawning site protections is due to enforcement failures or biological phenomena—i.e., Allee effects, discussed by Allee (42), Gascoigne and Lipcius (43), and Sadovy de Mitcheson and Erisman (3)—it is also possible that the lack of examples stems, at least in part, from challenges associated with accurately estimating population size. Our work provides clear evidence that Nassau grouper FSAs can recover through concerted management action and effective enforcement and compliance (see SI Appendix, Supplement A, Table S1 for management timeline).
Nearly all studies presenting evidence for FSA overfishing rely on estimates of spawner abundance or catch at FSAs as a proxy for population status. That is, the change in the abundance or catch of spawners at the FSA is assumed to correlate with a change in stock status (2, 14, 54, 64, 65). Multiple lines of evidence support this assumption. First, in areas with long-term catch records (e.g., Bermuda, Cuba), the collapse of catch at FSAs coincides with region-wide, persistent stock collapse (66–68). Second, multiple studies of aggregating grouper species have demonstrated high FSA site fidelity across spawning seasons, suggesting FSA attendants represent a persistent catchment of regional stocks (69–72). Finally, several tagging studies have demonstrated that the vast majority of reproductively mature individuals attend FSAs each year (69, 73). Taken together, these findings strongly support the assumption that changes in the size of an FSA are a proxy for changes in the regional stock of spawners. For the purposes of this study, we assume that island-specific FSAs reflect island-specific stocks, because 1) prior tagging studies indicate that few (2), if any (69, 74), Nassau grouper cross deep water (>250 m) during reproductive migrations, and 2) none of our tagged fish from any year or island in our study were resighted outside the island on which they were tagged.
Regardless of the modeling methods or data sources used, the size of Nassau grouper FSAs on Little Cayman and Cayman Brac have increased. The increase in FSA size on Little Cayman is particularly apparent, where Nassau grouper have approximately tripled in number since 2009 (Fig. 2). The Cayman Brac FSA also appears to have increased in abundance over this window of time (Fig. 3), although this finding is driven almost exclusively by observations from 2018. As such, it is not clear whether the rapid 2018 population increase is real or a manifestation of sparse observation coverage between 2008 and 2018. Ultimately, the 10-y gap in observations on Cayman Brac demands that we temper our conclusions regarding the ongoing recovery of Nassau grouper on that island. While the existing data are certainly suggestive of population growth, future observations will allow us to build confidence that Cayman Brac’s population is, in fact, recovering. Based on our findings from 2018, the current population of Nassau grouper on Cayman Brac is roughly one-third that of Little Cayman, despite having a similar total area of habitat available (Fig. 1). It is therefore reasonable to assume that the population on Cayman Brac has potential for considerable future growth.
Rather than reflecting a lack of effort, the sparsity of data from Cayman Brac reflects the challenges of in situ observations at some FSA sites. FSAs, particularly for large-bodied grouper and snapper species, tend to occur at locations that are difficult to reach. While the Little Cayman Nassau grouper aggregation is arguably one of the most accessible in the Caribbean, it exists on an exposed shelf break, requires a 30-min boat transit to the site, and can only be surveyed in good conditions by research divers with the skills and certification to regularly conduct 30-m dives. Access to the Cayman Brac aggregation requires triple the transit time, and the aggregation typically forms below safe scientific research diving limits (>33 m). Conditions at Grand Cayman’s aggregation site preclude sustained research diving activities. The logistical challenges associated with collecting the types of data we present here over a decade or more of sampling will likely preclude the replication of our approach and findings at many, if not most, FSAs. On the other hand, emerging technologies, such as passive and active acoustics (75–78), hold promise for providing alternative forms of fisheries-independent observations to document conservation success elsewhere.
While we observed increases in FSA size on both Little Cayman and Cayman Brac, in neither case was the growth immediate or constant through time. The integrated population model applied to data from Little Cayman suggests that the abundance of Nassau grouper at the FSA may have declined somewhat between 2005 and 2008, despite spawning-site protections beginning in 2003. The cause of the apparent 5-y delay in recovery following management action is unclear. However, the surprisingly high interannual variability in estimates of FSA size on Little Cayman, particularly when considering the mark–resight only model results (Figs. 2 and 3), may provide a clue as to the cause of this delay. A dramatic year-over-year increase in Little Cayman FSA abundance (e.g., 2016 to 2017) may be due to episodic recruitment, given that 1) the species has a pelagic larval duration of between 35 to 45 d (21, 22), so stochastic oceanography may play a substantial role in establishing a successful year class, and 2) the proximal populations of Nassau grouper in the region have been fished to collapse (23, 24, 79), so consistent larval supply from elsewhere is nonexistent. Therefore, the observed 2005 to 2008 decline may be attributed to a lack of strong year classes in the previous years.
This notion of episodic recruitment is supported by the fact that we observed a large recruitment event of 1-y-old fish on both Little Cayman and Cayman Brac in 2012 (80). Nassau grouper reach sexual maturity between 4 to 8 y of age (22), and, as such, the subadult Nassau grouper observed in 2012 should have matured and recruited to the FSA between 2015 and 2018. This timing coincides with the large population increases in the latter 3 y of this study (Fig. 2). The hypothesis of recruitment limitation due to region-wide overfishing fits within the broader notion that Allee effects act to suppress aggregating species following steep population declines (81–83). Other potential mechanisms contributing to recruitment-related Allee effects include a breakdown in spawning behavior at small FSAs (10) or a breakdown in the social transmission of FSA locations (e.g., “learning where to spawn”) at low population sizes (10, 29). Regardless of the specific mechanisms leading to the apparent delayed recovery, it is clear that persistent long-term protections have the potential to ultimately foster recovery.
While we were unable to collect the tagging and video census data necessary to include Grand Cayman in our analysis, the management actions taken by the Cayman government encompass all 3 islands. Although exceptionally coarse, observational evidence from 2012 and 2018 suggests that the FSA has remained highly depressed (500 or less fish) over the same time frame that Little Cayman and Cayman Brac showed marked growth. The apparent failure of the Grand Cayman FSA to respond to management action may reflect the Allee effects described above. However, there is no doubt that a high level of reserve-boundary fishing observed between 2003 and 2016 contravened any potential population gains. In addition, the CI-DoE prosecuted multiple instances of poaching inside FSA reserve boundaries during this time. These issues, in part, led to the development and passage of the legislation establishing complete seasonal closures on the fishery in 2016. It remains to be seen whether this adaptive management will lead to sustained recovery on Grand Cayman. Nonetheless, our findings from Grand Cayman, while sparse, highlight the challenges of motiving FSA recovery in areas near large human populations.
This study quantitatively demonstrates sustained, multidecadal Nassau grouper recovery following conservation actions precipitated by fishing-induced collapse. The only other study we are aware of that documents recovery of a Nassau grouper population comes from the US Virgin Islands Grammanik bank, were divers estimated a change in FSA size from ∼30 to ∼100 fish over a 5-y window of time (based on diver observations such as those we present here for Grand Cayman). Beyond Nassau grouper, studies have documented Red Hind (Epinephelus guttatus) FSA recovery on the Grammanik bank based on both length–frequency analysis (63) and abundance (70). In the Pacific, researchers used transect methods to document increased abundance of multiple grouper species at a multispecies FSA site 5 y after community-based marine protected-area establishment (84). Across these studies, seasonal and place-based protections emerge as common management actions for success.
The Cayman Island government has taken a multifaceted management approach by enacting a closed season, creating year-round FSA site closures, and implementing gear restrictions, slot limits, and bag limits during the open season. Even with these aggressive management actions, it is clear that population recovery takes considerably longer than time-to-maturity for the species, indicating a multigenerational timeline is necessary for conservation success. In Little Cayman, for instance, after 15 y of protections, the population has only very recently recovered to near the estimated size of the FSA when discovered in 2001. Furthermore, despite having the same level of protection, the population on Cayman Brac appears to have only very recently undergone population expansion. Other countries with FSAs of Nassau grouper and other aggregating species should thus expect that management actions aimed at recovering FSA stocks, while ultimately effective, may take decades or longer to meet management targets.
Supplementary Material
Acknowledgments
We thank the many research divers who have participated in and supported the REEF/CI-DoE Grouper Moon Project. We also thank the generous support and logistical assistance provided by local Cayman businesses and residents (including, but not limited to, Little Cayman Beach Resort, Southern Cross Club, and Guy Harvey). Funding was provided in part by the Lenfest Ocean Program, the Disney Conservation Fund, National Oceanic and Atmospheric Administration International Coral Reef Conservation Program Grant NA04NOS4630287, P. Hillenbrand, and the J. Edward Mahoney Foundation. L.W. was funded by Sea Grant and National Marine Fisheries Service Fisheries-Sea Grant Fellowship NA13OAR4170110 E/PD-11 and the Philanthropic Educational Organization Scholar Award.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All data and code used to develop the results reported in this paper are available on GitHub (https://github.com/WaterLynn) and Zenodo (DOI: 10.5281/zenodo.3585864).
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917132117/-/DCSupplemental.
References
- 1.Coleman F. C., Koenig C. C., Collins L. A., Reproductive styles of shallow-water groupers (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environ. Biol. Fishes 47, 129–141 (1996). [Google Scholar]
- 2.Sala E., Ballesteros E., Starr R. M., Rapid decline of nassau grouper spawning aggregations in Belize: Fishery management and conservation needs. Fisheries 26, 23–30 (2001). [Google Scholar]
- 3.Sadovy de Mitcheson Y., Erisman B. E., “Fishery and biological impications of fishing spawning aggregations, and the social and economic importance of aggregating fishes” in Reef Fish Spawning Aggregations: Biology, Research and Management, Sadovy de Mitcheson Y., Colin P. L., Eds. (Springer Netherlands, Dordrecht, The Netherlands, 2012), pp. 225–284. [Google Scholar]
- 4.Sadovy De Mitcheson Y., et al. , A global baseline for spawning aggregations of reef fishes. Conserv. Biol. 22, 1233–1244 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Sherman K. D., Dahlgren C. P., Knowles L. C., Nassau Grouper (Epinephelus striatus) (Conservation Management Plan, 2018). [Google Scholar]
- 6.Sadovy de Mitcheson Y., et al. , Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 14, 119–136 (2013). [Google Scholar]
- 7.Heppell S. A., et al. , Documenting recovery of a spawning aggregation through size frequency analysis from underwater laser calipers measurements. Biol. Conserv. 155, 119–127 (2012). [Google Scholar]
- 8.Bush P. G., et al. , “The Nassau grouper spawning aggregation fishery of the Cayman Islands–An historical and management” in Proceedings of the 57th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2006), pp. 515–524. [Google Scholar]
- 9.Colin P. L., Reproduction of the Nassau grouper, Epinephelus striatus (Pisces: Serranidae) and its relationship to environmental conditions. Environ. Biol. Fishes 34, 357–377 (1992). [Google Scholar]
- 10.Colin P. L., Longevity of some coral reef fish spawning aggregations. Copeia 1, 189–192 (1996). [Google Scholar]
- 11.Coleman F. C., et al. , Long-lived reef fishes: The grouper-snapper complex. Fisheries 25, 14–21 (2000). [Google Scholar]
- 12.Rose G. A., Kulka D. W., Hyperaggregation of fish and fisheries: How catch-per-unit-effort increased as the northern cod (Gadus morhua) declined. Can. J. Fish. Aquat. Sci. 56, 118–127 (1999). [Google Scholar]
- 13.Erisman B. E., et al. , The illusion of plenty: Hyperstability masks collapses in two recreational fisheries that target fish spawning aggregations. Can. J. Fish. Aquat. Sci. 68, 1705–1716 (2011). [Google Scholar]
- 14.Sadovy Y., Domeier M., Are aggregation-fisheries sustainable? Reef fish fisheries as a case study. Coral Reefs 24, 254–262 (2005). [Google Scholar]
- 15.Heppell S. A., et al. , “Behavior, hyperstability, and population declines of an aggregating marine fish” in Proceedings of the 66th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2013), pp. 379–380. [Google Scholar]
- 16.Hamilton R. J., et al. , Hyperstability masks declines in bumphead parrotfish (Bolbometopon muricatum) populations. Coral Reefs 35, 751–763 (2016). [Google Scholar]
- 17.Sadovy Y., “The case of the disappearing grouper: Epinephelus striatus, the nassau grouper, in the caribbean and Western Atlantic” in Proceedings of the 45th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 1997), pp. 5–22. [Google Scholar]
- 18.Sadovy Y., Aguilar-Perera A., Sosa-Cordero E., Epinephelus striatus: The IUCN Red List of Threatened Species 2018 (International Union for Conservation of Nature and Natural Resources, 2018), report no. e.T7862A46909843.
- 19.National Oceanic and Atmospheric Administration , Endangered and threatened wildlife and plants: Final listing determination on the proposal to list the Nassau grouper as threatened under the Endangered Species Act. Fed. Reg. 81, 42268–42285 (2016). [Google Scholar]
- 20.Whaylen L., Pattengill-Semmens C. V., Semmens B. X., Bush P. G., Boardman M. R., Observations of a Nassau grouper, Epinephelus striatus, spawning aggregation site in Little Cayman, Cayman Islands, including multi-species spawning information. Environ. Biol. Fishes 70, 305–313 (2004). [Google Scholar]
- 21.Colin P. L., Laroche W. A., Brothers E. B., Ingress and settlement in the nassau grouper, Epinephelus striatus (Pisces: Serranidae), with relationship to spawning occurrence. Bull. Mar. Sci. 60, 656–667 (1997). [Google Scholar]
- 22.Sadovy Y., Eklund A., Synopsis of Biological Data on the Nassau Grouper, Epinephelus striatus (Bloch, 1792), and the Jewfish, E. itajara (Lichtenstein, 1822) (Tech Rep. NMFS 146, National Oceanic and Atmospheric Administration National Marine Fisheries Service, 1999).
- 23.Jackson A. M., et al. , Population structure and phylogeography in Nassau grouper (Epinephelus striatus), a mass-aggregating marine fish. PLoS One 9, e97508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernard A. M., et al. , The ups and downs of coral reef fishes: The genetic characteristics of a formerly severely overfished but currently recovering Nassau grouper fish spawning aggregation. Coral Reefs 35, 273–284 (2016). [Google Scholar]
- 25.Paris C. B., Cowen R. K., Claro R., Lindeman K., Larval transport pathways from Cuban spawning aggregattions (Snappers; Lutjanidae) based on biophysical modeling. Mar. Ecol. Prog. Ser. 296, 93–106 (2005). [Google Scholar]
- 26.Semmens B. X., et al. , “Charting a course for nassau grouper recovery in the caribbean: What we’ve learned and what we still need to know” in Proceedings of the 60th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2007), pp. 607–609. [Google Scholar]
- 27.Smith C. L., A spawning aggregation of nassau grouper, Epinephelus striatus (Bloch). Trans. Am. Fish. Soc. 101, 257–261 (1972). [Google Scholar]
- 28.Stump K., Dahlgren C. P., Sherman K. D., Knapp C. R., Nassau grouper migration patterns during full moon suggest collapsed historic fish spawning aggregation and evidence of an undocumented aggregation. Bull. Mar. Sci. 93, 375–389 (2017). [Google Scholar]
- 29.Bolden S. K., Long-distance movement of a Nassau grouper (Epinephelus striatus) to a spawning aggregation in the central Bahamas. Fish. Bull. U.S. 98, 642–645 (2000). [Google Scholar]
- 30.Claro R., Sadovy de Mitcheson Y., Lindeman K. C., García-Cagide A. R., Historical analysis of Cuban commercial fishing effort and the effects of management interventions on important reef fishes from 1960-2005. Fish. Res. 99, 7–16 (2009). [Google Scholar]
- 31.Sadovy Y., Cheung W. L., Near extinction of a highly fecund fish: The one that nearly got away. Fish Fish. 4, 86–99 (2003). [Google Scholar]
- 32.Sadovy de Mitcheson Y., Colin P. L., “Species case studies” in Reef Fish Spawning Aggregations: Biology, Research and Management, Sadovy de Mitcheson Y., Colin P. L., Eds. (Springer, 2012), pp. 405–565. [Google Scholar]
- 33.Erisman B. E., Mckinney-Lambert C., Sadovy De Mitcheson Y., “Sad farewell to C. Lavett-Smith’s iconic nassau spawning aggregation site” in Proceedings of the 66th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2013), pp. 421–422. [Google Scholar]
- 34.Aguilar-Perera A., Disappearance of a Nassau grouper spawning aggregation off the southern Mexican Caribbean coast. Mar. Ecol. Prog. Ser. 327, 289–296 (2006). [Google Scholar]
- 35.Ehrhardt N. M., Deleveaux V. K. W., The Bahamas’ Nassau grouper (Epinephelus striatus) fisheryTwo assessment methods applied to a dataDeficient coastal population. Fish. Res. 87, 17–27 (2007). [Google Scholar]
- 36.Ray G. C., McCormick-Ray M. G., Layman C. A., Silliman B. R., Investigations of Nassau grouper breeding aggregations at High Cay, Andros: Implications for a conservation strategy (Department of Fisheries, Nassau, The Bahamas, 2000).
- 37.Benedetti L. S., “Marine protected areas (MPAS) as a fisheries management tool for the Nassau grouper (Epinephelus Striatus) in Belize,” Master’s thesis, Ryerson University, Toronto, Canada (2013).
- 38.Chiappone M., Sluka R., Sealey K. M. S., Groupers (Pisces: Serranidae) in fished and protected areas of the Florida Keys, Bahamas and northern Caribbean. Mar. Ecol. Prog. Ser. 198, 261–272 (2000). [Google Scholar]
- 39.Cheung W. W. L., Sadovy de Mitcheson Y. J., Braynen M. T., Gittens L. G., Are the last remaining Nassau grouper Epinephelus striatus fisheries sustainable? Status quo in the Bahamas. Endanger. Species Res. 20, 27–39 (2012). [Google Scholar]
- 40.Sherman K. D., et al. , Historical processes and contemporary anthropogenic activities influence genetic population dynamics of nassau grouper (Epinephelus striatus) within the Bahamas. Front. Mar. Sci. 4, 393 (2017). [Google Scholar]
- 41.Schärer-Umpierre M., et al. , “Nassau Grouper epinephelus striatus fish spawning aggregations in the US Caribbean” in Proceedings of the 66th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2014), pp. 408–412. [Google Scholar]
- 42.Allee W. C., Animal aggregations, a Study in General Sociology (The University of Chicago Press, Chicago, IL, 1931). [Google Scholar]
- 43.Gascoigne J., Lipcius R. N., Allee effects in marine systems. Mar. Ecol. Prog. Ser. 269, 49–59 (2004). [Google Scholar]
- 44.Russ G. R., Lou D. C., Ferreira B. P., Temporal tracking of a strong cohort in the population of a coral reef fish, the coral trout, (Plectropomus leopardus) Serranidae: Epinephelinae, in the central Great Barrier Reef, Australia. Can. J. Fish. Aquat. Sci. 53, 2745–2751 (1996). [Google Scholar]
- 45.Caley M. J., et al. , Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 27, 477–500 (1996). [Google Scholar]
- 46.Cayman Islands , The National Conservation Law, 2013 (Law 24 of 2013) (Cayman Islands, 2016).
- 47.Waterhouse L., Semmens B. X., Data from “IntegratedStateSpace_tagvideodata.” GitHub. https://github.com/WaterLynn/IntegratedStateSpace_tagvideodata. Deposited 8 October 2019.
- 48.Waterhouse L., Semmens B. X., Data from “Data and Code for - Recovery of critically endangered Nassau grouper (Epinephelus striatus) in the Cayman Islands following targeted conservation actions.” Zenodo. https://zenodo.org/record/3585864#.XfzpzmRKiUk. Deposited 19 December 2019. [DOI] [PMC free article] [PubMed]
- 49.Schaub M., Abadi F., Integrated population models: A novel analysis framework for deeper insights into population dynamics. J. Ornithol. 152, S227–S237 (2011). [Google Scholar]
- 50.Semmens B. X., et al. , “An in situ visual mark-recapture method to assess the abundance of spawners at an aggregation site” in Proceedings of the 64th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2011), pp. 224–226. [Google Scholar]
- 51.Devore J. L., Probability and Statistics for Engineering and the Sciences (Cengage Learning, ed. 9, 2016). [Google Scholar]
- 52.R Core Team , R: A language and environment for statistical computing, version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria, 2018).
- 53.Su Y.-S., Yajima M., R2jags: Using R to run ‘JAGS,’ version 0.5-7 (2015). https://CRAN.R-project.org/package=R2jags. Accessed 10 October 2019.
- 54.Erisman B. E., et al. , Fish spawning aggregations: Where well-placed management actions can yield big benefits for fisheries and conservation. Fish Fish. 18, 1–17 (2015). [Google Scholar]
- 55.Evermann B. W., Marsh M. C., Fishes and fisheries of Porto Rico. Bull. United States Fish Comm. 20, 49–350 (1900). [Google Scholar]
- 56.Cushion N. M., Sullivan-Sealey K., “Landings, effort and socio-economics of a small-scale commercial fishery in The Bahamas” in Proceedings of the 60th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2008), pp. 162–166. [Google Scholar]
- 57.Hayes M. C., Peterson M. N., Heinen-Kay J. L., Langerhans R. B., Tourism-related drivers of support for protection of fisheries resources on Andros Island, The Bahamas. Ocean Coast. Manage. 106, 118–123 (2015). [Google Scholar]
- 58.Rudd M. A., Tupper M. H., The impact of nassau grouper size and abundance on scuba diver site selection and MPA economics. Coast. Manage. 30, 37–41 (2002). [Google Scholar]
- 59.Mumby P. P. J., et al. , Fishing down a Caribbean food web relaxes trophic cascades. Mar. Ecol. Prog. Ser. 445, 13–24 (2012). [Google Scholar]
- 60.Stallings C. D., Indirect effects of an exploited predator on recruitment of coral-reef fishes. Ecology 89, 2090–2095 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Mumby P. J., Harborne A. R., Brumbaugh D. R., Kappel C., Brumbaugh D. D. R., Grouper as a natural biocontrol of invasive lionfish. PLoS One 6, e21510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maljković A., Van Leeuwen T. E., Cove S. N., Predation on the invasive red lionfish, Pterois volitans (Pisces: Scorpaenidae), by native groupers in the Bahamas. Coral Reefs 27, 501 (2008). [Google Scholar]
- 63.Beets J., Friedlander A., Evaluation of a conservation strategy: A spawning aggregation closure for red hind, Epinephelus guttatus, in the U.S. Virgin Islands. Environ. Biol. Fishes 55, 91–98 (1999). [Google Scholar]
- 64.Grüss A., Robinson J., Heppell S. S., Heppell S. A., Semmens B. X., Conservation and fisheries effects of spawning aggregation marine protected areas: What we know, where we should go, and what we need to get there. ICES J. Mar. Sci. 71, 1515–1534 (2014). [Google Scholar]
- 65.Graham R. T., Carcamo R., Rhodes K. L., Roberts C. M., Requena N., Historical and contemporary evidence of a mutton snapper (Lutjanus analis Cuvier, 1828) spawning aggregation fishery in decline. Coral Reefs 27, 311–319 (2008). [Google Scholar]
- 66.Claro R., Sadovy de Mitcheson Y., Lindeman K. C., García-Cagide A. R., Historical analysis of Cuban commercial fishing effort and the effects of management interventions on important reef fishes from 1960–2005. Fish. Res. 99, 7–16 (2009). [Google Scholar]
- 67.Luckhurst B. E., “Trends in commercial fishery landings of groupers and snappers in Bermuda from 1975 to 1992 and associated fishery management issues” in Biology, Fisheries and Culture of Tropical Groupers and Snappers, ICLARM Conf. Proc. 48 (International Center for Living Aquatic Resources Management, 1996), pp. 286–297. [Google Scholar]
- 68.Johannes R. E., Squire L., Graham T., Sadovy Y., Renguul H., Spawning Aggregations of Groupers (Serranidae) in Palau (Nature Conservancy Marine Research Series Publication, The Nature Conservancy, 1999), No. 1, pp. 1–10, 88–106. [Google Scholar]
- 69.Starr R. M., Sala E., Ballesteros E., Zabala M., Spatial dynamics of the Nassau grouper Epinephelus striatus in a Caribbean atoll. Mar. Ecol. Prog. Ser. 343, 239–249 (2007). [Google Scholar]
- 70.Nemeth R. S., Population characteristics of a recovering US Virgin Islands red hind spawning aggregation following protection. Mar. Ecol. Prog. Ser. 286, 81–97 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeller D., Spawning aggregations:patterns of movement of the coral trout Plectropomus leopardus (Serranidae) as determined by ultrasonic telemetry. Mar. Ecol. Prog. Ser. 162, 253–263 (1998). [Google Scholar]
- 72.Bijoux J., et al. , Spawning aggregation dynamics of brown-marbled grouper and camouflage grouper at a remote Indian Ocean atoll. Endanger. Species Res. 22, 145–157 (2013). [Google Scholar]
- 73.Semmens B. X., et al. , “The spatial ecology of a Remnant Nassau Grouper Stock on Cayman Brac, Cayman Islands” in Proceedings of the 61st Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2009), pp. 98–105. [Google Scholar]
- 74.Semmens B. X., et al. , “Investigating the reproductive migration and spatial ecology of Nassau grouper (Epinephelus striatus) on Little Cayman Island using acoustic tags – An Overview” in Proceedings of the 58th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2007), pp. 191–198. [Google Scholar]
- 75.Egerton J. P., et al. , Hydroacoustics for the discovery and quantification of Nassau grouper (Epinephelus striatus) spawning aggregations. Coral Reefs 36, 589–600 (2017). [Google Scholar]
- 76.Rowell T., Nemeth R., Schärer M., Appeldoorn R., Fish sound production and acoustic telemetry reveal behaviors and spatial patterns associated with spawning aggregations of two Caribbean groupers. Mar. Ecol. Prog. Ser. 518, 239–254 (2015). [Google Scholar]
- 77.Semmens B. X., et al. , “Linking recordings of fish vocalization with observations of spawning behavior on a multi-species fish spawning aggregation” in Proceedings of the 66th Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute, Marathon, FL, 2014), pp. 413–414. [Google Scholar]
- 78.Rowell T. J., et al. , Estimating fish abundance at spawning aggregations from courtship sound levels. Sci. Rep. 7, 3340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cowen R. K., Paris C. B., Srinivasan A., Scaling of connectivity in marine populations. Science 311, 522–527, (2006). [DOI] [PubMed] [Google Scholar]
- 80.Camp E. F., et al. , Microhabitat associations of late juvenile Nassau grouper (Epinephelus striatus) off Little Cayman, BWI. Bull. Mar. Sci. 89, 571–581 (2013). [Google Scholar]
- 81.Lundquist C. J., Botsford L. W., Model projections of the fishery implications of the Allee effect in broadcast spawners. Ecol. Appl. 14, 929–941 (2004). [Google Scholar]
- 82.Stoner A. W., Davis M., Booker C. J., Negative consequences of allee effect are compounded by fishing pressure: Comparison of queen conch reproduction in fishing grounds and a marine protected area. Bull. Mar. Sci. 88, 89–104 (2012). [Google Scholar]
- 83.Hutchings J. A., Thresholds for impaired species recovery. Proc. Biol. Sci. 282, 20150654 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamilton R. J., Potuku T., Montambault J. R., Community-based conservation results in the recovery of reef fish spawning aggregations in the Coral Triangle. Biol. Conserv. 144, 1850–1858 (2011). [Google Scholar]
- 85.Cayman Islands, The Economics & Statistics Office–Grand Cayman , Population and Vital Statistics (2019). https://www.eso.ky/populationandvitalstatistics.html#4. Accessed 26 September 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.