Significance
Although small interfering RNAs (siRNAs) are promising agents for treating many diseases, cellular delivery difficulties have prevented their widespread application. The use of spherical nucleic acids (SNAs) as single-entity gene regulation agents could overcome this limitation, since SNAs naturally and rapidly enter over 50 cell types. Consequently, siRNA-SNAs have been extensively used for effecting knockdown in both research and clinical settings. However, the mechanism of gene regulation by siRNA-SNAs is not well understood. Herein, we study cytosolic processing of siRNA-SNAs and how it leads to gene knockdown. Informed by this mechanism, we designed an SNA architecture with an order-of-magnitude-higher siRNA density. Importantly, this higher density reduces the cellular toxicity of SNAs without loss in RNA interference performance.
Keywords: spherical nucleic acids, siRNA processing, gene regulation
Abstract
Spherical nucleic acids (SNAs) are nanostructures formed by chemically conjugating short linear strands of oligonucleotides to a nanoparticle template. When made with modified small interfering RNA (siRNA) duplexes, SNAs act as single-entity transfection and gene silencing agents and have been used as lead therapeutic constructs in several disease models. However, the manner in which modified siRNA duplex strands that comprise the SNA lead to gene silencing is not understood. Herein, a systematic analysis of siRNA biochemistry involving SNAs shows that Dicer cleaves the modified siRNA duplex from the surface of the nanoparticle, and the liberated siRNA subsequently functions in a way that is dependent on the canonical RNA interference mechanism. By leveraging this understanding, a class of SNAs was chemically designed which increases the siRNA content by an order of magnitude through covalent attachment of each strand of the duplex. As a consequence of increased nucleic acid content, this nanostructure architecture exhibits less cell cytotoxicity than conventional SNAs without a decrease in siRNA activity.
Small interfering RNAs (siRNAs) are 22-nucleotide-long RNAs that are broadly utilized agents for gene silencing in biomedical research and disease treatment. Silencing of disease-causing genes by RNA interference (RNAi) offers the ability to target genetic diseases that cannot be regulated with small-molecule drugs, estimated to be 85 to 90% of the protein-coding genome (1). Additionally, since the physicochemical properties of small RNAs are largely sequence-independent, siRNA therapeutics, in principle, should enable the more rapid development of drugs. However, to be effective as drugs, delivery issues involving siRNAs must be overcome (2). Specifically, one must be able to deliver the siRNA in active form to the tissues and cells that matter. Since siRNAs, when systemically injected, distribute predominately to the liver and spleen and are rapidly degraded in serum, RNAi drug development still faces challenges (3).
To overcome these problems, we have explored the use of spherical nucleic acids (SNAs) as therapeutic modalities. SNAs typically comprise a spherical nanoparticle densely functionalized with highly oriented oligonucleotides (4–7). Remarkably, SNAs, unlike linear nucleic acids, are rapidly and actively internalized by a wide variety of mammalian cells, and the unusual architecture inhibits oligonucleotide degradation (8–12). SNAs have been used to treat many disease conditions in the clinic, including glioblastoma multiforme, psoriasis, and a variety of cancers (13–18). In addition, they have shown promise in mouse models for treating diabetic ulcers and spinal muscular atrophy. The prototypical SNA involves a gold nanoparticle chemically modified with thiol-terminated siRNA duplexes. In this architecture, the siRNA sense (passenger) strand is covalently anchored to the gold surface through thiol adsorption, and the antisense (guide) strand is noncovalently base-paired to the passenger strand. Although these structures can function as potent inhibitors of gene expression, no one has probed the mechanism of action of these SNAs (13–16). Specifically, it is not clear whether dissociation of RNA from the SNA is required for gene silencing and whether the SNA acts through the canonical RNAi pathway.
To probe the mechanism of SNA action, we have chosen Drosophila as a model system since it provides the ability to genetically and biochemically manipulate the RNAi pathway (19). Moreover, the Drosophila RNAi pathway is well understood and is virtually identical to the mammalian pathway (20). It begins with the introduction of double-stranded RNA (dsRNA) into cells. When dsRNA is detected in the cytoplasm, it is processed into 22-nucleotide duplex siRNAs by the ribonuclease Dicer-2 (Dcr-2), which is in complex form with Loquacious (Loqs)—a Drosophila ortholog of TRBP (19, 21). Each duplex siRNA product is then released, and thereafter the siRNA duplex binds to a complex composed of Dcr-2 and R2D2, another TRBP ortholog. This forms the R2D2-Dcr-2 Initiator (RDI) complex (22, 23). The RDI complex then recruits a Taf11 protein tetramer to form the RNA-induced silencing complex (RISC) Loading Complex (RLC) (22, 24). The duplex siRNA then transfers its binding from RLC to the protein Argonaute 2 (Ago2), creating the pre-RISC complex composed of siRNA duplex and Ago2 (25). Ago2 carries out specific cleavage of the passenger strand of the duplex siRNA, and only the guide strand is retained with Ago2 to form the mature RISC complex (25–27). RISC is the effector for RNAi by cleaving any messenger RNA (mRNA) that anneals to the siRNA guide strand. A simplified version of this pathway is shown in Fig. 1A (blue shaded region).
Fig. 1.
The RNAi pathway in Drosophila and possible mechanisms for SNA processing. (A) The blue shade marks the canonical RNAi pathway in Drosophila: Dicer-2 and Loqs bind to long dsRNA and cleave it into a 22-nucleotide duplex siRNA. Dicer-2 and R2D2 bind to this siRNA and form the RDI complex. Then Ago2 is recruited, which cleaves the passenger strand, forming active RISC. Pathway 1 is characterized by the desorption of the siRNA from the surface of the SNA, which then behaves like free linear siRNA in its fate after desorption. Pathway 2 initiates with a Dicer-2/R2D2 complex binding to siRNA on the surface of the SNA to form a bound RDI complex. It then recruits Ago2 to the SNA, which forms RISC with the guide strand and is free to dissociate. Pathway 3 is characterized by Ago2 directly binding and processing the siRNA on the surface of the SNA and forms a “minimal RISC.” Finally, pathway 4 initiates with a Dicer-2/Loqs complex cleaving the SNA-bound siRNA, and the liberated siRNA then enters the canonical RNAi pathway. Common entry points into the pathway are shown in dashed boxes, while the catalytically active endpoints are shown in solid boxes. Solid and dashed arrows depict experimentally verified and hypothesized steps, respectively. Shading illustrates steps that involve linear (blue) or spherical (green) forms of RNA. (B) Injection of SNAs into Drosophila embryos effectively knocks down bicoid mRNA levels as measured by RT-qPCR. The targeting SNAs and siRNAs exhibit comparable knockdown efficiency with equimolar amounts of nucleic acid injected per embryo. A control SNA with heterologous sequence composition to bicoid does not significantly inhibit the mRNA level of bicoid (***P < 0.001). Each bar shows the mean of at least 3 independent experiments and 3 replicate RT-qPCR measurements. Error bars show aggregate SEM of biological and technical replicates. ns, not significant.
Since SNAs cannot be clearly categorized as containing either dsRNA or bona fide duplex siRNA, we considered 5 possible mechanisms for explaining how SNAs silence gene expression (Fig. 1A): 1) Modified siRNA duplexes on the nanoparticle surface are passively released through the desorption of the gold-thiolate bond followed by entry into the RNAi pathway as RDI complexes. 2) RDI, RLC, and pre-RISC complexes serially assemble on the nanoparticle, and active RISC is released with the guide strand after unwinding. 3) Ago2 directly binds to the modified siRNA duplexes on the nanoparticle, and Ago2 is released with the guide strand after unwinding. 4) Dcr-2/Loqs complexes bind to the modified siRNA duplexes on the nanoparticle and cleave the siRNA, and the resulting duplex siRNAs are released for entry into the pathway as RDI complexes. 5) The SNAs use a mechanism independent of RNAi.
Here, we describe a set of experiments that evaluate these possibilities, and from them we have determined that the fourth pathway (Dcr-2 cleavage of siRNA from the nanoparticle) accounts for how SNAs silence gene expression. Importantly, an understanding of this process has created a blueprint for synthesizing a generation of SNAs with an order-of-magnitude-increased siRNA loading, which effectively increases the weighted contribution of the nucleic acid in the therapeutic candidate.
Results
Validation of the SNA Activity in Drosophila Embryos.
First, we wanted to determine whether Drosophila embryos are a valid system for testing siRNA-SNAs. To assess if SNAs can silence genes in this system, we injected Drosophila embryos with SNAs and measured the knockdown of expression for an endogenous target gene called bicoid. The SNAs induced silencing of bicoid expression that was highly comparable to silencing induced by injection of equimolar amounts of a linear siRNA duplex against bicoid composed of the same sequence (Fig. 1B). Furthermore, there was no silencing of bicoid expression with an SNA composed of a nonspecific siRNA. Thus, SNAs act as specific inhibitors of gene expression in the Drosophila system.
Release of siRNA from SNAs Is Dcr-2–Dependent.
Next, we sought to understand how siRNA-SNAs act within Drosophila cells. To differentiate between the 5 mechanisms described above, we dissected the mechanism using biochemical assays derived from Drosophila embryo extract. We assessed which, if any, factors are required for the release of siRNA from the nanoparticle surface. To this end, we incubated SNAs containing radioactively labeled guide strands with Drosophila embryo extract. We then separated the reaction mixtures using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) to denature protein complexes without dehybridizing siRNAs. We quantified the band intensities for the SNA, free duplex RNA, and free guide strand from 3 independent experiments (Fig. 2A, with a sample gel shown in Fig. 2B). Clearly, siRNA was released from the SNAs as a duplex when incubated with wild-type (WT) embryo extract. There was no detectable single-stranded guide strand released in the WT reaction. This result is consistent with previous observations that ∼1% of guide strand is rendered single-stranded by Drosophila embryo extracts within the time frame of this assay (25). Strikingly, duplex siRNA was not released when incubated with extract from embryos with the dcr-2 gene knocked out (labeled KO), nor was it released when incubated with extract from embryos in which Dcr-2 is catalytically inactivated by a single amino acid mutation (labeled CI). Knocking out or catalytically inactivating Ago2 had no effect on siRNA release (Fig. 2A). This result shows that the duplex cleavage step described in mechanism 4 takes place and suggests that further processing of the liberated siRNA might proceed through the canonical RNAi pathway
Fig. 2.
Release of siRNA from the SNA is Dcr-2 dependent. We incubated WT or mutant Drosophila embryo extracts with SNAs containing a radioactive guide strand. We analyzed the release of radioactive siRNA from the nanoparticle using an SDS/PAGE gel. (A) The quantification of the band intensities for 3 independent experiments for duplex RNA and guide strand RNA as a percentage of total radioactive signal. WT extract causes significant release of duplex siRNA from the SNA, indicating that a factor from the extract is required for siRNA processing. This release is abolished when extracts from Dcr-2 KO or CI embryos are used. Ago2 KO or CI extracts do not affect duplex siRNA release. Release of guide strand is not significant among any of the groups (***P < 0.001, ****P < 0.0001). Error bars show SEM of 3 independent experiments. (B) A sample SDS/PAGE gel used to quantify the bands for SNA, duplex, and guide strand. Left lane, no embryo extract control reaction. Right 2 lanes, single-stranded (ss) guide siRNA and duplex (ds) siRNA were run alone on the gel.
After Release, the siRNA Follows the Canonical RNAi Pathway.
The liberation of duplex siRNA from the SNA surface by Dcr-2 would suggest that liberated RNA might generate an RDI complex composed of duplex siRNA, Dcr-2, and R2D2. We previously characterized the RDI, as well as other complexes in the RNAi pathway, by biochemical assays using Drosophila embryo extracts (19, 22, 25). To determine whether these complexes form after the release of siRNA from the SNA as suggested by hypothesis 4, we incubated radioactively labeled SNAs with Drosophila embryo extract and resolved various complexes by native gel electrophoresis after removal of the SNAs by centrifugation. When WT extract was incubated with either linear siRNA duplexes or SNA-siRNAs, complexes corresponding to RDI and RISC were detected in both reactions (Fig. 3). The formation of these complexes from SNA-siRNA substrates indicates that after siRNA is released from SNAs the siRNA enters the RNAi pathway similar to linear siRNA duplexes. When either linear siRNAs or SNA-siRNAs were incubated with extracts from Dcr-2 KO or Dcr-2 CI mutant embryos, RDI and RISC complex formation was minimized (Fig. 3). Thus, siRNA incorporation into RISC from SNA substrates requires Dcr-2, which is also a property of linear siRNAs.
Fig. 3.
Complexes formed after the release of siRNA from SNAs are similar to those formed from linear siRNA duplexes. WT or mutant Drosophila embryo extracts were incubated with linear siRNA duplex (A) or SNAs (B) containing radioactive siRNA duplex. The SNAs were removed by centrifugation and the complexes formed were then separated on a native PAGE gel. Please note that both panels are from the same gel image with different brightness values, as SNAs complexes have weaker intensities. The asterisk indicates radioactivity in the well that did not enter the gel.
The formation of faint bands in these mutant extracts may be due to Dcr-1, which has been shown to partially compensate for Dcr-2 in Drosophila melanogaster (19). Additionally, higher intensities for RDI and RISC bands were observed from linear siRNA compared to SNAs. Since Dcr-2 cleavage is the only step that differs between the SNA and the linear siRNA pathways, this result suggests that the Dcr-2 cleavage of siRNA from SNAs is the rate-limiting step. We have previously demonstrated that nuclease activity is slower on SNAs compared to linear oligonucleotides (8–10, 12). The difference in RDI band intensities suggests that the RNase III activity of Dcr-2 is slower for SNAs than for linear siRNA. To test if the slower cleavage of SNAs impact gene silencing kinetics, we tested gene knockdown of SNAs and linear siRNA over time (SI Appendix, Fig. S1). Importantly, SNAs yield levels of knockdown similar to those of the linear siRNA, although it takes 2.6 times longer to achieve maximum knockdown levels; the mRNA half-lives for linear siRNA and SNA are 7.7 and 19.6 h, respectively.
Dcr-2 Is Necessary for SNA-Mediated Gene Silencing.
The in vitro experiments show that Dcr-2 is required for siRNA release from SNAs, and the released siRNA duplex forms RISC complexes through the canonical RNAi pathway. However, it is possible that more than one of the pathways we considered are operative in cells and generate RISC. To determine if any other of the proposed pathways generate active RISC, we injected dcr-2 KO or dcr-2 CI mutant embryos with SNAs and measured bicoid gene silencing by qPCR. If removal of Dcr-2 dicing activity blocked gene knockdown by SNAs, it would indicate that the only mechanism that generates active RISC is the Dcr-2 cleavage of siRNA from the surface of SNAs.
SNAs against bicoid achieved 65% gene silencing in WT embryos (Fig. 1B). However, when Dcr-2 KO or Dcr-2 CI embryos were injected with SNAs gene silencing activity was completely abolished (Fig. 4). This result shows that Dcr-2 cleavage activity is necessary for the gene silencing activity of siRNA-SNAs in cells. Since catalytic activity of Dcr-2 is necessary for the SNA function, the only possible pathway by which active RISC can be generated from SNAs is pathway 4, where RDI forms on the SNA and cleaves the siRNA from the nanoparticle surface and the RISC is subsequently formed from this linear siRNA-RDI complex. Surprisingly, dcr-2 KO mutant embryos showed weak but significant gene silencing when injected with linear siRNA duplexes (Fig. 4). This was not expected since previous studies found that Dcr-2 is essential for linear siRNA duplexes to load into RISC. However, the extent of silencing was impaired in dcr-2 KO embryos. Most likely, this effect is due to Dcr-1 compensation, an observation we previously noted (19).
Fig. 4.
Dcr-2 is necessary for gene silencing in vivo by SNAs. Injection of linear siRNA duplexes or SNAs into Drosophila embryos with dcr-2 KO or CI genotypes, followed by quantification of bicoid gene knockdown. In both mutants, the targeting SNA shows no silencing activity, indicating that the SNA function is dependent on Dcr-2 catalytic activity (*P < 0.05). Each bar shows the mean of 3 independent experiments and 3 replicate qPCR measurements. Error bars show aggregate SEM of biological and technical replicates.
An SNA Architecture Inspired by the Silencing Mechanism.
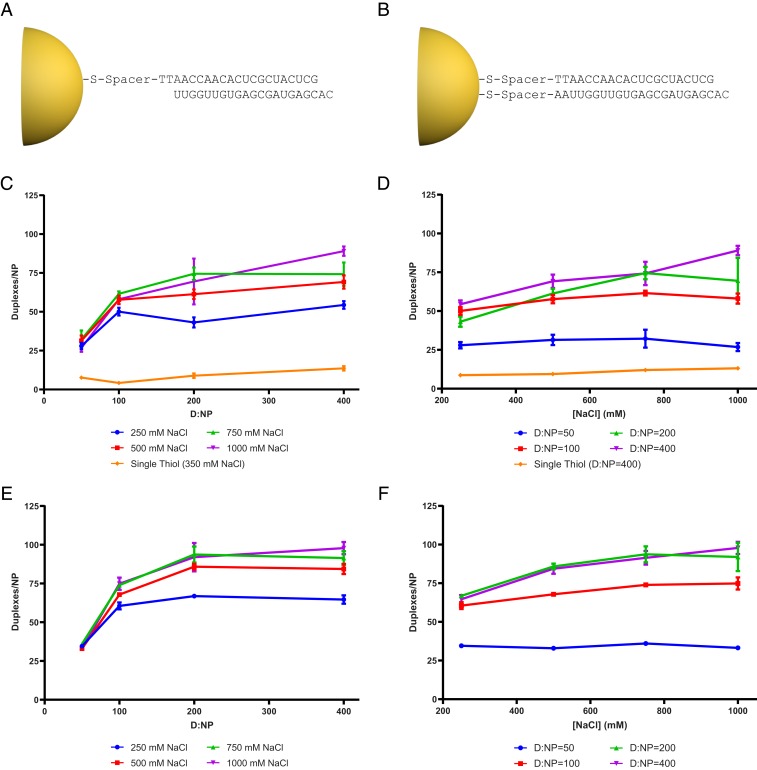
The mechanism of SNA action suggested a means to improve the SNA architecture for more efficient gene silencing. In previous SNA designs, the guide strand was hybridized to the passenger strand that was attached to the nanoparticle core (Fig. 5A). However, SNAs with this architecture had issues with duplex loading and stability. The loading density is governed by the electrostatic repulsion of oligonucleotides on the SNA, which results in dehybridization of duplexes at high oligonucleotide densities in these architectures (28, 29). This dehybridization is undesirable as it releases the therapeutic guide strand, which wastes material and lowers the amount of the active drug on the SNA. Previous work with this architecture typically achieved 40 duplexes per nanoparticle with <50% of the strand being duplexed, that is, >80 attached passenger strands per nanoparticle, with only 40 of these hybridized to their complement (15, 16). In addition, it is unknown if dehybridization occurs once SNAs are injected into animals, which could shed even more of the 40 duplexes before the SNAs reach their target tissues.
Fig. 5.
Doubly thiolated SNAs increase siRNA duplex loading on SNAs. Structure of (A) single-thiol and (B) doubly thiolated SNAs. Plots show duplex density as a function of D:NP ratio and NaCl concentration with disulfide duplex (C and D, respectively) and with thiol duplex (E and F, respectively). For both thiol and disulfide duplexes, increasing the D:NP ratio increases the duplex density until the electrostatic limit of oligonucleotide repulsion is reached for a given NaCl concentration. This limit can be overcome by addition of NaCl, which will increase duplex density until the oligonucleotides are depleted, or the absolute maximum density for a given sequence is reached. Even though both the thiol and disulfide forms of the duplex reach the absolute maximum loading density, using the thiol form of the duplex in the synthesis allows it to be reached with a lower D:NP ratio and NaCl concentration. Each data point shows the mean of 3 independent synthesis replicates and 3 independent duplex density measurements. The error bars show aggregated SEM of synthetic and technical replicates.
However, if Dicer cleaves the SNA-siRNA enzymatically, then both strands could be covalently attached to the nanoparticle and still function to silence genes. Therefore, we hypothesized that attachment of both strands would allow for better SNA design in terms of stability and loading. To test our hypothesis, we designed a nanoparticle architecture called a doubly thiolated SNA where both strands are thiolated such that they both covalently bind to the nanoparticle (Fig. 5B).
First, we wanted to estimate the duplex density achievable for this architecture. Estimation of duplex density on single-thiol SNAs relies on dehybridization of the guide strand with urea, which is not possible with doubly thiolated SNAs (12, 30). Therefore, we developed an assay that quantifies duplexes selectively (SI Appendix, Fig. S2). In this assay, we dissolved the gold core with potassium cyanide (KCN), digested the released RNA with an RNase mixture specific for single-stranded RNA, and quantified the remaining dsRNA with Picogreen reagent. Using this assay, we measured duplex densities for doubly thiolated SNAs synthesized under various conditions. Three parameters were varied combinatorially in the synthesis: 1) duplex to gold nanoparticle (D:NP) ratio, 2) NaCl concentration in the salt aging step, and 3) presence of a 3-hydroxypropyl disulfide group on the oligonucleotide terminus. These parameters affect loading of single-stranded thiolated oligonucleotides and the dehybridization of the duplex for the old architecture (31).
We synthesized SNAs in triplicate with all combinations of 250, 500, 750, and 1,000 mM NaCl; 50, 100, 200, and 400 D:NP ratio; and with oligonucleotides in free thiol form. We then measured duplex density per nanoparticle (Fig. 5 C–F and SI Appendix, Fig. S3). Duplex density was much greater for SNAs synthesized with the new architecture relative to the old architecture (Fig. 5 C and D). This enhancement was observed for all synthesis conditions. Our previous work suggests that this difference stems from the dehybridization of the duplex during synthesis for the single-thiol SNAs, which results in lower duplexing efficiencies. Note that duplex density is sequence- and environment-dependent within SNAs, and for the single thiol SNAs studied herein the maximum duplex density was on average 13 duplexes per particle. However, for the new architecture, the average duplex density was 98 duplexes per particle, at maximum loading.
In general, duplex density increases as a function of the D:NP ratio, and the magnitude of increase is dependent on NaCl concentration (Fig. 5 C and D). Since NaCl shields the charges of neighboring oligonucleotides, once the electrostatic limit for duplex density is reached for a given NaCl concentration, increasing the D:NP ratio does not increase loading any further. Single-thiol SNAs did not show an appreciable increase in loading with higher D:NP ratios, suggesting that the maximum loading equilibrium between the 2 strands had already been reached at the lowest D:NP ratio.
We also tested siRNA loading in which the disulfide bond on the oligonucleotide was reduced. Although this did not influence the maximum loading compared to the disulfide form, maximum loading was reached at lower NaCl concentrations and D:NP ratios for the reduced duplex (Fig. 5 E and F). This result suggests that even though 3-mercaptopropanol molecules adsorb onto the surface they can be replaced by oligonucleotides by increasing the D:NP ratio and NaCl concentration during synthesis. For both thiol and disulfide forms of the duplex, the maximum duplex density was almost an order-of-magnitude higher than single-thiol SNAs. Taken together, these results indicate that the duplex loading on doubly thiolated SNAs is solely governed by electrostatic interactions of the oligonucleotides as we hypothesized, and very high duplex densities can be achieved with the doubly thiolated SNA architectures.
To ensure that these trends were not sequence-dependent, we repeated these experiments with another oligonucleotide, which showed the same trend (SI Appendix, Fig. S4). However, for the second sequence, the maximum duplex density reached 136 duplexes per particle, compared to 98 duplexes per particle for the first sequence, suggesting that the maximum duplex density is also a function of sequence.
Duplex Density Does Not Influence siRNA Function.
We wanted to determine if doubly thiolated SNAs can still silence genes, and if so, assess the relationship between duplex loading and siRNA silencing. First, we compared doubly thiolated SNAs to SNAs with single thiols at the same density (40 duplexes per particle) and therefore at the same siRNA concentration. We observed that the doubly thiolated SNAs achieve similar levels of knockdown of the HER2 gene in mammalian cells as single-thiol SNAs (Fig. 6A). This result demonstrates that the mechanism of siRNA processing on SNAs is conserved between flies and mammals, as the covalently attached guide strand from doubly thiolated SNAs must have been cleaved for the siRNA to access RISC.
Fig. 6.
Doubly thiolated SNAs show gene knockdown activity and decreased cytotoxicity. (A) SKOV-3 cells were treated with SNAs targeting HER2 and matching linear siRNA controls, and target gene expression was assayed by qPCR. All HER2 targeting sequences show significant knockdown compared to nontargeting sequences (****P < 0.0001). However, none of the targeting sequences are statistically different. Each bar shows the mean of 3 independent experiments and 3 replicate qPCR measurements. Error bars show aggregate SEM of biological and technical replicates. (B) We quantified the relationship between gene knockdown efficiency of doubly thiolated SNAs and duplex density. We plotted the gene knockdown vs. duplex density between 40 and 160 duplexes per particle and fit each dataset (SI Appendix, Fig. S5). For both targeting and nontargeting SNAs, the average slope is zero, indicating that higher duplex densities do not hinder Dicer cleavage and hence SNA function. Each bar shows the mean of 4 independent experiments and 3 replicate qPCR measurements. Error bars show aggregate SEM of biological and technical replicates. (C) We treated cells with different concentrations of SNAs for 48 h and measured ATP in each well as a measure of cytotoxicity. Plots for the targeting sequence are shown here. The doubly thiolated SNA curve is shifted to the right, indicating that cytotoxicity is induced at higher siRNA concentrations. Each data point shows the mean of 4 biological replicates; error bars show the SEM of this mean. Dashed lines show the 95% confidence intervals for the fits. (D) We plotted the LD50 values from curves in C and SI Appendix, Fig. S8. For both the targeting and the nontargeting SNAs the LD50 value shifts by an order of magnitude, indicating that doubly thiolated SNAs are much less toxic to cells (****P < 0.0001). Error bars show SEM.
An additional concern with doubly thiolated SNAs is whether duplex density affects SNA function. It is possible that at very high duplex densities Dicer cleavage might become sterically hindered. To probe the relationship between duplex density and gene knockdown, we treated SKOV-3 cells with 4 SNAs having siRNA densities between ∼40 to ∼160 duplexes per particle. We adjusted the doses of SNAs per treatment such that there was a constant level of siRNAs introduced per treatment. Since our previous results have shown that the cellular uptake of SNAs is dependent on oligonucleotide density, we transfected the SNAs to ensure that the same amount of SNA is delivered to the cytoplasm in all cases (8, 11). We analyzed the resulting knockdown efficiency vs. duplex density plots with linear regression (SI Appendix, Fig. S5). The slope of the curve is a quantitative measure of how duplex density affects knockdown efficiency. Averaging the slopes of multiple independent experiments, we measured slopes that are statistically indistinguishable from zero (Fig. 6B). This result demonstrates that the varying duplex loading density does not detrimentally affect gene silencing. Interestingly, increasing the duplex density did not increase SNA potency, suggesting that the gene knockdown efficiency is a function of SNA concentration, instead of the siRNA concentration (SI Appendix, Fig. S6). This observation is consistent with the SNA’s being the active entity in gene knockdown. Since Dcr-2 cleavage is slower for SNAs compared to linear siRNA (Fig. 3 and SI Appendix, Fig. S1), it is probably the rate-limiting step in the SNA pathway. Hence, the overall rate of gene knockdown should be limited by SNA concentration regardless of architecture, since the siRNA released from SNAs are similar regardless of architecture. However, this result suggests that doubly thiolated SNAs may cause more durable gene knockdown than singly thiolated SNAs, as the higher duplex density would cause the release of siRNA for a longer duration than the lower density singly thiolated SNAs.
Doubly Thiolated SNAs with High Duplex Densities Reduce SNA Cytotoxicity.
SNAs can exhibit nonspecific cytotoxicity due to the gold nanoparticle if too many SNAs are taken up by cells. Since doubly thiolated SNAs allow more active siRNA to be loaded per gold nanoparticle, we hypothesized that this would decrease cytotoxicity without impacting efficacy of gene silencing. To test this hypothesis, we treated SKOV-3 cells with single-thiol and doubly thiolated SNAs for 24 or 48 h and measured the amount of ATP in cells as an indicator of cytotoxicity (Fig. 6C and SI Appendix, Fig. S7). Qualitatively, doubly thiolated SNAs show less toxicity compared to single-thiol SNAs at both time points. For 48 h, we quantified the cytotoxicity with a 4-parameter logistic fit and calculated median lethal doses (LD50) from these curves. We found that doubly thiolated SNAs decreased cytotoxicity of both targeting and nontargeting SNAs by an order of magnitude (Fig. 6D).
Discussion
The results reveal that siRNAs attached to gold nanoparticles are cleaved by Dicer, released, and processed through the canonical RNAi pathway. Using this knowledge, we designed an SNA architecture that achieves nearly an order-of-magnitude-higher duplex loading and thus increases the active siRNA agent within the SNA particle. These SNAs are promising for treating disease for multiple reasons. First, the higher loading of siRNA allows the treatment of cells with fewer nontherapeutic components, such as the nanoparticle core and single-stranded RNA. As demonstrated here, the reduction in these components decreases the cytotoxicity of the SNAs. The lower cytotoxicity might reduce side effects at a given siRNA dose compared to previous generations of SNAs, or allow treatment with a higher dose of siRNA in cases where toxicity limits the maximum therapeutic dose. In addition, siRNA stability can be increased with doubly thiolated SNAs. Due to the covalent attachment of both strands to the nanoparticle in the doubly thiolated SNAs, once the duplex attaches to the surface the release of either siRNA strand is expected to be minimal. Finally, prior work demonstrated how higher oligonucleotide density leads to increased cellular uptake and decreased degradation by nucleases (8–10, 12). These observations suggest that the increased duplex density of doubly thiolated SNAs will also result in higher cellular uptake and slower degradation of the siRNA. Taken together, these advances will increase SNA gene knockdown efficacy while reducing toxicity, thereby making such structures attractive options as gene regulatory materials for the treatment of disease.
Methods
Oligonucleotide Synthesis.
RNA oligonucleotides were synthesized using 2′-O-triisopropylsilyloxymethyl–protected phosphoramidites (Chemgenes). DNA bases were added as standard cyanoethyl phosphoamidites (Glen Research). For thiol modification of oligonucleotides, 1-O-dimethoxytrityl-propyl-disulfide,1′-succinyl-lcaa-controlled pore glass beads or S-trityl-6-mercaptohexyl-1-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite were used for 3′ and 5′ end modification, respectively (Glen Research). For spacer 18 modification, 18-O-dimethoxytritylhexaethyleneglycol,1-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite was used (Glen Research). The 5′ modified oligonucleotides were synthesized on 1,000-Å UnyLinker solid support (Chemgenes). Oligonucleotides were synthesized on a MerMaid 12 system and deprotected as recommended by the manufacturers (Bioautomation). Deprotected oligonucleotides were purified with reverse-phase high-performance liquid chromatography (HPLC) on a C18 column using 0.1 M triethylammonium acetate and acetonitrile as the solvent system. After HPLC, the 5′-DMT group was removed in 20% acetic acid at room temperature for 1 h, and the solution was extracted 3 times with ethyl acetate. The aqueous phase of this solution was lyophilized and resuspended in sterile water. The oligonucleotide sequences used in this work are listed in SI Appendix, Table S1.
Synthesis of SNAs.
Thirteen-nanometer gold nanoparticles (AuNPs) were synthesized using the Turkevich–Frens method, with the modification that chloroauric acid was reduced with sodium citrate in water (32). For single-thiol SNA synthesis, purified siRNA strands were duplexed in duplex buffer (30 mM Hepes, pH 7.5, 100 mM potassium acetate, and 2 mM magnesium acetate) at 100 µM siRNA concentration. The solution was heated to 95 °C for 5 min and the heater was turned off to slowly cool down the solution. For SNA synthesis, siRNA was added to a solution of 10 nM AuNP, 0.2% Tween-20 (vol/vol), and 150 mM NaCl to a final concentration of 4 µM duplex. This solution was incubated at room temperature with shaking overnight, and NaCl was added to 350 mM the next morning. After an additional 4-h incubation, the SNAs were purified in Amicon Ultra 100K molecular weight cutoff (MWCO) spin filters by washing with 10× volume of 1× PBS 4 times (MilliporeSigma).
Characterization of SNAs.
To measure the AuNP concentration of the purified SNAs, absorbance spectra of the SNAs were obtained in a Cary-5000 spectrophotometer (Agilent). The AuNP concentration was calculated using the absorbance value at 520 nm with an extinction coefficient of 2.27 × 108 M−1⋅cm−1. The guide strands on the nanoparticles were measured as described previously (30). The duplex densities of SNAs were calculated as the ratio of guide strand concentration to AuNP concentration.
Injection of Drosophila Embryos.
Injections into Drosophila embryos were performed as described previously, with modifications (33). Embryos were collected for 30 min, lined up for injections, and desiccated for 9 min in a closed chamber with Drierite. After injection, the embryos were incubated in a humidified chamber for 45 min and moved to a test tube and the tubes were stored on dry ice. For targeting and nontargeting SNAs, siRNA targeting the bicoid or luciferase mRNAs were used, respectively (SI Appendix, Table S1). Each biological replicate contained 15 embryos. The genotypes for the embryos are listed in SI Appendix.
mRNA Quantification in Drosophila Embryos.
mRNA was purified from embryos by lysing them in 5 µL of TRIzol per embryo (Thermo Fisher Scientific); 0.5 µL of chloroform per embryo was added to the TRIzol solution, incubated on ice for 15 min, and centrifuged to separate the aqueous and organic phases. The organic phase was transferred to a new tube and mixed with 3.2 µL of isopropanol per embryo. The mixture was incubated at −80 °C overnight and centrifuged at 16,000 × g for 15 min. The pellet was washed with 0.4 mL of 70% ethanol, air-dried, and resuspended in water. Complementary DNA was prepared from the RNA using SuperScript III as the reverse transcriptase (Thermo Fisher Scientific). The Bicoid and β-tubulin mRNA levels were quantified with qPCR using Power SYBR Green Master Mix (Thermo Fisher Scientific). Primer sequences are shown in SI Appendix, Table S2. The data were analyzed by the Pfaffl method to determine fold differences (34).
Radioactive Labeling of siRNA.
Thirty picomoles of HER2 guide strand was reacted with 32P-labeled ATP (6,000 Ci/mol; PerkinElmer) using 10 units of T4 polynucleotide kinase (New England Biolabs). The reaction was incubated at 37 °C for 1 h and the labeled oligonucleotide was run on a 20% denaturing urea-PAGE gel. The band for the labeled oligonucleotide was cut out and incubated in 400 µL of elution buffer (0.5M sodium acetate, pH 5.2, 1 mM EDTA, 2.5 mM Tris, pH 7.5, and 0.1% SDS) at room temperature for 8 h. After 3 elutions, the fractions were combined, and 3.3 mL of ethanol was added to the solution. The mixture was incubated at −20 °C overnight, followed by a 30-min incubation at −80 °C, and centrifuged at 16,000 × g for 15 min at 4 °C. The pellet was air-dried and resuspended in water. For the synthesis of labeled siRNA, 10 pmol of labeled strand was mixed with 10 pmol of unlabeled passenger strand in duplex buffer (30 mM Hepes, pH 7.5, 100 mM potassium acetate, and 2 mM magnesium acetate) in 10 µL, heated to 95 °C for 2 min, and slowly cooled to room temperature.
Synthesis of Radioactive Labeled SNAs.
The SNAs with only HER2 passenger strand were synthesized as described above, using only the passenger strand instead of the duplex. Ten picomoles of radioactively labeled guide strand was mixed with 90 pmol of passenger strand on this SNA in 300 mM NaCl, heated to 60 °C for 10 min, and slowly cooled to room temperature. To remove the unbound guide strand, the solution was centrifuged at 16,000 × g for 10 min at room temperature. The supernatant was removed and replaced with 200 mM NaCl, followed by 2 more washes. In the last 2 washes, the SNA was resuspended in 150 mM NaCl.
siRNA Release and Complex Formation Assays.
Drosophila embryo extracts were prepared as previously described, with 2 modifications (35). Dithiothreitol (DTT) and Pefabloc SC were replaced by 5 mM Tris(2-carboxyethyl)phosphine (TCEP) and 1 mM phenylmethylsulfonyl fluoride. The pH of the TCEP stock solution was adjusted to 7.5 before preparing the extract buffer. The reactions were performed as described previously, followed by electrophoresis in a 15% SDS/PAGE gel for the siRNA release assay, or 4% native PAGE gel for complex formation assays (22). For the native gels, reactions were set up in twice the volume (16 µL after heparin addition). The reactions were centrifuged at 16,000 × g for 10 min and 12 µL of the supernatant was removed. The supernatant was centrifuged again and 8 µL was removed and run on the gel. The band intensities for siRNA release gels were quantified in ImageJ.
Synthesis of Doubly Thiolated SNAs.
Doubly thiolated SNAs were synthesized as described above, with a few modifications. Thiolated guide strand was used in the duplexing of the siRNA (SI Appendix, Table S1). After the initial synthesis, NaCl was added up to 1 M in multiple steps of 250, 500, 750, and 1,000 mM, with a 2-h incubation at room temperature between additions. To determine duplex loading and reaction conditions, the SNAs were synthesized in high throughput starting with 200 µL of AuNP solution and purified using AcroPrep Omega 100K MWCO spin filters (Pall Corporation). To synthesize SNAs with duplexes terminated with free thiol, the duplex was reduced with 100 mM DTT and purified with a NAP-10 column according to the manufacturer’s instructions (GE Life Sciences). For these experiments, each SNA was synthesized in triplicate.
Characterization of Doubly Thiolated SNAs.
SNAs were characterized with spectrophotometry as described above for AuNP concentration. To determine duplex density we developed an assay, as Oligreen reagent does not allow differentiation between single- and double-stranded oligonucleotides. First, 2 µL SNAs at ∼100 to 300 nM by AuNP concentration were mixed with 5 µL of 40 mM KCN in 1× Tris-ethylenediaminetetraacetic acid (TE) + 300 mM NaCl. After a 15-min incubation at room temperature, 1 µL of RNase mixture (500 U/mL RNase A and 20,000 U/mL RNase T1) in 22 µL of 1× TE + 300 mM NaCl was added to digest single-stranded RNA (Ambion). The solution was incubated at 37 °C for 20 min, 70 µL of 1× Picogreen solution in 1× TE + 300 mM NaCl was added to this mixture, and the fluorescence was measured in a plate reader. The fluorescence readings were compared to a standard curve of the same duplex under the same reaction conditions. Each measurement was calculated in triplicate. The ratio of the duplex concentration to the AuNP concentration was reported as the duplex loading.
Quantification of Gene Knockdown in Mammalian Cells by qPCR.
SNAs targeting HER2 or Luciferase were transfected into SKOV-3 cells with RNAiMAX in 12-well plates using the manufacturer’s instructions. All treatments were done at 100 nM siRNA concentration. The cells were transfected for 48 h without changing the media, and the RNA was prepared from the cells using Purelink RNA Mini Kit (Thermo Fisher Scientific). The mRNA levels were quantified in triplicate with qScript One-Step RT-qPCR Kit (Quanta Biosciences). Taqman probes were ordered from Thermo Fisher Scientific and used per the manufacturer’s instructions (HER2: Hs01001580_m1, GAPDH: Hs03929097_g1). The CT values were normalized to untreated cells using the Pfaffl method (34). To compare SNA function, SNAs with 40 duplexes per particle were used with both SNAs architectures. To quantify gene knockdown at different densities, SNAs synthesized under the following conditions were used: 50 D:NP, 250 mM NaCl; 100 D:NP, 250 mM NaCl; 200 D:NP, 500 mM NaCl; 400 D:NP, 1,000 mM NaCl, which corresponded to duplex densities between 40 and 120.
Quantification of Gene Knockdown in Mammalian Cells by in-Cell Western.
SNAs targeting HER2 were transfected into SKOV-3 cells with RNAiMAX in 96-well plates using the manufacturer’s instructions. All treatments were done with 1.5 µL of RNAiMAX per well. The cells were transfected for 48 h without changing the media. Cells were washed with 1× phosphate-buffered saline (PBS) 3 times and fixed in methanol at −20 °C for 15 min. The wells were washed with 1× PBS 3 times and blocked with Odyssey blocking buffer (LI-COR) for 90 min with shaking. The cells were incubated with HER2 antibody (29D8, diluted 1:200 in Odyssey blocking buffer; Cell Signaling) for 2 h. The wells were washed with 1× PBS 3 times and incubated with 2 µg/mL IRDye 800CW donkey anti-rabbit secondary antibody (LI-COR) and 400 nM CellTag 700 (LI-COR) in Odyssey blocking buffer. The plate was washed 3 times in 1× PBS and imaged on an Odyssey CLx system (LI-COR).
Cytotoxicity of SNAs.
SNA solutions at 100 nM by AuNP were prepared in OptiMEM and serially diluted in half-log steps. These solutions were incubated with SKOV-3 cells in 96-well plates in quadruplicate for 24 or 48 h. The wells were washed 3 times with PBS to remove any SNAs and resuspended in 50 µL of PBS and the cytotoxicity was quantified using equal volume of CellTiter-Glo 2.0 reagent per the manufacturer’s instructions (Promega). The measurements were normalized to OptiMEM-only treatment.
Statistics.
All statistics were done using GraphPad Prism. All error bars shown are SEM. All P values were multiplicity adjusted to account for multiple comparisons. In cases where multiple independent experiments with multiple technical measurements in each experiment were performed (e.g., qPCR experiments), the SDs were calculated by propagating the technical error of the measurement into the SD of independent experiments with the following formula:
The means in these types of experiments were compared with an ordinary one-way ANOVA with Tukey’s multiple comparison test. The slopes for the gene knockdown vs. duplex densities plots were compared with an unpaired t test using Welch’s correction. The significance for the in vitro siRNA release assays was assessed with an ordinary one-way ANOVA, with Tukey’s multiple comparisons test. The cytotoxicity assay results were fit with a 4-parameter logistic curve using a least squares fit. LD50 values and the SEs for these curves were compared with an ordinary one-way ANOVA, with Tukey’s multiple comparisons test.
Data Availability Statement.
All data within this paper will be made available to readers upon reasonable request.
Supplementary Material
Acknowledgments
The work was supported by National Cancer Institute grants U54CA199091, P50CA221747, and R01CA208783 awarded to C.A.M. and National Institute of General Medical Sciences grant R35GM118144 awarded to R.W.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The project was also supported by the Prostate Cancer Foundation and the Movember Foundation (17CHAL08) awarded to C.A.M. G.Y. gratefully acknowledges support from the Chemistry of Life Processes T32GM105538 at Northwestern University. D.M.P. was supported by a Pew Latin American Postdoctoral Fellowship. G.Y. thanks Kevin Nyberg and Shelby Blythe for their help with the Drosophila embryo injections. This work made use of the Integrated Molecular Structure Education and Research Center and Keck Biophysics Facility at Northwestern University, which has received support from Northwestern University and the State of Illinois. The content is solely the responsibility of the authors and does not necessarily represent the official views of Northwestern University.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915907117/-/DCSupplemental.
References
- 1.Russ A. P., Lampel S., The druggable genome: An update. Drug Discov. Today 10, 1607–1610 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Conde J., Artzi N., Are RNAi and miRNA therapeutics truly dead? Trends Biotechnol. 33, 141–144 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Haussecker D., Kay M. A., RNA interference. Drugging RNAi. Science 347, 1069–1070 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirkin C. A., Letsinger R. L., Mucic R. C., Storhoff J. J., A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382, 607–609 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Rosi N. L., et al. , Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312, 1027–1030 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Banga R. J., et al. , Cross-linked micellar spherical nucleic acids from thermoresponsive templates. J. Am. Chem. Soc. 139, 4278–4281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler J. I., et al. , Polyvalent nucleic acid nanostructures. J. Am. Chem. Soc. 133, 9254–9257 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giljohann D. A., et al. , Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 7, 3818–3821 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seferos D. S., Prigodich A. E., Giljohann D. A., Patel P. C., Mirkin C. A., Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 9, 308–311 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prigodich A. E., Alhasan A. H., Mirkin C. A., Selective enhancement of nucleases by polyvalent DNA-functionalized gold nanoparticles. J. Am. Chem. Soc. 133, 2120–2123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayan S. P., et al. , The sequence-specific cellular uptake of spherical nucleic acid nanoparticle conjugates. Small 11, 4173–4182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnaby S. N., et al. , Design considerations for RNA spherical nucleic acids (SNAs). Bioconjug. Chem. 27, 2124–2131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giljohann D. A., Seferos D. S., Prigodich A. E., Patel P. C., Mirkin C. A., Gene regulation with polyvalent siRNA-nanoparticle conjugates. J. Am. Chem. Soc. 131, 2072–2073 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng D., et al. , Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc. Natl. Acad. Sci. U.S.A. 109, 11975–11980 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen S.A., et al. , Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 5, 209ra152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randeria P. S., et al. , siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. U.S.A. 112, 5573–5578 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewandowski K. T., et al. , Topically delivered tumor necrosis factor-α-targeted gene regulation for Psoriasis. J. Invest. Dermatol. 137, 2027–2030 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemati H., et al. , Using siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation in psoriasis. J. Control. Release 268, 259–268 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Lee Y. S., et al. , Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117, 69–81 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Carthew R. W., Sontheimer E. J., Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marques J. T., et al. , Loqs and R2D2 act sequentially in the siRNA pathway in Drosophila. Nat. Struct. Mol. Biol. 17, 24–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham J. W., Pellino J. L., Lee Y. S., Carthew R. W., Sontheimer E. J., A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell 117, 83–94 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Pham J. W., Sontheimer E. J., Molecular requirements for RNA-induced silencing complex assembly in the Drosophila RNA interference pathway. J. Biol. Chem. 280, 39278–39283 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Liang C., et al. , TAF11 assembles the RISC loading complex to enhance RNAi efficiency. Mol. Cell 59, 807–818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K., Lee Y. S., Carthew R. W., Conversion of pre-RISC to holo-RISC by Ago2 during assembly of RNAi complexes. RNA 13, 22–29 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matranga C., Tomari Y., Shin C., Bartel D. P., Zamore P. D., Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell 123, 607–620 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Rand T. A., Petersen S., Du F., Wang X., Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell 123, 621–629 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Randeria P. S., et al. , What controls the hybridization thermodynamics of spherical nucleic acids? J. Am. Chem. Soc. 137, 3486–3489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong L. K., Wang Z., Schatz G. C., Luijten E., Mirkin C. A., The role of structural enthalpy in spherical nucleic acid hybridization. J. Am. Chem. Soc. 140, 6226–6230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnaby S.N., Lee A., Mirkin C.A., Probing the inherent stability of siRNA immobilized on nanoparticle constructs. Proc. Natl. Acad. Sci. U.S.A. 111, 9739–9744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurst S. J., Lytton-Jean A. K., Mirkin C. A., Maximizing DNA loading on a range of gold nanoparticle sizes. Anal. Chem. 78, 8313–8318 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimling J., et al. , Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 110, 15700–15707 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Kennerdell J. R., Yamaguchi S., Carthew R. W., RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E. Genes Dev. 16, 1884–1889 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl M. W., A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuschl T., Zamore P. D., Lehmann R., Bartel D. P., Sharp P. A., Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 13, 3191–3197 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data within this paper will be made available to readers upon reasonable request.