Significance
Small-molecule inhibitors of enzymes are commonly derived from native ligands and represent invaluable and extensively applied tools in chemical biology. In addition to the well-known issue of selectivity, paradoxical curiosities were described over decades that have wide implications in drug discovery and treatment. Of particular interest are observations where low concentrations of inhibitors may activate their targets instead of causing partial inhibition. Our conceptual analysis explains the general phenomenon of “activation by inhibition” as a ligand-induced transition between the T state and the R state in the classic Monod–Wyman–Changeux model of activation, in which the inhibitory ligand acts as an activator.
Keywords: HtrA proteases, allostery, cooperativity, HTRA1, inhibitor
Abstract
Startling reports described the paradoxical triggering of the human mitogen-activated protein kinase pathway when a small-molecule inhibitor specifically inactivates the BRAF V600E protein kinase but not wt-BRAF. We performed a conceptual analysis of the general phenomenon “activation by inhibition” using bacterial and human HtrA proteases as models. Our data suggest a clear explanation that is based on the classic biochemical principles of allostery and cooperativity. Although substoichiometric occupancy of inhibitor binding sites results in partial inhibition, this effect is overrun by a concomitant activation of unliganded binding sites. Therefore, when an inhibitor of a cooperative enzyme does not reach saturating levels, a common scenario during drug administration, it may cause the contrary of the desired effect. The implications for drug development are discussed.
The activity of complex enzymes is precisely regulated by sophisticated molecular mechanisms that are described by the fundamental biochemical principles of allostery, cooperativity, and oligomerization. Allostery is defined as the interaction of binding sites at a distance, allowing for the regulation of catalytic activity. Thus, a ligand bound to one site affects the affinity of another site for the same or a different ligand by inducing transitions between distinct conformational states. In “classic” biochemistry, the couplings of binding sites are differentiated into functional interactions. Identical binding sites and homotropic ligands are the basis of cooperativity, while a more general functional interaction of individual binding sites and heterotropic ligands is known as allostery. Allosteric effects may be observed between individual domains of monomeric proteins or between protomers of oligomeric protein complexes. Moreover, the allosteric switch between the resting and the active conformations can be accompanied by rather dramatic events such as changes in oligomeric states. While well-known studies have been performed on, e.g., hemoglobin, the oxygen binding protein of red blood cells, and aspartate transcarbamoylase, a key enzyme in pyrimidine synthesis (1, 2), recent evidence suggests that prokaryotic DegP represents an exceptionally suitable model for addressing the underlying mechanisms of allostery, cooperativity, and activation by oligomerization (3, 4).
The heat shock factor DegP functions as a conformation-specific protease/chaperone complex that channels substrates into repair, assembly, or degradation pathways (3, 5). DegP is a cage-forming protease, where the size of the cage is determined by the number of assembled trimeric subcomplexes. DegP protomers consist of a serine protease domain and two C-terminal PDZ domains. PDZ domains are protein modules that bind the C-terminal 3–4 residues of target proteins (6). Recent studies revealed how ligands of the PDZ1 domain serve as allosteric activators inducing positive cooperativity by triggering conformational changes and large structural rearrangements, including switches between various oligomeric states where hexamers represent the resting state while higher oligomers represent the active state (7–10). DegP has two binding sites per protomer, the catalytic site and the peptide binding site of the PDZ1 domain, that are linked by an allosteric circuit. In addition, activation domains that are shared between adjacent protomers mediate concerted activation of the catalytic sites within the trimeric subcomplexes. This architecture is the basis of positive cooperativity where ligand binding to one binding site of one protomer not only increases the affinity of ligands for the second site within the same protomer but in addition increases the affinity for ligand binding to the neighboring protomer.
Results
These mechanistic considerations suggested a hypothesis where substoichiometric binding of a substrate-derived inhibitor would activate DegP instead of causing partial inhibition. This behavior can be explained because the ligand-bound active site is structurally connected to neighboring protomers and their unoccupied active sites and PDZ1 domains. Conceptually, these allosteric interactions should cause increased affinity of unoccupied sites for their ligands, ultimately resulting in increased enzymatic activity. To initially test this model of regulation, we used the synthetic substrate SPMFKGV-pNA to measure DegP activity in combination with the peptidic boronic acid inhibitor DPMFKLV-B(OH)2 that targets the active-site Ser residue (4, 11). The steady-state rate of substrate hydrolysis varied sigmoidally as a function of the substrate concentration, characteristic of substrate-induced allostery (SI Appendix, Fig. S1A). Kinetically, each time course showed a significant lag phase before activation of the proteolytic activity, while increases in DegP concentration reduced the length of the lag phase and both the steepness and extent of the activation (SI Appendix, Fig. S1A). This observation is consistent with a previously established mechanism in which DegP undergoes large changes in the oligomeric state, i.e., from the resting state hexamer, which dissociates into trimers and reassembles to form active 12-mer and even 24-mer assemblies (12, 13). This drastic change in oligomeric states normally occurs in response to the binding of the substrate to the active site or the binding of allosteric peptides to the PDZ1 domain. The presence of the lag phase (SI Appendix, Fig. S1A) reflects the rather slow interconversion of DegP between the resting conformation and the allosterically activated states due to the absence of a heterotropic allosteric activator binding to the PDZ1 domains and also the relatively weak binding of the peptide substrate (SPMFKGV-pNA) used here. Note that SPMFKGV-pNA does not bind to the PDZ domain because this substrate does not contain a C-terminal carboxylate, which is essential for binding (6).
Activation by Substoichiometric Inhibition.
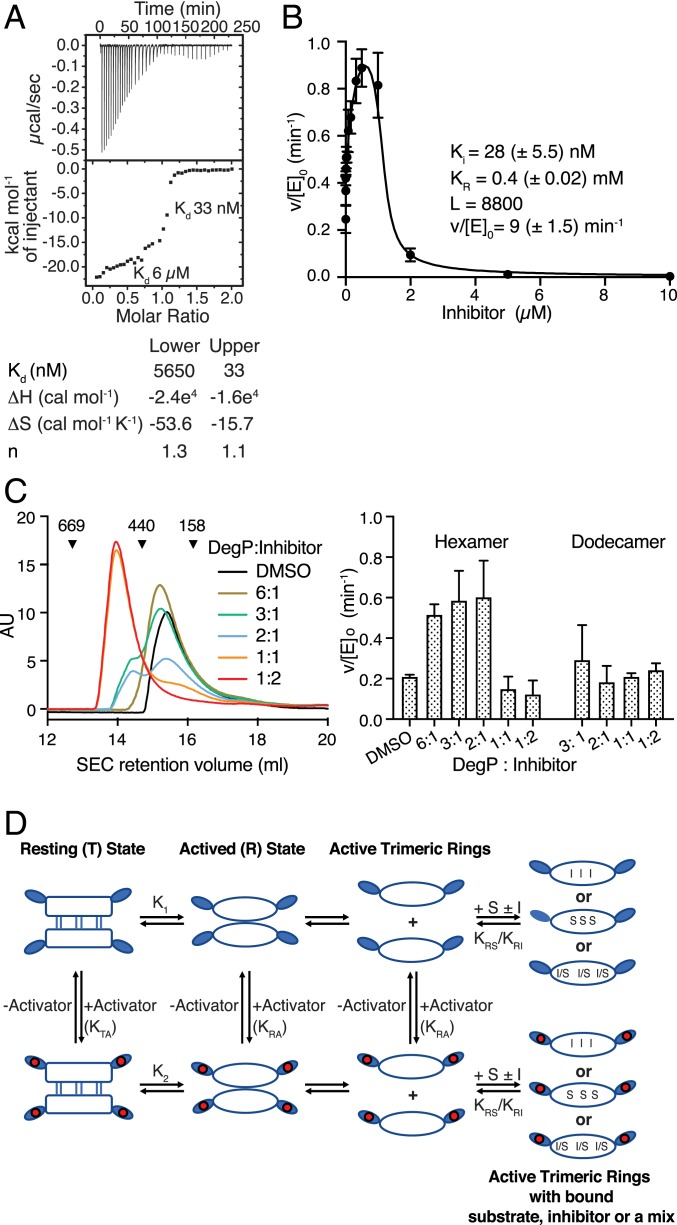
Binding of DPMFKLV-B(OH)2 was investigated using isothermal titration calorimetry (ITC). Measurements indicated a two-step binding mode of the inhibitor to DegP, expected for a cooperative enzyme. The weaker-binding phase (Kd = 6 µM) likely reflects the binding of the inhibitor to the resting state conformation, followed by a second, much tighter binding event (Kd = 33 nM) reflecting inhibitor binding to the fully activated proteolytic sites (Fig. 1A). Binding of the inhibitor to the active site of a cooperative enzyme is expected to promote the conformational switch to a tighter binding conformation, thereby resulting in activation of the enzyme at substoichiometric concentrations. To quantify activation by substoichiometric inhibition, DegP was treated with various concentrations of DPMFKLV-B(OH)2, ranging from 1 nM to 10 µM, before measuring DegP activity using SPMFKGV-pNA as a substrate. In agreement with our hypothesis, DegP activity is increased by up to threefold at substoichiometric concentrations of the inhibitor (Fig. 1B). To show that the observed activation is not substrate specific, the proteolytic assays were repeated using the periplasmic domain of the antisigma factor RseA from Escherichia coli as a substrate (14), revealing similar effects (SI Appendix, Fig. S1B). As activation of DegP is usually connected to a change in oligomeric state, DPMFKLV-B(OH)2 was titrated to DegP, and the oligomeric state was determined by size exclusion chromatography and chemical cross-linking. Interestingly, a switch from hexamer to dodecamer was only observed at above twofold excess of the inhibitor, i.e., concentrations where DegP is inhibited (Fig. 1C and SI Appendix, Fig. S1C), suggesting that activation by substoichiometric inhibition can occur in the hexamer. Control experiments show that the catalytically inactive DegP S210A mutant does not bind DPMFKLV-B(OH)2, as determined by ITC (SI Appendix, Fig. S1D), and changes in oligomeric states do not occur even at high inhibitor concentrations (SI Appendix, Fig. S1C). To rule out that the observed activation results from unspecific biochemical effects such as aggregation (15), we applied a nonbinding inhibitor derivative carrying a d configuration at the valine boronic acid residue that had no or only minor effects on DegP activity (SI Appendix, Fig. S1E).
Fig. 1.
Inhibition of DegP by DPMFKLV-B(OH)2. (A) ITC thermogram of binding of DPMFKLV-B(OH)2 inhibitor to wild-type (wt) DegP. Here, 400 µM inhibitor was titrated into the sample cell containing 40 μM DegP. Kd of the two distinct binding steps are indicated. (B) Kinetic parameters of DegP (1 µM) activity in the presence of the DPMFKLV-B(OH)2 concentration indicated using the chromogenic substrate SPMFKGV-pNA (500 µM). Error bars = SD of experimental data (n = 3). Data were fitted to the Monod-Wyman-Changeux (MWC) model as described in SI Appendix. L, equilibrium constant T/R in the absence of ligand; Ki, affinity of the inhibitor to activated DegP; KR, dissociation constant for substrate binding to activated DegP; v/[E]0, maximum turnover rate for the activated DegP species; parentheses, 95% confidence limit of the fit. (C) size-exclusion chromatography (SEC) analysis of DegP (100 µM) preincubated with the ratios of DPMFKLV-B(OH)2 indicated. Retention volumes and size (kDa) of calibration proteins thyroglobulin (669 kDa), ferritin (440 kDa), and aldolase (158 kDa) are indicated (Left). Activity of eluted DegP fractions was determined using 500 µM SPMFKGV-pNA as substrate (Right). Error bars indicate SD (n = 3). (D) Cartoon of the core allosteric mechanism. The T state is the inactive hexamer. It exists in equilibrium with the dissociated trimers (R state), which is competent to bind either substrate (S) or inhibitor (I) to the active site. Although the equilibrium in the absence of S or I lies toward the T state (i.e., K1 is small), the binding of S or I pulls the equilibrium toward the R state by thermodynamic coupling. Alternatively, the inactive T state binds activator to the PDZ1 domains moderately, but the R state PDZ1 domains bind activator more tightly (i.e., KRA >> KTA), leading to activation because once in the R state it can bind S or I.
Activation by Substoichiometric Inhibition in Combination with Allosteric Activators.
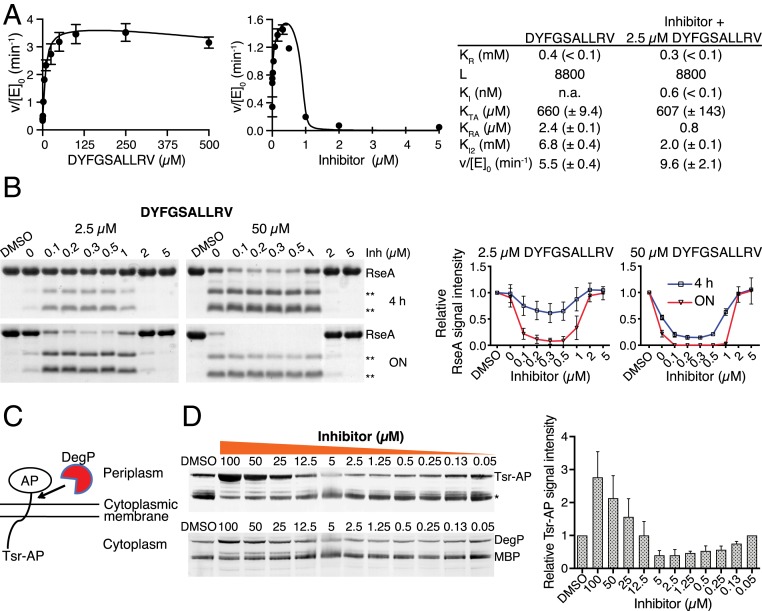
DegP is allosterically activated by the C termini of misfolded proteins which bind to its PDZ1 domain (Fig. 1D) (16). We therefore tested whether allosteric peptides would modulate activation by substoichiometric inhibition. Two allosteric peptides displaying a 100-fold difference in affinity toward DegP's PDZ1 domain were selected. The Kd values for the binding of DYFGSALLRV, corresponding to the C terminus of E. coli EfeB (17), and the synthetic peptide DNRDGNVYFF that was characterized previously (9) were 1.2 and 135 µM, respectively (SI Appendix, Figs. S2A and S3A). Titration experiments indicated sequence-specific differences in the potency of allosteric activation ranging between about 10- and 3-fold when using SPMFKGV-pNA as a substrate (Fig. 2A and SI Appendix, Figs. S2B and S3B). To subsequently test the combined effects of allosteric activators and the inhibitor, the concentrations of the two allosteric peptides, DYFGSALLRV (2.5 µM) and DNRDGNVYFF (500 µM), were kept constant. Subsequent titration of inhibitor caused an up to fourfold further activation of DegP at substoichiometric inhibitor concentrations (Fig. 2A and SI Appendix, Figs. S2B and S3B). Similar results were obtained using RseA of E. coli as a substrate. DPMFKLV-B(OH)2 was titrated to DegP in the presence of 2.5 and 50 µM of the allosteric activator DYFGSALLRV, and degradation of RseA was followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) at various time points. Again, activation was observed at substoichiometric levels of inhibitor (Fig. 2B). Similar data were obtained using DNRDGNVYFF (SI Appendix, Fig. S3C). Furthermore, analysis of kinetic data indicated that the Ki of the inhibitor changed in the presence of the PDZ activator from 28 to 0.6 nM for DYFGSALLRV and to 20 nM for DNRDGNVYFF, respectively (compare Figs. 1B and 2A and SI Appendix, Fig. S3B). Moreover, to demonstrate that the detected activation coincided with increased affinity of allosteric peptides, ITC experiments were performed at various inhibitor concentrations. As expected, Kd of both peptides showed sixfold higher affinities, i.e., for DYFGSALLRV from 1.2 µM in the absence of the inhibitor to 0.2 µM in its presence and for DNRDGNVYFF from 135 to 23 µM, respectively (SI Appendix, Figs. S2A and S3A).
Fig. 2.
Activation of DegP by substoichiometric inhibition. (A) Activity of DegP (1 µM) using chromogenic SPMFKGV-pNA (500 µM) as a substrate in the presence of various (Left) or fixed (Right) concentrations of DYFGSALLRV (2.5 µM, corresponding to twofold Kd) and various concentrations of the inhibitor DPMFKLV-B(OH)2 (Inh). Error bars indicate SD of experimental data (n = 3). Data were fitted to the MWC model as described in SI Appendix. L, equilibrium constant between T and R conformations in the absence of ligand; KR, dissociation constant for substrate binding to activated DegP; Ki, affinity of the inhibitor to activated DegP; KTA and KRA, the dissociation constants for the interaction between the activator and the T state conformation and R state conformation, respectively; Ki2, affinity of the activator for the active site; v/[E]0, maximum turnover rate for the activated DegP species; parentheses, 95% confidence limit of the fit. (B) Representative images of the digestion of the periplasmic domain of RseA (20 µM) by DegP (1 µM) in the presence of fixed concentrations of DYFGSALLRV (2.5 and 50 µM) and concentrations of DPMFKLV-B(OH)2 indicated (Upper). **, RseA cleavage products. Quantification of DegP activity using the signal intensity of RseA relative to the DMSO control (Lower). Error bars indicate SD (n = 3). (C) DegP activity in vivo. Cartoon of cell-based DegP reporter assay. Alkaline phosphatase (AP) is tethered to the N-terminal 164 amino acids of the Tsr protein. DegP cleaves the Tsr-AP hybrid protein near the fusion joint. (D) Inhibitor-mediated activation of DegP activity in vivo. E. coli cells expressing the tsr-phoA fusion were grown overnight (ON) at 30 °C in rich medium with either DMSO (2%) or various concentrations of DPMFKLV-B(OH)2. Whole-cell extracts of equivalent numbers of cells were subjected to SDS/PAGE and Western blotting using antibodies against AP. *, Tsr-AP degradation products. Additional Western blots of the same samples using MBP-DegP antibodies (Lower). Quantification of DegP activity using the signal intensity of the Tsr-AP band relative to the DMSO control (Upper). Error bars indicate SD (n = 3).
Activation by Substoichiometric Inhibition In Vivo.
To further substantiate our results, we tested whether activation at substoichiometric concentrations of inhibitor occurred in living cells. For these assays, we used the experimental system that led to the discovery of the degP gene (Fig. 2C) (18). When a tsr-phoA fusion is expressed in E. coli, the Tsr-AP hybrid protein is cleaved near the fusion joint mainly by DegP but only marginally by other cell envelope proteases (SI Appendix, Fig. S4). Therefore, these cells represent a suitable reporter system to measure DegP activity in vivo. Growing cells expressing the plasmid-derived tsr-phoA fusion and native chromosomal degP were treated with DPMFKLV-B(OH)2 at various concentrations ranging between 50 nM and 100 µM. Subsequently, proteolytic processing of the Tsr-AP hybrid protein by DegP was determined by Western blotting (Fig. 2D). The pattern of Tsr-AP bands indicated that at high inhibitor concentrations (25 to 100 µM), proteolytic processing of Tsr-AP was reduced because DegP is inhibited. At intermediate inhibitor concentrations (0.5 to 5 µM), processing of Tsr-AP was increased, while at lower concentrations of inhibitor (0.13 to 0.05 µM) processing of Tsr-AP was comparable to the dimethylsulphoxide (DMSO) control. Densitometry of the signal intensity of the Tsr-AP band relative to the DMSO control indicated an up to 2.5-fold increase in DegP activity at 2.5 and 5 µM inhibitor concentrations, respectively (note that the reduced amount of DegP at low inhibitor concentrations is likely the result of autoproteolysis caused by its activation). Therefore, the in vivo reporter system supported the results obtained by biochemical assays using purified proteins. These data also showed that even moderate activation of DegP can significantly affect processing of target proteins in vivo.
Activation by Inhibition in Human HTRA1.
To demonstrate that activation by inhibition is not a phenomenon that is specific to the bacterial enzyme DegP and its unique structural properties, we tested the established model using human HTRA1 for which X-ray crystallography has shown that DPMFKLV-B(OH)2 binds to the active site in a canonical manner (4). While HTRA1 shares structural and functional features with DegP, such as a trimeric arrangement of protomers and the conserved activation domain, it differs in several aspects, such as surface accessible active sites and a mode of activation that is not strictly coupled to changes in the oligomeric state.
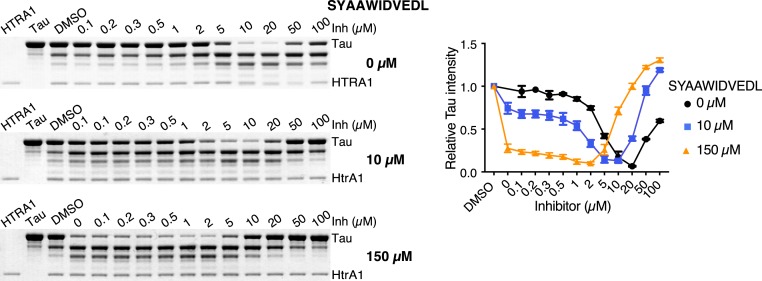
ITC measurements of DPMFKLV-B(OH)2 binding to HTRA1 indicated a Kd of 0.8 µM (SI Appendix, Fig. S5A). To test whether HTRA1 can be activated by DPMFKLV-B(OH)2, we performed proteolytic digests using its native substrate tau and various concentrations of inhibitor. An up to 15-fold increase in HTRA1 activity was observed in the presence of the inhibitor (Fig. 3). However, in contrast to DegP, the highest activation of HTRA1 was not observed at substoichiometric concentrations of the inhibitor, but at 20-fold excess. This effect might be best explained by the 24-fold lower affinity of the inhibitor for HTRA1. To overcome this limitation, HTRA1 was preincubated with an allosteric ligand of the PDZ domain, SYAAWIDVEDL (Kd = 32 µM, SI Appendix, Fig. S5B), at concentrations threefold below (10 µM) and fivefold above (150 µM) its Kd. Subsequently, DPMFKLV-B(OH)2 was titrated to determine the effects of the inhibitor on HTRA1's activity. At both concentrations of the allosteric PDZ domain ligand SYAAWIDVEDL, HTRA1 was activated by the inhibitor up to 7-fold and 10-fold, respectively (Fig. 3 and SI Appendix, Fig. S5C). As expected, in the presence of 150 µM SYAAWIDVEDL, the inhibitor concentrations where highest activation could be observed shifted from 20 to 1 to 2 µM. In agreement with these considerations, the presence of the allosteric activator caused stronger inhibition at high inhibitor concentrations. In addition, ITC measurements revealed, in a concentration-dependent manner, an up to fourfold increase of the affinity of the allosteric peptide in the presence of the inhibitor, i.e., from 32 to 8 µM (SI Appendix, Fig. S5B).
Fig. 3.
Activation of HTRA1 by substoichiometric inhibition. Proteolysis of tau (10 µM) by HTRA1 (1 µM) in the absence or presence of the fixed concentrations of the allosteric peptide SYAAWIDVEDL (10 and 150 µM) and various concentrations of the inhibitor DPMFKLV-B(OH)2 (Inh) after 5 h incubation at 37 °C (Left). Quantification of the tau signal intensity using densitometry (n = 3) relative to the DMSO control; error bars indicate SD (Right). An additional 2 h time point of tau digests is shown in SI Appendix, Fig. S5C.
Discussion
Ligand-derived synthetic inhibitors of enzymes are widely used in basic research and as drugs. For inhibitors interacting with single binding sites of monomeric enzymes, dose–response correlations are hyperbolic. The response of the enzyme system to inhibition changes fundamentally when an enzyme has multiple binding sites that are allosterically connected. Here, regulation of activity is more complex and can therefore cause effects that may seem paradoxical at first sight. HtrA proteases such as DegP belong to the S1 family of serine proteases, prominent members of which include, e.g., trypsin, chymotrypsin, thrombin, and elastase. The latter are typically activated by zymogen conversion. This process involves proteolytic processing of N-terminal segments stabilizing conformations via a disorder–order transition of the activation domain comprising loops L1, L2, and LD. As the formation of the active site is conformationally linked with binding of the newly formed N terminus into a preformed pocket, allostery is an integral part of the activation mechanism of these classic proteases (19). Even though activation of DegP does not include zymogen processing, the principles described above are conserved. However, the molecular mechanisms regulating DegP are more complex. Its loop LD reaches over from an adjacent protomer, and the activation domain is extended by loop L3 and PDZ domain 1 to sense allosteric ligands of the PDZ domain. Moreover, activation by PDZ ligands is coupled to oligomer conversion, i.e., from the resting hexamer to active larger oligomers (9). The events triggered by a strong active site ligand such as DPMFKLV-B(OH)2 involve the same elements, the interaction of which proceeds in reverse order; i.e., initial interaction of the ligand with the substrate specificity pockets and loop L2 triggers the rearrangement of loops L1 and LD* as well as of loop L3, leading to a structural rearrangement within the PDZ domain and a concomitant increase of affinity for its ligands. Therefore, high concentrations of the inhibitor have the same effect as allosteric ligands of the PDZ domain, i.e., triggering the conversion of hexamers into larger oligomers. Together, these allosteric communications across protomers result in the structural rearrangement of substrate-binding pockets, proper positioning of the catalytic triads, and formation of the oxyanion holes in structurally connected active sites and, ultimately, in positive cooperativity. These processes can be considered a ligand-induced transition between the T state and the R state in the classic Monod–Wyman–Changeux model of activation, in which the inhibitory ligand acts as an activator (20) (Fig. 1D). Therefore, activation does not require occupation of all allosteric sites.
Our model of activation of an enzyme by substoichiometric occupancy of an inhibitor is reminiscent of other inhibitor-activated systems. One prominent example is the activation of the mitogen-activated protein kinase pathway when wild-type BRAF is targeted by inhibitors such as PLX4032 (21). The paradoxical result has been readily explained by inhibitor-driven dimerization with unliganded CRAF causing allosteric disruption of autoinhibition and transactivation of CRAF, leading to increased pathway activity (22). Therefore, the BRAF PLX4032–unliganded CRAF complex represents another example of activation by substoichiometric inhibition. Consistently, similar effects are observed with kinase-dead mutants of BRAF (23). Moreover, additional allosteric events involving RAS–RAF interactions that are not RAS allele specific may come into play (22, 24), promoting additional activation, and are thus comparable to those observed with allosteric peptides and HTRA1 and DegP. A similar example involving small-molecule modulators of mammalian kinases describes activation by substoichiometric inhibitor concentrations of PERK, a kinase of the unfolded protein response of the endoplasmic reticulum (ER) (25). It seems that this mode of regulation is widespread. For example, low ratios of the inhibitors maleate and N-phosphonacetyl-l-aspartate to aspartate transcarbamoylase cause an increase in enzymatic activity (20, 26). In addition, an engineered E. coli lipoprotein containing a hydrophobic C terminus displayed a concentration-dependent pattern of activation and inhibition of DegP similar to DPMFKLV-B(OH)2; however, the binding site of the lipoprotein-derived inhibitor and thus the underlying molecular mechanism remain to be elucidated (27).
The molecular mechanisms described here have wide implications for drug development. If an inhibitor that targets a cooperative enzyme is not equally distributed across all tissues, reflecting the well-known problem of bioavailability, the inhibitor will be efficient in tissues where distribution is good, but it will activate the target protein in tissues where concentrations are low, causing the opposite of the desired effect. Thus, allosteric effects are not only important for basic research, but they have also considerable importance for clinical applications. In general, our work supports the notion that a careful consideration of classic biochemical principles is likely to significantly reduce side effects and failed efforts in drug discovery (28).
Materials and Methods
The synthetic substrate SPMFKGV-pNA of DegP and the peptidic boronic acid inhibitor DPMFKLV-B(OH)2 were prepared and used as described (4, 11). The cell-based assays of DegP activity employing a Tsr-AP hybrid protein were done as described (18). Methods for protein purification and ITC measurements followed previously described protocols. They are described in detail in the SI Appendix, which includes materials and methods and figures.
Data Availability.
All data are included in the paper and supporting information.
Supplementary Material
Acknowledgments
M.E. and M.K. were supported by Deutsche Forschungsgemeinschaft (Collaborative Research Centre 1093).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918721117/-/DCSupplemental.
References
- 1.Bohr C., Hasselbach K. A., Krogh A., Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Skand. Arch. Physiol. 16, 401–412 (1904). [Google Scholar]
- 2.Gerhart J., From feedback inhibition to allostery: The enduring example of aspartate transcarbamoylase. FEBS J. 281, 612–620 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Clausen T., Kaiser M., Huber R., Ehrmann M., HTRA proteases: Regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 12, 152–162 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Truebestein L., et al. , Substrate-induced remodeling of the active site regulates human HTRA1 activity. Nat. Struct. Mol. Biol. 18, 386–388 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Spiess C., Beil A., Ehrmann M., A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97, 339–347 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Ernst A., et al. , A structural portrait of the PDZ domain family. J. Mol. Biol. 426, 3509–3519 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Krojer T., Garrido-Franco M., Huber R., Ehrmann M., Clausen T., Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature 416, 455–459 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Krojer T., et al. , Structural basis for the regulated protease and chaperone function of DegP. Nature 453, 885–890 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Merdanovic M., et al. , Determinants of structural and functional plasticity of a widely conserved protease chaperone complex. Nat. Struct. Mol. Biol. 17, 837–843 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Krojer T., Sawa J., Huber R., Clausen T., HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat. Struct. Mol. Biol. 17, 844–852 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Hauske P., et al. , Selectivity profiling of DegP substrates and inhibitors. Bioorg. Med. Chem. 17, 2920–2924 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Krojer T., et al. , Interplay of PDZ and protease domain of DegP ensures efficient elimination of misfolded proteins. Proc. Natl. Acad. Sci. U.S.A. 105, 7702–7707 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson N. J., Merdanovic M., Ehrmann M., van Duijn E., Heck A. J., Substrate occupancy at the onset of oligomeric transitions of DegP. Structure 22, 281–290 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Hasenbein S., et al. , Conversion of a regulatory into a degradative protease. J. Mol. Biol. 397, 957–966 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Shoichet B. K., Screening in a spirit haunted world. Drug Discov. Today 11, 607–615 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meltzer M., et al. , Allosteric activation of HtrA protease DegP by stress signals during bacterial protein quality control. Angew. Chem. Int. Ed. Engl. 47, 1332–1334 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Liu X., et al. , Crystal structure and biochemical features of EfeB/YcdB from Escherichia coli O157: ASP235 plays divergent roles in different enzyme-catalyzed processes. J. Biol. Chem. 286, 14922–14931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauch K. L., Beckwith J., An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc. Natl. Acad. Sci. U.S.A. 85, 1576–1580 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauske P., Ottmann C., Meltzer M., Ehrmann M., Kaiser M., Allosteric regulation of proteases. Chembiochem 9, 2920–2928 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Monod J., Wyman J., Changeux J. P., On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12, 88–118 (1965). [DOI] [PubMed] [Google Scholar]
- 21.Weeraratna A. T., RAF around the edges–The paradox of BRAF inhibitors. N. Engl. J. Med. 366, 271–273 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Jin T., et al. , RAF inhibitors promote RAS-RAF interaction by allosterically disrupting RAF autoinhibition. Nat. Commun. 8, 1211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidorn S. J., et al. , Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su F., et al. , RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 366, 207–215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendez A. S., et al. , Endoplasmic reticulum stress-independent activation of unfolded protein response kinases by a small molecule ATP-mimic. eLife 4, e05434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foote J., Schachman H. K., Homotropic effects in aspartate transcarbamoylase. What happens when the enzyme binds a single molecule of the bisubstrate analog N-phosphonacetyl-L-aspartate? J. Mol. Biol. 186, 175–184 (1985). [DOI] [PubMed] [Google Scholar]
- 27.Park H., Kim Y. T., Choi C., Kim S., Tripodal lipoprotein variants with C-terminal hydrophobic residues allosterically modulate activity of the DegP protease. J. Mol. Biol. 429, 3090–3101 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Swinney D. C., Biochemical mechanisms of drug action: What does it take for success? Nat. Rev. Drug Discov. 3, 801–808 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the paper and supporting information.