Significance
Parkinson’s disease, Lewy body dementia, and other synucleinopathies are characterized by the accumulation of abnormally aggregated α-synuclein (α-syn) protein, which is the principal component of Lewy bodies. Immunotherapeutic approaches aimed at halting the propagation of both central and peripheral extracellular α-syn aggregates may be a promising therapeutic avenue for synucleinopathies. Our study demonstrates that natural killer (NK) cells act as efficient scavengers of abnormal α-syn aggregates without becoming aberrantly activated. We demonstrate that systemic depletion of NK cells significantly exacerbates synuclein pathology. Overall, our results provide strong evidence for a protective role of NK cells in synuclein-related neurodegenerative diseases.
Keywords: Parkinson’s disease, natural killer cell, α-synuclein, Lewy bodies, synucleinopathies
Abstract
The pathological hallmark of synucleinopathies, including Lewy body dementia and Parkinson’s disease (PD), is the presence of Lewy bodies, which are primarily composed of intracellular inclusions of misfolded α-synuclein (α-syn) among other proteins. α-Syn is found in extracellular biological fluids in PD patients and has been implicated in modulating immune responses in the central nervous system (CNS) and the periphery. Natural killer (NK) cells are innate effector lymphocytes that are present in the CNS in homeostatic and pathological conditions. NK cell numbers are increased in the blood of PD patients and their activity is associated with disease severity; however, the role of NK cells in the context of α-synucleinopathies has never been explored. Here, we show that human NK cells can efficiently internalize and degrade α-syn aggregates via the endosomal/lysosomal pathway. We demonstrate that α-syn aggregates attenuate NK cell cytotoxicity in a dose-dependent manner and decrease the release of the proinflammatory cytokine, IFN-γ. To address the role of NK cells in PD pathogenesis, NK cell function was investigated in a preformed fibril α-syn–induced mouse PD model. Our studies demonstrate that in vivo depletion of NK cells in a preclinical mouse PD model resulted in exacerbated motor deficits and increased phosphorylated α-syn deposits. Collectively, our data provide a role of NK cells in modulating synuclein pathology and motor symptoms in a preclinical mouse model of PD, which could be developed into a therapeutic for PD and other synucleinopathies.
The pathological hallmark of synucleinopathies, including dementia with Lewy bodies (DLB), Parkinson’s disease (PD), and multiple system atrophy, is the presence of aggregated forms of α-synuclein (α-syn) in Lewy bodies (LBs) (1, 2) that can induce pathology in healthy cells (3–5). α-Syn is a 140-amino acid (aa) natively unfolded endogenous protein, comprising 1% of total cytosolic proteins (6). This small protein is primarily located in neuronal presynaptic terminals (7) and in the nucleus (8). α-Syn can self-assemble to form ordered fibrillary aggregates characterized by a cross β-sheet structure (9). In the process of fibril formation, various intermediate forms of α-syn develop, including initially soluble oligomeric forms of α-syn that gradually become insoluble and coalesce into fibrils and then into LBs (6). The general consensus in the field is that aggregation is the main pathogenic feature of α-syn (6). While α-syn is typically considered an intracellular protein, it is normally present in extracellular biological fluids, including human cerebrospinal fluid (CSF) and blood plasma (10–14). LB-like pathology has also been found in various peripheral neurons, including in the enteric nervous system in premotor phases of the disease (15). In the central nervous system (CNS), extracellular preformed fibrils of α-syn (PFF α-syn) act as a damage-associated molecular pattern (DAMP) and activate microglia (16). Microglia are capable of clearing extracellular α-syn aggregates through Toll-like receptor (TLR) 2 (17) and TLR4 pathways (18). However, α-syn mediates activation of microglia and triggers the secretion of proinflammatory cytokines and reactive oxygen species (18, 19).
Natural killer (NK) cells are granular lymphocytes of the innate immune system that serve as the first line of defense. NK cells selectively recognize and destroy tumor cells or virus-infected cells without prior sensitization via the interplay between the inhibitory and activating receptors (reviewed in ref. 20). Recently, it has been shown that NK cell function extends beyond merely the recognition and elimination of transformed cells with several studies indicating diverse roles for NK cells in antimicrobial defense (21, 22), senescent cell clearance (23), resolution of inflammation (24, 25), and modulation of adaptive immunity (26, 27). NK cells have been utilized for immunotherapy in the cancer field because of their capacity to selectively target malignant cells (28–30). Particularly, the human NK cell line, NK92, has been safely infused in patients with cancer as an allogeneic cell therapeutic (28, 30–32) and was not tumorigenic (33, 34). Recently, NK92 cells that are genetically modified to express chimeric antigen receptors have been generated to target human glioblastoma (35–37), highlighting NK cells’ potential as a promising therapeutic strategy for brain disorders. The role of NK cells in neurological disorders has been investigated in experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis (38). These studies revealed that NK cells homing to the CNS were essential in ameliorating disease in experimental autoimmune encephalomyelitis (38), suggesting that NK cells exert protective effects. The presence of NK cells in the brain has been described by recent transcriptomic analyses of mouse brain myeloid cells in both healthy and disease states (39, 40), implicating their potential role in homeostasis and neurological disorders in the CNS.
The number of NK cells is increased in the blood of PD patients and have increased expression of the inhibitory receptor NKG2A, and no modifications of the activating receptor NKG2D (41–43). However, there have been no further efforts made to investigate the role of NK cells in PD. Here, we aimed to evaluate the bidirectional effects between extracellular α-syn and NK cells. Our data demonstrate that NK cells are able to internalize and degrade extracellular α-syn without becoming aberrantly activated. Next, we evaluated the effect of NK cell depletion on synuclein pathology in vivo. Our data demonstrate that systemic depletion of NK cells results in exacerbated motor symptoms and synuclein pathology in a preclinical mouse model of synucleinopathies.
Results
NK Cells Are Found in the Brains of Synucleinopathy Patients and the PFF α-Syn Mouse Model.
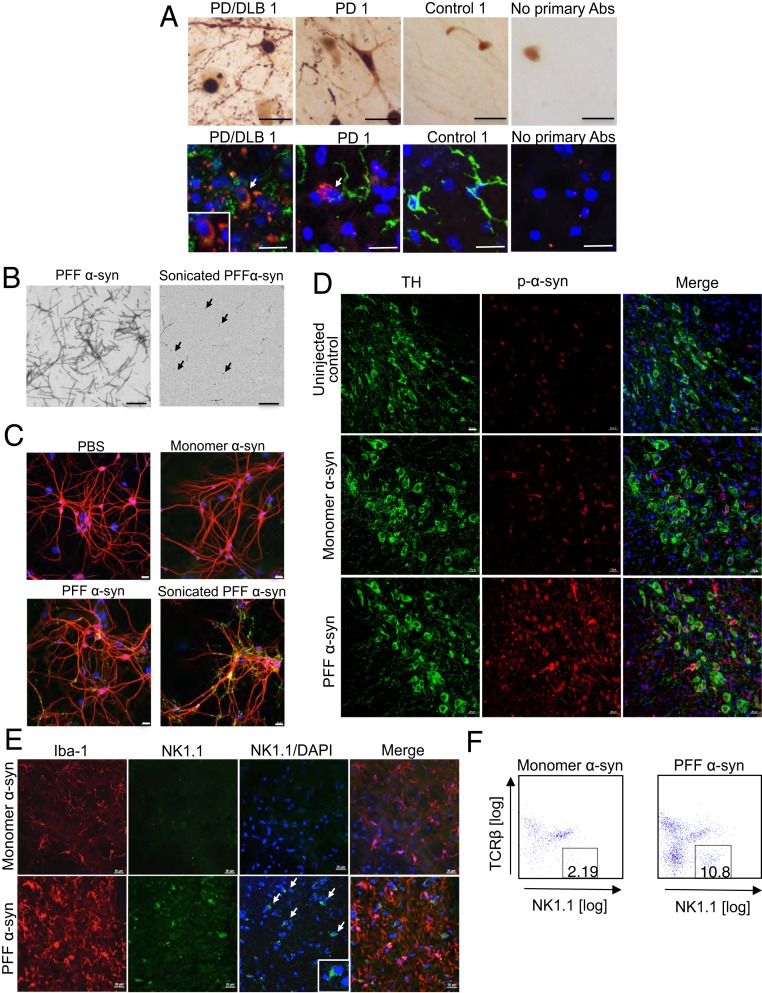
To determine if NK cells are present in the brains of healthy control or synucleinopathy patients, we performed immunohistochemistry (IHC) for a human NK cell marker, 2B4, in the substantia nigra (SN). We confirmed robust levels of staining for α-syn phosphorylated at serine 129 (p-α-syn) within the SN of PD and PD/DLB but not in age-matched controls (Fig. 1A and SI Appendix, Fig. S1). Importantly, we illustrated the presence of 2B4+ NK cells within the SN of both healthy and PD/DLB brains (Fig. 1A and SI Appendix, Fig. S1). Of 11 synucleinopathy patient samples analyzed, 8 revealed positive staining for the NK cell marker 2B4 (Fig. 1A and SI Appendix, Fig. S1). Three age-matched control samples were analyzed and 1 showed positive 2B4 staining (Fig. 1A and SI Appendix, Fig. S1). To determine if NK cells are present in mouse brain with synuclein pathology, we utilized an intrastriatal injection of PFF α-syn in M83 transgenic (Tg) mice overexpressing the human α-syn transgene with an A53T point mutation (44). To do so, we generated recombinant human α-syn (monomer α-syn), assembled the protein into PFF α-syn, and then sonicated immediately prior to addition in neuronal cultures or stereotaxic injections, as described previously (45, 46). The conformations of PFF α-syn and sonicated PFF α-syn were confirmed by transmission electron microscopy (Fig. 1B). The seeding and transduction of p-α-syn inclusions by sonicated PFF α-syn but not PBS and monomer α-syn was confirmed in primary mouse hippocampus neuronal cultures (Fig. 1C). Nonsonicated PFF α-syn also has the capacity to transduce p-α-syn inclusions but to a lesser extent (Fig. 1C). Intrastriatal injection of sonicated PFF α-syn in M83 Tg mice induced robust p-α-syn inclusions in the SN pars compacta (SNpc) compared to the monomer or uninjected animals (Fig. 1D). We observed low levels of p-α-syn+ staining within the SNpc of uninjected M83 Tg controls as M83 Tg mice intrinsically develop α-syn pathology. Notably, we performed IHC for a mouse NK cell marker, NK1.1, in the SNpc of PFF α-syn injected M83 mice and confirmed the presence of NK cells (Fig. 1E). To further confirm the presence of NK cells, we performed flow cytometry analysis and observed a 5-fold increase of NK cell infiltration into the CNS of the PFF α-syn–injected mice compared to monomer α-syn–injected control mice (10.19% vs. 2.19%) (Fig. 1F).
Fig. 1.
NK cells are present in the brains of humans and mice with α-syn pathology. (A) IHC images of α-syn pathology and human NK cells in the SNs of postmortem PD, PD/DLB, or age-matched control brains using antibodies for p-α-syn (Upper), the human NK cell marker, 2B4 (red)/Iba-1 (green), and DAPI (blue) for nuclei staining (Lower). (Scale bars, 20 μm.) (B) Representative transmission electron microscopy images confirmed the conformation of α-syn fibrils (Left) and sonicated α-syn (Right). (Scale bars, 500 nm.) The black arrows indicate sonicated PFF α-syn. (C) Immunocytochemistry images of primary mouse neurons using antibodies against p-α-syn (green) and MAP-2 (red) to confirm PFF α-syn seeding and transduction capacity. On day 7 of neuronal culture, neurons were treated with PBS, 1 µg/mL of monomer α-syn, nonsonicated PFF α-syn, or sonicated PFF α-syn for 7 d, then were fixed on day 14. (Scale bars, 20 μm.) (D) Immunohistological images in the SNpc of uninjected M83 Tg mice and M83 mice stereotaxically injected with PFF α-syn or monomer α-syn at 10 wk postinjection. Antibodies against p-α-syn (red), TH (green), and DAPI (blue) for nuclei staining were used. (Scale bars, 20 μm.) (E) Immunohistological images in the SNpc of M83 Tg mice intrastriatally injected with PFF α-syn or α-syn monomers at 10 wk postinjection. Antibodies against Iba-1 (red), NK1.1 (green), and DAPI (blue) were used. (Scale bars, 20 μm.) The white arrows indicate NK1.1+ cells. (F) Flow cytometry analysis of NK cells (CD45+CD3−NK1.1+) within the brain of monomer (Left) or PFF α-syn (Right) injected mice.
NK Cells Efficiently Internalize and Degrade Extracellular α-Syn.
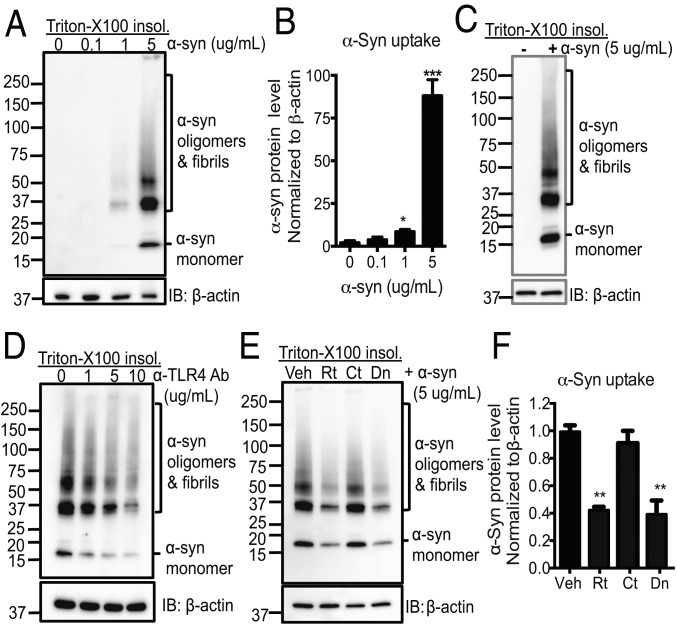
In addition to antitumor and antiviral functions, NK cells are involved in antimicrobial defense and the clearance of senescent cells (21–23). To assess whether NK cells internalize extracellular α-syn species, we utilized NK92 cells and primary human NK cells from healthy individuals. NK cells were treated with various concentrations of α-syn aggregates and the internalization of insoluble α-syn species in NK cells was analyzed by immunoblot analyses. We noted that there was no detectable endogenous α-syn in NK cells. Our results showed that NK92 cells efficiently internalized various sizes of α-syn (monomers, oligomers, and higher molecular weight fibrils) in a dose-dependent manner (Fig. 2 A and B). Importantly, we also showed that primary human NK cells from healthy individuals could internalize α-syn (Fig. 2C). To investigate the mechanism by which NK cells internalized α-syn, we tested if TLR2, TLR4, or heparan sulfate proteoglycans (HSPGs), which have been shown to mediate α-syn uptake by microglia and other cells (17, 18, 47), mediated α-syn uptake by NK cells. As TLR4, TLR2, and HSPGs are expressed on NK cells (48–50), NK92 cells were preincubated with neutralizing antibodies against TLR4, TLR2, or heparan for 1 h prior to α-syn treatments and then incubated with α-syn aggregates for 1 h. We observed antibodies against human TLR4 blocked α-syn uptake in a dose-dependent manner (Fig. 2D). Antibodies against human TLR2 also inhibited α-syn uptake but to a lesser extent. Heparan treatment did not affect α-syn uptake (SI Appendix, Fig. S2). Although NK cells are not professional phagocytic cells, their endocytosis machineries are highly functional (51). To explore the endocytosis mechanism of α-syn, we tested inhibitors that are involved in various endocytosis mechanisms: Rottlerin (30 μM; an inhibitor of protein kinase C [PKC]), cytochalasin D (1 μM; an actin polymerase inhibitor), and dynasore (80 μM; a dynamin inhibitor). Rottlerin and dynasore significantly inhibited α-syn uptake, while cytochalasin D-treated cells showed α-syn uptake similar to that of vehicle treated controls, implicating a mechanism by which the NK cells internalize α-syn is mediated by PKC-dependent, dynamin-mediated endocytosis (Fig. 2 E and F).
Fig. 2.
NK cells efficiently internalize α-syn via TLR4-mediated, PKC, and dynamin-dependent endocytosis. (A) Western blot analysis of internalized α-syn in NK92 cells. NK92 cells were incubated with various concentrations of α-syn as indicated for 1 h. (B) Quantitative analysis of the internalized α-syn aggregates. (C) Western blot analysis of internalization of α-syn in primary human NK cells. Primary human NK cells were isolated from healthy donors and cells were incubated with 5 µg/mL α-syn for 1 h. (D) Western blot analysis of internalization of α-syn with treatments of neutralizing antibodies for TLR4 in NK92 cells. NK92 cells were preincubated with different concentrations of TLR4-neutralizing antibodies for 1 h and then incubated with α-syn for 1 h. (E) Western blot analysis of internalization of α-syn following pretreatments with endocytosis inhibitors in NK92 cells. NK92 cells were preincubated with vehicle, rottlerin (30 μM), cytochalasin D (1 μM), or dynasore (80 μM) for 1 h and then incubated with α-syn (5 µg/mL) for 1 h. (F) Quantitative analysis of the internalized total α-syn. For all protein samples, Triton X-100 insoluble pellet fractions were analyzed by Western blot for α-syn and β-actin. Data were analyzed using one-way ANOVA. Error bars represent ± SEM. *P < 0.05; **P < 0.01, ***P < 0.001. The data shown are representative of 3 independent experiments. IB, immunoblot.
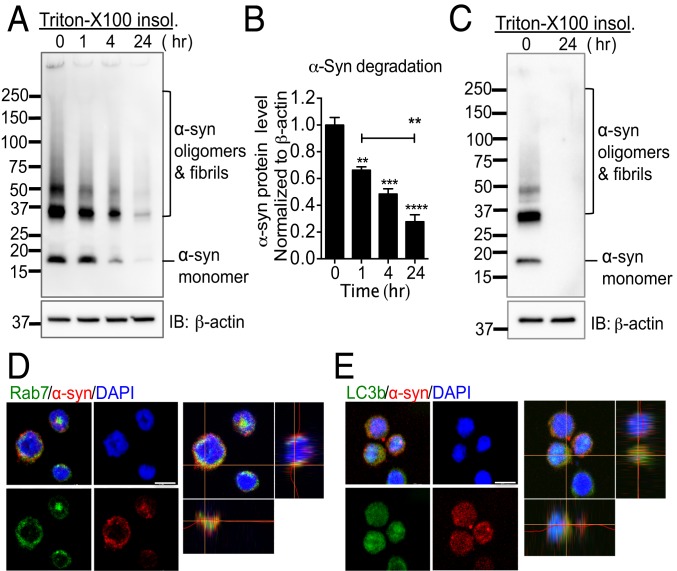
To monitor the degradation of α-syn within NK cells, we incubated NK92 and primary human NK cells with extracellular α-syn species for 1 h, and then cells were washed 3 times and incubated additional times as indicated. Internalized α-syn species were rapidly degraded by as early as 1 h and completely degraded by 24 h in both NK92 cells and primary human NK cells (Fig. 3 A–C). To determine whether internalized α-syn was detected in the cytoplasm and the pathways of degradation, we performed immunocytochemistry using a Ras-related protein Rab7 antibody (endosomal marker) (52) and a microtubule-associated protein 1 light chain 3β (LC3B) antibody (lysosomal/autophagy marker) (53). Our data showed that internalized α-syn was found within the cytoplasmic compartment of NK cells. Furthermore, α-syn was colocalized with both Rab7 and LC3B, implicating that NK cells degrade α-syn via the endosome and lysosome pathways (Fig. 3 D and E). Taken together, these data strongly implicate that NK cells are able to scavenge extracellular α-syn and may be critical for regulating and restraining synuclein pathology in synucleinopathies.
Fig. 3.
NK cells efficiently degrade extracellular α-syn aggregates. (A) Western blot analysis of degradation of α-syn in NK92 cells. NK92 cells were treated with α-syn aggregates for 1 h. Then, cells were washed 3 times with fresh medium. Cells were then incubated in fresh medium for up to 24 h. (B) Quantitative analysis of α-syn fibril degradation. Data were analyzed using one-way ANOVA. Error bars represent ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001. (C) Western blot analysis of degradation of α-syn in primary human NK cells. Primary NK cells isolated from the blood of healthy donors and cells were incubated with or without α-syn for 1 h. Then, cells were washed 3 times with PBS. Cells were then incubated in fresh medium for 24 h. For all protein samples, Triton X-100 insoluble fractions were analyzed by Western blot for α-syn and β-actin. (D and E) Representative images of colocalization of α-syn with the endosome and lysosome markers. NK92 cells were treated with α-syn aggregates (5 μg/mL) for 30 min. Immunocytochemistry was performed for Rab7 (endosome marker; green) and α-syn (red) (D), and LC3b (lysosome/autophagy marker; green) and α-syn (red) (E). (Scale bars, 10 μm.) The data shown are representative of 3 independent experiments.
Extracellular α-Syn Aggregates Attenuate Cytotoxicity and IFN-γ Production of NK Cells.
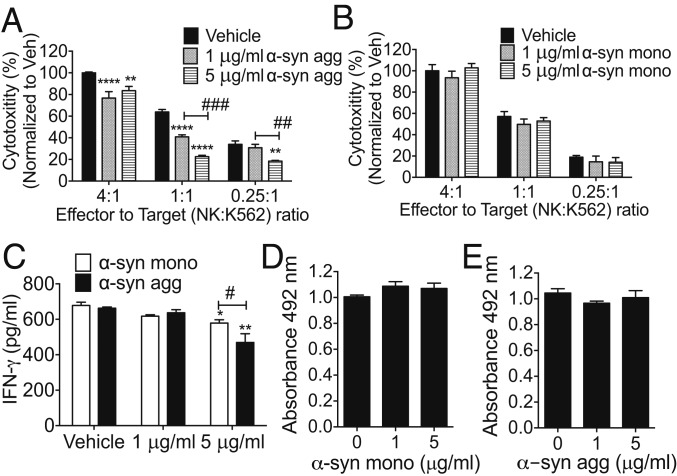
Microglia and macrophages are able to internalize and degrade extracellular α-syn (18, 19) and present α-syn peptides to T cells to mount an adaptive immune response (54). However, this results in increased release of proinflammatory cytokines and increased oxidative stress (55). To examine whether extracellular α-syn species modulate NK cell functions, we examined NK cell cytotoxicity and cytokine production. We measured NK cell cytotoxicity against fluorescently labeled K562 leukemia target cells in the presence of various concentrations of α-syn species. Our results demonstrated that only α-syn aggregates significantly attenuated NK cell cytotoxicity in a dose-dependent manner (Fig. 4A), while monomeric α-syn alone had no effect on NK cell cytotoxicity (Fig. 4B). NK cells mainly produce the proinflammatory cytokine IFN-γ, which is a critical regulator of both innate and adaptive immune responses (reviewed in ref. 56). We measured IFN-γ production by NK92 cells treated with extracellular α-syn aggregates and found that extracellular α-syn aggregates significantly decreased IFN-γ secretion and monomeric α-syn inhibited IFN-γ secretion to a lesser extent (Fig. 4C). We showed that there was no cell death or additional proliferation upon α-syn treatments (Fig. 4 D and E).
Fig. 4.
α-Syn aggregates attenuate NK cytotoxicity and IFN-γ production by NK cells. (A and B) NK cell cytotoxicity assays to measure the effects of α-syn. Human NK92 cells were treated with various concentrations of α-syn agg (A) or monomeric α-syn (B) for 1 h. Calcein AM (10 μM) labeled K562 target cells were plated in 96-well plates with NK cells (effector) to K562 (target) (E:T) ratio as indicated for 4 h. Fluorescence intensity of supernatants was measured to evaluate NK cell cytotoxicity. All data were analyzed using 2-way ANOVA followed by the Bonferroni post hoc test. Error bars represent ± SEM; **P < 0.01, ****P < 0.0001, ##P < 0.01, or ###P < 0.001. (C) IFN-γ production of NK cells. NK92 cells were treated with indicated concentrations of α-syn for 24 h. The supernatants were collected and IFN-γ was measured using eBioscienceTM Human IFN-γ ELISA Ready-SET-Go! Kit. Data were analyzed using 2-way ANOVA with Bonferroni post hoc test (A) *P < 0.05, **P < 0.01, #P < 0.05. Error bars represent ± SEM. (D and E) NK cell viability assays. NK92 cells were treated with indicated concentration of α-syn monomer (D) or α-syn agg (E) for 72 h. MTS solution (Promega) was added during the last 4 h of incubation, and cell viability was determined by measuring the absorbance at 490 nm. Data were analyzed using one-way ANOVA. Error bars represent ± SEM. All data shown are representative of 3 independent experiments.
Here, we also examined whether α-syn altered surface expression levels of NK cell receptors including CD107 (NK activation marker), NKG2A (an inhibitory receptor), and NKG2D (an activating receptor) that show altered expressions in PD patients (41, 42). The levels of CD107, NKG2A, and NKG2D receptors on NK cells were not altered by extracellular α-syn aggregate treatments (SI Appendix, Fig. S3).
NK Cell Depletion Augments Motor Symptoms and Disease Incidence in a Preclinical Mouse Model of PD.
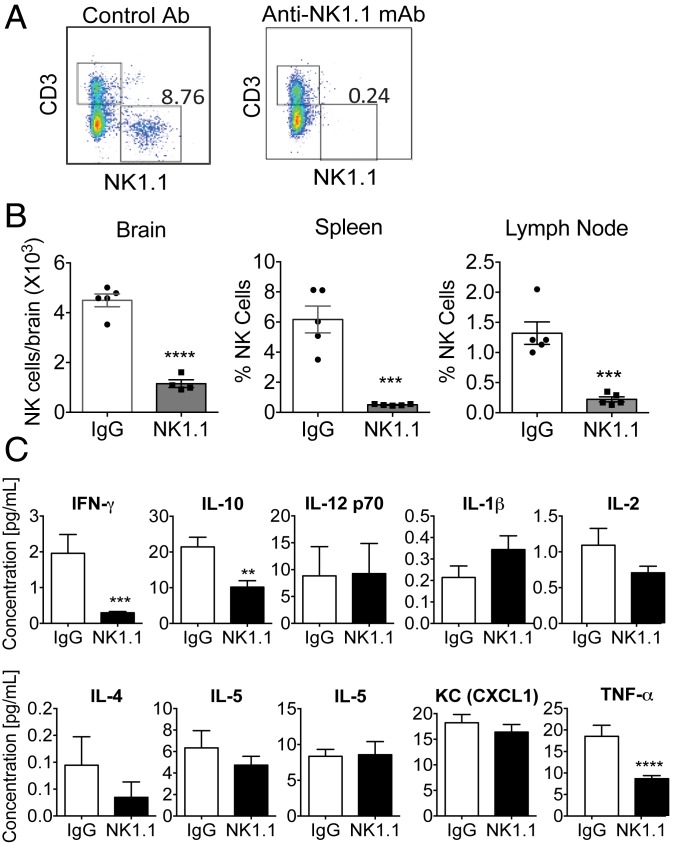
To address the role of NK cells in vivo, we systemically depleted NK cells by utilizing an anti-NK1.1 monoclonal antibody (mAb) (PK136 clone) (57), as described in SI Appendix, SI Materials and Methods. Depletion of NK cells (CD45+CD3−NK1.1+) was determined by flow cytometry analysis (Fig. 5A and SI Appendix, Fig. S4). Our data confirmed a significant reduction of NK cells within the brain, spleen, and inguinal lymph nodes of animals treated with anti-NK1.1 mAb (Fig. 5B). Multiplex proinflammatory cytokine analysis showed that NK1.1 mAb-injected mice displayed significantly decreased IFN-γ levels in serum compared to IgG controls, further validating our systemic NK cell depletion method (Fig. 5C). Our data also showed that the levels of TNF-α and IL-10 were lower in NK cell-depleted mice; however, there were no additional significant alterations in the serum cytokine profile (Fig. 5C).
Fig. 5.
Anti-NK1.1 mAb injection induces systemic NK cell depletion in mice. (A) Representative flow cytometry data to show the depletion of NK cells in mice injected with NK1.1 mAb. Mice were injected with NK1.1 mAb or control IgG2a mAbs (intraperitoneally) as described in SI Appendix, SI Materials and Methods. Quantification of NK cells (CD45+CD3−NK1.1+) in the spleen after injections of NK1.1 or IgG2a mAbs (intraperitoneally). Black boxes represent CD45+CD3+NK1.1− T cells (top left) and CD45+CD3−NK1.1+ NK cells (bottom right) as gating strategy described in SI Appendix, Fig. S4. (B) Quantification of NK cells (CD45+CD3–NK1.1+) in the brain, spleen, and lymph nodes by flow cytometry analysis after injections of NK1.1 or IgG2a mAbs (intraperitoneally), ***P < 0.001, ****P < 0.0001. (C) Multiplex proinflammatory cytokine analysis. Serum samples were collected from mice injected with IgG or NK1.1 mAbs (intraperitoneally) for 10 wk (n = 8 to 10 mice per group) and cytokines were analyzed. Data were analyzed using the Student t test. Error bars represent ± SEM, **P < 0.01, ***P < 0.001, ****P < 0.0001.
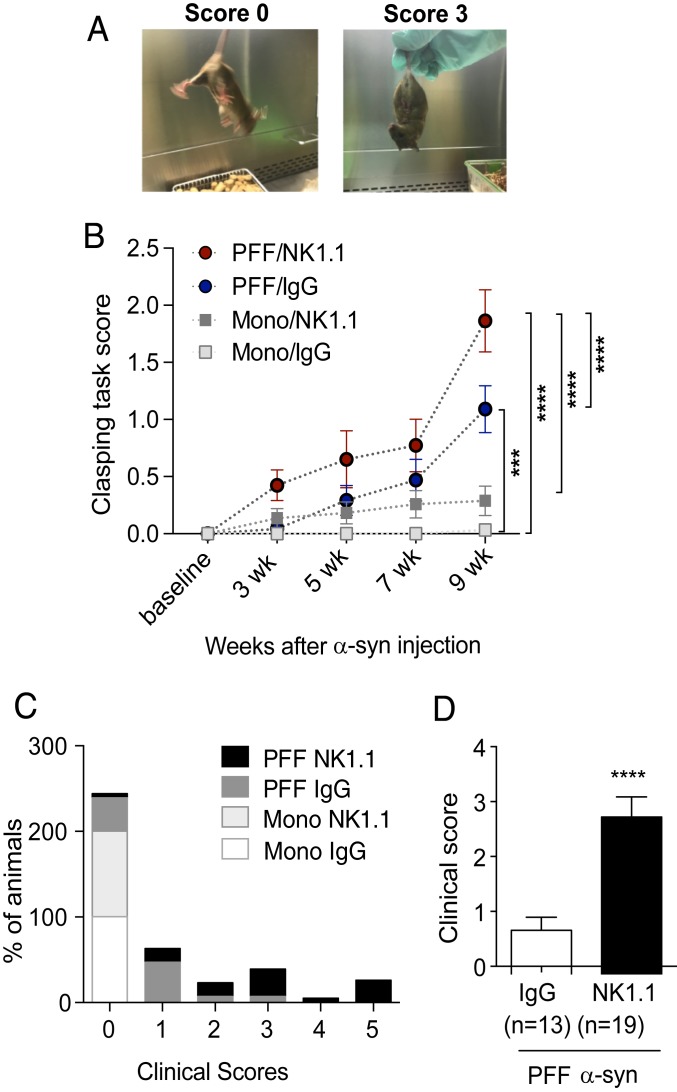
To determine the role of NK cells in a mouse model of PD, we utilized a systemic NK cell-depletion strategy in PFF α-syn M83 Tg mice. To generate this model, we injected PFF α-syn or monomer α-syn as a control into the dorsal striatum of M83 Tg mice as described previously (45). To deplete NK cells, mice began receiving injections of mAbs to NK1.1 or IgG2a 2 d prior to stereotaxic inoculation of PFF or monomer α-syn. Mice were aged for 10 wk while behavioral tasks were performed throughout. To evaluate motor and postural abnormalities as a basic neurological assessment, we conducted the clasping task (Fig. 6A). PFF α-syn–injected M83 Tg mice, but not monomer α-syn–injected M83 Tg mice, displayed observable deficits in hind limb clasping as early as 3 wk after inoculation and continued to display increased motor deficits evidenced by increasing clasping scores coinciding with progression of the disease (Fig. 6B). Monomer α-syn–injected M83 Tg mice did not develop deficits in motor coordination. Importantly, NK cell depletion significantly increased hind limb clasping and therefore exacerbated motor deficits and motor function in PFF α-syn–injected M83 Tg mice compared to PFF α-syn IgG control (Fig. 6B). Nine to 10 weeks after α-syn inoculations we observed obvious clinical motor deficits: That is, unstable gaits and hunched posture with extensive hind limb retraction in some of the PFF α-syn–injected mice. We assigned animals clinical symptom scores from 0 (no symptoms) to 5 (dead) as described in the SI Appendix, SI Materials and Methods. Representative examples of mice with clinical scores of 2 and 4 are provided in Movies S1 and S2. As the clinical symptoms progressed rapidly, all animals’ motor symptoms were evaluated at 10-wk postinjection and then animals were immediately killed for further histological analysis. Here, we showed the percentage of animals in each experimental group assigned to each clinical score (Fig. 6C) (χ2 test, P < 0.0001). Our data demonstrate that NK cell depletion induced significantly more severe clinical motor deficits in PFF α-syn M83 Tg mice compared to the IgG treated PFF α-syn M83 Tg mice (mean scores of 2.868 ± 0.368 vs. 0.808 ± 0.237, respectively) (Fig. 6D) (Table 1). Furthermore, overall percentages of mice showing clinical symptoms were significantly higher in the NK cell-depleted group compared to the IgG treated PFF α-syn M83 Tg mice (94% vs. 61%, respectively) (Table 1). We noted that none of the monomer α-syn–injected M83 Tg mice developed any clinical symptoms (summarized in Fig. 6C and Table 1). Overall, our data indicate that NK cell depletion augments motor deficits implicating a protective role of NK cells in a PFF α-syn–induced mouse model of PD.
Fig. 6.
NK cell depletion augments motor symptoms and disease incidence of PFF α-syn M83 Tg mice. (A) Representative images of the clasping task scores of 0 and 3. (B) Limb clasping tasks were performed on mice every 2 wk (n = 10 to 11 mice per group). Data were analyzed with a 2-way ANOVA followed by the Bonferroni’s post hoc test. ***P < 0.001, ****P < 0.0001. Error bars represent ± SEM. (C) Percentages of animals in each experimental group assigned to each clinical score as described in SI Appendix, SI Materials and Methods at 10 wk postinjection. Data were analyzed using the χ2 test. (D) Average clinical symptom scores of PFF α-syn M83 Tg mice at 10 wk postinjection. Data were analyzed with Student’s t test. ****P < 0.0001. Error bars represent ± SEM.
Table 1.
Summary of clinical motor symptom incidences and scores in mice
| Treatment groups | Incidence of clinical score ≥1 (%) | Mean clinical score (±SEM) |
| Mono α-syn + IgG | 0/11 (0%)a | N/A |
| Mono α-syn + NK1.1 | 0/11 (0%)a | N/A |
| PFF α-syn + IgG | 8/13 (61%)b | 0.808 (±0.237) |
| PFF α-syn + NK1.1 | 18/19 (94%)c | 2.868 (±0.368)*** |
Data are pooled from 3 independent experiments. Incidence of clinical motor symptom score. Different letters are statistically significantly different from each other, determined by ordinary one-way ANOVA with Tukey post hoc test. Mean of clinical score compared PFF α-syn + NK1.1 group to PFF α-syn + IgG, ***P < 0.001, determined by Mann–Whitney U test. N/A, not applicable.
NK Cell Depletion Exacerbates Synuclein Pathology and Neuroinflammation in a Preclinical Mouse Model of PD.
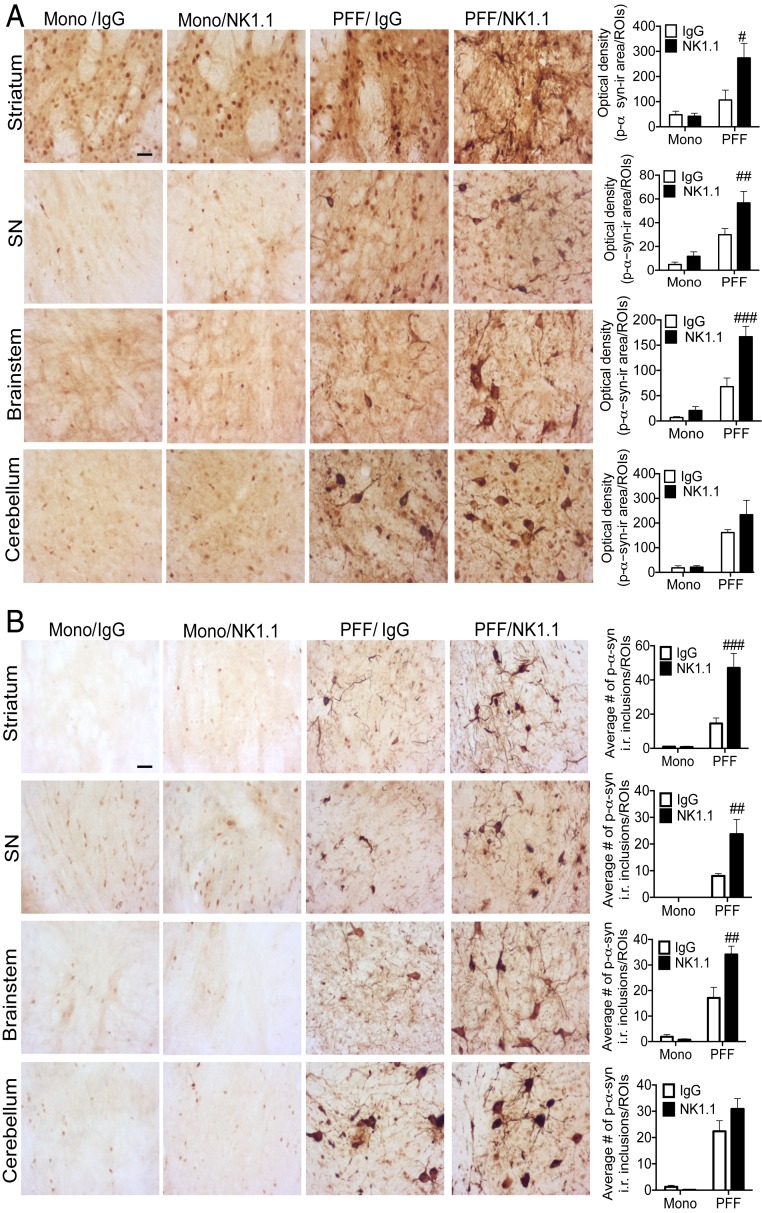
To examine whether NK cell depletion alters CNS pathology, we performed immunohistological analyses for p-α-syn inclusions throughout the CNS of these mice. We confirmed abundant p-α-syn inclusions developed in the striatum, SNpc, cerebellum, and brainstem of mice that received PFF α-syn injection, as previously demonstrated (58). Importantly, NK cell-deficient PFF α-syn M83 Tg mice displayed significantly increased p-α-syn inclusions within the striatum, SNpc, and brainstem but not in the cerebellum compared to control IgG treated PFF α-syn M83 Tg mice (Fig. 7A). To quantify insoluble p-α-syn inclusions, brain sections were treated with proteinase K (PK) and IHC analyses were performed for PK insoluble p-α-syn inclusions in the striatum, SNpc, cerebellum, and brainstem. Monomer α-syn M83 Tg mice did not display PK insoluble p-α-syn inclusions at 10 wk postinjection (Fig. 7B). However, NK cell-deficient PFF α-syn M83 Tg mice displayed significantly increased number of PK-resistant p-α-syn inclusions within the striatum, SNpc, and brainstem, but not in the cerebellum compared to control IgG treated PFF α-syn M83 Tg mice (Fig. 7B). We also interrogated the neuroinflammatory status within brain regions where we observed pathological p-α-syn inclusions by performing IHC analysis for astrocytes (GFAP) and microglia (Iba1) (SI Appendix, Fig. S5). Monomer α-syn M83 Tg mice displayed similar immunoreactivity (i.r.) for GFAP and Iba1 as uninjected M83 Tg mice (SI Appendix, Fig. S5). NK cell-deficient PFF α-syn M83 Tg mice displayed significantly increased i.r. for GFAP within the striatum and the SNpc but not in the brainstem and cerebellum compared to control IgG treated PFF α-syn M83 Tg mice (SI Appendix, Fig. S5A). Furthermore, NK cell depletion in PFF α-syn M83 Tg mice displayed increased i.r. for Iba1 within the striatum, SNpc, and brainstem compared to control IgG-treated PFF α-syn M83 Tg mice (SI Appendix, Fig. S5B).
Fig. 7.
NK cell depletion exacerbates synuclein pathology of PFF α-syn M83 Tg mice. (A) Representative immunohistological images and quantitation data of p-α-syn in the striatum, SNpc, brainstem, and cerebellum (n = 6 to 8 mice per group) at 10 wk postinjection. (Scale bar, 10 μm.) Graphs represent average optical density of positive p-α-syn within the striatum, SNpc, brainstem, and cerebellum. (B) Representative immunohistological images and quantitation of PK-resistant, p-α-syn+ inclusions within the striatum, SNpc, brainstem, and cerebellum. (Scale bar, 10 μM.) Graphs represent average number of PK-resistant, p-α-syn inclusions (≥7 µm)/regions-of-interest within the striatum, SNpc, brainstem, and cerebellum. All data were analyzed with a 2-way ANOVA followed by the Bonferroni post hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001 comparing IgG vs. NK1.1 groups. Error bars represent ± SEM.
NK Cell Depletion Induces Dopaminergic Striatal Degeneration but Not Dopaminergic Neurodegeneration in the SN in a Preclinical Mouse Model of PD.
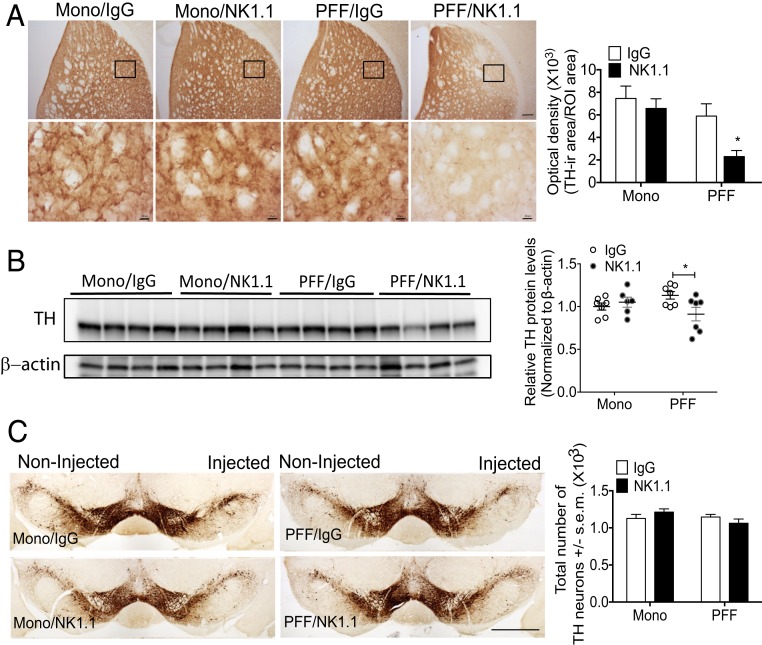
To evaluate nigrostriatal degeneration, we measured the optical density of tyrosine hydroxylase (TH)-positive staining in the striatum, conducted Western blot analysis for TH in the striatum, and performed stereological cell counts of total dopaminergic (DA) neurons in the SNpc. Monomer α-syn M83 Tg mice did not display alterations of TH+ staining within the dorsolateral striatum as measured by OD (Fig. 8A). Importantly, NK cell-deficient PFF α-syn M83 Tg mice displayed significantly decreased TH+ staining within the dorsolateral striatum compared to control IgG treated PFF α-syn M83 Tg mice, implicating striatal degeneration (Fig. 8A). To further interrogate TH levels in the striatum, Western blot analysis was performed. A significant reduction of TH in the striatum of NK cell-deficient PFF α-syn M83 Tg mice compared to control IgG-treated PFF α-syn M83 Tg mice was confirmed by Western blot analysis (Fig. 8B). However, unbiased stereological cell counts performed throughout the entire SNpc did not reveal any differences in the total number of TH+ neurons between groups (Fig. 8C).
Fig. 8.
NK cell depletion induces dopaminergic degeneration in the striatum of PFF α-syn M83 Tg mice. (A) Representative immunohistological images of TH+ staining in the striatum. (Scale bar, 100 μm [low-magnification images] and 10 μm [high-magnification pictures].) Graph represents optical density analysis of TH+ staining in the striatum. The black boxes indicate where high-magnification pictures below are taken from. Error bars represent ± SEM. (B) Western blot analyses of striatal protein using anti-TH or β-actin antibodies. Graph represents the relative TH levels compared to IgG treated monomer M83 Tg in the striatum normalized to β-actin (n = 6 to 7 mice per group). (C) Representative immunohistological staining of TH+ neurons in the SNpc. (Scale bar, 1 mm.) The graph represents the quantification of TH+ neurons by unbiased stereo-investigator. All data were analyzed with a 2-way ANOVA followed by the Bonferroni post hoc test. *P < 0.05 comparing IgG vs. NK1.1 groups. Error bars represent ± SEM.
Discussion
Although the CNS was once considered to be largely devoid of immune entities other than microglia, the central dogma of absolute impermeability of the CNS to immune cells has been refuted during the last decade (59). In conditions of chronic inflammation, like PD, the blood brain barrier becomes disrupted, thus allowing immune cells to extravasate into the brain (60). Our data illustrate the presence of NK cells in the brains of synucleinopathy patients in brain regions associated with robust p-α-syn pathology. Furthermore, we detected NK cell presence in the adult mouse brain, with the number of NK cells increasing with synuclein pathology. Our findings compliment recent transcriptomic analyses demonstrating the diversity of immune cells in the brain and that NK cells are one of the distinct populations within the brain along with B cells, T cells, and dendritic cells in both homeostatic and disease statuses (39, 40). NK cells have been suggested to be involved in neurological diseases (reviewed in ref. 61), particularly in multiple sclerosis (38, 62), yet the functional analysis of NK cells in other neurodegenerative diseases remains elusive. Therefore, we aimed to investigate the role of NK cells in the context of α-syn pathology by utilizing in vitro NK cell culture and an in vivo preclinical mouse model of PD. Our data demonstrate that NK cells clear α-syn without aberrant activation. Importantly, systemic depletion of NK cells led to exacerbated synuclein pathology and motor deficits in vivo suggesting a protective role of NK cells in LB-related neurodegenerative diseases.
Extracellular α-syn species have been detected in the plasma and CSF of PD and multiple system atrophy patients and have been shown to modulate immune responses in the CNS and the periphery (12, 13, 63, 64). Possible therapeutic approaches to stop LB formation and protect from neurodegeneration include inhibiting the production of α-syn, preventing aggregation within the cytoplasm, and promoting clearance in the cytoplasm. Importantly, α-syn can propagate extracellularly (reviewed in refs. 4, 65, and 66), so immunotherapeutic approaches targeting extracellular α-syn aggregates might be promising therapeutic approaches for synucleinopathies. In the CNS, neurons and glial cells can take up extracellular α-syn; however, α-syn internalization will result in inclusions in neurons and a proinflammatory response in glial cells (4, 67). Particularly, extracellular α-syn acts as a DAMP due to its ability to induce expression of TLRs 1, 2, 3, and 7, TNF, and IL-1 (16). Oligomeric α-syn produced by neurons are phagocytosed by microglia and activate neighboring microglia via the TLR2 signaling pathway (19), indicating that the effect of α-syn on immune cells is not simply mediated by phagocytosis of nonspecific protein debris but by specific receptors and their downstream pathways. Here we provide evidence that human NK cells efficiently scavenge extracellular α-syn species in a specific manner, mediated by TLR4 and TLR2 pathways. Furthermore, our data show that extracellular α-syn aggregates do not hyperactivate NK cell effector functions, but instead α-syn reduces the production of proinflammatory IFN-γ and their cytotoxic activity.
To address the physiologic role of NK cells in PD, we utilized an NK cell depletion strategy in a preclinical α-syn mouse model of PD. The PFF α-syn non-Tg mouse model of PD exhibits many clinically relevant hallmarks of PD, including DA cell loss, motor deficits, and synucleinopathies (45). M83 Tg mice overexpress human A53T α-syn (44) and display features of α-syn pathologies both in the CNS and the periphery, including the enteric nervous system, which resembles the pathophysiological conditions of PD. However, their neurodegenerative phenotypes manifest at 8 to 12 mo of age or later and it is not clear if nigrostriatal neurodegeneration is linked to their pathologies [summarized by Koprich et al. (68)]. These mice efficiently display α-syn pathology both in the CNS and periphery in a relatively short period of time (i.e., 3 to 4 mo) (58). In this study, we induced pathology by inoculating PFF α-syn in M83 Tg mice, as previously described (58). By utilizing this PFF α-syn in M83 Tg mouse model of synucleinopathy, we demonstrated that systemic depletion of NK cells leads to exacerbation of motor deficits and robust α-syn burden within the CNS, implicating a protective role of NK cells. Although we could not directly relate motor deficits to DA neurodegeneration in the SNpc, our data clearly suggest that the depletion of NK cells augments the accumulation of pathological α-syn and motor deficits. Indeed, the spread of pathological α-syn may interfere with neural networks, which could explain neurological decline in patients with synucleinopathies. One potential mechanism by which NK cells exert protection is through their ability to clear α-syn inclusions. Another potential mechanism by which NK cells exert protection could be via producing IFN-γ, the key cytokine required for activation or differentiation of antigen-presenting cells, including microglia, which are involved in resolving extracellular α-syn burden. Our data support the notion that there was a substantial decrease of IFN-γ in NK cell-depleted mice, confirming that NK cells are a major source of IFN-γ. It will be necessary to investigate the status of α-syn pathology within the periphery, as bidirectional transfer of α-syn aggregates between the CNS and periphery via the vagal nerve has been shown in vivo (69–72). Furthermore, as immune cells are adept to directly communicate with neurons through the vagal nerve within the gut (69), our future studies will be focused on how NK cells affect α-syn pathology in the periphery, particularly within the gut.
In the cancer field, NK cells have been utilized as a promising immunotherapy because of their capacity to selectively target cancer stem-like cells (73, 74). In particular, NK92 cells, which are highly cytotoxic against a broad range of malignant cells, are infused and well tolerated in patients with cancer (31). Recently, several studies have exploited NK cells’ cytotoxicity against brain cancers, including neuroblastoma and glioblastoma (35, 37, 73, 74). Our data support the idea of utilizing NK cells as a potential cell-based therapy for LB diseases. We have demonstrated that NK cells internalize and clear α-syn without aberrant activation and systemic depletion of NK cells led to exacerbated synuclein pathology in vivo. Our data suggest that NK cells have the potential to become the basis of a cell-therapeutic strategy to stop or slow abnormal protein pathogenesis in PD and possibly other synuclein-related neurodegenerative diseases.
Materials and Methods
Preparation of Recombinant Proteins and Aggregates.
Recombinant human α-syn (>98% purity) proteins were expressed in BL21(DE3)/RIL Escherichia coli and purified by size-exclusion chromatography and Mono Q ion-exchange chromatography, as previously described (46). To further remove endotoxin contamination, it was purified by High S support cation-exchange chromatography, as described previously (75, 76). Final endotoxin tests resulted in less than 0.5 EU/mg. Detailed methods can be found in SI Appendix, SI Materials and Methods.
Animals.
Transgenic mice (8- to 10-wk-old male and females) overexpressing human A53T α-syn mutant protein (M83 Tg) were purchased from Jackson Laboratory (004479). Experimental procedures involving the use of animals and animal tissue were performed in accordance with NIH guidelines for animal care and use and approved by the Institutional Animal Care and Committee at the University of Georgia.
Stereotaxic Surgery.
Animals received a unilateral injection of human PFF or monomer α-syn (5 µg in 1 µL) into the right striatum using stereotaxic coordinates relative to bregma and the dural surface at AP, +0.3 mm; ML, + 2.3 mm; DV, −3.5 mm bregma at the rate of 0.2 μL/min. Detailed methods can be found in SI Appendix, SI Materials and Methods.
Primary Human NK Cell Isolation.
The human subject studies were performed following the guidelines of the World Medical Association's Declaration of Helsinki. Human subjects were recruited at the University of Georgia to donate blood for NK cell preparation. The study protocol, including the human blood protocol (UGA# 2012-10769-06) and the consent form, was reviewed and approved by the Institutional Review Board of the University of Georgia. Enrolled healthy volunteers provided informed consent. Detailed human NK cell isolation method can be found in SI Appendix, SI Materials and Methods.
Immunohistochemistry.
Postmortem human brain tissues were acquired from Emory University Center for Neurodegenerative Disease (Atlanta, GA). PD or PD/DLB patients and control subject did not differ significantly for mean age at death (patients [n = 11], 72.7 ± 2.6 y: Healthy controls [n = 3], 58.7 ± 10.2 y, mean ± SEM; P > 0.05, unpaired t test). A detailed IHC method for mouse brain can be found in SI Appendix, SI Materials and Methods.
Additional Materials and Methods.
Additional materials and methods can be found in SI Appendix, SI Materials and Methods.
Data Availability.
Additional data can be found in SI Appendix, Figs. S1–S5 and Movies S1 and S2 and raw IHC images and analysis data have been deposited in the Open Science Framework (https://osf.io/8gqtk/).
Supplementary Material
Acknowledgments
We thank Dr. Nikolay Filipov, Dr. Jesse Schank, Mr. James Barber, Dr. Michelle Lewis, Ms. Ruth Davis, and Ms. Mary Ard at the University of Georgia for the valuable discussions and/or technical assistance. This work was supported by the start-up funds from the Department of Physiology and Pharmacology at the University of Georgia (to J.-K.L.), a Georgia Partners in Regenerative Medicine Seed grant (to J.-K.L. and L.B.W.), and a Michael J. Fox Foundation Target Validation grant (to J.-K.L.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The raw IHC images and analysis data reported in the paper have been deposited to the Open Science Framework (https://osf.io/8gqtk/).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909110117/-/DCSupplemental.
References
- 1.Croisier E., Moran L. B., Dexter D. T., Pearce R. K., Graeber M. B., Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J. Neuroinflammation 2, 14 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., Goedert M., Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clavaguera F., et al. , Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desplats P., et al. , Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J. L., Lee V. M., Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 286, 15317–15331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanis L., α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S., Seo J. H., Suh Y. H., Alpha-synuclein, Parkinson’s disease, and Alzheimer’s disease. Parkinsonism Relat. Disord. 10 (suppl. 1), S9–S13 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Yu S., et al. , Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience 145, 539–555 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Chiti F., Dobson C. M., Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Borghi R., et al. , Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson’s disease and normal subjects. Neurosci. Lett. 287, 65–67 (2000). [DOI] [PubMed] [Google Scholar]
- 11.El-Agnaf O. M., et al. , Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 17, 1945–1947 (2003). [DOI] [PubMed] [Google Scholar]
- 12.El-Agnaf O. M., et al. , Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 20, 419–425 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Lee P. H., et al. , The plasma alpha-synuclein levels in patients with Parkinson’s disease and multiple system atrophy. J. Neural Transm. (Vienna) 113, 1435–1439 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Tokuda T., et al. , Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson’s disease. Biochem. Biophys. Res. Commun. 349, 162–166 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Lee H. J., Bae E. J., Lee S. J., Extracellular α-synuclein—a novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 10, 92–98 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Béraud D., et al. , Microglial activation and antioxidant responses induced by the Parkinson’s disease protein α-synuclein. J. Neuroimmune Pharmacol. 8, 94–117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H. J., Suk J. E., Bae E. J., Lee S. J., Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem. Biophys. Res. Commun. 372, 423–428 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Fellner L., et al. , Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim C., et al. , Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 4, 1562 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam V. C., Lanier L. L., NK cells in host responses to viral infections. Curr. Opin. Immunol. 44, 43–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small C. L., et al. , NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J. Immunol. 180, 5558–5568 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Schmidt R. L., Filak H. C., Lemon J. D., Potter T. A., Lenz L. L., A LysM and SH3-domain containing region of the Listeria monocytogenes p60 protein stimulates accessory cells to promote activation of host NK cells. PLoS Pathog. 7, e1002368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagiv A., et al. , Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 32, 1971–1977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorén F. B., et al. , Human NK Cells induce neutrophil apoptosis via an NKp46- and Fas-dependent mechanism. J. Immunol. 188, 1668–1674 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Waggoner S. N., Kumar V., Evolving role of 2B4/CD244 in T and NK cell responses during virus infection. Front. Immunol. 3, 377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martín-Fontecha A., et al. , Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5, 1260–1265 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Vitale M., et al. , NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood 106, 566–571 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Williams B. A., et al. , A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 8, 89256–89268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonn T., Becker S., Esser R., Schwabe D., Seifried E., Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J. Hematother. Stem Cell Res. 10, 535–544 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Arai S., et al. , Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 10, 625–632 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Tonn T., et al. , Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 15, 1563–1570 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Suck G., et al. , NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 65, 485–492 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam Y. K., Miyagawa B., Ho V. C., Klingemann H. G., Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J. Hematother. 8, 281–290 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Yan Y., et al. , Antileukemia activity of a natural killer cell line against human leukemias. Clin. Cancer Res. 4, 2859–2868 (1998). [PubMed] [Google Scholar]
- 35.Zhang C., et al. , ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J. Natl. Cancer Inst., 108, djv375 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Genßler S., et al. , Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. OncoImmunology 5, e1119354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J., et al. , CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 5, 11483 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao J., et al. , Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J. Exp. Med. 207, 1907–1921 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q., et al. , Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223 e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Hove H., et al. , A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Mihara T., et al. , Natural killer cells of Parkinson’s disease patients are set up for activation: A possible role for innate immunity in the pathogenesis of this disease. Parkinsonism Relat. Disord. 14, 46–51 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Niwa F., Kuriyama N., Nakagawa M., Imanishi J., Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr. Gerontol. Int. 12, 102–107 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Cen L., et al. , Peripheral lymphocyte subsets as a marker of Parkinson’s disease in a Chinese population. Neurosci. Bull. 33, 493–500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giasson B. I., et al. , Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34, 521–533 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Luk K. C., et al. , Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volpicelli-Daley L. A., Luk K. C., Lee V. M., Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 9, 2135–2146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmes B. B., et al. , Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. U.S.A. 110, E3138–E3147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez J., Huang X., Yang Y., Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 6, e1000811 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brusilovsky M., et al. , Regulation of natural cytotoxicity receptors by heparan sulfate proteoglycans in -cis: A lesson from NKp44. Eur. J. Immunol. 45, 1180–1191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mian M. F., Lauzon N. M., Andrews D. W., Lichty B. D., Ashkar A. A., FimH can directly activate human and murine natural killer cells via TLR4. Mol. Ther. 18, 1379–1388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masilamani M., Peruzzi G., Borrego F., Coligan J. E., Endocytosis and intracellular trafficking of human natural killer cell receptors. Traffic 10, 1735–1744 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bottger G., Nagelkerken B., van der Sluijs P., Rab4 and Rab7 define distinct nonoverlapping endosomal compartments. J. Biol. Chem. 271, 29191–29197 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Tanida I., Ueno T., Kominami E., LC3 and autophagy. Methods Mol. Biol. 445, 77–88 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Harms A. S., et al. , MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci. 33, 9592–9600 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q. S., Heng Y., Yuan Y. H., Chen N. H., Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 265, 30–37 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Schoenborn J. R., Wilson C. B., Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 96, 41–101 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Koo G. C., Peppard J. R., Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma 3, 301–303 (1984). [DOI] [PubMed] [Google Scholar]
- 58.Luk K. C., et al. , Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louveau A., et al. , Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garretti F., Agalliu D., Lindestam Arlehamn C. S., Sette A., Sulzer D., Autoimmunity in Parkinson’s disease: The role of α-synuclein-specific T cells. Front. Immunol. 10, 303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poli A., et al. , NK cells in central nervous system disorders. J. Immunol. 190, 5355–5362 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Liu Q., et al. , Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat. Neurosci. 19, 243–252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mollenhauer B., et al. , α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 10, 230–240 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Tokuda T., et al. , Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75, 1766–1772 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Angot E., Brundin P., Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism Relat. Disord. 15 (suppl. 3), S143–S147 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Danzer K. M., et al. , Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 7, 42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee E. J., et al. , Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J. Immunol. 185, 615–623 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Koprich J. B., Kalia L. V., Brotchie J. M., Animal models of α-synucleinopathy for Parkinson disease drug development. Nat. Rev. Neurosci. 18, 515–529 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Houser M. C., Tansey M. G., The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 3, 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braak H., Rüb U., Gai W. P., Del Tredici K., Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna) 110, 517–536 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Holmqvist S., et al. , Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Uemura N., et al. , Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 13, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delgado D. C., et al. , Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 70, 9554–9561 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang R. K., et al. , Intratumoral treatment of smaller mouse neuroblastoma tumors with a recombinant protein consisting of IL-2 linked to the hu14.18 antibody increases intratumoral CD8+ T and NK cells and improves survival. Cancer Immunol. Immunother. 62, 1303–1313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giasson B. I., Murray I. V., Trojanowski J. Q., Lee V. M., A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J. Biol. Chem. 276, 2380–2386 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Rutherford N. J., et al. , Studies of lipopolysaccharide effects on the induction of α-synuclein pathology by exogenous fibrils in transgenic mice. Mol. Neurodegener. 10, 32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data can be found in SI Appendix, Figs. S1–S5 and Movies S1 and S2 and raw IHC images and analysis data have been deposited in the Open Science Framework (https://osf.io/8gqtk/).