Abstract
Fasting is defined as the abstinence from consuming food and/or beverages for different periods of time. Both traditional and modern healthcare systems recommend fasting as a therapeutic intervention for the management of several chronic, non-infectious diseases. Exercising during a fasting state increases lipolysis in adipose tissue while also stimulating peripheral fat oxidation, resulting in increased fat utilization and weight loss. A key focus of this review is to assess whether endurance training performed while fasting induces specific training adaptations, where increased fat oxidation improves long-term endurance levels. Fasting decreases body weight, lean body and fat content in both trained and untrained individuals. Several studies indicate a broader impact of fasting on metabolism, with effects on protein and glucose metabolism in sedentary and untrained subjects. However, there are conflicting data regarding the effects of fasting on glucose metabolism in highly trained athletes. The effects of fasting on physical performance indicators also remain unclear, with some reporting a decreased performance, while others found no significant effects. Differences in experimental design, severity of calorie restriction, duration, and participant characteristics could, at least in part, explain such discordant findings. Our review of the literature suggests that there is little evidence to support the notion of endurance training and fasting-mediated increases in fat oxidation, and we recommend that endurance athletes should avoid high intensity training while fasting.
Keywords: fasting state, calorie restriction, metabolic adaptation fat oxidation, glucose metabolism, endurance performance, Ramadan
Introduction
The high prevalence of overweight and obesity continues to be alarming, as such conditions are usually accompanied by health complications resulting from metabolic diseases, diabetes, cancer and cardiovascular disease.1,2 This situation is expected to worsen with the rampant spread of physical inactivity. However, fasting and exercise training are non-pharmacological and inexpensive ways to help manage obesity- and overweight-related complications.3 Many people fast for health, religious or cultural reasons.2 Fasting is defined as the abstinence from consuming food and/or beverages for different periods of time, which can last from several hours a day to a few weeks.4,5 There is no clear definition of when fasting begins after the last intake of food/drink.6 Fasting is associated with increased longevity because of its many roles in modifying human health and ageing.2 Moreover, this unique intervention is considered effective for the management of chronic and acute diseases in both traditional and modern healthcare systems.7 Fasting is a lifestyle management strategy that benefits several chronic, non-infectious diseases.7 Fasting and/or physical exercise is often used to investigate the regulation of intermediary metabolism.6 While prolonged periods of fasting harms health and physical performance, it remains unclear whether shorter or more prolonged periods of intermittent fasting are necessarily harmful.6
The effect of fasting on the performance of elite athletes started to be investigated in 2007,8–12 stimulated by the belief by many athletes and coaches that fasting negatively impacts on sports performance.8,13 Although various forms of fasting were evaluated to explore the effects on sports performance, Ramadan fasting (as prescribed by the Islamic faith) is garnering increased prominence that is largely due to its unique features.2,8 For example, interest in Ramadan-style intermittent fasting was renewed during the London 2012 Olympic Games and also the FIFA 2014 Soccer World Cup as both events were staged during the month of Ramadan.6,8,14 In this narrative review, we discuss the effects of a combination of physical training and fasting on body composition changes, metabolic adaptations and sport performance in untrained and trained subjects.
Literature Search Methodology
A literature search was conducted using four electronic databases (PubMed, ISI Web of Knowledge, Web of Science and SPORTDiscus) from inception until January 2019. The following key terms (and synonyms searched for by the MeSH database) were included and combined using the operators “AND”, “OR”, “NOT”: “fasting”*“body weight”, “body mass”, “body fat”, “weight loss”, “health”, “training”, “exercise”, “endurance”, “performance”, “metabolism”, “glucose”, “lipid”, “protein”, “obesity”, “cardiovascular disease”, “caloric restriction”, “diet”, “restricted feeding”, “Ramadan”. Our search identified 6671 records. Only randomized controlled trials that examined the effects of fasting on performance and selected health indices in healthy sedentary individuals, untrained and trained subjects were eligible for inclusion. Included articles had to be written in English and published in peer-reviewed journals. In addition, the reference lists and citations (Google Scholar) of the identified studies were explored in order to detect further relevant research papers. The final screening was based on the relevance of the identified items for assessing the effect of fasting on health indices and physical capacities of sedentary people and trained subjects.
Study Selection
The final screening done by two investigators was based on the relevance of the identified items for assessing the effect of fasting and training on health indices and physical performances of sedentary or trained people using PICO criteria (Table 1). Overall, our search identified 6,671 records, from which1,261 studies were included after title and abstract screening. 71 articles were included after duplicate and risk of bias checking.
Table 1.
PICOS Criteria
| PICO Title | Description |
|---|---|
| Population | Healthy Sedentary Human, Untrained, Trained |
| Intervention | Fasting, Physical Training |
| Comparison | Control Intervention, No Intervention |
| Outcomes | Health Indices, Physical Performances |
| Study Design | Randomized Controlled Trial |
Quality Assessment
The methodological qualities of the studies were assessed by using the PEDro scale with the criteria of >7 in a 11-point PEDro scale (http://www.pedro.fhs.usyd.edu.au) which has been shown to have good reliability and validity. Two independent researchers assessed the quality of the studies, and in cases of disagreement, a third researcher assessed the studies and made the final decision on the quality of the studies.
Fasting
The most frequent form of dietary restriction studied is daily caloric restriction, while other modes such as intermittent fasting is also widely practiced. However, research focusing on intermittent fasting and caloric restriction regimes is not as extensive as religious fasting.15 Intermittent fasting entails abstinence from food for parts of the day or the week. Energy restriction is the common factor for all the various forms of fasting, even if it is not practiced on a daily basis. Frequently practiced intermittent fasting can be classified into three categories; intermittent calorie restriction (ICR), alternate day fasting (ADF), and time restricted feeding (TRF), with each possessing varying periods of feeding and fasting.15
Intermittent Calorie Restriction
Intermittent calorie restriction (ICR), also known as whole-day fasting, is the simplest form of intermittent fasting and involves fasting for more than 24h two or three times a week together with ad libitum food intake on the other days, and separated from the next cycle by at least one week.16 There are two forms of intermittent fasting: 2:5 (caloric restriction for 2 days a week, and a regular diet for 5 days) or 3:4 (caloric restriction for 3 days a week, and a regular diet for 4 days). However, a number of protocols permit food consumption of about 25% (400–600 kcal/day) of total energy expenditure on fasting days.
Alternate Day Fasting
As the practice of intermittent fasting is gaining widespread popularity, there is also increased promotion of alternate day fasting (ADF).17,18 The ADF program involves alternating non-fasting days where participants consume food ad libitum followed by fasting days where only 25% of the usual dietary intake is consumed (~500 kcal).18,19 Of note, a number of ADF protocols do not allow any caloric intake on fast days.20
Time Restricted Feeding
While fasting requires abstinence from food consumption, a number of intermittent fasting protocols allow for the intake of relatively small quantities of food (~25% of daily caloric needs) during fasting times.16 Time restricted feeding (TRF) involves a set number of hours of daily fasting, while feeding is permissible during the remaining hours. TRF has three variants: 16/8 (16-h fast, 8-h feeding window), 18/6 (18-h fast, 6-h feeding window), and 20/4 (20-h fast, 4-h feeding window). The rationale for TRF centers around circadian rhythms as recent work outlined the importance of chrono-nutrition, which is the interaction between meal timing and the circadian system that regulates physiology, metabolism and behavior.21 Fasting during the month of Ramadan is one of the five pillars of Islam and is an extensively studied example of a timed dietary approach that falls under the TRF umbrella.7 Ramadan is the ninth month of the Islamic lunar calendar and daily abstinence from food and fluid intake occurs from dawn to sunset for the duration of this month.19 The duration of the fasting period is significantly influenced by the geographical location and season, and commonly lasts from 10 to 18h.19
The main differences between these protocols are the severity of caloric restriction, the abstinence from food/beverages per day and the frequency of caloric restriction per week. For ADF, an individual alternates ad libitum feeding days with fasting days, which typically consist of one meal consumed at lunchtime that contains approximately 25% of the baseline caloric needs for the individual. For most TRF protocols, a person fasts for a number of hours (16–20 h) and feeding occurs during the remaining hours (4–8 h) in a day. Whole day fasting protocols involve complete fasting or severe caloric restriction for one or two non-consecutive days per week.15
Acute Effects of Fasting
While fasting is associated with a coordinated set of metabolic changes designed to conserve carbohydrates and increase the reliance on fat as a substrate for energy supply, there is also a decline in cognitive behavior.22 There are marked metabolic changes that occur while fasting. Glucose levels are elevated during and about 6 h after eating but remain low for the remaining 16 h until the next meal. The rate of blood glucose utilization by tissues is ~ 2 mg/kg/min in the post-absorptive state. A modest reduction in serum glucose levels can occur within a few hours of fasting (fasting glucose levels of 3.3 and 3.9 mmol/L; normal range 3.5–5.5 mmol/L), likely due to attenuated hepatic glycogen synthesis and glycolysis. Such modifications occur due to decreased insulin concentrations and also increased glucagon levels together with enhanced sympathetic activity.23 During the fasting window the systemic levels of free fatty acids (FFA) and ketones are increased together with an activation of gluconeogenesis (from amino acids, glycerol and ketone bodies).6 Fasting also attenuates circulating insulin and insulin-like growth factor-1 levels and raises circulating glucagon levels due to hepatic gluconeogenesis. In fasting conditions, FFA and ketones are the main sources of energy for cells and this transition is called intermittent metabolic switching or the glucose-ketone (G-to-K) switchover. Inverse switching, i.e., ketone-glucose (K-to-G) occurs after eating a meal.6 By contrast, starvation (short term) increases levels of acylcarnitine species and the oxidized amino acid dimer cystine, and decreases plasma tryptophan, choline phosphate, hippuric acid and glycerophosphocholine levels. Moreover, although protein metabolism constitutes a relatively minor contribution to energy supply with normal feeding, its proportion markedly increases with severe calorie restriction. A decline in cognitive function during fasting may be due to decreased blood glucose levels.19,22
Chronic Effects of Fasting
Fasting Protocols and Body Composition
Details of fasting studies are summarized in Tables 2–5 according to the various categories used. The findings from six ICR studies are summarized in Table 2, and represents a combination of intermittent fasting and caloric restriction (hypocaloric diet), or modified fasting that allows for a small quantity of food intake even on fasting days. There were significant decreases in body weight,24,25 and body fat,26,27 with the exception of the study by Fitzgerald et al (2018).28 Such variability in ICR was likely due to a number of factors: not all subjects in ICR studies fast the same number of days per week, as some fasted one day per week,26 while others fasted for 2 days that were either consecutive,28 or nonconsecutive.24,25 The data generated thus did not suggest which of these ICR protocols elicited the greatest impact. Additional confounding factors included age, weight and gender, as ICR studies generally included middle-aged or older adults.15
Table 3.
Effect of Alternate Day Fasting on Body Composition
| Author | Study Population | Study Design | Main Findings |
|---|---|---|---|
| Oh et al (2018)30 | 45 overweight and obese (26 Females −19 Males): -ADCR, n=13 -Exercise, n=10 -Exercise + ADCR, n=12) -CG, n=10
|
For 8 weeks Exercise-ADCR and ADCR groups: → 3 Alternately fasted days (25% of daily energy intake, food consumed between 12 pm and 2 pm) → 4 Feed days (ad libitum) |
In ADCR group a significant reduction in: ↓ Body weight:-2.4 ± 3.1 kg (p<0.05) ↓ BMI: −0.9 ± 1.3 kg/m2 (p<0.05) ↓ Waist circumference: −2.2 ± 3.6 cm (p<0.05) ↓ Percent of body fat: −1.3 ± 2.4% (p<0.05). |
| Trepanowski et al (2017, 2018)18,31 | 100 obese adults (86 Females- 14 Males): - ADF, n=34 - CR, n=35 - CG, n=31
|
For 1 year (6-month weight-loss + 6-month weight-maintenance): →ADF: 25% of energy needs on fast days; 125% of energy needs on alternating “fast days” →CR: 75% of energy needs every day →CG: no-intervention |
↓ Fat free mass/total mass in both ADF (0.03 ± 0.00) and CR (0.03 ± 0.01) compared to the control group (P<0.01). NS differences between the intervention groups. |
| Catenacci et al (2016)36 | 26 adults with obesity (19 Females- 6 Males): -ADF, n=14 -CR, n=12
|
For 8 weeks → ADF: alternate between fasting days (zero-calorie) and ad libitum feeding days. → CR: ↓−400 kcal/day |
NS differences in Weight and Body composition between ADF and CR groups. In ADF group: ↓ Body weight: 94.8 ± 4.4 to 86.5 ± 4.4 kg (p < 0.001). ↓ BMI: 35.8 ± 1.4 to 33.6 ± 1.4 kg/m2 (p<0.001). ↓ Fat mass: 37.3 ± 2.6 to 33.9 ± 2.5 Kg (p<0.001). ↑ Lean mass: 35.2 ± 2.8 to 50 ± 2.7 kg (p<0.001). |
| Varady et al (2013)35 | 32 normal-weight and overweight (22 Females- 8 Males): - ADF, n=15 -CG, n=15
|
For 12 weeks →ADF group alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
↓ Body weight −5.2 ± 0.9 kg (P<0.001) ↓ Body fat −3.6 ± 0.7 kg (P<0.001) NC in fat free mass |
| Klempel et al (2013)39 | 32 obese women: -ADF-HF, n=17-ADF-LF, n=18
|
For 8 weeks Alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. The two diets: →ADF–HF (45% fat, 40% carbohydrate and 15% protein), →ADF–LF (25% fat, 60% carbohydrate and 15% protein). |
↓ Body weight (P<0.0001) −4.3±1.0 kg in the ADF–HF and −3.7±0.7 kg in ADF–LF group. ↓ Fat mass (P<0.0001) −5.4±1.5 kg and −4.2±0.6 kg in the ADF–HF and ADF–LF groups respectively. NC in Fat free mass (ADF–HF: 1.1±1.3 kg; ADF–LF: 0.5±0.7 kg). |
| Bhutani et al (2013)32 | 83 obese (80 Females - 3 Males) -E-ADF, n = 18 -ADF, n = 25 -Exercise, n = 24 -CG, n = 16
|
For 12 weeks →ADF and E-ADF groups alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
In ADF: ↓Body weight: 94 ± 3 to 91 ± 3 kg (p<0.001). ↓BMI: 35±1 to 34±1 kg/m2 (p<0.001). ↓Waist circumference: 100 ± 2 to 95 ± 2 cm (p < 0.001). ↓Fat mass: 43 ± 2 to 41 ± 2 kg (p=0.008). ↓Fat free mass: 51 ± 2 to 50 ± 2 kg (p=0.03). |
| Eshghinia & Mohammadzadeh (2013)33 | 15 overweight and obese women.
|
For 6 weeks, On 3 weekly fasting days; Saturday, Monday, and Thursday (25%– 30% of energy needs) On 4 feeding days (1700–1800 kcal/d) |
↓Body weight: 84.3 ± 11.4 to 78.3 ± 10.2 kg (P<0.001). ↓BMI: 33.16 ± 5.02 to 30.72 ± 4.62 kg/M2 (P<0.001). ↓Fat mass: 45.82 ± 4.16 to 42.98 ± 4.01% (P<0.001). |
| Varady et al (2011)40 | 59 overweight and obese (50 Females- 9 Males): ADF, n=13 CR, n=12 Exercise, n=12 Control, n=12
|
For 12 weeks: ADF and E-ADF groups alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
↓ Body weight (P<0.001) in: ADF: −5.2 ± 1.1% CR: −5.0 ± 1.4% EX: −5.1 ± 0.9% |
| Varady et al (2009)34 | 60 obese (12 Females, 4 Males)
|
For 8 weeks: Alteration between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
↓ weight −5.6 ± 1.0 kg (P<0.001) ↓ Body fat-5.4 ± 0.8 kg (P<0.01) |
Abbreviations: ADCR, Alternate Day Caloric Restriction ADF, Alternate Day Fasting; ADF-HF, Alternate Day Fasting with High Fat ADF-LF, Alternate Day Fasting with Low Fat; BMI, Body Mass Index; CR, Caloric Restriction; E-ADF, Exercise With Alternate Day Fasting; EX, Exercise; n, number; NC, No Change; NS, Non-Significant.
Table 2.
Intermittent Caloric Restriction and Body Composition
| Author | Study Population | Study Design | Main Findings |
|---|---|---|---|
| Schübel et al (2018)29 | 150 overweight and obese (50% Males – 50% Females): - CCR, n=49 - ICR, n=49 - CG, n=52.
|
→ICR: 5 days without energy restriction and 2days with 75% ↓ in energy needs → CCR: 20% daily ↓ in energy needs →CG: NC in calorie intake (12 weeks intervention phase((12 weeks maintenance phase)) 26 weeks follow-up phase). |
Body weight during intervention phase: ↓ ICR: 7.1%±0.7% (P<0.001) ↓CCR: 5.2%±0.6% (P=0.053). ↓ CG:3.3%±0.6% (NS) At the final follow-up assessment (week 50), weight loss was ↓ ICR: 5.2%±1.2% (P=0.01) ↓ CCR: 4.9%±1.1% (P=0.89) ↓ CG:1.7% ± 0.8% (NS) |
| Fitzgerald et al (2018)28 | 31 subjects with multiple sclerosis(7 Males - 24 Females): - DCR, n=11 -ICR, n=11 -CG, n=9.
|
For 8 weeks: →DCR: 22% daily ↓ in energy needs → ICR:75% ↓ in energy needs for2 days/week and 0% ↓for 5 days/week →CG:NC in energy needs. |
NS weight changes in the ICR group |
| Teng et al (2013)25 | 56 Men: - FCR, n=28 - CG, n=28.
|
For 12 weeks: → FCR: 300–500 kcal/d↓ in caloric intake (For 2 non-consecutive days per week). →CG: NC in energy needs |
A significant interaction effect in →Body weight (P<0.001) →BMI (P<0.01) →Fat percentage (P<0.001) →Fat mass (P<0.05). All these variables ↓ in the FCR group |
| Hussin et al (2013)24 | 32 healthy males: - FCR, n=16 - CG, n=16
|
For 12 weeks: → FCR: 300–500 kcal/d ↓ in caloric intake (For 2 non-consecutive days per week). →CG: NC in energy needs |
In FCR group: ↓ Body weight: −3.8% (74.2 ± 7.8 kg to 71.4 ± 7.2 kg) (P=0.000) ↓BMI: −3.7% (26.7 ± 1.8 to 71.4 ± 7.2) (P=0.043) ↓Percent body fat: −5.7% (26.4 ± 2.4% to 24.9 ± 2.5%) (P=0.001). |
| Klempel et al (2012)26 | 54 obese women: - IFCR-L, n= 28 - IFCR-F, n=26
|
For 8 weeks: →6 days each week anisocaloric diet with a 30% energy restriction →24 hFasting (120 kcal intakes from juice powder). |
↓Body weight(P=0.04): IFCR-L group (3.9 ± 1.4 kg) versus IFCR-F group (2.5 ± 0.6 kg). ↓Fat mass (P<0.0001): IFCR-L (2.8 ± 1.2 kg) versus IFCR-F (1.9 ± 0.7 kg). |
| Teng et al (2011)27 | 25 healthy men: -FCR, n=12 -CG (n=13)
|
A three-month clinical trial on CR (↓ of 300 to 500 kcal/day) combined with two non-consecutive days/week of fasting (FCR) | In the FCR ↓Body weight: −3.14% (71.6 ± 6.0 to 69.3 ± 6.0 kg) (P<0.001) ↓Body fat: −6.35% (26.4 ± 3.9 to 25.3 ± 3.8 kg) (P<0.05) ↓Fat-free mass: −0.9% (P<0.05) |
Abbreviations: BMI, Body Mass Index; CCR, Continuous Caloric Restriction; CG, Control Group; CR, Caloric Restriction; DCR, Daly Caloric Restriction; FCR, Fasting Caloric Restriction; ICR, Intermittent Caloric Restriction; IFCR-F, Intermittent Fasting Caloric Restriction food based; IFCR-l, Intermittent Fasting Caloric Restriction Liquid based; n, number; NS, Non-significant.
Table 5.
Effects of Ramadan Fasting on Body Composition
| Authors | Study Population | Main Findings |
|---|---|---|
| Alsubheen et al (2017)47 | 17 healthy males: →FG: n=9, Age: 32.2 ± 7.8 years →NFG: n=8, Age: 35 ± 9.4years. |
↓ Body mass and adiposity (P< 0.05) NC in lean mass |
| Nugraha et al (2017)51 | 50 healthy males: →FG: n=25, Age: 26.12 ± 0.98 years →NFG: n=25, Age: 26.2 ± 0.98 years |
↓Body weight (p<0.001) ↓Body mass index (p<0.001) ↓Skeletal muscle mass (p<0.01) ↓ Fat free mass (p<0.01) |
| Aliasghari et al (2017)37 | 83 patients with NAFLD (57 Males – 26 Females): →FG: n=42, Age: 37.59 ± 7.06 years →NFG: n=41, Age: 35.8 ± 7.33 years. |
↓Body weight, ↓BMI, ↓Waist and hip circumferences, ↓Waist-to-hip ratio (P<0.001) ↓Body fat percentage in males (P=0.31) and female (P=0.4) |
| Latiri et al (2016)49 | 29 healthy males Age: 27 ± 1 years | NS effect on weight (P=0.98) or BMI (P =0 0.97) |
| Sezen et al (2016)52 | 70 healthy men Age: 37±7 years | ↓BMI, ↓Waist-Hip ratio, ↓Body water rate, ↓Body fat mass, and ↓Visceral fat mass (p<0.05 each) |
| Gnanou et al (2015)53 | 20 healthy men Age: 19–23 years. |
↓ Body weight (2.4%, p < 0.001) ↓ Body mass index (5.5%, p < 0.01) |
| Hammouda et al (2014)54 | 12 males, professional soccer players Age 17.52 ± 0.2 years |
↓Body mass (P<0.001) ↓Fat mass (P<0.01) |
| Hammouda et al (2013)55 | 15 males, professional soccer players Age: 17.3 ± 0.3 years |
↓Body mass (P< 0.05) ↓ Fat mass (P<0.05). |
| Bouhlel et al (2013)56 | 20 trained men: →FG, n=10 →NFG, n=10 Age: 21.8 ± 1.9 years |
↓Body weight (P < 0.001) ↓BMI (p < 0.01) ↓Fat mass percentage (P<0.03). |
| Zarrouk et al (2013)57 | 8 trained males karate players Age: 17.2± 0.5 years |
NC |
| Mirzaei et al (2012)58 | 14 males collegiate wrestlers Age: 20.12±2.5 years |
↓ Body mass (P=0.001) ↓Fat free mass (P=0.019) ↓Fat percentage (P=0.001) |
| Racinais et al (2012)38 | 11 males Age: 31 ± 3 years |
NC |
| Kacimi et al (2012)59 | 50 healthy subjects (21 Males aged: 18–49 years and 29 females aged: 18–51 years) | ↓ Body weight (P<0.01) ↓BMI (P<0.01) ↓ body fat percentage (P<0.01) |
| Trabelsi et al (2011)60 | 18 active men →FG; n=10, Age: 26.6 years →NFG; n=8, Age: 27.2 years |
↓Body weight −1.9% (P<0.001) ↓Body fat percentage −6.2% (P=0.003) In NFG: ↑Body weight +2.2% (P<0.001) ↑ Body fat percentage +10.2% (P=0.001) |
| Güvenç (2011)61 | 16 male soccer players (Age: 17.4±1.2 years) | NS changes in body mass, percentage of body fat, fat-free mass ↓Skinfold thicknesses (p<0.05). |
| Asl (2011)46 | 15 male athletes Age: 20–25 years |
↓Body weight, ↓BMI, ↓Free fat mass and ↓Body fat (P < 0.05). |
| Lotfi et al (2010)62 | 9 male resistance athletes Age: 23 ± 3 years |
NS changes |
| Chennaoui et al (2009)63 | 8 male middle-distance athletes Age: 25.0 ± 1.3 years |
NS changes |
| Chaouachi et al (2008)64 | 15 male elite judo athletes Age: 18 ± 1 years |
↓ Body mass (P<0.001) ↓BMI (P<0.001) ↓Body fat (%) (P<0.05) ↓ Fat mass (P<0.05) NS modification in Fat free mass. |
| Meckel et al (2008)11 | 19 male soccer players Age: 14–16 years |
↓sum of skinfolds (P<0.05). NS change in body weight. |
| Aksungar et al (2007)65 | 68 healthy males: →FG, n=40, Age: 20–39 years →NFG, n=28, Age: 20–40years |
NS changes |
| Al-Hourani & Atoum (2007)66 | 57 students women | ↓Body mass, ↓Body fat, ↓muscle mass, ↓body water, ↓BMI (P<0.05 each). |
| Karli et al (2007)9 | 10 male elite power athletes (2 wrestlers, 7 sprinters and 1 thrower) Age: 20–24 years |
NS changes |
| Bouhlel et al (2006)67 | 9 trained men (Age: 19±2 years). |
↓Body mass, ↓BMI, ↓Fat mass, ↓% Lean mass (P<0.01 each). NS change in hip and waist circumferences. |
| Lamine et al (2006)68 | 9 males, 21 females | NC |
| Yucel et al (2007)69 | 21 males, 17 females Age 20–45years. |
NC |
| Ramadan (2002)70 | 16 sedentary adult male | NS changes |
| Ramadan et al (1999)71 | 13 healthy adult men. | NS changes |
Abbreviations: BMI, Body Mass Index; FG, Fasting Group; NFG, Non Fasting Group; RF, Ramadan Fasting; n, Number; NC, No Change; NS, Non Significant.
The data in Table 4 show that body weight,30,31 fat mass,32,33 and also fat free mass,32 are significantly lowered by ADF. Moreover, ADF-mediated weight loss occurred in obese and overweight individuals,30,34 and also in normal-weight persons.35 Variations in results may be related to differences in study design, duration of fasting, subject characteristics, as well as differences in weight (normal weight, overweight, obese), age, and gender. Older adults, obese individuals and women can experience a greater reduction in weight and loss of percentage fat body after fasting. In addition, some studies allowed no caloric intake on fasting days,36 while others allowed for reductions in caloric intake by up to 70–75%.37,38 Regulating macronutrient intake and adherence to dietary instructions can also influence study outcomes. Another factor to consider was that the majority of ADF studies were completed in animals,37,38 likely due to the difficulty in controlling all parameters within humans.21
Table 4.
Effect of Time Restricted Feeding on Body Composition
| Author | Study Population | Study Design | Main Findings |
|---|---|---|---|
| Moro et al (2016)42 | 34 resistance-trained males: -TRF, n= 17 -ND, n= 17
|
For 8 weeks: →TRF: 100% of their energy needs in an 8-h each day → three meals at 1 p.m., 4 p.m., and 8 p.m. and fasting period = 16h (2826 ± 412.3 kcal/day, carbohydrates 53.2 ± 1.4%, fat 24.7 ± 3.1%, protein 22.1 ± 2.6%) →ND: 100% of their energy needs three meals consumed at 8 a.m., 1 p.m., and 8 p.m. (3007 ± 444.7 kcal/day, carbohydrates 54.7 ± 2.2%, fat 23.9 ± 3.5 %, protein 21.4 ± 1.8). |
↓Fat mass (−16.4% TRF versus −2.8% ND) (p=0.0448). NC in Free fat mass. |
| Le Cheminant et al (2013)41 | 29 healthy men
|
→For 2 weeks: Night eating restriction intervention (elimination of energy intake from 19.00 to 06.00 hrs) →For 2 weeks: control condition, counterbalanced and separated by a 1-week washout period. |
↓ Body weight −1% (P<0.05). |
| Soeters et al (2009)45 | 8 lean healthy men: -IF, n=8 -Standard diet, n=8 Age: 20 to30 years BMI: 18.2 to 24.7 kg/m2 |
For 2 weeks 4h TRF every other day | NS changes |
| Stote et al (2007)43 | 69 healthy (14 Females −7 Males)
|
Two 8 Weeks treatment periods: all of the calories needed for weight maintenance in either 3 meals/d or 1 meal/d. | After 1 meal/d ↓Body weight −1.4 kg (p<0.05) ↓Body fat mass −2.1 kg, (P<0.05) NS differences in fat-free mass and total body water. NS change after 3 meals/d |
| Halberg et al (2005)44 | 8 healthy men
|
For 14 day 4h TRF every other day. Each fasting period (22:00 to18:00 h the following day). | NS changes |
Abbreviations: BMI, Body Mass Index; IF, Intermittent Fasting; ND, Normal Diet; TRF, Time Restricted Feeding; n, number; NC, No Change; NS, Non-Significant.
The outcomes of TRF studies in humans are shown in Table 4, which does not contain data from religious fasting. The results vary from significant decreases in body weight and fat mass,41–43 to no significant changes.44,45 There are a number of reasons for such differences as fasting periods lasted either for two46 or eight weeks,42,43 with variations in the number of fasting hours, participant weights (normal weight, overweight, obese), age, gender and also caloric intake.
Several studies reported decreases in body weight with fasting during Ramadan47 (Table 5). A systematic review of 35 studies48 reported that Ramadan fasting produced a fairly small but significant weight loss (−1.24 kg; 95% confidence interval:)- 1.60, - 0.88 kg() in both sexes, with most of the weight loss then restored within a few weeks after Ramadan.49 However, others failed to observe significant changes in body weight during Ramadan,49 while some even described weight gain during this period49 (Table 5); these inconsistencies may be due to varied eating routines, socioeconomic status, differences in the number of fasting hours, ethnicity, gender, and the health status and the medical history of participants.37,48,50
Fasting Protocols and Metabolic Adaptations
It is clear that that intermittent fasting, especially in the case of Ramadan fasting, benefits lipid metabolism in healthy subjects,72–74 as was also the case for other TRF protocols. Changes in protein, lipid and glucose metabolism and associated hormonal responses were studied during Ramadan fasting (see Table 6). Fasting attenuates serum low-density lipoprotein-cholesterol (LDL-C), total cholesterol (TC), triglycerides (TG), while increasing high-density lipoprotein-cholesterol (HDL-C) levels.54 Other studies reported little or no changes in TC47 or TG75 after Ramadan fasting, or in HDL-C and LDL-C levels following an ADF protocol.35 For example, Beltaifa et al (2002)76 reported no changes in plasma lipids after Ramadan fasting, while Shephard (2013)77 found decreased HDL-C and increased LDL-C and TG levels. A meta-analysis by Kul et al (2014)78 showed that Ramadan fasting decreased LDL-C levels, but did not change HDL-C and TG levels. Furthermore, two studies related to ICR and Ramadan fasting found no changes in lipid metabolism in obese and overweight subjects.29,37 However, this was not the case for ADF where some changes were measured.18,32,40
Table 6.
Effect of Fasting on Metabolic Adaptations in Trained and Untrained Subjects
| Author | Study Population | Study Design | Main Findings | ||
|---|---|---|---|---|---|
| Lipid Metabolism | Glucose Metabolism | Protein Metabolism | |||
| Schübel et al (2018)29 | 150 overweight and obese (50% Male – 50% female) - CCR, n=49 - ICR, n=49 - CG, n=52.
|
→ICR: 5 days without energy restriction and 2days with 75% ↓ in energy needs→ CCR: 20% daily ↓ in energy needs →CG: NC in calorie intake (12 weeks intervention phase) (12 weeks maintenance phase) (26 weeks follow-up phase). |
NS change | ↓ The glucose for CCR compared with ICR (P< 0.01) (at week 12). NS difference between 3 groups (at week s 24–50) |
NM |
| Oh et al (2018)30 | 45 overweight and obese (26 Females- 19 Males): -ADCR, n=13 -Exercise, n=10 -Exercise + ADCR, n=12) -CG, n=10
|
For 8 weeks Exercise-ADCR and ADCR groups: → 3 Alternately fasted days (25% of daily energy intake, food consumed between 12 pm and 2 pm) → 4 Feed days (ad libitum) |
In ADCR group NS changes | In ADCR group NS changes | NM |
| Trepanowski et al (2017)18 | 100 obese adults (86 Females- 14 Males): - ADF, n=34 - CR, n=35 - CG, n=31
|
For 1 year (6-month weight-loss + 6-month weight-maintenance): →ADF: 25% of energy needs on fast days; 125% of energy needs on alternating “feast days” →CR: 75% of energy needs every day →CG: no-intervention |
NS change in TC and TG. Just At month 6, ↑ HDL-C in ADF versus the daily CR (P<0.05) Just at month 12, ↑ LDL-C in the ADF group (P<0.05) |
NS changes. | NM |
| Aliasghari et al (2017)37 | 83 patients with NAFLD (57 Males – 26 Females): -FG: n=42, Age: 37.59 ± 7.06 years -NFG: n=41, Age: 35.8 ± 7.33 years. |
Ramadan fasting | NS changes | ↓ Fasting blood glucose (P<0.01) | NM |
| Alsubheen et al (2017)47 | 17 healthy males: -FG: n=9, Age: 32.2 ± 7.8 years -NFG: n=8, Age: 35 ± 9.4years. |
Ramadan fasting | NS change in TC | NS changes | NM |
| Bak et al (2016)79 | 18 healthy males -Lean, n=9, BMI:19 to 23 kg/m2 -Obese, n=9, BMI: 32 to 40 kg/m2
|
Examination on two occasions separated by a minimum of 21 days: 1) after an overnight fast of 12 h 2) after 72 h of fasting. |
In obese: ↑ whole body lipolysis | ↓ in glucose for both groups (P < 0.01) | Obese:↓ urea and amino acid fluxes both in the basal and 72-h fasted state. ↓ forearm muscle protein breakdown per 100 mL of forearm tissue, differences that persisted after 72 h of fasting |
| Syam et al (2016)80 | 43 volunteers (7 Males- 36 Females)
|
Ramadan fasting | NM | NM | NS change in Protein body mass (−0.049 ± 0.170 kg, P=0.561) |
| Moro et al (2016)42 | 34 resistance-trained males: -TRF, n= 17 -ND, n= 17
|
For 8 weeks: →TRF: 100% of their energy needs in an 8-h each day → three meals at 1 p.m., 4 p.m., and 8 p.m. and fasting period = 16h (2826 ± 412.3 kcal/day, carbohydrates 53.2 ± 1.4%, fat 24.7 ± 3.1%, protein 22.1 ± 2.6%) →ND: 100% of their energy needs three meals consumed at 8 a.m., 1 p.m., and 8 p.m. (3007 ± 444.7 kcal/day, carbohydrates 54.7 ± 2.2%, fat 23.9 ± 3.5%, protein 21.4 ± 1.8). |
In TRF: ↓ HDL-C (45.11 ± 5.89 to 58.06 ± 6.11 mg/dl) (p=0.014) ↓TG (54.11 ± 15.12 to 115.23 ± 11.77 mg/dl) (p=0.0052). NS changes in TC and LDL-C. |
In TRF: ↓Glucose (96.64 ± 85.92 to 85.92± 7.13 mg/dl) (p=0.0011) |
NM |
| Gnanou et al (2015)53 | 20 healthy men Age: 19–23 years. |
Ramadan fasting | NM | ↓ Blood glucose (p< 0.01) | NM |
| Varady et al (2013)35 | 32 normal-weight and overweight (22 Females- 8 Males): - ADF, n= 15 -CG, n=15
|
For 12 weeks →ADF group alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
In ADF group: ↓Triacylglycerol (20 ± 8%, P < 0.05) ↑ LDL particle size (4 ± 1 Å, P < 0.01) NC in LDL-C, HDL-C |
NM | NM |
| Bhutani et al (2013)32 | 83 obese (80 Females - 3 Males) -E-ADF, n=18 -ADF, n=25 -Exercise, n=24 -CG, n=16
|
For 12 weeks →ADF and E-ADF groups alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
↓ TC (p =0.053). NS change in TG, HDL-C, LDL-C. |
NS change in blood glucose | NC in C-reactive protein. |
| Eshghinia & Mohammadzadeh (2013)33 | 15 overweight and obese women.
|
For 6 weeks, On 3 weekly fasting days; Saturday, Monday, and Thursday (25%– 30% of energy needs) On 4 feeding days (1700–1800 kcal/d) |
NS changes | NM | NM |
| Teng et al (2013)25 | 56 Men: - FCR, n=28 - CG, n=28.
|
For 12 weeks: → FCR: 300–500 kcal/d ↓ in caloric intake (For 2 non-consecutive days per week). →CG: NC in energy needs |
A significant interaction effect in →TC (p<0.001) →LDL-C (p<0.05) → (TC)/HDL (P<0.05) NS effect in HDL and TG |
NS change | NM |
| Klempel et al (2013)39 | 32 obese women: -ADF-HF, n=17-ADF-LF, n=18
|
For 8 weeks Alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. The two diets: →ADF–HF (45% fat, 40% carbohydrate and 15% protein), →ADF–LF (25% fat, 60% carbohydrate and 15% protein). |
↓ TC: 13 ± 2 and 16 ± 2% (P<0.0001) in the ADF–HF and ADF–LF. ↓ LDL-C: 18 ± 5 and 25 ± 3% (P<0.0001) in the ADF–HF and ADF–LF. ↓ TG: 14 ± 5 and 14 ± 4% (P<0.001) in the ADF–HF and ADF–LF. NC in HDL-C in either group. |
NM | NM |
| Mirzaei et al (2012)58 | 14 male collegiate wrestlers (Age: 20.12±2.5 years). |
Ramadan fasting | ↓TC (P=0.011) ↓ LDL-C (P=0.001). ↑ HDL-C (P=0.045). |
↓ Blood glucose (p=0.001). | NM |
| Shehab et al (2012)75 | 65 subjects -42 Males (BMI: 28.1±4.4 kg/m2) - 18 Females (BMI: 27.2 ±5.5 kg/m2).
|
Ramadan fasting | ↑HDL-C (P<0.001) ↓LDL-C (P< 0.001) NS change in TC and TG |
NM | NM |
| Nematy et al (2012)74 |
- 38 Males (Age: 29–70 years) - 44 Females (Age: 54.0 ± 10 years) |
Ramadan fasting | ↓TC(P = 0.02) ↓TG (P <0.001) ↓VLDL-C (P <0.001) ↓ LDL-C (P <0.001) ↓TC/HDL (P <0.001) ↓LDL/HDL ratio (P <0.001) ↑HDL-C (P <0.001) |
NS modification. | NM |
| Varady et al (2011)40 | 59 overweight and obese (50 Females- 9 Males): ADF, n=13 CR, n=12 Exercise, n=12 Control, n=12
|
For 12 weeks: ADF and E-ADF groups alternated between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
In ADF: NC in TC and HDL-C ↓LDL-C (P < 0.05) (10 ± 4%) ↓TG (P < 0.05) (17 ± 5%) |
NM | NM |
| Varady et al (2009)34 | 60 obese (12 Females, 4 Males)
|
For 8 weeks: Alteration between fasting days (25% of kcal needs as determined by Mifflin equation; consumed between 12 pm and 2 pm) and ad libitum feeding days. |
↓TC −21 ± 4% (P < 0.01) ↓LDL-C −25 ± 10% (P< 0.01) ↓Triacylglycerol −32 ± 6% (P< 0.01) NC in HDL-C |
NM | NM |
| Chaouachi et al (2008)64 | 15 male elite judo athletes
|
Ramadan fasting | ↓TC (3.34 ± 0.26 to 3.72 ± 0.31 mmol • l –1) (P< 0.05) ↑HDL-C (1.30 ± 0.31 to 1.42 ± 0.28 mmol • l –1) (P < 0.01) NC in LDL-C and TG. |
NC | ↓ Protein from 77.1± 4.4 to 70.1 ± 4.3 g.l-1 (P < 0.01) |
| Ziaee et al (2006)81 | 81 healthy subjects (41 Males - 40 Females).
|
Ramadan fasting | ↓HDL-C (P=0.001) ↑ LDL-C (P=0.045) NS change in TC, TG and VLDL. |
↓ Glucose (p=0.000) | NM |
| Bouhlel et al (2006)67 | 9 trained men
|
Ramadan fasting | NM | NC | ↑ Hemoglobin (P < 0.01) |
| Heilbronn et al (2005)82 | 16 healthy (8 Males- 8 Females)
|
ADF: fasting every other day for 22 days | NM | For females: ↓ Glucose (p= 0.01) NC in insulin response For males NC in glucose ↓Insulin response (p= 0.03). |
NM |
| Fakhrzadeh et al (2003)73 | 91 subjects: -50 Males(Age: 19.9±1.8 years and BMI: 21.8 ± 2.6 kg/m2) -41 Females(Age: 21.9±3.9 years and BMI: 24.0 ± 4.5kg/m2) |
Ramadan fasting | ↓TC (P< 0.001) ↓ TG (P<0.001 for men, P < 0.02 for woman) ↓LDL-C (P < 0.001) ↑HDL-C (P < 0.001) |
↓Glucose in both men (P<0.0001) and women (P<0.0001). | NM |
| Larijani et al (2003)83 | 67 male, 48 female adults
|
Ramadan fasting | NM | ↓Glucose (88.4 ± 9.0 mg/dl to 62.9 ± 7.7 mg/dl) (p < 0.001). | NM |
| Beltaifa et al (2002)76 | 26 athletes and 32 sedentary individuals | Ramadan fasting | NS modifications | NC | No significant change |
| Adlouni et al (1997)72 | 32 healthy men (Age: 25–50 years) |
Ramadan fasting | ↓TC: −7.9% (P< 0.001), ↓TG: −30% (P< 0.001) ↓LDL-C: −11.7% (P< 0.001) ↑HDL-C: −14.3%(P< 0.001). |
↓ Blood glucose: 5.1 to 4.38 mmol/L (P < 0.001) | NM |
Abbreviations: ADCR, Alternate Day Caloric Restriction ADF, Alternate Day Fasting; ADF-HF, Alternate Day Fasting with High Fat ADF-LF, Alternate Day Fasting with Low Fat; BMI, Body Mass Index; CR, Caloric Restriction; CCR, Continuous Caloric Restriction; E-ADF, Exercise With Alternate Day Fasting; EX, Exercise; FCR, Fasting Caloric Restriction; FG, Fasting Group; HDL-C, High Density Lipoprotein; ICR, Intermittent Caloric Restriction; LDL-C, Low Density Lipoprotein; n, number; NC, No Change; ND, Normal Diet; NFG, Non Fasting Group; NM, Not Mentioned; NS, Non Significant; TC, Total Cholesterol; TG, Triglycerides; TRF, Time Restricted Feeding.
There may be a number of reasons for the differences reported on the effects of IF. Studies varied in experimental design (ICR, ADF, TRF, “Ramadan”), fasting duration, and participant characteristics (normal weight, overweight, obese, age, and gender).15 Macronutrient intake was also not controlled in most studies,15 while there were disparities in dietary habits depending on cultural rituals and the number of fasting hours, which can also affect metabolic regulation.75 It was evident that the data were related to the feeding behavior or biochemical responses to fasting.54 In fact, some biochemical parameters (lactate dehydrogenase, blood glucose, aspartate aminotransferase, alanine aminotransferase creatine kinase, lactate) were higher in the evening than in the morning, and their responses to exercise were higher in the evening. However, no diurnal variations were observed in resting values of the selected biochemical parameters during the fourth week of Ramadan.80
Alterations occur in carbohydrate metabolism during IF. The post-absorptive period occurs 8 to 16 h after eating, and represents an adaptation to fasting to ensure sufficient glucose supply to the brain and other vital organs. Several studies reported decreased glucose metabolism in healthy subjects after ADF72,82 and after Ramadan fasting.53,72,73,81,83 Some found a reduction in blood glucose levels after 12 h and 72 h of fasting,79 while others reported no changes.25,74 However, the meta-analysis by Kul et al78 concluded that Ramadan fasting reduced blood glucose levels. As summarized in Table 6, there were no significant changes in blood glucose levels of obese individuals after intermittent fasting.18,30,32 However, some studies found that highly trained athletes displayed improved glucose metabolism after intermittent fasting,58 while other studies failed to confirm this.67,76
Changes in protein metabolism during IF are not as well studied. Three studies indicated no reductions in plasma protein concentrations in healthy subjects during Ramadan (Table 6).70,76,79 However, ADF elicited no effects on protein metabolism in obese subjects (Table 6), while other studies reported loss of fat free mass3,84 which may be related to protein catabolism. Gluconeogenesis is the main cause of lean tissue loss in athletes. The work by Chaouachi et al (2008)64 demonstrated that when Judoka athletes ingested regular amounts of protein (1.6 g/kg), there was a 0.6 kg decline in lean tissue mass after Ramadan. In support of these findings is a study of nine Tunisian rugby players reporting decreases in plasma protein levels by the end of Ramadan at rest and after exercise.67 However, Soeters et al (2009)45 reported that short term ADF (alternating between 20 h fasting and 28 h feeding) did not influence whole body protein metabolism in lean healthy men, as they suggested that protein catabolism started on the third day of fasting, while energy utilized during the first 2–3 days of fasting was largely derived from glycogen and fat metabolism.15 Overall, the results varied between significant to non-significant modifications in protein metabolism, and the reasons were likely similar to studies to assess glucose and lipid metabolism.
The body undergoes transient metabolic adaptations during Ramadan, including improvements in lipid profiles and decreased glycemia. Chennaoui et al (2009)63 investigated eight middle-distance athletes (aged 25.0 ± 1.3 years and who trained 6 to 10 times per week for at least 3 years) and measured the maximal aerobic velocity 5 days before, during, and after Ramadan. Blood samples were collected before and after Ramadan fasting. This study confirmed increases in circulating FFA levels at the end of Ramadan fasting (from 87±20 to 203±90 µmol.L–1) (P<0.05) in endurance athletes, although other metabolic parameters such as TG (from 0.97±0.08 to 0.90±0.13 mmol.L–1), TC (from 4.29±0.23 to 3.83±0.12 mmol.L–1), HDL-C (from 1.63±0.16 to 1.75±0.18 mmol.L–1) and LDL-C (from 2.22±0.17 to 1.67±0.21 mmol.L–1) remained unaltered. There were significant changes in hormonal and inflammatory markers, for example increased catecholamine and IL-6 concentrations and lower melatonin levels. Concentrations of IL-6 correlated positively with catecholamine and FFA levels, but negatively with insulin levels. Chennaoui et al (2009)63 suggested that the IL-6 and catecholamine responses are influenced by alterations in the sleep and eating patterns inherent to Ramadan.
The effects of fasting on sportsmen versus sedentary individuals were investigated by Ba et al (2005)85 in a study of 30 participants (average age of 25 years) who were randomized to form two groups of 15 subjects each. All participants followed the same eating routine and glycemia was measured twice; during the second fortnight of Ramadan (15 mins before eating), and two months after Ramadan (at least 4 h after the last meal). Resting glycemia in the sportsmen (4.6±0.15 mmol/L) was similar to normal (non-trained) subjects (4.5±0.01 mmol/L) during the month of Ramadan. Moreover, a similar pattern was observed in glycemic levels after normal dietary intake (4.8±0.2 mmol/L and 4.8±0.4 mmol/L for sportsmen and normal subjects, respectively). Participants in the sedentary group returned to normal dietary intakes and also had an increased weight gain after Ramadan, leading the authors to conclude that Ramadan fasting elicited little effect on glucose metabolism in endurance athletes compared to inactive subjects, suggesting that athletes were better able to regulate their glucose metabolism.
Fasting Protocols and Performance
There are a limited number of studies on the effects of fasting on the endurance performance of trained athletes (Table 7), with findings of decreases,63,86 or no changes87,88 in endurance performances after Ramadan fasting. To the best of our knowledge, only a study by Asl (2011)46 reported a small increase in endurance performance, while other studies suggested that Ramadan fasting negatively affected the endurance performances of athletes.63,89 The reasons for such contradictory findings remain unclear, but a combination of factors such as sleep deprivation or fatigue during Ramadan are important considerations. A study by Aziz et al (2010)89 reported that Ramadan fasting did not influence all individuals equally, with relatively fitter individuals better able to resist the physiological and psychological perturbations sometimes observed. This may also help to explain discordant findings such as by Brisswaleter et al (2011)87 and Png et al (2014)88 who reported limited changes in endurance performance after Ramadan fasting. The study by Brisswaleter et al (2011)87 reported decreased muscular performance and increased oxygen kinetics with Ramadan fasting, but without changes in VO2max or performance in middle-distance runners. By contrast, Asl (2011)46 investigated the effects of Ramadan fasting on endurance running performance in male athletes, finding that Ramadan fasting elicited positive effects on endurance performance. The participants were tested twice (30 mins running on the track), with the first test performed one week before Ramadan and the second in the middle of the month of fasting. There were no significant changes in body weight, body mass index, fat free mass, body fat, urinary density, mean heart rate and lactate threshold (all p>0.05). Moreover, the finding revealed that Ramadan fasting had a small but significant impact on endurance running performance.46
Table 7.
Effects of Fasting on Endurance Performances in Endurance Trained Athletes
| Author | Participants | Study Design | Main Findings |
|---|---|---|---|
| Png et al (2014)88 | 12 active male runners Age: 27.9 ± 7.2 years | 60 min of continuous run during the Ramadan month after ingesting → Low glycemic index → Normal mixed carbohydrate food as the sahur meal (post 12 hrs). |
NS variations in metabolic and physiological measures. ↓Distance ran was in low glycemic index versus control meal trial |
| Brisswalter et al (2011)8 | 18 well trained males, middle-distance runners -Ramadan fasting, n=9 - CG, n=9 Age: 23.6 ± 2.9 years |
→The maximal running test →Maximal voluntary contraction of knee extensor →2 rectangular submaximal exercises on treadmill for 6 mins →Running performance test (5000 m) (Before and at the last week of Ramadan) |
NC in running efficiency or MAP. |
| Asl (2011)46 | 15 male endurance runners Age: 22.5 ± 1.14 years |
In the first week and in the middle of Ramadan The aerobic power was measured by using30 mins running in stationary bike. |
Small significant effect (P< 0.05) |
| Aziz et al (2010)89 | 10 moderately men trained runners Age: 27.3±7.2 years |
Comparing the subjects (60 min runs on a treadmill) during the Ramadan month in the fasted state and non-fasted conditions. The 60 min continuous endurance running: 30 min preloading run at 65% maximum oxygen consumption intensity speed + 30 min where subjects adjusted their speeds |
↑in the second 30 min in the control compared to Ramadan condition (5649±715 versus 5448±847 m, P=0.023). ↓ Blood glucose (4.5±0.3 versus 4.9±0.4 mmol/l, p=0.003) at the start of exercise in the Ramadan condition. NS changes in HR, blood lactate and RPE between the two conditions. |
| Chennaoui et al (2009)63 | 8 male middle-distance athletes Age: 25.0 ± 1.3 years |
The MAV test: 5 days before Ramadan and on days 7 and 21 of Ramadan. | ↓MAV values at days 7 and 21 (p < 0.05) |
| Loon et al (2004)90 | 8 male cyclists Age: 22.8 ± 0.8 years |
Following an overnight fast, subjects were studied at rest, during 120 min of moderate intensity exercise (60% maximal oxygen uptake) and 120 min of post-exercise recovery. | ↑Free fatty acid oxidation rates increased during exercise. ↓Use of other fat sources and muscle glycogen with the duration of exercise (P<0.001) ↑Plasma glucose production and utilization (P < 0.001). |
| Mehdioui, et al (1996)86 | 10 male distance runners | The maximal oxygen intake was measured in the beginning and the end of Ramadan | NC in maximal oxygen intake, ↑Endurance effort |
Abbreviations: HR, Heart Rate; MAP, Maximal Aerobic Power; MAV, Maximal Aerobic Velocity; n, number, NC, No Change; NS, Non-Significant; RPE, Rating Perceived Exertion.
There are only a limited number of studies on the effects of fasting on endurance performances in untrained and non-endurance-trained athletes but with conflicting findings (as summarized in Table 8). Some observed no changes62,64,91 while others reported increased,9,10,92 or impaired performances.11,54,92 For example, Chaouachi et al (2008)64 reported no changes in endurance performances of judo athletes, although the lack of information on total energy and macronutrient intake in some studies makes it difficult to compare the findings with others. Moreover, studies by Fouad (2008)91 and Chaouachi et al (2009)64 did not observe differences in the performance of soccer players measured during a maximal aerobic test or endurance time performance at 85% of maximal oxygen uptake (VO2max). However, the authors suggested that the small (but insignificant) increase in endurance time maybe related to the preferential use of lipids over carbohydrates; however carbohydrate metabolism in this instance was associated with muscle fatigue and hyperventilation, leading to dyspnea.8,91 As aerobic performance was not influenced by fasting, Fouad (2008)91 suggested that sportsmen adapt to a new physiological regulation of metabolism after three weeks of fasting.
Table 8.
Effects of Fasting on Physical Performances in Untrained Subjects and Non-Endurance Athletes
| Author | Participants | Study Design | Main Findings |
|---|---|---|---|
| Hammouda et al (2014)54 | 12 males, professional soccer players Age 17.52 ± 0.2 years |
The Yo-Yo test level 1(07:00 h and 17:00 h): one week before Ramadan, and in the second and fourth week. | ↓Total distance just in the evening (P<0.05). |
| Lotfi et al (2010)62 | 9 athletes Age: 23 ± 3 years |
Running 1000m test took place 3 times: before and in the first and fourth weeks of Ramadan. | NS modifications in physical performance (1000m running). ↓ RRT(p<0.001) ↓ TRT (p<0.0001) |
| Chaouachi et al (2009)8 | 15 healthy male elite judo athletes Age: 18 ± 1 years |
A multistage fitness test was applied in the beginning and the end of Ramadan | NC of multistage shuttle-run score |
| Meckel et al (2008)11 | 19 male soccer players Age: 14–16 years |
The endurance test “3,000 m run” the week before and during the last week of the Ramadan. | ↓Aerobic capacity: 3,000 m run time: 812.8 ± 73.3s versus 819.9 ± 73.4s (P < 0.001) |
| Kirkendall et al (2008)10 | 85 soccer male players -2 teams for each morning and afternoon testing (fasting group and non-fasting group) Age: 18 years |
20-m multistage shuttle run test took place 3 weeks: before and in the second and fourth weeks of Ramadan. | ↑Running distance in the 4thweek of Ramadan (P<0.05) and Post Ramadan (P<0.001) No time day effect. |
| Fouad (2008)91 | 30 male soccer players Age: 15 to 17 years |
To measure the aerobic exercise performance a Leger test was applied in the beginning and the end of Ramadan | NC of Leger-test score or endurance at 85% of maximal oxygen intake during Ramadan |
| Karli et al (2007)9 | 16 male soccer players (Age: 17.4 ± 1.2 years) |
20- multistage shuttle run test took place 3 times: before, in the beginning and in the end of Ramadan. | During Ramadan: ↑RPE (P<0.05). End of Ramadan: ↓Blood lactate (p<0.05) ↓HR responses (p<0.05) ↑Peak running performance (p<0.05) ↑running velocity at anaerobic threshold (p<0.05) |
| Sweileh (1992)92 | Sedentary subjects | The maximal oxygen uptake measured in the beginning and the end of Ramadan | The maximal oxygen uptake: ↓ in the first week of Ramadan ↑last week of Ramadan |
Abbreviations: HR, Heart Rate; NC, No Change; NS, Non-significant; RPE, Rating Perceived Exertion; RRT, Recognition Reaction Time; TRT, Total Reaction Time.
Other studies indicated decreased endurance performances in response to Ramadan fasting.11,54,92 For example, Hammouda et al (2014)54 demonstrated a significant decrease in distance covered during the Yo-Yo level 1 test (measures ability to perform the longest distance covered during repeated interval runs) after Ramadan fasting in male professional soccer players. This can largely be explained by fuel substrate selection during the exercise period, level of physical conditioning and regular physical training. At the same time, it should also be stressed that there was an increased tendency to consume calorie-rich dietary intake and energy-dense drinks by some athletes after breaking their fast.62 There was also a decreased aerobic capacity in young soccer players as noted by increases in their mean 3,000 m running times after the Ramadan fast,11 which may be related to the training program of the soccer players that was modified during Ramadan fasting. There was a tendency of favoring lower intensity and reduced tactical training during Ramadan fasting. Collectively, such changes in daily routines will decrease physical capacity of athletes by the end of Ramadan.
Training and Fasting
It is now increasingly recognized that physical training and fasting have beneficial effects on body composition and health.93,94 Aerobic exercise training and fasting are two well-known strategies to increase lipolysis in adipose and muscle tissue, and thereby reduces the amount of body fat mass. This is important for athletes, as they need to control their body composition to optimize the balance between lean and fat body mass so as to improve their performances.95,96
Fat and carbohydrate are the most important fuel substrates for skeletal muscle ATP synthesis during aerobic metabolism.90 Endurance capacity is determined by maximal cardiac output together with the oxidation of fat and carbohydrate stores. Exercise duration is limited by skeletal muscle metabolism and the relatively limited glycogen storage depots. Fatigue occurs when the rate of fat utilization is insufficient to meet energy demands. To counter this, athletes may consider an improved metabolic training regimen for events lasting for more than 2 hrs with the aim of increasing glycogen storage and enhancing fat oxidation capacity.97 We next discuss the physiological effects of exercise when fasting.
Untrained Subjects
Exercise with Fasting: Metabolic Regulation
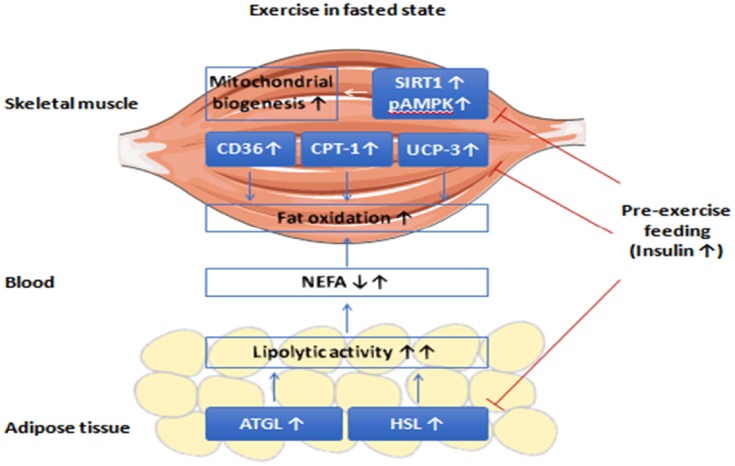
Fasting lowers circulating insulin levels and increases hepatic glycogen breakdown,6 suggesting that endurance training during this state leads to greater fat utilization compared to the fed state.4,98 Fat is the main fuel source used during exercise when fasting, when plasma glycerol and FFA levels increase due to activation of lipolysis in adipocytes. Fat burning pathways are activated by catecholamines (binding to beta-adrenergic receptors) and glucagon, and is inhibited by insulin. This makes blood glycerol and FFA levels useful markers of adipocyte lipolysis. Plasma levels of glycerol increase slightly at low levels of exercise, but are significantly greater during fasting. During low intensity exercise, glycerol levels during a fed-state is 5.5 mmol/kg/min while these reach 8.5 mmol/kg/min during a fasting state.4,5 Similarly, with low intensity exercise plasma FFA concentrations reach 0.20 mM in the fed-state and 0.45 mM in the fasting state.4,5 Exercising during the fed state attenuates fat oxidation due to higher post-prandial insulin concentrations96,99 (Figure 1).
Figure 1.
Exercise in fasted compared to fed states. Aerobic exercise performed in the fasted state induces higher fat oxidation than exercise performed in the fed state.
Abbreviations: NEFA, non-esterified fatty acids; ATGL, adipose triglyceride lipase; HSL, hormone-sensitive lipase; CD36, fatty acid translocase; CPT-1, carnitine palmitoyltransferase-1; UCP-3, uncoupling protein 3; AMPK, AMP-activated protein kinase; SIRT1, sirtuin-1.
By contrast, exercising during the fasting state increases adipose tissue lipolysis and peripheral fat oxidation via higher plasma adrenaline and cortisol concentrations and lower circulating insulin levels.10,12–15 These stress hormones interact with adipose tissue to phosphorylate adipose triglyceride lipase and hormone-sensitive lipase enzymes, which triggers lipolysis to increase circulating FFA levels. By contrast, exercise training in a fasting state leads to up-regulation of genes involved in fatty acid transport (e.g. fatty acid translocase/CD36; carnitine palmitoyltransferase-1) and β-oxidation (e.g. uncoupling protein 3, AMP-activated protein kinase) in muscle fibers,73 thereby resulting in enhanced skeletal muscle fat oxidation.83 In support, there is an increased rate of fat oxidation at rest after training while in a fasting state (versus the same exercise performed post-prandially), and leading to decreased body fat content.8 In contrast, feeding before exercise increases insulin levels (which can remain elevated for about 3 h) and can attenuate the metabolic responses (such as lipolysis enzymes, fatty acid transports and fat oxidation) induced by exercise during a fasting state (Figure 1).
These findings support the concept that performing endurance training while fasting increases fat oxidation and promotes long-term adaptations that are beneficial to overall health and well-being.100 Of note, hepatic glycogen stores are mostly depleted after fasting periods if no exogenous carbohydrates are supplied. Dysregulation of glucose metabolism and hypoglycemia can also occur with prolonged exercise.100–102 However, regular exercise when fasting can induce specific physiological adaptations to facilitate glucose homeostasis despite a limited hepatic glycogen availability.100 Thus many endurance athletes perform their training sessions after an overnight fast, hoping to increase performances by changing their fuel substrate selection.100 However, it should be kept in mind that there are only a few studies that have examined the effects of fasting on physical performances and the results obtained so far are inconclusive.8
Exercise with Fasting: Performance
The effects of short-term fasting on exercise performances have been extensively investigated6,103–110 and suggest that decreased physical performances occur during the fasting state.8 This could be explained (at least in part) by the fasting periods used (>24 to 55 h), dehydration,109 prolonged exhaustive exercise testing,103,104,107 and/or very high-intensity levels of exercise.104,110 However, others108,109,111,112 failed to record significant decreases in performance after shorter periods of fasting (11–24 h). For example, studies by Van Proeyen (2011)100 and Stannard et al (2010)113 indicated that habitual fasted training on sedentary and untrained subjects was a positive strategy to stimulate physiological adaptations in muscle that could improve endurance exercise performance. It is important to note that the majority of these studies investigated recreationally active or untrained subjects8 making it difficult to extrapolate these findings to highly trained athletes.Others reported significant reductions in running distance by the second week of Ramadan, although the distances returned or exceeded the baseline values by the fourth week.10 This was dissimilar to the findings Meckel et al (2008)11 who suggested that reduced endurance when fasting was likely due to the decline of soccer training intensity. Of note, in the study by Kirkendall et al (2008)10 all players trained on the same days and the number, intensity, and duration of training was similar to the rest of the year. Their data showed an initial decline in performance that returned to, or even exceeded pre-fasting values by the end of Ramadan. This phenomenon may be related to alterations in training, lifestyle, diet, and sleep patterns after the first two weeks of Ramadan.10
It is possible that modifications in endurance performances during Ramadan are not limited to altered training patterns. The physiological adaptations in fasting subjects during the month of Ramadan could also be account for by improved athletic performances. In fact, Sweileh et al (1992) reported that metabolism in fasting individuals slows down during Ramadan to likely conserve energy stores.92 Serum sodium, chloride, and protein levels increased during the first week of Ramadan (implying that the subjects were likely dehydrated during this period), with subjects losing ~ 1.13 kg body weight without changes in percent fat. These acute biochemical changes prevent catecholamine release and decreases venous return, thereby resulting in reductions in sympathetic tone, blood pressure, heart rate, and cardiac output.92 Such physiological adaptations can also influence physical work capacity and athletic performances as shown by the significant decrease in VO2max during the first week of Ramadan. These biochemical changes returned to baseline values during the last week of Ramadan while VO2max returned to pre-fasting levels.
Trained Subjects
Most reviews on fasting and athletic performance focus on fat and carbohydrate metabolism in healthy untrained subjects,96,114,115 with some also evaluating body composition changes in untrained subjects. Moreover, studies on exercise by highly trained athletes did not take body composition changes into account.
Exercise with Fasting Protocols: Metabolic Regulation
Fat and carbohydrate are the most important fuel substrates for skeletal muscle ATP synthesis during aerobic metabolism.90 Endurance capacity is associated with maximal cardiac output together with the oxidation of both fat and carbohydrate stores. The duration of exercise is limited by skeletal muscle metabolism and the relatively limited glycogen storage depots within the body. Fatigue occurs when the rate of fat utilization is insufficient to meet energy demands. To counteract this, athletes may consider an improved metabolic training regimen for competitive events lasting for more than 2 hrs, aimed at increasing glycogen storage and enhancing fat oxidation in this case.97
Although the studies cited above were conducted on healthy, sedentary subjects, some studies explored the effects of fasting on lipid metabolism in highly trained subjects. For example, Chaouachi et al (2008)64 found modifications in serum lipids (increased HDL-C, LDL-C and TC) in elite judo athletes. Moreover, Mirzaei et al (2012)58 reported increased HDL-C levels together with lower LDL-C and TC levels in wrestlers at the end of Ramadan fasting. In support, others found improvements in HDL-C and LDL-C in resistance-trained subjects after TRF.42
Exercise with Fasting Protocols: Performance
Endurance training while fasting represents a novel strategy to induce specific training adaptations by increasing fat oxidation during exercise and by enhancing physical performances in the long-term.100 Endurance training in a fasted state can trigger positive effects on endurance performance in untrained100,103 and endurance trained athletes116 (Table 9).
Table 9.
Effect of Endurance Training on Fast State on Endurance Performance
| Authors | Participants | Study Design | Main Findings |
|---|---|---|---|
| Bouguerra et al (2017)11 | 24 male middle- and long-distance runners Afternoon group, n=8 (14,00 to 16.00 h) Morning group, n=8(09,00 to 11.00 h) Evening group, n=8 (22,00 to 24.00 h) Age, 28.5 ± 10.6 years |
Maximal aerobic velocity, time to exhaustion, performance in running 3000-m (Before, at middle, and after Ramadan fasting training) |
↑MAV and maximal oxygen uptake (P< 0.01) in the afternoon and morning groups compared to the evening group. Before and after Ramadan ↑ Time of 3000-m running exercise (P < 0.001) ↓Time to (p <0.001) in the evening and Morning groups compared to the afternoon groups |
| Van Proeyen et al (2011)100 | 20 healthy males -Training group in fasted state “overnight fasting” (n=10) -Training group in fed state (n=10, carbohydrates before (∼160 g) and during (1 g.kg body wt−1·h−1) the training sessions) Age, 18–25 years |
6 weeks of endurance training program (1–1.5 h cycling at ∼70% of maximal oxygen uptake 4 days/wk) | ↑ Maximal oxygen uptake (+9%) (P < 0.01) ↑Performance in a 60-min simulated time examination (+8%) in both groups (P < 0.01). In fasting, intramyocellular lipid breakdown was enhanced in type I fibers (P < 0.05), tended to be ↑ in type IIa fibers (P=0.07). |
| Stannard et al (2010)113 | 8 females and 6 males untrained, -Training groups, overnight-fasted -Training groups, acutely fed state Age, 26.6 ± 5.8 years |
For4 weeks of 5 days per week endurance cycle ergometer training | In fast, ↑Maximal oxygen uptake and resting muscle glycogen concentration (P=0.014) than fed (P=0.047) NS gender interaction. |
Abbreviations: MAV, Maximal Aerobic Velocity; n, number; NS, Non-Significant.
In fact, regular exercise training in the fasted state stimulates the contribution of intramyocellular lipids to energy provision through fasting endurance exercise.100 Training in a fasted state also increases muscular oxidative capacity to a greater extent than other comparable exercise intensities and duration with sufficient exogenous carbohydrate supply. A study by Bouguerra et al (2017)116 investigated the effects of Ramadan fasting on maximal aerobic velocity, time to exhaustion, and performance for a 3000 m exercise in three different groups of highly trained runners: a) group who trained in the afternoon (between 2:00 and 4:00 pm), b) in the morning (between 09:00 and 11:00 am), and c) in the evening (between 10:00 and 12:00 pm). Their findings revealed that maximal aerobic velocity and VO2max were significantly higher (p< 0.01) in the afternoon and morning compared to the evening before, when measured mid-, and post-Ramadan. The performances for the 3000 m running exercise (before and after Ramadan) were higher (p < 0.01) and the time to exhaustion lower (p < 0.001) in the evening and morning compared to the afternoon. The authors concluded that training in the afternoon during Ramadan can more effectively enhance aerobic performance compared to morning or evening training.116 The superior physical outcomes during the afternoon could be due to the normal diurnal or circadian rhythm of sport performance,116 increased muscle temperature,117 a greater mobilization of glycogen and an increased use of FFAs as a fuel substrate during afternoon exercise sessions.118 However, additional research is needed to confirm that endurance training performed while fasting may indeed increase endurance performances in highly trained athletes.
Practical Applications
The available data suggest that exercise in a fasting state decreases body weight, free fat mass and fat mass, although more controlled studies are needed for definitive recommendations to be made. A moderate intensity exercise during fasting is recommended for the prevention of hypoglycemia. Training in the evening while fasting may be more effective in enhancing aerobic performance, compared to training in the morning. Athletes may wish to train in the fasting state in the pre-season as fasting increases the activity of fat-burning enzymes. However, fasting can elicit negative effects on performance in some events, and hence modifications to the training schedules (eg fasting during the pre-season) may minimize such effects.
Conclusions
This review of the literature identified that the effects of fasting on endurance athletic performances requires further investigation. Different fasting programs influences human physiological and biochemical parameters that are important for athletic performances. The collective data suggest that different fasting practices (ICR, ADF and TRF) decrease body weight and fat7,27 in both trained and untrained subjects. Moreover, several studies demonstrated that fasting alters protein, lipid and glucose metabolism and associated hormonal responses. However, the conflicting findings related to glucose metabolism in response to fasting in highly trained athletes require further investigation. Studies on the effects of fasting on indicators of physical performance have generated conflicting data. For example, some studies reported decreased performances while others showed no effect. There are a number of reasons for such differences, including variations in experimental design, fasting duration, and unique participant characteristics. We suggest that athletes train at relatively low intensities (and not at high intensity levels) when fasting to ensure that they recover adequately to optimize performances in competitive events. Our analyses also revealed a major weakness in that most fasting studies recruited sedentary subjects or low-level athletes, often without well-matched controls. We recommend that well-controlled studies are required to improve our understanding of the effects of exercise in fasting athletes, and to help uncover novel insights into the mechanisms driving changes in energetic pathways and physical performances in trained endurance athletes that choose to fast.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579. doi: 10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- 2.Persynaki A, Karras S, Pichard C. Unraveling the metabolic health benefits of fasting related to religious beliefs: a narrative review. Nutrition. 2017;35:14–20. doi: 10.1016/j.nut.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Wallis GA, Gonzalez JT. Is exercise best served on an empty stomach? Proc Nutr Soc. 2019;78(1):110–117. doi: 10.1017/S0029665118002574 [DOI] [PubMed] [Google Scholar]
- 4.Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am J Physiol Endocrinol Metab. 1997;273(4):E768–E775. doi: 10.1152/ajpendo.1997.273.4.E768 [DOI] [PubMed] [Google Scholar]
- 5.Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Substrate metabolism when subjects are fed carbohydrate during exercise. Am J Physiol Endocrinol Metab. 1999;276(5):E828–E835. doi: 10.1152/ajpendo.1999.276.5.E828 [DOI] [PubMed] [Google Scholar]
- 6.Maughan R, Fallah J, Coyle EF. The effects of fasting on metabolism and performance. Br J Sports Med. 2010;44(7):490–494. doi: 10.1136/bjsm.2010.072181 [DOI] [PubMed] [Google Scholar]
- 7.Michalsen A, Li C. Fasting therapy for treating and preventing disease-current state of evidence. Complementary Med Res. 2013;20(6):444–453. doi: 10.1159/000357765 [DOI] [PubMed] [Google Scholar]
- 8.Chaouachi A, Coutts AJ, Chamari K, et al. Effect of Ramadan intermittent fasting on aerobic and anaerobic performance and perception of fatigue in male elite judo athletes. J Strength Cond Res. 2009;23(9):2702–2709. doi: 10.1519/JSC.0b013e3181bc17fc [DOI] [PubMed] [Google Scholar]
- 9.Karli U, Guvenc A, Aslan A, Hazir T, Acikada C. Influence of Ramadan fasting on anaerobic performance and recovery following short time high intensity exercise. J Sports Sci Med. 2007;6(4):490. [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkendall DT, Leiper JB, Bartagi Z, Dvorak J, Zerguini Y. The influence of Ramadan on physical performance measures in young Muslim footballers. J Sports Sci. 2008;26(S3):S15–S27. doi: 10.1080/02640410802422199 [DOI] [PubMed] [Google Scholar]
- 11.Meckel Y, Ismaeel A, Eliakim A. The effect of the Ramadan fast on physical performance and dietary habits in adolescent soccer players. Eur J Appl Physiol. 2008;102(6):651–657. doi: 10.1007/s00421-007-0633-2 [DOI] [PubMed] [Google Scholar]
- 12.Zerguini Y, Kirkendall D, Junge A, Dvorak J. Impact of Ramadan on physical performance in professional soccer players. Br J Sports Med. 2007;41(6):398–400. doi: 10.1136/bjsm.2006.032037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiper JB, Junge A, Maughan RJ, Zerguini Y, Dvorak J. Alteration of subjective feelings in football players undertaking their usual training and match schedule during the Ramadan fast. J Sports Sci. 2008;26(S3):S55–S69. doi: 10.1080/02640410802538176 [DOI] [PubMed] [Google Scholar]
- 14.Aloui A, Chtourou H, Hammouda O, et al. Effects of Ramadan on the diurnal variations of physical performance and perceived exertion in adolescent soccer players. Biol Rhythm Res. 2013;44(6):869–875. doi: 10.1080/09291016.2013.780697 [DOI] [Google Scholar]
- 15.Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015;73(10):661–674. doi: 10.1093/nutrit/nuv041 [DOI] [PubMed] [Google Scholar]
- 16.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–1059. doi: 10.1016/j.cmet.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Toledo FW, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS One. 2019;14(1):e0209353. doi: 10.1371/journal.pone.0209353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177(7):930–938. doi: 10.1001/jamainternmed.2017.0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherif A, Roelands B, Meeusen R, Chamari K. Effects of intermittent fasting, caloric restriction, and Ramadan intermittent fasting on cognitive performance at rest and during exercise in adults. Sports Med. 2016;46(1):35–47. doi: 10.1007/s40279-015-0408-6 [DOI] [PubMed] [Google Scholar]
- 20.Stockman M-C, Thomas D, Burke J, Apovian CM. Intermittent fasting: is the wait worth the weight? Curr Obes Rep. 2018;7(2):172–185. doi: 10.1007/s13679-018-0308-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoni R, Johnston KL, Collins AL, Robertson MD. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc. 2017;76(3):361–368. doi: 10.1017/S0029665116002986 [DOI] [PubMed] [Google Scholar]
- 22.Solianik R, Sujeta A, Terentjevienė A, Skurvydas A. Effect of 48 h fasting on autonomic function, brain activity, cognition, and mood in amateur weight lifters. Biomed Res Int. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azizi F. Islamic fasting and health. Ann Nutr Metab. 2010;56(4):273–282. doi: 10.1159/000295848 [DOI] [PubMed] [Google Scholar]
- 24.Hussin N, Shahar S, Teng N, Ngah W, Das S. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674–680. doi: 10.1007/s12603-013-0344-9 [DOI] [PubMed] [Google Scholar]
- 25.Teng NIMF, Shahar S, Rajab NF, Manaf ZA, Johari MH, Ngah WZW. Improvement of metabolic parameters in healthy older adult men following a fasting calorie restriction intervention. Aging Male. 2013;16(4):177–183. doi: 10.3109/13685538.2013.832191 [DOI] [PubMed] [Google Scholar]
- 26.Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. 2012;11(1):98. doi: 10.1186/1475-2891-11-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng NIMF, Shahar S, Manaf ZA, Das SK, Taha CSC, Ngah WZW. Efficacy of fasting calorie restriction on quality of life among aging men. Physiol Behav. 2011;104(5):1059–1064. doi: 10.1016/j.physbeh.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald KC, Vizthum D, Henry-Barron B, et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord. 2018;23:33–39. doi: 10.1016/j.msard.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schübel R, Nattenmüller J, Sookthai D, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):933–945. doi: 10.1093/ajcn/nqy196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh M, Kim S, An K-Y, et al. Effects of alternate day calorie restriction and exercise on cardio-metabolic risk factors in overweight and obese adults: an exploratory randomized controlled study. BMC Public Health. 2018;18(1):1124. doi: 10.1186/s12889-018-6009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trepanowski JF, Kroeger CM, Barnosky A, et al. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr. 2018;37(6):1871–1878. doi: 10.1016/j.clnu.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. 2013;21(7):1370–1379. doi: 10.1002/oby.v21.7 [DOI] [PubMed] [Google Scholar]
- 33.Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12(1):4. doi: 10.1186/2251-6581-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90(5):1138–1143. doi: 10.3945/ajcn.2009.28380 [DOI] [PubMed] [Google Scholar]
- 35.Varady KA, Bhutani S, Klempel MC, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12(1):146. doi: 10.1186/1475-2891-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catenacci VA, Pan Z, Ostendorf D, et al. A randomized pilot study comparing zero‐calorie alternate‐day fasting to daily caloric restriction in adults with obesity. Obesity. 2016;24(9):1874–1883. doi: 10.1002/oby.21581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aliasghari F, Izadi A, Gargari BP, Ebrahimi S. The effects of Ramadan fasting on body composition, blood pressure, glucose metabolism, and markers of inflammation in NAFLD patients: an observational trial. J Am Coll Nutr. 2017;36(8):640–645. doi: 10.1080/07315724.2017.1339644 [DOI] [PubMed] [Google Scholar]
- 38.Racinais S, Périard J, Li C, Grantham J. Activity patterns, body composition and muscle function during Ramadan in a Middle-East Muslim country. Int J Sports Med. 2012;33(08):641–646. doi: 10.1055/s-0032-1304645 [DOI] [PubMed] [Google Scholar]
- 39.Klempel M, Kroeger C, Varady K. Alternate day fasting increases LDL particle size independently of dietary fat content in obese humans. Eur J Clin Nutr. 2013;67(7):783. doi: 10.1038/ejcn.2013.83 [DOI] [PubMed] [Google Scholar]
- 40.Varady KA, Bhutani S, Klempel MC, Kroeger CM. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011;10(1):119. doi: 10.1186/1476-511X-10-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeCheminant JD, Christenson E, Bailey BW, Tucker LA. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr. 2013;110(11):2108–2113. doi: 10.1017/S0007114513001359 [DOI] [PubMed] [Google Scholar]
- 42.Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. doi: 10.1186/s12967-016-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–988. doi: 10.1093/ajcn/85.4.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halberg N, Henriksen M, Soderhamn N, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol. 2005;99(6):2128–2136. doi: 10.1152/japplphysiol.00683.2005 [DOI] [PubMed] [Google Scholar]
- 45.Soeters MR, Lammers NM, Dubbelhuis PF, et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am J Clin Nutr. 2009;90(5):1244–1251. doi: 10.3945/ajcn.2008.27327 [DOI] [PubMed] [Google Scholar]
- 46.Asl NS. The effects of Ramadan fasting on endurance running performance in male athletes. Int J Sport Stud. 2011;1(1):18–22. [Google Scholar]
- 47.Sana’a AA, Ismail M, Baker A, et al. The effects of diurnal Ramadan fasting on energy expenditure and substrate oxidation in healthy men. Br J Nutr. 2017;118(12):1023–1030. doi: 10.1017/S0007114517003221 [DOI] [PubMed] [Google Scholar]
- 48.Sadeghirad B, Motaghipisheh S, Kolahdooz F, Zahedi MJ, Haghdoost AA. Islamic fasting and weight loss: a systematic review and meta-analysis. Public Health Nutr. 2014;17(2):396–406. doi: 10.1017/S1368980012005046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latiri I, Sandid S, Fennani MA, et al. The effects of Ramadan fasting on the spirometric data of healthy adult males. Am J Mens Health. 2017;11(4):1214–1223. doi: 10.1177/1557988316675091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazidi M, Rezaie P, Chaudhri O, Karimi E, Nematy M. The effect of Ramadan fasting on cardiometabolic risk factors and anthropometrics parameters: a systematic review. Pak J Med Sci. 2015;31(5):1250. doi: 10.12669/pjms.315.7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nugraha B, Ghashang SK, Hamdan I, Gutenbrunner C. Effect of Ramadan fasting on fatigue, mood, sleepiness, and health-related quality of life of healthy young men in summer time in Germany: A prospective controlled study. Appetite. 2017;111:38–45. doi: 10.1016/j.appet.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 52.Sezen Y, Altiparmak IH, Erkus ME, et al. Effects of Ramadan fasting on body composition and arterial stiffness. J Pak Med Assoc. 2016;66:1522–1527. [PubMed] [Google Scholar]
- 53.Gnanou JV, Caszo BA, Khalil KM, Abdullah SL, Knight VF, Bidin MZ. Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J Diabetes Metab Disord. 2015;14(1):55. doi: 10.1186/s40200-015-0183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammouda O, Chtourou H, Aloui A, et al. Does Ramadan fasting affect the diurnal variations in metabolic responses and total antioxidant capacity during exercise in young soccer players? Sport Sci Health. 2014;10(2):97–104. doi: 10.1007/s11332-014-0179-8 [DOI] [Google Scholar]
- 55.Hammouda O, Chtourou H, Aloui A, et al. Concomitant effects of Ramadan fasting and time-of-day on apolipoprotein AI, B, Lp-a and homocysteine responses during aerobic exercise in Tunisian soccer players. PLoS One. 2013;8(11):e79873. doi: 10.1371/journal.pone.0079873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouhlel H, Shephard RJ, Gmada N, et al. Effect of Ramadan observance on maximal muscular performance of trained men. Clin J Sport Med. 2013;23(3):222–227. doi: 10.1097/JSM.0b013e318275d213 [DOI] [PubMed] [Google Scholar]
- 57.Zarrouk N, Hug F, Hammouda O, et al. Effect of Ramadan intermittent fasting on body composition and neuromuscular performance in young athletes: a pilot study. Biol Rhythm Res. 2013;44(5):697–709. doi: 10.1080/09291016.2012.730891 [DOI] [Google Scholar]
- 58.Mirzaei B, Rahmani-Nia F, Moghadam MG, Ziyaolhagh SJ, Rezaei A. The effect of ramadan fasting on biochemical and performance parameters in collegiate wrestlers. Iran J Basic Med Sci. 2012;15(6):1215. [PMC free article] [PubMed] [Google Scholar]
- 59.Kacimi S, Ref’at A, Fararjeh MA, Bustanji YK, Mohammad MK, Salem ML. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. 2012;32(12):947–955. doi: 10.1016/j.nutres.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 60.Trabelsi K, El Abed K, Trepanowski JF, et al. Effects of Ramadan fasting on biochemical and anthropometric parameters in physically active men. Asian J Sports Med. 2011;2(3):134. doi: 10.5812/asjsm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Güvenç A. Effects of Ramadan fasting on body composition, aerobic performance and lactate, heart rate and perceptual responses in young soccer players. J Hum Kinet. 2011;29:79–91. doi: 10.2478/v10078-011-0042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lotfi S, Madani M, Abassi A, Tazi A, Boumahmaza M, Talbi M. CNS activation, reaction time, blood pressure and heart rate variation during ramadan intermittent fasting and exercise. World J Sports Sci. 2010;3(1):37–43. [Google Scholar]
- 63.Chennaoui M, Desgorces F, Drogou C, et al. Effects of Ramadan fasting on physical performance and metabolic, hormonal, and inflammatory parameters in middle-distance runners. Appl Physiol Nutr Metab. 2009;34(4):587–594. doi: 10.1139/H09-014 [DOI] [PubMed] [Google Scholar]
- 64.Chaouachi A, Chamari K, Roky R, et al. Lipid profiles of judo athletes during Ramadan. Int J Sports Med. 2008;29(4):282–288. doi: 10.1055/s-2007-965338 [DOI] [PubMed] [Google Scholar]
- 65.Aksungar FB, Topkaya AE, Akyildiz M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann Nutr Metab. 2007;51(1):88–95. doi: 10.1159/000100954 [DOI] [PubMed] [Google Scholar]
- 66.Al-Hourani H, Atoum M. Body composition, nutrient intake and physical activity patterns in young women during Ramadan. Singapore Med J. 2007;48(10):906. [PubMed] [Google Scholar]
- 67.Bouhlel E, Salhi Z, Bouhlel H, et al. Effect of Ramadan fasting on fuel oxidation during exercise in trained male rugby players. Diabetes Metab. 2006;32(6):617–624. doi: 10.1016/S1262-3636(07)70317-8 [DOI] [PubMed] [Google Scholar]
- 68.Lamine F, Bouguerra R, Jabrane J, et al. Food intake and high density lipoprotein cholesterol levels changes during ramadan fasting in healthy young subjects. Tunis Med. 2006;84(10):647–650. [PubMed] [Google Scholar]
- 69.Yucel A, Degirmenci B, Acar M, Albayrak R, Haktanir A. The effect of fasting month of Ramadan on the abdominal fat distribution: assessment by computed tomography. Tohoku J Exp Med. 2004;204(3):179–187. doi: 10.1620/tjem.204.179 [DOI] [PubMed] [Google Scholar]
- 70.Ramadan J. Does fasting during Ramadan alter body composition, blood constituents and physical performance? Med Principles Pract. 2002;11(Suppl. 2):41–46. doi: 10.1159/000066413 [DOI] [PubMed] [Google Scholar]
- 71.Ramadan J, Telahoun G, Al-Zaid NS, Barac-Nieto M. Responses to exercise, fluid, and energy balances during Ramadan in sedentary and active males. Nutrition. 1999;15(10):735–739. doi: 10.1016/S0899-9007(99)00145-8 [DOI] [PubMed] [Google Scholar]
- 72.Adlouni A, Ghalim N, Benslimane A, Lecerf JM, Saïle R. Fasting during Ramadan induces a marked increase in high-density lipoprotein cholesterol and decrease in low-density lipoprotein cholesterol. Ann Nutr Metab. 1997;41(4):242–249. doi: 10.1159/000177999 [DOI] [PubMed] [Google Scholar]
- 73.Fakhrzadeh H, Lariiani B, Sanjari M, Baradar-Jalili R, Amini M. Effect of Ramadan fasting on clinical and biochemical parameters in healthy adults. Ann Saudi Med. 2003;23(3–4):223–226. doi: 10.5144/0256-4947.2003.223 [DOI] [PubMed] [Google Scholar]
- 74.Nematy M, Alinezhad-Namaghi M, Rashed MM, et al. Effects of Ramadan fasting on cardiovascular risk factors: a prospective observational study. Nutr J. 2012;11(1):69. doi: 10.1186/1475-2891-11-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shehab A, Abdulle A, El Issa A, Al Suwaidi J, Nagelkerke N. Favorable changes in lipid profile: the effects of fasting after Ramadan. PLoS One. 2012;7(10):e47615. doi: 10.1371/journal.pone.0047615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beltaifa L, Bouguerra R, Ben CS, et al. Food intake, and anthropometrical and biological parameters in adult Tunisians during fasting at Ramadan. East Mediterr Health J. 2002;8(4–5):603–611. [PubMed] [Google Scholar]
- 77.Shephard RJ. Ramadan and sport: minimizing effects upon the observant athlete. Sports Med. 2013;43(12):1217–1241. doi: 10.1007/s40279-013-0080-7 [DOI] [PubMed] [Google Scholar]
- 78.Kul S, Savaş E, Öztürk ZA, Karadağ G. Does Ramadan fasting alter body weight and blood lipids and fasting blood glucose in a healthy population? A meta-analysis. J Relig Health. 2014;53(3):929–942. doi: 10.1007/s10943-013-9687-0 [DOI] [PubMed] [Google Scholar]
- 79.Bak AM, Møller AB, Vendelbo MH, et al. Differential regulation of lipid and protein metabolism in obese vs. lean subjects before and after a 72-h fast. Am J Physiol Endocrinol Metab. 2016;311(1):E224–E235. doi: 10.1152/ajpendo.00464.2015 [DOI] [PubMed] [Google Scholar]
- 80.Syam AF, Sobur CS, Abdullah M, Makmun D. Ramadan fasting decreases body fat but not protein mass. Int J Endocrinol Metab. 2016;14(1):e29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ziaee V, Razaei M, Ahmadinejad Z, et al. The changes of metabolic profile and weight during Ramadan fasting. Singapore Med J. 2006;47(5):409. [PubMed] [Google Scholar]
- 82.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81(1):69–73. doi: 10.1093/ajcn/81.1.69 [DOI] [PubMed] [Google Scholar]
- 83.Larijani B, Zahedi F, Sanjari M, et al. The effect of Ramadan fasting on fasting serum glucose in healthy adults. Med J Malaysia. 2003;58(5):678–680. [PubMed] [Google Scholar]
- 84.Vicente-Salar N, Otegui AU, Collado ER. Endurance training in fasting conditions: biological adaptations and body weight management. Nutr Hosp. 2015;32(6):2409–2420. doi: 10.3305/nh.2015.32.6.9488 [DOI] [PubMed] [Google Scholar]
- 85.Ba A, Samb A, Seck D, et al. Comparative study of the effect of fasting during Ramadan on the glycaemia at rest in sportsmen and sedentaries. Dakar Med. 2005;50(1):22–25. [PubMed] [Google Scholar]
- 86.Mehdioui H, Aberkane A, Bouroubi O, Bougrida M, Benhlassa L, Beltrache C. Influence de la pratique du jeûne Ramadan sur l’endurance maximale aérobie des coureurs de fond (Effects of Ramadan fasting on the maximal aerobic endurance of distance runners). J Alger Med. 1996;6(1):1–5. [Google Scholar]
- 87.Brisswalter J, Bouhlel E, Falola JM, Abbiss CR, Vallier JM, Hauswirth C. Effects of Ramadan intermittent fasting on middle-distance running performance in well-trained runners. Clin J Sport Med. 2011;21(5):422–427. doi: 10.1097/JSM.0b013e3182293891 [DOI] [PubMed] [Google Scholar]
- 88.Png W, Bhaskaran K, Sinclair AJ, Aziz AR. Effects of ingesting low glycemic index carbohydrate food for the sahur meal on subjective, metabolic and physiological responses, and endurance performance in Ramadan fasted men. Int J Food Sci Nutr. 2014;65(5):629–636. doi: 10.3109/09637486.2014.886187 [DOI] [PubMed] [Google Scholar]
- 89.Aziz AR, Wahid MF, Png W, Jesuvadian CV. Effects of Ramadan fasting on 60 min of endurance running performance in moderately trained men. Br J Sports Med. 2010;44(7):516–521. doi: 10.1136/bjsm.2009.070425 [DOI] [PubMed] [Google Scholar]
- 90.van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance‐trained males in a fasted state. J Physiol. 2003;553(2):611–625. doi: 10.1113/jphysiol.2003.052431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fouad C. Effets Du Jeune De Ramadhan Sur L’aptitude Aerobie Et Les Parametres Anthropometriques Et Biochimiques Chez Des Footballeurs (15–17 Ans) [Effects of Ramadan on aerobic fitness, anthropometric and biochemical parameters in footballers]. Rev Sci Hum. 2008;30:25–41. [Google Scholar]
- 92.Sweileh N, Schnitzler A, Hunter G, Davis B. Body composition and energy metabolism in resting and exercising muslims during Ramadan fast. J Sports Med Phys Fitness. 1992;32(2):156–163. [PubMed] [Google Scholar]
- 93.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99(3):1193–1204. [DOI] [PubMed] [Google Scholar]
- 94.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 95.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52(9):2191–2197. doi: 10.2337/diabetes.52.9.2191 [DOI] [PubMed] [Google Scholar]
- 96.Vieira AF, Costa RR, Macedo RCO, Coconcelli L, Kruel LFM. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br J Nutr. 2016;116(7):1153–1164. doi: 10.1017/S0007114516003160 [DOI] [PubMed] [Google Scholar]
- 97.Andersson Hall U, Edin F, Pedersen A, Madsen K. Whole-body fat oxidation increases more by prior exercise than overnight fasting in elite endurance athletes. Appl Physiol Nutr Metab. 2015;41(4):430–437. [DOI] [PubMed] [Google Scholar]
- 98.De Bock K, Richter EA, Russell A, et al. Exercise in the fasted state facilitates fibre type‐specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol. 2005;564(2):649–660. doi: 10.1113/jphysiol.2005.083170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44(1):87–96. doi: 10.1007/s40279-014-0154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Proeyen K, Szlufcik K, Nielens H, Ramaekers M, Hespel P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J Appl Physiol. 2010;110(1):236–245. doi: 10.1152/japplphysiol.00907.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coyle E, Hagberg J, Hurley B, Martin W, Ehsani A, Holloszy J. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J Appl Physiol. 1983;55(1):230–235. doi: 10.1152/jappl.1983.55.1.230 [DOI] [PubMed] [Google Scholar]
- 102.Coyle EF, Coggan AR, Hemmert M, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61(1):165–172. doi: 10.1152/jappl.1986.61.1.165 [DOI] [PubMed] [Google Scholar]
- 103.Hagan RD, Upton SJ, Wong L, Whittam J. The effects of aerobic conditioning and/or caloric restriction in overweight men and women. Med Sci Sports Exerc. 1986;18(1):87–94. doi: 10.1249/00005768-198602000-00015 [DOI] [PubMed] [Google Scholar]
- 104.Loy SF, Conlee RK, Winder WW, Nelson AG, Arnall DA, Fisher AG. Effects of 24-hour fast on cycling endurance time at two different intensities. J Appl Physiol. 1986;61(2):654–659. doi: 10.1152/jappl.1986.61.2.654 [DOI] [PubMed] [Google Scholar]
- 105.Nieman DC, Carlson KA, Brandstater ME, Naegele RT, Blankenship JW. Running endurance in 27-h-fasted humans. J Appl Physiol. 1987;63(6):2502–2509. doi: 10.1152/jappl.1987.63.6.2502 [DOI] [PubMed] [Google Scholar]
- 106.Gleeson M, Greenhaff P, Maughan R. Influence of a 24 h fast on high intensity cycle exercise performance in man. Eur J Appl Physiol Occup Physiol. 1988;57(6):653–659. doi: 10.1007/BF01075984 [DOI] [PubMed] [Google Scholar]
- 107.Keim NL, Barbieri TF, Van Loan MD, Anderson BL. Energy expenditure and physical performance in overweight women: response to training with and without caloric restriction. Metabolism. 1990;39(6):651–658. doi: 10.1016/0026-0495(90)90035-B [DOI] [PubMed] [Google Scholar]
- 108.Zinker BA, Britz K, Brooks GA. Effects of a 36-hour fast on human endurance and substrate utilization. J Appl Physiol. 1990;69(5):1849–1855. doi: 10.1152/jappl.1990.69.5.1849 [DOI] [PubMed] [Google Scholar]
- 109.Gueye L, Samb A, Seck D, Cissé F, Camara K, Martineaud J. Influence of a 12 hours-fast on maximal exercise. Script Medica (Brno). 2004;77:5–8. [Google Scholar]
- 110.Aragón-Vargas LF. Effects of fasting on endurance exercise. Sports Med. 1993;16(4):255–265. doi: 10.2165/00007256-199316040-00004 [DOI] [PubMed] [Google Scholar]
- 111.Ferguson LM, Rossi KA, Ward E, Jadwin E, Miller TA, Miller WC. Effects of caloric restriction and overnight fasting on cycling endurance performance. J Strength Cond Res. 2009;23(2):560–570. doi: 10.1519/JSC.0b013e31818f058b [DOI] [PubMed] [Google Scholar]
- 112.Knapik JJ, Jones BH, Meredith C, Evans WJ. Influence of a 3.5 day fast on physical performance. Eur J Appl Physiol Occup Physiol. 1987;56(4):428–432. doi: 10.1007/BF00417770 [DOI] [PubMed] [Google Scholar]
- 113.Stannard SR, Buckley AJ, Edge JA, Thompson MW. Adaptations to skeletal muscle with endurance exercise training in the acutely fed versus overnight-fasted state. J Sci Med Sport. 2010;13(4):465–469. doi: 10.1016/j.jsams.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 114.Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I. Health benefits of fasting and caloric restriction. Curr Diab Rep. 2017;17(12):123. doi: 10.1007/s11892-017-0951-7 [DOI] [PubMed] [Google Scholar]
- 115.Vicente-Salar N, Urdampilleta Otegui A, Roche Collado E. Entrenamiento aeróbico en ayunas: adaptaciones biológicas y efectos en el control de peso. Nutr Hosp. 2015;32(6):2409–2420. doi: 10.3305/nh.2015.32.6.9488 [DOI] [PubMed] [Google Scholar]
- 116.Bouguerra L, Ben Abderrahman A, Chtourou H, Zouhal H, Tabka Z, Prioux J. The effect of time-of-day of training during Ramadan on physiological parameters in highly trained endurance athletes. Biol Rhythm Res. 2017;48(4):541–555. doi: 10.1080/09291016.2016.1276271 [DOI] [Google Scholar]
- 117.Chtourou H, Souissi N. The effect of training at a specific time of day: a review. J Strength Cond Res. 2012;26(7):1984–2005. doi: 10.1519/JSC.0b013e31825770a7 [DOI] [PubMed] [Google Scholar]
- 118.Mujika I. Intense training: the key to optimal performance before and during the taper. Scand J Med Sci Sports. 2010;20:24–31. doi: 10.1111/sms.2010.20.issue-s2 [DOI] [PubMed] [Google Scholar]