Abstract
Pancreatic islets produce pulses of insulin and other hormones that maintain normal glucose homeostasis. These micro-organs possess exquisite glucose-sensing capabilities, allowing for precise changes in pulsatile insulin secretion in response to small changes in glucose. When communication among these cells is disrupted, precision glucose sensing falters. We measured intracellular calcium patterns in 6-mM-steps between 0 and 16 mM glucose, and also more finely in 2-mM-steps from 8 to 12 mM glucose, to compare glucose sensing systematically among intact islets and dispersed islet cells derived from the same mouse pancreas in vitro. The calcium activity of intact islets was uniformly low (quiescent) below 4 mM glucose and active above 8 mM glucose, whereas dispersed beta-cells displayed a broader activation range (2-to-10 mM). Intact islets exhibited calcium oscillations with 2-to-5-min periods, yet beta-cells exhibited longer 7–10 min periods. In every case, intact islets showed changes in activity with each 6-mM-glucose step, whereas dispersed islet cells displayed a continuum of calcium responses ranging from islet-like patterns to stable oscillations unaffected by changes in glucose concentration. These differences were also observed for 2-mM-glucose steps. Despite the diversity of dispersed beta-cell responses to glucose, the sum of all activity produced a glucose dose-response curve that was surprisingly similar to the curve for intact islets, arguing against the importance of “hub cells” for function. Beta-cells thus retain many of the features of islets, but some are more islet-like than others. Determining the molecular underpinnings of these variations could be valuable for future studies of stem-cell-derived beta-cell therapies.
Keyterms: type 2 diabetes, islets, beta cells, oscillations, pulsatility, intracellular calcium, glucose sensitivity, hubs, hub cells
Graphical Abstract:

1. Introduction
The pancreatic beta-cell is an endocrine cell found within islets of Langerhans that produces and secretes insulin in response to rising glucose concentration within the body [1–3]. In the simplest model of insulin secretion, the ‘consensus model,’ glucose is transported into the beta-cell where it undergoes glycolysis to generate pyruvate, which is further oxidized in the tricarboxylic acid (TCA) cycle and oxidative phosphorylation to produce a rise in ATP/ADP. This leads to closure of ATP-sensitive potassium channels, cell membrane depolarization, activation of voltage-gated calcium channels, and a subsequent rise in calcium, leading to SNARE-dependent insulin release [4–6]. This classic pathway leads to what is known as first-phase insulin release, or the triggering pathway, in which a rapid spike of prepackaged insulin granules is exocytosed from beta-cells [7]. In order to achieve optimal euglycemic conditions, a second phase of insulin release follows which maintains a sustained response [8], and it is here that ‘amplifying pathways’ augment glucose-stimulated insulin secretion in both phases of insulin secretion [9,10]. Through these pathways, beta-cells are highly sensitive to small changes in glucose concentrations to allow for the precise and tight minute-to-minute control in which insulin is secreted in a pulsatile fashion [11,12].
Early concepts of islet function assumed that beta-cells behaved as a homogeneous unit throughout the islet due to gap junctional coupling, however beta-cell heterogeneity has also been shown to exist [13–17]. Recent studies have made it clear that there are subpopulations of beta-cells that differ in their gene expression patterns, sensitivities to glucose, metabolic activity, and secretory output [17–19]. Work done by Dorrell et al demonstrated functional heterogeneity among beta-cells such that four unique subtypes can be identified having different genes and transcription factors resulting in changes in their insulin secretion [20]. For example, beta-cell subtypes expressing ST8SIA1 are less capable of secreting insulin compared to their ST8SIA1-lacking counterparts. Other transcription factors that can differentiate beta-cell subtypes include SIX3, MAFB, NEUROD1 and RFX6 [20]. Differential expression of these transcription factors may explain, in part, the observation that the glucose sensitivity and threshold for insulin release vary between populations of beta-cells within an islet [14]. Individual beta cells can become active over a wider range of glucose concentrations in comparison to intact islets, as demonstrated in studies from both rodents [14,21] and humans [22].
The vast difference between the response to glucose levels seen in individual beta-cells and islets suggests that communication throughout the islet is critical to the regulation of insulin release [17,23]. Studies have shown that beta-cells that remain in contact with at least one other beta-cell or an alpha cell exhibit significantly more robust glucose sensing and insulin release [22]. An important contributor to such differences may be the oscillatory nature of insulin release by beta-cells. After glucose elevation, cytosolic free calcium oscillates, and these oscillations are highly synchronized by gap junctions that couple beta-cells to one another, resulting in pulsatile insulin secretion [17,24]. These gap junctions are formed from individual proteins known as connexins, and connexin 36 (Cx36) is the major connexin expressed on beta-cells [25]. In the absence of Cx36, individual beta-cells retain their oscillatory calcium, but oscillations are no longer synchronous throughout the beta-cell population, resulting in disrupted insulin release [26]. As a consequence of lost beta-cell synchrony, the amplitude of the first phase of insulin release is reduced and second-phase insulin release loses pulsatility, leading to glucose intolerance [27].
Some recent studies have reported that specialized beta-cells known as “hubs” appear to be responsible for orchestrating insulin release in a controlled manner within islet cellular network [28], although the concept is highly controversial. Lei et al demonstrated disruption in normal calcium oscillations when the most metabolically active cells within a glucose-stimulated islet were inhibited [24]. However, those highly active cells were not hubs in the sense of having the demonstrably higher connectivity to other cells described in Stozer et al, raising questions about the relationship between network properties and pace-making roles [29].
We revisit here the issue of intact islets versus dispersed beta-cells with respect to oscillations triggered by the glucose-dose-response curve. We systematically examined differences in the intracellular free calcium patterns of intact islets vs. dispersed beta-cells obtained from the same mouse pancreas. We recorded and analyzed free calcium oscillations in response to 6 mM glucose steps and more subtle changes in response to 2 mM glucose steps. Our findings confirm that islets have a more uniform glucose sensitivity compared to individual beta-cells. Analysis of the patterns of calcium activity seen in dispersed beta-cells further revealed variation in their capacity to respond to glucose: some had activity that was modulated by small changes in glucose, similar to the case of intact islets, while others did not change their oscillatory patterns despite experiencing large shifts in glucose concentration. Nonetheless, the summed response of dispersed beta-cells was quite similar to the summed responses of intact islets, provided care was taken to compare cells and islets that were taken from the same mouse.
2. Materials and Methods.
2.1. Mice.
Adult male CD1 mice at 8–20 weeks of age were used for all studies. Mice were purchased from Charles River Laboratories (Wilmington, MA) or Envigo (Indianapolis, IN) and housed in a pathogen-free facility at the University of Virginia or at Ohio University. All protocols used in these studies were approved by the respective Institutional Animal Care and Use Committees of the two institutions.
2.2. Islet isolation
Pancreatic islets were isolated and cultured as described previously [30]. Following isolation, islets were cultured overnight in RPMI-1640 media (Invitrogen) with 11mM glucose, 10% fetal bovine serum, and 1% penicillin/streptomycin. All drug treatments or supplements were made up in RPMI-1640, and all experiments utilizing intact islets were conducted on the day after isolation. Note that islets must be provided sufficient recovery time from cleaved surface proteins and other stresses of the isolation process before they will exhibit normal function. Overnight recovery permits the possibility of transcriptional changes that could impact islet function. However, we have observed that islets retain many aspects of their in vivo environment in their response to glucose following isolation and overnight incubation [31,32].
2.3. Islet cell dispersion
Islets were dispersed into dissociated islet cell cultures following overnight recovery from islet isolation. One day prior to islet dispersion, glass coverslips were coated with poly-D-lysine per manufacturer’s instructions and dried overnight. Islets were dispersed into single cells and cultured as described previously [30].
2.4. Intracellular Calcium
Islets were loaded with 1 uM fura-2AM in modified KRB solution containing 134.5 mM NaCl, 3 mM CaCl2, 5mM KCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.4); and 0, 2, 4, or 8 mM glucose. NaCl was reduced proportionally (1:2) to the addition of glucose to maintain osmolarity. For each experiment, the glucose concentration for loading fura-2 was the same starting concentration for the experiment. Islets were loaded for 30 min at 37 degrees C and 5% C02 and then transferred to the recording chamber for an additional 10-min. Islets were thus exposed to the starting glucose concentration (0, 2, 4, or 8 mM depending on the experimental protocol) for ~40 min prior to the start of the recording. Intracellular calcium was measured using the ratiometric calcium indicator fura-2 AM as described previously for experiments at the University of Virginia [33,34] or Ohio University [35,36].
2.5. Analysis of intracellular calcium patterns
The Cluster8 pulse detection algorithm was used to detect peaks and nadirs within islet calcium traces [37]. Within Cluster8, parameters were chosen appropriately to identify pulses while filtering out random pulse-like noise within the traces. The following parameters were used: four points required for each peak or nadir, a t-score of two to detect an increase or decrease, and a t-score of four to detect outliers. A standard deviation of 0.04 was used to reduce collection of false positives due to noise. Plateau fractions were determined by dividing the time islets and beta-cells were in active phase oscillations by the total time recorded. Specifically, the plateau fraction equals the ‘peak width’ estimated by Cluster8 divided by peak interval.
The threshold for activation was determined by identifying the lowest glucose concentration at which calcium levels increased over basal calcium levels. Since recordings generally drifted upward due to gradual fura-2 depletion and other factors, calcium increases that followed the basal upward trajectory were also not considered to reach threshold unless at least one transient burst of increased calcium was observed (see Figure 1A). A drop in calcium was not considered to cross threshold (see Figure 1B). In some cases, activity was considered at threshold in the initial glucose condition (0, 2, or 4mM) if transient increases in calcium above basal levels were observed (see Figure 1D).
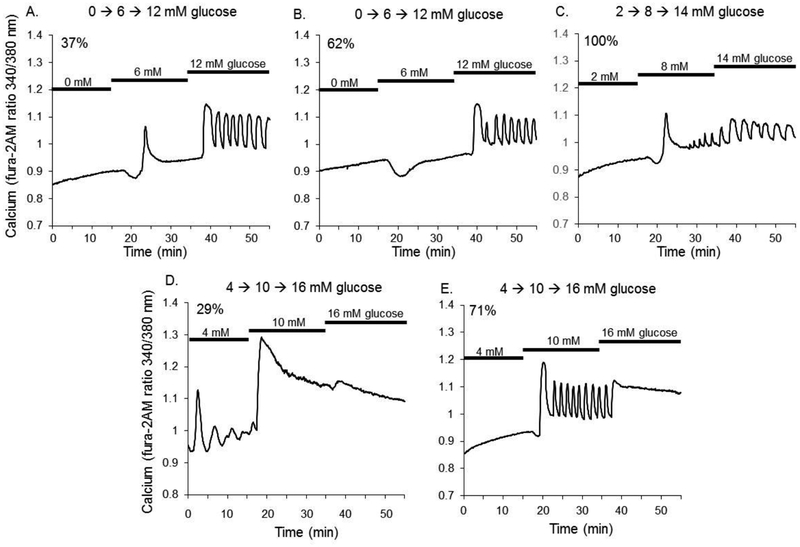
Figure 1.
Islet calcium patterns during 6mM glucose steps. (A-D) Islets were quiescent for all starting steps of 0, 2, or 4 mM glucose. (A) When stepped from 0 to 6mM glucose, 37.5% (40 out of 108) of islets showed a first phase spike and mildly elevated plateau following the spike (B) 62.5% (68 out of 108) of islets did not become active until the second step from 6 to 12mM glucose. (C) 100% of islets (161 out of 161) starting at 2 mM glucose were quiescent and became active for steps to 8mM and then to 14mM glucose. (D-E) 29% of islets starting in 4mM glucose displayed oscillations during this starting glucose step and 71% were quiescent. All islets starting in 4mM glucose responded to both the first 6mM step and again to the second 6mM step, indicating a high degree of glucose sensitivity across this range of glucose concentrations.
To identify beta-cells within dispersed islet cell cultures, we looked for some form of increase in calcium in response to increased glucose load. Such responses to glucose include an increase in fura-2 ratio, an increase in oscillation amplitude, or an increase in plateau fraction. We also identified cells with an inverse calcium relationship to glucose as putative alpha-cells and cells with small intermittent calcium transients at all glucose concentrations as putative delta-cells (see Sections 3.6 and 4.5 for additional details).
2.6. Insulin staining
Islet cells on glass coverslips were fixed in 4% paraformaldehyde for 20 min, washed in PBS, permeabilized with 0.5% Triton for 10-min and maintained in PBS. Samples were wrapped in parafilm to avoid evaporation until they were stained. Insulin and glucagon staining were performed using an anti-insulin antibody (Abcam, #ab7842, guinea pig polyclonal to insulin, IgG isotype) and anti-glucagon antibody (Abcam #ab10988, mouse monoclonal to glucagon, IgG1 isotype) at a 1/200 dilution using the manufacturer’s protocol.
2.7. Data analysis
A two-tailed or paired Student’s t-test was used for all other comparisons unless stated otherwise. A Fisher Exact test (2×2 contingency) was used to compare the percentages of oscillating vs. non-oscillating islets and beta cells and for threshold of activation for islets vs. dispersed beta-cells.
3. Results
3.1. Intracellular calcium patterns of intact islets had finely tuned responses to glucose.
The oscillatory patterns of intact islets displayed the precise modulation to glucose concentration that has been well reported in the literature [38–42]. We demonstrated this precision by examining responses to steps of 6 mM glucose. As shown in Figure 1, islet intracellular calcium was quiescent in low glucose, whether runs were started at glucose concentrations of 0 or 2 mM. Islets were then stepped to two additional glucose concentrations in three sequential experiments as follows: Protocol A: 0 to 6 to 12 mM, Protocol B: 2 to 8 to 14 mM, and Protocol C: 4 to 10 to 16 mM. This was done to determine at which concentration of glucose islets displayed oscillatory calcium activity and demonstrated the precision regulation involved in insulin release.
As shown in Figure 1A, when glucose was stepped from 0 to 6 mM 37% of islets (40 out of 108 islets) responded with a large amplitude first-phase spike in calcium and a mildly elevated plateau following the spike (second-phase response). Calcium increased further from 6 mM to 12 mM glucose, resulting in oscillations in calcium and a higher baseline calcium level. As shown in Figure 1B, 62% of islets (68 out of 108) did not show an increase in calcium in response from 0 to 6 mM glucose stimulation, although a decrease in calcium was observed. This calcium drop is consistent with metabolically-induced sequestration of calcium into the endoplasmic reticulum observed in rodent [43–45] and human islets [46], but without a sufficient enough metabolic increase in some instances to trigger KATP-channel closure and subsequent increase in calcium influx and insulin release. This dip was not considered a response. 100% of islets that did not show a spike at 6 mM became active following the step from 6 to 12 mM glucose (Figure 1B). Steps from 2 mM glucose to 8 mM produced a first-phase spike (Figure 1C), and steps from 4 to 10 mM glucose produced the characteristic second-phase plateau 100% of the time (Figure 1D–E). Steps to still higher glucose resulted in oscillations of larger size (Figure 1C), as observed by increased amplitude and/or plateau fraction, or a continuous plateau of elevated calcium (Figure 1D–E) in all of the islets tested. 29% of islets in Figure 1D displayed oscillatory activity in the starting glucose concentration of 4 mM. These islets were derived from 2 of the 8 mice; only one other islet showed such activity among all islets recorded from the other 6 mice.
3.2. Dispersed islet cells displayed a mix of responses to glucose stimulation.
We next looked at dispersed beta-cells that were generated from islets isolated from the same mice used to produce the data shown in Figure 1. After dispersion and overnight recovery, intracellular calcium measurements were made as described for Figure 1. A subset of these cell preparations was stained for insulin and glucagon in order to estimate the expected percentage of beta cells in each preparation. In Figure 2, 71 islet cells or cell clusters are displayed in a brightfield image (Figure 2A) in which 56 cells or cell clusters stained positively for insulin are green (Figure 2B), and 4 stained for glucagon are red (Figure 2C). Using several of these images, we estimated that the vast majority (>90%) of the recorded cells were isolated beta cells or else clusters containing beta cells along with other islet cell types.
Figure 2.
Dispersed islet cells are majority beta-cells. (A) Example of brightfield image of a field of islet cells prior to recording calcium. (B) Same field of cells imaged for immunofluorescence for insulin staining (green). (C) Same field of cells imaged for immunofluorescence for glucagon staining (red). Among 305 cells examined, 209 were stained for insulin (69%), 46 stained red for glucagon (15%: 29 of 46 were in contact with insulin stained cells as clusters), and 50 did not stain (16%).
3.3. Dispersed islet cells had a broader range of activation thresholds for calcium activity.
Dispersed islet cells showed much more variable responses to glucose stimulation than islets. In these recordings, calcium activity is defined as a glucose-dependent increase in intracellular calcium between glucose steps or calcium fluctuations observed in the lowest glucose condition for that cell. About 10% of beta cells were active in 2mM glucose (Figure 3A, representing 29 of 263 cells), whereas ~25% of dispersed beta cells showed no activity at 8mM glucose (Figure 3B, representing 67 of 263 cells). In contrast, not a single intact islet showed activity in 2 mM glucose out of 118 tested, but 100% of 118 islets tested were active in 8mM glucose. These data show that there is a broader range of glucose sensitivities among dispersed beta cells as compared to intact islets.
Figure 3.
Individual islet-cells show variations in calcium patterns during 6mM glucose steps. A) Example of a beta-cell with a low threshold of activation with calcium oscillations appearing at 2mM glucose and continuing to 14mM glucose with changes in calcium in a dose-dependent manner. (B) Example of a beta-cell with a high threshold of activation with calcium oscillations appearing only at 14mM glucose. (C) Example of a beta-cell which demonstrate no dose-dependent changes in calcium level with changes in glucose concentration. (D) Example of a dispersed beta-cell that was quiescent in 2mM and 4mM glucose and became active by the second step to 14 mM and 16mM glucose and displayed a dose-dependent increase in calcium level and activity from each step. This is similar to the patterns demonstrated in intact islets
Note that the cell in Figure 3B displays a sustained drop in calcium in going from 2 to 8mM glucose. Because this response results in lower, not higher, calcium, it is not considered to be a response that would drive insulin secretion. In stepping from 8 to 14 mM glucose, this cell shows a strong increase in calcium, so this cell is considered active in 14 mM glucose. Had this cell not responded with an increase in calcium from 8 to 14 mM glucose, this cell would have been considered a non-beta-cell and would have been excluded from analysis.
Dispersed beta cells also did not always show altered responses when glucose was stepped. As shown in Figure 3C, this representative example had robust calcium oscillations in 4mM, 10mM, and 16mM glucose, without substantial change in period, amplitude, or plateau fraction. Approximately one third of beta cells produced a pattern that could be categorized as “not modulated” by glucose between 6mM glucose steps. Note that only a small number of cells (~8%) showed no change in calcium pattern across two 6mM steps (a 12 mM range); most not-islet-like cells were quiescent in the starting glucose concentration, responded to the first step, and showed no apparent change in pattern during the second step. As shown in Figure 3D, a subset of beta cells showed islet-like behavior, meaning that calcium levels and/or calcium oscillations displayed glucose-dependent changes in activity similarly to islets. This representative beta cell was quiescent in 4mM glucose, showed a distinct biphasic response to 10mM glucose and had robust oscillations in 16mM glucose.
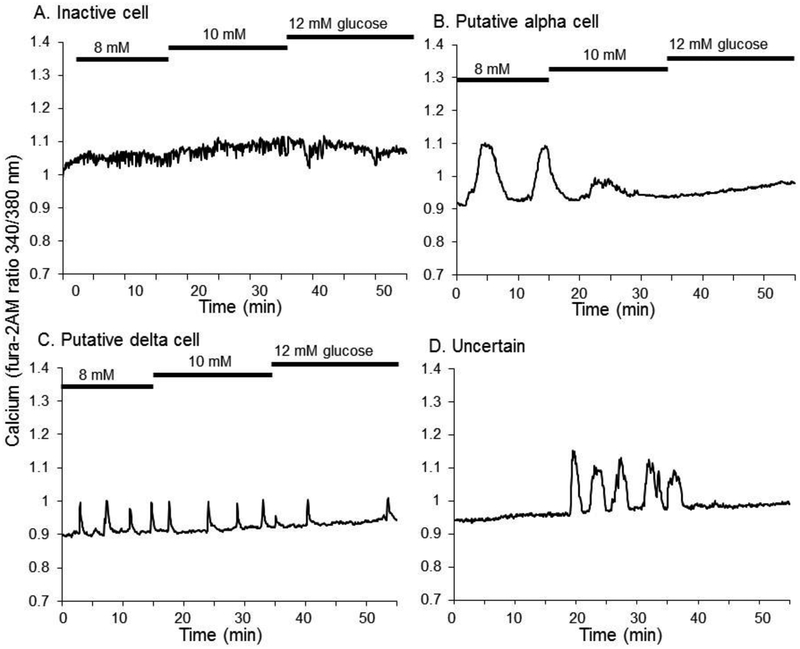
One last category that we defined as “Other” included islets that decreased their activity as glucose was increased or that showed other unusual patterns of activity (see Figure 8). Cells that completely lacked calcium activity of any sort were excluded to avoid the possibility of including obviously sick or dead cells in the analysis.
Figure 8.
Other types of cell responses to glucose. (A) Example of a cell that was not active within the range of 8–12 mM glucose. (B) Example of a putative alpha cell response to glucose. (C) Example of a putative delta-cell response to glucose. (D) Example of an enigmatic form of glucose sensitivity.
We combined calcium traces from islets isolated from several mice to produce an “aggregate islet response” constructed from ~40 islets per mouse (range, 27–53 islets). Likewise, we combined all the dispersed beta-cell calcium traces (after excluding completely non-responsive cells) derived from several mice, totaling approximately 100 dispersed islets from each mouse (range, 65–153 cells). As shown in Figure 4A–C, the aggregate islet response produced similar responses to mean islet cell responses in terms of glucose sensing for each of the three sets of glucose steps. The calcium responses of dispersed islet cells to glucose were generally higher than those seen in intact islets, although this was statistically significant only for first-phase responses to 8 mM glucose (Figure 4B).
Figure 4.
Aggregate islet calcium is remarkably similar to aggregate islet-cell responses. (A-C) Average fluorescence from beta-cells (dashed) and islets (solid) for (A) 0→6→12G, (B) 2→8→14G, (C) 4→10→16G (G = mM glucose). Mean data from N=8 mice for each panel. (D) Mean fluorescence level for islets and dispersed beta-cells in panels A – C pooled and normalized to response at 16 mM glucose as 100%. (E) Threshold for activation for islets and dispersed beta-cells in panels A – C pooled. P<0.05, **P<0.01, N.S. = not significant. (F-G) Threshold for activation for islets (F) and beta cells (G) presented for each of 8 individual mice.
We combined the data from the three sets of glucose stimulations to produce a glucose dose-response curve of mean calcium responses, with 2 mM glucose intervals ranging from 0 to 16 mM glucose. To adjust for differences between islets and beta-cells in terms of the amplitudes of their respective calcium responses, we normalized mean calcium levels per mouse as a percent of the values we observed in response to 16 mM glucose. This accounts for increased dye penetration in individual islet-cells which can impact the recorded calcium levels. As shown in Figure 4D, islets and dispersed beta-cells had very similar glucose sensitivities, with no significant differences noted across the entire glucose dose-response curve. We also examined the threshold for activation (the lowest glucose concentration for which islets or beta-cells displayed baseline calcium increases, spikes, or oscillations). As shown in Figure 4E, the threshold for glucose-stimulated calcium activity for islets was generally between 4 and 8 mM glucose. All islets lacked activity at 2 mM glucose and below. In contrast, 3% of dispersed islet cells displayed some sort of calcium activity in 0 mM glucose and ~20% in 2 mM glucose. A majority of islets and beta cells reached the threshold for activation at 4–6 mM. While 100% of islets were active at 8 mM glucose and above, 100% activation of individual beta cells was not achieved until 12 mM glucose was applied (Figure 4E).
When viewed at a mouse-to-mouse level, these differences are even more evident. As shown in Figure 4F, islet calcium responses are plotted for each of the 8 CD1 mice used as a source of pancreatic tissue. Islets from 2 mice activated at fairly low glucose concentrations, with no active islets in 2 mM glucose, but nearly all islets active in 4 mM glucose. Islets from 2 mice became active between 4 and 6 mM glucose, and islets from 4 mice became active between 6 and 8mM glucose (Figure 4F). In contrast, the threshold for activation among dispersed beta cells from these same 8 mice was not nearly as sharp. Cells from all 8 mice showed some activity in 2mM glucose, and beta cells from only one mouse reached 100% activation by 8 mM glucose. In fact, the shift in glucose needed to go from <25% active to >75% activity was 2.25 +/−0.27 mM glucose for islets and 5.50 +/− 0.67 mM glucose for beta cells (P<0.001). Our data show there is a much broader range of glucose sensitivities among dispersed beta cells that are uncoupled compared to those of intact islets.
3.4. Mathematical modeling of glucose dose-response curves for calcium
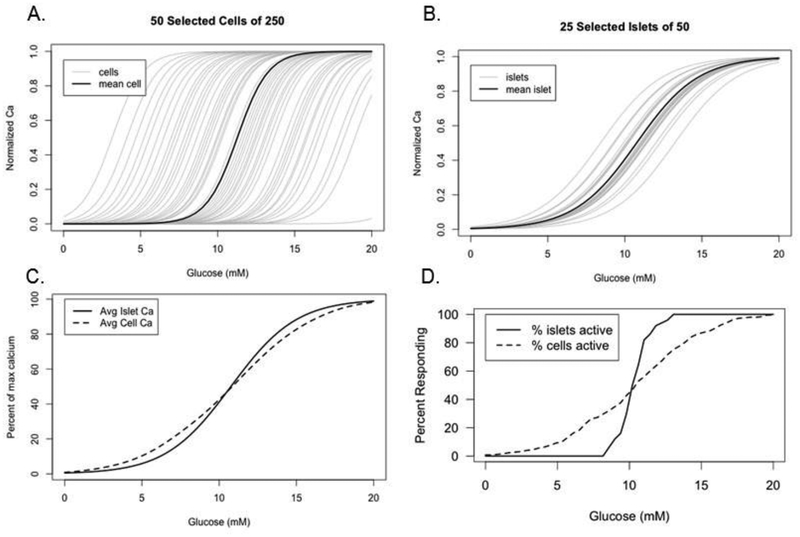
We carried out simulations with a simplified model to check whether the population behavior we reported in Figure 4D–E can be accounted for by a plausible set of assumptions about the properties of individual cells and islets. Specifically, we assumed that both dispersed cells and intact islets had sigmoidally increasing dose response curves for activity, which we identified with normalized calcium, and that the half-activation levels of glucose, which determined the threshold, were normally distributed. For simplicity, we assumed that the steepness of the sigmoids was the same for all cells and all islets but could differ between the two preparations.
Figure 5A shows a sample of randomly generated individual cells with broadly distributed thresholds, and Figure 5B shows a random sample of islets. The islets are assumed to have a tighter distribution of thresholds than the cells because they benefit from averaging over the intrinsic properties of many cells. At the same time the islets are assumed to have shallower dose response curves because they are composed of cells with heterogeneous thresholds, which is only partially mitigated by electrical coupling. The net result is that the islets all turn on at physiologically reasonable levels of glucose, whereas many cells turn on at very low or very high levels of glucose. Figure 5C shows the averaged activity, corresponding to the normalized calcium in Figure 5D. The tighter distribution of thresholds for islets balances the shallower curves for individual islets to produce a population curve in Figure 5C that is very similar to that for the individual cells, in agreement with Figure 4D. Figure 5D shows the percent of responding islets and cells, defined as individual units with activity above a threshold level. As in the corresponding experimental plot in Figure 4E, the islet curve is markedly steeper than the cell curve. This is because the nonlinear process of thresholding, in contrast to the linear process of averaging, is more sensitive to the narrow distribution of thresholds for islets than it is to the shallowness of the individual curves.
Figure 5.
Mathematical model of beta-cell and islet responses to glucose concentration. Activity (“normalized Ca”) is modeled as sigmoidal function of glucose, f(G) = 1/(1 + exp((θ − G)/k)), with threshold θ and scale factor k; θ is distributed normally with mean μ and standard deviation σ. (A) Randomly generated individual beta-cells with a broad distribution of thresholds (μ = 11 mM, σ = 4 mM) and k = 1 mM. (B) Randomly generated islets with a tighter distribution of thresholds (μ = 11 mM, σ = 1.2 mM) but shallower response curves than isolated cells due to heterogeneity within each islet (k = 2 mM). (C) Average activity of the islets and individual beta-cells from A, B. (D) Percentages of islets and individual beta-cells from A, B with activity above 0.4.
3.5. Smaller glucose steps confirm uniformity among islets and heterogeneity among dispersed beta cells.
To further examine the differential glucose responses of intact islets and dispersed islet cells, we studied the actions of much smaller glucose steps by examining calcium responses to 8, 10, and 12 mM glucose. As shown in Figure 6A, a representative islet in 8 mM glucose showed robust pulsatility with a period of ~4-min and a plateau fraction of 46%. When glucose was raised to 10 mM, the plateau fraction increased to 65% without much effect on period. When glucose was raised to 12 mM, the islet showed a further increase in plateau fraction to 90%. The period throughout the recording was 241 +/− 12 sec (265 seconds in 8mM, 225 seconds in 10 mM, and 235 seconds in 12 mM glucose.
Figure 6.
Smaller steps reveal much more sophisticated modulation of responses in islets compared to dispersed beta-cells. (A) Example of an intact islets displaying glucose-induced changes in oscillatory intracellular calcium activity with each 2-mM glucose step from 8 to 10 and from 10 to 12mM. (B) Example of an oscillatory pattern in a dispersed beta-cell that appear unaffected by the same 2mM step changes in glucose which is considered non-glucose sensitive. (C) Example of oscillatory calcium activity of an individual beta-cell that glucose sensitive and is more in line with islet calcium patterns.
Dispersed islet cells produced a much greater degree of variation compared to islets. As shown in Figure 6B, some islet cells displayed oscillations in calcium that persisted without any apparent change in their pattern across glucose concentrations. In Figure 6C, the representative beta cell shown was oscillatory in 8 mM glucose, produced a broader plateau fraction in 10 mM glucose, and displayed a nearly continuous plateau in response to 12 mM glucose. The glucose-modulated pattern shown in Figure 6C is more similar to intact islets.
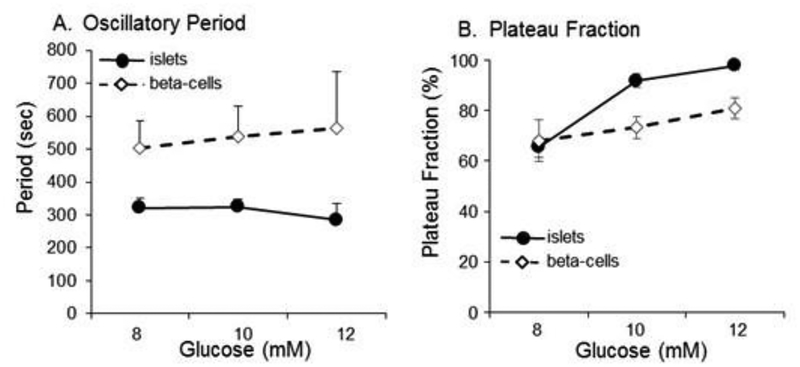
As shown in Figure 7A, period of calcium oscillations of islets on the whole remained steady at roughly 5 min, whereas the period of dispersed islet cells was significantly greater (period ~8 – 10 min). This is consistent with our previous published observations [31,47], although it should be noted that even we do not always observe slower periods of oscillation for beta-cells [16,48], which may be due to complex interactions among multiple types of oscillatory processes [49,50]. As shown in Figure 7B, plateau fraction increased markedly from 8 to 10 mM glucose for islets and still further in 12 mM glucose, whereas dispersed islet cells showed a more attenuated dose-dependent increase in plateau fraction. Similar results were reported previously using islets and beta-cells cells isolated from Swiss-Webster mice [47].
Figure 7.
Differences in small step oscillations. (A) Period of oscillatory activity for each glucose step. (B) Plateau fraction of oscillatory activity for each glucose step.
3.6. Possible alpha cells, delta cells, and unclassifiable responses within the “Other” category.
Approximately one third of islet cells that were sequentially exposed to 8, 10, and 12 mM glucose did not follow a pattern that could be easily described. Some cells did not show any change in calcium activity (Figure 8A), suggesting that these cells did not reach the threshold for activation or that they were completely unresponsive to glucose because of either cell type or poor health. Some cells showed patterns that are not typical of beta cells. For example, the cell in Figure 8B showed robust oscillations in 8 mM glucose that declined in amplitude and or disappeared entirely with increasing glucose. This is consistent with the inverse glucose sensitivity of glucagon-secreting alpha cells [51], although it should be noted that the inverse glucose sensitivity of alpha cells is not necessarily reflected by the changes in intracellular calcium [52]. Another subset of islet cells displayed calcium spikes that were too brief to be considered beta-cell-like oscillations (Figure 8C). Such spikes might indicate somatostatin-secreting delta-cells, as others have reported [51,53]. Figure 8D shows an enigmatic cell that was inactive in 8 mM glucose, displayed elevated calcium and/or oscillations in 10 mM glucose, and became inactive again in 12 mM glucose. We summarize in Figure 9 the percentage of cells that were classified as putative beta-cells, alpha-cells, delta-cells, and “uncertain” among all recordings from 0–16 mM glucose and 8–12 mM glucose that showed some response (a total of 410 cells). The percentage of beta cells that we observed falls within the expected range of islet cell distributions for the mouse endocrine pancreas and with previous estimations from pancreatic sections [54] or dispersed beta-cell cultures [55].
Figure 9.
Estimate of the percentage of each islet cell type based on calcium responses as described for the 410 cells remaining after eliminating non-responsive cells shown in Figure 8.
4. Discussion
Our findings confirm the long-standing observation that individual beta cells display much greater heterogeneity in their thresholds for activation by glucose than intact islets. We expand upon this observation by providing two important additional findings. First, we show that individual beta-cells vary in how finely their activity is tuned or modulated by glucose. Second, we show that, in spite of the great heterogeneity we encountered, summing the individual activities of many dispersed beta cells closely reproduced the glucose-induced calcium responses of intact islets.
4.1. Beta-cell heterogeneity.
The study of beta-cell heterogeneity has received increasing attention over the last several years, and ongoing studies have demonstrated that beta-cells exhibit morphological, functional and molecular heterogeneity [23,56,57]. It has been demonstrated that single beta-cells display a wide range of thresholds for glucose-stimulated insulin release [15,21] although the exact reason for such differences is less clear. It is well known that differences in relative glucokinase activity can left shift or right shift the glucose threshold for activation for islets [28,58,59]. It has been suggested that variations in glucose response among beta-cells may be due to differences in glucose phosphorylation rather than transport of glucose into the cell [60]. It was shown that both high responsive and low responsive beta-cells, those with high or low proinsulin biosynthesis and insulin release respectively, express the GLUT2 glucose transporter similarly. However, glucokinase mRNA level and glucokinase activity were significantly elevated in the highly responsive beta-cell population [60]. Another study demonstrated that when glucokinase is overexpressed using a doxycycline-dependent transcriptional transactivator in a beta-cell population, 90% of maximal insulin release was seen by 6mM of glucose while those cells with normal glucokinase function showed 75% of maximal insulin release taking place between 6 and 12 mM glucose, indicating that increased glucokinase shifts the threshold to the left [61]. A similar result was observed in diabetic islets from db/db mice [58] and rats [62]. The variation in glucose thresholds we observed among isolated cells thus may result from differences in glucokinase expression, a concept that warrants further study.
Another possible explanation for the differences between isolated beta cells and intact islets is gap junctional coupling. Several studies have highlighted the importance of connexin 36 to islet function [25–27,63,64]. Of particular interest to the present study, Bennginer et al. specifically compared islets with and without connexin 36 for differences in glucose dose-response curves [65]. Similar to our results, the absence of connexin 36 led to an elevation in calcium and insulin release in low glucose. However, the increase in beta-cell activity in low glucose was much more pronounced for islets lacking connexin 36 compared to what we observed for dissociated beta cells. Despite these difference, both studies conclude that cell-cell communication enhances islet function by reducing the heterogeneous responses of constituent beta cells.
4.2. Support from Mathematical Modeling.
Our finding that isolated cells can oscillate but with altered thresholds and dose response curves from the islets they composed has been anticipated by mathematical models of coupled and uncoupled islets. Smolen et al simulated heterogeneous islets in which a number of cell parameters were randomized and found that most cells could oscillate at some level of glucose, but only a minority at any given level of glucose [66]. In other words, each beta cell has a range of glucose concentrations that permit oscillations, but the upper and lower limits of this range may differ markedly from beta-cell to beta-cell. The oscillatory range of glucose concentrations that a given beta cell responds to may vary greatly, however, the same basic physiological mechanisms appear to govern the responses of each beta cell. Those simulations, though at variance with our observations that at some glucose concentrations many cells were oscillating, suggested that the oscillatory activity of islets is built out of the activity of their component cells and does not require additional inputs or specialized pacemaker cells. This is quite different from other excitable tissues, such as the heart, which has a spatially localized and electrically isolated pacemaker region in the sino-atrial node with distinct physiological properties.
The model based on simple input-output curves shown in Figure 5 further supports the hypothesis that individual beta cells have similar oscillatory capabilities as islets but differ quantitatively in having a broad distribution of activation thresholds. Those simulations do not take into account explicitly the electrical coupling of cells within islets, but the coupling is indirectly reflected in the parameters representing steepness of the curves and the width of the distribution of the thresholds.
4.3. Mouse-to-mouse imprinting of calcium patterns.
A key feature that distinguishes this study from previous ones is that we compared single cells and islets from the same mouse. Islets from the same mouse exhibit little variation in calcium oscillations, but islets of different mice display greater variation, a phenomenon known as “islet imprinting” [31]. It has been argued that each islet from the same mouse shares a unique oscillatory signature, a characteristic oscillatory pattern which occurs by unknown mechanisms [32]. This phenomenon may have reduced the observed differences between islets and beta-cells in our study and made it easier to discern the similarities between the two.
4.4. Hub Cells
Previous work has argued that highly connected cells known as “hubs,” constituting 1 – 10% of the beta cells in an islet, are responsible for controlling insulin release throughout the islet, and that silencing those cells can shut down islet oscillations [28]. Disruption to a different kind of specialized beta cell with high levels of glucokinase was shown in islet simulations to similarly shut down islet oscillations [24]. Our study argues against this hypothesis, as we found that the majority of the dispersed beta-cells were still capable of producing calcium oscillations in response to glucose independent of coupling to other cells. Note that single cells differed from each other and from the islets they came from with regard to the range of glucose at which they were active; at any given level of glucose, only a minority of cells may have had oscillations. Nonetheless when combined, these cells produced a collective calcium response to glucose stimulation that is quite similar to the responses from intact islets. One key difference between our study and that of Johnston et al. is that our studies were conducted a full day after dispersing islets into individual islet cells and small cell clusters, whereas ablating a hub cell has an immediate impact [28]. It is thus possible that islet behavior is dominated by hubs on short time scales, but that many beta-cells possess the glucose-sensing capacity to emerge as new hubs over time. If this is the case, islets would be resilient to the loss of hub cells.
4.5. The Importance of ‘Islet-Like’ Beta-Cells
The two main patterns we observed represent coarse and fine-tuned sensitivities, respectively, to glucose with direct relevance to current attempts to derive functional beta-cells from pluripotent stem-cells and other precursor cells [67–69]. To date, there have been numerous successes in creating insulin-producing cells [70,71], but there is substantial variability in glucose sensitivity. There are two key elements to deriving an ideal beta-cell-like cell line: 1) the capacity to produce and secrete insulin and 2) the ability to secrete insulin proportionally to glucose load. It is the latter element that is more difficult to achieve at the single-cell level. Glucose-stimulated changes in intracellular calcium can be used as an indicator for glucose-stimulated insulin secretion, although there is not always a perfect correlation between the two [72–74]. Novel approaches have already been developed to identify insulin-producing cells and other islet cell types based on calcium responses to glucose stimulation [75]. In addition, sophisticated computer software has recently been developed to help to identify key features of beta-cell calcium responses to glucose stimulation for potential use in determining the most “beta-cell-like” stem-cell-derived cell lines with regard to function [76]. The present study suggests that those are beta-cells that possess the same sort of fine-tuned modulation of glucose responses as intact islets. Moreover, this study suggests that electrically coupling cells, while desirable, may not be essential. Stem cells with these “glucose-modulated” responses, in addition to producing insulin, should be viewed as ideal targets for development as therapeutics.
The use of glucose-stimulated calcium measurements may also be valuable in identifying and characterizing other islet cell types, particularly alpha and delta-cells. We identified a number of cells, for example, that showed an inverse response to glucose stimulation, which we identified as putative alpha cells. We also observed a small number of cells displaying brief sharp calcium spikes or transients that occurred intermittently regardless of glucose concentration, which we attributed to somatostatin-secreting delta cells. These observations are consistent with previous observations of alpha-cell and delta-cell calcium patterns [53,75]. We note, however, that these assumptions have not been confirmed by immunostaining to positively identify the cell type. This issue is discussed in Section 4.6.
4.6. Limitations
There are limitations to this study that should be noted. First, we did not examine the activity of individual cells within islets. The entire islet was recorded as a single region of interest. Therefore, we are unable to determine whether the sharper threshold for activation in islets is due to activation of all beta cells by hub cells [24,28,77], a product of gap junctional coupling [27,63,64], a gradual recruitment of beta-cells based on glucose sensitivity [78,79,56], or some combination of these. However, our data from dissociated beta-cells suggest that the continuum of calcium responses to glucose average out to be similar to the response of an intact islet. Cell-to-cell communication appears to sharpen this response.
A second concern is that we identified islet cell types by their physiological responses to glucose stimulation without definitively identifying recorded cells by immunostaining. There is some concern that cells active in 0, 2, or 4 mM glucose, in particular, could be misidentified as beta-cells. We did, however, stain for insulin and glucagon in a subset of our studies in order to identify beta-cell populations. We found that 69% of cells/clusters were exclusively insulin positive (209 of 305), and 15% were stained for glucagon (29 of these 46 cells also stained for insulin within the same small cluster). Thus, 78% of cells/clusters contained some amount of insulin staining (in line with 77% reported as beta-cells by calcium response). Glucagon-only-stained cells were ~6% (in line with the 5.3% reported by calcium response). An additional 50 cells (16%) did not stain for insulin or glucagon; these could be delta cells or other pancreatic cells that may or may not be glucose-responsive. The cells identified by calcium response as delta cells (2.7%) or uncertain (14.8%) are very close to the estimate of 16% ‘unlabeled’ (not stained) among cells co-stained for insulin and glucagon. These represent remarkably similar estimates of islet cell type by two different methods.
It is still possible that some cells may have been mislabeled based on calcium responses to glucose. Because alpha cells appear to lose glucose-induced inhibition when contact with beta-cells is lost [80], our method of identifying alpha cells may not be accurate, especially among putative alpha cells that are not in contact with insulin-positive cells. However, our estimates of cell type percentages appear to be reasonably in line with several other studies involving calcium imaging [29,42,75]. Overall, based on the similar percentages of cell types identified by physiology vs. immunohistochemistry and the literature, the number of misidentified beta cells is likely low.
5. Conclusion
Aristotle’s concept, “The whole is greater than the sum of its parts”, is particularly apropos to the pancreatic beta-cell, which secretes insulin in response to glucose stimulation at the single-cell level, but functions differently when incorporated into an islet. Our data suggest, however, modifying this dictum to “greater, but not much greater”. We have shown that (1) beta-cells are heterogeneous in their calcium responses to glucose stimulation, (2) despite this heterogeneity, the summation of many dispersed beta-cells produces an aggregate response similar to that of the intact islet, and (3) a subset of beta-cell appears to have nearly the degree of fine-tuned glucose sensitivity as in the intact islet. These glucose-modulated beta-cells may hold the key to designing de novo better cells for beta-cell replacement.
Highlights.
--When removed from intact islet structures, insulin-producing beta-cells are heterogeneous in their calcium responses to glucose stimulation.
--The summation of responses from many dispersed beta-cells produces an aggregate response similar to that of the intact islet.
--A subset of beta cells possesses the fine-tuned glucose sensitivity found in the intact islet.
--Islets from the same mouse have similar glucose responses, but islet responses to glucose differs widely from mouse to mouse in vitro.
Acknowledgments:
The authors thank the Research and Scholarly Advancement Fellowship (RSAF) program and the Heritage College of Osteopathic Medicine (HCOM) at Ohio University for providing R.S. the opportunity to conduct a portion of this research. This work was funded, in part, by R01 DK046409 to L.S.S. and R15 DK121247 to C.S.N, as well as funding from HCOM and the Osteopathic Heritage Foundation.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Conflict of Interest Statement:
There are no conflicts of interest with any co-author of this manuscript with regard to financial or other affiliations that relate to the contact of this manuscript.
References
- [1].Lacy PE, The pancreatic beta cell. Structure and function., N. Engl. J. Med 276 (1967) 187–195. doi: 10.1056/NEJM196701262760401. [DOI] [PubMed] [Google Scholar]
- [2].Reaven GM, Role of Insulin Resistance in Human Disease, Diabetes. 37 (1988) 1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- [3].Fu Z, Gilbert ER, Liu D, Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes., Curr. Diabetes Rev 9 (2013) 25–53. [PMC free article] [PubMed] [Google Scholar]
- [4].Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P, Stimulus-secretion coupling in pancreatic beta cells, J. Cell. Biochem 55 Suppl (1994) 54–65. [DOI] [PubMed] [Google Scholar]
- [5].Sherman A, Contributions of modeling to understanding stimulus-secretion coupling in pancreatic beta-cells, Am J Physiol 271 (1996) E362–72. [DOI] [PubMed] [Google Scholar]
- [6].Wang Z, Thurmond DC, Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins., J. Cell Sci 122 (2009) 893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henquin JC, Triggering and amplifying pathways of regulation of insulin secretion by glucose, Diabetes. 49 (2000) 1751–1760. [DOI] [PubMed] [Google Scholar]
- [8].Rorsman P, Renstrӧm E, Insulin granule dynamics in pancreatic beta cells, Diabetologia. 46 (2003) 1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- [9].Henquin J-C, The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells, Diabetes Res. Clin. Pract 93 (2011) S27–S31. doi: 10.1016/S0168-8227(11)70010-9. [DOI] [PubMed] [Google Scholar]
- [10].Jensen MV, Gooding J, Ferdaoussi M, Dai X-Q, MacDonald PE, Newgard CB, Metabolomics Applied to Islet Nutrient Sensing Mechanisms, Diabetes Obes. Metab 19 (2017) 90–94. doi: 10.1111/dom.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Porksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O, Pulsatile insulin secretion: detection, regulation, and role in diabetes, Diabetes. 51 Suppl 1 (2002) S245–54. [DOI] [PubMed] [Google Scholar]
- [12].Nunemaker CS, Satin LS, Episodic hormone secretion: a comparison of the basis of pulsatile secretion of insulin and GnRH, Endocrine. 47 (2014) 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Salomon D, Meda P, Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells, Exp. Cell Res 162 (1986) 507–520. doi: 10.1016/0014-4827(86)90354-X. [DOI] [PubMed] [Google Scholar]
- [14].Pipeleers DG, Heterogeneity in pancreatic beta-cell population, Diabetes. 41 (1992) 777–781. [DOI] [PubMed] [Google Scholar]
- [15].Van Schravendijk CF, Kiekens R, Pipeleers DG, Pancreatic beta cell heterogeneity in glucose-induced insulin secretion., J. Biol. Chem 267 (1992) 21344–21348. [PubMed] [Google Scholar]
- [16].Zhang M, Goforth P, Bertram R, Sherman A, Satin L, The Ca2+ Dynamics of Isolated Mouse β-Cells and Islets: Implications for Mathematical Models, Biophys. J 84 (2003) 2852–2870. doi: 10.1016/S0006-3495(03)70014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benninger RKP, Hodson DJ, New Understanding of β-Cell Heterogeneity and In Situ Islet Function, Diabetes. 67 (2018) 537–547. doi: 10.2337/dbi17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roscioni SS, Migliorini A, Gegg M, Lickert H, Impact of islet architecture on β-cell heterogeneity, plasticity and function, Nat. Rev. Endocrinol 12 (2016) 695–709. doi: 10.1038/nrendo.2016.147. [DOI] [PubMed] [Google Scholar]
- [19].Nasteska D, Hodson DJ, The role of beta cell heterogeneity in islet function and insulin release, J. Mol. Endocrinol 61 (2018) R43–R60. doi: 10.1530/JME-18-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, Grompe M, Human islets contain four distinct subtypes of β cells, Nat. Commun 7 (2016). doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jonkers FC, Henquin JC, Measurements of cytoplasmic Ca2+ in islet cell clusters show that glucose rapidly recruits beta-cells and gradually increases the individual cell response, Diabetes. 50 (2001) 540–550. [DOI] [PubMed] [Google Scholar]
- [22].Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D, Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts, Diabetologia. 51 (2008) 1843. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- [23].Benninger RKP, Piston DW, Cellular Communication and Heterogeneity in Pancreatic Islet Insulin Secretion Dynamics, Trends Endocrinol. Metab. TEM. 25 (2014) 399–406. doi: 10.1016/j.tem.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lei C-L, Kellard JA, Hara M, Johnson JD, Rodriguez B, Briant LJB, Beta-cell hubs maintain Ca2+ oscillations in human and mouse islet simulations, Islets. 10 (2018) 151–167. doi: 10.1080/19382014.2018.1493316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Umrani MR, Joglekar MV, Somerville Glover E, Wong W, Hardikar AA, Connexins and microRNAs: Interlinked players in regulating islet function?, Islets. 9 (2017) 99–108. doi: 10.1080/19382014.2017.1331192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ravier MA, Guldenagel M, Charollais A, Gjinovci A, Caille D, Sohl G, Wollheim CB, Willecke K, Henquin J-C, Meda P, Loss of Connexin36 Channels Alters -Cell Coupling, Islet Synchronization of Glucose-Induced Ca2+ and Insulin Oscillations, and Basal Insulin Release, Diabetes. 54 (2005) 1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- [27].Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RKP, Connexin-36 Gap Junctions Regulate In Vivo First- and Second-Phase Insulin Secretion Dynamics and Glucose Tolerance in the Conscious Mouse, Diabetes. 61 (2012) 1700–1707. doi: 10.2337/db11-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, Berishvili E, Duncanson P, Watkinson M, Broichhagen J, Trauner D, Rutter GA, Hodson DJ, Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose, Cell Metab 24 (2016) 389–401. doi: 10.1016/j.cmet.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stožer A, Gosak M, Dolenšek J, Perc M, Marhl M, Rupnik MS, Korošak D, Functional Connectivity in Islets of Langerhans from Mouse Pancreas Tissue Slices, PLoS Comput. Biol 9 (2013) e1002923. doi: 10.1371/journal.pcbi.1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS, A Practical Guide to Rodent Islet Isolation and Assessment, Biol. Proced. Online. 11 (2009) 3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nunemaker CS, Dishinger JF, Dula SB, Wu R, Merrins MJ, Reid KR, Sherman A, Kennedy RT, Satin LS, Glucose Metabolism, Islet Architecture, and Genetic Homogeneity in Imprinting of [Ca2+]i and Insulin Rhythms in Mouse Islets, PLoS ONE. 4 (2009) e8428. doi: 10.1371/journal.pone.0008428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nunemaker CS, Zhang M, Wasserman DH, McGuinness OP, Powers AC, Bertram R, Sherman A, Satin LS, Individual Mice Can Be Distinguished by the Period of Their Islet Calcium Oscillations: Is There an Intrinsic Islet Period That Is Imprinted In Vivo?, Diabetes. 54 (2005) 3517–3522. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- [33].Corbin KL, Hall TE, Haile R, Nunemaker CS, A novel fluorescence imaging approach for comparative measurements of pancreatic islet function in vitro, Islets. 3 (2011) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jahanshahi P, Wu R, Carter JD, Nunemaker CS, Evidence of Diminished Glucose Stimulation and Endoplasmic Reticulum Function in Nonoscillatory Pancreatic Islets, Endocrinology. 150 (2009) 607–615. doi: 10.1210/en.2008-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Whitticar NB, Strahler EW, Rajan P, Kaya S, Nunemaker CS, An Automated Perifusion System for Modifying Cell Culture Conditions over Time., Biol. Proced. Online. 18 (2016) 19. doi: 10.1186/s12575-016-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gelin L, Li J, Corbin KL, Jahan I, Nunemaker CS, Metformin Inhibits Mouse Islet Insulin Secretion and Alters Intracellular Calcium in a Concentration-Dependent and Duration-Dependent Manner near the Circulating Range., J. Diabetes Res 2018 (2018) 9163052. doi: 10.1155/2018/9163052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Veldhuis JD, Johnson ML, Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection, Am. J. Physiol.-Endocrinol. Metab 250 (1986) E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- [38].Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M, Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets, Pflüg. Arch 418 (1991) 417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- [39].Sanchez-Andres JV, Gomis A, Valdeolmillos M, The electrical activity of mouse pancreatic beta-cells recorded in vivo shows glucose-dependent oscillations, J Physiol 486 (Pt 1) (1995) 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS, Glucose Modulates [Ca2+]i Oscillations in Pancreatic Islets via Ionic and Glycolytic Mechanisms, Biophys. J 91 (2006) 2082–2096. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dyachok O, Idevall-Hagren O, Sågetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjärvi G, Gylfe E, Tengholm A, Glucose-Induced Cyclic AMP Oscillations Regulate Pulsatile Insulin Secretion, Cell Metab. 8 (2008) 26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- [42].Stozer A, Dolensek J, Rupnik MS, Glucose-stimulated calcium dynamics in islets of Langerhans in acute mouse pancreas tissue slices., PloS One. 8 (2013) e54638. doi: 10.1371/journal.pone.0054638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gylfe E, Hellman B, Glucose-stimulated sequestration of Ca2+ in clonal insulin-releasing cells. Evidence for an opposing effect of muscarinic-receptor activation., Biochem. J 233 (1986) 865–870. doi: 10.1042/bj2330865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grapengiesser E, Gylfe E, Hellman B, Glucose-induced oscillations of cytoplasmic Ca2+ in the pancreatic beta-cell, Biochem Biophys Res Commun 151 (1988) 1299–1304. [DOI] [PubMed] [Google Scholar]
- [45].Roe MW, Mertz RJ, Lancaster ME, W. JF 3rd, Dukes ID, Thapsigargin inhibits the glucose-induced decrease of intracellular Ca2+ in mouse islets of Langerhans, Am. J. Physiol 266 (1994) E852–62. [DOI] [PubMed] [Google Scholar]
- [46].Martin F, Soria B, Glucose-induced [Ca2+]i oscillations in single human pancreatic islets, Cell Calcium 20 (1996) 409–414. [DOI] [PubMed] [Google Scholar]
- [47].Nunemaker CS, Zhang M, Bertram R, et al. , Mouse beta-cells and islets differ in glucose responsiveness, in: Diabetes, San Diego, CA, 2005: pp. A407–A407. [Google Scholar]
- [48].Nunemaker CS, Satin LS, Comparison of metabolic oscillations from mouse pancreatic beta cells and islets, Endocrine. 25 (2004) 61–67. [DOI] [PubMed] [Google Scholar]
- [49].Tengholm A, Gylfe E, Oscillatory control of insulin secretion., Mol. Cell. Endocrinol 297 (2009). doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- [50].Bertram R, Satin LS, Sherman AS, Closing in on the Mechanisms of Pulsatile Insulin Secretion., Diabetes. 67 (2018) 351–359. doi: 10.2337/dbi17-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nadal A, Quesada I, Soria B, Homologous and heterologous asynchronicity between identified alpha-, beta- and delta-cells within intact islets of Langerhans in the mouse., J. Physiol 517 (Pt 1) (1999) 85–93. doi: 10.1111/j.1469-7793.1999.0085z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li J, Yu Q, Ahooghalandari P, Gribble FM, Reimann F, Tengholm A, Gylfe E, Submembrane ATP and Ca2+ kinetics in alpha-cells: unexpected signaling for glucagon secretion., FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 29 (2015) 3379–3388. doi: 10.1096/fj.14-265918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Soria B, Tudurí E, González A, Hmadcha A, Martin F, Nadal A, Quesada I, Pancreatic islet cells: a model for calcium-dependent peptide release, HFSP J 4 (2010) 52–60. doi: 10.2976/1.3364560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A, The unique cytoarchitecture of human pancreatic islets has implications for islet cell function, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].O’Neill CM, Lu C, Corbin KL, Sharma PR, Dula SB, Carter JD, Ramadan JW, Xin W, Lee JK, Nunemaker CS, Circulating Levels of IL-1B+IL-6 Cause ER Stress and Dysfunction in Islets From Prediabetic Male Mice, Endocrinology. 154 (2013) 3077–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ling Z, Wang Q, Stange G, In’t Veld P, Pipeleers D, Glibenclamide treatment recruits beta-cell subpopulation into elevated and sustained basal insulin synthetic activity., Diabetes. 55 (2006) 78–85. [PubMed] [Google Scholar]
- [57].Gutierrez GD, Gromada J, Sussel L, Heterogeneity of the Pancreatic Beta Cell., Front. Genet 8 (2017) 22. doi: 10.3389/fgene.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Corbin KL, Waters CD, Shaffer BK, Verrilli GM, Nunemaker CS, Islet Hypersensitivity to Glucose Is Associated With Disrupted Oscillations and Increased Impact of Proinflammatory Cytokines in Islets From Diabetes-Prone Male Mice, Endocrinology. 157 (2016) 1826–1838. doi: 10.1210/en.2015-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jahan I, Corbin KL, Bogart AM, Whitticar NB, Waters CD, Schildmeyer C, Vann NW, West HL, Law NC, Wiseman JS, Nunemaker CS, Reducing glucokinase activity restores endogenous pulsatility and enhances insulin secretion in islets from db/db mice, Endocrinology. (2018). doi: 10.1210/en.2018-00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Heimberg H, De Vos A, Vandercammen A, Van Schaftingen E, Pipeleers D, Schuit F, Heterogeneity in glucose sensitivity among pancreatic beta-cells is correlated to differences in glucose phosphorylation rather than glucose transport., EMBO J. 12 (1993) 2873–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang H, Iynedjian PB, Modulation of glucose responsiveness of insulinoma β-cells by graded overexpression of glucokinase, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 4372–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhou Y-P, Cockburn BN, Pugh W, Polonsky KS, Basal insulin hypersecretion in insulin-resistant Zucker diabetic and Zucker fatty rats: Role of enhanced fuel metabolism, Metab. - Clin. Exp 48 (1999) 857–864. doi: 10.1016/S0026-0495(99)90219-6. [DOI] [PubMed] [Google Scholar]
- [63].Calabrese A, Zhang M, Serre-Beinier V, Caton D, Mas C, Satin LS, Meda P, Connexin 36 controls synchronization of Ca2+ oscillations and insulin secretion in MIN6 cells, Diabetes. 52 (2003) 417–424. [DOI] [PubMed] [Google Scholar]
- [64].Cigliola V, Chellakudam V, Arabieter W, Meda P, Connexins and beta-cell functions, Diabetes Res. Clin. Pract 99 (2013) 250–259. [DOI] [PubMed] [Google Scholar]
- [65].Benninger RKP, Head WS, Zhang M, Satin LS, Piston DW, Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet., J. Physiol 589 (2011) 5453–5466. doi: 10.1113/jphysiol.2011.218909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Smolen P, Rinzel J, Sherman A, Why pancreatic islets burst but single beta cells do not. The heterogeneity hypothesis, Biophys J 64 (1993) 1668–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Soria B, Skoudy A, Martin F, From stem cells to beta cells: new strategies in cell therapy of diabetes mellitus., Diabetologia. 44 (2001) 407–415. doi: 10.1007/s001250051636. [DOI] [PubMed] [Google Scholar]
- [68].Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA, Generation of functional human pancreatic β cells in vitro, Cell. 159 (2014) 428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YHC, Johnson JD, Kieffer TJ, Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells, Nat. Biotechnol 32 (2014) 1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- [70].Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE, Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo, Nat. Biotechnol 26 (2008) 443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- [71].Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA, Generation of stem cell-derived β-cells from patients with type 1 diabetes, Nat. Commun 7 (2016) 11463. doi: 10.1038/ncomms11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Draznin B, Intracellular calcium, insulin secretion, and action., Am. J. Med 85 (1988) 44–58. doi: 10.1016/0002-9343(88)90397-x. [DOI] [PubMed] [Google Scholar]
- [73].Gilon P, Ravier MA, Jonas JC, Henquin JC, Control mechanisms of the oscillations of insulin secretion in vitro and in vivo, Diabetes. 51 Suppl 1 (2002) S144–51. [DOI] [PubMed] [Google Scholar]
- [74].Heart E, Corkey RF, Wikstrom JD, Shirihai OS, Corkey BE, Glucose-dependent increase in mitochondrial membrane potential, but not cytoplasmic calcium, correlates with insulin secretion in single islet cells, Am J Physiol Endocrinol Metab 290 (2006) E143–E148. [DOI] [PubMed] [Google Scholar]
- [75].Kenty JHR, Melton DA, Testing pancreatic islet function at the single cell level by calcium influx with associated marker expression., PloS One. 10 (2015) e0122044. doi: 10.1371/journal.pone.0122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wills QF, Boothe T, Asadi A, Ao Z, Warnock GL, Kieffer TJ, Johnson JD, Statistical approaches and software for clustering islet cell functional heterogeneity, Islets. 8 (2016) 48–56. doi: 10.1080/19382014.2016.1150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kolic J, Johnson JD, Specialized Hub Beta Cells Trade Maximal Insulin Production for Perfect Timing., Cell Metab 24 (2016) 371–373. doi: 10.1016/j.cmet.2016.08.022. [DOI] [PubMed] [Google Scholar]
- [78].Jonkers FC, Guiot Y, Rahier J, Henquin JC, Tolbutamide stimulation of pancreatic beta-cells involves both cell recruitment and increase in the individual Ca(2+) response, Br J Pharmacol 133 (2001) 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schuit FC, In’t Veld PA, Pipeleers DG, Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells., Proc. Natl. Acad. Sci. U. S. A 85 (1988) 3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Reissaus CA, Piston DW, Reestablishment of Glucose Inhibition of Glucagon Secretion in Small Pseudoislets., Diabetes. 66 (2017) 960–969. doi: 10.2337/db16-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]