Abstract
The advent of porous materials, in particular zeolitic nanoparticles, has opened up unprecedented putative research avenues in nanomedicine. Zeolites with intracrystal mesopores are low framework density aluminosilicates possessing a regular porous structure along with intricate channels. Their unique physiochemical as well as physiological parameters necessitate a comprehensive overview on their classifications, fabrication platforms, cellular/macromolecular interactions, and eventually their prospective biomedical applications through illustrating the challenges and opportunities in different integrative medical and pharmaceutical fields. More particularly, an update on recent advances in zeolite-accommodated drug delivery and the prevalent challenges regarding these molecular sieves is to be presented. In conclusion, strategies to accelerate the translation of these porous materials from bench to bedside along with common overlooked physiological and pharmacological factors of zeolite nanoparticles are discussed and debated. Furthermore, for zeolite nanoparticles, it is a matter of crucial importance, in terms of biosafety and nanotoxicology, to appreciate the zeolite-bio interface once the zeolite nanoparticles are exposed to the bio-macromolecules in biological media. We specifically shed light on interactions of zeolite nanoparticles with fibrinogen and amyloid beta which had been comprehensively investigated in our recent reports. Given the significance of zeolite nanoparticles’ interactions with serum or interstitial proteins conferring them new biological identity, the preliminary approaches for deeper understanding of administration, distribution, metabolism and excretion of zeolite nanoparticles are elucidated.
Keywords: zeolite, mesoporous, nanostructure, biosafety, biomedical applications
Introduction
Porous materials are promisingly sustainable solutions for some global concerns including rising energy demands along with the need for stricter environmental approvals for industrial contaminants, depleting resources, and improving health. The capacity of these porous materials, physically and chemically, is an attraction point due to demonstrating differential local atomic environment once exposed to solid versus bulk surfaces. Thus, a greater number of surface atoms, or in better words, an increased specific surface area of porous solids will lead to higher material reactivity and improved efficacy in relevant applications. Three strategies have been utilized for this purpose in order to aid size reduction of dense solids, to generate the bulk of the particles with an open pore structure, or to combine these two approaches by preparing nanoscale particles comprising accessible and homogeneous nanopores maximizing the fraction of atoms exposed to the surface.
According to the International Union of Pure and Applied Chemistry (IUPAC), the porous structures are sorted into three groups based on their pore diameter: microporous structures with pore diameters up to 2 nm, mesoporous materials containing pores in the range of 2 −50 nm, and macroporous solids with pores larger than 50 nm.1 Depending on their structural and compositional characteristics, each of the microporous and mesoporous classes is further divided into subfamilies of porous materials.
The size of particle itself may be another factor for classification. In general, particles with a size of up to 100 nm, in at least one dimension, are considered nanosized porous solids that may contain only the microporous and mesoporous pore ranges.2 Recently developed crystals with a size that does not exceed 15 nm are known as ultra-small NPs. Porous solids with uniform, periodic pore structures are referred to as ordered porous materials. Porous particles with periodic structures have increasingly gained attention because they can constitute putative tools in sensing, separation, storage, and transformation of small molecules.3 In particular, zeolites are broadly employed in different areas of industry pertaining to catalysis, adsorption, ion-exchange, etc.4 Due to the large collected number of publications on synthesis and application of zeolite NPs and their expanding outreach in biology and medicine, the goal of this review is to give a summary of recent biomedical applications of the different groups of zeolite porous nanomaterials.
Zeolites are a class of minerals with an ordered porous structure having a microporous pore range primarily found in nature. From the viewpoint of chemical composition, zeolites are composed of crystalline metal oxides whose building blocks consist of a tetrahedral atom (eg, Si, P, Al, Ti, B, Ga, Ge, Fe, etc.) bound to four or two oxygen atoms, where each oxygen connects to two tetrahedral atoms.4 The general chemical formula of these porous structures can be represented by the following formula:
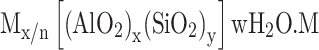 |
where “M” represents an alkali/alkaline earth cation with valence of n, “w” is the number of water molecules, and “x” and “y” are the molar concentrations of tetrahedra in the zeolite structure, of which the y/x ratio usually has a value of 1 to 5. However, this ratio can be increased to 100 in the siliceous zeolites.
Zeolite NPs possess conspicuous advantages in comparison with other nanostructured porous materials including: (1) low cytotoxicity which can limit the undesirable effects on normal cells/tissues, (2) adjustable, high payload capacity which is favorable for increasing the loading content of zeolite pores, and (3) improved intracellular targeting specificity and efficacy which benefits drug delivery applications.
The main advantages of applying NPs as compared to traditional therapeutics include at least three items: (1) decreased adverse effects of delivered drugs, (2) higher level of formulation versatility (eg, conjugation to drugs, antibodies, peptides, DNA, carbohydrates, etc.), and (3) destruction of inflammatory or tumoral cells thanks to their electrical, magnetic, or optical characteristics (eg, by the means of magnetic or optical hyperthermia) used to motivate an effective response.
The three-dimensional structure of natural zeolites, ie, those with aluminosilicate composition, encompasses pores and cavities that are captured by water molecules and alkali or alkali-earth metals. Despite the abundance of zeolites, their industrial application is limited due to the presence of impurities and chemical composition diversity in some natural zeolites. Accordingly, there have been comprehensive attempts to improve the characteristics of existing zeolite materials which have led to preparing novel types of zeolitic frameworks with compositions extended far beyond the restrictions enforced by the nature. Synthetic zeolite-type solids demonstrate a high degree of diversity in the Si/Al ratios as compared to natural minerals and contain other framework elements including but not limited to Zn, Ga, Ge, P, B, and Be. The non-classical (non-aluminosilicate) zeolitic frameworks are comprised of all-silica, galo-, alumino-, and transition metal phosphates along with gallium, zinc, and germanium silicates.5–8 These new structures/compositions, organic structure-directing agent(s) (SDA) are generally utilized to direct synthesis of new alumino-silicate zeolites by increasing the Si/Al ratio for preparing high- or pure-silica zeolites and to promote the synthesis of novel zeolitic framework forms from synthetic gels comprising framework atoms other than Si and Al.8–10 New framework types are regularly approved by the Crystallographic Commission of the International Zeolite Association, and as of January 2018, there are 235-approved zeolite framework forms.11
The approach in the research and design of zeolite solids in comparison with other classes of porous materials, such as metal-organic frameworks, has become even more application-driven.4 Based on a citation network analysis, low impact factor journals commonly publish articles related to evaluation of the acidic/basic properties and reaction mechanism of zeolites; whereas, research on biomass generation, nanosheet synthesis, and photo/electrocatalysts have been published by high impact factor journals.4 And this is understandable from the viewpoint of applications. Principally, zeolites are valuable materials for catalysis, and more sophisticated investigation fields are reviewed in literature.
It must be taken into consideration that the structure-property relation is important to be realized in the present situation. The widespread application of zeolites stems from their solid acid and ion-exchange efficiencies owing to their robust frameworks rooted in their inorganic-based structures. The diameter of micropores in zeolites is typically less than 0.7 nm, which is applicable for molecular sieving. Furthermore, incorporation of isolated metal atoms into the silicate frameworks and/or introduction of cations via ion-exchange display defining characteristics. Production of designable zeolites regarding the structural and physical characteristics will be a challengeable issue in the future.4
Herein, the present paper encompasses the following sections:
The first section briefly introduces biomaterials, biomedicine, and natural and synthetic mesoporous zeolitic structures along with their subclasses. The second section highlights and discusses the relevant approaches for fabrication of these porous materials. The third section presents some important biomedical applications of these promising nanoplatforms. And the fourth section covers the most recent advances of zeolite structures and the challenges encountered in the field of biomedicine.
High Throughput Methods for Fabrication of Zeolitic Porous Biomaterials with a Focus on Hydrogel-Based Systems
As noted in the introduction section, zeolitic materials are synthesized by various methods in five synthesis systems, ie, the conventional hydrogel system, the confined-space synthesis templates, microreactors, top-down approaches, and zeolite nanosheet synthesis systems.2 Scaling up the production of zeolites, however, depends on hydrogel-based systems comprising bulk solid and liquid phases, where the zeolite backbone is formed around charged templating species, ie, alkali-metal and cation-water complexes or organic molecules.12 The production of zeolites through hydrogel systems is based on a strategy, where the amorphous materials undergo chemical and structural reorganization until achieving a zeolite arrangement.13–16 Zeolite nuclei form in the solid portion of the system, while the zeolite framework propagates to the gel network until the crystallites are released from the solid matrix and proceed developing within the mother liquor. Thus, zeolite production through a hydrogel system can be considered to take place in three steps: (1) nucleation at gel-liquid interface, (2) growth and release of zeolite nuclei from gel matrix, and (3) growth within the mother liquor until completion to the ultimate, fully crystalline zeolite product.
Mass zeolite production in hydrogel compositions exhibits a considerable degree of heterogeneity. The heterogeneous virtue of zeolite nucleation process has been consensually approved, and there is typically an agreement that the nucleation of zeolite deviates from the classical crystallization process featuring crystal production from supersaturated solutions.17,18 This is probably one of the most intricate products of hydrothermal crystallization, where usually one-unit cell is formed from several hundred atoms. So, the control of zeolite expansion in such systems is more complicated. The high intricacy and heterogeneity of events in the solid and liquid phases of the system also create a critical, challengeable issue in monitoring the mechanism of zeolite production. However, a considerable advancement has been obtained in recent years, and there is a broad agreement over the fundamental events occurred during a zeolite nucleation process.19,20 For example, it has become obvious that the size of the precursor gel particles in the system is determined by alkaline hydroxide, eg, in case of producing FAU-type zeolites with industrial applications.
The diameter of the final zeolite materials can be regulated by careful and systematic control of the starting gel chemistry which facilitates formation of ultrasmall-, nanometer-, or micrometer-sized zeolite solids with uniform particle size distribution. Closed systems are used in order to synthesize zeolite crystals, where the components of the initial gel react together, so that this reaction ultimately leads to nucleation and further growth of the kinetically most favorable phase. Under these circumstances, controlling the zeolite growth permits regulating the final-crystal size. Simply put, there is a restriction in the nutrient resources, and the growth of the solid terminates after the exhaustion of building components. There is a reverse relationship between nucleation process and crystal size, so that increasing the number of nuclei causes a decrease in the ultimate crystallite size.21 Therefore, accurate control of the zeolite nucleation events and simultaneous growth of crystals is needed for regulation of the zeolite particle scale. The latter is especially critical when the purpose is production of nanosized particles with narrow size distribution, in which case the product homogeneity arises from the temporal and spatial uniformity of the nucleation process.
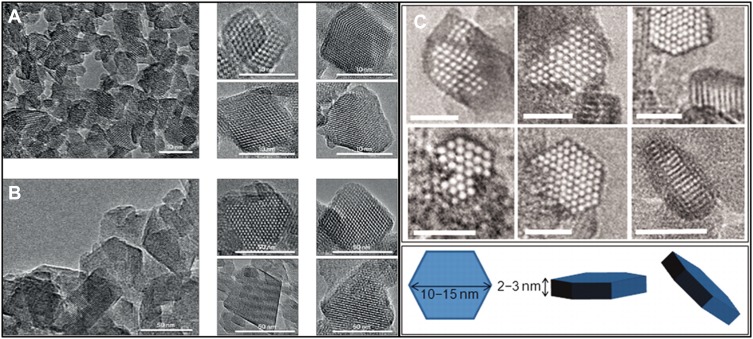
Different research teams have focused on FAU-type zeolite materials because of the enormous influence of these zeolite types on the petrochemical industry. In the 1990s, FAU-type nanocrystals were produced, by utilizing huge quantities of tetramethylammonium hydroxide and 15-crown-5 ether, and considerably reduced Na2O amount in the initial system.22,23 This was followed by several attempts to increase the production yield.24,25 Valtchev and Bozhilov illustrated the possibility of synthesizing FAU-type nanomaterials through an organic-template-free system.18 Hu et al produced submicrometer-sized zeolite Y (a subfamily of FAU-type) using organic-template-free hydrogels,26 and the procedure was optimized by Kim et al.27 In 2012, the production of template-free nanosized EMT-type zeolites was reported by Ng et al. After, in 2015, the fabrication of FAU-type zeolites was reported by Awala et al. These zeolite materials possessed extraordinary properties, such as high-efficiency crystalline (above 80%), micropore volumes (0.30 cm3 g−1) comparable to their conventional counterparts (micrometer-sized crystals), very small particles (10–15 nm) with narrow size distribution, extraordinary thermal stability, and Si/Al ratios that were modifiable between 1.1 and 2.1 values (zeolites X or Y) (Figure 1).28,29
Figure 1.
TEM images of FAU-type Y-10 (9 nm) (A) and Y-70 (38 nm) (B) nanosized zeolites. The corresponding high-magnification image of a single nanocrystal is shown as insets. Ultrasmall EMT-type zeolite was synthesized by Mintova group from template-free precursor suspension at 30°C for 36 hrs (C)28,29. (A) Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature; Nature Materials; Template-free nanosized faujasite-type zeolites. Awala H, Gilson J-P, Retoux R, et al. 2015;14(4):447–451. Copyright 2015.28 (B)From Ng E-P, Chateigner D, Bein T, et al. Capturing ultrasmall EMT zeolite from template-free systems. Science. 2012;335(6064):70–73. Reprinted with permission from AAAS. Copyright 2012 The American Association for the Advancement of Science.29
Ultrasmall EMT-type material, another solid with a significant capacity in catalytic and separation reactions, was obtained through an organic-template-free system.29 This nanomaterial was stabilized at nearly ambient temperature conditions under which ultrasmall nanocrystals (7–15 nm) were assembled and then were enlarged to 50–70 nm under-prolonged crystallization. In addition to the very tiny size, the uniform particle size distribution in a sodium-rich environment and low production cost are great achievements that make this synthesis technique unique.
Besides FAU and EMT, zeolite types of BEA, LTI, MFI, and MOR are some of the other industrially most important framework types.2 In biological and medical fields, the use of zeolites dates back to the early 1990s, when the capability of FAU (X, Y), LTA (Linde Type A, zeolite A), and MOR frameworks to be applied as magnetic resonance imaging (MRI) contrast agents and/or bactericidal agents30,31 along with their potential for dental or pharmacological applications32,33 were realized. Since then, advances in synthesis of different zeolitic frameworks have progressively paved the way for expanding their exploitation in biomedical research and industry sectors. The most applied zeolite types in this area are FAU, EMT, clinoptilolite, BEA, FER, LTA, LTL (Linde Type L, zeolite L), MFI, MOR, and ZSM, whose applications are reviewed in the following sections of this review.
Biosafety and Cytotoxicity
The earliest time when the natural zeolite NPs were administered to the human bodies dates back to the late 1920s, when a series of unprecedented translational and clinical experiments authenticated by the Cuban Quality Control Agency were accomplished. This huge and long-lasting research, which include the basic in vitro experiments followed by the translational and clinical trials including acute diarrheic participants whose gastrointestinal (GI) track is infected by food intoxication, was set out to investigate the safety and pharmacological efficacy of the Entrex natural zeolite for diarrheic conditions and their proposed mechanism of action. Further insights on the underlying mechanism of action for these natural zeolites are given in the 4.3. section which overviews zeolites applicability in anti-diarrhea drugs.
Regarding the diverse fabrication methods of zeolitic NPs’ platforms in the realm of biomaterial engineering, as well as the number of scientific publications built upon biocompatibility of zeolite NPs, it is worthwhile to appreciate the cytotoxicity and biocompatibility assessment methods that constitute crucial steps prior to any clinical interventions. Despite the recent advances made in fabrication methods, functionalization, and biomedical applications of zeolitic NPs, the exact mechanism of their toxicity is still vague. Regarding their benefits in various biomedical applications, it seems necessary to assess the cellular toxicity of these molecular sieves. In comparison with other mesoporous NPs, there are a poor number of studies reporting physiological toxicity of zeolitic NPs. Specifically, it seems that the biosafety of these porous materials is influenced by some of the structural features including but not limited to external surface area, pore size, surface charge, functional groups, and crystallinity. In the recent literature, some researchers have raised concerns regarding the cytotoxicity effects of zeolitic NPs on a multitude of yet understudied cell lines. To this end, a series of 150-diverse nanozeolites with different shapes, sizes, dosages, and surface compositions were synthesized, and through an in vitro investigation, their dose/time-dependent/independent cytotoxicity against human cervix carcinoma (HeLa) cells were quantitatively and qualitatively evaluated via exploring the cellular death by either a necrosis or apoptosis pathway. The main conclusion of their experiments underlined the nontoxicity of the “pure silica nanozeolite silicalite-1” with spherical morphology, while the toxicity of other alumina containing nanozeolites was non-linearly associated with the alumina content. In addition, it was gathered that nanozeolites with a cubic morphology were more toxic for HeLa cells as compared to those with a spherical morphology.34
The important parameters for determining the toxicity of nanosized zeolites have been disclosed as particle scale and crystalline structure. A conclusive priority for cellular necrosis was exhibited over a programmed cell death via apoptotic processes for the materials with various scales and morphologies.35
In a study reported in 2011, contrary to the purely siliceous zeolite, the nanosized zeolites comprising aluminum and silicalite groups demonstrated a noticeable dose-dependent toxicity. Variations in morphology was also determined to have a profound influence on zeolites toxicity. Additionally, it was proposed that the differences in surface charge of the zeolites could slightly impact their toxicity. Kihara et al evaluated the cytotoxicities of nanoscaled zeolites including MFI (both ZSM-5 and silicalite-1), LTA and LTL types against the cell lines of HeLa, human embryonic kidney 293 (HEK-293), and RAW264.7 macrophages. Results demonstrated that the toxicities of zeolite nanomaterials depended on their Si/Al ratio, size (30–500 nm), and morphology. Pure-silica zeolite with MFI-type structure (silicalite-1) showed no toxicity; however, the other three nanoscale zeolites comprising aluminum group exhibited a dose-dependent toxicity (Figure 2).34
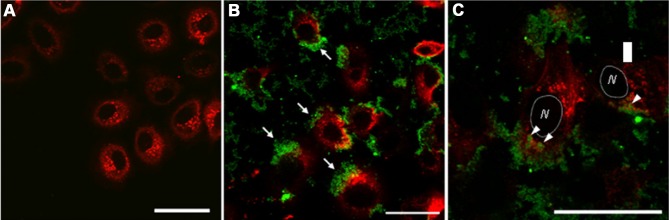
Figure 2.
Confocal microscopy photos of HeLa cells treated with nanozeolite LTL. HeLa cells (A) and HeLa cells treated with 10 μg.mL−1 of green fluorescent protein (GFP) adsorbed nanozeolite LTL-90 (B, C). GFP adsorbed nanozeolite attached to the surface of cultured cells (B, arrows) and thimbleful adhered nanozeolites were internalized into the cells (C, arrowheads). Green color shows GFP adsorbed nanozeolite, and red color exhibits actin filaments of cells. The cell nucleus part (N) has been lined via a circle (C). Scale bar is 50 μm.Reprinted from Kihara T, Zhang Y, Hu Y, et al. Effect of composition, morphology and size of nanozeolite on its in vitro cytotoxicity. J Biosci Bioeng. 2011;111(6):725–730. Copyright 2011, with permission from Elsevier.34
In a study done in 2013, HeLa cells were employed to assess the possible toxicity of zeolite materials against tumor cells in vitro. It was revealed that coatings of different charges led to different outcomes regarding serum protein adsorption, toxicity, agglomeration in media, and cell internalization. Moreover, it was shown that the disc-shaped zeolite L crystals could penetrate the tumor cells through multiple pathways.36
It has been reported that functionalization of zeolites could demonstrate different cytotoxicity levels depending on the presence of aluminum, and amine modification of zeolites could significantly increase their cytotoxicity.5 Regarding the serious impacts of free radicals on human body, the antioxidant and pro-oxidant properties of well-dispersed nanozeolites were measured. TS-1 zeolite (Ti-containing MFI-type zeolite) displayed a significantly comparable or even more antioxidant capacity in comparison with cerium oxide. Also, it was concluded that the cytotoxicity of zeolites depended on type of the cell line utilized in the research emphasizing that in vitro investigations must employ representative methodologies to achieve reliable data.37
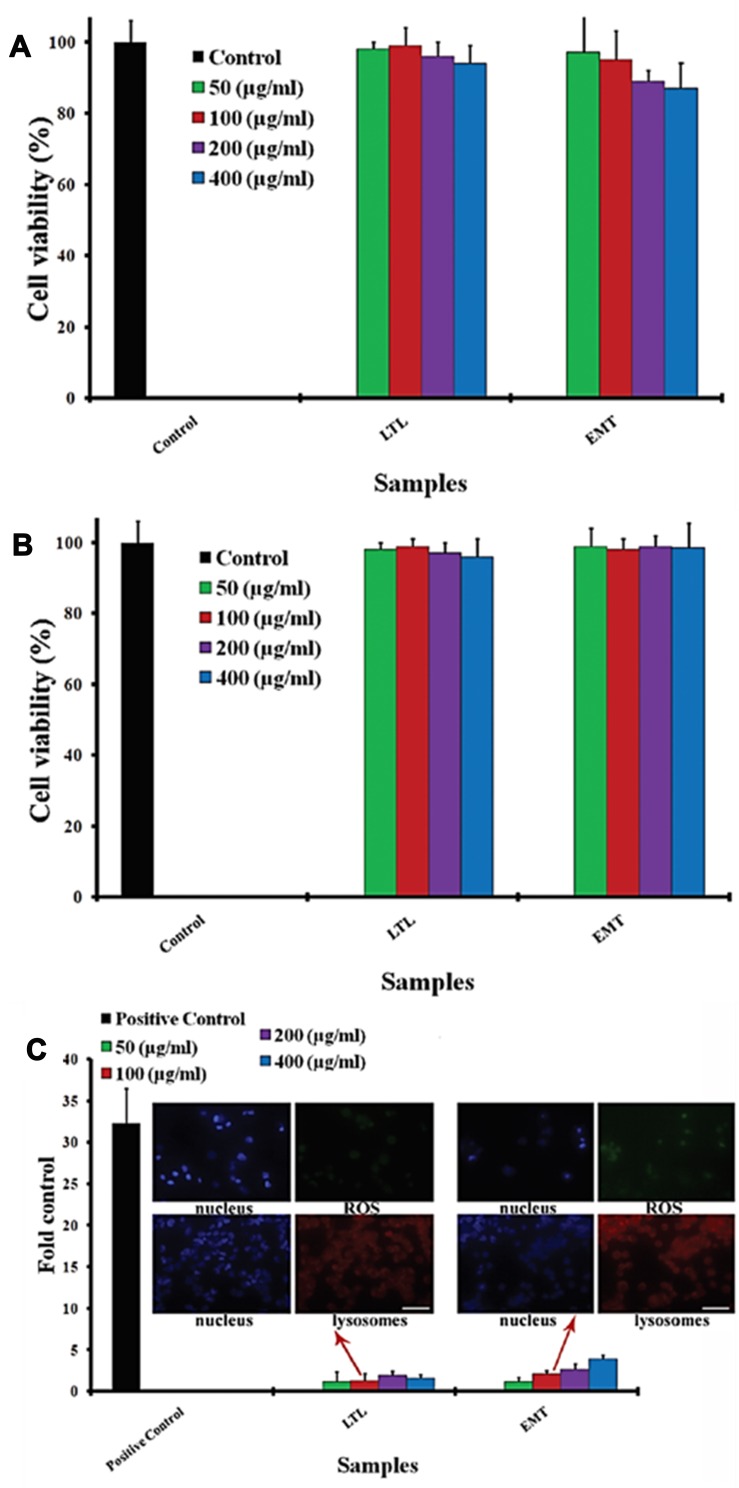
In our previous research, we investigated the toxicity of both LTL and EMT types of zeolites, categorized in the common class of molecular sieves, against HeLa cells. The HeLa cell interaction with both types of zeolite NPs was assessed by cell viability, reactive oxygen species (ROS), and cell cycle assays. It was perceived that various doses of nanozeolites possessed trivial influence on the cell cycle. Furthermore, no detectable oxidative stress was induced in the presence of zeolite nanocrystals in the cells. Despite the significant cellular uptake of zeolites, none of the employed ultra-small EMT and LTL zeolites, at any concentrations, induced cytotoxicity (Figure 3).38
Figure 3.
(A) HeLa cell viabilities before and after incubation with diverse concentrations of zeolites. (B) HeLa cell viabilities before and after incubation with diverse concentrations of surface saturated zeolites. (C) ROS generation for zeolites at different concentrations (ie 50–400 μg.mL−1) on HeLa cells after 6 hrs’ incubation; confocal photos (scale bars are 50 μm) indicate the induced lysosomes (the nucleus and lysosomes are shown as blue and red fluorescence, respectively) and induced ROS level (the nucleus and ROS level are shown as blue and green fluorescence, respectively) gained by the incubation of HeLa cells with zeolites (concentration of 100 μg.mL Republished with permission of Royal Society of Chemistry, from Laurent S, Ng E-P, Thirifays C, et al. Corona protein composition and cytotoxicity evaluation of ultra-small zeolites synthesized from template free precursor suspensions. Toxicol Res (Camb). 2013;2(4):270–279. Copyright 2013; permission conveyed through Copyright Clearance Center, Inc.38
By the intervention of synthesis methods, the concern around cytotoxicity has shifted towards nanoscale materials. Microscale zeolites have been demonstrated to be safe and non-toxic; whereas, zeolite NPs have not yet been completely scrutinized regarding their cytotoxicity.39
Biomedical Application of Zeolitic Materials
Given the unique structure and favorable physiochemical advantages over other mesoporous nanomaterials, such as lower cytotoxicity, higher payload capacity, and improved intracellular targeting specificity and efficacy, zeolite NPs are recognized for their improved biocompatibility as well as adjusted biomolecular delivery capacity.
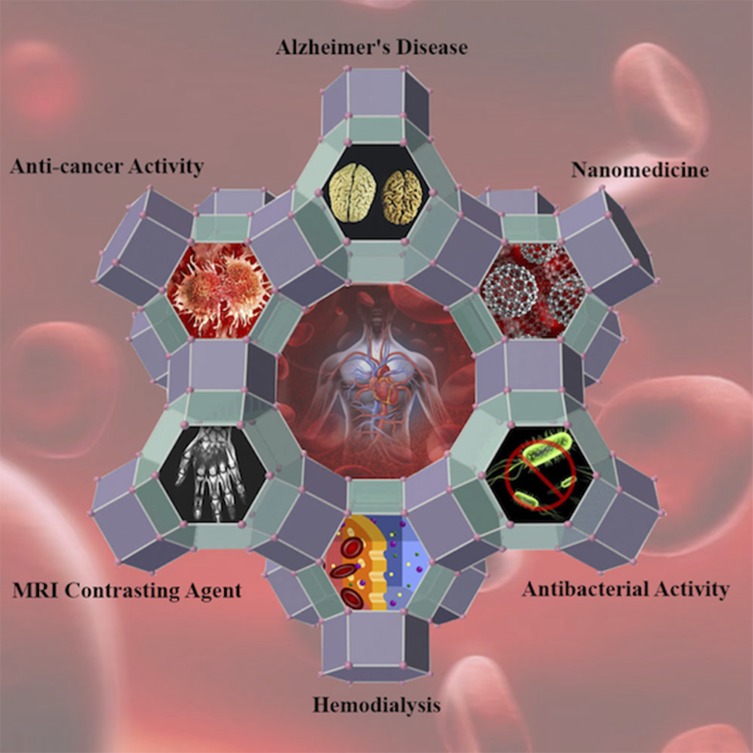
Zeolites encompass a broad range of biomedical applications, eg, utilization as antidiarrheal agents, antitumor adjuvants, antibacterial agents, MRI contrast agents, their employment in studies on bone formation, Alzheimer’s disease development, and their hemodialytic, drug delivery, and dental applications (Scheme 1).
Scheme 1.
Schematic diagram of zeolite’s biomedical applications.
Zeolite and Bone Formation
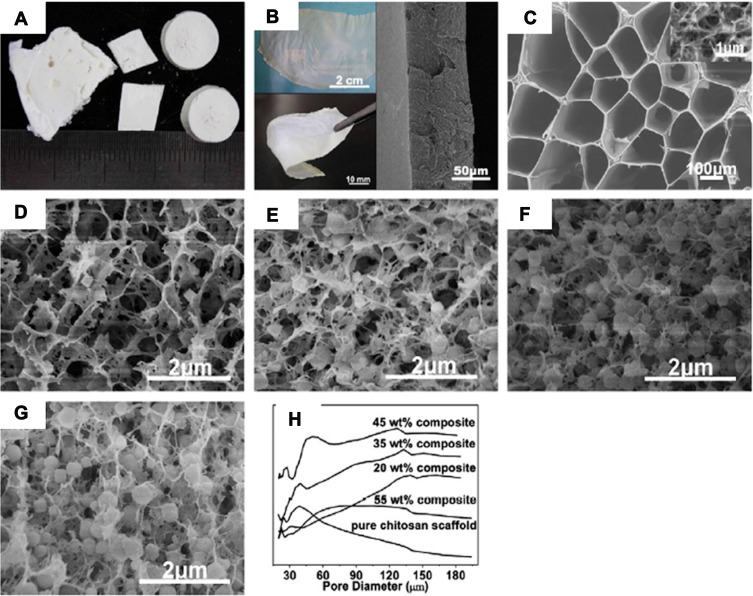
Osteoblast proliferation and differentiation are considered as significant factors that must be taken into account during the bone regeneration investigations in implants.40 In order to replacing conventional materials, zeolite crystals can be utilized as implants because their 3D microstructures form a great network of sub-nanometer pores, and their special topography turns them into an appropriate substance for bone cell attachment, proliferation, and expansion.40–42 For instance, the application of a new composite containing zeolite–hydroxyapatite, coated on stainless steel and titanium alloys in regenerative medicine, has been proposed.43 The zeolite–hydroxyapatite composite is super-hydrophilic, and the zeolite coating removes the elastic modulus mismatch between the coating and the bone. Therefore, it may benefit a quicker post-surgery recovery. Because of the ability of zeolite A to stimulate bone formation, it possesses therapeutic advantages for individuals experiencing osteoporosis.33 A significant enhancement in the proliferation, differentiation, and generation of transforming growth factor h (TGF h) has been detected by applying the zeolite A to the normal, adult human osteoblast-like cells in vitro. In addition, the DNA synthesis in these cells has raised dependently to the applied dose of zeolite A. Moreover, zeolite A has been able to increase the release of osteocalcin and the activity of alkaline phosphatase enzyme. Also, the steady-state mRNA levels of TGF h1 have increased through the treatment with zeolite A.44 The suppressive impact of zeolite A on bone-resorption activity has been discovered in the highly purified avian osteoclasts by Schütze et al.45 However, zeolite MFI coatings on titanium alloys seem to promote attachment of osteoblasts and induction of osteointegration. Moreover, MFI coating enhances the osteoinductive characteristics in comparison with bare titanium.40 Regarding the importance of antimicrobial activity in the tissue engineering framework, the zeolite-A/chitosan hybrid materials also nullify the immunological infection after operation, on top of their osteoinductive properties previously mentioned (Figure 4).46
Figure 4.
(A) Photographs of zeolite-A/chitosan hybrid composites. (B) Photograph exhibiting the transparency (up left) and flexibility (left down) of the zeolite-A/chitosan hybrid film and SEM image of the cross-section of the film (right). (C) The internal architecture of the pure chitosan scaffold with diverse magnifications. The internal microstructure of zeolite-A/chitosan hybrid composites with diverse zeolite percentages of 20 wt.% (D), 35 wt.% (E), 45 wt.% (F) and 55 wt.% (G). (H) The pore size distribution diagram of the pure chitosan scaffold and zeolite-A/chitosan hybrid composites with diverse zeolite percentages. Reprinted from Yu L, Gong J, Zeng C, et al. Preparation of zeolite-A/chitosan hybrid composites and their bioactivities and antimicrobial activities. Mater Sci Eng C. 2013;33(7):3652–3660. Copyright 2013, with permission from Elsevier.46
Zeolite and Hemodialysis
A significant challenge in improving a genuinely portable, regenerable hemodialysis system is the ammonia elimination from a recirculating dialysate flow.47 Due to the impurity removal features of zeolites, they are employed as a filter medium throughout hemodialysis instead of conventional polymer membranes for elimination of uremic toxins using the adsorption reaction.47–49 Some zeolites including clinoptilolite, type F, and type W possess an excellent potential for hemodialysis application in ammonia ion–exchange systems. These systems can be controlled by washing the column with a 2 M sodium chloride solution after each ion-exchange run. Also, based on the results of atomic absorption spectroscopy of the column eluent, no detectable Si or Al leaches from the zeolite.47 Furthermore, a lot of effort have been placed on improvement of the adsorption efficiency of zeolites versus toxins in various media.50–52 Besides, in patients undergoing dialysis with chronic renal failure, the ability of zeolite (FAU13× and FERCP914C) to reduce ROS production in extracorporal circuits was suggested as valuable as antioxidant supplementation (ie, vitamin E) leading to a lower mortality.53
Zeolite and GI Tract Illnesses
Some natural zeolites had been initially found as effective antidiarrheal effectors and stepped into the realm of clinical trials complying with the Cuban Drug Quality Agency standards. Based on the results of several physiochemical, microbiological, translational, and clinical experiments, purified natural clinoptilolite-Enterex has been introduced as a new antidiarrheal drug. Despite the initial hypothesis, where the anti-diarrheic effects of zeolites were ascribed to its bactericidal activity and/or ability to shortening the intestinal transit ingest similarly to that of the effects observed in the animal GI tract, no significant antibacterial activity and reduction in intestinal transit ingest was observed. The authors of the paper proposed a pharmacological mechanism for the efficacy of natural zeolites against diarrhea mediated by adsorbing of bile acid, aflatoxin B1, and glucose.54 Nevertheless, the anti-diarrheic essence of the natural zeolites (clinoptilolite tuff particles) was recently reviewed from pharmacodynamic and pharmacokinetic standpoints, where the M-cell intestinal epithelium cells accommodate the internalization, lumen-to-apical transportation, and then exposing clinoptilolite tuff particles to immunological cells residing in the Peyer’s patch. The Peyer’s patch hosts a multitude of T cells, macrophages, and IgA secreting B and plasma cells. These cells are activated once they contact the transported clinoptilolite tuff particles on the apical surface and consequently elevate their products including IgA and anti-microbe peptides on the luminal mucosa of the intestine. The IgA and anti-microbial peptides in the mucosa encompass a physical and chemical barrier preventing the gut microbiota from a direct contact with the intestinal lumen membrane, and from this point of view, the oral administration of natural safe zeolites can ameliorate the diarrhea’s symptoms indirectly.55,56 In addition, in a randomized, double-blind, placebo-controlled, pilot clinical trial including 23 participants, the pharmacological capacity and activity of the Absorbatox™, as a potentiated clinoptilolite, against NSAID-induced gastric mucosal erosion severity was assessed. The patients who received 1500 mg of Absorbatox orally three times a day were compared to the placebo control patients and demonstrated to be leveraged with more gastroprotection through a suggested – but unstudied – mechanism, wherein the binding to hydrogen ions and biologically active amines is the main deriver of this pharmacological property.57
Zeolite and Contrast in MRI
MRI is recognized as a method that produces an image of the target tissue in the body by detecting the nuclear-spin reorientations in an applied magnetic field.58 Possessing low contrast is known as a challenge in MRI technique. Therefore, contrast agents are administered in high doses to improve the image contrast.59 All nano-sized porous materials have demonstrated the potential to be applied as MRI contrast agents,2 so a bright future has been pictured for zeolites to improve the quality of MRI images. Zeolites encompass high-spin metals capable of binding to water molecules giving rise to producing significantly faster proton spin relaxation times.60 Besides, the accumulation of zeolites at specific targets is desirable for MRI application, which is possible either by adding biologically particular portions to the surface of zeolites by trialkoxysilane condensation process or functionalizing zeolites by molecular targeting vectors, such as aptamers and peptides that possess a highly specific binding affinity to cell surface receptors.61
Although gadolinium ions (Gd3+) are considered as suitable MRI contrast agents, they cannot be administered directly because of their intrinsic toxicity.62 However, Gd3+ loaded zeolites31,63 have been examined as MRI contrast agents for application in the digestive system. Moreover, FAU-type zeolite materials appear to be perfect carriers for Gd3+ ions owing to their ion-exchange capability.61 After several safety and efficacy evaluations, microparticulate Gd3+ loaded zeolite NaY has been verified as an oral contrast agent.64 Based on clinical Phase II/III multicenter research, it is concluded that the oral forms of gadolite are considerably efficient, well tolerated, and safe gastrointestinal contrast agents that can be used for clinical MRI applications.65 It is noteworthy that no gadolinium was observed in urine or blood samples, and no remarkable side effects were mentioned according to clinical trials’ reports.66
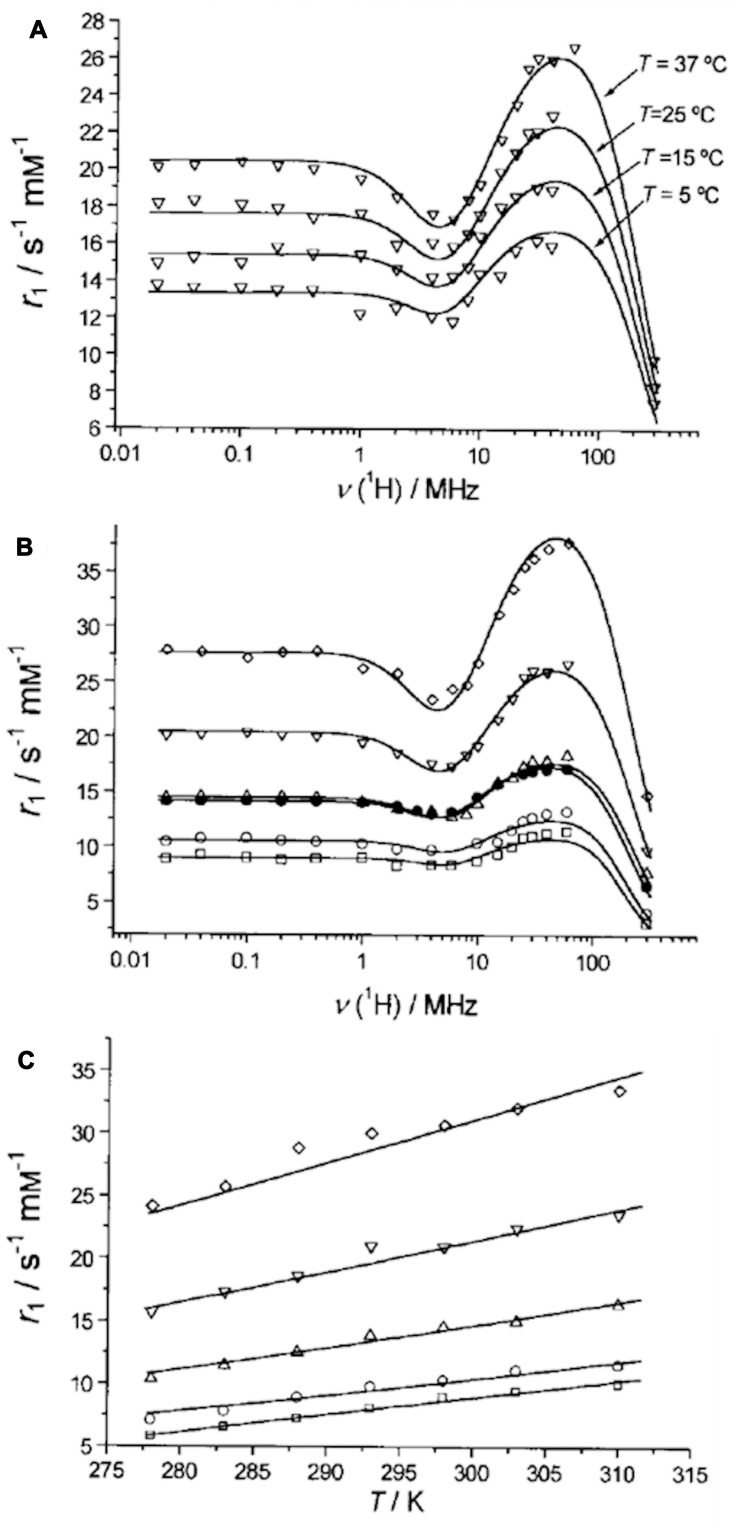
Recently, the application of Gd3+ loaded NaY NPs as an MRI contrast agent has been reported (Figure 5).67 Gd3+ loaded NaY NPs possess a desirably higher relaxivity as compared to the micrometer-sized zeolite.68 Additionally, since a more efficient water exchange rate through the pores leads to higher relaxivity of porous materials, nanozeolites with large cavities are desired for utilization as MRI contrast agents.69 In addition to Gd3+ loaded zeolites, Ln3+ loaded zeolites are also considered as promising MRI contrast agents that have been studied by Peters and Djanashvili.70
Figure 5.
(A) The magnetic field dependence of longitudinal proton relaxation (NMRD profiles) of GdNaY-2.3 recorded at different temperatures. (B) NMRD profiles at different Gd3+ loadings for the GdNaY samples evaluated at 37 ºC. (C) Temperature dependence of the proton relaxivity at 20 MHz: GdNaY-1.3 ( ), GdNaY-2.3 (
), GdNaY-2.3 ( ), GdNaY-3.6 (
), GdNaY-3.6 ( ), GdNaY-5.0 (
), GdNaY-5.0 ( ) and GdNaY-5.4 (
) and GdNaY-5.4 ( ) and La-2.8-GdNaY-3.3 (
) and La-2.8-GdNaY-3.3 ( ). Reprinted with permission from Platas-Iglesias C, Vander Elst L, Zhou W, et al. Zeolite GdNaY nanoparticles with very high relaxivity for application as contrast agents in magnetic resonance imaging. Chem Eur J. 2002;8(22):5121–5131. Copyright 2002, John Wiley and Sons.67
). Reprinted with permission from Platas-Iglesias C, Vander Elst L, Zhou W, et al. Zeolite GdNaY nanoparticles with very high relaxivity for application as contrast agents in magnetic resonance imaging. Chem Eur J. 2002;8(22):5121–5131. Copyright 2002, John Wiley and Sons.67
Zeolite and Antitumor Adjuvants
Regarding the regulatory role of zeolites in the immune system, they hold a promising application as antitumor adjuvants.71 Recently, the natural clinoptilolite zeolite has been considered as a useful adjuvant in cancer treatment.72 Tumor size reduction, survival time enhancement, and overall health improvement have been reported during the oral administration of natural clinoptilolite to mice and dogs having various types of tumors. Based on in vitro studies, the fine powder of clinoptilolite provokes the expression of p21WAF1/CIP1 and p27KIP1 tumor suppressor proteins. Furthermore, it suppresses the protein kinase B (c-Akt) and thus blocks cancer cell growth.73 Besides, results of in vivo studies on TMA-zeolite orally administered to mice and dogs and in vitro studies of its effect on tissue culture, accomplished by another research group, endorse the results obtained for clinoptilolite zeolite.74 In addition, administration of activated TMA-zeolite to cancer and diabetic patients leads to the reduction of oxidative stress which is associated with the general-health improvement.75
Furthermore, micronized zeolite clinoptilolite has been employed as an adjuvant for doxorubicin, a conventional chemotherapy medicine. This combined orally-administered treatment gently improved the efficacy of doxorubicin as an anticancer drug, and it also decreased the amount of cancer cells metastasized to the lungs in cancer-bearing mice and dogs.76
Zeolite and Antibacterial Agents
Compounds offering antibacterial activity eradicate bacteria (bactericides) or impede their growth (bacteriostatic) without possessing toxicity for the nearby tissues. The most well-known antibacterial agents are modified versions of natural compounds. Regarding the evolutionary concept of emerging inheritable bacterial resistance to conventionally prescribed organic antibacterial agents, medical pathologists are trying to find new strategies to overcome the emergence of multi-drug resistant bacteria. Among them, nanoscale solids, due to their high surface area to volume ratio and less toxicity, are recognized as effective antibacterial agents.77–79 Besides, inorganic antibacterial materials not only do offer more thermal and chemical stability during nanocomposite preparation, but also possess more flexible structures in alloys, nanocomposites, and coatings.80 Silver (Ag) has been highlighted among other inorganic metals due to introducing the most well-established antibacterial properties. To guarantee the long-term antibacterial activity of Ag NPs, they were incorporated to zeolite media,79,81–90 as zeolite particles could hold silver ions and protect their bacteriostatic properties which lie on their ion-exchangeable sites, and this depends on composition of the selected zeolite, eg, A zeolite, X zeolite, Y zeolite, etc.30 Based on a research by Dong et al on the antibacterial properties of Ag-containing nanozeolites, E. coli cells were immediately destroyed upon exposure to water forms of ultra-small EMT-type zeolite containing Ag0 or Ag+ ion.88
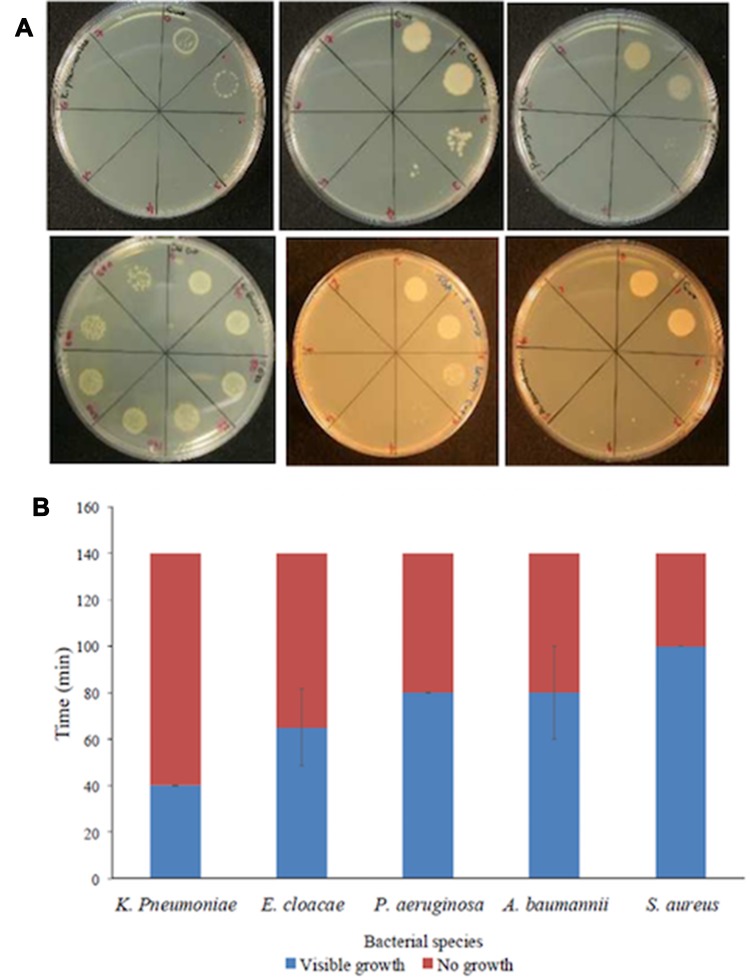
In addition to silver, the antibacterial property of copper (Cu) was also enhanced by its introduction to the zeolite immobilized in a solid matrix. Controlled release of Cu ions during a period of time and adjustable regulation of Cu ion concentration in the system were accounted as advantages of employing Cu-FAU zeolite. Cu-FAU zeolite has demonstrated antibacterial activity, especially against gram-negative bacteria, eg, Pseudomonas aeruginosa, Acinetobacter baumanii, and Klebsiella pneumoniae. Furthermore, applying the colloidal forms of Cu-FAU on surfaces of healthcare settings was suggested as an alternative for antibiotic treatment against ESKAPE pathogens including Acinetobacter baumanii, Klebsiella pneumoniae, Staphylococcus aurous, Pseudomonas aeruginosa, Enterococcus species, and Enterobacter species (Figure 6).91
Figure 6.
(A) Spot inoculation of ESKAPE microorganisms following treatment with the Cu-FAU suspension. Every drawn part on the plates above corresponds to 20-min sampling time (40 mins for E. faecalis); total sampling time 0–140 mins (0–280 mins for E. faecalis). Microorganisms (clockwise): K. pneumoniae, E. cloacae, P. aeruginosa, A. baumannii, S. aureus and E. faecalis. (B) Summary of the average killing times calculated in the semi-quantitative assays. Reprinted from Redfern J, Goldyn K, Verran J, et al. Application of Cu-FAU nanozeolites for decontamination of surfaces soiled with the ESKAPE pathogens. Microporous Mesoporous Mater. 2017;253:233–238. Copyright 2017, with permission from Elsevier.91
Zeolite and Dental Application
Dental application of zeolite polymer for orthodontic purposes was achieved by using inorganic agents, such as zeolite NPs, for continued release of the active ingredient, eg, silver, into the mouth cavity over a long time. Due to long-lasting effects of zeolites in the mouth environment, even in a prolonged contact with saliva, they were recognized as the most effective delivery platform for antimicrobial agents.92 Furthermore, the inclusion of zeolite into the cement dental composite ingredients was performed, and as a result, zeolite increased the resistance of the composite material against acidic environments bred by inflamed dental tissues and also against alkali-aggregate expansion caused by thermal treatment.93 For root-end fillings, various substances were utilized including amalgam, resin-modified glass ionomers, glass ionomer cement (GIC), and mineral trioxide aggregate (MTA).94–97
The in vitro antibacterial activity of two-experimental GICs, recommended for endodontic applications,32,98,99 combined with silver zeolite (SZ) at 0.2% and 2% mass fraction concentrations was reported on Streptococcus milleri, Staphylococcus aureus, and Enterococcus faecalis by carrying out the agar diffusion inhibitory test. It was concluded that adding SZ increased the antibacterial effects in glass ionomer cement proportionally to its concentration.100
Besides, adding a small amount of SZ composition into MTA was reported to possess an inhibitory effect on Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, etc. The purpose of the research was to assess whether adding SZ to MTA would improve the antibacterial effect of MTA, and it was concluded that SZ had the potential to enhance MTA’s antibacterial virtue.101
Zeolite and Excipient Functionality
Undergoing a significant evolution, the traditional basics of excipients changed from chemically simple and pharmacologically inert vehicles to a vital adjuvant with the aim of assuring and optimizing the efficiency of the modern medicinal material. Previously, the attention of the pharmaceutical industry and Regulatory Authorities was almost addressed to control the property of the active components rather than excipients, but due to the rapid progression of economic, scientific, technological, and regulatory factors, a considerable amount of attention has been focused on physical features of excipients, as well as their role in drug formulation in order to release the active component in a controlled manner.102
The functionality of a natural zeolite modified with cationic surfactants was investigated as a drug formulation excipient. Profiles related to in vitro drug release from these composites suggested that drug release maintenance was achievable over 8 hrs. Additionally, given the outcomes of drug uptake and drug release analysis, it was revealed that the zeolite compositions were capable of being used as advanced excipients in drug formulations.103
The extended release of diclofenac sodium (DS) from three natural zeolites (NZ) with cetylpyridinium chloride (CPC) composites and also from a physical mixture comprising ZCPC-10 and DS was attained over 8 hrs. The kinetic analysis verified that the drug release profiles almost fitted the Korsmeyer-Peppas and Bhaskar release models emphasizing a combination of drug diffusion and ion exchange as the principal release mechanisms in the dissolution medium.104
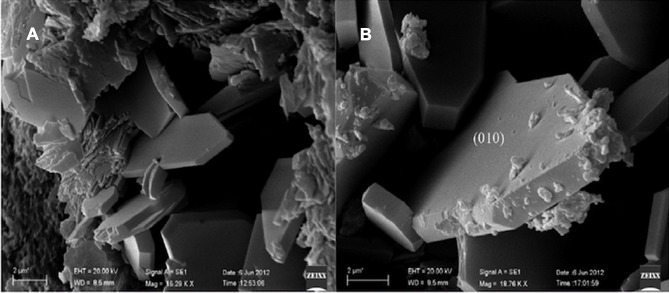
Recently, to expand natural zeolites based on pharmaceuticals for oral administration of drugs, a study initiated by the “Italian Ministry for Education, University and Research” has proposed to consider the 90 wt.% of Na-clinoptilolite as a drug excipient. Also, based on the recommended experimental protocols by European, US, and Japanese Pharmacopoeias performed for “bentonite” as the most available similar reference for Na-clinoptilolite, bentonite has been evaluated as comparable to marketed ingestible drugs containing clinoptilolite (Figure 7).105
Figure 7.
SEM observation of clinoptilolite crystals in the bulk rock (A, B). Zeolites of the clinoptilolite series demonstrate a thorough cleavage parallel to the (010) plane. The particle size might be important to correlate the intensity of intestinal irritation and inflammation of the different dimension of the administered clinoptilolite-rich powder. Reprinted from Cerri G, Farina M, Brundu A, et al. Natural zeolites for pharmaceutical formulations: preparation and evaluation of a clinoptilolite-based material. Microporous Mesoporous Mater. 2016;2231335 (Supplement C):58–67. Copyright 2016, with permission from Elsevier.105
Zeolite and Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disorder that is progressive with an approximate prevalence rate of 5 million new patients per year, and 33 million patients are now suffering from it. Prevalence of AD correlates with ageing, so that 0.6–8% of adults between 60 and 85 years old are exposed to AD. This prevalence rate will turn into a critical challenge by the increasing number of older adults.106
In 1983, various concentrations of both fine (10 nm) and coarse (less than 5 microns) silica particles were delivered into the brains of mice and rats employing different methods of administration. Brain reactions to the presence of the silica were observed by light and electron microscopy up to one-year post-injection. The intracerebral injection of silica materials in rat led to a neurotoxic inflammatory response, while it did not cause lesions similar to those which characterize Alzheimer’s disease. Hence, it remains opaque whether silica is involved in neuritic (senile) plaque development or not.107 In 1992, different model aluminosilicate materials were proposed to promote the in vitro generation of microglia-derived reactive oxygen metabolites (ROM) implying that the potential cellular mechanism, by which the proposed analogous aluminosilicate deposits in the core of senile plaques, might play an essential role in the aetiopathogenesis of Alzheimer’s.108 In terms of free radical biology, in vivo aluminosilicate-induced and phagocyte-mediated oxidative stress were found to conduct the pathogenic mechanism in Alzheimer’s disease development.109
On contrary, in parallel studies, the traditional paradigm changed, so that Memo and Manna claimed to prepare an activated clinoptilolite zeolite possessing a neuroprotective effect, specifically when it comes to its antioxidant activity in the mitochondria and thus its benefit in neuropathologies, eg, Alzheimer’s or Parkinson’s disease, where the reactive oxygen species (ROS) play an important role.110
AD is usually distinguished by many symptoms, such as amyloid-β deposition in amyloid plaques, neurofibrillary tangles, neuronal loss, and cognitive deficits. The verified hypothesis for AD pathology is the amyloid-β deposition in the brain which causes disruption of neuronal cell membranes, imbalance in neuronal circuit hemostasis, and eventually the neuron demise.111 Therefore, according to the amyloid-β hypothesis, targeting these amyloid structures and preventing amyloid formation have come to the attention of many nanomaterial scientists as a treatment strategy for AD through this framework.112
By emphasizing the antioxidant effects of the micronized zeolite (MZ) for ageing-related neurodegeneration, administration of MZ to a transgenic mice model of AD resulted in no toxicity or adverse effects even in a long-term period. Unexpectedly, MZ treatment seemed to prevent amyloidogenic processing of Aβ due to a considerable decrease in Aβ42 levels in MZ-treated transgenic mice.113
Lately, fibrinogen protein was detected in the brain of AD patients which could be due to the blood brain barrier (BBB) impairment occurring as a result of AD. Fibrinogen, as a soluble plasma glycoprotein, plays important roles in blood clotting, thrombosis, and inflammation. In regards to the new etiology of AD development, targeting the Aβ−fibrinogen interaction has been hypothesized as a practical therapeutic approach for either prevention or treatment of AD.114,115
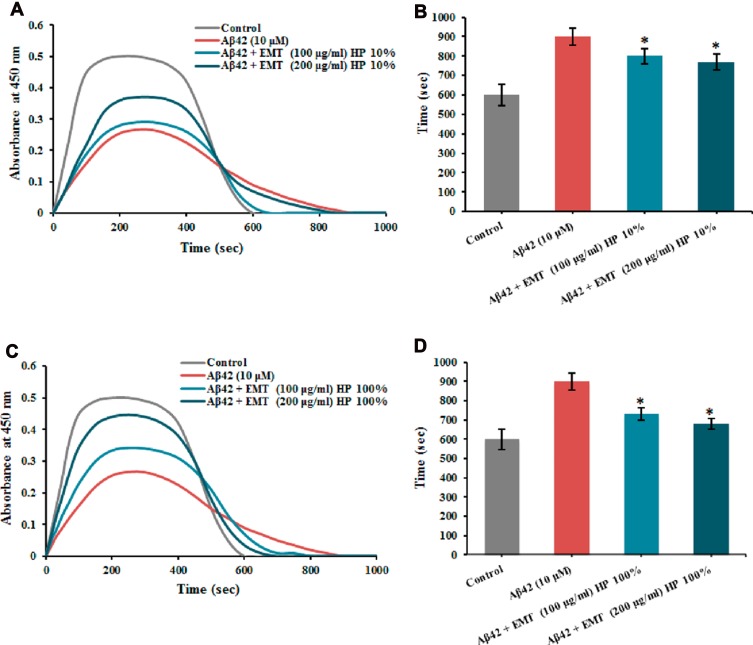
Recently, we studied EMT-type zeolite nanocrystals that could bind to the fibrinogen molecules in contact with the Aβ proteins. EMT zeolite suppressed the Aβ−fibrinogen interaction with abnormal clots, and the template-free zeolite NPs demonstrated the advantages of being nontoxic and biocompatible in physiological media. By employing both Aβ (1−42) and Aβ (25−35) to interact with Aβ−fibrinogen, it was also determined that the effect of zeolite NPs did not rely on Aβ sequences. The computational and experimental results were both in accordance with each other revealing the significant residues and interactome effectors contributing to the interaction of Aβ with fibrinogen (Figure 8).116
Figure 8.
Total time determined for clot formation and breakdown in the presence of Aβ 1–42 and corona coated EMT zeolite NPs achieved from (A and B) 10% and (C and D) 100% plasmas. * P< 0.1. Reprinted with permission from Derakhshankhah H, Hajipour MJ, Barzegari E, et al. Zeolite nanoparticles inhibit Aβ–fibrinogen interaction and formation of a consequent abnormal structural clot. ACS Appl Mater Interfaces. 2016;8(45):30768–30779. Copyright © 2016, American Chemical Society.116
Zeolite nanomaterials have been applied not only in the treatment of AD but also in developing diagnostic instruments and biosensors for AD. Following the merits and shortcomings of zeolite-based solid-phase extraction from the crude extract of Corydalis yanhusuo, 14 acetylcholinesterase (ACE) inhibitors were screened, among which 10 (ACE) inhibitors were newly discovered.117
Zeolite’s Protein Corona Composition and Biological Interactions
Nanomaterials have been employed in multiple targeting, diagnostics, and therapeutic applications depending on their size. Once a nanomaterial is introduced to a physiological medium, eg, blood, it is rapidly covered by a layer of proteins (ie, protein corona) giving it a new biological identity. Efforts to determine the rules that govern the corona composition and its consequent biological identity could change their fate.118–120 Most of the rules determining the interaction of proteins with biomaterials could be applied to interactions of proteins with nanomaterials. However, it should be noted that due to highly curved surface of nanomaterials relative to proteins and also widely distribution of nanomaterials throughout the body, they may act differently in diverse media according to their physicochemical features.121 The biological identity of corona protein could vary depending on distinctive protein types belonging to diverse individual physiological compartments and their concentrations along with the nanomaterial’s characteristics including shape, size, and surface chemistry. The biological identity of a nanomaterial undergoes evolving after being exposed to a new physiological medium, eg, after migration of the nanomaterial from blood to other physiological compartments, such as the cytoplasm.120,122–124
Protein corona of two types of ultra-small zeolites, with LTL- and EMT-type structures, after exposure to 10% and 100% human plasma was analyzed via the nLC-MS/MS-PEAKS analysis, so that 99 and 66 kinds of proteins in LTL-corona and EMT-corona were verified, respectively. Among them, EMT-corona incubated with 100% human plasma displayed a very high affinity for fibrinogen and a very low affinity for albumin.38
Zeolites that have been utilized for numerous years by US forces in Iraq and Afghanistan to induce coagulation and to stabilize life-threatening injuries, in the form of Ca-zeolite, have demonstrated to be dressed by protein corona, abundantly with thrombin. Their discovery emphasized the connection of the biomolecules in the protein corona with the procoagulant activity of the zeolite particles picturing a bright future for the hemostatic mechanisms of zeolite nanomaterials.125
Also, the NP-induced inflammatory cytokines were shown to rely on the different protein concentrations during incubation of three NPs including hydrophobic sulfonated-modified polystyrene NPs, hydrophilic amorphous SiO2, and hydrophilic crystalline EMT zeolite with human plasma. Nevertheless, among them, the EMT zeolite remarkably exhibited no toxicity independent of the plasma protein concentrations.126
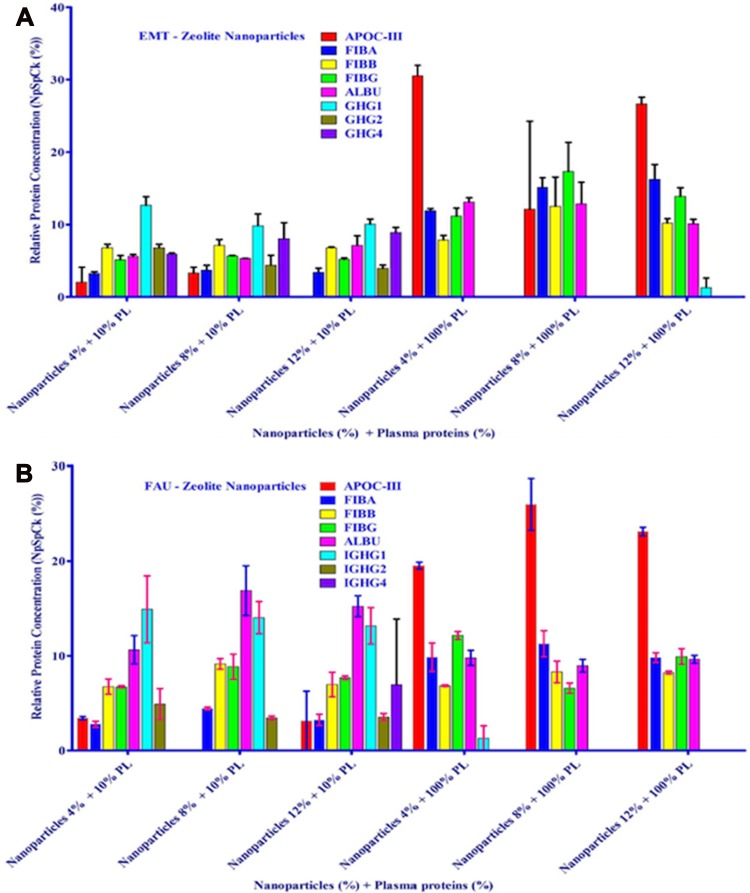
To open the possibility of applying zeolite’s protein corona for therapeutic purposes, their protein corona compositions after exposure to 10% and 100% plasma were explored. The protein corona composition is dominantly related to protein concentration and chemical identity of zeolites but less significantly to NPs concentration. Protein corona compositions were identified through semi-quantitative assessment of the amount of proteins. Regarding the fact that albumin and immunoglobulin G (IgG) 38–40 are the most abundant proteins in plasma, the composition of EMT- and FAU-corona, exposed to 10% plasma, was merely consisted of albumin and IgG. More strikingly, the number of existing proteins in the corona of zeolite NPs exposed to 100% plasma was less than that of the NPs exposed to 10% plasma which could be explained by the competition theory, ie, proteins compete to occupy the surface of the NPs. It was also concluded that since EMT-zeolite are more hydrophilic than FAU-zeolites, proteins are adsorbed more on EMT-zeolite’s surface. Regarding zeolite NPs capability to quicken the time of blood clot formation, they may be offered for the use of hemophilic patients (ie, hemophilia A (F-VIII deficient) and hemophilia B (F-IX deficient)) with a risk of bleeding. Besides, since APOC-III would be selectively captured, it could minimize the activation of lipoprotein lipase inhibition while undergoing hypertriglyceridemia treatment (Figure 9).127
Figure 9.
nLC-MS/MS test of corona-associated proteins on EMT-zeolite (A) and FAU-zeolite (B) NPs. Apolipoprotein C-III (APOC-III), fibrinogen alpha chain (FIBA), fibrinogen beta chain (FIBB), fibrinogen gamma chain (FIBG), albumin (ALBU), IGHG1, IGHG2 and IGHG4. Reprinted from Rahimi M, Ng E-P, Bakhtiari K, et al. Zeolite nanoparticles for selective sorption of plasma proteins. Sci Rep. 2015;5:17259. Copyright © 2015, Springer Nature.127
Zeolites and Drug Delivery
Initially, inorganic materials had shown not enough biocompatibility to be employed in drug delivery systems. However, recently, the results of successful experiments on nano-scale zeolites with a larger external surface area, up to an order of magnitude as compared to micron-sized zeolites, indicate their applicability for a wide spectrum of applications in drug delivery systems.128–131
Additionally, the interaction of both purified and modified natural clinoptilolites with metronidazole and sulfamethoxazole demonstrated that the organic molecules did not exhibit degradation after contacting with different zeolite products at any pH levels indicating their promising advantage for drug delivery applications.132
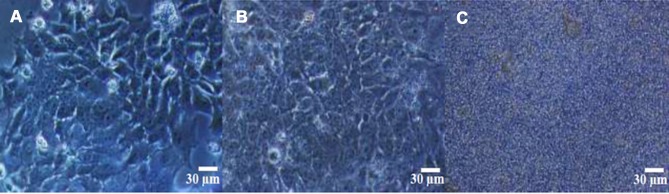
The application of various NPs containing zeolite in drug delivery systems have been categorized in Table 1 in a chronological order. Only two-early studies131,133 focus on oral administration of two types of zeolite NPs to assess their capacity for sustained release and enhanced delivery of cyclophosphamide and fenbendazole, respectively. The other studies demonstrate the efficiency of various zeolite NPs to deliver different drugs in vitro. It is noticeable that due to the unique properties of zeolite-L nanocrystals, intracellular delivery of DAPI probes paved the way for future drug delivery systems targeting intercellular macro molecules (Figure 10).134 The chemotherapeutic doxorubicin is the most abundantly loaded drug into the zeolite NPs. Due to obvious advantages of zeolites, eg, enhancing bioavailability of drugs even in gastrointestinal route, their application in oral delivery of drugs that target gastric cancer and intestinal worms is a possibility in a foreseeable future.
Table 1.
Application of Various Nanoparticles Containing Zeolite in Drug Delivery Systems Based on Indicators Including NP’s Physicochemical Properties, Loaded Drug-Substances, Type of Study (in vitro or in vivo), Biological Applications and Advantages of These Nanoparticles Containing Zeolite
| Nanoparticles Type | Particles Size (nm) | Loaded Drug-Substances | Type of Study | Biological Applications | Advantages | Ref |
|---|---|---|---|---|---|---|
| Cu-X zeolite | N.A | Cyclophosphamide | In vivo | Tumor therapy | Continual maintenance concentration of Cyclophosphamide via oral administration | [131] |
| Zeolite Y | 2 µm | Fenbendazole | In vivo | Anti-parasites (Intestinal Worms) | Efficient slow release | [133] |
| Zeolite rock | N.A | Zn+2 Erythromycin |
In vitro | Acne treatment | Sustained treatment | [135] |
| Magnetic FAU-zeolite | 20 to 600 nm | Doxorubicin | In vitro | Chemotherapy | Simple preparation, avoiding agglomeration of the magnetite nanoparticles to a huge extent | [136] |
| Zeolite Y | 0.4–0.6 µm | Ibuprofen | In vitro | Pain, Fever, Inflammation | Accelerated Ibuprofen delivery rate | [137] |
| Mixture of Zeolite X and Zeolite A | 4.5 µm | Ketoprofen | In vitro | Inflammation Gastrointestinal pathology |
Lower risk of adverse effects related to NSAIDs oral administration | [138] |
| Zeolite/polymer composites (Chitosan-Gelatin-alginate) | ~70–250 μm | Cefalexin and Gentamaycin | In vitro | Homeostatic treatment, Antibiotics | Prolonged release | [139] |
| Zeolite HY | N. A | Aspirin | In silico | Pain, Fever, Inflammation | Optimized SiO2/Al2O3 ratio | [140] |
| Zeolite-L nanocrystals | 60 nm | Peptide Nucleic Acid (PNA) probes like DAPI | In vitro | Cancer | Intercellular drug delivery | [141] |
| MOR (mordenite), Zeolite Y | N. A | Temozolomide (TMZ) | In vitro | Glioblastoma brain tumors | Lower systematic toxicity | [134] |
| Zinc-clinoptilolite/Graphene Oxide (Zn-Clin/GO) hybrid nanostructure | 20–30 nm | Doxorubicin | In vitro | Chemotherapy | Homogeneous and stable nanocomposite | [142] |
| Magnetite–zeolite | 30 nm | 5-fluorouracil (5-FU) | In vitro | Gastric cancer | Efficient delivery | [143] |
| Zeolite beta (BEA) | N. A | Nifedipine (NIF) | In vitro | Hypertension and Angina | Dissolution enhancement for oral bioavailability | [144] |
| Microporous zeolites: NaX, ZSM and BEA | 2 μm | Indomethacin | In vitro | Severe osteoarthritis, rheumatoid arthritis, Gouty arthritis | Fine-tuned drug release | [145] |
| MFI-type borosilicate zeolites | 41–55 nm | Doxorubicin | In vitro | Chemotherapy | Excellent biocompatibility noticeable cytotoxic effect |
[146] |
| Clinoptilolite-rich rock from California (CLI_CA), surface modified with cetylpyridinium chloride (CP) | (805 ± 131) μm | Diclofenac sodium (DS) | In vitro | Inflammation, Gastrointestinal pathology | Satisfactory dosage uniformity, suitable flowability as excipient carrier, meeting pharmacopeia regulations | [147] |
Figure 10.
Optical microscopy images demonstrating U251 cells treated with (A) 0 mg/mL of drug delivery system (DDS), (B) 0.05 mg/mL of temozolomide (TMZ) containing mordenite zeolites (MOR) (TMZ0.026-MOR) and (C) 0.75 mg/mL of TMZ0.026-MOR under 100x magnification and found that above 0.75 mg mL1 there is high rate of cell dead, probably because from this concentration the cells are completely coated by the zeolite, which compromise the cell-nutrient exchange with the culture media. Reprinted with permission from Ref [134] Copyright (2011), ROYAL SOCIETY OF CHEMISTRY.
Conclusion
Zeolites are crystalline porous materials known for a decade and have recently become very attractive for researchers due to their biomedical applications, especially when it comes to safety and non-toxicity of some natural zeolites whose oral administration was found to safely and effectively exert gastro-beneficial effects for the patients suffering from diarrhea and other diseases. Significant prominence of nanozeolites as compared to other nanostructured porous material lies in their low cytotoxicity, high payload capacity, and efficient delivery. According to the importance of zeolite nanocrystals’ characteristics, most of the research focus on engineering the framework composition, size, morphology, and secondary porosity of these NPs. In the relevant approaches for fabrication of zeolite NPs, there is still a serious challenge regarding the heterogeneity of the NPs, and controlling the zeolite nucleation process plays a key role in achieving the product homogeneity. For example, for FAU- and EMT-type zeolites, it has been shown that the optimal condition results in great achievements in costly production of particles with a uniform size distribution. Nonetheless, controlling zeolite nucleation and growth in such systems is difficult and needs to be further investigated.
Complexity of the fabrication of zeolite NPs and, more importantly, the intricacies regarding corona-protein biological identity of zeolite NPs yet remain poorly understood. The latter is of great significance in the context of predicting the pharmacodynamics and pharmacokinetics of the corona-covered zeolite NPs whose physiological activities are dictated by their biomolecular corona composition. Due to the fact that some NPs are intrinsically able or chemically modified to cross a physiological barrier, such as the BBB, different corona proteins with different patterns of composition could mask the NPs and consequently confer them a different biological identity. The biological identity of the zeolite-protein corona directly depends on the type and amount of individual proteins residing on the corona layer and indirectly on other corona-interacting proteins, such as receptor proteins on the cell membranes whose occupation and activation by zeolite corona proteins manifest different signaling and cellular toxicity readouts. Thereby, given the fast accumulative breakthroughs in the bioanalytical field, in particular within proteomics, the future of zeolite NPs translational research is dependent on the advancement of the tools applied for probing and exploring the diseases-specific zeolite corona proteins. For example, for deep understanding of the zeolite NPs metabolism and physiological fate, researchers may take advantage of the corona-dependent biological identity and manipulate its composition, so that it allows the receptors on the cell membrane to internalize the zeolite NPs readily. In addition, the intracellular incidents following internalization can be tracked and probed. Thereby, it seems feasible to integrate a probe protein to the corona composition in order to accurately spot zeolite NPs’ localization inside the cells.
In this review, our focus was specifically on the application of zeolite NPs and their expanding outreach in biology and medicine. We know that the cytotoxicity and bio-applicability assessments of each molecule are a decisive topic prior to its use in clinical practices. Hence, although cytotoxicity studies on zeolite NPs are less than other mesoporous NPs, most of the papers report low cytotoxicity of these frameworks. As presented, we aimed to provide an overview of the widespread biomedical applications of zeolite NPs as antidiarrheal agents, antitumor adjuvants, antibacterial agents, and MRI contrast agents along with their utilization in studies on bone formation, Alzheimer’s disease development, excipient functionality, protein corona composition, biological interactions, and their hemodialytic, drug delivery, and dental applications. We hope the researchers find the content of this review helpful for their future works.
Acknowledgment
This work was supported by Kermanshah University of Medical Sciences, Kermanshah, Iran.
Highlights
Significant prominence of nanozeolites as compared to another nanostructured porous materials are their low cytotoxicity, high payload capacity, and efficient delivery.
The control of the zeolite nucleation process plays a key role in achieving homogeneous nanoparticles.
The size and uniformity of zeolite nanoparticles with improved biocompatibility lead to great achievements in biomolecular delivery capacity.
The application of zeolite nanoparticles and their expanding outreach in biology and medicine.
Zeolites nanoparticles as promising antidiarrheal agents, antitumor adjuvants, antibacterial agents, drug carriers, and MRI contrast agents due to their favorable cytotoxicity, excipient functionality, protein corona composition, and biological interactions.
Abbreviations
NPs, Nanoparticles; Aβ, Amyloid beta; IUPAC, International Union of Pure and Applied Chemistry; SDA, Structure-Directing Agent; FCC, Fluid Catalytic Cracking; MRI, Magnetic Resonance Imaging; GI, Gastrointestinal; HeLa, human cervix carcinoma cells; TGF h, Transforming growth factor h; Ag, Silver; GIC, Glass Ionomer Cement; MTA, Mineral Trioxide Aggregate; SZ, Silver Zeolite; DS, Diclofenac Sodium; NZ, Natural Zeolites; CPC, Cetylpyridinium Chloride; ROM, Reactive Oxygen Metabolites; MZ, Micronised Zeolite; BBB, Blood Brain Barrier; ACE, acetylcholinesterase; IgG, Immunoglobulin G.
Disclosure
The authors declare they have no competing interests.
References
- 1.Everett DH. Manual of symbols and terminology for physicochemical quantities and units, appendix II: definitions, terminology and symbols in colloid and surface chemistry. Pure Appl Chem. 1972;31(4):577–638. doi: 10.1351/pac197231040577 [DOI] [Google Scholar]
- 2.Valtchev V, Tosheva L. Porous nanosized particles: preparation, properties, and applications. Chem Rev. 2013;113(8):6734–6760. doi: 10.1021/cr300439k [DOI] [PubMed] [Google Scholar]
- 3.Cho J, Ishida Y. Macroscopically oriented porous materials with periodic ordered structures: from zeolites and metal-organic frameworks to liquid-crystal-templated mesoporous materials. Adv Mater. 2017;29(25):1605974. doi: 10.1002/adma.201605974 [DOI] [PubMed] [Google Scholar]
- 4.Ogawa T, Iyoki K, Fukushima T, et al. Landscape of research areas for zeolites and metal-organic frameworks using computational classification based on citation networks. Materials (Basel). 2017;10(12):1428. doi: 10.3390/ma10121428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrer RM. Hydrothermal Chemistry of Zeolites. London: Academic Press; 1982. [Google Scholar]
- 6.Cundy CS, Cox PA. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem Rev. 2003;103(3):663–702. doi: 10.1021/cr020060i [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Yu J, Corma A. Extra-large-pore zeolites: bridging the gap between micro and mesoporous structures. Angew Chem Int Ed Engl. 2010;49(18):3120–3145. doi: 10.1002/anie.v49:18 [DOI] [PubMed] [Google Scholar]
- 8.Szostak R. Handbook of Molecular Sieves. New York: Van Nostrand Reinhold; 1992. [Google Scholar]
- 9.Barrer RM. Zeolites and Clay Minerals as Sorbents and Molecular Sieves. London: Academic Press; 1978. [Google Scholar]
- 10.Breck D. Zeolite Molecular Sieves. New York: John Wiley and Sons; 1974. [Google Scholar]
- 11.Cheng H, Zhang L, He J, et al. Integrated nanozymes with nanoscale proximity for in vivo neurochemical monitoring in living brains. Anal Chem. 2016;88(10):5489–5497. doi: 10.1021/acs.analchem.6b00975 [DOI] [PubMed] [Google Scholar]
- 12.Cundy CS, Cox PA. The hydrothermal synthesis of zeolites: precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005;82(1–2):1–78. doi: 10.1016/j.micromeso.2005.02.016 [DOI] [Google Scholar]
- 13.de Moor -P-PEA, Beelen TPM, Komanschek BU, et al. Imaging the assembly process of the organic-mediated synthesis of a zeolite. Chem Eur J. 1999;5(7):2083–2088. doi: 10.1002/(ISSN)1521-3765 [DOI] [Google Scholar]
- 14.Hould ND, Lobo RF. Nanoparticle precursors and phase selectivity in hydrothermal synthesis of zeolite β. Chem Mater. 2008;20(18):5807–5815. doi: 10.1021/cm800884q [DOI] [Google Scholar]
- 15.Davis TM, Drews TO, Ramanan H, et al. Mechanistic principles of nanoparticle evolution to zeolite crystals. Nat Mater. 2006;5:400–408. doi: 10.1038/nmat1636 [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Wang Z, Penn RL, et al. A structural resolution cryo-TEM study of the early stages of MFI growth. J Am Chem Soc. 2008;130(51):17284–17286. doi: 10.1021/ja8063167 [DOI] [PubMed] [Google Scholar]
- 17.Hould ND, Kumar S, Tsapatsis M, et al. Structure and colloidal stability of nanosized zeolite beta precursors. Langmuir. 2010;26(2):1260–1270. doi: 10.1021/la902445c [DOI] [PubMed] [Google Scholar]
- 18.Valtchev VP, Bozhilov KN. Transmission electron microscopy study of the formation of FAU-Type zeolite at room temperature. J Phys Chem B. 2004;108(40):15587–15598. doi: 10.1021/jp048341c [DOI] [Google Scholar]
- 19.Valtchev VP, Bozhilov KN. Evidences for zeolite nucleation at the solid-liquid interface of gel cavities. J Am Chem Soc. 2005;127(46):16171–16177. doi: 10.1021/ja0546267 [DOI] [PubMed] [Google Scholar]
- 20.Valtchev VP, Tosheva L, Bozhilov KN. Synthesis of zeolite nanocrystals at room temperature. Langmuir. 2005;21(23):10724–10729. doi: 10.1021/la050323e [DOI] [PubMed] [Google Scholar]
- 21.Valtchev V, Mintova S. Encyclopedia of Inorganic Chemistry. Chichester, UK: John Wiley & Sons Ltd.; 2008:543. [Google Scholar]
- 22.Schoeman BJ, Sterte J. Colloidal zeolite-preparation, properties and applications. KONA. 1997;15:150–158. doi: 10.14356/kona.1997019 [DOI] [Google Scholar]
- 23.Mintova S, Valtchev V. Synthesis of nanosized FAU-type zeolite. Stud Surf Sci Catal. 1999;125:141–148. [Google Scholar]
- 24.Song W, Grassian VH, Larsen SC, High yield method for nanocrystalline zeolite synthesis. Chem Commun (Camb). 2005;23:2951–2953. doi: 10.1039/b501768h [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Wang K, Dong D, et al. Synthesis of hierarchical porous zeolite NaY particles with controllable particle sizes. Microporous Mesoporous Mater. 2010;127:167–175. doi: 10.1016/j.micromeso.2009.07.026 [DOI] [Google Scholar]
- 26.Hu D, Xia Q-H, Lu X-H, et al. Synthesis of ultrafine zeolites by dry-gel conversion without any organic additive. Mater Res Bull. 2008;43:3553−3561. doi: 10.1016/j.materresbull.2008.01.008 [DOI] [Google Scholar]
- 27.Kim YC, Jeong JY, Hwang JY, et al. Influencing factors on rapid crystallization of high silica nano-sized zeolite Y without organic template under atmospheric pressure. J Porous Mater. 2009;16(3):299–306. doi: 10.1007/s10934-008-9200-4 [DOI] [Google Scholar]
- 28.Awala H, Gilson J-P, Retoux R, et al. Template-free nanosized faujasite-type zeolites. Nat Mater. 2015;14(4):447–451. doi: 10.1038/nmat4173 [DOI] [PubMed] [Google Scholar]
- 29.Ng E-P, Chateigner D, Bein T, et al. Capturing ultrasmall EMT zeolite from template-free systems. Science. 2012;335(6064):70–73. doi: 10.1126/science.1214798 [DOI] [PubMed] [Google Scholar]
- 30.Hagiwara Z, Hoshino S, Ishino H, et al. Zeolite particles retaining silver ions having antibacterial properties. Google Patents. 1990
- 31.Balkus KJ, Bresinska I, Kowalak S, Young SW. The application of molecular sieves as magnetic resonance imaging contrast agents. MRS Online Proc Lib Arch. 1991;233. [Google Scholar]
- 32.Ray H, Seltzer S. A new glass ionomer root canal sealer. J Endod. 1991;17(12):598–603. doi: 10.1016/S0099-2399(06)81832-7 [DOI] [PubMed] [Google Scholar]
- 33.Cefali EA, Nolan JC, McConnell WR, et al. Pharmacokinetic study of zeolite A, sodium aluminosilicate, magnesium silicate, and aluminum hydroxide in dogs. Pharm Res. 1995;12(2):270–274. doi: 10.1023/A:1016291228957 [DOI] [PubMed] [Google Scholar]
- 34.Kihara T, Zhang Y, Hu Y, et al. Effect of composition, morphology and size of nanozeolite on its in vitro cytotoxicity. J Biosci Bioeng. 2011;111(6):725–730. doi: 10.1016/j.jbiosc.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 35.Petushkov A, Intra J, Graham JB, et al. Effect of crystal size and surface functionalization on the cytotoxicity of silicalite-1 nanoparticles. Chem Res Toxicol. 2009;22(7):1359–1368. doi: 10.1021/tx900153k [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Hüve J, Krampe C, et al. Internalization pathways of anisotropic disc-shaped zeolite L nanocrystals with different surface properties in HeLa cancer cells. Small. 2013;9(9–10):1809–1820. doi: 10.1002/smll.201201702 [DOI] [PubMed] [Google Scholar]
- 37.Mellaerts R, Delvaux J, Levêque P, et al. Screening protocol for identifying inorganic oxides with anti-oxidant and pro-oxidant activity for biomedical, environmental and food preservation applications. RSC Adv. 2013;3(3):900–909. doi: 10.1039/C2RA20921G [DOI] [Google Scholar]
- 38.Laurent S, Ng E-P, Thirifays C, et al. Corona protein composition and cytotoxicity evaluation of ultra-small zeolites synthesized from template free precursor suspensions. Toxicol Res (Camb). 2013;2(4):270–279. doi: 10.1039/c3tx50023c [DOI] [Google Scholar]
- 39.Lehman SE, Larsen SC. Zeolite and mesoporous silica nanomaterials: greener syntheses, environmental applications and biological toxicity. Environ Sci Nano. 2014;1(3):200–213. [Google Scholar]
- 40.Bedi RS, Zanello LP, Yan Y. Osteoconductive and osteoinductive properties of zeolite MFI coatings on titanium alloys. Adv Funct Mater. 2009;19(24):3856–3861. doi: 10.1002/(ISSN)1616-3028 [DOI] [Google Scholar]
- 41.Taton TA. Nanotechnology: boning up on biology. Nature. 2001;412(6846):491–492. doi: 10.1038/35087687 [DOI] [PubMed] [Google Scholar]
- 42.Balasundaram G, Webster TJ. A perspective on nanophase materials for orthopedic implant applications. J Mater Chem. 2006;16(38):3737–3745. doi: 10.1039/b604966b [DOI] [Google Scholar]
- 43.Bedi RS, Chow G, Wang J, et al. Bioactive materials for regenerative medicine: zeolite-hydroxyapatite bone mimetic coatings. Adv Eng Mater. 2012;14(3):200–206. doi: 10.1002/adem.201100170 [DOI] [Google Scholar]
- 44.Keeting PE, Oursler MJ, Wiegand KE, et al. Zeolite a increases proliferation, differentiation, and transforming growth factor β production in normal adult human osteoblast-likecells in vitro. J Bone Miner Res. 1992;7(11):1281–1289. doi: 10.1002/jbmr.5650071107 [DOI] [PubMed] [Google Scholar]
- 45.Schütze N, Oursler MJ, Nolan J, et al. Zeolite a inhibits osteoclast-mediatedbone resorption in vitro. J Cell Biochem. 1995;58(1):39–46. doi: 10.1002/(ISSN)1097-4644 [DOI] [PubMed] [Google Scholar]
- 46.Yu L, Gong J, Zeng C, et al. Preparation of zeolite-A/chitosan hybrid composites and their bioactivities and antimicrobial activities. Mater Sci Eng C. 2013;33(7):3652–3660. doi: 10.1016/j.msec.2013.04.055 [DOI] [PubMed] [Google Scholar]
- 47.Patzer JF, Yao SJ, Wolfson S. Zeolitic ammonium ion exchange for portable hemodialysis dialysate regeneration. ASAIO J. 1995;41(2):221–226. doi: 10.1097/00002480-199506000-00019 [DOI] [PubMed] [Google Scholar]
- 48.Eventov V, Andrianova MY, Palyulina M. Water purification for hemodialysis. Biomed Eng. 1999;33(2):76–81. doi: 10.1007/BF02386173 [DOI] [PubMed] [Google Scholar]
- 49.Wernert V, Schäf O, Ghobarkar H, et al. Adsorption properties of zeolites for artificial kidney applications. Microporous Mesoporous Mater. 2005;83(1):101–113. doi: 10.1016/j.micromeso.2005.03.018 [DOI] [Google Scholar]
- 50.Bergé-Lefranc D, Vagner C, Calaf R, et al. In vitro elimination of protein bound uremic toxin p-cresol by MFI-type zeolites. Microporous Mesoporous Mater. 2012;153:288–293. doi: 10.1016/j.micromeso.2011.11.024 [DOI] [Google Scholar]
- 51.Narasimhan L, Boulet P, Kuchta B, et al. Adsorption of paracresol in silicalite-1 and pure silica faujasite. A comparison study using molecular simulation. Appl Surf Sci. 2010;256(17):5470–5474. doi: 10.1016/j.apsusc.2009.12.142 [DOI] [Google Scholar]
- 52.Wernert V, Schäf O, Faure V, et al. Adsorption of the uremic toxin p-cresol onto hemodialysis membranes and microporous adsorbent zeolite silicalite. J Biotechnol. 2006;123(2):164–173. doi: 10.1016/j.jbiotec.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 53.Pellegrino P, Mallet B, Delliaux S, et al. Zeolites are effective ROS-scavengers in vitro. Biochem Biophys Res Commun. 2011;410(3):478–483. doi: 10.1016/j.bbrc.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Fuentes G, Barrios MA, Iraizoz A, et al. Enterex: anti-diarrheic drug based on purified natural clinoptilolite. Zeolites. 1997;19(5–6):441–448. doi: 10.1016/S0144-2449(97)00087-0 [DOI] [Google Scholar]
- 55.Cabrera G, Vercelli C, Burzyn D, et al. Increases in IgA(+) B cells in Peyer’s patches during milk-borne mouse mammary tumor virus infection are influenced by Toll-like receptor 4 and are completely dependent on the superantigen response. J Gen Virol. 2010;91(Pt11):2814–2820. doi: 10.1099/vir.0.023358-0 [DOI] [PubMed] [Google Scholar]
- 56.Kraljevic Pavelic S, Simović Medica J, Gumbarević D, et al. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front Pharmacol. 2018;9:1350. doi: 10.3389/fphar.2018.01350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potgieter W, Samuels CS, Snyman JR. Potentiated clinoptilolite: artificially enhanced aluminosilicate reduces symptoms associated with endoscopically negative gastroesophageal reflux disease and nonsteroidal anti-inflammatory drug induced gastritis. Clin Exp Gastroenterol. 2014;7:215–220. doi: 10.2147/CEG.S51222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Della Rocca J, Lin W. Nanoscale metal–organic frameworks: magnetic resonance imaging contrast agents and beyond. Eur J Inorg Chem. 2010;2010(24):3725–3734. doi: 10.1002/ejic.201000496 [DOI] [Google Scholar]
- 59.Aime S, Crich SG, Gianolio E, et al. High sensitivity lanthanide (III) based probes for MR-medical imaging. Coord Chem Rev. 2006;250(11):1562–1579. doi: 10.1016/j.ccr.2006.03.015 [DOI] [Google Scholar]
- 60.Lauffer RB. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem Rev. 1987;87(5):901–927. doi: 10.1021/cr00081a003 [DOI] [Google Scholar]
- 61.Mintova S, Jaber M, Valtchev V. Nanosized microporous crystals: emerging applications. Chem Soc Rev. 2015;44(20):7207–7233. doi: 10.1039/C5CS00210A [DOI] [PubMed] [Google Scholar]
- 62.Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging. 1990;8(4):467–481. doi: 10.1016/0730-725X(90)90055-7 [DOI] [PubMed] [Google Scholar]
- 63.Balkus KJ, Sherry AD, Young SW. Zeolite-enclosed transistion and rare earth metal ions as contrast agents for the gastrointestinal tract. Google Patents. 1992
- 64.Young SW, Qing F, Rubin D, et al. Gadolinium zeolite as an oral contrast agent for magnetic resonance imaging. J Magn Reson Imaging. 1995;5(5):499–508. doi: 10.1002/(ISSN)1522-2586 [DOI] [PubMed] [Google Scholar]
- 65.Rubin DL, Falk KL, Sperling MJ, et al. A multicenter clinical trial of gadolite oral suspension as a contrast agent for MRI. J Magn Reson Imaging. 1997;7(5):865–872. doi: 10.1002/(ISSN)1522-2586 [DOI] [PubMed] [Google Scholar]
- 66.Pavelic K, Hadzija M. Medical Applications of zeolites. Handbook of Zeolite Science and Technology. New York: Dekker; 2003:1143–1174. [Google Scholar]
- 67.Platas-Iglesias C, Vander Elst L, Zhou W, et al. Zeolite GdNaY nanoparticles with very high relaxivity for application as contrast agents in magnetic resonance imaging. Chem Eur J. 2002;8(22):5121–5131. doi: [DOI] [PubMed] [Google Scholar]
- 68.Bresinska I, Balkus KJJ. Studies of Gd (III)-exchanged Y-type zeolites relevant to magnetic resonance imaging. J Phys Chem. 1994;98(49):12989–12994. doi: 10.1021/j100100a029 [DOI] [Google Scholar]
- 69.Tosheva L, Valtchev V. Nanozeolites: synthesis, crystallization mechanism, and applications. Chem Mater. 2005;17(10):2494–2513. doi: 10.1021/cm047908z [DOI] [Google Scholar]
- 70.Peters JA, Djanashvili K. Lanthanide loaded zeolites, clays, and mesoporous silica materials as MRI probes. Eur J Inorg Chem. 2012;2012(12):1961–1974. doi: 10.1002/ejic.v2012.12 [DOI] [Google Scholar]
- 71.Pavelic K, Katic M, Sverko V, et al. Immunostimulatory effect of natural clinoptilolite as a possible mechanism of its antimetastatic ability. J Cancer Res Clin Oncol. 2002;128(1):37–44. doi: 10.1007/s00432-001-0301-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colic M, Pavelic K. Molecular mechanisms of anticancer activity of natural dietetic products. J Mol Med. 2000;78(6):333–336. doi: 10.1007/s001090000121 [DOI] [PubMed] [Google Scholar]
- 73.Pavelić K, Hadžija M, Bedrica L, et al. Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. J Mol Med. 2001;78(12):708–720. doi: 10.1007/s001090000176 [DOI] [PubMed] [Google Scholar]
- 74.Ivkovic S, Bendzko P, Schulz J. Tribomechanically activated zeolite (TMAZ) as an adjuvant cancer treatment-Promising results from clinical case observations. Free Radical Biol Med. 2004;37:S173. [Google Scholar]
- 75.Ivkovic S, Zabcic D. Antioxidative Therapy: Nanotechnology Product, TMA-Zeolite Reduces Oxidative Stress in Cancer and Diabetic Patients. In Free Radical Biology and Medicine. Kidlington, Oxford: Pergamon-Elsevier Science Ltd The Boulevard; 2002. [Google Scholar]
- 76.Zarcovic N, Zarkovic K, Kralj M, et al. Anticancer and antioxidative effects of micronized zeolite clinoptilolite. Anticancer Res. 2003;23(2):1589–1596. [PubMed] [Google Scholar]
- 77.Hajipour MJ, Fromm KM, Akbar Ashkarran A, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 78.Alswat AA, Ahmad MB, Saleh TA, et al. Effect of zinc oxide amounts on the properties and antibacterial activities of zeolite/zinc oxide nanocomposite. Mater Sci Eng C. 2016;68:505–511. doi: 10.1016/j.msec.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 79.Alswat AA, Ahmad MB, Saleh TA. Preparation and characterization of zeolite\zinc oxide-copper oxide nanocomposite: antibacterial activities. Colloid Interface Sci Commun. 2017;16:19–24. doi: 10.1016/j.colcom.2016.12.003 [DOI] [Google Scholar]
- 80.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34(34):8533–8554. doi: 10.1016/j.biomaterials.2013.07.089 [DOI] [PubMed] [Google Scholar]
- 81.Alswat AA, Ahmad MB, Hussein MZ, et al. Copper oxide nanoparticles-loaded zeolite and its characteristics and antibacterial activities. J Mater Sci Technol. 2017;33(8):889–896. doi: 10.1016/j.jmst.2017.03.015 [DOI] [Google Scholar]
- 82.Tosheva L, Brockbank A, Mihailova B, et al. Micron-and nanosized FAU-type zeolites from fly ash for antibacterial applications. J Mater Chem. 2012;22(33):16897–16905. doi: 10.1039/c2jm33180b [DOI] [Google Scholar]
- 83.Lalueza P, Monzón M, Arruebo M, et al. Antibacterial action of Ag-containing MFI zeolite at low Ag loadings. Chem Commun. 2011;47(2):680–682. doi: 10.1039/C0CC03905E [DOI] [PubMed] [Google Scholar]
- 84.Ferreira L, Fonseca AM, Botelho G, et al. Antimicrobial activity of faujasite zeolites doped with silver. Microporous Mesoporous Mater. 2012;160:126–132. doi: 10.1016/j.micromeso.2012.05.006 [DOI] [Google Scholar]
- 85.Saint-Cricq P, Kamimura Y, Itabashi K, et al. Antibacterial activity of silver-loaded “Green zeolites”. Eur J Inorg Chem. 2012;2012(21):3398–3402. doi: 10.1002/ejic.v2012.21 [DOI] [Google Scholar]
- 86.Chiericatti C, Basílico JC, Basílico MLZ, et al. Antifungal activity of silver ions exchanged in mordenite. Microporous Mesoporous Mater. 2014;188:118–125. doi: 10.1016/j.micromeso.2013.12.033 [DOI] [Google Scholar]
- 87.Zhou Y, Deng Y, He P, et al. Antibacterial zeolite with a high silver-loading content and excellent antibacterial performance. RSC Adv. 2014;4(10):5283–5288. doi: 10.1039/c3ra44750b [DOI] [Google Scholar]
- 88.Dong B, Belkhair S, Zaarour M, et al. Silver confined within zeolite EMT nanoparticles: preparation and antibacterial properties. Nanoscale. 2014;6(18):10859–10864. doi: 10.1039/C4NR03169E [DOI] [PubMed] [Google Scholar]
- 89.Hanim SAM, Malek NANN, Ibrahim Z. Amine-functionalized, silver-exchanged zeolite NaY: preparation, characterization and antibacterial activity. Appl Surf Sci. 2016;360:121–130. doi: 10.1016/j.apsusc.2015.11.010 [DOI] [Google Scholar]
- 90.Singh VV, Jurado-Sánchez B, Sattayasamitsathit S, et al. Multifunctional silver-exchanged zeolite micromotors for catalytic detoxification of chemical and biological threats. Adv Funct Mater. 2015;25(14):2147–2155. doi: 10.1002/adfm.v25.14 [DOI] [Google Scholar]
- 91.Redfern J, Goldyn K, Verran J, et al. Application of Cu-FAU nanozeolites for decontamination of surfaces soiled with the ESKAPE pathogens. Microporous Mesoporous Mater. 2017;253:233–238. [Google Scholar]
- 92.Barry JE, Trogolo JA, Pastecki EA. Antimicrobial dental products. Google Patents. 2001
- 93.Saghiri MA, Lotfi M, Aghili H. Dental cement composition. Google Patents. 2014
- 94.Dorn SO, Gartner AH. Retrograde filling materials: a retrospective success-failure study of amalgam, EBA, and IRM. J Endod. 1990;16(8):391–393. [DOI] [PubMed] [Google Scholar]
- 95.Maeda H, Hashiguchi I, Nakamuta H, et al. Histological study of periapical tissue healing in the rat molar after retrofilling with various materials. J Endod. 1999;25(1):38–42. [DOI] [PubMed] [Google Scholar]
- 96.Pertot W-J, Stephan G, Tardieu C, Proust JP. Comparison of the intraosseous biocompatibility of Dyract and Super EBA. J Endod. 1997;23(5):315–319. [DOI] [PubMed] [Google Scholar]
- 97.Pelliccioni GA, Vellani CP, Gatto MR, et al. Proroot mineral trioxide aggregate cement used as a retrograde filling without addition of water: an in vitro evaluation of its microleakage. J Endod. 2007;33(9):1082–1085. [DOI] [PubMed] [Google Scholar]
- 98.Patel V, Santerre JP, Friedman S. Suppression of bacterial adherence by experimental root canal sealers. J Endod. 2000;26(1):20–24. doi: 10.1097/00004770-200001000-00005 [DOI] [PubMed] [Google Scholar]
- 99.Thom DC, Davies JE, Santerre JP, et al. The hemolytic and cytotoxic properties of a zeolite-containing root filling material in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(1):101–108. doi: 10.1067/moe.2003.90 [DOI] [PubMed] [Google Scholar]
- 100.Çınar Ç, Ulusu T, Özçelik B, et al. Antibacterial effect of silver-zeolite containing root-canal filling material. J Biomed Mater Res B Appl Biomater. 2009;90(2):592–595. doi: 10.1002/jbm.b.v90b:2 [DOI] [PubMed] [Google Scholar]
- 101.Odabaş ME, Çinar Ç, Akça G, et al. Short-termantimicrobial properties of mineral trioxide aggregate with incorporatedsilver-zeolite. Dent Traumatol. 2011;27(3):189–194. doi: 10.1111/edt.2011.27.issue-3 [DOI] [PubMed] [Google Scholar]
- 102.Pifferi G, Santoro P, Pedrani M. Quality and functionality of excipients. Il Farmaco. 1999;54(1):1–14. doi: 10.1016/S0014-827X(98)00101-3 [DOI] [PubMed] [Google Scholar]
- 103.Krajišnik D, Milojević M, Malenović A, et al. Cationic surfactants-modified natural zeolites: improvement of the excipients functionality. Drug Dev Ind Pharm. 2010;36(10):1215–1224. doi: 10.3109/03639041003695121 [DOI] [PubMed] [Google Scholar]
- 104.Krajišnik D, Daković A, Malenović A, et al. An investigation of diclofenac sodium release from cetylpyridinium chloride-modified natural zeolite as a pharmaceutical excipient. Microporous Mesoporous Mater. 2013;167(Supplement C):94–101. doi: 10.1016/j.micromeso.2012.03.033 [DOI] [Google Scholar]
- 105.Cerri G, Farina M, Brundu A, et al. Natural zeolites for pharmaceutical formulations: preparation and evaluation of a clinoptilolite-based material. Microporous Mesoporous Mater. 2016;223(Supplement C):58–67. doi: 10.1016/j.micromeso.2015.10.034 [DOI] [Google Scholar]
- 106.Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 107.Rees S, Cragg B. Is silica involved in neuritic (senile) plaque formation? Acta Neuropathol. 1983;59(1):31–40. doi: 10.1007/BF00690314 [DOI] [PubMed] [Google Scholar]
- 108.Evans PH, Peterhans E, Bürge T, et al. Aluminosilicate-induced free radical generation by murine brain glial cells in vitro: potential significance in the aetiopathogenesis of alzheimer’s dementia. Dement Geriatr Cogn Disord. 1992;3(1):1–6. doi: 10.1159/000106985 [DOI] [Google Scholar]
- 109.Evans PH, Klinowski J, Yano E, et al. Alzheimer’s disease: a pathogenic role for aluminosilicate-induced phagocytic free radicals. Free Radic Res Commun. 1989;6(5):317–321. doi: 10.3109/10715768909055157 [DOI] [PubMed] [Google Scholar]
- 110.Memo M, Manna S. Zeolites having a neuro-protective effect. Google Patents. 2013
- 111.Castello MA, Soriano S. On the origin of alzheimer’s disease. Trials and tribulations of the amyloid hypothesis. Ageing Res Rev. 2014;13:10–12. doi: 10.1016/j.arr.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 112.Mahmoudi M, Quinlan-Pluck F, Monopoli MP, et al. Influence of the physiochemical properties of superparamagnetic iron oxide nanoparticles on amyloid β protein fibrillation in solution. ACS Chem Neurosci. 2013;4(3):475–485. doi: 10.1021/cn300196n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Montinaro M, Uberti D, Maccarinelli G, et al. Dietary zeolite supplementation reduces oxidative damage and plaque generation in the brain of an alzheimer’s disease mouse model. Life Sci. 2013;92(17–19):903–910. doi: 10.1016/j.lfs.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 114.Mosesson M. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894–1904. doi: 10.1111/jth.2005.3.issue-8 [DOI] [PubMed] [Google Scholar]
- 115.Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. [DOI] [PubMed] [Google Scholar]
- 116.Derakhshankhah H, Hajipour MJ, Barzegari E, et al. Zeolite nanoparticles inhibit Aβ–fibrinogen interaction and formation of a consequent abnormal structural clot. ACS Appl Mater Interfaces. 2016;8(45):30768–30779. doi: 10.1021/acsami.6b10941 [DOI] [PubMed] [Google Scholar]
- 117.Tao Y, Jiang Y, Li W, et al. Zeolite based solid-phase extraction coupled with UPLC-Q-TOF-MS for rapid analysis of acetylcholinesterase binders from crude extract of Corydalis yanhusuo. RSC Adv. 2016;6(100):98476–98486. doi: 10.1039/C6RA24585D [DOI] [Google Scholar]
- 118.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19(3):311–330. doi: 10.1096/fj.04-2747rev [DOI] [PubMed] [Google Scholar]
- 119.Lynch I, Dawson KA. Protein-nanoparticle interactions. Nano Today. 2008;3(1):40–47. doi: 10.1016/S1748-0132(08)70014-8 [DOI] [Google Scholar]
- 120.Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442 [DOI] [PubMed] [Google Scholar]
- 121.Anderson JM, Rodriguez A, Chang DT. Foreign Body Reaction to Biomaterials. In Seminars in Immunology. Elsevier; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lundqvist M, Stigler J, Cedervall T, et al. The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5(9):7503–7509. doi: 10.1021/nn202458g [DOI] [PubMed] [Google Scholar]
- 123.Walkey CD, Chan WC. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41(7):2780–2799. doi: 10.1039/C1CS15233E [DOI] [PubMed] [Google Scholar]
- 124.Monopoli MP, Walczyk D, Campbell A, et al. Physical− chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133(8):2525–2534. doi: 10.1021/ja107583h [DOI] [PubMed] [Google Scholar]
- 125.Li Y, Liao X, Zhang X, et al. In situ generated thrombin in the protein corona of zeolites: relevance of the functional proteins to its biological impact. Nano Res. 2014;7(10):1457–1465. doi: 10.1007/s12274-014-0505-0 [DOI] [Google Scholar]
- 126.Tirtaatmadja N, Mortimer G, Ng E-P, et al. Nanoparticles-induced inflammatory cytokines in human plasma concentration manner: an ignored factor at the nanobio-interface. J Iran Chem Soc. 2015;12(2):317–323. doi: 10.1007/s13738-014-0486-7 [DOI] [Google Scholar]
- 127.Rahimi M, Ng E-P, Bakhtiari K, et al. Zeolite nanoparticles for selective sorption of plasma proteins. Sci Rep. 2015;5:17259. doi: 10.1038/srep17259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Song W, Woodworth JF, Grassian VH, et al. Microscopic and macroscopic characterization of organosilane-functionalized nanocrystalline NaZSM-5. Langmuir. 2005;21(15):7009–7014. doi: 10.1021/la050559i [DOI] [PubMed] [Google Scholar]
- 129.Barquist K, Larsen SC. Chromate adsorption on amine-functionalized nanocrystalline silicalite-1. Microporous Mesoporous Mater. 2008;116(1):365–369. doi: 10.1016/j.micromeso.2008.04.025 [DOI] [Google Scholar]
- 130.Zhan B-Z, White MA, Lumsden M. Bonding of organic amino, vinyl, and acryl groups to nanometer-sized NaX zeolite crystal surfaces. Langmuir. 2003;19(10):4205–4210. doi: 10.1021/la026737e [DOI] [Google Scholar]
- 131.Uglea CV, Albu I, Vatajanu A, et al. Drug delivery systems based on inorganic materials: I. Synthesis and characterization of a zeolite-cyclophosphamide system. J Biomater Sci Polym Ed. 1995;6(7):633–637. doi: 10.1163/156856294X00572 [DOI] [PubMed] [Google Scholar]
- 132.Farí T, Ruiz-Salvador A, Rivera A. Interaction studies between drugs and a purified natural clinoptilolite. Microporous Mesoporous Mater. 2003;61(1):117–125. doi: 10.1016/S1387-1811(03)00391-3 [DOI] [Google Scholar]
- 133.Dyer A, Morgan S, Wells P, et al. The use of zeolites as slow release anthelmintic carriers. J Helminthol. 2000;74(2):137–141. doi: 10.1017/S0022149X00000184 [DOI] [PubMed] [Google Scholar]
- 134.Martinho O, Vilaça N, Castro PJG, et al. In vitro and in vivo studies of temozolomide loading in zeolite structures as drug delivery systems for glioblastoma. RSC Adv. 2015;5(36):28219–28227. doi: 10.1039/C5RA03871E [DOI] [Google Scholar]
- 135.De GM, Cerri G, Caramella C, Bonferoni MC. Pharmaceutical zeolite-based compositions containing zinc and erythromycin, to be used in the treatment of acne. Google Patents. 2002
- 136.Arruebo M, Fernández-Pacheco R, Irusta S, et al. Sustained release of doxorubicin from zeolite–magnetite nanocomposites prepared by mechanical activation. Nanotechnology. 2006;17(16):4057. doi: 10.1088/0957-4484/17/16/011 [DOI] [PubMed] [Google Scholar]
- 137.Horcajada P, Márquez-Alvarez C, Rámila A, et al. Controlled release of Ibuprofen from dealuminated faujasites. Solid State Sci. 2006;8(12):1459–1465. doi: 10.1016/j.solidstatesciences.2006.07.016 [DOI] [Google Scholar]
- 138.Rimoli MG, Rabaioli MR, Melisi D, et al. Synthetic zeolites as a new tool for drug delivery. J Biomed Mater Res A. 2008;87(1):156–164. doi: 10.1002/jbm.a.v87a:1 [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y, Xu C, He Y, et al. Zeolite/polymer composite hollow microspheres containing antibiotics and the in vitro drug release. J Biomater Sci Polym Ed. 2011;22(4–6):809–822. doi: 10.1163/092050610X496242 [DOI] [PubMed] [Google Scholar]
- 140.Datt A, Fields D, Larsen SC. An experimental and computational study of the loading and release of aspirin from zeolite HY. J Phys Chem C. 2012;116(40):21382–21390. doi: 10.1021/jp3067266 [DOI] [Google Scholar]
- 141.Bertucci A, Lülf H, Septiadi D, et al. Intracellular delivery of peptide nucleic acid and organic molecules using zeolite-L nanocrystals. Adv Healthc Mater. 2014;3(11):1812–1817. doi: 10.1002/adhm.v3.11 [DOI] [PubMed] [Google Scholar]
- 142.Khatamian M, Divband B, Farahmand-zahed F. Synthesis and characterization of Zinc (II)-loaded Zeolite/Graphene oxide nanocomposite as a new drug carrier. Mater Sci Eng C. 2016;66(Supplement C):251–258. doi: 10.1016/j.msec.2016.04.090 [DOI] [PubMed] [Google Scholar]
- 143.Sağir T, Huysal M, Durmus Z, et al. Preparation and in vitro evaluation of 5-flourouracil loaded magnetite–zeolite nanocomposite (5-FU-MZNC) for cancer drug delivery applications. Biomed Pharmacother. 2016;77(Supplement C):182–190. doi: 10.1016/j.biopha.2015.12.025 [DOI] [PubMed] [Google Scholar]
- 144.Karavasili C, Kokove L, Kontopoulou I, et al. Dissolution enhancement of the poorly soluble drug nifedipine by co-spray drying with microporous zeolite beta. J Drug Deliv Sci Technol. 2016;35(Supplement C):91–97. doi: 10.1016/j.jddst.2016.06.004 [DOI] [Google Scholar]
- 145.Karavasili C, Amanatiadou EP, Kontogiannidou E, et al. Comparison of different zeolite framework types as carriers for the oral delivery of the poorly soluble drug indomethacin. Int J Pharm. 2017;528(1):76–87. doi: 10.1016/j.ijpharm.2017.05.061 [DOI] [PubMed] [Google Scholar]
- 146.Khatamian M, Yavari A, Akbarzadeh A, et al. Synthesis and characterization of MFI-type borosilicate zeolites and evaluation of their efficiency as drug delivery systems. Mater Sci Eng C. 2017;78(Supplement C):1212–1221. doi: 10.1016/j.msec.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 147.Serri C, de Gennaro B, Quagliariello V, et al. Surface modified zeolite-based granulates for the sustained release of diclofenac sodium. Eur J Pharm Sci. 2017;99(Supplement C):202–208. doi: 10.1016/j.ejps.2016.12.019 [DOI] [PubMed] [Google Scholar]