Abstract
Aims
To the best of our knowledge, no study has tried to quantitatively summarize the published evidence regarding the effect of hesperidin supplementation on blood glucose control. The present systematic review and meta‐analysis of randomized controlled trials aimed to determine the effectiveness of hesperidin supplementation in improving blood glucose control in adults.
Methods
Electronic databases including PubMed, ISI Web of Science, Scopus, and Google Scholar were searched up to February 2019. The risk of bias in individual studies was assessed using the Cochrane collaboration's tool. The overall estimates and their 95% confidence intervals (CIs) were calculated using a random‐effects model.
Results
Six trials with 318 participants were reviewed in the present systematic review. The results showed that hesperidin had no significant effect on serum fasting blood glucose (weighted mean difference [WMD] = −1.10 mg/dL, 95% CI: −3.79, 1.57), plasma insulin (WMD = −0.01 μU/mL, 95% CI: −1.20, 1.19), glycated haemoglobin A1c (WMD = −0.04%, 95% CI: −0.14, 0.04), homeostasis model assessment for insulin resistance (WMD = 0.117, 95% CI: −0.06, 0.29) and quantitative insulin sensitivity check index (WMD = 0.135; 95% CI: −0.13, 0.39), with no significant between‐study heterogeneity. Subgroup analyses also indicated that the effects were not different based on the studies' design and duration, or the health status of the participants.
Conclusion
Although several animal studies have proposed that hesperidin supplementation might improve blood glucose control, the present study could not confirm this benefit in humans.
Keywords: hesperidin, citrus flavonoid, glucose, insulin, systematic review, meta‐analysis
What is already known on the subject
Several animal studies have revealed that hesperidin supplementation might beneficially affect blood glucose control. However, clinical trials have led to inconsistent results.
What this study adds
The present systematic review and meta‐analysis revealed that hesperidin supplementation does not significantly affect different markers of blood glucose control including fasting blood glucose, plasma insulin, glycated haemoglobin A1c, homeostasis model assessment for insulin resistance and quantitative insulin sensitivity check index in human adults.
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a common chronic disease characterized by abnormal glucose metabolism.1 The dramatic increase in T2DM prevalence is considered a global public health concern.2 As reported by the International Diabetes Federation (IDF), approximately 415 million people suffer from diabetes mellitus in 2015, and this number is expected to rise to 642 million by 2040, with the largest increase in developing countries.3 Moreover, T2DM is associated with a high prevalence of cardiovascular complications.4
Lifestyle modifications and medical therapy are considered critical in the prevention and control of T2DM.5 Patient education and self‐care practices might also help in the management of T2DM.6, 7 Dietary interventions play essential roles in improving the hyperglycaemic state and of its resulting complications.8 For instance, several dietary supplements including resveratrol, cinnamon, ginger and cumin are proposed to be effective in blood glucose control.9, 10, 11, 12 A meta‐analysis also showed that fasting glucose levels are reduced after supplementation with flavonols.13
Flavonoids represent a very diverse group of biologically active compounds synthesized during plant metabolism.14, 15 Fifteen carbon atoms and 2 benzene rings bearing 1 or more hydroxyl groups joined by a linear 3‐carbon chain are present in the flavonoids' structure.16 Flavonoids are classified into 6 main classes: flavonols, flavan‐3‐ols, flavones, flavanones, anthocyanins and isoflavones.17
Hesperidin is an important flavanone that is mainly found in citrus fruits such as lemons, clementine, grapefruit, mandarins and oranges.18 It is suggested that hesperidin plays effective roles in oxidative stress, inflammation, nitric oxide synthesis, hypertension, infection and apoptosis.19 However, recent systematic reviews and meta‐analyses have shown that hesperidin supplementation might not significantly affect blood pressure, lipid profile or inflammatory markers.20 The effect of hesperidin intake on glycaemic parameters have also been evaluated in animal and human studies; however, previous studies have led to different findings in this regard. For instance, 1 study indicated that hesperidin might not significantly improve the hyperglycaemia and HbA1c levels in male Wistar rats.21 In contrast, other studies have shown that hesperidin administration might reduce glucose levels and improve glycaemic control in animals with diabetes (mainly rats and mice), possibly through several mechanisms including increased hepatic glucokinase activity, glycolysis and glycogen synthesis.22, 23, 24 The clinical trials have also reported different findings on the efficacy of hesperidin supplementation on blood glucose control.25, 26, 27
Indeed, although several studies have assessed the effect of hesperidin supplementation on blood glucose markers, we are not aware of any study trying to summarize the published evidence. Therefore, the present systematic review and meta‐analysis of randomized controlled trials (RCTs) aimed to investigate the possible effect of hesperidin supplementation on blood glucose control and insulin sensitivity in adults.
2. MATERIALS AND METHODS
The preferred reporting items for systematic reviews and meta‐analyses (PRISMA) was considered to report the current systematic review and meta‐analysis.28 The study protocol was also registered in the international prospective register of systematic reviews (PROSPERO; registration code: CRD42017058859).29
2.1. Search strategy
To find potentially eligible articles, electronic databases including PubMed (http://www.pubmed.com; National Library of Medicine), Scopus (http://www.scopus.com/), ISI Web of Science (http://www.thomsonreuters.com) and Google Scholar (http://www.scholar.google.com) were searched up to February 2019. To ensure maximum sensitivity, searches were not restricted by language or date of publication. Medical subject headings (MeSH) and non‐MeSH terms were used to search the databases for relevant publications. The Patient/Population, Intervention, Comparison, Outcome and Study types (PICOS) with their related keywords are provided in Table 1.
Table 1.
The patient/population, intervention, comparison, outcome, study types (PICOS) framework for keywords used in the search strategy
| Criteria (PICOS) | Definition (keywords) |
|---|---|
| Population (P) | Adults aged >18 years (no specific keywords) |
| Intervention (I) | Hesperidin supplementation (“hesperidin”, “hesperitin”, “citrus flavonoid”, “orange juice”) |
| Control or comparison (C) | A separate group who received placebo or control intervention (no specific keywords) |
| Outcome (O) | Blood glucose control markers including fasting blood glucose, serum insulin, HbA1c, HOMA‐IR, QUICKI (“glycemic”, “glycemic indices”, “glucose”, “blood glucose”, “blood sugar”, “fast plasma glucose”, “FPG”, “fasting blood sugar”, “FBS”, “L‐glucose”, “D‐glucose”, “dextrose”, “insulin”, “hyperinsulinism”, “hyperinsulinemia”, “novolin”, “iletin”, “proinsulin”, “C‐peptide”, “C peptide”, “insulin resistance”, “insulin sensitivity”, “hemoglobin A", “glycosylated”, “glycosylated hemoglobin”, “glycated hemoglobins”, “glycosylated hemoglobin A", “glycohemoglobin A", “HbA1”, “Hb A1c”, “Hb A1b”, “hyperglycemia”, “hyperglycemias”, “hypoglycemia”, “hypoglycemias”, “fasting hypoglycemia", “glycemic load”, “glucose tolerance test”, “oral glucose tolerance test”, “OGTT”) |
| Study type (S) | Randomized controlled clinical trials (“intervention”, “trial”, “randomized”, “random”, “randomly”, “placebo”, “assignment”, “clinical trial”, “RCT”, “cross‐over”, “parallel”) |
Two investigators (S.Z. and M.M.) independently scanned the titles and abstracts to exclude any irrelevant studies. The full text of the remaining articles were carefully checked to determine whether the remaining articles pass the eligibility criteria. Moreover, the reference lists of the eligible articles were scanned for any other potentially related study.
2.2. Eligibility criteria
All published RCTs were included if they assessed the effect of hesperidin supplementation on at least 1 of blood glucose parameters [fasting blood glucose (FBG), insulin, glycated hemoglobin (HbA1c), homeostasis model assessment for insulin resistance (HOMA‐IR), quantitative insulin sensitivity check index (QUICKI)] as primary or secondary outcome. Exclusion criteria were as follows: studies conducted among participants aged under 18 years; studies assessing the acute effect of hesperidin; and trials in which hesperidin supplementation was not the only difference between the treatment and the control groups.
2.3. Data extraction
The data extraction was independently done by 2 investigators (M.A.M. and S.S.R.). Other investigators were responsible for verifying the data extraction process (A.S.A. and M.M.). We collected the following information from each of the included studies: the last name of the first author, the year of publication, the country in which the study was implemented, the design of the study (crossover or parallel), the details of the intervention including the exact amount of consumed hesperidin (mg/d), the type of intervention carried out in the control group, the intervention period, the total number of participants by sex, the mean/range of age for study participants, the baseline health status of participants and the number of participants who completed the follow‐up period.
2.4. Risk of bias assessment
The risk of bias in each included study was independently assessed by 2 reviewers (S.S.R. and N.R.J.) using the Cochrane Collaboration risk of bias tool.30 Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias were used to summarize the quality of studies. Each domain was regarded as a low risk of bias, high risk of bias or unclear risk of bias. The last category shows either lack of information or uncertainty over the potential for bias. By considering the 6 mentioned domains as the key domains, a summary of overall assessment (low risk, low for all key domains; high risk, high for 1 or more key domains; and unclear risk, unclear for 1 or more key domains) was provided.
2.5. Statistical analysis
The outcome variables were as follows: FBG, insulin, HbA1c, HOMA‐IR, and QUICKI. The serum glucose values were converted into mg/dL and the serum insulin values were converted into μU/mL before calculating the effect sizes. For parallel trials, the mean change and its corresponding standard deviation (SD) from baseline to follow‐up for the intervention and the control groups and for crossover trials, the same values for the intervention and control periods were calculated. For studies in which the SDs of mean change from baseline were not provided, these estimates were calculated if P values reported for the comparison between baseline and after follow‐up mean values or they were imputed by using estimated correlation coefficients between baseline and after follow‐up values. We calculated the correlation coefficients between baseline and after follow‐up values by using SD of baseline mean, SD of after mean and SD of mean change (r = 0.47 for FBG and r = 0.76 for HOMA‐IR) from studies that readily reported these values. The SD of change in HbA1c and QUICKI for intervention and control groups were calculated by considering a correlation coefficient of 0.5. To check the robustness of the meta‐analysis to the imputed SDs, all analyses were repeated by the use of correlation coefficients of 0.2 and 0.8. Finally, the difference in mean change between intervention and control groups/periods and its corresponding standard error were calculated to be used as effect size.
The overall weighted mean difference (WMD) and its 95% confidence interval (CI) was calculated by using a random‐effects model which takes the between‐study heterogeneity into account. The statistical heterogeneity was assessed using Cochran's Q test and the I2 statistic.31 P‐values <.05 for Cochran's Q test were considered as high levels of heterogeneity.32 I2 values would vary between 0 and 100% with higher values representing greater degrees of heterogeneity (0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; 75–100%: considerable heterogeneity).30 To explore the potential sources of between‐study heterogeneity, subgroup analyses were done to evaluate whether results were different by the study design (parallel/cross‐over), treatment duration (≤6 weeks/>6 weeks) and baseline health status of the participants (healthy/with diabetes or metabolic syndrome). Sensitivity analyses were performed to test the robustness of the meta‐analyses results by removing the trials 1 by 1 and recalculating the overall effects with the remaining studies.33 To evaluate the potential publication bias visual inspection of funnel plots and also Begg's and Egger's asymmetry tests were conducted.34 STATA software, version 11.2 (Stata Corp, College Station, TX, USA) was used for the statistical analyses. P values <.05 were considered as statistically significant.
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,35 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.36
3. RESULTS
3.1. Selection and characteristics of the included trials
The study selection process is presented in a flow chart suggested by PRISMA (Figure 1). A total of 6 RCTs that enrolled 318 participants were eligible to be included in the present systematic review and meta‐analysis.26, 27, 37, 38, 39, 40 The detailed characteristics of the studies included in the systematic review and meta‐analysis are presented in Table 2. Three studies had a crossover design38, 39, 40 and the others were parallel RCTs.26, 27, 37 Two studies were performed in Asian populations,26, 27 whereas the remaining studies were done in European countries,37, 38, 39, 40 published between 2011 and 2018. The duration of the studies varied from 3 to 12 weeks. The supplementation dose ranged from 292 to 500 mg/d. In the majority of included studies, starch or cellulose were used as the placebo for the control groups/periods.26, 27, 37, 39, 40 One study was conducted with the use of juice containing high polyphenol concentration (582 mg hesperidin) for the interventions and normal polyphenol concentration (237 mg hesperidin) for the control group.38 Five studies included both sexes,26, 27, 37, 38, 39 and 1 of them only included male subjects.40 The age of the participants ranged from 18 to 65 years. Three studies were conducted among healthy overweight/obese individuals,37, 38, 40 and other trials included patients with metabolic syndrome39 or T2DM.26, 27
Figure 1.
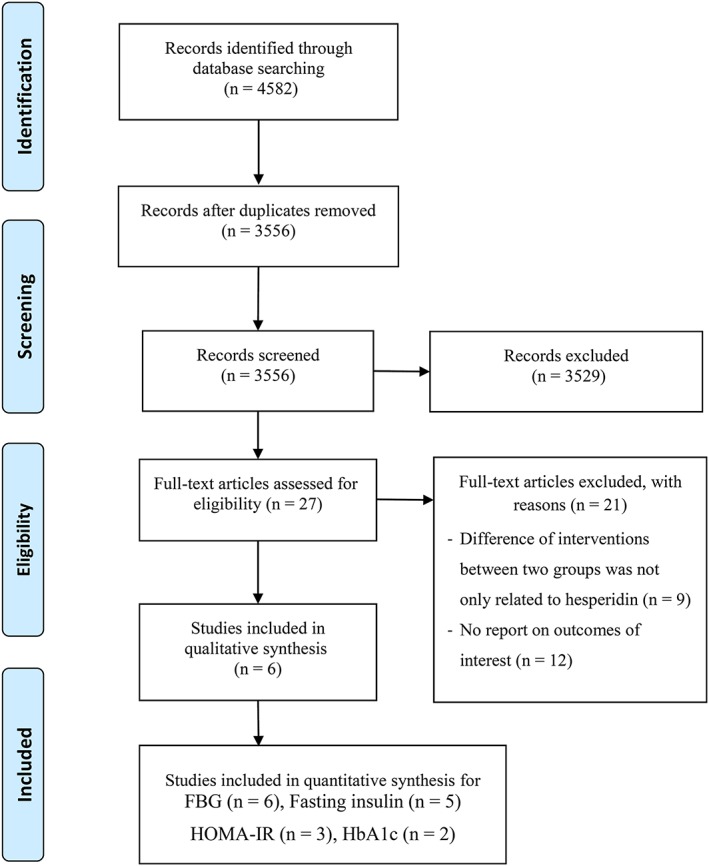
PRISMA flow chart for the study selection process
Table 2.
Characteristics of randomized clinical trials that were included in the systematic review
| Study, yref | Country | Number, sex (F/M) 1 | Age (y) | RCT1 design | Duration | Intervention group | Control group | Reported data | Notes about participants |
|---|---|---|---|---|---|---|---|---|---|
| Homayouni et al., 201827 | Iran | 60 (32F/28M) | 30–65 | Parallel | 6 wk | 500 mg/d hesperidin | 500 mg/d starch | FBG1 HOMA‐IR1 | Patients with diabetes |
| int1: 51.3 | |||||||||
| con1: 54 | |||||||||
| Salden et al., 201637 | Germany | 68 (39F/29M) | 18–65 | Parallel | 6 wk | 450 mg/d hesperidin | 500 mg/d cellulose | FBG insulin QUICKI1 | Healthy overweight individuals |
| Int: 54 | |||||||||
| Con: 53 | |||||||||
| Rangel‐Huerta et al., 201538 | Spain | 100 F/M | 18–65 | Cross‐over | 12 wk | 500 ml/d high polyphenol juice hesperidin: 582.5 mg | 500 ml/d normal polyphenol juice hesperidin: 237 mg | FBG insulin HOMA‐IR | Healthy overweight/obese individuals |
| Eghtesadi et al., 201526 | Iran | 45 F/M | Int: 53.2 | Parallel | 8 wk | 500 mg/d hesperidin | 500 mg/d cellulose | FBG insulin HOMA‐IR HbA1c1 | Patients with diabetes |
| Con: 53.4 | |||||||||
| Morand et al., 201140 | France | 24 (24 M) | 50–65 | Cross‐over | 4 wk | 500 ml of the control drink +292 mg hesperidin | 500 ml of the control drink +292 mg starch | FBG insulin | Healthy overweight individuals |
| Rizza et al., 201139 | Italy | 24 (9F/15M) | 21–65 | Cross‐over | 3 wk | 500 mg/d hesperidin | 500 mg/d cellulose | FBG insulin QUICKI HbA1c | Patients with metabolic syndrome |
| Int: 53 | |||||||||
| Con: 50 |
F: female, M: male, RCT: randomized controlled trial, Int: intervention, Con: control, FBG: fasting blood glucose, HOMA‐IR: homeostatic model assessment for insulin resistance, QUICKI: quantitative insulin sensitivity check index, HbA1c: haemoglobin A1c
3.2. Risk of bias assessment
The articles included in this review were assessed for their quality using the Cochrane collaboration' tool (Table 3 ). All of the trials were categorized as low risk of bias for blinding of participants and personnel and selective outcome reporting. Allocation concealment method was described in 1 study,27 but a lack of information was observed for allocation concealment in the other studies.26, 37, 38, 39, 40 Due to clear reporting of randomization methods, we considered 4 studies to be low risk of selection bias.27, 37, 38, 40 Two articles were blinded for outcome assessment,37, 40 but the rest were unclear about blinding the outcome assessment.26, 27, 38, 39 Incomplete outcome data were addressed in the majority of the studies.26, 27, 37, 38, 40 All included studies were unclear risk of bias for at least 1 of the 6 key domains; therefore, the overall quality of all included studies was considered to be moderate or unclear risk.
Table 3.
Risk of bias assessment according to the Cochrane collaboration tool
| Study, yref | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Summary of overall assessment |
|---|---|---|---|---|---|---|---|
| Homayouni et al., 201827 | Low | Low | Low | Unclear | Low | Low | Unclear |
| Salden et al., 201637 | Low | Unclear | Low | Low | Low | Low | Unclear |
| Eghtesadi et al., 201526 | Unclear | Unclear | Low | Unclear | Low | Low | Unclear |
| Rangel et al., 201538 | Low | Unclear | Low | Unclear | Low | Low | Unclear |
| Morand et al., 201140 | Low | Unclear | Low | Low | Low | Low | Unclear |
| Rizza et al., 201139 | Unclear | Unclear | Low | Unclear | Unclear | Low | Unclear |
3.3. Meta‐analysis
As shown in Figures 2 and 3, hesperidin supplementation did not significantly affect serum levels of FBG (WMD = −1.10 mg/dL, 95% CI: −3.79, 1.57, P = .418; Cochran Q test, P = .826, I2 = 0%, n = 6) and fasting insulin (WMD = −0.01 μU/mL, 95% CI: −1.20, 1.19, P = .991; Cochran Q test, P = .844, I2 = 0%, n = 5), respectively. The pooled results also indicated no significant effect on HbA1c (WMD = −0.04%, 95% CI: −0.14, 0.04, P = .321; Cochran Q test, P = .356, I2 = 0%, n = 2), as well as HOMA‐IR (WMD = 0.117, 95% CI: −0.06, 0.29, P = .208; Cochran Q test, P = .651, I2 = 0%, n = 3) and QUICKI (WMD = 0.135, 95% CI: −0.13, 0.39, P = .319; Cochran Q test, P < .001, I2 = 99.7%, n = 2) levels compared with the placebo. A significant heterogeneity was found among studies assessing the effect of hesperidin on QUICKI, but there was no between‐study heterogeneity noticed for the meta‐analysis of other outcomes.
Figure 2.
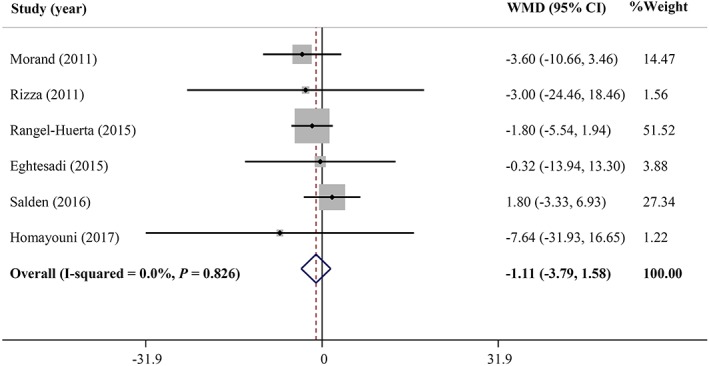
Forest plot illustrating the meta‐analysis of clinical trials investigating the effect of hesperidin supplementation on serum fasting blood glucose levels. CI, confidence interval; WMD, weighted mean difference
Figure 3.
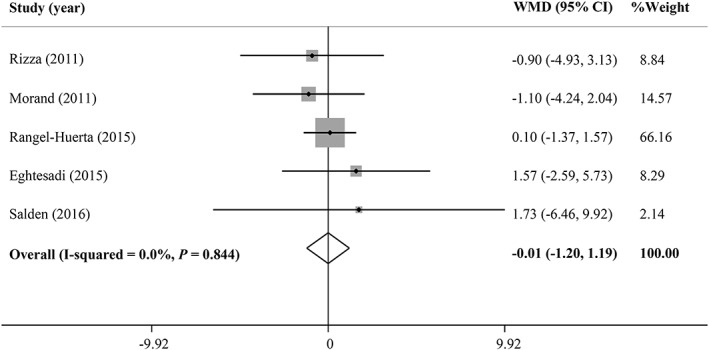
Forest plot describing the meta‐analysis of clinical trials investigating the effect of hesperidin supplementation on serum insulin levels CI, confidence interval; WMD, weighted mean difference
The funnel plots were symmetrical (Figure 4), and Begg's and Egger's asymmetry tests did not provide sufficient evidence on publication bias in the present meta‐analysis (P > .05).
Figure 4.
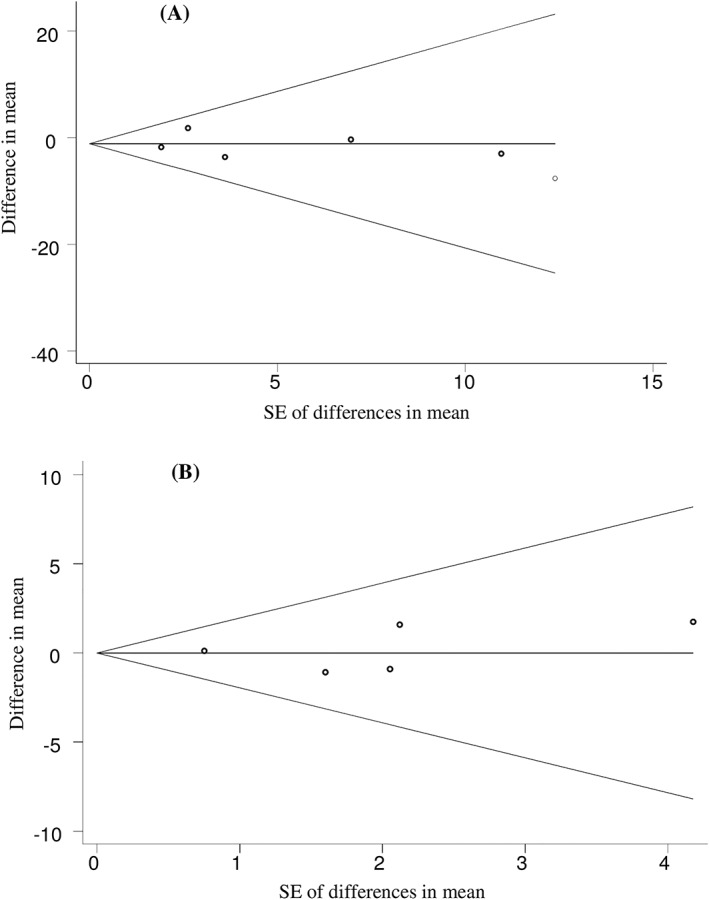
Begg's funnel plots (with pseudo 95% confidence intervals) of difference in means vs the standard error (SE) of mean difference for clinical trials investigating the effects of hesperidin supplementation on serum levels of fasting blood glucose (A), and insulin (B)
3.4. Subgroup and sensitivity analyses
We conducted subgroup analyses to explore the possible different effects of hesperidin supplementation by study design (parallel/cross‐over), treatment duration (≤6 wk/>6 wk), and baseline health status of the participants (healthy/diabetes or metabolic syndrome). Our findings showed that the pooled effects of hesperidin on serum levels of FBG and insulin were not influenced by study design, treatment duration, or baseline health status of the participants. Indeed, no beneficial effect of hesperidin supplementation on glycaemic indices was observed in these subgroups. The results of subgroup analyses are summarized in Table 4. The sensitivity analysis also indicated that the 1‐by‐1 removal of trials from the meta‐analyses did not significantly change the overall estimates.
Table 4.
Meta‐analysis showing the effects of hesperidin supplementation on glycaemic markers (all analyses were conducted using random effects model)
| Meta‐analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|
| Markers | No. of studies | WMD (95% CI) | P effect | Q statistic | P within group | I 2 (%) | P between group |
| FBG (mg/dL) | |||||||
| Study design | |||||||
| Parallel | 3 | 1.19 (−3.52, 5.90) | .620 | 0.61 | .737 | 0 | .245 |
| Cross‐over | 3 | −2.21 (−5.47, 1.05) | .184 | 0.20 | .905 | 0 | |
| Study duration | |||||||
| ≤6 weeks | 4 | −0.37 (−4.39, 3.64) | .853 | 1.89 | .595 | 0 | .633 |
| >6 weeks | 2 | −1.69 (−5.30, 1.90) | .357 | 0.04 | .837 | 0 | |
| Health status | |||||||
| Healthy | 3 | −1.02 (−3.80, 1.75) | .470 | 1.84 | .399 | 0 | .818 |
| Diabetes or MetS | 3 | −2.28 (−12.68, 8.10) | .666 | 0.27 | .873 | 0 | |
| Overall | 6 | −1.10 (−3.79, 1.57) | .418 | 2.16 | .826 | 0 | ‐ |
| Insulin (μU/mL) | |||||||
| Study design | |||||||
| Parallel | 2 | 1.60 (−2.10, 5.31) | .397 | 0.00 | .973 | 0 | .369 |
| Cross‐over | 3 | −0.19 (−1.45, 1.07) | .764 | 0.59 | .744 | 0 | |
| Study duration | |||||||
| ≤ 6 weeks | 3 | −0.79 (−3.16, 1.57) | .511 | 0.40 | .817 | 0 | .450 |
| > 6 weeks | 2 | 0.26 (−1.12, 1.65) | .710 | 0.43 | .514 | 0 | |
| Health status | |||||||
| Healthy | 3 | −0.06 (−1.38, 1.24) | .918 | 0.65 | .722 | 0 | .822 |
| Diabetes or MetS | 2 | 0.29 (−2.59, 3.18) | .841 | 0.70 | .403 | 0 | |
| Overall | 5 | −0.01 (−1.20, 1.19) | .991 | 1.40 | .844 | 0 | ‐ |
WMD: weighted mean difference; FBG: fasting blood glucose; MetS: metabolic syndrome
4. DISCUSSION
To the best of our knowledge, the current systematic review and meta‐analysis examined the effect of hesperidin supplementation on the markers of blood glucose control for the first time. Our meta‐analysis showed that hesperidin supplementation did not significantly affect serum levels of fasting glucose, insulin, HbA1c, as well as HOMA‐IR and QUICKI levels. The overall effects were stable in the sensitivity analysis. The subgroup analyses also demonstrated that the pooled effects of hesperidin on FBG and fasting insulin concentrations were not influenced by study design (parallel/cross‐over), treatment duration (≤6 wk/>6 wk), and baseline health status of the participants (healthy/diabetes or metabolic syndrome).
The present systematic review is not consistent with the suggestion made by a recent systematic review on the effects of dietary polyphenols on metabolic syndrome features in humans, which claimed the beneficial effects of hesperidin supplementation on glycaemia and insulin resistance.41 The mentioned review did not apply meta‐analytic methods to quantify the results. Furthermore, Amiot et al. included just 2 articles in their review,41 while the present systematic review includes 6 related RCTs.
It has been proposed that hesperidin supplementation might be effective in glycaemic control by influencing these pathways: (i) increased hepatic https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2798 activity23; (ii) increased hepatic glycolysis and glycogen synthesis23; (iii) increased https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5012 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5015 production23; and (iv) inducing antioxidant effects, in animal models.22, 24 High activity of phosphoenolpyruvate carboxykinase and glucose‐6‐phosphatase and low activity of glucokinase in a diabetic state seems to be associated with increased hepatic glucose production, as well as decreased hepatic glycolysis and glycogen synthesis, which are the main symptoms in patients with T2DM.42, 43, 44 Furthermore, some evidence revealed that the plasma leptin and insulin levels are positively correlated, because leptin synthesis and secretion is stimulated by insulin, which helps to control the glucose metabolism in the adipocytes.23, 45, 46 Some hypotheses might explain why the hesperidin supplementation does not significantly affect glycaemic control in human adults. It should be noted that glycaemic control mechanism in rats and mice differs from that in humans, and the findings observed in animal models must be confirmed in the relevant clinical trials. Furthermore, in the majority of animal studies, the blood glucose lowering effect of hesperidin has been observed in the dosages between 100 and 200 mg/kg. Indeed, all the studies included in our systematic review have used 292–582.5 mg/d; therefore, the plasma hesperidin might not reach the concentrations seen in the animal models.
The current meta‐analysis had some limitations that should be noted. First, despite wide systematic search, we found a few relevant studies to include in the meta‐analysis. Second, after hesperidin consumption, the accurate concentration that appeared in the blood is not specified, because the trials did not assess the bioavailability of hesperidin. Third, the intervention durations were ≤3 months in all of the included studies. The American Diabetes Association has stated that measurement of HbA1c has several advantages over assessing the oral glucose tolerance test or fasting glucose levels, including fewer day‐to‐day perturbations, lower between and within‐subject variations, greater preanalytical stability, and more convenience because fasting is not necessary.47 Since the levels of HbA1c reflect average blood glucose levels during the past 2 or 3 months,47 the follow‐up periods of >3 months might be more suitable for studies that evaluate the effects of dietary factors on glycaemic control. In contrast to the limitations mentioned above, there was no evidence of heterogeneity between the included studies and no evidence of publication bias was found. Besides, sensitivity analyses expressed the robustness of the overall results.
In conclusion, the present study shows that hesperidin supplementation might not significantly improve glycaemic control, and subgroup analyses consistently indicated similar results. More high‐quality RCTs with follow‐up longer than 3 months are needed to further clarify the effects of hesperidin on blood glucose control, especially among patients with T2DM.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
The authors' responsibilities were as follows: A.S.A., M.M. and N.R.J. developed the study concept and designed the research; S.Z. and M.M. conducted the electronic searches and study selection; M.A.M. and S.S.R. conducted data extraction and tabulated data; M.M. and A.S.A. conducted the data analysis and interpretation of results; S.S.R., N.R.J. and M.M. wrote the first draft of the manuscript; A.S.A. performed the critical review and revised the manuscript; and all authors read and approved the final version.
ACKNOWLEDGEMENTS
The study was funded by Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Shams‐Rad S, Mohammadi M, Ramezani‐Jolfaie N, Zarei S, Mohsenpour M, Salehi‐Abargouei A. Hesperidin supplementation has no effect on blood glucose control: A systematic review and meta‐analysis of randomized controlled clinical trials. Br J Clin Pharmacol. 2020;86:13–22. 10.1111/bcp.14120
REFERENCES
- 1. Oboh G, Ogunruku OO, Ogidiolu FO, Ademiluyi AO, Adedayo BC, Ademosun AO. Interaction of some commercial teas with some carbohydrate metabolizing enzymes linked with Type‐2 diabetes: a dietary intervention in the prevention of Type‐2 diabetes. Adv Prev Med. 2014;2014:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang C, Huang Z, Sun K, Hu Y, Bao X. Comparing the economic burden of type 2 diabetes mellitus patients with and without medical insurance: a cross‐sectional study in China. Med Sci Monit: Int Med J Exp Clin Res. 2018;24:3098‐3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. [DOI] [PubMed] [Google Scholar]
- 4. Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281(14):1291‐1297. [DOI] [PubMed] [Google Scholar]
- 5. Rawal LB, Tapp RJ, Williams ED, Chan C, Yasin S, Oldenburg B. Prevention of type 2 diabetes and its complications in developing countries: a review. Int J Behav Med. 2012;19(2):121‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CDC . National Diabetes Statistics Report. Estimates of Diabetes and Its Burden in the United States. In.
- 7. Norris SL, Engelgau MM, Venkat Narayan KM. Effectiveness of self‐management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561‐587. [DOI] [PubMed] [Google Scholar]
- 8. Makrilakis K, Liatis S, Grammatikou S, Perrea D, Katsilambros N. Implementation and effectiveness of the first community lifestyle intervention programme to prevent type 2 diabetes in Greece. The DE‐PLAN study. Diabet Med. 2010;27(4):459‐465. [DOI] [PubMed] [Google Scholar]
- 9. Liu K, Mi M‐T, Zhou R, Wang B. Effect of resveratrol on glucose control and insulin sensitivity: a meta‐analysis of 11 randomized controlled trials. Am J Clin Nutr. 2014;99(6):1510‐1519. [DOI] [PubMed] [Google Scholar]
- 10. Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta‐analysis. J Med Food. 2011;14(9):884‐889. [DOI] [PubMed] [Google Scholar]
- 11. Jafarnejad S, Keshavarz SA, Mahbubi S, et al. Effect of ginger (Zingiber officinale) on blood glucose and lipid concentrations in diabetic and hyperlipidemic subjects: a meta‐analysis of randomized controlled trials. J Funct Foods. 2017;29:127‐134. [Google Scholar]
- 12. Jafarnejad S, Tsang C, Taghizadeh M, Asemi Z, Keshavarz SA. A meta‐analysis of cumin (Cuminum cyminim L.) consumption on metabolic and anthropometric indices in overweight and type 2 diabetics. J Funct Foods. 2018;44:313‐321. [Google Scholar]
- 13. Menezes R, Rodriguez‐Mateos A, Kaltsatou A, et al. Impact of Flavonols on Cardiometabolic biomarkers: a meta‐analysis of randomized controlled human trials to explore the role of inter‐individual variability. Nutrients. 2017;9(2):117. [Google Scholar]
- 14. Heim K, Tagliaferro A, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure‐activity relationships. J Nutr Biochem. 2002;13(10):572‐584. [DOI] [PubMed] [Google Scholar]
- 15. Ivey KL, Hodgson JM, Croft KD, Lewis JR, Prince RL. Flavonoid intake and all‐cause mortality. Am J Clin Nutr. 2015;101(5):1012‐1020. [DOI] [PubMed] [Google Scholar]
- 16. Lagerweij GR, de Wit GA, Moons KG, et al. A new selection method to increase the health benefits of CVD prevention strategies. Eur J Prev Cardiol. 2018;25(6):642‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulvihill EE, Huff MW. Antiatherogenic properties of flavonoids: implications for cardiovascular health. Can J Cardiol. 2010;26(Suppl A):17a‐21a. [DOI] [PubMed] [Google Scholar]
- 18. Wu GA, Terol J, Ibanez V, et al. Genomics of the origin and evolution of citrus. Nature. 2018;554(7692):311‐316. [DOI] [PubMed] [Google Scholar]
- 19. Olszanecki R, Gebska A, Kozlovski VI, Gryglewski RJ. Flavonoids and nitric oxide synthase. J Physiol Pharmacol. 2002;53:571‐584. [PubMed] [Google Scholar]
- 20. Lorzadeh E, Ramezani‐Jolfaie N, Mohammadi M, Khoshbakht Y, Salehi‐Abargouei A. The effect of hesperidin supplementation on inflammatory markers in human adults: a systematic review and meta‐analysis of randomized controlled clinical trials. Chem Biol Interact. 2019;307:8‐15. [DOI] [PubMed] [Google Scholar]
- 21. Franke SIR, Molz P, Mai C, et al. Influence of hesperidin and vitamin C on glycemic parameters, lipid profile, and DNA damage in rats treated with sucrose overload. An Acad Bras Cienc. 2018;90(2 suppl 1):2203‐2210. [DOI] [PubMed] [Google Scholar]
- 22. Toumi M, Merzoug S, Boutefnouchet A, Tahraoui A, Ouali K, Guellati MA. Hesperidin, a natural citrus flavanone, alleviates hyperglycaemic state and attenuates embryopathies in pregnant diabetic mice, 2009.
- 23. Jung UJ, Lee M‐K, Jeong K‐S, Choi M‐S. The hypoglycemic effects of hesperidin and Naringin are partly mediated by hepatic glucose‐regulating enzymes in C57BL/KsJ‐db/db mice. J Nutr. 2004;134(10):2499‐2503. [DOI] [PubMed] [Google Scholar]
- 24. Iskender H, Dokumacioglu E, Sen TM, Ince I, Kanbay Y, Saral S. The effect of hesperidin and quercetin on oxidative stress, NF‐κB and SIRT1 levels in a STZ‐induced experimental diabetes model. Biomed Pharmacother. 2017;90:500‐508. [DOI] [PubMed] [Google Scholar]
- 25. Lee M‐K, Jeong K‐S, Jung UJ, Choi M‐S. The hypoglycemic effects of hesperidin and Naringin are partly mediated by hepatic glucose‐regulating enzymes in C57BL/KsJ‐db/db mice. J Nutr. 2004;134:2499‐2503. [DOI] [PubMed] [Google Scholar]
- 26. Eghtesadi S, Mohammadi M, Vafa M, Heidari I, Salehi M, Khadem HH, Amiri F, Alipour R, Eghtesadi M. Effects of hesperidin supplementation on glycemic control, lipid profile and inflammatory factors in patients with type 2 diabetes: a randomized, double‐blind and placebo‐controlled clinical trial. In: World Congress on Clinical Trials in Diabetes: BioScientifica, 2016.
- 27. Homayouni F, Haidari F, Hedayati M, Zakerkish M, Ahmadi K. Hesperidin supplementation alleviates oxidative DNA damage and lipid peroxidation in type 2 diabetes: a randomized double‐blind placebo‐controlled clinical trial. Phytother Res. 2017;31(10):1539‐1545. [DOI] [PubMed] [Google Scholar]
- 28. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zarei S, Salehi‐Abargouei A, Mohammadi M, Ramezani‐Jolfaie N. Effects of hesperidin and orange juice supplementation on glycemic control: a systematic review and meta‐analysis of clinical trials. PROSPERO 2017 CRD42017058859. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php? ID=CRD42017058859. 2017.
- 30. Higgins JPT, Green S. (Eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 []. The Cochrane Collaboration; 2011. Available from http://handbook.cochrane.org. [Google Scholar]
- 31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed). 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egger M, Davey‐Smith G, Altman D. Systematic reviews in health care: meta‐analysis in context. London: John Wiley & Sons; 2001. [Google Scholar]
- 34. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol. 2017;145(Suppl 1):S272‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salden BN, Troost FJ, De Groot E, et al. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals1,2. Am J Clin Nutr. 2016;104(6):1523‐1533. [DOI] [PubMed] [Google Scholar]
- 38. Rangel‐Huerta OD, Aguilera CM, Martin MV, et al. Normal or high polyphenol concentration in Orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr. 2015;145(8):1808‐1816. [DOI] [PubMed] [Google Scholar]
- 39. Rizza S, Muniyappa R, Iantorno M, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(5):E782‐E792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morand C, Dubray C, Milenkovic D, et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. 2011;93(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 41. Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17(7):573‐586. [DOI] [PubMed] [Google Scholar]
- 42. Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595‐1607. [DOI] [PubMed] [Google Scholar]
- 43. Guignot L, Mithieux G. Mechanisms by which insulin, associated or not with glucose, may inhibit hepatic glucose production in the rat. Am J Physiol Endocrinol Metab. 1999;277(6):E984‐E989. [DOI] [PubMed] [Google Scholar]
- 44. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta‐cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37(6):667‐687. [DOI] [PubMed] [Google Scholar]
- 45. Ahren B, Larsson H, Wilhelmsson C, Nasman B, Olsson T. Regulation of circulating leptin in humans. Endocrine. 1997;7(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 46. Wabitsch M, Jensen PB, Blum WF, et al. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45(10):1435‐1438. [DOI] [PubMed] [Google Scholar]
- 47. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67‐S74. [DOI] [PMC free article] [PubMed] [Google Scholar]


