Abstract
Recurrent miscarriage (RM) and vasculoplacental disorders, such as preeclampsia (PE), affect women of childbearing age worldwide. Vascular endothelial dysfunction and immunological impairment are associated with both RM and PE. To date, there is no effective or optimal therapeutic approach for these conditions. Notably, aspirin use is only partially effective in the prevention of PE. Hydroxychloroquine (HCQ) has demonstrated beneficial effects on disease flares, pregnancy outcomes and cardiovascular impairment in systemic erythaematosus lupus (SLE) through its immunomodulatory, vasculoprotective and antithrombotic properties. Here, in the context of the underlying physiological dysregulation associated with PE and RM, the beneficial properties and potential therapeutic efficacy of HCQ are reviewed in anticipation of the results of current and future trials. Two related trials addressing RM in the absence of maternal autoimmune disease are ongoing. Other trials addressing pregnancy outcomes in the presence of maternal autoimmune disease are forthcoming. In this review, we hypothesise that the immunological and endothelial effects of HCQ may be beneficial in the context of PE and RM, regardless of the maternal autoimmune status.
Keywords: hydroxychloroquine, pregnancy, preeclampsia, recurrent miscarriage
1. INTRODUCTION
Recurrent miscarriage (RM), defined as ≥3 consecutive miscarriages, is a frequent condition in reproductive medicine, and it affects 1 to 2% of fertile couples. To date, there is no effective treatment for preventing the recurrence of pregnancy loss. Similarly, preeclampsia (PE), defined as concomitant arterial hypertension and significant proteinuria after 20 weeks of gestation (WG), affects approximately 5% of pregnant women worldwide. The only treatment for PE is delivery, which needs to be induced early. PE causes maternal and fetal complications, such as premature birth. PE has a high recurrence rate (approximately 20%)1, 2 and can be only partially prevented by aspirin use.
2. PATHOPHYSIOLOGY OF PREECLAMPSIA
PE is a complex disease with a multifaceted presentation. Some authors have hypothesised the existence of two types of PE: early onset and late‐onset PE.3 These types share the same pathogenesis but have different inherent maternal characteristics.4, 5 The precise pathogenesis of PE is unclear. However, the placenta is the organ that triggers PE, and an imbalance in early angiogenesis seems to be a key risk factor for PE.
Early onset PE can be explained by impaired early placentation due to an imbalance between growing fetal trophoblasts and maternal endothelial vascular remodelling. Early onset PE is mediated by innate immune mechanisms. This was suggested by a study reporting that deficient stimulation of uterine natural killer (NK) cells is correlated with the inadequate expression of paternal‐fetal HLA surface‐cell markers.6 Under physiological conditions, the invading cytotrophoblasts adopt a vascular adhesion phenotype. The defect in vascular remodelling is the consequence of the deficient transformation of cytotrophoblast surface integrins and adhesion molecules.7
Late‐onset PE may be linked to a pre‐existing maternal endothelial dysfunction that is associated with obesity, diabetes, chronic hypertension or age >35 years.8, 9 Long‐term cardiovascular complications are associated with late‐onset PE. High placental growth factor (PlGF) levels at PE diagnosis have been reported in late‐onset PE compared to non‐hypertensive pregnancies.10 These high PlGF levels have been associated with an increased risk of coronary disease more than 10 years after the PE diagnosis.10 Late‐onset PE is the most predictable type of PE; thus, its prevention should be a standard therapeutic strategy in the care of nulliparous women.
In late pregnancy, placenta‐induced hypoxemia and its related complications become apparent in both types of PE. This placenta‐induced hypoxemia leads to the release of numerous placental factors, such as antiangiogenic factors and trophoblastic debris (e.g., syncytiotrophoblast membrane microparticles, fetal soluble DNA and RNA, cytotrophoblast cells), into the maternal circulation.11, 12 Upregulated antiangiogenic factors (soluble fms‐like tyrosine kinase 1 [sFlt1] and soluble endoglin [sEng]) bind to angiogenic factors (VEGF and PlGF) and reduce their bioavailability.13 The severity and timing of this angiogenic imbalance, combined with inherent maternal factors, may be the determinants of the clinical presentation of PE.4 Moreover, placenta‐induced hypoxaemia leads to placental oxidative stress,14 resulting in mitochondrial dysfunction, NADPH1 upregulation,15 and elevated levels of free radicals and oxidised lipids. Lastly, placenta‐induced hypoxaemia induces apoptosis and adiponecrosis in the placenta.
The above effects of placenta‐induced hypoxia trigger the following maternal responses: (i) a systemic inflammatory response with proinflammatory cytokine production,13, 14, 15, 16 lysosomal (toll‐like receptor [TLR]2, 4) and extralysosomal (TLR3, 7, 9) TLR activation,17, 18, 19 alterations in the Th1/Th2 balance, the production of agonistic angiotensin II type 1 receptor antibodies20 (and hence vasoconstriction via endothelin 1,21 the stimulation of NADPH oxidase22 and sFlt1 production23) and the activation of the complement system; (ii) the activation of maternal oxidative stress, resulting in endothelial NADPH2 upregulation in women with PE24; and (iii) maternal endothelial dysfunction with increased production of vasoconstrictor molecules25 (endothelin 1, thromboxane A2), increased activation of the renin‐angiotensin system, a decrease in NO, and the overexpression of adhesion molecules (ICAM, VCAM) (leading to the adhesion of monocytes to the endothelium).
The clinical presentation of maternal endothelial dysfunction is vasoconstriction in the mother manifesting as hypertension and glomerular dysfunction. In addition, severe symptoms can occur either in the mother (HELLP syndrome, disseminated intravascular coagulation, placental abruption, eclampsia) or in the fetus (intrauterine growth restriction [IUGR], stillbirth).
To date, the only therapeutic option for the prevention of PE is aspirin use. Thus, aspirin given at a daily dose of 75–150 mg26, 27, 28 from the end of the first trimester to the end of pregnancy may reduce the risk of PE onset by 30%.27 In women with a history of early onset PE (<34 WG), the gestational time is most often prolonged by approximately five weeks in the subsequent pregnancy.29 Given the severity of the disease, identifying additional treatments for women with a history of early onset PE remains essential.
3. PATHOPHYSIOLOGY OF RECURRENT MISCARRIAGE
In RM, standard investigations (parental karyotypes, uterine cavity exploration, screening for antiphospholipid [aPL] antibodies) fail to reveal an apparent cause for RM in ~50% of women. However, on the basis of animal models and clinical studies, several hypotheses have been proposed: an altered ovarian reserve,30, 31 a progesterone defect,32 thrombotic and/or endothelial dysfunction,33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 immunological disturbances,40, 49, 50, 51, 52, 53, 54, 55, 56 and chronic endometritis.57, 58, 59, 60 Some of these possible mechanisms may be occur simultaneously in the same patient, e.g., in obese women at high risk for RM.61
Consequently, a variety of therapeutics, targeting haemostasis (LMWH, aspirin),62, 63 immune mechanisms (polyvalent immunoglobulins, small doses of corticosteroids),64 or endocrine mechanisms (e.g., progesterone), have been tested.32, 65 To date, no treatment seems to be effective. This is even true in the presence of well‐known risk factors of RM, such as aPL antibody positivity, which is detected in 5–15% of women with RM,66 as demonstrated by the HEPASA study.67 For women of childbearing age with a history of RM, the current recommendations for their follow‐up rely on cocooning (i.e., nonpharmacologic interventions, such as psychological support, or dietary therapy for the management of obesity or vitamin deficiencies).49
A basal prothrombotic state was previously observed in nonpregnant women with previous RM who were negative for aPL antibodies and appeared to be without inherited thrombophilia.33, 34, 68 This prothrombotic state can be a sign of endothelial damage. In this subgroup of women, endothelial damage was demonstrated by increased levels of circulating endothelial markers, such as microparticles.35 The EPIC‐Heidelberg cohort study suggested that RM is a strong sex‐specific predictor of myocardial infarction (fivefold greater risk after adjusting for age and usual risk factors).36 An association between RM and some types of inherited thrombophilia was also reported (factor V Leiden, mutation G20210A in prothrombin, protein S deficiency).37, 38 However, the clinical relevance of this association remains to be determined. It has been shown that women with a history of RM have reduced levels of annexin A5 and resistance to anticoagulant activity of annexin A5.41 The M2 haplotype in the annexin A5 gene might be a risk factor for obstetric complications such as RM and PE.42 In mice, some components of the haemostatic system may participate in placental development, independent of the coagulation process itself. Some fetal regulators of coagulation, expressed by trophoblast cells (thrombomodulin, EPCR, tissue factor [TF]), interact with soluble maternal regulators of coagulation and platelets for normal placental development independent of the coagulation process.43, 44, 45, 46, 47, 48 However, overactivation of the placental coagulation process is harmful, and TF has been identified as a crucial mediator of fetal and placental damage in mouse models of RM.39
With regard to aPL‐induced RM, vascular endothelial cell dysfunction, mediated by aPL antibody binding to endothelial cell β2GP1 receptors and subsequent complement activation, are the key mechanisms in APS pathogenesis.69 aPL antibodies can inhibit endothelial nitric oxide synthase (eNOS) in endothelial cells in vitro and in vivo.70 This results in impaired endothelial function.
Furthermore, normal placental development requires a well‐functioning uteroplacental interface microenvironment. In women with RM, a positive correlation was found between the density of NK cell infiltration into the uterine lining and the number of previous miscarriages.49 An increase in circulating NK cells has been reported in these women.50 Uterine NK cells contribute to the cytokine response and the Th1/Th2 balance during pregnancy. Women with RM tend to produce a predominant Th1‐type response.56 Moreover, the overexpression of TLR, particularly TLR4 and TLR9, in the decidua is observed in women with RM.51
An in vitro study suggested that obstetric morbidity in APS might be linked to the placental inflammatory response mediated by the binding of aPL antibodies to β2GP1 receptors on the surface of placental trophoblasts.52 aPL antibodies activate TLR4, which results in the production of proinflammatory interleukin (Il)8 and Il1β. NF‐KB pathway activation leads to the release of proinflammatory cytokines such as Il6, the overexpression of ICAM1 and VCAM1, and the production of TF.53, 54 In addition, aPL antibodies induce the activation of the classical complement pathway.55 Finally, aPL antibodies reduce the levels of annexin A5 on placental villous syncytiotrophoblasts.40
4. INTRODUCTION TO HYDROXYCHLOROQUINE (MOLECULE AND CLINICAL USE)
Hydroxychloroquine (HCQ), a molecule derived from quinacrine, was first used as an antimalarial agent in 1955.71 Shortly thereafter, HCQ was commonly used as an anti‐inflammatory and immunomodulatory agent at a dose of 200–400 mg/day for the treatment of autoimmune diseases, such as rheumatoid arthritis (RA) and systemic erythaematosus lupus (SLE). HCQ was briefly used at a high dose (600–1200 mg/day) as a thromboembolic prophylactic agent after total hip replacement in the 1970s in some centres, before the routine use of LMWH.72, 73, 74 HCQ is currently being given as an adjuvant treatment at 600 mg/day for some intracellular infections, such as Whipple disease.75
At a physiologic pH of 7.4, a small fraction of HCQ (a more water‐soluble agent than chloroquine [CQ]) becomes monoprotonated; some of the HCQ molecules are lipid soluble and can cross the cell membrane.76 Due to this ability, HCQ can coexist with nucleic acids in endosomes, such as melanosomes. When biprotonated, as occurs in lysosomes at a pH of 4–5, HCQ is sequestered and cannot cross into the cytoplasm. This increases the pH within the lysosome and endosome and thereby alters intracellular processes, such as protein degradation, the assembly of macromolecules and the posttranslational modification of proteins.77 CQ only changes the chemical environment of DNA without any effect on the DNA primary structure (i.e., hinders nucleic acid assembly or the availability of TLR binding sites).78
The pleiomorphic clinical effects of HCQ are of high interest among scientists. Some properties of HCQ, such as its vasculoprotective effects, are well known in the management of SLE. The life expectancy of SLE patients has been significantly extended since HCQ began to be routinely used.
5. SAFETY OF HCQ IN PREGNANCY
The pharmacokinetic properties of HCQ (good oral absorption, long plasma elimination half‐life of ~40 days due to its high volume of distribution at ~44 000 L,79 slow 50% renal clearance) confer the following. HCQ crosses the placental barrier and is found at similar concentrations in both the umbilical cord blood and maternal blood. However, CQ (a pharmacologic analogue of HCQ) prophylaxis for malaria has been reported to be safe by numerous studies that have included more than 10 000 pregnant women.80, 81 This is even true with regard to the neurological development of children and their 1‐year visual acuity.81 Similarly, cumulative clinical data in pregnant women with autoimmune disorders, such as SLE, have suggested no HCQ‐related adverse effect on the fetus, with the exception of one meta‐analysis that noted an increased rate of spontaneous abortion. However, this analysis was potentially biased by the higher underlying disease activity and older age of the HCQ‐exposed group.82, 83, 84, 85, 86, 87 Tarfaoui et al.88 analysed the results of nine studies enrolling 246 new‐borns who were exposed to HCQ in utero and screened at birth for ocular damage. They found no clinical ocular toxicity but suspected preclinical retinal toxicity on electrophysiological explorations of four new‐borns. Moreover, other studies have reported reassuring data with regard to fetuses and new‐borns (especially for ocular and hearing deficits) for more than 400 pregnancies exposed to HCQ in utero.82, 85, 89 A good risk‐to‐benefit balance regarding a pregnant woman's health condition and pregnancy outcomes in terms of HCQ treatment is supported in the context of SLE90 and in RA91 by the EULAR and British Society of Rheumatology, respectively.
HCQ is proven to be safe and effective in treating the immunological impairment and chronic endothelial dysfunction of pregnant women with SLE. In a recent study92 of 251 SLE patients with 263 pregnancies, HCQ was associated with a lower risk (OR = 0.3, 95% CI = 0.1–0.7, P = 0.01) of adverse pregnancy outcomes (stillbirth, premature birth, IUGR). Since 1983, case reports and case series on HCQ use during pregnancy and breast‐feeding have not reported an increased incidence of abnormalities in fetal outcomes or in early childhood development.93, 94, 95, 96, 97 Given the reported safety profile of HCQ in pregnancy, there has been a rise in HCQ use during pregnancy in the past 13 years (from 6.3% in 2005 to 60.9% in 2017).92
6. MOLECULAR AND CELLULAR EFFECTS OF HCQ
HCQ has well‐known anti‐inflammatory and immunomodulatory effects (Figure 1).77, 98, 99, 100, 101, 102 It has an impact on innate immune mechanisms through the inhibition of some TLRs (3, 7, 9).78, 103, 104 HCQ decreases the levels of circulating Il1, Il2,105 Il6,100 TNFα100, 106 and interferon‐γ100 and thus promotes the TH2 processes of a normal pregnancy immunological state. Moreover, HCQ lowers aPL plasma levels107 and interferes with both endothelial cell activation and TNFα production, two key pathways involved in APS.108, 109, 110
Figure 1.
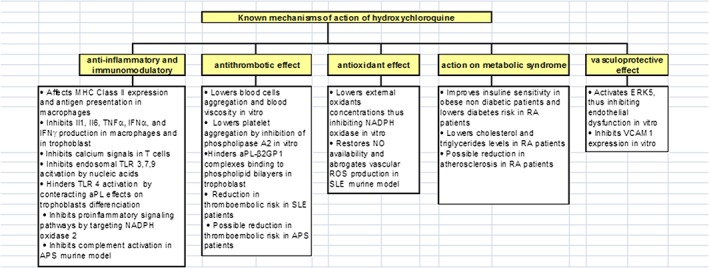
Known mechanisms of action of hydroxychloroquine
The antithrombotic activity of HCQ in patients without autoimmune diseases is not as well known (Figure 1).111, 112 Randomised trials72, 73, 74 on approximately 10 000 patients have concluded that HCQ can prevent venous thromboembolism (VTE) after orthopaedic surgery.72 Furthermore, HCQ can reduce the size of induced thrombi in mice previously treated with monoclonal aPL antibodies.113 In a study of 272 patients with SLE,114 an 83% reduction in VTE risk was observed in HCQ‐exposed patients compared to unexposed patients. A cohort study of 1930 patients with SLE found a 38% reduction in VTE risk.115 In a recent non‐randomised study,116 20 APS patients without SLE were treated with HCQ combined with direct oral anticoagulants (DOACs) and compared with 20 controls treated with only DOACs for three years. The VTE recurrence rates in the treatment and control patients were 0% and 30%, respectively. This suggests that HCQ could be used for VTE prophylaxis. Further randomised studies are warranted to confirm these results.
Moreover, HCQ can be an effective treatment for endothelial dysfunction through the following mechanisms: ERK5 protein kinase activation, anti‐diabetic actions, lipid lowering effects and antioxidant actions117, 118, 119 (Figure 1). ERK5 is a mitogen‐activated protein kinase with transcriptional activity that inhibits endothelial inflammation and dysfunction. In an in vitro model of cultured human and bovine endothelial cells, Le et al.120 demonstrated that HCQ was a strong ERK5 activator and inhibited VCAM‐1 expression in an ERK5‐dependent manner.
The antioxidant effects of HCQ were demonstrated in murine studies on adjuvant arthritis.121 In human neutrophils, HCQ reduces the concentration of external oxidants and decreases the phosphorylation of protein kinase C, thus regulating NADPH oxidase activation on the plasma membrane. Additionally, Virdis et al.122 highlighted that HCQ prevents the development of endothelial dysfunction (i.e., ROS overload) in a murine model of SLE via an antioxidant effect. In this experiment, HCQ restored NO availability and suppressed NADPH‐oxidase‐induced vascular ROS overload.
Furthermore, HCQ has beneficial metabolic actions. HCQ improves insulin sensitivity in obese nondiabetic subjects.117 In a prospective observational cohort of 4905 RA patients, the adjusted relative risk to develop diabetes was reduced by 77% in patients treated with HCQ118 compared to HCQ‐unexposed patients. HCQ reduced cholesterol and triglyceride levels in RA patients, regardless of concomitant steroid administration.119 In this study, the total cholesterol and LDL cholesterol levels were lowered in patients treated with HCQ, but there were no differences in the HDL cholesterol levels.
Finally, in vitro, HCQ has protective effects on human placenta exposed to aPL. HCQ can reverse aPL‐mediated inhibition of trophoblast IL6 secretion and limit aPL‐mediated inhibition of cell migration.123 HCQ can also hinder the binding of aPL‐b2GP1 complexes to phospholipid bilayers and protect annexin A5 from disruption by aPL in trophoblasts.124, 125 Lastly, HCQ‐induced TLR4 activation can restore the trophoblastic differentiation affected by aPL.126
7. POTENTIAL MECHANISMS OF HYDROXYCHLOROQUINE IN THE PREVENTION OF PREECLAMPSIA OR RECURRENT MISCARRIAGE
In light of the above, we hypothesised that HCQ might also be beneficial in all‐comer women who present with PE or a history of RM. These prevalent clinical disorders may share some underlying mechanisms, namely, vascular endothelial dysfunction and immunological impairment (Figure 2).
Figure 2.
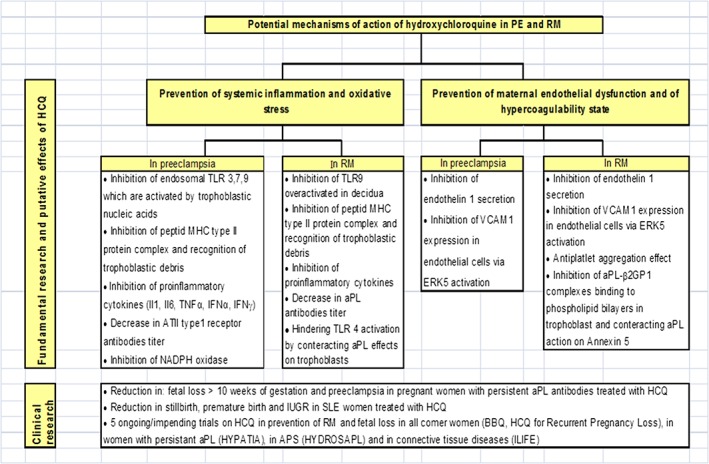
Potential mechanisms of action of hydroxychloroquine (HCQ) in preeclampsia (PE) and recurrent miscarriage (RM) and clinical studies
HCQ can prevent the occurrence of systemic inflammation in PE and RM by inhibiting lysosomal and extralysosomal TLR. During PE, TLR9 activation is increased due to the presence of nucleic acids from trophoblasts released into the maternal circulation in response to placental hypoxemia.19 In addition, TLR4 is overexpressed in the syncytium in PE.17 TLR overexpression disrupts maternal‐fetal immune tolerance. This is mediated by the activation of caspase 8/3 and its effect on T reg cell survival during RM.51 TLR9 activation can be induced by the injection of fetal DNA in mice.19 This effect can be inhibited by CQ, a known inhibitor of TLR9 activation.19 This is mediated by the effect of CQ on the chemical environment of DNA (CQ hinders nucleic acid assembly and the availability of TLR binding sites)78 (Figure 3).
Figure 3.
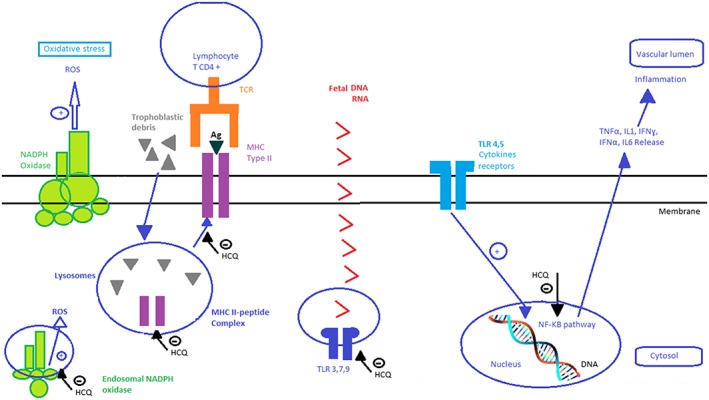
Potential effects of hydroxychloroquine on monocytes and macrophages in pregnant women
Other potential properties of HCQ are summarised below. Inhibition of the immune response to auto‐antigenic peptides (such as trophoblastic debris) via inhibition of peptide‐MHC type II protein complex formation can be beneficial in PE and RM.11, 12, 35 HCQ can also inhibit Il1, IL6, TNFα, IFNα and IFNα production in PE and RM, thus restoring the Th1/Th2 balance by favouring the Th2 immune response13, 16, 56 (Figure 3). HCQ can reduce the levels of angiotensin II type 1 receptor antibodies, mitigating then the clinical symptoms of PE (antibody titre is correlated with the severity of PE127). Moreover, HCQ can reduce aPL titres107 and hence be beneficial in obstetrical APS. Furthermore, HCQ may inhibit oxidative stress in preeclamptic women by targeting endosomal NADPH oxidase 2 and plasma membrane NADPH oxidase.78
Moreover, HCQ may also prevent endothelial dysfunction in both PE and RM through the inhibition of TNFα‐induced endothelin 1 secretion (Figure 4)128 and inhibition of VCAM‐1 expression to prevent monocyte adhesion to the endothelium.120 In addition, the HCQ‐related antiplatelet aggregation effect, which lowers blood viscosity,111, 112 might be beneficial to women with RM who demonstrate a basal prothrombotic state.
Figure 4.
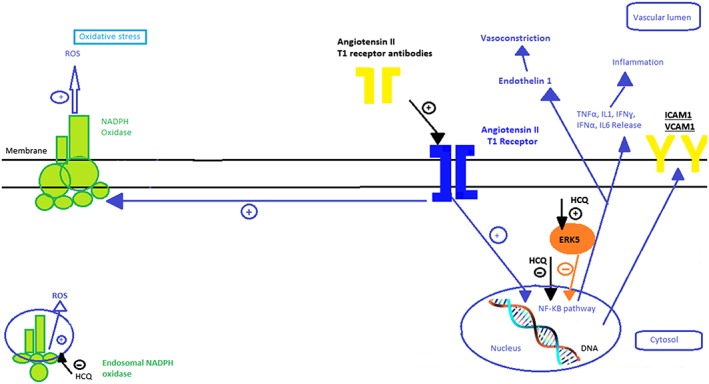
Potential effects of hydroxychloroquine on endothelial cells in pregnant women
In RM associated with aPL, the in vitro ability of HCQ to reduce the binding of aPL‐β2GPI complexes to phospholipid bilayers in trophoblasts and monocytes124 could be clinically relevant (Figure 5). Moreover, in vitro, HCQ inhibits platelet aggregation and arachidonic acid release from aPL‐activated platelets.129 In addition, HCQ inhibits the complement activation that is partly responsible for placental ischaemia102 in a mouse model of obstetrical APS.
Figure 5.
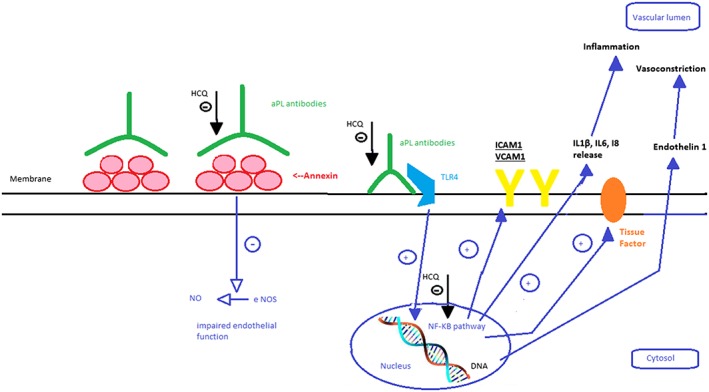
Potential effects of hydroxychloroquine on endothelial cells in the presence of aPL antibodies in pregnant women
Another possible HCQ‐related beneficial effect on RM can be anticipated from its well‐known anti‐infection activity (especially against intracellular bacterial infections) in chronic endometritis. Indeed, chronic endometritis is another possible underlying mechanism of RM.59 Finally, HCQ can ameliorate the metabolic syndrome associated with PE and RM.
8. CLINICAL STUDIES ON HCQ IN RM AND E
Clinical studies on HCQ in PE or RM have mostly involved women with autoimmune diseases. Interestingly, in a retrospective study of 170 pregnancies in 96 women with persistent positivity for aPL antibodies, Sciascia et al.89 found that HCQ (200–400 mg/day) reduced adverse pregnancy outcomes (OR = 2.2; 95% CI = 1.2–136), especially fetal losses at > 10 WG (2% vs. 11%; P = 0.05) and placenta‐mediated complications, such as PE, placental abruption and IUGR (2% vs 11%; P = 0.05). The pregnancy duration was longer for HCQ‐treated women (27.6 vs. 21.5 weeks; P = 0.03), and the new‐born birth weight was higher (3 kg vs. 2.3 kg; P = 0.04). Similarly, a retrospective Chinese study of 152 pregnancies in 122 women with SLE reported an 89% reduction in PE in HCQ‐treated women vs. non‐HCQ‐treated women.130
Two phase‐3 multicentre double‐blind randomised clinical trials are ongoing and are investigating the preventive effect of HCQ on fetal loss in women with a history of RM. One trial is a French study, namely, “HCQ for Prevention of RM or BBQ” (http://ClinicalTrials.gov: NCT03165136; estimated study completion date: February 2023131), which compares HCQ to a placebo in women with RM (three or more losses in the first trimester of pregnancy) regardless of their thrombophilia status. HCQ is orally administered before conception onset at a daily dose of 400 mg and is stopped at the end of 10 WG. The primary outcome is a live and viable birth. The other trial is a Danish study, namely, “HCQ for Recurrent Pregnancy Loss”, of women with RM without aPL antibody positivity (http://ClinicalTrials.gov: NCT03305263; estimated study completion date: January 2023).
Three upcoming trials (not yet recruiting) on women with autoimmune diseases will aim to evaluate the impact of HCQ in addition to conventional therapy in the prevention of obstetrical complications (ILIFE trial: NCT03671174; HYDROSAPL132; and HYPATIA133).
In the near future, the results of these five studies will help address the following question: “Could hydroxychloroquine prevent preeclampsia and recurrent miscarriage?”
9. CONCLUSION
In addition to the treatment of malaria, HCQ has many indications that are based on its anti‐inflammatory, immunomodulating, antithrombotic and vasculoprotective properties. The use of HCQ for autoimmune diseases, such as SLE, has a good safety record and improves pregnancy outcomes. Based on this and on our current insights into the pathophysiology of PE and RM, clinical studies on the potential benefit of HCQ in PE and RM are warranted.
COMPETING INTERESTS
The authors declare no conflicts of interest.
de Moreuil C, Alavi Z, Pasquier E. Hydroxychloroquine may be beneficial in preeclampsia and recurrent miscarriage. Br J Clin Pharmacol. 2020;86:39–49. 10.1111/bcp.14131
REFERENCES
- 1. Hernandez‐Diaz S, Toh S, Cnattingius S. Risk of pre‐eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Rijn BB, Hoeks LB, Bots ML, Franx A, Bruinse HW. Outcomes of subsequent pregnancy after first pregnancy with early‐onset preeclampsia. Am J Obstet Gynecol. 2006;195(3):723‐728. [DOI] [PubMed] [Google Scholar]
- 3. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592‐1594. [DOI] [PubMed] [Google Scholar]
- 4. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre‐eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang M, Niu J, Zhang L, et al. Gene expression profiling reveals different molecular patterns in G‐protein coupled receptor signaling pathways between early‐ and late‐onset preeclampsia. Placenta. 2016;40:52‐59. [DOI] [PubMed] [Google Scholar]
- 6. Redman CWG, Sargent IL. Immunology of pre‐eclampsia. Am J Reprod Immunol. 2010;63(6):534‐543. [DOI] [PubMed] [Google Scholar]
- 7. Zhou Y, McMaster M, Woo K, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duckitt K, Harrington D. Risk factors for pre‐eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365‐1370. [DOI] [PubMed] [Google Scholar]
- 10. Cassidy A, Chiuve SE, Manson JE, Rexrode KM, Girman CJ, Rimm EB. Potential role for plasma placental growth factor in predicting coronary heart disease risk in women. Arterioscler Thromb Vasc Biol. 2009;29(1):134‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672‐683. [DOI] [PubMed] [Google Scholar]
- 12. Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122(5):478‐487. [DOI] [PubMed] [Google Scholar]
- 13. Redman CWG, Sargent IL. Pre‐eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24(Suppl A):S21‐S27. [DOI] [PubMed] [Google Scholar]
- 14. Bilodeau J‐F, Hubel CA. Current concepts in the use of antioxidants for the treatment of preeclampsia. J Obstet Gynaecol Can. 2003;25(9):742‐750. [DOI] [PubMed] [Google Scholar]
- 15. Cui X‐L, Brockman D, Campos B, Myatt L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta. 2006;27(4‐5):422‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40(2):102‐111. [DOI] [PubMed] [Google Scholar]
- 17. Tangerås LH, Silva GB, Stødle GS, et al. Placental inflammation by HMGB1 activation of TLR4 at the syncytium. Placenta. 2018;72‐73:53‐61. [DOI] [PubMed] [Google Scholar]
- 18. Chatterjee P, Weaver LE, Doersch KM, et al. Placental toll‐like receptor 3 and toll‐like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS ONE. 2012;7(7):e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scharfe‐Nugent A, Corr SC, Carpenter SB, et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188(11):5706‐5712. [DOI] [PubMed] [Google Scholar]
- 20. Herse F, Verlohren S, Wenzel K, et al. Prevalence of agonistic autoantibodies against the angiotensin II type 1 receptor and soluble Fms‐like tyrosine kinase 1 in a gestational age‐matched case study. Hypertension. 2009;53(2):393‐398. [DOI] [PubMed] [Google Scholar]
- 21. Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res. 2013;113(1):78‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dechend R, Viedt C, Müller DN, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107(12):1632‐1639. [DOI] [PubMed] [Google Scholar]
- 23. Zhou CC, Ahmad S, Mi T, et al. Autoantibody from women with preeclampsia induces soluble Fms‐like tyrosine kinase‐1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T‐cells signaling. Hypertension. 2008;51(4):1010‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim R, Acharya R, Delpachitra P, et al. Activin and NADPH‐oxidase in preeclampsia: insights from in vitro and murine studies. Am J Obstet Gynecol. 2015;212(1):86.e1‐86.e12. [DOI] [PubMed] [Google Scholar]
- 25. Verdonk K, Saleh L, Smilde JE, et al. 8C.03: a key role for endothelin‐1 in the pathogenesis of preeclampsia and the associated suppression of the renin‐angiotensin‐aldosterone system. J Hypertens. 2015;33(Suppl 1):e110. [DOI] [PubMed] [Google Scholar]
- 26. Askie LM, Duley L, Henderson‐Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre‐eclampsia: a meta‐analysis of individual patient data. Lancet. 2007;369(9575):1791‐1798. [DOI] [PubMed] [Google Scholar]
- 27. Tolcher MC, Chu DM, Hollier LM, et al. Impact of USPSTF recommendations for aspirin for prevention of recurrent preeclampsia. Am J Obstet Gynecol. 2017;217(3):365.e1‐365.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613‐622. [DOI] [PubMed] [Google Scholar]
- 29. Seeho SK, Algert CS, Roberts CL, Ford JB. Early‐onset preeclampsia appears to discourage subsequent pregnancy but the risks may be overestimated. Am J Obstet Gynecol. 2016;215(6):785.e1‐785.e8. [DOI] [PubMed] [Google Scholar]
- 30. Atasever M, Soyman Z, Demirel E, Gencdal S, Kelekci S. Diminished ovarian reserve: is it a neglected cause in the assessment of recurrent miscarriage? A cohort study. Fertil Steril. 2016;105(5):1236‐1240. [DOI] [PubMed] [Google Scholar]
- 31. Pils S, Promberger R, Springer S, Joura E, Ott J. Decreased ovarian reserve predicts inexplicability of recurrent miscarriage? A retrospective analysis. PLoS ONE. 2016;11(9):e0161606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst Rev. 2018;10:CD003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laude I, Rongieres‐Bertrand C, Boyer‐Neumann C, et al. Circulating procoagulant microparticles in women with unexplained pregnancy loss: a new insight. Thromb Haemost. 2001;85(01):18‐21. [PubMed] [Google Scholar]
- 34. de Saint Martin L, Duchemin J, Bohec C, et al. Increased thrombin generation measured in the presence of thrombomodulin in women with early pregnancy loss. Fertil Steril. 2011;95(5):1813.e1‐1815.e1. [DOI] [PubMed] [Google Scholar]
- 35. Pasquier E, de Saint Martin L, Bohec C, Collet M, Dignat George F, Mottier D. Unexplained pregnancy loss: a marker of basal endothelial dysfunction? Fertil Steril. 2013;100(4):1013‐1017. [DOI] [PubMed] [Google Scholar]
- 36. Kharazmi E, Dossus L, Rohrmann S, Kaaks R. Pregnancy loss and risk of cardiovascular disease: a prospective population‐based cohort study (EPIC‐Heidelberg). Heart. 2011;97(1):49‐54. [DOI] [PubMed] [Google Scholar]
- 37. Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta‐analysis. Lancet. 2003;361(9361):901‐908. [DOI] [PubMed] [Google Scholar]
- 38. Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132(2):171‐196. [DOI] [PubMed] [Google Scholar]
- 39. Girardi G. Role of tissue factor in pregnancy complications: crosstalk between coagulation and inflammation. Thromb Res. 2011;127(Suppl 3):S43‐S46. [DOI] [PubMed] [Google Scholar]
- 40. Rand JH, Wu XX, Guller S, et al. Reduction of annexin‐V (placental anticoagulant protein‐I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;171(6):1566‐1572. [DOI] [PubMed] [Google Scholar]
- 41. Rand JH, Arslan AA, Wu X‐X, et al. Reduction of circulating annexin A5 levels and resistance to annexin A5 anticoagulant activity in women with recurrent spontaneous pregnancy losses. Am J Obstet Gynecol. 2006;194(1):182‐188. [DOI] [PubMed] [Google Scholar]
- 42. Tiscia G, Colaizzo D, Chinni E, et al. Haplotype M2 in the annexin A5 (ANXA5) gene and the occurrence of obstetric complications. Thromb Haemost. 2009;102(2):309‐313. [DOI] [PubMed] [Google Scholar]
- 43. Sood R, Kalloway S, Mast AE, Hillard CJ, Weiler H. Fetomaternal cross talk in the placental vascular bed: control of coagulation by trophoblast cells. Blood. 2006;107(8):3173‐3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sood R, Sholl L, Isermann B, Zogg M, Coughlin SR, Weiler H. Maternal Par4 and platelets contribute to defective placenta formation in mouse embryos lacking thrombomodulin. Blood. 2008;112(3):585‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sood R. Thrombophilia and fetal loss: lessons from gene targeting in mice. Thromb Res. 2009;123(Suppl 2):S79‐S84. [DOI] [PubMed] [Google Scholar]
- 46. Weiler‐Guettler H, Christie PD, Beeler DL, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101(9):1983‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li W, Zheng X, Gu J‐M, et al. Extraembryonic expression of EPCR is essential for embryonic viability. Blood. 2005;106(8):2716‐2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Isermann B, Sood R, Pawlinski R, et al. The thrombomodulin‐protein C system is essential for the maintenance of pregnancy. Nat Med. 2003;9(3):331‐337. [DOI] [PubMed] [Google Scholar]
- 49. Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601‐611. [DOI] [PubMed] [Google Scholar]
- 50. Jeve YB, Davies W. Evidence‐based management of recurrent miscarriages. J Hum Reprod Sci. 2014;7(3):159‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kang X, Zhang X, Liu Z, et al. Excessive TLR9 signaling contributes to the pathogenesis of spontaneous abortion through impairment of Treg cell survival by activation of Caspase 8/3. Int Immunopharmacol. 2015;29(2):285‐292. [DOI] [PubMed] [Google Scholar]
- 52. Mulla MJ, Brosens JJ, Chamley LW, et al. Antiphospholipid antibodies induce a pro‐inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009;62(2):96‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lopez‐Pedrera C, Buendia P, Cuadrado MJ, et al. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF‐κB/Rel proteins via the p38 mitogen‐activated protein kinase pathway, and of the MEK‐1/ERK pathway. Arthritis Rheum. 2006;54(1):301‐311. [DOI] [PubMed] [Google Scholar]
- 54. Vega‐Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up‐regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005;52(5):1545‐1554. [DOI] [PubMed] [Google Scholar]
- 55. Pierangeli SS, Girardi G, Vega‐Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody‐mediated thrombophilia. Arthritis Rheum. 2005;52(7):2120‐2124. [DOI] [PubMed] [Google Scholar]
- 56. Ahmadi M, Aghdam SA, Nouri M, et al. Intravenous immunoglobulin (IVIG) treatment modulates peripheral blood Th17 and regulatory T cells in recurrent miscarriage patients: non randomized, open‐label clinical trial. Immunol Lett. 2017;192:12‐19. [DOI] [PubMed] [Google Scholar]
- 57. Cicinelli E, Mateo M, Tinelli R, et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci. 2014;21(5):640‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kitaya K. Prevalence of chronic endometritis in recurrent miscarriages. Fertil Steril. 2011;95(3):1156‐1158. [DOI] [PubMed] [Google Scholar]
- 59. McQueen DB, Perfetto CO, Hazard FK, Lathi RB. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil Steril. 2015;104(4):927‐931. [DOI] [PubMed] [Google Scholar]
- 60. Bouet P‐E, El Hachem H, Monceau E, Gariepy G, Kadoch I‐J, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105(1):106‐110. [DOI] [PubMed] [Google Scholar]
- 61. Cavalcante MB, Sarno M, Peixoto AB, Araujo Júnior E, Barini R. Obesity and recurrent miscarriage: a systematic review and meta‐analysis. J Obstet Gynaecol Res. 2019;45(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 62. Pasquier E, de Saint Martin L, Bohec C, et al. Enoxaparin for prevention of unexplained recurrent miscarriage: a multicenter randomized double‐blind placebo‐controlled trial. Blood. 2015;125(14):2200‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rodger MA, Hague WM, Kingdom J, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open‐label randomised trial. Lancet. 2014;384(9955):1673‐1683. [DOI] [PubMed] [Google Scholar]
- 64. Egerup P, Lindschou J, Gluud C, Christiansen OB, ImmuReM IPD Study Group . The effects of intravenous immunoglobulins in women with recurrent miscarriages: a systematic review of randomised trials with meta‐analyses and trial sequential analyses including individual patient data. PLoS ONE. 2015;10(10):e0141588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coomarasamy A, Williams H, Truchanowicz E, et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med. 2015;373(22):2141‐2148. [DOI] [PubMed] [Google Scholar]
- 66. Branch DW, Gibson M, Silver RM. Clinical practice. Recurrent miscarriage. N Engl J Med. 2010;363(18):1740‐1747. [DOI] [PubMed] [Google Scholar]
- 67. Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA trial. J Rheumatol. 2009;36(2):279‐287. [DOI] [PubMed] [Google Scholar]
- 68. Rai R, Tuddenham E, Backos M, et al. Thromboelastography, whole‐blood haemostasis and recurrent miscarriage. Hum Reprod. 2003;18(12):2540‐2543. [DOI] [PubMed] [Google Scholar]
- 69. Corban MT, Duarte‐Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. Antiphospholipid syndrome: role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J Am Coll Cardiol. 2017;69(18):2317‐2330. [DOI] [PubMed] [Google Scholar]
- 70. Ramesh S, Morrell CN, Tarango C, et al. Antiphospholipid antibodies promote leukocyte‐endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J Clin Invest. 2011;121(1):120‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wallace DJ. The history of antimalarials. Lupus. 1996;5(Suppl 1):S2‐S3. [PubMed] [Google Scholar]
- 72. Carter AE, Eban R. Prevention of postoperative deep venous thrombosis in legs by orally administered hydroxychloroquine sulphate. Br Med J. 1974;3(5923):94‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johansson E, Forsberg K, Johnsson H. Clinical and experimental evaluation of the thromboprophylactic effect of hydroxychloroquine sulfate after total hip replacement. Haemostasis. 1981;10(2):89‐96. [DOI] [PubMed] [Google Scholar]
- 74. Wu TK, Tsapogas MJ, Jordan FR. Prophylaxis of deep venous thrombosis by hydroxychloroquine sulfate and heparin. Surg Gynecol Obstet. 1977;145(5):714‐718. [PubMed] [Google Scholar]
- 75. Rolain J‐M, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30(4):297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Browning DJ, Lee C. Relative sensitivity and specificity of 10‐2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathy. Clin Ophthalmol. 2014;8:1389‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23(2 Suppl 1):82‐91. [DOI] [PubMed] [Google Scholar]
- 78. Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794‐7804. [DOI] [PubMed] [Google Scholar]
- 79. Rainsford KD, Parke AL, Clifford‐Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231‐269. [DOI] [PubMed] [Google Scholar]
- 80. Garner P, Gulmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev. 2006;4:CD000169. [DOI] [PubMed] [Google Scholar]
- 81. Villegas L, McGready R, Htway M, et al. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double‐blind, placebo‐controlled trial. Trop Med Int Health. 2007;12(2):209‐218. [DOI] [PubMed] [Google Scholar]
- 82. Costedoat‐Chalumeau N, Amoura Z, Huong DLT, Lechat P, Piette J‐C. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun Rev. 2005;4(2):111‐115. [DOI] [PubMed] [Google Scholar]
- 83. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795‐810. [DOI] [PubMed] [Google Scholar]
- 84. Buchanan NM, Toubi E, Khamashta MA, Lima F, Kerslake S, Hughes GR. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55(7):486‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Clowse MEB, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54(11):3640‐3647. [DOI] [PubMed] [Google Scholar]
- 86. Sperber K, Hom C, Chao CP, Shapiro D, Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kaplan YC, Ozsarfati J, Nickel C, Koren G. Reproductive outcomes following hydroxychloroquine use for autoimmune diseases: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;81(5):835‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tarfaoui N, Autret‐Leca E, Mazjoub S, Cissoko H, Jonville‐Bera A‐P. Hydroxychloroquine during pregnancy: a review of retinal toxicity in the newborns. Therapie. 2013;68(1):43‐47. [DOI] [PubMed] [Google Scholar]
- 89. Sciascia S, Hunt BJ, Talavera‐Garcia E, Lliso G, Khamashta MA, Cuadrado MJ. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol. 2016;214(2):273.e1‐273.e8. [DOI] [PubMed] [Google Scholar]
- 90. Bertsias G, Ioannidis JPA, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67(2):195‐205. [DOI] [PubMed] [Google Scholar]
- 91. Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—Part I: standard and biologic disease modifying anti‐rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55(9):1693‐1697. [DOI] [PubMed] [Google Scholar]
- 92. Zhan Z, Yang Y, Zhan Y, Chen D, Liang L, Yang X. Fetal outcomes and associated factors of adverse outcomes of pregnancy in southern Chinese women with systemic lupus erythematosus. PLoS ONE. 2017;12(4):e0176457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Suhonen R. Hydroxychloroquine administration in pregnancy. Arch Dermatol. 1983;119(3):185‐186. [PubMed] [Google Scholar]
- 94. Costedoat‐Chalumeau N, Amoura Z, Aymard G, et al. Evidence of transplacental passage of hydroxychloroquine in humans. Arthritis Rheum. 2002;46(4):1123‐1124. [DOI] [PubMed] [Google Scholar]
- 95. Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum. 2003;48(11):3207‐3211. [DOI] [PubMed] [Google Scholar]
- 96. Motta M, Tincani A, Faden D, et al. Follow‐up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J Perinatol. 2005;25(2):86‐89. [DOI] [PubMed] [Google Scholar]
- 97. Gaffar R, Pineau CA, Bernatsky S, Scott S, Vinet E. Risk of ocular anomalies in children exposed in utero to antimalarials: a systematic literature review. Arthritis Care Res (Hoboken). 2019;71(12):1606‐1610. [DOI] [PubMed] [Google Scholar]
- 98. Bertolaccini ML, Contento G, Lennen R, et al. Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J Autoimmun. 2016;75:30‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Karres I, Kremer JP, Dietl I, Steckholzer U, Jochum M, Ertel W. Chloroquine inhibits proinflammatory cytokine release into human whole blood. Am J Physiol. 1998;274(4 Pt 2):R1058‐R1064. [DOI] [PubMed] [Google Scholar]
- 100. van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor‐alpha, interleukin 6, and interferon‐gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24(1):55‐60. [PubMed] [Google Scholar]
- 101. Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. 2000;95(11):3460‐3466. [PubMed] [Google Scholar]
- 102. Muller‐Calleja N, Manukyan D, Canisius A, Strand D, Lackner KJ. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann Rheum Dis. 2017;76(5):891‐897. [DOI] [PubMed] [Google Scholar]
- 103. Lafyatis R, York M, Marshak‐Rothstein A. Antimalarial agents: closing the gate on Toll‐like receptors? Arthritis Rheum. 2006;54(10):3068‐3070. [DOI] [PubMed] [Google Scholar]
- 104. Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA—evidence of an inhibitory effect on Toll‐like receptor signaling. Nat Clin Pract Rheumatol. 2006;2(9):458‐459. [DOI] [PubMed] [Google Scholar]
- 105. Bygbjerg IC, Svenson M, Theander TG, Bendtzen K. Effect of antimalarial drugs on stimulation and interleukin 2 production of human lymphocytes. Int J Immunopharmacol. 1987;9(4):513‐519. [DOI] [PubMed] [Google Scholar]
- 106. Weber SM, Levitz SM. Chloroquine interferes with lipopolysaccharide‐induced TNF‐α gene expression by a nonlysosomotropic mechanism. J Immunol. 2000;165(3):1534‐1540. [DOI] [PubMed] [Google Scholar]
- 107. Broder A, Putterman C. Hydroxychloroquine use is associated with lower odds of persistently positive antiphospholipid antibodies and/or lupus anticoagulant in systemic lupus erythematosus. J Rheumatol. 2013;40(1):30‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Raschi E, Borghi MO, Grossi C, Broggini V, Pierangeli S, Meroni PL. Toll‐like receptors: another player in the pathogenesis of the anti‐phospholipid syndrome. Lupus. 2008;17(10):937‐942. [DOI] [PubMed] [Google Scholar]
- 109. Döring Y, Hurst J, Lorenz M, et al. Human antiphospholipid antibodies induce TNFα in monocytes via Toll‐like receptor 8. Immunobiology. 2010;215(3):230‐241. [DOI] [PubMed] [Google Scholar]
- 110. Berman J, Girardi G, Salmon JE. TNF‐α is a critical effector and a target for therapy in antiphospholipid antibody‐induced pregnancy loss. J Immunol. 2005;174(1):485‐490. [DOI] [PubMed] [Google Scholar]
- 111. Ernst E, Rose M, Lee R. Modification of transoperative changes in blood fluidity by hydroxychloroquine: a possible explanation for the drug's antithrombotic effect. Pharmatherapeutica. 1984;4(1):48‐52. [PubMed] [Google Scholar]
- 112. Jancinova V, Nosal R, Petrikova M. On the inhibitory effect of chloroquine on blood platelet aggregation. Thromb Res. 1994;74(5):495‐504. [DOI] [PubMed] [Google Scholar]
- 113. Edwards MH, Pierangeli S, Liu X, Barker JH, Anderson G, Harris EN. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96(12):4380‐4384. [DOI] [PubMed] [Google Scholar]
- 114. Mok MY, Chan EYT, Fong DYT, Leung KFS, Wong WS, Lau CS. Antiphospholipid antibody profiles and their clinical associations in Chinese patients with systemic lupus erythematosus. J Rheumatol. 2005;32(4):622‐628. [PubMed] [Google Scholar]
- 115. Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi‐ethnic cohort. Ann Rheum Dis. 2009;68(2):238‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schmidt‐Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11(10):1927‐1929. [DOI] [PubMed] [Google Scholar]
- 117. Mercer E, Rekedal L, Garg R, Lu B, Massarotti EM, Solomon DH. Hydroxychloroquine improves insulin sensitivity in obese non‐diabetic individuals. Arthritis Res Ther. 2012;14(3):R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wasko MCM, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187‐193. [DOI] [PubMed] [Google Scholar]
- 119. Wallace DJ, Metzger AL, Stecher VJ, Turnbull BA, Kern PA. Cholesterol‐lowering effect of hydroxychloroquine in patients with rheumatic disease: reversal of deleterious effects of steroids on lipids. Am J Med. 1990;89(3):322‐326. [DOI] [PubMed] [Google Scholar]
- 120. Le N‐T, Takei Y, Izawa‐Ishizawa Y, et al. Identification of activators of ERK5 transcriptional activity by high‐throughput screening and the role of endothelial ERK5 in vasoprotective effects induced by statins and antimalarial agents. J Immunol. 2014;193(7):3803‐3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jančinová V, Pažoureková S, Lucová M, et al. Selective inhibition of extracellular oxidants liberated from human neutrophils—a new mechanism potentially involved in the anti‐inflammatory activity of hydroxychloroquine. Int Immunopharmacol. 2015;28(1):175‐181. [DOI] [PubMed] [Google Scholar]
- 122. Virdis A, Tani C, Duranti E, et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Res Ther. 2015;17(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Albert CR, Schlesinger WJ, Viall CA, et al. Effect of hydroxychloroquine on antiphospholipid antibody‐induced changes in first trimester trophoblast function. Am J Reprod Immunol. 2014;71(2):154‐164. [DOI] [PubMed] [Google Scholar]
- 124. Rand JH, Wu X‐X, Quinn AS, Chen PP, Hathcock JJ, Taatjes DJ. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody‐beta2‐glycoprotein I complexes to phospholipid bilayers. Blood. 2008;112(5):1687‐1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rand JH, Wu X‐X, Quinn AS, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood. 2010;115(11):2292‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Marchetti T, Ruffatti A, Wuillemin C, de Moerloose P, Cohen M. Hydroxychloroquine restores trophoblast fusion affected by antiphospholipid antibodies. J Thromb Haemost. 2014;12(6):910‐920. [DOI] [PubMed] [Google Scholar]
- 127. Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension. 2010;55(2):386‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rahman R, Murthi P, Singh H, et al. The effects of hydroxychloroquine on endothelial dysfunction. Pregnancy Hypertens. 2016;6(4):259‐262. [DOI] [PubMed] [Google Scholar]
- 129. Pierangeli SS, Vega‐Ostertag M, Harris EN. Intracellular signaling triggered by antiphospholipid antibodies in platelets and endothelial cells: a pathway to targeted therapies. Thromb Res. 2004;114(5‐6):467‐476. [DOI] [PubMed] [Google Scholar]
- 130. Seo MR, Chae J, Kim YM, et al. Hydroxychloroquine treatment during pregnancy in lupus patients is associated with lower risk of preeclampsia. Lupus. 2019;28(6):722‐730. [DOI] [PubMed] [Google Scholar]
- 131. Pasquier E, de Saint‐Martin L, Marhic G, et al. Hydroxychloroquine for prevention of recurrent miscarriage: study protocol for a multicentre randomised placebo‐controlled trial BBQ study. BMJ Open. 2019;9(3):e025649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mekinian A, Vicaut E, Cohen J, Bornes M, Kayem G, Fain O. Hydroxychloroquine to obtain pregnancy without adverse obstetrical events in primary antiphospholipid syndrome: French phase II multicenter randomized trial, HYDROSAPL. Gynecol Obstet Fertil Senol. 2018;46(7‐8):598‐604. [DOI] [PubMed] [Google Scholar]
- 133. Schreiber K, Breen K, Cohen H, et al. HYdroxychloroquine to improve Pregnancy outcome in women with AnTIphospholipid Antibodies (HYPATIA) protocol: a multinational randomized controlled trial of hydroxychloroquine versus placebo in addition to standard treatment in pregnant women with antiphospholipid syndrome or antibodies. Semin Thromb Hemost. 2017;43(6):562‐571. [DOI] [PubMed] [Google Scholar]


