Abstract
Aims
Despite a significant increase in using cannabis for medical purposes, current evidence on its safety in real‐world clinical practice is still poorly characterised. By a case‐by‐case analysis of spontaneous reports of suspected adverse events (AEs) collected in Tuscany within the Italian Phytovigilance database, the aim of the present study was to describe AEs occurred in patients exposed to medical cannabis.
Methods
We evaluated all reports of cannabis‐related suspected AEs collected within the Phytovigilance database up to December 2018. Information regarding cannabis therapy, patient's demographic and clinical characteristics, concomitant medications, AE description according to the Medical Dictionary for Regulatory Activities (MedDRA) classification, AE seriousness and AE outcome, were collected. The causality assessment was performed following World Health Organisation–Uppsala Monitoring Centre criteria.
Results
Fifty‐three cannabis‐related AE reports were analysed. The majority of patients were females (77.3%), with a mean age of 61.9 years. Thirty‐nine (73.6%) cases were defined as nonserious and the majority of them (86.9%) showed a complete resolution or improvement. Forty‐six (86.8%) cases were judged as probably related to cannabis consumption. The most frequently reported system organ class was psychiatric and nervous system disorders, and a potential drug–drug interaction was present in 16 cases.
Conclusion
Cannabis was generally well tolerated and the majority of AEs were mild and transient. Our analysis highlighted important safety issues for clinical practice, in particular the need for an accurate prescription monitoring during the titration phase, particularly in the presence of concomitant medications.
Keywords: adverse drug reactions, clinical pharmacology, drug safety, general practice, medical cannabis
What is already known about this subject
Cannabis is a therapeutic option in multiple clinical conditions, including chronic pain, AIDS, cancer, glaucoma, Tourette syndrome and multiple sclerosis. Despite a significant increase in medical cannabis use, its safety in clinical practice is poorly characterised.
What this study adds
This study shows that medical cannabis is generally well tolerated and the majority of observed AEs are mild and transient. Important safety issues for clinical practice include the need for accurate prescription and monitoring during the titration phase, particularly in presence of concomitant medications.
1. INTRODUCTION
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main active substances in Cannabis sativa, and have several pharmacological targets that include both cannabinoid (CB) receptors (CB1 and CB2), the calcitonin gene‐related peptide orphan receptor, the ligand‐gated ion channels of human 5‐HT3A receptors, and other additional ionic channels and enzymes.1, 2 The action of THC and CBD on these targets is responsible for their therapeutic properties, ranging from pain relief to antiemetic, antiepileptic and anticraving effects.3
Recent evidence shows the positive effects of medical cannabis use both as first‐line treatment and as adjuvant therapy in several clinical conditions, identifying cannabis as a valid therapeutic option. In a study published by Bellnier and colleagues, the use of cannabis has led patients with chronic pain to discontinue their opioids, or to reduce opioids doses by approximately 75%.4 In patients affected by Dravet syndrome, CBD administration have reduced monthly drop seizure frequency from baseline by 43.9%, with the occurrence of mild or moderate adverse events (AEs).5 In a systematic review and meta‐analysis performed to evaluate the efficacy and safety of cannabinoids in different clinical settings, 79 trials for a total of 6462 participants have been included. Compared with placebo, a higher number of patients treated with cannabinoids have shown: (i) nausea and vomiting complete response; (ii) greater reduction in numerical rating scale pain assessment; (iii) greater reduction in the Ashworth spasticity scale. The study has highlighted an increased risk of short‐term AEs with cannabinoids, including those classified as serious.6 Another study by Nugent and colleagues7 reviewed the benefits of cannabis preparations for treating chronic pain in adults and the harms of cannabis use in chronic pain and general adult populations. Authors performed a systematic review of 27 chronic pain trials, 11 systematic reviews and 32 primary studies, concluding that cannabis may alleviate neuropathic pain in some patients, but insufficient evidence exists for other types of chronic pain. They also highlighted that cannabis is associated with an increased risk for adverse mental health effects.
In studies evaluating the safety of medical cannabis over all indications, it has been found to be safe and well tolerated,8 and its AEs have been considered less severe than those of other prescribed drugs.9 Nevertheless, different suspected AEs have been reported following cannabis consumption, and despite the significant increase in use, the current evidence on safety of medical cannabis in real‐world clinical practice are is poorly characterised.
Italy legally recognised medical use of cannabis since 2006, when the Ministry of Health authorised cannabis import from the Netherlands. In 2016, Italian production of cannabis was authorised and the Military Pharmaceutical Chemical Works (Florence) started growing and processing cannabis in a controlled and standardised setting, according to Good Manufacturing Practice. Medical cannabis can be prescribed as magistral preparations, such as oil extracts, decoction filter bags, and bags for inhalation through an authorised device. Medical use of cannabis is authorised in Italy and reimbursed within the National Health System for selected medical conditions (as reported in the Official Gazette no. 279 from 30 November 2015): (i) analgesia in diseases involving spasticity associated with pain (multiple sclerosis, spinal cord injury) resistant to conventional therapies; (ii) analgesia in chronic pain (with particular reference to neurogenic pain) in which treatment with nonsteroidal anti‐inflammatory drugs or with cortisone or opioid drugs has proved to be ineffective; (iii) anti‐motion sickness and antiemetic effect in nausea and vomiting, caused by chemotherapy, radiotherapy, human immunodeficiency virus therapies, which cannot be obtained with traditional treatments; (iv) appetite stimulating effect in cachexia, anorexia, loss of appetite in cancer patients or patients with AIDS and in anorexia nervosa, which cannot be obtained with standard treatments; (v) hypotensive effect in glaucoma resistant to conventional therapies; (vi) reduction of involuntary body and facial movements in Tourette syndrome, which cannot be achieved with standard treatments.10, 11, 12
Spontaneous reports of suspected AEs to natural health products (including galenic preparations containing herbals) are collected in Italy within the Italian Phytovigilance system coordinated by the National Institute of Health.13
In this context, the aim of the present study was to describe the safety profile of cannabis for medical use, with a case‐by‐case analysis of spontaneous reports of suspected AEs collected in Tuscany (Italy) within the Italian Phytovigilance database.
2. METHODS
We considered and evaluated all spontaneous reports of cannabis‐related suspected AEs collected within the Italian Phytovigilance database (coordinated by the Italian National Institute of Health) since 2006 (date of cannabis for medical use approval) until December 2018. All reports associated with registered products or magistral preparations containing cannabis were extracted from the database. From the reporting form (available online at: http://www.epicentro.iss.it/fitosorveglianza/pdf/scheda_fito.pdf), we collected all information on: (i) patient's demographical (i.e. age, sex etc.) and clinical status; (ii) AE, codified using the Medical Dictionary for Regulatory Activities (MedDRA) classification, and described using the preferred terms (PT) and system organ class (SOC) most frequently reported14, 15; (iii) AE degree of seriousness, classified according to the World Health Organisation criteria as fatal, life‐threatening, or requiring hospitalisation of the patient, or causing serious/permanent disability, or causing congenital abnormalities, or other clinically relevant conditions (https://apps.who.int/iris/bitstream/handle/10665/67378/WHO_EDM_QSM_2002.2.pdf;jsessionid=EF4F54EFF6FBD634E21D2C075ADCA3C0?sequence=1; 29661234); (iv) AE outcome; (v) ongoing cannabis therapy (i.e. product type, indication, dosage, length of exposure, and administration route); (vi) concomitant medications.
The causality assessment (categorised as certain, probable/likely, possible, unlikely or unclassifiable) was performed using the World Health Organisation–Uppsala Monitoring Centre system for standardised case causality assessment (https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf).
Moreover, a multidisciplinary group of experts in pharmacology, phytotherapy, clinical toxicology, pharmacovigilance and phytovigilance fields performed a case‐by‐case clinical evaluation by, with the aim of identifying potential factors (i.e. presence of drug–drug interaction, DDI), which may have contributed to the AEs collected.
Drug targets reported in the present manuscript conform to the IUPHAR/BPS Guide to PHARMACOLOGY nomenclature classification.16
Data a presented as number and percentages or, for continuous variables, as mean and standard deviation (SD).
3. RESULTS
During the study period (2006–2018), a total of 103 suspected AE reports concerning medical cannabis use in Italy have been collected in the Italian Phytovigilance database, of which 61 (59%) were reported by Tuscany healthcare professionals. We have excluded 8 reports due to lacking information which did not allow us to perform their clinical evaluation.
Table 1 and Table 2 show general patients' characteristics and a case‐by‐case clinical description of the 53 evaluated reports, respectively. The majority of patients were female (n = 41, 77.3%), with a mean age of 61.9 (±SD, 15.9) years. Out of all reports evaluated, 39 (73.6%) were defined as nonserious and only in 2 cases AE seriousness was not specified. Most AE reports (n = 45, 84.9%) showed complete resolution or improvement as AE outcome. Only 2 cases were still unresolved at the time of AE reporting. Neuropathic pain and chronic or nonspecified pain were the most reported indication for medical cannabis use, amounting to 23 (43.4%) and 21 (39.6%) cases, respectively. Indication for fibromyalgia and multiple sclerosis were reported in 5 and 2 cases, respectively. Only 1 patient took his medication by inhalation, while all other patients used cannabis orally, at a mean dose of 138.5 (±SD, 139.0) mg per day. Length of exposure before AE onset largely vary among cases, ranging from 1 day to 37 months (mean 149.3 ± 242.4 days). Thirty‐eight cases (71.7%) reported the presence of concomitant medications, mainly represented by pregabalin, gabapentin and other pain medication, such as paracetamol/codeine or oxycodone/naloxone fixed association.
Table 1.
General patients' characteristics
| Cases characteristics | No. of cases |
|---|---|
| n = 53 (%) | |
| Patient age, y | |
| 19–64 | 25 (47.2) |
| 65–79 | 24 (45.3) |
| ≥80 | 4 (7.5) |
| Mean ± standard deviation, y | 61.9 ± 15.9 |
| Sex | |
| Female | 41 (77.3) |
| Male | 12 (22.7) |
| Cannabis administration route | |
| Oral | 52 (98.1) |
| Inhalation | 1 (1.9) |
| Mean dose ± standard deviation, mg | 138.5 ± 139.0 |
| No. of concomitant medications | |
| None | 15 (28.3) |
| 1 | 13 (24.5) |
| ≥2 | 25 (47.2) |
| Potential drug–drug interaction | |
| No | 37 (69.8) |
| Yes | 16 (30.2) |
| Seriousness | |
| Nonserious | 39 (73.6) |
| Serious | 12 (22.6) |
| Not reported | 2 (3.8) |
| Outcome | |
| Complete resolution | 36 (67.9) |
| Improvement | 9 (17.0) |
| Still unresolved | 2 (3.8) |
| Not reported | 6 (11.3) |
| Causality assessment | |
| Certain | 1 (1.9) |
| Probable | 46 (86.8) |
| Possible | 5 (9.4) |
| Not classifiable | 1 (1.9) |
Table 2.
Case‐by‐case clinical description of all evaluated reports
| Case | Age (y) | M/F | Adverse events (PT) | Seriousness | Outcome | Product | Indication | Dosage | Exposure length | Administration route | Concomitant drugs | Causality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M |
Anxiety Panic attack Acute psychosis |
Serious | Complete resolution | Bedrocan | Neuropathic pain | 50 mg | 110 days | Inhaled | Alprazolam | Probable |
| 2 | 72 | F |
Dysphoria Visual hallucinations Drowsiness Accidental overdose |
Serious | Complete resolution | Bedrocan | Neuropathic pain | 800 mg | 70 days | Oral |
Pregabalin Irbesartan Salbutamol Fluticasone |
Probable |
| 3 | 46 | F |
Altered mental status Mental confusion Lipothymia Vomiting |
Serious | Complete resolution | Bedrocan | Neuropathic pain | 150 mg daily | 125 days | Oral | Fentanyl | Probable |
| 4 | 71 | F | Mental confusion | Serious | Complete resolution | Cannabis inflorescences | Neuropathic pain | 10 mg daily | 1 day | Oral | Tapentadole | Probable |
| 5 | 22 | F |
Lack of efficacy Drowsiness |
Nonserious | Complete resolution | Cannabis inflorescences | Facial neuralgia | 150 mg daily | 4 months | Oral | ‐‐ | Probable |
| 6 | 80 | F |
Drowsiness Balance disorder |
Nonserious | Complete resolution | Bedrocan | Chronic pain | 200 mg daily | 130 days | Oral |
Paracetamol/codeine association Metformin Esomeprazole |
Probable |
| 7 | 75 | F | Epistaxis | Nonserious | Improvement | Cannabis inflorescences | NR | NR | 3 months | NR |
Acetylsalicylic acid Paracetamol/oxycodone association Omeprazole Pregabalin |
Possible |
| 8 | 45 | M |
Difficulty speaking Dizziness |
Nonserious | NR | Bedrocan | Neuropathic pain, neuralgia | 400 mg daily | 2 days | Oral | ‐‐ | Probable |
| 9 | 69 | F |
Mental confusion Dizziness |
Nonserious | Complete resolution | Bedrocan | Neuropathic pain | 100 mg | 20 days | Oral |
Codeine Pregabalin Anxiolytics (not specified) |
Probable |
| 10 | 61 | F |
Mental confusion Xerophthalmia Xerostomia |
Nonserious | Complete resolution | Bedrocan | Chronic pain | 50 mg | 3 weeks | Oral | ‐‐ | Probable |
| 11 | 78 | F |
Dizziness Fall Amnesia |
Nonserious | Complete resolution | Bedrocan | Neuropathic pain | 50 mg | 5 days | Oral | ‐‐ | Probable |
| 12 | 50 | F |
Headache Nausea |
Nonserious | Complete resolution | Bedrocan | Neuropathic pain | 25 mg | 4 days | Oral | ‐‐ | Probable |
| 13 | 50 | M |
Major depression Suicidal ideation |
Serious | Complete resolution | Bedrocan | Neuropathic pain, headache | 300 mg | 7 months | Oral | Valproic acid | Probable |
| 14 | 66 | M |
Bradyarrhythmia Lipothymia Balance disorder |
Serious | Complete resolution | Bedrocan | Chronic neuropathic pain | 150 mg | 1 day | Oral |
Tramadol Ibuprofen |
Probable |
| 15 | 80 | F | Drowsiness | Nonserious | Complete resolution | Bedrocan | Chronic pain | 50 mg | 20 days | Oral | ‐‐ | Probable |
| 16 | 72 | F |
Supraventricular tachycardia Aphasia Agnosia |
Nonserious | Complete resolution | Bedrocan | Neuropathic pain | 50 mg daily | 4 months | Oral |
Acetylsalicylic acid Losazide |
Probable |
| 17 | 78 | F | Mental confusion | Nonserious | Complete resolution | FM2 | Chronic pain | 30 mg | NR | Oral |
Pregabalin Oxycodone/naloxone association |
Not classifiable |
| 18 | 64 | F | Drowsiness | Nonserious | NR | Bedrocan | Chronic pain | 150 mg daily | 30 months | Oral |
Colchicine Paracetamol/acetylsalicylic acid/caffeine association Etoricoxib |
Probable |
| 19 | 79 | F | Mental confusion | Nonserious | Complete resolution | Bediol | Chronic pain | 60 mg daily | 20 days | Oral | ‐‐ | Probable |
| 20 | 77 | F |
Nausea Lack of appetite |
Nonserious | NR | Bedrocan | Chronic pain | 100 mg daily | 49 days | Oral |
Nimesulide Pregabalin |
Possible |
| 21 | 55 | F |
Drowsiness Mental confusion Biliary vomiting |
Nonserious | Improvement | Cannabis inflorescences | Neuropathic pain (autoimmune neuropathy) | NR | 15 days | NR |
Insulin Pantoprazole Duloxetine Pregabalin Escitalopram Alprazolam Oxycodone |
Probable |
| 22 | 50 | F |
Asthma Diarrhoea Gastritis |
Nonserious | Complete resolution | Bedrocan | Chronic pain | 90 mg | NR | Oral | ‐‐ | Probable |
| 23 | 57 | F |
Mental confusion Balance disorder |
Nonserious | Complete resolution | Bedrocan | Pain (nonresponsive to conventional therapies) | 90 mg daily | NR | Oral | ‐‐ | Probable |
| 24 | 71 | F | Smell alteration | NR | Still unresolved | Bedrocan | Chronic neuropathic pain | 120 mg | NR | Oral | Acetylsalicylic acid | Probable |
| 25 | 53 | M |
Tachycardia Pain Lack of efficacy Dulling |
Nonserious | Complete resolution | Bediol oil (magistral preparation) | Chronic pain | 175 mg | 200 days | Oral | ‐‐ | Probable |
| 26 | 69 | F |
Memory loss Balance disorder |
Nonserious | NR | FM2 oil (magistral preparation) | Chronic pain | 400 mg | 132 days | Oral | ‐‐ | Probable |
| 27 | 69 | F | Bronchospasm | Nonserious | Complete resolution | FM2 oil (magistral preparation) | Chronic pain (nonresponsive to conventional therapies) | 200 mg | 36 days | Oral | ‐‐ | Probable |
| 28 | 72 | F |
Dulling Swoon Hypoxia Frequent hospitalisation |
Serious | NR | FM2 oil (magistral preparation) | NR | 300 mg | 1 month | Oral |
Pregabalin Fentanyl Amitriptiline |
Probable |
| 29 | 22 | F |
Lack of efficacy Drowsiness |
Nonserious | Complete resolution | Bedrocan | Headache and facial neuralgia | 150 mg | 15 months | Oral |
Etoricoxib Colchicine Paracetamol/acetylsalicylic acid/caffeine association |
Probable |
| 30 | 80 | F |
Balance disorder Dulling Drowsiness |
Nonserious | Complete resolution | Bediol oil (magistral preparation) | Chronic pain | 200 mg | 100 days | Oral |
Metformin Esomeprazole Paracetamol/codeine association |
Probable |
| 31 | 72 | M | Drowsiness | Nonserious | Complete resolution | Bedrocan | Chronic pain | 120 mg daily | 1 month | Oral |
Levothyroxine Apixaban Lacidipine Flecainide |
Probable |
| 32 | 55 | F |
Major depression Psychotic crisis |
Nonserious | Complete resolution | Bediol oil 1 g/10 mL (magistral preparation) | Fibromyalgia and chronic pain, headache | 150 mg daily | 37 months | Oral | Pregabalin | Probable |
| Duloxetina | ||||||||||||
| Smilax aspera | ||||||||||||
| 33 | 68 | F |
Body altered perception Catastrophic thoughts Tachyarrhythmia Hypertension |
Nonserious | Complete resolution | Bedrocan oil 10% (magistral preparation) | Fibromyalgia and mixed pain with vertebral canal stenosis | 100 mg daily | 2 months | Oral |
Oxycodone Pregabalin |
Probable |
| 34 | 74 | F |
Leg swelling Leg itching |
Nonserious | Improvement | FM2 | Chronic pain | 50 mg | 6 days | Oral | Pregabalin | Probable |
| 35 | 83 | M |
Myasthenic crisis General muscle weakness and related eye disorder |
Nonserious | Complete resolution | Bedrocan oil 10% (magistral preparation) | Neuropathic pain and myelopathy | 100 mg | 49 days | Oral | Not specified drugs for myasthenia treatment | Possible |
| 36 | 77 | F |
Increased appetite Weight gain |
Nonserious | Improvement | Bedrocan | Chronic pain | 30 mg | 18 months | Oral | Pregabalin | Probable |
| 37 | 31 | F | Tachycardia | Nonserious | Improvement | Bedrocan | Chronic pain | 30 mg | 18 months | Oral |
Duloxetine Gabapentin |
Probable |
| 38 | 26 | F |
Drowsiness Dizziness Balance disorder |
Nonserious | Improvement | Pedanios oil (magistral preparation) | Fibromyalgia and neuropathic pain | 100 mg | 6 days | Oral |
Pregabalin Cyclobenzaprine |
Probable |
| 39 | 79 | F | Anxious depressive syndrome | Nonserious | Complete resolution | Bedrocan | Chronic pain | 60 mg daily | 2 months | Oral |
Enalapril Methylprednisolone |
Probable |
| 40 | 44 | F |
Tachyarrhythmia Dizziness Dysphoria |
Serious | Complete resolution | Bedrocan oil 10% (magistral preparation) | Fibromyalgia and neuropathic pain | 150 mg daily | 111 days | Oral |
Magnesium Pregabalin Duloxetine |
Probable |
| 41 | 47 | F |
Dysphoria Hallucinations Panic attack |
Nonserious | Complete resolution | Bedrocan oil 10% (magistral preparation) | Fibromyalgia and neuropathic pain | 100 mg | 43 days | Oral |
Pregabalin Duloxetine |
Probable |
| 42 | 64 | F |
Palpitation Nausea |
NR | Complete resolution | Magistral preparation 15% THC | Trigeminal neuralgia | 80 gtt/die | 1 day | Oral | Insulin | Probable |
| 43 | 77 | M | Mental confusion | Nonserious | Complete resolution | Cannabis inflorescences | Neuropathic pain | 50 mg | NR | Oral | ‐‐ | Probable |
| 44 | 52 | F | Supraventricular tachycardia | Serious | Complete resolution | Bedrocan | Neuropathic pain | 150 mg daily | 107 weeks | Oral | Venlafaxine | Probable |
| 45 | 63 | F |
Abdominal pain Gastroesophageal reflux |
Serious | Improvement | Bedrocan | Neuropathic pain | 175 mg daily | 11 days | Oral | Lansoprazole | Probable |
| 46 | 66 | M | Bradycardia | Nonserious | NR | Bedrocan oil (magistral preparation) | Neuropathic pain | 450 mg daily | 5 months | Oral | ‐‐ | Probable |
| 47 | 44 | M |
Anxiety Lipothymia Hyperhidrosis Blurred vision |
Nonserious | Improvement | Cannabis inflorescences | Pain | NR | 1 day | NR |
Ramipril Oxycodone/naloxone association Paracetamol/codeine association |
Probable |
| 48 | 45 | M |
Mental confusion Pharmacological interaction Memory impairment Tachycardia Lipothymia Shaking Urinary retention |
Serious | Improvement | Cannabis inflorescences | Neuropathic pain | NR | 6 months | NR | Gabapentin | Probable |
| 49 | 74 | F | Lack of efficacy | Nonserious | Complete resolution | FM2 | Multiple sclerosis | 60 mg | 1 month | Oral |
Paracetamol/codeine association Warfarin |
Probable |
| 50 | 44 | F |
Lack of efficacy Lower limb spasticity |
Serious | Complete resolution | Bediol | Multiple sclerosis | 60 mg | 2 months | Oral | Vitamin D | Probable |
| 51 | 51 | F | Dizziness | Nonserious | Still unresolved | Nor specified cannabis | Chronic pain | 100 mg | 1 month | Oral | Gabapentin | Probable |
| 52 | 78 | M | Mental confusion | Nonserious | Complete resolution | Cannabis inflorescences | Neuropathic pain, neuralgia | 50 mg | When needed | Oral | ‐‐ | Probable |
| 53 | 71 | F |
Hallucinations Nausea |
Nonserious | Complete resolution | Bediol | Chronic pain | 100 mg daily | 9 months | Oral |
Acetylsalicylic acid Ranitidine Cyclosporine Metoprolol Prednisolone Potassium kanrenoate |
Possible |
Regarding the causality assessment, 46 (86.8%) and 5 (9.4%) cases were judged as probably or possibly related to cannabis consumption, respectively. Only 1 case was defined as certainly associated to cannabis administration, while for 1 case, information has not been sufficient to assess a causality relationship, thus, it has been defined as not classifiable.
Overall, 53 AE reports referred to a total of 118 AEs, whose distribution is reported according to SOC classification (Figure 1). Following MedDRA hierarchy, the 5 most frequently reported SOC are: psychiatric disorders (26.3%, n = 31 out of 118 AEs), followed by nervous system disorders (22.0%, n = 26), ear and labyrinth disorders (10.2%, n = 12), gastrointestinal disorders (10.2%, n = 12), general disorders and administration site condition (8.5%, n = 10) and cardiac disorders (8.5%, n = 10). Consequently, within each SOC the most frequently reported PT were (see also Table 2): mental confusion, dysphoria, and anxiety (among psychiatric disorders); drowsiness and lipothymia (among nervous system disorders); dizziness and balance disorders (among ear and labyrinth disorders); nausea and vomiting (among gastrointestinal disorders); lack of efficacy and dulling (among general disorders and administration site condition); tachycardia and tachyarrhythmia, and bradycardia (among cardiac disorders).
Figure 1.
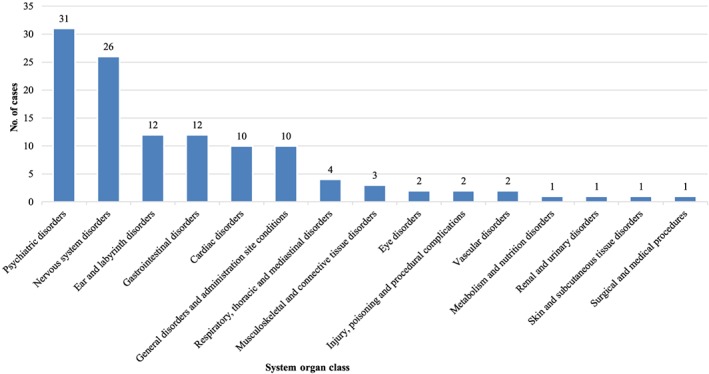
Distribution of medical cannabis‐related adverse events according to system organ classes
4. DISCUSSION
Since 2006 (date of cannabis for medical use approval) until December 2018, the Italian National Institute of Health collected within its Phytovigilance database a total of 103 AE reports following cannabis for medical use administration, mainly coming from Tuscany (61 reports).17 The high number of AE reports collected in this Italian region could be related to the well‐established use of medical cannabis18 and to the clinicians' knowledge of AE reporting procedures.19 To the best of our knowledge this is the first case series study describing all medical cannabis‐related AEs observed in the general population (all age groups).
Our study showed that cannabis treatment was not associated with a high number of AEs, the majority of which were nonserious and resolved completely without sequelae. Moreover, our results were in line with those published in the study by Whiting and colleagues,6 who found a statistically significant association between cannabis use and the occurrence of dizziness, dry mouth, nausea, fatigue, somnolence, euphoria, vomiting, diarrhoea, drowsiness, disorientation, confusion, loss of balance and hallucination. Moreover, in their systematic review, a statistically significant association was highlighted for the following SOCs: gastrointestinal, psychiatric and nervous system disorders, general disorders and administration site conditions, ear and labyrinth disorders, and renal and urinary disorders.6 Following, the description of our case‐by‐case clinical evaluation and the biological explanation of observed AEs, reported according to the SOCs most frequently reported (Table 2).
4.1. Psychiatric and nervous system disorders
Among people who use cannabis for recreational purposes, desired psychotropic THC‐related acute effects of mild euphoria, relaxation and general pleasant feelings are well characterised. By contrast, recreational use of cannabis, especially in naïve individuals, can cause motor coordination and executive function impairment, anxiety, panic attacks and psychotic episodes. Despite AEs of therapeutic cannabis resemble to those experienced during the recreational use, there are major differences both in terms of spectrum and intensity of these effects.20
In our sample, psychiatric disorders include several AEs (reported as PT), such as: mental confusion; depression and suicidal ideation; anxiety; acute psychosis; body altered perception; and panic attacks. In only 2 cases did patients report episodes of hallucinations. These transient psychotic‐like experiences are described in the literature21 and, differently to psychotic disorders, they resolve within a few hours and rarely cause distress. A study published in 2004 and performed on healthy volunteers underlines the occurrence of both positive (i.e. hallucinations, delusions, racing thoughts etc.) and negative symptoms (i.e. apathy, lack of emotion, poor or nonexistent social functioning etc.), similar to those experienced by schizophrenic patients. Nevertheless, no serious AEs has occurred during this study.21 Moreover, neurological or psychiatric AEs seems to increase with higher THC concentrations, suggesting a potential relationship between cannabis dosages and AEs seriousness,22 as in our case of accidental overdose (case 2).
A systematic review has collected data from 11 studies on psychosis highlighting an increased risk of psychotic outcome in individuals who never used cannabis, and an increased risk of psychotic outcome and depression in those who frequently used cannabis. Four of the included studies reported an association between cannabis use and increased risk of suicidal ideation, while the other 7 studies emphasised an association with anxiety outcomes.23 Another systematic review of randomised controlled trials of medical cannabinoids confirmed a significant higher rate in patients exposed to cannabis both for nervous system and psychiatric disorders.24
The hypothesis of the association between the use of cannabinoids and the development of psychosis is still debated. Epidemiological studies have consistently demonstrated that cannabis use is associated with an increased risk of schizophrenia‐like psychoses. This risk varies according to the dose and duration of use, on top of age use and genetic factors, including partially shared genetic predisposition with schizophrenia.25
Considering patients' average age in our sample, it is important to note that cannabis‐induced nervous system disorders are not specific to older adults, even if, during clinical trials, sedation‐like symptoms such as drowsiness, tiredness and somnolence are more reported in older subjects exposed to cannabis than in controls.26 Clinicians should always take these kind of AEs into account, particularly to better manage patients' adherence to cannabis prescriptions. This aspect is also important because it has been found that experiencing a cannabis‐related AE (i.e. dizziness, fatigue, mild anxiety and feeling “weird”) is more common among nonadhering patients than among adhering ones.27 Thus, improving patients' education on cannabis treatments, could improve their therapy compliance, and reduce nonadherence affects, in particular psychiatric and nervous system AEs.
4.2. Ear and labyrinth disorders
Among chronic users of cannabis, a decrease in maximum amplitude on torsion swing and an increase in incidence of nystagmus in supine positions have been observed since the 1970s.28 Following studies, based on immunohistochemistry, demonstrate that CB1 and CB2 receptors are localised in the central nervous system and cover areas involved in movement control (i.e. basal ganglia, cerebral cortex, and cerebellum). Endocannabinoids are also involved in setting the baseline activity of the spinal locomotor circuitry, where CB1 activation or antagonism results in an increase and decrease of locomotor frequency, respectively.29 Moreover, it has been demonstrated that CB1 receptors exist in significant densities in the vestibular nucleus complex and are likely to contribute to the neurochemical control of vestibular reflexes.30 Given that CB1 receptors have apparently an exclusive presynaptic localisation, in the vestibular nucleus complex they could be situated on presynaptic glutamatergic axon terminals where they would reduce glutamate release during vestibular stimulation.31 In this frame, the activation of these receptors could explain the occurrence of loss of balance and labyrinth disorders as cannabis‐related AEs.30
In chronic users of cannabis, the cerebellum presents neuroanatomical alterations that impair its ability to execute postural adjustments, resulting in trembling.32, 33 Results from a study published in 2017 suggest that cannabis users show subtle changes in gait, primarily in open‐chain components of walking gait, but not in balance.34
The main clinical concern refers to cannabis use in older patients, as those in our sample, who are notably susceptible to AEs due to physiological changes, comorbidities, concurrent medication use and cognitive impairment.35 Even if this assumption has not been already confirmed, sedation‐like neurological AEs of cannabis may expose the elderly to an increased risk of falls. In this frame, a recent study examines the effect of THC on balance and gait in old demented adults using cannabinoids. THC administration in elderly significantly increases sway during standing and stride length and trunk sway during walking.36 Another systematic review and meta‐analysis included 7 randomised controlled trials that reported data about hearing AEs after cannabis use. The combined risk for all hearing‐related AEs has been significantly higher among cannabis users compared to placebo‐exposed patients, with tinnitus, loud noise, ringing in the ears, and vertigo as the most commonly reported AEs.37
4.3. Gastrointestinal disorders
In our sample, gastrointestinal disorders include several AEs (reported as PT), such as: nausea, vomiting, and gastritis. A systematic review and meta‐analysis published by Aviram and colleagues37 has collected evidence on gastrointestinal AEs in patients treated with cannabis‐based medicines for chronic and postoperative pain management. From 20 randomised controlled trials, the combined risk ratio for all gastrointestinal AEs was significantly higher in patients exposed to cannabinoids than in controls, especially in those who used oromucosal and oral cannabis preparations.37 Furthermore, cannabis‐related nausea and vomiting appear to be THC‐dose‐dependent AEs: at low doses, THC is characterised by antiemetic properties, but at high doses, is proemetic.38
Despite antiemetic effects of cannabis are well described, as in animal models cannabinoids show both an antiemetic/emetic response,39 a paradoxical syndrome of hyperemesis resultant from cannabinoids exposure has been already defined also in humans. This syndrome is labelled cannabinoid hyperemesis syndrome (CHS), and consists of nausea, vomiting and abdominal pain particularly associated with chronic use.40 A recent retrospective observational study shows that, among cannabis users, gastrointestinal symptoms, including CHS, are the most common cause of cannabis‐related emergency departments visits.41 Two main hypothesis involving THC try to explain this phenomenon. The first suggests that THC directly activates cannabinoid receptors in the enteric nervous system, reducing gastric motility, and promoting a proemetic state. A second explanation of this phenomenon has taken into account (i) the impairing in thermoregulation and (ii) the alteration of the hypothalamic–pituitary–adrenal axis caused by cannabinoids, which reduces body temperature and prolactin, gonadotropin, and growth hormone release, and increases corticotrophin secretion.42 Impaired body temperature and hypothalamic–pituitary–adrenal axis alterations have been also associated with the development of CHS, which is characterised by 3 phases: prodromal, hyperemetic and recovery phase. Patients usually access emergency departments during the hyperemetic phase, presenting profuse vomiting.43 Even if mild, nausea and vomiting could have a deep impact on patients' morbidity and quality of life, and an economic burden for hospitals and healthcare systems. Therefore, clinicians should consider cannabis as a cause of cyclic nausea and vomiting, especially in chronic cannabis users and in patients treated with oral cannabis preparations, particularly in oncological patients.
4.4. General disorders and administration site condition
In our sample, among general disorders and administration site condition, lack of efficacy is the most frequently reported PT. Such event can derive from (i) the prescription of an inadequate dosage of prescribed/administered cannabis, (ii) presence of interactions that can reduce the effectiveness of the preparation, (iii) incorrect intake, or (iv) personal sensitivity.
In 2015 the Italian Ministry of Health has recommended clinicians to start a therapy based on cannabis with the minimum dose needed and, if necessary, to increase the dosage gradually.44 Moreover, clinicians have to declare in their prescription which type of cannabis should be used for the preparation, according to the proper level of THC and CBD, and the total dose of cannabis should be adapted according to patient's clinical characteristics (i.e. age, sex, weight, comorbidity and presence of concomitant medications). Due to the need for a titration phase and to the accumulation in adipose tissue, until the circulating active fraction of THC and CBD raises, first administrations may result ineffective.45 Of note, standard dosages for cannabis are currently not available from guidelines. Evidences provided by clinical trials allowed to establish the maximum daily doses recommended in clinical practice that ranges, from an initial dose of 25 mg to around 130 mg of cannabis with 19% of THC, both for chronic pain and multiple sclerosis, respectively.46
In our sample we encountered a total of 5 cases of lack of efficacy, and its onset differed among them. In particular, 3 patients (cases 5, 25 and 29) are affected by chronic neuropathic pain and 2 women (cases 49 and 50) are affected by multiple sclerosis. All of them have been treated with oral preparation of cannabis within the recommended therapeutic dose range. Moreover, in cases 29 and 49, patients were treated with cannabis concomitantly with other drugs, in particular analgesics (paracetamol/acetylsalicylic acid/caffeine and paracetamol/codeine fixed associations), etoricoxib, colchicine and warfarin. Since patients with multiple sclerosis (cases 49 and 50) have been treated with very low doses of cannabis (60 mg daily) for a short period (1 and 2 months, respectively), we hypothesise that their titration phase was still ongoing when the AE lack of efficacy was reported. Considering that the length of medical cannabis treatment was very heterogeneous among these patients, and given the lack of information about individual factors, such as comorbidities, concomitant treatment, and genetic factors, we were not able to evaluate the possible relationship between length of medical cannabis exposure and the event lack of efficacy. Further investigations, with a larger sample of patients, could be performed to better clarify this issue. Moreover, to date no interaction between cannabinoids and etoricoxib and colchicine has been clearly identified. However, given that THC and CBD are both substrates and modulators of cytochrome (CYP) P450,47 DDIs could not be excluded, particularly with drugs at low therapeutic index as warfarin.48
4.5. Cardiac disorders
In our sample, we also observed some cardiovascular (CV) AEs, in particular (reported as PT): bradyarrhythmia (case 14) and bradycardia (case 46), tachycardia and tachyarrhythmia (cases 25, 33, 37, 40, and 48), supraventricular tachycardia (cases 16 and 44), and palpitations (case 42).
Regarding CV AEs related to medical cannabis (e.g. atrial fibrillation, acute coronary syndromes, ventricular tachycardia, and even sudden death), available evidence appears to be insufficient to draw a definitive conclusion on the effects of cannabis on these kind of manifestations and its possible mechanism.49 It is well known that CB2 receptors are located in cardiac myocyte and in the smooth muscles of blood vessels. Nevertheless, the exact mechanism of the various vascular effect of cannabis is not yet clear.50 Furthermore, in the study published by Whiting and colleagues, the authors did not find any statistically significant association between cannabis use and the occurrence of CV AEs.6
4.6. Drug‐drug interactions
In the majority of cases reported in our sample, patients are administered with at least another concomitant prescribed medication over their cannabis prescription. Although DDIs are a major concern for physicians, few studies have examined the effects of DDIs involving cannabis preparations. Most available evidence derives only from preclinical studies and currently the extent of cannabis‐related DDIs in real‐world practice remains poorly investigated.
As mentioned above, oral cannabinoids are metabolised by the enzyme family of CYP450, and THC and CBD are inhibitors of CYP2C9, CYP3A4, and CYP2C19, while smoked cannabis is demonstrated to induce CYP1A1 and CYP1A2.51 Moreover, CBD inhibits P‐glycoprotein mediated transports in a dose‐dependent manner.52 Established cannabis‐related DDIs involve THC and psychotropic agents,51 CBD and anticancer agents,53 CBD and gabapentin,54 and cannabinoids and warfarin.48 In general, clinicians should always take into account that medical cannabis could bidirectionally interact with concomitant administered agents by affecting membrane transporters and/or metabolising enzymes.47
In our sample, among patients treated with at least another prescribed medication over cannabis, only in 1 case (case 48) did the physician specifically report pharmacological interaction as PT. However, from our case‐by‐case analysis, we have identified other 16 cases in which the presence of DDI could be involved in the AE. Alteration of the mental status, dysphoria, dizziness, drowsiness and balance disorders are reported concomitantly to the use of opioids, such as fentanyl (cases 3 and 28), tapentadol (case 4), codeine (alone or in association, cases 6, 9, 30 and 47), oxycodone and naloxone (cases 17, 21, 33 and 47), and concomitantly to the use of pregabalin (cases 2, 9, 17, 21, 28, 33, 38 and 40) and gabapentin (case 48). One patient (case 21) has shown as concomitant medications alprazolam, escitalopram, oxycodone and pregabalin, together with duloxetine, insulin and pantoprazole. Furthermore, we observed a patient concomitantly exposed to cannabis and cyclosporine (case 53), after a renal transplant, who experienced hallucinations and nausea. This case is particularly relevant due to the fact that the concomitant exposure to cannabis and cyclosporine could be related to a transplant failure.55
These results allow us to make some considerations. Considering that the AEs reported in all these cases are common to cannabis and opioid derivatives, benzodiazepines and antiepileptic agents, and that are listed as adverse drug reactions in the summary of products characteristics for all the concomitant medications registered, we believe that all reported AEs may be due to a synergistic interaction between cannabis and other agents. Moreover, in a clinically complex condition such those represented by some of our cases and considering the excellent analgesic properties of cannabis, 4 it should be advisable to gradually reduce the administration of opioids, up to interrupt it when possible, in order to reduce the potential inappropriate prescription. Finally, according to pharmacovigilance and phytovigilance guidelines,56, 57 the quite low number of DDIs identified and reported in our sample, probably highlights the need to increase physicians' awareness about this clinically relevant event as cannabis‐related cause of AEs.
5. STRENGTHS AND LIMITATIONS
This study has some limitations and strengths. First, our analysis is based on AE reports that are affected by limits that include inaccurate and incomplete information, mainly related to lack of clinical data. Given that, the absence of information that was not listed in AE reports and that might have influenced the clinical evaluation of each report (i.e. the lack of information on previous and/or current patient medical conditions which could affect the clinical evaluation of each case) could not always be excluded. Moreover, data concerning medical cannabis prescriptions in Tuscany among the study period were not available.
Despite these limitations, this is the first study describing all medical cannabis‐related AEs observed in the general population. Furthermore, considering that medical cannabis‐related AEs reporting is mandatory for clinicians, we can affirm that our case series includes the totality of patients who experienced a cannabis‐related AE in Tuscany within the study period.
6. CONCLUSIONS
Our case‐by‐case assessment of AEs following medical cannabis treatment underlined several potential safety issues of great importance for clinical practice.
Cannabis was well tolerated and the majority of AEs were mild and transient. Nevertheless, prescribing cannabis, clinicians should be aware that, especially during the titration phase, cannabis could result ineffective and that the occurrence of psychiatric and gastrointestinal AEs, mainly paradoxical nausea and vomiting, may increase as cannabis dosages increase too fast and if cannabis is administered orally. Moreover, clinicians should be aware of the possible occurrence of cannabis‐related AEs in older or complex patients, exposed to concomitant synergic medications, for whom phenomena can occur not only of interaction, but also of inappropriate prescribing. Our results have also highlighted that cannabis was used as adjunct treatment in a relevant proportion of cases, particularly in pain management, thus justifying the importance of monitoring prescriptions.
The risks associated with long‐term use of medical cannabis are still poorly characterised in published clinical trials and observational studies. A good vigilance of cannabis‐related AEs may improve our knowledge of cannabis safety profile, filling the gaps of clinical studies. Moreover, these results could ameliorate current clinical practice and lead to correct use of cannabis exploiting its full potential.
CONTRIBUTORS
Study design was contributed by GC, NL, VM, and AV, with assistance from the rest of the authors. Data acquisition was performed by FMI, RDC, MDL, EG and FF. Data interpretation was performed by GC, NL, VM, and AV, with assistance from the other authors. The manuscript was written primarily by GC and NL, with assistance from the other authors, and revised by AB, AM, and AV. All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Crescioli G, Lombardi N, Bettiol A, et al. Adverse events following cannabis for medical use in Tuscany: An analysis of the Italian Phytovigilance database. Br J Clin Pharmacol. 2020;86:106–120. 10.1111/bcp.14140
Giada Crescioli and Niccolò Lombardi are co‐first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Italian National Institute of Health (Istituto Superiore di Sanità, ISS). Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of the Italian National Institute of Health (Istituto Superiore di Sanità, ISS).
REFERENCES
- 1. Perez‐Reyes M, White WR, McDonald SA, Hicks RE, Jeffcoat AR, Cook CE. The pharmacologic effects of daily marijuana smoking in humans. Pharmacol Biochem Behav. 1991;40(3):691‐694. [DOI] [PubMed] [Google Scholar]
- 2. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9‐tetrahydrocannabinol, cannabidiol and delta9‐tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonini SA, Premoli M, Tambaro S, et al. Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol. 2018;227:300‐315. [DOI] [PubMed] [Google Scholar]
- 4. Bellnier T, Brown GW, Ortega TR. Preliminary evaluation of the efficacy, safety, and costs associated with the treatment of chronic pain with medical cannabis. The Mental Health Clinician. 2018;8(3):110‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet (London, England). 2018;391:1085‐1096. [DOI] [PubMed] [Google Scholar]
- 6. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta‐analysis. JAMA. 2015;313(24):2456‐2473. [DOI] [PubMed] [Google Scholar]
- 7. Nugent SM, Morasco BJ, O'Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med. 2017;167:319‐331. [DOI] [PubMed] [Google Scholar]
- 8. Harris D, Jones RT, Shank R, et al. Self‐reported marijuana effects and characteristics of 100 San Francisco medical marijuana club members. J Addict Dis. 2000;19(3):89‐103. [DOI] [PubMed] [Google Scholar]
- 9. Swift W, Gates P, Dillon P. Survey of Australians using cannabis for medical purposes. Harm Reduct J. 2005;2(18):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bettiol A, Lombardi N, Crescioli G, et al. Galenic preparations of therapeutic Cannabis sativa differ in cannabinoids concentration: a quantitative analysis of variability and possible clinical implications. Front Pharmacol. 2019;9:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Md, S . Uso medico della Cannabis. Available on‐line: http://wwwsalutegovit/portale/temi/p2_6jsp?lingua=italiano&id=4587&area=sostanzeStupefacenti&menu=vuoto 2018; last access: 04/03/2019.
- 12. Zaami S, Di Luca A, Di Luca NM, Montanari VG. Medical use of cannabis: Italian and European legislation. Eur Rev Med Pharmacol Sci. 2018;22(4):1161‐1167. [DOI] [PubMed] [Google Scholar]
- 13. Crescioli G, Lombardi N, Bettiol A, et al. Acute liver injury following Garcinia cambogia weight‐loss supplementation: case series and literature review. Intern Emerg Med. 2018;13(6):857‐872. [DOI] [PubMed] [Google Scholar]
- 14. Lombardi N, Crescioli G, Bettiol A, et al. Characterization of serious adverse drug reactions as cause of emergency department visit in children: a 5‐years active pharmacovigilance study. BMC Pharmacol Toxicol. 2018;19(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lombardi N, Crescioli G, Bettiol A, et al. Vaccines safety in children and in general population: a pharmacovigilance study on adverse events following anti‐infective vaccination in Italy. Front Pharmacol. 2019;10:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexander SP, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2017/18: overview. Br J Pharmacol. 2017;174(Suppl 1):S1‐S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Istituto Superiore di Sanità CNPLRELVPECDF. Segnalazioni di sospette reazioni avverse a preparazioni magistrali di cannabis per uso medico. Available on‐line: https://wwwepicentroissit/farmaci/pdf/Relazione%20semestrale%20ADR%20Cannabis%20(ISS)%20I%20semestre%202018pdf 2018; Last access: 04/03/2019.
- 18. Bifulco M, Pisanti S. Medicinal use of cannabis in Europe: the fact that more countries legalize the medicinal use of cannabis should not become an argument for unfettered and uncontrolled use. EMBO Rep. 2015;16(2):130‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Medicines Utilization Monitoring Centre . National Report on Medicines use in Italy 2016. Rome: Italian Medicines Agency; 2017. [Google Scholar]
- 20. Cohen K, Weinstein AM. Synthetic and non‐synthetic cannabinoid drugs and their adverse effects‐a review from public health prospective. Front Public Health. 2018;6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta‐9‐tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2004;29(8):1558‐1572. [DOI] [PubMed] [Google Scholar]
- 22. Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo‐controlled, crossover trial of cannabis cigarettes in neuropathic pain. The Journal of Pain: Official Journal of the American Pain Society. 2008;9(6):506‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore TH, Zammit S, Lingford‐Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet (London, England). 2007;370:319‐328. [DOI] [PubMed] [Google Scholar]
- 24. Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ: Canadian Medical Association Journal = Journal de l'Association Medicale Canadienne. 2008;178:1669‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krebs MO, Kebir O, Jay TM. Exposure to cannabinoids can lead to persistent cognitive and psychiatric disorders. Eur J Pain (London, England). 2019;23(7):1225‐1233. [DOI] [PubMed] [Google Scholar]
- 26. Beauchet O. Medical cannabis use in older patients: update on medical knowledge. Maturitas. 2018;118:56‐59. [DOI] [PubMed] [Google Scholar]
- 27. Zolotov Y, Baruch Y, Reuveni H, Magnezi R. Adherence to medical cannabis among licensed patients in Israel. Cannabis and Cannabinoid Research. 2016;1(1):16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spector M. Chronic vestibular and auditory effects of marijuana. Laryngoscope. 1974;84:816‐820. [DOI] [PubMed] [Google Scholar]
- 29. El Manira A, Kyriakatos A, Nanou E, Mahmood R. Endocannabinoid signaling in the spinal locomotor circuitry. Brain Res Rev. 2008;57(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 30. Ashton JC, Zheng Y, Liu P, Darlington CL, Smith PF. Immunohistochemical characterisation and localisation of cannabinoid CB1 receptor protein in the rat vestibular nucleus complex and the effects of unilateral vestibular deafferentation. Brain Res. 2004;1021(2):264‐271. [DOI] [PubMed] [Google Scholar]
- 31. Smith PF, Ashton JC, Darlington CL. The endocannabinoid system: a new player in the neurochemical control of vestibular function? Audiol Neurootol. 2006;11(4):207‐212. [DOI] [PubMed] [Google Scholar]
- 32. Lorenzetti V, Solowij N, Yucel M. The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry. 2016;79(7):e17‐e31. [DOI] [PubMed] [Google Scholar]
- 33. Bolbecker AR, Apthorp D, Martin AS, et al. Disturbances of postural sway components in cannabis users. Drug Alcohol Depend. 2018;190:54‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pearson‐Dennett V, Todd G, Wilcox RA, Vogel AP, White JM, Thewlis D. History of cannabis use is associated with altered gait. Drug Alcohol Depend. 2017;178:215‐222. [DOI] [PubMed] [Google Scholar]
- 35. Davies EA, O'Mahony MS. Adverse drug reactions in special populations ‐ the elderly. Br J Clin Pharmacol. 2015;80(4):796‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van den Elsen GA, Tobben L, Ahmed AI, et al. Effects of tetrahydrocannabinol on balance and gait in patients with dementia: a randomised controlled crossover trial. J Psychopharmacol. 2017;31(2):184‐191. [DOI] [PubMed] [Google Scholar]
- 37. Aviram J, Samuelly‐Leichtag G. Efficacy of cannabis‐based medicines for pain management: a systematic review and meta‐analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755‐E796. [PubMed] [Google Scholar]
- 38. Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin‐induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology (Berl). 2004;174(2):254‐259. [DOI] [PubMed] [Google Scholar]
- 39. Parker LA, Kwiatkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium‐induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology (Berl). 2004;171(2):156‐161. [DOI] [PubMed] [Google Scholar]
- 40. Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170(8):531‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc. 2012;87(2):114‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goyal H, Singla U, Gupta U, May E. Role of cannabis in digestive disorders. Eur J Gastroenterol Hepatol. 2017;29:135‐143. [DOI] [PubMed] [Google Scholar]
- 44. Md, S . Decreto uso medico Cannabis (GU 30.11.2015), GAZZETTA UFFICIALE DELLA REPUBBLICA ITALIANA. Available on‐line: https://wwwepicentroissit/farmaci/pdf/Decreto%20uso%20medico%20Cannabis%20(GU%2030112015)%20pdf 2015; Last access: 04/03/2019.
- 45. Ospedaliera SIdF . Linee di indirizzo per l'utilizzo dei medicinali a base di cannabinoidi a carico del SSR. Bollettino SIFO 2017; Available on‐line at: http://www.bollettinosifo.it/r.php?v=2826&a=28560&l=332778&f=allegati/02826_2017_05/fulltext/Suppplemento%20SIFO%205-2017%201b.pdf.
- 46. Firenzuoli, F , Epifani, F , Loiacono, I . Cannabis. «Erba» medica. Norme, preparazioni galeniche, attualità e prospettive di cura. Edra 2015. ISBN: 8821439577.
- 47. Alsherbiny MA, Li CG. Medicinal cannabis‐potential drug interactions. Medicines (Basel). 2018;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Damkier P, Lassen D, Christensen MMH, Madsen KG, Hellfritzsch M, Pottegard A. Interaction between warfarin and cannabis. Basic Clin Pharmacol Toxicol. 2019;124(1):28‐31. [DOI] [PubMed] [Google Scholar]
- 49. Ravi D, Ghasemiesfe M, Korenstein D, Cascino T, Keyhani S. Associations between marijuana use and cardiovascular risk factors and outcomes: a systematic review. Ann Intern Med. 2018;168:187‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rezkalla S, Kloner RA. Cardiovascular effects of marijuana. Trends Cardiovasc Med. 2019;29(7):403‐407. [DOI] [PubMed] [Google Scholar]
- 51. Rong C, Carmona NE, Lee YL, et al. Drug‐drug interactions as a result of co‐administering Delta(9)‐THC and CBD with other psychotropic agents. Expert Opin Drug Saf. 2018;17(1):51‐54. [DOI] [PubMed] [Google Scholar]
- 52. Zhu HJ, Wang JS, Markowitz JS, et al. Characterization of P‐glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther. 2006;317(2):850‐857. [DOI] [PubMed] [Google Scholar]
- 53. Opitz BJ, Ostroff ML, Whitman AC. The potential clinical implications and importance of drug interactions between anticancer agents and Cannabidiol in patients with cancer. J Pharm Pract. 2019. 10.1177/0897190019828920 [DOI] [PubMed] [Google Scholar]
- 54. Atwal N, Casey SL, Mitchell VA, Vaughan CW. THC and gabapentin interactions in a mouse neuropathic pain model. Neuropharmacology. 2019;144:115‐121. [DOI] [PubMed] [Google Scholar]
- 55. Pondrom S. Transplantation and marijuana use. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16:1‐2. [DOI] [PubMed] [Google Scholar]
- 56. Mazzitello C, Esposito S, De Francesco AE, Capuano A, Russo E, De Sarro G. Pharmacovigilance in Italy: an overview. J Pharmacol Pharmacother. 2013;4(Suppl 1):S20‐S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sessa M, di Mauro G, Mascolo A, et al. Pillars and pitfalls of the new pharmacovigilance legislation: consequences for the identification of adverse drug reactions deriving from abuse, misuse, overdose, occupational exposure, and medication errors. Front Pharmacol. 2018;9:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Italian National Institute of Health (Istituto Superiore di Sanità, ISS). Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of the Italian National Institute of Health (Istituto Superiore di Sanità, ISS).


