Abstract
Karyolysis is the complete dissolution of nuclear components of a dying cell. However, the generation mechanism has not been clarified. We studied a necrotic DNA fragmentation factor DNase γ (also known as DNase1L3) and previously found that karyolysis was inhibited in DNase γ deficient (DNase γ−/−) mice. To confirm this, we transiently expressed DNase γ in the liver of DNase γ−/− mice and caused hepatocyte necrosis by acetaminophen overdose. As expected, karyolysis was induced in the necrotic hepatocytes. We also found that the depletion of Kupffer cells from wild type mice reduced the expression and activity of DNase γ in the liver. Thus, we concluded that DNase γ produced from Kupffer cells caused karyolysis of necrotic hepatocytes.
Keywords: DNA fragmentation, DNase γ, DNase1L3, karyolysis, Kupffer cell
Karyolysis is disintegration and dissolution of the nucleus of a necrotic cell [1]. Although it is a typical morphological change, the generation mechanism is not well understood. We studied an endonuclease, DNase γ (also known as DNase1L3), that is a secreting protein of the DNase I family and causes DNA fragmentation in necrosis [8]. Previously, we generated DNase γ gene deficient (DNase γ−/−) mice and induced hepatocyte necrosis by acetaminophen (APAP) overdose [7]. We found that karyolysis was inhibited in the treated hepatocyte, suggesting that DNase γ was essential for the process. However, it was not determined whether the expression of DNase γ was sufficient for karyolysis. Thus, in this experiment, to answer to this question, we transiently expressed DNase γ in hepatocytes of DNase γ−/− mice and determined whether karyolysis occurred after necrosis induction. We also investigated which cells produced DNase γ−although macrophages, especially Kupffer cells in liver, were expected to produce DNase γ, no detailed analysis has been reported [11].
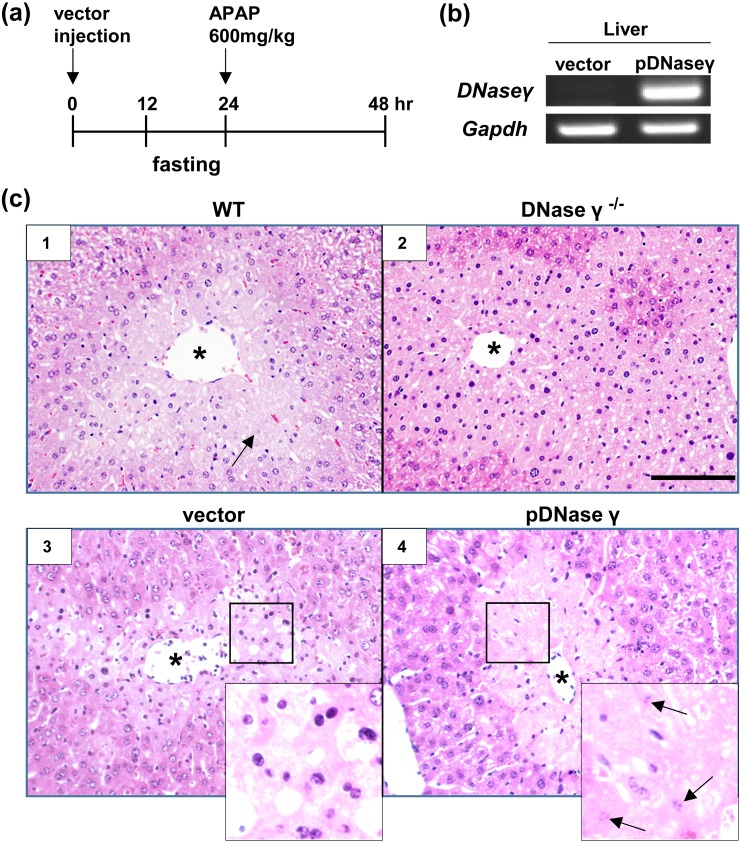
To express DNase γ transiently in the liver of DNase γ−/− mice, we used the hydrodynamic injection method [5]. We injected 30 µg of DNase γ expression vector (pDNase γ) or a control vector (pcDNA3) (Invitrogen, Carlsbad, CA, USA) in 2 ml of phosphate buffered saline from the tail vein within 5 sec [10]. As controls, we used non-injected mice, both C57BL/6 wild type (WT) and DNase γ−/− mice. Mouse fasting was started 12 hr later and then, after 24 hr, APAP was intraperitoneally injected into the mice (600 mg/kg body weight) to induce hepatocyte necrosis (Fig. 1a) [3, 8]. Mice were sacrificed at 24 hr after APAP injection to collect blood and liver samples. The DNase γ expression was confirmed by reverse transcription (RT)-PCR analysis (Fig. 1b). The expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as the control. Total RNA was extracted from the excised liver using TRIzol (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The RNA (10 µg) was reverse-transcribed into cDNA using Superscript II with oligo (dT) primers (Invitrogen). Thereafter, the resultant cDNA was amplified with GoTaq DNA polymerase (Promega, Madison, WI, USA) using gene-specific primer pairs: DNase γ-mNGSP5, 5ʹ-CACGTACAAAGAGCAGTATGCCTTCG-3ʹ; DNase γ-mGSP1, 5ʹ-CGAATGTTCTGCCAGGCCTTCTTGGGG-3ʹ; Gapdh-F, 5ʹ-GGAGAAACCTGCCAAGTATGA-3ʹ; and Gapdh-R, 5ʹ-CCCTGTTGCTGTAGCCGATATT-3ʹ.
Fig. 1.
Inducing karyolysis by DNase γ expression. (a) Time course of hydrodynamic injection and acetaminophen (APAP) treatment. DNase γ−/− mice were injected with expression vectors, control vector or DNase γ expression vector (pDNase γ), intravenously at 0 hr and APAP intraperitoneally at 24 hr, and then sacrificed at 48 hr. Mice were fasted for 12 hr before APAP injection. As the control, mice without plasmid injection were used. (b) DNase γ expression in a pDNase γ-injected mouse was confirmed by RT-PCR (right). No expression was detected in a control-vector-injected mouse (left). Gapdh expression was the loading control. (c) Hematoxylin and eosin (H&E) staining of mouse liver sections from WT (panel1) and DNase γ−/− (panels 2–4) mice. All mice were treated with APAP and some mice (panels 3 and 4) were pre-injected with expression vectors, control vector (panel 3) or pDNase γ (panel 4). Mice without plasmid injection were the control (panels 1 and 2). All treated liver showed necrosis of hepatocytes surrounding central veins. Karyolysis was induced in the hepatocytes of WT mice (panel 1, arrow) and those of DNase γ−/− mice injected with pDNase γ (panel 4, arrows). Magnified images were shown in insets. Asterisk (*), central vein. Scale bar, 200 µm.
A part of liver was fixed with 10% formalin and embedded in paraffin. Liver sections (5 µm) prepared from the paraffin block were followed by hematoxylin and eosin (H&E) staining to examine karyolysis (nuclear dissolution and fading) (Fig. 1c). In non-injected control mice, we detected nuclear fading of cells surrounding a central vein in WT (panel 1, arrow) but not in DNase γ−/− mice (panel 2), and found that the injection of pDNase γ (panel 4) but not control vector (panel 3) caused nuclear dissolution and fading in the hepatocytes of DNase γ−/− mice (panel 4, arrows), confirming that DNase γ expression was sufficient for karyolysis. Probably, chromatin is a structural component in the nucleus and DNase γ-dependent DNA fragmentation is required to dissolve the necrotic nucleus.
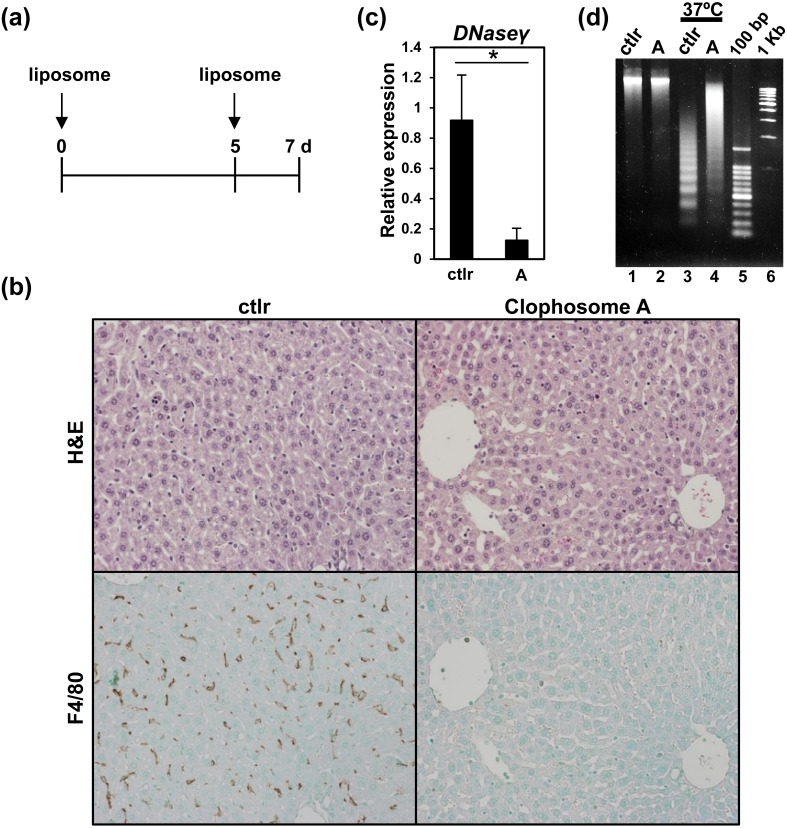
Next, we determined the cells producing DNase γ. Previous reports suggested that DNase γ was secreted from phagocytic cells, i.e., macrophage or dendritic cells [11]. We expected that Kupffer cells might express DNase γ in liver. Then, we depleted Kupffer cells with clodronate liposome and examined the expression and activity of DNase γ (Fig. 2) [6]. Clophosome A and control-liposome (ctlr) (FormuMax Scientific Inc., Sunnyvale, CA, USA) were injected twice (at day 0 and 5) into WT mice (100 µl/mouse) and then the mice were sacrificed at day 7 after the first injection (Fig. 2a). Mouse liver was collected and further analyzed. Formalin-fixed liver sections (5 µm) were followed by H&E staining (Fig. 2b, top) or immunostaining (Fig. 2b, bottom). Kupffer cells were stained with anti-F4/80 antibody (eBioscience, San Diego, CA, USA) and then treated with Simple stain mouse Max-PO (Rat) (Nichirei, Tokyo, Japan) [4]. All sections were counterstained with methyl green and diamino benzidine (Nichirei) was used for visualizing Kupffer cells (Fig. 2b, bottom). The Clophosome A treatment caused the depletion of Kupffer cells (Fig. 2b, bottom right).
Fig. 2.
Kupffer cells produce DNase γ. (a) Time course of liposome treatments. C57BL/6 WT mice were injected with liposomes, Clophosome A or control-liposome, at day 0 and 5, and sacrificed at day 7. (b) H&E staining (top) and immunostaining (bottom) of mouse liver sections from the treated mice. Serial sections of a control-liposome treated mouse (ctlr) are shown in left panels and those of a Clophosome A-treated mouse in right panels. Kupffer cells were detected by immunostaining with anti-F4/80 antibody. (c) q-PCR analysis of DNase γ expression in a liposome-treated mouse liver. Relative expressions to Gapdh are shown. Left, control-liposome treated sample (ctlr); Right, Clophosome A-treated sample (A). Asterisk (*): P<0.01, n=4, Student’s t-test. (d) DNase γ activities in mouse liver. Control-liposome (ctlr, lanes 1 and 3) and Clophosome A-treated samples (A, lanes 2 and 4) are shown. Half of the samples were incubated at 37°C for 1 hr to determine DNase γ activities (lanes 3 and 4). Non-incubated samples were the control (lanes 1 and 2). 100 bp, 100 bp DNA marker. 1 kb, 1 kb DNA marker.
Next, DNase γ expression was examined using quantitative PCR (q-PCR) and its significant reduction was detected after the Clophosome A treatment (P<0.01, n=4, Student’s t-test) (Fig. 2c). The RNA and cDNA preparation were mentioned before, and the cDNA was amplified in a thermal cycler (7500 Fast, Applied Biosystems, Foster City, CA, USA) with THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan) and primers. Primers used in this analysis follow: DNase γ-F, 5ʹ-TGCTTAAGCCATCAGCATGTC-3ʹ; DNase γ-R, 5ʹ-CTGTTGGAGCATCCAAAGTGAG-3ʹ; Gapdh-F, 5ʹ-GGAGAAACCTGCCAAGTATGA-3ʹ; and Gapdh-R, 5ʹ-CCCTGTTGCTGTAGCCGATATT-3ʹ.
Finally, we examined DNase γ activities in the Clophosome A-treated livers (Fig. 2d). A part of excised liver was mashed with a biomasher (Nippi, Tokyo, Japan) and 3.7 mg of the sample was suspended in 60 µl of Dulbecco’s Modified Eagle’s Medium with 25 mM HEPES. We prepared samples with or without incubation for 1 hr at 37°C. Then DNA was purified with the Wizard DNA purification resin (Promega) as previously described [8]. The DNA was separated in 1.5% agarose gel. Without incubation, we could not detect DNase activity in both ctlr- and Clophosome A-treated liver (Fig. 2d, lanes 1 and 2). With incubation, we could detect differential DNase activities and the Clophosome A-treated liver showed decreased DNase activity (Fig. 2d, lanes 3 and 4), suggesting that Kupffer cell depletion reduced DNase γ activity. Thus, we confirmed that Kupffer cells were the cells producing DNase γ.
As mentioned before, karyolysis is a general morphological change in necrotic cells. However, the generation mechanism has not been clarified. In this study, we showed that DNase γ produced from Kupffer cells caused karyolysis of necrotic hepatocytes. This suggested that chromatin is a component of nuclear architecture and its cleavage is required for dissolution of the nucleus. The delay of nuclear degradation may prolong inflammation and increases the possibility of generating self-antibodies and, notably, DNase γ deficiency causes autoimmune diseases in both human and mouse [2, 9, 11]. Thus, DNase γ-dependent DNA fragmentation and karyolysis is essential for maintaining homeostasis in vivo.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (C) 17K08891 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (to RM). All experimental procedures using mice were approved by the Institutional Animal Care and Use Committee at the Tokyo University of Science.
REFERENCES
- 1.Abdelhalim M. A., Jarrar B. M.2011. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 10: 166. doi: 10.1186/1476-511X-10-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Mayouf S. M., Sunker A., Abdwani R., Abrawi S. A., Almurshedi F., Alhashmi N., Al Sonbul A., Sewairi W., Qari A., Abdallah E., Al-Owain M., Al Motywee S., Al-Rayes H., Hashem M., Khalak H., Al-Jebali L., Alkuraya F. S.2011. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat. Genet. 43: 1186–1188. doi: 10.1038/ng.975 [DOI] [PubMed] [Google Scholar]
- 3.Arai T., Koyama R., Yuasa M., Kitamura D., Mizuta R.2014. Acrolein, a highly toxic aldehyde generated under oxidative stress in vivo, aggravates the mouse liver damage after acetaminophen overdose. Biomed. Res. 35: 389–395. doi: 10.2220/biomedres.35.389 [DOI] [PubMed] [Google Scholar]
- 4.Endo-Umeda K., Nakashima H., Komine-Aizawa S., Umeda N., Seki S., Makishima M.2018. Liver X receptors regulate hepatic F4/80+ CD11b+ Kupffer cells/macrophages and innate immune responses in mice. Sci. Rep. 8: 9281. doi: 10.1038/s41598-018-27615-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim M. J., Ahituv N.2013. The hydrodynamic tail vein assay as a tool for the study of liver promoters and enhancers. Methods Mol. Biol. 1015: 279–289. doi: 10.1007/978-1-62703-435-7_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi Y., Kiguchi N., Fukazawa Y., Saika F., Maeda T., Kishioka S.2015. Macrophage-T cell interactions mediate neuropathic pain through the glucocorticoid-induced tumor necrosis factor ligand system. J. Biol. Chem. 290: 12603–12613. doi: 10.1074/jbc.M115.636506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama R., Arai T., Kijima M., Sato S., Miura S., Yuasa M., Kitamura D., Mizuta R.2016. DNase γ, DNase I and caspase-activated DNase cooperate to degrade dead cells. Genes Cells 21: 1150–1163. doi: 10.1111/gtc.12433 [DOI] [PubMed] [Google Scholar]
- 8.Mizuta R., Araki S., Furukawa M., Furukawa Y., Ebara S., Shiokawa D., Hayashi K., Tanuma S., Kitamura D.2013. DNase γ is the effector endonuclease for internucleosomal DNA fragmentation in necrosis. PLoS One 8: e80223. doi: 10.1371/journal.pone.0080223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozçakar Z. B., Foster J., 2nd., Diaz-Horta O., Kasapcopur O., Fan Y. S., Yalçınkaya F., Tekin M.2013. DNASE1L3 mutations in hypocomplementemic urticarial vasculitis syndrome. Arthritis Rheum. 65: 2183–2189. doi: 10.1002/art.38010 [DOI] [PubMed] [Google Scholar]
- 10.Shiokawa D., Tanaka M., Kimura T., Hashizume K., Takasawa R., Ohyama H., Fujita K., Yamada T., Tanuma S.2000. Characterization of two DNase gamma-specific monoclonal antibodies and the in situ detection of DNase gamma in the nuclei of apoptotic rat thymocytes. Biochem. Biophys. Res. Commun. 275: 343–349. doi: 10.1006/bbrc.2000.3249 [DOI] [PubMed] [Google Scholar]
- 11.Sisirak V., Sally B., D’Agati V., Martinez-Ortiz W., Özçakar Z. B., David J., Rashidfarrokhi A., Yeste A., Panea C., Chida A. S., Bogunovic M., Ivanov I. I., Quintana F. J., Sanz I., Elkon K. B., Tekin M., Yalçınkaya F., Cardozo T. J., Clancy R. M., Buyon J. P., Reizis B.2016. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166: 88–101. doi: 10.1016/j.cell.2016.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]