Abstract
Brachycephalic airway syndrome (BAS) is a common disease in certain “flat-faced” dog breeds. This syndrome includes stenotic nares, elongated and thickened soft palate, laryngeal collapse, and tracheal hypoplasia. Pharyngeal collapse is also commonly observed, but it is unclear if laryngopharynx motions are merely sequelae or actually contribute to BAS respiratory symptoms. Laryngopharynx motion was imaged using dynamic four-dimensional computed tomography (4D-CT) during spontaneous respiration in four dogs with different BAS types. Dynamic 4D-CT showed laryngopharynx motion in the following order during inspiration: pharyngeal collapse, contraction, and laryngospasm. We concluded that dynamic 4D-CT is a highly-detailed diagnostic approach for detecting laryngopharynx motion. Pharyngeal contraction during inspiration appears to contribute toward the worsening of clinical respiratory signs of BAS.
Keywords: brachycephalic airway syndrome, dog, laryngospasm, pharyngeal collapse, pharyngeal contraction
Brachycephalic airway syndrome (BAS) is a common disease in brachycephalic dog breeds, including the English Bulldog, French Bulldog, Pug, Pekingese, and Shih Tzu. The clinical features of BAS include stenotic nares, elongated and thickened soft palate, laryngeal collapse, and tracheal hypoplasia [2, 12]. In addition, Pollard et al. reported pharyngeal collapse in multiple brachycephalic breeds with BAS (2 of 8 Boston Terriers, 19 of 22 English Bulldogs, 18 of 21 French Bulldogs, and 18 of 27 Pugs) [6]. For dynamic imaging, fluoroscopy is considered to be the gold standard for the diagnosis of laryngopharynx motion. Fluoroscopy findings are classified as complete pharyngeal collapse (complete loss of the nasopharyngeal airway lumen) and partial pharyngeal collapse (>50% estimated decrease in lumen diameter). In most dogs with BAS, coughing and stertor are the common clinical signs that occur when main-stem bronchial or tracheal collapse is concomitant with pharyngeal collapse. Therefore, pharyngeal collapse could be a sequela of long-term negative pressure gradients and both anatomic and functional abnormalities [9].
A high-speed computed tomography (CT) technique called dynamic CT with three-dimensional (3D) internal rendering was recently introduced, enabling the evaluation of both the morphological shape of the larynx and the severity of the laryngeal collapse [10]. This technique can be used to distinguish the three stages of laryngeal collapse as defined by Leonard: eversion of the laryngeal saccules (stage I), loss of rigidity and medial displacement of the cuneiform processes of the arytenoid cartilage (stage II), and collapse of the corniculate processes of the arytenoid cartilage with loss of the dorsal arch of the rima glottidis (stage III) [4]. Therefore, the evaluation of laryngopharynx motion in BAS may be feasible with dynamic 4D-CT imaging from free-breathing 3D-CT images in dogs. In the present study, we examined four dogs with several types of BAS. Pharyngeal contraction was suspected to worsen the respiratory states of the dogs examined in this study.
Subjects of this study were four client-owned dogs with suspected worsening of mild BAS (increasing throat noise) evaluated at the Animal Medical Center, Nihon University from 2016 to 2018. After an informed consultation, the owners who were averse to diagnosis using surgery under general anesthesia approved diagnostic imaging under sedation. The dogs were sedated using intramuscular injections of 0.4 mg/kg butorphanol (Vetorphal, Meiji Seika Pharma Co., Ltd., Tokyo, Japan) and 2.5 µg/kg medetomidine (Domitor, Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) in the femoral region to facilitate sufficient imaging time. Additionally, this sedation could be reversed quickly using antagonist agents if necessary [7]. The dogs were positioned in sternal recumbency. Images were acquired for 6 sec using a dynamic 4D-CT system equipped with 320 detector rows, each 0.5 mm in width with a gantry rotation time of 0.275 sec, covering a 16 cm range (24 cm field of view) per rotation (Aquilion ONE, Toshiba Medical Systems, Tokyo, Japan).
CASE 1 was an 11-year-old female French Bulldog (neutered, 9.4 kg) with a body conditioning score (BCS) of 3/5, difficulty in breathing when excited, stertorous upper airway noise during sleep, and moderately stenotic nostrils. CASE 2 was a 3-year-old male French Bulldog (10.7 kg) with unknown BCS, inspiratory difficulty, and sleep impairment. CASE 3 was a 6-year-old male French Bulldog (neutered, 11.6 kg) with a BCS of 3/5, daytime coughing, stertorous upper airway noise, difficulty in breathing during sleep, and mild stenotic nostrils. CASE 4 was a 7-year-old male French Bulldog (neutered, 10.4 kg) with a BCS of 4/5, stertorous upper airway noise, and both difficulty in breathing and vomiting during sleep. CASES 2 and 4 had undergone corrective surgery for stenotic nares and elongated soft palate at 1 and 4 years of age, respectively, and were prescribed antibiotics, corticosteroids, and bronchodilators by their local veterinarians. One owner (CASE 3) chose both rhinoplasty and staphylectomy, and another owner (CASE 4) chose only resection of the elongated soft palate as corrective surgery after the second informed consultation following the results of dynamic 4D-CT. After surgery, the dogs were hospitalized for three days, following which corticosteroids, bronchodilators, and gastrointestinal agents (antacids, antiemetics, and prokinetics) were administrated for several weeks after discharge (Table 1). According to the owners, ptyalism and airway noise had decreased a month later. CASE 3 showed increased deep sleep and in CASE 4, retching, vomiting, and snoring during sleep had disappeared.
Table 1. Characteristics and clinical profiles in four dogs with brachycephalic airway syndrome.
| CASE1 | CASE2 | CASE3 | CASE4 | |
|---|---|---|---|---|
| Species | French Bulldog | French Bulldog | French Bulldog | French Bulldog |
| Age (years) | 11 | 3 | 6 | 7 |
| Body weight (kg) | 9.4 | 10.7 | 11.6 | 10.4 |
| Gender | Neutered female | Male | Neutered male | Neutered male |
| Body conditioning score | 3/5 | Not recoded | 3/5 | 4/5 |
| Stenotic nares | Moderate | Normalized at 1 year of age | Mild | Normalized at 4 years of age |
| Clinical signs | Difficulty breathing during excitement and stertorous upper airway noise during sleep | Inspiratory difficulty and sleep impairment | Daytime coughing, stertorous upper airway noise, and difficulty breathing during sleep | Stertorous upper airway noise and both difficulty breathing and vomiting during sleep |
| Current treatment in referring hospital | None | None | None | Theophylline, as needed; amoxicillin, as needed; prednisolone, as needed |
| Treatments | Commitment: to avoid heat and excitement, decrease body weight, and stay in a cool place. | Commitment: to avoid heat and excitement, decrease body weight, and stay in a cool place. | Surgery: rhinoplasty & staphylectomy. | Surgery: resection of the elongated soft palate. |
| Post surgery: Cefovecin, 8 mg/kg, sc. | Post surgery: Cefovecin, 8 mg/kg, sc. | |||
| At discharge: famotidine, 1 mg/kg, po, bid, 1 week; metoclopramide, 5 mg/kg, po, bid, 1 week; maropitant, 2 mg/kg, po, sid, 1 week; prednisolone 1 mg/kg, po, sid, 1 week. | At discharge: famotidine, 1 mg/kg, po, bid, 4 weeks; mosapride 0.5 mg/kg, po, bid, 4 weeks; theophylline 10 mg/kg, po, bid, 2 weeks; prednisolone 0.5 mg/kg, po, bid, 2 weeks; ambroxol 1.5 mg/kg, po, bid, 2 weeks. | |||
Dynamic 4D-CT (Figs. 1 and 2) revealed that a flaccid and thick soft palate stretched and elongated during inspiration, similar to an elastic band. At the beginning of inspiration, the elongated soft palate touched lightly against the epiglottis during pharyngeal collapse. After collapse, both thickening of the pharyngeal constrictor muscles and backward movement of the larynx as pharyngeal contraction occurred. In turn, this contraction intercepted the opening of the arytenoid cartilage and induced laryngospasm. In addition, the nasopharyngeal airway space at the end of inspiration was maintained to reduce the thickness of stretched and elongated soft palates in CASES 2, 3 and 4. In this study, laryngeal collapse (Stage I) was observed only in CASE 2, and it was difficult to confirm the same due to lamination among the soft palate, epiglottis, and arytenoid cartilage by pharyngeal contraction. Table 2 summarizes the anatomical and functional characteristics visualized in the chest dynamic 4D-CT of supplementary examination. There were no side effects, such as bradycardia, respiratory depression, observed in any of the dogs.
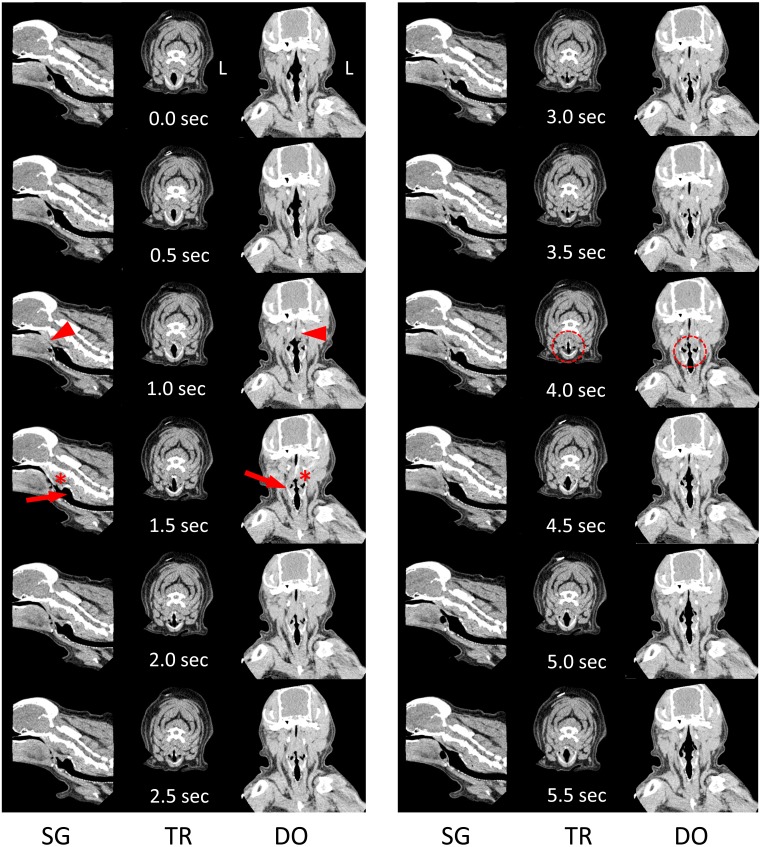
Fig. 1.
Dynamic four-dimensional computed tomography (4D-CT) images with time elapsed (sec) during one breath in CASE 1 (Rf=10). Images at 0.0 sec show the start of inspiration. The pharyngeal airway collapses at 1.0 sec (arrowheads). Thickening of the pharyngeal muscle (asterisks) and backward movement of the larynx (arrows) as pharyngeal contraction begins at 1.5 sec. Interference of larynx open (dotted circles) continues until the end of inspiration. Images at 4.5 sec depict the start of expiration. Rf: respiratory frequency (breaths/m); L: left; SG: sagittal plane; TR: transverse plane at laryngeal level; DO: dorsal plane at nasopharyngeal level.
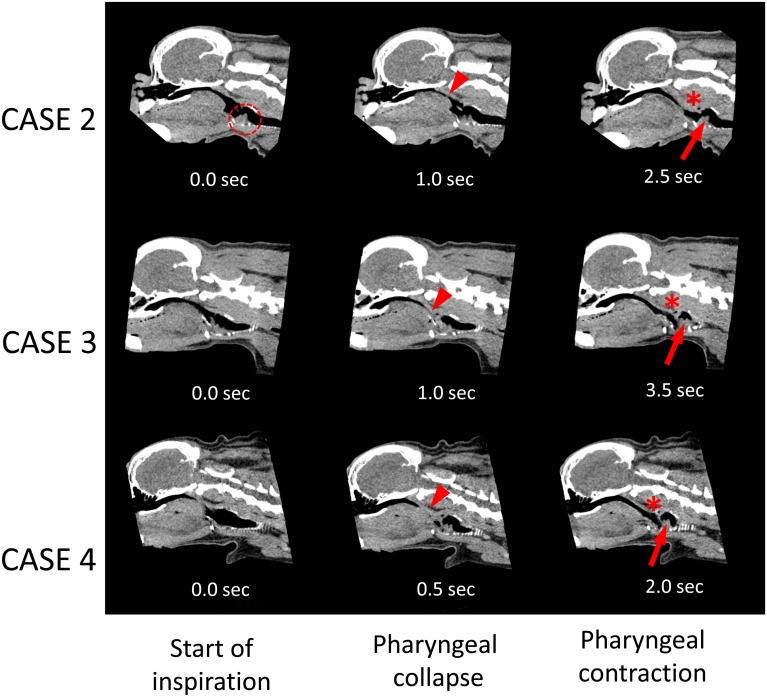
Fig. 2.
Selected dynamic 4D-CT images (sagittal plane) of the pharynx and larynx with time elapsed (sec) in CASES 2 (Rf=15), 3 (Rf=12) and 4 (Rf=24). The left column shows the start of inspiration, the center column shows pharyngeal collapse (arrowheads) after the start of inspiration, and the right column shows pharyngeal contraction as thickening of the pharyngeal muscle (asterisks) and backward movement of the larynx (arrows) after pharyngeal collapse. Eversion of the laryngeal saccules (dotted circle) was detected before pharyngeal collapse. Rf: respiratory frequency (breaths/m).
Table 2. Anatomical and functional characteristics in four-dimensional chest computed tomography.
| CASE 1 | CASE 2 | CASE 3 | CASE 4 | |
|---|---|---|---|---|
| Hypoplastic trachea (TI/TD) | No (0.22) | No (0.30) | No (0.23) | No (0.28) |
| Tracheal collapse (grade, location) | Yes (I, thorax) | No | No | No |
| Main-stem bronchial collapse (location) | Yes (left) | No | No | Yes (left) |
| Pectus excavatum | Yes | No | No | Yes |
TI/TD: ratio of the tracheal lumen diameter to the thoracic inlet distance; Grade I: 25% reduction in airway diameter.
There are several limitations to this study, such as the small sample size, only one breed examined (French Bulldog), and unpredictable effects of sedation on laryngopharynx motion due to relaxed smooth muscles in the laryngopharyngeal region. In fact, pharmacological sedation has been used to prevent the development of gag reflexes in dental patients [8]. Additionally, the temporal resolution of dynamic 4D-CT is lower than videofluoroscopy for real-time imaging. Despite these limitations, the current study provides valuable information on the functional changes in BAS, because 3D-CT images generally provide superior anatomic detail and more clearly image the causes of airway obstructions (e.g., tumor and foreign body) than 2D images obtained by videofluoroscopy.
Pharyngeal contraction contributes to reflex systems in the throat, such as swallowing and gag reflexes [3, 5]. The gag reflex is a physiologically normal function and an oral protective reflex that prevents foreign bodies from entering the airway [3, 11]. Nasal obstruction, postnasal drip, sinusitis, nasal polyps, and congestion of the oral, nasal, and pharyngeal mucosa can also cause gag reflex [3, 11]. The gag reflex is a brief elevation of the soft palate and bilateral contraction of the constrictor muscles of the pharynx by intraoral stimulation [3]. However, no elevation of the soft palate was observed in three cases in this study because the posterior soft palate was stretched to the larynx by inspiratory effort. The following five areas are known to serve as “trigger zones”: palatoglossal and palatopharyngeal folds, base of tongue, palate, uvula, and posterior pharyngeal wall [1]. Therefore, we believe that pharyngeal collapse also stimulates the palate, uvula, and pharyngeal wall and induces pharyngeal contraction and a transient laryngospasm for each breath together with other disorders of the upper airway. If the laryngospasm continues without relief, it can lead to pulmonary edema, cardiac dysrhythmias, cardiac arrest, and ultimately death, which are also frequent in dogs with BAS.
Rhinoplasty, turbinectomy, and staphylectomy are corrective surgeries for BAS that decrease airway resistance and prevent suction of the soft palate into the trachea. Clinical signs, such as difficulty in breathing, due to transient pharyngeal contraction and laryngospasm, were likely eliminated after surgeries in two cases. Therefore, pharyngeal collapse in BAS may be secondarily caused by excessive negative pressure of the airway. Respiratory dyspnea improved with open-mouth breathing only when nasal stenosis was involved, and the severity of dyspnea by elongated soft palate and laryngeal collapse depended on the degree of additional upper airway abnormalities in BAS [2, 12]. Gagging and retching are common even after corrective surgery and it is thought that the pharyngeal reflex does not function normally in during postoperative period [2]. Postoperative gagging and retching were prevented in our cases and follow-up dynamic 4D-CT was not available. Therefore, it is necessary to use dynamic 4D-CT to confirm whether surgical corrections reduce the incidences of pharyngeal collapse, contraction and laryngospasm.
In conclusion, dynamic 4D-CT is a highly-detailed diagnostic approach for examining laryngopharynx motion and pharyngeal contraction after pharyngeal collapse during inspiration. Pharyngeal contraction seems to worsen the clinical respiratory signs of BAS, such as stenotic nares, elongated and thickened soft palate, laryngeal collapse, and tracheal hypoplasia.
Acknowledgments
We thank all the staff of the Animal Medical Center, Nihon University, for the data collection.
REFERENCES
- 1.Bassi G. S., Humphris G. M., Longman L. P.2004. The etiology and management of gagging: a review of the literature. J. Prosthet. Dent. 91: 459–467. doi: 10.1016/j.prosdent.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 2.Harvey C. E.1981. Upper airway obstruction surgery. II. Soft palate resection surgery in brachycephalic dogs. J. Am. Anim. Hosp. Assoc. 18: 538–544. [Google Scholar]
- 3.Kook P. H.2017. Gagging. pp. 152–154. In: Textbook of Veterinary Internal Medicine, 8th ed., Saunders Elsevier, Philadelphia. [Google Scholar]
- 4.Leonard H. C.1960. Collapse of the larynx and adjacent structures in the dog. J. Am. Vet. Med. Assoc. 137: 360–363. [PubMed] [Google Scholar]
- 5.Marks S. L.2017. Diseases of the pharynx and esophagus. pp. 1476–1490. In: Textbook of Veterinary Internal Medicine, 8th ed., Saunders Elsevier, Philadelphia. [Google Scholar]
- 6.Pollard R. E., Johnson L. R., Marks S. L.2018. The prevalence of dynamic pharyngeal collapse is high in brachycephalic dogs undergoing videofluoroscopy. Vet. Radiol. Ultrasound 59: 529–534. doi: 10.1111/vru.12655 [DOI] [PubMed] [Google Scholar]
- 7.Puighibet Z., Costa-Farré C., Santos L., Canfrán S., Gómez de Segura I. A.2015. The sedative effects of intramuscular low-dose medetomidine in combination with butorphanol or methadone in dogs. Vet. Anaesth. Analg. 42: 590–596. doi: 10.1111/vaa.12256 [DOI] [PubMed] [Google Scholar]
- 8.Reshetnikov A. P., Kasatkin A. A., Urakov A. L., Baimurzin D. Y.2017. Management of exaggerated gag reflex in dental patients using intravenous sedation with dexmedetomidine. Dent Res J (Isfahan) 14: 356–358. doi: 10.4103/1735-3327.215967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin J. A., Holt D. E., Reetz J. A., Clarke D. L.2015. Signalment, clinical presentation, concurrent diseases, and diagnostic findings in 28 dogs with dynamic pharyngeal collapse (2008–2013). J. Vet. Intern. Med. 29: 815–821. doi: 10.1111/jvim.12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadler K., Hartman S., Matheson J., O’Brien R.2011. Computed tomographic imaging of dogs with primary laryngeal or tracheal airway obstruction. Vet. Radiol. Ultrasound 52: 377–384. doi: 10.1111/j.1740-8261.2011.01816.x [DOI] [PubMed] [Google Scholar]
- 11.Stefos S., Zoidis P., Nimmo A.2019. Managing gag reflex during removable partial denture treatment: a review and a clinical report. J. Prosthodont. 28: 618–622. doi: 10.1111/jopr.12957 [DOI] [PubMed] [Google Scholar]
- 12.Trappler M., Moore K.2011. Canine brachycephalic airway syndrome: pathophysiology, diagnosis, and nonsurgical management. Compend. Contin. Educ. Vet. 33: E1–E4, quiz E5. [PubMed] [Google Scholar]