Abstract
Recently, a mixture of medetomidine, midazolam and butorphanol (MMB) has been used as an injectable general anesthetic agent for laboratory animals. The purpose of this study was to establish data to encourage practical usage of MMB, and to clarify the effects of MMB on the respiratory function in rats. To compare the anesthetic efficacy between the injection routes, the anesthetic effects of MMB by subcutaneous (s.c.) or intraperitoneal (i.p.) injection were evaluated in rats. To assess the respiratory function, the blood gas parameters and electrolytes were assessed in serial venous blood samples collected from before s.c. injection of MMB to 270 min after the injection. Recovery from anesthesia and the respiratory changes after atipamezole injection at 30 min after MMB injection was also examined. Subcutaneous injection of MMB was associated with more rapid induction and a longer duration of anesthesia as compared to i.p. injection. The blood gas analysis findings showed MMB had effects on respiratory function, that is, elevations of the partial pressures of carbon dioxide and bicarbonate and reduction of the blood pH. Atipamezole injection resulted in recovery from the MMB-induced anesthetic effect as well as respiratory depression. In conclusion, MMB provides more effective anesthesia administered by s.c. injection compared to i.p. injection and induces respiratory change. These changes were counteracted by atipamezole. Therefore, we recommend MMB administered by s.c. injection for anesthesia, followed by injection of atipamezole after the operative procedure to allow recovery.
Keywords: anesthesia, blood gas, butorphanol, medetomidine, midazolam
Inhalational anesthetics are recommended for general anesthesia in animals, because regulation of the anesthetic depth is easy with their use [25]. However, an injectable anesthetic agent is often applied when a large number of animals need to be anesthetized at the same time or a mask for inhalation cannot be used, e.g., in cases requiring surgery of the head and neck region. Injectable anesthetics for inducing general anesthesia commonly consist of a combination of more than two drugs, including a sedative, analgesic and a muscle relaxant. In recent years, the general anesthetic consisting of the combination of medetomidine, midazolam and butorphanol (MMB) has been used in Japan [8]. The use of an injectable agent for inducing general anesthesia entails difficulty in controlling the anesthetic depth and duration, but MMB anesthesia has the advantage that its effects can be rapidly reversed by the injection of atipamezole [10, 11, 13,14,15]. Use of MMB for inducing general anesthesia has not become widespread in other countries, where ketamine/xylazine and isoflurane are commonly applied; therefore, experimental data regarding MMB anesthesia are still limited. Differences in the anesthetic effects of MMB depending on the injection route are not still clear, even though several studies have been conducted in rodents [6, 10, 15].
To understand the underlying physiological changes is important while selecting an anesthetic agent. General anesthesia, including that induced by MMB, is often associated with depressions of respiration and circulation, and reduction of the general motor activity, neuronal activity and body temperature. In addition, MMB anesthesia causes inhibition of insulin secretion from the islet cells of the pancreas [12], and has been reported to induce hyperglycemia in mice [14], rats [15, 22] and hamsters [13].
In this study, we focused on the effect of MMB anesthesia on the respiratory function, because this aspect remains unclear, especially in small rodents. Respiratory function during anesthesia is assessed by measuring the respiratory rate, blood oxygen saturation, respirometry and capnometry in human and animals; although the latter two methods are not commonly used in rodents. Conflicting results have been reported on the influence of MMB anesthesia on the respiratory rate in rodents [1, 10, 11, 23]. Blood oxygen saturation is measured by pulse oximetry or blood gas analysis. Although MMB anesthesia was found to be associated with a decrease of the blood oxygen saturation measured with a pulse oximeter (SpO2) in mice [10, 23] and rats [11], accurate SpO2 measurement is difficult in animals under general anesthesia, because of the difficulty in detecting the arterial pulse under the condition of poor tissue perfusion [4]. Blood gas analysis is a useful method to evaluate the respiratory function, and the influences of numerous anesthetic agents on the results of blood gas analysis have been reported [21, 26, 27]. The effect of MMB on the respiratory function monitored by blood gas analysis has been reported in dogs [16], but not yet in rodents.
One of the purposes of this study was to examine the differences in the anesthetic effect of MMB depending on the injection route, to establish data for practical usage of MMB anesthesia in rats. Another was to perform venous blood gas analysis to evaluate the effect of MMB anesthesia on the respiratory function; furthermore, recovery from the anesthesia induced by atipamezole was elucidated by the anesthetic depth and blood gas analysis.
MATERIALS AND METHODS
Animals, housing conditions and ethics statement
Thirty four male Sprague-Dawley rats (Crl:CD(SD)) were purchased from a commercial breeder (Charles River Laboratories Japan, Inc., Yokohama, Japan). These rats were housed individually in a rat TPX single cage (Tokiwa Kagaku Kikai Co., Ltd., Tokyo, Japan) or in groups of 2 or 3 in Ekon cages (CLEA Japan, Inc., Tokyo, Japan) containing a bedding material (white-flakes, Oriental Yeast Co., Ltd., Tokyo, Japan) and environmental enrichment (rat retreats, Bio-Serv, Flemington, NJ, USA). Commercially available rodent food (MF, Oriental Yeast Co., Ltd.) and water were provided to the animals ad libitum. The cages, bedding material and environmental enrichment were changed once a week and the water bottles were changed three times a week. The animal room was maintained at a temperature of 20 to 26°C and relative humidity of 30 to 70%. Lights were switched on at 7:15 am and switched off at 19:15 pm (12/12 hr cycle). The rats were acclimatized to the housing conditions and husbandry procedures for at least a week prior to the start of the experiments and provided for the experiments at 8 weeks of age.
The animal housing and experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee at Taisho Pharmaceutical Co., Ltd.
Drugs and their preparations
Medetomidine hydrochloride (Dorbene®, Kyoritsuseiyaku Corp., Tokyo, Japan), midazolam (Dormicum®, Astellas Pharma Inc., Tokyo, Japan), butorphanol (Vetorphale®, Meiji Seika Pharma Co., Ltd., Tokyo, Japan) and sodium pentobarbital (Somnopentyl®, Kyoritsuseiyaku Corp.) were used as the anesthetic agents. Atipamezole (Antisedan®, Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) was used as an antagonist of medetomidine.
Medetomidine, midazolam and butorphanol were mixed and diluted with saline (Otsuka Normal Saline®, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) to concentrations of 0.03, 0.4 and 0.5 mg/ml, respectively [11]. Pentobarbital was diluted with saline to a concentration of 10 mg/ml. Atipamezole was diluted with saline to concentrations of 0.03 and 0.15 mg/ml. The anesthetics and atipamezole were administered at the dosing volume of 5 ml/kg. In the case of need for additional anesthesia, the respective anesthetic agents were administered at the dose volume of 1 ml/kg until complete anesthesia was induced (up to an anesthesia score of 5, as described below).
Experimental procedures
Experiment 1: Comparison of the anesthetic effects of MMB and pentobarbitaladministered by s.c. or i.p. injection. Fourteen rats were used for Experiment 1. In the first experiment, MMB was administered at 0.15, 2 and 2.5 mg/kg of medetomidine, midazolam and butorphanol, respectively, by s.c. (n=7) or i.p. (n=7) injection. In the second experiment, all 14 rats received pentobarbital at a dose of 50 mg/kg by i.p. injection 2 weeks after the first MMB experiment. The induction time, anesthesia duration and necessity for additional anesthesia were compared between the animals given s.c. and i.p. injections of MMB or between those given i.p. injections of MMB and pentobarbital. The animals were not heated up by heat mat or heat board during experiments.
Experiment 2: Effects of MMB administered by s.c. injection on the physiological functions and recovery from anesthesia by atipamezole. Twenty rats were used for Experiment 2. The rats were assigned at random to 4 experimental groups consisting of 5 animals each. The animals in the control group were given saline by s.c. injection. Another 15 animals were given MMB by s.c. injection at the same dose as in Experiment 1, and 30 min thereafter, received atipamezole by s.c. injection at the dose of 0.15 mg/kg (low ATI group) or 0.75 mg/kg (high ATI group) for reversal of anesthesia, or did not receive any atipamezole (MMB group). The recovery time, rectal temperature, venous blood gas parameters and blood electrolyte levels were measured. The animals were not heated up by heat mat or heat board during experiments.
Evaluation of the anesthetic effects
The depth of anesthesia was confirmed by examination of 5 reflexes [11]. Briefly stated, the tail pinch reflex and pedal withdrawal reflex of a forelimb and hindlimb were evaluated using blunt forceps to pinch the proximal tail and the interdigital web of the limbs lightly. The corneal or eyelid reflex was evaluated by blowing air on the eyes using a pipette. The righting reflex was evaluated by turning the rat on its back and any attempt of the animal to right itself from the dorsal to the sternal recumbency position was judged as a positive reflex response. Each reflex was scored as 0 or 1, depending on whether any motor response or reactive movement was observed or not. An anesthesia score was assigned to each animal (from 0 to 5): 0 meant arousal, and 4 or more was defined as surgical anesthesia [9]. The anesthesia score was evaluated at 5-min intervals after the injection of the anesthetic agents or at 1-min intervals after the injection of atipamezole until arousal. The time until the score reached 4 or more was defined as the induction time. The duration of surgical anesthesia was defined as the anesthesia duration. The time until the score returned to 0 after injection of atipamezole was defined as the recovery time.
Evaluation of the rectal temperature, venous blood gases, electrolytes and other parameters
Rectal temperature was measured with a digital thermometer (TD-300, Shibaura Electronics Co., Ltd., Saitama, Japan) every 5 min after the injection of MMB, or every 30 min under arousal. The probe was inserted up to about 3 cm from the anus.
Venous blood samples (0.2 ml) were collected from the external jugular vein before administration of MMB, and 20, 70 and 270 min after injection of the anesthetic agent. The samples were analyzed immediately using a portable blood gas analyzer (i-STAT®, Abbott Japan, Tokyo, Japan) with cartridges (CG8+, Abbott Japan) for measuring and calculating the hydrogen potential (pH), venous carbon dioxide partial pressure (PvCO2), venous oxygen partial pressure (PvO2), base excess (BE), bicarbonate ion (HCO3), total carbon dioxide (TCO2), venous oxygen saturation (SvO2), sodium (Na), potassium (K), ionized calcium (iCa), glucose (Glu), and hemoglobin (Hgb) concentrations, and the hematocrit (Hct). Because PvCO2 and pH were measured at 37°C in the i-STAT® system, they were corrected with the rectal temperature as follows [2].
Corrected pH (pHt) = measured pH + 0.0147 × (37– rectal temperature)
Corrected PvCO2 (PvCO2t) = measured PCO2 × 10 ^ 0.019 (rectal temperature – 37)
Statistical analysis
Statistical analysis was performed using EXSUS ver. 7.7.1 (CAC Croit Corp., Tokyo, Japan). Statistical significances of differences between two groups were analyzed for homogeneity of variance by the F-test, followed by Student’s t-test when the variance was homogeneous or Welch’s t-test when the variance was heterogeneous. Statistical significances of differences in the rectal temperature (30, 60, 90, 120, 150, 180, 210, 240 and 270 min), venous blood gases and other parameters (20, 70 and 270 min) were analyzed by repeated measures analysis of variance (ANOVA). In case of significant differences in interaction of group and time or even only between groups, Tukey’s test were performed at each time point. Data were expressed as means ± standard deviation (SD) and the P value for statistical significance was set at 0.05.
RESULTS
There was no mortality in any of the experiments, and all the rats recovered from anesthesia.
Experiment 1: Comparison of the anesthetic effects of MMB and pentobarbital administered by s.c. or i.p. injection
Anesthesia score. Following s.c. injection of MMB, the rats immediately lost their reflexes and reached the surgical anesthesia state by 5 min and were completely anesthetized by 10 min after the injection, without the need for any additional anesthesia (Fig. 1). On the other hand, since 5 of 7 rats administered MMB by i.p. injection failed to be anesthetized completely, these 5 animals needed additional anesthesia at 10 min after the first injection (Table 1). Furthermore, one of them needed another additional anesthesia once again at 25 min, and finally, all the i.p.- injected rats became completely anesthetized by 30 min after the first injection. Following i.p. injection of pentobarbital, 5 of 14 rats failed to be anesthetized completely, 3 of them needed additional anesthesia, of which 2 required it twice. All the rats given pentobarbital by i.p. injection reached the anesthesia score of 5 at once, however, 6 of the 14 animals recovered their reflexes immediately, although some of these animals reached the anesthesia score of 5 again.
Fig. 1.
Changes in the anesthesia score after administration of a mixture of medetomidine, midazolam and butorphanol (MMB) by s.c./ i.p. injection (n=7), or of pentobarbital at a dose of 50 mg/kg by i.p. injection (n=14) in rats. MMB: 0.15, 2 and 2.5 mg/kg of medetomidine, midazolam and butorphanol, respectively. Data represent means ± SD.
Table 1. Induction time, anesthesia duration and number of additional anesthetic injections after administration of a mixture of medetomidine, midazolam and butorphanol (MMB) by s.c. or i.p. injection, or of pentobarbital by i.p. injection in rats.
| Anesthesia | Route | N | Age (week) |
Body weight (g) |
Induction time (min) |
Anesthesia duration (min) |
Times of additional anesthetic injections (number of rats) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Shortest | Longest | 1 | 2 | ||||
| MMB | i.p. | 7 | 8 | 349.2 ± 13.7 | 13.6 ± 3.8 | 69.3 ± 14.0 | 45 | 80 | 4 | 1 |
| MMB | s.c. | 7 | 8 | 367.8 ± 30.9 | 5.0 ± 0.0# | 135.7 ± 21.7* | 105 | 160 | 0 | 0 |
| Pentobarbital | i.p. | 14 | 10 | 423.2 ± 31.4 | 10.0 ± 3.4* | 37.9 ± 18.7* | 10 | 75 | 3 | 2 |
Data represent the means ± SD. N: number of rats. #P<0.05 and *P<0.05 as compared to the MMB group administered MMB by i.p. injection (Welch’s t-test and Student’s t-test, respectively).
Induction time. The anesthesia induction time after s.c. injection of MMB (5.0 ± 0.0 min) was significantly shorter than that after i.p. injection (13.6 ± 3.8 min), and the anesthesia induction time after i.p. injection of pentobarbital (10.0 ± 3.4 min) was significantly shorter than that after i.p. injection of MMB (Table 1).
Anesthesia duration. The duration of anesthesia after s.c. injection of MMB (135.7 ± 21.7 min) was significantly longer than that after i.p. injection (69.3 ± 14.0 min), and that after i.p. injection of pentobarbital (37.9 ± 18.7 min) was significantly shorter than that after i.p. injection of MMB (Table 1).
Experiment 2: Effects of MMB administered by s.c. injection on the physiological functions and recovery from anesthesia by atipamezole
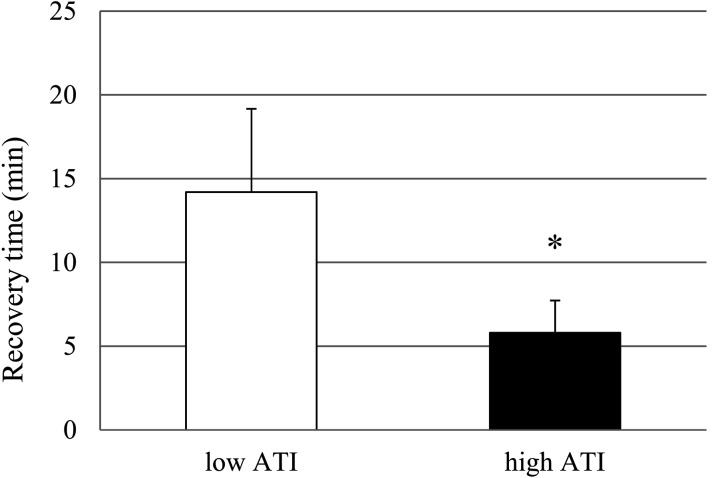
Recovery time from MMB anesthesia. Following atipamezole injection at 30 min after the MMB injection, all rats, at either dose of atipamezole, showed a gradual decrease of the anesthesia score. The high ATI group showed more effective reversal from the MMB anesthesia than the low ATI group, and the recovery time in the high ATI group (5.8 ± 1.9 min) was significantly shorter than that in the low ATI group (14.2 ± 5.0 min) (Fig. 2). Furthermore, in the low ATI group, the sedation persisted even after the anesthesia score returned to 0, whereas the animals of the high ATI group became more stable and conscious than those of the low ATI group after the anesthesia score returned to 0 (data not shown).
Fig. 2.
Recovery time after administration of atipamezole by s.c. injection at 0.15 mg/kg (low ATI group) or 0.75 mg/kg (high ATI group) at 30 min after s.c. injection of a mixture of medetomidine, midazolam and butorphanol in rats. Data represent means ± SD of 5 rats. *P<0.05 as compared with the low ATI group (Student’s t-test).
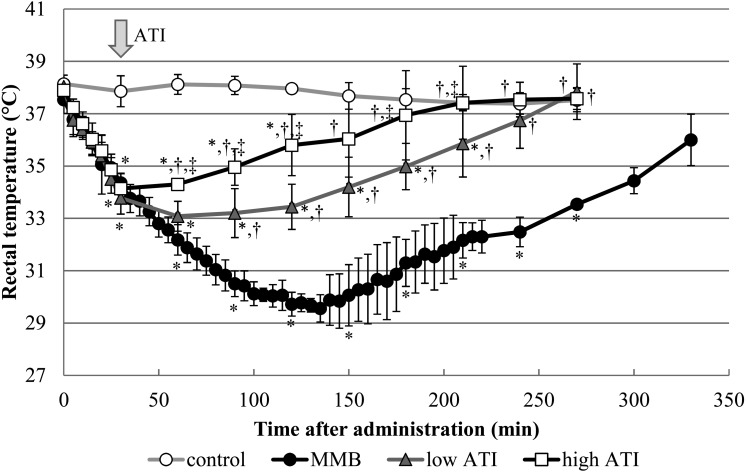
Effects on the rectal temperature. In all the rats that received MMB, the rectal temperature began to fall immediately after the injection (Fig. 3). Repeated measures ANOVA showed a significant difference in interaction of group and time. Tukey’s test indicated that significant declines of the rectal temperature in the MMB, low ATI and high ATI groups compared to the control group continued from 30 to 270, 210 and 120 min, respectively, and by that time the anesthesia score had already returned to 0 in all the groups. When atipamezole was injected at 30 min after MMB, the rectal temperature began to recover. The rectal temperatures in the low ATI and high ATI groups were significantly higher as compared to those in the MMB group from 90 to 270 min and from 60 to 270 min after the MMB injection, respectively. The rectal temperature in the high ATI group was higher than that in the low ATI group from 60 to 210 min, except at 150 min.
Fig. 3.
Changes in the rectal temperature after administration of atipamezole by s.c. injection at 0.15 mg/kg (low ATI group) or 0.75 mg/kg (high ATI group) at 30 min after s.c. injection of a mixture of medetomidine, midazolam and butorphanol (MMB), and in the absence of atipamezole (natural reversal of anesthesia; the MMB group) in rats. Arrow indicates the time of atipamezole injection. Pre-values represent 0 time. Data represent means ± SD of 5 rats. *P<0.05, †P<0.05 and ‡P<0.05 as compared with the control, MMB and low ATI groups at each time point, respectively (repeated measures ANOVA followed by Tukey’s test).
Effect on the venous blood gases, electrolytes and other parameters. The mean venous blood gases, pH, electrolytes and biochemical parameters are summarized in Table 2. In the course of the experiments, the blood gases in one rat of the control group at all the sampling points and another rat of the low ATI group at 70 min could not be measured due to errors in the portable blood gas analyzer. In pHt, PvCO2t, PvO2, TCO2, K, iCa, Glu, Hct, and Hgb, repeated measures ANOVA showed a significant difference in interaction of group and time. In BE, HCO3 and Na, repeated measures ANOVA showed a significant difference between groups, but not in interaction of group and time. Tukey’s test about those parameters were performed at each time point. In SvO2, no significant difference in interaction of group and time and between groups was observed by repeated measures ANOVA, then subsequent analysis was not performed.
Table 2. Changes in the venous blood gas parameters, pHt and other biochemical parameters after administration of atipamezole by s.c. injection at 0.15 mg/kg (low ATI group) or 0.75 mg/kg (high ATI group) at 30 min after s.c. injection of a mixture of medetomidine, midazolam and butorphanol (MMB), and in the absence of atipamezole (natural reversal of anesthesia; the MMB group) in rats.
pHt: Significant declines as compared to the control group were observed in the MMB and high ATI groups from 20 to 70 min after the MMB injection, and in the low ATI group at 20 min. The blood pHt in the low ATI and high ATI groups was higher than that in the MMB group at 70 min.
PvCO2t: Significant elevations as compared to the control group were observed in the MMB group from 20 to 270 min after the MMB injection, and in the low ATI and high ATI groups, from 20 to 70 min after the MMB injection. The PvCO2t values in the low ATI and high ATI groups were lower than that in the MMB group at 70 min.
PvO2: Significant elevation as compared to the control group was observed in the MMB group at 70 min after the MMB injection. On the other hand, as compared to the MMB group, significant decreases were observed in the low ATI and high ATI groups at 70 min.
HCO3: Significant elevations compared to the control group were observed in the MMB group from 20 to 270 min after the MMB injection, and the low ATI and high ATI groups from 20 to 70 min.
SvO2: Although a tendency towards transient declines of the SvO2 were observed in MMB administered groups at 20 min, these changes were not statistically significant.
Glu: A significant elevation compared to the control group was observed in the MMB group from 70 to 270 min after the MMB injection. Significant elevation in the MMB group as compared to the low ATI group was observed at 70 min, and as compared to the high ATI group was observed at 70 and 270 min.
Other parameters: In the MMB group, significant elevations as compared to the control group were observed in the K at 20 min, in the TCO2 and iCa from 20 to 270 min, Na at 70 and 270 min, and in the Hct and Hgb at 270 min. No significant alteration of the BE was seen in the MMB group. The changes of the TCO2, Na, K and iCa in the low ATI and high ATI groups were similar to those in the MMB group. No significant alteration of the Hct or Hgb were seen in the low ATI and high ATI groups as compared to control group.
DISCUSSION
We found different anesthetic efficacies of MMB between s.c. and i.p. injections, and showed that MMB administered by s.c. injection induced rapid surgical anesthesia and a longer duration of anesthesia than MMB administered by i.p. injection in rats. The elevation of PvCO2 and decline of the venous blood pH were suggestive of respiratory depression in MMB anesthesia. Furthermore, hyperglycemia and a tendency towards hemoconcentration were also observed. Atipamezole rapidly reversed the MMB-induced anesthesia, and minimized the aforementioned physiological alterations induced by MMB.
The present study clearly showed that MMB administered by s.c. injection exerted a more rapid, complete and stable anesthetic effect as compared to MMB administered by i.p. injection. MMB administered by s.c. injection for induction of anesthesia is recommended from some studies in rodents [10, 15], however, the difference in the duration of anesthesia between the two injection routes has not yet been clarified. In mice, MMB administered by s.c. injection tended to be associated with an extended duration of anesthesia as compared to MMB administered by i.p. injection [10], and MMB administered by i.p. injection did not consistently induce surgical anesthesia in all of the mice studied [6]. In rats, the difference in the duration of anesthesia depending on the injection route of MMB has not yet been demonstrated. In this study, a single s.c. injection of MMB induced surgical anesthesia within 5 min after the injection, and the surgical anesthesia was sustained for 136 min, which was approximately twice as long as that induced by i.p. injection of MMB. On the other hand, a single i.p. injection of MMB failed to produce surgical anesthesia in approximately 70% of the animals, in the absence of additional injection. One of the reasons for the different anesthetic effect observed between s.c. and i.p. injections of MMB could be the differences in the metabolic pathways between the two routes of injection; some drugs administered by i.p. injection are absorbed into the portal system and subjected to the hepatic first-pass effect [5]. Another reason is a possibility of loss the agent into gastrointestinal tract [5]. Misinjection is a consistent limitation of i.p. injection, the rate of misinjection is reported from 6 to 20% [28]. In this study, additional injection was needed for some animals with i.p. injection of both MMB and pentobarbital, but not needed for ones with s.c. injection. In the aspects of both anesthesia effects and animal welfare, s.c. injection seems to be preferred for injection route of MMB.
Blood gas analysis was used to assess respiratory depression under MMB anesthesia in this study, because poor tissue perfusion does not affect the results of blood gas analysis. The results of our analysis showed that MMB anesthesia produced respiratory depression. Contradictory reports have been published on the effects of MMB anesthesia on respiration. It has been reported, based on counting of the chest or abdominal wall movements, that MMB reduces the respiratory rate [23], however, assessment by pulse oximetry failed to reveal significant effect on respiratory rate [10, 11]. While decline of SpO2 was observed in all of these studies, the reported magnitude of decline of SpO2 varies in the range of 10% to 30%. Pulse oximetry detects volumetric changes associated with pulsatile arterial blood flow to measure oxygen saturation. Arterial pulse is difficult to detect under poor tissue perfusion conditions [4], and medetomidine is known to produce peripheral vasoconstriction [20]. Usually, arterial blood samples are obtained for blood gas analysis. However, venous blood gas analysis reflects the metabolic status of the tissues more accurately than arterial blood gas analysis [16, 24], therefore, we decided to evaluate the respiratory function by venous blood gas analysis. The results of the venous blood gas analysis in this study revealed that MMB induces respiratory depression. Elevation of the PvCO2 immediately after MMB injection indicated respiratory acidosis as a result of reduction of alveolar ventilation. Increase of the HCO3 implied metabolic alkalosis in compensation for the acidosis. The blood pH reduced from 7.45 to 7.30 as a result of these changes. The present study indicated that MMB-induced respiratory depression is not severe because no significant changes were observed in the SvO2 throughout the experiment despite these changes.
MMB induces several physiological changes. In this study, the rectal temperature dropped by approximately 8°C as compared to the pre-value after MMB administration in rats, the reduced temperature persisted continued even after the rats awakened from anesthesia. MMB seemed to cause hypothermia via causing CNS depression and via muscle relaxation induced by medetomidine [20], predominantly through the α2A-adrenoceptor [7]. Cutaneous heat loss is large, especially in small animals, due to the large body surface area. It is necessary to prevent body heat loss and carefully ensure that small animals are kept warm when under MMB anesthesia. Medetomidine increases the blood glucose concentration transiently by inhibiting insulin secretion from the pancreatic islets [12], and MMB has been reported to induce hyperglycemia in laboratory animals [13,14,15, 22]. Hyperglycemia associated with MMB anesthesia has been attributed to the actions of medetomidine. In this study, significant increase of the blood glucose, by approximately 140% of the pre-value, was observed after recovery from the anesthesia; this increase was slighter than that reported previously [13,14,15]. Ionization of calcium increases under an acid environment [3], and the increase in the iCa concentration in the present study could have resulted from balancing of the ionization/protein binding ratio of calcium. The iCa ratio increased with decrease of the protein binding ratio under the low blood pH environment. Blood Na, Hct and Hgb concentrations increased with time after the MMB injection and these changes were thought to be a result of hemoconcentration, secondary to dehydration following diuresis. Medetomidine exerts a diuretic effect via increasing the plasma natriuretic peptide concentration [18] and inhibiting antidiuretic hormone release in rats [17]. The fact that the animals could not drink sufficient water during the experiment could also have contributed to the hemoconcentration.
Atipamezole induced recovery, not only from the anesthetic effect, but also from the respiratory depression, hypothermia and hyperglycemia; in addition, it also induced some improvement in the hemoconcentration induced by MMB in this study. Atipamezole is an α2-adrenergic antagonist with high selectivity and is known to counteract the anesthetic effect of MMB. Recovery from the anesthetic effect and hypothermia was dose-dependent, and after a low dose of atipamezole, that is, the same dose as medetomidine, the sedation persisted, even after all the reflexes recovered. Therefore, administration of atipamezole at the same dose as medetomidine is not sufficient to induce recovery from MMB anesthesia. One of the reasons why atipamezole can counteract MMB-induced anesthesia is that MMB exerts its anesthetic effect mainly through the α2-adrenoceptor; moreover, midazolam exerts a significant synergistic action with dexmedetomidine, an α2-adrenergic agonist [19]. Furthermore, it has been reported that atipamezole can also counteract the anesthetic effect of the combination of medetomidine, butorphanol and alfaxalone, a neurosteroid anesthetic, as well as it can counter the anesthetic effect of MMB [6].
The recommended protocol for anesthesia is to induce sufficient anesthesia for the operation and then recover from anesthesia immediately after the end of the procedure. We recommend MMB anesthesia by s.c. injection to perform the operation, and counteracted the anesthetic effect by injection of atipamezole immediately after the procedure; this recommendation is based on our present study showing that MMB administration by s.c. injection induced more effective anesthesia than that by i.p injection. We also demonstrated by blood gas analysis, that MMB induced respiratory depression in the rats. While atipamezole injection is known to counteract the anesthetic effect of MMB, it also counteracts the respiratory changes induced by MMB.
Acknowledgments
The authors thank Yu Kajiyama for suggestions about statistical analysis, Hiroki Tanigami, Tomoko Hata, Ayako Ishigaki, Yutaka Nakamura, Kohei Yamamoto and Takayuki Konno for their technical supports.
REFERENCES
- 1.Bellini L., Banzato T., Contiero B., Zotti A.2014. Evaluation of three medetomidine-based protocols for chemical restraint and sedation for non-painful procedures in companion rats (Rattus norvegicus). Vet. J. 200: 456–458. doi: 10.1016/j.tvjl.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 2.Burnett R. W., Noonan D. C.1974. Calculations and correction factors used in determination of blood pH and blood gases. Clin. Chem. 20: 1499–1506. [PubMed] [Google Scholar]
- 3.Calvi L. M., Bushinsky D. A.2008. When is it appropriate to order an ionized calcium? J. Am. Soc. Nephrol. 19: 1257–1260. doi: 10.1681/ASN.2007121327 [DOI] [PubMed] [Google Scholar]
- 4.Goldman J. M., Petterson M. T., Kopotic R. J., Barker S. J.2000. Masimo signal extraction pulse oximetry. J. Clin. Monit. Comput. 16: 475–483. doi: 10.1023/A:1011493521730 [DOI] [PubMed] [Google Scholar]
- 5.Hedenqvist P., Roughan J. V., Flecknell P. A.2000. Sufentanil and medetomidine anaesthesia in the rat and its reversal with atipamezole and butorphanol. Lab. Anim. 34: 244–251. doi: 10.1258/002367700780384762 [DOI] [PubMed] [Google Scholar]
- 6.Higuchi S., Yamada R., Hashimoto A., Miyoshi K., Yamashita K., Ohsugi T.2016. Evaluation of a combination of alfaxalone with medetomidine and butorphanol for inducing surgical anesthesia in laboratory mice. Jpn. J. Vet. Res. 64: 131–139. [PubMed] [Google Scholar]
- 7.Hunter J. C., Fontana D. J., Hedley L. R., Jasper J. R., Lewis R., Link R. E., Secchi R., Sutton J., Eglen R. M.1997. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br. J. Pharmacol. 122: 1339–1344. doi: 10.1038/sj.bjp.0701520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 9.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Kurosawa T.2013. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2015. Anesthetic effects of a three-drugs mixture--comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp. Anim. 64: 39–47. doi: 10.1538/expanim.14-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2016. Effects of an anesthetic mixture of medetomidine, midazolam, and butorphanol in rats-strain difference and antagonism by atipamezole. Exp. Anim. 65: 27–36. doi: 10.1538/expanim.15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodera S. Y., Yoshida M., Dezaki K., Yada T., Murayama T., Kawakami M., Kakei M.2013. Inhibition of insulin secretion from rat pancreatic islets by dexmedetomidine and medetomidine, two sedatives frequently used in clinical settings. Endocr. J. 60: 337–346. doi: 10.1507/endocrj.EJ12-0308 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T., Karakida N., Dantsuka A., Ichii O., Elewa Y. H. A., Kon Y., Nagasaki K. I., Hattori H., Yoshiyasu T.2017. Effects of a mixture of medetomidine, midazolam and butorphanol on anesthesia and blood biochemistry and the antagonizing action of atipamezole in hamsters. J. Vet. Med. Sci. 79: 1230–1235. doi: 10.1292/jvms.17-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochiai Y., Iwano H., Sakamoto T., Hirabayashi M., Kaneko E., Watanabe T., Yamashita K., Yokota H.2016. Blood biochemical changes in mice after administration of a mixture of three anesthetic agents. J. Vet. Med. Sci. 78: 951–956. doi: 10.1292/jvms.15-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochiai Y., Baba A., Hiramatsu M., Toyota N., Watanabe T., Yamashita K., Yokota H., Iwano H.2018. Blood biochemistry and hematological changes in rats after administration of a mixture of three anesthetic agents. J. Vet. Med. Sci. 80: 387–394. doi: 10.1292/jvms.17-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pypendop B., Verstegen J.1999. Cardiorespiratory effects of a combination of medetomidine, midazolam, and butorphanol in dogs. Am. J. Vet. Res. 60: 1148–1154. [PubMed] [Google Scholar]
- 17.Roman R. J., Cowley A. W., Jr., Lechene C.1979. Water diuretic and natriuretic effect of clonidine in the rat. J. Pharmacol. Exp. Ther. 211: 385–393. [PubMed] [Google Scholar]
- 18.Ruskoaho H., Leppäluoto J.1989. The effect of medetomidine, an α 2-adrenoceptor agonist, on plasma atrial natriuretic peptide levels, haemodynamics and renal excretory function in spontaneously hypertensive and Wistar-Kyoto rats. Br. J. Pharmacol. 97: 125–132. doi: 10.1111/j.1476-5381.1989.tb11932.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salonen M., Reid K., Maze M.1992. Synergistic interaction between α 2-adrenergic agonists and benzodiazepines in rats. Anesthesiology 76: 1004–1011. doi: 10.1097/00000542-199206000-00022 [DOI] [PubMed] [Google Scholar]
- 20.Sinclair M. D.2003. A review of the physiological effects of α2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 44: 885–897. [PMC free article] [PubMed] [Google Scholar]
- 21.Szczȩsny G., Veihelmann A., Massberg S., Nolte D., Messmer K.2004. Long-term anaesthesia using inhalatory isoflurane in different strains of mice-the haemodynamic effects. Lab. Anim. 38: 64–69. doi: 10.1258/00236770460734416 [DOI] [PubMed] [Google Scholar]
- 22.Tsubokura Y., Kobayashi T., Oshima Y., Hashizume N., Nakai M., Ajimi S., Imatanaka N.2016. Effects of pentobarbital, isoflurane, or medetomidine-midazolam-butorphanol anesthesia on bronchoalveolar lavage fluid and blood chemistry in rats. J. Toxicol. Sci. 41: 595–604. doi: 10.2131/jts.41.595 [DOI] [PubMed] [Google Scholar]
- 23.Tsukamoto A., Serizawa K., Sato R., Yamazaki J., Inomata T.2015. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp. Anim. 64: 57–64. doi: 10.1538/expanim.14-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weil M. H., Rackow E. C., Trevino R., Grundler W., Falk J. L., Griffel M. I.1986. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N. Engl. J. Med. 315: 153–156. doi: 10.1056/NEJM198607173150303 [DOI] [PubMed] [Google Scholar]
- 25.Wenger S.2012. Anesthesia and analgesia in rabbits and rodents. J. Exot. Pet Med. 21: 7–16. doi: 10.1053/j.jepm.2011.11.010 [DOI] [Google Scholar]
- 26.Whelan G., Flecknell P. A.1994. The use of etorphine/methotrimeprazine and midazolam as an anaesthetic technique in laboratory rats and mice. Lab. Anim. 28: 70–77. doi: 10.1258/002367794781065735 [DOI] [PubMed] [Google Scholar]
- 27.Wixson S. K., White W. J., Hughes H. C., Jr., Lang C. M., Marshall W. K.1987. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab. Anim. Sci. 37: 736–742. [PubMed] [Google Scholar]
- 28.Zatroch K. K., Knight C. G., Reimer J. N., Pang D. S.2017. Refinement of intraperitoneal injection of sodium pentobarbital for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet. Res. 13: 60. doi: 10.1186/s12917-017-0982-y [DOI] [PMC free article] [PubMed] [Google Scholar]