Abstract
Tobacco smoke is a complex mixture that includes thousands of compounds. Previously we have found that gestational exposure to the complex mixture of tobacco smoke extract caused long-term neurobehavioral impairments. In this study, we examined the interaction of two of the most biologically active, nicotine and benzo[a]pyrene (BaP). Developmental effects were determined in Sprague-Dawley rats prenatally exposed to low doses of BaP and nicotine (0.03 mg/kg/day of BaP and 2 mg/kg/day of nicotine) via maternal osmotic minipumps throughout gestation. Behavioral function was assessed in the offspring via a battery of tests through adolescence into adulthood. There were sex-selective effects in four of the behavioral tests. In the elevated plus maze, there was a significant interaction of BaP and sex, where BaP-treated males showed a trend for increased activity. In the novelty suppressed feeding test, there were significant sex selective effects in males such that the normal sex difference in the behavior in this test was eliminated. Male offspring with prenatal exposure to either nicotine or BaP showed significant reductions in fear response. In the Figure-8 locomotor activity test, BAP-exposed male offspring were significantly hyperactive. This also eliminated the sex difference typically seen in this test. This effect persisted into adulthood. In the attention task, males exposed to nicotine during gestation showed a significant percent hit impairment. BaP reversed this effect. No significant effects were seen with percent correct rejection. These data show that both nicotine and BaP cause persisting sex-selective behavioral effects that persist into adulthood.
Keywords: Behavioral Teratogenesis, Nicotine, Benzo[a]pyrene, BaP, Development
1. Introduction
Maternal tobacco smoking during pregnancy has been significantly associated with persisting adverse neurobehavioral impairments in the offspring in a wide variety of studies (Huizink & Mulder, 2006; Polanska et al., 2015; Stroud et al., 2018). Commonly reported behavioral symptoms include impulsive, disruptive, and inattentive behaviors, learning difficulties, affective symptoms and reduced IQ (Clifford et al., 2012; Gaysina et al., 2013; Kovess et al., 2015). In addition, children with fetal tobacco exposure show elevated risk for psychiatric issues, including attention deficit hyperactivity disorder (ADHD) and conduct disorder (Han et al., 2015; Ramsay et al., 2016; Talati et al., 2017). Although the association between maternal smoking and offspring behavior has been well demonstrated, many questions remain as to the exact mechanisms by which tobacco smoke impacts the developing brain. These questions are all the more important with the rise of electronic cigarettes and the changing landscape of fetal nicotine exposure (England et al., 2015), which deliver full doses of nicotine without the combustion products like BaP.
In addition to nicotine, combustion of tobacco releases a variety of combustion products, including polycyclic aromatic hydrocarbons (PAHs) such as benzo[a]pyrene (BaP). PAHs are of broad concern, as they are environmental pollutants that are independently associated with adverse developmental outcomes, separate from their roles in tobacco smoke effects (Chepelev et al., 2015). Although much less studied than nicotine, BaP exposure during development is similarly associated with long-term changes in neural organization and signaling (McCallister et al., 2008; Peterson et al., 2015) and a range of behavioral effects, including ADHD-risk, effects on activity and affective functions, and cognition (Chen, 2012 et al.; Jedrychowski et al., 2015; Peterson et al., 2015; Xia et al., 2011).
Based on these findings, it is hypothesized that the profile of behavioral and psychiatric outcomes in children with fetal tobacco smoke exposure results from the individual and interactive toxicities of multiple tobacco smoke constituents, including nicotine and PAHs. However, it remains to be demonstrated whether the potentially overlapping effects produced by those constituents are redundant or whether co-exposure may lead to additive or supra-additive effects on behavioral function. The current study was conducted to determine the interactive effects of nicotine and BaP in a rat model of neurobehavioral function across development. Female rats were exposed to vehicle, nicotine, BaP or their combination across the full length of gestation using subcutaneous osmotic minipumps. One male and one female offspring from each litter were run in a behavioral battery which contained assays of psychomotor, affective and cognitive outcomes across adolescent development and into adulthood.
2. Methods
Subjects and Housing Conditions:
Subjects in the present study consisted of Sprague Dawley (Charles Rivers Labs, Raleigh, NC, USA) and their offspring. Sprague-Dawley rats were chosen to facilitate comparison to our previous studies with prenatal nicotine effects. (Cauley et al., 2018; Hall et al., 2016; Levin et al., 1993; Levin et al., 1996; Seidler et al., 1992; Slotkin et al., 2019; Slotkin et al., 2015). All animals were maintained on a 12:12 reversed day-night cycle with ad libitum access to food and water, except in select behavioral procedures which required food restriction to motivate feeding behaviors (see below). Developmental monitoring and behavioral testing were conducted under low-ambient lighting conditions during the dark phase of the reversed light cycle (800h-1700h). All procedures were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Duke University.
Exposures and Behavioral Testing:
Adult female Sprague-Dawley rats were anaesthetized using ketamine (60 mg/kg) and dexdormitor (15 mg/kg) and implanted with Alzet osmotic infusion pumps (2ML4, Durect Inc, Cupertino, CA, USA). These pumps were selected because they deliver a reliable steady exposure to the chemicals under study and have proven usefull in our previous studies of gestational nicotine exposure (Cauley et al., 2018; Hall et al., 2016; Levin et al., 1993; Levin et al., 1996; Seidler et al., 1992; Slotkin et al., 2019; Slotkin et al., 2015). Pumps delivered one of the following: the DMSO (dimethyl sulfoxide) vehicle, 2 mg/kg/day of nicotine, or 0.03 mg/kg/day of BaP. The 0.03 mg/kg/day dose of BaP was chosen because it is at the lower end of the dose range that has been shown to cause neurobehavioral alterations after developmental exposure (McCallister et al., 2008). The 2 mg/kg/day nicotine infusion dose was selected to model maternal smoking of 10–20 cigarettes per day and has been found to cause persistent neurobehavioral alterations in the offspring without impairing fertility or growth retardation (Fewell et al., 2001; Levin & Slotkin, 1998; Trauth et al., 2000). Each rat was implanted with two pumps such that there were four treatment groups: Control, Nicotine, BaP or Nicotine + BaP. For dams exposed to either nicotine or BAP alone (not in combination), the second pump contained vehicle. These females were then bred with untreated male rats to generate 9–10 litters per condition. At weaning, 1 male and 1 female from each litter were used for behavioral testing. Remaining pups from the litters were separately housed and used for neurochemical analysis, which is presented elsewhere (Slotkin et al., 2019). Behavioral animals acclimated to their new housing for one week after weaning (3 weeks of age), then began the behavioral battery at 4 weeks of age. The behavioral battery included the following tests:
Elevated Plus Maze (week 4):
The rats were tested on the elevated plus maze (Med Associates, St Albans, VT, USA) to assess their anxiety like behavior vs. risk-taking like behavior. The maze measured 142 cm × 104 cm × 76 cm high and consisted of two arms with 15 cm high enclosed walls and two open arms with low 2 cm railings. Each rat was assessed individually on the elevated plus maze for a single five-min session. The percent time the rat spent in the open vs. enclosed arms of the maze was calculated, as well as the number of crossings across the center.
Figure-8 Locomotor Activity Apparatus (week 5 and adult):
Locomotor activity was assessed in an enclosed maze in the shape of a figure-8. The Figure-8 apparatus consisted of a continuous alley that measured 10 cm × 10 cm, with the entire maze measuring 70 cm × 42 cm. Animals were allowed to freely explore and locomotor activity was assessed by the crossing of eight photobeams located at equal points in the alley. Each locomotor test session lasted 1 h, and photobeam breaks were tallied in 5 min blocks across the session. Subjects were tested in the Figure-8 maze at two time points, 5 weeks of age and following the end of attention testing in adulthood.
Novelty Suppressed Feeding (week 6):
To assess fear responsivity, the offspring rats were tested for suppression of feeding in a novel environment. The rats had food restriction for 24 h prior to the novelty suppressed feeding test. A novel environment consisted of a plastic rectangular cage (different from the home cage) placed in the middle of a brightly lit testing room. There was no cage top or bedding in the cage. Twelve standard rat-chow pellets were weighed before testing and then spread across the floor of the cage in 4 rows of 3 pellets each. The sessions were 10 min long and the latency for the rat to begin eating, the duration of eating and the bouts of eating were recorded. Eating was defined as the act of chewing the food and not merely sniffing, holding, or carrying the food around in the mouth. The food pellets which remained after the test session were weighed to determine the amount of food eaten. A composite score representing the latency to eat, time spent eating and amount of food eaten was also generated (percent of control mean).
Novel Object Recognition (week 7):
To test attention and memory in a low-motivational state recognition of a novel vs. familiar object was determined. Tests were conducted in opaque plastic enclosures measuring 70 cm × 41 cm × 33 cm. Objects consisted of plastic, glass, or ceramic material and were randomized for each animal. Animals were first habituated to the apparatus in two consecutive 10 min sessions over the course of two days. Testing began on day 3 with a 10 min “information” session in which two identical objects (A/A) were placed in the cage for the animal to explore. The A/A session was then followed by a 15 min, 1 h, or 6 h delay period spent in the animal’s home cage. The animal was then placed back in the enclosure with one object from the A/A session and with another, dissimilar, “novel” object (A/B session). The time spent actively exploring each object was recorded. Between sessions, the objects were wiped clean with a solution of 10% acetic acid in order to avoid odor recognition cues by the rats.
16-Arm Radial Maze (weeks 8–11):
To index spatial learning and spatial memory the offspring rats were tested in 16-arm radial mazes. The mazes were black painted wood with a central platform and 16 arms placed radially from the central platform. The center was 50 cm in diameter, and each arm was 10 cm across × 60 cm long. A food cup was 2 cm from the end of each arm. Visual cues (cardboard shapes) were placed on the walls of the testing room to facilitate spatial orientation. Each rat was habituated in the maze in two 10 min sessions in which they were placed on the central platform inside a large, round, opaque cylinder with halves of sugar-coated cereal (Froot Loops®; Kellogg’s Inc, Battle Creek MI, USA) available to consume. Food cups of twelve of the arms of the maze were baited at the beginning of each session to test working memory performance and the remaining four arms were always left unbaited to test reference memory. The baited arms of the maze for each rat remained constant throughout series of testing sessions but the choice of arms baited was randomized among the different rats. Each trial began by placing the rat on the central platform inside the opaque cylinder for 10 s. Then, the cylinder was lifted and the rat was allowed to move freely. Each session lasted ten min or until the rat had entered all twelve baited arms, whichever came first. Each rat was trained for 18 sessions and working and reference memory errors were assessed. Working memory errors were defined as repeated entries into a baited arm, and reference memory errors were defined as entry into one of the arms that was never baited. Latency was calculated as the total session time divided by the number of arm entries. After each session, the maze was cleaned with a solution of 10% acetic acid with a damp cloth.
Operant Visual Signal Detection Task (weeks 11+):
To assess attention, the operant visual detection task was used. The task was conducted as described in detail previously (Hall et al., 2016). Briefly, each rat inside an operant chamber was trained to press one of two retractable levers in response to a visual cue-light that was illuminated for a duration of 500 ms. If the cue-light became illuminated (“signal” trial), the animal needed to press one of the two levers to receive a 20 mg food pellet reward. If the cue-light did not illuminate (“blank” trial) the animal needed to press the opposite lever in the chamber to receive the reward. The choice of “signal” and “blank” levers was randomized among the rats. If the rat made no response within 5 s of insertion of the response levers into the chamber, both levers retracted and a response “failure” was recorded. Each “signal” and “blank” pair was considered one test trial, and each test session consisted of 240 trials.
Social and Sexual Behavior and Figure-8 Locomotor Activity Apparatus (Adulthood):
To assess social interactions with conspecifics of the opposite sex, including sexual behavior, the study subjects were placed in a glass holding tank (50 cm × 25cm × 30 cm, fitted with a wire mesh lid) with an untreated Sprague Dawley rat of the opposite sex for 30 minutes. Females were not hormonally primed or staged prior to the interaction. The room was kept under red light, which rendered the room nearly dark for the rats but allowed the session to be recorded by a digital video recorder. The experimenter did not remain in the room during the session. Later, the videos were manually scored for social interaction and social behaviors, as described by Nugent et al (2015). Behavioral endpoints for females included anogenital sniffing, darts and hops, and lordosis. Behavioral endpoints for the males included anogenital sniffing, mounts, latency to first mount, mounts with thrusts, intromissions and ejaculations. These endpoints were analyzed separately for treated animals and their partners. Experimenters scoring the videos were blinded to which rat was from an experimental group and what their treatment was. The holding tank was wiped clean with a solution of 10% acetic acid solution between sessions to remove scent cues.
Data Analysis:
The dependent measures for each test were evaluated for statistical significance by the analysis of variance. Litter was the unit of variance for treatment effects. Nicotine and BaP treatments were between subjects factors. Sex was a within litter factor and repeated measures for each test (e.g., test period, trial and error type) were within subjects factors. Interactions p<0.10 were followed up by tests of the simple main effects within the interaction as recommended by Snedecor and Cochran (1967). The cut-off for statistical significance was p<0.05, two-tailed.
3. Results
Both nicotine and BaP exposure individually during gestation caused significant persisting behavioral effects. These toxicant-induced behavioral effects were sex-selective effects in several of the functional tests. Males were particularly sensitive to the persisting neurobehavioral effects of nicotine and BaP. These persisting behavioral effects were seen with nicotine and BaP doses that did not cause significant changes in pregnancy success, maternal weight gain, litter size, sex ratio or birth or weaning weight of the pups (Table 1).
Table 1.
Developmental health measurements of conception, maternal and offspring weight gain, litter size and sex ratio (mean ± sem).
| Number | %Male | Male | Female | Male | Female | |
|---|---|---|---|---|---|---|
| Control | 13.6±0.5 | 46.8±4.4 | 7.8±0.3 | 7.4±0.3 | 54.9±2.5 | 53.2±2.3 |
| Nicotine | 12.0±0.8 | 43.7±3.8 | 8.0±0.4 | 7.8±0.4 | 56.8±1.6 | 55.3±1.5 |
| BaP | 12.8±0.8 | 50.1±3.8 | 7.9±0.3 | 7.6±0.3 | 55.4±2.1 | 55.3±1.5 |
| Nic+BaP | 12.7±1.2 | 51.5±3.7 | 7.7±0.3 | 7.1±0.3 | 56.0±1.4 | 54.3±1.1 |
Elevated Plus Maze
There was a significant (F(1,33) = 5.71, p < 0.025) interaction of BaP exposure x sex. BaP treated males showed a trend for increased activity in this test (Fig.1). However, none of the simple main effects for each sex or treatment were significant significantly different from control.
Figure 1.
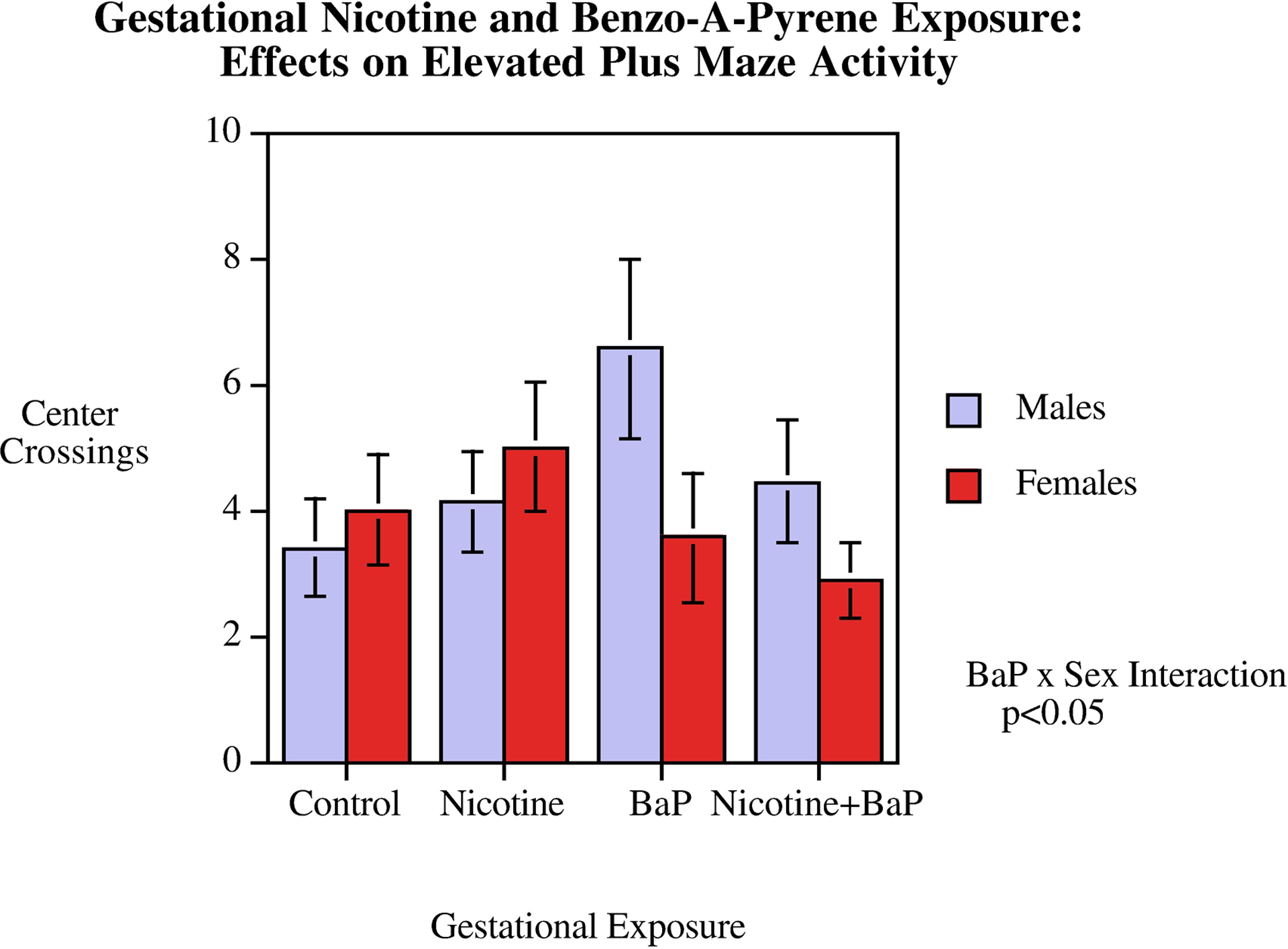
Elevated Plus Maze: Center Crossings (mean ± sem). There was a significant (p < 0.025) BaP exposure x sex interaction. BaP treated males showed a trend for increased activity, but none of the simple main effects for each sex or treatment were significantly different from controls.
Figure-8 Apparatus
During adolescence, locomotor activity as measured in the Figure-8 apparatus was significantly affected by gestational exposure to nicotine and BaP differentially in male and female offspring as indicated by a significant interaction of nicotine x BaP x sex (F(1,33) = 5.26, p < 0.05). As shown in figure 2, in the Figure-8 locomotor activity test there was a significant degree of hyperactivity relative to controls caused by gestational BaP exposure in male, but not female rats (p < 0.05). This eliminated the typical sex difference seen in this test of locomotor activity where females are more active than males in the controls. With vehicle exposed controls there was a significant (p < 0.025) sex difference with females being faster than males (Fig. 2). This sex effect in locomotor activity was not seen in any of the nicotine and/or BaP exposure groups. In adulthood, this locomotor activity test was repeated. The significant (p<0.05) locomotor hyperactivity in BaP treated male offspring was still seen (Fig. 3).
Figure 2.
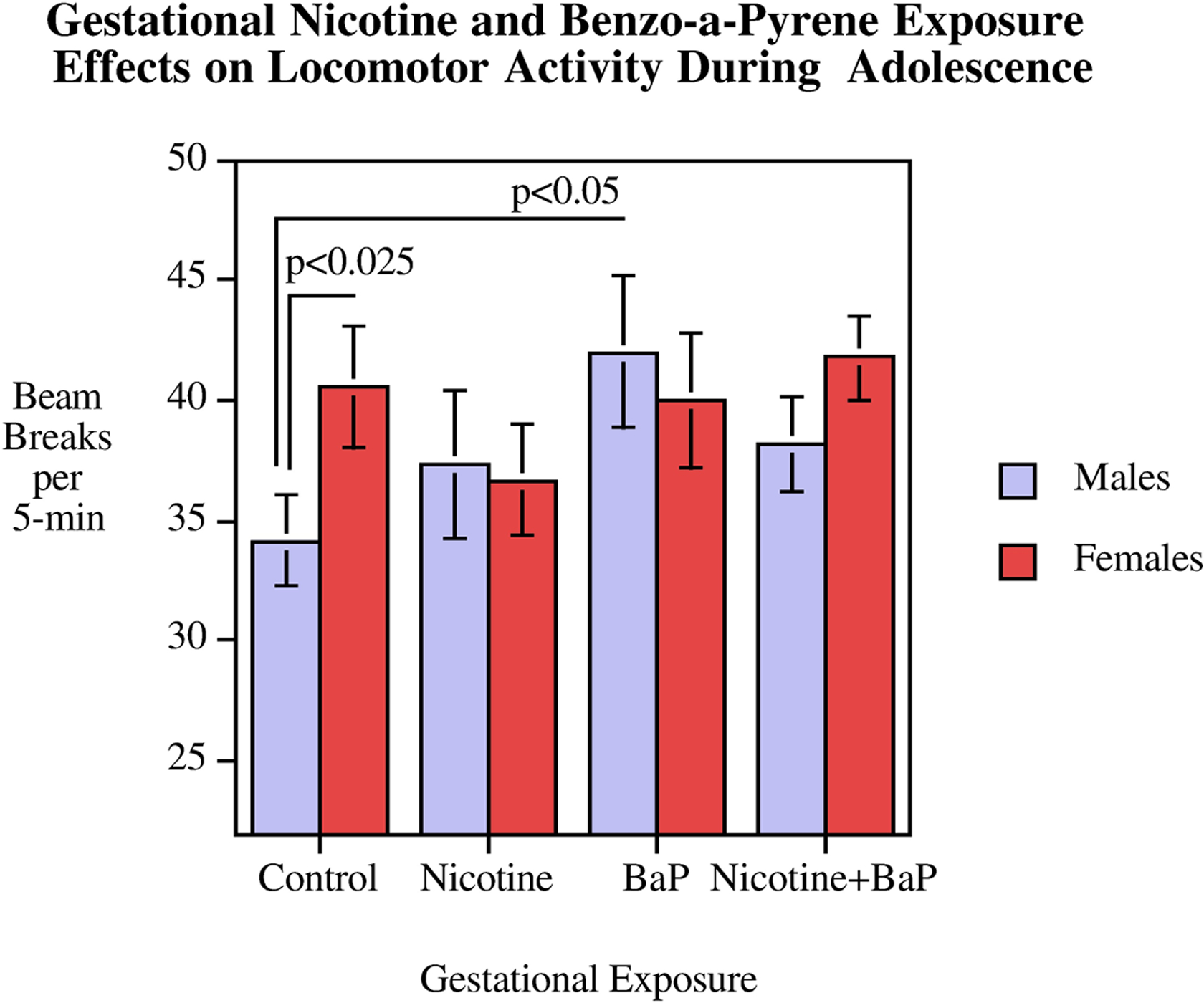
Figure-8 Apparatus: Adolescent mean locomotor activity (mean ± sem). There was a significant interaction of nicotine x BaP x sex (p < 0.05). In controls, but not any of the exposed groups, there was a significant (p < 0.025) sex effect in which females were more active than males. BaP exposed males were significantly (p < 0.05) hyperactive relative to control males.
Figure 3.
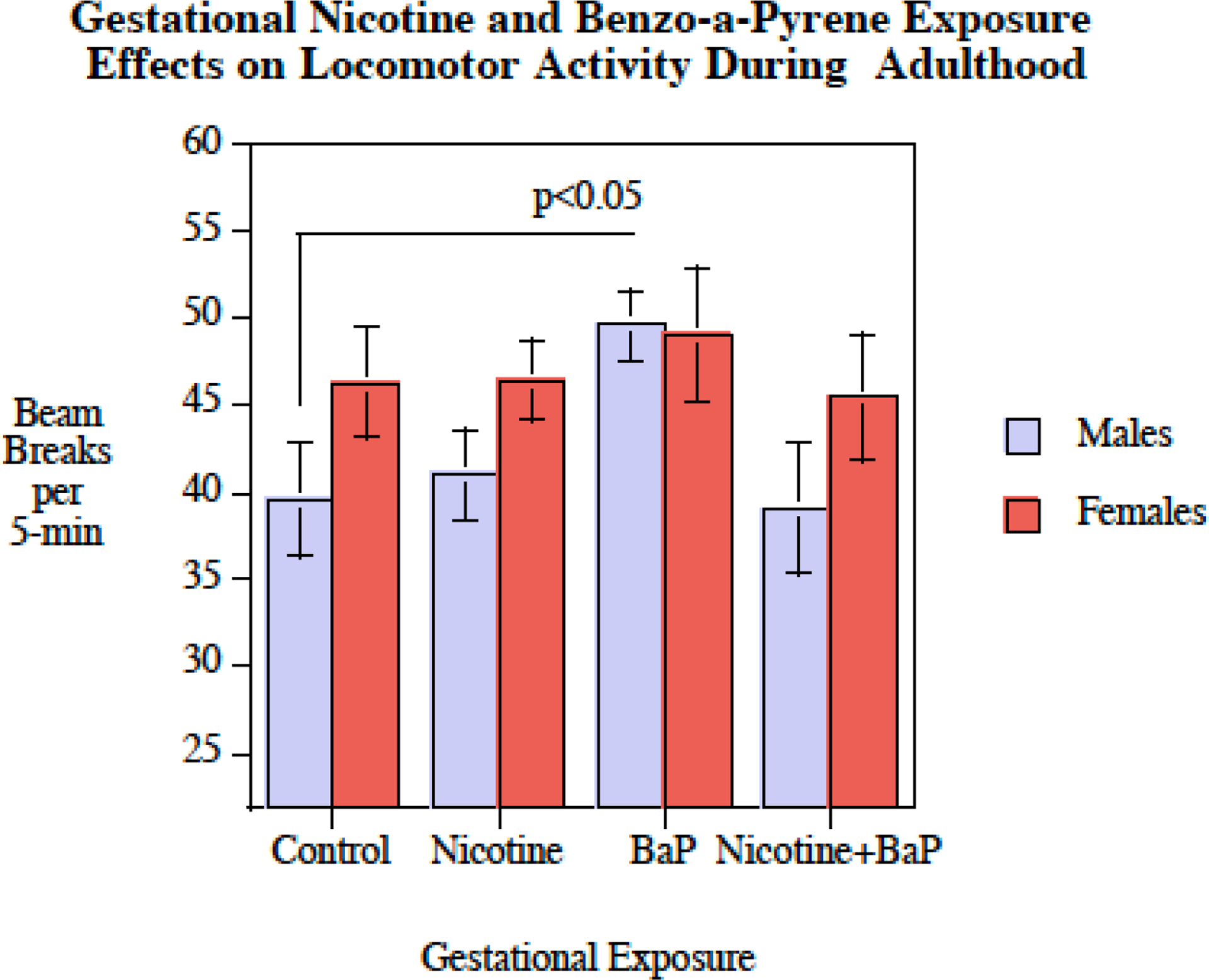
Figure-8 Apparatus: Adult mean locomotor activity (mean ± sem). The adult male with gestational exposure to BaP were significantly hyperactive relative to control males (p < 0.05).
Novelty Suppressed Feeding
In this test of fear response there was a significant main effect of BaP exposure (F(1,33) = 6.55, p < 0.025), with the rats with BaP exposure (78.3 ± 5.1) having less fearful response than those with no BaP exposure (97.5 ± 4.8). There was a significant sex effect in controls with females showing less fear-like behavior than males (p < 0.025). This differential effect was not seen in any of the exposure groups. Males showed a significant decrease in fear response with either nicotine (p < 0.05) or BaP (p < 0.025) exposure. There were significant sex-selective effects in males with both nicotine and BaP causing a significant reduction in fear responses such that the typical sex difference in the behavior in this test with controls was eliminated (Fig. 4).
Figure 4.
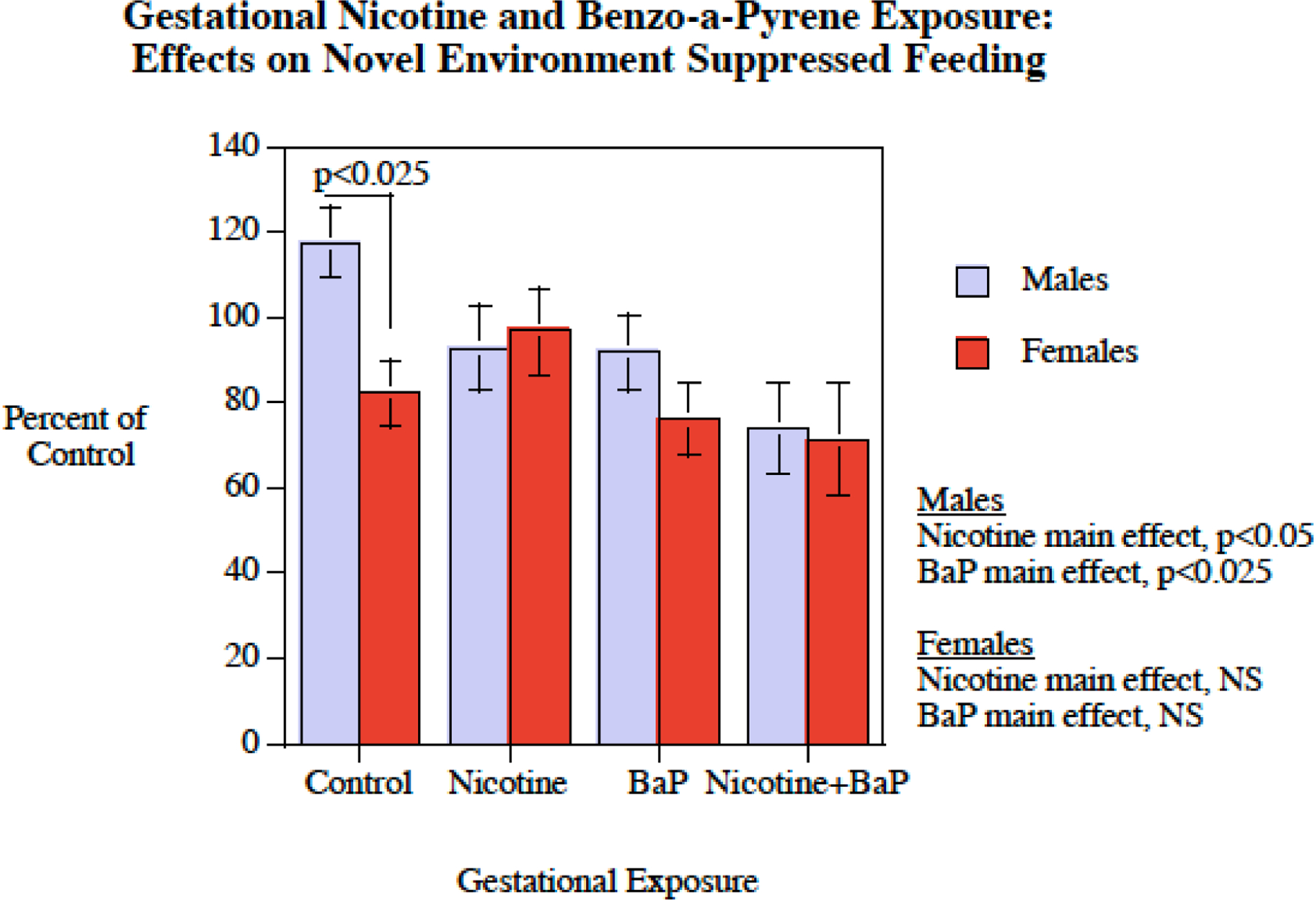
Novel Environment Suppressed Feeding: Percent of control behavior (mean ± sem). In controls there was a significant sex difference in which females showed less inhibition of feeding in the novel environment. This sex-difference was not seen in any of the exposed groups.
Novel Object Recognition
In this task there were interactions of nicotine and BaP exposure with phase of testing (F(1,33) = 8.08, p < 0.01) as well with familiar/novel object and sex (F(1,33) = 2.97, p < 0.10) and with familiar/novel object and test phase (F(1,33) = 4.12, p < 0.06) that prompted follow-up tests of nicotine and BaP effects in these different aspects of the test. There was also a test phase x familiar/novel x BaP interaction (F(1,33) = 5.49, p < 0.05) that prompted follow-up tests. However, there were no significant simple main effects of nicotine or BaP in these follow-up tests.
The Radial-Arm Maze
Training on this test of spatial learning did not show signs of learning in the controls. Training for only six sessions on the radial-arm maze appeared to be too brief to index learning. Therefore, changes over the course of training could not be used as a test of learning. There was the typical sex difference with males having significantly fewer errors than females (F(1,33) = 5.13, p <0 0.05) and there was also the normal significant difference in working and reference memory errors (F(1,33) = 554.42, p < 0.0005). No significant nicotine or BaP effects were seen with average working and reference memory errors. With response latency on the radial-arm maze, there was a significant nicotine x BaP x sex interaction (F(1,33) = 10.82, p < 0.005). Follow-up tests of the simple main effects of nicotine and BaP treatments in males and females showed that the male offspring exposed during gestation to both nicotine and BaP (30.3 ± 3.4 s/arm entry) had significantly longer latencies than control males (19.4 ± 2.0 s/arm entry). No significant effects on radial-arm maze response latency in males were seen with either nicotine or BaP exposure alone or with any of the treatments in females.
The Operant Visual Signal Detection Test
For percent hit response, the nicotine x BaP interaction was significant (F(1,64) = 4.01, p < 0.05). The BaP x sex interaction was also significant (F(1,64) = 9.90, p < 0.01). Finally, the nicotine x BaP x sex interaction (F(1,64) = 3.66, p < 0.07) prompted follow-up tests of the simple main effects of the BaP and nicotine exposed condition and control for each sex. This test of attention showed a significant (p < 0.05) nicotine-induced impairment in percent hit accuracy in males (Fig. 5). Interestingly, this gestational nicotine-induced attentional impairment was significantly reversed by BaP co-treatment (p < 0.001). The exposure effects on percent hit accuracy appeared to be rather specific, inasmuch as there were no significant effects of either nicotine or BaP exposure on percent correct rejection accuracy.
Figure 5.
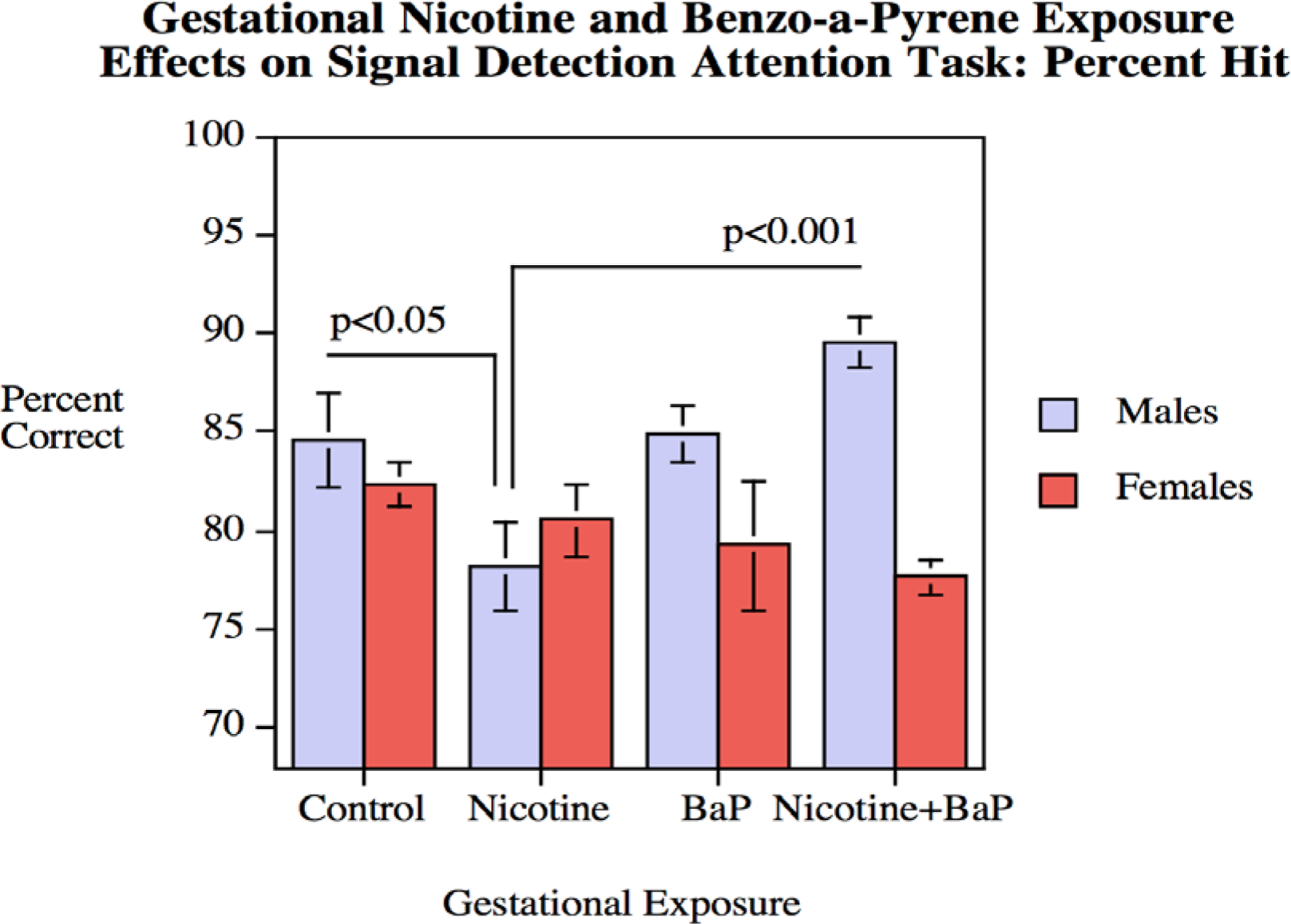
Signal Detection Attention Task: Percent hit performance (mean ± sem). Percent correct hit performance in males was significantly (p < 0.05) impaired by gestational nicotine exposure, an effect significantly reversed by the addition of BaP (p < 0.001).
Social Interaction and Sexual Behavior
With the rats in the study, the incidence of sexual reproductive behaviors (intromissions/ejaculations, lordosis) were rare with all groups of rats. Among the exposed males, only ten of the thirty-eight (26%) rats exhibited at least one intromission and only three (8%) rats had at least one ejaculation. Among exposed females, only eight of the forty (25%) rats exhibited at least one lordosis. Similar levels of sexual behavior were observed in the partners of these rats (intromissions: 24%, ejaculations: 11%, lordosis: 25%). Therefore, the analysis focused on mounting and anogenital sniffing. The number or latency of mounts was not significantly affected by treatment, either for the treated males or for the male partners of the treated females. For anogenital sniffing, there was a significant main effect of sex for both treated, (F(1,70)=109.94, p<0.001), and control partners (F(1,70)=72.95, p<0.001); males exhibited greater amounts of anogenital sniffing than their partner. However, there were no main effects of nicotine or BaP treatment or interactions for these outcomes.
4. Discussion
The present study was conducted to assess the individual and interactive effects of prenatal exposure to nicotine and BaP on neurobehavioral function in the offspring. The doses selected did not cause any overt effects on the health of the exposed dams or their offspring (Table 1), but rather produced behavioral effects that persisted into adulthood. Behavioral effects were heavily driven by sex differences, expressed as either attenuation of typical sex differences or selective effects in male offspring. In no case did combined exposure to nicotine and BaP significantly enhance the behavioral impairment produced by these toxicants alone. Rather, there were multiple cases where a deficit was apparent in offspring exposed to one toxicant, but not the combination of both toxicants.
There were sex-selective effects in four of the behavioral tests, each of which pertained to male offspring. In the elevated plus maze, there was a significant interaction of BaP and sex with BaP treated males showing a trend for increased activity. In the Figure-8 maze, prenatal BaP led to significant hyperactivity in males, both in adolescence and in adulthood. In the novel environment suppressed feeding test, males exposed to either nicotine or BaP showed a weakened fear response. In the attention task, by contrast, prenatal nicotine led a significant impairment in choice accuracy in males when the stimulus light was presented (percent hit), but co-treatment with BaP significantly reversed this effect. Additionally, baseline sex differences in behavior were disrupted by prenatal treatment in two tasks. Control males exhibit lower levels of activity than females in the figure-8 maze during adolescence, and this effect was disrupted by prenatal exposure to nicotine, BaP or their combination. Control males also show less suppression of feeding behaviors than females in the novel environment suppressed feeding test, an effect which was also disrupted by prenatal exposure to nicotine, BaP or their combination.
In short, prenatal nicotine exposure was associated with attention deficits and reduced fear-like responding among males, while BaP exposure was associated with hyperactivity and reduced fear-like responding, also among males. These findings are in agreement with previous nicotine work and suggest a key area of risk for BaP exposure. Fetal nicotine exposure has been previously associated with poor rodent attention tasks, such as the 5-choice serial response time task (Schneider et al., 2011; Schneider et al., 2012) and the object-based attention test (Alkam et al., 2013; Zhu et al., 2017). This particular attention task had not previously been found to be sensitive to prenatal nicotine effects when used by Hall et al. (2016) although that dataset did find deficits in novel object recognition, which is believed to rely on another form of attention. Nicotine exposure has also been associated with impulse control deficits or reduced anxiety-like responding (Schneider et al., 2011; Schneider et al., 20112), which is in agreement with the enhanced feeding behavior by a novel environment in the present study. A similar effect was not observed on the elevated plus maze, so the impaired response may be related to the higher motivation level induced by food deprivation. As for BaP, the present figure-8 maze data closely mirror previous findings from Chen et al (2012) which detected BAP-induced hyperactivity in the open field test during adolescence and adulthood. Taken together, the present activity and fear-response data also complement a growing body of work showing that early BaP exposure may impact a variety of distinct behavioral functions, as evidenced by clinical associations with ADHD-characteristics (Perera et al., 2012; Perera et al., 2014), cognitive or affective perturbations in rodent models (Chen et al., 2012; Grova et al., 2008; Xia et al., 2011), and altered behaviors in non-rodent models such as zebrafish or killifish (Brown et al., 2016; Knecht et al., 2017).
Interestingly, some of the specific effects in males exposed to either nicotine or BAP alone were not evident in offspring exposed to the combination of both compounds. In fact, the attentional deficits produced by nicotine alone were significantly reversed by co-administration with BaP. Although these differences may appear to be a discrepancy, they coincide with notable differences between the neurochemical effects of prenatal exposure to these compounds alone or in combination, as measured in the siblings of these subjects by Slotkin et al (2019).
Slotkin et al. (2019) recently reported that prenatal exposure to nicotine and BaP had overlapping impacts on the brain, albeit through different mechanisms, and a differing profile of effects when exposed together. Prenatal exposure to each compound led to reduced presynaptic acetylcholine (ACh) activity and a compensatory upregulation of receptors that may offset those cholinergic effects. Prenatal nicotine exposure led to upregulation of nAChRs and both 5HT receptor subtypes, while BaP led to a selective increase in 5HT2 receptors. Although the combination of nicotine and BaP led to supra-additive effects on ACh function, the combined effects of the two compounds led to a reduction or elimination of compensatory upregulations of nACh or 5HT receptors. Of these effects, certain aspects were sex dependent. Presynaptic Ach activity was indicated by ratios between choline acetyltransferase (ChAT), an enzyme responsible for the synthesis of acetylcholine, and HC3 binding, which indicates choline reuptake. ChAT was elevated in male offspring with fetal exposure to nicotine, and similarly but significantly less so, following BaP and the combination of both compounds. Prenatal nicotine also led to elevated binding of a choline reuptake inhibitor (HC3) among males, while BaP had the opposite effect in females.
Taken together, the behavioral and neurochemical data identifying male-specific deficits are in line with previous work suggesting that males are more susceptible to certain aspects of developmental neurotoxicity than their female counterparts (DiPietro et al., 2017). Although the exact mechanisms of male-specific vulnerability remain unclear, the consequences of that vulnerability were observable at both behavioral and neurochemical levels for nicotine and BaP. However, it should be noted that the present behavioral data differ from the corresponding mechanistic work in one important aspect. Slotkin et al. (2019) concluded that the combination of nicotine and BaP more closely mimicked the critical consequences of fetal exposure to tobacco smoke extract (TSE) than the individual components alone. However, the behavioral effects of combined nicotine and BaP exposure did not closely mirror those of TSE exposure. Hall et al. (2016) reported that fetal TSE exposure was associated with hyperactivity during adolescence regardless of sex, elevated food consumption in a novel environment among males and impaired novel object recognition. None of these areas were significantly affected by nicotine and BaP co-exposure. Also, in contrast to the present findings, TSE exposure was found to produce effects of higher magnitude than nicotine alone. The neurochemical data do suggest a mechanism by which co-exposure might produce less behavioral disruption than the compounds alone, a lack of compensatory upregulations of neurotransmitter receptors, but that connection may not capture the basis of behavioral deficits following TSE exposure. Therefore, nicotine and BaP-induced deficits in behavior in the context of tobacco smoke may be mediated by the presence of additional neurotoxicants, such as additional PAHs or heavy metals like cadmium.
Beyond the primary deficits observed in male offspring, the present study detected an added set of effects not reported by Hall et al. (2016). Developmentally typical rats tend to show baseline sex differences in a variety of non-reproductive behaviors, including multiple used in the present battery. On these tasks, male and female offspring form two overlapping distributions positioned in a reliable direction from one another. In the present study, two baseline behaviors had significant sex differences. Males were less active than females in the figure-8 maze and exhibited less anxiety-like suppression of feeding in a novel environment. Offspring with prenatal exposure to nicotine, BaP or their combination failed to show these characteristic sex differences, regardless of whether there was a significant effect of treatment on male offspring. The low correspondence between this disruption and sex-specific effects suggests that these compounds may interact with additional mechanisms that drive differences between males and female offspring.
Male and female nervous systems follow a similar developmental trajectory, but are uniquely influenced by a series of influential events involving gonadal hormones (Forger et al., 2016; Lenz et al., 2012). The sexualization of the brain impacts not only subtle neurochemical endpoints, but also higher order outcomes such as the size and cellular organization of neural structures. Nicotine and BaP exposures have each been shown to influence gonadal health and lead to reproductive deficits in the offspring (Camlin et al., 2016; Holloway et al., 2006; Wong et al., 2015). Such effects may be highly relevant to fetal tobacco effects, as estrogen receptors are present in multiple, behaviorally relevant areas of the brain with prominent cholinergic activity, such as the hippocampus and basal forebrain (Bora et al., 2005; Gibbs et al., 2010; Towart et al., 2003). Additionally, BaP exposure is associated with a variety of estrogenic effects and interactions with estrogen and androgen receptors (Bolden et al., 2017; Vondráček et al., 2018), any of which may have organizational or physiological effects during development. Toxicant-induced changes in sexualization of the brain may account for the disruption of sexually dimorphic behavioral patterns in the present study, although additional studies are needed to clearly identify such a mechanism.
The present data suggest multiple aspects of behavioral function that are sensitive to prenatal exposure to tobacco smoke constituents. Further, these data suggest that nicotine and BaP have distinct patterns of risk with respect to male subjects and similar risks with respect to the disruption of typical sex differences. These data also emphasize the importance of testing specific mixture effects with respect to neurodevelopment. The consequences of a combined exposure were difficult to predict based upon the individual actions of the constituents. For example, the hyperactivity produced by BaP exposure appeared more similar to the effects of TSE exposure than the specific combination of nicotine and BaP. By the same token, the consequences of a single compound when isolated from a mixture may be equally difficult to predict. For example, exposure to nicotine alone led to attention deficits which were not specifically associated with either TSE or the nicotine-BaP mixture. In short, the context of a neurotoxic exposure is an important component of its toxicity, and investigations of mixture effects may be necessary to fully capture the risk presented by a neurotoxicant of interest. This may be particularly important for projecting the change in neurodevelopmental risk as smoking trends shift towards non-combustible tobacco products, such as electronic cigarettes, which present nicotine in novel and variable chemical mixtures.
Acknowledgements
Sponsored by the Children’s Environmental Health and Disease Prevention Research Center. U.S. Environmental Protection Agency (US EPA) grant RD83543701 & National Institute for Environmental Health Sciences (NIEHS) grant ES022831. EPA support does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Alkam T, Kim HC, Mamiya T, Yamada K, Hiramatsu M, & Nabeshima T (2013). Evaluation of cognitive behaviors in young offspring of C57BL/6J mice after gestational nicotine exposure during different time-windows. Psychopharmacology, 230(3), 451–463. [DOI] [PubMed] [Google Scholar]
- Bolden AL, Rochester JR, Schultz K, & Kwiatkowski CF (2017). Polycyclic aromatic hydrocarbons and female reproductive health: a scoping review. Reproductive Toxicology, 73, 61–74. [DOI] [PubMed] [Google Scholar]
- Bora SH, Liu Z, Kecojevic A, Merchenthaler I, & Koliatsos VE (2005). Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Experimental Neurology, 194(2), 506–522. [DOI] [PubMed] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, & Di Giulio RT (2016). Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicology & Teratology, 53, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camlin NJ, Sobinoff AP, Sutherland JM, Beckett EL, Jarnicki AG, Vanders RL, Hansbro PM, McLaughlin EA, & Holt JE (2016). Maternal smoke exposure impairs the long-term fertility of female offspring in a murine model. Biology of Reproduction, 94(2), 39–31. [DOI] [PubMed] [Google Scholar]
- Cauley M, Hall B, Abreu-Villaça Y, Junad S, White H, Kiany A, Slotkin TA, & Levin ED (2018). Critical developmental periods for effects of low-level tobacco smoke exposure on behavioral performance. NeuroToxicology, 68, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tang Y, Jiang X, Qi Y, Cheng S, Qiu C, Chen C, Tang Y, Jiang X, Qi Y, Cheng S, Qiu C, Peng B, & Tu B (2012). Early postnatal benzo[a]pyrene exposure in Sprague-Dawley rats causes persistent neurobehavioral impairments that emerge postnatally and continue into adolescence and adulthood. Toxicological Sciences, 125(1), 248–261. [DOI] [PubMed] [Google Scholar]
- Chepelev NL, Moffat ID, Bowers WJ, & Yauk CL . (2015). Neurotoxicity may be an overlooked consequence of benzo[a]pyrene exposure that is relevant to human health risk assessment. Mutation Research/Reviews in Mutation Research, 764, 64–89. [DOI] [PubMed] [Google Scholar]
- Clifford A, Lang L, & Chen R (2012). Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicology & Teratology, 34(6), 560–570. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, & Voegtline KM (2017). The gestational foundation of sex differences in development and vulnerability. Neuroscience, 342, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England LJ, Bunnell RE, Pechacek TF, Tong VT, & McAfee TA (2015). Nicotine and the developing human: a neglected element in the electronic cigarette debate. American Journal of Preventive Medicine, 49(2), 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, & Ng VKY (2001). Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. Journal of Applied Physiology, 90(5), 1968–1976. [DOI] [PubMed] [Google Scholar]
- Forger NG, Strahan JA, & Castillo-Ruiz A (2016). Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Frontiers in Neuroendocrinology, 40, 67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Elam KK, Natsuaki MN, Neiderhiser JM, & Harold GT (2013). Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry, 70(9), 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB (2010). Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocrine Reviews, 31(2), 224–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grova N, Schroeder H, Farinelle S, Prodhomme E, Valley A, & Muller CP (2008). Sub-acute administration of benzo[a]pyrene (BaP) reduces anxiety-related behaviour in adult mice and modulates regional expression of N-methyl-D-aspartate (NMDA) receptors genes in relevant brain regions. Chemosphere, 71(3), S295–S302. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Cauley M, Burke DA, Kiany A, Slotkin TA, & Levin ED . (2016). Cognitive and behavioral impairments evoked by low-level exposure to tobacco smoke components: comparison with nicotine alone. Toxicological Sciences, 151(2), 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Kwon HJ, Ha M, Paik KC, Lim MH, Lee SG, Yoo SJ, & Kim EJ (2015). The effects of prenatal exposure to alcohol and environmental tobacco smoke on risk for ADHD: A large population-based study. Psychiatry Research, 225(1–2), 164–168. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Kellenberger LD, & Petrik JJ (2006). Fetal and neonatal exposure to nicotine disrupts ovarian function and fertility in adult female rats. Endocrine, 30(2), 213–216. [DOI] [PubMed] [Google Scholar]
- Huizink AC, & Mulder EJ (2006). Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience & Biobehavioral Reviews, 30(1), 24–41. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R & Sowa A (2015). Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environmental Science and Pollution Research, 22(5), 3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AL, Truong L, Marvel SW, Reif DM, Garcia A, Lu C, Simonich MT, Teeguarden JG & Tanguay RL (2017). Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo [a] pyrene in zebrafish. . Toxicology and Applied Pharmacology, 329, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovess V, Keyes KM, Hamilton A, Pez O, Bitfoi A, Koç C, Goelitz D, Kuijpers R, Lesinskiene S, Mihova Z and Otten R (2015). Maternal smoking and offspring inattention and hyperactivity: results from a cross-national European survey. European Child & Adolescent Psychiatry, 24(8), 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, & McCarthy MM (2012). Sexual differentiation of the rodent brain: dogma and beyond. Frontiers in Neuroscience, 6(26), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, & Rose JE (1993). Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicology and Teratology, 15, 251–260. [DOI] [PubMed] [Google Scholar]
- Levin ED, & Slotkin TA (1998). Developmental neurotoxicity of nicotine In Slikker WW Jr & Chang LW (Eds.), Handbook of Developmental Neurotoxicology (pp. 587–615). San Diego: Academic Press. [Google Scholar]
- Levin ED, Wilkerson A, Jones JP, Christopher NC, & Briggs SJ (1996). Prenatal nicotine effects on memory in rats: Pharmacological and behavioral challenges. Developmental Brain Research, 97(2), 207–215. [DOI] [PubMed] [Google Scholar]
- McCallister MM, Maguire NM, Ramesh A, Aimin Q, Liu S, Khoshbouei H, Aschner M, Ebner FF, & Hood DB (2008). Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology, 29, 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister MM, Maguire M, Ramesh A, Aimin Q, Liu S, Khoshbouei H, Aschner M, Ebner FF, & Hood DB (2008). Prenatal exposure to benzo[a]pyrene impairs later-life cortical neuronal function. Neurotoxicology, 29(5), 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, & McCarthy MM (2015). Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience, 16(6), 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, Huang TJ, Miller RL, Wang S, & Rauh V (2014). Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. . PLoS One, 9(11), e111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Camann D, & Rauh V (2012). Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environmental Health Perspectives, 120(6), 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, Semanek D, & Perera F (2015). Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry, 72(6), 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Jurewicz J, & Hanke W (2015). Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment–A review of epidemiological studies. . International Journal of Occupational Medicine and Environmental Health, 28(3), 419–443. [DOI] [PubMed] [Google Scholar]
- Ramsay H, Barnett JH, Murray GK, Mäki P, Hurtig T, Nordström T, Miettunen J, Kiviniemi V, Niemelä S, Pausova Z, & Paus T (2016). Smoking in pregnancy, adolescent mental health and cognitive performance in young adult offspring: results from a matched sample within a Finnish cohort. BMC Psychiatry, 16(1) 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Bizarro L, Asherson PJE, & Stolerman IP (2012). Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharmacology, 223(4), 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Ilott N, Brolese G, Bizarro L, Asherson PJ, & Stolerman IP (2011). Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology, 36(5), 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Levin ED, Lappi SE, & Slotkin TA (1992). Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat brain regions. Developmental Brain Research, 69(2), 288–291. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Ko A, Levin ED, & Seidler FJ (2019). The developmental neurotoxicity of tobacco smoke can be mimicked by a combination of nicotine and benzo[a]pyrene: Effects on cholinergic and serotonergic systems. Toxicological Sciences, 167, 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Levin ED, & Seidler FJ (2015). Prenatal nicotine changes the response to postnatal chlorpyrifos: Interactions targeting serotonergic synaptic function. Brain Research Bulletin, 111, 4–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, & Cochran WG (1967). Statistical Methods. Iowa State University Press, Ames, IA, USA. [Google Scholar]
- Stroud LR, McCallum M, & Salisbury AL (2018). Impact of maternal prenatal smoking on fetal to infant neurobehavioral development. Development and Psychopathology, 30(3), 1087–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati A, Wickramaratne PJ, Wesselhoeft R, & Weissman MM (2017). Prenatal tobacco exposure, birthweight, and offspring psychopathology. Psychiatry Research, 252, 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, & Milner TA (2003). Subcellular relationships between cholinergic terminals and estrogen receptor‐α in the dorsal hippocampus. Journal of Comparative Neurology, 463(4), 390–401. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, & Slotkin TA (2000). An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Research, 867(1–2), 29–39. [DOI] [PubMed] [Google Scholar]
- Vondráček J, Pivnička J, & Machala M (2018). Polycyclic aromatic hydrocarbons and disruption of steroid signaling: History, recent advances and open questions. . Current Opinion in Toxicology, 11–12, 27–34. [Google Scholar]
- Wong MK, Barra NG, Alfaidy N, Hardy DB, & Holloway AC (2015). Adverse effects of perinatal nicotine exposure on reproductive outcomes. Reproduction, 150(6), R185–R193. [DOI] [PubMed] [Google Scholar]
- Xia Y, Cheng S, He J, Liu X, Tang Y, Yuan H, He L, Lu T, Tu B, & Wang Y (2011). Effects of subchronic exposure to benzo [a] pyrene (B [a] P) on learning and memory, and neurotransmitters in male Sprague–Dawley rat. Neurotoxicology, 32, 188–198. [DOI] [PubMed] [Google Scholar]
- Zhu J, Fan F, McCarthy DM, Zhang L, Cannon EN, Spencer TJ, Biederman J and Bhide PG (2017). A prenatal nicotine exposure mouse model of methylphenidate responsive ADHD-associated cognitive phenotypes. International Journal of Developmental Neuroscience, 58, 26–34. [DOI] [PubMed] [Google Scholar]


