Abstract
Effective means to identify the role of reactive oxygen species (ROS) mediating several diseases including cancer, ischemic heart disease, stroke, Alzheimer’s and other inflammatory conditions in in vivo models would be useful. The cyclic nitrone 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) is a spin trap frequently used to detect free radicals in vitro using Electron Paramagnetic Resonance (EPR) spectroscopy. In this study, we synthesized 13C-labeled DMPO for hyperpolarization by dynamic nuclear polarization, in which 13C NMR signal increases more than 10000-fold. This allows in vivo 13C MRI to investigate the feasibility of in vivo ROS detection by the 13C-MRI. DMPO was 13C-labeled at C5 position, and deuterated to prolong the T1 relaxation time. The overall yield achieved for 5-13C-DMPO-d9 was 15%. Hyperpolarized 5-13C-DMPO-d provided a single peak at 76 ppm in the 13C-spectrum, and the T1 was 60 s in phosphate buffer making it optimal for in vivo 13C MRI. The buffered solution of hyperpolarized 5-13C-DMPO-d9 was injected into a mouse placed in a 3 T scanner, and 13C-spectra were acquired every 1 s. In vivo studies showed the signal of 5-13C-DMPO-d9 was detected in the mouse, and the T1 decay of 13C signal of hyperpolarized 5-13C-DMPO-d9 was 29 s. 13C-chemical shift imaging revealed that 5-13C-DMPO-d9 was distributed throughout the body in a minute after the intravenous injection. A strong signal of 5-13C-DMPO-d9 was detected in heart/lung and kidney, whereas the signal in liver was small compared to other organs. The results indicate hyperpolarized 5-13C-DMPO-d9 provided sufficient 13C signal to be detected in the mouse in several organs, and can be used to detect ROS in vivo.
Keywords: hyperpolarized 13C-MRI, spin trapping, DMPO: reactive oxygen species
Graphical Abstract:

INTRODUCTION:
Nitrone and nitroso based spin traps have been used to trap short lived free radicals with life times in the order of nanoseconds to stabilize as radical spin-adducts with longer half-lives (minutes) which can be detected by Electron Spin Resonance (EPR) spectroscopy (1). From the EPR spectra of the spin-adducts which accumulate to detectable levels, the identity of the short-lived intermediates can be confirmed (2). The EPR-spin trapping methodology has been used to detect, characterize and quantify short-lived intermediates generated in various radiolytic, photochemical, sonochemical, and enzymatic reactions (3–6). Nitrone based spin traps such as DMPO, PBN (N-tert-Butyl-α-phenylnitrone) and POBN (α-(4-Pyridyl N-oxide)-N-tert-butylnitrone) have been widely used in chemical and cellular studies to detect free radical adducts using EPR spectroscopy (1,7–9). Newer derivatives of DMPO such as 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide (DEPMPO), 2-ethoxycarbonyl-2-methyl-3,4-dihydro-2H-pyrrole-1-oxide (EMPO), 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO) have been developed for enhanced sensitivity in detecting radicals where the spin adducts are longer lived (4,10–16). Nitrones have been shown to have minimal to no toxicity in vitro and in vivo (~200 mg/kg in mice, rats) and were effective as antioxidants in vitro and in vivo against various types of oxidative stress (5,17–20). Free radical adducts of nitrones such as PBN and POBN were detected after in vivo administration in mice which were exposed to whole body ionizing radiation and the protective effects were attributed to their radical scavenging ability (18,19,21). While EPR spectroscopy has been widely used to detect free radical intermediates, NMR based techniques and immune-spin trapping techniques have enabled the use of nitrones using NMR (22–24), immunology and MRI methods for the detection of free radical based end products in vitro and in vivo (23–28). Of the nitrones developed for spin trapping applications, the PBN derivative NXY-059 has been evaluated for clinical use in protecting against post ischemic reperfusion injury in ischemic stroke in brain (21,29–31).
Carmichael et al., conducted a systematic study to quantify the EPR-detectable radical adducts formed when a known quantity of free radicals were generated using ionizing radiation (32). Free radicals with poor reactivity such as superoxide were trapped with > 90% efficiency generating the corresponding spin-adduct (4,14) (33). Superoxide radical reactions occur primarily at the nitrone bond of DMPO yielding the spin adducts which themselves are short lived and are converted to the DMPO-OH radical which is a more persistent radical (Eq 1, 2).
| (Eq 1) |
| (Eq 2) |
Conversely, nitrone traps yielded radical adducts of the hydroxyl radical in the range of 14 – 35% in spite of diffusion limited rates of reaction (Eq 3).
| (Eq 3) |
The low yield of the spin adducts of these highly reactive radicals, regardless of the efficient rates of reaction were attributed to non-selective reaction on the spin trap other than at the nitrone bond, which are not detectable by EPR (Eq 4).
| (Eq 4) |
Greater than 60% of the reactions of short lived radicals with nitrones result in intermediateswhich are also transient and ultimately form stable diamagnetic end products (Eq 5) (32).
| (Eq 5) |
To monitor such transient intermediates of DMPO reaction with highly reactive radicals, which are rapidly converted to diamagnetic end products, hyperpolarized MRI methods may be applied since the hyperpolarized spin states in DMPO may survive the brief paramagnetic state of the intermediates. While the paramagnetic intermediates cannot be detected, the diamagnetic end-products produced in reactive oxygen species (ROS) can be detected by 13C MRI. The injected 5–13C-DMPO and the ROS mediated diamagnetic end-products will have distinct 13C chemical shifts from which are distinguishable.
Hyperpolarization of the nuclear spin states using dynamic nuclear polarization (DNP) in 13C labeled at sites with T1 ~ 30 s provided > 10,000 fold enhancement in sensitivity for 13C MRI and allowed metabolic studies of the conversion of these substrates to their products in vivo in pre-clinical and clinical settings. Requirements for a candidate molecule suitable for in vivo hyperpolarized MRI include: a) to be non-toxic at high bolus concentrations for in vivo administration; b) have a carbon atom in the molecule with T1 longer than 20 s; c) high aqueous solubility (> 5 M); d) form a glass when mixed with the paramagnetic trityl radicals and frozen to enable DNP. Molecules which satisfy these conditions can be detected with high sensitivity after DNP by in vivo 13C MRI. Endogenous molecules such as pyruvate, fumarate, ketoglutarate etc., are examples of molecules where the carbonyl carbon atoms have been enriched with 13C and polarized to provide 4 orders of magnitude increase in sensitivity. This enhanced sensitivity allowed detection and imaging of these substrates and their metabolic products in vivo. With the 4–5 orders of sensitivity enhancement provided by DNP, it is possible in practice to detect and image ~ 5 uM of ROS-induced diamagnetic end products in vivo.
DMPO is liquid at room temperature and it forms glass when it is frozen with trityl radicals, making it suitable for DNP. In this study, we identified C-5 as a site in the molecule with a long T1 for C atom, synthesized 13C-labeled DMPO, and investigated feasibility of hyperpolarized 13C-MRI and established conditions to image its biodistribution in vivo in a mouse. In vivo models of oxidative stress where high levels of reactive oxygen species (ROS) are generated focally, hyperpolarized MRI methods may become practical for the detection and imaging of free radical processes.
MATERIALS AND METHODS
Reagents
DMPO was purchased from Dojindo Laboratories. OX063 and the symmetric trityl radical (commonly known as the “Finland radical”) were purchased from GE Healthcare. Tris, ethylendiaminetetraacetic acid (EDTA), hypoxanthine, Fe-EDTA, SOD, and catalase were purchased from Sigma-Aldrich. Xanthine oxidase (XO) was purchased from Roche Molecular Biochemicals (Indianapolis, IN, USA). Isotopically labeled compounds were obtained from Cambridge Isotope Laboratories, Inc., MA. All other reagents and solvents were obtained from Sigma Aldrich, Inc. and used as received unless otherwise noted. Flash chromatography was performed on a Teledyne Isco CombiFlash Companion instrument with UV detection at 220 and 254nm. Analytical HPLC analysis was performed on an Agilent 1200 Series instruments equipped with multi wavelength detectors using an Agilent SB C18 column (4.6 × 50mm, 3.5 µm) with a flow rate of 1 mL/min. Solvent A was 0.05 % TFA in water, solvent B was 0.05 % TFA in ACN, and a linear gradient of 5% B to 95% B over 10 min was used. APCI mass spectrometry was performed on 6130 Quadrupole LC/MS Agilent Technologies instrument equipped with diode array detector. HRMS data were acquired using Waters XEVO G2-XS Q-Tof in ESI positive mode. 1H and 13C NMR spectra were recorded with a Varian spectrometer operating at 400MHz and 101 MHz respectively. Chemical shifts are reported in parts per million (δ) and are referenced to tetramethylsilane (TMS).
Synthesis of 5-13C-DMPO-d9
Schemes of 5-13C-DMPO-d9 synthesis are summarized in Figure 1.
Figure 1:

Summary of schemes of 5-13C-DMPO-d9 synthesis.
Acetone-[2-13C, d6] (3):
A 3N LiOD solution in D O (0.625 mL) was added to Acetone-[2-13C] (10.0 g, 170 mmol) and D2O (20.0 mL). The reaction mixture was stirred for 72h at RT. The acetone was distilled via short path distillation. The distillate was subjected to the same reaction conditions except that the stirring was for 48 h. The process was repeated after second and third distillation. At the end of four cycles, 98.6 % deuteration of compound 3 (9.87 g, 152 mmol) was obtained in a 89% yield. 13C NMR (101 MHz, D O) δ215.5 (C–2)
Isopropanol-[2-13C, d7] (4):
Ruthenium on carbon (5%, 4.8 g) was added to a solution of acetone-[2-13C, d6] compound 3 (9.8 g, 150.6 mmol) in D2O (40.0 mL) in a Parr reactor vessel. Air from the vessel was evacuated and replaced with deuterium gas at 45 PSI pressure. The reaction vessel was shaken for 16 h keeping the pressure of deuterium at 45 PSI. A small aliquot was analyzed by 13C NMR and the reaction was found to be complete. The reaction mixture was filtered over a celite pad and the residue washed with deionized water. The combined filtrate was distilled via a short path distillation. The distillate obtained was the pure desired product 4 (9.11 g, 136 mmol) in an 90% yield. 13C NMR (101 MHz, D2O) δ 63.51 (C–2, t, J = 22.2 Hz)
2-Bromopropane-[2-13C, d7] (5):
A solution of 48 % DBr in D2O (50.0 mL) was slowly added to Isopropanol-[2-13C, d7] compound 4 (9.10 g, 136 mmol) at 0°C. The reaction mixture was stirred for 16h at RT. After a short path distillation the product 5 (13.2 g, 103 mmol) was obtained in a 76% yield. 13C NMR (101 MHz, CDCl3) δ 44.85 (C–2, t, J = 23.3 Hz)
2-Nitropropane-[2-13C, d7] (6):
Silver nitrite (16.20 g, 105.3 mmol) was added to a mixture of molecular sieves (4Å, 5g) and 2-bromopropane-[2-13C, d7] compound 5, (11.5 g, 88.4 mmol) in 1,4-dioxane (100.0 mL). The mixture was stirred in the dark for 48 h. It was then analyzed by 13C NMR and found to be complete. The reaction mixture was filtered and the residue washed with ether. The filtrates were combined and the solvent concentrated at reduced pressure, while keeping the temperature of water bath below 20°C. The dried product 6 (5.10 g, 53.6 mmol) was obtained in a 60% yield. 13C NMR (101 MHz, DMSO) δ 78.21 (C–2, t, J = 22.8 Hz)
Methyl-4-methyl-4-nitro pentanoate-[2-13C, d9] (8):
Methyl acrylate-2,3,3-d3 compound 7 (5.0 g; 53.6 mmol) was added to a solution of 2-nitropropane-[2-13C, d7] compound 6, (5.1 g; 52.5 mmol) and Triton-B (40% in H2O, 2.0 mL) in 1,4-dioxane (100.0 mL) at 70°C for 60 min. The temperature of the reaction was then raised to 85°C and maintained for 3 h. The solution was cooled to RT, diluted with 200 mL DCM and washed with 1 N HCl (300ml). The aqueous phase was then washed with DCM (3×100 mL). All organic fractions were combined, dried with magnesium sulfate and concentrated. The oil 8 (5.39g, 29.1 mmol) was obtained in a 52% yield. δ13C NMR (101 MHz, CDCl3)15 δ 86.8 (C–4), 51.72(–OCH3), 28.4 (C–3), 24.8 (C–2)
4-Methyl-4-nitro pentanal-[2-13C, d9] (9):
DIBAL-H (1M in DCM, 32 mL, 32 mmol) was added over 30 min. to a mixture of 4 Å molecular sieves (2.0 g), and nitropentanoate 8 (4.74 g; 25.6 mmol) in DCM (45 mL) at –78°C. The reaction mixture was stirred for 1h at –78°C under an atmosphere of Ar. It was then quenched with addition of wet silica (20 g silica + 10 g water). The temperature was raised to RT. The reaction mixture was then filtered and the residue washed with 3 × 50 mL DCM. All DCM fractions were combined and washed with 300 mL 1N HCl solution. Aqueous phases were extracted with 2 × 100 mL DCM. Organic fractions were combined, dried with MgSO4, and concentrated provide product 9 (3.59 g, 23.1 mmol) in an 90% yield. 13C NMR (101 MHz, CDCl3) δ 199.8 (–CHO), 86.70 (C–4); Lit16: 13C NMR (75.47 MHz, CDCl3) δ 25.61 (–CH3), 32.09 (C–3), 38.60 (C–2), 87.09 (C–4), 199.68 (–CHO).
Dimethyl pyrroline-N-oxide-[5-13C, d9] (10):
Acetic acid-d4 (5.98 g, 93.5 mmol) was added over 1 h to a mixture of activated zinc dust (3.05 g; 47.7 mmol, 2.1 eq.) and pentanal (3.45 g, 22.2 mmol, 1.0 eq.) in ethanol-d (50 mL) at 0°C under Ar. The reaction mixture was then stirred at 4°C for 44 h. It was then diluted with cold ethanol (20 mL) and filtered. The residue was washed with 4×75 mL cold ethanol. The ethanol phases were combined and concentrated. The crude syrup obtained was chromatographed twice on a normal phase silica (100 % DCM to 9:1 DCM-MeOH) to provide DMPO 10 (0.891 g, 7.23 mmol) in a 32% yield. 13C NMR (101 MHz, CDCl3) δ 73.1 (C–5); Lit17 : 13C NMR (101 MHz, CDCl3): δ 23.9 (C–3), 25.2 (–CH3), δ 33.8 (C–4), δ 72.8 (C–5), 129.0 (nitronyl). ESI-MS: 124.1 (M+).
The NMR data is as follows: 1H NMR (400 MHz) CDCl3 δ 6.79 (dd, J = 6.7, 2.7 Hz, 1H), 2.55 (d, J = 4.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 73.1 (C–5); Lit17 : 13C NMR (101 MHz, CDCl3): δ 23.9 (C–3), 25.2 (–CH3), δ 33.8 (C–4), δ 72.8 (C–5), 129.0 (nitronyl). HRMS (ESI/Q-TOF) m/z: [M+H]+ Calcd for C513CH3D9NO+ 124.1512; Found 124.1513
Animal studies
All animal experiments were carried out in compliance with the Guide for the care and use of laboratory animal resources (National Research Council, 1996) and approved by the National Cancer Institute Animal Care and Use Committee (NCI-CCR-ACUC (Bethesda). Female C3H/Hen mice were supplied by the Frederick Cancer Research Center, Animal Production (Frederick, MD).
The mice were anesthetized with isoflurane (4% for induction and 1.5–2.5 % for maintaining anesthesia) in medical air (500 mL/min) and were positioned prone in a MRI scanner. During MRI scans, the breathing rate of the mouse was monitored with a pressure transducer (SA Instruments Inc., Stony Brook, NY) and was maintained at 70 ± 15 breaths per minute. Core body temperature was also monitored with a non-magnetic rectal temperature probe (FISO Technologies, INC., Quebec, Canada) and was maintained at 36 ± 1°C with a flow of warm water. For administration of 5-13C-DMPO-d9, a 30-gauge needle was cannulated into the tail vein and was extended using polyethylene tubing (PE-10).
Hyperpolarized 13C-MRI studies
MRI scans were performed on a 3 T scanner controlled with EasyScan and PowerScan (MR Solutions, Acton, MA), a 4.7 T scanner controlled with ParaVision 5.1 (Bruker Bio-Spin MRI GmbH), or a 3 T scanner (Achieva 3T-TX, Philips Healthcare, Best, Nertherland). A 17 mm home-built 13C solenoid coil placed inside of a saddle coil for 1H and a 35 mm 13C-1H saddle coil was used for phantom and a mouse, respectively. In the MRI scans of mice body, T2-weighted anatomical images were obtained using a fast spin echo sequence (FSET2) with TE of 13 ms, TR of 2,500 ms, 8 slices, 2 mm thickness, resolution of 0.25 × 0.25 mm. 5-13C-DMPO-d9 (30 µL) containing 15 mM symmetric trityl and 2.5 mM gadolinium chelate ProHance (Bracco Diagnostics, Milano, Italy) was hyperpolarized using the Hypersense DNP polarizer (Oxford Instruments, Abingdon, UK) as described previously. After 60–90 min, the hyperpolarized sample was rapidly dissolved in 4.5 mL of PBS containing 100 mg/L EDTA. The hyperpolarized 5-13C-DMPO-d9 solution (60 mM) was intravenously injected through a catheter placed in the tail vein of the mouse (12 µL/g body weight). For the dynamic study, 13C spectra in mouse body were acquired every 1s for 240 s. 13C two-dimensional spectroscopic images were also acquired 25–47 s after the beginning of the DMPO injection, with a 32 × 32 mm, field of view in a 8 mm coronal slice through the body, a matrix size of 16 × 16, spectral width of 3330 Hz, repetition time of 85 ms, and excitation pulse with a flip angle of 10º. The total time required to acquire each image was 23 s. In the MRI scans of mice head, 5-13C-DMPO-d9 was hyperpolarized using the SPINlab (GE Healthcare) for 3–4 hours, and the scans were performed using the Philips 3 T scanner. 13C two-dimensional spectroscopic images were acquired 30 s after the beginning of the DMPO injection, with a 28 × 28 mm, field of view in a 10 mm axial slice through the head, a matrix size of 14 × 14, spectral width of 3333 Hz, repetition time of 86 ms, and excitation pulse with a flip angle of 3º.
RESULTS
Synthesis of 13C-labeled DMPO (Deepak)
Preliminary 13C NMR spectroscopy studies conducted using a 4. 7 T MRI scanner identified that of all carbon atoms, the carbon atom at the 5-position of DMPO (Figure 2A) with a chemical shift of 76 ppm (Figure 2B) had the longest T1(Figure 2C). Since DMPO is amphipathic, we tested the polarization efficiency using, the Finland-COOH radical, which is also an amphipathic paramagnetic probe. A frozen glass mixture of the DMPO and the Finland-COOH radical was polarized for 60 minutes and rapidly dissolved to room temperature and measuring its NMR spectrum immediately. A significant enhancement of the signal intensity of the 13C NMR signal was observed (Figure 2D) compared to the signals from DMPO polarized with OX063 (Figure 2B). Based on these observations, DMPO enriched with 13C-labeled at C5 position and deuterated was synthesized to further prolong the T1 relaxation time.
Figure 2:

(A) Position of 13C-labeled in DMPO which has the longest T1. (B) 13C-MR spectrum of unlabeled DMPO hyperpolarized with OX063. Single peak was detected at 76 ppm which was from 13C atom at the 5-position of DMPO. (C) The signal decay after dissolution in PBS. T1 was calculated to be 26 s from the decay curve. (D) 13C-MR spectrum of unlabeled DMPO with natural abundance 13C, hyperpolarized with Finland-COOH. The signal to noise ratio of DMPO peak was remarkably increased by using Finland-COOH.
Since the initial synthesis of unlabeled DMPO (34,35) several other researchers have synthesized DMPO and its analogs using various routes (36–42). Based on works of Beth et al. (43) and Janzen et al. (38), synthesis of labeled DMPO as a spin trap agent was first explored by Pou et al. (37). In order to prepare DMPO with 13C at position C5 and deuterated protons on neighboring carbons (to prolong the T1 relaxation times) we followed the procedure of Le et al. (41) and Leinisch et al. (42). We found that the 13C-C2 deuterated three carbon starting material could be effectively prepared from, the h-d exchange on acetone 13C-C2 as described in the literature (44) under basic conditions. Per-deuterated acetone-13C-C2 was then reduced to isopropanol-13C-C2 with Pd/C under D2 atmosphere. Labeled isopropanol was brominated with DBr, converted to 2-nitropropane with silver nitrite and then alkylated with methyl acrylate-2,3,3-d3.
The resulting nitropentanoate was reduced to aldehyde with DIBAL-H. This was followed by treatment with activated zinc and acetic acid to yield the cyclic nitrone, DMPO. However, the resulting DMPO was not fully deuterated due to the DIBAL reduction. The overall yield achieved for DMPO-d9 was 15%. Figure 1 shows the overall synthetic scheme followed.
Hyperpolarization of 13C-DMPO
For successful polarization, the molecule to be polarized should dissolve at high concentrations (~ 5 M) and form a homogenous glass with the trityl radical when frozen. Of the four trityl radical analogs for hyperpolarization of 13C-DMPO, namely OX063, Finland trityl, OX063-HCl, Finland-COOH, Finland-COOH was found to be the most suited for glass formation and polarization of DMPO. Figure 3A shows the polarization build-up of DMPO with natural abundance 13C (open circles) and 5-13C-DMPO-d9 (closed circles) with 15 mM Finland-COOH and 2.5 mM Gd chelate ProHance. The time constant of polarization build-up in the hyperpolarizer was found to be ~ 23 min. After the polarization was maximal, the hyperpolarized samples were dissolved in PBS containing EDTA, and 13C-spectra were measured every 1 s using the 4.7 T scanner with 10 deg flip angle. The T1 relaxation time of non-enriched/non-deuterated DMPO and 5-13C-DMPO-d9 was 26 s and 60 s, respectively, suggesting that deuteration of DMPO extended the T1 significantly (Figure 3B). The T1 of 5-13C-DMPO-d9 was similar to that of 1-13C-pyruvic acid, which is the most frequently used probe in hyperpolarized 13C-MRI in vivo, suggesting 13C-DMPO has sufficiently long T1 to permit in vivo studies. With the known toxicological data of DMPO from prior studies where large bolus doses have no adverse effects (20) and with the long T1 of the 5-C atom, the results suggest that imaging of hyperpolarized DMPO in vivo monitoring its biodistribution and its transformation is feasible.
Figure 3:

(A) Hyperpolarization build-up of unlabeled DMPO (○) and 13C-labeled DMPO-d9 (●) with 15 mM Finland-COOH and 2.5 mM Gd chelate ProHance. The build-up was monitored by an NMR spectroscopy in the hyperpolarizer. (B) The decay of 13C-signal at 76 ppm of unlabeled DMPO (○) and 13C-labeled DMPO-d9 (●). Deuteration of DMPO extended the T1 of the 13C signal from 26 s to 60 s.
In Vivo 13C MRI of hyperpolarized 5-13C-DMPO-d9
The in vivo behavior of DMPO was tested in a mouse where the DMPO after dissolution of the hyperpolarized mixture was administered through a tail vein cannula of a mouse placed in a double tuned 1H/13C coil and the 13C signal monitored every 1 s using the 3 T scanner with 10 deg flip angle pulse. The signal of 5-13C-DMPO-d9 was detected in the mouse body immediately after its injection and detectable for more than 150 s (Figure 4A). Figure 4B shows the signal intensity curve of 5-13C-DMPO-d9 after iv administration of the hyperpolarized sample in the mouse. From the signal intensity curve, the T1 decay of 5-13C-DMPO-d9 in mouse was estimated to be 29s. The persistence of the hyperpolarized signal in vivo for this duration points to the feasibility of imaging DMPO biodistribution and possible use for monitoring its transformation either enzymatically or through reactive oxygen species.
Figure 4:
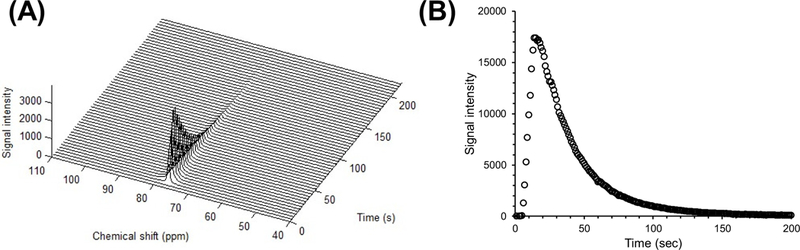
(A) Dynamic 13C-MR spectra in a mouse body after hyperpolarized 5-13C DMPO-d9 injection. The spectra were acquired every 1 s for 240 s. DMPO peak (76 ppm) was obserbed. (B) Signal intensity curve of DMPO calculated from the spectra. The T1 was estimated to be 29 s from the curve.
We carried out 13C-chemical shift imaging in a mouse body after iv administration of hyperpolarized 5-13C-DMPO-d9. Figure 5A shows consecutive anatomical slices (2 mm thickness each) of the mouse body. Figure 5B shows the 13C-CSI overlaid on the anatomic image obtained from 8mm slice thickness of the mouse body 25–47 s after hyperpolarized 5-13C-DMPO-d9 injection. The DMPO map obtained from the chemical shift image (Figure 5C) revealed that 13C-DMPO was distributed through the mouse body within a minute of its injection. The signal was higher in the heart/lung region and kidney, whereas the signal from liver was relatively low compared to the other region (Figure 5D, E). To obtain a higher signal in the mouse liver, we did slice selected 13C-MRS by averaging 100 transients (Figure 5 F). Signal to noise ratio was much better compared to 13C-CSI. The relative levels of DMPO in the heart, kidney and liver regions are shown in Figure 5G. No metabolites were detected in the normal mouse liver suggesting that enzymatic conversion of DMPO was minimal.
Figure 5:

(A) T2-weighted anatomical images of a mouse body (4 adjacent slices). The thickness of each slice was 2 mm. (B) 13C-chemical shift image (CSI) in the mouse body acquired 25 s after hyperpolarized 5-13C-DMPO-d9 injection. The 13C-CSI was obtained from 8 mm thickness slice, and the size of each pixel was 2 mm × 2 mm. (C) Distribution of DMPO in the mouse body calculated from the 13C-CSI. The positions of heart/lung, liver, and kidney were obtained from the T2-weighted anatomy. (D, E) 13C-MR spectrum of heart and left kidney obtaied from the 13C CSI. (F) 13C-MR spectrum from a mouse liver with 100 times averaging. (G) Averaged signal inteisty of 13C-DMPO in each organ calculated form the DMPO distribution map.
Several studies showed that DMPO exhibits anti-oxidative and anti-inflammatory effects in vivo (45,46). A recent study showed that DMPO injected directly into the intranigral region in rats afforded protection against 6-OH-Dopamine mediated neurotoxicity (10). With the enhanced sensitivity provided by hyperpolarization, it is possible to examine the biodistribution of DMPO in the brain following systemic administration intravenously. A tail vein cannula was placed in an anesthetized mouse placed in a doubly tuned resonator encompassing the head region. Figure 6A shows the 13C-CSI overlayed on the anatomic image in an axial profile. DMPO biodistribution in the brain was monitored immediately after hyperpolarized DMPO was administered (Figure 6B). The 13C image shows evidence of DMPO accumulation in the brain region suggesting that it penetrates the blood-brain barrier rapidly and accumulates at effective concentrations while retaining polarization. This image represents the first imaging evidence for the localization of DMPO in the brain and may explain the observed antioxidant effects it exerts.
Figure 6:

(A) 13C-CSI in the mouse head acquired 30 s after hyperpolarized 5-13C-DMPO-d9 injection. The CSI was obtained from 10 mm thickness slice, and overlaied on a 2 mm thickness of T2-weighted anatomical image. (B) Distribution of DMPO in the mouse head calculated from the 13C-CSI.
DISCUSSION:
DNP of low γ nuclei in molecules enriched with 13C, 15N has allowed imaging of endogenous molecules and their biochemical transformations in vivo. 13C enriched pyruvate, fumarate, urea, glucose, gluconolactate, bicarbonate, have been successfully imaged in vivo in various animal models to obtain information pertaining to metabolism, perfusion and pH. Exogenous molecules such as ascorbate, glycerate, methyl glutamine were also developed and tested for probe for perfusion, pH. Most probes report on biochemical, physiologic, and microenvironmental features. Dehydroascorbate has been used to probe the levels of ROS by monitoring its transformation to ascorbate and determining the ration of DHA/AA (47). However, this transformation being a 2-electron process, the ratio may also be significantly influenced by the tissue redox status. The search for hyperpolarizable antioxidants which can report on oxidative stress and provide noninvasive capabilities to monitor these reactions led to considering nitrones as candidate probes for the following reasons: a) Nitrones can have carbon atoms which can have potentially long 13C T1s. b) Nitrones are non-toxic even at high bolus doses typically used for HP MRI; c) NXY-059, a nitrone derivative has been characterized to have anti-oxidant activity and has been developed and used for human applications to limit post-ischemic reperfusion injury (21). DMPO shares many of these features and has been successfully polarized. DMPO has long been used as a spin trap to scavenge reactive oxygen species, identify the intermediates and quantify them in simple chemical systems using EPR spectroscopy. However, in cellular studies, it was of limited use since the spin adducts undergo rapid reduction to diamagnetic products which are EPR invisible. NMR-Spin trapping overcame some of these limitations. In vivo detection of ROS was demonstrated by EPR by administering nitrone spin traps and analyzing the tissue extracts (22,24). Direct in vivo detection of spin adducts was also demonstrated using POBN by using low-frequency EPR by exposing tumors to high radiation doses (19). These approaches however, cannot be translated to in vivo imaging because of poor sensitivity.
HP-MRI with 5-13C-DMPO-d9 has the potential to provide imaging assessment of reactive oxygens species because: a) the signals of the original molecule can be significantly enhanced by the DNP method; b) the molecule biodistribution in vivo is effective to have adequate global concentrations in organs/regions of interest; c) appropriate models of ROS mediated conversion of the DMPO to stable diamagnetic products proceeds with sufficient efficiency to have detectable levels of products with characteristic chemical shifts. The hyperpolarized DMPO biodistribution monitored by 13C MRI revealed that significant levels were attained in heart, kidney, brain with detectable levels in the liver and muscle. The demonstration of the in vivo biodistribution of DMPO, by 13C MRI using hyperpolarization techniques sets the stage for oxidative stress evaluation in vivo. With suitable in vivo models of focal ROS generation at high levels such as post-ischemic reperfusion injury or systemic ROS generation such as with lipopolysaccharide challenge, this method can be applied for in vivo imaging of oxidative stress.
Supplementary Material
Figure S1: 1H NMR spectra of DMPO.
Figure S2: 13C NMR spectra of DMPO.
Highlights.
Spin traps/radical scavengers cannot be currently imaged by MRI
13C labeled spin trap DMPO can be imaged in vivo after hyperpolarization
Its biodistribution was followed in vivo after hyperpolarization.
13C MRI with hyperpolarized DMPO can be used for in vivo free radical detection/imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Janzen EG. Spin Trapping. Accounts Chem Res 1971;4(1):31-& 10.1021/ar50037a005. [DOI] [Google Scholar]
- 2.Buettner GR. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med 1987;3(4):259–303. [DOI] [PubMed] [Google Scholar]
- 3.Villamena FA, Zweier JL. Detection of reactive oxygen and nitrogen species by EPR spin trapping. Antioxid Redox Signal 2004;6(3):619–29 10.1089/152308604773934387. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Joseph J, Vasquez-Vivar J, Karoui H, Nsanzumuhire C, Martasek P, et al. Detection of superoxide anion using an isotopically labeled nitrone spin trap: potential biological applications. FEBS Lett 2000;473(1):58–62. [DOI] [PubMed] [Google Scholar]
- 5.Halpern HJ, Yu C, Barth E, Peric M, Rosen GM. In situ detection, by spin trapping, of hydroxyl radical markers produced from ionizing radiation in the tumor of a living mouse. Proc Natl Acad Sci U S A 1995;92(3):796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riesz P, Kondo T, Carmichael AJ. Sonochemistry of acetone and acetonitrile in aqueous solutions. A spin trapping study. Free Radic Res Commun 1993;19 Suppl 1:S45–53. [DOI] [PubMed] [Google Scholar]
- 7.McCormick ML, Buettner GR, Britigan BE. The spin trap alpha-(4-pyridyl-1-oxide)-N-tert-butylnitrone stimulates peroxidase-mediated oxidation of deferoxamine. Implications for pharmacological use of spin-trapping agents. J Biol Chem 1995;270(49):29265–9. [DOI] [PubMed] [Google Scholar]
- 8.Buettner GR, Britigan BE. The spin trapping of superoxide with M4PO (3,3,5,5-tetramethylpyrroline-N-oxide). Free Radic Biol Med 1990;8(1):57–60. [DOI] [PubMed] [Google Scholar]
- 9.Janzen EG. Esr Detection and Identification of Short-Lived Free Radicals by Spin Trapping. Appl Spectrosc 1971;25(1):139-&. [Google Scholar]
- 10.Kitamura Y, Kamibayashi M, Inden M, Yanagida T, Shibaike T, Takata K, et al. Detoxification of 6-hydroxydopamine-induced Parkinsonian neurodegeneration by G-CYPMPO, a novel radical trapper. Neurochem Int 2011;58(6):721–7 10.1016/j.neuint.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Kalyanaraman B. Synthesis and ESR studies of a novel cyclic nitrone spin trap attached to a phosphonium group-a suitable trap for mitochondria-generated ROS? Free Radic Res 2007;41(1):1–7 10.1080/10715760600911147. [DOI] [PubMed] [Google Scholar]
- 12.Kamibayashi M, Oowada S, Kameda H, Okada T, Inanami O, Ohta S, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO). Free Radic Res 2006;40(11):1166–72 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 13.Clement JL, Finet JP, Frejaville C, Tordo P. Deuterated analogues of the free radical trap DEPMPO: synthesis and EPR studies. Org Biomol Chem 2003;1(9):1591–7. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Joseph J, Zhang H, Karoui H, Kalyanaraman B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: a relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic Biol Med 2001;31(5):599–606. [DOI] [PubMed] [Google Scholar]
- 15.Timmins GS, Liu KJ, Bechara EJ, Kotake Y, Swartz HM. Trapping of free radicals with direct in vivo EPR detection: a comparison of 5,5-dimethyl-1-pyrroline-N-oxide and 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide as spin traps for HO* and SO4*. Free Radic Biol Med 1999;27(3–4):329–33. [DOI] [PubMed] [Google Scholar]
- 16.Frejaville C, Karoui H, Tuccio B, Le Moigne F, Culcasi M, Pietri S, et al. 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: a new efficient phosphorylated nitrone for the in vitro and in vivo spin trapping of oxygen-centered radicals. J Med Chem 1995;38(2):258–65. [DOI] [PubMed] [Google Scholar]
- 17.Villamena FA, Das A, Nash KM. Potential implication of the chemical properties and bioactivity of nitrone spin traps for therapeutics. Future Med Chem 2012;4(9):1171–207 10.4155/fmc.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young HK, Floyd RA, Maidt ML, Dynlacht JR. Evaluation of nitrone spin-trapping agents as radioprotectors. Radiat Res 1996;146(2):227–31. [PubMed] [Google Scholar]
- 19.Lai EK, Crossley C, Sridhar R, Misra HP, Janzen EG, McCay PB. In vivo spin trapping of free radicals generated in brain, spleen, and liver during gamma radiation of mice. Arch Biochem Biophys 1986;244(1):156–60. [DOI] [PubMed] [Google Scholar]
- 20.Khan N, Wilmot CM, Rosen GM, Demidenko E, Sun J, Joseph J, et al. Spin traps: in vitro toxicity and stability of radical adducts. Free Radic Biol Med 2003;34(11):1473–81. [DOI] [PubMed] [Google Scholar]
- 21.Floyd RA, Kopke RD, Choi CH, Foster SB, Doblas S, Towner RA. Nitrones as therapeutics. Free Radic Biol Med 2008;45(10):1361–74 10.1016/j.freeradbiomed.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khramtsov VV, Reznikov VA, Berliner LJ, Litkin AK, Grigor’ev IA, Clanton TL. NMR spin trapping: detection of free radical reactions with a new fluorinated DMPO analog. Free Radic Biol Med 2001;30(10):1099–107. [DOI] [PubMed] [Google Scholar]
- 23.Berliner LJ, Khramtsov V, Fujii H, Clanton TL. Unique in vivo applications of spin traps. Free Radic Biol Med 2001;30(5):489–99. [DOI] [PubMed] [Google Scholar]
- 24.Khramtsov V, Berliner LJ, Clanton TL. NMR spin trapping: detection of free radical reactions using a phosphorus-containing nitrone spin trap. Magn Reson Med 1999;42(2):228–34. [DOI] [PubMed] [Google Scholar]
- 25.Towner RA, Saunders D, Smith N, Towler W, Cruz M, Do S, et al. Assessing long-term neuroinflammatory responses to encephalopathy using MRI approaches in a rat endotoxemia model. Geroscience 2018;40(1):49–60 10.1007/s11357-018-0009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason RP. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol 2016;8:422–9 10.1016/j.redox.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towner RA, Smith N, Saunders D, Lupu F, Silasi-Mansat R, West M, et al. In vivo detection of free radicals using molecular MRI and immuno-spin trapping in a mouse model for amyotrophic lateral sclerosis. Free Radic Biol Med 2013;63:351–60 10.1016/j.freeradbiomed.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Towner RA, Smith N, Saunders D, De Souza PC, Henry L, Lupu F, et al. Combined molecular MRI and immuno-spin-trapping for in vivo detection of free radicals in orthotopic mouse GL261 gliomas. Biochim Biophys Acta 2013;1832(12):2153–61 10.1016/j.bbadis.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 29.de Souza PC, Smith N, Pody R, He T, Njoku C, Silasi-Mansat R, et al. OKN-007 decreases VEGFR-2 levels in a preclinical GL261 mouse glioma model. Am J Nucl Med Mol Imaging 2015;5(4):363–78. [PMC free article] [PubMed] [Google Scholar]
- 30.de Souza PC, Balasubramanian K, Njoku C, Smith N, Gillespie DL, Schwager A, et al. OKN-007 decreases tumor necrosis and tumor cell proliferation and increases apoptosis in a preclinical F98 rat glioma model. J Magn Reson Imaging 2015;42(6):1582–91 10.1002/jmri.24935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutinho de Souza P, Smith N, Atolagbe O, Ziegler J, Njoku C, Lerner M, et al. OKN-007 decreases free radical levels in a preclinical F98 rat glioma model. Free Radic Biol Med 2015;87:157–68 10.1016/j.freeradbiomed.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmichael AJ, Makino K, Riesz P. Quantitative aspects of ESR and spin trapping of hydroxyl radicals and hydrogen atoms in gamma-irradiated aqueous solutions. Radiat Res 1984;100(2):222–34. [PubMed] [Google Scholar]
- 33.Ben-Hur E, Carmichael A, Riesz P, Rosenthal I. Photochemical generation of superoxide radical and the cytotoxicity of phthalocyanines. Int J Radiat Biol Relat Stud Phys Chem Med 1985;48(5):837–46. [DOI] [PubMed] [Google Scholar]
- 34.Brown RFC, Clark VM, Todd A. DELTA-1-PYRROLINE N-OXIDES. Proceedings of the Chemical Society of London 1957(3):97–8. [Google Scholar]
- 35.Bonnett R, Clark VM, Giddey A, Todd A. EXPERIMENTS TOWARDS THE SYNTHESIS OF CORRINS .1. THE PREPARATION AND REACTIONS OF SOME DELTA-1-PYRROLINES - A NOVEL PROLINE SYNTHESIS. Journal of the Chemical Society 1959(JUN):2087–93 10.1039/jr9590002087. [DOI] [Google Scholar]
- 36.Janzen EG, Zhang YK, Arimura M. SYNTHESIS AND SPIN-TRAPPING CHEMISTRY OF 5,5-DIMETHYL-2-(TRIFLUOROMETHYL)-1-PYRROLINE N-OXIDE. Journal of Organic Chemistry 1995;60(17):5434–40 10.1021/jo00122a021. [DOI] [Google Scholar]
- 37.Pou S, Rosen GM, Wu YX, Keana JFW. SYNTHESIS OF DEUTERIUM-CONTAINING AND N-15-CONTAINING PYRROLINE 1-OXIDES - A SPIN TRAPPING STUDY. Journal of Organic Chemistry 1990;55(14):4438–43 10.1021/jo00301a042. [DOI] [Google Scholar]
- 38.Janzen EG, Zhang YK, Haire DL. SYNTHESIS OF A NOVEL NITRONE, 2-PHENYL-5,5-DIMETHYL-1-PYRROLINE N-OXIDE (NITRONYL-C-13), FOR ENHANCED RADICAL ADDEND RECOGNITION AND SPIN ADDUCT PERSISTENCE. Journal of the American Chemical Society 1994;116(9):3738–43 10.1021/ja00088a009. [DOI] [Google Scholar]
- 39.Haire DL, Hilborn JW, Janzen EG. A MORE EFFICIENT SYNTHESIS OF DMPO-TYPE (NITRONE) SPIN TRAPS. Journal of Organic Chemistry 1986;51(22):4298–300 10.1021/jo00372a039. [DOI] [Google Scholar]
- 40.Golubev VA, Shilov GV, Sen VD. Synthesis of new functionalized 5,5-dimethyl-1-pyrroline 1-oxides and their investigation as spin traps. Russian Chemical Bulletin 2010;59(11):2081–5 10.1007/s11172-010-0358-y. [DOI] [Google Scholar]
- 41.Le DD, Zhang YS, Nguyen D, Moravek J. Synthesis of 5,5-dimethyl-1-pyrroline-N-oxide-2-C-14. Journal of Labelled Compounds & Radiopharmaceuticals 2000;43(11):1107–11 [DOI] [Google Scholar]
- 42.Leinisch F, Jiang J, Deterding LJ, Mason RP. Simplified synthesis of isotopically labeled 5,5-dimethyl-pyrroline N-oxide. Molecules 2011;16(10):8428–36 10.3390/molecules16108428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beth AH, Venkataramu SD, Balasubramanian K, Dalton LR, Robinson BH, Pearson DE, et al. N-15-SUBSTITUTED AND H-2-SUBSTITUTED MALEIMIDE SPIN LABELS - IMPROVED SENSITIVITY AND RESOLUTION FOR BIOLOGICAL ELECTRON-PARAMAGNETIC-RES STUDIES. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences 1981;78(2):967–71 10.1073/pnas.78.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulsen PJ, Cooke WD. PREPARATION OF DEUTERATED SOLVENTS FOR NUCLEAR MAGNETIC RESONANCE SPECTROMETRY. Analytical Chemistry 1963;35(10):1560-& 10.1021/ac60203a072. [DOI] [Google Scholar]
- 45.Munoz MD, Della Vedova MC, Bushel PR, Ganini da Silva D, Mason RP, Zhai Z, et al. The nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide dampens lipopolysaccharide-induced transcriptomic changes in macrophages. Inflamm Res 2018;67(6):515–30 10.1007/s00011-018-1141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai Z, Gomez-Mejiba SE, Ramirez DC. The nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide affects stress response and fate of lipopolysaccharide-primed RAW 264.7 macrophage cells. Inflammation 2013;36(2):346–54 10.1007/s10753-012-9552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keshari KR, Sai V, Wang ZJ, Vanbrocklin HF, Kurhanewicz J, Wilson DM. Hyperpolarized [1–13C]dehydroascorbate MR spectroscopy in a murine model of prostate cancer: comparison with 18F-FDG PET. J Nucl Med 2013;54(6):922–8 10.2967/jnumed.112.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: 1H NMR spectra of DMPO.
Figure S2: 13C NMR spectra of DMPO.


