Abstract
Commensal microbes inhabit barrier surfaces, providing a first line of defense against invading pathogens, aiding in metabolic function of the host, and playing a vital role in immune development and function. Several recent studies have demonstrated that commensal microbes influence systemic immune function and homeostasis. For patients with extramucosal cancers, or cancers occurring distal to barrier surfaces, the role of commensal microbes in influencing tumor progression is beginning to be appreciated. Extrinsic factors such as chronic inflammation, antibiotics, and chemotherapy dysregulate commensal homeostasis, and drive tumor-promoting systemic inflammation through a variety of mechanisms including disruption of barrier function and bacterial translocation, release of soluble inflammatory mediators, and systemic changes in metabolic output. Conversely, it has also been demonstrated that certain immune therapies, immunogenic chemotherapies, and checkpoint inhibitors rely on the commensal microbiota to facilitate anti-tumor immune responses. Thus, it is evident that the mechanisms associated with commensal microbe facilitation of both pro- and anti-tumor immune responses are context dependent and rely upon a variety of factors present within the tumor microenvironment and systemic periphery. The goal of this review is to highlight the various contexts during which commensal microbes orchestrate systemic immune function with a focus on describing possible scenarios where the loss of microbial homeostasis enhances tumor progression.
Keywords: Commensal microbiota, cancer, inflammation, metabolism, dysbiosis
1. Introduction
Commensal microbes, comprised of bacteria, archaea, viruses, and eukaryotes, inhabit all mucosal barrier surfaces, providing a physical barrier in defense against invading pathogens. Additionally, commensal microbes play essential roles in the maintenance of local tissue and immune homeostasis within the gastrointestinal tract [1–5], the skin [6, 7], the urogenital tract [8, 9] and the oral/respiratory tract [10–13]. Colonization with commensal microbes at birth is critical for the postnatal development and function of mucosal immunity [14, 15]. However, commensal-mediated immune conditioning extends beyond mucosal surfaces, impacting both systemic immune function and homeostasis. Changes in commensal homeostasis are dynamic and occur gradually during aging or from changes in diet. Acute disturbances resulting from antibiotic usage, infection, or chemotherapy can also drastically alter established commensal equilibrium, rapidly culminating in a loss of immune homeostasis. Loss of commensal homeostasis, or commensal dysbiosis, can lead to increased inflammation and immune pathology that ultimately affects the systemic periphery. In this context, alterations to commensal equilibrium induce pathological inflammation that is supportive of tumor growth. Although we do not yet have a firm understanding of the precise microbial populations that associate with tumor-promoting inflammation, it is evident that commensal microbes influence the outcome of extramucosal tumors.
Over three decades ago, scientists began to observe that certain gram-negative commensal species influence myelopoiesis and the emergence of granulocyte precursors from the bone marrow [16–18], suggesting that commensal microbes influence immune function through undefined interactions with distal sites such as the bone marrow. Germ-free mice have a deficit in the myeloid compartment of bone marrow resulting in increased susceptibility to infection with Listeria. However, restoration of immune function is achieved through recolonization of germ-free mice with fecal contents from conventional mice, enabling clearance of Listeria [19]. Additional studies demonstrated that commensal products, such as lipopolysaccharide (LPS) or peptidoglycan provide a tonic level of stimulation through Toll-like receptors (TLR) and other innate receptors expressed by myeloid cells, driving myelopoiesis [20] and enhancing myeloid clearance of bacterial [21] and viral pathogens [22, 23]. Commensal microbes are also associated with the development of mucosal-associated and peripheral lymphocytes such as Foxp3+ regulatory T cells [24–26], IL-17-producing αβ T cells [27, 28] and γδ T cells [29], and invariant NKT cells [30, 31]. Data from the Human Functional Genomics Project supports much of what has been elucidated in mice, demonstrating that distinct commensal or metabolic signatures are associated with both innate and adaptive cytokine response patterns [32]. These studies underscore the complex immunoregulatory influence that commensal microbes have on local and systemic immune homeostasis in healthy individuals.
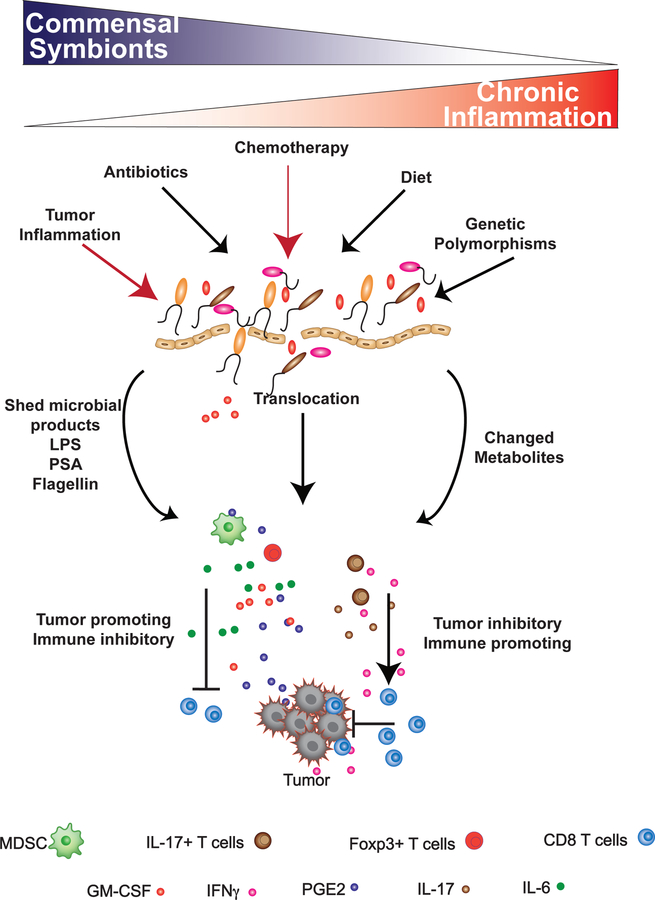
Cancer is a systemic disease: inflammatory immune cells, chemokines and cytokines distally influence tumor growth and metastatic progression. Cancer can impact the composition of commensal microbes locally within affected tissues [33–36] or distally within the intestines [37, 38], altering the immune environment in favor of tumor growth and global immune suppression [39]. The relationship between commensal microbes, inflammation, and oncogenesis is well-documented for colorectal cancer [40–42], which is locally influenced by dysregulation of commensal homeostasis as a result of chronic antibiotic exposure, diet, age, infection, and genetic polymorphisms that drive inflammation and oncogenesis. Cancer patients may also have disruptions in commensal homeostasis as a result of chemotherapy, administration of antibiotics, whole body irradiation, cachexia, and/or systemic tumor-promoting inflammation (Figure 1). Several recent studies have begun to link changes within the composition of commensal microbes to global modulation of tumor-promoting inflammatory cytokines [39] and have identified certain microbes that facilitate enhancement of anti-tumor immune responses during immunotherapy [43–45] and chemotherapy [43, 46]. Thus, patients with extramucosal tumors, occurring distal to mucosal surfaces, are also influenced by alterations in commensal composition. In this review, we will highlight mechanisms associated with commensal-induced pathological inflammation with a focus on detailing how microbes, microbial products, and/or disruptions in commensal homeostasis impact extramucosal cancer progression.
Figure 1: Loss of commensal equilibrium results in systemic inflammation and context-dependent modulation of extramucosal tumor progression.
Factors such as antibiotic usage, diet, or genetics (black arrows) can effectively decrease the biodiversity of the commensal microflora, leading to a higher incidence of chronic inflammation. In tumor-bearing individuals, cancer therapy and tumor-derived inflammation (red arrows) can also modulate the composition of the commensal ecosystem. A reduction in commensal biodiversity (dysbiosis), antibiotics, and chemotherapy can directly damage the integrity of the mucosal epithelium, resulting in bacterial translocation, commensal shedding of inflammatory products, such as LPS, PSA, and flagellin, and a change in metabolic output from the commensal ecosystem. This can lead to an enhancement in tumor-promoting inflammation due to the induction of IL-6, GM-CSF, and TGFβ: factors which enhance the recruitment of immune suppressive myeloid-derived suppressor cells and regulatory T cells into the tumor microenvironment. In the presence of these cells, the ability of T cells to effectively eliminate the tumor are inhibited. Translocation of commensals from mucosal surfaces can also induce the activation of IFNγ-producing Th-17 cells, which in certain contexts, can facilitate T cell-mediated killing of tumors.
2. Alterations to microbiome associated with inflammation and cancer
In healthy adults, the abundance of certain commensal species is associated with the functional ability of both myeloid and lymphoid cells to produce inflammatory cytokines such as TNFα, IL-6, IL-1β, IFNγ, IL-17 and IL-22 [32]. These cytokines are all capable of influencing tumor progression through multiple mechanisms, including the promotion of tumor growth through the recruitment of suppressive immune cells into the tumor microenvironment via TNFα, IL-6, IL-1β or facilitating enhanced tumor immune surveillance via IFNγ and IL-17. However, microbial populations associated with inflammation may be altered during states of dysbiosis, defined as an imbalance of commensal homeostasis and resultant outgrowth of pathological microbial species. It is well-accepted that commensal dysbiosis can drive pathologies within barrier surfaces, but dysbiosis also results in systemic damage to distal organs due to aberrant inflammation and metabolic dysregulation [47]. Dysbiosis can be induced by multiple mechanisms, including diet or genetic-induced dysbiosis, antibiotic-induced dysbiosis, and dysbiosis due to tumor-promoting chronic inflammation, all of which associate with a more unfavorable outcome during cancer.
2.1. Inflammation, dysbiosis, and cancer
There are several studies linking dysbiosis with cancer and inflammation, although it remains relatively undefined whether dysbiosis directly impacts tumor progression or serves as a biomarker of oncogenesis. Dysbiosis has been demonstrated in patients with advanced breast cancer, with breast tumors having reduced microbial diversity compared to normal breast tissue [34]. In these patients, reduced diversity of tumor-associated commensal species corresponds with reduced expression of inflammatory innate signaling receptors such as TLR2, TLR5, and nucleotide-binding oligomerization domain–containing protein (NOD)1 and NOD2 [34]. These innate recognition receptors may serve a protective role in breast tissue, as TLR5 signaling and activation of MAIP1S has been shown to inhibit breast tumor growth through the induction of autophagy and tumor cell death [48, 49]. These studies suggest that dysbiosis within the breast tissue may occur through dysregulation of innate signaling receptors, promoting the outgrowth of inflammatory or DNA-damaging bacterial species. Indeed, Urbaniak et al. determined that Escherichia coli and Staphylococcus epidermidis isolates from dysbiotic breast tissues are able to directly induce DNA damage in a tumor cell line [50]. Microbial sequencing of an additional cohort of breast tissue specimens found a tumor-specific increase in Fusobacterium [51], a genus of bacteria which harbors the species F. nucleatum, a bacterium directly associated with driving inflammation and carcinogenesis in colorectal cancer [52, 53].
Changes in the composition of commensal microbes within the reproductive tract are also associated with increased inflammation. Women with endometrial cancer have dysbiosis in the vagina, cervix, and endometrium that is associated with malignant progression [54]. Protein analysis of cervical samples revealed that severe cervical dysbiosis correlates with elevated levels of both proinflammatory cytokines and enzymes associated with proteolysis and alterations to the cervical mucosa and cytoskeleton [55]. Furthermore, alterations in the composition of bacteria within the reproductive tract are associated with increased levels of GM-CSF, TNFα, IFNγ, and IL-1β [56]: cytokines that promote myeloid infiltration within tumor beds. Together, these studies suggest that severe dysbiosis within the reproductive tract leads to an increase in inflammation and pathology, resulting in damage to mucosal surfaces. Importantly, these studies highlight that the location, composition and function of the normal microflora within each unique niche serves a specific homeostatic role.
2.2. Diet-induced dysbiosis and cancer
Diet-induced dysbiosis and obesity are prevalent health issues in developed countries. Morbidities associated with obesity include insulin resistance, cardiovascular disease, and an increased incidence of several types of cancers [57–60]. In a healthy individual, commensal metabolic byproducts help to stabilize commensal equilibrium, preventing the growth of inflammatory species which compete with the host for nutrients. In homeostatic conditions, commensal microbes also salvage potentially toxic nutritional byproducts such as bile, which can accumulate, cause toxicity, and in some instances, induce DNA damage leading to oncogenic transformation [61]. A previous study comparing the bacterial composition of obese and non-obese individuals found that obese individuals have significantly reduced gene richness and compositional diversity within their microbiota, with a predominance of Bacteroides spp. occurring within the dysbiotic gastrointestinal tract [62]. Functionally, this study found that commensal bacteria from obese individuals have microbial gene signatures associated with inflammation and mucosal damage, including increased proportions of inflammatory bacterial species, a reduced capacity to produce immune regulatory butyrate, an increase in mucus degrading proteins, and an increased capacity to handle oxidative stress [62]. Diet-induced changes in microbial diversity can therefore result in systemic inflammation and a disruption in homeostasis due to altered metabolite output and/or a disruption in mucosal integrity, leading to bacterial translocation and systemic distribution of shed microbial products (Figure 1), which we will discuss in greater detail below.
In mice, a high-fat diet results in changes within the composition of commensal microbes, promoting decreased production of immunoregulatory metabolites and localized inflammation within the stomach epithelium, leading to cancer [63]. Fecal transplant experiments have demonstrated that transfer of the microbiome from mice fed a high-fat diet was sufficient to increase cancer incidence in recipient animals [63], providing evidence that diet-induced changes to the composition of commensal microbes is sufficient to influence cancer progression independent of the physiologic manifestations associated with obesity. Administration of the immune regulatory short-chain fatty acid butyrate, which was reduced in people and animals with diet-induced changes to commensal equilibrium [62, 63], was also sufficient to reduce histopathology and tumor progression in animals fed a high-fat diet [63]. Butyrate directly inhibits the growth of multiple tumor types through inhibition of histone deacetylases and potent anti-inflammatory activity [64], suggesting that dietary intervention through consumption of fiber-rich foods could provide therapeutic benefit during tumor progression. The effects of butyrate will be discussed in further detail in section 4.1.
Diets high in fat and low in fiber also result in the accumulation of metabolites associated with increased toxicity. For example, obesity has been linked to increased systemic levels of deoxycholic acid [61]. Mechanistically, circulation of deoxycholic acid through the hepatic portal vein induces an inflammatory and pro-tumorigenic senescence-associated secretory phenotype in hepatic stellate cells, resulting in inflammation-induced damage of adjacent hepatic cells and resultant oncogenesis [65]. Supporting a role for this DNA-damage inducing metabolite, rats fed a diet containing high amounts of deoxycholic acid developed pre-neoplastic liver lesions [66]. Alterations in commensal microbe composition resulting from dietary changes may also influence additional bacterial metabolites as highlighted in Table 1.
Table 1:
Bacteria and associated metabolites that influence cancer and anti-tumor immunity.
| Bacterial metabolite | Bacteria that produces | Function in context of cancer | Reference |
|---|---|---|---|
| Short-chain fatty acids: butyrate, propionate, and acetate | Butyrate: Firmicutes Propionate and acetate: Bacteroidetes. |
Pro-tumor: HDAC inhibitors. Suppresses inflammation. Induces Tregs, eliminates activated T cells through upregulation of Fas. Induces production of PGE2. Anti-tumor: anti-proliferative and pro-apoptotic effects on cancer lines. Inhibits angiogenesis. Suppresses CCL2 production. |
[146, 148, 151, 163–166, 168, 170–174, 176, 177, 209] |
| Deoxycholic acid | Many | Pro-tumor: DNA damage | [182, 183, 210] |
| Superoxide | E. faecalis | Pro-tumor: DNA damage | [184] |
| Colibactin | E. coli | Pro-tumor: DNA damage | [186, 187] |
| Hydrogen sulfide | Sulfate-reducing bacteria | Pro-tumor: DNA damage | [211] |
| Polyamines | Many |
Pro-tumor: suppression of lymphocyte proliferation and IL-2 production. Promote cancer cell proliferation. Anti-tumor: protect against DNA damage. |
[188, 189, 191, 195–198, 212] |
| β-glucuronidase | Many | Pro-tumor: Increases estrogen levels, pro-tumor effects on both estrogen-receptor positive and negative cancers. | [202, 213, 214] |
| Fragilysin | B. fragilis | Pro-tumor: Stimulates cellular proliferation and E-cadherin degradation, degrades intestinal barrier function. | [215, 216] |
| Heptose-1,7-bisphosphate (HBP) | Neisseria | Pro-tumor: Activates NF-κB pathway and promotes inflammation. | [217] |
| Kynurenine | Indole-positive bacteria | Pro-tumor: Tryptophan catabolite, suppresses T cell function. | [218, 219] |
2.3. Antibiotic-induced dysbiosis
Although antibiotics have significant health benefits for individuals with microbial infections, excessive antibiotic exposure can negatively impact commensal biodiversity and immune function [67–69]. According to the CDC, greater than 5 out of 6 individuals are prescribed antibiotics annually in the United States [70], often unnecessarily. Additionally, cancer patients are frequently prescribed prophylactic antibiotics due to severe immune suppression and susceptibility to infectious diseases. Several studies have demonstrated associations between chronic/prolonged antibiotic use and increased cancer risk. In one study, women prescribed 11 or more courses of the same antibiotics had an increased association with developing breast cancer [71]. While a large-scale retrospective study of over 2 million women found only a slight increase in hazard risk of breast cancer in women using all classes of antibiotics for more than 101 days, women prescribed 3 or more courses of tetracyclines over a span of more than 101 days have a significantly greater risk of breast cancer than women given other types of antibiotics [72, 73]. Similarly, in a large multi-cancer health database survey, it was found that the risk for lung, esophageal, gastric and renal cancers significantly increases in individuals prescribed penicillins more than 5 times. The risk for lung and renal cancers is significantly associated with multiple courses of macrolides [73]. This study also confirmed that long-term exposure to tetracyclines increases the risk of breast cancer [73]. Overall, these retrospective studies suggest that although a single exposure to antibiotics does not significantly influence cancer risk, multiple and long-term exposures to single classes of antibiotics increase the incidence of numerous cancers. Although the mechanisms linking antibiotic use and cancer development and progression are unknown, one might speculate that chronic antibiotic exposure drives prolonged dysbiosis, inflammation, and tissue damage that leads to an increased risk for oncogenic transformation in susceptible tissues. However, undefined host or environmental factors may result in the need for frequent antibiotic courses and may also be associated with an increased risk of cancer. Further studies are necessary to determine whether there is a direct relationship between antibiotics and cancer.
Antibiotics, especially tetracyclines [74], are also capable of directly influencing tumor progression through the induction of mitochondrial dysfunction, increased production of reactive oxygen species (ROS), and corresponding DNA damage [75]. Antibiotics have been shown to directly influence immune cell function in addition to modulating the composition of commensal microbes, resulting in the attenuation of immune surveillance. Metastatic growth of Lewis lung carcinoma and B16F10 melanoma is enhanced in the lungs of antibiotic-treated mice which is mediated by a reduction in lung-protective IL-17-producing γδ T cells. This increase in metastatic growth was lost upon administration of recombinant IL-17 [76], demonstrating that the systemic influence of commensal microbes on the induction of immunoregulatory IL-17 T cells can serve a protective role in the establishment of anti-tumor immunity. However, other studies have demonstrated that antibiotic treatment induces the production of immune-suppressive and tumor-promoting mediators, through the induction of dysbiosis. Antibiotic-induced dysbiosis promotes the outgrowth of Candida species in the lungs, resulting in an increase in prostaglandin E2 (PGE2) production and polarization of macrophages into an M2 suppressive phenotype [77]. These studies highlight the contextual relationship between commensal microbes and the immune system. By further understanding the functional consequences of alterations to microbial diversity, we may be able to develop unique probiotic cocktails to attenuate various pathological conditions, especially in immune compromised patients that require antibiotic treatment.
2.4. Genetic mutations associated with effects on bacterial composition/tumorigenesis
Analysis of data from the Human Microbiome Project demonstrated a significant association between certain single nucleotide polymorphisms (SNP) and the composition of commensal microbes at several barrier sites [78]. Polymorphisms associated with immunological pathways and commensal-driven diseases significantly impact microbial composition [78]. This human data supports several mouse studies demonstrating that single genes associated with microbial sensing are significantly able to impact the composition of commensal microbes and overall host physiology [79–81].
Approximately 7% of the general population harbors a dominant-negative SNP mutation within the flagellin-binding domain of TLR5, resulting in ablation of TLR5 signaling [82]. This polymorphism was shown to have an influence on the outcome and survival of patients with various extramucosal malignancies [39]. Mechanistically, TLR5 signaling through interactions with commensal microbes results in systemic elevation of IL-6 and the recruitment of myeloid-derived suppressor cells (MDSC) into the tumor microenvironment. MDSCs secreting adenosine induce galectin-1 expression in γδ T cells, resulting in cumulative suppression of tumor-associated immune responses and more rapid progression of ovarian tumors and sarcomas [39]. This study was the first to suggest that genetic polymorphisms present within the human population, mediated through interactions with commensal microbes and the immune system, could influence the outcome of extramucosal cancers.
A recent study has demonstrated that retinoic acid-inducible gene-I (RIG-I), a viral RNA receptor capable of recognizing double-stranded RNA viruses, regulates the composition of commensal microbes through the production of IgA and the downstream induction of Reg3γ, an antimicrobial peptide important in controlling the composition of commensal microbes [83]. RIG-I-deficient mice have increased dysbiosis and emergence to colorectal cancer upon treatment with AOM/DSS. Clinically, these findings are relevant, as human colorectal cancer specimens have significantly reduced levels of RIG-I, suggesting that downregulation of this receptor results in dysbiosis and susceptibility to colorectal cancer [83]. Expression of NOD2 on the colonic mucosa was also found to influence the composition of commensal microbes and the inflammatory microenvironment within the intestines, leading to an increased incidence of colitis-associated cancer in NOD2-deficient mice [84]. Although most studies have focused upon the role of NOD receptors in modulating intestinal malignancies, a study using NOD-deficient breast tumor cell lines demonstrated that breast tumors lacking NOD were more resistant to TNFα-induced apoptosis and were more sensitive to estrogen-mediated tumor growth [85]. Thus, similar to what has been reported in colorectal cancer, NOD expression on the tumor epithelium may have a protective role in extramucosal cancers, such as breast cancer. However, the attenuation of tumor growth in this model is primarily mediated through NOD-dependent activation of caspase 8, resulting in apoptosis [85, 86]. Because these studies were performed using in vitro models, this mechanism may be mediated independently of commensal microbiota within the breast tissue.
3. Microbial products associated with inflammation and tumor progression
While most bacteria comprising the human microbiota are compartmentalized on the skin and in the gastrointestinal and female reproductive tracts, recent studies have demonstrated that additional sites contain regional microbiota. Organs previously considered to be sterile in healthy individuals, such as lymph nodes [87–89] and the bladder [90–93], are now known to contain bacteria under homeostatic conditions without any observable pathological consequence. Additionally, some bacterial-derived products reach systemic circulation [94, 95], which may influence tumor progression. For example, disruption in the compartmentalization of commensal microbes or enhanced microbial shedding of inflammatory ligands during pathological conditions has the potential to drive systemic tumor-promoting inflammation, induce DNA damage, and promote cellular proliferation; all of which promote the progression of extramucosal tumors.
3.1. Bacterial components circulating in the periphery
Bacterial LPS, also known as endotoxin, is the best-characterized bacterial component found in systemic circulation. A cell wall component of gram negative bacteria, LPS is the major ligand for TLR4 and stimulates proinflammatory cytokine production through binding the receptor. Additionally, LPS has pro-angiogenic effects [96–98]. In the context of wound healing, pro-inflammatory and pro-angiogenic functions of acute LPS exposure are considered beneficial, but chronic LPS-mediated inflammation could be detrimental during tumorigenesis and metastasis. Importantly, though gram negative bacteria shed LPS as a normal part of cell division, antibiotics further promote LPS shedding through their bactericidal effects [99, 100], suggesting that during dysbiosis, LPS levels may be elevated in the periphery.
In addition to promoting proinflammatory cytokine production, LPS also drives myeloid cells to produce ROS [101, 102]. The superoxide radicals produced by this process cause direct DNA damage, potentially contributing to mutations leading to cellular transformation [103]. The resultant inflammation from LPS exposure contributes both to hepatic injury and the induction of acute pancreatitis in mice [104, 105]. This inflammation accelerates the development of pancreatic cancer in mice with a genetic driver mutation in K-ras [106, 107]. As mutated K-ras has been identified in a substantial proportion of healthy individuals, increased levels of circulating endotoxin may synergize with K-ras induced transformation, driving tumorigenesis [108, 109]. Furthermore, systemic exposure to LPS enhances metastasis to both the lungs and liver in several different tumor models [110–112]. LPS may act directly on tumor cells, eliciting production of mediators that influence epithelial mesenchymal transition and promote enhanced metastasis through the induction of cell adhesion molecules that facilitate invasion, as demonstrated using in vitro cell culture spheroid models [113, 114]. Additionally, TLR4 expression associates with metastasis to distal lymph nodes in patients with breast cancer [115]. In vitro stimulation of breast tumor cell lines with LPS induces a significant increase in tumor-specific production of matrix metalloproteinases and cytokines associated with angiogenesis, such as VEGF and IL-6. In immunocompromised mice, this ultimately results in increased metastatic dissemination to the liver [115].
Another bacterial product associated with systemic inflammatory effects is flagellin. The only known ligand for TLR5, flagellin is the main protein component of flagellum on flagellated bacteria. Though it has been reported to be detectable in healthy human serum, systemic concentrations of flagellin in the serum increase in the context of inflammation and injury [116–118]. Flagellin has been reported to have both pro- and anti-tumor effects. Multiple publications have demonstrated immunosuppressive functions of flagellin and TLR5 signaling through the induction of Th2 responses [119, 120]. Dendritic cells stimulated with flagellin secrete low levels of Th1-supportive cytokines, such as IL-12p40 and p70, IL-6 and TNFα, resulting in Th2 polarization of CD4 T cells [119]. Additionally, flagellin stimulation of myeloid precursors induces the generation of CXCR4-expressing MDSCs [120]. These studies suggest that depending upon the cell type (mature DC versus immature myeloid precursor) and the immunological context of flagellin activation, stimulation through TLR5 may have varying immune modulatory effects. Flagellated bacteria are a common commensal type found on murine skin, and the presence of flagellin exacerbates tumor development in a model of skin cancer [7, 121]. In vitro, flagellin enhances proliferation and migration of several types of cancer cell lines, including multiple myeloma, gastric cancer, and salivary gland adenocarcinoma [122–124]. However, other studies have shown that flagellin promotes anti-tumor activity through suppression of cancer cell proliferation and migration [48, 125, 126]. These contrasting findings may depend on several factors, including the antigenicity of the tumor, the timing of the flagellin stimulus [127], the cytokine and immune composition of the tumor microenvironment, and perhaps even the stage of tumor initiation or progression.
Two additional bacterial components, peptidoglycan and polysaccharide A (PSA), affect systemic inflammation through the ligation of TLR2. Peptidoglycan is a bacterial cell wall component that is shed through cell division. Present in human plasma, peptidoglycan has been shown to promote invasiveness, adhesion, and pro-inflammatory cytokine production of cancer cells in vitro [128, 129]. PSA, a capsule component of Bacteroides fragilis, drives the differentiation of immunosuppressive IL-10-producing Foxp3+ regulatory T cells both in mice and humans [130–132]. The broad expression of TLRs 2, 4, and 5 on cells of various human cancer types underline the potential effects of bacteria and/or their components on the tumor microenvironment (reviewed in [133]).
3.2. Bacterial Translocation
While resident bacterial communities are present in extramucosal organs of healthy individuals, intestinal barrier permeability is additionally increased in the context of inflammation, enhancing translocation of bacteria and bacterial components from the gut to distal locations [134]. For example, intestinal permeability and systemic levels of LPS are enhanced in cases of alcohol abuse and are often detected in patients with metabolic disorders influenced by obesity [95, 135–137]. Considering the strong correlations between obesity, alcohol use, and increased cancer risk, bacterial translocation and/or systemic bacterial products likely play a large role in promoting the systemic inflammation seen in these conditions. Cancer treatments such as chemotherapy, radiation, and checkpoint blockade also result in mucosal damage, increasing intestinal permeability [94]. Total body irradiation, used to lymphodeplete recipients of both bone marrow transplants and adoptive T cell therapy, results in elevated serum LPS and proinflammatory cytokines in mice [94, 138]. Thus, while bacteria may play a role in tumorigenesis, cancer treatment may additionally exacerbate this effect by increasing bacterial translocation to extramucosal sites.
Bacterial translocation is not always associated with tumor-promoting inflammation, and may also be associated with the enhancement of anti-tumor immunity. Cyclophosphamide induces immunogenic cell death in cancer cells and promotes the differentiation of Th17 and Th1 cells which enhance therapeutic efficacy [139, 140]. Cyclophosphamide also results in increased intestinal permeability and the translocation of bacteria from the intestines to lymph organs. Unexpectedly, cyclophosphamide-induced bacterial translocation of gram positive bacterial species results in the generation of Th1 memory T cells and the differentiation of IFNγ-producing Th17 cells (Figure 1) which are critical for anti-tumor immune responses during therapy [46]. Because this study demonstrates that antibiotics diminish the efficacy of cyclophosphamide-induced tumor immune surveillance, it is important to consider how antibiotic administration during treatment with certain chemotherapies may impair therapeutic efficacy. Treatment with the checkpoint inhibitor anti-CTLA-4 is also known to induce mucosal pathology and disrupt commensal homeostasis. However, Vetizou et al. demonstrated a clear relationship between colonization with certain Bacteroides species, such as B. fragilis and B. thetaiotaomicron, and reduced mucosal pathology during treatment with a-CTLA-4. Additionally, they showed that T cells recognizing these bacterial species associate with responsiveness to therapy in both murine models and in patients with melanoma [45]. As T cells recognizing specific bacterial species mediate the efficacy of anti-CTLA-4 therapy, it is possible that shared antigens exist between commensal species and tumor neoantigens.
4. Systemic effects of commensal metabolites during cancer
Another mechanism by which bacteria contribute to systemic inflammation is through the production of metabolites. The microbiome, defined as the combined genetic material of all microbes within the human body, outnumbers human genes by roughly 150-fold [141]. This vast quantity of genetic material codes for a wide variety of different proteins that impact bacterial metabolism. Commensal bacteria have key roles in human digestion, including the extraction of nutrients and the synthesis of biologically relevant metabolites (reviewed in [142]). Several groups have shown striking differences in the concentration and composition of circulating metabolites in germ-free compared to conventional mice, demonstrating the contributions of commensal microbes to systemic metabolic products [143, 144]. This could be relevant in the development of immune function as germ-free animals have poorly developed lymphoid structures and a defect in granulopoiesis, as discussed previously [19, 20].
4.1. Short-Chain Fatty Acids
One of the best-studied families of microbial metabolites is the short-chain fatty acids (SCFA), comprised of butyrate, propionate, and acetate. Produced by anaerobic bacterial fermentation of dietary fiber, SCFAs are broadly anti-inflammatory. SCFAs are ligands for G protein-coupled receptors, and butyrate and propionate exert their anti-inflammatory effects through their actions as histone deacetylase inhibitors. These effects include the modulation of NF-кB pathways, leukocyte trafficking, and suppression of various cytokines and chemokines [145–153]. Additionally, SCFAs promote tight junction integrity in the intestines, reducing translocation of bacteria and bacterial products [154–156]. Germ-free mice have very low concentrations of SCFAs in their intestines, demonstrating the importance of commensal microbes in the production of these metabolites [157]. SCFAs have mainly been investigated for their anti-inflammatory effects on colitis [158]. However, as they reach systemic circulation after their derivation in the colon, SCFAs may have effects in the context of extramucosal cancers [159–161].
One well-known function of SCFAs is the effect they exert on populations of T cells. Butyrate promotes both the induction of regulatory T cells and their resultant production of IL-10 in addition to driving the elimination of activated T cells through the upregulation of Fas [162–166]. In the context of intestinal inflammation, these effects would be beneficial to the host. However, in the context of cancer, an increase in Tregs and concurrent decrease in effector T cells would facilitate a reduction in tumor immune surveillance and anti-tumor immunity. This effect of butyrate extends beyond the gut, as mice on a high-fiber or SFCA-supplemented diet showed both suppressed colonic inflammation as well as diminished allergic airway disease as a result of increased suppressive activity of Tregs in the lungs [167]. Additionally, SCFAs induce the production of PGE2, a potent tumor-promoting mediator, from human monocytes. This effect is enhanced in the presence of LPS, demonstrating the complex effects of commensal bacteria on systemic inflammation [168].
However, other studies have shown anti-tumor effects of butyrate and other SCFAs. For example, oral administration of dietary fiber as a prebiotic reduced the incidence of carcinogen-induced mammary cancer [169]. Several potential mechanisms of SCFAs may account for this effect. Butyrate exerts anti-proliferative and pro-apoptotic effects on several cancer cell lines in vitro [170–175], primarily through the induction of oxidative stress within tumor cells and modulation of the expression of genes associated with cellular proliferation and growth. Additionally, butyrate inhibits angiogenesis through downregulation of VEGF gene expression [176]. Another potential anti-tumor effect of butyrate is its ability to suppress CCL2 production, both in vivo and in vitro from tumor cell lines, through impairment of phosphorylation of the ERK1/2 and Akt inflammatory signaling pathways [177–179]. As CCL2 has been implicated in tumor progression and metastasis through recruitment of tumor-associated macrophages, SCFAs may inhibit this process [180]. Finally, it was demonstrated that butyrate in combination with 5-azacytidine, a DNA methyltransferase inhibitor, reduces the growth of breast cancer stem cells [181], suggesting that using probiotics to enrich for butyrate-producing bacteria or increasing the dietary consumption of fiber may have a protective role against certain types of cancers.
4.2. Metabolites that influence DNA damage
In the context of gastrointestinal cancers, some bacterial metabolites impact tumorigenesis through direct DNA damage. Commensal bacteria convert primary bile acids produced by the liver to secondary bile acids. As previously mentioned, the secondary bile acid deoxycholic acid causes DNA damage through the induction of ROS [182, 183]. Additional bacterial products, such as Enterococcus faecalis-derived superoxide and Escherichia coli-derived colibactin also induce DNA strand breaks [184–187]. Repetitive DNA damage leads to the acquisition of mutations that can drive cellular transformation and the development of cancer.
Polyamines, such as putrescine, spermidine, and spermine, are additional metabolites that are produced by commensal bacteria. Unlike the metabolites mentioned above, polyamines actively protect against DNA damage by scavenging free radicals and influencing the structure of DNA [188–192]. However, polyamines have also been implicated in the suppression of anti-tumor immune responses. Polyamines are increased in the urine and serum of cancer patients and have been shown to promote cancer cell proliferation [193–195]. Additionally, they suppress lymphocyte proliferation and IL-2 production, presumably through metabolic constraints on activated T cells, eventually resulting in decreased anti-tumor immunity [196]. This effect can be inhibited, as depletion of polyamines restricted tumor growth in a T cell-dependent mechanism [197]. Antibiotic treatment decreases polyamine concentrations, demonstrating the impact of commensal bacteria on this metabolic product [198]. Polyamines can also directly enhance the production of tumor-derived proteases and matrix metalloproteinases, resulting in increased extravasation and invasion of tumor cells. Combined with their function in immune suppression, polyamines promote enhanced tumor progression and metastasis [199].
4.3. Metabolites and hormone production
Microbes also play a significant role in the metabolism of hormones. This is particularly important in the context of hormone-receptor positive cancers. Estrogen, specifically, is one driving factor in the development of hormone-receptor positive breast cancer and promotes tumor growth [200, 201]. Estrogens are conjugated with glucuronic acid in the liver, allowing them to be excreted. Some bacteria produce an enzyme, β-glucuronidase, that deconjugates estrogen from glucuronic acid, promoting its reabsorption into circulation, thereby enhancing estrogen levels in the host [202]. In humans, differences in estrogen metabolism correlate with variability in gut microbial diversity, and antibiotic treatment associates with an increase in excretion of conjugated estrogens [203–205]. Despite its significance in hormone-receptor positive cancer, estrogen has also recently been shown to affect the progression of different estrogen-insensitive tumor models. Svoronos et al. showed that estrogen drives MDSC mobilization as well as their suppressive activity, enhancing tumor progression [206]. This demonstrates that hormones directly impact both tumor growth and immune function.
Additional bacterial metabolites have potential roles in promoting systemic inflammation and tumor progression. These are described in Table 1.
5. Conclusions and future perspectives
The microbiome is often thought of as the “forgotten organ” [207]. Work from the last twenty years has demonstrated critical roles for commensal microbes in the development and progression of many disease states, including cancer. The widespread use of antibiotics in our society makes this topic relevant in the context of cancer, as cancer patients are frequently prescribed prophylactic antibiotics to combat infections associated with chemotherapy-induced immunosuppression. While chronic antibiotic exposure increases the risk of tumorigenesis in multiple cancer types, antibiotic use also impacts commensal homeostasis, decreasing circulating bacterial components and metabolites and shaping systemic immune function. As discussed previously, many secreted bacterial factors act in context-dependent mechanisms, and depending upon the type and stage of cancer, the class of antibiotics prescribed, the duration of antibiotic exposure, and the initial composition of commensal microorganisms, antibiotic use during cancer therapy has the potential to both promote and to inhibit cancer progression. It was recently hypothesized that the impact of persistent use of antibiotics is compounded across generations, resulting in the gradual loss of diversity and emergence into dysbiotic states [67]. Thus, further understanding of the potential direct effects of antibiotics during tumor progression is required to better inform clinical decisions for the treatment of cancer patients.
In tumor-bearing individuals, antibiotics are not the only factor influencing the composition of commensal microbes. Chemotherapy influences microbial composition and induces translocation of commensal microbes from barrier surfaces due to damage of the proliferating cells within the mucosal epithelium. Depending upon the chemotherapeutic agent and the mechanisms associated with tumor-progression, commensal microbes may facilitate tumor regression or result in toxicity, as discussed previously. Multiple studies have found that certain immune therapies enhance tumor immune surveillance through triggering of innate receptors and inflammasomes with microbial products, such as LPS, complexed DNA, and cellular RNA, whereas inhibition of TLR3 and AIM2 inflammasome activation are protective against radiation-induced cytotoxicity [208]. Thus, it will be critical to understand the relevant contexts in which commensal microbes and the activation of relevant signaling pathways influence tumor progression and anti-tumor immunity.
Commensal disequilibrium results in a range of pathologies, and in the context of cancer can have a direct influence on tumor proliferation and cytokine secretion (Figure 1). Systemically, commensal products and metabolic function provoke changes to immune function that may result in either tumor-promoting inflammation or enhanced anti-tumor immunity. The role of commensal microbes during cancer is dependent upon many factors, demonstrating that a more comprehensive understanding of the responses of individual microbes and the collective ecosystem to the tumor environment is needed. Conversely, it will be important to understand how tumors and the tumor microenvironment are impacted by changes in commensal equilibrium. Several studies have already identified that certain commensal microbes are required to facilitate cancer therapy: through checkpoint inhibition of PD-1 [44] and CTLA-4 [45] and during treatment with immunostimulatory immune therapy [43] or chemotherapy [46]. These and future studies could pave the way for engineering probiotic cocktails associated with the restoration of anti-tumor immune responses.
Acknowledgments
This work was supported by the American Cancer Society IRG grant 81-001-29 and the National Cancer Institute P30 award 4P30CA044579-25. CBR is supported by the National Institutes of Health 5 T32 AI007496-22. We would like to thank Raegan R. Bostic and Tzu-Yu Feng for critical reading and editing of the manuscript.
Abbreviations:
- LPS
lipopolysaccharide
- TLR
Toll-like receptor
- NKT cells
natural killer T cells
- NOD
nucleotide-binding oligomerization domain–containing protein
- SNP
single nucleotide polymorphism
- MDSC
myeloid-derived suppressor cell
- PSA
polysaccharide A
- RIG-I
retinoic acid-inducible gene-I
- SCFA
short-chain fatty acid
- CDC
Centers for Disease Control and Prevention
- PGE2
prostaglandin E2
References
- [1].Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R, Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis, Cell 118(2) (2004) 229–241. [DOI] [PubMed] [Google Scholar]
- [2].Mazmanian SK, Round JL, Kasper DL, A microbial symbiosis factor prevents intestinal inflammatory disease, Nature 453(7195) (2008) 620–625. [DOI] [PubMed] [Google Scholar]
- [3].Silva MJ, Carneiro MB, dos Anjos Pultz B, Pereira Silva D, Lopes ME, dos Santos LM, The multifaceted role of commensal microbiota in homeostasis and gastrointestinal diseases, J Immunol Res 2015 (2015) 321241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D, Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis, Mucosal immunology 3(2) (2010) 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D, Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine, The Journal of experimental medicine 205(10) (2008) 2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nakamizo S, Egawa G, Honda T, Nakajima S, Belkaid Y, Kabashima K, Commensal bacteria and cutaneous immunity, Commensal bacteria and cutaneous immunity, Springer, 2015, pp. 73–80. [DOI] [PubMed] [Google Scholar]
- [7].Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y, Compartmentalized control of skin immunity by resident commensals, Science 337(6098) (2012) 1115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whiteside SA, Razvi H, Dave S, Reid G, The microbiome of the urinary tract [mdash] a role beyond infection, Nature Reviews … (2015). [DOI] [PubMed]
- [9].Mirmonsef P, Gilbert D, Zariffard MR, The effects of commensal bacteria on innate immune responses in the female genital tract, American journal of … (2011). [DOI] [PMC free article] [PubMed]
- [10].Avila M, Ojcius DM, Yilmaz Ö, The oral microbiota: living with a permanent guest, DNA and cell biology (2009). [DOI] [PMC free article] [PubMed]
- [11].Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R, The oral metagenome in health and disease, The ISME … (2012). [DOI] [PMC free article] [PubMed]
- [12].Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO, Disordered microbial communities in asthmatic airways, PLoS One 5(1) (2010) e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clarke TB, Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands, Infection and immunity (2014). [DOI] [PMC free article] [PubMed]
- [14].Koch MA, Reiner GL, Lugo KA, Kreuk LS, Stanbery AG, Ansaldo E, Seher TD, Ludington WB, Barton GM, Maternal IgG and IgA Antibodies Dampen Mucosal T Helper Cell Responses in Early Life, Cell 165(4) (2016) 827–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dimmitt RA, Staley EM, Chuang G, The role of postnatal acquisition of the intestinal microbiome in the early development of immune function, Journal of pediatric … (2010). [DOI] [PMC free article] [PubMed]
- [16].Goris H, Boer DF, der Waaij VD, Myelopoiesis in experimentally contaminated specific-pathogen-free and germfree mice during oral administration of polymyxin, Infection and immunity (1985). [DOI] [PMC free article] [PubMed]
- [17].Tada T, Yamamura S, Kuwano Y, Abo T, Level of myelopoiesis in the bone marrow is influenced by intestinal flora, Cellular immunology (1996). [DOI] [PubMed]
- [18].Nicaise AGP, Sandre C, Forestier F, Kergot R, Quero A-M, and Labarre C, Influence of intestinal microflora on murine bone marrow and spleen macrophage precursors, Scand. J. Immunol 48 (1998) 585–591. [DOI] [PubMed] [Google Scholar]
- [19].Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Gut microbiota promote hematopoiesis to control bacterial infection, Cell host & … (2014). [DOI] [PMC free article] [PubMed]
- [20].Balmer ML, Schürch CM, Saito Y, Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling, The Journal of … (2014). [DOI] [PubMed]
- [21].Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN, Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity, Nature Medicine 16(2) (2010) 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abt M, Osborne L, Monticelli L, Doering T, Alenghat T, Sonnenberg G, Paley M, Antenus M, Williams K, Erikson J, Wherry E, Artis D, Commensal bacteria calibrate the activation threshold of innate antiviral immunity, Immunity 37(1) (2012) 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A, Microbiota regulates immune defense against respiratory tract influenza A virus infection, Proceedings of the National Academy of Sciences of the United States of America 108(13) (2011) 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lathrop S, Bloom S, Rao S, Nutsch K, Lio C-W, Santacruz N, Peterson D, Stappenbeck T, Hsieh C-S, Peripheral education of the immune system by colonic commensal microbiota, Nature 478(7368) (2011) 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, Deroos P, Liu H, Cross J, Pfeffer K, Coffer P, Rudensky A, Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation, Nature (2013). [DOI] [PMC free article] [PubMed]
- [26].Furusawa Y, Obata Y, Fukuda S, Endo T, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda N, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke J, Topping D, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H, Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells, Nature (2013). [DOI] [PubMed]
- [27].Shaw MH, Kamada N, Kim Y-GG, Núñez G, Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine, The Journal of experimental medicine 209(2) (2012) 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ivanov I, Atarashi K, Manel N, Brodie E, Shima T, Karaoz U, Wei D, Goldfarb K, Santee C, Lynch S, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman D, Induction of intestinal Th17 cells by segmented filamentous bacteria, Cell 139(3) (2009) 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duan J, Chung H, Troy E, Kasper DL, Microbial Colonization Drives Expansion of IL-1 Receptor 1-Expressing and IL-17-Producing γ/δ T Cells, Cell Host & Microbe 7(2) (2010) 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Olszak T, An D, Zeissig S, Vera M, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS, Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function, Science 336(6080) (2012) 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wei B, Wingender G, Fujiwara D, Chen D, McPherson M, Brewer S, Borneman J, Kronenberg M, Braun J, Commensal Microbiota and CD8+ T Cells Shape the Formation of Invariant NKT Cells, The Journal of Immunology 184(3) (2010) 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A, Fu J, Joosten LA, Zhernakova A, Huttenhower C, Wijmenga C, Netea MG, Xavier RJ, Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity, Cell 167(4) (2016) 1125–1930850304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gagniere J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, Bringer MA, Pezet D, Bonnet M, Gut microbiota imbalance and colorectal cancer, World J Gastroenterol 22(2) (2016) 501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xuan C, Shamonki JM, Chung A, DiNome ML, Microbial dysbiosis is associated with human breast cancer, PloS one (2014). [DOI] [PMC free article] [PubMed]
- [35].Chase D, Goulder A, Zenhausern F, Monk B, The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment, Gynecologic … (2015). [DOI] [PubMed]
- [36].Zeng XT, Deng AP, Li C, Xia LY, Niu YM, Leng WD, Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies, PLoS One 8(10) (2013) e79017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, Falk RT, Gail MH, Shi J, Ravel J, Feigelson HS, Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study, Journal of the National Cancer Institute 107(8) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cozen W, Yu G, Gail MH, Ridaura VK, Nathwani BN, Hwang AE, Hamilton AS, Mack TM, Gordon JI, Goedert JJ, Fecal microbiota diversity in survivors of adolescent/young adult Hodgkin lymphoma: a study of twins, Br J Cancer 108(5) (2013) 1163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG, Zhang R, Salatino M, Tchou J, Rabinovich GA, Conejo-Garcia JR, Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation, Cancer cell 27(1) (2015) 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grivennikov S, Wang K, Mucida D, Stewart C, Schnabl B, Jauch D, Taniguchi K, Yu G-Y, Osterreicher C, Hung K, Datz C, Feng Y, Fearon E, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M, Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth, Nature 491(7423) (2012) 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M, IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer, Cancer Cell 15(2) (2009) 103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, Gallini CA, Beal C, van Hylckama-Vlieg JE, Ballal SA, Morgan XC, Glickman JN, Gevers D, Huttenhower C, Garrett WS, Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission, ISME J 8(7) (2014) 1403–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS, Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment, Science 342(6161) (2013) 967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF, Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy, Science 350(6264) (2015) 1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L, Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota, Science 350(6264) (2015) 1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L, The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide, Science 342(6161) (2013) 971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Littman DR, Pamer EG, Role of the commensal microbiota in normal and pathogenic host immune responses, Cell host & microbe (2011). [DOI] [PMC free article] [PubMed]
- [48].Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D, Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth, Cancer Res 71(7) (2011) 2466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shi M, Yao Y, Han F, Li Y, Li Y, MAP1S controls breast cancer cell TLR5 signaling pathway and promotes TLR5 signaling-based tumor suppression, PLoS One 9(1) (2014) e86839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G, The Microbiota of Breast Tissue and Its Association with Breast Cancer, Appl Environ Microbiol 82(16) (2016) 5039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, Xiao J, Radisky DC, Knutson KL, Kalari KR, Yao JZ, Baddour LM, Chia N, Degnim AC, The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease, Sci Rep 6 (2016) 30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bashir A, Miskeen AY, Hazari YM, Asrafuzzaman S, Fazili KM, Fusobacterium nucleatum, inflammation, and immunity: the fire within human gut, Tumour Biol 37(3) (2016) 2805–10. [DOI] [PubMed] [Google Scholar]
- [53].Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS, Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment, Cell Host Microbe 14(2) (2013) 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Walther-Antonio MR, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, Keeney GL, Creedon DJ, Nelson H, Mariani A, Chia N, Potential contribution of the uterine microbiome in the development of endometrial cancer, Genome Med 8(1) (2016) 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Borgdorff H, Gautam R, Armstrong SD, Xia D, Ndayisaba GF, van Teijlingen NH, Geijtenbeek TB, Wastling JM, van de Wijgert JH, Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier, Mucosal Immunol 9(3) (2016) 621–33. [DOI] [PubMed] [Google Scholar]
- [56].Anderson BL, Cu-Uvin S, Raker CA, Fitzsimmons C, Hillier SL, Subtle perturbations of genital microflora alter mucosal immunity among low-risk pregnant women, Acta Obstet Gynecol Scand 90(5) (2011) 510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Scholz C, Andergassen U, Hepp P, Schindlbeck C, Friedl TWP, Harbeck N, Kiechle M, Sommer H, Hauner H, Friese K, Obesity as an independent risk factor for decreased survival in node-positive high-risk breast cancer, Breast cancer research and treatment 151(3) (2015) 569–576. [DOI] [PubMed] [Google Scholar]
- [58].Liu Z, Zhang TT, Zhao JJ, Qi SF, Du P, Liu DW, Tian QB, The association between overweight, obesity and ovarian cancer: a meta-analysis, Jpn J Clin Oncol 45(12) (2015) 1107–15. [DOI] [PubMed] [Google Scholar]
- [59].Onstad MA, Schmandt RE, Lu KH, Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment, J Clin Oncol 34(35) (2016) 4225–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jochem C, Leitzmann M, Obesity and Colorectal Cancer, Recent Results Cancer Res 208 (2016) 17–41. [DOI] [PubMed] [Google Scholar]
- [61].Ridlon JM, Kang D-JJ, Hylemon PB, Bile salt biotransformations by human intestinal bacteria, Journal of lipid research 47(2) (2006) 241–259. [DOI] [PubMed] [Google Scholar]
- [62].Chatelier LE, Nielsen T, Qin J, Prifti E, Hildebrand F, Richness of human gut microbiome correlates with metabolic markers, Nature (2013). [DOI] [PubMed]
- [63].Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, Salinas-Riester G, Bock A, Alpert C, Blaut M, Polson SC, Brandl L, Kirchner T, Greten FR, Polson SW, Arkan MC, High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity, Nature 514(7523) (2014) 508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Canani R, Costanzo M, Leone L, The epigenetic effects of butyrate: potential therapeutic implications for clinical practice, Clinical Epigenetics 4(1) (2012) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N, Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome, Nature 499(7456) (2013) 97–101. [DOI] [PubMed] [Google Scholar]
- [66].Kitazawa S, Denda A, Tsutsumi M, Tsujiuchi T, Hasegawa K, Tamura K, Maruyama H, Konishi Y, Enhanced preneoplastic liver lesion development under ‘selection pressure’ conditions after administration of deoxycholic or lithocholic acid in the initiation phase in rats, Carcinogenesis 11(8) (1990) 1323–8. [DOI] [PubMed] [Google Scholar]
- [67].Blaser MJ, Antibiotic use and its consequences for the normal microbiome, Science 352(6285) (2016) 544–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Keeney KM, Yurist-Doutsch S, Arrieta MC, Finlay BB, Effects of antibiotics on human microbiota and subsequent disease, Annu Rev Microbiol 68 (2014) 217–35. [DOI] [PubMed] [Google Scholar]
- [69].Peterson CT, Sharma V, Elmén L, Peterson SN, Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota, Clinical and experimental immunology 179(3) (2015) 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH, Schrag SJ, US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 60(9) (2015) 1308–1316. [DOI] [PubMed] [Google Scholar]
- [71].Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH, Antibiotic use in relation to the risk of breast cancer, JAMA 291(7) (2004) 827–35. [DOI] [PubMed] [Google Scholar]
- [72].Friedman GD, Oestreicher N, Chan J, Quesenberry CP Jr., Udaltsova N, Habel LA, Antibiotics and risk of breast cancer: up to 9 years of follow-up of 2.1 million women, Cancer Epidemiol Biomarkers Prev 15(11) (2006) 2102–6. [DOI] [PubMed] [Google Scholar]
- [73].Boursi B, Mamtani R, Haynes K, Yang YX, Recurrent antibiotic exposure may promote cancer formation--Another step in understanding the role of the human microbiota?, Eur J Cancer 51(17) (2015) 2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, Jovaisaite V, Frochaux MV, Quiros PM, Deplancke B, Houtkooper RH, Auwerx J, Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research, Cell Rep (2015). [DOI] [PMC free article] [PubMed]
- [75].Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS, Collins JJ, Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells, Sci Transl Med 5(192) (2013) 192ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cheng M, Qian L, Shen G, Bian G, Xu T, Xu W, Shen G, Hu S, Microbiota modulate tumoral immune surveillance in lung through a gammadeltaT17 immune cell-dependent mechanism, Cancer Res 74(15) (2014) 4030–41. [DOI] [PubMed] [Google Scholar]
- [77].Kim Y-GG, Udayanga KG, Totsuka N, Weinberg JB, Núñez G, Shibuya A, Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE₂, Cell host & microbe 15(1) (2014) 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, Spector TD,Keinan A, Ley RE, Gevers D, Clark AG, Host genetic variation impacts microbiome composition across human body sites, Genome Biol 16 (2015) 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT, Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5, Science 328(5975) (2010) 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA, Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity, Nature 482(7384) (2012) 179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH, Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system, Cell 131(1) (2007) 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, Ozinsky A, Smith KD, Aderem A, A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires’ disease, J Exp Med 198(10) (2003) 1563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhu H, Xu WY, Hu Z, Zhang H, Shen Y, Lu S, Wei C, Wang ZG, RNA virus receptor Rig-I monitors gut microbiota and inhibits colitis-associated colorectal cancer, J Exp Clin Cancer Res 36(1) (2017) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, Gaillot O, Schreiber S, Lemoine Y, Ryffel B, Hot D, Nunez G, Chen G, Rosenstiel P, Chamaillard M, NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer, J Clin Invest 123(2) (2013) 700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].da Correia J, Miranda Y, Austin-Brown N, Hsu J, Mathison J, Xiang R, Zhou H, Li Q, Han J, Ulevitch RJ, Nod1-dependent control of tumor growth, Proceedings of the National Academy of Sciences of the United States of America 103(6) (2006) 1840–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].da Silva Correia J, Miranda Y, Leonard N, Hsu J, Ulevitch RJ, Regulation of Nod1-mediated signaling pathways, Cell Death Differ 14(4) (2007) 830–9. [DOI] [PubMed] [Google Scholar]
- [87].Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, Kagiyama Y, Nochi T, Yuki Y, Fukuyama Y, Mukai A, Shinzaki S, Fujihashi K, Sasakawa C, Iijima H, Goto M, Umesaki Y, Benno Y, Kiyono H, Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis, Proceedings of the National Academy of Sciences of the United States of America 107(16) (2010) 7419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D, Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria, Science (New York, N.Y.) 336(6086) (2012) 1321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Nunez G, Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens, Immunity 44(3) (2016) 647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dong Q, Nelson DE, Toh E, Diao L, Gao X, Fortenberry JD, Van der Pol B, The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens, PloS one 6(5) (2011) e19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, Huang ST, Ljungberg I, Sprague BM, Lucas SK, Torralba M, Nelson KE, Groah SL, Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury, Journal of translational medicine 10 (2012) 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC, Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder, Journal of clinical microbiology 52(3) (2014) 871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi JR, Drake MJ, The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults, Frontiers in cellular and infection microbiology 3 (2013) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP, Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling, The Journal of clinical investigation 117(8) (2007) 2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Fukui H, Brauner B, Bode JC, Bode C, Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay, Journal of hepatology 12(2) (1991) 162–9. [DOI] [PubMed] [Google Scholar]
- [96].Li WW, Grayson G, Folkman J, D’Amore PA, Sustained-release endotoxin. A model for inducing corneal neovascularization, Investigative ophthalmology & visual science 32(11) (1991) 2906–11. [PubMed] [Google Scholar]
- [97].Mattsby-Baltzer I, Jakobsson A, Sorbo J, Norrby K, Endotoxin is angiogenic, International journal of experimental pathology 75(3) (1994) 191–6. [PMC free article] [PubMed] [Google Scholar]
- [98].Harmey JH, Bucana CD, Lu W, Byrne AM, McDonnell S, Lynch C, Bouchier-Hayes D, Dong Z, Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion, International journal of cancer 101(5) (2002) 415–22. [DOI] [PubMed] [Google Scholar]
- [99].Tanabe S, Yoshioka M, Hinode D, Grenier D, Subinhibitory concentrations of tetracyclines induce lipopolysaccharide shedding by Porphyromonas gingivalis and modulate the host inflammatory response, J Periodontal Res 49(5) (2014) 603–8. [DOI] [PubMed] [Google Scholar]
- [100].Eng RH, Smith SM, Fan-Havard P, Ogbara T, Effect of antibiotics on endotoxin release from gram-negative bacteria, Diagn Microbiol Infect Dis 16(3) (1993) 185–9. [DOI] [PubMed] [Google Scholar]
- [101].Hsu HY, Wen MH, Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression, The Journal of biological chemistry 277(25) (2002) 22131–9. [DOI] [PubMed] [Google Scholar]
- [102].Simon F, Fernandez R, Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells, Journal of hypertension 27(6) (2009) 1202–16. [DOI] [PubMed] [Google Scholar]
- [103].Glukhov IL, Sirota NP, Kuznetsova EA, DNA damage in human mononuclear cells induced by bacterial endotoxin, Bulletin of experimental biology and medicine 146(3) (2008) 301–3. [DOI] [PubMed] [Google Scholar]
- [104].Monden K, Arii S, Itai S, Sasaoki T, Adachi Y, Funaki N, Higashitsuji H, Tobe T, Enhancement and hepatocyte-modulating effect of chemical mediators and monokines produced by hepatic macrophages in rats with induced sepsis, Research in experimental medicine. Zeitschrift fur die gesamte experimentelle Medizin einschliesslich experimenteller Chirurgie 191(3) (1991) 177–87. [DOI] [PubMed] [Google Scholar]
- [105].Ding SP, Li JC, Jin C, A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide, World journal of gastroenterology 9(3) (2003) 584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y, Badar S, Hajdu CH, Frey AB, Bar-Sagi D, Miller G, MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells, The Journal of experimental medicine 209(9) (2012) 1671–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, Cruz-Monserrate Z, Wang H, Ji B, Logsdon CD, An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice, The Journal of clinical investigation 122(4) (2012) 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yan L, McFaul C, Howes N, Leslie J, Lancaster G, Wong T, Threadgold J, Evans J, Gilmore I, Smart H, Lombard M, Neoptolemos J, Greenhalf W, Molecular analysis to detect pancreatic ductal adenocarcinoma in high-risk groups, Gastroenterology 128(7) (2005) 2124–30. [DOI] [PubMed] [Google Scholar]
- [109].Yakubovskaya MS, Spiegelman V, Luo FC, Malaev S, Salnev A, Zborovskaya I, Gasparyan A, Polotsky B, Machaladze Z, Trachtenberg AC, et al. , High frequency of K-ras mutations in normal appearing lung tissues and sputum of patients with lung cancer, International journal of cancer 63(6) (1995) 810–4. [DOI] [PubMed] [Google Scholar]
- [110].Yan L, Cai Q, Xu Y, The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis, Clinical cancer research : an official journal of the American Association for Cancer Research 19(17) (2013) 4706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pidgeon GP, Harmey JH, Kay E, Da Costa M, Redmond HP, Bouchier-Hayes DJ, The role of endotoxin/lipopolysaccharide in surgically induced tumour growth in a murine model of metastatic disease, British journal of cancer 81(8) (1999) 1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA, Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro, Journal of the National Cancer Institute 88(3–4) (1996) 198–205. [DOI] [PubMed] [Google Scholar]
- [113].Simiantonaki N, Jayasinghe C, Kirkpatrick CJ, Effect of pro-inflammatory stimuli on tumor cell-mediated induction of endothelial cell adhesion molecules in vitro, Experimental and molecular pathology 73(1) (2002) 46–53. [DOI] [PubMed] [Google Scholar]
- [114].Simiantonaki N, Jayasinghe C, Kirkpatrick CJ, Differential endothelial CAM-expression after stimulation with supernatants of LPS- and cytokine-stimulated HT-29 and ST-ML-12 tumor cells growing as monolayer cultures and multicellular spheroids, Anticancer research 22(5) (2002) 2641–9. [PubMed] [Google Scholar]
- [115].Yang H, Wang B, Wang T, Xu LJ, He CY, Wen HY, Yan J, Su HH, Zhu XM, Toll-Like Receptor 4 Prompts Human Breast Cancer Cells Invasiveness via Lipopolysaccharide Stimulation and Is Overexpressed in Patients with Lymph Node Metastasis, Plos One 9(10) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Svard J, Paquin-Proulx D, Buggert M, Noyan K, Barqasho B, Sonnerborg A, Nowak P, Role of translocated bacterial flagellin in monocyte activation among individuals with chronic HIV-1 infection, Clinical immunology (Orlando, Fla.) 161(2) (2015) 180–9. [DOI] [PubMed] [Google Scholar]
- [117].Ziegler TR, Luo M, Estivariz CF, Moore DA 3rd, Sitaraman SV, Hao L, Bazargan N, Klapproth JM, Tian J, Galloway JR, Leader LM, Jones DP, Gewirtz AT, Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome, American journal of physiology. Regulatory, integrative and comparative physiology 294(2) (2008) R402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Grimes L, Doyle A, Miller AL, Pyles RB, Olah G, Szabo C, Hoskins S, Eaves-Pyles T, Intraluminal Flagellin Differentially Contributes to Gut Dysbiosis and Systemic Inflammation following Burn Injury, PloS one 11(12) (2016) e0166770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Kraehenbuhl JP, Sirard JC, Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response, J Immunol 172(11) (2004) 6922–30. [DOI] [PubMed] [Google Scholar]
- [120].Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schafer I, Wecker I, Neri D, Wirth A, Mays L, Zundel S, Fuchs J, Handgretinger R, Stern M, Hogardt M, Doring G, Riethmuller J, Kormann M, Hartl D, Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease, J Immunol 190(3) (2013) 1276–84. [DOI] [PubMed] [Google Scholar]
- [121].Hoste E, Arwert EN, Lal R, South AP, Salas-Alanis JC, Murrell DF, Donati G, Watt FM, Innate sensing of microbial products promotes wound-induced skin cancer, Nature communications 6 (2015) 5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Cho HY, Lee SW, TLR5 activation by flagellin induces doxorubicin resistance via interleukin-6 (IL-6) expression in two multiple myeloma cells, Cellular immunology 289(1–2) (2014) 27–35. [DOI] [PubMed] [Google Scholar]
- [123].Song EJ, Kang MJ, Kim YS, Kim SM, Lee SE, Kim CH, Kim DJ, Park JH, Flagellin promotes the proliferation of gastric cancer cells via the Toll-like receptor 5, International journal of molecular medicine 28(1) (2011) 115–9. [DOI] [PubMed] [Google Scholar]
- [124].Park JH, Yoon HE, Kim DJ, Kim SA, Ahn SG, Yoon JH, Toll-like receptor 5 activation promotes migration and invasion of salivary gland adenocarcinoma, Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 40(2) (2011) 187–93. [DOI] [PubMed] [Google Scholar]
- [125].Rhee SH, Im E, Pothoulakis C, Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer, Gastroenterology 135(2) (2008) 518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhou H, Chen JH, Hu J, Luo YZ, Li F, Xiao L, Zhong MZ, High expression of Toll-like receptor 5 correlates with better prognosis in non-small-cell lung cancer: an anti-tumor effect of TLR5 signaling in non-small cell lung cancer, Journal of cancer research and clinical oncology 140(4) (2014) 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sfondrini L, Rossini A, Besusso D, Merlo A, Tagliabue E, Menard S, Balsari A, Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer, Journal of immunology (Baltimore, Md. : 1950) 176(11) (2006) 6624–30. [DOI] [PubMed] [Google Scholar]
- [128].Xu XL, Lee RT, Fang HM, Wang YM, Li R, Zou H, Zhu Y, Wang Y, Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p, Cell host & microbe 4(1) (2008) 28–39. [DOI] [PubMed] [Google Scholar]
- [129].Xie W, Huang Y, Xie W, Guo A, Wu W, Bacteria peptidoglycan promoted breast cancer cell invasiveness and adhesiveness by targeting toll-like receptor 2 in the cancer cells, PloS one 5(5) (2010) e10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK, The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota, Science (New York, N.Y.) 332(6032) (2011) 974–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Round JL, Mazmanian SK, Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota, Proceedings of the National Academy of Sciences of the United States of America 107(27) (2010) 12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, Begum-Haque S, Kasper DL, Kasper LH, A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function, Gut microbes 6(4) (2015) 234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sato Y, Goto Y, Narita N, Hoon DS, Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment, Cancer microenvironment : official journal of the International Cancer Microenvironment Society 2 Suppl 1 (2009) 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hietbrink F, Besselink MG, Renooij W, de Smet MB, Draisma A, van der Hoeven H, Pickkers P, Systemic inflammation increases intestinal permeability during experimental human endotoxemia, Shock 32(4) (2009) 374–8. [DOI] [PubMed] [Google Scholar]
- [135].Bode C, Kugler V, Bode JC, Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess, Journal of hepatology 4(1) (1987) 8–14. [DOI] [PubMed] [Google Scholar]
- [136].Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Fruhbeck G, Burcelin R, Fernandez-Real JM, Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance, International journal of obesity (2005) 36(11) (2012) 1442–9. [DOI] [PubMed] [Google Scholar]
- [137].Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M, Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes, Molecular and cellular biochemistry 388(1–2) (2014) 203–10. [DOI] [PubMed] [Google Scholar]
- [138].Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP, Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells, The Journal of experimental medicine 202(7) (2005) 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L, Immunomodulatory effects of cyclophosphamide and implementations for vaccine design, Semin Immunopathol 33(4) (2011) 369–83. [DOI] [PubMed] [Google Scholar]
- [140].Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, Soria JC, Marty V, Vielh P, Robert C, Chaput N, Zitvogel L, Cyclophosphamide induces differentiation of Th17 cells in cancer patients, Cancer Res 71(3) (2011) 661–5. [DOI] [PubMed] [Google Scholar]
- [141].Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J, A human gut microbial gene catalogue established by metagenomic sequencing, Nature 464(7285) (2010) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Brestoff JR, Artis D, Commensal bacteria at the interface of host metabolism and the immune system, Nat Immunol 14(7) (2013) 676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G, Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites, Proceedings of the National Academy of Sciences of the United States of America 106(10) (2009) 3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Boren J, Oresic M, Backhed F, The gut microbiota modulates host energy and lipid metabolism in mice, Journal of lipid research 51(5) (2010) 1101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ, The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids, The Journal of biological chemistry 278(13) (2003) 11312–9. [DOI] [PubMed] [Google Scholar]
- [146].Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA, Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts, Gut 52(10) (2003) 1442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, Biagi E, Andersen MH, Brigidi P, Odum N, Litman T, Woetmann A, The effect of short-chain fatty acids on human monocyte-derived dendritic cells, Scientific reports 5 (2015) 16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, Kotani J, Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells, Nutrition research (New York, N.Y.) 28(5) (2008) 321–8. [DOI] [PubMed] [Google Scholar]
- [149].Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R, Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils, The Journal of nutritional biochemistry 22(9) (2011) 849–55. [DOI] [PubMed] [Google Scholar]
- [150].Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly YM, Stephens L, Hawkins PT, Curi R, SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor, PloS one 6(6) (2011) e21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Chang PV, Hao L, Offermanns S, Medzhitov R, The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition, Proceedings of the National Academy of Sciences of the United States of America 111(6) (2014) 2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V, Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases, The Journal of biological chemistry 285(36) (2010) 27601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ, Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis, Nature medicine 20(2) (2014) 159–66. [DOI] [PubMed] [Google Scholar]
- [154].Kinoshita M, Suzuki Y, Saito Y, Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation, Biochemical and biophysical research communications 293(2) (2002) 827–31. [DOI] [PubMed] [Google Scholar]
- [155].Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H, Bifidobacteria can protect from enteropathogenic infection through production of acetate, Nature 469(7331) (2011) 543–7. [DOI] [PubMed] [Google Scholar]
- [156].Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP, Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function, Cell host & microbe 17(5) (2015) 662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Hoverstad T, Midtvedt T, Short-chain fatty acids in germfree mice and rats, The Journal of nutrition 116(9) (1986) 1772–6. [DOI] [PubMed] [Google Scholar]
- [158].Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR, Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43, Nature 461(7268) (2009) 1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].van der Beek CM, Bloemen JG, van den Broek MA, Lenaerts K, Venema K, Buurman WA, Dejong CH, Hepatic Uptake of Rectally Administered Butyrate Prevents an Increase in Systemic Butyrate Concentrations in Humans, The Journal of nutrition 145(9) (2015) 2019–24. [DOI] [PubMed] [Google Scholar]
- [160].Nilsson AC, Ostman EM, Knudsen KE, Holst JJ, Bjorck IM, A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning, The Journal of nutrition 140(11) (2010) 1932–6. [DOI] [PubMed] [Google Scholar]
- [161].Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, Delcour JA, Verbeke KA, Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study, The Journal of physiology 595(2) (2017) 541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V, Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis, Immunity 40(1) (2014) 128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS, The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis, Science (New York, N.Y.) 341(6145) (2013) 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H, Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells, Nature 504(7480) (2013) 446–50. [DOI] [PubMed] [Google Scholar]
- [165].Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY, Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation, Nature 504(7480) (2013) 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K, Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells, American journal of physiology. Gastrointestinal and liver physiology 302(12) (2012) G1405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, Chevalier N, Tan JK, Marino E, Moore RJ, Wong L, McConville MJ, Tull DL, Wood LG, Murphy VE, Mattes J, Gibson PG, Mackay CR, Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites, Nature communications 6 (2015) 7320. [DOI] [PubMed] [Google Scholar]
- [168].Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou CC, Lundell D, Jenh CH, Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines, World journal of gastroenterology 15(44) (2009) 5549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kassayova M, Bobrov N, Strojny L, Kiskova T, Mikes J, Demeckova V, Orendas P, Bojkova B, Pec M, Kubatka P, Bomba A, Preventive effects of probiotic bacteria Lactobacillus plantarum and dietary fiber in chemically-induced mammary carcinogenesis, Anticancer research 34(9) (2014) 4969–75. [PubMed] [Google Scholar]
- [170].Bonnotte B, Favre N, Reveneau S, Micheau O, Droin N, Garrido C, Fontana A, Chauffert B, Solary E, Martin F, Cancer cell sensitization to fas-mediated apoptosis by sodium butyrate, Cell death and differentiation 5(6) (1998) 480–7. [DOI] [PubMed] [Google Scholar]
- [171].Fung KY, Brierley GV, Henderson S, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove L, Butyrate-induced apoptosis in HCT116 colorectal cancer cells includes induction of a cell stress response, Journal of proteome research 10(4) (2011) 1860–9. [DOI] [PubMed] [Google Scholar]
- [172].Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA, The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation, The Journal of nutrition 132(5) (2002) 1012–7. [DOI] [PubMed] [Google Scholar]
- [173].Kim YH, Park JW, Lee JY, Kwon TK, Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of transcription from the DR5 gene promoter through Sp1 sites in colon cancer cells, Carcinogenesis 25(10) (2004) 1813–20. [DOI] [PubMed] [Google Scholar]
- [174].Qiu J, Gao Z, Shima H, Growth of human prostate cancer cells is significantly suppressed in vitro with sodium butyrate through apoptosis, Oncology reports 27(1) (2012) 160–7. [DOI] [PubMed] [Google Scholar]
- [175].Mandal M, Kumar R, Bcl-2 expression regulates sodium butyrate-induced apoptosis in human MCF-7 breast cancer cells, Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research 7(3) (1996) 311–8. [PubMed] [Google Scholar]
- [176].Prasanna Kumar S, Thippeswamy G, Sheela ML, Prabhakar BT, Salimath BP, Butyrate-induced phosphatase regulates VEGF and angiogenesis via Sp1, Archives of biochemistry and biophysics 478(1) (2008) 85–95. [DOI] [PubMed] [Google Scholar]
- [177].Pulliam SR, Pellom ST Jr., Shanker A, Adunyah SE, Butyrate regulates the expression of inflammatory and chemotactic cytokines in human acute leukemic cells during apoptosis, Cytokine 84 (2016) 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Aguilar EC, Leonel AJ, Teixeira LG, Silva AR, Silva JF, Pelaez JM, Capettini LS, Lemos VS, Santos RA, Alvarez-Leite JI, Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFkappaB activation, Nutrition, metabolism, and cardiovascular diseases : NMCD 24(6) (2014) 606–13. [DOI] [PubMed] [Google Scholar]
- [179].Simeoli R, Mattace Raso G, Pirozzi C, Lama A, Santoro A, Russo R, Montero-Melendez T, Berni Canani R, Calignano A, Perretti M, Meli R, An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis, British journal of pharmacology (2016). [DOI] [PMC free article] [PubMed]
- [180].Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW, CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis, Nature 475(7355) (2011) 222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Pathania R, Ramachandran S, Mariappan G, Thakur P, Shi H, Choi J-H, Manicassamy S, Kolhe R, Prasad PD, Sharma S, Combined inhibition of DNMT and HDAC blocks the tumorigenicity of cancer stem-like cells and attenuates mammary tumor growth, Cancer research 76(11) (2016) 3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Bernstein H, Payne CM, Bernstein C, Schneider J, Beard SE, Crowley CL, Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate, Toxicology letters 108(1) (1999) 37–46. [DOI] [PubMed] [Google Scholar]
- [183].Craven PA, Pfanstiel J, DeRubertis FR, Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation, The Journal of clinical investigation 77(3) (1986) 850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Huycke MM, Moore DR, In vivo production of hydroxyl radical by Enterococcus faecalis colonizing the intestinal tract using aromatic hydroxylation, Free radical biology & medicine 33(6) (2002) 818–26. [DOI] [PubMed] [Google Scholar]
- [185].Wang X, Huycke MM, Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells, Gastroenterology 132(2) (2007) 551–61. [DOI] [PubMed] [Google Scholar]
- [186].Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E, Escherichia coli induces DNA double-strand breaks in eukaryotic cells, Science (New York, N.Y.) 313(5788) (2006) 848–51. [DOI] [PubMed] [Google Scholar]
- [187].Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrede JP, Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells, Proceedings of the National Academy of Sciences of the United States of America 107(25) (2010) 11537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Feuerstein BG, Pattabiraman N, Marton LJ, Molecular mechanics of the interactions of spermine with DNA: DNA bending as a result of ligand binding, Nucleic acids research 18(5) (1990) 1271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Fujisawa S, Kadoma Y, Kinetic evaluation of polyamines as radical scavengers, Anticancer research 25(2a) (2005) 965–9. [PubMed] [Google Scholar]
- [190].Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, Diegelman P, Kisiel N, Porter CW, Soleimani M, Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest, American journal of physiology. Cell physiology 292(3) (2007) C1204–15. [DOI] [PubMed] [Google Scholar]
- [191].Johansson VM, Oredsson SM, Alm K, Polyamine depletion with two different polyamine analogues causes DNA damage in human breast cancer cell lines, DNA and cell biology 27(9) (2008) 511–6. [DOI] [PubMed] [Google Scholar]
- [192].Douki T, Bretonniere Y, Cadet J, Protection against radiation-induced degradation of DNA bases by polyamines, Radiation research 153(1) (2000) 29–35. [DOI] [PubMed] [Google Scholar]
- [193].Durie BG, Salmon SE, Russell DH, Polyamines as markers of response and disease activity in cancer chemotherapy, Cancer research 37(1) (1977) 214–21. [PubMed] [Google Scholar]
- [194].Russell DH, Levy CC, Schimpff SC, Hawk IA, Urinary polyamines in cancer patients, Cancer research 31(11) (1971) 1555–8. [PubMed] [Google Scholar]
- [195].Pegg AE, Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy, Cancer research 48(4) (1988) 759–74. [PubMed] [Google Scholar]
- [196].Chamaillard L, Catros-Quemener V, Delcros JG, Bansard JY, Havouis R, Desury D, Commeurec A, Genetet N, Moulinoux JP, Polyamine deprivation prevents the development of tumour-induced immune suppression, British journal of cancer 76(3) (1997) 365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hayes CS, Shicora AC, Keough MP, Snook AE, Burns MR, Gilmour SK, Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment, Cancer immunology research 2(3) (2014) 274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, Ivanisevic J, Cho K, Wick EC, Hechenbleikner EM, Uritboonthai W, Goetz L, Casero RA Jr., Pardoll DM, White JR, Patti GJ, Sears CL, Siuzdak G, Metabolism links bacterial biofilms and colon carcinogenesis, Cell metabolism 21(6) (2015) 891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Soda K, The mechanisms by which polyamines accelerate tumor spread, J Exp Clin Canc Res 30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH, 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 20(10) (2006) 1622–34. [DOI] [PubMed] [Google Scholar]
- [201].Furth PA, Cabrera MC, Diaz-Cruz ES, Millman S, Nakles RE, Assessing estrogen signaling aberrations in breast cancer risk using genetically engineered mouse models, Annals of the New York Academy of Sciences 1229 (2011) 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P, Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria, FEMS microbiology ecology 66(3) (2008) 487–95. [DOI] [PubMed] [Google Scholar]
- [203].Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, Goedert JJ, Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women, The Journal of clinical endocrinology and metabolism 99(12) (2014) 4632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, Gajer P, Ravel J, Goedert JJ, Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study, Journal of translational medicine 10 (2012) 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Adlercreutz H, Pulkkinen MO, Hamalainen EK, Korpela JT, Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones, Journal of steroid biochemistry 20(1) (1984) 217–29. [DOI] [PubMed] [Google Scholar]
- [206].Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ, Nguyen JM, Curiel TJ, Cadungog MG, Singhal S, Eruslanov EB, Zhang P, Tchou J, Zhang R, Conejo-Garcia JR, Tumor Cell-Independent Estrogen Signaling Drives Disease Progression through Mobilization of Myeloid-Derived Suppressor Cells, Cancer discovery 7(1) (2017) 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].O’Hara AM, Shanahan F, The gut flora as a forgotten organ, EMBO Rep 7(7) (2006) 688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Roy S, Trinchieri G, Microbiota: a key orchestrator of cancer therapy, Nature reviews. Cancer (2017). [DOI] [PubMed]
- [209].Macfarlane S, Macfarlane GT, Regulation of short-chain fatty acid production, The Proceedings of the Nutrition Society 62(1) (2003) 67–72. [DOI] [PubMed] [Google Scholar]
- [210].Ridlon JM, Kang DJ, Hylemon PB, Bile salt biotransformations by human intestinal bacteria, Journal of lipid research 47(2) (2006) 241–59. [DOI] [PubMed] [Google Scholar]
- [211].Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR, Abundance and diversity of mucosa-associated hydrogenotrophic microbes in the healthy human colon, The ISME journal 6(1) (2012) 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Schneider J, Wendisch VF, Biotechnological production of polyamines by bacteria: recent achievements and future perspectives, Applied microbiology and biotechnology 91(1) (2011) 17–30. [DOI] [PubMed] [Google Scholar]
- [213].Nakamura J, Kubota Y, Miyaoka M, Saitoh T, Mizuno F, Benno Y, Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces, Microbiology and immunology 46(7) (2002) 487–90. [DOI] [PubMed] [Google Scholar]
- [214].McBain AJ, Macfarlane GT, Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites, Journal of medical microbiology 47(5) (1998) 407–16. [DOI] [PubMed] [Google Scholar]
- [215].Wu S, Rhee KJ, Zhang M, Franco A, Sears CL, Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage, Journal of cell science 120(Pt 11) (2007) 1944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Obiso RJ Jr., Azghani AO, Wilkins TD, The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells, Infection and immunity 65(4) (1997) 1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Gaudet RG, Sintsova A, Buckwalter CM, Leung N, Cochrane A, Li J, Cox AD, Moffat J, Gray-Owen SD, INNATE IMMUNITY. Cytosolic detection of the bacterial metabolite HBP activates TIFA-dependent innate immunity, Science (New York, N.Y.) 348(6240) (2015) 1251–5. [DOI] [PubMed] [Google Scholar]
- [218].Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Fioretti MC, Puccetti P, Tryptophan catabolism generates autoimmune-preventive regulatory T cells, Transplant immunology 17(1) (2006) 58–60. [DOI] [PubMed] [Google Scholar]
- [219].Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P, The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells, Journal of immunology (Baltimore, Md. : 1950) 176(11) (2006) 6752–61. [DOI] [PubMed] [Google Scholar]