Abstract
Background
Glioblastoma multiforme (GBM) is a very aggressive form of brain cancer that carries with it a tragically poor prognosis. As with many other forms of cancer, the extracellular environment near GBM tumors is acidified and is relevant to the pathogenesis of GBM because decreased pH promotes tumor cell invasion, increases angiogenesis, decreases immune surveillance, and increases resistance to possible treatments. Recently, vacuolar ATPase (v‐ATPase), a proton pump that helps maintain the acidic environment in endosomes and lysosomes (hereafter referred to endolysosomes) as well as proton gradients across the plasma membrane, was identified as a novel therapeutic target for GBM. However, information is lacking about cancer cell and tissue pH of endolysosomes, cytosol, and extracellular fluid.
Aims
Here, we measured endolysosome, cytosolic, and extracellular pH in U87MG cells in the absence and presence of the v‐ATPase inhibitor bafilomycin A1.
Methods
In vitro measurements of U87MG cells were conducted using LysoSensor dye and a Lysosome‐RFP dye for lysosome pH, BCECF‐AM for cytosolic pH, and a pH‐sensitive microprobe for extracellular pH.
Results
Bafilomycin A1 increased endolysosome pH from 5.28 to 5.57, decreased cytosolic pH from 7.01 to 6.46, and increased extracellular pH from 7.18 to 7.40.
Conclusions
Here, we report the ability to make pH measurements in U87MG glioblastoma cells and discuss these results in the context of GBM pathogenesis and possible treatment. This might be of some importance in understanding the pathogenesis of GBM because the highly regulated stores of hydrogen (H+) ions in endolysosomes can influence cytosolic and extracellular pH as well as the distribution, numbers, and sizes of endolysosomes.
Keywords: Cytosolic pH, Endolysosome pH, Endolysosomes, Extracellular pH, Glioblastoma, v‐ATPase
1. INTRODUCTION
Glioblastoma multiforme (GBM), a very aggressive and highly invasive primary brain cancer with limited treatment options and a very poor prognosis for the patient, has at least one thing in common with other cancers: The extracellular environment near GBM tumors is acidic. Extracellular acidification is particularly relevant to tumorgenesis and the concept of tumor cell dormancy because of findings that acidic pH increases resistance to apoptosis and autophagy, promotes tumor cell invasion, increases angiogenesis, obscures immune surveillance, and promotes resistance to drugs and radiotreatment.1 Extracellular acidification is influenced by a number of associated factors including nutrient starvation, oxidative stress, hypoxia, and high levels of aerobic glycolysis that increases levels of lactic acid. Accordingly, mitochondria have been implicated, and mitochondrial abnormalities have been described in GBM.2 The origin of the hydrogen (H+) ions that promote acidification of cytosol and extracellular spaces can originate from acidic organelles, especially endolysosomes.
Endolysosomes are further implicated in GBM because of findings that proton pumping vacuolar ATPase (v‐ATPase) not only regulates endolysosome acidity but also might be a novel therapeutic target for GBM.3 To understand better the role of pH in GBM, it was important for us to measure endolysosome, cytosolic, and extracellular pH. Accordingly, here we focus on the ability to measure the pH of endolysosomes, cytoplasm, and the extracellular milieu in U87MG glioblastoma cells and discuss possible contributions of pH to the pathological development of glioblastoma and to its possible treatment.
2. MATERIALS AND METHODS
2.1. Cell culture
U87MG glioblastoma cells were cultured in 1× DMEM (Invitrogen) supplemented with 10% fetal calf serum and 1% penicillin/streptomycin (Invitrogen). Cells were grown to confluence in T75 flasks or to about 40% confluence in 35‐mm2 dishes in an environment 5% CO2 at 37°C. Cells were only used at passage numbers of 10 or less.
2.2. Endolysosome pH
As previously described,4, 5, 6 endolysosome pH was measured using a ratiometric indicator dye, LysoSensor Yellow/Blue DND‐160, a dual excitation dye that allows for the measurement of acidic organelles independent of dye loading efficiency. U87MG cells were loaded with 10 μM DND‐160 for 5 minutes at 37°C. Postincubation, dye‐containing media were removed and PBS was added to the cells prior to them being taken for imaging. CellLight Lysosome‐RFP (Thermo Fisher), incubated in cells overnight, was used to label lysosomes in combination with LysoSensor DND‐160 for selectivity of lysosomal labeling. Light emitted at 520 nm in response to excitation at 340 and 380 nm was measured for 2 milliseconds every 10 seconds using a filter‐based imaging system (Zeiss, Germany). The ratios of light excited (340/380 nm) versus light emitted (520 nm) were converted to pH using a calibration curve established with 10 μM of the H+/Na+ ionophore monensin and 20 μM of the H+/K+ ionophore nigericin; both were dissolved in 20 mM 2‐(N‐morpholino) ethane sulfonic acid (MES), 110 mM KCl, and 20 mM NaCl adjusted to pH 3.0 to 7.0 with HCl/NaOH, and the linear range is 4.5 to 6.0 pH.
2.3. Cytosolic pH
2′,7′‐Bis‐(2‐carboxyethyl)‐5‐(and‐6)‐carboxyfluorescein, acetoxymethyl ester (BCECF‐AM) was used as a measure of cytosolic pH. Cytosolic pH was determined from the pH‐dependent ratio of emission intensity (535 nm) when the dye is excited at 488 nm (pH‐dependent) versus the emission intensity when excited at its isobestic point of 440 nm (non pH‐dependent) using a multiwell plate reader (Molecular Devices, SpectraMax GeminiEM). As previously described,7, 8 a standard curve was calibrated with BCECF‐AM‐loaded dye over multiple pH values, and the linear range is 6.0 to 8.0 pH. Cells were incubated with 1 μM BCECF‐AM for 20 minutes at 37°C and then washed three times with PBS prior to pH measurements.
2.4. Extracellular pH
Using U87MG cells grown in 35‐mm2 dishes, extracellular pH was measured using a microprobe‐based pH system (Micro pH Electrode, Thermo Fisher). The electrode was calibrated with pH standards and placed in the media of the 35‐mm2 dishes containing the cells. pH measurements were made using an Accumet Ab150 pH meter (Fisher Scientific).
2.5. Morphology of endolysosomes
U87MG cells treated with vehicle (0.1% DMSO) or 200 nM bafilomycin A1 were incubated at 37°C for 60 minutes. Cells were washed three times with PBS, fixed with 4% paraformaldehyde (for 15 min at room temperature), permeabilized with 0.01% Triton X‐100, and incubated with blocking solution (3% BSA) for 90 minutes. Cells were incubated with antibodies to LAMP1 (1:250, Catalog #9091, Cell Signaling) for late endosomes and lysosomes and α‐tubulin for cell boundary (1:250, Catalog #T9026, Sigma Aldrich) overnight. Secondary antibodies were Alexa goat anti‐rabbit 488 (1:250, Catalog #A‐11034) and Alexa goat anti‐mouse 594 (1:250, Catalog #A‐11032) from Thermo Fisher. Cells were then washed and mounted on frosted glass slides (Fisher Scientific) using ProLong Gold antifade with DAPI (Catalog #P36935, Thermo Fisher). Images were acquired with a Zeiss LSM800 laser confocal microscope system (Zeiss, Germany). Images were then reconstructed in Imaris software (Bitplane, Zurich, Switzerland) using the Cell and Spots module. LAMP1‐positive vesicle sizes, numbers, and volumes were calculated using the Imaris reconstructed images.
2.6. Statistical analysis
Experiments were conducted a minimum of three independent times in triplicate, and data were reported as means ± SD. Statistical significance between two groups was analyzed with a Student's t test, and statistical significance among multiple groups was analyzed with one‐way ANOVA plus a Tukey post hoc test. P < .05 was considered to be statistically significant.
3. RESULTS AND DISCUSSION
Endolysosomes are acidic organelles, a feature regulated by v‐ATPase proton pumps.9 The acidic nature of endolysosomes predates the discovery of lysosomes by about 60 years,10 and this acidity is essential for the activity of endolysosome‐containing hydrolases that help degrade macromolecules into cellular building blocks, the fusing of autophagosomes with lysosomes to form autophagolysosomes that control autophagy,11 and the establishment of transmembrane proton gradients that exist in endolysosomes, Golgi complex, secretory granules as well as on the plasma membrane.12 H+ ions released from endolysosomes cannot only result in deacidification of endolysosomes, but can also cause cytosol acidification and changes in extracellular pH. This is an issue of some importance for the pathogenesis of GBM because the GBM tumor microenvironments are acidic,13, 14 and endolysosome pH specifically affects oncogenic signaling in GBM15; the “necrotic zone” has pH values less than or equal to 3.4, the surrounding “pseudo‐palisading cell zone” has pH values less than 5.5, the “cellular tumor zone” has pH values of 6.2 to 7.0, and the “leading edge zone” has a normal pH.16 It is also an issue of possible importance to GBM therapeutics because v‐ATPase is highly expressed by high‐grade gliomas compared with normal brain and less aggressive grade II tumors, and v‐ATPase has been identified as a novel therapeutic target for GBM.3
Factors that influence extracellular acidification in cancer include nutrient starvation, oxidative stress, hypoxia, and high levels of aerobic glycolysis resulting in increased levels of lactic acid, factors that are abnormal in GBM and increase the progression of GBM.2 , 16 However, the role of pH in cancer dynamics is made difficult by the lack of reagents capable of studying pH dynamics between organellar compartments, the cytoplasm, and the extracellular environment.17 Accordingly, we established methodology with which to measure, in tandem, levels of pH in endolysosomes, cytoplasm, and the extracellular mileau. In order to do so, we first calibrated pH standard curves for endolysosomes (Figure 1) and cytosol (Figure 2) as previously described.4, 5, 6, 7, 8 Therefore as illustrated, we were able to make such measurements and found that when v‐ATPase was inhibited by bafilomycin A1, endolysosomes and extracellular spaces were deacidified while cytoplasm was acidified (Figure 3). Our findings with bafilomycin A1 are consistent with the presence of v‐ATPase in endolysosomes and plasma membranes and how H+ ions flux through v‐ATPase subunits (Figure 4). Our findings are also consistent with two previous studies that found that bafilomycin A1 increased extracellular pH and decreased cytosolic pH.18, 19 Moreover, v‐ATPase is highly expressed by high‐grade gliomas compared with normal brain and less aggressive grade II tumors,3 and this might provide further reason to investigate v‐ATPase inhibitors as GBM therapeutic agents (Figure 5).
Figure 1.

Fluorescense intensity ratios vs pH for measuring lysosome pH. Standard curve of fluorescense intensity ratios of the LysoSensor Yellow/Blue DND‐160 vs pH. The fluorescence emission of 520 nm was measured for excitations at 340 and 380 nm and was found to be linear between 4.5 and 6.0 pH
Figure 2.

Fluorescense intensity ratios vs pH for measuring cytosolic pH. Standard curve of fluorescense intensity ratios of BCECF‐AM vs pH. The fluorescence emission of 535 nm was measured at excitations at 488 and 440 nm and was found to be linear between 6.0 and 8.0 pH
Figure 3.

Bafilomycin A1 deacidified endolysosomes in U87MG cells: (A) U87MG cells treated for 60 minutes with 200 nM bafilomycin A1 (Baf) exhibited significantly increased levels of endolysosome pH as determined using Lysosensor DND‐160, (B) U87MG cells treated for 60 minutes with 200 nM Baf exhibited significantly decreased levels of cytosolic pH as determined using BCECF, and (C) U87MG cells treated for 60 minutes with 200 nM Baf exhibited increased levels of extracellular pH as determined using a microprobe pH meter. The vehicle control for Baf in these studies was 0.1% DMSO (n = 5, ***P < .01, **P < .02, *P < .05)
Figure 4.
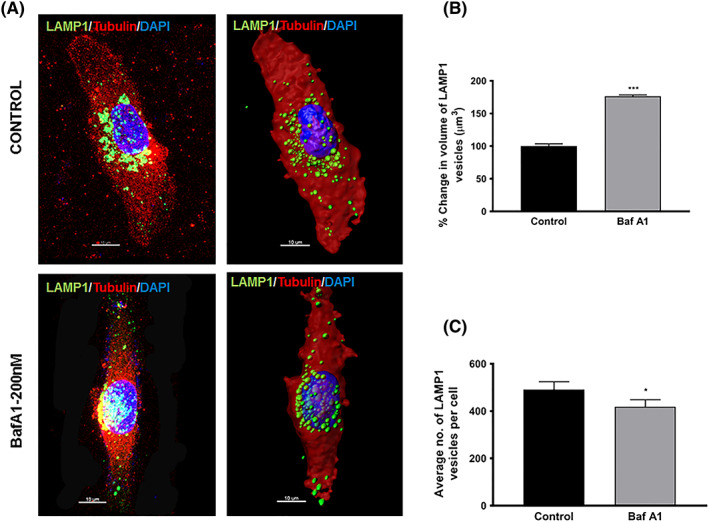
Effects of bafilomycin A1 on endolysosome number and volume in U87MG cells: (A) representative confocal (left) and Imaris (right) images of U87MG cells treated with vehicle (control, 0.1% DMSO) or bafilomycin A1 (Baf A1, 200 nM) for 60 minutes. Cells were fixed and stained for LAMP1 (green) for late endosomes and lysosomes, tubulin (red) for cytoplasm, and DAPI (blue) for nucleus. Images on the left show confocal images (Zeiss LSM800), and those on the right show the same image reconstructed in Imaris software (Bitplane) using the Cell and Spots module. Scale bar = 10 μm. LAMP1‐positive vesicle sizes and numbers were calculated using the Imaris reconstructed images; (B) LAMP1‐positive endolysosome average volumes were increased by Baf A1 treatment as compared with control (n = 3, ***P < .01); and (C) LAMP1‐positive endolysosome average numbers were increased by Baf A1 treatment as compared with control (n = 3, *P < .05). Bars indicate mean ± SD
Figure 5.

Model of v‐ATPase regulation of endolysosome, cytosolic, and extracellular space pH: v‐ATPase is a proton pump located on endolysosomes and plasma membranes. In endolysosomes, v‐ATPase regulates the flow of protons into the lumen of endolysosomes, and in plasma membranes, it regulates the flow of protons into the extracellular space. Accordingly, inhibition of v‐ATPase with, for example, bafilomycin A1 (Baf A1) deacidifies endolysosomes and the extracellular space thus validating the effects of Baf A1 in U87MG cells shown above in Figure 3
During transformation of normal cells into cancer cells, lysosomes undergo morphological changes including increased size, increased fragility, and altered intracellular distribution patterns.20, 21, 22 In GBM, there is a tight correlation between tumor grade and staining intensity for the lysosomal marker LAMP1.23, 24 Similar to the findings of others, we found that bafilomycin A1 significantly affected morphological features of endolysosomes including affecting their subcellular distribution patterns, decreasing their numbers, and increasing their volumes in U87MG glioblastoma cells (Figure 3).
In GBM, cancer cells acquire resistance to cell death via apoptosis, but do retain lysosomal cell death pathways. Because of this, lysosome destabilizing drugs including those that deacidify endolysosomes might be used therapeutically against GBM.25 Indeed, changes in endolysosome pH have been found to precede endolysosome permeabilization in immortalized and transformed glioblastoma cells.23 Further, the weak base antimalarial drug chloroquine and chloroquine‐like drugs were found to increase the antiglioma effects of temozolomide,26, 27 and chloroquine has been promoted as an adjuvent therapeutic against GBM.28 Similar to chloroquine, the v‐ATPase inhibitor bafilomycin A1 was toxic to glioma primary cultures, was synergistic with temozolomide and the tetracyclic triterpene alcohol euphol in activating autophagy‐associated cell death,29 decreased the viability of primary GBM neurospheres, and decreased the viability of GBM organotypic cultures.3 Accordingly, it is not surprising that investigators have conducted clinical trials to determine anti‐GBM effects of chloroquine and analogs of chloroquine.30, 31
Endolysosomes are acidic organelles that have been implicated in the pathogenesis of GBM. Further, possible therapeutic agents that affect endolysosomes and associated autophagolysosomes continue to be tested because glioblastoma cells die not from apoptosis, but rather from autophagy. Central to pathological features of endolysosomes in U87MG glioblastoma cells is the regulation of pH. The findings of others and us suggest strongly that greater attention be paid to understanding better the effects of GBM therapeutic strategies on endolysosomes and levels of H+ ions in acidic organelles, cytoplasm, and extracellular environments.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHORS' CONTRIBUTION
All authors had full access to the data in the study and take responsibliltiy for the integrity of the data and the accuracy of the data analysis. Conceptualization, J.D.G., P.H., N.K.; Methodology, X.C., G.D., P.H., N.K.; Writing ‐ Original Draft, J.D.G., P.H.; Writing ‐ Review and Editing, P.H., J.D.G., J.E.O.; Funding Acquisition, J.D.G., X.C., J.E.O.
ACKNOWLEDGEMENTS
Research reported in this publication from the Geiger Laboratory was supported by the National Institute of General Medical Sciences under award numbers P30GM100329 and U54GM115458, the National Institute of Mental Health under award numbers R01MH100972 and R01MH105329, and the National Institute of Neurological Diseases and Stroke under award number R01NS065957. Research reported in this publication from the Ohm Laboratory was supported by the National Institute of Environmental Health Sciences under award number R01ES022030.
Halcrow PW, Khan N, Datta G, Ohm JE, Chen X, Geiger JD. Importance of measuring endolysosome, cytosolic, and extracellular pH in understanding the pathogenesis of and possible treatments for glioblastoma multiforme. Cancer Reports. 2019;2:e1193. 10.1002/cnr2.1193
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Peppicelli S, Andreucci E, Ruzzolini J, et al. The acidic microenvironment as a possible niche of dormant tumor cells. Cell Mol Life Sci. 2017;74(15):2761‐2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chinopoulos C, Seyfried TN. Mitochondrial substrate‐level phosphorylation as energy source for glioblastoma: review and hypothesis. ASN Neuro. 2018;10. 1759091418818261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrea Di Cristofori SF, Bertolini I, Gaudioso G, et al. The vacuolar H+ ATPase is a novel therapeutic target for glioblastoma. Oncotarget. 2015;6:17514‐17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD. Endolysosome involvement in HIV‐1 transactivator protein‐induced neuronal amyloid beta production. Neurobiol Aging. 2013;34(10):2370‐2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hui L, Chen X, Geiger JD. Endolysosome involvement in LDL cholesterol‐induced Alzheimer's disease‐like pathology in primary cultured neurons. Life Sci. 2012a;91(23–24):1159‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hui L, Chen X, Haughey NJ, Geiger JD. Role of endolysosomes in HIV‐1 Tat‐induced neurotoxicity. ASN Neuro. 2012b;4(4):243‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gores GJ, Nieminen AL, Wray BE, Herman B, Lemasters JJ. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989;83(2):386‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grant RL, Acosta D. Ratiometric measurement of intracellular pH of cultured cells with BCECF in a fluorescence multi‐well plate reader. In Vitro Cell Dev Biol Anim. 1997;33(4):256‐260. [DOI] [PubMed] [Google Scholar]
- 9. Perera RM, Zoncu R. The lysosome as a regulatory hub. Annu Rev Cell Dev Biol. 2016;32(1):223‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Metchnikoff . Lectures on the Comparative Pathology of Inflammation. New York: Dover; 1893. [Google Scholar]
- 11. Sharon A, Tooze I. Autophagy captures the Nobel prize. Cell. 2016;167(6):1433‐1435. [DOI] [PubMed] [Google Scholar]
- 12. Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74(1):69‐86. [DOI] [PubMed] [Google Scholar]
- 13. Honasoge A, Shelton KA, Sontheimer H. Autocrine regulation of glioma cell proliferation via pHe‐sensitive K(+) channels. Am J Physiol Cell Physiol. 2014;306(5):C493‐C505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671‐677. [DOI] [PubMed] [Google Scholar]
- 15. Kondapalli KC, Llongueras JP, Capilla‐González V, et al. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat Commun. 2015;6(1):6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. John S, Sivakumar KC, Mishra R. Extracellular proton concentrations impacts LN229 glioblastoma tumor cell fate via differential modulation of surface lipids. Front Oncol. 2017;7(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajendran M, Claywell B, Haynes EP, Scales U, Henning CK, Tantama M. Imaging pH dynamics simultaneously in two cellular compartments using a ratiometric pH‐sensitive mutant of mCherry. ACS Omega. 2018;3(8):9476‐9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McSheehy PMJ, Troy H, Kelland LR, Judson IR, Leach MO, Griffiths JR. Increased tumour extracellular pH induced by bafilomycin A1 inhibits tumour growth and mitosis in vivo and alters 5‐fluorouracil pharmacokinetics. Eur J Cancer. 2003;39(4):532‐540. [DOI] [PubMed] [Google Scholar]
- 19. Heming TA, Traber DL, Hinder F, Bidani A. Effects of bafilomycin A1 on cytosolic pH of sheep alveolar and peritoneal macrophages: evaluation of the pH‐regulatory role of plasma membrane V‐ATPases. J Exp Biol. 1995;198(8):1711. [DOI] [PubMed] [Google Scholar]
- 20. Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholipid metabolism: a target in cancer cells? J Cell Biochem. 2003;90(3):525‐533. [DOI] [PubMed] [Google Scholar]
- 21. Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434‐6451. [DOI] [PubMed] [Google Scholar]
- 22. Ono K, Kim SO, Han J. Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor alpha‐induced cell death. Mol Cell Biol. 2003;23(2):665‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen SS, Aaberg‐Jessen C, Christensen KG, Kristensen B. Expression of the lysosomal‐associated membrane protein‐1 (LAMP‐1) in astrocytomas. Int J Clin Exp Pathol. 2013a;6(7):1294‐1305. [PMC free article] [PubMed] [Google Scholar]
- 24. Sarafian VS, Koev I, Mehterov N, Kazakova M, Dangalov K. LAMP‐1 gene is overexpressed in high grade glioma. APMIS. 2018;126(8):657‐662. [DOI] [PubMed] [Google Scholar]
- 25. Jensen SS, Petterson SA, Halle B, Aaberg‐Jessen C, Kristensen BW. Effects of the lysosomal destabilizing drug siramesine on glioblastoma in vitro and in vivo. BMC Cancer. 2017;17(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golden EB, Cho HY, Hofman FM, Louie SG, Schonthal AH, Chen TC. Quinoline‐based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015;38(3):E12. [DOI] [PubMed] [Google Scholar]
- 27. Golden EB, Cho HY, Jahanian A, et al. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg Focus. 2014;37(6):E12. [DOI] [PubMed] [Google Scholar]
- 28. Steven M, Toler DN, Sharma A. Selective enhancement of cellular oxidative stress by chloroquine: implications for the treatment of glioblastoma multiforme. Neurosurg Focus. 2006;15;21(6):E10. [DOI] [PubMed] [Google Scholar]
- 29. Silva VAO, Rosa MN, Miranda‐Gonçalves V, et al. Euphol, a tetracyclic triterpene, from Euphorbia tirucalli induces autophagy and sensitizes temozolomide cytotoxicity on glioblastoma cells. Invest New Drugs. 2018. [DOI] [PubMed] [Google Scholar]
- 30. Johannessen T‐CA, Prestegarden L, Grudic A, Hegi ME, Tysnes BB, Bjerkvig R. The DNA repair protein ALKBH2 mediates temozolomide resistance in human glioblastoma cells. Neuro Oncol. 2013;15(3):269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Munshi A. Chloroquine in glioblastoma—new horizons for an old drug. Cancer. 2009;115(11):2380‐2383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


