Abstract
Background:
Long non-coding RNAs (lncRNAs) are emerging as important regulators in the modulation of virus infection by targeting mRNA transcription. However, their roles in chronic hepatitis B (CHB) remain to be elucidated.
Objective:
The study aimed to explore the lncRNAs and mRNA expression profiles in CHB and asymp-tomatic HBsAg carriers (ASC) and construct mRNA-lncRNA co-expression profile and ceRNA net-works to identify the potential targets of diagnosis and treatment in CHB.
Methods:
We determined the expression profiles of lncRNAs and mRNAs in CHB and ASC using mi-croarray analysis. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) path-way enrichment analyses were performed to explore their function. We also constructed co-expression, cis-regulatory, and competing endogenous RNA (ceRNA) networks with bioinformatics methods.
Results:
We identified 1634 mRNAs and 5550 lncRNAs that were differentially expressed between CHB and ASC. Significantly enriched GO terms and pathways were identified, many of which were linked to immune processes and inflammatory responses. Co-expression analysis showed 1196 relation-ships between the top 20 up/downregulated lncRNAs and mRNA, especially 213 lncRNAs interacted with ZFP57. The ZFP57-specific ceRNA network covered 3 lncRNAs, 5 miRNAs, and 17 edges. Cis-correlation analysis showed that lncRNA T039096 was paired with the most differentially expressed gene, ZFP57. Moreover, by expending the clinical samples size, the qRT-PCR results showed that the expression of ZFP57 and T039096 increased in CHB compared to ASC.
Conclusion:
Our study provides insights into the roles of mRNA and lncRNA networks in CHB, high-lighting potential applications of lncRNA-T039096 and mRNA-ZFP57 for diagnosis and treatment.
Keywords: lncRNA, mRNA, chronic hepatitis B, HBV, co-expression, ceRNA
1. INTRODUCTION
Chronic hepatitis B virus (HBV) infection continues to be a major health burden globally, especially in China. It is estimated that there are 280 million individuals living with chronic hepatitis B (CHB) worldwide and approximately 90 million in China [1, 2]. According to the serological profile, HBV infection can be divided into two well-distinguished subsets of subjects: asymptomatic HBV carriers (ASC) and CHB patients. ASC are a cohort of patients who are in an immune tolerance phase with normal serum alanine aminotransferase (ALT) levels despite massive antigen levels and high-level of HBV replication. In contrast, CHB refers to hepatitis B virus-positive patients with the clinical manifestations of chronic hepatitis [3]. Compared with ASC, the development of HBV-mediated liver disease in CHB is more likely to lead to liver fibrosis, cirrhosis and, eventually, hepatocellular carcinoma [4]. In additional, since the hepatitis D virus (HDV) is a defective RNA virus in hepatocytes, its replication and proliferation require HBV assistance. Previous research has found that HBV infection co-exists with HDV infection, resulting in co-infection or superinfection. Some research works have revealed that if both HBV and HDV were infected at the same time, there was a cumulative and additive effect on liver damage [5]. Stroffolini et al., found that HDV infection jointly affects both HBeAg status and HBV DNA could lead to severity of the liver. However, the researchers proved that in the HBV and HDV co-infection, decreased serum levels of HBsAg, HBeAg and HBV-DNA were found to be resulted from the HDV replication which could inhibit HBV replication. So, clinicians emphasized the superinfection of HDV and HBV, and paid attention to the biochemical examination of HBV-DNA and HDAg [6, 7]. Despite extensive research, the HBV immunopathogenesis is not completely understood. Viral persistence and clinical outcomes following HBV infection depend on viral and host factors; including genetic factors that determine a host's immune mechanisms. The primary goal of HBV treatment is to eradicate HBV or to at least maintain the suppression of HBV replication. Despite recent advances in anti-viral agents (pegylated interferon or a nucleos(t)ide analogue) for chronic HBV infection, complete eradication of the virus is difficult to achieve. Further, anti-viral agents could not be extensively used because of the generally low income and limited resources in China [8, 9].
The recent advent of next-generation RNA sequencing (RNA-Seq) and publication of reference genomes for many organisms have allowed researchers to study transcription profiles. The majority of the mammalian genomes are transcribed, of which only 2-3% are protein-coding RNAs (mRNAs) and the rest are noncoding RNAs [10]. Long non-coding RNAs (lncRNA) are a class of non-coding RNAs longer than 200 nucleotides that make up the majority of the transcriptome. Accumulating evidence suggests that lncRNAs play critical roles in various biological processes, including chromatin modification, regulation of transcription, influence of nuclear architecture, and regulation of gene expression at the post-transcriptional level, and are also reported to interact with mRNA and proteins during these processes [11]. lncRNAs have now emerged as critical regulators of gene expression in the modulation of immune responses upon viral infection. Infected cells may express viral lncRNAs, cellular lncRNAs, and chimeric lncRNAs formed by viral and cellular sequences. Some viruses express viral lncRNAs whose function is essential for viral viability. Expression of cellular lncRNAs may be altered in response to viral replication or viral protein expression [12, 13].
In addition, Salmena et al. proposed the one particular breakthrough-ceRNA hypothesis, which refers to the regulatory model where RNAs can regulate each others’ expression by competing for common miRNA response elements at post-transcriptional levels [14]. The ceRNA network links the function of protein-coding genes (mRNAs) with the functions of non-coding RNAs (such as lncRNA) and plays an important role in a diverse range of biological processes [15]. Understanding this novel ceRNA network will lead to significant insight into gene regulatory networks and have implications in the pathogenesis of diseases, such as tumor [16], immunopathy [17], and genetic disease [18]. Lu et al., found that lncRNA BC032469 could function as a ceRNA to impair miR-1207-5p-dependent hTERT regulation, suggesting that it may be clinically valuable as a poor prognostic biomarker of gastric cancer [19]. Three key lncRNAs revealed could be used as potential diagnostic biomarkers and therapeutic targets for rheumatoid arthritis by ncRNA-miRNA-mRNA network analysis based on ceRNA [20]. Moreover, Zhang et al., indicated that a potential link between Alzheimer's disease and circRNA-associated-ceRNA network and circRNA-associated-ceRNA networks could profoundly affect the diagnosis and treatment of Alzheimer's disease in the future [21]. All the evidence proved that the ceRNA mechanism participated in the maintenance of normal physiological state, occurrence and development of diseases. However, the role of cellular lncRNAs and the ceRNA regulatory network in HBV infection is not well clear.
In this study, we performed a microarray analysis to detect the lncRNAs and mRNA expression profiles in patients with CHB and in ASC individuals. In addition, we performed a comprehensive analysis of lncRNAs and mRNAs with associated co-expression, cis-regulation, and competing endogenous RNA (ceRNA) networks in CHB. We identified the imprinted lncRNA T039096 and ZFP57 gene targets in CHB, which might be associated with CHB development. Our findings may provide a new avenue for investigating the pathogenesis of CHB.
2. MATERIALS AND METHODS
2.1. Experimental Subjects and Samples
The study group included 30 ASC patients and 30 CHB patients who were recruited from the First Affiliated Hospital, College of Medicine, Zhejiang University, between 2014 and 2017. All of the patients were definitively diagnosed according to the American Association for the Study of Liver Diseases (AASLD) 2016 Practice Guidelines for Treatment of Chronic Hepatitis B [22], and all patients were not administered anti-viral agents before diagnosis. Clinical data of patients are listed in Table 1. Among the 60 samples, 3 ASC and 3 CHB were conducted for sequence analysis. The selected criteria were as follows: 1) All the patients were female and between the ages of 30 and 40; 2) All of the subjects were first diagnosed with hepatitis B virus infection, received no antiviral treatment, and excluded immunity, infection and tumor diseases; 3) CHB samples had fatigue, anorexia, poor mental condition, insomnia and other symptoms. Physical examination revealed yellow staining of the skin and enlargement of the liver. Whereas, there was no clinical manifestation of ASC; 4) HsAg (+),HeAg (+), and HcAb (+) for CHB and ASC. HBV-DNA > 10 E+5 for CHB samples, whereas no HBV-DNA for ASC. ALT > 100 U/ml and AST > 100 U/ml for CHB, whereas no ALT and AST for ASC. Total bilirubin (TB) >51 for CHB samples, whereas no TB for ASC. 5) Other biochemical tests were normal for both CHB and ASC. The study was approved by the Clinical Research Ethics Committee of the College of Medicine, Zhejiang University, and all the patients provided written informed consent for participation. After venous whole blood collection, peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Hypaque-Ficoll (GE Healthcare Bio-sciences AB, Uppsala, Sweden) according to the manufacturer’s protocol. Then, the PBMC samples were lysed with TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) and stored at -80°C. RNA quantity and quality were measured with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The RNA integrity was assessed by standard denaturing agarose gel electrophoresis.
Table 1. Clinical data of patients.
| Clinical Characteristics | CHB | ASC |
|---|---|---|
| Number of patients | 30 | 30 |
| Sex number (%) | ||
| Female | 9 | 11 |
| Male | 21 | 19 |
| Age | ||
| Median | 36 | 41 |
| Range | 26-49 | 23-64 |
| HBV DNA (IU/ml) | ||
| Median | 2.82E+07 | — |
| Range | 2.97E+03-9.58E+08 | negative-1.08E+10 |
| ALT (U/ml) | ||
| Median | 187 | 25 |
| Range | 65-239 | 8-38 |
| AST (U/ml) | ||
| Median | 155 | 25 |
| Range | 60-178 | 7-45 |
| HBsAg (ng/ml) | ||
| Median | 4543 | — |
| Range | 350-822278 | negative-6366 |
| HBeAg (PEIU/ml) | ||
| Median | 466 | — |
| Range | 13-1514 | negative-36 |
| HDAg | ||
| Median | — | — |
| Range | — | |
| anti-HDV | ||
| Median | — | — |
| Range | — | — |
2.2. LncRNA and mRNA Microarrays
An Arraystar Human LncRNA Microarray V4.0 (Array-Star, Inc., Rockville, MD, USA) was used to detect the global profiles of human lncRNAs and protein-coding transcripts. This array contained 32,667 LncRNA probes and 20,730 coding transcript probes, and they were collected from the most authoritative databases, including Refseq, UCSC known genes, Ensembl, as well as landmark publications. The Agilent Array platform was used for the microarray analysis. The sample preparation, RNA quality control, labeling reaction, microarray hybridization, slide washing, scan, and data extraction were performed according to the standard Arraystar protocols with minor modifications. Agilent Feature Extraction software (version 11.0.1.1, Agilent Technologies) was employed to analyse the acquired array images. Quantile normalization and subsequent data processing were performed by the GeneSpring GX v12.0 software package (Agilent Technologies, Inc.). Differentially expressed lncRNAs and mRNAs with statistical significance between two groups were identified through scattering plot and volcano plot filtering with a threshold of fold-change >2 and P<0.05. Scatter plot and volcano plot filtering were used to distinguish the significantly differentially expressed LncRNAs and mRNAs between the two groups. Significantly different expressions of LncRNAs and mRNAs were revealed through Fold Change filtering. The microarray work was performed by KangChen Bio-tech (Shanghai, China).
2.3. GO and KEGG Pathway Analysis
The GO (www.geneontology.org) enrichment was calculated to assess the biological process, cellular component and molecular function of the differential expression genes found [23]. The differentially expressed mRNAs were mapped to terms in the GO database, and the number of genes of each term was calculated. The P<0.05 denoted the significance of GO term enrichment in the deregulated expressed genes. Pathway analysis was used to investigate the differentially expressed mRNAs according to the Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) database [24]. The p-value indicated the significance of the pathway correlated with the conditions. P<0.05 was considered statistically significant. Fisher's exact test was used to select significant GO terms and pathways.
2.4. Correlation Analysis Between lncRNA and mRNA
A network between lncRNA and mRNA was constructed based on the correlation analysis among DE lncRNAs and protein-coding genes. For each pair, the Pearson correlation coefficient was calculated to assess the correlation. Pairs with absolute values of Pearson correlation coefficients not less than 0.90 were selected to design the network using Cytoscape (National Resource for Network Biology).
2.5. Cis-correlation Analysis
The cis-regulatory regions were identified by the following procedures. For each lncRNAs, we identified the mRNAs as “cis-regulated mRNAs” when: (1) the mRNA’s locus was within 300k windows up- and downstream of the given lncRNA, (2) the Pearson correlation coefficient of lncRNA-mRNA expression was significant (P value of correlation less than 0.05).
2.6. ceRNA Analysis
According to the ceRNA hypothesis, lncRNAs compete for the same miRNA response elements and act as ‘molecular sponges’ for miRNAs, thereby regulating the de-repression of all target genes of the respective miRNA family. The MiRNA targets on mRNA 3′ untranslated regions (UTR) and lncRNA were calculated using the PITA algorithm (http://genie.weizmann.ac.il/pubs/mir07) and the ceRNA network was built by using Cytoscape software.
2.7. Real-time Quantitative PCR Validation Analysis
Total RNA was extracted from the PBMCs collected for microarray assay by using TRIzol reagent (Invitrogen). Then, the extracted RNA was reverse transcribed using the PrimeScript RT reagent kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Dalian, China) according to the manufacturer’s recommendations. The primer sequences for the selected lncRNAs and protein-coding genes are listed in Supplementary Table 1 (2MB, pdf) . The qPCR was performed using SYBRGreen (TaKaRa), and GAPDH was employed as an internal control. The expression of each lncRNA is represented as fold change using 2-ΔΔCt. Student’s t-test was utilized to analyze the significance of the expression differences between CHB and ASC. The value of P<0.05 was considered significant.
2.8. ELISA
The serum ZFP57 protein level was quantified using a commercially available ELISA kit (Shanghai Elisa Biotech, China)) according to the manufacturer’s instructions. The optical density was measured at 450 nm using a Model 680 microplate reader (Bio-Rad Laboratory, CA).
2.9. Statistical Analysis
The fold change and Student's t-test were analyzed for statistical significance of the microarray results. The false discovery rate (FDR) was calculated to correct the P-value. The threshold value used to designate differentially expressed lncRNAs and mRNAs was a fold change of >2.0. P<0.05 was considered to indicate a statistically significant result. An unpaired Student t-test was also conducted to test the association between expression levels of ZFP57 and T039096 and clinical signatures of CHB. Statistical analysis was performed by SPSS 21.0 (SPSS Inc., Chicago, IL).
3. RESULTS
3.1. Identification of DE mRNA and lncRNA
We performed a genome-wide analysis on 3 CHB patients and 3 ASC individuals. DE mRNAs and lncRNAs were selected when they were altered by a fold change greater than 2.0 (P value < 0.05). A total of 1634 mRNAs and 5550 lncRNAs were found to be DE between CHB and ASC. Among them, 3325 lncRNAs were upregulated and 2225 were downregulated (Fig. 1A), whereas 675 mRNAs were upregulated and 959 were downregulated (Fig. 1B). Concerning the mRNA expression, there were 5 upregulated and 8 downregulated genes with greater than 10-fold change. The top 20 DE gene (DEGs) mRNAs are summarized in Table 2. ZFP57 was found to be the most DE mRNA whose fold change reached 320. Similar to the DE genes, 15 upregulated lncRNAs and 21 down-regulated lncRNAs were identified with expression changes greater than 10-fold change. The top 20 DE lncRNAs (DELs) are summarized in Table 3. The DELs and DEGs were further screened by heat maps (Fig. 1C, 1D). The analysis revealed that the CHB patients could be distinguished from ASC cases in terms of a different heat signs evident in each case.
Fig. (1).
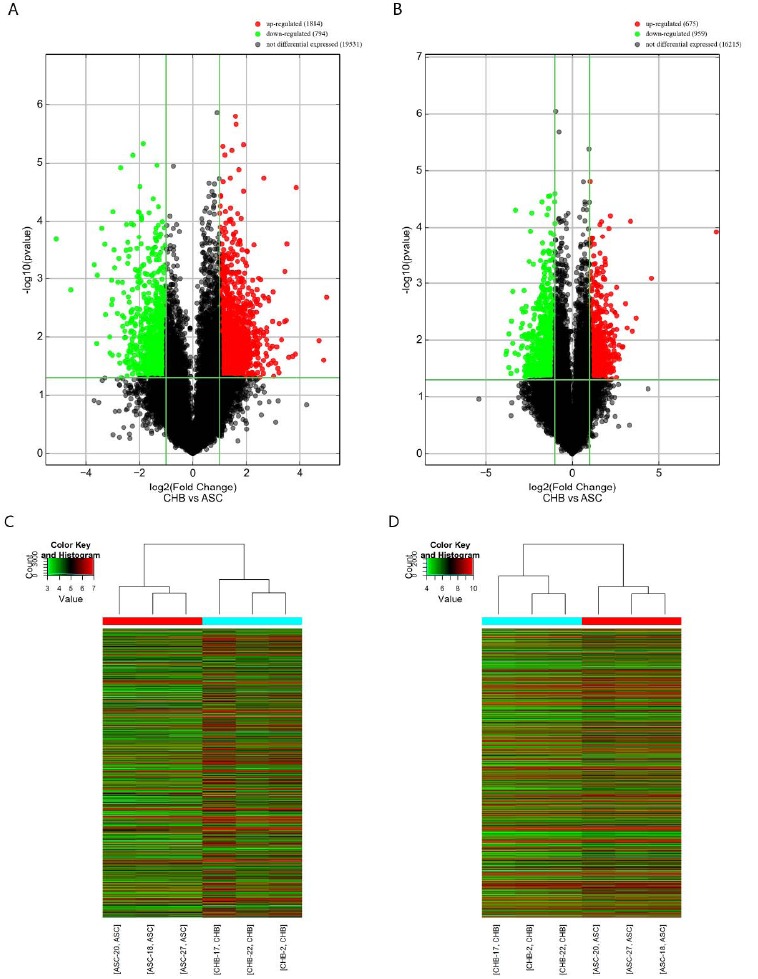
Differentially expressed lncRNAs and mRNAs. Volcano plot of differentially expressed lncRNAs (A) and mRNAs (B). Heat map showing hierarchical clustering of differentially expressed lncRNAs (C) and mRNAs (D). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 2. Top 20 downregulated mRNAs detected using microarray assays.
| Upregulated mRNA | Fold-change | Downregulated mRNA | Fold-change |
|---|---|---|---|
| ZFP57 | 320.0690793 | PDE4D | 14.2864131 |
| TRIM64B | 23.8886022 | ATP6V0C | 13.7441076 |
| IFIT1 | 12.94737 | KDM2A | 12.6422952 |
| NOG | 11.0887566 | CTD-2054N24.2 | 12.6253085 |
| CCL2 | 10.3518678 | BAG4 | 11.6855495 |
| TRIM49D1 | 8.9890274 | SERTAD2 | 11.4104957 |
| ABCD2 | 8.4682723 | C16orf80 | 10.8164539 |
| ATP8A2 | 7.5641813 | ZNF683 | 10.8078429 |
| ZNF613 | 7.4106288 | NR4A2 | 9.974607 |
| ST8SIA4 | 6.5840689 | FAM46B | 9.7873591 |
| ZNF626 | 6.5236618 | FAM46C | 9.2136941 |
| DPYSL4 | 6.3293118 | NR4A3 | 8.6363487 |
| SETX | 6.2286018 | AC005606.1 | 7.8296143 |
| CCL8 | 5.974498 | MED9 | 7.749753 |
| IFIT3 | 5.94552 | PMP22 | 7.4325852 |
| DIP2C | 5.9369192 | HIPK1 | 7.3956708 |
| IGIP | 5.9103333 | RPRD1B | 7.3006256 |
| KMO | 5.6836129 | CRY2 | 7.2847231 |
| C3AR1 | 5.6529898 | TMEM176A | 7.1065918 |
| CCDC122 | 5.5687108 | LINGO2 | 6.8759258 |
3.2. GO and KEGG Pathway Analysis
GO enrichment analysis of DEGs provided a measure of functional significance: the more the enrichment, the more the specificity. GO analysis revealed upregulated DEGs related to positive regulation of immune responses, regulation of defense responses, immune effector processes, and inflammatory responses (Fig. 2), and downregulated DEGs are mostly related to biological regulation, metabolic processes, regulation of biological processes, and regulation of cellular processes in biological functions (Supplementary Fig. 4A (2MB, pdf) ). Upregulated DEGs are involved in cellular components related to intrinsic and integral components of membranes (Fig. 2B). Downregulated DEGs are related to intracellular, cell components, membrane-bounded organelles, and intracellular organelles (Supplementary Fig. 4B (2MB, pdf) ). The enrichment of upregulated DEGs was observed to be associated with molecular functions, largely related to G-protein coupled receptor binding and transferase activity, transferring glycosyl groups (Fig. 2C). Downregulated DEGs were related to protein binding and organic cyclic compound binding (Supplementary Fig. 4C (2MB, pdf) ). Additionally, KEGG pathway analysis indicated that 19 pathways were significantly enriched among the DEGs. These included the TGF-β signaling pathway and complement and coagulation cascades in upregulated DEGs, (Fig. 2D, Supplementary Fig. 4D (2MB, pdf) ), which are important in viral infection immune processes.
Fig. (2).
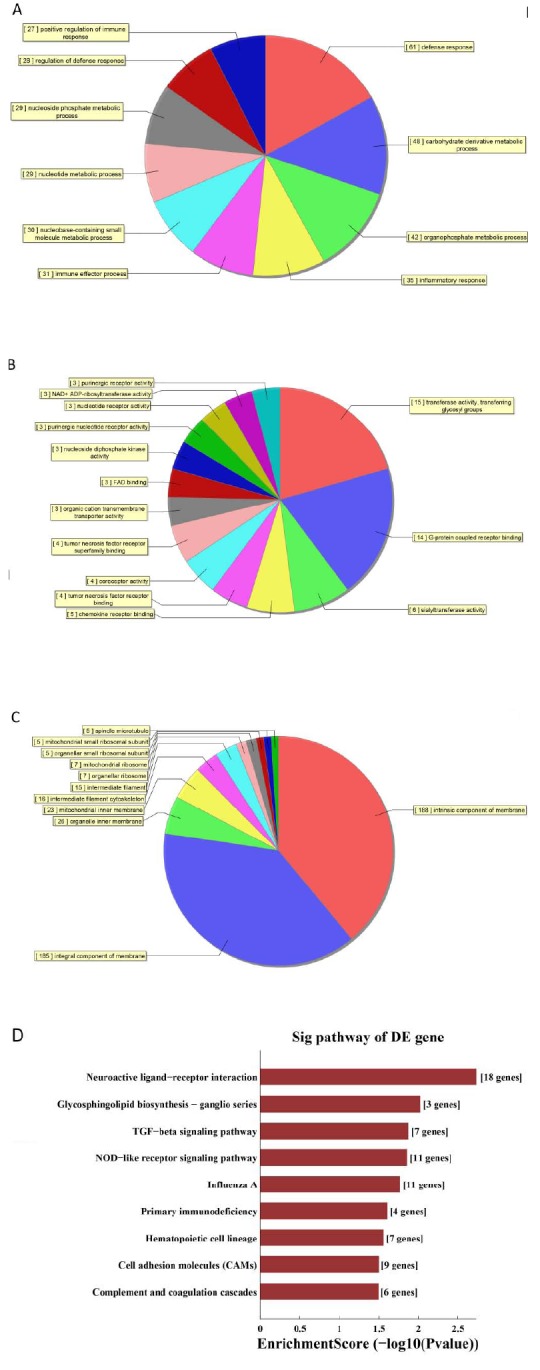
GO and KEGG pathway analyses. Enrichment of biological process (A), cellular component (B) and molecular function (C) in upregulated mRNA. (D) KEGG pathway enrichment analysis of upregulated mRNAs. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.3. Co-expression of lncRNAs and mRNAs
To further explore the potential regulatory mechanisms of the DELs, an lncRNA and mRNA network was constructed based on correlation analysis. We chose the top 20 up-/downregulated lncRNAs and mRNAs to construct co-expression networks according to the degree of correlation. Only the strong correlations of Pearson correlation coefficients (≥0.90) and threshold of FDR less than 0.05 were selected to construct the network, and Cytoscape was used to construct the co-expression network. The co-expression network consisted of 1196 co-expression relationships between the top 20 up/downregulated lncRNAs and mRNAs (Fig. 3A). As ZFP57 was found to be the most DEG, we analyzed the lncRNAs that correlated specifically with ZFP57. We found 213 lncRNAs that correlated with ZFP57 (Fig. 3B), indicating possible regulatory roles of many DELs for ZPF57. In KEGG analyses, we revealed that the TGF-β signaling pathway and complement and coagulation cascades involving the DEGs were viral infections immune process-related. Complement and coagulation cascades contained 1266 co-expression pairs, involving 5 DEGs and 798 DELs (Supplementary Fig. 1A (2MB, pdf) ). The TGF-β signaling pathway contained 1819 co-expression pairs, involving 7 DEGs and 1251 DELs (Supplementary Fig. 1B (2MB, pdf) ). The findings suggested that these DELs might also play important roles during viral infection immune processes. An lncRNA-pathway network was constructed to identify the possible functions of lncRNAs, thus supporting that the pathway is regulated by the corresponding lncRNAs. Eleven lncRNAs were linked with 11 enriched pathways, including lncRNA T039096, which was linked with the TGF-β signaling pathway (Fig. 3C). Besides, the mRNA-pathway network was constructed to identify the mRNAs connecting the pathways. The results showed 147 mRNAs to be linked with 21 pathways (Fig. 3D). TGF-beta signaling pathway and complement and coagulation cascades were connected by HLA-DOB, CD19, CR2 and CREBBP. The outcome suggesting these DEGs and DELs participating in the pathways play a central role in regulating the CHB.
Fig. (3).
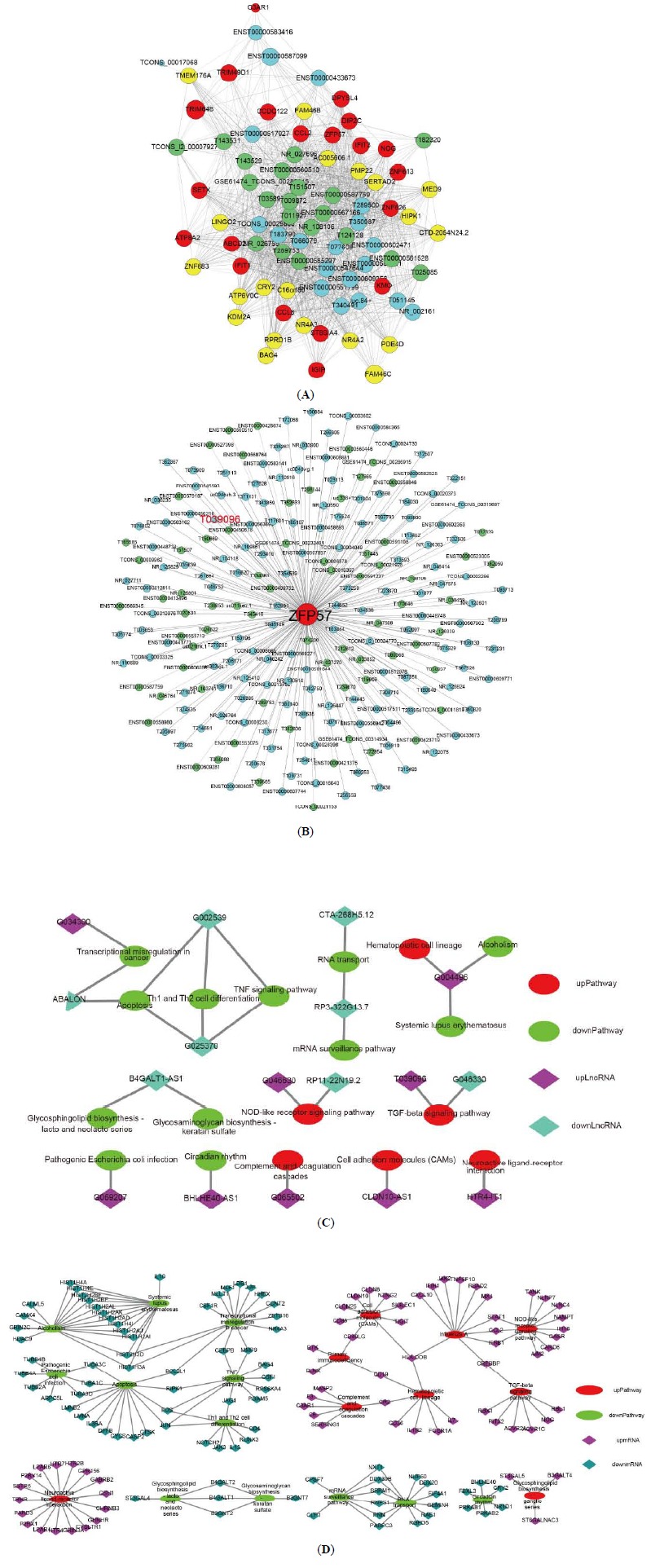
lncRNA-mRNA co-expression network. (A) Co-expression relationships between top 20 up/downregulated lncRNAs and mRNAs. (B) The differentially expressed lncRNAs that correlated with ZFP57. Blue nodes, and green nodes represent upregulated lncRNAs, and downregulated lncRNAs, respectively. (C) lncRNA-pathway network. (D) mRNA-pathway network. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4. Cis-regulation Analysis
As lncRNAs are physically linked to the loci encoding them, they may have the ability to function during or immediately following the process of transcription. To understand the cis-regulation of lncRNAs in CHB, the lncRNAs and the associated protein-coding genes that were screened by Pearson correlation coefficient analysis were further mapped to their genomic loci. We examined the top 10 up/downregulated lncRNAs to identify their nearby coding genes. Each lncRNA had a different number of neighboring coding genes. The number of adjacent coding genes ranged from 0 to 5 (Fig. 4A). These networks provide valuable clues about these lncRNAs and their flanking coding genes. In addition, we noticed that several of the DELs targeted ZFP57, the most upregulated expressed DEG. These included uc.362, HLA-F-AS1, and T039096 (Fig. 4B). Interestingly, we found that T039096 was also correlated with ZFP57 in lncRNA and mRNA co-expression analyses (Fig. 2B), indicating a possible regulatory role of T039096 for ZFP57.
Fig. (4).
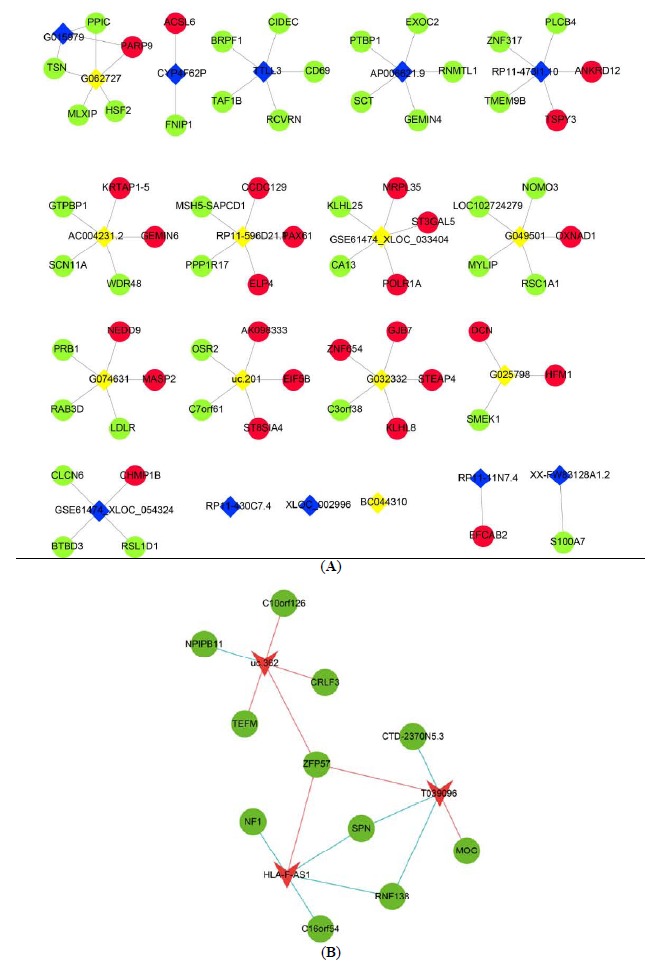
Cis-regulation prediction among lncRNAs. (A) 10 up/downregulated lncRNAs and their potential cis-regulated adjacent genes. Red nodes, yellow nodes, blue nodes, and green nodes represent upregulated mRNAs, downregulated mRNAs, upregulated lncRNAs, and downregulated lncRNAs, respectively. (B) Cis-correlated with ZFP57. Red triangles represent upregulated lncRNA, green nodes represent mRNAs. The red line represents positive correlation, the blue line represents negative correlation. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.5. Construction of the ceRNA Networks
We predicted ceRNAs for the top 10 up/downregulated lncRNAs, and the ceRNAs were intersected with the DE mRNAs and miRNA (Supplementary Fig. 2 (2MB, pdf) ). The result revealed 275 mRNAs and 632 miRNAs acting as ceRNAs of the top 10 up/downregulated lncRNAs. Because ZFP57 was the most upregulated expressed DEG, we constructed ZFP57-related ceRNA networks based on lncRNA/miRNA and miRNA/mRNA interactions. The networks represented the ZFP57-specific ceRNA interactome, and the ceRNA network covered 3 lncRNAs and 5 miRNAs, with 20 edges (Fig. 5). lncRNAs shared a common binding site involving the miRNA response element. lncRNAs such as ENST00000525233, were the ceRNA of hsa-miR-508-5p, hsa-miR-766-3p, hsa-miR-6849-3p, and hsa-miR-6720-5p; ENST00000399123 was the ceRNA of hsa-miR-766-3p, hsa-miR-6849-3p, and hsa-miR-6720-5p too. The DE ceRNAs might provide clues to functions of ZFP57 in CHB.
Fig. (5).
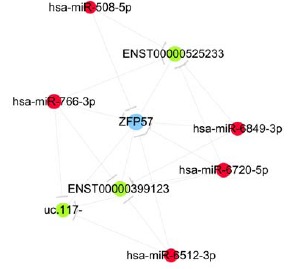
ZFP57-specific ceRNA networks. Red nodes and green nodes represent miRNA and lncRNA, respectively. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.6. Validation and Association Analysis
Since ZFP57 was mostly found to be the DEG, whose fold change reached 320, T039096 was correlated with ZFP57 in lncRNA and mRNA co-expression, cis-regulation, and in lncRNA-pathways network analyses. Hence, we further validated the expression of ZFP57 and T039096 in 30 CHB and 30 ASC patients using qRT-PCR. The results showed that the expression of ZFP57 and T039096 increased in CHB compared to ASC (Fig. 6). Our results demonstrated the level of ZFP57 and T039096 expression by qRT-PCR which were consistent with the microarray analysis. Moreover, serum ZFP57 levels were found to be significantly higher in CHB than in ASC by ELISA methods analysis (Supplementary Fig. 5 (2MB, pdf) ). In addition,the expressions of ZFP57 and T039096 in 30 CHB and ASC sample were certified by qRT-PCR, and we found that there was no significantly different expression of lncRNA-T039096 and mRNA-ZFP57 between 30 CHB or 30 ASC samples. The similar results were found in three CHB and ASC samples analysis. (Supplementary Fig. 3 (2MB, pdf) ). Furthermore, based on the qRT-PCR result, we revealed that mRNA-ZFP57 was associated with ALT in the connection between mRNA-ZFP57 and clinical signature. CHB patients with increased ALT had a higher expression of mRNA-ZFP57 (Supplementary Table 2 (2MB, pdf) ). All the results demonstrated the specificity and accuracy of ZFP57 and T039096, which could serve as biomarkers for CHB diagnosis and treatment.
Fig. (6).
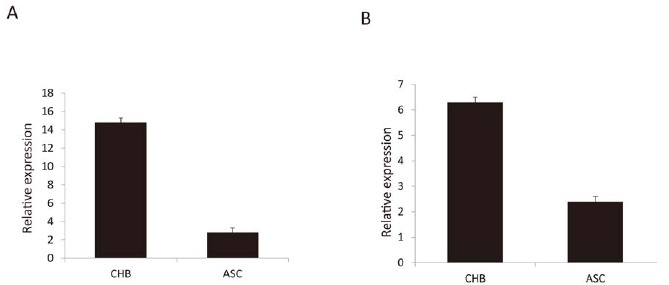
Validation of the expression of ZFP57 and T039096 by qRT-PCR. (A) ZFP57, (B) T039096, P < 0.05. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
lncRNAs are now emerging as regulators of diverse biological functions. Few studies have confirmed that lncRNAs play an important role in viral infection and inflammatory responses [25]. However, most studies have focused on HBV-related liver cancer and cirrhosis. There has been little research on HBV infection, only HBV or HBX can affect the expression of some lncRNAs in host cells [26]. In this research, we identified 5550 lncRNAs that were DE. By co-expression, cis-regulatory, and lncRNA-pathway network analyses, we identified that T039096 was correlated with ZFP57, which was predominantly DEG. By extending samples in 30 CHB and 30 ASC, the qPCR analysis revealed the expression of T039096 to be higher in CHB compared to ASC, suggesting that lncRNAs have great potential in elucidating the pathogenesis of, and targeted biomarker in CHB. Huang et al., found that the level of lncRNA DBH-AS1 was positively correlated with HBsAg, suggesting that DBH-AS1 might be related to HBV infection [27]. By receiver operating characteristic and area under the curve analyses, Lu et al., found that lncRNA uc003wbd and lncRNA AF085935 were observed at aberrant serum levels in healthy individuals and in HBV patients, which demonstrates that both lncRNA uc003wbd and lncRNA AF085935 are potential biomarkers of health and for HBV screening. All this research supports that lncRNAs represent important mechanisms in CHB and could be potential targets used in the diagnosis and treatment [28]. Throughout the microarray and bioinformatics analysis, we found two outstanding targets, lncRNA-T039096 and mRNA-ZFP57 which could serve as biomarkers in further research, diagnosis and treatment in CHB. However, owing to the small sample size in our study, these results regarding correlations should be treated with caution.
GO analysis revealed upregulated DEGs related to positive regulation of immune responses, regulation of defense responses, immune effector processes, and inflammatory responses in biological processes, which have close relationships with immunological processes. It is now well recognized that efficient control of viral infections requires the coordinated action of both innate and adaptive immune responses. During HBV infection, the presence of HBV-specific antibody-producing B-cells and functional HBV-specific T-cells ultimately determines HBV infection outcomes [29]. The difference between ASC and CHB is the host immune response to HBV infection, nevertheless, the mechanism of the immune response to this host is not clear. Some research revealed that bacterial-induced epigenetic deregulations may affect the host immune function either to promote host defense or to allow pathogen persistence [30]. So, we hypothesized that lncRNA could participate in the host immune response during HBV persistent infection. Our findings are in accordance with the studies on HBV infection and the immune process, in which the main enrichments were those corresponding to the innate and adaptive immunity responses. Additionally, we evaluated these DEGs by KEGG pathway analysis and identified 19 associated pathways, including the TGF-β signaling pathway and complement and coagulation cascades, which are linked with cirrhosis in HBV infection and in hepatocellular carcinoma [31, 32]. These results indicated that DGEs participate in HBV-mediated progression of CHB to liver cirrhosis and hepatocellular carcinoma.
Comprehensive analysis of lncRNA and mRNA expression profiles gives us a better understanding of the biological functions of DEGs and DELs in CHB. Therefore, we performed a correlation analysis of the DEGs and DELs by bioinformatics methods (co-expression, cis-regulation and lncRNA-pathway networks), and identified abundant correlations between the transcripts of the two groups. The correlations between lncRNAs and mRNAs suggest a possibility that lncRNAs function through upregulated or downregulated expression of their associated mRNAs. Importantly, we found that ZFP57, the most upregulated DEG, was associated with T039096. In addition, qPCR result showed that CHB patients had a higher expression of ZFP57, suggesting the potential for clinical diagnostic use. Although the results of the present study require further experimental verification, they provide insight into the significant role that lncRNAs have in the physiological and biochemical responses to mRNA in CHB.
Increasing evidence indicates the crucial role of lncRNAs in the ceRNA network in terms of modulating other RNA transcripts [33]. Despite the role of lncRNAs as ceRNAs having been extensively studied in HBV-hepatocellular carcinoma [34], the relevance of lncRNAs serving as ceRNAs in CHB is unknown, but expected. Our present study identified a ZFP57-specific ceRNA interactome, and the ceRNA network covered 3 lncRNAs, 5 miRNAs, and 20 edges. Interestingly, all the 5 miRNAs were found to be DE amongst CHB patients and ASC individuals in our previous research [35]. We suspect that the 3 lncRNAs play important roles involving ZFP57 by competing with the 5 miRNAs. However, further research is needed to test whether ZFP57 functions through these ceRNAs in HBV during CHB infection.
CONCLUSION
In summary, our comprehensive analysis provide novel knowledge on mRNAs and lncRNAs at the transcriptomic level regarding CHB. These results and conclusions will serve as important resources for future experiments that further investigate the role and regulation of lncRNAs and mRNAs in CHB. Furthermore, these findings suggest that lncRNA-T039096 and mRNA-ZFP57 may be the novel targets for the diagnosis and treatment of CHB.
Table 3. Top 20 downregulated lncRNAs detected using microarray assays.
| Upregulated lncRNA | Fold-change | Downregulated lncRNA | Fold-change |
|---|---|---|---|
| ENST00000587099 | 31.9073982 | G035138 | 34.6300604 |
| NR_002161 | 29.7776101 | G035138 | 34.6300604 |
| T350987 | 26.42205 | G035138 | 34.6300604 |
| ENST00000433673 | 14.5030437 | G033377 | 23.7035516 |
| TCONS_00029808 | 14.2370539 | G033377 | 23.7035516 |
| T289500 | 13.3070189 | G033377 | 23.7035516 |
| T051145 | 12.0540713 | G033377 | 23.7035516 |
| ENST00000583416 | 11.4584055 | G033377 | 23.7035516 |
| T066079 | 11.2554843 | G033377 | 23.7035516 |
| ENST00000583141 | 10.8858793 | G033377 | 23.7035516 |
| ENST00000551799 | 10.7708094 | G033377 | 23.7035516 |
| ENST00000551799 | 10.7708094 | G033377 | 23.7035516 |
| ENST00000551799 | 10.7708094 | LINC01135 | 12.8882496 |
| ENST00000551799 | 10.7708094 | G005485 | 12.055096 |
| ENST00000551799 | 10.7708094 | G005485 | 12.055096 |
| T077608 | 9.9606089 | G005485 | 12.055096 |
| T183790 | 9.3742262 | G005485 | 12.055096 |
| T340491 | 9.34115 | G005485 | 12.055096 |
| ENST00000602471 | 9.0651417 | G005485 | 12.055096 |
| ENST00000609356 | 8.7903786 | G067750 | 11.8474174 |
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- lncRNAs
Long non-coding RNAs
- CHB
Chronic Hepatitis B
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- ceRNA
Competing endogenous RNA
- HBV
Chronic Hepatitis B Virus
- ASC
Asymptomatic HBV Carriers
- ALT
Alanine Aminotransferase
- mRNAs
Protein-coding RNAs
- lncRNA
Long non-coding RNAs
- AASLD
American Association for the Study of Liver Diseases
- PBMCs
Peripheral Blood Mononuclear Cells
- DE
Differentially Expressed
- UTR
Untranslated Regions
- DEGs
Differentially Expressed Genes
- DELs
Differentially Expressed lncRNAs
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Clinical Research Ethics Committee of the College of Medicine, Zhejiang University, China (Approval no. 2018967).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the experimental procedures on patients were definitively diagnosed according to the American Association for the Study of Liver Diseases (AASLD) 2016, Practice Guidelines for Treatment of Chronic Hepatitis B and were also in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All the patients provided written informed consent for participation.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the NCBI GEO database at (https://www.ncbi.nlm.nih.gov/gds/) with accession number GSE136343.
FUNDING
This work was supported by funds received from the National Natural Science Foundation of China (No. 81571953), the major national S&T projects for infectious diseases (2018ZX10301401-006, 2017ZX10202202).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ren H. The experience of management of chronic hepatitis B in China. J. Viral Hepat. 2017;24(Suppl. 1):4–5. doi: 10.1111/jvh.12793. [http://dx.doi.org/10.1111/jvh.12793]. [PMID: 29082643]. [DOI] [PubMed] [Google Scholar]
- 2.Soriano V., Young B., Reau N. Report from the International Conference on Viral Hepatitis - 2017. AIDS Rev. 2018;20(1):58–70. [http://dx.doi.org/10.24875/AIDSRev.M17000012]. [PMID: 29369303]. [PubMed] [Google Scholar]
- 3.Pungpapong S., Kim W.R., Poterucha J.J. Natural history of hepatitis B virus infection: An update for clinicians. Mayo Clin. Proc. 2007;82(8):967–975. doi: 10.4065/82.8.967. [http://dx.doi.org/10.4065/82.8.967]. [PMID: 17673066]. [DOI] [PubMed] [Google Scholar]
- 4.Busch K., Thimme R. Natural history of chronic hepatitis B virus infection. Med. Microbiol. Immunol. (Berl.) 2015;204(1):5–10. doi: 10.1007/s00430-014-0369-7. [http://dx.doi.org/10.1007/s00430-014-0369-7]. [PMID: 25540037]. [DOI] [PubMed] [Google Scholar]
- 5.Romeo R., Petruzziello A., Pecheur E.I., Facchetti F., Perbellini R., Galmozzi E., Khan N.U., Di Capua L., Sabatino R., Botti G., Loquercio G. Hepatitis delta virus and hepatocellular carcinoma: An update. Epidemiol. Infect. 2018;146(13):1612–1618. doi: 10.1017/S0950268818001942. [http://dx.doi.org/10.1017/S0950268818001942]. [PMID: 29991359]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagnelli E., Felaco F.M., Rapicetta M., Stroffolini T., Petruzziello A., Annella T., Chionne P., Pasquale G., Filippini P., Peinetti P. Interaction between HDV and HBV infection in HBsAg-chronic carriers. Infection. 1991;19(3):155–158. doi: 10.1007/BF01643238. [DOI] [PubMed] [Google Scholar]
- 7.Stroffolini T., Sagnelli E., Rapicetta M., Felaco F.M., Filippini P., Annella T., Petruzziello A., Chionne P., Sarrecchia B., Piccinino F. Hepatitis B virus DNA in chronic HBsAg carriers: Correlation with HBeAg/anti-HBe status, anti-HD and liver histology. Hepatogastroenterology. 1992;39(1):62–65. [PubMed] [Google Scholar]
- 8.You C.R., Lee S.W., Jang J.W., Yoon S.K. Update on hepatitis B virus infection. World J. Gastroenterol. 2014;20(37):13293–13305. doi: 10.3748/wjg.v20.i37.13293. [http://dx.doi.org/10.3748/wjg.v20.i37.13293]. [PMID: 25309066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu R., Fan R., Hou J. Chronic hepatitis B virus infection: Epidemiology, prevention, and treatment in China. Front. Med. 2014;8(2):135–144. doi: 10.1007/s11684-014-0331-5. [http://dx.doi.org/10.1007/s11684-014-0331-5]. [PMID: 24810645]. [DOI] [PubMed] [Google Scholar]
- 10.Alexander R.P., Fang G., Rozowsky J., Snyder M., Gerstein M.B. Annotating non-coding regions of the genome. Nat. Rev. Genet. 2010;11(8):559–571. doi: 10.1038/nrg2814. [http://dx.doi.org/10.1038/nrg2814]. [PMID: 20628352]. [DOI] [PubMed] [Google Scholar]
- 11.Ponting C.P., Oliver P.L., Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006. [http://dx.doi.org/10.1016/j.cell.2009.02.006]. [PMID: 19239885]. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang J., Hu J., Chen J.L. lncRNAs regulate the innate immune response to viral infection. Wiley Interdiscip. Rev. RNA. 2016;7(1):129–143. doi: 10.1002/wrna.1321. [http://dx.doi.org/10.1002/wrna.1321]. [PMID: 26667656]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortes P., Morris K.V. Long noncoding RNAs in viral infections. Virus Res. 2016;212:1–11. doi: 10.1016/j.virusres.2015.10.002. [http://dx.doi.org/10.1016/j.virusres.2015.10.002]. [PMID: 26454188]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [http://dx.doi.org/10.1016/j.cell.2011.07.014]. [PMID: 21802130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi: 10.1038/nature12986. [http://dx.doi.org/10.1038/nature12986]. [PMID: 24429633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karreth F.A., Pandolfi P.P. ceRNA cross-talk in cancer: When ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [http://dx.doi.org/10.1158/2159-8290.CD-13-0202]. [PMID: 24072616]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L.J., Zhao W., Tao S.S., Leng R.X., Fan Y.G., Pan H.F., Ye D.Q. Competitive endogenous RNA network: Potential implication for systemic lupus erythematosus. Expert Opin. Ther. Targets. 2017;21(6):639–648. doi: 10.1080/14728222.2017.1319938. [http://dx.doi.org/10.1080/14728222.2017.1319938]. [PMID: 28406715]. [DOI] [PubMed] [Google Scholar]
- 18.Lai K., Jia S., Yu S., Luo J., He Y. Genome-wide analysis of aberrantly expressed lncRNAs and miRNAs with associated co-expression and ceRNA networks in β-thalassemia and hereditary persistence of fetal hemoglobin. Oncotarget. 2017;8(30):49931–49943. doi: 10.18632/oncotarget.18263. [http://dx.doi.org/10.18632/oncotarget.18263]. [PMID: 28624809]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lü M.H., Tang B., Zeng S., Hu C.J., Xie R., Wu Y.Y., Wang S.M., He F.T., Yang S.M. Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene. 2016;35(27):3524–3534. doi: 10.1038/onc.2015.413. [http://dx.doi.org/10.1038/onc.2015.413]. [PMID: 26549025]. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H., Ma R., Zou S., Wang Y., Li Z., Li W. Reconstruction and analysis of the lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveal functional lncRNAs in rheumatoid arthritis. Mol. Biosyst. 2017;13(6):1182–1192. doi: 10.1039/c7mb00094d. [http://dx.doi.org/10.1039/C7MB00094D]. [PMID: 28470264]. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S., Zhu D., Li H., Feng C., Zhang W. Characterization of circRNA-associated-ceRNA networks in a senescence-accelerated mouse prone 8 brain. Molecular therapy. J. Am. Soc. Gene Ther. 2017;25(9):2053–2061. doi: 10.1016/j.ymthe.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrault N.A., Bzowej N.H., Chang K.M., Hwang J.P., Jonas M.M., Murad M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156. [http://dx.doi.org/10.1002/hep.28156]. [PMID: 26566064]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huntley R.P., Harris M.A., Alam-Faruque Y., Blake J.A., Carbon S., Dietze H., Dimmer E.C., Foulger R.E., Hill D.P., Khodiyar V.K., Lock A., Lomax J., Lovering R.C., Mutowo-Meullenet P., Sawford T., Van Auken K., Wood V., Mungall C.J. A method for increasing expressivity of Gene Ontology annotations using a compositional approach. BMC Bioinformatics. 2014;15(1):155. doi: 10.1186/1471-2105-15-155. [http://dx.doi.org/10.1186/1471-2105-15-155]. [PMID: 24885854]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M., Goto S., Sato Y., Kawashima M., Furumichi M., Tanabe M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014;42(Database issue):D199–D205. doi: 10.1093/nar/gkt1076. [http://dx.doi.org/10.1093/nar/gkt1076]. [PMID: 24214961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kambara H., Niazi F., Kostadinova L., Moonka D.K., Siegel C.T., Post A.B., Carnero E., Barriocanal M., Fortes P., Anthony D.D., Valadkhan S. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res. 2014;42(16):10668–10680. doi: 10.1093/nar/gku713. [http://dx.doi.org/10.1093/nar/gku713]. [PMID: 25122750]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyo B., Nicholson S.A., Arbuthnot P.B. The role of long non-coding RNAs in hepatitis B virus-related hepatocellular carcinoma. Virus Res. 2016;212:103–113. doi: 10.1016/j.virusres.2015.07.025. [http://dx.doi.org/10.1016/j.virusres.2015.07.025]. [PMID: 26239319]. [DOI] [PubMed] [Google Scholar]
- 27.Huang J.L., Ren T.Y., Cao S.W., Zheng S.H., Hu X.M., Hu Y.W., Lin L., Chen J., Zheng L., Wang Q. HBx-related long non-coding RNA DBH-AS1 promotes cell proliferation and survival by activating MAPK signaling in hepatocellular carcinoma. Oncotarget. 2015;6(32):33791–33804. doi: 10.18632/oncotarget.5667. [http://dx.doi.org/10.18632/oncotarget.5667]. [PMID: 26393879]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J., Xie F., Geng L., Shen W., Sui C., Yang J. Investigation of serum lncRNA-uc003wbd and lncRNA-AF085935 expression profile in patients with hepatocellular carcinoma and HBV. Tumour Biol. 2015;36(5):3231–3236. doi: 10.1007/s13277-014-2951-4. [http://dx.doi.org/10.1007/s13277-014-2951-4]. [PMID: 25501706]. [DOI] [PubMed] [Google Scholar]
- 29.Bertoletti A., Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: Towards restoration of immune control of viral infection. Gut. 2012;61(12):1754–1764. doi: 10.1136/gutjnl-2011-301073. [http://dx.doi.org/10.1136/gutjnl-2011-301073]. [PMID: 22157327]. [DOI] [PubMed] [Google Scholar]
- 30.Bierne H., Hamon M., Cossart P. Epigenetics and bacterial infections. Cold Spring Harb. Perspect. Med. 2012;2(12):a010272. doi: 10.1101/cshperspect.a010272. [http://dx.doi.org/10.1101/cshperspect.a010272]. [PMID: 23209181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y.H., Ai X., Liu F.Y., Liang H.F., Zhang B.X., Chen X.P. c-Jun N-terminal kinase inhibitor favors transforming growth factor-β to antagonize hepatitis B virus X protein-induced cell growth promotion in hepatocellular carcinoma. Mol. Med. Rep. 2016;13(2):1345–1352. doi: 10.3892/mmr.2015.4644. [http://dx.doi.org/10.3892/mmr.2015.4644]. [PMID: 26648552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L., Lei Q.S., Kong L.N., Zhang S.J., Qin B. Gene expression profile after knockdown of USP18 in Hepg2.2.15 cells. J. Med. Virol. 2017;89(11):1920–1930. doi: 10.1002/jmv.24819. [http://dx.doi.org/10.1002/jmv.24819]. [PMID: 28369997]. [DOI] [PubMed] [Google Scholar]
- 33.Ulitsky I. Interactions between short and long noncoding RNAs. FEBS Lett. 2018;592(17):2874–2883. doi: 10.1002/1873-3468.13085. [http://dx.doi.org/10.1002/1873-3468.13085]. [PMID: 29749606]. [DOI] [PubMed] [Google Scholar]
- 34.Fan H., Lv P., Mu T., Zhao X., Liu Y., Feng Y., Lv J., Liu M., Tang H. LncRNA n335586/miR-924/CKMT1A axis contributes to cell migration and invasion in hepatocellular carcinoma cells. Cancer Lett. 2018;429:89–99. doi: 10.1016/j.canlet.2018.05.010. [http://dx.doi.org/10.1016/j.canlet.2018.05.010]. [PMID: 29753758]. [DOI] [PubMed] [Google Scholar]
- 35.Hou X., Liang Y., Chen J., Wei Y., Zeng P., Wang L., Lu C., Diao H. Expression profiling of cellular MicroRNA in asymptomatic HBsAg carriers and chronic hepatitis B patients. BioMed Res. Int. 2017:20176484835. doi: 10.1155/2017/6484835. [http://dx.doi.org/10.1155/2017/6484835]. [PMID: 28913356]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
The data supporting the findings of the article is available in the NCBI GEO database at (https://www.ncbi.nlm.nih.gov/gds/) with accession number GSE136343.


