Abstract
CB1 receptor antagonists disrupt operant responding for food and drug reinforcers, and cue-induced reinstatement of cocaine and heroin seeking. Conversely, enhancing endocannabinoid signaling, particularly 2-arachidonyl glycerol (2-AG), by inhibition of monoacyl glycerol lipase (MAGL), may facilitate some aspects of reward seeking. To determine how endocannabinoid signaling affects responding to reward-predictive cues, we employed an operant task that allows us to parse the incentive motivational properties of cues. Rats were required to nosepoke during an intermittent audiovisual incentive cue (IC) to obtain a 10% sucrose reward. The CB1 receptor antagonist, rimonabant, dose-dependently decreased the response ratio (rewarded ICs/total presented) and active nosepokes per IC, while it increased the latency to respond to the cue and obtain the reward, indicating an overall decrease in both the choice and vigor of responding. Yet rats persisted in entering the reward cup. Using a modified version of the task, the novel MAGL inhibitor MJN110 increased the response ratio, decreased the latencies to respond to the IC and enhanced active nosepokes per IC, indicating a facilitation of cue-induced reward seeking. These effects were blocked by a subthreshold dose of rimonabant. Finally, MJN110 did not alter consumption of freely available sucrose within volumes obtained in the operant task. Together these data demonstrate blocking endocannabinoid tone at the CB1 receptor attenuates the ability of cues to induce reward seeking, while some aspects of motivation for the reward are retained. Conversely, enhancing 2-AG signaling at CB1 receptors facilitates IC responding and increases the motivational properties of the IC.
Keywords: CB1 receptor, endocannabinoid, incentive cue, MAGL inhibitor, MJN110, rimonabant, operant, reward predictive cues
1. Introduction
Previously neutral cues that are repeatedly paired with a hedonic reward, such as food or drugs of abuse, can themselves acquire incentive properties and elicit reward- or drug-seeking behaviors (Robinson et al., 2014). The incentive salience attributed to the cues depends heavily on mesolimbic dopamine neurons that project from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) (Berridge, 2007). Natural rewards and drugs of abuse, including opiates, alcohol, nicotine, amphetamine, cocaine, and cannabinoids, excite dopamine cells in the VTA and subsequently increase dopamine levels in the NAc (Di Chiara and Imperato, 1988; Hernandez and Hoebel, 1988; Cheer et al., 2004). Consequently, NAc dopamine release facilitates responding to reward-predictive information, while blockade of NAc dopamine receptors, as well as chemogenetic activation of inhibitory VTA gamma-aminobutyric acid (GABA) neurons, decreases operant cue responding (Di Ciano et al., 2001; Wakabayashi et al., 2004; Nicola, 2010; Wakabayashi et al., 2018).
Mounting evidence indicates that endocannabinoids (eCBs) regulate dopaminergic induced reward seeking by increasing burst activity of dopaminergic neurons in the VTA (Riegel and Lupica, 2004; Perra et al., 2005; Matyas et al., 2008; Pan et al., 2008; Oleson et al., 2012; Oleson and Cheer, 2012). Cannabinoid type 1 (CB1) receptors are densely expressed in reward-related brain regions, including the VTA and NAc (Herkenham et al., 1991; Glass et al., 1997; Wang et al., 2003). Although VTA dopamine neurons do not express CB1 receptors, they are regulated indirectly by eCBs via CB1 receptors on glutamatergic and GABAergic inputs (Matyas et al., 2008). Under normal conditions, VTA dopamine neurons are under tonic GABA-mediated inhibition (Johnson and North, 1992). When animals are presented with reward-predictive incentive stimuli, VTA dopamine neurons fire at high frequency (Schultz et al., 1997). Consequently, eCBs, particularly the more abundant 2-arachidonyl glycerol (2-AG), are released on demand from dopamine neurons and retrogradely activate CB1 receptors on presynaptic GABA terminals, leading to suppressed GABAergic neurotransmission in the VTA and disinhibition of postsynaptic dopamine neurons (Wilson and Nicoll, 2001; Szabo et al., 2002; Freund et al., 2003). The resulting increase in NAc dopamine concentrations can be blocked by the CB1 receptor antagonists rimonabant and AM251 (Cheer et al., 2007; Sperlagh et al., 2009). Exogenous cannabinoids including Δ9-tetrahydrocannabinol (Δ9-THC) also enhance dopamine release in the NAc (Chen et al., 1991; Tanda et al., 1997).
The prevailing theory is that exogenous and endocannabinoid activation of VTA CB1 receptors indirectly enhances dopamine neurotransmission by disinhibition of GABA neurons resulting in facilitation of reward-seeking behaviors. However, behavioral studies are much less clear. Previous work has demonstrated that augmenting 2-AG tone by systemic JZL184, a monoacyl glycerol lipase (MAGL) inhibitor, increases the breakpoint in a progressive ratio food self-administration task, and decreases the latency to respond in an intracranial self-stimulation (ICSS) model (Oleson et al., 2012). Further, intra-VTA infusions of rimonabant decreases appetitive food seeking, while intra-VTA JZL184 modestly decreases the latency to respond in an ICSS model (Oleson et al., 2012). Still others have shown attenuation or no change in ICSS responding by MAGL inhibitors (Wiebelhaus et al., 2015). Rimonabant and Δ9-THC also produced inconsistent findings in rat and mice ICSS studies (De Vry et al., 2004; Vlachou et al., 2007; Katsidoni et al., 2013; Wiebelhaus et al., 2015). To determine how endocannabinoid signaling specifically affects cue processing, we tested rimonabant and the novel MAGL inhibitor, MJN110, in an operant behavioral model that is dependent entirely on cue processing in order to obtain the reward (10% sucrose). We decided to use the novel MAGL inhibitor MJN110 as it is much more efficacious at enhancing 2-AG levels in the brain than JZL184, and has less activity at other serine hydrolase systems (Niphakis et al., 2013). We hypothesized that the CB1 receptor antagonist rimonabant will disrupt responding to reward-predictive incentive cues (ICs), while elevation of 2-AG levels by MJN110 and the subsequent increase in tone at CB1 receptors will enhance the motivational properties of the cues and facilitate responding. In this cue dependent operant task, we can characterize multiple aspects of cue processing, including the choice and vigor of responding to the cue. Thus, our study will help determine whether the more potent and specific MAGL inhibitor, MJN110, is effective in regulating reward seeking by modulating incentive cue processing, and if this potential therapeutic may have efficacy for treating psychiatric disorders characterized by low eCB tone.
2. Material and methods
2.1. Subjects
Male Long Evans rats (~280-300 g) were obtained from Envigo (Indianapolis, IN, USA). The animals were individually housed under controlled ambient conditions (21-22 °C, 55 ± 10% relative humidity, inverted 12 h/12 h light/dark cycle, lights on at 3 PM). Behavioral experiments were conducted between 9 AM and 11 AM. Food and water were available ad libitum. All procedures complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865–23) and were approved by the Institutional Care and Use Committee at the University at Buffalo.
2.2. Drugs
MJN110 was provided by B. F. Cravatt and rimonabant was acquired from the National Institute on Drug Abuse Drug Supply Program (Rockville, MD, USA). MJN110 and rimonabant were freshly prepared on each treatment day and dissolved in a vehicle (V) solution of ethanol, emulphor (Solvay, Princeton, NJ, USA), and saline in a ratio of 1:1:18 (see (Niphakis et al., 2013)).
2.3. Behavioral apparatus
Behavioral testing occurred in operant chambers (Med-Associates, Georgia, VT) within a soundattenuating cubicle, equipped with two illuminated infrared nosepoke ports surrounding a centrally located liquid receptacle equipped with an infrared entry detector. The left port was designated as the active, the right as the inactive port. A dim white houselight and a speaker were located on the opposite wall. Each chamber was equipped with a pump to deliver 10% sucrose.
2.4. Training and behavioral paradigm
2.4.1. IC task training
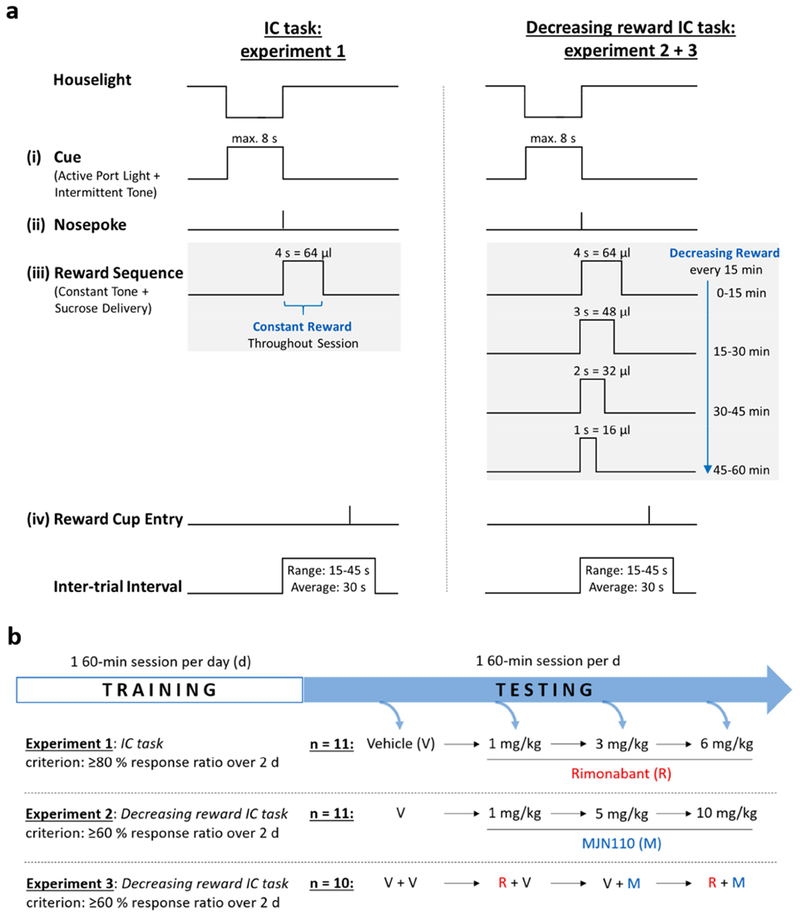
The IC task (Figure 1a, left panel) is modified from a previously described operant task (Wakabayashi et al., 2004; Yun et al., 2004; Ambroggi et al., 2011). Briefly, rats were first trained to nosepoke into the active nosepoke port to receive ~64 μl 10% sucrose. Initially, a tone and light combination (consisting of an intermittent 2.9 kHz, ~80 dB tone with a 25/20 ms tone-on/off pulse, illumination of the active port while the houselight was turned off) was present during the entire session, except when the rat entered the port. This combination of stimuli comprised the IC. After a correct nosepoke into the active port, the syringe pump was activated for 4 s. During this time, the nosepoke light was turned off, the houselight illuminated, and the audio cue changed from an intermittent to a constant tone; this constituted a conditioned stimulus (CS) distinct from the IC. There was no time-out period. The session ended when the rat received 130 rewards or after 1 h. Once rats received a minimum of 100 rewards for two consecutive days, the IC was put on a variable inter-trial interval (ITI) 30-s schedule, where it was presented for up to 30 s. Intervals between cue presentations were randomly selected from a Gaussian distribution (upper and lower limits 45 s and 15 s, respectively). Nosepokes into the active port during the IC terminated the interval and resulted in reward delivery and presentation of the CS. Nosepokes into the active port during the ITI, or during reward delivery, as well as entries into the inactive port, were not rewarded. Once rats responded to 80% or more ICs during two consecutive daily sessions, the IC length was shortened to 8 s, where rats had the opportunity to receive ~100 rewards during the 1-h daily session. Rats were run 5 days a week, and trained to a performance criterion of 80% responding to the ICs.
Figure 1: Schematic representation of one trial of the operant testing schedule in the incentive cue (IC) task and the decreasing reward IC task (a), and outline of the operant experiments in this study (b).
The sequence of events during the session includes (i) presentation of a reward-predictive IC, (ii) a nosepoke response, (iii) a sucrose reward that is kept constant in the IC task, but progressively decreased in the modified IC task, and (iv) collection of the reward.
2.4.2. Decreasing reward IC task
In the normal IC task, the sucrose reward was kept constant (64 μl) throughout the 1-h session. Under these conditions, rats generally respond to the majority of ICs (>85%), which presents a high ceiling that is suitable for measuring decreases in responding. To avoid this ceiling and examine treatments that may produce increases in responding, we used a modified version of the standard task (Figure 1a, right panel, decreasing reward IC task) that produced a lower overall baseline in responding. This was accomplished by progressively decreasing the reward volume every 15 min from ~64 μl to 48 μl, 32 μl, and finally 16 μl in the 1-h session. We controlled the volume by altering the time the pump was activated, from 4 s to 3 s, 2 s, and 1 s, respectively. During all trials, the CS duration matched the pump-on time, but the length of the IC remained constant (8 s). Rats were trained until they reached a stable performance of ≥60% responding to the ICs in the modified decreasing reward IC task.
2.5. Experimental design
Testing started when the rats showed a stable baseline performance of ≥80% (IC task; experiment 1) or ≥60% (modified IC task; experiment 2 and 3) responding to the ICs over two consecutive days. Each test day was followed by at least two days of training to re-establish baseline performance and ensure adequate washout of the drug. The test session parameters were identical to those used during the training. MJN110 (1, 5, and 10 mg/kg) and rimonabant (1, 3, and 6 mg/kg) were injected in an ascending manner starting with vehicle at a volume of 1 ml/kg bodyweight intraperitoneally (i.p.) 30 min prior to start of the behavioral sessions on test days (Figure 1b). Doses were based on previous studies (De Vries et al., 2001; Niphakis et al., 2013) and the ascending dose response was chosen to minimize the potential to develop tolerance. In experiment 1, the effects of rimonabant were examined under normal IC task conditions (Figure 1a, left panel) in rats (n = 11), while in experiment 2 the effects of MJN110 were tested in a separate group of rats (n = 11) in the decreasing reward IC task (Figure 1a, right panel). The decreasing reward IC task was also used in experiment 3 to test whether a subthreshold dose of rimonabant (0.3 mg/kg) blocks the effects of MJN110 (5 mg/kg). We employed a third cohort of rats (n = 10) for the experiment, and each received the CB1 receptor antagonist 15 min prior to administration of the MAGL inhibitor, followed by start of the behavioral session 30 min later. The following treatments were tested in a counterbalanced fashion: vehicle + vehicle (V+V), vehicle + rimonabant (V+R), vehicle + MJN110 (V+M), and rimonabant + MJN110 (R+M).
2.6. Free drinking
Another fourth group of subjects (n = 8) were tested in free drinking sessions with 10% sucrose to determine whether effects on appetite contribute to MJN110-induced alteration in operant responding in the IC task. One-hour free drinking sessions were conducted in the same operant chambers as the IC task. Before each session, 30-ml syringes containing 10% sucrose were loaded into syringe pumps. Sucrose was delivered during head entry detection and experimenters replaced empty syringes with pre-loaded syringes during sessions if necessary. Animals underwent at least three baseline intake sessions followed by one day off before drug tests. Once rats were acclimated to the task, i.p. injections of MJN110 (5 mg/kg) and vehicle (counterbalanced) were given 30 min prior to free intake testing sessions. Treatment sessions were separated by two days off to allow for washout. The dependent measure was the amount of sucrose dispensed (ml) in each session.
2.7. Measures and statistical analysis
In experiment 1-3, several metrics were investigated including the response ratio to the IC designating availability of sucrose (i.e. the ratio of active nosepokes resulting in a reward divided by the total number of ICs presented during a session). The nosepoke latency is the time to emit a rewarded nosepoke after the start of the IC presentation (i → ii, Figure 1a). The reward cup latency is the time to enter the receptacle after a rewarded nosepoke was emitted (iii → iv, Figure 1a). The ratio of active nosepokes per IC presented was also measured, and includes both rewarded and unrewarded nosepokes in the active port. The number of unrewarded active nosepokes per IC (i.e. those occurring during the ITI), the number of inactive nosepokes per IC (i. e. those performed in the inactive port), and the number of cup entries per reward were also determined. Latencies were measured only when a response was emitted and not when rats failed to respond to the IC.
Data are presented as means ± the standard error of the mean (±SEM). Statistical analyses were conducted by the software GraphPad Prism (version 8 for Windows). Statistical comparisons were conducted using one-way repeated measures (RM) ANOVAs (experiment 1 and 3) and two-way RM ANOVAs (experiment 2). All post-hoc pairwise comparisons were conducted using Holms-Sidak correction where the overall ANOVAs revealed statistical significance. Significance was set to α = 0.05. Cumulative free drinking data were analyzed using two-way RM ANOVA, Holm-Sidak post-hoc pairwise comparisons, while total volume obtained was analyzed with paired-samples t-test.
3. Results
3.1. Rimonabant attenuates responding to incentive cues.
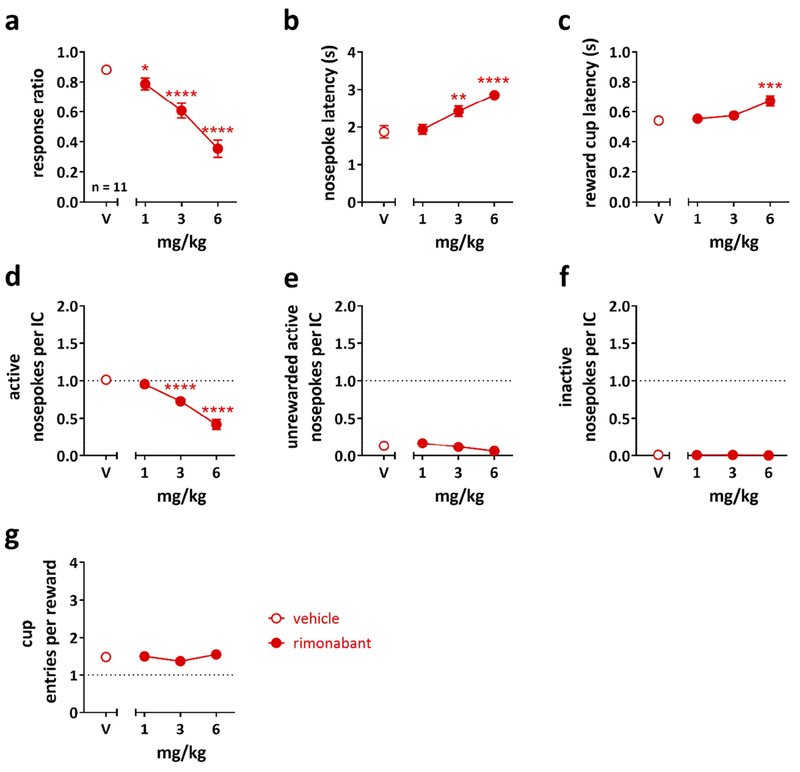
To examine whether CB1 receptor antagonism reduces responding to reward-predictive ICs, in experiment 1, rats (n = 11) were trained to nosepoke in response to the IC to receive a 64 μl sucrose reward. This volume of reward was kept constant over the course of the session. Rats were trained to a baseline response ratio of ≥80%. As depicted in Figure 2, the CB1 receptor antagonist rimonabant dose-dependently decreased responding to the ICs (Figure 2a, one-way RM ANOVA, main effect of treatment: F(3,30) = 48.87, P < 0.0001), increased the latencies to respond to the cue (Figure 2b, one-way RM ANOVA, main effect of treatment: F(3,30) = 20.07, P < 0.0001) and enter the reward cup after a rewarded nosepoke (Figure 2c, one-way RM ANOVA, main effect of treatment: F(3,30) = 6.9, P = 0.0011), and also reduced the number of active nosepokes per IC (Figure 2d, one-way RM ANOVA, main effect of treatment: F(3,30) = 40.79, P < 0.0001). One-way RM ANOVA yielded a main effect of treatment for the number of unrewarded active nosepokes per IC (Figure 2e, F(3,30) = 3.697, P = 0.0224). However, post-hoc analysis did not reveal a significant dose effect. We did not observe an effect on the number of inactive nosepokes (Figure 2f) indicating that the rats distinguished between inactive and active ports. Cup entries per reward (Figure 2g) were also unchanged by rimonabant treatment, indicating that sucrose-seeking persisted even as the response ratio dropped.
Figure 2: Cannabinoid type 1 (CB1) receptor antagonism decreased responding to incentive cues, while some aspects of motivation for the sucrose reward were retained.
Effects of the CB1 receptor antagonist rimonabant on (a) the response ratio, (b) the nosepoke latency, (c) the reward cup latency, (d) the number of active nosepokes per IC, (e) the number of unrewarded active nosepokes per IC, (f) the number of inactive nosepokes per IC, and (g) the number of cup entries per reward in the IC task in rats (n = 11). Data is expressed as mean ± SEM of an entire 1-h session. Statistically significant differences between drug dose and vehicle (V) as control treatment are indicated by asterisks (one-way repeated measures ANOVA, post-hoc Holm-Sidak correction, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
3.2. MJN110 enhances responding in the decreasing reward IC task.
In the modified version of the standard IC task used in experiment 2, the reward volume is decreased progressively every 15 min throughout the session from 64 μl to 48 μl, 32 μl, and 16 μl. This progression brings overall responding down and allowed us to investigate whether the MAGL inhibition increases responding to the IC in a second group of rats (n = 11). In the decreasing reward IC task, rats acquire lower baseline response ratios of ~60-70% for the entire 1-h session, compared to 80-90% baseline responding in the standard IC task.
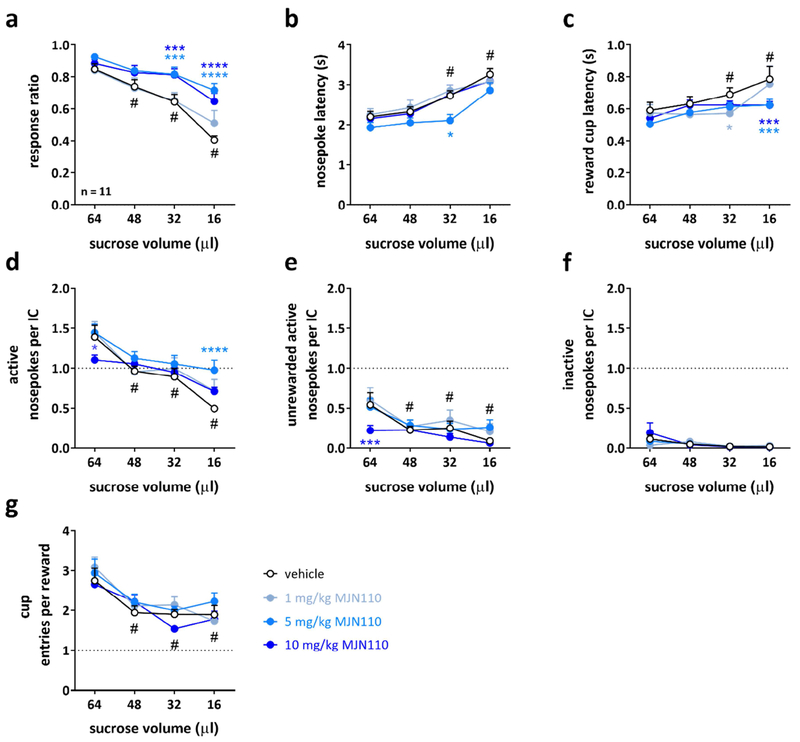
We first examined how different sucrose volumes alter responding in the decreasing reward IC task (Figure 3). Analysis of the vehicle data revealed an overall significant decrease in response ratio, increases in nosepoke and reward cup entry latencies, and decreases in active nosepokes per IC, unrewarded nosepokes per IC, and cup entries per reward at the lower volumes (e.g. 32 and 16 μl) compared to the 64 μl training volume (signified by a # in Figure 3). Together, these data indicate that the rats’ choice and motivation to respond to the IC depends upon, and is often proportional to the volume of the sucrose reward they receive. In both vehicle- and MJN110-treated rats, the response ratio was reduced at the lower reward volumes (e.g. 32 and 16 μl), but this decrease was attenuated after MJN110 treatment. Further, 5 mg/kg and 10 mg/kg MJN110 robustly increased responding to the ICs, particularly at the smaller reward volumes (Figure 3a, two-way RM ANOVA, main effect of treatment: F(3,30) = 11.07, P < 0.0001; main effect of sucrose volume: F(3,30) = 32.33, P < 0.0001; significant treatment x sucrose volume interaction: F(9,90) = 2.081, P = 0.0394). MJN110 5 mg/kg and 10 mg/kg significantly increased the response ratio at 32 μl and at 16 μl of sucrose compared to vehicle-treated animals. The lowest dose of MJN110 tested (1 mg/kg) did not change the response ratio. The MAGL inhibitor also decreased the nosepoke latency at 32 μl after 5 mg/kg MJN110 pretreatment (Figure 3b, two-way RM ANOVA, main effect of treatment: F(3,30) = 4.087, P = 0.0151; main effect of sucrose volume: F(3,30) = 44.88, P < 0.0001). The reward cup latency was also significantly decreased at 32 μl after 1 mg/kg, and at 16 μl after 5 mg/kg and 10 mg/kg MJN110 (Figure 3c, two-way RM ANOVA, main effect of sucrose volume: F(3,30) = 10.64, P < 0.0001; significant treatment x sucrose volume interaction: F(9,90) = 2.070, P = 0.0404). Together these data indicate that MJN110 increases the speed to respond to the IC primarily at the 5 mg/kg dose, and to obtain the reinforcer at the 5 and 10 mg/kg doses, with most of the enhancement observed at the lower reward volumes.
Figure 3: Elevating 2-arachidonyl glycerol levels increases responding to incentive cues and decreases latencies to respond and obtain the reward at the low reward sizes.
Effects of 1, 5, and 10 mg/kg MJN110 on (a) the response ratio, (b) the nosepoke latency, (c) the reward cup latency, (d) the number of active nosepokes per IC, (e) the number of unrewarded active nosepokes per IC, (f) the number of inactive nosepokes per IC, and (g) the number of cup entries per reward in the decreasing reward IC task in rats (n = 11). Data is expressed as mean ± SEM in 15-min bins. Statistically significant differences between drug and vehicle as control treatment are indicated by asterisks (two-way repeated measures ANOVA, post-hoc Holm-Sidak correction, * P < 0.05, *** P < 0.001, **** P < 0.0001), while # indicates significant differences between sucrose volumes compared to 64 μl after vehicle treatment only.
We next examined the type of responses emitted during the session. MJN110 increased the total number of active nosepokes per IC at 16 μl sucrose at 5 mg/kg (Figure 3d: two-way RM ANOVA, main effect of sucrose volume: F(3,30) = 32.07, P < 0.0001; significant treatment x sucrose volume interaction: F(9,90) = 2.604, P = 0.0102) while reducing the number of unrewarded active nosepokes per IC at 64 μl at 10 mg/kg (Figure 3e: two-way RM ANOVA, main effect of dose: F(3,30) = 3.174, P = 0.0384; main effect of sucrose volume: F(3,30) = 13.69, P < 0.0001). We did not observe an effect on the number of inactive nosepokes per IC (Figure 3f), which were rare events in all conditions. The overall number of cup entries per reward were sucrose volume sensitive, but MJN110 treatment had no effect on them.
3.3. Rimonabant blocks MJN110-induced increases in IC responding under decreasing reward volume conditions.
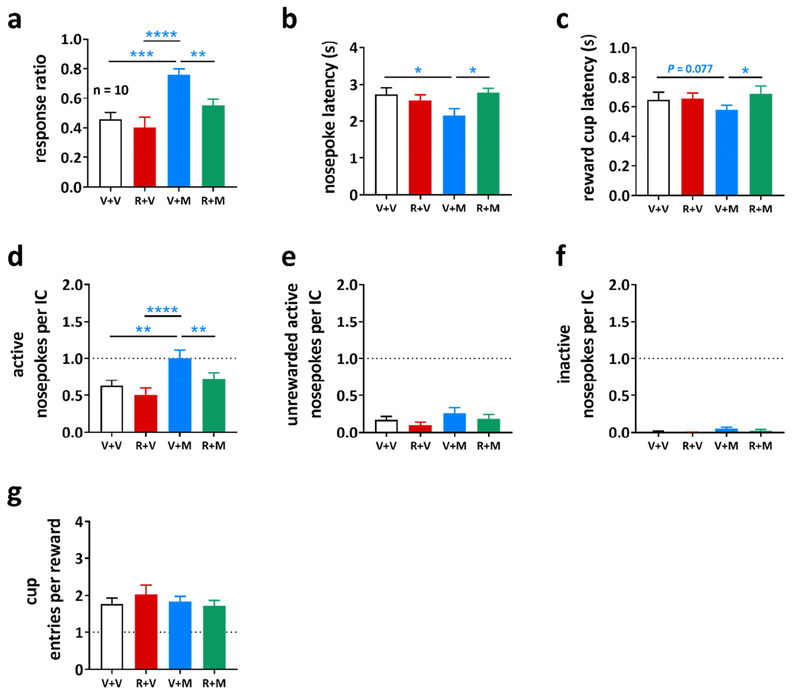
In experiment 3, we examined whether a CB1 receptor antagonist can attenuate the effect of MAGL inhibition on IC responding. For that purpose, we pretreated a third group of animals (n = 10) with a subthreshold dose of rimonabant (0.3 mg/kg) 15 min priot to administration of MJN110 (5 mg/kg). Doses were based on preliminary data (unpublished observations) and our findings in experiment 2. Given that the effects of MJN110 were mostly attributable to enhancement at the lower reward volumes in experiment 2, we conducted a planned comparison between pretreatments using the lower 32 and 16 μl volumes. We found that pretreatment with rimonabant blocked the ability of MJN110 to increase the response ratio (Figure 4a, one-way RM ANOVA, main effect of treatment: F(3,27) = 11.31, P < 0.0001), and the MJN110-induced decreases in both nosepoke (Figure 4b, one-way RM ANOVA, main effect of treatment: F(3,27) = 3.529, P = 0.0281) and reward cup latencies (Figure 4c, one-way RM ANOVA, main effect of treatment: F(3,27) = 3.382, P = 0.0326), however, while there was a trend towards a decrease in reward cup latency (P = 0.077), it did not reach significance. Likewise, rimonabant attenuated the MJN110-induced decrease in the total number of active nosepokes per IC (Figure 4d, one-way RM ANOVA, main effect of treatment: F(3,27) = 9.157, P = 0.0002). The other metrics were not affected by MAGL inhibition, or the combination of rimonabant and MJN110 (Figure 4e–g).
Figure 4: Effects of MJN110 on incentive cue (IC) responding are mediated through the cannabinoid type 1 receptor.
Effects of vehicle (V+V) rimonabant (R+V, 0.3 mg/kg), MJN110 (V+M, 5 mg/kg), and the combined treatment with rimonabant + MJN110 (R+M) on the (a) response ratio, (b) nosepoke latency, (c) reward cup latency, (d) number of active nosepokes per IC, (e) number of unrewarded active nosepokes per IC, (f) number of inactive nosepokes per IC, and (g) number of cup entries per reward in the modified IC task in rats (n = 10). Collapsed data for 16 + 32 μl sucrose volumes is expressed as mean ± SEM. Statistically significant differences to V+M are indicated by asterisks (one-way repeated measures ANOVA, post-hoc Holm-Sidak correction, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
3.4. MJN110-induced enhancement of responding to reward-predictive cues is not due to an increase in appetite.
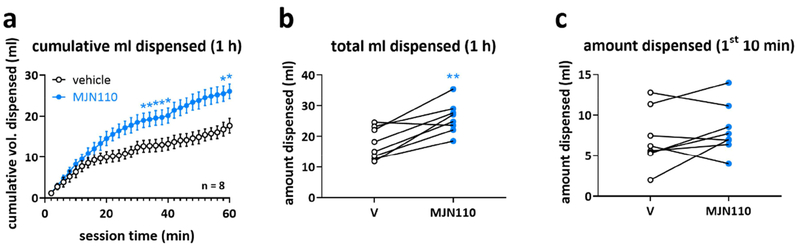
To determine whether appetite stimulation contributed to increased responding to ICs during the operant task, we examined the impact of 5 mg/kg MJN110 on the free intake of 10% sucrose in a fourth group of rats (n = 8). We chose the 5 mg/kg i.p. dose as it was most effective in altering the multiple metrics of responding in the IC task (Figure 3a, b, d). MJN110 treatment 30 min prior to the start of the session increased the 1-hr cumulative consumption of sucrose compared to vehicle (Figure 5a, two-way RM ANOVA, main effect of treatment: F(1,7) = 18.35, P = 0.0036). However, Holm-Sidak post-hoc analyses showed no significant differences in consumption between treatments until ~30 min after the start of the drinking session, at which point, rats had consumed much more sucrose (~10-20 ml) than is available during the operant sessions (max of ~7ml). Thus, MJN110 induced an increase in overall sucrose consumed in the one hour session compared to vehicle treatment (Figure 5b, 26.06 ml ± 1.79 vs. 17.68 ml ± 1.81, respectively, paired-samples t-test: t(7) = 4.73, P = 0.0021). However, there was no significant difference in consumption between treatment conditions during the first 10 min of the session (Figure 5c, paired-samples t-test: t(7) = 1.347, P = 0.2214), in which rats consume approximately the same volume of sucrose as obtained during the IC task under vehicle conditions (~4.5 and ~5.5 ml of 10% sucrose in the decreasing reward and standard IC task, respectively). Therefore, while 5 mg/kg MJN110 did increase overall intake of sucrose during the 1-hr free drinking session, it did not change consumption at volumes comparable to those obtained during the 1-hr IC task.
Figure 5: MJN110-induced stimulated sucrose consumption over time.
Effects of MJN110 and vehicle (V) on a) the cumulative consumption and b) the total consumption of 10 % sucrose in a 1-h free drinking paradigm as well as on c) the amount of sucrose dispensed in the first 10 min of the 1-h session. Note that circles in b) and c) represent individual subjects. Statistically significant differences between drug and vehicle are indicated by asterisks (a, two-way repeated measures ANOVA, post-hoc Holm-Sidak correction; b, paired-samples t-test; * p < 0.05, ** p < 0.01).
4. Discussion
The present study sought to determine the contribution of eCB signaling on responding to reward-predictive ICs. Despite mixed results in other reward-seeking models, we predicted that MAGL inhibition would have more impact in an operant task that requires cue processing to perform correctly. Further, we sought to characterize the effects of the novel MAGL inhibitor, MJN110, which has therapeutic potential on reward processing. We found that MAGL inhibition by MJN110 robustly enhanced both the choice and vigor of responding.
Our findings substantiate a critical role of the eCB system in reward-seeking behavior through direct modulation of the incentive motivational properties of cues. Several studies have shown that CB1 receptor agonists increase food-seeking behaviors (Solinas and Goldberg, 2005; Oleson et al., 2012), while CB1 receptor antagonists reduce motivation for palatable foods (Arnone et al., 1997; Thornton-Jones et al., 2005). However, these effects have often been attributed to modulation of appetite and satiety at both centrally and peripherally located CB1 receptors (Di Marzo and Matias, 2005). Some studies indicate a role of the cannabinoid system in cue-induced reward-seeking behaviors, while others have shown no effect. For example, even though Δ9-THC clearly facilitates dopamine release in the NAc (Chen et al., 1991), results from both rat and mouse ICSS studies have most often demonstrated attenuation or no change in responding, or enhancement under a narrow range at low doses (Vlachou et al., 2007; Katsidoni et al., 2013; Wiebelhaus et al., 2015). However, operant food seeking is a multimodal process, involving a variety of neural systems mediating appetite, satiety, motivation, and cue processing. Thus, it may be possible that these discrepancies can be ascribed to differences between operant models, which rely more on distinct individual component processes.
Given the role of NAc dopamine in cue and reward processing, we correctly predicted that an operant model entirely dependent upon IC processing would be sensitive to 2-AG regulation of reward-seeking. Additionally, the IC task allows us to characterize multiple aspects of operant responding, including the choice to respond to the IC (response ratio), the vigor of responding to the IC (nosepoke latency), and the vigor to obtain the reward (reward cup latency). Within the context of these metrics, our data clearly show that rimonabant decreases both the choice and vigor of responding to the IC. Rimonabant also dose-dependently attenuated the number of active nosepokes per IC, another index of a reduced motivation for the cue. The decrease in response vigor for the reward was more modest compared to the decreased vigor to the cue. However, reward cup latency may be more resistant to drug manipulations, since once a response is emitted in the active port in response to the IC, subsequent entry into the adjacent reward cup likely becomes rote. Interestingly, even though rimonabant decreased the response ratio, the reward cup entries were maintained, indicating that the rats checked the reward cup even though they did not complete the correct response sequence. Therefore, while rimonabant impairs cue processing, some aspects of sucrose seeking are still intact.
We anticipated that increasing 2-AG levels via MAGL inhibition would enhance responding to ICs. However, the response ratio in the standard task is close to maximal (>85%), and this ceiling does not allow observation of increases in responding. We therefore modified the IC task by decreasing the volume of sucrose reward every 15 min from 64 to 48, 32, and 16 μl Of sucrose. In this modified task, rats responded successfully to approximately 60% of the ICs over the course of the 1-h session (Figure 3), compared to >85% in the standard task. Under vehicle conditions, we observed a clear proportional relationship between sucrose volume and all metrics in this modified task (Figure 3). For example, vehicle-treated rats responded to the preponderance of the ICs when the sucrose reward was 64 μl, the same volume used in the standard task. Yet the response ratio significantly decreased proportionally as the sucrose volume decreased. Further, there was an inverse relationship between the sucrose volume and latencies to respond to the IC and obtain the sucrose. Together, these data demonstrate that the magnitude of the reward dictates how frequently and quickly the rats respond, indicating that the reinforcing efficacy of the sucrose and incentive cues are proportional to the volume of each reinforcer.
MJN110 significantly enhanced the response ratio at both the 5 and 10 mg/kg doses (Figure 3), while 5 mg/kg also increased the active nosepokes per IC, and decreased nosepoke and reward cup latencies in the decreasing reward IC task, most notably at the lowest reinforcer volumes. Thus, MJN110 increased the choice to respond to the IC, and the vigor of the response and obtaining the reinforcer. However, it should be noted that the decreasing reward task potentially engages additional neurobiological processes than the standard IC task, such as adapting to new reward contingencies or negative incentive contrast, in which behavioral performance decreases after the reward value downshifts compared to the preceding higher reward outcome trials. Thus, we cannot rule out that MJN110 alters other neurobiological mechanisms specific to the decreasing reward IC task. Further, the effects of MJN110 were not always dose responsive. Five and 10 mg/kg doses enhanced the response ratio, while 1, 5 and 10 mg/kg decreased the latency of reward cup entry. However, only 5 mg/kg MJN110 decreased nosepoke latency and the number of nosepokes per IC. There are three possible explanations for disparate results between 5 and 10 mg/kg doses. First the 10 mg/kg dose of MJN110 may elevate 2-AG to levels that can induce decreases in coordination and activity, common cannabinoid effects. Secondly, since we used a within-subjects design, it is possible that the two previous MJN110 exposures (1 and 5 mg/kg) induced tolerance, such that 10 mg/kg was then less effective. While we cannot rule tolerance out, the decrease in extraneous active nosepokes per IC induced by 10 mg/kg MJN110 support the notion that this dose produces cannabimimetic effects (e.g. decreased motor activity). The fact that some studies show low doses of Δ9-THC enhance ICSS responding while higher doses do not, suggests that a higher degree of CB1 receptor activation may counteract the facilitation of ICSS responding observed at lower doses. For example, Δ9-THC and high doses of JZL184 both decrease locomotor activity. The more potent MJN110 may evoke enough 2-AG to inhibit locomotor or fine motor activity, resulting in slower responding, even while motivation is higher. While one study observed no changes in locomotion from 5 to 20 mg/kg of MJN110 in rats (Parker et al., 2015), the activity needed for operant responding may be more sensitive to such effects. Nonetheless, further examination to fully understand the dose-effect relationship of MJN110 in this task should be conducted. Regardless, the rats in this study clearly responded faster and more frequently to the cue after treatment with 5 mg/kg MJN110, particularly at the lower reward volumes when motivation is normally decreased under vehicle conditions. Finally, a third possibility is that 10 mg/kg of MJN110 may affect the activity at other lipases at this higher dose.
We also explored whether the enhancement of cue processing induced by 5 mg/kg of MJN110 occurs primarily through the CB1 receptor. Pretreatment with a subthreshold dose of rimonabant (0.3 mg/kg), which alone does not alter any of the IC response metrics (Figure 4), attenuated the ability of MJN110 to alter cue responding. Specifically, rimonabant blocked the MJN110 induced increases in response ratio, and active nosepokes per IC. While the effects of MJN110 on nosepoke and reward cup entry latencies were less profound in this experiment, rimonabant pretreatment nonetheless attenuated them. Therefore, the MJN110 enhancement in IC responding appears to occur through a CB1 receptor mechanism. Our results are in line with previous studies in which rimonabant blocks the effects of the MAGL inhibitor JZL184 on nicotine withdrawal in mice (Muldoon et al., 2015) and anxiety in rats (Sciolino et al., 2011).
Recently, the effects of MJN110 on feeding were assessed in a model of stress induced anorexia (Sticht et al., 2018). MJN110 decreased homeostatic feeding at 10 mg/kg in unstressed animals. It seems unlikely that an anorectic effect would lead to the increases in responding and decreases in latencies in our task. Nonetheless, we conducted a free-drinking test with sucrose and observed that 5 mg/kg MJN110 increased total sucrose consumption, indicating that MJN110 increases hedonic feeding at this dose within one hour of administration. However, we observed no difference in consumption in the first 10 minutes of free drinking compared to controls. Since the volumes of sucrose consumed during this time are similar to the volumes earned in the IC task, these findings support the notion that the MJN110 enhancement of consumption, or decrease in satiety, alone does not produce an enhancement of operant IC responding. However, free-drinking and operant responding to obtain sucrose are procedurally very different, with the operant task restraining and pacing consumption over the 1-hr operant session. Thus, a independent evaluation of appetitive and consummatory aspects of motivation is not possible using these models. Further investigations, using the runway paradigm for example, may help to dissociate MJN110’s effects on appetitive and consummatory behaviors directly.
It might be possible that changes in cue responding are driven by inappropriate responding, but this also seems unlikely given the lack of an effect on unrewarded active or inactive nosepokes after MAGL inhibition. Moreover, 10 mg/kg of MJN110 actually inhibits extraneous active nosepokes. Erroneous responding also does not account for disrupted cue responding following CB1 receptor antagonism, as we did not observe any change in spurious active or inactive nosepokes. Interestingly, the metric of reward cup entries per IC was unchanged by either rimonabant (Figure 2g) or MJN110 (Figure 3g), even though these drugs produced a decrease and increase in overall responding to the IC, respectively. Together these data suggest that regulation of the endocannabinoid system (via CB1 antagonism or MAGL inhibition) may impact cue processing more than motivation to seek the reward.
The reinforcing effects of natural rewards, abused drugs, and the incentive motivational properties of reward predictive cues are greatly influenced by their ability to elevate NAc dopamine levels (Di Chiara and Imperato, 1988; Hernandez and Hoebel, 1988; Cheer et al., 2004; Saunders and Robinson, 2012). NAc dopamine also invigorates environmentally cued reward-seeking behavior (du Hoffmann and Nicola, 2014). In our study, antagonism of CB1 receptors via rimonabant likely attenuated increases in NAc dopamine, and thus reduced the incentive salience attributed to the reward-predictive cues. Endocannabinoid signaling modulates these processes, for example, amphetamine-induced increases of NAc dopamine transmission are blocked by rimonabant (Covey et al., 2016). Rimonabant also decreases NAc neural activity and dopamine release associated with cue presentation and reward delivery in rats (Hernandez and Cheer, 2012; Oleson et al., 2012). Within the VTA, CB1 receptors are located presynaptically on glutamatergic and GABAergic synapses with DA neurons. Retrograde 2-AG signaling from DA neurons may inhibit either glutamatergic and GABAergic transmission. Inhibition of glutamate would likely decrease excitability of DA neurons and subsequent DA release in the NAc, while inhibition of GABA would enhance excitability of DA neur ons (Lupica and Riegel, 2005). Our behavioral results are consistent with a 2-AG modulation of GABAergic synapses with DA neurons, in that CB1 agonism inhibits the incentive motivational properties of reward predictive cues in our model. Conversely, MAGL inhibition, and subsequent enhancement of 2-AG, increases the incentive motivation of the cues, likely through disinhibition of GABAergic tone. Indeed, we recently demonstrated that chemogenic activation of VTA GABA neurons in the incentive cue task produced the same reduction of response ratio, and increased nosepoke and reward cup entry latencies (Wakabayashi et al., 2018) we observe in the current study. While this supports the notion that endocannabinoids regulate the incentive motivational properties of cues through a VTA GABA mechanism, further experiments examining MAGL inhibition in specific mesolimbic regions will provide useful mechanistic insights into eCB regulation of incentive motivation.
MAGL inhibitors are a promising therapeutic class of compounds with potential to reduce pain and inflammatory responses, while displaying limited cannabimimetic activity compared with direct CB1 receptor agonists (Niphakis et al., 2013; Ignatowska-Jankowska et al., 2015). Our findings demonstrate that the selective MAGL inhibitor MJN110 increases responding to incentive reward-predictive cues, through CB1 receptor activation by 2-AG. Therefore, MJN110 may also prove effective in treating psychiatric disorders involving motivational deficits, especially in situations where the drive triggered by environmental cues is weak. In conclusion, we clearly demonstrate bidirectional control of IC responding, through CB1 receptor antagonism and MAGL inhibition. Enhanced 2-AG signaling regulates responding to reward-predictive ICs by enhancing the choice to respond and the vigor of responding. Antagonism of CB1 receptors attenuates processing reward-predictive cues by decreasing response choice and IC vigor. In both cases, some aspects of motivation for the reward are preserved.
Highlights.
Rats were tested in an operant task that allows parsing motivation for incentive cues from other aspects of operant responding.
Rimonabant dose-dependently reduced responding to reward-predictive incentive cues
MJN110 increased incentive cue responding by activation of CB1 receptors
CB1 receptor antagonism preferentially decreases motivation for incentive cues
Enhancing 2-AG signaling increases motivation for both the cue and the reward
Acknowledgements
We thank Micah J. Niphakis for providing consultation to the investigators on the study, and Thomas Bassett, Heather Bloom, and Anna Fimmel for technical assistance.
Funding
This work was supported by the State University of New York BRAIN Network of Excellence Postdoctoral Fellow program and T32 AA007583 (K.T.W), the Whitehall Foundation 2017-1298 (C.E.B.), as well as R01 AA024112 and R21 DA043190 (C.E.B). The authors report no financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no financial interests or potential conflicts of interest.
References
- Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL (2011) Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:6820–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G (1997) Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132:104–106. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 191:391–431. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:4393–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM (2007) Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Lowinson JH, Gardner EL (1991) Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: an in vivo microdialysis study. Neurosci Lett 129:136–180. [DOI] [PubMed] [Google Scholar]
- Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA (2016) Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. The European journal of neuroscience 43:1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN (2001) A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7:1151–1154. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, Jentzsch KR (2004) Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol 483:55–63. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ (2001) Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:9471–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Matias I (2005) Endocannabinoid control of food intake and energy balance. Nat Neurosci 8:585–589. [DOI] [PubMed] [Google Scholar]
- du Hoffmann J, Nicola SM (2014) Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:14349–14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL (1997) Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299–318. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. The Journal of neuroscience : the official journal of the Society for Neuroscience 11:563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Cheer JF (2012) Effect of CB1 receptor blockade on food-reinforced responding and associated nucleus accumbens neuronal activity in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:11467–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42:1705–1712. [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska B, Wilkerson JL, Mustafa M, Abdullah R, Niphakis M, Wiley JL, Cravatt BF, Lichtman AH (2015) Selective monoacylglycerol lipase inhibitors: antinociceptive versus cannabimimetic effects in mice. J Pharmacol Exp Ther 353:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA (1992) Two types of neurone in the rat ventral tegmental area and their synaptic inputs. The Journal of physiology 450:455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G (2013) Biphasic effects of Delta9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol 16:2273–2284. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC (2005) Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48:1105–1116. [DOI] [PubMed] [Google Scholar]
- Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, Katona I (2008) Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology 54:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon PP, Chen J, Harenza JL, Abdullah RA, Sim-Selley LJ, Cravatt BF, Miles MF, Chen X, Lichtman AH, Damaj MI (2015) Inhibition of monoacylglycerol lipase reduces nicotine withdrawal. Br J Pharmacol 172:869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM (2010) The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of rewardseeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:16585–16600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphakis MJ, Cognetta AB 3rd, , Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, Cravatt BF (2013) Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci 4:1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF (2012) A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, Lichtman AH, Cheer JF (2012) Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron 73:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS (2008) Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:1385–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Niphakis MJ, Downey R, Limebeer CL, Rock EM, Sticht MA, Morris H, Abdullah RA, Lichtman AH, Cravatt BF (2015) Effect of selective inhibition of monoacylglycerol lipase (MAGL) on acute nausea, anticipatory nausea, and vomiting in rats and Suncus murinus. Psychopharmacology (Berl) 232:583–593. [DOI] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni Al, Gessa GL, Pistis M (2005) Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 183:368–377. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR (2004) Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:11070–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT (2014) On the motivational properties of reward cues: Individual differences. Neuropharmacology 76 Pt B:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE (2012) The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. The European journal of neuroscience 36:2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG (2011) Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res 64:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR (2005) Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology 30:2035–2045. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Windisch K, Ando RD, Sylvester Vizi E (2009) Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem Int 54:452–457. [DOI] [PubMed] [Google Scholar]
- Sticht MA, Lau DJ, Keenan CM, Cavin JB, Morena M, Vemuri VK, Makriyannis A, Cravatt BF, Sharkey KA, Hill MN (2018) Endocannabinoid regulation of homeostatic feeding and stress-induced alterations in food intake in male rats. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I (2002) Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. The European journal of neuroscience 15:2057–2061. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG (2005) The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl) 179:452–460. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G (2007) Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol 18:311–319. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Fields HL, Nicola SM (2004) Dissociation of the role of nucleus accumbens dopamine in responding to reward-predictive cues and waiting for reward. Behav Brain Res 154:19–30. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Feja M, Baindur AN, Bruno MJ, Bhimani RV, Park J, Hausknecht K, Shen RY, Haj-Dahmane S, Bass CE (2018) Chemogenetic activation of ventral tegmental area GABA neurons, but not mesoaccumbal GABA terminals, disrupts responding to reward-predictive cues. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E, Hurd YL (2003) Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118:681–694. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, Niphakis MJ, Vann RE, Cravatt BF, Wiley JL, Negus SS, Lichtman AH (2015) Delta9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. J Pharmacol Exp Ther 352:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM (2004) The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:2923–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]