Abstract
Objective:
To assess the efficacy of triclabendazole (TCBZ) in porcine cysticercosis.
Methods:
Eighteen naturally infected cysticercosis pigs were divided into 3 groups of 6 individuals each. The first group was treated orally with TCBZ at a single dose of 30 mg/kg of body weight, the second group was treated orally with oxfendazole at a single dose of 30 mg/kg of body weight and the third group received a placebo (control group). All animals were kept under the same management conditions. The pigs were euthanized 17 wk post-treatment and the number of surviving cysts in muscles was assessed and compared between groups.
Results:
All pigs treated with oxfendazole had only degenerated cysts in their carcasses. In contrast, TCBZ had very little effect against the parasitic cysts. Cysts from pigs in the TCBZ group looked apparently normal after treatment. However, histological evaluation showed a mild to moderate degree of inflammation.
Conclusions:
TCBZ is not an efficacious drug against Taenia solium cysticercosis in swine using a single dose.
Keywords: Taenia solium, Cysticercosis, Triclabendazole, Oxfendazole, Treatment
1. Introduction
The disease complex, taeniasis/cysticercosis caused by the pork tapeworm Taenia solium (T. solium), is a very important zoonotic disease in developing countries [1,2]. The adult T. solium tapeworm localizes in the small intestine of humans, the definitive host. The eggs of T. solium are eliminated into gravid proglottids with the faeces, and then they are ingested by pigs, the intermediate host. The T. solium metacestode then develops in different organs of the pig, principally muscles [3,4]. However, humans can act as accidental hosts when they ingest eggs in contaminated food [3,5].
During the last decade, a variety of strategies have been performed for the control of T. solium cysticercosis including animal inspection, massive chemotherapy for T. solium in humans and pigs from endemics areas, health education, and pig vaccination [2,6-9]. For this purpose, many studies have been performed to evaluate the efficacy of different drugs against porcine cysticercosis [10]. Of these drugs, albendazole and oxfendazole (OFZ), members of the benzimidazole drugs, are efficacious for the treatment of the porcine cysticercosis [11]. Albendazole has been used in humans since 1979, and currently it is considered the drug of preference in humans for its availability, low cost and efficacy [5,11]. Albendazole does not seem to be a good option for field interventions due to the requirement for multiple doses [10]. OFZ, used in a single oral dose of 30 mg/kg, is the drug of choice for the treatment of porcine cysticercosis [12]. The widely used triclabendazole (TCBZ), another member of the benzimidazole family, is efficacious against adults and larval stages of a variety of helminthic parasites [13,14]. The aim of this study was to compare the efficacy of a single oral dose of TCBZ or OFZ against T. solium cysticercosis in naturally infected pigs.
2. Materials and methods
2.1. Study design and settings
The study was conducted at the animal facilities of the Laboratory of Veterinary Epidemiology and Economics, School of Veterinary Medicine, National University of San Marcos in Lima, Peru. Pigs were acquired in a cysticercosis-endemic area and transported to the animal facilities in Lima. Pigs were randomized in three similar groups (TCBZ, OFZ, and placebo) using a random numbers table. All pigs were coded and labelled with ear tags.
2.2. Animals
Eighteen adult pigs were acquired in Huancayo, a cysticercosis-endemic area in the Peruvian highlands [15]. All animals were positive to cysticercosis by tongue examination, and confirmed by antibody detection on serum enzyme-linked immunoelectro transfer blot [16,17]. Only animals with more than one cyst in the tongue and more than four antigen bands in enzyme-linked immunoelectro transfer blot were included in the study. All pigs were vaccinated against hog cholera immediately after purchase.
2.3. Treatment protocol
The pigs were randomly placed in three different groups of six pigs each. Pigs in group 1 received a single oral dose of 30 mg/kg body weight of TCBZ (Trisan 12%, Montana S.A., Peru). Pigs in group 2 were treated orally with OFZ (Synanthic® 9.06%, Fort Dodge, Mexico), using a single dose of 30 mg/kg body weight. Control pigs (group 3) received sugar water as a placebo. Each treatment group was housed in a single pen.
2.4. Necropsy
All animals were euthanized 17 wk after treatment, and necropsied at the School of Veterinary Medicine facilities in Lima, Peru. Pigs were anesthetized and sacrificed by intramuscular injection of a combination of ketamine (20 mg/kg) and xylazine (2 mg/kg) followed by intravenous injection of pentobarbital sodium overdose (60 mg/kg). Necropsy was performed immediately after euthanasia, and muscles from arms and legs were dissected, and all cysts were counted and classified. Cysts were classified as viable if a defined cystic structure with clear liquid content was still present, and degenerated if this had been replaced by semi-solid contents or an inflammatory scar (calcified nodule).
2.5. Histological analysis
Five randomized cysts from the muscles of each pig were fixed in 10% formalin, embedded in paraffin, then sectioned at 5 μm and stained with haematoxylin and eosine (H&E), as well as Von Kossa stain to assess the presence of calcium.
2.6. Data analysis
Parasitic load was expressed in median with their respective ranges. A non-parametric one-way analysis of variance (Kruskal–Wallis) test was used to estimate the difference in the number of cysts between treatment groups. Data was analysed using STATA 10 statistical software (v10.0; StataCorp LP, College Station, TX). Differences were considered statistically significant at P-value <0.05.
2.7. Ethical approval
The study was approved by the Animal Ethics Committee of the School of Veterinary Medicine, National University of San Marcos, Lima, Peru.
3. Results
During treatment and follow up, pigs from the treated groups involved in this experiment were apparently normal without apparent adverse events. All pigs used in this study were positive to cysticercosis at tongue examination as well as positive to 4 or more antibody bands enzyme-linked immunoelectro transfer blot. After necropsy, the overall parasite load including viable or degenerated cysts for each group was 1658 (131–2575) (median, interquartile range) cysts for control group, 1414 (511–4052) cysts for TCBZ treated group, and 259 (38–1953) cysts for OFZ treated group (Table 1). All remaining cysts in the OFZ pigs were evidently degenerated. Furthermore, the numbers of cysts were statistically significantly smaller in OFZ group compared with the TCBZ and control groups (P < 0.01).
Table 1.
Median, range and feature of larval stage of T. solium after treatment of pigs.
| Indexes | Group | ||
|---|---|---|---|
| TCBZ | OFZ | Control | |
| Animals | 6 | 6 | 6 |
| Male | 2 | 2 | 2 |
| Weight (kg)a | 77 (67–86) | 95 (66–117) | 81 (49–119) |
| Number of viable cysts | 1414 (511–4052)b | 0b | 1658 (131–2575)b |
| Number of degenerated cysts | 0 | 259 (38–1953) | 0 |
Mean and range
Significant difference between control and treatment (Kruskal–Wallis test, P < 0.01).
TCBZ had very little effect against the parasitic cysts. Cysts from animals in the TCBZ group looked apparently normal after treatment. However, histological evaluation showed a mild to moderate degree of inflammation composed of eosinophils, macrophages, lymphocytes, plasmocytes, and fibroblasts with connective tissue in the surrounding parenchyma (Figure 1) in all samples from this group. On the other hand, OFZ killed 100% of cysts in treated pigs, and all animals in this group showed only degenerated cysts. The inflammatory reaction around cysts from the OFZ group was severe, and also demonstrated areas of dystrophic calcification (Figure 2). No inflammatory reaction was observed in pericystic tissue samples from pigs in the control group.
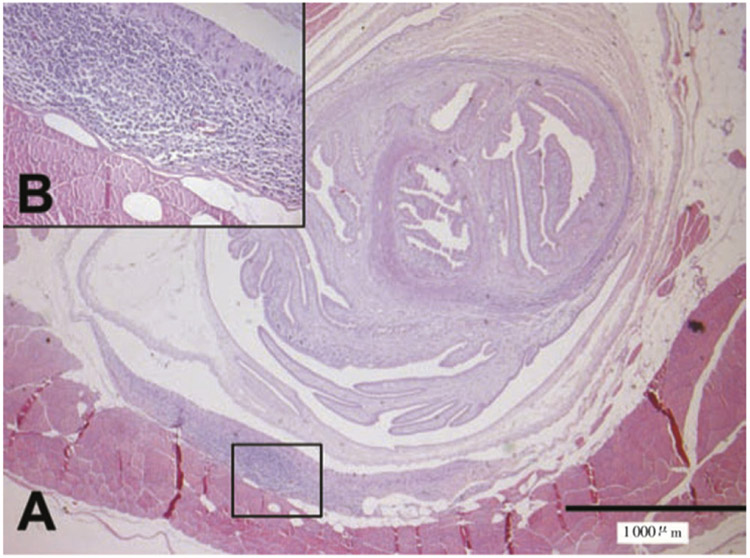
Figure 1.
Histological section of cysts from a pig treated with TCBZ (H&E stain).
It showed a significant inflammatory reaction. Multifocal lymphocytic myositis (A). Presence of lymphocytes, eosinophils, macrophages, plasmocytes, and fibroblasts (B).
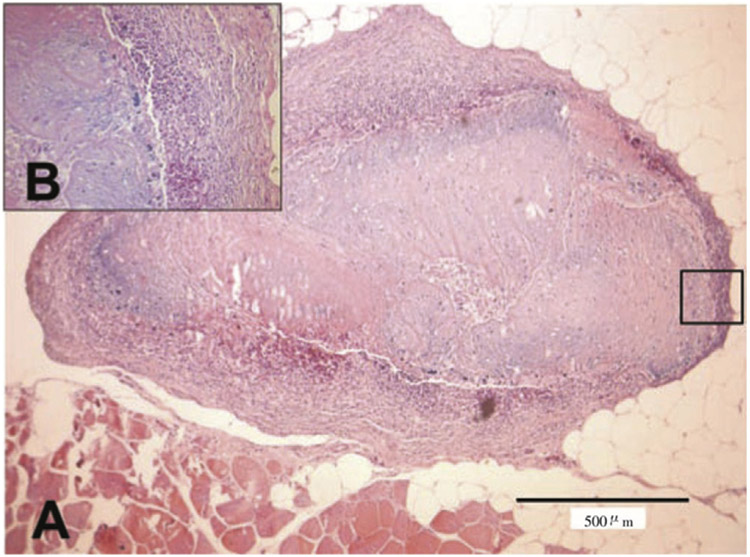
Figure 2.
Histological section of cysts from a pig treated with OFZ (H&E stain).
It showed moderate diffuse lymphocytic myositis and dystrophic calcification (A). Presence of lymphocytes, fibroblasts, connective tissue, tissue mineralization with basophilic granules (B).
4. Discussion
T. solium cysticercosis remains a public health issue in many developing countries, and therefore, the evaluation of new alternative drugs for its control is urgently required. A very large variety of antiparasitic drugs have been tested against cysticercus so far [10]. Although almost all these anthelmintic drugs are active against adult stages of cestodes, nematodes and trematodes, only a few drugs that have a positive effect against its larval stage [11,18].
TCBZ, a benzimidazole drug, has a potent effect against adult and larval stages of some tissue helminths such as Fasciola and Paragonimus [14]. Likewise, studies in vitro have been shown effective activity in some nematodes and cestodes [13,19]. On these bases, we evaluated the efficacy of TCBZ against porcine cysticercosis. TCBZ in a single dose of 30 mg/kg was not effective against cysticercosis in this group of naturally infected pigs. On necropsy, there were no apparent differences in cyst burden or cyst appearance between pigs treated with TCBZ and pigs in the control group.
Microscopic evaluation of cysts from treated pigs with TCBZ evidenced mild to moderate inflammatory reaction with presence of different types of leukocytes. This indicates that cysts are not found completely healthy, as observed at necropsy. This mild inflammatory reaction, macroscopically imperceptible, suggests that TCBZ has a slight effect against cysticerci raising the question of whether higher or multiple doses could be efficacious against cystic cestode larvae, as shown by Richter et al. [19]. In that study, TCBZ had a positive activity against Echinococcus multilocularis metacestodes in vitro, using a 20-d treatment regime. Increased doses or multiple doses would still make TCBZ poorly practical for use in porcine cysticercosis in field conditions.
Several antihelminthic drugs (i.e. albendazole, flubendazole, fenbendazole, nitazoxanide, and praziquantel) have been tested for the treatment of porcine cysticercosis. Albendazole was evaluated using a single (50 mg/kg) and multiple doses (30 mg/kg) in experimentally infected pigs, and it was effective using multiple doses (3 consecutive days). However, some pigs treated to single and multiple doses showed secondary effects as anorexia, lethargy, prostration and death [11]. Nitazoxanide was tested in naturally infected pigs using multiple doses (150 mg/kg) by 7 consecutive days, and it was not effective against cysts. Some pigs treated with nitazoxanide developed prostatic hypertrophy and bladder distention [11]. In this experiment, single doses of 30 mg/kg of either OFZ or TCBZ did not produce any adverse effects in pigs. They were apparently normal and did not show any signs of toxicity during and after treatment.
Flubendazole and fenbendazole have show efficacy against porcine cysticercosis when used in multiple doses, 40 mg/kg for 10 d and 5 mg/kg for 7 d, respectively, without secondary effects. Furthermore, praziquantel has been shown to have efficacy against cysticerci using daily doses of 50 mg/kg for 15 consecutive days [11,20]. However, animal treatments using multiple doses are impractical in field conditions and not suitable for mass chemotherapy campaigns in endemic zones. In summary our findings do not support the use of TCBZ for porcine cysticercosis, and confirm the efficacy of a single dose of OFZ for muscle cysticercosis cysts.
Acknowledgements
Partial support from the Fogarty International Center/NIH (training grants D43 TW008273-03 and D43 TW001140) is acknowledged. Luis A. Gomez-Puerta's PhD study, Ana Vargas Calla's MSc study, and Juan Calcina's MSc study are supported by the Fogarty International Center/NIH (D43 TW008273-03>). Hector H. Garcia is supported by a Wellcome Trust Senior International Research Fellowship in Public Health and Tropical Medicine. Many thanks are also due to Dr. Narry Tiao for the comments and critique of the manuscript.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
References
- [1].Flisser A State of the art of Taenia solium as compared to Taenia asiatica. Korean J Parasitol 2013; 51(1): 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gilman RH, Gonzalez AE, Llanos-Zavalaga F, Tsang VC, Garcia HH. Cysticercosis Working Group in Peru. Prevention and control of Taenia solium taeniasis/cysticercosis in Peru. Pathog Glob Health 2012; 106(5): 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Del Brutto OH, Garcia HH. Neurocysticercosis. Handb Clin Neurol 2013; 114: 313–325. [DOI] [PubMed] [Google Scholar]
- [4].Handali S, Pawitan Y. Verifying elimination programs with a special emphasis on cysticercosis endpoints and postelimination surveillance. J Parasitol Res 2012; 2012: 974950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol 2011; 7(10): 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Flisser A, Rodriguez-Canul R, Willingham AL 3rd. Control of the taeniasis/cysticercosis complex: future developments. Vet Parasitol 2006; 139(4): 283–292. [DOI] [PubMed] [Google Scholar]
- [7].Gauci CG, Jayashi CM, Gonzalez AE, Lackenby J, Lightowlers MW. Protection of pigs against Taenia solium cysticercosis by immunization with novel recombinant antigens. Vaccine 2012; 30(26): 3824–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lightowlers MW. Control of Taenia solium taeniasis/cysticercosis: past practices and new possibilities. Parasitology 2013; 140(13): 1566–1577. [DOI] [PubMed] [Google Scholar]
- [9].O'Neal S, Winthrop K, Gonzalez A. Cysticercosis control: bringing advances to the field. J Glob Infect Dis 2011; 3(2): 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mkupasi EM, Sikasunge CS, Ngowi HA, Johansen MV. Efficacy and safety of anthelmintics tested against Taenia solium cysticercosis in pigs. PLoS Negl Trop Dis 2013; 7(7): e2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gonzalez AE, Bustos JA, Jimenez JA, Rodriguez ML, Ramirez MG, Gilman RH, et al. Efficacy of diverse antiparasitic treatments for cysticercosis in the pig model. Am J Trop Med Hyg 2012; 87(2): 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gonzales AE, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Bernal T, et al. Effective, single-dose treatment or porcine cysticercosis with oxfendazole. Am J Trop Med Hyg 1996; 54(4): 391–394. [DOI] [PubMed] [Google Scholar]
- [13].Coles GC. Anthelmintic activity of triclabendazole. J Helminthol 1986; 60(3): 210–212. [DOI] [PubMed] [Google Scholar]
- [14].Keiser J, Engels D, Buscher G, Utzinger J. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin Investig Drugs 2005; 14(12): 1513–1526. [DOI] [PubMed] [Google Scholar]
- [15].Garcia HH, Gilman RH, Gonzalez AE, Verastegui M, Rodriguez S, Gavidia C, et al. Hyperendemic human and porcine Taenia solium infection in Peru. Am J Trop Med Hyg 2003; 68(3): 268–275. [PubMed] [Google Scholar]
- [16].Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 1989; 159(1): 50–59. [DOI] [PubMed] [Google Scholar]
- [17].Gonzalez AE, Cama V, Gilman RH, Tsang VC, Pilcher JB, Chavera A, et al. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg 1990; 43(2): 194–199. [DOI] [PubMed] [Google Scholar]
- [18].Coles TB, Lynn RC. Antiparasitic drugs In: Bowman DD, editor. Georgis' parasitology for veterinarians. 10th ed. Missouri: Elsevier; 2014, p. 264–325. [Google Scholar]
- [19].Richter D, Richter J, Gruner B, Kranz K, Franz J, Kern P. In vitro efficacy of triclabendazole and clorsulon against the larval stage of Echinococcus multilocularis. Parasitol Res 2013; 112(4): 1655–1660. [DOI] [PubMed] [Google Scholar]
- [20].Flisser A, Gonzalez D, Shkurovich M, Madrazo I, Correa D, Rodriguez-Carbajal J, et al. Praziquantel treatment of porcine brain and muscle Taenia solium cysticercosis. 1. Radiological, physiological and histopathological studies. Parasitol Res 1990; 76(3): 263–269. [DOI] [PubMed] [Google Scholar]