Abstract
Background
The purpose of the present study was to evaluate the regulatory effects of acetyl-L-carnitine (ALCAR) on atherosclerosis in Wister rats and to explore its anti-atherosclerotic mechanism.
Material/Methods
We randomly divided 32 Wister rats into 4 groups: a normal diet group (control group, n=8), a normal diet+ALCAR group (ALCAR group, n=8), an atherosclerosis group (AS group, n=8), and an atherosclerosis+ALCAR group (AS+ALCAR group, n=8). The serum lipid distribution, oxidative stress, inflammatory factors and adiponectin (APN) in the blood, and heart and aortic tissues were determined using the standard assay kits, xanthine oxidase method, and ELISA, respectively. HE staining was performed to observe aortic pathology structure change, and the level of angiotensin II (AngII) in the aorta was assessed using radioimmunoassay. In addition, real-time quantitative PCR and Western blot analysis were applied to detect the expression of iNOS, IL-1β, TNF-α, and CRP in the aortic and heart tissues.
Results
Compared with the AS group, the levels of serum TC, TG, LDL, and VLDL in rats decreased significantly, while HDL level significantly increased in the AS+ALCAR group. ALCAR administration enhanced the SOD and GSH-Px activities and decreased MDA activity. APN level was significantly elevated in the AS group, but ALCAR had no significant effect on APN. Further, ALCAR reduced the expressions of inflammation factors TNF-α, IL-1β, iNOS, and CRP, and the concentration of AngII in serum, aortic, and heart tissues.
Conclusions
ALCAR can inhibit the expressions of inflammatory factors and antioxidation to suppress the development of atherosclerosis by adjusting blood lipid in the myocardium of AS rats.
MeSH Keywords: Acetylcarnitine, Antioxidants, Atherosclerosis
Background
Atherosclerosis (AS) is an arterial disease characterized by focal thickening of the intima of the artery wall associated with fatty deposits. High-fat or high-cholesterol diet plays a significant role in the pathogenesis of AS. Several studies have shown that dyslipidemia is an important risk factor for various cardiovascular diseases, characterized by decrease in plasma high-density lipoprotein (HDL) cholesterol, and increase in plasma triglyceride, low-density lipoprotein (LDL) cholesterol, and total cholesterol (TC) [1]. Among these lipids, LDL has a pivotal role and is a predictive marker of atherosclerosis. Elevation of LDL leads to the development and progression of AS, whereas elevation of HDL attenuates the progression by blocking the atherogenic effects of LDL [2,3]. These results suggest that improving blood lipid profile may prevent cardiovascular dysfunction preventing the formation of pathogenic atherosclerotic plaques and blood vessel injury.
Oxidative stress and inflammation are major features in the development of AS. The pathogenesis of AS involves activation of pro-inflammatory factors, expression of cytokines/chemokines, and increased oxidative stress [4]. The generation of reactive oxygen species (ROS) increased oxidative stress, which plays a vital role in inflammatory responses, apoptosis, cell growth, and alteration in vascular tone, as well as in oxidation of LDL-cholesterol; therefore, LDL is thought to be more important that native-LDL in AS [5,6]. AS is caused by the release of various cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and nitric oxide (NO) [7,8]. TNF-α and IL-1β stimulate the expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) in endothelial cells [9], and promote the interaction between endothelial cells and monocytes. This induces the transmigration of circulating monocytes to the intima and the maturation of monocytes to macrophages. Additionally, these macrophages accumulate lipids and become foam cells, which cause the expression of inflammatory genes and formation of atheroma plaques [10].
As a natural derivative of carnitine (3-hydroxy-4-N-trinethylaminobutyric acid), acetyl-L-carnitine (ALCAR) is widely distributed in mammalian tissues, particularly in liver and skeletal muscles. It has been reported that, when added to the diet, this antioxidant reverses age-related alterations in metabolic rate, fatty acid profiles, and cardiolipin levels [11]. Some studies have also shown that ALCAR can provide protection against oxidative stress in several different situations [12–14]. The present study aimed to determine whether ALCAR is able to prevent atherosclerosis by regulating lipid profile, the antioxidant system, and inflammatory markers.
Material and Methods
Experimental animals
Male Wister rats (weight, 200–250 g) were used for the present study and all the procedures performed on these animals were in accordance with the guidelines of Institutional Animal Care and Use Committee at Weifang People’s Hospital. Rats were maintained at a specific pathogen-free housing facility and given ad libitum access to food and water. A total of 32 Wister rats were adaptively fed for 1 week, and then randomly divided into 4 groups: a control group (n=8), an ALCAR group (n=8), an atherosclerosis (AS) group (n=8), and an AS+ALCAR group (n=8). Rats fed a routine diet in the ALCAR group were given oral ALCAR (200 mg/kg/d), and rats in the control group were given an oral equivalent amount of drinking water. Rats in the AS group and AS+ALCAR group received intramuscular injection of 3×105 U/kg of vitamin D3, and the aortic balloon injury in rats fed with high-fat diet was treated by surgery. Rats in the AS+ALCAR group were given oral ALCAR (200 mg/kg/d).
AS rat models were built by feeding a high-fat diet, intramuscular injection 3×105 U/kg of vitamin D3 in the right lower limb once every 4 weeks for 4 times, and artery balloon injury surgery 1 week after injection. The specific surgery methods were as follows: 1% sodium pentobarbital intraperitoneal injection (50 mg/kg) was used as anesthesia, and then the left carotid artery was exposed and separated from the organization. Next, the common carotid artery segment was ligated at a distal line, and the line was slightly tightened to the proximal end. A small incision was made between the 2 lines with a pair of scissors. A Boston 2.0×15 mm balloon catheter was gently inserted into the aorta and reached the aortic arch. The catheter, with rotation, was slowly pulled and ballooned to continue injury of the entire common carotid artery 4 times. After drug withdrawal, the left common carotid artery was ligated and sutured. Then, 8×104 U gentamicin was injected intramuscularly to prevent infection once a day for 3 days. Body weights and food intake were measured every week at regular intervals.
Samples preparation
After 16 weeks, all rats were euthanized and blood samples were collected. Blood samples were centrifuged at 3500 rpm at 4°C for 15 min to collect the supernatant for subsequent analysis of lipid profile and antioxidant and anti-inflammatory levels. A portion of the aorta tissues was removed and preserved for histological examination. Subsequently, the cardiac and aortic tissue were homogenized in 50 mM phosphate buffer (pH 7.2) and centrifuged for 15 min. The supernatant was then collected and used for biochemical analysis. The protein concentration in each fraction was determined using the method described by Bradford [15] and crystalline bovine serum albumin was used as a standard.
Effect of ALCAR on serum lipid profile
Lipid profile included the contents of triglycerides (TG), total cholesterol (TC), very low-density lipoprotein cholesterol (VLDL), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL). Standard assay kits were used to determine the serum concentration of these lipids and the units were expressed as mg/dl.
Effects of ALCAR on expressions of reactive oxygen species (ROS)
The expression of reactive oxygen species (ROS) in the serum and the homogenate of aorta and heart tissues were determined. The xanthine oxidase and the dithio dinitrotoluene acid methods were used for the determination of rat superoxide dismutase (SOD) activity and the rat glutathione peroxidase activity (GSH-Px), respectively, and the thiobarbituric acid colorimetric method was performed to determine the content of malondialdehyde (MDA). All procedures were performed with commercial kits according to the manufacturer’s instructions.
Effects of ALCAR on expressions of Ang II in aorta tissue
Radioimmunoassay was used to assess the level of angiotensin II in the aorta. The concentration was analyzed with Ang II kit according to the manufacturer’s instructions.
Effects of ALCAR on expressions of inflammation factors and adipocytokines in serum
Enzyme-linked immune sorbent assay (ELISA) was used to assess the concentrations of serum TNF-α, IL-1β, CRP, and adiponectin (APN). All procedures followed the instructions of the corresponding kits.
Western blot analysis
Proteins were extracted from the aortic and heart tissue samples. Next, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% standard gel) and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% fat-free milk powder for 2 h at room temperature, the membranes were incubated overnight at 4°C with primary antibodies against CRP (#14316, dilution 1: 1000), IL-1β (#31202, dilution 1: 1000), TNF-α (#11948, dilution 1: 1000), iNOS (#2982, dilution 1: 1000) from Cell Signaling Technology, Inc., and β-actin (sc-130656, dilution 1: 1000) from Santa Cruz Biotechnology. After washing, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. Signals were detected using an enhanced chemiluminescence (ECL) Western blot detection system. β-actin was used to normalize total proteins expression. Image J software was used to assess the densities of the protein bands.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted from the aortic and cardiac tissue samples using a Trizol kit (Takara) according to manufacturer’s protocol. For RT-qPCR, RNA was reverse-transcribed using PrimeScript Reverse Transcription Supermix (Bio-Rad). The reverse-transcribed RNA was analyzed using SsoFast Eva Green Supermix (Bio-Rad). The reverse transcription conditions were 10 min at 25°C, 30 min at 48°C, and a final step for 5 min at 95°C. The primer sequences for amplifying rat genes were as shown in Table 1. The qPCR reactions (25 μl) were conducted as follows: 95°C for 2 min, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. Samples were amplified in triplicates, and a standard curve was constructed using serial dilutions of a reference sample. The relative copy numbers were obtained from the standard curve and data were normalized to β-actin expression by the comparative CT method [16].
Table 1.
Primer sequences for RT-qPCR.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| iNOS | TTTGGAGCAGAAGTGCAGTCTC | GATCAGGAGGGATTTCAAAGACCT |
| TNF-α | ATGAGCACAGAAAGCATG | TCACAGAGCAATGACTCC |
| IL-1β | ATGGCAACTGTTCCTGAAC | TTAGGAAGACACGGATTC |
| CRP | GGGTGGTGCTGAAGTACGAT | AAACATTGGGGCTGAGTGTC |
| β-actin | CCAACCGCGAGAAGATGA | CCAGAGGCGTACAGGGATAG |
Histopathological staining
Slices of fresh aorta tissues were cut and fixed with buffered neutral formalin fixative for 24 h. The fixed tissue slices were then washed and dehydrated with a series of alcohol. After clearing the tissues in methyl salicylate, they were infiltrated with wax. Subsequently, sections were cut to 4-μm thickness and stained with aqueous hematoxylin and eosin. The sections were observed under an optical microscope.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software. Data are presented as the mean±SD. Direct comparisons were made using a one-way ANOVA and Tukey’s test for multiple comparison. The differences between 2 groups were assessed using the minimum significant difference (LSD) test. If the variance was not homogeneous, we used Dunnett’s T3 test. If data were not normally distributed, we used the Mann-Whitney test. P<0.05 was set as the level of statistical significance.
Results
ALCAR regulates serum lipid profiles
To investigate the effects of ALCAR on serum lipid profiles, standard assay kits were used to determine the serum concentration of these lipids. As shown in Table 2 and Figure 1, compared with the control group, the serum levels of TC, TG, LDL, and VLDL of rats in the AS group increased sharply (P<0.01) and the level of HDL decreased significantly (P<0.05). Compared with the AS group, the serum levels of TC, TG, LDL, and VLDL of rats in the AS+ALCAR group were remarkably lower (P<0.01), while the level of HDL clearly higher (P<0.01). after giving ALCAR, the serum TC, TG, LDL and VLDL content of normal-diet rats had certain changes, but without a significant statistical difference (P>0.05); however, HDL levels were significantly elevated (P<0.05). These results indicated that ALCAR regulated the serum lipid profiles in the rats with AS.
Table 2.
Effects of ALCAR on serum parameters in AS rats (χ̄±s, mg/dL).
| Group | TC | TG | LDL | HDL | VLDL |
|---|---|---|---|---|---|
| Control | 82.21±7.72 | 80.23±6.47 | 21.21±2.21 | 42.44±5.17 | 15.11±1.52 |
| AS | 325.34±37.32** | 180.12±15.69** | 205.21±22.12** | 38.46±2.32* | 50.61±4.91** |
| ALCAR | 86.33±15.10 | 75.64±6.56 | 23.02±2.82 | 48.45±4.83* | 13.32±1.33 |
| AS+ALCAR | 235.18±15.44**## | 130.32±8.82**## | 127.21±12.62**## | 49.56±5.23**## | 33.33±1.85**## |
Compared with control group,
P<0.05 and
P<0.01;
Compared with AS group,
P<0.01.
Figure 1.

Effects of ALCAR on serum lipid profiles in rats with AS. The levels of serum TC, TG, LDL, HDL and VLDL in the control, AS, ALCAR and AS+ALCAR groups. Compared with the control group, * P<0.05 and ** P<0.01; Compared with the AS group, ## P<0.01.
ALCAR attenuated the oxidative stress
To evaluate the effects of ALCAR on expression of reactive oxygen species, we used xanthine oxidase method, thiobarbituric acid colorimetric method, and dithio dinitrotoluene acid method. In AS rats, the activities of SOD, GSH-Px, and MDA in serum (Table 3), aortic tissue (Table 4) and heart tissue (Table 5) were weaker than those in the control group (P<0.01), while the MDA level was increased significantly compared with the control group (P<0.01). By contrast, in the AS+ALCAR group when compared with the AS group, the levels of SOD and GSH-Px in serum (Table 3) and in aortic tissue (Table 4) and heart tissue (Table 5) were remarkably increased (P<0.05 or P<0.01), whereas the MDA level was significantly decreased (P<0.01). These results suggested that ALCAR enhanced the SOD and GSH-Px activities and decreased the MDA level, thereby inhibiting the oxidative stress response in rats with AS.
Table 3.
Effects of ALCAR on reactive oxygen species in serum of AS rats (χ̄±s).
| Group | SOD (U/ml) | GHS-Px (U/ml) | MDA (nmol/ml) |
|---|---|---|---|
| Control | 62.15±8.21 | 146.38±16.52 | 1.94±0.21 |
| AS | 38.13±4.59** | 115.52±15.31** | 2.66±0.29** |
| ALCAR | 65.88±7.82 | 162.89±18.46 | 1.86±0.26 |
| AS+ALCAR | 44.18±4.28# | 133.62±14.76# | 2.13±0.19## |
Compared with control group,
P<0.01;
Compared with AS group,
P<0.05 and
P<0.01.
Table 4.
Effects of ALCAR on reactive oxygen species in aortic tissue of AS rats (χ̄±s).
| Group | SOD (U/mg·prot) | GHS-Px (U/mg·prot) | MDA (nmol/mg·prot) |
|---|---|---|---|
| Control | 66.75±7.74 | 165.42±17.62 | 1.97±0.25 |
| AS | 38.72±4.22** | 113.54±12.19** | 6.95±0.73** |
| ALCAR | 71.54±8.12 | 168.87±19.33 | 2.14±0.32 |
| AS+ALCAR | 59.34±6.16## | 148.71±15.80## | 4.26±0.51## |
Compared with control group,
P<0.01;
Compared with AS group,
P<0.01.
Table 5.
Effects of ALCAR on reactive oxygen species in heart tissue of AS rats (χ̄±s).
| Group | SOD (U/mg·prot) | GHS-Px (U/mg·prot) | MDA (nmol/mg·prot) |
|---|---|---|---|
| Control | 70.33±9.74 | 174.32±21.62 | 2.83±0.35 |
| AS | 47.42±7.22** | 124.32±16.62** | 5.33±0.43** |
| ALCAR | 88.67±10.42 | 196.94±23.11 | 2.64±0.32 |
| AS+ALCAR | 65.74±8.16## | 145.84±18.91# | 3.84±0.37## |
Compared with control group,
P<0.01;
Compared with AS group,
P<0.05 and
P<0.01.
ALCAR regulated the levels of inflammation factors and adiponectin (APN) in serum
To measure the concentrations of TNF-α, IL-1β, CRP, and APN in serum, ELISA was conducted. As shown in Table 6, compared with the control group, the concentrations of TNF-α, IL-1β, and CRP in serum were distinctly increased in AS rats (P<0.01), and the concentration of APN was significantly decreased (P<0.01). However, the concentrations of TNF-α, IL-1β, and CRP in the AS+ALCAR group were noticeably decreased in comparison with the AS group (P<0.05 or P<0.01), and the concentration of APN was slightly decreased, but without statistical significance (P>0.05). Therefore, ALCAR decreased the concentrations of TNF-α, IL-1β, and CRP, but had no significant effect on APN level.
Table 6.
Effects of ALCAR on inflammation factors and adiponectin in serum of AS rats (χ̄±s).
| Group | TNF-α (ng/L) | IL-1β (ng/L) | CRP (ug/L) | APN (ug/L) |
|---|---|---|---|---|
| Control | 245.63±38.45 | 45.86±6.83 | 543.77±88.73 | 148.94±13.94 |
| AS | 402.98±33.24** | 82.19±10.57** | 882.61±94.33** | 83.67±12.65** |
| ALCAR | 227.85±43.16 | 43.25±4.68 | 505.64±73.22 | 159.32±21.64 |
| AS+ALCAR | 318.07±36.34## | 71.86±5.74# | 622.34±84.19## | 75.94±8.55 |
Compared with control group,
P<0.01;
Compared with AS group,
P<0.05 and
P<0.01.
ALCAR suppressed the mRNA and protein levels of inflammatory markers in aortic and heart tissues
RT-qPCR was applied to analyze the gene expression of inflammatory markers in aortic and heart tissues. As shown in Figure 2, AS rats exhibited higher mRNA levels of TNF-α, iNOS, IL-1β, and CPR in the aortic (Figure 2A) and heart (Figure 2B) tissues when compared to the control group (P<0.01). However, ALCAR significantly reduced the mRNA levels of TNF-α, iNOS, IL-1β, and CPR in the aorta tissues of AS rats when compared with the AS group (Figure 2A, P<0.01). Similarly, ALCAR significantly downregulated the protein expression of TNF-α, IL-1β, iNOS, and CRP in heart tissues both in the AS rats (Figure 2B, P<0.01) and in normal rats (Figure 2B, P<0.05 or P<0.01). These results suggest the mechanism underlying the action of ALCAR on lipid deposition in the aorta and heart tissues.
Figure 2.
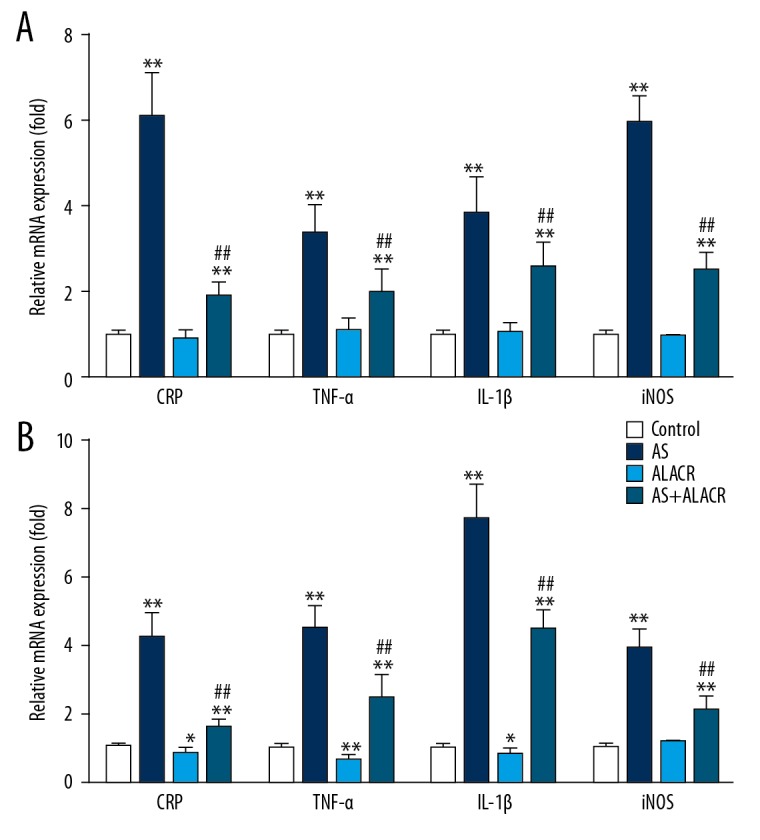
Effects of ALCAR on the mRNA expressions of CRP, TNF-α, IL-1β, and iNOS in aorta and heart tissues of AS rats. (A) The relative mRNA expression of CRP, TNF-α, IL-1β, and iNOS in the aorta tissue among the control, AS, ALCAR, and AS+ALCAR groups. (B) The relative mRNA expression of CRP, TNF-α, IL-1β, and iNOS in the heart tissue among the control, AS, ALCAR, and AS+ALCAR groups. Compared with the control group, * p<0.05 and ** P<0.01; Compared with the AS group, ## P<0.01.
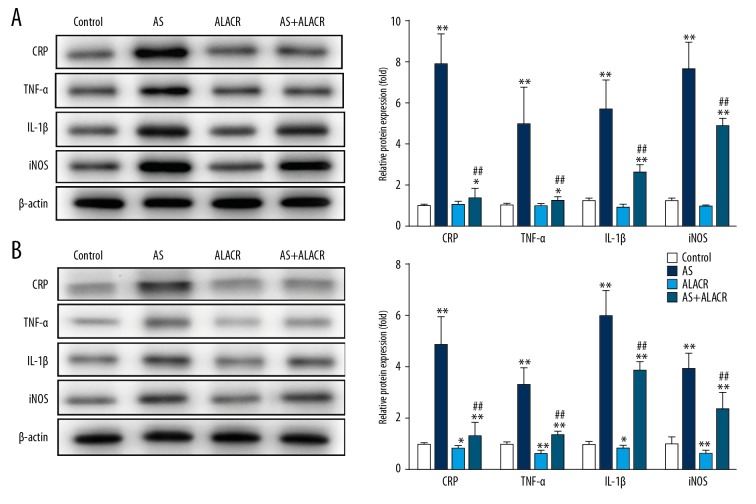
To investigate the effects of ALCAR on CRP, TNF-α, IL-1β, and iNOS in the aorta and heart tissues, Western blot assay was performed to detect the protein expression of TNF-α, IL-1β, iNOS, and CPR. It was revealed that the protein expression of TNF-α, IL-1β, iNOS, and CRP in the aorta (Figure 3A) and heart (Figure 3B) tissues were significantly higher in rats with AS than in those in the control group (P<0.01), while the protein expressions of CRP, TNF-α, IL-1β, and iNOS in the AS+ALCAR group were distinctly lower in the aorta tissues in comparison to the AS group (Figure 3A, P<0.01). In addition, ALCAR significantly downregulated the protein expression of TNF-α, IL-1β, iNOS, and CRP in heart tissues both in rats with AS (Figure 3B, P<0.01) and in normal rats (Figure 3B, P<0.05 or P<0.01). Therefore, ALCAR can significantly decrease the protein expression of TNF-α, IL-1β, iNOS, and CRP in rats with AS.
Figure 3.
Effects of ALCAR on the protein expressions of CRP, TNF-α, IL-1β, and iNOS in aorta and heart tissues of AS rats. (A) The relative protein expression of CRP, TNF-α, IL-1β and iNOS in the aorta tissue among the control, AS, ALCAR, and AS+ALCAR groups. (B) The relative protein expression of CRP, TNF-α, IL-1β, and iNOS in the heart tissue among the control, AS, ALCAR, and AS+ALCAR groups. Compared with the control group, * p<0.05 and ** P<0.01; Compared with the AS group, ## P<0.01.
ALCAR reduced the concentration of angiotensin II (Ang II) in aorta
To investigate the effects of ALCAR on the expression of Ang II in the aorta, radioimmunoassay using the Ang II kit was used to detect the concentration of angiotensin II in the aorta. As shown in Figure 4A, the concentration of Ang II was significantly increased in rats with AS when compared with the control group (P<0.01). On the contrary, the concentration of Ang II in the AS+ALCAR group was significantly decreased compared with the AS group (P<0.01). Accordingly, these results demonstrated that ALCAR significantly reversed the high expression of Ang II in the aorta induced by atherosclerosis.
Figure 4.
Effects of ALCAR on Ang II content and histological patterns arterial tissue in AS rats. (A) The AngII content in the control, AS, ALCAR, and AS+ALCAR groups. (B) Histological examination in aorta (HE staining, ×400). Compared with the control group, ** P<0.01; Compared with the AS group, ## P<0.01.
ALCAR improved structural integrity of aortas in AS rats
As shown in Figure 4B, in the control group and the ALCAR group, the aorta vascular lumen of the rats was large with thin walls, and its intimal structure was clear and smooth, having intact internal elastic membrane, muscle cells were smooth and regularly arranged, with parallel internal elastic membranes. The aorta vascular lumen of atherosclerotic rats was narrow with thickening wall, scarce endothelial cells, widened endothelial gap, ruptured and wavy internal elastic membrane, and disorderly arranged middle elastic membrane and smooth muscle cells. Additionally, some smooth muscle cells proliferated, and the plaque showed numerous foam cells and lipid deposition. The lumen area of the AS+ALCAR group was significantly larger than that of the AS group, and the severity of the plaque was far lower than that of the AS group. These results confirmed that ALCAR improves the structural integrity of the aorta and indicates that ALCAR has anti-atherosclerotic activity against aortic vascular lesions.
Discussion
Establishing the animal model of AS is one of the basic conditions of this study. Rats are easily obtained and omnivorous animals, and are similar to humans in anatomy and physiology; therefore, the rat AS model was established for this study. Since rats have lipids with natural anti-atherosclerosis ability, especially cholesterol, it is difficult to establish the AS model, and this is only possible in rats fed a high-fat diet. Although hyperlipidemia is easy to form, it is not easy to bring about damage to artery and plaque. Moreover, ingestion of large doses of vitamin D3 can cause calcium overload in vivo, vascular endothelial injury, and facilitation of the cholesterol invasion, which needs a long time to develop. Further, the application of high-fat diet, vitamin D3, and mechanical balloon vascular endothelium injury accelerate the invasion of lipid deposition and promote the formation of atherosclerosis to establish a stable model of AS.
Modern research has shown that L-carnitine can enter the mitochondrial membrane through long-chain acyl coenzyme A (coenzyme A CoA) to oxidize fatty acid beta energy, accelerate the production of ATP, and increase the level of ATP in treatment of myocardial cells in heart failure [17]. However, for treatment of coronary heart disease and AS, research is still at a preliminary stage. Although there are many similarities, L-carnitine did not decrease or enhance oxidative damage, while ALCAR did decrease MDA, nitrotyrosine, and oxo8dG/oxo8G in old rat brains. These data suggest that ALCAR is a more effective dietary supplement than L-carnitine [13]. To further explore the therapeutic effect of ALCAR-induced AS and coronary artery disease induced, the research group observed the formation of ALCAR anti-atherosclerosis in rats. The results showed that the morphology of the aortas in AS rats after ALCAR intervention was healthier than that of AS rats, and the severity of atherosclerotic plaque was significantly reduced. The pathological study suggested that the ALCAR intervention could improve the pathological changes of aortas in AS rats. In addition, further experiments proved that ALCAR could prevent and treat AS by regulating the level and distribution of lipid in vivo, reducing the level of inflammatory factors, and improving the antioxidant capacity of the body.
High-fat diet can deposit cholesterol in the form of cholesterol esters in the blood vessels and other parenchymal organ tissue [18], leading to the formation of AS. Low-density lipoprotein (LDL) plays a key role in AS, and LDL is a lipid component related to the development of AS, which has a pivotal role in AS. Currently, LDL overexpression is considered as one of the risk factors of atherosclerosis. LDL is transported to the damaged vascular endothelium, and oxidized low-density lipoprotein (OX-LDL) is formed under the attack of reactive oxygen species (ROS). OX-LDL causes vasodilation and contraction dysfunction [19], activates apoptotic pathways, has cytotoxic effects, and causes EC damage. The uptake of lectins like OX-LDL receptor-1 plays an important role in the formation of foam cells [20]. It is considered that the overexpression of LDL is one of the most dangerous factors for AS, and regulating serum LDL can effectively control the occurrence and development of AS [21]. Medical research has shown that HDL is a key factor in reversing AS [22]. For instance, the decrease of HDL leads to the increase of free cholesterol in plasma, the decline of lipid clearance function, the formation of vascular plaques, and the acceleration of AS. High-fat feeding can result in the disorders of serum lipid metabolism in rats, as well as increasing levels of serum TG, TC, and LDL and the decreasing HDL levels. The results of the present experiment showed that ALCAR is one of the pathogenic factors of lipid disorder in rats induced by high-fat diet, had a good mitigatory effect, significantly reducing the levels of TC, TG, and LDL in serum of rats, and increasing the level of HDL in serum. In normal-diet rats, ALCAR had no significant changes in TC, TG, or LDL contents of lipid composition. Furthermore, our results suggested that ALCAR in hyperlipidemia can improve the distribution of liposomes, including lowering the level of LDL and elevating the level of HDL, through which ALCAR plays a critical role in AS. Our study also found that there was no obvious change in serum TG and LDL levels, while HDL level was significantly increased after ALCAR intervention in normal animals. Therefore, these results suggest that ALCAR is a general expression of the upregulation of HDL expression.
Vascular oxidative stress leads to disordered lipid metabolism, which can further aggravate AS, and the vascular endothelial cells in hyperlipidemia environment produce severe lipid peroxidation damage, induced by AS or further aggravated by it [23]. Oxygen free radicals play an important role in the development of AS [24,25]. MDA is the product of lipid peroxidation, whose levels can indirectly reflect the degree of lipid peroxidation. High-fat diet can lead to increased production of free radicals, lipid peroxidation, and elevated levels of MDA. In the dietary management of aged rats, a combination of ALCAR and r-α-lipoic acid, or r-α-lipoic acid alone, decreased the content of MDA in brain tissue of aged rats, and oral administration of ALCAR hydrochloride and α lipoic acid reduced the MDA level in the liver [13]. The decline of their antioxidation ability might be one of the factors contributing to atherosclerosis; thus, the selection of antioxidants may be an important way to treat AS. This study found that ALCAR could effectively decrease MDA in serum and aortic tissue, reduce lipid peroxidation production, and increase the level of antioxidants, suggesting that ALCAR can strengthen the body’s own free radical defense system through its antioxidants, and alleviate the oxidative damage of DNA, protein, and lipid free radicals. Combined with aortic pathomorphological detection, ALCAR can significantly alleviate the symptoms of atherosclerosis in rats with AS, and the antioxidant capacity of aortic tissue was significantly improved after administration of ALCAR. Our results suggest that ALCAR exerts an anti-AS effect through the intervention of LDL oxidation to OX-LDL.
The inflammation theory states that AS is a chronic inflammatory process. To explore whether ALCAR can exert an anti-AS effect by reducing the high expression of inflammatory factors in the environment of high blood lipids, we assessed the levels of TNF-α, IL-1β, C-reactive protein (CRP), and mRNA in serum and aortic tissues. CRP is an important marker of inflammation, and is clinically used to predict the severity of aorta AS [26]. CRP promotes oxidative stress in vascular endothelial cells, and OX-LDL stimulates expression of adhesion molecules and promotes the differentiation and development of plaque-mediated migration of vascular smooth muscle cells (VSMC) [27]. Additionally, CRP activates VSMC nuclear transcription factor kappa B (NF-κB) to induce monocyte chemotaxis protein, IL-6, and iNOS expression [28,29]. Treatment with IL-1β platelet-derived growth factor stimulates the differentiation phenotype of VSMC and enhances the proliferation and migration of VSMCs [30]. TNF-α can upregulate the expression of syndecan-4 protein, thereby promoting the infiltration, polymerization, and proliferation of VSMC intimal [31]. At the same time, we found the levels of iNOS were closely associated with inflammation. In recent years, researchers have attached more importance to the role of nitric oxide (NO) in inflammatory diseases. iNOS is widely involved in chemotaxis expression of inflammatory factors and ROS/reaction of nitride product (RNS), further demonstrating the key role of nitric oxide in the development of inflammation. In the present study, we found that ALCAR can significantly reduce the concentration of inflammatory factors TNF-α, IL-1β, and CRP in atherosclerotic rats. After atherosclerosis formation and ALCAR intervention, TNF-α, IL-1β, CRP, and mRNA levels of iNOS and protein expression were significantly decreased compared to the AS group, showing that the anti-inflammatory action may be one of the mechanisms underlying the effect of ALCAR in treating AS.
Previous research has shown that the level of plasma APN in patients with AS was significantly lower than that in the negative control function of physiological anti-AS [32], and it decreased with the increase of AS degree, which was negatively related to stenosis and coronary artery [33]. Here, the serum APN in high-fat diet group rats was significantly lower than that in the normal-diet group, compared with the previous research results. However, there was no obvious change in serum APN level in rats of the ALCAR group, suggesting that the APN function of ALCAR has no significant effect on adipocyte secretion.
The renin-angiotensin-aldosterone system (RAAS) exists widely in vascular endothelial tissue. Studies have shown that Ang II is associated with the development of atherosclerosis, and the increase of Ang II concentration in the aortic tissue is one of the main mechanism of AS formation[34]. According to the results of the present study, ALCAR can play an anti-atherosclerosis role by reducing the expression of Ang II in aortic tissue.
Based on observing the preventive effect of ALCAR on atherosclerotic plaque formation, inflammatory factors in heart tissue and antioxidant capacity of heart tissue were detected. The results showed that, on the basis of the formation of the atherosclerosis, the expression of inflammatory factor gene and protein was significantly increased in myocardial tissue, whereas the antioxidant capacity decreased. The expression of inflammatory factor gene and protein in the AS+ALCAR group was significantly lower than that in the AS group, and the antioxidant capacity was higher than that in the AS group. AS is a systemic vascular disease related to heart disease. High-fat diet and vitamin D3 injection can cause chronic coronary artery disease and mild atherosclerotic symptoms, resulting in mild myocardial ischemia, cardiac oxidative stress, and chronic inflammation. Analysis of the causes suggested that ALCAR can reduce inflammation and antioxidants to prevent coronary heart disease. Studies have found that fat itself can cause inflammation and oxidative damage, and the reduction of inflammatory factors may be involved in the anti-atherosclerosis and direct myocardial protection of ALCAR [35]. We found that ALCAR significantly decreased the expression of inflammatory cytokines in myocardial tissue of rats fed a normal diet compared with the control group. The intervention of ALCAR in heart inflammation may not depend exclusively on the anti-atherosclerotic effect. A recent study [36] showed that levocarnitine was able to upregulate the expression of TIMP-1, inhibit the expression of ICAM-1, and had a myocardial protection effect. From the level of myocardial cells protection, it may be one of the crucial reasons to further reduce the severity of inflammation.
In recent years, many studies have found that regulation of intestinal microbial metabolism is important, especially the trimethylamine (TMA) oxides of intestinal flora in the liver, which can increase the risk of cardiovascular disease. L-carnitine is an important source of TMA production in intestinal flora. Koeth [37] found that giving large doses of L-carnitine to mice accelerated the process of AS, which was in conflict with our study. In their study, after adding 1.3% levocarnitine to the drinking water (equivalent to 600–800 mg/kg dose) for a long time, mice were healthy. However, our findings used AS model rats, and the model was in high blood lipids, high oxidation, and high inflammatory state, and given low doses of L-carnitine. The conflicting research showed that, in atherosclerosis rats with high-fat diet, a certain dose of levocarnitine could improve the process of coronary AS. An editorial published by McCarty [38] also explicitly mentioned the one-sided study of Koeth.
Conclusions
ALCAR inhibits the development of atherosclerosis by regulating blood lipids and inhibiting the gene expression of inflammatory factors and oxidant stress. ALCAR can reduce the level of mRNA and protein of CPR, TNF-α, IL-1β, and iNOS in the aorta and heart tissues of rats with AS, which may be related to anti-coronary artery atherosclerosis and myocardial protection. Further in-depth research on the mechanism underlying ALCAR is needed.
Footnotes
Source of support: Projects of Medical and Health Science and Technology Development Program in Shandong Province (2016WS0643)
Conflict of interests
None.
References
- 1.Iqbal J, Al Qarni A, Hawwari A, et al. Metabolic syndrome, dyslipidemia and regulation of lipoprotein metabolism. Curr Diabetes Rev. 2018;14:427–33. doi: 10.2174/1573399813666170705161039. [DOI] [PubMed] [Google Scholar]
- 2.Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044:275–83. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 3.Funk CD, Cyrus T. 12/15-lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med. 2001;11:116–24. doi: 10.1016/s1050-1738(01)00096-2. [DOI] [PubMed] [Google Scholar]
- 4.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 5.Peluso I, Morabito G, Urban L, et al. Oxidative stress in atherosclerosis development: the central role of LDL and oxidative burst. Endocr Metab Immune Disord Drug Targets. 2012;12:351–60. doi: 10.2174/187153012803832602. [DOI] [PubMed] [Google Scholar]
- 6.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–31. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Jeong TS, Kim DY, et al. Hematein inhibits atherosclerosis by inhibition of reactive oxygen generation and NF-kappaB-dependent inflammatory mediators in hyperlipidemic mice. J Cardiovasc Pharmacol. 2003;42:287–95. doi: 10.1097/00005344-200308000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Luoma JS, Stralin P, Marklund SL, et al. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arterioscler Thromb Vasc Biol. 1998;18:157–67. doi: 10.1161/01.atv.18.2.157. [DOI] [PubMed] [Google Scholar]
- 9.Lee WJ, Ou HC, Hsu WC, et al. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg. 2010;52:1290–300. doi: 10.1016/j.jvs.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 10.Libby P1, Ridker PM, Hansson GK Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: From pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagen TM, Liu J, Lykkesfeldt J, et al. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci USA. 2002;99:1870–75. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boerrigter ME, Franceschi C, Arrigoni-Martelli E, et al. The effect of L-carnitine and acetyl-L-carnitine on the disappearance of DNA single-strand breaks in human peripheral blood lymphocytes. Carcinogenesis. 1993;14:2131–36. doi: 10.1093/carcin/14.10.2131. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Head E, Kuratsune H, et al. Comparison of the effects of L-carnitine and acetyl-L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann NY Acad Sci. 2004;1033:117–31. doi: 10.1196/annals.1320.011. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese V, Ravagna A, Colombrita C, et al. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: Involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79:509–21. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Shang R, Sun Z, Li H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: A systematic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:88. doi: 10.1186/1471-2261-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodis HN, Crawford DW, Sevanian A. Cholesterol feeding increases plasma and aortic tissue cholesterol oxide levels in parallel: Further evidence for the role of cholesterol oxidation in atherosclerosis. Atherosclerosis. 1991;89:117–26. doi: 10.1016/0021-9150(91)90051-4. [DOI] [PubMed] [Google Scholar]
- 19.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Chen XP, Zhang TT, Du GH. Lectin-like oxidized low-density lipoprotein receptor-1, a new promising target for the therapy of atherosclerosis? Cardiovasc Drug Rev. 2007;25:146–61. doi: 10.1111/j.1527-3466.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 21.Cao Q, Cui X, Wu R, et al. Myeloid deletion of alpha1AMPK exacerbates atherosclerosis in LDL receptor knockout (LDLRKO) mice. Diabetes. 2016;65:1565–76. doi: 10.2337/db15-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakamata H, Miyazaki A, Sakai M, et al. Species difference in cholesteryl ester cycle and HDL-induced cholesterol efflux from macrophage foam cells. Arterioscler Thromb. 1994;14:1860–65. doi: 10.1161/01.atv.14.11.1860. [DOI] [PubMed] [Google Scholar]
- 23.Shah P, Bajaj S, Virk H, et al. Rapid progression of coronary atherosclerosis: A review. Thrombosis. 2015;2015 doi: 10.1155/2015/634983. 634983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antelava NA, Pachkoriia KZ, Kezeli TD, et al. [Major pathogenic links of atherosclerosis]. Georgian Med News. 2005;(128):72–79. [in Russian] [PubMed] [Google Scholar]
- 25.Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: Myth or fact? Arch Biochem bBiophys. 2003;420:217–21. doi: 10.1016/j.abb.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Momiyama Y, Ohmori R, Fayad ZA, et al. Associations between plasma C-reactive protein levels and the severities of coronary and aortic atherosclerosis. J Atheroscler Thromb. 2010;17:460–67. doi: 10.5551/jat.2931. [DOI] [PubMed] [Google Scholar]
- 27.Calabro P, Golia E, Yeh ET. CRP and the risk of atherosclerotic events. Semin Immunopathol. 2009;31:79–94. doi: 10.1007/s00281-009-0149-4. [DOI] [PubMed] [Google Scholar]
- 28.Devaraj S, Yun JM, Duncan-Staley C, Jialal I. C-reactive protein induces M-CSF release and macrophage proliferation. J Leukoc Biol. 2009;85:262–67. doi: 10.1189/jlb.0808458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Stevenson MJ, Brown JM, et al. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: Mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 30.Chen CN, Li YS, Yeh YT, et al. Synergistic roles of platelet-derived growth factor-BB and interleukin-1beta in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:2665–70. doi: 10.1073/pnas.0510973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng W, Zhao Y, Wang S, Jiang F. Tumor necrosis factor-related apoptosis-inducing ligand in vascular inflammation and atherosclerosis: A protector or culprit? Vascul Pharmacol. 2014;63:135–44. doi: 10.1016/j.vph.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Chakraborti CK. Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity. World J Diabetes. 2015;6:1296–308. doi: 10.4239/wjd.v6.i15.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamczak M, Wiecek A, Funahashi T, et al. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16:72–75. doi: 10.1016/s0895-7061(02)03197-7. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki M, Sakonjo H, Takai S. Anti-atherosclerotic effects of an angiotensin converting enzyme inhibitor and an angiotensin II antagonist in Cynomolgus monkeys fed a high-cholesterol diet. Br J Pharmacol. 1999;128:523–29. doi: 10.1038/sj.bjp.0702833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng C, Liu JL, Du AL. Cardioprotective effect of resveratrol on atherogenic diet-fed rats. Int J Clin Exp Pathol. 2014;7:7899–906. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SX, Tan L, Wang J, Zhong JQ. Effect of levocarnitine on TIMP-1, ICAM-1 expression of rats with coronary heart disease and its myocardial protection effect. Asian Pac J Trop Med. 2016;9:269–73. doi: 10.1016/j.apjtm.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarty MF. L-carnitine consumption, its metabolism by intestinal microbiota, and cardiovascular health. Mayo Clin Proc. 2013;88:786–89. doi: 10.1016/j.mayocp.2013.06.004. [DOI] [PubMed] [Google Scholar]