Abstract
Objectives:
To determine if hospitalization, testing, diagnosis and management of suspected gastroesophageal reflux, and follow-up visits decreased since introduction of American Academy of Pediatrics guidelines for brief resolved unexplained events (BRUE) s.
Study design:
We performed a retrospective cohort study of infants with BRUE evaluated at Boston Children’s Hospital in the year before and after guideline implementation to determine if practice patterns have changed. Outcomes included hospitalization rates, frequency of swallow assessments, other diagnostic testing, and reflux diagnoses, cost of care and number of repeat visits. Groups were compared based on whether they presented before or after guideline implementation.
Results:
359 subjects (186 pre-, 173 post-guidelines) were identified. There were no significant differences in practice patterns or outcomes before or after guideline implementation. Subjects had mean age 2.53±0.15 months and 80% were hospitalized for 2.49±0.26 days. Each subject had 2.47 diagnostic tests performed and 89% were noncontributory. Despite only13% having videofluoroscopic swallow study performed, 72% showed aspiration/penetration. No subject had gastroesophageal reflux testing, yet reflux was implicated as the cause for admission in 40% of subjects, resulting in increased odds of discharge on acid suppressing medications (OR 2.88, 95% CI 1.68-4.92, P = .0001). In follow-up, 28% of subjects had repeat hospitalizations or emergency room visits for persistent symptoms.
Conclusions:
Infants with BRUE continue to undergo low-yield diagnostic testing and after admission remain symptomatic and frequently re-present to medical care. Swallow testing remains infrequent despite its high-yield, reflux continues to be implicated and children are still being discharged on acid suppression despite lack of efficacy.
Keywords: brief resolved unexplained event, oropharyngeal dysphagia, aspiration, gastroesophageal reflux, videofluoroscopic swallow study
Brief resolved unexplained events (BRUE) are frightening episodes characterized by choking, pallor, cyanosis, and limpness in previously healthy infants. Numerous studies have attempted to outline appropriate management strategies but a major limitation of this research is that the definition remains subjective and includes a heterogeneous patient population,1, 2 Previously known as apparent life-threatening events (ALTE), they were re-conceptualized as BRUE in 2016 American Academy of Pediatrics (AAP) clinical practice guidelines; with this reconceptualization a management algorithm was proposed, recommending limited testing based on history and physical examination3-6.
Although gastroesophageal reflux disease (GERD) is frequently implicated in these patients, pediatric gastroenterologists are often not involved in the initial diagnosis or management of BRUE patients, but are commonly involved in their follow-up care, which often includes un-doing or reversing the diagnosis of GERD and changing the management plan in these patients who typically have recurrent symptoms1, 2, 7-9. In fact, gastroenterologists are much more likely to diagnose swallowing dysfunction which has been shown to be the most common modifiable diagnosis that can improve outcome in infants with BRUE10, 11.
It is understandable that these patients are misdiagnosed with GERD because the symptoms of swallowing dysfunction are indistinguishable from GERD and include gagging, choking, coughing and blue spells 12, 13. Furthermore, because 80% of aspiration is silent in infants, there are no historical clues to make the diagnosis8, 9. In fact, in infants undergoing swallowing assessments for BRUE using videofluoroscopic swallow study (VFSS), 73% had evidence of aspiration during swallowing, making this is the highest yield test of any performed in infants with BRUE10. Unfortunately, the AAP BRUE guideline only recommends an assessment of feeding difficulties if suggested by the patient’s presentation but we now know this is impossible because of the high rate of silent aspiration4. Making the incorrect diagnosis of GERD has significant implications; it can result in inappropriate treatment with acid suppression medications which actually can worsen outcomes, and delays the correct diagnosis in infants who are very symptomatic10, 14.
Therefore, the goal of this study was to determine if publication of the AAP BRUE algorithm resulted in a reduction in diagnostic testing and hospitalizations; an increased incorporation of swallow testing in evaluation of these patients; a decrease in the number diagnoses of suspected GERD and acid suppression use; and a reduction in readmissions and emergency room visits.
Methods
We reviewed records of infants with BRUE evaluated at Boston Children’s Hospital between June 2015-May 2016 (pre-algorithm) and June 2016-May 2017 (post-algorithm). We selected these 2 periods to compare differences in diagnostic evaluations and outcomes before and after guideline publication and subsequent implementation. Subjects were identified with Informatics for Integrating Biology and the Bedside (i2b2) software using ICD codes for ALTE (R68.13), cyanosis (R23.0), and apnea (R06.81) for any child under 1 year15. Charts were reviewed to confirm that each presentation was consistent with the BRUE definition; any subjects not meeting the definition were excluded prior to completing in-depth chart review4. Subjects with significant medical diagnoses prior to BRUE presentation (eg, seizure disorder, cyanotic heart disease, metabolic disorder) were excluded.
Charts were reviewed to determine baseline characteristics, prior visits for similar symptoms, hospitalization rates at presentation, length of stay for initial presentation, frequency of diagnostic testing and diagnostic yield of tests, treatment with acid suppressing medications, including H2 receptor antagonists (H2RA) and proton pump inhibitors (PPI), based on prescriptions in charts and medical record documentation of home medications, whether discharge summaries or consultant documentation included reference to or discharge diagnosis of GERD, and number of repeat visits for similar symptoms (e.g. choking, color changes, change in tone). To tally repeat visits, we included hospital admissions, emergency department (ED) visits, combined hospital admission and ED (because there were some subjects that came to the ED for persistent symptoms and were not admitted but were admitted at later points for persistent or recurrent symptoms) and clinic visits at Boston Children’s Hospital in the 6-months following BRUE. This 6-month follow-up period was selected because most subjects would have been beyond the risk of repeat BRUE at that point. Reasons for repeat visits were identified based on chart reviews, discharge diagnoses and billing codes. Diagnostic tests were considered contributory if the results could provide an explanation for BRUE symptoms.
Swallow evaluations were reviewed to determine if subjects had VFSS or clinical feeding evaluation (CFE). VFSS were considered abnormal if there was evidence of aspiration or laryngeal penetration for any consistency. Laryngeal penetration was considered abnormal because these patients have similar outcomes to patients with overt aspiration and respond to thicking16-18. CFEs were performed by speech language pathologists (SLP); VFSS were performed as previously described8, 19-22.
Hospital billing records were reviewed to determine total charges for BRUE visits and follow-up charges for the 6-months following. Outliers, with charges totaling over $1 million, were excluded from this analysis.
Proportions were compared with the Fisher exact test and continuous outcomes with t-tests. VFSS and CFE were compared using the McNemar test. Cox proportional hazards models were used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for time to repeat admission/ED visit. Covariates adjusted for in the model included age at BRUE, sex, premature status at birth, and total number of diagnostic tests performed during BRUE hospitalizations. All statistical tests were 2-sided with P<0.05 considered statistically significant. All data were analyzed using SPSS Statistics version 23 and SAS version 9.4 (SAS Institute, Cary, NC, USA).
The present study was approved by the Institutional Review Board at Boston Children’s Hospital.
Results
Subject Characteristics
Table I shows presenting characteristics; there were no differences in the periods before and after the guidelines. Figure 1 (available at www.jpeds.com) is a flow diagram of the study population. Patient demographics showing the high proportion of local patients are shown in Figure 2 (available at www.jpeds.com). BRUE admissions were evenly distributed throughout all seasons of the year.
Table 1. Subject Characteristics.
Subject characteristics for the cohort, showing no difference between year the before and the year after BRUE guidelines, including similar proportion with prior visits, admission rates and lengths of stay.
| Pre- Algorithm (n=186) |
Post- Algorithm (n=173) |
p-value | |
|---|---|---|---|
| Subject Characteristics | |||
| Age (months) | 2.36 ± 0.19a | 2.70 ± 0.23 | 0.25 |
| Premature | 49 (26) | 33 (19) | 0.10 |
| Gestational Age (weeks) | 32.88 ± 0.46 | 33.78 ± 0.44 | 0.16 |
| Female Sex | 108 (58) | 95 (55) | 0.60 |
| Breastfed | 83 (45) | 88 (51) | 0.13 |
| Prior Visits | 38 (20) | 24 (14) | 0.38 |
| Prior ED | 11 (6) | 7 (4) | 0.48 |
| Prior Clinic for Symptoms | 27 (15) | 17 (10) | 0.20 |
| Admitted to Hospital | 153 (82) | 135 (78) | 0.35 |
| Not Admitted | 33 (18) | 38 (22) | 0.35 |
| Length of Stay (days) | 2.65 ± 0.40 | 2.31 ± 0.32 | 0.50 |
mean ± standard error or n (%)
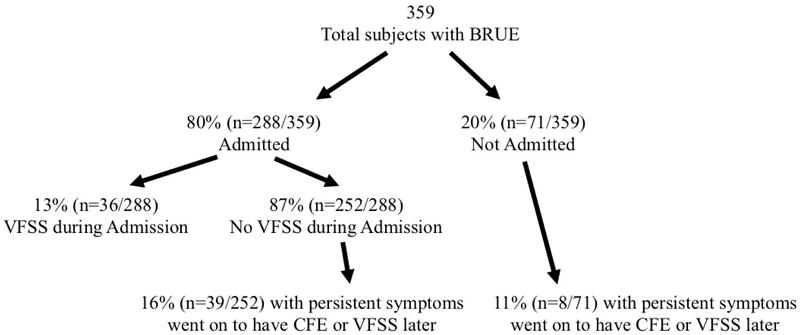
Figure 1 (online). Flow Diagram.
Flow diagram of study population showing proportion of BRUE subjects admitted and seen in ED, along with clinical feeding evaluation (CFE) and videofluoroscopic swallow study (VFSS) testing rates during and after initial BRUE evaluation.
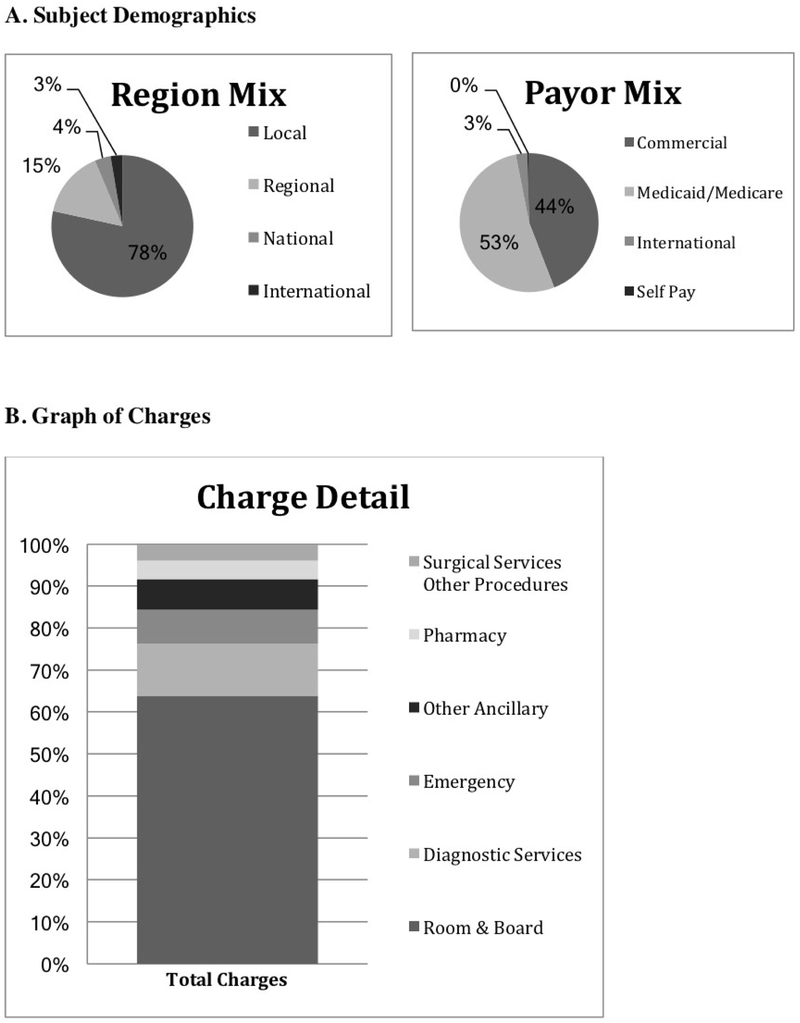
Figure 2 (online). A. Subject Demographics and B. Graph of Charges.
Part A shows that the patient population was primarily local with a mix of commercial and state insurance. Part B shows the distribution of charges for all BRUE presentations, consisting largely of charges for room and board, diagnostic testing and ED care.
Length of stay and proportion of patients hospitalized did not change significantly after guideline publication, as shown in Table 1. Premature subjects had longer length of stay compared with full term subjects (3.73 ± 0.68 vs 2.12 ± 0.26, p=0.028). There were two patient deaths in the second year (0.6% of total cohort), one from unexplained causes during follow-up and another from cerebral hemorrhage related to suspected neuro-metabolic disorder diagnosed during BRUE hospitalization.
Subsequent Visits
Table 2 shows subsequent visits after the initial admission; 28% of subjects were either readmitted or seen back in the ED (without hospital admission) for similar symptoms. Repeat ED and hospital admission diagnoses that were found to increase in the second year; most commonly billed diagnoses were feeding difficulties (1% vs 9%, p=0.007), respiratory symptoms (14% vs 35%, p=0.001), and vomiting (3% vs 19%, p=0.0002). Premature infants were more likely to have subsequent readmissions compared with full term infants; 23% of preterm infants were readmitted vs 13% of term infants, p=0.035.
Table 2. Subsequent Visits.
Subsequent visits for symptoms similar to BRUE during follow-up period, showing no significant change in subsequent admissions, admission nights, or repeat ED visits between the two years.
| Pre- Algorithm (n=186) |
Post- Algorithm (n=173) |
p- value |
|
|---|---|---|---|
| Subsequent Visits | |||
| Repeat Admission | 33 (18) a | 23 (13) | 0.31 |
| Repeat ED without Admit | 32 (17) | 25 (15) | 0.56 |
| Repeat Admit or ED | 58 (31) | 41 (24) | 0.13 |
| 1+ Clinic Visits for Symptoms | 82 (44) | 66 (38) | 0.28 |
| 2+ Clinic Visits for Symptoms | 60 (32) | 46 (27) | 0.25 |
| Mean Admits | 0.26 ± 0.06 | 0.20 ± 0.05 | 0.40 |
| Admit Nights | 4.97 ± 1.47 | 3.26 ± 0.55 | 0.28 |
| Mean ED Visits | 0.30 ± 0.06 | 0.20 ± 0.04 | 0.17 |
| Mean Clinic Visits | 2.2 ± 0.31 | 1.35 ± 0.21 | 0.02 |
n (%)
Swallow Evaluations
Table 3 (available at www.jpeds.com) shows results of CFEs performed during and after the hospitalization. In a comparison between SLP recommendations before and after the algorithm, fewer subjects were sent for confirmatory VFSS in the second year despite the high rates of silent aspiration which limits the sensitivity of the CFE. There was poor correlation between CFE and VFSS results for subjects that had both evaluations, with 33% of subjects with reassuring CFE ultimately found to have aspiration/penetration on VFSS (p<0.0005).
Table 3. Clinical Feeding Evaluation Results.
Proportion with CFE, findings on CFE, and change in management based on CFE results, showing a significant increase in proportion for which change in bottle flow rate was recommended, decrease in recommendation for thickening, and fewer subjects sent for confirmatory VFSS.
| Pre- Algorithm (n=186) |
Post- Algorithm (n=173) |
p-value | ||
|---|---|---|---|---|
| Clinical Feeding Evaluation | ||||
| CFE Performed | Ever | 68/186 (37)a | 57/173 (33) | 0.51 |
| During Admission | 48/186 (26) | 41/173 (24) | 0.71 | |
| After Admission | 19/186 (10) | 16/173 (9) | 0.86 | |
| CFE Results | Normal | 31/68 (46) | 27/57 (47) | 0.58 |
| Concern for Aspiration/Penetration | 16/68 (24) | 12/57 (21) | 0.53 | |
| Discoordination | 14/68 (21) | 18/57 (32) | 0.51 | |
| Change in Management | 33/68 (49) | 30/57 (53) | 0.36 | |
| Change in Flow Rate | 8/33 (24) | 25/30 (83) | <0.001 | |
| Thickening | 3/33 (9) | 0/30 (0) | 0.10 | |
| Recommend VFSS | 15/33 (46) | 5/30 (17) | 0.003 | |
n (%)
VFSS results are in Table 4. Specific consistencies recommended as a result of VFSS are shown in Table 5 (available at www.jpeds.com). Only 13% of subjects had VFSS performed during their admission and 72% of these studies showed aspiration/penetration. 42% of subjects that underwent VFSS were breastfed and 58% were bottle fed. Of the breastfed infants, 58% of VFSS were abnormal. Of the patients that were bottle fed, 61% had an abnormal VFSS.
Table 4. Videofluoroscopic Swallow Study Results.
Proportion with VFSS testing, findings on VFSS, and change in management based on VFSS results. All subjects with aspiration on VFSS had silent aspiration.
| Pre- Algorithm (n=186) |
Post- Algorithm (n=173) |
p-value | ||
|---|---|---|---|---|
| VFSS Results | ||||
| VFSS Performed | Ever | 44/186 (24)a | 35/173 (20) | 0.45 |
| During Admission | 24/186 (13) | 12/173 (7) | 0.12 | |
| After Admission | 20/186 (11) | 23/173 (13) | 0.62 | |
| Months from Admit to VFSS | 2.23 ± 0.72 | 2.72 ± 0.56 | 0.60 | |
| 2+ Admits Prior to VFSS | 7/44 (16) | 4/35 (11) | 0.55 | |
| VFSS Results | Normal | 15/44 (34) | 17/35 (49) | 0.25 |
| Aspiration | 15/44 (34) | 7/35 (20) | 0.13 | |
| Silent | 15/15 (100) | 7/7 (100) | 1.0 | |
| Penetration | 14/44 (32) | 11/35 (31) | 1.0 | |
| Change in Management | 27/44 (61) | 17/35 (49) | 0.36 | |
| Change in Flow Rate | 7/27 (26) | 5/17 (29) | 0.15 | |
| Thickening | 14/27 (52) | 12/17 (71) | 1.0 | |
| Made NPO | 6/27 (22) | 0/17 (0) | 1.0 | |
mean ± standard error or n (%)
Table 5. Liquid Consistencies Recommended after VFSS.
Liquid consistencies recommended after VFSS in the year before and the year after BRUE guidelines.
| Pre- Algorithm (n=44 with VFSS) |
Post- Algorithm (n=35 with VFSS) |
p-value | |
|---|---|---|---|
| Thin liquid Consistency | 24 (54)a | 23 (65) | 0.36 |
| Half-nectar Consistency | 6 (14) | 8 (23) | 0.38 |
| Nectar Consistency | 7 (16) | 1 (3) | 0.07 |
| Half-honey Consistency | 0 (0) | 1 (3) | 0.44 |
| Honey Consistency | 1 (2) | 2 (6) | 0.58 |
| NPO after VFSS | 6 (14) | 0 (0) | 0.03 |
n (%)
In the cohort overall, 15% of subjects had 2 or more ED visits or hospitalizations for BRUE before undergoing a VFSS. 100% of patients with aspiration on VFSS had silent aspiration. At the time of the BRUE admission, premature infants were more likely to undergo clinical feeding evaluations and VFSS and also more likely to have aspiration/penetration on VFSS, compared with full term infants (74% vs 50% abnormal, p=0.038).
Figure 1 shows VFSS testing rates and repeat visits. Of the subjects that were not admitted or did not have VFSS during their BRUE admission, 15% went on to have CFE and/or VFSS in follow-up due to persistent symptoms. Subjects that had VFSS during their initial admission had fewer combined ED visits and admissions in the six months following the index admission compared with subjects that had VFSS later (0.46 ± 0.15 vs 1.29 ± 0.30, p=0.017).
Half of subjects were breastfed and 30% of these subjects had lactation evaluation while hospitalized. 31% of lactation consultations resulted in change in management, including change in position/latch adjustments to decrease rapid flow. There was no significant difference in repeat hospitalization rates between breastfed and bottle fed infants in either year (p>0.29).
Other Diagnostic Testing
Diagnostic testing remained common after the guidelines, as in Table 6. A mean of 2.47±0.11 tests were performed for each subject with 45% of patients having 3 or more tests. 89% of tests yielded negative, noncontributory results. VFSS was the highest yield test ordered with 72% of results abnormal. There was no significant difference in diagnostic testing rate or overall yield of diagnostic tests for premature infants. There was no difference in diagnostic yield of any individual diagnostic test (all p>0.221).
Table 6. Diagnostic Testing Rates and Yields.
All testing performed during BRUE evaluation, including proportion that had each test and proportion for which each test contributed to evaluation. EKG was most commonly performed but low-yield while VFSS was infrequently performed but highest-yield.
| Pre- Algorithm (n=186) |
Post- Algorithm (n=173) |
p-value | ||
|---|---|---|---|---|
| Mean Diagnostic Tests Performed | 2.31 ± 0.16a | 2.64 ± 0.17 | 0.15 | |
| Mean Test Contribution Rate | 0.12 ± 0.02 | 0.11 ± 0.02 | 0.68 | |
| EKG | Performed | 105 (57) | 111 (64) | 0.16 |
| Contributory | 0 (0) | 5 (5) | 0.06 | |
| Lab Testing | Performed | 59 (32) | 71 (41) | 0.08 |
| Contributory | 3 (5) | 2 (3) | 0.66 | |
| Chest x-ray | Performed | 67 (36) | 59 (34) | 0.74 |
| Contributory | 3 (5) | 6 (10) | 0.30 | |
| Infectious Testing | Performed | 40 (22) | 45 (26) | 0.32 |
| Contributory | 5 (13) | 9 (20) | 0.39 | |
| EEG | Performed | 24 (13) | 30 (17) | 0.30 |
| Contributory | 2 (8) | 7 (23) | 0.27 | |
| Echocardiogram | Performed | 20 (11) | 27 (16) | 0.21 |
| Contributory | 1 (5) | 1 (4) | 1.00 | |
| VFSS | Performed | 24 (13) | 12 (7) | 0.08 |
| Contributory | 20 (83) | 6 (50) | 0.05 | |
| Flexible Laryngoscopy | Performed | 20 (11) | 15 (9) | 0.59 |
| Contributory | 4 (20) | 2 (13) | 1.00 | |
| Head Ultrasound | Performed | 10 (5) | 16 (9) | 0.22 |
| Contributory | 1 (10) | 1 (6) | 1.00 | |
| Abdominal X-ray | Performed | 12 (7) | 10 (6) | 0.83 |
| Contributory | 0 (0) | 0 (0) | 1.00 | |
| Brain MRI | Performed | 6 (3) | 15 (9) | 0.04 |
| Contributory | 2 (33) | 3 (20) | 1.00 | |
| Sleep Study | Performed | 8 (4) | 6 (4) | 0.79 |
| Contributory | 1 (13) | 1 (17) | 0.58 | |
| Abdominal Ultrasound | Performed | 5 (3) | 8 (5) | 0.40 |
| Contributory | 1 (20) | 1 (13) | 1.00 | |
| Head CT | Performed | 4 (2) | 6 (4) | 0.53 |
| Contributory | 1 (25) | 2 (33) | 1.00 | |
| Upper GI | Performed | 3 (2) | 5 (3) | 0.49 |
| Contributory | 0 (0) | 0 (0) | 0.44 | |
mean ± standard error or n (%)
Hospital Charges
Combined charges and charge breakdown for all BRUE presentations are shown in Figure 2. This value increased from the first year to the second, from $2,198,121 to $2,588,703, an increase of 18% in charges and admissions actually decreased by 7%. Mean charges were $12,144 per patient for the year before and $15,409 per patient for the year after guidelines. Follow-up charges for the 6-months following BRUE totaled $2.4 million. This included $7,834 per patient before and $5,268 per patient after the guidelines (p=0.174). Post-discharge charges consisted largely of room and board for repeat admissions (42%), diagnostic testing (22%) and ED care for subsequent visits (11%).
Reflux Attribution and Treatment
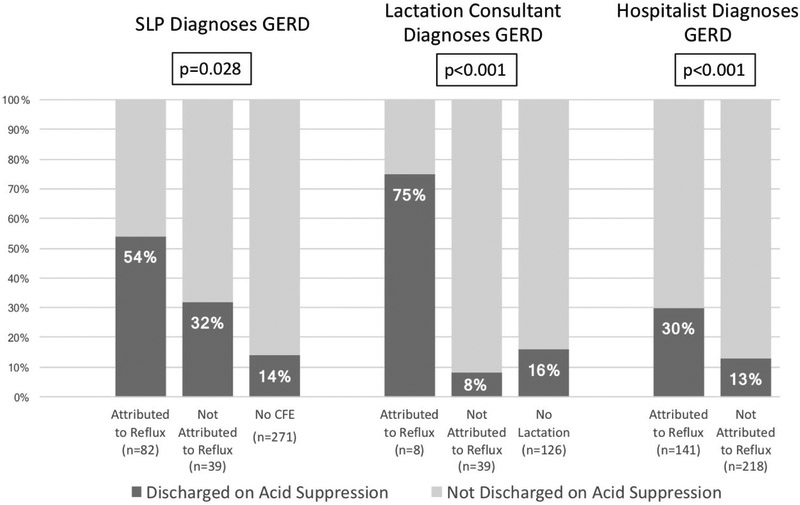
No patient in either year had reflux testing by pH/impedance. Only 5% of subjects had a gastroenterology consult. Despite lack of GI involvement, 33% of CFEs, 17% of lactation consultants and 40% of discharge documents attributed the event to reflux. The proportion of patients discharged on any acid suppression decreased from 24% to 15% (p=0.046) from the first to the second year, but the proportion of patients treated during and after admission (i.e. ever treated) with any acid suppression following BRUE admission (i.e. started by pediatricians or specialists) remained constant at 31%. 8% were discharged on PPI in both years (p=0.84). 18% were discharged on H2RA in the year before the guidelines, compared with 8% discharged on H2RA in the year after the guidelines (p=0.005). 15% were ever treated with PPI in both years (p=0.38) and 24% were ever treated with H2RA in both years (p=0.62). Subjects were more likely to have been discharged on acid suppression if the event was attributed to reflux by any of the consultants who saw the patient (OR 2.88, 95% CI 1.68-4.92, p=0.0001), as shown in Figure 3. Subjects were also more likely to ever be treated with acid suppression following BRUE if the event was attributed to reflux (OR 2.43, 95% CI 1.53-3.83, p=0.0002). Premature subjects were significantly more likely to be placed on acid suppression medications compared with full term subjects (32% vs 16% at discharge with p=0.002, and 45% vs 27% ever following BRUE, p=0.003).
Figure 3. Proportion of Subjects Discharged on Acid Suppression Varies by Attribution to Reflux.
Association between whether BRUE was attributed to reflux and whether subjects were discharged on acid suppression, showing that attribution to reflux by SLP in clinical feeding evaluation, lactation consultant or hospitalist team in discharge summary are all associated with increased odds of discharge on acid suppression.
Table 7 (available at www.jpeds.com) shows results of Cox proportional hazards analysis comparing time with repeat ED visit and/or hospitalization for subjects that were or were not discharged on or ever treated with acid suppression, showing no improvement in outcomes for infants treated with acid suppression (HR 0.76, 95% CI 0.47-1.25, p=0.28 for subjects discharged on acid suppression and HR 1.42, 95% CI 0.96-2.11, p=0.08 for subjects ever on acid suppression). Figure 4 (available at www.jpeds.com) shows Kaplan-Meier curves showing no improvement in risk of subsequent admission or ED visit for subjects discharged on acid suppression or ever treated with acid suppression.
Table 7. Association between Acid Suppression and Repeat Admission or Emergency Room Visit.
Results of Cox proportional hazards analysis comparing time to repeat ED visit and/or hospitalization for subjects that were or were not discharged on or ever treated with acid suppression, showing no significant difference in hazard ratio for any comparison group.
| Exposure | na/nb | Univariate | Multivariated | ||
|---|---|---|---|---|---|
| HRc (95%CI) | P value |
HR (95%CI) | P value |
||
| Discharged on Acid Suppression | |||||
| No | 102/289 | 1.00 | 1.00 | ||
| Yes | 21/70 | 0.83(0.52,1.32) | 0.43 | 0.76(0.47,1.25) | 0.28 |
| Ever on Acid Suppression | |||||
| No | 76/248 | 1.00 | 1.00 | ||
| Yes | 47/111 | 1.48 (1.03,2.13) | 0.04 | 1.42(0.96,2.11) | 0.08 |
number with admission or ED visit
total number at risk
HR=hazard ratio
Multivariate adjustment for age at initial presentation, sex, premature status at birth, and total number of diagnostic tests performed during initial hospitalization.
Figure 4 (online). Kaplan-Meier Curves for Treatment with Acid Suppression and Risk of Repeat Admission or Emergency Room Visit.
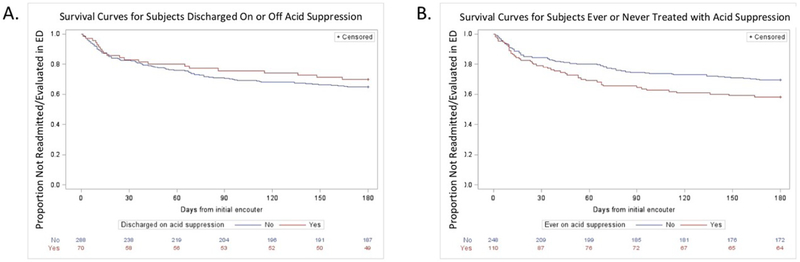
Kaplan-Meier curves showing risk of subsequent admission or ED visit for subjects discharged on acid suppression (p=0.28) in (A) and subjects ever treated with acid suppression (p=0.08) in (B), showing no benefit of acid suppression in BRUE.
Discussion
We compared management and outcomes for BRUE patients to determine if practice patterns have changed and outcomes have improved since introduction of the AAP guidelines, and if swallowing dysfunction remains common in these patients. Our results suggest 5 key findings: 1) BRUE admissions remain common, 2) BRUE management continues to be expensive and involves a great deal of low-yield testing, 3) the highest-yield test, VFSS, remains underutilized, 4) the diagnosis of GERD is often made, resulting in prescription of ineffective medications during and after admission based on this diagnosis, and 5) patients remain symptomatic after discharge and frequently re-present to care. Our results suggest that practice patterns and outcomes have changed very little in the year after publication of the BRUE algorithm. Management of these patients remains expensive. It is important to acknowledge that many providers continue to attribute these events to reflux, but our results suggest this approach might not only be unhelpful, this misattribution might also be harmful. Misdiagnosis can result in the inappropriate prescription of acid suppression and a failure to order a videofluoroscopic study to assess swallow function.
Despite the prevalence of low-yield testing, VFSS remains underutilized in this population despite its high yield in this study and prior studies showing that aspiration is silent and cannot be detected by history or observed feedings8-10. A major advance resulting from close collaborations and research emanating from aerodigestive centers is the recognition that oropharyngeal dysphagia with aspiration puts patients at risk for symptoms such as BRUE8, 10, 11, 23-26. This recognition of swallowing dysfunction is critical in all patients with BRUE, and in infants in general, as the prevalence of oropharyngeal dysphagia is increasing in the pediatric population, which may be due to increased survival of premature infants and children with chronic disease12, 27, 28. It may also be because of increased recognition of swallowing difficulties since many of these patients were previously misdiagnosed as having GERD.
Considering all of the other low-yield testing patients with BRUE undergo, performance of the VFSS as the first-line study for these infants could result in cost savings. Based on $5,231 in mean daily charges for subjects that had VFSS during admission, if VFSS were performed on the first day of the admission instead of the fourth, an estimated $24,010 may have been saved.
Providers might not obtain swallow studies for patients presenting with BRUE for a variety of reasons, including concerns about radiation exposure, concerns for whether mild abnormalities on VFSS warrant intervention, and the assumption that history and observed feedings can reliably replace fluoroscopic studies. First, we and others have shown that radiation exposure is relatively low with VFSS and significantly less than upper GI series and less than many of the tests the infants are undergoing as part of the BRUE evaluation8, 29. Second, regarding the question of if swallowing abnormalities (aspiration/penetration) are normal, we have also previously shown that infants with even mild abnormalities of swallow function (i.e. isolated laryngeal penetration), have improved outcomes including a reduction in hospitalizations for respiratory exacerbations after treatment with feeding interventions18. Finally, we and others have consistently shown that aspiration cannot be diagnosed by observed clinical feeding or by symptoms; 100% of patients in the present study who had aspiration on VFSS had silent aspiration, suggesting that neither CFE nor characterization of symptoms during feedings could have further stratified the risk of aspiration among patients with BRUE or predicted the VFSS results. Additionally, 33% of subjects with a reassuring CFE were ultimately found to have aspiration/penetration on VFSS, confirming that the assumption that VFSS might be unnecessary is incorrect8, 29.
The symptoms of oropharyngeal dysphagia overlap completely with gastroesophageal reflux and our study shows that GERD continues to be inappropriately diagnosed in BRUE, despite multiple prior studies showing that GERD is not a causative factor in these patients 4,30-35. Diagnosing GERD has significant implications and, as shown in this study, a variety of providers are making this diagnosis. Patients diagnosed with GERD in this study were twice as likely to have been discharged on or ever be treated with acid suppression if the BRUE was attributed to reflux. This is an important reminder that labeling a child with this diagnosis can be harmful, elegantly illustrated by reports that just the use of the GERD diagnosis made parents interested in reflux medicines even after being told they would likely be ineffective36. It is critical that providers and consultants avoid inappropriately labeling patients with GERD given implications on acid suppression prescribing. Not only are these medications unhelpful because the majority of infant reflux is non-acidic, many adverse effects have been associated with acid suppression and national recommendations emphasize limiting use of anti-reflux medicines in infants14, 37-43. In the present study, we found no benefit of acid suppression on subsequent visits, reinforcing that they should not be prescribed for suspected reflux in BRUE.
A final misconception about BRUE is that these patients have no recurrent symptoms. However, we found that patients continue to present with similar and/or ongoing symptoms with costs totaling up to $7000 per patient in the 6 months following BRUE. More than a quarter of patients were either readmitted or seen in the ED for similar symptoms during follow-up, contradicting the assumption that the initial BRUE hospitalization might serve a reassuring role. Others have suggested that BRUE patients are not at increased risk for increased mortality44. In our cohort, however, there were 2 deaths, representing 0.6% of the presenting cohort.
Almost one-third of the subjects in our study were premature and notably, these subjects were even more likely to have abnormal swallow study results at the time of the BRUE presentation or after, a longer length of stay, and increased risk of repeat hospitalization. Premature subjects were also more likely to be treated with acid suppression. The AAP guidelines do report higher rates of BRUE in premature infants that are born at less than 32 weeks’ gestational age and suggest that such patients fall into the higher risk BRUE category4. Our results support this additional consideration for premature infants presenting after BRUE and highlight that this group is at particular risk for oropharyngeal dysphagia and may benefit from early performance of VFSS.
Recognizing the high rate of re-presentation for persistent symptoms and recognizing the high costs associated with care of these patients, we feel that the current guideline would be strengthened by recommending objective assessment of swallowing and feeding interventions along with removal of GERD from consideration as an explanatory model for BRUE, changes which are low risk interventions with potential to reduce hospitalization and cost. Our recommended approach for these patients would include testing with VFSS and/or empiric thickening of feeds in collaboration with feeding experts while awaiting VFSS, particularly at centers where coordination of VFSS can be more of a challenge and might significantly delay discharge. We also recommend based on this study and others in the past that acid suppression should be avoided as its use does not improve outcomes14. Future studies will be needed to show that treatment of swallowing dysfunction improves outcomes and decreases the incidence of BRUE.
There are several potential limitations to the present study. It is possible that there were unmeasured differences in the patient populations in the years before and after AAP guideline publication. However, for both years we used ICD-10 codes and also reviewed charts to make sure all subjects fit the AAP definition. We found no differences in any characteristics to suggest the populations differed. Additionally, although Boston Children’s Hospital is a tertiary referral center and the possibility exists that our patients with BRUE may not represent the same population as those seen in other hospitals, more than 78% of patients came from the Boston metropolitan area, suggesting this population is not a referral population. It is also important to recognize that there might be a delay between the time of guideline publication and the widespread implementation of the guideline into practice. At our institution, the AAP guidelines were reviewed and a new clinical practice approach agreed upon in the spring of 2016 but it is possible that not all providers immediately incorporated the recommendations into their clinical approach to patients with BRUE. Lastly, it is possible that decisions about obtaining VFSS or other testing may have been affected by breastfeeding status or other unmeasured factors including family preferences; this must be assessed by a prospective multicenter study randomized trial with inclusion of all infants to reduce bias.
Acknowledgments
Supported by The Translational Research Program at Boston Children’s Hospital, NIH R01 DK097112-01, and NIH T32 DK007477-33. The authors declare no conflicts of interest.
List of Abbreviations
- AAP
American Academy of Pediatrics
- ALTE
Apparent life threatening event
- BRUE
Brief resolved unexplained event
- GERD
Gastroesophageal reflux disease
- VFSS
Videofluoroscopic swallow study
Footnotes
Portions of this study were presented in part at Digestive Diseases Week, June 2-5, 2018, Washington, DC; and NASPGHAN, October 24-27, 2018, Hollywood, Florida.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tieder JS, Altman RL, Bonkowsky JL, Brand DA, Claudius I, Cunningham DJ, et al. Management of apparent life-threatening events in infants: a systematic review. The Journal of pediatrics. 2013;163:94–9.e1-6. [DOI] [PubMed] [Google Scholar]
- [2].McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Archives of disease in childhood. 2004;89:1043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Piumelli R, Davanzo R, Nassi N, Salvatore S, Arzilli C, Peruzzi M, et al. Apparent Life-Threatening Events (ALTE): Italian guidelines. Italian journal of pediatrics. 2017;43:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tieder JS, Bonkowsky JL, Etzel RA, Franklin WH, Gremse DA, Herman B, et al. Brief Resolved Unexplained Events (Formerly Apparent Life-Threatening Events) and Evaluation of Lower-Risk Infants. Pediatrics. 2016;137. [DOI] [PubMed] [Google Scholar]
- [5].Arane K, Claudius I, Goldman RD. Brief resolved unexplained event: New diagnosis in infants. Canadian family physician Medecin de famille canadien. 2017;63:39–41. [PMC free article] [PubMed] [Google Scholar]
- [6].National Institutes of Health Consensus Development Conference on Infantile Apnea and Home Monitoring, Sept 29 to Oct 1, 1986. Pediatrics. 1987;79:292–9. [PubMed] [Google Scholar]
- [7].Jarasvaraparn C, Gallegos MBR, Mulekar MS, Bin W, Gremse DA, Crissinger KD. Short article: The endoscopic and histologic findings of infants who have experienced brief resolved unexplained events. European journal of gastroenterology & hepatology. 2018;30:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Duncan DR, Mitchell PD, Larson K, Rosen RL. Presenting Signs and Symptoms do not Predict Aspiration Risk in Children. The Journal of pediatrics. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weir KA, McMahon S, Taylor S, Chang AB. Oropharyngeal aspiration and silent aspiration in children. Chest. 2011;140:589–97. [DOI] [PubMed] [Google Scholar]
- [10].Duncan DR, Amirault J, Mitchell PD, Larson K, Rosen RL. Oropharyngeal Dysphagia Is Strongly Correlated With Apparent Life-Threatening Events. Journal of pediatric gastroenterology and nutrition. 2017;65:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hasenstab KA, Jadcherla SR. Respiratory events in infants presenting with apparent life threatening events: is there an explanation from esophageal motility? The Journal of pediatrics. 2014;165:250–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vaquero-Sosa E, Francisco-Gonzalez L, Bodas-Pinedo A, Urbasos-Garzon C, Ruizde-Leon-San-Juan A. Oropharyngeal dysphagia, an underestimated disorder in pediatrics. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. 2015;107:113–5. [PubMed] [Google Scholar]
- [13].Rubin BK. "The cruelest lies are often told in silence". Chest. 2011;140:567. [DOI] [PubMed] [Google Scholar]
- [14].Duncan DR, Mitchell PD, Larson K, McSweeney ME, Rosen RL. Association of proton pump inhibitors with hospitalization risk in children with oropharyngeal dysphagia. JAMA Otolaryngology–Head & Neck Surgery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). Journal of the American Medical Informatics Association : JAMIA. 2010;17:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gurberg J, Birnbaum R, Daniel SJ. Laryngeal penetration on videofluoroscopic swallowing study is associated with increased pneumonia in children. International journal of pediatric otorhinolaryngology. 2015;79:1827–30. [DOI] [PubMed] [Google Scholar]
- [17].Serel Arslan S, Demir N, Karaduman AA. Both pharyngeal and esophageal phases of swallowing are associated with recurrent pneumonia in pediatric patients. The clinical respiratory journal. 2016. [DOI] [PubMed] [Google Scholar]
- [18].Duncan DR, Larson K, Davidson K, May K, Rahbar R, Rosen RL. Feeding Interventions are Associated with Improved Outcomes in Children with Laryngeal Penetration. Journal of pediatric gastroenterology and nutrition. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arvedson JC, Lefton-Greif MA. Instrumental Assessment of Pediatric Dysphagia. Seminars in speech and language. 2017;38:135–46. [DOI] [PubMed] [Google Scholar]
- [20].Hiorns MP, Ryan MM. Current practice in paediatric videofluoroscopy. Pediatric radiology. 2006;36:911–9. [DOI] [PubMed] [Google Scholar]
- [21].Jadcherla SR, Stoner E, Gupta A, Bates DG, Fernandez S, Di Lorenzo C, et al. Evaluation and management of neonatal dysphagia: impact of pharyngoesophageal motility studies and multidisciplinary feeding strategy. Journal of pediatric gastroenterology and nutrition. 2009;48:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rangarathnam B, McCullough GH. Utility of a Clinical Swallowing Exam for Understanding Swallowing Physiology. Dysphagia. 2016;31:491–7. [DOI] [PubMed] [Google Scholar]
- [23].DeBoer EM, Kinder S, Duggar A, Prager JD, Soden J, Deterding RR, et al. Evaluating the yield of gastrointestinal testing in pediatric patients in aerodigestive clinic. Pediatric pulmonology. 2018. [DOI] [PubMed] [Google Scholar]
- [24].Greifer M, Santiago MT, Tsirilakis K, Cheng JC, Smith LP. Pediatric patients with chronic cough and recurrent croup: the case for a multidisciplinary approach. International journal of pediatric otorhinolaryngology. 2015;79:749–52. [DOI] [PubMed] [Google Scholar]
- [25].Boesch RP, Balakrishnan K, Acra S, Benscoter DT, Cofer SA, Collaco JM, et al. Structure and Functions of Pediatric Aerodigestive Programs: A Consensus Statement. Pediatrics. 2018. [DOI] [PubMed] [Google Scholar]
- [26].Boesch RP, Balakrishnan K, Grothe RM, Driscoll SW, Knoebel EE, Visscher SL, et al. Interdisciplinary aerodigestive care model improves risk, cost, and efficiency. International journal of pediatric otorhinolaryngology. 2018;113:119–23. [DOI] [PubMed] [Google Scholar]
- [27].Horton J, Atwood C, Gnagi S, Teufel R, Clemmens C. Temporal Trends of Pediatric Dysphagia in Hospitalized Patients. Dysphagia. 2018;33:655–61. [DOI] [PubMed] [Google Scholar]
- [28].Bhattacharyya N The prevalence of pediatric voice and swallowing problems in the United States. The Laryngoscope. 2015;125:746–50. [DOI] [PubMed] [Google Scholar]
- [29].Hersh C, Wentland C, Sally S, de Stadler M, Hardy S, Fracchia MS, et al. Radiation exposure from videofluoroscopic swallow studies in children with a type 1 laryngeal cleft and pharyngeal dysphagia: A retrospective review. International journal of pediatric otorhinolaryngology. 2016;89:92–6. [DOI] [PubMed] [Google Scholar]
- [30].McFarlin A What to Do when Babies Turn Blue: Beyond the Basic Brief Resolved Unexplained Event. Emergency medicine clinics of North America. 2018;36:335–47. [DOI] [PubMed] [Google Scholar]
- [31].Mittal MK, Donda K, Baren JM. Role of pneumography and esophageal pH monitoring in the evaluation of infants with apparent life-threatening event: a prospective observational study. Clin Pediatr (Phila). 2013;52:338–43. [DOI] [PubMed] [Google Scholar]
- [32].Smits MJ, van Wijk MP, Langendam MW, Benninga MA, Tabbers MM. Association between gastroesophageal reflux and pathologic apneas in infants: a systematic review. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:1527–38. [DOI] [PubMed] [Google Scholar]
- [33].Puntis JW, Booth IW. ALTE and gastro-oesophageal reflux. Archives of disease in childhood. 2005;90:653; author reply [PMC free article] [PubMed] [Google Scholar]
- [34].Zimbric G, Bonkowsky JL, Jackson WD, Maloney CG, Srivastava R. Adverse outcomes associated with gastroesophageal reflux disease are rare following an apparent life-threatening event. Journal of hospital medicine. 2012;7:476–81. [DOI] [PubMed] [Google Scholar]
- [35].Mousa H, Woodley FW, Metheney M, Hayes J. Testing the association between gastroesophageal reflux and apnea in infants. Journal of pediatric gastroenterology and nutrition. 2005;41:169–77. [DOI] [PubMed] [Google Scholar]
- [36].Scherer LD, Zikmund-Fisher BJ, Fagerlin A, Tarini BA. Influence of "GERD" label on parents" decision to medicate infants. Pediatrics. 2013;131:839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stark CM, Nylund CM. Side Effects and Complications of Proton Pump Inhibitors: A Pediatric Perspective. The Journal of pediatrics. 2016;168:16–22. [DOI] [PubMed] [Google Scholar]
- [38].Rosen R, Hu L, Amirault J, Khatwa U, Ward DV, Onderdonk A. 16S community profiling identifies proton pump inhibitor related differences in gastric, lung, and oropharyngeal microflora. The Journal of pediatrics. 2015;166:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Freedberg DE, Lamouse-Smith ES, Lightdale JR, Jin Z, Yang YX, Abrams JA. Use of Acid Suppression Medication is Associated With Risk for C. difficile Infection in Infants and Children: A Population-based Study. Clin Infect Dis. 2015;61:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brown KE, Knoderer CA, Nichols KR, Crumby AS. Acid-Suppressing Agents and Risk for Clostridium difficile Infection in Pediatric Patients. Clin Pediatr (Phila). 2015;54:1102–6. [DOI] [PubMed] [Google Scholar]
- [41].Rosen R, Amirault J, Liu H, Mitchell P, Hu L, Khatwa U, et al. Changes in gastric and lung microflora with Acid suppression: Acid suppression and bacterial growth. JAMA Pediatr. 2014;168:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of pediatric gastroenterology and nutrition. 2018;66:516–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duncan DR, Amirault J, Johnston N, Mitchell P, Larson K, Rosen RL. Gastroesophageal Reflux Burden, Even in Children That Aspirate, Does Not Increase Pediatric Hospitalization. Journal of pediatric gastroenterology and nutrition. 2016;63:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brand DA, Fazzari MJ. Risk of Death in Infants Who Have Experienced a Brief Resolved Unexplained Event: A Meta-Analysis. The Journal of pediatrics. 2018. [DOI] [PubMed] [Google Scholar]