Abstract
Objective
To establish the non-inferior efficacy of vonoprazan versus lansoprazole in the treatment of Asian patients with erosive oesophagitis (EO).
Design
In this phase III, double-blind, multicentre study, patients with endoscopically confirmed EO were randomised 1:1 to receive vonoprazan 20 mg or lansoprazole 30 mg, once daily for up to 8 weeks. The primary endpoint was EO healing rate at 8 weeks. The secondary endpoints were EO healing rates at 2 and 4 weeks. Safety endpoints included treatment-emergent adverse events (TEAEs).
Results
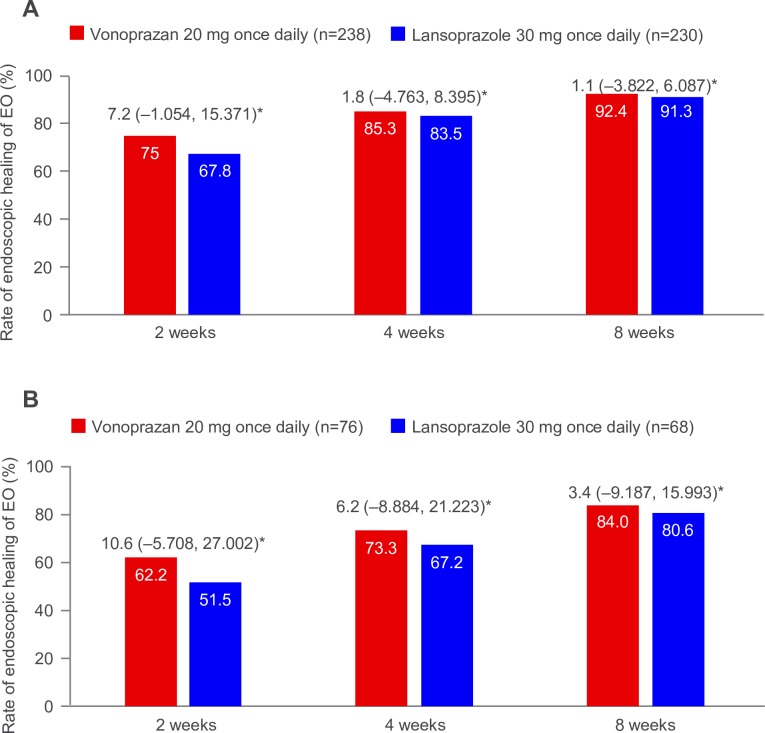
In the vonoprazan (n=238) and lansoprazole (n=230) arms, 8-week EO healing rates were 92.4% and 91.3%, respectively (difference 1.1% (95% CI –3.822% to 6.087%)). The respective 2-week EO healing rates were 75.0% and 67.8% (difference 7.2% (95% CI –1.054% to 15.371%)), and the respective 4-week EO healing rates were 85.3% and 83.5% (difference 1.8% (95% CI –4.763% to 8.395%)). In patients with baseline Los Angeles classification grade C/D, 2-week, 4-week and 8-week EO healing rates were higher with vonoprazan versus lansoprazole (2 weeks: 62.2% vs 51.5%, difference 10.6% (95% CI –5.708% to 27.002%); 4 weeks: 73.3% vs 67.2%, difference 6.2% (95% CI –8.884 to 21.223); and 8 weeks: 84.0% vs 80.6%, difference 3.4% (95% CI –9.187% to 15.993%)). Overall, EO healing rates appeared higher with vonoprazan versus lansoprazole. TEAE rates were 38.1% and 36.6% in the vonoprazan and lansoprazole group, respectively.
Conclusion
Our findings demonstrate the non-inferior efficacy of vonoprazan versus lansoprazole in terms of EO healing rate at 8 weeks in this population. Safety outcomes were similar in the two treatment arms.
Trial registration number
Keywords: erosive oesophagitis, proton pump inhibition, gastric acid, gastro-oesophageal reflux disease
Significance of this study.
What is already known on this subject?
Conventional proton pump inhibitors such as lansoprazole are used as a first-line therapy to treat acid-related diseases worldwide.
Vonoprazan is currently indicated for the treatment of gastric and duodenal ulcers, reflux oesophagitis, and Helicobacter pylori eradication, and for the prevention of low-dose aspirin-related or non-steroidal anti-inflammatory drug-related gastric and duodenal ulcer recurrence in Japan.
Previous studies have demonstrated the non-inferiority of vonoprazan to lansoprazole 30 mg in Japanese patients.
Although vonoprazan 20 mg is the approved dose in Japan, no study has examined the efficacy and safety of vonoprazan 20 mg versus lansoprazole 30 mg outside of Japan.
What are the new findings?
The aim of this study was to demonstrate the non-inferiority of vonoprazan 20 mg to lansoprazole 30 mg in Asian patients with erosive oesophagitis (EO), predominantly in mainland China, and from Malaysia, South Korea and Taiwan.
Vonoprazan 20 mg was shown to be effective and non-inferior to lansoprazole 30 mg in terms of endoscopic EO healing rate at 8 weeks in the population studied.
EO healing rates at 2 and 4 weeks were slightly higher with vonoprazan 20 mg versus lansoprazole 30 mg treatment.
The safety profile of vonoprazan 20 mg was similar to that of once-daily lansoprazole 30 mg in the populations studied.
Significance of this study.
How might it impact on clinical practice in the foreseeable future?
Vonoprazan provides a consistent, rapid-onset and durable acid suppression.
Vonoprazan is a meaningful and effective treatment option for EO in Chinese patients.
Similar efficacy trends were observed in Korean and Malaysian patients.
Introduction
GORD is characterised by symptoms such as heart burn and acid regurgitation resulting from the reflux of gastric contents into the oesophagus.1 A recent systematic review and meta-analysis of approximately 100 studies examining gastro-oesophageal reflux symptoms reported a pooled prevalence of 13.3% worldwide, 17.1% in Europe, 15.4% in North America, 10.0% in Asia and 2.5% in China.2 Patients with GORD are categorised as having non-erosive reflux disease or erosive oesophagitis (EO), which are the two main phenotypes.3 Using endoscopic examination, EO is graded by the severity of the mucosal injury, also referred to as mucosal breaks according to the Los Angeles (LA) classification system, which ranges from grade A (mild) to grade D (severe).4
The main aim of EO treatment is to relieve symptoms, heal and maintain remission of EO, prevent complications, and improve health-related quality of life (HRQoL).5 Proton pump inhibitors (PPIs) are considered the standard treatment for EO and have an established efficacy and safety profile.3 6–10 The high potency of PPIs results from their ability to inhibit the gastric enzyme hydrogen potassium adenosine triphosphatase (H+, K+ ATPase), which is responsible for gastric acid secretion by the parietal cells in the gastric mucosa.3 However, many early-generation PPIs have a slow, cumulative onset of action whereby several doses may be required to achieve maximum acid suppression and symptom relief.11
Vonoprazan (TAK-438) belongs to a class of acid-inhibitory agents called potassium-competitive acid blockers, which, unlike PPIs such as lansoprazole, reversibly inhibit H+, K+ ATPase independently of acid pH.12–15 Of note, vonoprazan is stable in the presence of acid,16 is water-soluble and does not require a specific pharmacological preparation such as an enteric coating, unlike PPIs,17 which suggests that vonoprazan may have less variation in time to onset of action than PPIs. Furthermore, in contrast to PPIs that take 3–5 days to produce their maximum acid-inhibitory effects,18 19 vonoprazan demonstrates maximum acid-inhibitory effects from the first day of administration.20 Vonoprazan was approved and marketed in Japan in February 2015 for the treatment of acid-related GI disorders, including EO, gastric ulcer, duodenal ulcer, peptic ulcer, reflux oesophagitis and Helicobacter pylori eradication. Recent phase II and III clinical trials in Japan have demonstrated that vonoprazan 20 mg and 40 mg is effective, well-tolerated and non-inferior to lansoprazole 30 mg with respect to EO healing at 8 weeks.5 21 In a long-term maintenance study over 52 weeks, EO recurred in fewer than 10% of patients treated with once-daily vonoprazan 10 mg or 20 mg dose.5
Vonoprazan 20 mg is the approved dose in Japan for the treatment of EO.22 However, the non-inferior efficacy and safety of vonoprazan 20 mg to lansoprazole 30 mg with respect to healing EO has not been demonstrated in Asian patients. This phase III study was conducted to meet the regulatory registration requirements in mainland China, South Korea and Taiwan. The primary objective of the study was to demonstrate the non-inferior efficacy of vonoprazan 20 mg to lansoprazole 30 mg at 8 weeks in Asian patients with EO. The secondary objectives were to demonstrate the non-inferior efficacy of vonoprazan 20 mg to lansoprazole 30 mg at 2 and 4 weeks, and to compare the safety of treatment with vonoprazan versus lansoprazole in this patient population.
Methods
Study design
This was a randomised, double-blind, double-dummy, parallel-group, multicentre study conducted in 56 sites across Asia. Patient baseline EO symptoms were recorded during an initial observation phase of 3–7 days. An interactive web response system (IWRS) program was used to manage inventory, assist the site in dispensing the investigational drug to the patients, record accountability, and support the return to sponsor or designee of investigational drugs after study completion. Eligible patients were stratified according to the LA classification grade A/B or C/D and randomised 1:1 via the IWRS to receive oral vonoprazan 20 mg once daily or oral lansoprazole 30 mg once daily for up to 8 weeks. Patients self-administered the study medications each day after breakfast, except on day 1 when they were administered at the study site before the patient’s visit was concluded. Patients in the vonoprazan group received a 20 mg active vonoprazan tablet or a placebo capsule for 30 mg lansoprazole, while patients in the lansoprazole group received a 30 mg active lansoprazole capsule or a placebo tablet for 20 mg vonoprazan. Patients with healed EO at either 2, 4 or 8 weeks after the start of the study were considered completed cases and were invited to enrol in the longer term maintenance study (TAK-438_305, NCT02388737), which was ongoing at the time of writing. A total of six visits were scheduled during the study: at the start of the observation phase (visit 1), at the start of the treatment phase after randomisation (visit 2), and then after 2 (visit 3), 4 (visit 4), 6 (visit 5; liver function testing only) and 8 (visit 6) weeks of treatment.
The study was conducted between March 2015 and July 2017 and is registered at ClinicalTrials.gov.
Participants
Eligible patients had endoscopically confirmed EO (LA classification grades A–D) within 14 days of randomisation (treated as outpatients or temporarily admitted) and aged ≥18 years. Key exclusion criteria were prior exposure to vonoprazan or lansoprazole within 84 days of the observation phase, or exposure to vonoprazan at any time in a previous clinical trial or as a therapeutic agent; hypersensitivity or allergy to vonoprazan, its excipients or to PPIs; significant history of central nervous system, cardiovascular, pulmonary, hepatic, renal, metabolic, GI, urological, endocrine or haematological disease; presence of comorbidities or medical or surgical history that could affect the oesophagus; acute upper GI bleeding or gastric or duodenal ulcer within 30 days of the observation phase; history of or treatment for malignancy within 5 years of visit 1; and creatinine >2 mg/dL (>177 μmol/L), or alanine aminotransferase (ALT), aspartate aminotransferase (AST) or total bilirubin greater than the upper limit of normal at the start of the observation phase.
Study endpoints and assessments
The primary efficacy endpoint was EO healing rate at 8 weeks. The secondary efficacy endpoints were EO healing rates at 2 and 4 weeks of treatment. Endoscopy was performed at the start of the screening period and at weeks 2, 4 and 8 (or on early termination) under fasted conditions and classified in terms of LA classification grades (A–D or no mucosal breaks (grade O)). EO healing was defined as ‘no mucosal breaks’. During the 8-week treatment period, healed EO was confirmed from any endoscopy test performed at visit 3, 4 or 6. During the 2-week treatment period, healed EO was confirmed at visit 3 and during the 4-week treatment period at visit 3 or 4.
Additional efficacy endpoints included the subjective symptoms of EO as recorded in patient diaries (eg, heart burn, gastric acid regurgitation), HRQoL over 8 weeks and the percentage of days without rescue medication during treatment. Safety endpoints included adverse events (AEs), laboratory test values, ECG, vital signs, serum gastrin values and pepsinogen I/II values. AEs and treatment-emergent adverse events (TEAEs) were coded using the Medical Dictionary for Regulatory Activities V.18.0.
Statistical analyses
Assuming an 8-week healing rate of 94.7%21 23 in both treatment arms and a 20% dropout rate, it was estimated that 160 patients per group would provide 90% power to establish non-inferiority with a –10% margin using a two-sided 95% CI. A sample size of 240 subjects per group was planned to provide more subjects with healed EO for the subsequent maintenance study (TAK-438_305) and to provide adequate subjects to meet the regulatory requirements in each of the countries in which the study was being conducted. The proportion of patients with LA classification grade C/D was planned to be ≥30%.
Primary and secondary efficacy endpoints were analysed using the full analysis set (FAS), defined as randomised patients who received at least one dose of the study drug and had at least one postbaseline endoscopy. Safety was analysed using the safety analysis set (SAS), defined as patients who received at least one dose of the study drug. No statistical tests or inferential statistics were generated for the safety data. For the primary (EO healing rate at 8 weeks) and secondary (EO healing rates at 2 and 4 weeks) efficacy endpoints, two-sided 95% CIs were calculated for the difference between the vonoprazan and lansoprazole treatment arms. Non-inferiority was claimed if the lower bound of the CIs was ≥–10%. If non-inferiority was demonstrated for the primary efficacy endpoint, the secondary efficacy endpoint of EO healing rate at week 2 was tested for superiority between the vonoprazan and lansoprazole treatment arms. The Cochran-Mantel-Haenszel test was used to adjust for covariates in the FAS with baseline LA classification as a stratification factor. The primary and secondary efficacy endpoints were evaluated in a prespecified subgroup analysis in patients with LA classification grade A/B or C/D.
Results
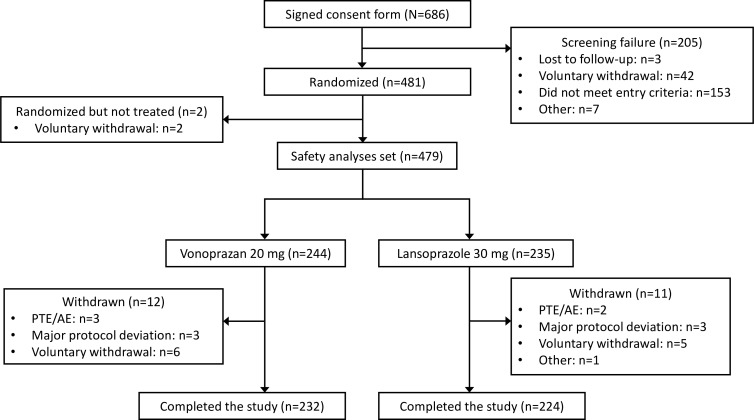
Patients were enrolled from March 2015 to June 2017 across Asia (mainland China, 27 sites; South Korea, 12; Taiwan, 11; Malaysia, 6). A total of 686 patients were screened and 481 patients (70.1%) were randomised. Figure 1 illustrates patient disposition. The proportion of randomised patients completing treatment was similar in both treatment arms (95.1% (232/244) in the vonoprazan group and 94.5% (224/237) in the lansoprazole group). ‘Completers’ included patients with healed EO at 2, 4 or 8 weeks, or those without healing who completed 8 weeks of treatment. Demographics and baseline characteristics of the randomised patients are described in table 1. The majority of patients were enrolled in mainland China (n=276, 57.4%). Approximately 30% of patients had LA classification grade C/D in each arm.
Figure 1.
Patient disposition. AE, adverse event; PTE, pretreatment event.
Table 1.
Patient demographics and baseline characteristics (randomised set)
| Vonoprazan | Lansoprazole | Total | |
| (n=244) | (n=237) | (n=481) | |
| Country/area, n (%) | |||
| Mainland China | 143 (58.6) | 133 (56.1) | 276 (57.4) |
| South Korea | 52 (21.3) | 55 (23.2) | 107 (22.2) |
| Taiwan | 28 (11.5) | 25 (10.5) | 53 (11.0) |
| Malaysia | 21 (8.6) | 24 (10.1) | 45 (9.4) |
| Age*, years, mean (SD) | 54.1 (13.16) | 53.8 (12.53) | 53.9 (12.84) |
| Male, n (%) | 176 (72.1) | 179 (75.5) | 355 (73.8) |
| Height, cm, mean (SD) | 166.1 (8.24) | 166.3 (8.80) | 166.2 (8.52) |
| Weight, kg, mean (SD) | 68.48 (12.311) | 70.26 (12.133) | 69.35 (12.243) |
| BMI, kg/m2, mean (SD) | 24.70 (3.389) | 25.31 (3.430) | 25.00 (3.419) |
| Smoking status, n (%) | |||
| Never smoked | 157 (64.3) | 137 (57.8) | 294 (61.1) |
| Current smoker | 48 (19.7) | 64 (27.0) | 112 (23.3) |
| Ex-smoker | 39 (16.0) | 36 (15.2) | 75 (15.6) |
| Consumption of alcohol, n (%) | |||
| Every day | 13 (5.3) | 12 (5.1) | 25 (5.2) |
| Two days a week | 32 (13.1) | 40 (16.9) | 72 (15.0) |
| Two days a month | 57 (23.4) | 48 (20.3) | 105 (21.8) |
| Never | 142 (58.2) | 137 (57.8) | 279 (58.0) |
| Consumption of caffeine†, n (%) | |||
| Yes | 58 (23.8) | 52 (21.9) | 110 (22.9) |
| No | 185 (75.8) | 185 (78.1) | 370 (76.9) |
| LA classification, n (%) | |||
| Grade A | 76 (31.1) | 83 (35.0) | 159 (33.1) |
| Grade B | 92 (37.7) | 84 (35.4) | 176 (36.6) |
| Grade C | 58 (23.8) | 58 (24.5) | 116 (24.1) |
| Grade D | 18 (7.4) | 10 (4.2) | 28 (5.8) |
*At the date of informed consent.
†One patient with unknown status in the vonoprazan group.
BMI, body mass index; LA, Los Angeles.
The FAS comprised 238 patients in the vonoprazan group and 230 patients in the lansoprazole group. A total of 13 patients (6 in the vonoprazan group and 7 in the lansoprazole group) did not have postbaseline endoscopy and were excluded from the FAS. With the exception of two patients in the lansoprazole group who withdrew prior to study drug administration, the remaining 11 patients were included in the SAS. The SAS (n=479) comprised 244 patients in the vonoprazan and 235 patients in the lansoprazole group. The majority of patients in both treatment arms fully complied (≥90%) with the study medication; the mean treatment compliance was 93.30% (SD: 6.315) and similar between treatment arms.
Efficacy
Non-inferiority (determined based on the lower CI being ≥–10%) of vonoprazan compared with lansoprazole in terms of EO healing rate at 8 weeks was demonstrated and thus the primary efficacy endpoint was met (figure 2A). The 8-week healing rate was 92.4% (n=220) with vonoprazan and 91.3% (n=210) with lansoprazole (difference: 1.1% (95% CI –3.822% to 6.087%)). The 2-week healing rate was 75.0% (n=177) in the vonoprazan group and 67.8% (n=154) in the lansoprazole group (difference: 7.2% (95% CI –1.054% to 15.371%)) (figure 2A). The healing rate at 4 weeks was 85.3% (n=203) in the vonoprazan group and 83.5% (n=192) in the lansoprazole group (difference: 1.8% (95% CI –4.763% to 8.395%)) (figure 2A). Treatment differences with respect to endoscopic healing of EO at 2 and 4 weeks were not statistically significant. The superiority of vonoprazan 20 mg treatment to lansoprazole 30 mg treatment was not demonstrated at 2 weeks.
Figure 2.
Rate of endoscopic healing of EO during the 2-week, 4-week and 8-week treatment period for the full analysis set (A) and patients with LA classification grade C/D (B). *Treatment difference between arms (95% CI). EO, erosive oesophagitis; LA, Los Angeles.
In patients with baseline LA classification grade C/D, 2-week, 4-week and 8-week EO healing rates were numerically higher with vonoprazan versus lansoprazole: 62.2% vs 51.5% (difference: 10.6% (95% CI –5.708% to 27.002%)); 73.3% vs 67.2% (difference: 6.2% (95% CI –8.884% to 21.223%)); and 84.0% vs 80.6% (difference: 3.4% (95% CI –9.187% to 15.993%)), respectively (figure 2B). In patients with baseline LA classification grade A/B, 2-week, 4-week and 8-week EO healing rates with vonoprazan versus lansoprazole were 80.9% vs 74.5% (difference: 6.3% (95% CI –2.724% to 15.384%)); 90.8% vs 90.2% (difference: 0.6% (95% CI –5.755% to 6.982%)); and 96.3% vs 95.7% (difference: 0.6% (95% CI –3.634% to 4.861%)), respectively.
For the additional efficacy endpoints (subjective symptoms, HRQoL and days without rescue medication), there were no statistically significant differences between the treatment arms. The proportion of patients without heart burn at week 8 was numerically higher in the vonoprazan group at 70.5% compared with 66.0% in the lansoprazole group, and a similar trend was observed for the Chinese subgroup at 77.8% vs 69.2% for vonoprazan versus lansoprazole treatment.
Safety
Approximately 50% of patients received vonoprazan or lansoprazole treatment for up to 2 weeks, and approximately 21% of patients received treatment for longer than 4 weeks. The incidence of TEAEs was similar between the two arms: 38.1% (93/244) in the vonoprazan group and 36.6% (86/235) in the lansoprazole group, of whom 31.1% and 30.2% reported only mild TEAEs, 5.7% and 5.5% reported a TEAE of moderate severity, and 1.2% and 0.9% had severe TEAE, respectively (table 2). Of these, the majority of patients reported TEAEs that were not related to vonoprazan (61.3%) or lansoprazole (68.6%) treatment. Serious adverse events (SAEs) were experienced by three patients in each group and were not related to the study drugs.
Table 2.
Overview of TEAEs and SAEs (safety analysis set)
| Vonoprazan 20 mg (n=244) | Lansoprazole 30 mg (n=235) | |||
| Events | n (%) | Events | n (%) | |
| TEAEs | 162 | 93 (38.1) | 162 | 86 (36.6) |
| Related* | 70 | 36 (14.8) | 49 | 27 (11.5) |
| Not related | 92 | 57 (23.4) | 113 | 59 (25.1) |
| Mild | 144 | 76 (31.1) | 140 | 71 (30.2) |
| Moderate | 15 | 14 (5.7) | 17 | 13 (5.5) |
| Severe | 3 | 3 (1.2) | 5 | 2 (0.9) |
| Leading to discontinuation | 6 | 5 (2.0) | 4 | 4 (1.7) |
| Related* | 3 | 3 (1.2) | 2 | 2 (0.9) |
| Not related | 3 | 2 (0.8) | 2 | 2 (0.9) |
| Liver function abnormalities | 0 | 0 | 2 | 2 (0.9) |
| SAEs | 3 | 3 (1.2) | 3 | 3 (1.3) |
| Related | 0 | 0 | 0 | 0 |
| Not related | 3 | 3 (1.2) | 3 | 3 (1.3) |
| Leading to discontinuation | 2 | 2 (0.8) | 1 | 1 (0.4) |
| Significant TEAEs† | 29 | 24 (9.8) | 47 | 34 (14.5) |
| Deaths | 0 | 0 | 0 | 0 |
*An adverse event that followed a reasonable temporal sequence from administration of study drug (including the course after withdrawal of the drug) or for which possible involvement of the drug could be argued, although factors other than the drug, such as underlying diseases, complications, concomitant drugs and concurrent treatments, may also have been responsible.
†Any TEAE (excluding serious TEAEs) that led to an intervention, including withdrawal of treatment, dose increase, dose reduction or additional concomitant therapy.
SAEs, serious adverse events; TEAEs, treatment-emergent adverse events.
Four drug-related severe TEAEs were reported by one patient in the lansoprazole group, namely increased ALT, increased AST, increased blood alkaline phosphatase and increased gamma-glutamyl transferase; another patient in the same group also experienced arrhythmia. There were no drug-related severe TEAEs reported in the vonoprazan group. GI disorders (diarrhoea, abdominal distention and so on) were the most frequently reported system organ class, with a similar incidence in the vonoprazan (18.4%, 45/244) and lansoprazole (19.1%, 45/235) group (table 3). The most frequently reported TEAE occurred in the investigations system organ class (preferred term, blood gastrin increased), which was reported with a higher incidence in the vonoprazan group (5.3%, 13/244) compared with the lansoprazole group (1.7%, 4/235), all of which, with the exception of one patient in the vonoprazan group, were considered to be drug-related. The increase in blood gastrin level in the vonoprazan group was evident from week 2 but did not increase further over time (to week 8).
Table 3.
TEAEs occurring in ≥2% of patients in either treatment group (safety analysis set)
| Preferred term | Patients*, n (%) | |
| Vonoprazan (n=244) | Lansoprazole (n=235) | |
| Patients with any TEAE | 93 (38.1) | 86 (36.6) |
| GI disorders | 45 (18.4) | 45 (19.1) |
| Diarrhoea | 7 (2.9) | 9 (3.8) |
| Abdominal distension | 5 (2.0) | 6 (2.6) |
| Investigations | 26 (10.7) | 21 (8.9) |
| Blood gastrin increased | 13 (5.3) | 4 (1.7) |
| Enzyme level increased† | 9 (3.7) | 2 (0.9) |
| Pepsinogen I increased | 9 (3.7) | 1 (0.4) |
| Alanine aminotransferase increased | 4 (1.6) | 5 (2.1) |
| Nervous system disorders | 8 (3.3) | 10 (4.3) |
| Headache | 2 (0.8) | 8 (3.4) |
*A patient was counted once, even if the patient reported the same event more than once. Adverse events were coded using MedDRA V.18.0.
†Increased enzyme levels were associated with 10 events in 9 patients in the vonoprazan treatment group: raised serum pepsinogen 2, increased pepsinogen II (×5), increased pepsinogen I/II (×2) and high pepsinogen II (×2); and 2 events in 2 patients in the lansoprazole treatment group: pepsinogen I/II increase and high pepsinogen.
MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse events.
A total of nine patients experienced 10 TEAEs that led to study drug discontinuation: five patients (2.0%) in the vonoprazan group reported diarrhoea (drug-related), bile duct stone (SAE), increased ALT and increased AST (drug-related; both experienced by one patient), cerebral infarction (SAE), and headache (drug-related); four patients (1.7%) in the lansoprazole group reported arrhythmia, gastric dilatation (drug-related), gastroenteritis (SAE) and abnormal liver function test (drug-related). In addition to those resulting in discontinuation, additional SAEs included colon adenoma (experienced by one patient in the vonoprazan group), and glaucoma and large intestine polyp (one patient each in the lansoprazole group). Generally, there were no clinically relevant changes in laboratory, ECG and vital signs, and there were no on-study deaths.
Discussion
This is the first study to demonstrate the non-inferior efficacy and safety of once-daily vonoprazan 20 mg to once-daily lansoprazole 30 mg with respect to healing EO at 8 weeks in Asian patients, predominantly from mainland China, and from Malaysia, South Korea and Taiwan (92.4% vs 91.3%, respectively; difference: 1.1% (95% CI –3.822% to 6.087%)). These findings are consistent with the results reported in a previous phase III study which demonstrated the non-inferiority of vonoprazan 20 mg to lansoprazole 30 mg with respect to healing EO at 8 weeks (99% vs 95.5%; difference: 3.5% (95% CI 0.362% to 6.732%)).5 Of note, there were differences in the 8-week EO healing rate reported by Ashida et al and the present study, which could be attributed to treatment compliance.5 Ashida et al 5 reported a 98% treatment compliance rate, whereas in the present study 93% of Asian patients were treatment-compliant, which could have resulted in the lower EO healing rate reported in our findings. Although our findings differ from the aforementioned pivotal study in Japan, the healing rate with lansoprazole reported in this study was similar to those reported in China.24
There was a rapid response to treatment with both the overall population and those with LA classification grade C/D demonstrating a healing rate of >60% at 2 weeks with vonoprazan treatment. In the overall population, the EO healing rate at 2 weeks was numerically higher with vonoprazan than that observed with lansoprazole (75.0% vs 67.8%), which may be indicative of a more potent and faster clinical effect with vonoprazan than with lansoprazole treatment resulting from the rapid and strong suppression of gastric acid secretion.25 The results of a previous phase III study conducted in Japan5 reported higher healing rates with vonoprazan 20 mg and lansoprazole 30 mg treatment (90.7% vs 81.9%; difference: 8.8% (95% CI 2.105% to 15.448%)) at 2 weeks. As previously mentioned, the differences observed could be attributed to the differences in treatment compliance and the overall low EO healing rates in patients treated with PPIs among Asians, excluding Japanese patients.24
The prevalence of GORD has increased in some Asian countries over the past few decades,26 27 largely owing to the ageing population and Westernisation. Despite the presence of a wide choice of therapeutic modalities and improvements in the therapeutic management of GORD, there remain several areas of unmet need, including but not limited to the treatment of advanced grades of EO, maintenance treatment of EO and refractory GORD.28 At present, PPIs are considered the gold standard for the treatment of GORD; however, healing rates in patients with advanced grades of EO have been limited.29 In the present study, subgroup analysis in patients with baseline LA classification grade C/D showed persistently higher EO healing rates at 2, 4 and 8 weeks with vonoprazan, compared with lansoprazole treatment.
It has been predicted that a 100% healing rate in EO can be achieved after 4 weeks with a pH >4 holding time ratio (HTR) of ≥90%, and after 8 weeks with a pH >4 HTR of ≥75%.30 Results from a published study have reported that the HTR with vonoprazan 20 mg was more than 83% at gastric pH ≥4 on day 7,31 suggesting that EO could be successfully treated especially in patients with severe EO (LA classification grades C/D) as it effectively controls both daytime and night-time acid secretion.32 In another study, EO healing rates in patients with LA classification grade C/D were 96% vs 82.6% at week 2, 100% vs 87% at week 4, and 100% vs 93.5% at week 8 with vonoprazan 20 mg versus lansoprazole 30 mg treatment, respectively.21 Furthermore, the results of a third study also demonstrated higher EO healing rates with vonoprazan treatment in patients with baseline LA classification grade C/D compared with lansoprazole after 8 weeks (98.7% vs 87.5%, respectively).33
Patients self-administered study medications, including lansoprazole, each day after breakfast as the prescribing information for lansoprazole in China34 and Japan35 did not specify dose timing with respect to mealtimes. Of note, published phase I studies suggested that the gastric inhibitory effect of lansoprazole following 7 days of daily dosing was unaffected by food intake.36 37 There is lack of evidence to suggest differences in efficacy related to the timing of lansoprazole dosing between Asian and non-Asian patients.
From this study, 312 patients with endoscopically confirmed healed EO at 2, 4 or 8 weeks were enrolled in the randomised, multicentre, 24-week maintenance study (TAK-438_305) designed to evaluate the recurrence of EO after 12 and 24 weeks of treatment with vonoprazan 10 mg and 20 mg compared with lansoprazole 15 mg (NCT02388737; at the time of writing, the study is ongoing).
Vonoprazan and lansoprazole were similarly well tolerated in the populations studied. The incidences of TEAEs were similar between the treatment arms. Drug-related TEAEs occurred slightly more frequently with vonoprazan (36/244, 14.8%) than with lansoprazole (27/235, 11.5%) treatment. Of note, there were no new safety signals and no deaths were reported during the study.
The sensitivity of vonoprazan in ethnic variations had been examined by looking at effects on pharmacokinetics linearity, metabolism by hepatic cytochrome (CY)P450 enzymes, therapeutic index and drug accumulation with repetitive dosing. Although vonoprazan exhibits non-linear pharmacokinetic characteristics, the dose-normalised exposure (maximum concentration and area under the concentration-time curve) for Chinese and Japanese subjects is similar, indicating that the non-linearity does not contribute to potential differences in exposure. CYP2C19 phenotypes do not affect vonoprazan pharmacokinetic parameters,25 38 39 indicating that ethnic differences in phenotypical distribution of CYP2C19 are not expected to play a major role in contributing to metabolic differences among Chinese, Japanese and other ethnic populations. There is evidence that vonoprazan exhibits a wide therapeutic index; it has been demonstrated that a single dose of vonoprazan 120 mg was well tolerated.40 There is also evidence that multiple doses of 40 mg per day were well tolerated.41 Interaction of vonoprazan and clarithromycin, an inhibitor of CYP3A4, resulted in an almost twofold increase in oral bioavailability, but this was not associated with a significant alteration in efficacy and/or safety42 as plasma concentrations remained within the therapeutic window. In addition, given that the dosing interval of vonoprazan is 24 hours and that the accumulation factor is two when the dosing interval is equal to the half-life, which is 9.4 hours, vonoprazan accumulation under CYP3A4 inhibition will be considerably lower than twofold. Taken together, this evidence suggests that vonoprazan may be insensitive to differences in ethnicity.
One limitation of this study was that CYP2C19 genotyping was not evaluated in Asian patients and thus we were unable to examine the efficacy of vonoprazan in patients with the extensive metaboliser phenotype. However, given the similarity of EO healing rates between the present study and the previously published pivotal study conducted in Japanese patients, the proportion of patients receiving vonoprazan with healed EO in the present study is expected to be higher in patients identified as CYP2C19 extensive metabolisers compared with patients receiving lansoprazole. Future vonoprazan studies investigating EO should also include night-time symptoms, such as heart burn and night-time awakening.
Conclusion
In conclusion, these results demonstrated that vonoprazan 20 mg once daily was non-inferior to lansoprazole 30 mg once daily for healing EO at 8 weeks and was well tolerated, thus indicating that vonoprazan is effective for the treatment of EO in Chinese patients. Similar efficacy trends were observed in Korean and Malaysian patients.
Acknowledgments
The authors would like to thank the patients who have participated in this trial and their families. The authors would like to thank all patients and their families, all investigators, and the Takeda and IQVIA (CRO) 303 study team for their valuable involvement in this study. Writing assistance from Sabah Farooq of FireKite, an Ashfield company, part of UDG Healthcare, was used during the development of this manuscript, which was funded by Takeda Pharmaceutical Company, in compliance with Good Publication Practice 3 ethical guidelines (Battisti et al, Ann Intern Med 2015;163:461–4).
Footnotes
Contributors: YX contributed to study design, acquisition and interpretation of data, and critically reviewed and edited the manuscript. SZ contributed to the enrolment of patients, and critically reviewed and edited the manuscript. ND contributed to acquisition of data, revision of the manuscript and final approval of the manuscript. GF contributed to the enrolment of patients and reviewed the manuscript. K-LG contributed to the enrolment of patients, acquisition and analysis of data, and revision of the manuscript. HJC contributed to the acquisition and analysis of data and drafting the final manuscript. B-SS contributed to the enrolment of patients in Taiwan, data analysis and revision of the manuscript. CFC contributed to study design, acquisition and interpretation of the data, and critically reviewed and edited the manuscript. NF contributed to data analysis and interpretation, and critically reviewed and edited the manuscript. WZ contributed to study design, and critically reviewed and edited the manuscript. MC contributed to study design, acquisition and analysis of data, and critically reviewed and edited the manuscript. All authors read and approved the final manuscript, and all authors agree to be accountable for all aspects of the work, which include ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This study was funded by Takeda Pharmaceutical Company. The sponsor was involved in study design, as well as data collection, analysis and interpretation of the data, as well as reviewing the paper and providing funding support for medical writing assistance. The final decision to submit the paper, however, lay with the authors.
Competing interests: MC received speaker honorarium from Xian Janssen, AstraZeneca China, Ipsen Tianjin, Takeda China and CMS China. CFC is an employee of Takeda Development Center Asia, and stock shareholder in Air Liquide and Abbott Laboratories. NF is an employee of Takeda Pharmaceutical Company, and WZ is a former employee of Takeda Pharmaceutical Company. K-L G received fees for participating in an advisory committee or review panel and speaking and chairing for Takeda Pharmaceutical Company. All other authors declare no competing interests.
Patient consent for publication: Obtained.
Ethics approval: The study protocol, informed consent form and other regulation-specified documents were reviewed and approved by the Independent Ethics Committees at Nakakinen Clinic. The study was conducted in accordance with the principles of the World Medical Association Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, the International Conference for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, ethical guideline for clinical research, institutional review board regulations, and all applicable local regulations at each participating centre (mainland China, Korea, Taiwan and Malaysia). Written informed consent was obtained from patients before study commencement.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Takeda makes patient-level, de-identified data sets and associated documents available after applicable marketing approvals and commercial availability have been received, an opportunity for the primary publication of the research has been allowed, and other criteria have been met as set forth in Takeda’s Data Sharing Policy (see https://www.takedaclinicaltrials.com/ for details). To obtain access, researchers must submit a legitimate academic research proposal for adjudication by an independent review panel, who will review the scientific merit of the research and the requestor’s qualifications and conflict of interest that can result in potential bias. Once approved, qualified researchers who sign a data sharing agreement are provided access to these data in a secure research environment.
References
- 1. Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20. quiz 1943 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 2. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018;67:430–40. 10.1136/gutjnl-2016-313589 [DOI] [PubMed] [Google Scholar]
- 3. Maradey-Romero C, Fass R. New and future drug development for gastroesophageal reflux disease. J Neurogastroenterol Motil 2014;20:6–16. 10.5056/jnm.2014.20.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–80. 10.1136/gut.45.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 2016;43:240–51. 10.1111/apt.13461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freedberg DE, Kim LS, Yang Y-X. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American gastroenterological association. Gastroenterology 2017;152:706–15. 10.1053/j.gastro.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 7. Fuchs KH, Babic B, Breithaupt W, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc 2014;28:1753–73. 10.1007/s00464-014-3431-z [DOI] [PubMed] [Google Scholar]
- 8. Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-Based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol 2016;51:751–67. 10.1007/s00535-016-1227-8 [DOI] [PubMed] [Google Scholar]
- 9. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28. quiz 29 10.1038/ajg.2012.444 [DOI] [PubMed] [Google Scholar]
- 10. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver 2017;11:27–37. 10.5009/gnl15502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tytgat GN. Shortcomings of the first-generation proton pump inhibitors. Eur J Gastroenterol Hepatol 2001;13 Suppl 1:S29–33. [PubMed] [Google Scholar]
- 12. Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther 2010;335:231–8. 10.1124/jpet.110.170274 [DOI] [PubMed] [Google Scholar]
- 13. Hori Y, Matsukawa J, Takeuchi T, et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 2011;337:797–804. 10.1124/jpet.111.179556 [DOI] [PubMed] [Google Scholar]
- 14. Matsukawa J, Hori Y, Nishida H, et al. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011;81:1145–51. 10.1016/j.bcp.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 15. Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H, K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther 2011;339:412–20. 10.1124/jpet.111.185314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Otake K, Sakurai Y, Nishida H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther 2016;33:1140–57. 10.1007/s12325-016-0345-2 [DOI] [PubMed] [Google Scholar]
- 17. Inatomi N, Matsukawa J, Sakurai Y, et al. Potassium-Competitive acid blockers: advanced therapeutic option for acid-related diseases. Pharmacol Ther 2016;168:12–22. 10.1016/j.pharmthera.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 18. Piche T, Galmiche JP. Pharmacological targets in gastro-oesophageal reflux disease. Basic Clin Pharmacol Toxicol 2005;97:333–41. 10.1111/j.1742-7843.2005.pto_273.x [DOI] [PubMed] [Google Scholar]
- 19. Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther 2006;23 Suppl 2:2–8. 10.1111/j.1365-2036.2006.02943.x [DOI] [PubMed] [Google Scholar]
- 20. Andersson K, Carlsson E. Potassium-Competitive acid blockade: a new therapeutic strategy in acid-related diseases. Pharmacol Ther 2005;108:294–307. 10.1016/j.pharmthera.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 21. Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 2015;42:685–95. 10.1111/apt.13331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeda Pharmaceutical Company Ltd Takecab® (Vonoprazan fumarate), package insert [Japanese] 2018.
- 23. Takeda Pharmaceutical Company Ltd Investigation of the efficacy and safety of TAK-390MR for erosive esophagitis. public disclosure synopsis 2010.
- 24. Zheng R-N. Comparative study of omeprazole, lansoprazole, pantoprazole and esomeprazole for symptom relief in patients with reflux esophagitis. World J Gastroenterol 2009;15:990–5. 10.3748/wjg.15.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 2015;6:e94 10.1038/ctg.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim K-M, Cho YK, Bae SJ, et al. Prevalence of gastroesophageal reflux disease in Korea and associated health-care utilization: a national population-based study. J Gastroenterol Hepatol 2012;27:741–5. 10.1111/j.1440-1746.2011.06921.x [DOI] [PubMed] [Google Scholar]
- 27. Tan VP-Y, Wong BC, Wong WM, et al. Gastroesophageal reflux disease: cross-sectional study demonstrating rising prevalence in a Chinese population. J Clin Gastroenterol 2016;50:e1–7. 10.1097/MCG.0000000000000304 [DOI] [PubMed] [Google Scholar]
- 28. Dickman R, Maradey-Romero C, Gingold-Belfer R, et al. Unmet needs in the treatment of gastroesophageal reflux disease. J Neurogastroenterol Motil 2015;21:309–19. 10.5056/jnm15105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higuchi K, Joh T, Nakada K, et al. Is proton pump inhibitor therapy for reflux esophagitis sufficient?: a large real-world survey of Japanese patients. Intern Med 2013;52:1447–54. 10.2169/internalmedicine.52.0349 [DOI] [PubMed] [Google Scholar]
- 30. Yuan Y, Hunt RH. W1100 intragastric pH holding time of pH. Gastroenterology 2010;138:S651 10.1016/S0016-5085(10)62998-8 [DOI] [Google Scholar]
- 31. Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015;41:636–48. 10.1111/apt.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunt RH, Scarpignato C. Potassium-Competitive acid blockers (P-CABs): are they finally ready for prime time in acid-related disease? Clin Transl Gastroenterol 2015;6:e119 10.1038/ctg.2015.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwakiri K, Umegaki E, Hiramatsu N, et al. Tu1059 a phase 3, randomized, double-blind, multicenter study to evaluate the efficacy and safety of TAK-438 (20 Mg once-daily) compared to lansoprazole (30 Mg once-daily) in patients with erosive esophagitis. Gastroenterology 2014;146:S-741 10.1016/S0016-5085(14)62682-2 [DOI] [Google Scholar]
- 34. Takeda Pharmaceutical Company Ltd Takepron®Lansoprazole enteric-coated capsules. package insert 2017.
- 35. Takeda Pharmaceutical Company Ltd Takepron® lansoprazole delayed-release capsules j.p., product information 2018.
- 36. Brummer RJ, Geerling BJ, Stockbrügger RW. Initial and chronic gastric acid inhibition by lansoprazole and omeprazole in relation to meal administration. Dig Dis Sci 1997;42:2132–7. 10.1023/A:1018891106425 [DOI] [PubMed] [Google Scholar]
- 37. Moules I, Garrett A, Brocklebank D, et al. Gastric acid inhibition by the proton pump inhibitor lansoprazole is unaffected by food. Br J Clin Res 1993;4:153–61. [Google Scholar]
- 38. Oshima T, Miwa H. Potent potassium-competitive acid blockers: a new era for the treatment of acid-related diseases. J Neurogastroenterol Motil 2018;24:334–44. 10.5056/jnm18029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 2016;43:1048–59. 10.1111/apt.13588 [DOI] [PubMed] [Google Scholar]
- 40. Mizokami Y, Oda K, Funao N, et al. Vonoprazan prevents ulcer recurrence during long-term NSAID therapy: randomised, lansoprazole-controlled non-inferiority and single-blind extension study. Gut 2018;67:1042–51. 10.1136/gutjnl-2017-314010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Astruc B, Jenkins H, Jenkins R. Effect of therapeutic and supratherapeutic doses of Vonoprazan on the QT/QTc interval in a phase I randomized study in healthy subjects. Clin Transl Sci 2017;10:208–16. 10.1111/cts.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jenkins H, Jenkins R, Patat A. Effect of multiple oral doses of the potent CYP3A4 inhibitor clarithromycin on the pharmacokinetics of a single oral dose of vonoprazan: a phase I, open-label, sequential design study. Clin Drug Investig 2017;37:311–6. 10.1007/s40261-016-0488-6 [DOI] [PubMed] [Google Scholar]