Abstract
Objective
This study aimed to develop a novel therapeutic vaccine based on a unique B cell epitope and investigate its therapeutic potential against chronic hepatitis B (CHB) in animal models.
Methods
A series of peptides and carrier proteins were evaluated in HBV-tolerant mice to obtain an optimised therapeutic molecule. The immunogenicity, therapeutic efficacy and mechanism of the candidate were investigated systematically.
Results
Among the HBsAg-aa119-125-containing peptides evaluated in this study, HBsAg-aa113-135 (SEQ13) exhibited the most striking therapeutic effects. A novel immunoenhanced virus-like particle carrier (CR-T3) derived from the roundleaf bat HBV core antigen (RBHBcAg) was created and used to display SEQ13, forming candidate molecule CR-T3-SEQ13. Multiple copies of SEQ13 displayed on the surface of this particulate antigen promote the induction of a potent anti-HBs antibody response in mice, rabbits and cynomolgus monkeys. Sera and purified polyclonal IgG from the immunised animals neutralised HBV infection in vitro and mediated efficient HBV/hepatitis B virus surface antigen (HBsAg) clearance in the mice. CR-T3-SEQ13-based vaccination induced long-term suppression of HBsAg and HBV DNA in HBV transgenic mice and eradicated the virus completely in hydrodynamic-based HBV carrier mice. The suppressive effects on HBsAg were strongly correlated with the anti-HBs level after vaccination, suggesting that the main mechanism of CR-T3-SEQ13 vaccination therapy was the induction of a SEQ13-specific antibody response that mediated HBV/HBsAg clearance.
Conclusions
The novel particulate protein CR-T3-SEQ13 suppressed HBsAg effectively through induction of a humoural immune response in HBV-tolerant mice. This B cell epitope-based therapeutic vaccine may provide a novel immunotherapeutic agent against chronic HBV infection in humans.
Keywords: hepatitis B, drug development, immunotherapy
Significance of this study.
What is already known on this subject?
Achievement of hepatitis B virus surface antigen (HBsAg) loss is an ideal endpoint of chronic hepatitis B (CHB) treatment, and it is rarely achieved by current approved anti-HBV drugs.
High load of HBsAg directly inhibit both adaptive and innate immune functions and ultimately leads to specific tolerance that prevents the host from eradicating HBV infection. A long-term suppression of HBsAg hopefully allows for restoration of anti-HBs B cell response and HBV specific T cell function.
Most HBV therapeutic vaccines based on the native HBsAg and HBcAg could induce specific T cells in HBV carrier mice, even in patients with CHB, but there was no significant effective antibody response that mediates viral clearance, thus exhibited limited inhibitory effect on HBsAg levels.
The antibodies recognise the sA epitope (HBsAg-aa119-125) and exhibit more remarkable and prolonged HBsAg suppression effects than antibodies binding to other epitopes as we described previously.
Significance of this study.
What are the new findings?
Among the HBsAg-aa119-125-containing peptides evaluated in this study, HBsAg-aa113-135 (SEQ13) exhibited the most striking therapeutic effects. A novel immuno-enhanced virus-like particle carrier (CR-T3) derived from the roundleaf bat HBV core antigen (RBHBcAg) was created and used to display SEQ13, forming the novel particulate protein CR-T3-SEQ13.
Multiple copies of SEQ13 displayed on the surface of this particulate antigen promote the induction of a potent antibody response in mice, rabbits and cynomolgus monkeys, and dominant antibodies recognise HBsAg-aa119-125, which exhibit broad spectrum of activity to mediate HBsAg clearance in vivo and neutralise HBV infection in vitro.
CR-T3-SEQ13-based vaccination showed a long-term suppression of HBsAg and HBV DNA in HBV transgenic mice and eradicated the virus completely in hydrodynamic-based HBV carrier mice by induction of a SEQ13-specific antibody response that mediated HBV/HBsAg clearance.
How might it impact on clinical practice in the foreseeable future?
CR-T3-SEQ13 is a superior candidate based on a unique B cell eiptope for developing therapeutic vaccine against HBV, which will be tested in the clinic alone or in combination with current available anti-HBV drugs to treat patients with CHB, and aims to increase the HBsAg loss rate.
If successful, CR-T3-SEQ13-based immunotherapy will provide a novel anti-HBV strategy to achieve long-term inhibition of the HBsAg levels and improve the clinical management of CHB.
Introduction
Currently, approximately 250–340 million individuals are chronically infected with HBV, which is one of the most prevalent chronic viral infections worldwide and have a 15%–25% risk of dying from HBV-related disease.1–3 The current approved anti-HBV drugs, including nucleoside/nucleotide analogues and PEG interferons, can suppress viral replication and limit hepatitis B but cannot reduce the viral antigen load effectively. Achieving loss of hepatitis B virus surface antigen (HBsAg) with or without anti-HBs seroconversion, which is the optimal endpoint of current treatment, is rare.4–6 Thus, new antiviral strategies against chronic HBV infection are urgently required. Why is chronic hepatitis B (CHB) so difficult to cure? Generally, there are two major obstacles to overcome. The first obstacle is the stable presence of covalently closed circular DNA (cccDNA), which is the template for HBV production and forms a mini-chromosome in the hepatocyte nucleus.7 However, the mechanisms underlying the maintenance and degradation of cccDNA are not fully understood, and no convincing theory has guided the discovery of drugs targeting cccDNA.7 The second main obstacle is the high HBsAg load, which has been proposed to directly inhibit both adaptive and innate immune functions and ultimately leads to specific tolerance that prevents the host from eradicating HBV infection.8 9 Several studies have indicated that the appearance of immune tolerance to chronic viral infections is highly associated with the viral antigen load.10 11 Viruses with high viral loads, such as HBV, HIV, HCV and lymphocytic choriomeningitis mammarenavirus (LCMV) cause a severe loss of T cell numbers and/or functions.11 Furthermore, a high ratio of antigen to antigen-specific B cells can lead to terminal differentiation of B cells and ultimately loss of effective IgG responses.12 Consistently, prolonged inhibition of the HBsAg level can promote reversal of B cell tolerance to produce an anti-HBs antibody response.13 14 In hepatitis B e antigen (HBeAg)-negative patients, the lower HBsAg level predicts a higher probability of achieving HBsAg loss.15 HBsAg seroclearance achieved after therapy has been associated with a significantly lower hepatocellular carcinoma incidence or mortality rate.16 These studies indicate that the HBsAg levels are associated with progression of liver disease. Therefore, the development of new drugs that can effectively reduce the HBsAg level may restore specific immune responses and achieve a higher functional cure rate.
Therapeutic vaccine is one of the most promising biologicals against chronic viral infections. However, most therapeutic vaccine candidates designed to induce T cell responses or based on native HBsAg have missed their primary endpoints in clinical trials.17–22 In the presence of high HBsAg levels in HBV carriers, T cells targeting the dominant epitopes of HBsAg are believed to be exhausted, which would represent a big challenge for stimulation of an effective cellular immune response without removal of the abundant HBsAg. Induction of an anti-HBs antibody response may be one promising approach to reduce the circulating HBsAg level.23 Therefore, we propose that a functional B cell epitope derived from HBsAg must be found, and an immunogenic virus-like particle (VLP) must be designed to display hundreds copies of this epitope. A chimeric VLP displaying multiple epitopes will stimulate stronger epitope-specific antibody responses that can mediate HBsAg clearance in vivo. In our previous study, we found that not all antibodies against HBsAg demonstrated an equal ability to clear HBV in vivo; only E6F6-like antibodies recognising the unique sA epitope (HBsAg-aa119-125 as the core motif) could potently mediate HBsAg clearance.24–26 Previous findings suggest that the epitope is the key factor that determines the efficacy of anti-HBsAg antibodies, and the sA epitope has potential to be a therapeutic target against CHB. On one hand, monoclonal antibody (mAb)-based immunotherapy represents a promising avenue for treatment of CHB; on the other hand, the sA epitope can be used to develop a novel therapeutic vaccine that is expected to primarily induce humoral immune responses.
In this study, an optimised peptide (SEQ13, HBsAg-aa113-135) based on the sA epitope was designed for displaying on the spikes of a novel immuno-enhanced VLP carrier (CR-T3) derived from the roundleaf bat hepatitis B virus core antigen (RBHBcAg); the candidate molecule was named CR-T3-SEQ13. Multiple copies of epitopes on the surface of this particulate antigen promote the production of a potent sA epitope-specific antibody response and then mediate long-term HBsAg clearance and suppress the viral load in HBV transgenic (HBV-Tg) mice and hydrodynamic-based HBV (HDI-HBV) carrier mice. We also found good safety and immunogenicity of CR-T3-SEQ13 in cynomolgus monkeys. Induction of a similar immune response in patients with CHB will represent a promising method to achieve long-term inhibition of the HBsAg levels and a higher functional cure rate.
Materials and methods
Animals
The HBV-Tg mice were provided by Pei-Jer Chen (NTU, Taiwan) and bred at the Laboratory Animal Centre of Xiamen University27; this study used HBV-Tg mice aged 8–10 weeks. The hydrodynamic injection (HDI)-based HBV carrier models were developed as previously described using the ‘B6-Tg(AAVS1)A1Xob/J’ strain and the pAAV-HBV1.2 plasmid.28 29 BALB/c mice (6–8 weeks old) were purchased from Shanghai Lingchang Biotechnology Co, Ltd. The New Zealand White rabbits used for immunisation were purchased from the Shanghai SLAC Laboratory Animal Co, Ltd. All mice were maintained under specific pathogen-free conditions in the Laboratory Animal Centre of Xiamen University. The experiments in mice and rabbits were conducted under approval of the Institutional Animal Care and Use Committee at Xiamen University and were in accordance with the Guide for the Care and Use of Laboratory Animals.
Four male and three female cynomolgus monkeys were used in the immunogenicity study, which was conducted at WuXi AppTec (Suzhou) Co, Ltd. All experiments in this study were in full compliance with the protocol and conformed to the following regulations and guidelines regarding animal care and welfare: Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and National Institutes of Health guidelines as reported in the ‘Guide for the Care and Use of Laboratory Animals’ and the National Research Council – ILAR, Revised 2011. The study was reviewed and approved by WuXi AppTec’s Institutional Animal Care and Use Committee prior to any activities involving animals.
Blood from the patients with CHB
This study used 25 blood samples from hospitalised patients with CHB. Written informed consent was obtained from all patients.
More details of vaccine preparation, epitope mapping of antibodies, detection of HBV markers, cryo electron microscopy (cryo-EM) and three-dimensional (3D) reconstruction of CR-T3-SEQ13, combinational treatment experiments, in in vivo potency of anti-CR-T3-SEQ13 sera or purified polyclonal IgG and in vitro neutralising assay are provided in the online supplementary materials and methods and tables.
Results
Construction of a unique B cell epitope-based VLP vaccine: CR-T3-SEQ13
For a recombinant protein therapeutic vaccine designed to induce an epitope-specific B cell response, the key factors associated with efficacy include functional epitopes, immunogenic carriers and adjuvants. In this study, experiments were performed to systematically optimise the epitopes and carriers. According to our previous research, HBsAg-aa119-125 was proposed as an initial target.24
To obtain an optimised epitope, we selected five polypeptides of different lengths derived from HBsAg that contained HBsAg-aa119-125; then, the coding sequences of the peptides were inserted into the truncated HBcAg carrier (HBC149) and expressed in an Escherichia coli system (online supplementary figure 1A). All five chimeric proteins and the carrier protein spontaneously assembled into spherical particles with a diameter of approximately 30 nm, and we obtained high-purity particles displaying these peptides on the surface (online supplementary figure 1B). Then, we evaluated the immunogenicity of these antigens in HBV-free BALB/c mice. The data showed that all five particulate antigens containing HBsAg-aa119-125 could induce strong anti-HBs and anti-carrier antibody responses (online supplementary figure 2A, B), with the HBC149-S113-135-immunised mice showing slightly higher anti-HBs antibody levels than the other groups (online supplementary figure 2A). Furthermore, we evaluated the immunogenicity and efficacy of these antigens in HBV-Tg mice. The results showed that these antigens could induce strong anti-carrier antibody responses comparable with those of the HBV-free mice (online supplementary figure 2D), although the anti-HBs antibody response was significantly weaker, as expected (online supplementary figure 2C). The highest anti-HBs antibody levels were detected in the mice after HBC149-S113-135 treatment; consistently, the HBsAg and HBV DNA levels of this group of mice decreased significantly compared with those of the other mouse groups (online supplementary figure 2E, F). Therefore, we selected HBsAg-aa113-135, which is a 23-amino acid polypeptide, as an optimal candidate for further study, and we named it SEQ13.
gutjnl-2018-317725supp003.pdf (1.5MB, pdf)
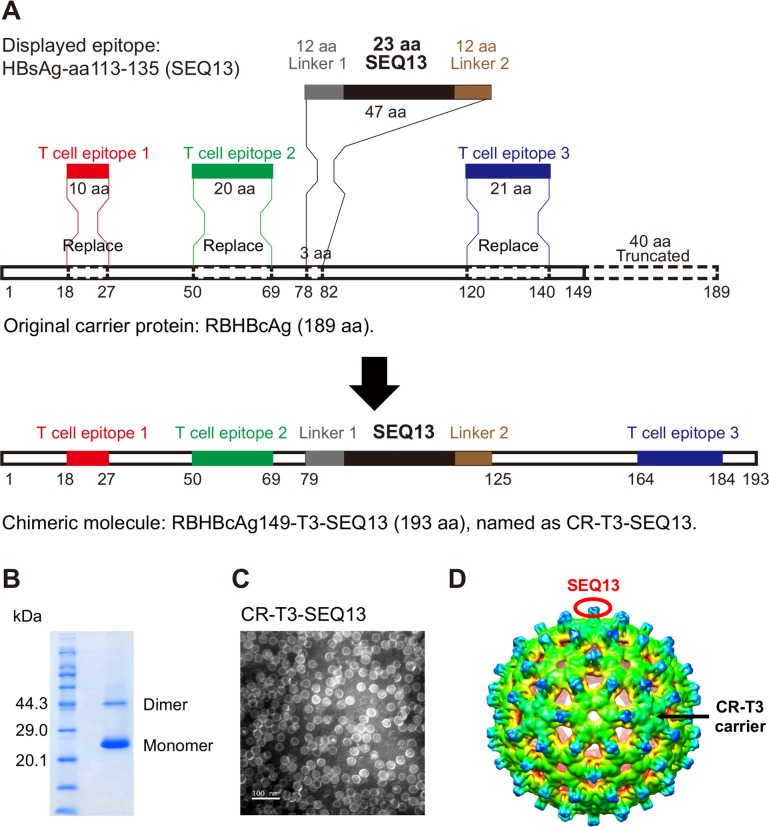
To obtain the optimised carrier, a series of VLP carriers based on viral capsids were evaluated, including HBcAg carriers with different mutations, a woodchuck hepatitis B virus core antigen (WHBcAg) carrier, a human papillomavirus L1 carrier and bat hepadnavirus capsids. Finally, RBHBcAg, which is the capsid protein of the intermediate roundleaf bat (Hipposideros larvatus) hepatitis B virus (RBHBV, NCBI GenBank Accession Number: KC790373.1), was selected as the lead candidate.30 The full-length RBHBcAg consists of 189 amino acids and has a topological domain similar to that of HBcAg that contains an arginine-rich domain at the C-terminus. The arginine-rich region binds to host nucleic acids non-specifically; thus, RBHBcAg-aa150-189 was truncated to prevent its interference with protein purification. The truncated carrier, which was named RBHBcAg149, can spontaneously assemble into VLPs in an E. coli system. In addition to the importance of particulate shape for immunogenicity, helper T cell responses are also very important for effective B cell responses, and CD8+ T cell responses are beneficial for eradication of intracellular viruses. Therefore, we were trying to integrate the well-studied CD4+ T cell epitopes and CD8+ T cell epitopes into the RBHBcAg149 carrier. We think that the modified molecule containing human T cell epitopes can be used as candidate molecule by satisfying the following three points: (1) it still has the characteristics of spontaneous assembly into VLPs; (2) it has the therapeutic effects comparable with or better than CR-SEQ13 in HBV carrier mice, which indicate that the immunogenicity is not damaged; and (3) the selected epitopes can bind to as many HLA alleles as possible to cover a larger population.31 The modified molecule can enhance the interferon gamma (IFNγ) releasing in the antigen-stimulated whole blood culture system derived from patients with CHB. The experiments were carried out for these three points. Finally, one CD8+ T cell epitope and two helper CD4+ T cell epitopes derived from HBcAg were introduced into RBHBcAg149 by homologous replacement (figure 1A), including the CD8+ T cell epitope HBcAg-aa18-27 (FLPSDFFPSV)31 32 and the CD4+ T cell epitopes HBcAg-aa50-69 (PHHTALRQAILCWGELMTLA)31 33 and HBcAg-aa120-140 (VSFGVWIRTPPAYRPPNAPIL).31 34 The sequence of SEQ13 polypeptide and linker was inserted into modified carrier (CR-T3) by replacement of RBHBcAg-aa79-81 to form a chimeric molecule with a length of 193 amino acids, designated CR-T3-SEQ13. We obtained the candidate therapeutic protein with a purity higher than 95% and a molecular weight of 20.4 kDa after purification by column chromatography (figure 1B). This protein could still spontaneously assemble into VLPs (figure 1C, online supplementary figure 3A). We further used cryo-EM and 3D image reconstruction methods to investigate the structure of CR-T3-SEQ13 VLPs. The 6.3 Å resolution cryo-EM map revealed a T=3 icosahedral capsid structure with a diameter of ~36 nm, which was highly similar to HBV core particle35 (figure 1D). The SEQ13 polypeptide was located on the top of each protrusion (figure 1D), thus was presumed to behave potential to elicit strong immune response. The efficacy of CR-T3-SEQ13 and CR-SEQ13 to reduce HBsAg levels were evaluated in both male and female HBV-TG mice. The data showed that the HBsAg decline in CR-T3-SEQ13-treated mice were comparable with those of CR-SEQ13 (online supplementary figure 3B). In addition, we used IFNγ release assay to test T cell responsiveness to antigens in whole blood of patients with CHB; the data showed that the level of IFNγ in CR-T3-SEQ13 stimulated blood samples were significantly higher than that of CR-SEQ13 and the negative control group (online supplementary figure 3C). These results suggested that CR-T3-SEQ13 could be used as a candidate molecule for further investigation.
Figure 1.
Construction of a unique B cell epitope-based virus-like particulate antigen: CR-T3-SEQ13. (A) Schematic diagram of the design from RBHBcAg to CR-T3-SEQ13; RBHBcAg-aa150-189 was truncated, HBcAg-aa18-27, HBcAg-aa50-69 and HBcAg-aa120-140 were introduced into the RBHBcAg149 by homologous replacement, sequence of SEQ13 polypeptide and linker was inserted by replacement of RBHBcAg-aa79-81, forming a chimeric molecule with a length of 193 amino acid, designated CR-T3-SEQ13. (B) Coomassie blue staining of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and (C) electron microscopy (EM) picture of recombinant CR-T3-SEQ13 protein. (D) Cryo-EM structure of CR-T3-SEQ13 particle, SEQ13 epitopes are displayed on the spikes of particle. Cryo-EM, cryoelectron microscopy.
CR-T3-SEQ13 induces sA epitope-specific antibodies with broad-spectrum activity to mediate HBsAg clearance
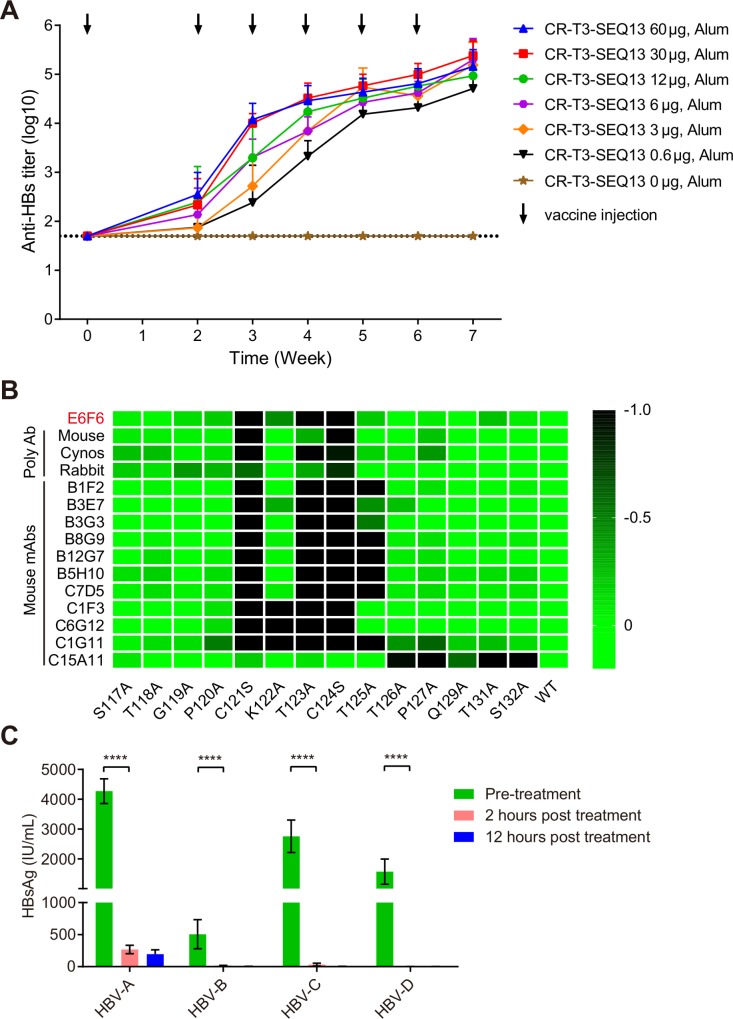
To evaluate whether CR-T3-SEQ13 could induce specific antibodies against HBsAg, BALB/c mice were used to assess the dose–response relationship. Seven groups of BALB/c mice were immunised with a vaccine containing a fixed dose of aluminium adjuvant (126 µg/dose) and variant doses of CR-T3-SEQ13 via intramuscular injection (0 µg, 0.6 µg, 3 µg, 6 µg, 12 µg, 30 µg and 60 µg). The data showed that CR-T3-SEQ13 induced a potent HBsAg-specific antibody response. The antibody titres gradually increased after the booster immunisations, whereas no specific antibody response was observed in the adjuvant control group (figure 2A). The serum anti-HBs antibody titres showed a dose–response relationship at weeks 2, 3 and 4. The antibody levels of the 60 µg group were comparable with those of the 30 µg group, whereas the other dose groups showed lower doses and antibody titres. The results suggest that a higher antigen dose can induce a stronger and faster antibody response in the early phase.
Figure 2.
CR-T3-SEQ13 induces sA epitope-specific antibodies with broad-spectrum activity to mediate HBsAg clearance. (A) The kinetics of anti-HBs antibody response level in BALB/c mice immunised with a series of vaccine formulations containing fixed alum adjuvant dose (840 µg/mL) and different antigen doses, including 60 µg, 30 µg, 12 µg, 6 µg, 3 µg, 0.6 µg and 0 µg, respectively. (B) Alanine scanning epitope mapping strategy was used to identify the key residues for binding activity of polyclonal antiserum or monoclonal antibodies derived from mice, rabbits and cynomolgus monkeys that immunised with CR-T3-SEQ13. The value means the fold change of binding activity caused by each amino acid mutation. (C) The dynamic change of HBsAg levels in the four genotypes of HBV carrier mice after treatment with polyclonal antiserum derived from BALB/c mice immunised with CR-T3-SEQ13. The data represent mean±SEM, n=4. Significant differences between groups are indicated on the top. ****P<0.0001; two-tailed unpaired t-tests. HBsAg, hepatitis B virus surface antigen.
To identify the epitopes of antibodies induced by CR-T3-SEQ13 vaccination, both polyclonal antibodies from the sera and mAbs from a mouse hybridoma were analysed. The sera were collected from mice, rabbits and cynomolgus monkeys immunised with CR-T3-SEQ13, and a total of 11 mouse mAbs recognising SEQ13 were obtained. Alanine-scanning mutations of SEQ13 were used to identify key residues for the antibody binding activity. The results showed that all of the polyclonal antibodies from the three species were sensitive to mutations located in the HBsAg-aa119-125 region, especially C121 and C124, which are the key amino acids of the sA epitope recognised by E6F6 (figure 2B). Among the mAbs, 10 of 11 recognised the HBsAg-aa119-125 region and only one antibody recognised the C-terminus of HBsAg-aa113-135, which was more sensitive to amino acid mutations in HBsAg-aa126-132 (figure 2B). The results indicated that the dominant antibodies induced by CR-T3-SEQ13 were similar to those of E6F6, suggesting that the antibodies would be able to functionally mediate HBsAg clearance in vivo.
A total of 10 HBV genotypes have been identified worldwide, among which genotype A, B, C and D are the most prevalent. To evaluate the broad-spectrum anti-HBV effects of polyclonal antibodies induced by CR-T3-SEQ13, sera were collected from BALB/c mice immunised with CR-T3-SEQ13 and transferred to mice carrying HBV genotypes A, B, C and D. Blood was collected from the mice at pretreatment (0) and at 2 and 12 hours post-treatment, and short-term dynamic changes in the HBsAg levels were monitored. The results showed that the HBsAg levels in the carrier mice for the four HBV genotypes were rapidly and significantly reduced to very low levels within 2 hours (figure 2C). This result shows that the CR-T3-SEQ13-induced antibodies can effectively suppress the globally predominant HBV genotypes.
CR-T3-SEQ13 significantly inhibits HBsAg and HBV DNA in HBV-Tg mice in a dose-dependent manner
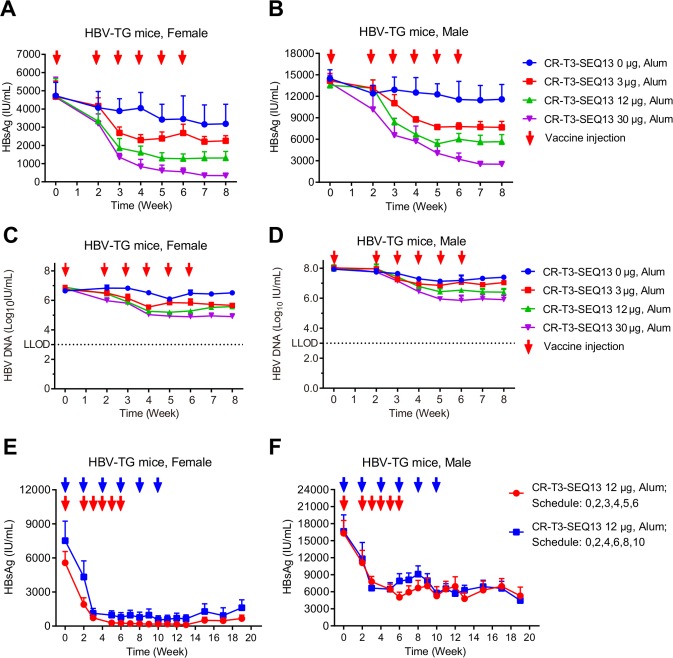
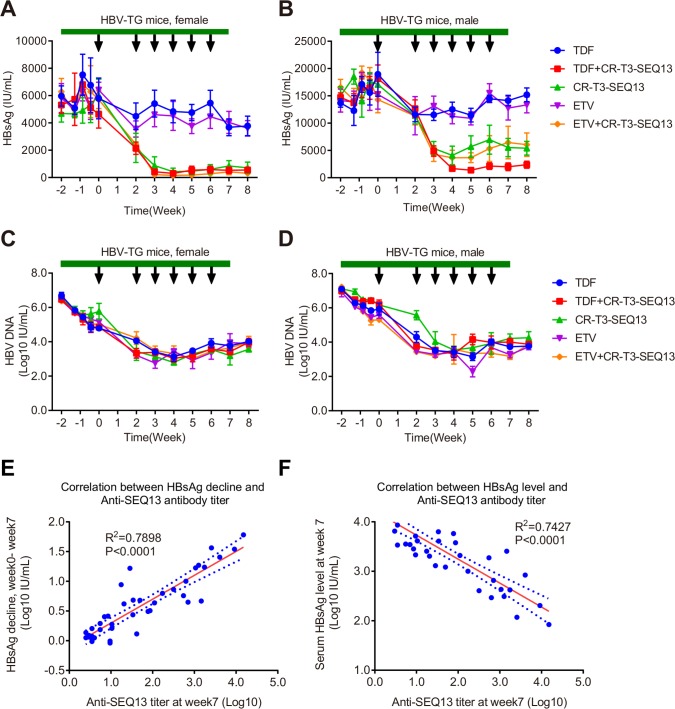
HBV-Tg mice were used to evaluate the anti-HBV efficacy of CR-T3-SEQ13 vaccination. To evaluate the relationship between efficacy and the antigen dose, formulations with three different antigen doses were prepared with 30 µg, 12 µg and 3 µg of CR-T3-SEQ13. The same dose of alum adjuvant without antigen was included as the control group. Four groups of HBV-Tg mice (four female and four male mice per group) were intramuscularly injected with 150 µL of the vaccine formulations at 0, 2, 3, 4, 5 and 6 weeks. Compared with those of the control mice treated with adjuvant alone, significant dose-dependent inhibitory effects on serum HBsAg and HBV DNA were observed in the CR-T3-SEQ13-treated mice (figure 3A–D). With the antigen dose of 30 µg, the average HBsAg level decreased from approximately 4600 IU/mL to 300 IU/mL in the female mice (figure 3A) and from approximately 14000 IU/mL to 2500 IU/mL in the male mice (figure 3B), for an inhibition rate of greater than 90%. The average HBV DNA level decreased from 6.44×106 IU/mL to 7.60×104 IU/mL in the female mice (figure 3C) and from 1.10×108 IU/mL to 8.00×105 IU/mL in the male mice (figure 3D), for an inhibition rate of 99%. Therefore, the efficacy of the therapeutic vaccine described in this study was correlated with the CR-T3-SEQ13 antigen dose, and the higher antigen doses were associated with better inhibitory effects on HBV. Furthermore, to verify whether different immunisation procedures would affect the vaccine efficacy, two different treatment schedules were tested (0, 2, 3, 4, 5 and 6 weeks and 0, 2, 4, 6, 8 and 10 weeks). Significant inhibition of the HBsAg levels was achieved by both immunisation procedures in the male and female mice, with no significant differences (figure 3E, F). We monitored the HBsAg level until 19 weeks and found that the inhibitory effects were sustained without apparent viral rebound (figure 3E, F). The results indicated that the CR-T3-SEQ13-based therapeutic vaccine could induce specific immune responses and mediate a potent reduction of HBsAg and HBV DNA in the HBV-Tg mice, which were highly tolerant to HBV. We also evaluated the anti-HBV effects of a vaccine consisting of recombinant natural HBsAg plus HBcAg in the HBV-Tg mice, the formulation of which include 12 µg of HBsAg and 12 µg of HBcAg per dose, with alum adjuvant (840 µg/mL of aluminium) that is the same with CR-T3-SEQ13 vaccine. The data showed that no significant decrease in the HBsAg and HBV DNA levels was observed in mice treated by the HBsAg plus HBcAg vaccine; in contrast, the viral loads in the CR-T3-SEQ13-treated mice were suppressed and were significantly lower at the endpoint of treatment (online supplementary figure 4).
Figure 3.
CR-T3-SEQ13 can significantly inhibit HBsAg and HBV DNA in HBV transgenic mice in a dose-dependent manner and sustain the suppression effects for a long time. (A–D) HBV transgenic mice were intramuscular injected with vaccine formulations containing fixed alum adjuvant dose (840 µg/mL) and different antigen doses, including 30 µg, 12 µg and 3 µg and 0 µg, respectively. A total of 6 doses were injected at 0, 2, 3, 4, 5 and 6 weeks. (A) Dynamics of HBsAg level in female HBV-Tg mice; (B) dynamics of HBsAg level in male HBV-Tg mice; (C) dynamics of HBV DNA level in female HBV-Tg mice; and (D) dynamics of HBV DNA level in male HBV-Tg mice. (E–F) HBV-Tg mice were injected with vaccine formulations containing 840 µg/mL of alum adjuvant and 12 µg of CR-T3-SEQ13 at 0, 2, 3, 4, 5 and 6 weeks and 0, 2, 4, 6, 8 and 10 weeks, respectively. (E) Dynamics of HBsAg level in female HBV-Tg mice vaccinated by different schedule; (B) dynamics of HBsAg level in male HBV-Tg mice vaccinated by different schedule. The data represent mean±SEM, n=4. HBsAg, hepatitis B virus surface antigen.
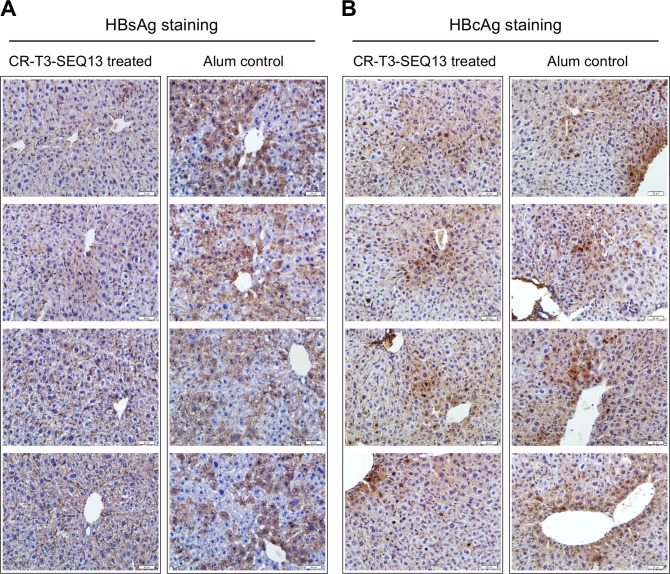
Furthermore, the intrahepatic HBsAg and HBcAg levels were analysed by immunohistochemistry at week 8 post-treatment. The data showed that the cytosolic HBsAg level in the hepatocytes of the CR-T3-SEQ13-treated mice was much lower than that in the alum-treated mice (figure 4A) and that the intrahepatic HBcAg levels were comparable between the two groups (figure 4B). We also measured the HBsAg and HBcAg in liver lysate of these mice quantitatively. The data were consistent with the observed results of immunohistochemistry. The HBsAg of the CR-T3-SEQ13 treated group was significantly lower than that of the control group, which was about 30% of the control group, and the level of HBcAg was not significantly different between the two groups (online supplementary figure 5). This observation was similar to the intrahepatic anti-HBV effects of E6F6 that we reported previously.24
Figure 4.
Immunohistochemical staining of HBsAg and HBcAg in the livers of HBV-Tg mice (n=4) after CR-T3-SEQ13 treatment. Assays were performed at week 8 after treatment. The scale bar is 20 µm. HBsAg, hepatitis B virus surface antigen.
CR-T3-SEQ13 vaccination eradicated HBsAg and HBV DNA in hydrodynamic injection-based HBV carrier mice
To further evaluate the potent ability of CR-T3-SEQ13 to induce an anti-HBs antibody response and eliminate HBsAg, the HDI-HBV carrier mouse model was used. HDI-HBV mice have been demonstrated to have acquired immune tolerance to HBV, which is similar to the situation experienced by most patients with CHB who develop immune tolerance due to HBV exposure at an early age. We developed HDI-HBV mice based on the ‘B6-Tg(AAVS1)A1Xob/J’ strain as described previously.29 Mice were screened according to the HBsAg level at 6 weeks after hydrodynamic injection of the pAAV-HBV1.2 plasmid. Mice with HBsAg levels higher than 2000 IU/mL were selected for this study. Ten mice with comparable HBsAg levels were selected and divided into two groups, which were treated with the CR-T3-SEQ13 vaccine or the alum adjuvant control. A total of five doses were injected at weeks 0, 2, 3, 4 and 5. The data showed that CR-T3-SEQ13 vaccination could induce an anti-HBs antibody response (figure 5B) together with a decline in HBsAg to the lower limit of detection that was sustained at undetectable levels for more than 40 weeks after drug treatment had ceased. In contrast, mice in the control group still continued to carry HBsAg, with an average level higher than 100 IU/mL (figure 5A). The results suggested that CR-T3-SEQ13 could effectively stimulate the SEQ13-specific antibody response and then mediate HBsAg clearance, which was similar to the ‘functional cure’ situation defined as HBsAg loss and/or anti-HBs seroconversion.
Figure 5.
CR-T3-SEQ13 vaccination could eradicate HBsAg and HBV DNA in hydrodynamic injection-based HBV carrier mice. A total of five doses of CR-T3-SEQ13 were injected at week 0, 2, 3, 4 and 5, respectively. (A) Dynamics of HBsAg level and (B) kinetics of anti-HBs antibody level in HDI-HBV carrier mice (genotype A, n=5). The data represent mean±SEM. HBsAg, hepatitis B virus surface antigen.
Combinational therapy with CR-T3-SEQ13 plus nucleoside analogues
Nucleoside/nucleotide analogues and interferons are currently approved anti-HBV drugs. Entecavir (ETV) and tenofovir disoproxil fumarate (TDF) are first-line oral anti-HBV drugs. The effect of CR-T3-SEQ13 in combination with either ETV or TDF was investigated. Five groups were designed (ETV alone, TDF alone, RHBPI alone, ETV plus RHBPI and TDF plus RHBPI). In the combination therapy groups, the vaccination was followed by 2 weeks of oral administration of the analogues. The serum HBsAg level was significantly decreased in the mice treated with CR-T3-SEQ13 plus ETV, CR-T3-SEQ13 plus TDF or CR-T3-SEQ13 alone (figure 6A–D). The inhibitory effect of CR-T3-SEQ13 plus TDF treatment on the serum HBsAg level of the male mice was slightly better than that of CR-T3-SEQ13 plus ETV or CR-T3-SEQ13 treatment alone (figure 6B); in contrast, no obvious change in HBsAg was observed in the mice treated with ETV or TDF alone (figure 6A, B). The data suggest that there was no interference between the first-line anti-HBV nucleoside analogues and CR-T3-SEQ13. TDF may have the potential to improve the therapeutic effect of CR-T3-SEQ13 in this model.
Figure 6.
Combinational therapy with CR-T3-SEQ13 plus nucleoside analogues. HBV transgenic mice were treated with TDF alone, ETV alone, CR-T3-SEQ13 alone, CR-T3-SEQ13plus ETV and CR-T3-SEQ13 plus TDF. In the combinational therapy group, 2 weeks after oral administration of the analogues, the CR-T3-SEQ13 was injected at weeks 0, 2, 3, 4, 5, 6. Dynamics change of serum HBsAg levels in (A) male and (B) female HBV-Tg mice, and HBV DNA levels in (C) male and (D) female HBV-Tg mice. (E) The correlation between HBsAg decline and anti-SEQ13 antibody titre in female mice treated with CR-T3-SEQ13, TDF plus CR-T3-SEQ13 and ETV plus CR-T3-SEQ13. (F) The correlation between HBsAg decline and Anti-SEQ13 antibody titre in female mice treated with CR-T3-SEQ13, TDF plus CR-T3-SEQ13 and ETV plus CR-T3-SEQ13. Data are presented as mean±SEM, n=4. The arrow refers to injection of CR-T3-SEQ13, and the green band refers to nucleotide analogue. ETV, entecavir; HBsAg, hepatitis B virus surface antigen; TDF, tenofovir disoproxil fumarate.
To analyse the correlation between the anti-SEQ13 antibody titre and the decline in HBsAg, serum anti-SEQ13 antibodies of all mice were measured by indirect ELISA. The kinetics of anti-SEQ13 antibody titres indicated that female mice have a stronger antibody response than male mice (online supplementary figure 6). The data showed a strong positive correlation between the HBsAg decline (weeks 0–7) and the anti-SEQ13 antibody titre at week 7 (R2=0.7898, p<0.0001) (figure 6E). Meanwhile, the HBsAg level was strongly negatively correlated with the anti-SEQ13 antibody titre at week 7 (R2=0.7427, p<0.0001) (figure 6F). The strong correlation indicates that the anti-HBV effects of CR-T3-SEQ13 vaccination are highly dependent on the induction of an anti-SEQ13 antibody response, which is consistent with our hypothesis.
Immunogenicity of CR-T3-SEQ13 in cynomolgus monkeys
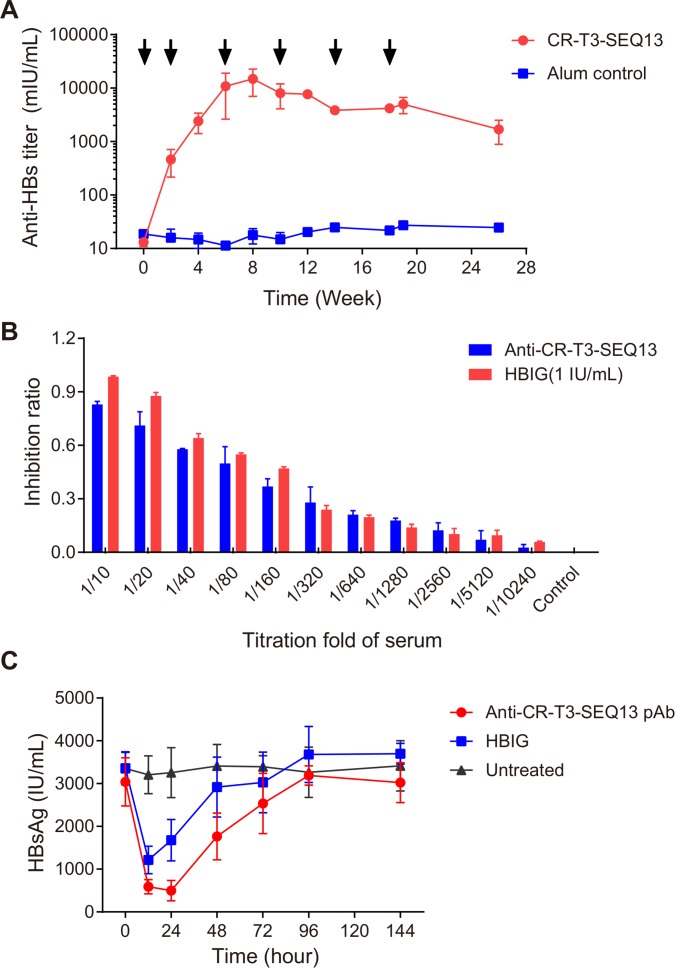
The immunogenicity of alum-adjuvanted CR-T3-SEQ13 and the time course of the immune response were evaluated in cynomolgus monkeys. Seven cynomolgus monkeys were used in this study. The immunisation schedule was six injections over the time course of 0, 2, 6, 10, 14 and 18 weeks. Venous blood was collected every 2 weeks out to 26 weeks to monitor the dynamics of the antibody response. The HBsAg-specific antibody titre increased significantly and reached a plateau at 6 weeks following the three injections (figure 7A). The peak response occurred at 8 weeks, with an anti-HBs antibody titre of approximately 14 800 mIU/mL; human hepatitis B immunoglobulin (HBIG) was used as the active standard (figure 7A). This result indicated that CR-T3-SEQ13 had good immunogenicity in cynomolgus monkeys.
Figure 7.
Immunogenicity of CR-T3-SEQ13 in cynomolgus monkeys and potency of antiserum. (A) The kinetics of anti-HBs antibody tittr in monkeys immunised with CR-T3-SEQ13 vaccine and alum control. Two male and two female monkeys received 1 mL/dose of formulation containing CR-T3-SEQ13 (20 µg/dose) and alum adjuvant (420 µg/mL), by intramuscular (IM) injection in the deltoid muscle of the upper arm. The control group (two male and one female monkeys) received alum adjuvant only. The immunisation schedule was six injections over the time course of 0, 2, 6, 10, 14 and 18 weeks. (B) In vitro neutralising activity of serum derived from monkeys vaccinated with CR-T3-SEQ13, HBIG (1 IU/mL) was used as control group. (C) Serum HBsAg dynamics of HBV-Tg mice after infusions of polyclonal antibodies (pAb). CR-T3-SEQ13 pAb and HBIG were injected into HBV-Tg mice (n=4) at the same dosage (10 IU/dose). Untreated mice served as controls. The data were expressed as the mean±SEM. HBIG, human hepatitis B immunoglobulin; HBsAg, hepatitis B virus surface antigen.
Potency of the anti-HBV polyclonal antibodies induced by CR-T3-SEQ13
To evaluate the in vitro neutralising activity of the anti-HBs antibodies induced by CR-T3-SEQ13 in cynomolgus monkeys, HepG2 cells stably expressing human Na+-taurocholate cotransporting polypeptide (NTCP) (named the HepG2-hNTCP-2B1 cell line) were used. Cynomolgus monkey sera collected at 12 weeks from vaccinated monkeys were used as the test sample, and HBIG (1 IU/mL) was used as a control. A twofold serum dilution series was prepared from 1/10 to 1/10240. The polyclonal antibodies from the monkeys immunised with CR-T3-SEQ13 showed good neutralisation activity comparable with that of 1 IU/mL of HBIG (figure 7B). Generally, good protective effects can be achieved if the anti-HBs titre is above 10 mIU/mL, suggesting that the antibodies generated by CR-T3-SEQ13 have protective effects and thus may play a role in protection of HBV-free hepatocytes from HBV infection or block the spread of HBV in the liver.
To further evaluate the in vivo anti-HBV efficacy of the antibodies induced by CR-T3-SEQ13 in cynomolgus monkeys, polyclonal antibodies were purified from sera from the immunised monkeys. We measured the anti-HBs titres of the purified polyclonal antibodies and diluted them to 10 IU/mL, which was equivalent to the HBIG titre. The same amount of polyclonal anti-CR-T3-SEQ13 antibodies and HBIG (10 IU/mL, 1 mL) was injected into the HBV-Tg mice. Both the anti-CR-T3-SEQ13 polyclonal antibodies and HBIG significantly reduced the serum HBsAg levels in the mice (figure 7C). The serum HBsAg was more significantly reduced in the mice in the anti-CR-T3-SEQ13 treatment group than in those in the HBIG group, and the duration of suppression was also longer in the anti-CR-T3-SEQ13 treatment group. The results indicated that the antibodies induced by CR-T3-SEQ13 in cynomolgus monkeys could potently mediate clearance of HBsAg, which also suggested that the main mechanism of CR-T3-SEQ13 treatment was to induce anti-HBsAg specific antibody responses and mediate clearance of HBsAg.
Discussion
Dozens of preclinical and clinical efforts have been failed to generate an effective therapeutic vaccine against CHB,20 indicating that it is indeed difficult to overcome immune tolerance in patients with CHB, and it is particularly difficult to produce an anti-HBs antibody response that can mediate HBsAg clearance. In this study, we constructed a VLP displaying the candidate epitope based on a B cell epitope (HBsAg-aa119-125) that was recognised by the superior candidate therapeutic antibody E6F6 described in our previous study.24 First, we used HBC149 as an epitope-displaying carrier and HBV-Tg mice to screen the candidate molecules. A total of 23 epitope peptides in the hydrophilic region of HBsAg were prepared and evaluated, and finally the optimised epitope SEQ13 was obtained. Although HBC-SEQ13 could induce an anti-HBs response and suppressed HBsAg significantly in the female HBV-Tg mice, it did not function effectively in the male HBV-Tg mice. As a vaccine based on a B cell epitope, the carrier protein should be another crucial factor in achieving a strong immune response. Therefore, we tested dozens of carriers. Finally, in this study, we used a carrier derived from the capsid of a bat hepatitis B virus. The therapeutic effects of the molecule displayed by SEQ13 on the roundleaf bat HBV core (RBHBcAg149) was greatly improved and effectively inhibited the HBsAg level in the male HBV-Tg mice. To further optimise the RBHBcAg149 carrier, one human cytotoxic T lymphocyte (CTL) epitope and two helper T cell epitopes were introduced by replacement to obtain the candidate molecule CR-T3-SEQ13 (figure 1).
CR-T3-SEQ13 induced a specific antibody response to the SEQ13 epitope in the HBV carrier mice and significantly inhibited HBsAg and HBV DNA in the HBV-Tg mice in a dose-dependent manner (figure 3). In contrast, the vaccine based on HBsAg plus HBcAg did not exhibit a significant inhibitory effect on viral antigens (online supplementary figure 3). The efficacy of the vaccine used alone or in combination with first-line anti-HBV drugs was evaluated. The inhibitory effect on HBsAg was slightly better in the CR-T3-SEQ13+TDF (figure 6A–D) or CR-T3-SEQ13+interferon a (online supplementary figure 7) treatment regimen, although the difference was not statistically significant. In the acquired immune tolerant HDI-HBV model, CR-T3-SEQ13 vaccination inhibited HBsAg to a level below the detection limit for more than 40 weeks (figure 5). The serological markers were similar to those of a functional cure. In the non-human primate model, we demonstrated that CR-T3-SEQ13 had excellent immunogenicity and could induce cynomolgus monkeys to produce high-titre sA epitope antibodies, which could neutralise HBV infection in the HepG2-NTCP cell model in vitro and effectively eliminate HBsAg by passive transfer to HBV-Tg mice (figure 7). Anti-SEQ13 polyclonal antibodies from BALB/c mice suppressed HBsAg in vivo with broad-spectrum activity (figure 2C). Consistently, the anti-HBV effects of CR-T3-SEQ13 were positively correlated with the anti-SEQ13 antibody titre (figure 5E, F). These data indicated that the major mechanism of CR-T3-SEQ13 treatment was dependent on the induction of anti-SEQ13 antibodies, which mediated clearance of HBsAg-containing viral particles.
A comparison for adjuvants and delivery routes is important for development of a therapeutic vaccine, thus we performed experiments to find the potential adjuvants and superior routes. Various adjuvants, including AddaVax, Quil-A, Chitosan, ODN 1585, ODN 1826 and conventional alum adjuvant (online supplementary figure 8A, were evaluated in wild type C57BL/6 mice and HBV-TG mice, the data showed that the immunogenicity and efficacy of vaccine formulated with Addavax is comparable with alum adjuvant, both of which were better than the other adjuvants (online supplementary figure 8B, C). We also compared three different delivery routes, including intramuscular injection (IM), intravenous injection and subcutaneous injection, the data showed that the efficacy of IM and subcutaneous injection were comparable, both of which were much better than intravenous injection (online supplementary figure 9), we think it is because of that antigen injected by intravenous injection would be cleared rapidly in the peripheral circulating blood.
Although CR-T3-SEQ13 could induce antibodies that efficiently mediate HBsAg clearance in HBV carrier mice, thus suppressing HBsAg levels and sustaining for a long period of time, has the potential to restore HBV-specific immune responses, but the mouse models do not equal to patients with CHB, they have different immune status against HBV and also different sensitivity to vaccination immune response. Therefore, we are unable to accurately predict what will happen in the patients based on the results obtained in the mouse models. In addition, since SEQ13 only contains a part of the B cell epitope of HBsAg, the induced antibody diversity might be relatively limited, which may face problems such as virus escape mutation or weak antibody responses. All of these challenges have to be verified in clinical trials.
Much evidence has indicated that the appearance of T cell exhaustion is highly associated with the pathogen burden of the host.11 36 Removal of circulating HBsAg by anti-HBs antibody in HBV carrier mice could gradually reduce tolerance and enhance subsequent therapeutic vaccination.13 Furthermore, data from the treatment cohort of patients with CBH also showed that HBV-specific T cell responses were negatively correlated with the HBsAg level.37 Consistently, clinical trial data for REP-2139 support the hypothesis that long-term suppression of HBsAg can promote HBsAg-specific B cell responses.14 38
Therefore, suppressing the high level of circulating HBsAg should be a key factor for immunotherapy of CHB.23 Combination of approaches that show efficient reduction of HBsAg have chance to restore the effective immune response against HBV. The viral antigens reducing treatments, such as therapeutic antibody, siRNA or Nucleic acid polymers (NAPs), may benefit for induction of humoural responses by therapeutic vaccine. This strategy will hopefully overcome immune tolerance in patients with CHB and allow the clinical efficacy of a therapeutic vaccination to cure HBV infection.
Taken together, our data show that CR-T3-SEQ13 is a superior candidate for further development towards a therapeutic vaccine for CHB treatment. Our findings provide new insights into understanding the approach of a therapeutic vaccine based on B cell epitopes against persistent viral infection. Considering the distinct anti-HBV effects provided by the CR-T3-SEQ13 with alum/another novel adjuvant-based therapeutic vaccine alone or in combination with interferon or nucleos(t)ide analogues, other anti-HBV candidates under development may provide promising strategies to promote the HBV cure.
gutjnl-2018-317725supp001.pdf (398.4KB, pdf)
gutjnl-2018-317725supp002.pdf (1.5MB, pdf)
Footnotes
T-YZ, X-RG and Y-TW contributed equally.
Contributors: TZ, QY, JZ and N-X had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: TZ, QY and N-X. Acquisition of data: TZ, XG, Y-TW, X-ZK, Q-BZ, R-YQ, B-BC, YL and SW. Analysis and interpretation of data: TZ, XG, Y-TW, QY, JZ and N-X. Drafting of the manuscript: TZ, QY and JZ. Critical revision of the manuscript for important intellectual content: Q-JZ, S-WL, S-XG, P-JC and N-X. Statistical analysis: TZ and QY. Technical or material support: MW, H-LX, JC, B-HZ, X-YQ, X-FH, YZ, TC, YC, Y-BW and M-JF. Study supervision: JZ, QY and N-X. Approval of the final version of the manuscript: JZ, QY and N-X.
Funding: This work was supported by the National Scientific and Technological Major project (2017ZX10202203-001/009), the National Natural Science Foundation of China (31730029, 81672023, 81871316 and 81702006) and the Xiamen University President Fund Project (20720160063).
Competing interests: We have read and understood BMJ policy on declaration of interests anddeclare that we have no competing interests.
Ethics approval: The study was approved by the Medical Ethics Committee of School of Public Health of Xiamen University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383–403. 10.1016/S2468-1253(18)30056-6 [DOI] [PubMed] [Google Scholar]
- 2. Huang YT, Jen CL, Yang HI, et al. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J Clin Oncol 2011;29:3643–50. 10.1200/JCO.2011.36.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Margolis HS, Coleman PJ, Brown RE, et al. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA 1995;274:1201–8. [PubMed] [Google Scholar]
- 4. EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;2017:370–98. [DOI] [PubMed] [Google Scholar]
- 5. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. 10.1002/hep.28156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015;64:1972–84. 10.1136/gutjnl-2015-309809 [DOI] [PubMed] [Google Scholar]
- 8. Tsai KN, Kuo CF, Ou JJ. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol 2018;26:33–42. 10.1016/j.tim.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kondo Y, Ninomiya M, Kakazu E, et al. Hepatitis B surface antigen could contribute to the immunopathogenesis of hepatitis B virus infection. ISRN Gastroenterol 2013;2013:1–8. 10.1155/2013/935295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Utzschneider DT, Alfei F, Roelli P, et al. High antigen levels induce an exhausted phenotype in a chronic infection without impairing T cell expansion and survival. J Exp Med 2016;213:1819–34. 10.1084/jem.20150598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell 2009;138:30–50. 10.1016/j.cell.2009.06.036 [DOI] [PubMed] [Google Scholar]
- 12. Zellweger RM, Hangartner L, Weber J, et al. Parameters governing exhaustion of rare T cell-independent neutralizing IgM-producing B cells after LCMV infection. Eur J Immunol 2006;36:3175–85. 10.1002/eji.200636087 [DOI] [PubMed] [Google Scholar]
- 13. Zhu D, Liu L, Yang D, et al. CLearing persistent extracellular antigen of hepatitis b virus: An immunomodulatory strategy to reverse tolerance for an effective therapeutic vaccination. J Immunol 2016;196:3079–87. 10.4049/jimmunol.1502061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al-Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive bangladeshi patients with hbeag+ chronic hepatitis b infection. PLoS One 2016;11:e0156667 10.1371/journal.pone.0156667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tseng TC, Liu CJ, Yang HC, et al. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology 2012;55:68–76. 10.1002/hep.24615 [DOI] [PubMed] [Google Scholar]
- 16. Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut 2014;63:1325–32. 10.1136/gutjnl-2013-305517 [DOI] [PubMed] [Google Scholar]
- 17. Fontaine H, Kahi S, Chazallon C, et al. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial-ANRS HB02 VAC-ADN. Gut 2015;64:139–47. 10.1136/gutjnl-2013-305707 [DOI] [PubMed] [Google Scholar]
- 18. Lok AS, Pan CQ, Han SH, et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol 2016;65:509–16. 10.1016/j.jhep.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 19. Xu DZ, Wang XY, Shen XL, et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol 2013;59:450–6. 10.1016/j.jhep.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 20. Kosinska AD, Bauer T, Protzer U. Therapeutic vaccination for chronic hepatitis B. Curr Opin Virol 2017;23:75–81. 10.1016/j.coviro.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 21. Lobaina Y, Michel ML. Chronic hepatitis B: Immunological profile and current therapeutic vaccines in clinical trials. Vaccine 2017;35:2308–14. 10.1016/j.vaccine.2017.03.049 [DOI] [PubMed] [Google Scholar]
- 22. Michel ML, Bourgine M, Fontaine H, et al. Therapeutic vaccines in treating chronic hepatitis B: the end of the beginning or the beginning of the end? Med Microbiol Immunol 2015;204:121–9. 10.1007/s00430-014-0381-y [DOI] [PubMed] [Google Scholar]
- 23. Dembek C, Protzer U, Roggendorf M. Overcoming immune tolerance in chronic hepatitis B by therapeutic vaccination. Curr Opin Virol 2018;30:58–67. 10.1016/j.coviro.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 24. Zhang TY, Yuan Q, Zhao JH, et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut 2016;65:658–71. 10.1136/gutjnl-2014-308964 [DOI] [PubMed] [Google Scholar]
- 25. Kang XZ, Guo XR, Chen BB, et al. The unique antibody suppresses HBV viremia and reduces hepatocarcinogenesis in HBV-transgenic mice. Hum Vaccin Immunother 2018;14:1779–81. 10.1080/21645515.2018.1449553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sureau C. A unique monoclonal antibody for therapeutic use against chronic hepatitis B: not all antibodies are created equal. Gut 2016;65:546–7. 10.1136/gutjnl-2015-310978 [DOI] [PubMed] [Google Scholar]
- 27. Wu MH, Ma WL, Hsu CL, et al. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med 2010;2:ra35 10.1126/scitranslmed.3001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang LR, Wu HL, Chen PJ, et al. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci U S A 2006;103:17862–7. 10.1073/pnas.0608578103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan L, Wang T, Zhang Y, et al. An HBV-tolerant immunocompetent model that effectively simulates chronic hepatitis B virus infection in mice. Exp Anim 2016;65:373–82. 10.1538/expanim.16-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drexler JF, Geipel A, König A, et al. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc Natl Acad Sci U S A 2013;110:16151–6. 10.1073/pnas.1308049110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Depla E, Van der Aa A, Livingston BD, et al. Rational design of a multiepitope vaccine encoding T-lymphocyte epitopes for treatment of chronic hepatitis B virus infections. J Virol 2008;82:435–50. 10.1128/JVI.01505-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Missale G, Redeker A, Person J, et al. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J Exp Med 1993;177:751–62. 10.1084/jem.177.3.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrari C, Bertoletti A, Penna A, et al. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J Clin Invest 1991;88:214–22. 10.1172/JCI115280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Milich DR, Hughes JL, McLachlan A, et al. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc Natl Acad Sci U S A 1988;85:1610–4. 10.1073/pnas.85.5.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu X, Jin L, Jih J, et al. 3.5Å cryoEM structure of hepatitis B virus core assembled from full-length core protein. PLoS One 2013;8:e69729 10.1371/journal.pone.0069729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol 2016;64:S71–S83. 10.1016/j.jhep.2016.01.026 [DOI] [PubMed] [Google Scholar]
- 37. Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215–25. 10.1128/JVI.02844-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bazinet M, Pântea V, Cebotarescu V, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877–89. 10.1016/S2468-1253(17)30288-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2018-317725supp003.pdf (1.5MB, pdf)
gutjnl-2018-317725supp001.pdf (398.4KB, pdf)
gutjnl-2018-317725supp002.pdf (1.5MB, pdf)