Abstract
These consensus guidelines were jointly commissioned by the British Society of Gastroenterology (BSG), the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and Public Health England (PHE). They provide an evidence-based framework for the use of surveillance colonoscopy and non-colonoscopic colorectal imaging in people aged 18 years and over. They are the first guidelines that take into account the introduction of national bowel cancer screening. For the first time, they also incorporate surveillance of patients following resection of either adenomatous or serrated polyps and also post-colorectal cancer resection. They are primarily aimed at healthcare professionals, and aim to address:
Which patients should commence surveillance post-polypectomy and post-cancer resection?
What is the appropriate surveillance interval?
When can surveillance be stopped?
two or more premalignant polyps including at least one advanced colorectal polyp (defined as a serrated polyp of at least 10 mm in size or containing any grade of dysplasia, or an adenoma of at least 10 mm in size or containing high-grade dysplasia); or
five or more premalignant polyps
The Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument provided a methodological framework for the guidelines. The BSG’s guideline development process was used, which is National Institute for Health and Care Excellence (NICE) compliant.
two or more premalignant polyps including at least one advanced colorectal polyp (defined as a serrated polyp of at least 10 mm in size or containing any grade of dysplasia, or an adenoma of at least 10 mm in size or containing high-grade dysplasia); or
five or more premalignant polyps
The key recommendations are that the high-risk criteria for future colorectal cancer (CRC) following polypectomy comprise either:
two or more premalignant polyps including at least one advanced colorectal polyp (defined as a serrated polyp of at least 10 mm in size or containing any grade of dysplasia, or an adenoma of at least 10 mm in size or containing high-grade dysplasia); or
five or more premalignant polyps
This cohort should undergo a one-off surveillance colonoscopy at 3 years. Post-CRC resection patients should undergo a 1 year clearance colonoscopy, then a surveillance colonoscopy after 3 more years.
Keywords: colorectal cancer, surveillance, colonoscopy, colonic polyps, colorectal adenomas
Introduction
Colorectal cancer (CRC) is a major cause of morbidity and mortality in the UK: more than 40 000 people are diagnosed and more than 16 000 people die from the disease each year.1 The vast majority of CRCs arise from premalignant polyps, a process that takes many years.2 Endoscopic polypectomy is effective in reducing CRC incidence and mortality.3
Some patients who have premalignant polyps (adenomas or serrated polyps) detected at colonoscopy are more likely to develop metachronous polyps or CRC.4–6 Endoscopic follow-up of patients with such polyps is referred to as a post-polypectomy surveillance colonoscopy. Likewise, people who have had a CRC resection may develop a metachronous CRC and are offered post-CRC resection colonoscopic surveillance. Surveillance aims to detect and resect metachronous premalignant polyps and to detect lesions not identified on the initial examination, thereby preventing cancer and reducing CRC mortality; however, no randomised trial has directly assessed the benefit of post-polypectomy or post-cancer resection surveillance.
Premalignant polyps are common, occurring in a quarter to a half of all people of screening age,7–10 yet only about 5% of the population will develop CRC during their life; thus, only a minority of people with polyps will develop CRC, meaning that most people will not benefit from post-polypectomy surveillance. Indeed, it is an increasingly held view that the greatest benefit in terms of CRC prevention is derived from the initial polypectomy, rather than from subsequent surveillance. It is possible to stratify individuals according to future CRC risk and identify cohorts of patients with persistently elevated CRC risk beyond index polypectomy,11 12 yet even with current risk stratification, surveillance places a considerable burden on patients and endoscopy services; approximately 15% of the half a million colonoscopies performed each year in the UK are performed for polyp surveillance.13
Aims and objectives
These guidelines were jointly commissioned by the British Society of Gastroenterology (BSG), the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the English Bowel Cancer Screening Programme (BCSP) (Public Health England; PHE) and supported by NHS England (NHSE).
These guidelines consider the use of surveillance colonoscopy and non-colonoscopic colorectal imaging in people aged 18 and over and are an update of current BSG/ACPGBI post-polypectomy and post-CRC resection colorectal surveillance guidelines (first published in 2002, last revised in 2010 (containing evidence up to 2006))14 15; they are the first guidelines that take into account the introduction of national bowel cancer screening. For the first time, they also incorporate surveillance of patients following resection of either adenomatous or serrated polyps, and serve as an update on the surveillance recommendations in the BSG 2017 position statement on serrated polyps in the colon and rectum.6 They are primarily aimed at healthcare professionals contributing to the management of such patients.
The high-level aims of the guidelines are to address:
Which patients should commence surveillance post-polypectomy and post-cancer resection?
What is the appropriate surveillance interval?
When can surveillance be stopped?
These guidelines do not address surveillance in patients affected by hereditary colorectal syndromes, guidelines for which have also been updated recently16; however, care has been taken to ensure consistency, avoid overlap and ensure that all patient cohorts are comprehensively covered by one of these guidelines.
Methods
The Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument provided a methodological framework for the development of the guidelines.17 The BSG’s guideline development process was used, which is National Institute for Health and Care Excellence (NICE) compliant.18
Guideline development group
A guideline development group (GDG)—including epidemiologists, gastroenterologists, endoscopists, colorectal surgeons, gastrointestinal (GI) pathologists, GI radiologists, patient representatives, charity representatives, representatives from the English BCSP, a health economist and a methodologist—was selected in accordance with BSG/NICE criteria to ensure wide ranging expertise across all relevant disciplines. The surgical and histopathological representatives were nominated by the ACPGBI and the Royal College of Pathologists, respectively. All members completed a conflicts of interest form at the outset; no significant conflicts were identified.
The guideline development process included meetings, telephone conferences, online discussions and voting among members of the GDG between September 2017 and June 2019.
Key guidelines questions
The initial step of the GDG process was to compile a long list of potential questions to be covered by the guidelines. These were subsequently discussed and revised in an iterative process, until the final list was produced:
What are the aims and principles of post-polypectomy and post-cancer resection surveillance?
-
Who should be commenced on post-polypectomy surveillance?
-
Which polyp factors confer higher future risk of CRC?
Multiplicity
Size
Morphology
Histological subtype (degree of villous component in adenomas)
Dysplasia grade
Colonic location
-
Which patient factors confer higher future risk of CRC?
Age
Sex
Body mass index (BMI)
Smoking
Family history of CRC
-
Which colonoscopic factors confer higher future risk of CRC?
Completion to caecum
Bowel prep quality
Endoscopist quality
Enhanced detection technologies
How should such factors be used to stratify risk and produce a composite surveillance strategy?
Can a risk threshold be set to determine who requires surveillance?
-
At what interval(s) should surveillance be performed?
-
Ongoing surveillance
Can the findings at index and first surveillance (S1) colonoscopies be used to determine who needs a second surveillance (S2)?
-
When (and in whom) can surveillance be stopped?
Relating to patient age/comorbidity
Relating to colonoscopy findings
-
Special situations
Are special considerations required for patients who are below the national bowel cancer screening lower age limit?
How does the quality of index colonoscopy affect surveillance recommendations?
-
Other surveillance cohorts
How should surveillance be performed following surgical resection of CRC?
How should surveillance be performed following endoscopic resection of CRC?
How can serrated polyp follow-up be incorporated into these guidelines?
How should these guidelines integrate with the BSG/ACPGBI Large Non-Pedunculated Colorectal Polyp (LNPCP) guidelines19 ?
How should these guidelines integrate with the BSG/ACPGBI Hereditary CRC guidelines?16
-
Other surveillance modalities
Can CT colonography (CTC) be used for surveillance?
Can faecal immunochemical testing (FIT) be used for surveillance?
Can colon capsule be used for surveillance?
How does the risk of cumulative radiation dose balance with CRC risk in CTC surveillance?
-
Other questions
What are the risks associated with surveillance colonoscopy?
What information should the patient receive and how should patients be involved in the surveillance process?
How should polyp size be measured?
How does optical diagnosis of polyp type align with the guidelines?
How should the guidelines be implemented?
What is the anticipated change in workload from these surveillance guidelines?
What are the key unanswered research questions?
128 clinical questions were created from the long-list of guidelines questions (online supplementary appendix 1).
gutjnl-2019-319858supp001.xlsx (20KB, xlsx)
PICOs
The key guidelines questions were, where appropriate, framed as PICO statements (Patients, Interventions, Controls and Outcomes) to structure subsequent literature searches. One hundred and seven PICOs were developed (online supplementary appendix 1).
Evidence synthesis
An expert evidence synthesis team (School of Health and Related Research (ScHARR), Sheffield University) was commissioned to provide systematic reviews, narrative summaries and evidence statements for the central questions (2 to 4 above; online supplementary appendix 2).
gutjnl-2019-319858supp002.pdf (1.4MB, pdf)
The systematic review was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A systematic literature search was performed to identify all published evidence relevant to the review questions. The search was undertaken in accordance with the parameters stipulated within the NICE guidelines manual. Databases were searched using relevant medical subject headings, free-text terms and study design filters (such as randomised controlled trial (RCT), systematic review and observational study), where appropriate. In addition to assessing the evidence from electronic database-searching, evidence reported in existing guidelines, which met the inclusion criteria, were checked for inclusion in the review.14 15 20 Returned abstracts and articles were reviewed for relevance with additional references obtained from cross-referencing of references and recommendations from the GDG. The consideration of articles published only in abstract form was only undertaken in exceptional circumstances (ie, where the article was of particular relevance in an area where evidence was scarce).
After identifying eligible studies for inclusion, methodological quality of the studies was assessed using the Quality In Prognosis Studies (QUIPS) tool for studies of prognostic factors, and a Cochrane risk of bias tool for non-randomised studies (ROBINS) of interventions, where applicable. Data were extracted into a piloted data extraction form by one reviewer and checked by a second reviewer. Information on study characteristics and methods, participant characteristics, interventions and comparators evaluated, and clinical outcomes was extracted.
A narrative summary of included studies was undertaken, including tabulation of relevant study information and a Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment of the evidence, from which a draft evidence statement was written.
Subgroups of the GDG performed similar systematic reviews, narrative summaries and evidence statements for the other questions. Additional relevant publications were considered at the discretion of the GDG, up until June 2019.
Details of the GDG search strategies are provided in online supplementary appendix 3.
gutjnl-2019-319858supp003.pdf (343.1KB, pdf)
Delphi consensus
The evidence statement, narrative summary and supporting references for each guideline question were uploaded onto a bespoke guidelines web platform (ECD Solutions, USA), which was used to facilitate the guideline development process.
Each evidence statement and narrative summary was reviewed and voted on in anonymised fashion by each member of the GDG using a 5-point scale (strongly agree, agree, neutral, disagree, strongly disagree) in a primary voting round. GDG members were encouraged to add comments and cite other relevant references. Consensus was considered reached when either ≥80% participants agreed or, in the final round, where≥50% agreed and <20% disagreed.
Two subsequent face-to-face meetings were held to discuss the results and, where appropriate, revise, merge or refine the statements. These statements were then re-voted on, leading to further statement modifications. In total, three rounds of voting were held for statements relating to the evidence.
Email discussions and two subsequent teleconferences were held to construct a draft surveillance algorithm and guidelines recommendations. These were subsequently voted on over three further consensus voting rounds, including at a final face-to-face meeting in June 2019.
GRADE
The GRADE tool was used to evaluate the guideline. Where an evidence GRADE was inappropriate, “Good Practice Recommendations” were made at the discretion of the GDG.21 This categorises both the strength of evidence and the strength of a recommendation following consensus by an expert panel. While the strength of recommendation may often reflect the evidence base, the GRADE system allows for occasions where this is not the case, for example where there appears good sense to make a recommendation in spite of an absence of high-quality scientific evidence such as a large RCT.
Definitions
The following definitions are used in these guidelines.
Serrated polyp—The umbrella term used to describe hyperplastic polyps, sessile serrated lesions (SSLs), SSLs with dysplasia (SSLd), traditional serrated adenomas (TSA) and mixed polyps.6
Premalignant polyp—The term includes both serrated polyps (excluding diminutive (1–5 mm) rectal hyperplastic polyps) and adenomatous polyps. It does not include other polyps such as post-inflammatory polyps.
Advanced serrated polyp—A serrated polyp of at least 10 mm in size or containing any grade of dysplasia.
Advanced adenomatous polyp (synonymous with advanced adenoma)—An adenoma of at least 10 mm in size or containing high-grade dysplasia. Note: international definitions also include tubulovillous or villous histology, but these are not part of the UK definition.
Advanced colorectal polyp—The term includes both advanced serrated polyps and advanced adenomatous polyps.
Advanced neoplasia—This term has been used historically to describe the combination of advanced adenomas and colorectal cancers. The GDG feels that the term is outmoded, first because it does not include lesions from the serrated pathway, and second because it combines two entities that have very different clinical significance. However, as advanced neoplasia has been used extensively in the past as an outcome measure, the term was used as an search term in the evidence synthesis.
Results
Surveillance principles
Some but not all colorectal polyps have malignant potential.
Some but not all patients with previous polyps are at increased risk of recurrent polyps and thus CRC.
The primary aim of post-polypectomy and post-CRC resection surveillance is to reduce CRC incidence in patients found to have prior colonic neoplasia, once neoplasia clearance has been achieved. This is achieved through the subsequent identification and resection of de novo and missed polyps, thereby preventing these lesions from progressing to CRC.
The secondary aim of post-polypectomy and post-CRC resection surveillance is to reduce CRC mortality. This is achieved both through the subsequent identification and resection of de novo and missed polyps, thereby preventing these lesions from progressing to CRC (ie, by reducing CRC incidence) and through the identification of CRC at an earlier stage when prognosis is better.
Surveillance should only be offered to individuals who remain at higher risk of developing CRC, beyond the reduction seen by index polyp clearance, as compared with the general population.
Surveillance should be undertaken at the minimum frequency required to deliver these aims.
Surveillance should not be continued unless there is evidence that ongoing surveillance is required to deliver these aims.
The need for post-polypectomy surveillance is best determined by comparing the long-term CRC risk of a defined cohort of post-polypectomy patients not undergoing surveillance with that of an age- and sex-matched general population comparator group.
The effectiveness of post-polypectomy surveillance is best determined by comparing the long-term CRC risk of a defined cohort of post-polypectomy patients undergoing surveillance with that of an age- and sex-matched general population comparator group.
Where long-term CRC data are not available, the findings at surveillance may be used as a surrogate means to determine the need for post-polypectomy surveillance, although this method is inferior.
Surveillance risk stratification is predicated on an assumption that the initial colonoscopy is of an acceptable minimum quality, defined as complete colonoscopy to the caecum with at least adequate bowel preparation, and with clearance of all identified premalignant polyps.
The findings at surveillance comprise both de novo pathology and pathology missed or incompletely excised at the prior colonoscopy.
Higher quality colonoscopy reduces the proportion of pathology that is missed or incompletely excised, hence reduces that patient’s future CRC risk.
The impact of surveillance in terms of CRC risk reduction should be balanced with the risks of harm (for example, colonoscopy complications or psychological distress) and the costs to both the health service and patients.
Patients should be offered surveillance based on the best available evidence. The benefits and risks of surveillance should be explained to patients, who should be involved in shared decision-making. The risks and benefits of non-adherence to surveillance should also be explained.
The GDG reached the above important principles and prerequisites underpinning colonoscopic surveillance by consensus. While reducing CRC mortality is an important aim of surveillance, the main aim of surveillance is to prevent CRC by resecting premalignant polyps.
Clearing the colon of premalignant polyps is a powerful tool in the prevention of CRC and probably more important than subsequent surveillance; as evidenced in these guidelines, many patients benefit from this alone and do not require surveillance. Reducing unnecessary surveillance colonoscopies benefits those patients by reducing their exposure to the inconvenience and risk of the procedure. Moreover, in a resource-constrained healthcare system, it frees up resource for others who would benefit more from undergoing colonoscopy. However, while this health economic aspect was a consideration, we did not consider it the primary one for these guidelines, which was to develop guidelines for those people who demonstrably benefit from surveillance. There is an important distinction to be made between performing a colonoscopy on a symptomatic patient, where the potential benefit is immediate, and performing a surveillance colonoscopy, where there is seldom any immediate benefit (the patient is asymptomatic and highly unlikely to have malignancy at the time of surveillance); rather the potential for benefit (future cancer prevention by removing asymptomatic premalignant polyps) is only realised many years (over a decade on average) into the future due to the slow polyp-cancer progression timeline.
While much of the literature on post-polypectomy surveillance analyses surrogate endpoints of advanced pathology found by surveillance, the GDG acknowledged that, where available, evidence relating to long-term CRC incidence (or mortality) should be afforded greater importance. The GDG considered setting a minimum threshold for advanced colorectal polyp yield at surveillance to indicate that such surveillance procedures were indeed worthwhile, with discussion suggesting that a yield of approximately 10% for advanced colorectal polyps might be sufficient to justify surveillance. However, although the GDG agreed that setting a threshold would be helpful, consensus was not reached.
The GDG considered it vital to stress the importance of a high-quality index colonoscopic procedure: improving the quality of mucosal visualisation at colonoscopy above the acceptable minimum results in increased detection of adenomas and sessile serrated lesions and a reduction in missed pathology. Where the bowel preparation is poor, or the colonoscopy incomplete, the clinician should aim for early re-examination, rather than relying on future surveillance to detect missed lesions. Careful polypectomy using optimal technique to ensure complete and safe excision is also an important aspect of a high-quality index colonoscopic procedure.22 The GDG considered that a shift in ethos to a higher quality index procedure with more selective and less frequent surveillance was desirable. There is clear evidence that patients of higher adenoma-detecting endoscopists have lower post-colonoscopy CRC incidence and mortality rates.23 24 Low-detecting endoscopists expose their patients to “double unprotection”: not only are lesions left in situ, but also the patient’s need for surveillance may be underestimated.25 26 High detection rates can only be achieved by a slow, meticulous inspection technique. Enhancement or modifications to colonoscopy technique (eg, chromoendoscopy) or technology (eg, artificial intelligence, or endoscopic caps and cuffs) may further reduce pathology miss-rates, although the clinical significance of the additional lesions detected by these enhanced techniques is uncertain and requires further research on the longer-term impact on post-colonoscopy CRC.
Patient views should be considered when determining the most appropriate surveillance strategy. The National Health Service (NHS) Constitution states that, where appropriate, patients should be involved in all decisions about their care and treatment. It has been shown that patients are more likely to attend for a procedure if they understand why it is being performed and what it will entail. It has been reported that endoscopists underestimate the value of clear communication and shared decision-making. The National Quality Board indicates strong links between being involved in the decision-making process and improved safety and better clinical outcomes. However, there is a paucity of evidence regarding patient views on, and experiences of, surveillance. This is consistent with the lack of research regarding patient views and experiences of endoscopy, and is an area that requires significant research.27 28
Patients should have the evidence for surveillance explained to them and risks and benefits of different strategies explained. The principles of shared decision-making and informed choice should be applied. Patients should also be made aware of any alternative strategy available, and a discussion should take place regarding risks and benefits. Patient needs and expectations should be kept in mind and addressed where possible.
Patients should be made aware of other evidence-based interventions that could reduce their risk of CRC and/or polyp recurrence. These could include lifestyle and behavioural modifications (eg, stopping smoking and reducing red meat consumption) as well as medications (eg, aspirin).
Information should be conveyed in a manner and language that is understandable, allowing patients to make informed choices. Information should be provided in clear written form and with clear verbal explanation and opportunity for reflection and discussion. Patients should be made aware of whom they can contact in the event of any subsequent questions about surveillance.
Surveillance guidelines recommendations
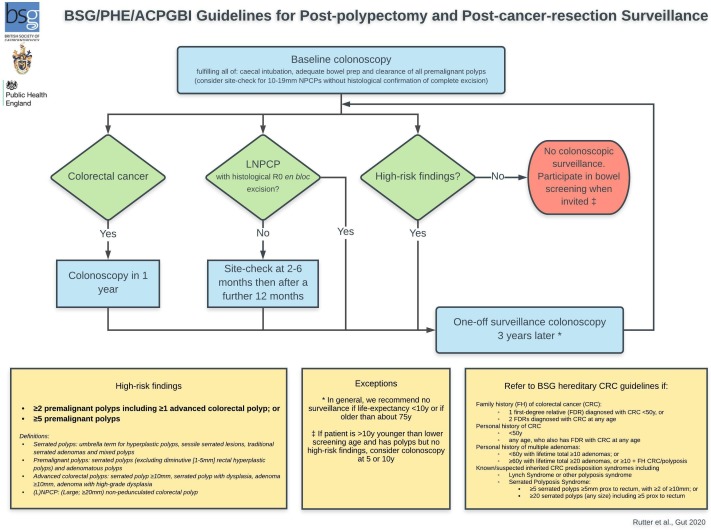
The following recommendations have been developed by the GDG, based on the predetermined surveillance principles, the underlying evidence and following detailed discussion and consensus voting. These recommendation are summarised in figure 1.
Figure 1.
British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England (BSG/ACPGBI/PHE) post-polypectomy and post-colorectal cancer resection surveillance guideline algorithm. LNPCP, large non-pedunculated colorectal polyp; NPCPs, non-pedunculated colorectal polyps; y, years.
We recommend that the high-risk criteria for future CRC comprise either:
two or more premalignant polyps including at least one advanced colorectal polyp (defined as a serrated polyp of at least 10 mm in size or containing any grade of dysplasia, or an adenoma of at least 10 mm in size or containing high-grade dysplasia); o r
five or more premalignant polyps.
GRADE of evidence: See later evidence sectionStrength of recommendation: Strong
The guidelines incorporate surveillance of patients following resection of either adenomatous or serrated polyps, aiming to simplify risk stratification of patients who may have both types of polyp. Surveillance following resection of CRC and LNPCPs have also been incorporated into the same algorithm in order to standardise surveillance across these broad cohorts of patients.
The high-risk criteria are based on the GRADE evidence synthesis detailed later in this manuscript, and follow detailed discussion among the GDG. The evidence suggests that while patient factors influence the likelihood of developing premalignant polyps in the first place, prediction of polyp recurrence is better determined by the index polyp findings, presumably because these crystallise the overall patient risk, and by the quality of the index colonoscopy. One of the key new criteria is that patients (except for the post-CRC/LNPCP cohorts) need to have had at least two premalignant polyps to qualify for surveillance.
Although there is evidence to suggest that index colonoscopy findings of adenoma with tubulovillous/villous histology is associated with an increased risk of advanced adenomas (AA), advanced neoplasia (AN) and CRC at first surveillance, tubulovillous/villous histology has not been included in the algorithm. Tubulovillous/villous histology has never been included in previous UK post-polypectomy guidelines, due to the well documented lack of inter-observer agreement among histopathologists in the assessment of villous architecture.29 30 The GDG felt the inclusion of tubulovillous/villous histology in the guidelines was not justified, given the additional surveillance workload that would be generated; this view is supported by the recent large study by Atkin et al 31 32 of individuals undergoing surveillance for intermediate grade adenomas detected in the symptomatic service, where tubulovillous/villous histology was not a risk factor for long-term CRC risk.
We suggest that where histological completeness of excision cannot be determined in patients with non-pedunculated polyps of 10–19 mm in size, or an adenoma containing high-grade dysplasia, or a serrated polyp containing any dysplasia, then a site-check should be considered within 2–6 months. The need for subsequent surveillance should then be determined based on the high-risk surveillance criteria.
GRADE of evidence: LowStrength of recommendation: Weak
Careful polypectomy using optimal technique to ensure complete and safe excision is an important aspect of a high-quality index colonoscopic procedure. The role of site checks for lesions <20 mm in size is less robust than for larger lesions; however, there are clear data that the risk of incomplete excision, even for endoscopists who know they are being observed, is higher than expected (10.1%), and that this risk is higher for large (10–20 mm) than small (5–9 mm) neoplastic polyps (17.3% vs 6.8%; relative risk (RR) 2.1), and that sessile serrated lesions are at higher risk of incomplete excision than adenomatous polyps (RR 3.7).22 Cancers thought to have arisen in sessile serrated lesions are also over-represented in interval cancer series, also suggesting that either missed or incompletely resected sessile serrated lesions may explain some post-colonoscopy CRCs (PCCRCs).33 In a recent study that looked at neoplasia within the same colonic segments after resection of a 10–20 mm sized polyp, the estimated rate of incomplete resection for non-pedunculated polyps was higher (18.3%, 95% CI 14.2 to 22.5) than for pedunculated polyps (3.5%, 95% CI –0.7 to –11.3).34 Thus, the GDG considered that a shift in ethos to more careful polypectomy, supported by selected site checks, may enable less frequent surveillance. It is hoped that ultimately this might also help drive better polypectomy and improved histological reporting.
We recommend that polyp size should be recorded as the largest dimension of neoplastic tissue (adenoma or serrated) as measured at histopathological examination. For piecemeal resection or where there has been fragmentation of tissue during retrieval, endoscopic assessment of size should be used.
GRADE of evidence: ModerateStrength of recommendation: Strong
Current evidence suggests that there is significant inter-observer variation between colonoscopists in estimation of polyp size and that size in situ is often underestimated by visual inspection.35 Various methods of improving size estimation in situ through comparison against endoscopic accessories of standard size have been proposed, though results have been conflicting.36 37 Polyp size estimation at CTC is also known to be variable in comparison to colonoscopic and histopathology size and subject to reader-related and technical factors.38 39 Studies have also demonstrated significant variation between the size in situ, pre-fixation in formalin and post-fixation at histopathology.40 The size of polyp that is considered most significant when assigning surveillance intervals is generally considered to be 10 mm, as most surveillance guidelines use this threshold as a major criterion for assignment of surveillance category41–43; therefore accurate recording of size is essential. Size of polyps resected en bloc as estimated at histopathology is considered to demonstrate the least variation in assignment of colonoscopic surveillance category,40 and each type of measurement (in situ, pre-fixation and post-fixation) will have a proportion of cases that will be assigned to a less or more frequent surveillance category in comparison. The phenomenon of terminal digit preference is also known to occur with both colonoscopic, radiologic as well as histopathologic estimation of polyp size whereby the precise polyp size tends to be rounded off to the nearest digit ending with zero.40 44 Piecemeal resection of polyps, and particularly larger polyps, is associated with difficulties in accurate estimation of polyp size, and is not considered to be relevant to this statement as it generally does not have an impact on assigning surveillance categories (since such polyps are usually at least 10 mm).
In conclusion, polyp size estimation at histopathology provides the least variation (narrower confidence intervals) in assignment of surveillance category, and most consistency for estimating size of polyps resected en bloc at colonoscopy. Therefore, the polyp size used for assigning surveillance intervals should be that of the neoplastic portion measured at histopathologic examination rather than from visual estimation at colonoscopy for polyps resected and retrieved en bloc.
We recommend that people with high-risk findings on index colonoscopy who are under the age of 75 years should have a surveillance colonoscopy performed after an interval of 3 years (note the one exception in the next statement).
GRADE of evidence: LowStrength of recommendation: Strong
We suggest that due to the long timeline from a clearance colonoscopy through the potential development of new polyps to the possible development of a symptomatic cancer, surveillance should only be performed in people whose life-expectancy is greater than 10 years, and in general not in people older than about 75 years.
GRADE of evidence: LowStrength of recommendation: Weak
We recommend that people with no high-risk findings on index colonoscopy should not undergo colonoscopic surveillance, but should be strongly encouraged to participate in their national bowel screening programme when invited (note the one exception in the next statement).
GRADE of evidence: LowStrength of recommendation: Strong
We suggest that people with premalignant polyps but no high-risk findings on index colonoscopy, who are more than 10 years younger than the national bowel screening programme lower age-limit, should be considered for a surveillance colonoscopy performed after an interval of 5 or 10 years, individualised to their age and other risk factors.
GRADE of evidence: LowStrength of recommendation: Weak
As outlined in our surveillance principles, not all patients with previous polyps are at increased risk of future CRC (indeed due to polyp clearance, many are at lower risk than the general population), and therefore not all patients benefit from colonoscopic surveillance. These guidelines are the first to be published since the introduction of general population bowel cancer screening in the UK. As stated in the previous iteration, participation in population screening will be sufficient for many people post-clearance colonoscopy, and is therefore appropriate management for people without high-risk criteria who are of screening age.
The GDG felt that with the tighter, newly redefined surveillance criteria, all qualifying patients should form a single surveillance cohort and should undergo surveillance at an interval of 3 years. The evidence underpinning the GDG’s selection of a 3 year interval is detailed later in these guidelines. The GDG notes that a large RCT (the EPOS trial; ClinicalTrials.gov Identifier NCT02319928) is underway to assess the suitability of a 5 year interval for high-risk cohorts.
While there is no direct evidence from surveillance studies for what age or estimated life-expectancy surveillance can be stopped without increasing the risk of future CRC development, there is ample evidence, cited throughout these guidelines, that following a clearance colonoscopy, the vast majority of patients will not go on to develop a subsequent cancer. Even when this occurs, the timeline from the development of new polyps, through the development of an advanced polyp, to the possible development of a cancer, and then for that cancer to cause symptoms (ie, to be clinically relevant) is at least 10 years for most patients.45–47 Given that the risks associated with undergoing colonoscopy increase with age and comorbidity, we recommend that it is good practice not to perform post-polypectomy surveillance routinely on patients over 75 years, or where comorbidity indicates that life expectancy is likely to be less than 10 years48—doing so will often result in overtreatment (removal of benign polyps that would not affect the patient’s health during their lifetime). Perhaps the most pertinent study is a large, retrospective cohort study of colonoscopic surveillance in the elderly,49 which showed a significantly lower CRC incidence among elderly patients (over 75 years) undergoing surveillance compared with non-elderly patients (HR for CRC 0.06, 95% CI 0.02 to 0.13; p<0.001), and that both age 75 years and older and Charlson score of 2 were independently associated with increased risk of postprocedure hospitalisation (adjusted odds ratio (OR) 1.28, 95% CI 1.07 to 1.53; p=0.006, and 2.54, 95% CI 2.06 to 3.14; p<0.001, respectively).
Life-expectancy tools (for example https://eprognosis.ucsf.edu/calculators.php) may be used to assist decision-making in such patients. Where ongoing surveillance is being considered despite this recommendation, the patient should have the opportunity to discuss the increased risks and reduced benefits of undergoing surveillance with an appropriate clinician.
NICE methods evaluate cost-effectiveness to determine how to allocate our finite NHS resources to maximise health outcomes. Health outcomes are usually measured in terms of quality adjusted life years (QALYs) which encompass both survival and health-related quality of life. Public preferences for NHS spending are to maximise QALY gains and it follows that it is more cost-effective to prevent a death in a younger person. A health economic analysis would be required to determine whether surveillance of persons over 75 is cost-effective. We do know, however, that the prevention of a CRC death in a young person is associated with a higher QALY gain than in an older person due to differences in life expectancies. Therefore, given the same future CRC risk, surveillance will be more cost-effective in a younger person due to the longer life expectancy and associated higher QALY gain.
Despite a declining incidence of CRC for people over the age of 55 years in the USA, the incidence has been increasing in younger people, a phenomenon also seen in Europe.50 51 The reasons for increasing CRC risk in people under 50 years of age (sometimes called early onset CRC) are likely to be multifactorial. As a result of these recent findings, CRC screening guidelines from the American Cancer Society now include a qualified recommendation to begin screening at age 45.52 The prevalence of adenomas in people aged under 50 years ranges between approximately 15–19% (under the age of 40 years) and 24–30% (40–49 years), and rates of colonoscopy are increasing in this age group.50 As described later in these guidelines, published evidence indicates that metachronous colorectal neoplasia risk is actually lower in younger age groups. However, there needs to be a balance between published epidemiological evidence and clinical concern about possible heterogeneous risk factors for CRC in a younger population who have adenomas. For example, some younger patients who have what appear to be sporadic adenomas, may benefit from additional surveillance due to hereditary or other risk factors which account for their young age of presentation.
Thus, the GDG suggests that for low-risk individuals (those with premalignant polyps but falling short of high-risk criteria) who are younger than about 40 years of age, surveillance should be considered on an individualised basis with other risk factors (such as family history) taken into consideration. Ideally, management should be agreed by the local surveillance lead (ie, a local expert) in conjunction with clinical genetics services. For example, if a patient with adenomas or serrated polyps (falling short of the high-risk criteria) is under 30 years of age or has a family history of CRC, a 5 year surveillance colonoscopy may be considered; if this surveillance colonoscopy is normal the patient could be discharged from colonoscopic surveillance.
The GDG acknowledges the role of the national bowel cancer screening programmes in providing ongoing screening of people who do not qualify for colonoscopic surveillance. The age range of national screening currently varies from nation to nation, and it is anticipated that the lower age limits will change over time; the guidelines have been written with this in mind, hence the “10 years younger than screening” cut-off will vary in different nations, and will change over time as the relevant screening programme for that patient alters.
Age-specific surveillance recommendations are shown in table 1.
Table 1.
Post-polypectomy surveillance recommendations by age
| Colonoscopy findings | High-risk criteria | Low-risk (premalignant polyp(s)but no high-risk criteria) | No polyps |
| Within national bowel screening age range or within 10 years of lower age limit | Colonoscopy after 3 years | Participate in national bowel screening when invited | Participate in national bowel screening when invited |
| More than 10 years younger than national bowel screening lower age limit | Colonoscopy after 3 years | Consider colonoscopy after 5 or 10 years, individualised to their age and other risk factors | Participate in national bowel screening when invited |
| At least 75 years old, or if life expectancy <10 years | In general, no colonoscopic surveillance recommended | ||
We recommend that patients who have undergone a potentially curative CRC resection should have a clearance colonoscopy within a year of their diagnosis
GRADE of evidence: LowStrength of recommendation: Strong
We recommend that once a clearance colonoscopy has been performed in the postoperative period in patients who have had a CRC resection, their next surveillance should be performed after an interval of 3 years. The need for further surveillance should then be determined in accordance with the post-polypectomy high-risk criteria.
GRADE of evidence: LowStrength of recommendation: Strong
A recent Cochrane Review and three subsequent RCTs comparing different intensity follow-up regimens (including colonoscopic surveillance, Carcino-Embryonic Antigen (CEA) and/or CTC surveillance) after potentially curative resection of CRC have not shown any significant effect on overall survival.53–56 Two further trials have demonstrated that colonoscopy independently influences outcomes in terms of overall survival, but not with regard to cancer-specific mortality or identification of recurrence.57 58
Large published observational studies59–63 including population-based cancer registry studies59 64 and those that specifically excluded patients with Lynch syndrome65 report an increased risk of metachronous CRC after curative resection. In a cancer registry cohort study from the Netherlands, CRC was diagnosed in 10 283 patients after 39 974 person-years of follow-up.59 The presence of synchronous CRC was the only significant risk factor for developing metachronous CRC (RR 13.9, 95% CI 4.7 to 41.0). However, a recent systematic review and meta-analysis shows that the absolute risk is substantially lower than 1%, ranging between 0.63% and 0.74% in the first 3 years of follow-up, further dropping to <0.5% after 36 months.66
It has been suggested that more than half of the apparent metachronous cancers are due to missed lesions, incompletely resected lesions or inadequate initial examination.65 Only just over 5% of metachronous cancers are thought to be due to newly developed cancers.65 There is some evidence that a single colonoscopy performed following resection may be associated with improved survival, possibly by colorectal ‘clearance’ of synchronous lesions, some of which may have been missed at the time of diagnosis of CRC.67 In a US study of 1002 patients, 5 year survival was higher (76.8%) for patients who had at least one follow-up exam than for patients who did not undergo follow-up (52.2%, p<0.001). However, others have questioned the value of the colonoscopy at 1 year, emphasising the necessity to have a clean colon before surveillance begins.
There is some low-quality evidence of the cost-effectiveness of early colonoscopic surveillance after cancer resection in a single study.68 In this study the number of early (1 year) colonoscopies needed to detect one CRC and to prevent one CRC-related death was 143 and 926, respectively. The incremental cost-effectiveness ratio was $40 313 per life-year gained by performing colonoscopy. However, the rate of metachronous cancers used in the analysis was generated from a pooled analysis of historical data.61
Regarding the intensity of colonoscopic follow-up, Wang et al 69 showed that there was no survival difference between routine (colonoscopy at 6, 30 and 60 months postoperatively) and intensive (colonoscopy at 3 month intervals for 1 year, at 6 month intervals for the next 2 years, and once a year subsequently) colonoscopic surveillance regimens.
The risk of metachronous cancer development is highest in the first 3 years after diagnosis of CRC.66 70 This increased risk is particularly common in older studies or in those where perioperative colonoscopy was not systematically performed.70 The risk of metachronous CRC compared with the general population appears to be of the same magnitude as that following resection of high-risk precancerous lesions.70 Hence it seems logical, once a high quality postoperative clearance colonoscopy has been performed, that further follow-up should be determined in accordance with the post-polypectomy surveillance guidelines schedule.
We recommend that as recurrence rates after pathologically en bloc R0 endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) of LNPCPs or early polyp cancers are low, no site checks are required, and the patient should undergo post-polypectomy surveillance after an interval of 3 years. The need for further surveillance should then be determined in accordance with the post-polypectomy high-risk criteria.
GRADE of evidence: LowStrength of recommendation: Strong
We recommend a site check is performed 2–6 months after piecemeal EMR or ESD of LNPCPs (at least 20 mm in size), in line with BSG/ACPGBI LNPCP guidelines. A further site check at 18 months from the original resection is recommended to detect late recurrence. Once no recurrence is confirmed patients should undergo post-polypectomy surveillance after an interval of 3 years. The need for further surveillance should then be determined in accordance with the post-polypectomy high-risk criteria
GRADE of evidence: LowStrength of recommendation: Strong
The 2015 BSG and ACPGBI guidelines on LNPCPs recommend a polypectomy site check at 2–6 months after piecemeal EMR19 for non-polypoid lesion ≥20 mm in size. The 2017 European Society of Gastrointestinal Endoscopy (ESGE) guidelines on polypectomy and EMR emphasised the role of en bloc resection either by EMR or ESD for lesions with a risk of submucosal invasion and noted high rates of recurrence in larger lesions ≥40 mm in size.71 Early recurrence was seen by 3 months in 76% of cases, and 96% of cases by 6 months where studies differentiated between 3 and 6 month site checks, suggesting 6 months may be the optimal time for a site check where the polyp is thought to be completely resected. Late recurrence is a recognised feature of piecemeal EMR, where recurrence can occur even if the initial site check and biopsies did not show residual polyp, with recurrence detected beyond 6–12 months in 5–9% of cases.72 73 Therefore, it may be prudent to reassess piecemeal EMR sites again at 12–18 months before returning to standard surveillance intervals. Some groups have attempted to use polyp and resection characteristics to stratify (Sydney Endoscopic Recurrence Tool—SERT), where low-risk lesions have a 12% recurrence rate versus 36% for higher risk lesions at 18 months.73 Most recurrence (>90%) can be managed endoscopically.
Rates of recurrence after en bloc resection either by EMR or ESD appear very much lower than after piecemeal EMR. Meta-analysis suggests that the overall rate of recurrence after piecemeal EMR was 22% (95% CI 15% to 31%), but only 3% (95% CI 1% to 6%) after en bloc EMR.72 In a Japanese multicentre cohort, overall recurrence rates were 2.3% for en bloc EMR versus 11.9% for piecemeal EMR. Similarly, recurrence at ESD was 19 times more common if a piecemeal resection was performed.74 This was confirmed in a meta-analysis of Asian and Western ESD studies, where recurrence after en bloc R0 resection (microscopically margin-negative resection) was 0.05% for 16 Asian studies and 0% for four western studies.75 Pathological R0 resection after endoscopic excision has been defined as at least 50 μm clearance between dysplasia and the cut edge of the lesion.76 Therefore, when a lesion is resected en bloc and confirmed as both endoscopically and pathologically R0, the risk of recurrence is sufficiently low that surveillance can simply be carried out at the standard surveillance interval dictated by size and number of polyps, without the additional site checks at 2–6 and 12–18 months required for piecemeal resection, assuming the colon was comprehensively cleared of other lesions.
In conclusion, LNPCPs that are resected en bloc and pathologically R0 have exceptionally low recurrence rates, and can safely return to standard surveillance. Piecemeal resection requires more intensive polypectomy site surveillance.
We recommend that the need for ongoing colonoscopic surveillance should be determined by the colonoscopic findings at each surveillance procedure, using the same high-risk criteria to stratify risk.
GRADE of evidence: LowStrength of recommendation: Strong
We recommend that people with high-risk findings on a surveillance colonoscopy should undergo a further surveillance colonoscopy at an interval of 3 years (with the same age-related caveats applied again).
GRADE of evidence: LowStrength of recommendation: Strong
We recommend that people with no high-risk findings on a surveillance colonoscopy should cease colonoscopic surveillance, but should participate in the national bowel screening programme when invited (with the same age-related caveats applied again).
GRADE of evidence: LowStrength of recommendation: Strong
The evidence for ongoing surveillance beyond the first surveillance colonoscopy is sparse and is summarised later in these guidelines. The GDG therefore felt that it was most appropriate to apply the same high-risk criteria to findings on surveillance. It is anticipated that this means that most people will undergo only one surveillance colonoscopy; this aligns with our guidelines’ surveillance principles of not continuing surveillance unless there is evidence that this is beneficial. An additional benefit is that this should make the guidelines easier to follow, as only one set of criteria is required and because clinicians need only consider the findings of the most recent surveillance episode.
The GDG considered extending second and subsequent surveillance intervals to 5 years, given the very low probability of missed pathology after both the index and first surveillance colonoscopy combined, and the slow polyp-cancer progression timeline. However, this option did not reach consensus. The EPOS trial should add further evidence for this question.
We recommend that surveillance colonoscopies should only be performed by colonoscopists who are either screening accredited, or whose colonoscopy performance measures (key performance indicators—KPIs) exceed the minimum standard as defined in the BSG lower GI quality standards publication
GRADE of evidence: LowStrength of recommendation: Strong
Evidence from many studies outlined in these guidelines, investigating the impact of colonoscopy quality, accuracy of polyp detection and completeness of polyp resection, suggests that in order to achieve optimal effectiveness from surveillance, operators need to meet the relevant KPIs. This requires endoscopy services to emphasise and monitor achievement of minimum KPIs for all colonoscopists. While these guidelines relate to surveillance colonoscopy, it is important, as stated in our surveillance principles, that high-quality colonoscopy should apply to all colonoscopic procedures and not just to surveillance procedures. Moreover, the minimum standards are just that—the minimally acceptable quality—and endoscopists and endoscopy units should strive to achieve the higher target standards.77
We recommend that when colonic surveillance is required after previous polypectomy, CTC is an acceptable alternative if colonoscopy is incomplete or not possible due to the patient’s clinical condition.
GRADE of evidence: Very lowStrength of recommendation: Strong
There is surprisingly little literature investigating the role of CTC for polyp surveillance specifically. A single high-quality multicentre prospective cohort study recruited high-risk patients, including a subset with previous colonic polyps.78 In the 343 patients with previous polypectomy, CTC was 84.2% sensitive and 85.3% specific for 6 mm+AN, and 90.8% sensitive for 10 mm+AN. Although many other CTC diagnostic test accuracy studies have recruited patients with prior polypectomy, few report the results solely for the post-polypectomy cohort. In one meta-analysis,79 studies were classified as recruiting patients at average (ie, a screening population) or high risk (mainly comprising a mix of patients with symptoms, positive faecal occult blood testing, previous polypectomy). A separate meta-analysis was not performed for the 41 studies recruiting high-risk patients, but overall pooled sensitivity was 67% for 6–9 mm polyps and 87% for 10 mm+polyps, with corresponding specificities of 92% and 96%.
The ultimate goal of colonic surveillance post-polypectomy is to reduce subsequent CRC incidence; we found no studies reporting longer-term clinical outcomes of using CTC for post-polypectomy surveillance. A recent systematic review and meta-analysis reported a rate of post-imaging CRC (PICRC) at a mean of 34 months follow-up of 0.61 PICRCs per 1000 patient-years, or 4.4% when expressed as a percentage of total cancers detected, which is similar to analogous rates for colonoscopy.80 However, again these studies derive from a mixed population of screening, symptomatic and high-risk patient cohorts rather than purely a surveillance population.
We recommend that when colonic surveillance is required after curative-intent resection of CRC, CTC should only be used for individuals in whom colonoscopy is contraindicated or not possible due to the patient’s clinical condition.
GRADE of evidence: ModerateStrength of recommendation: Strong
CTC is intuitively attractive for surveillance following curative-intent CRC resection, since it combines intraluminal assessment for metachronous polyps and cancer with evaluation of the extracolonic structures for locoregional recurrence and remote metastases, thereby simplifying follow-up pathways and potentially reducing costs.
A systematic review and meta-analysis of cohort studies81 showed that CTC was highly sensitive (95%, 18 of 19 cases detected) and 100% specific for anastomotic recurrence, as well as detecting 10 of 10 metachronous cancers. However, this article did not assess the diagnostic sensitivity of CTC for polyps or adenomas (as opposed to carcinoma).
Three single centre prospective cohort studies82–84 showed promising accuracy of CTC for polyps after prior CRC resection, the largest83 (550 patients) reporting a sensitivity of 81.8% for AN and a specificity of 93.1%. However, these studies were of variable quality, with incomplete84 or delayed83 comparison to reference standard tests such as colonoscopy for the presence/absence of polyps.
A recent high-quality multicentre prospective cross-sectional study85 86 recruited 231 patients scheduled for 1 year colonic surveillance following curative-intent resection of CRC and conducted both CTC and same-day colonoscopy with segmental unblinding (ie, sequential revelation of the CTC result to the colonoscopist on a segment-by-segment basis, thereby providing an enhanced reference standard). CTC was only 44.0% sensitive for 6 mm+polyps and 76.9% sensitive for 10 mm+polyps; the authors speculate this poor performance was due to colonic under-distension in patients with previous right hemicolectomy and thus no ileocaecal valve to permit optimal colonic distension. Although the negative predictive value was reasonable (85.8% for 6 mm+polyps and 98.5% for 10 mm+polyps), this may have been due to low prevalence rather than test accuracy. If 1000 patients underwent CTC instead of colonoscopic surveillance, although 922 colonoscopies would be avoided, 87 patients would have 6 mm+polyps missed, and only 69 patients with 6 mm+polyps would be identified; 30 patients would have 10 mm+polyps identified, but 13 patients would have 10 mm+polyps missed. Cost-effectiveness analysis of this risk-benefit trade-off has not yet been published.
We recommend that when post-polypectomy surveillance is indicated, the radiation risk of CTC is likely to be outweighed by its potential benefits.
GRADE of evidence: HighStrength of recommendation: Strong
The precise radiation dose from CTC varies between scanners, but in two international surveys from 2008 and 2012 the average effective radiation dose was estimated at 9.1 mSv for symptomatic/diagnostic scans and 5.7 mSv for screening/follow-up scans in 2008,87 and 7.6 mSv (symptomatic) and 4.4 mSv (screening) in 2012.88 This compares to average annual background radiation exposure of 2–3 mSv per annum in the UK. A 2012 single-centre report, using more modern CTC technology, which is now widely used in the UK, estimated doses to be around 2.5–3 mSv.89 Therefore, a single CTC examination is likely to incur approximately between 1 and 3 years’ worth of background radiation.
The risk of cancer induction associated with this level of radiation exposure is uncertain, but in one radiation modelling study90 the lifetime attributable risk (LAR) of cancer induction from a single scan at age 50 years was estimated to be 1 in 1670, and a risk of 1 in 670 for a 5-yearly CTC screening strategy. The authors estimated the benefit:risk ratio (ie, CRCs prevented:other cancers induced by radiation) for such a screening strategy to be approximately 24–35:1 depending on the colorectal carcinogenesis model used. However, they did not directly model a surveillance (rather than primary screening) population, and radiation doses were assumed to be higher than outlined above, at 8 mSv per CTC. We found no published data specifically modelling the risks of repeated CTC at a variety of surveillance intervals.
The risk of inducing cancer due to radiation exposure decreases with age. In England, the average age of individuals having CTC is approximately 70 years (data from the Diagnostic Imaging Dataset - https://did.hscic.gov.uk/; comparable data from other devolved nations are not available). The risk for a single 5 mSv scan at age 70 years is approximately 1 in 5000 (estimated via the National Cancer Institute Radiation Risk Assessment Tool,91 available at https://irep.nci.nih.gov/radrat). For repeated surveillance examinations, much will depend on the frequency such CTC surveillance is required; however, assuming 3-yearly repeated 5 mSv examinations starting at age 50 and ending at age 70 years, the additional risk of malignancy is approximately 1 in 525. As the inherent baseline risk of cancer is approximately 1 in 3, this is a very small relative increase, and is likely outweighed by the potential benefits of preventing CRC in patients who have been identified as high-risk (by virtue of their need for colonic surveillance). The risk must also be weighed against the alternative—that is, adverse incidents arising from repeated colonoscopic surveillance. Since radiation risks are greater in younger patients, in whom endoscopic perforation risks are smaller, and vice versa for older patients, the risk:benefit equation will vary according to patient factors including age, intensity of the surveillance programme and comorbidity.
Therefore, the radiation risk of cancer induction from CTC is very small and the risks are significantly outweighed by detection of cancer when colonic surveillance with CTC is indicated.
We do not recommend the use of faecal immunochemical testing (FIT) for surveillance after resection of premalignant colorectal polyps, as there is insufficient evidence.
GRADE of evidence: LowStrength of recommendation: Strong
Several studies report on the long-term performance of FIT-based population screening programmes for CRC, but evidence for surveillance after polypectomy is lacking in these studies. One study reports on participants recruited between January 2012 and December 2013 via the English BCSP.92 Men and women aged 60–72 years, deemed at intermediate-risk following adenoma removal after a positive guaiac faecal occult blood test, were offered quantitative FIT at 1, 2 and 3 years post-polypectomy. Participants testing positive with any FIT were referred for colonoscopy. Participants testing negative were offered colonoscopy at 3 years post-polypectomy (standard English BCSP surveillance). Of 8009 individuals invited, 5938 (74%) consented and returned a Round 1 FIT. In this group, uptake of FIT in Rounds 2 and 3 was 97%. Programme sensitivities of three FITs at 10 µg/g were 72% for CRC and 57% for AA. The use of FIT for surveillance could miss 30–40% of CRCs and 40–70% of AAs.
We do not recommend the use of colon capsule for surveillance after resection of premalignant colorectal polyps, as there is insufficient evidence.
GRADE of evidence: Very lowStrength of recommendation: Strong
A number of studies report on the diagnostic accuracy of colon capsule when compared with optical colonoscopy, but evidence for the use of capsule for surveillance after polypectomy is lacking—apart from one recent study of 180 patients, of whom only 43% had a satisfactorily complete capsule assessment and over half required subsequent lower GI endoscopy due to possible polyp identification.
Surveillance evidence statements
There is some, but inconsistent, evidence that adenomas with high-grade dysplasia at index colonoscopy are associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Moderate
Thirteen studies reported evidence relating to risks at first surveillance associated with the presence of high-grade dysplasia at index colonoscopy. There were fairly consistent positive associations of finding AA at surveillance, with generally low to moderate risk of bias. Five studies31 32 93–97 reported a statistically significantly increased odds of AA at first surveillance if high-grade dysplasia (HGD) was present at index colonoscopy. Atkin et al 31 32 reported an incidence of AA of 19.06% (OR 1.44, 95% CI 1.18 to 1.75), and Huang et al 95 reported an incidence of 27.7% (HR 1.61, 95% CI 1.07 to 2.42). ORs only were reported by Facciorusso et al 96 97 and Fairley et al,94 respectively, as 4.25 (95% CI 2.11 to 7.5) and 4.3 (95% CI 2.2 to 8.4), and Van Enckevort et al 93 reported an HR of 1.73 (95% CI 1.13 to 2.64).
Five studies12 98–101 only reported statistical analyses on AN, with four reporting no significant association between HGD at index and risk of AN at first surveillance, and the fifth study (Cubiella et al 100) reporting an OR of 0.7 (95% CI 0.5 to 0.98), consistent with reduced risk associated with HGD. Again, risk of bias was generally rated as moderate to low. Across these studies, where reported, the incidence ranged from 12.1%100 for AN up to 16% for AA and CRC 1.3%.12
The risk of CRC at surveillance was reported in three studies, all rated as having a low risk of bias,31 32 94 102 and again demonstrated consistent statistically significant associations between HGD at index and CRC incidence, although the number of events was very small. One of these studies reported the incidence of CRC as 3.1% (OR 2.09, 95% CI 1.29 to 3.37).31 32 102 The two other studies reported an OR of 13.2 (95% CI 2.8 to 62.1) for incidence of CRC,94 and an OR of 1.61 (95% CI 1.07 to 2.42) for interval CRC.102
Only one study reported evidence for HGD and long-term CRC incidence,31 32 demonstrating a significant association with HGD at index colonoscopy (OR 1·85, 95% CI 1.34 to 2.55). Although this study was large and was rated as having a low risk of bias, the number of events was very small.
No studies reported evidence for HGD and long-term CRC mortality.
Analysis of English BCSP surveillance data (n=43 131 for AA; n=28 468 for CRC) showed HGD was not significantly associated with subsequent diagnosis of CRC on univariable analysis. Moreover, although HGD was weakly associated with AA at first surveillance, this was not significant on multivariable analysis (OR 1.08, 95% CI 0.994 to 1.172).103–105
There is consistent evidence that adenomas with tubulovillous or villous histology at index colonoscopy are associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Moderate
Sixteen studies reported evidence relating to risks at first surveillance associated with the presence of tubulovillous or villous histology at index colonoscopy. Overall, the evidence suggests that risk for AA at first surveillance was increased if tubulovillous or villous components (rather than tubular) were identified at index, with consistent statistically significant associations reported across four studies31 32 94 95 106 rated as low to moderate risk of bias, and in one study107 rated as having a high risk of bias. Incidence of AA when tubulovillous components were identified at index was 17.5% (OR 1.93, 95% CI 1.59 to 2.34) and 25.1% (OR 3.03, 95% CI 2.33 to 3.95) when villous components were identified.31 32
The incidence of AA on surveillance when tubulovillous and villous histology were reported at index colonoscopy was reported as 21.2% by Nusko et al.107 Incidence for tubulovillous and villous histology combined was reported as 26.1% (HR 2.57, 95% CI 1.24 to 5.32) in Huang et al,95 and Fairley et al 94 reported an OR for villous histology of 3.7 (95% CI 2.9, 4.7). Laiyemo et al 106 presented risk ratios comparing AA with no AA at first surveillance and AA with no adenoma at first surveillance, reporting statistically significant risk ratios of 2.38 (95% CI 1.56 to 3.64) and 2.25 (95% CI 1.49 to 3.39), respectively. A further study108 reported no significant association when the index tubulovillous adenoma was <10 mm (OR 0.63, 5% CI 0.36 to 1.12), but the association was statistically significant when it was ≥10 mm (OR 2.11, 95% CI 1.40 to 3.19). Two further studies96 97 109 did not report statistically significant associations for villous components and incident AA.
When the outcome measure was detection of AN, at surveillance, the findings were similar, with five studies rated as low to moderate risk of bias12 98–101 identified. Four of the five studies reported significant associations, showing increased risk for AN at first surveillance if tubulovillous or villous components (rather than tubular) were identified at index. Incidence of AN when villous components were identified at index was reported as 15.5% (OR 1.4, 95% CI 1.1 to 1.7)100 and 11.9%,99 while Van Heijningen et al 101 reported an OR of 2.0 (95% CI 1.2 to 3.2) with incidence of AA of 13% and CRC of 3.8%. Where findings were reported for tubulovillous and villous components combined, Martinez et al 12 reported an OR of 1.28 (95% CI 1.07 to 1.52) with incidence of AA of 16.8% and CRC of 0.9%. In the final study,98 although the HR indicated an increased risk (HR 1.29, 95% CI 0.92 to 1.81), it was not statistically significant. A number of studies did not include data relating to the number of events.
The risk for CRC at first surveillance was reported in five studies rated as low risk of bias,31 32 94 102 108 110 with all five reporting statistically significant findings, showing that risk for CRC was increased at first surveillance if tubulovillous or villous histology (rather than tubular) was present at index. Atkin et al 31 32 reported an incidence of CRC of 1.83% (OR 1.76, 95% CI 1.00 to 3.09) if tubulovillous components were identified at index and 4.14% (OR 4.09, 95% CI 2.13 to 7.86) if villous components were identified. Fairley et al 94 reported a statistically significant OR of 7.4 (95% CI 2.5 to 21.5) for the risk of CRC if villous components were present at index. The incidence of CRC was reported as 2.9% (HR 1.51, 95% CI 1.02 to 2.23),110 and the odds for interval CRC was 1.38 (95% CI 1.03 to 1.85)102 if tubulovillous or villous components were present at index. Laish et al 108 reported the incidence of CRC at first surveillance if small tubulovillous adenomas (TVA) were present at index as 1.5%, and as 1.3% for large TVA.
One study, rated as having a low risk of bias, presented evidence on long-term CRC incidence31 32 showing, in univariable analyses, a statistically significant increased risk for long-term CRC (vs tubular) if tubulovillous components were present at index (HR 1.36, 95% CI 1.00 to 1.87) and if villous components were present (HR 1.65, 95% CI 1.03 to 2.64). There was no statistically significant increased risk for long-term CRC if tubulovillous or villous histology was present at index in multivariable analysis. One other study on long-term CRC mortality111 112 reported tubulovillous or villous histology was a statistically significant risk factor for CRC (HR 1.30, 95% CI 1.13 to 1.50).
Overall, there was fairly consistent moderate to high quality evidence suggesting that tubulovillous or villous histology at index is associated with an increased risk for AA or AN and CRC at next surveillance. Findings were reported across a number of studies, and, although there is some uncertainly due to the lack of absolute values available in some studies, the evidence was consistent, with a number of large studies rated as having a low risk of bias. Evidence for long-term CRC incidence and mortality was limited, being presented in only one study for each outcome; however, each was a large-scale study rated as either low or moderate risk of bias.
Analysis of English BCSP surveillance data showed villous histology was not statistically significantly associated with subsequent CRC incidence at surveillance. However, it was statistically significant for AA at first surveillance on univariable analysis: OR 1.38 for TVA (95% CI 1.296 to 1.487), OR 1.89 for villous adenoma (VA) (95% CI 1.654 to 2.170); and on multivariable analysis: OR 1 for tubular adenoma only, OR 1.37 for TVA (95% CI 1.277 to 1.472), OR 1.69 for VA (95% CI 1.475 to 1.952).103–105
There is consistent evidence that larger adenomas (of at least 20 mm) at index colonoscopy are associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Moderate
There is some, but inconsistent, evidence that larger adenomas (of at least 10 mm) at index colonoscopy are associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Moderate
Fifteen studies reported evidence relating to polyp size and were rated as having a moderate to low risk of bias. Risk for AA at first surveillance was increased if the size of the adenoma at index was ≥20 mm; this association was statistically significant in three studies,31 32 95–97 in which the incidence of AA at surveillance ranged from 21.4%31 32 to 81.1%.95
One study reporting the outcome AA,94 and three studies reporting the outcome AN12 98 101 at surveillance, found significant associations if the size of the adenoma was between 10 mm and 20 mm at index. One study reported an OR of 3.6 (95% CI 2.8 to 4.5),94 and one study reported a HR of 1.81 (95% CI 1.28 to 2.55).98 No other associations relating to adenomas at smaller sizes were statistically significant for either AA or AN.
Two studies,31 32 94 rated as having a low risk of bias, reported on the outcome of CRC incidence at next surveillance. Risk for CRC at first surveillance was statistically significantly associated with size of the adenoma at index of ≥20 mm in one of these studies.31 32 The other reported an OR of 5.2 (95% CI 1.8 to 15.1) for size of adenoma at index of ≥10 mm.94 One study on the outcome of interval CRC reported no statistically significant association when size of adenoma at index was ≥10 mm102; although the number of patients in this polyp category at index was small, the point estimate suggested an increased risk.
Only one study rated as low risk of bias presented evidence regarding long-term CRC incidence.31 32 Statistically significant associations were reported for adenomas 10–19 mm in size (incidence 2.1%) and for those ≥20 mm in size (incidence 1.7%).
The GDG found no evidence regarding associations between polyp size at index colonoscopy and long-term CRC mortality.
Analysis of English BCSP surveillance data showed polyp size was not statistically significant for CRC detection on univariable analysis; however, it was statistically significant for AA yield at first surveillance on univariable analysis: OR 1 for largest adenoma of 10–14 mm; OR 1.28 for 20–29 mm (95% CI 1.171 to 1.401); OR 1.57 for 30–39 mm (95% CI 1.369 to 1.804); OR 2.10 for ≥40 mm (95% CI 1.817 to 2.431); and was statistically significant for AA at first surveillance on multivariable analysis: OR 1.38 for non-pedunculated adenoma of ≥10 mm compared with none (95% CI 1.288 to 1.483).103–105
There is consistent evidence that multiplicity of adenomas at index colonoscopy is associated with an increased risk of AA and AN at first surveillance. There is some, but inconsistent, evidence for an association with an increased risk of CRC at first surveillance.
GRADE of evidence: Moderate
Twenty-one studies, rated from low to high risk of bias, presented evidence on risk associated with number of adenomas at index. Results were not consistently statistically significant. Having two adenomas (compared with one) at index was statistically significantly associated with increased risk of AA at next surveillance in two studies, in which the incidence of AA was 18.3%31 32 and 6.1%.95 Three studies reported statistically significant increased risks of AA for ≥3 adenomas at index, compared with 1 or 2 adenomas,94 95 113 and one further study reported a significant increased risk for ≥3 vs 1 or 2 non-advanced adenomas at index or if multiple AAs were present at index.108 Facciorusso et al 96 97 reported a significantly increased risk with the presence of more than one AA at index (OR 3.22, 95% CI 2.19 to 5.39) and Jang et al 114 reported a statistically significant increase in the odds of AA at first surveillance with increasing number of adenomas at index. However, Atkin et al 31 32 reported no significant association for three or four adenomas versus one adenoma, and three further studies reported no significant associations for any comparisons.106 109 115
Six studies, rated as having low to moderate risk of bias, reported evidence for the outcome AN. Four studies, ranging in size from 1414 to 9167 patients, showed significantly increased risks for AN with increasing numbers of adenomas at index, although the comparisons made in the studies varied.12 100 101 116 The other two studies did not find any associations between number of adenomas present at index and AN.99 117
Six studies, rated as having low to moderate risk of bias31 32 94 102 108 110 118 reported on the outcome CRC at next surveillance. Two of these found statistically significantly increased risks with increased numbers of adenomas at index94 118; this was seen for AA, but not non-advanced adenomas in one of the studies.118 Laish et al 108 reported increasing incidence of CRC with increasing number of non-advanced adenomas at index (1–2 non-advanced adenomas, 1.1%; ≥3, 2.7%), and higher incidence in those with multiple AAs at index (3.7%). Three studies reported no statistically significant increased risk of CRC incidence31 32 110 and one study found no association with interval CRC,102 with increasing numbers of adenomas. One study, rated as having a low risk of bias,31 32 found no statistically significant association with long-term CRC incidence.
One further large-scale study111 112 reported a statistically significant increased risk of CRC mortality if more than one adenoma was present at index (HR 1.30, 95% CI 1.10 to 1.55).
Analysis of English BCSP surveillance data showed that multiplicity was statistically significant for CRC on multivariable analysis (compared with one adenoma, HR 2.45 for 6–9 adenomas (95% CI 1.523 to 3.952), and HR 3.58 for ≥10 adenomas (95% CI 1.879 to 6.821)). Adenoma multiplicity was also statistically significant for AA at first surveillance on univariable analysis (compared with one adenoma, OR 1.62 for two adenomas (95% CI 1.469 to 1.798); OR 1.61 for three adenomas (95% CI 1.457 to 1.783); OR 2.02 for four adenomas (95% CI 1.806 to 2.262); OR 2.38 for five adenomas (95% CI 2.113 to 2.696); OR 2.75 for 6–9 adenomas (95% CI 2.475 to 3.076); and OR 3.82 for ≥10 adenomas (95% CI 3.278 to 4.473)). It was also statistically significant for AA at first surveillance on multivariable analysis: OR 1.56 for two adenomas (95% CI 1.407 to 1.736); OR 1.58 for three adenomas (95% CI 1.424 to 1.753); OR 1.90 for four adenomas (95% CI 1.692 to 2.136); OR 2.23 for five adenomas (95% CI 1.973 to 2.538); OR 2.47 for 6–9 adenomas (95% CI 2.208 to 2.773); and OR 3.03 for ≥10 adenomas (95% CI 2.578 to 3.570).103–105
There is some, but inconsistent, evidence that proximal adenomas at index colonoscopy are associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Low
Twelve studies, rated as having low to high risk of bias, presented evidence on the risk associated with the presence of proximal adenoma at index. The findings were inconsistent. Seven studies, ranging in size from 47 to 11 944 patients, presented evidence relating to the risk of AA at first surveillance.31 32 95 106 109 113–115 Only one of these106 reported a statistically significant increased risk for proximal adenomas at index versus either non-advanced adenoma or no adenoma (AA vs non-AA at first surveillance: RR 1.58, 95% CI 1.11 to 2.25; AA vs no adenoma at first surveillance: RR 1.84, 95% CI 1.31 to 2.59).
All three studies which presented data on the outcome AN reported statistically significant increased risks associated with proximal adenomas at index colonoscopy.12 99 101
There was no evidence for a statistically significant association with proximal adenomas and CRC incidence at next surveillance in two studies,31 32 118 but one of these31 32 reported a statistically significant association with risk for long-term CRC (HR 1.76, 95% CI 1.30 to 2.38). One further large-scale study111 112 reported no association between proximal adenomas at index and CRC mortality.
Analysis of English BCSP surveillance data showed presence of a proximal adenoma was statistically significant for CRC on univariable analysis (HR 1.53, 95% CI 1.159 to 2.027) but not on multivariable analysis. It was statistically significant for AA at first surveillance on univariable analysis (OR 1.67, 95% CI 1.574 to 1.782), but not significant on multivariable analysis.103–105
There is no consistent evidence that adenomas with sessile or flat morphology at index colonoscopy are associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Low
Four studies,95–97 100 113 three of which were rated as low risk of bias, and one as high risk, presented evidence on the risk associated with adenoma morphology at index. Of three studies which presented evidence on the risks for AA,95–97 113 the largest of which included 1356 patients, only one reported a statistically significant increased risk in patients with sessile (OR 1.96, 95% CI 1.12 to 2.43) or non-polypoid adenomas (OR 2.43, 95% CI 1.14 to 3.26) at index, relative to those with pedunculated adenomas.96 97 The other two studies reported non-significantly increased risk.
One study presented evidence relating to the outcome AN100 and found no statistically significant findings relating to adenoma morphology at index.
No evidence relating to CRC at the next surveillance, long-term CRC incidence, or long-term CRC mortality was identified.
There is some, but inconsistent, evidence that male sex is associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Very low
Twenty studies, with generally a low risk of bias, presented evidence on the association between male sex and outcomes at first surveillance. Eleven studies presented evidence relating to AA31 32 95–97 102 106 109 114 115 119–122; of these, four studies presented evidence showing a statistically significant increased risk of AA at first surveillance if the patient was male.95 102 114 122
Five studies presented evidence on the outcome AN,12 98 100 116 117 with one study12 reporting a statistically significant increased risk of AN at first surveillance in men (OR 1.40, 95% CI 1.19 to 1.69), with detection rates of 11.7% for AA and 0.8% for CRC.
Four studies presented evidence on detection rates of CRC at first surveillance, with only one study110 reporting male sex as a statistically significant risk factor (HR 1.69, 95% CI 1.26 to 2.27).
One study reported no association between male sex and long-term risk of CRC among 11 944 patients deemed to be at intermediate-risk according to the 2002 adenoma surveillance guidelines,31 32 while a further study reported no association between male sex and long-term CRC mortality.111 112
Analysis of English BCSP surveillance data showed that sex was not associated with CRC on univariable or multivariable analysis. However, men had a higher risk of AA at first surveillance in both univariable (OR 1.30, 95% CI 1.213 to 1.399) and multivariable analysis (OR 1.13, 95% CI 1.049 to 1.223).103–105
There is consistent evidence that a family history of CRC (which falls short of warranting family history surveillance in its own right) is not associated with an increased risk of AA, AN or CRC at first surveillance
GRADE of evidence: Moderate
We assessed the published evidence of the diagnostic yield of post-polypectomy surveillance in those with a family history of CRC. This evidence search did not include patients undergoing surveillance because of their family history per se, which has been reviewed for the hereditary CRC guidelines (in press). Seven studies, rated as moderate to low risk of bias,12 98 106 114 117 118 122 reported evidence on the risks associated with a family history of CRC. Two studies reported on the outcome AA,114 122 and three on the outcome AN.12 98 117 There was no statistically significant increased risk of either AA or AN at first surveillance for patients with a family history of CRC.
One study reported on the incidence of CRC at first surveillance118 and again there was no evidence for increased risk associated with a family history of CRC. Cottett et al 118 presented evidence separately for those with AA at index, and for those with non-AA at index. In those with an AA at index both those with a family history (standardised incidence ratio (SIR) 3.76, 95% CI 1.51 to 7.75) and those without (SIR 2.10, 95% CI 1.54 to 2.81) were at statistically significantly increased risk for CRC at first surveillance. A formal statistical test for difference between those with or without family history was not performed. In the non-AA group neither those with or without a family history of CRC were at increased risk of CRC, although as for the analysis above a formal statistical test for difference between those with or without family history was not performed.
Only one study reported on the long-term incidence of CRC,106 reporting that of new CRC cases diagnosed during follow-up, 44% (4/9) had a family history of CRC; however, no statistical analyses were presented.
No evidence on family history, surveillance and long-term CRC mortality was identified.
There is consistent evidence that younger age is associated with a decreased risk of AA, AN and CRC at first surveillance, and also a decreased risk of long-term CRC incidence and mortality.
GRADE of evidence: Moderate
Twenty studies, generally rated as low risk of bias12 31 32 93 95 98 100 102 106 109–118 120–123 presented evidence on the risks of neoplasia associated with increasing age, and demonstrated that there is relatively consistent evidence suggesting that younger people are at lower risk.
Ten studies31 32 93 95 106 109 113–115 120–122 reported on AA. Studies used a younger age range as the reference (eg, <55 years) and reported risks for a range of different older age groups, or presented the findings as the risk associated with continuous increasing age. Two studies reported increased risks for AA at first surveillance associated with older age but these were not statistically significant.109 114 In three of the remaining eight studies, compared with a younger age group, AA incidence at first surveillance was statistically significantly increased for those aged from 55 years to those over 80 years, with incidence ranging from 10.9% for the 50–60 year age group95 to 22.75% in the 80 years or older age group.31 32 Five further studies with increasing age presented as a continuous variable presented evidence showing statistically significant increased risks for AA at first surveillance93 106 115 120–122 with ORs ranging from 1.02 (95% CI 1.01 to 1.04)122 to 1.47 (95% CI 1.16 to 1.87).120 121
Six studies reported on AN.12 98 100 116 117 123 Three studies presented evidence per age group relative to the younger age group. Martinez et al 12 reported statistically significant increased risks for AN across three age groups (61–71; 80+years) compared with the younger age group (50–59 years), with incidence of AA at first surveillance as 12.2%, 14.5%, and 17.7%, respectively. Furthermore, Martinez et al also presented evidence on the younger than 40 age group, and the 40–49 age group relative to the 50–59 age group. For both age groups the ORs and 95% CIs were below 1, suggesting that younger age has a protective effect. Park et al 98 reported only the 70 year or older age group had statistically significantly increased risk for AN (HR 2.56, 95% CI 1.43 to 4.59), while for the 50–70 year age group there was no statistically significant association. The association reported by Cubiella et al 100 for the 60–69 year age group relative to the 50–59 year age group was not statistically significant. Two studies reported statistically significant increased risks for AN with increasing age: with age as a continuous variable the HR was 1.02 (95% CI 1.01 to 1.03)117 and with age increasing with yearly increments the OR was 1.04 (95% CI 1.01 to 1.07).116 Kim et al found no significant difference in AN on surveillance when comparing 20–29 year-olds with 40–44 and 45–49 year-olds.123
Four studies31 32 102 110 118 reported evidence on incidence of CRC at first surveillance. All four studies reported statistically significant associations between risk of CRC and increasing age, although it should be noted that there are a small number of events across each age group. All four studies used a younger age range as the reference (eg, <55 years; 50–60 years) and reported risks for a range of different older age groups. Relative to patients younger than 55 years, Atkin et al 31 32 reported only those 75 years or older had statistically significant increased risk for CRC at first surveillance, with incidence reported as 5.08% and 5.58%, respectively. Coleman et al 110 reported, relative to the under 50 year group, significantly increased risks for CRC for the 60–69 group, the 70–79 group, and the 80 years or older group, with incidence of CRC at first surveillance reported as 2.7% and 3.7–5.9% respectively, whereas there was no significantly increased risk in the 50–59 age group. Huang et al 102 reported on interval CRC showing a statistically significant increased risk for the 60 years or older age group (OR 1.34, 95% CI 1.08 to 1.92) relative to the under 50 year age group, but this comparison was not significant for the 50–60 year age group. Cottet et al 118 reported on the risk of CRC at first surveillance associated with increasing age groups for those with AA and those with non-AA at index separately. For each of the age groups the SIR was statistically significant for those with AA at index (<60 years: SIR 3.65, 95% CI 1.88 to 6.37; 60–79 years: SIR 1.75, 95% CI 1.18 to 2.50; ≥80 years: SIR 3.32, 95% CI 1.66 to 5.95). However, there was no increased risk associated with any of the three age groups when patients had a non-AA at index.
Long-term CRC incidence was reported on in one study31 32 reporting statistically significant increased risk in older patients, and two studies111 112 reported a higher long-term CRC mortality in older patients.
There is consistent evidence that increasing age is associated with an increased risk of AA, AN and CRC at first surveillance, and of long-term CRC incidence and mortality.
GRADE of evidence: Moderate
Studies assessing age as a prognostic risk factor using a younger age group as a reference are outlined in the previous section. Studies of people older than 75 years compared with younger cohorts demonstrated a similar pattern, with statistically significant increased risks in older cohorts compared with younger reference cohorts (<55 years), for AA31 32 and AN12 at first surveillance, for CRC incidence at first surveillance in two studies,31 32 110 yet only for those with an AA at index in a further study,118 for long-term CRC incidence31 32 and for long-term CRC mortality.111 112
Analysis of BCSP surveillance patients in England demonstrated a statistically significant risk for CRC in older people on multivariable analysis: HR 1 for age <65 at screening, HR 2.08 for age >69 at screening (95% CI 1.299 to 3.350), and a statistically significant increased risk for AA at first surveillance on univariable but not on multivariable analysis.103–105
There is no consistent evidence that current smoking status is associated with an increased risk of AA, AN or CRC at first surveillance.
GRADE of evidence: Low
Five studies, rated as having low to moderate risk of bias,12 98 115 117 122 presented evidence relating smoking status to the risks of neoplasia at first surveillance. Two studies presented evidence on the association between smoking and AA115 122 and three studies presented evidence on smoking and AN at first surveillance12 98 117; none of these studies found significant associations. No studies have examined the association between smoking status and detection rates of CRC at first surveillance, long-term CRC incidence, or long-term CRC mortality.
Analysis of English BCSP surveillance data showed that smoking status at index colonoscopy was not statistically significantly associated with subsequent CRC at first surveillance in univariable or multivariable analysis. However, it was statistically significant for AA at first surveillance on univariable analysis: compared with never smokers, OR 1.12 for ex-smoker (95% CI 1.048 to 1.201), OR 1.33 for current smoker (95% CI 1.225 to 1.462); and on multivariable analysis: OR 1.16 for current smoker versus never/ex-smoker (95% CI 1.064 to 1.264).103–105
There is no consistent evidence that high BMI is associated with an increased risk of AA, AN or CRC at first surveillance.
GRADE of evidence: Low
Seven studies, rated as having a low to moderate risk of bias, examined BMI in relation to AA, AN or CRC at first surveillance.12 98 106 114 115 117 122 Four studies examined the association between BMI and AA at first surveillance.106 114 115 122 One study106 reported that patients with AA at index colonoscopy together with a high BMI had an increased risk of AA at first surveillance (RR 1.62, 95% CI 1.01 to 2.57). The remaining three studies found no associations between BMI and AA at first surveillance.114 115 122 Three studies examined the association between BMI and AN at first surveillance12 98 117; none of these studies found an association. No studies have examined BMI and detection rates of CRC at first surveillance, long-term CRC incidence or long-term CRC mortality.
Analysis of English BCSP surveillance data showed high BMI at index colonoscopy was not associated with either CRC or AA at first surveillance on univariable or multivariable analysis.103–105
There is consistent evidence that inadequate bowel preparation at index colonoscopy is associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Low
Five studies, ranging from low to high risk of bias, reported on the risks associated with different levels of bowel preparation quality.31 32 96 97 100 101 114 Three studies reported evidence on the risks for AA at first surveillance.31 32 96 114 Relative to excellent or good bowel preparation, there were statistically significant associations between poor bowel preparation and the risk for AA in two studies (Atkin et al 31 32: OR 1.54, 95% CI 1.04 to 2.28; Jang et al 114: OR 2.21, 95% CI 1.24 to 4.66). There were no statistically significant associations between adequate, moderate or satisfactory bowel preparation (relative to excellent) and the risk for AA.
Two studies reported evidence on the risks for AN at first surveillance,100 101 with only one101 reporting a statistically significant increased risk for AN associated with insufficient bowel preparation relative to good bowel preparation with an incidence of AA of 17.7% and CRC of 2.2%. There was no increased risk when bowel preparation was reported as moderate in the same study, and also when bowel preparation was reported as adequate (relative to inadequate) in a second study.100
One study31 32 reported on incidence of CRC at first surveillance and presented similar findings in that there was only statistically significantly increased risk for CRC if bowel preparation was poor (OR 3.80, 95% CI 1.79 to 8.05) and not if it was satisfactory. The same study31 32 reported on long-term CRC incidence and again the associations were only statistically significant for poor bowel preparation (HR 2.09, 95% CI 1.19 to 3.67) and not for satisfactory bowel preparation.
No studies were identified which presented evidence on bowel preparation quality and long-term CRC mortality.
There is consistent evidence that an incomplete index colonoscopy is associated with an increased risk of AA, AN and CRC at first surveillance.
GRADE of evidence: Low
Three studies, ranging from low to high risk of bias, presented evidence on the risks associated with completeness of colonoscopy.31 32 100 101 Only one study31 32 reported on the risks for AA at first surveillance associated with incomplete colonoscopy at index, reporting a statistically significant increased risk for AA (OR 1.92, 95% CI 1.58 to 2.33).
Two studies100 101 reported on the risk for AN at first surveillance and found no statistically significant association between incomplete colonoscopy and AN.
One study31 32 presented evidence on the risk for CRC at first surveillance and found an increased risk if colonoscopy was incomplete at index (OR 4.28, 95% CI 2.61 to 7.03); the same study31 32 presented evidence on long-term CRC incidence, similarly finding an increased risk if colonoscopy was incomplete at index (HR 1.80, 95% CI 1.34 to 2.41).
No studies on colonoscopy completeness and long-term CRC mortality were identified.
Analysis of English BCSP surveillance data showed that a combination of either poor bowel preparation or incomplete colonoscopy to the caecum was not associated with CRC at first surveillance, but it was associated with AA in univariable (OR 1.30, 95% CI 1.098 to 1.553) and multivariable analyses (compared with complete colonoscopy with good or adequate prep, OR 1.46 for either poor prep or incomplete colonoscopy at screening, 95% CI 1.220 to 1.754).103–105
There is evidence to suggest that advanced serrated polyps are risk equivalent to AAs for future CRC risk, and surveillance should be as for AAs
GRADE of evidence: Low
Colonoscopic surveillance is predicated on the increased risk of CRC following adenoma removal; however, another major pathway to colorectal cancer, the “serrated pathway”, accounts for 15–30% of colorectal cancer and has serrated polyps as cancer precursors. The BSG position statement on serrated polyps in the colorectum recommended one-off surveillance colonoscopy at 3 years for patients with an advanced serrated polyp, defined as a sessile serrated lesion (SSL) ≥10 mm, SSL with dysplasia and traditional serrated adenomas.6 No prospective data to validate this recommendation exist; however, evidence strongly suggests that future CRC risk is increased in people with advanced serrated polyps to a level similar to that post adenoma detection. In a post-hoc analysis of the Norwegian flexible sigmoidoscopic screening study (NORCCAP), large (≥10 mm) serrated lesions were associated with the same future colorectal cancer risk as AAs (HR 4.2 vs 3.3, respectively).124 A recent larger study using three US cohorts combined with 122 899 patients showed a similar result with a significantly higher risk of CRC after resection of an AA or a large (≥10 mm) serrated polyp with hazard ratios of 4.07 and 3.35, respectively.125
In a large Danish cohort which re-analysed pathological samples using modern definitions of serrated polyps, serrated lesions alone were risk equivalent to adenomas alone for future cancer risk without considering size (adjusted OR 3.4 vs 2.5, respectively).126 Traditional serrated adenomas and SSL with dysplasia had a significantly higher risk of future CRC (adjusted OR 4.8 and 4.8, respectively).
There is evidence to suggest that the future CRC risk may be additive between serrated and adenomatous polyps and their numbers should be summated when determining surveillance intervals
GRADE of evidence: Low
There has been recent data on the future risk when adenomas and serrated lesions are found together. The risk of finding an AA at surveillance when an AA and serrated lesion were found together was fourfold higher than when an AA alone was the index lesion, suggesting that the risk may be more than additive between serrated and adenomatous lesions, with an OR for future risk with synchronous AAs and serrated lesions at index exam of 16.04 (95% CI 6.95 to 37) compared with an OR of 3.86 (95% CI 2.77 to 5.39) for AAs alone.127 A further similar study from Korea also suggests additive risk between adenomas and SSLs, with the risk of AN at 3 years follow-up for adenoma with synchronous serrated polyp being 17.9% versus 10.7% for adenoma alone (p<0.001). Audit data from an Australian CRC surveillance programme with 2157 patients followed up for a median of 50 months found additive risk of AN when serrated lesions and adenomas were found together (high-risk adenoma: HR 2.04, 95% CI 1.70 to 2.45; high-risk SSP + adenoma: HR 3.20, 95% CI 1.31 to 7.82; low-risk SSP + adenoma: HR 2.20, 95% CI 1.03 to 4.68).128 Older data from when serrated lesions were less recognised both endoscopically and pathologically (1994–1997) is supportive, but less definitive.129
There is evidence to suggest that serrated polyps <10 mm in size, except for rectal hyperplastic polyps, are risk equivalent to adenomas <10 mm in size for future CRC risk, and surveillance should be as for adenomas <10 mm in size.
GRADE of evidence: Low
The BSG position statement on serrated polyps in the colorectum recommended no surveillance for patients with one or more serrated lesions <10 mm in size who do not meet the criteria for serrated polyposis syndrome.6 No prospective data to validate this recommendation exist. In a US cohort from 1994 to 1997 a proximal serrated polyp alone was associated with an increased risk of any adenoma during surveillance (OR 3.14, 95% CI 1.59 to 6.20), but not AN (OR 2.09, 95% CI 0.44 to 9.87)129; however, this cohort included approximately 10% of serrated polyps ≥10 mm in size (25/248 proximal non-dysplastic serrated polyps). A US pathology based case–control study suggested that the rate of CRC was significantly higher in SSLs than in patients with adenomas or hyperplastic polyps over 13 years follow-up (12.5% vs 1.8% vs 1.8%, respectively).130 All serrated lesions with subsequent cancer were <10 mm in size; however, some serrated polyposis syndrome patients and patients with TSA were included, and it is not clear whether SSLs were resected comprehensively rather than biopsied.
No prospective data exist for the risk with multiple non-advanced serrated lesions; however, multiple serrated lesions are associated with synchronous AN (OR 4.86), though this may reflect the inclusion of some larger lesions. Given that non-advanced serrated lesions appear risk equivalent to non-advanced adenomas, their surveillance should be equivalent. Multiple studies suggest diminutive hyperplastic rectal polyps do not significantly increase risk of future CRC, therefore need not be resected.6
There is no consistent evidence to suggest that a surveillance interval of less than 3 years, when compared to 3 years, reduces CRC incidence or mortality
GRADE of evidence: Low
One study presented evidence directly comparing an interval of 3 years to less than 3 years. Atkin et al 31 32 made comparisons to an interval of less than 18 months to 3 years, and showed that although there was an association with AA it was not statistically significant (3 years: OR 1.06, 95% CI 0.83 to 1.34).
Two studies31 32 119 reported non-significant associations between risk for AA and increasing interval between index and first surveillance. Atkin et al 31 32 reported a non-significant but positive association, for the per year increase in risk for AA (univariable: OR 1.04, 95% CI 0.99 to 1.09; multivariable, OR 1.03, 95% CI 0.98 to 1.09). Again, when comparing the interval of less than 18 months to each time interval, there was no statistically significant association (2 years: OR 1.05, 95% CI 0.83 to 1.33; 3 years: OR 1.06, 95% CI 0.83 to 1.34; 4 years: OR 1.10, 95% CI 0.78 to 1.55; 5 years: OR 1.24, 95% CI 0.81 to 1.91; 6 years: OR 0.90, 95% CI 0.51 to 1.61) until the comparison with 6.5 years or more where the unadjusted odds were 1.94 (95% CI 1.26 to 2.98), although this association did not remain significant when adjusted for covariates.
Two studies reported on CRC incidence at next surveillance,31 32 131 132 although only Atkin et al presented statistical analyses which showed that a longer interval was significantly associated with increased odds of CRC at first surveillance after multivariable adjustment (per year increase in interval OR 1.21, 95% CI 1.08 to 1.37; p=0.0040). When comparing the interval between index and first surveillance, relative to an interval of less than 18 months, the odds of finding CRC at 2, 3 or 5 years was not statistically significant.
There was no evidence relating to surveillance interval and long-term CRC incidence and long-term CRC mortality.
There is insufficient evidence to determine whether a surveillance interval of 3 years is superior to a longer interval, in terms of CRC incidence and mortality
GRADE of evidence: Low
Three studies reported on a comparison of 3 or 4 years or less compared with a longer interval. Pinsky et al 119 reported no significant association for AA when they compared incidence at first surveillance of 4 years or less to incidence after an interval of more than 4 years. They showed that incidence of AA was 9.6% when surveillance was performed no more than 4 years after index, and incidence of AA was 8% when surveillance was performed more than 4 years after index, with the associated odds of 0.86 (95% CI 0.6 to 1.2).
Chung et al 133 reported on incidence of AA for the interval between index and first surveillance of less than 3 years compared with 3–5 years, for low-risk (7.1% vs 2.2%) and high-risk groups (10.1% vs 8.7%), but did not present statistical analyses. Contrary to these findings, one study115 reported that an interval between index and first surveillance of 3 or more years was an independent risk factor for AA, reporting an adjusted OR of 2.97 (95% CI 1.11 to 7.93, p=0.030), but there was no statistically significant association when the interval was 5 years or more (OR 2.30, 95% CI 0.80 to 6.67; p=0.124.) One study only looked at AN100 and showed no significant association when comparing first surveillance at less than 3 years (14.4%, 95% CI 12.6 to 16.2) to 3 or more years (13.3%, 95% CI 11.8 to 14.7), with an associated OR of 1.0 (95% CI 0.8 to 1.2).
Two studies31 32 119 reported non-significant associations between risk for AA and increasing interval between index and first surveillance, and two studies reported on CRC incidence at next surveillance31 32 131 132 as described in the previous section.
There was no evidence relating to long-term CRC incidence and long-term CRC mortality.
There is insufficient evidence to determine who may benefit from a second surveillance procedure.
GRADE of evidence: Low
Two publications relating to one large study with a low risk of bias provide relevant evidence.31 32 The study reported findings regarding associations with prognostic factors at first surveillance and outcomes at second surveillance. Although some statistically significant associations between risk of AA or CRC at second surveillance and prognostic factors identified at first surveillance (no adenoma as reference) were reported, for some prognostic factors the number of patients was small, and the reported associations were not statistically significant.
There was modest increased risk for AA at second surveillance among those with HGD at first surveillance colonoscopy (OR 1.19, 95% CI 0.48 to 2.74),31 32 although this was not statistically significant. The number of patients with HGD at first surveillance was small. A greater number of patients had low-grade dysplasia and there was a statistically significant association of low-grade dysplasia and AA at second surveillance (OR 1.70, 95% CI 1.19 to 2.43). There were no CRC events at second surveillance for patients with HGD.
Risk for AA at second surveillance was increased if tubulovillous (OR 1.73, 95% CI 1.01 to 2.93) or villous components (OR 2.23, 95% CI 1.09 to 4.54) were identified at first surveillance. There were no CRC events at second surveillance for patients with tubulovillous components. For villous components, there were few CRC cases and thus wide 95% CIs (OR 2.57, 95% CI 0.31 to 21.70).31 32
Risk for AA at second surveillance was statistically significantly increased if the size of the adenoma at first surveillance was ≥20 mm (OR 3.12, 95% CI 1.69 to 5.75). For adenomas of a smaller size the magnitude of the ORs suggests an increased CRC risk; however, the result was not statistically significant. There were no CRC events at second surveillance for patients with adenomas >10 mm. For patients with adenomas <10 mm there was an increased risk of CRC; however, the result was not statistically significant (OR 1.34, 95% CI 0.33 to 5.37).31 32
Risk for AA at second surveillance was statistically significantly increased if the number of adenomas at first surveillance was two (OR 2.57, 95% CI 1.50 to 4.39), although there was no statistically significant increased risk for AA if patients had one, three, four or five (or more) adenomas at first surveillance. There was no statistically significant association for any number of adenomas at first surveillance and risk of CRC at second surveillance.31 32 The number of events in each category was small.
Risk for AA at second surveillance was increased if an adenoma or polyp was identified at a proximal location at first surveillance (OR 1.87, 95% CI 1.25 to 2.82). There was no such association for CRC.31 32
There was no statistically significant association between male sex at first surveillance colonoscopy and risk for AA and CRC at second surveillance.31 32
There were no statistically significant associations between older age at first surveillance colonoscopy and risk for AA and CRC at second surveillance, although the general trend was for increased risk in most age groups compared with the reference category (<55 years).31 32
There is little evidence for an association between bowel preparation quality at first surveillance (reference excellent or good) and the risk of AA or CRC at second surveillance. There was only one statistically significant association reported showing the risk for AA was increased if bowel preparation quality was satisfactory (OR 2.66, 95% CI 1.53 to 4.60); all other associations were not statistically significant for AA or CRC incidence.31 32
There was no evidence for a statistically significant association between the risk of AA at second surveillance and completeness of the colonoscopy at first surveillance (reference complete colonoscopy); however, there was a significant association between the risk of CRC at second surveillance where the colonoscopy at first surveillance was reported as incomplete (OR 5.72, 95% CI 1.27 to 25.87).31 32
There was no evidence presented on the following prognostic factors for second surveillance: adenoma morphology, family history of CRC, smoking status, BMI, high quality colonoscopist, and high-adenoma-detecting technologies. There was no evidence for second surveillance and long-term CRC incidence or CRC mortality reported in this study.
There is insufficient evidence to determine the optimal interval between first and second surveillance.
GRADE of evidence: Low
Two studies examined the interval between first and second surveillance.31 32 134 Atkin et al demonstrated statistically significant increased odds for risk of AN per year increase (OR 1.11 95% CI 1 to 1.24). In multivariable models for AN, using an interval of less than 18 months as referent, a 2 year interval was not statistically significant, but a 3 year (OR 2.02, 95% CI 1.19 to 3.42), 4 year (OR 2.45, 95% CI 1.20 to 5.00), and more than 6.5 year (OR 5.95, 95% CI 2.15 to 16.46) interval was significant (an interval of 5 or 6 years was not significant). Miller et al (rated as having a moderate risk of bias) did not find an association between risk for AA and interval between first and second surveillance when the interval was 3 or more years, compared with an interval of less than 3 years.134 There was no evidence for interval between first and second surveillance and long-term CRC incidence or CRC mortality.
There is no consistent evidence from surveillance studies to determine at what age, or at what life-expectancy, surveillance can be stopped without increasing the risk of future CRC development.
GRADE of evidence: Very low
There is evidence that the risk of colonoscopy in a healthy patient is low, but that colonoscopy risks increase with comorbidity and advanced age.
GRADE of evidence: Moderate
Colonoscopy is an invasive procedure, and as such carries a risk. Complications may be GI (procedure-specific, for example, perforation or post-polypectomy bleeding) or non-GI (for example, renal impairment due to bowel preparation, cardiovascular or cerebrovascular). While these risks are in general very low, they need to be weighed against the potential benefit of post-polypectomy surveillance colonoscopy.
The risk of either perforation or bleeding is small with diagnostic colonoscopy but increases when polypectomy is performed. The BSG audit demonstrated an overall perforation rate of 0.04%.135 Diagnostic perforation rates of 0–0.2% are reported,136–138 the English BCSP figure being 0.03%.138 Polypectomy perforation rates of 0.06–0.65% are reported,139–143 the English BCSP polypectomy procedure perforation rate being 0.09%.138 Post-polypectomy bleeding (PPB) rates of 0.0.8–6.1% for polypectomies are reported,136 144 the English BCSP PPB rate (requiring transfusion) being at the lower end of this range at 0.08%.138
Colonoscopy complication rates are higher in non-screening populations, probably due to both patient factors and colonoscopist expertise.145
Colonoscopy risks also increase in older people and in people with comorbidity.146–149 Warren et al reported that risk for adverse events among persons undergoing colonoscopy increased with age and is significantly higher in patients 80 years or older compared with those 66 to 69 years. Relative to people aged 66 to 69 years, the adjusted predictive risk for adverse GI events was significantly higher for patients aged 80 years or older (risk per 1000 procedures in persons 80 to 84 years of age vs those 66 to 69 years of age: 8.8 (95% CI 6.9 to 10.7) vs 5.0 (95% CI 3.8 to 6.2) for serious GI events and 15.9 (95% CI 13.5 to 18.3) vs 6.9 (95% CI 5.6 to 8.2) for other GI events). Persons in the colonoscopy group were significantly more likely than their age-equivalent matched group to have adverse GI events. The risk for adverse cardiovascular events increased with age among persons undergoing colonoscopy, but these rates did not significantly differ from those in the age-equivalent matched group. In a large-scale, population-based, prospective observational study of over 1.3 million US Medicare beneficiaries, the excess 30 day risk for any adverse event in the colonoscopy group was 5.6 events per 1000 individuals (95% CI 4.4 to 6.8) in the 70–74 year age group and 10.3 per 1000 (95% CI 8.6 to 11.1) in the 75–79 year age group.
Perhaps the most pertinent study is a large, retrospective cohort study of colonoscopic surveillance in the elderly.49 This showed a significantly lower CRC incidence among elderly patients undergoing surveillance compared with non-elderly patients (0.24 per 1000 person-years vs 3.61 per 1000 person-years; p<0.001; HR for CRC 0.06, 95% CI 0.02 to 0.13; p<0.001). Moreover, both age 75 years and older and Charlson score of 2 were independently associated with increased risk of postprocedure hospitalisation (adjusted OR 1.28, 95% CI 1.07 to 1.53; p=0.006, and 2.54, 95% CI 2.06 to 3.14; p<0.001, respectively).
Of note, these populations (that is, older people and those with comorbidity) are also least likely to derive any benefit from post-polypectomy surveillance. This should be considered when deciding on the appropriateness of surveillance for an individual.
Lay summary
A lay summary to accompany these guidelines is provided in online supplementary appendix 4.
gutjnl-2019-319858supp004.pdf (212.1KB, pdf)
Hereditary cancer/polyposis risk
Management of patients with a higher than average risk of CRC due to hereditary CRC syndromes, serrated polyposis syndrome and other high familial CRC risk are covered in separate guidelines that have been developed by the BSG, ACPGBI and UK Cancer Genetics Group (UKCGG) (in press), and are not addressed within this guideline. Clinicians should refer to those guidelines in the following situations:
-
A family history of colorectal cancer:
A family history of one first degree relative diagnosed with CRC under 50 years, or
Two affected first-degree relatives diagnosed with CRC at any age.
A patient with personal history of CRC diagnosed under age 50 years (early onset CRC).
A patient with a personal history of CRC diagnosed at any age, who also has a first degree relative diagnosed with CRC at any age.
Patients with multiple polyps, specifically: patients under 60 years of age with at least 10 adenomas, or patients from 60 years of age with at least 20 adenomas or at least 10 adenomas and a family history of CRC or polyposis.
-
Patients with known or suspected inherited CRC predisposition syndromes including:
Lynch syndrome
Polyposis syndromes including serrated polyposis syndrome (SPS) and familial polyposis.
The separate hereditary guidelines will include recommendations for germline genetic testing for patients with early onset CRC, multiple polyps and SPS. The diagnostic criteria for SPS has been redefined in a 2019 update by the World Health Organization:
Either: At least five serrated polyps proximal to the rectum all being ≥5 mm in size, with two or more ≥10 mm in size; or
More than 20 serrated polyps of any size distributed throughout the large bowel, with at least five proximal to the rectum included in the final polyp count. The polyp count is cumulative over multiple colonoscopies.
Implementation of guidelines
It is important that these updated surveillance guidelines are effectively implemented to deliver benefits to patients and provide effective, higher value care. Not only will this ensure that patients receive appropriate surveillance, it will also ensure that patients who do not benefit from surveillance do not have unnecessary invasive procedures. Inappropriate surveillance also carries an opportunity cost, by preventing more effective use of endoscopy in a resource-constrained service, increasing the burden on endoscopy services and the NHS generally. The Joint Advisory Group on Gastrointestinal Endoscopy (JAG) will continue to assess that surveillance validation and waiting times are appropriate and linked to the unit’s endoscopy governance process. This is particularly important given historically poor adherence to surveillance guidelines.
Dissemination of the guidelines from national endoscopy stakeholders to the endoscopy workforce will be done using a variety of electronic communications and web-based technologies to reach the target audience. Conference presentations, workshops and other educational fora will support this dissemination. In addition, a visual summary of the surveillance algorithm has been developed to allow easy understanding and implementation of the guidelines (online supplementary appendix 5).
gutjnl-2019-319858supp005.pdf (698.8KB, pdf)
By combining the serrated and adenomatous polyp count, these guidelines will reduce the need for histology review before determining appropriate surveillance; nevertheless, surveillance decision-making will still be dependent on review of the polyp histology at times. The inevitable delay between the colonoscopy and the histology report becoming available often undermines this process, as evidenced by audits of the appropriateness of surveillance colonoscopy waiting lists.150 This longstanding systemic issue has been identified within the Getting It Right First Time (GIRFT) programme as an important quality improvement initiative, focused mainly on the process around clinical decision-making for surveillance. Part of the solution may come from improvements in IT software, to facilitate the review process, and the management of surveillance populations including call and recall of patients. However, the clinical review needs to be transparent (eg, within a virtual clinic) and to be recognised within job plans. The appointment of a trust surveillance lead, responsible for ensuring that robust systems are in place to deliver surveillance to other high-risk groups, may also improve guideline compliance.
In implementing these updated guidelines, healthcare organisations are advised to review patients already on surveillance waiting lists to determine concordance with new guidance. There will be some patients who no longer meet the criteria for colonoscopic surveillance and clear strategies for informing patients and their responsible clinicians should be agreed locally.
Optical diagnosis of diminutive polyps in context of the current guideline
Optical biopsy—the use of endoscopic appearances to decide on polyp histology—was approved by NICE in 2017, with the use of virtual chromoendoscopy for diminutive polyps ≤5 mm in size to guide the setting of surveillance intervals; however, stipulations for training, audit and accreditation were specified but have not yet been developed. The Bowel Scope programme also permitted in 2018 the use of a white light optical assessment to diagnose hyperplastic polyps in the rectum that may be left in situ, without formal training or accreditation.151 The ESGE also supported optical biopsy for diminutive polyps in 2014 with similar caveats to NICE, and a recent revision of that guideline with new data did not change their statement.152 153 There is therefore support for optical biopsy to avoid unnecessary resection in the rectum, and to set surveillance intervals.
Recent data from the DISCOUNT study from the Netherlands in their bowel cancer screening programme suggest at least a proportion (59%) of endoscopists can reach and maintain performance in optical biopsy adequate to replace histopathology after training and meet predefined performance thresholds.154 In the proportion of lost or uninterpretable polyps where a pathological diagnosis cannot be made, polyps are assumed to be adenomas. When this is taken into account, pathological accuracy is very similar to the diagnostic accuracy of optical biopsy.155 The remaining barrier to uptake of optical diagnosis in the UK is the lack of an available accreditation process. It is possible that computer vision/deep learning based approaches will become commercially available to support endoscopists in optical biopsy in the next few years.156 157
The current surveillance guidelines differ from previous guidelines in that small and diminutive serrated polyps and adenomas are considered together as risk equivalent for surveillance intervals, except for diminutive rectal hyperplastic polyps. Potentially this obviates the need for pathological review of diminutive lesions, which could simply be counted to determine whether surveillance was required or not. Such lesions ≤5 mm have a low risk of containing advanced pathology, and an exceptionally low risk of containing cancer, 5.6% and 0.07%, respectively.158 Diminutive SSLs also have a very low risk of containing dysplasia (≤5 mm, 0%; 6–9 mm, 6.0%; ≥10 mm, 13.6%).159 So called “DISCARD-lite” or “location based” strategies perform almost as well as traditional optical biopsy strategies in terms of setting surveillance intervals using older adenoma-only based guidelines, and their performance with the current guidelines is likely to be even better.160 161 Therefore, simply resecting and discarding, and counting the numbers of diminutive polyps, is potentially a safe and very cost-effective strategy to set surveillance intervals based on the current guidelines, requiring minimal “optical biopsy” for hyperplastic polyps in the rectum. This may encourage and facilitate the wider uptake of optical biopsy and DISCARD-type strategies in clinical practice, and avoid the need for accreditation.
Workload implications and impact of the new guidelines
Preliminary analysis of available datasets suggests that implementation of these new guidelines may reduce the number of people entering post-polypectomy surveillance to around a quarter to a third of that of the previous low-/intermediate-/high-risk cohort. This is primarily by removal of almost all the previous low-risk cohort, along with approximately half of the previous intermediate-risk cohort; all the previous high-risk cohort will enter surveillance. The new algorithm appears to discriminate well between those at low- and high-risk of long-term CRC and of AN on surveillance.
We estimate that around 10% of those undergoing an initial surveillance procedure will qualify for further surveillance. Overall, we estimate that colonoscopic surveillance workload for the post-polypectomy cohort will reduce to approximately 20% of the present level.
No formal cost-effectiveness analysis of the guidelines has been performed. It is clear, however, that implementation of these guidelines will free up colonoscopy capacity in the UK. The UK currently has constrained endoscopy capacity which, for example, limits the expansion of the screening programme to younger age groups. Additional capacity could be used in patient populations with higher pathology yields—for example, advanced neoplasia is found in 20–40% of FIT positive people undergoing screening,162 leading to a more cost-effective use of the current colonoscopy resource.
Key performance indicator
Adherence to post-polypectomy and post-cancer resection surveillance recommendations should be monitored at least annually. Non-compliant cases should be reviewed to determine whether the reason for deviation from surveillance recommendations was clearly documented and clinically appropriate.
Research questions
Throughout the guideline development process, the GDG identified some of the key unanswered research questions and needs, which are listed below:
Greater evidence of the effect of surveillance using long-term CRC incidence/mortality as the endpoints.
Evidence or consensus on minimum surveillance yield threshold of advanced premalignant polyps that justifies surveillance being worthwhile, for example, by correlation between surveillance yields and long-term CRC incidence.
More robust evidence of the effectiveness of surveillance in people with serrated polyps.
Evidence of the effectiveness of surveillance using a combined serrated plus adenomatous polyp count.
The impact of a high-quality (as opposed to “acceptable quality”) index colonoscopy on surveillance findings, and how this might be incorporated into future surveillance algorithms.
Further research on the impact of histological completeness of excision on surveillance yields, and whether this variable should be incorporated into future surveillance algorithms.
Development of a personalised surveillance prognostic algorithm.
Relevance of surveillance for those under the current screening age limit, especially the very young (eg, under 35 years old).
Relevance of surveillance for those above the current screening age limit (ie, 75 years or older).
Further research on “stop surveillance” criteria and on the benefit of ongoing surveillance beyond the first surveillance procedure.
Further evidence for the potential use of FIT, other technologies or biomarkers for surveillance.
Greater data on patient experience and patient preferences surrounding surveillance, including what affects compliance.
Future health economics evaluation: Collection of long-term outcomes data for the polypectomy population to enable estimation of expected lifetime QALYs. In a situation where endoscopy capacity is constrained (such as in the UK) it may be useful to consider the relative benefits (expected lifetime QALYs) of allocation of capacity between symptomatic referrals, screening and surveillance.
Guidelines review
We recommend that the guidelines should be considered for review 5–10 years from the date of publication. Any updates to the guidelines in the interim will be noted on the BSG and ACPGBI websites.
In memoriam
Professor Wendy Atkin, world-leading expert on cancer surveillance, author of original BSG/ACPGBI guidelines, mentor and friend to us all.
Acknowledgments
Professor Cathy Bennett provided methodological support and liaison with ECD Solutions. ScHARR team provided the core evidence synthesis. NHS England, in particular Robert Logan, provided support and advice on implementation. Kate Wooldrage and the other members of the Cancer Screening and Prevention Research Group at Imperial College London. Bowel Cancer UK (including Asha Kaur, Jess Lewington and Katherine Nash) provided input at the outset and reviewed and advised on the draft manuscript and lay summary. Cancer Research UK and Jodie Moffat reviewed and advised on the draft manuscript and lay summary. Professor James East was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Twitter: @sdolwani, @kevinjmonahan
Contributors: MDR was lead author for the guidelines and co-chaired the GDG. LS and AC were co-senior authors for the guidelines. BM co-chaired the GDG on behalf of PHE. MDR, BM, LS, Dr AC, BPS, ST-G, CJR, JE, SD, KM, MN, PVK, NC, JD, SW, AP and DJMT performed literature searches and evidence syntheses for the GDG, voted on the statements and reviewed/edited the manuscript. SB performed BCSP analyses, literature searches and evidence syntheses for the GDG. BH and JM were voting lay-representatives on the GDG and co-wrote the lay sections of the manuscript. AS and RW performed literature searches and evidence syntheses for the GDG.
Funding: Funding was secured from Public Health England for the evidence synthesis provided by ScHARR, for administrative support and for meetings. Particular thanks to Hazel Rudge-Pickard, Sam Mullins, John Marshall, Anne Mackie and Bob Steele. British Society of Gastroenterology and Simone Cort provided additional administrative support and funding for meetings. Stuart Gittens (ECD Solutions) provided the on-line guideline platform.
Competing interests: JE – Clinical advisory boards: Lumendi, Boston Scientific; Speaker fees: Olympus, FalkColin Rees – Research grant: ARC medical, Norgine, Olympus. Expert witness: ARC medical Matt Rutter – Speaker fees: SwissSCWeb, Pentax; Research Grant: Olympus; Consultancy: Norgine DJMT – Speaker fees: Bracco, Guerbet.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Cancer Research UK, 2019. Available: https://www.cancerresearchuk.org/about-cancer/bowel-cancer/about-bowel-cancer
- 2. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. 10.1016/0092-8674(90)90186-I [DOI] [PubMed] [Google Scholar]
- 3. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. 10.1056/NEJMoa1100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 2005;129:34–41. 10.1053/j.gastro.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 5. Cottet V, Jooste V, Fournel I, et al. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut 2012;61:1180–6. [DOI] [PubMed] [Google Scholar]
- 6. East JE, Atkin WS, Bateman AC, et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017;66:1181–96. 10.1136/gutjnl-2017-314005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. NEnglJMed 2000;343:162–8. [DOI] [PubMed] [Google Scholar]
- 8. Imperiale TF, Wagner DR, Lin CY, et al. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med Overseas Ed 2000;343:169–74. 10.1056/NEJM200007203430302 [DOI] [PubMed] [Google Scholar]
- 9. Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med Overseas Ed 2006;355:1863–72. 10.1056/NEJMoa054967 [DOI] [PubMed] [Google Scholar]
- 10. Hassan C, Pickhardt PJ, Kim DH, et al. Systematic review: distribution of advanced neoplasia according to polyp size at screening colonoscopy. Aliment Pharmacol Ther 2010;31:210–7. 10.1111/j.1365-2036.2009.04160.x [DOI] [PubMed] [Google Scholar]
- 11. Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–62. 10.1056/NEJM199203053261002 [DOI] [PubMed] [Google Scholar]
- 12. Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136:832–41. 10.1053/j.gastro.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cancer Research UK. Scoping the future: an evaluation of endoscopy capacity across the NHS in England. Cancer Research UK publication 2015.
- 14. Atkin WS, Saunders BP, et al. , British Society for Gastroenterology . Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002;51 Suppl 5:v6–9. 10.1136/gut.51.suppl_5.v6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–89. 10.1136/gut.2009.179804 [DOI] [PubMed] [Google Scholar]
- 16. Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditarycolorectal cancer from the British Society ofGastroenterology (BSG)/Association ofColoproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2019:gutjnl-2019-319915. [Google Scholar]
- 17. Brouwers MC, Kho ME, Browman GP, et al. Agree II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839–42. 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tham TCK, Gleeson D, Greenfield SM, et al. British Society of Gastroenterology policy and processes for the development of guidelines. Gut 2015;64:1184–5. 10.1136/gutjnl-2015-309164 [DOI] [PubMed] [Google Scholar]
- 19. Rutter MD, Chattree A, Barbour JA, et al. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut 2015;64:1847–73. 10.1136/gutjnl-2015-309576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hassan C, Quintero E, Dumonceau J-M, et al. Post-polypectomy colonoscopy surveillance: European Society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 2013;45:842–64. 10.1055/s-0033-1344548 [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Schünemann HJ, Djulbegovic B, et al. Guideline panels should not grade good practice statements. J Clin Epidemiol 2015;68:597–600. 10.1016/j.jclinepi.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 22. Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy--results of the Complete Adenoma Resection (CARE) study. Gastroenterology 2013;144:74–80. 10.1053/j.gastro.2012.09.043 [DOI] [PubMed] [Google Scholar]
- 23. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–306. 10.1056/NEJMoa1309086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med Overseas Ed 2010;362:1795–803. 10.1056/NEJMoa0907667 [DOI] [PubMed] [Google Scholar]
- 25. Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: a population-based case-control study. Ann Intern Med 2012;157:225–32. [DOI] [PubMed] [Google Scholar]
- 26. Mangas-Sanjuan C, Zapater P, Cubiella J, et al. Importance of endoscopist quality metrics for findings at surveillance colonoscopy: the detection-surveillance paradox. United European Gastroenterol J 2018;6:622–9. 10.1177/2050640617745458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tierney M, Bevan R, Rees CJ, et al. What do patients want from their endoscopy experience? the importance of measuring and understanding patient attitudes to their care. Frontline Gastroenterol 2016;7:191–8. 10.1136/flgastro-2015-100574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown S, Bevan R, Rubin G, et al. Patient-derived measures of Gi endoscopy: a meta-narrative review of the literature. Gastrointest Endosc 2015;81:1130–40. 10.1016/j.gie.2014.11.047 [DOI] [PubMed] [Google Scholar]
- 29. Foss FA, Milkins S, McGregor AH. Inter-observer variability in the histological assessment of colorectal polyps detected through the NHS bowel cancer screening programme. Histopathology 2012;61:47–52. 10.1111/j.1365-2559.2011.04154.x [DOI] [PubMed] [Google Scholar]
- 30. Mahajan D, Downs-Kelly E, Liu X, et al. Reproducibility of the villous component and high-grade dysplasia in colorectal adenomas <1 cm. Am J Surg Pathol 2013;37:427–33. 10.1097/PAS.0b013e31826cf50f [DOI] [PubMed] [Google Scholar]
- 31. Atkin W, Brenner A, Martin J, et al. The clinical effectiveness of different surveillance strategies to prevent colorectal cancer in people with intermediate-grade colorectal adenomas: a retrospective cohort analysis, and psychological and economic evaluations. Health Technol Assess 2017;21:1–536. 10.3310/hta21250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol 2017;18:823–34. 10.1016/S1470-2045(17)30187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. 10.1056/NEJMoa1301969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adler J, Toy D, Anderson JC, et al. Metachronous neoplasias arise in a higher proportion of colon segments from which large polyps were previously removed, and can be used to estimate incomplete resection of 10-20 mm colorectal polyps. Clin Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 35. Chaptini L, Chaaya A, Depalma F, et al. Variation in polyp size estimation among endoscopists and impact on surveillance intervals. Gastrointest Endosc 2014;80:652–9. 10.1016/j.gie.2014.01.053 [DOI] [PubMed] [Google Scholar]
- 36. Kim JH, Park SJ, Lee JH, et al. Is forceps more useful than visualization for measurement of colon polyp size? World J Gastroenterol 2016;22:3220–6. 10.3748/wjg.v22.i11.3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rex DK, Rabinovitz R. Variable interpretation of polyp size by using open forceps by experienced colonoscopists. Gastrointest Endosc 2014;79:402–7. 10.1016/j.gie.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 38. Liedenbaum MH, de Vries AH, Halligan S, et al. CT colonography polyp matching: differences between experienced readers. Eur Radiol 2009;19:1723–30. 10.1007/s00330-009-1328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta S, Durkalski V, Cotton P, et al. Variation of agreement in polyp size measurement between computed tomographic colonography and pathology assessment: clinical implications. Clin Gastroenterol Hepatol 2008;6:220–7. 10.1016/j.cgh.2007.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turner JK, Wright M, Morgan M, et al. A prospective study of the accuracy and concordance between in-situ and postfixation measurements of colorectal polyp size and their potential impact upon surveillance. Eur J Gastroenterol Hepatol 2013;25:562–7. 10.1097/MEG.0b013e32835d1f2d [DOI] [PubMed] [Google Scholar]
- 41. Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975;36:2251–70. 10.1002/cncr.2820360944 [DOI] [PubMed] [Google Scholar]
- 42. Martínez ME, Thompson P, Messer K, et al. One-year risk for advanced colorectal neoplasia: U.S. versus U.K. risk-stratification guidelines. Ann Intern Med 2012;157:856–64. 10.7326/0003-4819-157-12-201212180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Karsa L, Patnick J, Segnan N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition-executive summary. Endoscopy 2012;44:SE1–8. [DOI] [PubMed] [Google Scholar]
- 44. Plumb A, Nickerson C, Wooldrage K, et al. Terminal digit preference biases polyp size measurements at endoscopy, computed tomographic colonography, and histopathology. Endoscopy 2016;48:899–908. 10.1055/s-0042-108727 [DOI] [PubMed] [Google Scholar]
- 45. Strum WB, Adenomas C. Colorectal adenomas. N Engl J Med 2016;374:1065–75. 10.1056/NEJMra1513581 [DOI] [PubMed] [Google Scholar]
- 46. Stryker SJ, Wolff BG, Culp CE, et al. Natural history of untreated colonic polyps. Gastroenterology 1987;93:1009–13. 10.1016/0016-5085(87)90563-4 [DOI] [PubMed] [Google Scholar]
- 47. Sekiguchi M, Otake Y, Kakugawa Y, et al. Incidence of advanced colorectal neoplasia in individuals with untreated diminutive colorectal adenomas diagnosed by magnifying image-enhanced endoscopy. Am J Gastroenterol 2019. [DOI] [PubMed] [Google Scholar]
- 48. National life tables, UK: 2015 to 2017: Office of National Statistics. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2015to2017#quality-and-methodology
- 49. Tran AH, Man Ngor EW, BU W. Surveillance colonoscopy in elderly patients: a retrospective cohort study. JAMA internal medicine 2014;174:1675–82. [DOI] [PubMed] [Google Scholar]
- 50. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109 10.1093/jnci/djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68:1820–6. 10.1136/gutjnl-2018-317592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- 53. Jeffery M, Hickey BE, Hider PN, et al. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2016;3 10.1002/14651858.CD002200.pub3 [DOI] [PubMed] [Google Scholar]
- 54. Rosati G, Ambrosini G, Barni S, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol 2016;27:274–80. 10.1093/annonc/mdv541 [DOI] [PubMed] [Google Scholar]
- 55. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: Results of the randomized “CEAwatch” trial. Eur J Surg Oncol 2015;41:1188–96. 10.1016/j.ejso.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 56. Wille-Jørgensen P, Syk I, Smedh K, et al. Effect of more vs less frequent follow-up testing on overall and colorectal cancer-specific mortality in patients with stage II or III colorectal cancer: the COLOFOL randomized clinical trial. JAMA 2018;319:2095–103. 10.1001/jama.2018.5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pita-Fernández S, Alhayek-Aí M, González-Martín C, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol 2015;26:644–56. 10.1093/annonc/mdu543 [DOI] [PubMed] [Google Scholar]
- 58. Tjandra JJ, Chan MKY. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 2007;50:1783–99. 10.1007/s10350-007-9030-5 [DOI] [PubMed] [Google Scholar]
- 59. Mulder SA, Kranse R, Damhuis RA, et al. The incidence and risk factors of metachronous colorectal cancer: an indication for follow-up. Dis Colon Rectum 2012;55:522–31. [DOI] [PubMed] [Google Scholar]
- 60. Bouvier A-M, Latournerie M, Jooste V, et al. The lifelong risk of metachronous colorectal cancer justifies long-term colonoscopic follow-up. Eur J Cancer 2008;44:522–7. 10.1016/j.ejca.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 61. Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task force on colorectal cancer. Gastroenterology 2006;130:1865–71. 10.1053/j.gastro.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 62. Green RJ, Metlay JP, Propert K, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of intergroup 0089. Ann Intern Med 2002;136:261–9. 10.7326/0003-4819-136-4-200202190-00005 [DOI] [PubMed] [Google Scholar]
- 63. Yang J, Du XL, Li S, et al. The risk and survival outcome of subsequent primary colorectal cancer after the first primary colorectal cancer: cases from 1973 to 2012. BMC Cancer 2017;17:783 10.1186/s12885-017-3765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Samadder NJ, Curtin K, Wong J, et al. Epidemiology and familial risk of synchronous and metachronous colorectal cancer: a population-based study in Utah. Clin Gastroenterol Hepatol 2014;84:e1–2. [DOI] [PubMed] [Google Scholar]
- 65. le Clercq CM, Winkens B, Bakker CM, et al. Metachronous colorectal cancers result from missed lesions and non-compliance with surveillance. Gastrointest Endosc 2015. [DOI] [PubMed] [Google Scholar]
- 66. Fuccio L, Rex D, Ponchon T, et al. New and recurrent colorectal cancers after resection: a systematic review and meta-analysis of endoscopic surveillance studies. Gastroenterology 2019;156:1309–23. 10.1053/j.gastro.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 67. Rulyak SJ, Lieberman DA, Wagner EH, et al. Outcome of follow-up colon examination among a population-based cohort of colorectal cancer patients. Clin Gastroenterol Hepatol 2007;5:470–6. 10.1016/j.cgh.2006.11.027 [DOI] [PubMed] [Google Scholar]
- 68. Hassan C, Pickhardt PJ, Zullo A, et al. Cost-effectiveness of early colonoscopy surveillance after cancer resection. Dig Liver Dis 2009;41:881–5. 10.1016/j.dld.2009.03.016 [DOI] [PubMed] [Google Scholar]
- 69. Wang T, Cui Y, Huang W-S, et al. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointest Endosc 2009;69:609–15. 10.1016/j.gie.2008.05.017 [DOI] [PubMed] [Google Scholar]
- 70. Hassan C, Wysocki PT, Fuccio L, et al. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) guideline. Endoscopy 2019;51:266–77. 10.1055/a-0831-2522 [DOI] [PubMed] [Google Scholar]
- 71. Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2017;49:270–97. 10.1055/s-0043-102569 [DOI] [PubMed] [Google Scholar]
- 72. Belderbos T, Leenders M, Moons L, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388–402. 10.1055/s-0034-1364970 [DOI] [PubMed] [Google Scholar]
- 73. Tate DJ, Desomer L, Klein A, et al. Adenoma recurrence after piecemeal colonic EMR is predictable: the Sydney EMR recurrence tool. Gastrointest Endosc 2017;85:647–56. 10.1016/j.gie.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 74. Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015;110:697–707. 10.1038/ajg.2015.96 [DOI] [PubMed] [Google Scholar]
- 75. Akintoye E, Kumar N, Aihara H, et al. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open 2016;04:E1030–44. 10.1055/s-0042-114774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Toyonaga T, Man-i M, East JE, et al. 1,635 endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc 2013;27:1000–8. 10.1007/s00464-012-2555-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rees CJ, Thomas Gibson S, Rutter MD, et al. UK key performance indicators and quality assurance standards for colonoscopy. Gut 2016;65:1923–9. 10.1136/gutjnl-2016-312044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Regge D, et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA 2009;301:2453–61. 10.1001/jama.2009.832 [DOI] [PubMed] [Google Scholar]
- 79. Chaparro M, Gisbert JP, del Campo L, et al. Accuracy of computed tomographic colonography for the detection of polyps and colorectal tumors: a systematic review and meta-analysis. Digestion 2009;80:1–17. 10.1159/000215387 [DOI] [PubMed] [Google Scholar]
- 80. Obaro AE, Plumb AA, Fanshawe TR, et al. Post-imaging colorectal cancer or interval cancer rates after CT colonography: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018;3:326–36. 10.1016/S2468-1253(18)30032-3 [DOI] [PubMed] [Google Scholar]
- 81. Porté F, Uppara M, Malietzis G, et al. CT colonography for surveillance of patients with colorectal cancer: systematic review and meta-analysis of diagnostic efficacy. Eur Radiol 2017;27:51–60. 10.1007/s00330-016-4319-1 [DOI] [PubMed] [Google Scholar]
- 82. Amitai MM, Fidder H, Avidan B, et al. Contrast-enhanced CT colonography with 64-slice MDCT compared to endoscopic colonoscopy in the follow-up of patients after colorectal cancer resection. Clin Imaging 2009;33:433–8. 10.1016/j.clinimag.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 83. Kim HJ, Park SH, Pickhardt PJ, et al. CT colonography for combined colonic and extracolonic surveillance after curative resection of colorectal cancer. Radiology 2010;257:697–704. 10.1148/radiol.10100385 [DOI] [PubMed] [Google Scholar]
- 84. Neri E, Vagli P, Turini F, et al. Post-surgical follow-up of colorectal cancer: role of contrast-enhanced CT colonography. Abdom Imaging 2010;35:669–75. 10.1007/s00261-009-9596-6 [DOI] [PubMed] [Google Scholar]
- 85. Pickhardt PJ, Edwards K, Bruining DH, et al. Prospective trial evaluating the surgical anastomosis at one-year colorectal cancer surveillance: CT colonography versus optical colonoscopy and implications for patient care. Dis Colon Rectum 2017;60:1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weinberg DS, Pickhardt PJ, Bruining DH, et al. Computed tomography colonography vs colonoscopy for colorectal cancer surveillance after surgery. Gastroenterology 2018;154:927–34. 10.1053/j.gastro.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liedenbaum MH, Venema HW, Stoker J. Radiation dose in CT colonography–trends in time and differences between daily practice and screening protocols. Eur Radiol 2008;18:2222–30. 10.1007/s00330-008-0994-x [DOI] [PubMed] [Google Scholar]
- 88. Boellaard TN, Venema HW, Streekstra GJ, et al. Effective radiation dose in CT colonography: is there a downward trend? Acad Radiol 2012;19:1127–33. 10.1016/j.acra.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 89. Perisinakis K, Seimenis I, Tzedakis A, et al. Screening computed tomography colonography with 256-slice scanning: should patient radiation burden and associated cancer risk constitute a major concern? Invest Radiol 2012;47:451–6. 10.1097/RLI.0b013e318250a58c [DOI] [PubMed] [Google Scholar]
- 90. de González AB, Kim KP, Knudsen AB, et al. Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis. AJR Am J Roentgenol 2011;196:816–23. 10.2214/AJR.10.4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. de Gonzalez AB, Iulian Apostoaei A, Veiga LHS, et al. RadRAT: a radiation risk assessment tool for lifetime cancer risk projection. J Radiol Prot 2012;32:205–22. 10.1088/0952-4746/32/3/205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cross AJ, Wooldrage K, Robbins EC, et al. Faecal immunochemical tests (FIT) versus colonoscopy for surveillance after screening and polypectomy: a diagnostic accuracy and cost-effectiveness study. Gut 2019;68:1642–52. 10.1136/gutjnl-2018-317297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van Enckevort CC, de Graaf AP, Hollema H, et al. Predictors of colorectal neoplasia after polypectomy: based on initial and consecutive findings. Netherlands J Med 2014;72:139–45. [PubMed] [Google Scholar]
- 94. Fairley KJ, Li J, Komar M, et al. Predicting the risk of recurrent adenoma and incident colorectal cancer based on findings of the baseline colonoscopy. Clin Transl Gastroenterol 2014;5:e64 10.1038/ctg.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Huang Y, Gong W, Su B, et al. Recurrence and surveillance of colorectal adenoma after polypectomy in a southern Chinese population. J Gastroenterol 2010;45:838–45. 10.1007/s00535-010-0227-3 [DOI] [PubMed] [Google Scholar]
- 96. Facciorusso A, Di Maso M, Serviddio G, et al. Development and validation of a risk score for advanced colorectal adenoma recurrence after endoscopic resection. World J Gastroenterol 2016;22:6049–56. 10.3748/wjg.v22.i26.6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Facciorusso A, Di Maso M, Serviddio G, et al. Factors associated with recurrence of advanced colorectal adenoma after endoscopic resection. Clin Gastroenterol Hepatol 2016;14:1148–54. 10.1016/j.cgh.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 98. Park S-K, Kim NH, Jung YS, et al. Risk of developing advanced colorectal neoplasia after removing high-risk adenoma detected at index colonoscopy in young patients: a KASID study. J Gastroenterol Hepatol 2016;31:138–44. 10.1111/jgh.13167 [DOI] [PubMed] [Google Scholar]
- 99. Lee TJW, Nickerson C, Goddard AF, et al. Outcome of 12-month surveillance colonoscopy in high-risk patients in the National Health Service bowel cancer screening programme. Colorectal Dis 2013;15:e435–42. 10.1111/codi.12278 [DOI] [PubMed] [Google Scholar]
- 100. Cubiella J, Carballo F, Portillo I, et al. Incidence of advanced neoplasia during surveillance in high- and intermediate-risk groups of the European colorectal cancer screening guidelines. Endoscopy 2016;48:995–1002. 10.1055/s-0042-112571 [DOI] [PubMed] [Google Scholar]
- 101. van Heijningen E-MB, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology 2013;144:1410–8. 10.1053/j.gastro.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 102. Huang Y, Gong W, Su B, et al. Risk and cause of interval colorectal cancer after colonoscopic polypectomy. Digestion 2012;86:148–54. 10.1159/000338680 [DOI] [PubMed] [Google Scholar]
- 103. Bonnington SN, Sharp L, Rutter MD. Post-polypectomy surveillance in the English bowel cancer screening programme: multivariate logistic regression of factors influencing advanced adenoma detection at first surveillance (Abstract). Endoscopy 2019;51:S153. [Google Scholar]
- 104. Bonnington SN, Sharp L, Rutter MD. Post-polypectomy surveillance in the English bowel cancer screening programme: results of first surveillance (Abstract). Endoscopy 2019;51:S116. [DOI] [PubMed] [Google Scholar]
- 105. Bonnington SN, Sharp L, Rutter MD. Post-polypectomy surveillance in the English bowel cancer screening programme: results of second surveillance (Abstract). Endoscopy 2019;51:S138. [DOI] [PubMed] [Google Scholar]
- 106. Laiyemo AO, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med 2008;148:419–26. 10.7326/0003-4819-148-6-200803180-00004 [DOI] [PubMed] [Google Scholar]
- 107. Nusko G, Hahn EG, Mansmann U. Risk of advanced metachronous colorectal adenoma during long-term follow-up. Int J Colorectal Dis 2008;23:1065–71. 10.1007/s00384-008-0508-y [DOI] [PubMed] [Google Scholar]
- 108. Laish I, Seregeev I, Naftali T, et al. Surveillance after positive colonoscopy based on adenoma characteristics. Dig Liver Dis 2017;49:1115–20. 10.1016/j.dld.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 109. Solakoğlu T, Köseoğlu H, Özer Sarı S, et al. Role of baseline adenoma characteristics for adenoma recurrence in patients with high-risk adenoma. Turk J Med Sci 2017;47:1416–24. 10.3906/sag-1502-105 [DOI] [PubMed] [Google Scholar]
- 110. Coleman HG, Loughrey MB, Murray LJ, et al. Colorectal cancer risk following adenoma removal: a large prospective population-based cohort study. Cancer Epidemiol Biomarkers Prev 2015;24:1373–80. 10.1158/1055-9965.EPI-15-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Emilsson L, Løberg M, Bretthauer M, et al. Colorectal cancer death after adenoma removal in Scandinavia. Scand J Gastroenterol 2017;52:1377–84. 10.1080/00365521.2017.1377763 [DOI] [PubMed] [Google Scholar]
- 112. Løberg M, Kalager M, Holme Øyvind, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014;371:799–807. 10.1056/NEJMoa1315870 [DOI] [PubMed] [Google Scholar]
- 113. Huang Y, Li X, Wang Z, et al. Five-year risk of colorectal neoplasia after normal baseline colonoscopy in asymptomatic Chinese Mongolian over 50 years of age. Int J Colorectal Dis 2012;27:1651–6. 10.1007/s00384-012-1516-5 [DOI] [PubMed] [Google Scholar]
- 114. Jang HW, Park SJ, Hong SP, et al. Risk factors for recurrent high-risk polyps after the removal of high-risk polyps at initial colonoscopy. Yonsei Med J 2015;56:1559–65. 10.3349/ymj.2015.56.6.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lee JL, Cha JM, Lee HM, et al. Determining the optimal surveillance interval after a colonoscopic polypectomy for the Korean population? Intest Res 2017;15:109–17. 10.5217/ir.2017.15.1.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vemulapalli KC, Rex DK. Risk of advanced lesions at first follow-up colonoscopy in high-risk groups as defined by the United Kingdom post-polypectomy surveillance guideline: data from a single U.S. center. Gastrointest Endosc 2014;80:299–306. 10.1016/j.gie.2014.02.1029 [DOI] [PubMed] [Google Scholar]
- 117. Jung YS, Park DI, Kim WH, et al. Risk of advanced colorectal neoplasia according to the number of high-risk findings at index colonoscopy: a Korean Association for the Study of Intestinal Disease (KASID) study. Dig Dis Sci 2016;61:1661–8. 10.1007/s10620-016-4038-0 [DOI] [PubMed] [Google Scholar]
- 118. Cottet V, Jooste V, Fournel I, et al. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut 2012;61:1180–6. 10.1136/gutjnl-2011-300295 [DOI] [PubMed] [Google Scholar]
- 119. Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol 2009;7:86–92. 10.1016/j.cgh.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 120. Morelli M, Glowinski E, Juluri R, et al. Yield of the second surveillance colonoscopy based on the results of the index and first surveillance colonoscopies. Endoscopy 2013;45:821–6. 10.1055/s-0033-1344582 [DOI] [PubMed] [Google Scholar]
- 121. Imperiale TF, Juluri R, Sherer EA, et al. A risk index for advanced neoplasia on the second surveillance colonoscopy in patients with previous adenomatous polyps. Gastrointest Endosc 2014;80:471–8. 10.1016/j.gie.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 122. Tae CH, Moon CM, Jung S-A, et al. Higher body mass index is associated with an increased risk of multiplicity in surveillance colonoscopy within 5 years. Sci Rep 2017;7:14239 10.1038/s41598-017-14163-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kim HG, Cho Y-S, Cha JM, et al. Risk of metachronous neoplasia on surveillance colonoscopy in young patients with colorectal neoplasia. Gastrointest Endosc 2018;87:666–73. 10.1016/j.gie.2017.05.053 [DOI] [PubMed] [Google Scholar]
- 124. Holme Øyvind, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut 2015;64:929–36. 10.1136/gutjnl-2014-307793 [DOI] [PubMed] [Google Scholar]
- 125. He X, Hang D, Wu K, et al. Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology 2019. 10.1053/j.gastro.2019.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology 2016;150:895–902. 10.1053/j.gastro.2015.11.046 [DOI] [PubMed] [Google Scholar]
- 127. Anderson JC, Butterly LF, Robinson CM, et al. Risk of metachronous high-risk adenomas and large serrated polyps in individuals with serrated polyps on index colonoscopy: data from the New Hampshire Colonoscopy Registry. Gastroenterology 2018;154:117–27. 10.1053/j.gastro.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Symonds E, Anwar S, Young G, et al. Sessile serrated polyps with synchronous conventional adenomas increase risk of future advanced neoplasia. Dig Dis Sci 2019;64:1680–5. 10.1007/s10620-019-5454-8 [DOI] [PubMed] [Google Scholar]
- 129. Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010;139:1497–502. 10.1053/j.gastro.2010.06.074 [DOI] [PubMed] [Google Scholar]
- 130. FI L, van Niekerk de W, Owen D, et al. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol 2010;34:927–34. [DOI] [PubMed] [Google Scholar]
- 131. Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med Overseas Ed 1993;329:1977–81. 10.1056/NEJM199312303292701 [DOI] [PubMed] [Google Scholar]
- 132. Winawer SJ, Zauber AG, O'Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med Overseas Ed 1993;328:901–6. 10.1056/NEJM199304013281301 [DOI] [PubMed] [Google Scholar]
- 133. Chung SJ, Kim YS, Yang SY, et al. Five-Year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011;60:1537–43. 10.1136/gut.2010.232876 [DOI] [PubMed] [Google Scholar]
- 134. Miller J, Mehta N, Feldman M, et al. Findings on serial surveillance colonoscopy in patients with low-risk polyps on initial colonoscopy. J Clin Gastroenterol 2010;44:e46–50. 10.1097/MCG.0b013e3181a7ed2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gavin DR, Valori RM, Anderson JT, et al. The National Colonoscopy Audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2013;62:242–9. 10.1136/gutjnl-2011-301848 [DOI] [PubMed] [Google Scholar]
- 136. Nelson DB, McQuaid KR, Bond JH, et al. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc 2002;55:307–14. 10.1067/mge.2002.121883 [DOI] [PubMed] [Google Scholar]
- 137. Silvis SE, Nebel O, Rogers G, et al. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy survey. JAMA 1976;235:928–30. 10.1001/jama.235.9.928 [DOI] [PubMed] [Google Scholar]
- 138. Rutter M, Nickerson C, Rees C, et al. Risk factors for adverse events related to polypectomy in the English bowel cancer screening programme. Endoscopy 2014;46:90–7. 10.1055/s-0033-1344987 [DOI] [PubMed] [Google Scholar]
- 139. Gondal G, Grotmol T, Hofstad B, et al. The Norwegian Colorectal Cancer Prevention (NORCCAP) screening study: baseline findings and implementations for clinical work-up in age groups 50-64 years. Scand J Gastroenterol 2003;38:635–42. [DOI] [PubMed] [Google Scholar]
- 140. Heldwein W, Dollhopf M, Rösch T, et al. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic SNARE polypectomies. Endoscopy 2005;37:1116–22. 10.1055/s-2005-870512 [DOI] [PubMed] [Google Scholar]
- 141. Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med 2006;145:880–6. 10.7326/0003-4819-145-12-200612190-00004 [DOI] [PubMed] [Google Scholar]
- 142. Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc 2001;53:620–7. 10.1067/mge.2001.114422 [DOI] [PubMed] [Google Scholar]
- 143. Panteris V, Haringsma J, Kuipers EJ, et al. Mechanisms and outcome: from diagnostic to therapeutic colonoscopy. Endoscopy 2009;41:941–51. [DOI] [PubMed] [Google Scholar]
- 144. Rosen L, Bub DS, Reed JF. Hemorrhage following colonoscopic polypectomy. Dis Colon Rectum 1993;36:1126–31. [DOI] [PubMed] [Google Scholar]
- 145. Wang L, Mannalithara A, Singh G, et al. Low rates of gastrointestinal and non-gastrointestinal complications for screening or surveillance colonoscopies in a population-based study. Gastroenterology 2018;154:540–55. 10.1053/j.gastro.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 146. Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 2008;135:1899–906. 10.1053/j.gastro.2008.08.058 [DOI] [PubMed] [Google Scholar]
- 147. Singh H, Penfold RB, DeCoster C, et al. Colonoscopy and its complications across a Canadian regional health authority. Gastrointest Endosc 2009;69:665–71. 10.1016/j.gie.2008.09.046 [DOI] [PubMed] [Google Scholar]
- 148. Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849–57. 10.7326/0003-4819-150-12-200906160-00008 [DOI] [PubMed] [Google Scholar]
- 149. García-Albéniz X, Hsu J, Bretthauer M, et al. Effectiveness of screening colonoscopy to prevent colorectal cancer among Medicare beneficiaries aged 70 to 79 years. Ann Intern Med 2017;166:18–26. 10.7326/M16-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Djinbachian R, Dube AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy 2019;51:673–83. [DOI] [PubMed] [Google Scholar]
- 151. National Health Service No. 26A: NHS bowel scope screening programme: Public Health England, 2018. Available: https://www.england.nhs.uk/wp-content/uploads/2017/04/Gateway-ref-07848-180913-Service-specification-No.-26A-NHS-Bowel-Scope-screening-programme.pdf
- 152. Kamiński MF, Hassan C, Bisschops R, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2014;46:435–57. 10.1055/s-0034-1365348 [DOI] [PubMed] [Google Scholar]
- 153. Bisschops R, East J, Hassan C, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: updated ESGE guidelinein press. Endoscopy 2019. [Google Scholar]
- 154. Vleugels JLA, Dijkgraaf MGW, Hazewinkel Y, et al. Effects of training and feedback on accuracy of predicting rectosigmoid neoplastic lesions and selection of surveillance intervals by endoscopists performing optical diagnosis of diminutive polyps. Gastroenterology 2018;154:1682–93. 10.1053/j.gastro.2018.01.063 [DOI] [PubMed] [Google Scholar]
- 155. East JE, Wang LM. Response. Gastrointest Endosc 2016;83:675–6. 10.1016/j.gie.2015.11.034 [DOI] [PubMed] [Google Scholar]
- 156. Mori Y, Kudo S-ei, Misawa M, et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy. Ann Intern Med 2018;169:357–66. 10.7326/M18-0249 [DOI] [PubMed] [Google Scholar]
- 157. Byrne MF, Chapados N, Soudan F, et al. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut 2019;68:94–100. 10.1136/gutjnl-2017-314547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vleugels JLA, Hassan C, Senore C, et al. Diminutive polyps with advanced histologic features do not increase risk for metachronous advanced colon neoplasia. Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- 159. Sano W, Fujimori T, Ichikawa K, et al. Clinical and endoscopic evaluations of sessile serrated adenoma/polyps with cytological dysplasia. J Gastroenterol Hepatol 2018;33:1454–60. 10.1111/jgh.14099 [DOI] [PubMed] [Google Scholar]
- 160. Atkinson NS, East JE. Optical biopsy and sessile serrated polyps: is discard dead? Long live DISCARD-lite! Gastrointest Endosc 2015;82:118–21. 10.1016/j.gie.2015.01.059 [DOI] [PubMed] [Google Scholar]
- 161. von Renteln D, Kaltenbach T, Rastogi A, et al. Simplifying resect and discard strategies for real-time assessment of diminutive colorectal polyps. Clin Gastroenterol Hepatol 2018;16:706–14. 10.1016/j.cgh.2017.11.036 [DOI] [PubMed] [Google Scholar]
- 162. Moss S, Mathews C, Day TJ, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the National screening programme in England. Gut 2017;66:1631–44. 10.1136/gutjnl-2015-310691 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2019-319858supp001.xlsx (20KB, xlsx)
gutjnl-2019-319858supp002.pdf (1.4MB, pdf)
gutjnl-2019-319858supp003.pdf (343.1KB, pdf)
gutjnl-2019-319858supp004.pdf (212.1KB, pdf)
gutjnl-2019-319858supp005.pdf (698.8KB, pdf)