Abstract
Background/Aim: Dermal mesenchymal stem cells (DMSCs) are pluripotent stem cells found in the skin which maintain the thickness of the dermal layer and participate in skin wound healing. Materials and Methods: The MTT assay was performed to detect cell proliferation and cell-cycle progression and cell-surface markers were assessed by flow cytometry. The levels of proteins in related signaling pathways were detected by western blotting assay and the translocation of β-catenin into the nucleus were detected by immunofluorescence. Red oil O staining was performed to examine the differentiational ability of DMSCs. Results: Knockout of PRDX2 inhibited DMSC cell growth, and cell-cycle arrest at G0/G1 phase; p16, p21 and cyclin D1 expression levels in Prdx2 knockout DMSCs were significantly increased. Furthermore, AKT phosphorylation were significantly increased in Prdx2 knockout DMSCs, GSK3β activity were inhibited, result in β-Catenin accumulated in the nucleus. Conclusion: In conclusion, these results demonstrated that PRDX2 plays a pivotal role in regulating the proliferation of DMSCs, and this is closely related to the AKT/glycogen synthase kinase 3 beta/β-catenin signaling pathway.
Keywords: Peroxiredoxin II, cell cycle, mesenchymal stem cells, glycogen synthase kinase 3 beta/β-catenin signaling
Skin has a strong capacity for repair and regeneration due to the fact that it contains various stem cells, such as epidermal stem cells, skin-derived precursors and dermal mesenchymal stem cells (DMSCs) (1,2). DMSCs play two major roles, one is as a direct source of fibroblasts, and the other is to secrete a variety of cytokines which promote fibroblasts to produce collagen and elastin (3). Therefore, the function of DMSCs plays decisive role in skin development and wound repair (4).
Peroxiredoxin 2 (PRDX2) is wildly distributed in various tissues and cells, and functions as a scavenger of reactive oxygen species (ROS) (5,6). Our previous study showed that Prdx2 knockout mice had significant symptoms of skin aging, which are characterized by reduced skin thickness and thinning of the dermis (7), suggesting that PRDX2 may play a regulatory role in dermal cells. We also reported that Prdx2 knockout can induced cellular senescence of embryonic fibroblasts through ROS-dependent signaling pathway (7), however, the role of PRDX2 in the regulation of DMSC proliferation is not clear.
The Wnt signal is activated by Wnt ligand binding to a frizzled receptor (8). In the absence of Wnt ligands, the downstream signaling molecule β-catenin can be phosphorylated by glycogen synthase kinase 3 beta (GSK3β) and then phosphorylated β-catenin is degraded by ubiquitination (9).When Wnt ligand binds to the receptor, the phosphorylation of β-catenin by GSK3β is inhibited, which results in accumulation of β-catenin in the cytoplasm, it finally being transferred to the nucleus to induce the expression of target genes (10,11). Therefore, phosphorylation of β-catenin and GSK3β is a marker distinguishing the activation of the classical Wnt/β-catenin signal (12). Previous studies have shown that PRXs play a role in cell proliferation via Wnt signaling. It has been reported that knockdown of PRDX2 can inhibit the growth of colon cancer cells by inhibiting Wnt signaling pathway (13), but down-regulation of PRDX5 inhibited the growth of chondrocytes through activating the Wnt signaling pathway (14).These findings suggests that PRXs may participate in cell proliferation by modulating Wnt signaling in different ways. The role of PRDX2 and Wnt signaling in the regulation of DMSC proliferation has not been elucidated.
Therefore, in this study, we used both wild-type and Prdx2 knockout DMSCs to study the effect of PRDX2 on DMSC proliferations and molecular mechanisms, especially on activation of β-catenin signaling under normal cell culture conditions, in order to understand the regulatory function of PRDX2 in DMSC growth.
Materials and Methods
Animals. Prdx2+/+ (wild-type) and Prdx2–/– 129/SvJ mice were provided by the Korea Research Institute of Bioscience and Biotechnology (KRIBB). Mice were kept under the temperature at 20-22˚C, the humidity 50-60% and the 12-h-dark/light cycles conditions and provided food and water ad libitum. The Institutional Animal Care and Use Committee approved both the animal care and experiments.
Cell isolation and cell culture. The dorsal skin of newborn wild-type and Prdx2–/– mice was collected and spread in 3.5 cm culture dish with 2 ml 0.25% trypsin-EDTA (T/E; Solarbio, Beijing, China) for 4 h at 4˚C. The subcutaneous fat and blood vessels on skin were removed using tweezers. The skin was then treated for 1 h at 37 ˚C in T/E to Separation of dermis and epidermis. The epidermal-free tissue were dissected into 1.0 cm2 pieces and digested with 0.25% T/E for 1 h at 37˚C. After that, the cells were collected by centrifugation at 300 × g for 7 minutes and cultured in growth medium consisting of Dulbecco’s modified Eagle’s medium (Gibco-BRL, Grand Island, NY, USA) and Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (Gibco-BRL) with 2 mM L-glutamine and 1% non-essential amino acid solution (NEAA; Solarbio, Beijing, PR China), containing 10% fetal bovine serum (FBS; Solarbio) and 1% 100 U/ml penicillin-streptomycin (P/S; Solarbio). The cells were cultured at 37˚C under 5% CO2.
Detection of surface antigens. Prdx2+/+ (wild-type) and Prdx2–/– DMSCs from passage 3 were seeded in six-well plates at density of 3×105 cells/well. After cell adherence for 24 h, the cells were washed with phosphate buffer (PBS; Solarbio, Beijing, PR China) twice, permeabilized, and then immune stained with the following fluorochrome-conjugated antibodies overnight at 4˚C: anti-CD44-phycoerythrin (PE), anti-CD106-PE, anti-CD14-fluorescein isothiocyanate (FITC), anti-CD34-PE and anti-CD45-FITC (BioLegend, San Diego, CA, USA).The MSC phenotypes of DMSCs were characterized by flow cytometric analysis (FACScan; BD Biosciences, San Jose, CA, USA).
Multilineage differentiation potential of DMSCs. DMSCs from passage 3 were seeded in a 3.5 cm culture dish and cultured with osteogenic differentiation medium (Solarbio) when cells reached 70% confluence. The culture medium was changed every 2 days. After 21 days of differentiation, the cells were permeabilized with 70% alcohol for 10 min and stained with 0.1% alizarin red S pH 8.3 for 7 min (Sigma-Aldrich, St. Louis, MO, USA). Finally, images were obtained by fluorescence microscopy coupled with a camera (Leica DM2500, Germany).
The double types of DMSCs from passage 3 (3 P) were seeded in 3.5 cm culture dish and cultured with adipogenic differentiation medium (Solarbio) when cells reached 70% confluence. The culture medium was changed every 2 days. After 15 days of differentiation, the cells were permeabilized with 4% paraformaldehyde for 1 h, treated with 60% isopropanol for 2 min and stained with 0.5% Oil-red O for 20 min (Solarbio). Finally, images were obtained with a DM2500 camera (Leica, Wetzlar, Germany).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells (2.5×103 cells/well) were cultured in 96-well plates with growth medium at 37˚C for 1, 2, 5, and 7 days. MTT (10 μl of 5 mg/ml) was added at specific times during incubation for 4 h and formazan was dissolved using dimethyl sulfoxide (100 μl). A micro-plate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) was used to measure the absorbance of cells at 570 nm. Each condition was determined in sextuplicate, and all results were repeated at least three times.
Flow cytometric analysis. DMSCs from different passages (3, 6 and 12) were seeded in six-well plates at the same density (3×105 cells/well). After cell adherence for 24 h, the cells were washed with PBS twice and suspended in PBS (−20˚C) containing 70% ethyl alcohol for 24 h. Subsequently, the cells were stained with propidium iodide (PI)/RNase staining solution in the dark for 30 min at 37˚C, and analyzed using flow cytometry (FACScan; BD Biosciences, San Jose, CA, USA).
Western blot analysis. The cells were lysed in RIPA buffer, Lysates were incubated for 30 min on ice and centrifuged at 13,201 × g for 5 min at 4˚C Proteins were boiled for 5 min and separated on a 12% polyacrylamide gel. Protein expression and phosphorylation were monitored with specific antibodies and chemiluminescent horseradish peroxidase substrate (ZSGB-BIO, Beijing, PR China). Primary antibodies used in this study were as follows: anti-PRDX2 (Abfrontier, Seoul, Republic of Korea), anti-proliferating cell nuclear antigen (PCNA), anti-signal transducer and activator of transcription 3 (STAT3), anti p-STAT3, anti-p21, anti-p16, anti-cyclin D1, anti-AKT serine/threonine kinase 1 (AKT) anti-p-AKT, anti-GSK3β, anti-p-GSK3β, anti-β-catenin, anti-p-β-catenin and anti-α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Secondary antibodies used were, Goat anti-mouse and Goat anti-rabbit (ZSGB-BIO) and the images were quantified using Image J software (https://imagej.nih.gov/ij/index.html, National Institutes of Health, Bethesda, MD, USA).
Cellular immunofluorescence staining. DMSCs were cultured in 24-well plates at a density of 1.5×104 cells/well. After 24 h, cells were treated with 4% paraformaldehyde at room temperature to maintain cellular shape for 10 min and washed twice with PBS. After that, cells were incubated with β-catenin antibody (Beyotime, Haimen, PR China) for overnight. The cells were then treated with Alexa Fluor™ 633 dye (Invitrogen, Carlsbad, CA, USA) for 1 h and the nuclei were labeled with Hoechst 33342 (Invitrogen) and finally positive cells were detected by fluorescence microscopy.
Statistical analysis. All of the data were analyzed by Student t-test. All results are expressed as mean±SEM from at least three independent repeated experiments. Statistical significance was assumed for p<0.05.
Results
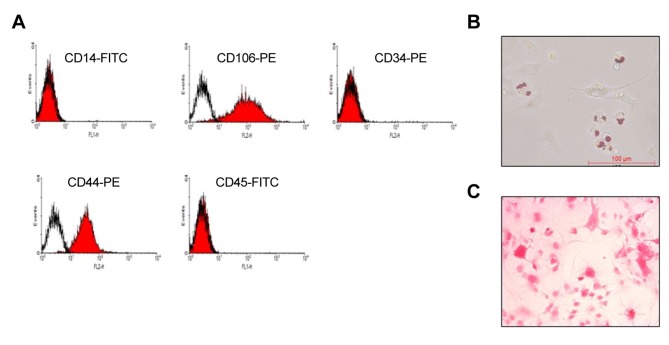
Isolation and characterization of primary DMSCs. The DMSCs were isolated through the protocol described in the Materials and Methods, and then characterized by staining for CD106, CD44 and negative marker of CD14, CD34 and CD45 (15-18). As shown in Figure 1A, the isolated cells strongly stained with antibodies to CD106 and CD44, and low binding affinity with CD14, CD34 and CD45 antibodies. Since DMSCs have stem cell characteristics, we also examined the differentiation potential of the DMSCs. The results show that the isolated cells were strongly stained by red oil red O and alizarin red (Figure 1 B and C), suggesting that the isolated DMSCs maintained stem cell characteristics, and were suitable for use in subsequent experiments.
Figure 1. Characterization of isolated dermal mesenchymal stem cells (DMSCs). A: Representative images from flow cytometry show the expression of surface markers of DMSCs isolated from newborn mice. Microscope images showing that isolated DMSCs can differentiate into adipocytes (B) and osteocytes (C). Scale bar: 100 μm. FITC: Fluorescein isothiocyanate; PE: phycoerythrin.
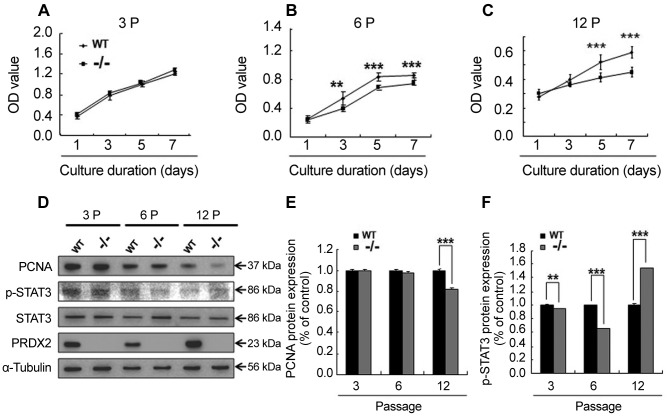
Knockout of PRDX2 inhibits DMSC cell growth. To understand the effect of Prdx2 deletion on DMSC proliferation, the wild-type and Prdx2 knockout DMSCs were cultured for 1, 3, 5 and 7 days). The results showed that in early passages (passage 3), there were no significant differences between the growth of wild-type and Prdx2–/– DMSCs (Figure 2A), while in the late passages (6 and 12), Prdx2–/– DMSCs exhibited delay cell growth (Figure 2B and C) as detected by MTT assay. Therefore, we also checked the expression of PCNA and STAT3 proteins which are involved in cell proliferation. As shown in Figure 2D-F, with increasing culture passage, expression of PCNA protein was down-regulated in primary Prdx2–/– DMSCs as was that of p-STAT3 protein.
Figure 2. Knockout of peroxiredoxin 2 (PRDX2) inhibits dermal mesenchymal stem cell (DMSC) growth. A-C: The growth curve of DMSCs from different passages (3 P, 6 P and 12 P). D: Western blot analysis showing that the proliferation-related signal in Prdx2+/+ (WT) and Prdx2–/– (–/–) DMSCs is inhibited at different passages. E and F: Quantification of western blot analysis by ImageJ software. Data are presented as the mean±SEM. Significantly different at *p<0.05, **p<0.01, and ***p<0.001. GSK3β: Glycogen synthase kinase 3 beta; PCNA: proliferating cell nuclear antigen; STAT3: signal transducer and activator of transcription 3.
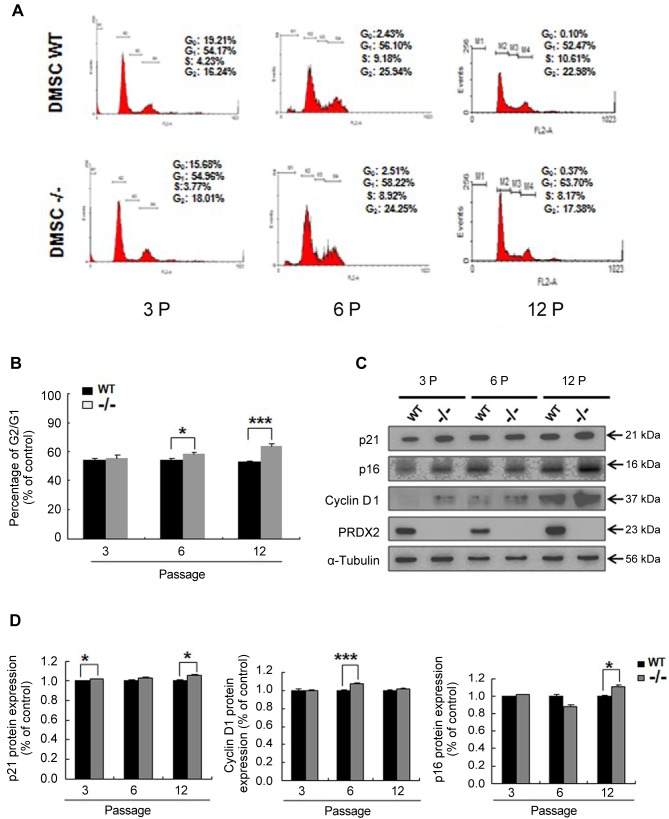
Deletion of Prdx2 increases G0/G1 cell-cycle arrest of DMSCs. Since Prdx2 deletion inhibited DMSC growth (Figure 2), we hypothesized that it may affect the cell cycle processing, which is a key point of regulation of cell proliferation. To verify this, wild-type and Prdx2 knockout primary DMSCs were stained with PI/RNase solution to examine the cell cycle. The results showed that late passage (6 and 12 passage) DMSCs exhibited significant cell-cycle arrest, marked by G0/G1 cell accumulation, but not in early passages (passage 3) (Figure 3A and B). This strongly supports the cell growth results shown in Figure 2. In order to understand the possible molecular regulatory mechanism of PRDX2 in cell-cycle arrest, we also compared the expression of cell cycle-related proteins between wild-type and Prdx2–/– DMSCs. The results showed that expression of p21 and p16 proteins, known as cell-cycle inhibitors, were significantly increased in Prdx2–/– DMSCs, as well as cyclin D1 (Figure 3C and D).
Figure 3. Deletion of peroxiredoxin 2 (Prdx2) increases cell-cycle arrest of dermal mesenchymal stem cells (DMSCs) in G0/G1. A: Flow cytometric analysis showing cell cycle of DMSCs at three distinct passages. B: Quantification of G0/G1 phase at three distinct passages. Deletion of Prdx2 (Prdx2–/–) led to G0/G1 cell-cycle arrest of DMSCs. C: Western blotting was used to measure the expression of cell cycle-associated proteins. D: Quantification of western blot analysis by ImageJ software. Significantly different at *p<0.05, and ***p<0.001. WT: Wild-type Prdx2+/+.
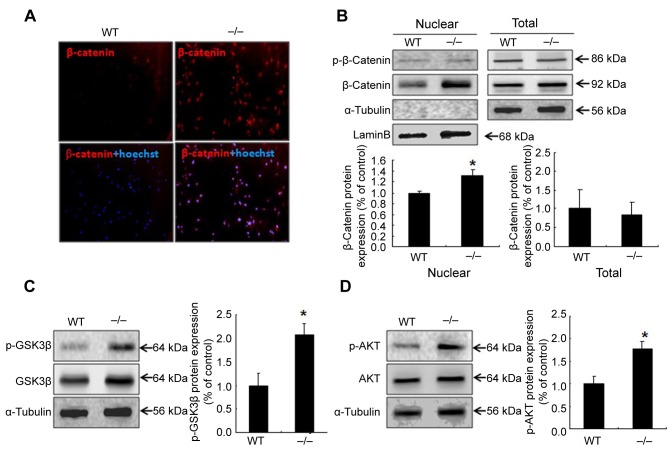
Up-regulation of GSK/β-catenin signaling in Prdx2–/– knockout DMSCs. GSK3β/β-catenin is involved in various aspects of cell growth and differentiations through regulating the cell cycle related protein expressions (19-22). Our findings suggest that PRDX2 gene knockout could dramatically inhibit the cell growth by affect the cell cycle related proteins expressions (Figure 3), we hypotheses that PRDX2 knockout may also affect the GSK/β-catenin signaling pathway. To verify this, we examined the β-catenin expression levels in the wild-type and Prdx2–/– DMSCs. The result showed that up-regulation of β-catenin in Prdx2–/– DMSCs compared with wild-type, as observed from immunocytochemistry (Figure 4A). Translocation of β-catenin is a mark of β-catenin activation, thus, we examined nuclear β-catenin protein expression between wild-type and Prdx2–/– DMSCs. The results showed that deletion of Prdx2 increased the expression of nuclear β-catenin compared with wild-type DMSCs, while there was no significant difference in total cell lysate (Figure 4B). Since β-catenin is activated by GSK phosphorylation, we also assessed p-GSK and p-AKT expression between wild-type and Prdx2–/– DMSCs. The results showed significant up-regulation of p-GSK and p-AKT in Prdx2–/– DMSCs compared with wild-type (Figure 4C and D).
Figure 4. Up-regulation of glycogen synthase kinase 3 beta (GSK3β)/β-catenin signaling in dermal mesenchymal stem cells (DMSCs) with knockout of peroxiredoxin 2 (Prdx2–/–). The expression of GSK/β-catenin signaling proteins analyzed with immunofluorescence (A) and western blot (B-D) implied that lack of Prdx2–/– inhibits DMSCs growth via GSK/β-catenin signaling. *Significantly different at p<0.05. WT: Wild-type Prdx2+/+.
Discussion
DMSCs are pluripotent stem cells in the dermis (23-25). DMSCs can activate fibroblasts, stimulate secretion of collagen and promote the proliferation of fibroblasts and epidermal cells by secreting a series of cytokines such as tumor growth factor β, vascular endothelial growth factor, platelet-derived growth factor and hepatocyte growth factor (26,27), therefore, the number and function of DMSCs play an important role in skin maintenance and homeostasis (28). DMSCs are a kind of seed cells suitable for tissue engineering because of their rapid proliferation, multi-directional differentiation and low immunogenicity (29). However, in vitro culture and proliferation of DMSCs is difficult, and it is easy to cause the loss of stemness, which limits its wide application. Therefore, there is a need to establish optimal culture conditions to study the key physiological and biochemical factors of DMSC self-renewal, so that DMSCs can maintain stemness and normal physiological functions after massive expansion to better research findings apply to clinical practice.
PRDX2 plays an important role in the proliferation and differentiation of many kinds of stem cells. Nitrosylation of PRDX2 can promote cardiac formation of mouse embryonic stem cells through X-box binding protein-1s/phosphati-dylinositol-4,5-bisphosphate 3-kinase (PI3K) signaling pathway (30); PRDX2 has also been shown to regulate the differentiation of embryonic stem cells into neurons (31). Compared with primary stem cells, the regulation of stem cell stemness by PRDX2 comes more from the study of cancer stem cells (6,32). Silencing PRDX2 gene resulted in the decrease of NANOG expression in colon cancer stem cells, and cell proliferation and migration were significantly reduced (6). The expression of SRY-related high-mobility-group-box protein-2 and octamer-binding transcription factor-4 was down-regulated in PRDX2-silenced Huh7-H-RasG12V hepatoma cells, and sphere formation efficiency and epithelial–mesenchymal transition were significantly inhibited (6). Mechanistic studies showed that PRDX2 can regulate stemness of cancer stem cells through a variety of signaling pathways, which contribute to the maintenance of the cancer stem cell properties of hepatocellular carcinoma via VEGF/EGFR/STAT3 signaling and RAS/FOXM1 signaling (33-35). In the process of isolation and expansion of DMSCs, we found that knockout of PRDX2 can significantly inhibit the proliferation of stem cells with the increasing cell passage. Prdx2–/– DMSCs exhibited cell-cycle arrest in the sixth and 12th passage; p16, p21 and cyclin D1 expression levels in Prdx2–/– DMSCs were also significantly increased, indicating that knockout of the PRDX2 in mesenchymal stem cells leads to cell-cycle arrest, thereby affecting the proliferation of stem cells.
β-Catenin is a key element in driving the Wnt/β-catenin signaling pathway, and GSK3β is a critical kinase regulating β-catenin phosphorylation (36,37). When the activity of GSK3β kinase is inhibited, unphosphorylated β-catenin can accumulate in the cytoplasm without being degraded by proteasomes, and be transferred into the nucleus, where it binds to transcription factors and forms transcriptionally active complexes that initiate transcription of genes such as c-MYC, cyclin D1, and CD44(38), thereby regulating cell proliferation and differentiation (39). Our results showed that the expression of p-GSK3β(Ser9) increased in Prdx2–/– DMSCs with increasing cell passage, and β-catenin accumulated in the nucleus, indicating that β-catenin in the cytoplasm could not be phosphorylated and degraded by ubiquitination. The results of this study show that knockout of Prdx2 in DMSCs can reduce the activity of GSK3β and promote the translocation of β-catenin into the nucleus, thereby activating the downstream signaling pathways. However, activation of this signaling pathway did not improve cell proliferation caused by PRDX2 knockout, high expression of cyclin D1 also did not prevent cell-cycle arrest (40).The kinase activity of GSK3β can be regulated by different signaling pathways, in which phosphorylated AKT can phosphorylate GSK3β to inactivate it (12,37,41). In Prdx2–/– DMSCs, the phosphorylation of AKT increased as the number of cell passages increased, this is most likely achieved through cross talk between the PRDX2, PDGF and PI3K signals (42).
We conclude that PRDX2 plays an important role in regulating the proliferation of DMSCs, and this regulation is closely related to the AKT/GSK3β/β-catenin signaling pathway. However, the specific regulatory mechanism involved remains to be further studied.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03028188), KRIBB-OGM5201922, and by the grants from the KRIBB Research Initiative Program (KGM5161914, KGM4251913).
Conflicts of Interest
The Authors declare that there are no conflicts of interest in regard to this study.
Authors’ Contributions
Y.H.H., M.H.J., T.K. and H.N.S. performed the experiments and wrote the article. Y.H.J., N.N.Y., J.L., Y.Q.Z., Y.D.C., A.G.W., D.S.L., S.U.K., J.S.K. and Y.H.H. performed the data analysis. Y.H.H., T.K., H.N.S. made substantial contributions to conception and design. All Authors read and approved the final article.
Acknowledgements
Support for the present study was provided by the Scientific Research Foundation of Heilongjiang Provincial Education Department of China (1253HQ008, 1253HQ011) and the Research Project of Heilongjiang Bayi Agricultural University (XYB2012-12).
References
- 1.Li X, Li J, Zhao X, Wang Q, Yang X, Cheng Y, Zhou M, Wang G, Dang E, Yang X. Comparative analysis of molecular activity in dermal mesenchymal stem cells from different passages. Cell and tissue banking. 2018;19(3):277–285. doi: 10.1007/s10561-017-9672-z. PMID: 29159500. DOI: 10.1007/s10561-017-9672-z. [DOI] [PubMed] [Google Scholar]
- 2.Guo L, Wang X, Yuan J, Zhu M, Fu X, Xu R-H, Wu C, Wu Y. Tsa restores hair follicle-inductive capacity of skin-derived precursors. Sci Rep. 2019;9(1):2867. doi: 10.1038/s41598-019-39394-w. PMID: 30814580. DOI: 10.1038/s41598-019-39394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beate H, Douaa D, Salita E, Yousef S, Hiroshi S, Shinya M, Koichi H. Role of mesenchymal stem cell-derived fibrinolytic factor in tissue regeneration and cancer progression. Cell Mol Life Sci. 2015;72(24):4759–4770. doi: 10.1007/s00018-015-2035-7. PMID: 26350342. DOI: 10.1007/s00018-015-2035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gundogdu G, Gundogdu K, Nalci KA, Demirkaya AK, Yilmaz Tasci S, Demirkaya Miloglu F, Senol O, Hacimuftuoglu A. The effect of parietin isolated from rheum ribes l on in vitro wound model using human dermal fibroblast cells. Int J Low Extrem Wounds. 2019;18(1):56–64. doi: 10.1177/1534734618819660. PMID: 30612496. DOI: 10.1177/1534734618819660. [DOI] [PubMed] [Google Scholar]
- 5.Moon EY, Han YH, Lee DS, Han YM, Yu DY. Reactive oxygen species induced by the deletion of peroxiredoxin II (PRXII) increases the number of thymocytes resulting in the enlargement of PRXII-null thymus. Eur J Immunol. 2004;34(8):2119–2128. doi: 10.1002/eji.200424962. PMID: 15259009. DOI: 10.1002/eji.200424962. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Wei J, Zhang S, Wu X, Guo J, Liu M, Du K, Xu J, Peng L, Lv Z. Peroxiredoxin 2 is essential for maintaining cancer stem cell-like phenotype through activation of hedgehog signaling pathway in colon cancer. Oncotarget. 2016;7(52):86816. doi: 10.18632/oncotarget.13559. PMID: 27894099. DOI: 10.18632/oncotarget.13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han YH, Kim HS, Kim JM, Kim SK, Yu DY, Moon EY. Inhibitory role of peroxiredoxin II (PRDX2 ) on cellular senescence. FEBS Lett. 2005;579(21):4897–4902. doi: 10.1016/j.febslet.2005.07.049. PMID: 16109412. DOI: 10.1016/j.febslet.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Fan X, Xing L, Tian F. Wnt signaling: A promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1):97–97. doi: 10.1186/s12964-019-0411-x. PMID: 31420042. DOI: 10.1186/s12964-019-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan V, Unda BK, Singh KK. Wnt signaling networks in autism spectrum disorder and intellectual disability. J Neurodev Disord. 2016;8:45. doi: 10.1186/s11689-016-9176-3. PMID: 27980692. DOI: 10.1186/s11689-016-9176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wodarz A, Nusse R. Mechanisms of wnt signaling in development. Ann Rev Cell Dev Biol. 1998;14(1):59–88. doi: 10.1146/annurev.cellbio.14.1.59. PMID: 9891778. DOI: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. PMID: 17081971. DOI: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Woodgett JR. Glycogen synthase kinase 3: A kinase for all pathways. Curr Top Dev Biol. 2017;123:277–302. doi: 10.1016/bs.ctdb.2016.11.011. PMID: 28236969. DOI: 10.1016/bs.ctdb.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Lu W, Fu Z, Wang H, Feng J, Wei J, Guo J. Peroxiredoxin 2 knockdown by rna interference inhibits the growth of colorectal cancer cells by downregulating wnt/β-catenin signaling. Cancer Lett. 2014;343(2):190–199. doi: 10.1016/j.canlet.2013.10.002. PMID: 24125860. DOI: 10.1016/j.canlet.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Li R, Zhang Y, Zhou L, Dai Y. Knockdown of peroxiredoxin 5 inhibits the growth of osteoarthritic chondrocytes via upregulating wnt/β-catenin signaling. Free Rad Biol Med. 2014;76:251–260. doi: 10.1016/j.freeradbiomed.2014.08.015. PMID: 25236745. DOI: 10.1016/j.freeradbiomed.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Chen L, Liu Y, Luo B, Xie N, Tan T, Song L, Erli P, Luo M. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am J Transl Res. 2016;8(11):4912. PMID: 27904691. [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao Y, Wang X, Zhang J, Qi Y, Gong H, Jiang D. Inhibiting function of human fetal dermal mesenchymal stem cells on bioactivities of keloid fibroblasts. Stem Cell Res Ther. 2017;8(1):170. doi: 10.1186/s13287-017-0624-0. PMID: 28720118. DOI: 10.1186/s13287-017-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim W-S, Park B-S, Sung J-H, Yang J-M, Park S-B, Kwak S-J, Park J-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24. doi: 10.1016/j.jdermsci.2007.05.018. PMID: 17643966. DOI: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Park J-R, Kim E, Yang J, Lee H, Hong S-H, Woo H-M, Park S-M, Na S, Yang S-R. Isolation of human dermis derived mesenchymal stem cells using explants culture method: Expansion and phenotypical characterization. Cell Tissue Banking. 2015;16(2):209–218. doi: 10.1007/s10561-014-9471-8. PMID: 25163610. DOI: 10.1007/s10561-014-9471-8. [DOI] [PubMed] [Google Scholar]
- 19.Santhosh D. Brain region-specific neurovascular signaling and germinal matrix hemmorhage. Dis Models Mech. 2019 doi: 10.1242/dmm.041228. PMID: 31601549. DOI: 10.1242/dmm.041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puelles L, Martínez-Marin R, Melgarejo-Otalora P, Ayad A, Valavanis A, Ferran JL. Patterned vascularization of embryonic mouse forebrain and neuromeric topology of major human subarachnoidal arterial branches: Prosomeric mapping. Front Neuroanat. 2019;13:59. doi: 10.3389/fnana.2019.00059. PMID: 31275117. DOI: 10.3389/fnana.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Hou J, Fu X, Chen X, Wu J, Han X. TPA promotes the proliferation of lung fibroblasts and activates the Wnt/beta-catenin signaling pathway in idiopathic pulmonary fibrosis. Cell Cycle. 2019;18:1–10. doi: 10.1080/15384101.2019.1669997. PMID: 31550972. DOI: 10.1080/15384101.2019.1669997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Shi Q, Nan W, Wang Y, Wang S, Yang F, Li G. Ginkgolide b and bilobalide promote the growth and increase beta-catenin expression in hair follicle dermal papilla cells of American minks. Biofactors. 2019 doi: 10.1002/biof.1562. PMID: 31520488. DOI:10.1002/biof.1562. [DOI] [PubMed] [Google Scholar]
- 23.Bartsch G Jr, Yoo JJ, De Coppi P, Siddiqui MM, Schuch G, Pohl HG, Fuhr J, Perin L, Soker S, Atala A. Propagation, expansion, and multilineage differentiation of human somatic stem cells from dermal progenitors. Stem Cells Dev. 2005;14(3):337–348. doi: 10.1089/scd.2005.14.337. PMID: 31520488. DOI: 10.1089/scd.2005.14.337. [DOI] [PubMed] [Google Scholar]
- 24.Feisst V, Brooks AE, Chen C-JJ, Dunbar PR. Characterization of mesenchymal progenitor cell populations directly derived from human dermis. Stem Cells Dev. 2013;23(6):631–642. doi: 10.1089/scd.2013.0207. PMID: 24325341. DOI: 10.1089/scd.2013.0207. [DOI] [PubMed] [Google Scholar]
- 25.Natesan S, Stone II R, Chan RK, Christy RJ. Mesenchymal stem cell–based therapies for repair and regeneration of skin wounds. In: A Roadmap to Non-Hematopoietic Stem Cell-based Therapeutics. Elsevier. 2019;In:173–222. DOI: 10.1016/b978-0-12-811920-4.00008-2. [Google Scholar]
- 26.Jiang Z, Liu G, Meng F, Wang W, Hao P, Xiang Y, Wang Y, Han R, Li F, Wang L. Paracrine effects of mesenchymal stem cells on the activation of keratocytes. Br J Ophthalmol. 2017;101(11):1583–1590. doi: 10.1136/bjophthalmol-2016-310012. PMID: 28844046. DOI: 10.1136/bjophthalmol-2016-310012. [DOI] [PubMed] [Google Scholar]
- 27.Pakdemirli A, Toksöz F, Karadağ A, Misirlioğlu Hk, Başbinar Y, Ellidokuz H, Açikgöz O. Role of mesenchymal stem cellderived soluble factors and folic acid in wound healing. Turk J Med Sci. 2019;49(3) doi: 10.3906/sag-1901-231. PMID: 31070342. DOI: 10.3906/sag-1901-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther. 2015;6(1):24. doi: 10.1186/s13287-015-0001-9. PMID: 25881077. DOI: 10.1186/s13287-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez J, Boucher F, Lequeux C, Josset-Lamaugarny A, Rouyer O, Ardisson O, Rutschi H, Sigaudo-Roussel D, Damour O, Mojallal A. Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Res Ther. 2015;6(1):241. doi: 10.1186/s13287-015-0238-3. PMID: 26645735. DOI: 10.1186/s13287-015-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu B, Yu H, Wang Y, Pan Z, Zhang Y, Li T, Li L, Zhang W, Ge L, Chen Y, Ho CK, Zhu D, Huang X, Lou Y. Peroxiredoxin-2 nitrosylation facilitates cardiomyogenesis of mouse embryonic stem cells via XBP-1S/PI3K pathway. Free Radic Biol Med. 2016;97:179–191. doi: 10.1016/j.freeradbiomed.2016.05.025. PMID: 27261193. DOI: 10.1016/j.freeradbiomed.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Fathi A, Hatami M, Vakilian H, Han CL, Chen YJ, Baharvand H, Salekdeh GH. Quantitative proteomics analysis highlights the role of redox hemostasis and energy metabolism in human embryonic stem cell differentiation to neural cells. J Proteomics. 2014;101:1–16. doi: 10.1016/j.jprot.2014.02.002. PMID: 24530625. DOI: 10.1016/j.jprot.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Kwon T, Bak Y, Park YH, Jang GB, Nam JS, Yoo JE, Park YN, Bak IS, Kim JM, Yoon DY. Peroxiredoxin II is essential for maintaining stemness by redox regulation in liver cancer cells. Stem Cells. 2016;34(5):1188–1197. doi: 10.1002/stem.2323. PMID: 26866938. DOI: 10.1002/stem.2323. [DOI] [PubMed] [Google Scholar]
- 33.Chandimali N, Jeong D, Kwon T. Peroxiredoxin II regulates cancer stem cells and stemness-associated properties of cancers. Cancers. 2018;10(9):305. doi: 10.3390/cancers10090305. PMID: 30177619. DOI: 10.3390/cancers10090305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang DH, Lee DJ, Lee KW, Park YS, Lee JY, Lee S-H, Koh YJ, Koh G-Y, Choi C, Yu D-Y. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Molecular Cell. 2011;44(4):545–558. doi: 10.1016/j.molcel.2011.08.040. PMID: 22099303. DOI: 10.1016/j.molcel.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 35.Park YH, Kim SU, Kwon TH, Kim JM, Song IS, Shin HJ, Lee BK, Bang DH, Lee SJ, Lee DS, Chang KT, Kim BY, Yu DY. Peroxiredoxin II promotes hepatic tumorigenesis through cooperation with RAS/forkhead box M1 signaling pathway. Oncogene. 2016;35(27):3503–3513. doi: 10.1038/onc.2015.411. PMID: 26500057. DOI: 10.1038/onc.2015.411. [DOI] [PubMed] [Google Scholar]
- 36.Mendoza-Reinoso V, Beverdam A. Epidermal YAP activity drives canonical Wnt16/β-catenin signaling to promote keratinocyte proliferation in vitro and in the murine skin. Stem Cell Res. 2018;29:15–23. doi: 10.1016/j.scr.2018.03.005. PMID: 29562208. DOI: 10.1016/j.scr.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Niu C, Yin L, Aisa H. Novel furocoumarin derivatives stimulate melanogenesis in B16 melanoma cells by up-regulation of MITF and Tyr family via AKT/GSK3β/β-catenin signaling pathways. Int J Mol Sci. 2018;19(3):746. doi: 10.3390/ijms19030746. PMID: 29509689. DOI: 10.3390/ijms19030746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–638. doi: 10.1038/382638a0. PMID: 8757136. DOI: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 39.Tao Y, Yang Q, Wang L, Zhang J, Zhu X, Sun Q, Han Y, Luo Q, Wang Y, Guo X. Β-catenin activation in hair follicle dermal stem cells induces ectopic hair outgrowth and skin fibrosis. J Mol Cell Biol. 2018;11(1):26–38. doi: 10.1093/jmcb/mjy032. PMID: 29771334. DOI: 10.1093/jmcb/mjy032. [DOI] [PubMed] [Google Scholar]
- 40.Burch PM, Heintz NH. Redox regulation of cell-cycle re-entry: Cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxid Redox Signal. 2005;7(5-6):741–751. doi: 10.1089/ars.2005.7.741. PMID: 15890020. DOI: 10.1089/ars.2005.7.741. [DOI] [PubMed] [Google Scholar]
- 41.Dema A, Schroeter MF, Perets E, Skroblin P, Moutty MC, Deak VA, Birchmeier W, Klussmann E. The AKAP GSKIP regulates beta-catenin through both its interactions with PKA and GSK3beta. J Biol Chem. 2016;291(37):19618–19630. doi: 10.1074/jbc.M116.738047. PMID: 27484798. DOI: 10.1074/jbc.M116.738047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi MH, Lee IK, Kim GW, Kim BU, Han Y-H, Yu D-Y, Park HS, Kim KY, Lee JS, Choi C. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435(7040):347. doi: 10.1038/nature03587. PMID: 15902258. DOI: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]