Abstract
Background/Aim: Radiation mitigator, GS-nitroxide, JP4-039, was evaluated for mitigation of total body irradiation (TBI) in Fanconi anemia (FA) Fancd2−/− (129/Sv), Fancg−/− (B6), and Fanca−/− (129/Sv) mice. Materials and Methods: JP4-039 dissolved in 30% 2-hydroxypropyl-β-cyclodextrin was injected intramuscularly 24 h after total body irradiation (9.25 Gy) into Fanca−/−, Fancd2−/− and Fancg−/− mice. Irradiation survival curves were performed in vitro using bone marrow stromal cell lines derived from Fanca−/−, Fancd2−/− and Fancg−/− mice. Results: FA mice demonstrate genotype specific differences in TBI mitigation by JP4-039. Radiation effects in derived bone marrow stromal cell lines in vitro were mitigated by drugs that block apoptosis, but not necroptosis or ferroptosis. Conclusion: FA mouse models are valuable for elucidating DNA repair pathways in cell and tissue responses to TBI, and the role of drugs that target distinct cell death pathways.
Keywords: Fanconi Anemia, genotype, JP4-039, total body irradiation
Fanconi anemia (FA) represents a disease associated with defect in one or more of the 26 genes involved in the scaffold of proteins that facilitate binding and activity of DNA double strand break repair enzymes (1-3). Patients with FA, as well as mouse models of FA, demonstrate radiosensitivity of explanted bone marrow stromal cells in vitro (1-4).
The mitochondrial targeted radiation mitigator GS-nitroxide, JP4-039, has been demonstrated to significantly ameliorate the effects of total body irradiation (TBI) of several mouse strains when delivered systemically up to 72 h after (5-11). Furthermore, in organ-specific radiation protection and mitigation experiments including the oral cavity, mice of three different genotypes on two different mouse background strains with DNA repair-deficient FA revealed therapeutic effects of JP4-039 (1-3,12-19). Radiation dose–response curves showed significant reduction in colony formation in vitro and a radiation-protective effect of JP4-039 in bone marrow stromal and hematopoietic progenitor cell lines as well as fresh bone marrow colony-forming progenitor cells consistent with FA genotype mouse genotype (1-3,12,15).
In the present studies, we evaluated the response to TBI with mice of each FA genotype, as well as wild-type littermates, using the TBI dose lethal for 50% of mice at 30 days (LD50/30). In these experiments, we looked at mitigation of irradiation damage as reflected in increased survival following irradiation by drugs that block apoptosis, necroptosis or ferroptosis.
Materials and Methods
Mice and animal care. Fanca−/− (129/Sv background) (3), Fancg−/− (C57BL/6J background) (3), and Fancd2−/− (C57BL/6J (2) or 129/Sv background) (1) mice were maintained at four mice per cage, according to University of Pittsburgh Institutional Animal Care and Use Committee regulations. The breeding genotyping of FA mice of each genotype, as well as normal control littermates from each breeding have been described previously (1-3). Serpinb3a−/− and Balb/c control littermates were bred and maintained according to published methods (20).
TBI. Mice received the LD50/30 dose of TBI for each background mouse strain using a cesium137 irradiator (JL Shepherd, San Fernando, CA, USA). The dose of TBI delivered was 8.5 Gy. This dose was shown to be the LD50/30 dose for FA mice and is consistent with the known differences in radiosensitivity of FA mice (1-3).The conditions for irradiation at 310 cGy/min, with filters removed from the cesium irradiator, and dosimetry carried out to ensure uniform dose distribution in mouse phantoms are described elsewhere (18). Conditions for irradiation using a pie-plate animal separator, and specific slots for irradiation, ensuring uniform dose distribution between animals are described therein.
Radiation mitigator drugs and administration. JP4-039 (provided by Dr. Peter Wipf of the University of Pittsburgh) (18) was dissolved in 30% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA) at 8 mg/ml and 50 μl was injected intramuscularly to give a final concentration of 20 mg/kg. For in vitro studies, JP4-039, necrostatin-1 (Sigma-Aldrich, St. Louis, MO, USA), and baicalein (Cayman Chemical, Ann Arbor, MI, USA) were dissolved in dimethyl sulfoxide at 10 mM, and then diluted 1:1000 in tissue culture medium for a final concentration of 10 μM for each drug.
Bone marrow stromal cell line irradiation and clonogenic survival curve analysis. Bone marrow stromal cell lines from each FA genotype and control littermates were passaged and cultured according to previous publications (1-3). The effect of JP4-039 (10 mM), necrostatin-1 (10 mM) or baicalein (10 mM) was tested by adding the drug to single-cell suspensions of each cell line immediately before irradiation. Cells were irradiated to doses between 0 and 8 Gy in conical centrifuge tubes according to previous publications (1-3). Cells were plated in Dulbecco’s modified Eagle’s medium for clonogenic survival assay at densities of between 500 and 3,000 cells per ml. Quadruplicate cultures were plated for each cell density. Cells and cultures were incubated in a high humidity incubator with 7% CO2 for 7-10 days after which time the colonies were stained with crystal violet. Colonies of greater than 50 cells were scored according to published methods (8). The calculation of the irradiation dose that reduced survival to 37% on the exponential portion of the survival curve (D0) and the extrapolation number of the linear portion of the survival curve to the y axis (ñ) was carried out according to previous publication (8).
Statistics. In vitro irradiation survival curves were analyzed using linear quadratic and single-hit multi-target models. Comparisons of D0 and ñ were performed with an Unpaired t-test. In vivo irradiation survival curves were analyzed using a log-rank test.
Results
Mitigation of effects of TBI by JP4-039 in Fanca−/− mice. Groups of Fanca−/− and control 129/Sv littermates (Figure 1), Fancg−/− mice and control C57BL/6 littermates (Figure 2), and Fancd2−/− and control 129/Sv littermates (Figure 3) were irradiated to the LD50/30 dose of TBI.
Figure 1. Total body irradiation (TBI) mitigation by JP4-039 in Fanca−/− mice. Thirty-four Fanca−/− mice (129/Sv background) were irradiated to 8.5 Gy TBI. Seventeen of the mice were injected with JP4-039 (20 mg/kg dissolved at 8 mg/ml in 30% 2-carboxypropy-β-cyclodextrin) (50 μl) 24 h after irradiation. The mice were followed for development of hematopoietic syndrome at which time they were sacrificed. Mice injected with JP4-039 had significantly increased survivaI.
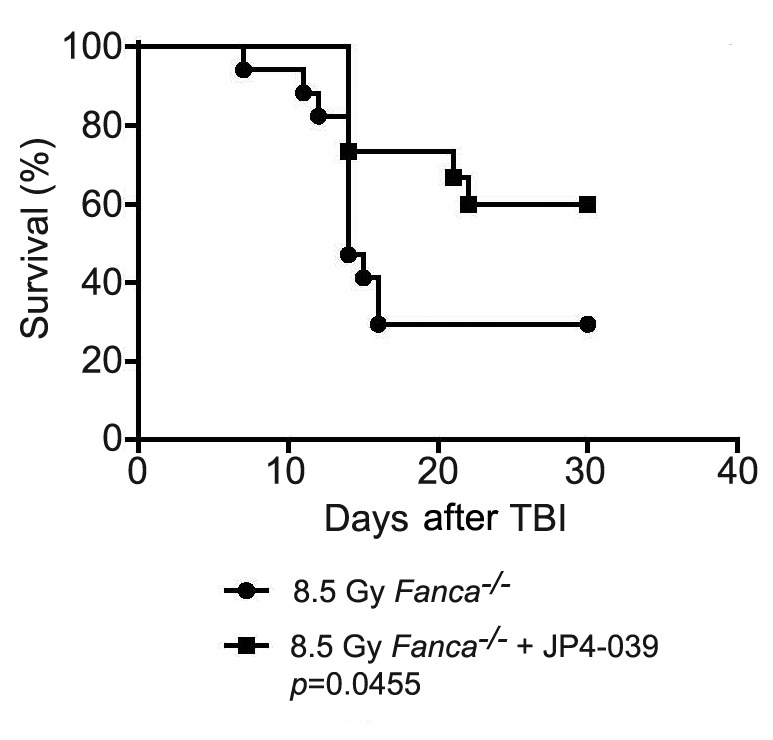
Figure 2. Lack of total body irradiation (TBI) mitigation by JP4-039 in Fancg−/− mice. Thirteen Fancg−/− mice (C57BL/6 background) were irradiated to 8.5 Gy TBI. Twenty-four hours later, seven of the mice were injected with 50 μl of JP4-039 dissolved in 30% 2-hydroxypropyl-β- cyclodextrin at a concentration of 8 mg/ml. The mice were followed for the development of hematopoietic syndrome at which time they were sacrificed. There was no significant difference in survival between the groups.
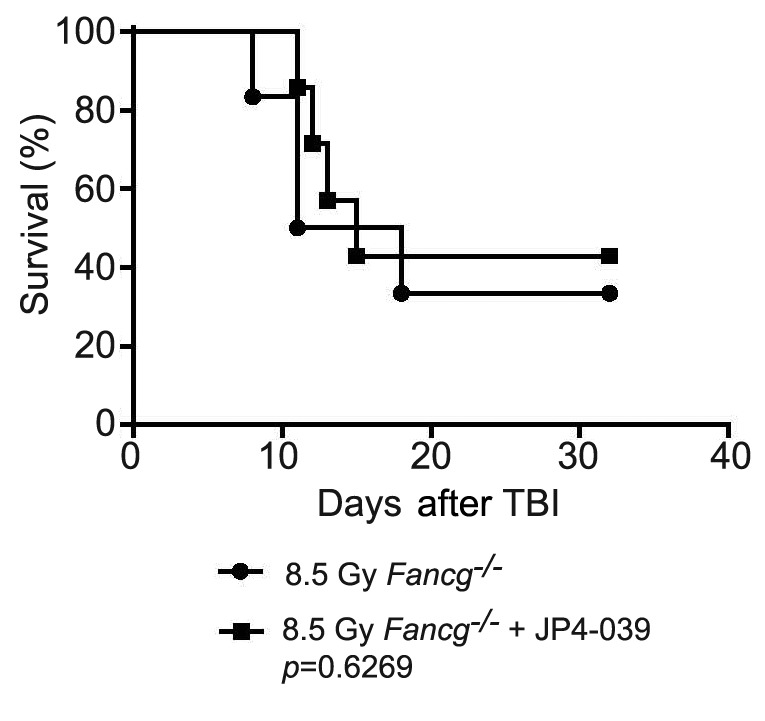
Figure 3. Lack of total body irradiation (TBI) mitigation by JP4-039 in Fancd2−/− mice. Ten Fancd2−/− on a 129/Sv background were irradiated to 8.5 Gy TBI, and five were injected (50 μl) 24 h later with JP4-039 (20 mg/kg) in 30% 2-hydroxypropyl-β-cyclodextrin (8 mg/ml). The mice were followed for development of hematopoietic syndrome. There was no significant difference in survival between the groups.
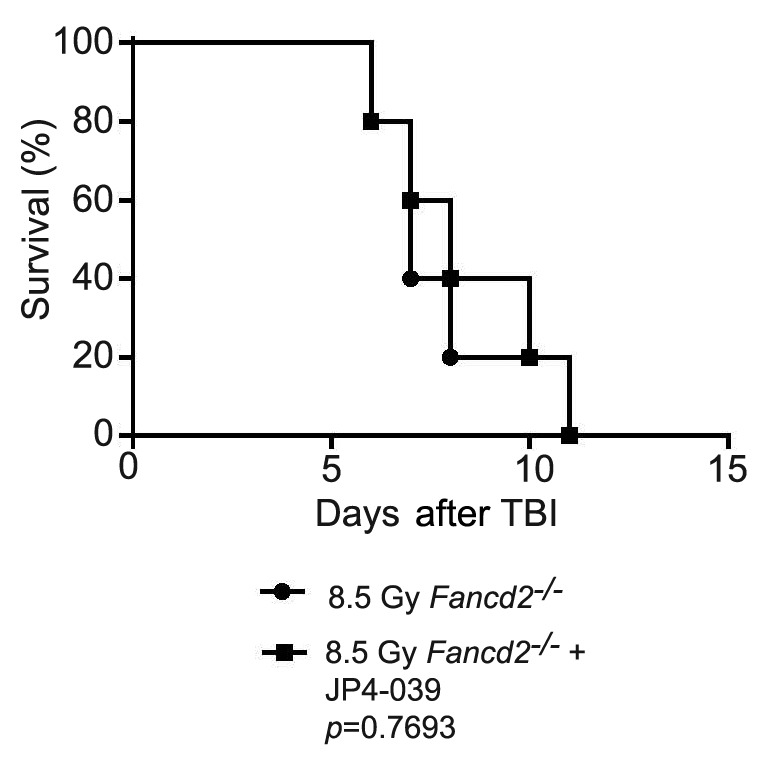
Fanca−/− mice that received JP4-039 24 h after TBI survived significantly longer than those that did not (Figure 1). In contrast, in Fancg−/− (Figure 2) and Fancd2−/− (Figure 3) mice, JP4-039 demonstrated no significant radiation mitigation and survival was not improved. JP4-039 demonstrated significant mitigation in normal littermate control strains on both the 129/Sv and C57BL/6J B6 (8) background. These results establish that there are genotypic differences in FA mice with respect to TBI mitigation by JP4-039.
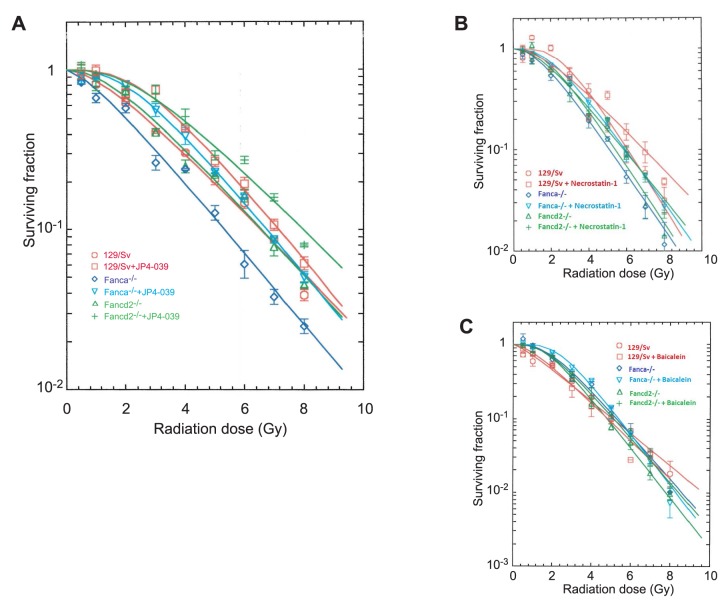
JP4-039 induces significant radioresistance in bone marrow stromal cell lines from FA mice. To determine whether differences in TBI response to JP4-039 were seen with irradiation of cell lines in vitro, bone marrow stromal cell lines of each genotype and control littermate were treated with JP4-039 in vitro immediately after irradiation to doses of 0 to 8 Gy. Colony formation by irradiated cells in continuous presence of JP4-039 over 7 days in culture was scored. As shown in Figure 4A, JP4-039 significantly mitigated the effects of radiation in all cell lines (Table I).
Figure 4. Radiosensitivity of Fanconi anemia cell lines is mitigated by JP4-039, but not by necrostatin-1 or baicalein. Mitigation by JP4-039 of radiation damage was detected in 129/Sv, Fanca−/−, and Fancd2−/− bone marrow stromal cells (A). In contrast, necrostatin-1 (B) or baicalein (C) mitigated irradiation damage in the 129/Sv cell line, but not the Fanca−/− or the Fancd2−/− cell lines.
Table I. Radioprotection of Fanconi anemia bone marrow stromal cell lines by small molecule drugs JP4-039, baicalein, and necrostatin-1. 129/Sv control, Fanca−/− and Fancd2−/− bone marrow stromal cell lines were used in irradiation survival curves. JP4-039, baicalein (10 μM) and necrostatin-1 (10 μM) were added 1 h before irradiation, and cells were then kept in culture for 7 days. The cells were stained with crystal violet and colonies greater than 50 cells counted. The data were analyzed using single-hit, multi-target or linear quadratic models. Significant p-values are shown.
*For D0 vs. Control.
These results establish that in vitro, JP4-039 was radiation-protective and -mitigating in bone marrow stromal cell lines derived from mice of two different FA genotypes. These results are in contrast to the in vivo mitigation by JP4-039 at 24 h after TBI which was observed only in Fanca−/− mice.
Lack of detectable radiation mitigation by necrostatin-1 and baicalein in FA mouse marrow stromal cell lines. Using clonogenic irradiation survival curves, we tested the anti-necroptosis drug necrostatin-1, and the anti-ferroptosis drug baicalein to determine whether Fanca−/− and Fancd2−/− bone marrow stromal cells were able to modulate the irradiation dose response. Each cell line was treated with each drug and evaluated in clonogenic survival assays. The results demonstrated no detectable mitigation by necrostatin-1 or baicalein for any of the FA bone marrow stromal cell lines of each genotype (Table I; Figure 4B and C). In contrast, necrostatin-1, or baicalein Ied to significant radiation mitigation in bone marrow stromal cell lines derived from control littermate (Table I). These results established that Fanca−/− and Fancd2−/− bone marrow stromal cell lines did not have increased radiation resistance when incubated with anti-necroptosis drug, necrostatin-1, and the anti-ferroptosis drug, baicalein.
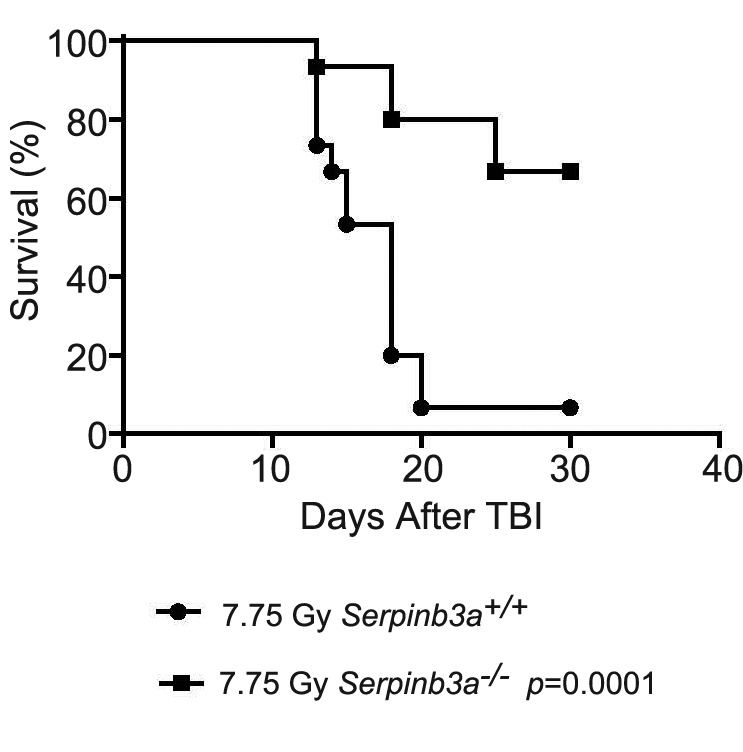
Gene deletion does not always result in increased radiation sensitivity. As a control experiment, we determined whether homologous recombinant deletion of another gene product accounted for the radiosensitivity of the FA mouse strains tested. As a control for the radiosensitivity of Fanca−/−, Fancg−/− and Fancd2−/− mice, we carried out TBI with a third background strain of radiosensitive Balb/c mice, and a genotypic variant (Serpinb3a−/−) (20) (Figure 5). In contrast to the data for FA mice, homologous recombinant negative Serpinb3a−/− mice exhibited radioresistance (Figure 5). Thus, homologous recombinant deletion of a gene does not uniformly produce the phenotype of radiosensitivity to TBI.
Figure 5. Radiation mitigation in Serpinb3a−/− mice compared to control Balb/c littermates. Fifteen Balb/c (Serpinb3+/+) and 15 Serpinb3a−/− mice received total body irradiation to 7.75 Gy, and were followed up for development of hematopoietic syndrome at which time they were sacrificed. Serpinb3a−/− mice had significantly increased survivaI.
Discussion
There are multiple cell death pathways initiated by exposure of cell lines, tissues, and organs to TBI (1,20). Radiation mitigator drugs delivered 24 h after irradiation, which target each of three cell death pathways (apoptosis, necroptosis, and ferroptosis), have been demonstrated to be more effective in increasing survival after TBI than the delivery of one mitigator or administration of two mitigators (18). Those studies also indicated that in TBI, the time of delivery of mitigators targeted to each cell death pathway would be optimized by identification of the biochemical targets for each drug (18).
To effectively mitigate against irradiation-induced apoptosis, JP4-039 was shown to be optimal when delivered at 24 h after TBI, although waves of apoptosis were still detectable up to 72 h, and delivery of this mitigator at that time was still effective (6). In contrast, necrostatin-1, a drug which targets necroptosis, was shown to be ineffective until delivered at the delayed time point of 48 h after TBI, and was associated with detectable up-regulation of tumor necrosis factor-α and interleukin-1, which represent biochemical markers for the initiation of necroptosis (18). Furthermore, the phosphorylation of receptor-interacting serine/threonine-protein kinase 3 in bone marrow and tissues was shown to be detectable at 48 h after TBI, but not earlier, and consistent with the optimal time of delivery of necrostatin-1.
A third cell death pathway, ferroptosis (21), has been shown to be initiated when the level of glutathione peroxidase-4 (mitochondrial active antioxidant) decreased, and when the acyl-CoA-synthase marker for the oxidized lipid changes associated with ferroptosis increased. Of three drugs known to block ferroptosis, baicalein was found to be the optimal mitigator for this cell death pathway, and best delivered at 24 h after TBI along with the anti-apoptosis drug, JP4-039 (18).
In the present experiments both background strain mice, 129/Sv and C57BL/6J, as well as their derived bone marrow stromal cell lines were radioresistant when treated with JP4-039. Furthermore, irradiation was also mitigated in control mouse cell lines in vitro by necrostatin-1 and baicalein. We tested whether radiation mitigators were also effective against TBI in FA mice.
Mice with FA of two different background strains and different genotypes were tested for amelioration of TBI-induced hematopoietic syndrome, using a dose of irradiation that is rescuable by bone marrow transplantation and by delivery of the anti-apoptotic drug, JP4-039. The results demonstrated that JP4-039 protected from TBI in Fanca−/− but not Fancg−/− or Fancd2−/− mice. The difference in TBI radiation mitigation by JP4-039 according to FA genotype was in contrast to the uniform in vitro radiation protection observed in bone marrow stromal cell lines from each genotype.
The present data are consistent with the model in which different cell phenotypes in tissues of mice, including bone marrow hematopoietic cells, intestinal stem cells, and cells of the liver and lung, may account for the radiosensitivity to TBI that is not ameliorated by systemic delivery of JP4-039 (12,18). In contrast to the data with FA mice, in wild-type littermates of each FA genotype, uniform radiation mitigation was induced by necrostatin-1 and baicalein, as well as JP4-039. The data are also consistent with the notion that different cell populations within tissues in some FA genotypes may be radiosensitive (12,22). The results support the non-uniform response of patients with FA to total or partial body irradiation used to prepare individuals for bone marrow transplantation. Results with different FA genotypes with respect to TBI sensitivity and amelioration by JP4-039 are consistent with other studies showing genotype specific differences in TBI sensitivity and known relative radioresistance of female compared to male mice (23,24), and between mouse strains (25).
The present studies establish the relative ineffectiveness of radiation mitigators targeting necroptosis (necrostatin-1) or ferroptosis (baicalein) (18) in cell lines from FA mice, while there was significant radiation protection/mitigation by the same drugs in control bone marrow stromal cell lines. These mouse models of FA should prove valuable for studies of the mechanisms of radiation toxicity and amelioration by drugs that target distinct cell death pathways.
Conflicts of Interest
There are no conflicts of interest.
Authors’ Contributions
Michael W. Epperly aided in the experimental design, irradiation, and analysis of data. Renee Fisher aided in the breeding of the mice, irradiation of the mice and drug administration. Xichen Zhang was responsible for maintaining the cells in vitro. Wen Hou aided in the survival curves and genotyping of the pups. Donna Shields prepared the in vitro survival curves. Peter Wipf made the JP4-039 while Hong Wang performed the statistical analysis. Stephanie Thermozier aided in the Serapinb3a−/− mouse experiments. Joel S. Greenberger participated in the experimental design and data analysis.
Acknowledgements
This study was supported by grant from the NIH/NIAID U19A168021, and the UPCI-Hillman Animal Research Core Facility award P30CA047904.
References
- 1.Berhane H, Shinde A, Kalash R, Xu K, Epperly MW, Goff J, Franicola D, Zhang X, Dixon T, Shields D, Wang H, Wipf P, Li S, Gao X, Greenberger JS. Amelioration of irradiation induced oral cavity mucositis and distant bone marrow suppression in Fancd2−/− (FVB/N) mice by intraoral JP4-039/F15. Radiat Res. 2014;182:35–49. doi: 10.1667/RR13633.1. PMID: 24932534. DOI: 10.1667/RR13633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinde A, Berhane H, Rhieu BH, Kalash R, Xu K, Goff J, Epperly MW, Franicola D, Zhang X, Dixon T, Shields D, Wang H, Wipf P, Parmar K, Ferris R, Li S, Greenberger JS. Intraoral mitochondrial-targeted GS-nitroxide, JP4-039, radioprotects normal tissue in tumor-bearing radiosensitive Fancd2−/− (C57BL/6) mice. Radiat Res. 2016;185:134–150. doi: 10.1667/RR14035.1. PMID: 26789701. DOI: 10.1667/RR14035.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willis J, Epperly MW, Fisher R, Zhang X, Shields D, Hou W, Wang H, Li S, Wipf P, Parmar K, Guinan E, Steinman J, Greenberger JS. Amelioration of head and neck irradiation-induced mucositis and distant marrow suppression in Fanca−/− and Fancg−/− mice by intraoral administration of GS-nitroxide (JP4-039) Radiat Res. 2018;189:560–578. doi: 10.1667/RR14878.1. PMID: 29584588. DOI: 10.1667/RR14878.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn TJ, Ding X, Li X, Wilson GD, Buelow K, Sivananthan A, Thermozier S, Henderson A, Epperly MW, Franicola D, Wipf P, Greenberger JS, Stevens CW, Kabolizadeh P. Amelioration of mucositis in proton therapy of fanconi anemia Fanca−/− mice by JP4-039. In Vivo. 2019;33(6):1757–1766. doi: 10.21873/invivo.11666. PMID: 31662500. DOI: 10.21873/invivo.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink M, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin-TEMPO conjugates: Novel mitochondria-targeted antioxidants. Crit Care Med. 2007;35(9):5461–5470. doi: 10.1097/01.CCM.0000279192.96303.E7. PMID: 17713394. DOI: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Belikova NA, Xiao J, Zhao Q, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemi-gramicidin S conjugate protects mouse embryonic cells against ƴ-irradiation. Int J Radiat Oncol Biol Phys. 2008;70(3):816–825. doi: 10.1016/j.ijrobp.2007.10.047. PMID: 18262096. DOI: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagan VE, Bayir A, Bayir H, Stoyanovsky D, Borisenko GG, Tyurina YY, Wipf P, Atkinson J, Greenberger JS, Chapkin RS, Belikova NA. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: A new strategy in anti-apoptotic drug discovery. Mol Nutr Food Res. 2009;53:104–114. doi: 10.1002/mnfr.200700402. PMID: 18979502. DOI: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, Franicola D, Dixon T, Frantz M-C, Wipf P, Tyurina Y, Kagan VE, Wang H, Greenberger JS. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80(3):860–868. doi: 10.1016/j.ijrobp.2011.01.059. PMID: 21493014. DOI: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Bernard ME, Epperly MW, Shen H, Amoscato A, Dixon TM, Doemling AS, Li S, Gao X, Wipf P, Wang H, Zhang X, Kagan VE, Greenberger JS. Amelioration of radiation esophagitis by orally administered p53/MDM2/MDM4 inhibitor (BEB55) or GS-nitroxide. In Vivo. 2011;25(6):841–849. PMID: 22021675. [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberger JS, Clump D, Kagan V, Bayir H, Lazo J, Wipf P, Li S, Gao X, Epperly MW. Strategies for discovery of small molecule radiation protectors and radiation mitigators. Front Radiat Oncol. 2012;1:59. doi: 10.3389/fonc.2011.00059. PMID: 22655254. DOI: 10.3389/fonc.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff J, Shields D, Wang H, Skoda E, Sprachman M, Wipf P, Lazo J, Atkinson J, Kagan V, Epperly M, Greenberger JS. Evaluation of potential ionizing irradiation protectors and mitigators using clonogenic survival of human umbilical cord blood hematopoietic progenitor cells. Exp Hematol. 2013;41(11):957–966. doi: 10.1016/j.exphem.2013.08.001. PMID: 23933481. DOI: 10.1016/j.exphem.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, Zhang X, Shields D, Houghton F, Wang H, Sprachman M, Wipf P, Li S, Gao X, Parmar K, Greenberger JS. Radiobiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi anemia (Fancd2−/−) mice. Radiat Res. 2014;181:76–89. doi: 10.1667/RR13405.1. PMID: 24397476. DOI: 10.1667/RR13405.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyurina YY, Poloyac SM, Tyurin VA, Kapralov AA, Jiang J, Anthonymuthus TS, Kapralova VI, Vikulina AS, Jung M-J, Epperly MW, Mohammadyani D, Klein-Seetharaman J, Jackson TC, Rochanek PM, Pitt BR, Greenberger JS, Vladimirov YA, Bayir H, Kagan VE. A mitochondrial pathway for biosynthesis of lipid mediators. Nat Chem. 2014;6(6):542–552. doi: 10.1038/nchem.1924. PMID: 24848241. DOI: 10.1038/nchem.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberger JS, Berhane H, Shinde A, Rhieu BH, Bernard M, Wipf P, Skoda EM, Epperly MW. Can radiosensitivity associated with defects in DNA repair be overcome by mitochondrial-targeted antioxidant radioprotectors. Front Radiat Oncol. 2014;4:24. doi: 10.3389/fonc.2014.00024. PMID: 24596683. DOI: 10.3389/fonc.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinde A, Epperly MW, Franicola D, Cao S, Goff J, Shields D, Wipf P, Wang H, Greenberger JS. Improved hematopoiesis in GS-nitroxide (JP4-039)-treated mouse long-term bone marrow cultures and radioresistance of derived bone marrow stromal cell lines. In Vivo. 2014;28(5):699–708. PMID: 25189880. [PMC free article] [PubMed] [Google Scholar]
- 16.Brand R, Epperly MW, Stottlemyer JM, Skoda E, Gao X, Li S, Huq S, Wipf P, Kagan VE, Greenberger JS, Falo LD Jr. A topical mitochondria-targeted redox cycling nitroxide mitigates oxidative stress induced skin damage. J Invest Dermatol. 2017;137(3):576–586. doi: 10.1016/j.jid.2016.09.033. PMID: 27794421. DOI: 10.1016/j.jid.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epperly MW, Sacher JR, Krainz T, Zhang X, Wipf P, Liang M, Fisher R, Li S, Wang H, Greenberger JS. Effectiveness of analogues of the GS-nitroxide, JP4-039, as total body radiation mitigators. In Vivo. 2017;31:39–44. doi: 10.21873/invivo.11022. PMID: 28064218. DOI: 10.21873/invivo.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman J, Epperly M, Hou W, Willis J, Wang H, Fisher R, Liu B, Bahar I, McCaw T, Kagan V, Bayir H, Yu J, Wipf P, Li S, Huq MS, Greenberger JS. Improved total-body irradiation survival by delivery of two radiation mitigators that target distinct cell death pathways. Radiat Res. 2018;189(1):68–83. doi: 10.1667/RR14787.1. PMID: 29140165. DOI: 10.1667/RR14787.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epperly MW, Wipf P, Fisher R, Franicola D, Beumer J, Li S, Brand R, Falo L, Greenberger JS. Evaluations of different formulations and routes for the delivery of the ionizing radiation mitigation GS-nitroxide (JP4-039) In Vivo. 2018;32:1009–1023. doi: 10.21873/invivo.11341. PMID: 30150422. DOI: 10.21873/invivo.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thermozier S, Zhang X, Hou W, Fisher R, Epperly MW, Liu B, Bahar I, Greenberger JS. Radioresistance of Serpinb3a−/− mice and derived hematopoietic and marrow stromal cell lines. Radiat Res. 2019;192(3):267–281. doi: 10.1667/RR15379.1. PMID: 31295086. PMID: 31295086. DOI: 10.1667/RR15379.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dar HH, Tyurina YY, Mikulska KA, Shrivastava I, Tyurin VA, Krieger J, St. Croix C, Watkins S, Bayir E, Ting H-C, Mao G, Ogunsola AF, Flitter BA, Freedman C, Gaston JR, Holman T, Pilewski J, Greenberger JS, Mallampalli R, Bahar I, Bomberger J, Bayir H, Kagan VE. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J Clin Invest. 2018;128(10):4639–4653. doi: 10.1172/JCI99490. PMID: 30198910. DOI: 10.1172/JC199490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, Houghton F, Shields D, Wang H, Bakkenist CJ, Frantz M-C, Wipf P, Greenberger JS. GS-nitroxide (JP4-039) mediated radioprotection of human Fanconi anemia cell lines. Radiat Res. 2011;176:603–612. doi: 10.1667/rr2624.1. PMID: 21939290. DOI: 10.1667/rr2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epperly MW, Smith T, Wang H, Schlesselman J, Franicola D, Greenberger JS. Modulation of total body irradiation induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Radiat Res. 2008;170(4):437–444. doi: 10.1667/rr1286.1. PMID: 19024650. DOI: 10.1667/rr1286.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epperly MW, Wang H, Jones J, Dixon T, Montesinos C, Greenberger JS. Antioxidant-chemoprevention diet ameliorates late effects of total body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat Res. 2011;175:759–765. doi: 10.1667/RR2398.1. PMID: 21466381. DOI: 10.1667/RR2398.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalash R, Berhane H, Goff J, Houghton F, Epperly MW, Dixon T, Zhang X, Sprachman MM, Wipf P, Franicola D, Wang H, Greenberger JS. Thoracic irradiation effects on pulmonary endothelial compared to alveolar type II cells in fibrosis-prone C57BL/6NTac mice. In Vivo. 2013;27:291–298. PMID: 23606683. [PMC free article] [PubMed] [Google Scholar]