Abstract
Background/Aim: Wire-guided localisation (WGL) has been the mainstay for localisation of clinically occult breast lesions before excision. However, it has restrictive scheduling requirements, and causes patient discomfort. This has prompted the development of various wireless alternatives. In this prospective study we shall evaluate the role of radiation-free wireless localisation using a radiofrequency identification (RFID) tag and a hand-held reader (LOCalizer™) in the management of occult breast lesions. Patients and Methods: This technique was evaluated in a prospective cohort of 10 patients. The evaluation focused on: i) successful deployment, ii) identification and retrieval, iii) the status of surgical margins and need for re-operation, iv) resected specimen weight, v) marker migration rates (>5mm), and vi) acceptance by patients, radiologists and surgeons. Results: RFID tags (n=11) were deployed under ultrasound guidance pre-operatively to localise occult breast lesions in 10 patients. The mean time for deployment of the RFID tag was 5.4 min (range=2-20). The mean distance from the lesion was 0.45 mm (range=0-3). The mean duration for retrieval was 10.2 min (range=6-20). Mean specimen weight was 19.6 g for malignant lesions (range=4.5-42). All tags were identified, and none had migrated. There were no positive margins, re-operations, nor complications. Patient feedback was highly positive. Both radiologists and surgeons rated the LOCalizer™ technique as better than WGL. Conclusion: Our study demonstrates that wireless localisation using RFID is an effective and time-efficient alternative to WGL, with low margin positivity and re-operation rates, and high patient, radiologist and surgeon acceptance.
Keywords: Breast cancer, tumour localisation, radio-frequency tags
The introduction of mammography revolutionised the diagnosis and treatment of breast cancer. The current form of mammography was pioneered at the MD Anderson Cancer Center during the fifties, most prominently by RL Egan (1). In the initial case series of 1,253 patients, it was noted that many patients with clinically normal breasts harboured an occult, otherwise undetectable breast cancer (2). With the increased acceptance of mammography as a screening tool, following the publication of randomised trials in the 1960s, these clinically occult breast lesions became an increasingly significant clinical issue (3).
Especially with the increased prevalence of breast conservation, localising impalpable lesions for wide excision required the development of localisation techniques. Wire-guided stereotactic localisation was the result of these endeavours. It was initially described by Frank et al. in the 1970s (4), and continues to be the predominant method for marking impalpable breast lesions for surgical excision today (5,6).
However, wire guide localization (WGL) has its limitations. It requires close coordination between the radiological and the surgical teams, which can be a significant obstacle in the initial setup of service, as the surgeon’s freedom for surgical access is limited by the radiologist’s approach of where the wire should be inserted. The wire itself could migrate, break, or cause needle-stick injuries to the surgeons. Furthermore, the placement of the wire protruding from the patient’s skin causes patients anxiety and discomfort (7).
The above state, as well as other issues, have provided an impetus for the development of alternatives to WGL. Predominantly, these alternatives are variations of wireless occult breast lesion localisation techniques, which may use a radioactive seed as a tracer (6), or an alternative non-radioactive tracer based on various other technologies (8).
In this study, we shall evaluate the role of the LOCalizer™ system (Hologic Inc., Santa Carla CA, USA), a wireless technique that uses a radiofrequency identification (RFID) tag and a handheld reader for the management of impalpable breast lesions.
Patients and Methods
This study was performed in the Princess Grace Hospital (London, UK) at the London Breast institute as part of clinical implementation of a new technology. Patients who required excision of impalpable breast lesions were recruited and decisions regarding treatment were made following a multidisciplinary discussion. Patients were counselled regarding their treatment and associated risk and consented to undergo wireless tumour localisation using the RFID tag and LOCalizer™ device, rather than WGL. The clinical and pathological parameters, the reported perceptions of the patient and the surgeons and the views of the radiologists were recorded. Specifically, the clinical and pathological evaluation focused on: i) successful deployment, ii) identification and retrieval, iii) the status of surgical margins and the need for re-operation, iv) the weight of resected specimens, v) migration rates (>5 mm) of the marker, and vi) acceptance by patients, radiologists and surgeons. This was an observational clinical evaluation; hence a formal ethical approval was not required. However, the use of these technologies was approved by the multidisciplinary breast cancer board of the London Breast Institute and the clinical governance team of The Princess Grace Hospital. All participants signed an informed written consent. Detailed patient information leaflets regarding this novel wireless technique were made available.
Results
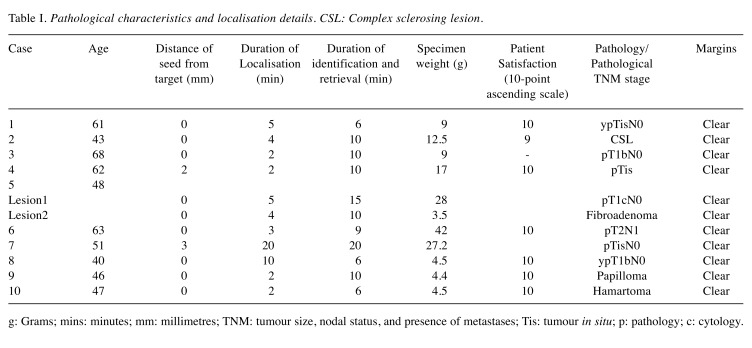
In total, 11 non-palpable lesions (7 malignant and 4 benign) in 10 patients (mean age=52.9 years, range=40-68 years) were included (Table I).
Table I. Pathological characteristics and localisation details. CSL: Complex sclerosing lesion.
g: Grams; mins: minutes; mm: millimetres; TNM: tumour size, nodal status, and presence of metastases; Tis: tumour in situ; p: pathology; c: cytology.
RFID tags (n=11) were deployed under ultrasound guidance within 7 days of surgery to guide the surgical excision of non-palpable breast lesions in 11 patients (Figure 1 and Figure 2). The mean duration of deployment of the RFID tag was 5.4 min (range=2-20 min) and mean distance between the deployed marker and lesion was 0.45 mm (range=0-3 mm). The identification/retrieval and migration rates were 100% and 0%, respectively.
Figure 1. Faxitron LOCalizer™ handheld reader device being used intra-operatively to locate the RFID tag implanted at the tumour site prior to surgery. RFID: Radio-frequency identification.
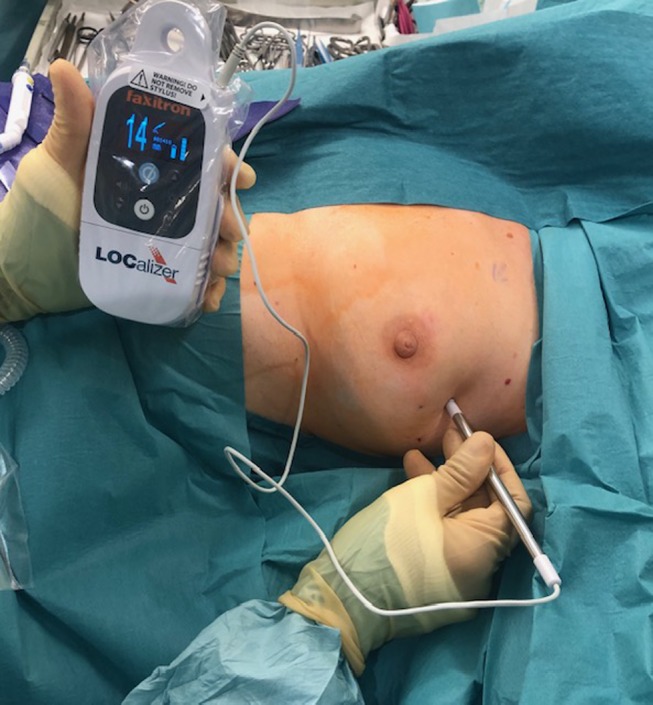
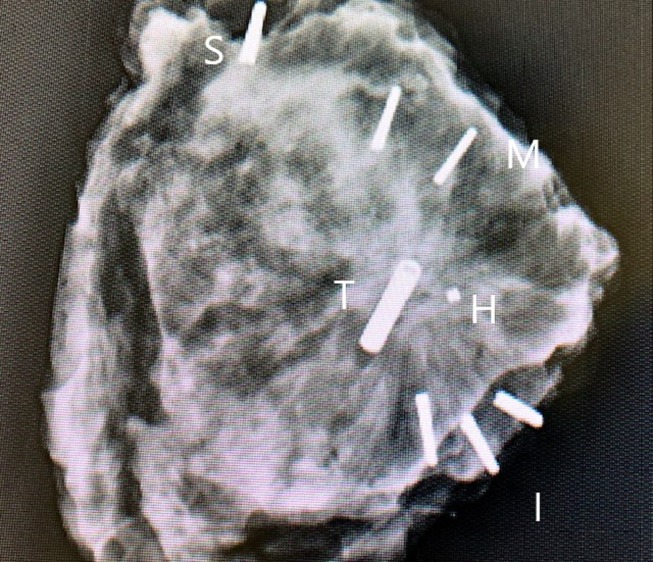
Figure 2. Specimen radiograph. It demonstrates the RFID tag (T) adjacent to the hydrogel marker (H) in a patient who received NACT. The histology showed that the DCIS and the margins were clear. Titanium clips have been placed for orientation: 1 for superior (S); 2 for medial (M); 3 for inferior (I). NACT: Neoadjuvant chemotherapy; RFID: radio-frequency identification.
The mean specimen weight was 19.6 g for malignant lesions (range=4.5-42 g). The mean duration of identification and retrieval was 10.2 min (range=6-20 min). All patients had clear surgical margins and none required reoperation.
We observed no preoperative, intraoperative or postoperative complications.
Patient feedback was obtained by 7 patients with a mean score of 9.9 out of 10 (range=9-10) using a linear visual analogue scale. Both radiologists and surgeons rated the LOCalizer™ technique as better compared to wire-guided localization.
There was an excellent correlation between the margin width measured by the LOCalizer™ compared to the measurement of the pathologist.
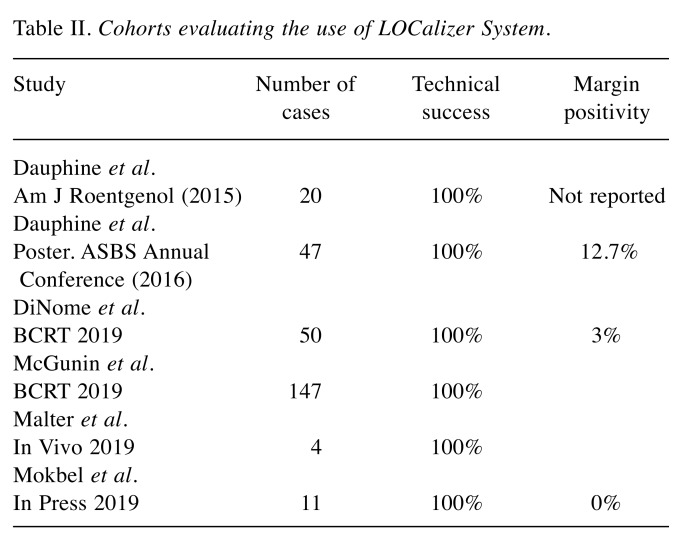
Currently, the RFID system has been evaluated in five cohorts (9-13). Our current cohort would be the sixth (Table II). The table demonstrates that in a total of 279 cases, the technical success and margin positivity (for malignant lesions) rates were 100% and 13.4%, respectively. The 100% technical success rate confirms the robustness and reliability of this type of technology.
Table II. Cohorts evaluating the use of LOCalizer System.
Discussion
WGL has been the mainstay for the localisation of impalpable breast lesions for excision in breast conserving surgery since the 1980s. The majority of breast surgeons are well-versed in this technique and it is relatively affordable. However, the procedure’s pitfalls are well-known: i) inflexible scheduling coupled with the surgery, ii) wire displacement, iii) wire fragmentation, iv) wire migration, v) patient discomfort and anxiety, vi) injury of other organs, vii) the risk of needle-stick injuries and viii) issues with cosmesis owing to the wire-placement procedure that dictates incision (14).
Dauway et al. in 1999 described the procedure of radioactive seed localisation (RSL) as an alternative to WGL (15). In this procedure, a titanium seed containing 125I is placed at the site of the lesion using radiological guidance, and is located intraoperatively by a gamma camera. In contrast to WGL, the localisation could be done within 5 days prior to the surgical excision, and it is well-tolerated as it spares the patient from the discomfort caused by the protruding wire (16). Subsequent studies have shown that RSL has low rates of margin positivity and a favourable learning curve (17). Furthermore, Zhang et al., have found RSL to be more cost-effective compared to WGL (18).
The limitation of RSL is that, due to its radioactivity, the seeds cannot be retained for more than 5 days. Also, the radioactive materials needed for this technique require special handling and disposal precautions, and carry a significant regulatory burden, which has to be considered when setting up an RSL service (19).
These limitations inherent to RSL have given an impetus to the development of alternative non-radioactive wireless occult breast lesion localisation techniques, which eschew the use of radioactive seeds. In these techniques, the radio-active seed is substituted by a non-radioactive tag or tracer, located by its specific detection apparatus intra-operatively. However, most systems available are proprietary, and vary on the basis of the underlying technology with specific limitations of their own (8).
The use of magnetic tracers was pioneered during the magnetic sentinel node and occult lesion localisation (MagSNOLL) trial, which was a prospective feasibility study concerning the use of a ferromagnetic suspension in the place of the radio-labelled tracer for sentinel node biopsy and tumour localisation (15). Said solution was injected into the tumour, and was detected intra-operatively by handheld magnetometers developed for the trial (20). The results of this trial resulted in the development of the Magseed system (Endomag limited, Cambridge, UK), which employs a 5mm seed that can be deployed using an 18-gauge introducer. This system has since been approved by the FDA for implantation in patients (21). Recent cohort studies have been encouraging in terms of margin positivity rates and tolerability (22,23). The Magseed marker can be deployed at any time prior to surgery and can be used to mark pathological lymph nodes prior to commencing neoadjuvant chemotherapy (NACT), thus, facilitating targeted axillary dissection (TAD).
However, the Magseed system has its limitations. The seed interferes with MRI, leaving a significant phantom artefact on the images. The tracer can be detected at a depth of up to 4 cm, but less reliably at greater depths. Also, when the magnetometer is in use, metal objects need to be removed from the surgical field (14).
MAgnetic MArker LOCalisation (MaMaLoc) (Sirius Medical, Eindhoven, Netherlands) is another wireless occult breast lesion localisation system based around a magnetic tracer. However, there is currently little literature available regarding its efficacy (24).
Another wireless occult breast lesion localisation system currently in use, is the SAVI Scout system (Cianna Medical, Aliso Viejo, CA, USA), in which a reflector is implanted into the tumour, and reflects a combination of infra-red and radar signals from a handheld detector to achieve tumour localisation. This device is FDA approved for long-term placement (25). It can be implanted at any time prior to surgery (26). A recent study comparing the SAVI Scout system to WGL and RSL found no difference in margin positivity, specimen volume or 30-day complication rate, but did find that the SAVI Scout device reduced the overall operating time (27). This system is also being evaluated for use in lung cancer (28). The main advantage of this technology is the minimal MRI void signals (less than 5mm), which makes it suitable for use in patients who require MRI to monitor responses to NACT. However, the Savi Scout technique is marred by its high initial cost of implementation and the larger than desired size of marker (12 mm), which limits its use in the axilla. In addition, its range is limited to 4cm in depth, and could be affected by blood pooling, which may interfere with infra-red detection, occasionally resulting in failed localization of the reflector (8,29).
Another approach to wireless occult breast lesion localisation is the use of radiofrequency identification (RFID) devices. These devices are ubiquitous in commercial settings, where they are typically deployed for use in biometric identification and inventory tracking. RFID devices have been cleared for implantation by the FDA. The RFID technology has been piloted for use in endotracheal tubes to monitor the tube’s position and detect its migration at the bedside (30). This methodology has been found highly applicable for breast tumour localisation. A pilot study of 20 patients using RFID devices for tumour localisation has been encouraging with regard to the feasibility and efficacy of the technique (10). The research in RFID localisation led to the development of the Faxitron LOCalizer™ system (Hologic Inc., Santa Clara, CA, USA), the most recent entrant into the wireless occult breast lesion localisation ecosystem. Whilst large scale trial data are still awaited, the LOCalizer™ system was trialled most recently by Malter et al., who investigated the technique in 4 benign cases and concluded that the procedure would be feasible for use in the European setting (27). Furthermore, the use of RFID technology means that the cost of initial deployment compares favourably to systems, such as Magseed or SAVI Scout, which require specialised technology for their detectors (11). In addition, the current licence in the European Union permits deployment of the RFID tag within 30 days of surgery, however, this duration has been extended to ‘long-term’ in the United States, which permits deployment at the time of diagnostic core biopsy in patients requiring NACT (9). However, large-scale comparative studies are still awaited.
Other wireless tumour localisation methods mentioned in the literature include intra-operative ultrasound (IOUS) guided lumpectomy, haematoma ultrasound guided (HUG), ROLL combined with methylene blue dye injection (RCML), and cryo-assisted localisation (CAL). These are regarded to be only of academic interest (7).
Our report represents the first European experience of using RFID technology in patients with breast cancer. We have observed a 100% technical success rate and 0% margin positivity for malignant lesions. The mean time for successful deployment in our study was 5.4 min, which is significantly shorter compared to what is reported for wire localization (10 min) (32). This results in time efficiency in the radiology suite with potential cost-effectiveness implications.
The mean specimen weight of the excised malignant lesions in our analysis was 19.6 g, which is lower compared to what is reported in a similar cohort (17). Moreover, the margin positivity and reoperation rates observed in our study (0% or malignant lesions) are lower compared to that reported for WGL (13-21%) (5,16). The real-time constant navigation facilitates centring of the lesion within the resection specimen, thus leading potentially to a smaller volume of resected tissue and a higher probability of achieving clear surgical margins.
The LOCalizer™ accurately estimates the distance from the tag to the probe, thus, it facilitates a more precise excision. However, the limitations include the presence of MRI phantom of approximately 2 cm in association with the marker (compared to 4 cm for Magseed and less than 5 mm for SAVI Scout), which limits its use in patients who require MRI for monitoring response to NACT. Contrast-enhanced mammography could be considered as an effective alternative to MRI for monitoring the response to NACT when Magseed or RFID markers are used (31). The technology can be improved further by reducing i) the width of the introducer needle (currently 12 gauge compared to 16 for SAVI Scout and 18 for Magseed), ii) the size of the marker (currently 10.5 mm compared to 5 mm for Magseed and 12 mm for SAVI Scout) and iii) by the development of MRI-compatible introducer needles from appropriate alloys to enable MRI-guided localisation of lesions.
The procedures in our series were performed by dedicated breast radiologists and a senior breast surgeon in an academic breast care facility and our results are, therefore, not generalizable to other institutions. Other limitations of our report include the small sample size and the lack of a direct comparison with WGL.
Quantitative cost-effectiveness analysis and potential improvement of the aesthetic outcome should be included in future studies. In conclusion, our study provides further evidence that wireless breast localisation using RFID technology is an effective and reliable alternative to WGL with time efficiency improvement in both the radiology suite and the operating room. It facilitates accurate surgical excision of non-palpable breast lesions and it is associated with acceptably low margin positivity rates and high acceptance by patients, radiologists and surgeons.
However, large prospective cohort studies comparing RFID with WGL, RSL and other localisation systems are required to better characterise the merits and pitfalls of this new technique.
Conflicts of Interest
The Authors have no conflicts of interest to report regarding this study.
Author’s Contributions
KM and MM supervised this study. KM, ST and UW conducted the data collection and analysis. UW and ST drafted the manuscript. UW, MM, AM, NP and KM proofed and finalised the manuscript.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Egan RL. Mammography and diseases of the breast. CA Cancer J Clin. 1968;18(5):279–283. doi: 10.3322/canjclin.18.5.279. PMID: 4321970. DOI: 10.3322/canjclin.18.5.279. [DOI] [PubMed] [Google Scholar]
- 2.Egan RL. Experience with mammography in a tumor institution. Evaluation of 1,000 studies. Radiology. 1960;75:894–900. doi: 10.1148/75.6.894. PMID: 13725888. DOI: 10.1148/75.6.894. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro S. Evaluation of periodic breast cancer screening with mammography. JAMA. 1966;195(9):731. DOI: 10.1001/jama.1966.03100090065016. [PubMed] [Google Scholar]
- 4.Frank HA, Hall FM, Steer ML. Preoperative localization of nonpalpable breast lesions demonstrated by mammography. N Engl J Med. 1976;295(5):259–260. doi: 10.1056/NEJM197607292950506. PMID: 934190. DOI: 10.1056/NEJM197607292950506. [DOI] [PubMed] [Google Scholar]
- 5.Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol. 2013;2013:793819. doi: 10.1155/2013/793819. PMID: 23986868. DOI: 10.1155/2013/793819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blichert-Toft M, Dyreborg U, Bogh L, Kiaer H. Nonpalpable breast lesions: mammographic wire-guided biopsy and radiologic-histologic correlation. World J Surg. 1982;6(1):119–125. doi: 10.1007/BF01656385. PMID: 7090389. DOI: 10.1007/bf01656385. [DOI] [PubMed] [Google Scholar]
- 7.Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev. 2015;(12):CD009206. doi: 10.1002/14651858.CD009206.pub2. PMID: 26718728. DOI: 10.1002/14651858.CD009206.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed M, Douek M. ROLL versus RSL: toss of a coin. Breast Cancer Res Treat. 2013;140(2):213–217. doi: 10.1007/s10549-013-2609-8. PMID: 23793603. DOI: 10.1007/s10549-013-2609-8. [DOI] [PubMed] [Google Scholar]
- 9.DiNome ML, Kusske AM, Attai DJ, Fischer CP, Hoyt AC. Microchipping the breast: an effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res Treat. 2019;175(1):165–170. doi: 10.1007/s10549-019-05143-w. PMID: 30689105. DOI: 10.1007/s10549-019-05143-w. [DOI] [PubMed] [Google Scholar]
- 10.Dauphine C, Reicher JJ, Reicher MA, Gondusky C, Khalkhali I, Kim M. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol. 2015;204(6):W720–W723. doi: 10.2214/AJR.14.13201. PMID: 26001262. DOI: 10.2214/AJR.14.13201. [DOI] [PubMed] [Google Scholar]
- 11.Malter W, Holtschmidt J, Thangarajah F, Mallmann P, Krug B, Warm M, Eichler C. First Reported Use of the Faxitron LOCalizer Radiofrequency Identification (RFID) System in Europe - A Feasibility Trial, Surgical Guide and Review for Non-palpable Breast Lesions. In Vivo. 2019;33(5):1559–1564. doi: 10.21873/invivo.11637. PMID: 31471405. DOI: 10.21873/invivo.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGugin C, Spivey T, Coopey S, Smith B, Kelly B, Gadd M, Hughes K, Dontchos B, Specht M. Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Cancer Res Treat. 2019;177(3):735–739. doi: 10.1007/s10549-019-05355-0. PMID: 31302856. DOI: 10.1007/s10549-019-05355-0. [DOI] [PubMed] [Google Scholar]
- 13.Dauphine C, Goldberger L, Schroeder J, Barone J. 0316 - A Multicenter Prospective Evaluation of a Radiofrequency Identification Tag in the Localization of Nonpalpable Breast Lesions. Dallas, TX. Columbia, MD2016. 17th Annual Meeting of the American Association of Breast Surgeons. 2016;April:13–17. [Google Scholar]
- 14.Cheang E, Ha R, Thornton CM, Mango VL. Innovations in image-guided preoperative breast lesion localization. Br J Radiol. 2018;91(1085):20170740. doi: 10.1259/bjr.20170740. PMID: 29271240. DOI: 10.1259/bjr.20170740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauway E, Saunders R, Friedland J. Innovative diagnostics for breast cancer: new frontiers for the new millennium using radioactive seed localization. Chicago, IL. 85th Annual American College of Surgeons Clinic Congress. American College of Surgeons. 1999 [Google Scholar]
- 16.Gray RJ, Salud C, Nguyen K, Dauway E, Friedland J, Berman C, Peltz E, Whitehead G, Cox CE. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol. 2001;8(9):711–715. doi: 10.1007/s10434-001-0711-3. PMID: 11597011. DOI:10.1007/s10434-001-0711-3. [DOI] [PubMed] [Google Scholar]
- 17.Velazco CS, Wasif N, Pockaj BA, Gray RJ. Radioactive seed localization for breast conservation surgery: Low positive margin rate with no learning curve. Am J Surg. 2017;214(6):1091–1093. doi: 10.1016/j.amjsurg.2017.08.025. PMID: 28947271. DOI: 10.1016/j.amjsurg.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Seely J, Cordeiro E, Hefler J, Thavorn K, Mahajan M, Domina S, Aro J, Ibrahim AM, Arnaout A, Gravel D, Nessim C. Radioactive seed localization versus wire-guided localization for nonpalpable breast cancer: A cost and operating room efficiency analysis. Ann Surg Oncol. 2017;24(12):3567–3573. doi: 10.1245/s10434-017-6084-z. PMID: 28913761. DOI: 10.1245/s10434-017-6084-z. [DOI] [PubMed] [Google Scholar]
- 19.Jakub J, Gray R. Starting a radioactive seed localization program. Ann Surg Oncol. 2015;22(10):3197–3202. doi: 10.1245/s10434-015-4719-5. PMID: 26254168. DOI: 10.1245/s10434-015-4719-5. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed M, Anninga B, Goyal S, Young P, Pankhurst QA, Douek M, Mag STG. Magnetic sentinel node and occult lesion localization in breast cancer (MagSNOLL Trial) Br J Surg. 2015;102(6):646–652. doi: 10.1002/bjs.9800. PMID: 25868072. DOI: 10.1002/bjs.9800. [DOI] [PubMed] [Google Scholar]
- 21.Hayes MK. Update on preoperative breast localization. Radiol Clin North Am. 2017;55(3):591–603. doi: 10.1016/j.rcl.2016.12.012. PMID: 28411682. DOI: 10.1016/j.rcl.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Zacharioudakis K, Down S, Bholah Z, Lee S, Khan T, Maxwell AJ, Howe M, Harvey J. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol. 2019 doi: 10.1016/j.ejso.2019.06.035. PMID: 31288944. DOI: 10.1016/j.ejso.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Harvey JR, Lim Y, Murphy J, Howe M, Morris J, Goyal A, Maxwell AJ. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat. 2018;169(3):531–536. doi: 10.1007/s10549-018-4709-y. PMID: 29453521. DOI: 10.1007/s10549-018-4709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schermers B, van der Hage JA, Loo CE, Vrancken Peeters M, Winter-Warnars HAO, van Duijnhoven F, Ten Haken B, Muller SH, Ruers TJM. Feasibility of magnetic marker localisation for non-palpable breast cancer. Breast. 2017;33:50–56. doi: 10.1016/j.breast.2017.03.003. PMID: 28282587. DOI: 10.1016/j.breast.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Cox CE, Garcia-Henriquez N, Glancy MJ, Whitworth P, Cox JM, Themar-Geck M, Prati R, Jung M, Russell S, Appleton K, King J, Shivers SC. Pilot study of a new nonradioactive surgical guidance technology for locating nonpalpable breast lesions. Ann Surg Oncol. 2016;23(6):1824–1830. doi: 10.1245/s10434-015-5079-x. PMID: 26847680. DOI: 10.1245/s10434-015-5079-x. [DOI] [PubMed] [Google Scholar]
- 26.Cox CE, Russell S, Prowler V, Carter E, Beard A, Mehindru A, Blumencranz P, Allen K, Portillo M, Whitworth P, Funk K, Barone J, Norton D, Schroeder J, Police A, Lin E, Combs F, Schnabel F, Toth H, Lee J, Anglin B, Nguyen M, Canavan L, Laidley A, Warden MJ, Prati R, King J, Shivers SC. A Prospective, single arm, multi-site, clinical evaluation of a nonradioactive surgical guidance technology for the location of nonpalpable breast lesions during excision. Ann Surg Oncol. 2016;23(10):3168–3174. doi: 10.1245/s10434-016-5405-y. PMID: 27469121. DOI: 10.1245/s10434-016-5405-y. [DOI] [PubMed] [Google Scholar]
- 27.Srour MK, Kim S, Amersi F, Giuliano AE, Chung A. Comparison of wire localization, radioactive seed, and Savi scout((R)) radar for management of surgical breast disease. Breast J. 2019 doi: 10.1111/tbj.13499. PMID: 31448530. DOI: 10.1111/tbj.13499. [DOI] [PubMed] [Google Scholar]
- 28.Cornella KN, Palafox BA, Razavi MK, Loh CT, Markle KM, Openshaw LE. SAVI SCOUT as a novel localization and surgical navigation system for more accurate localization and resection of pulmonary nodules. Surg Innov. 2019;26(4):469–472. doi: 10.1177/1553350619843757. PMID: 31027475. DOI: 10.1177/1553350619843757. [DOI] [PubMed] [Google Scholar]
- 29.Kalambo M, Parikh JR. Implementing the SAVI SCOUT system in community radiology practice. J Am Coll Radiol. 2017;14(9):1234–1238. doi: 10.1016/j.jacr.2017.04.036. PMID: 28601614. DOI: 10.1016/j.jacr.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Reicher J, Reicher D, Reicher M. Use of radio frequency identification (RFID) tags in bedside monitoring of endotracheal tube position. J Clin Monit Comput. 2007;21(3):155–158. doi: 10.1007/s10877-007-9069-9. PMID: 17406988. DOI: 10.1007/s10877-007-9069-9. [DOI] [PubMed] [Google Scholar]