Abstract
Background/Aim: The role of upper abdominal resection as part of debulking surgery for advanced-stage or relapsed ovarian cancer has been widely debated. The aim of this study was to investigate the safety and efficacy of upper abdominal resection as part of tertiary cytoreduction. Patients and Methods: Between 2005 and 2019, 11 cases presenting upper abdominal recurrences after surgically treated ovarian cancer were submitted to surgery with radical intent. Results: Complete debulking surgery was feasible in eight cases, optimal debulking was performed in two cases, while in one case a suboptimal resection was performed. The most commonly performed upper abdominal resections consisted of liver resection in seven cases, splenectomy in four cases, diaphragmatic resection in three cases, pancreatic tail resection in two cases and partial gastrectomy in another two cases. Postoperative complications were encountered in two cases, while postoperative mortality was null. Conclusion: Extended upper abdominal resection can be safely performed in order to increase the chances of optimal debulking surgery at the time of tertiary cytoreduction.
Keywords: Tertiary cytoreduction, upper abdomen, resection, ovarian cancer
Cytoreductive surgery remains the cornerstone in treating advanced-stage ovarian cancer; the most important objective is to achieve the state of no residual disease (1). This principle has been successfully applied in cases presenting locally advanced pelvic tumors, an improvement of the long-term outcomes being achieved. When it comes to cases presenting upper abdominal involvement in advanced-stage ovarian cancer, initially it was considered that these cases present a more aggressive tumor biology, responsible for the distant migration of tumor cells, which is incompatible with debulking surgery (2); however, this myth was rapidly destroyed when similar benefits in terms of survival were reported in cases presenting pelvic disease and upper abdominal involvement, once complete debulking was achievable (3-10). The aim of this study was the safety and effectiveness of upper abdominal resection as part of tertiary cytoreduction in patients diagnosed with recurrent ovarian cancer.
Patients and Methods
After receiving approval of the Ethics Committee (no. 34/2019), data of patients submitted to surgery between 2005 and 2019 for advanced-stage or relapsed ovarian cancer were retrospectively reviewed. Among the 167 cases presenting this diagnosis, we identified 32 submitted to tertiary cytoreduction, with 11 cases presenting upper abdominal involvement; data of these cases were retrospectively reviewed. Cytoreduction was defined as complete when no visible residual tumor was achieved, optimal when the residual tumoral volume was lower than 0.5 cm, and suboptimal when the residual tumoral volume was higher than 0.5 cm. Early postoperative complications were considered within the first 30 days and were classified according to Clavien–Dindo classification (11).
Results
The median time from secondary cytoreduction to tertiary cytoreduction was 20 months (range=17-38 months), while the median age at the time of conducting the study was 54 years (range=43-67 years). At the time of initial diagnostic, seven cases were classified with International Federation of Obstetrics and Gynecology (FIGO) stage IIIC disease, three with stage IV, and one with FIGO stage IIIB. Details of patients at the time of the initial diagnostic are shown in Table I.
Table I. Clinicopathological characteristics at the time of initial diagnosis.
Postoperatively, all patients were submitted to adjuvant platinum-based chemotherapy and were diagnosed with first relapse after a median interval of 32 months; the second relapse occurred after a median interval of 20 months.
At the time of tertiary cytoreduction attempt, all patients were submitted to surgery with curative intent. However, at that time, complete cytoreduction was feasible in only eight cases, optimal cytoreduction being achieved in two cases, while in the last case, the debulking procedure was suboptimal. The main locations of residual disease were the mesenteral surface (in two cases), and the liver pedicle (in one case). The intraoperative details at the time of tertiary cytoreduction are shown in Table II.
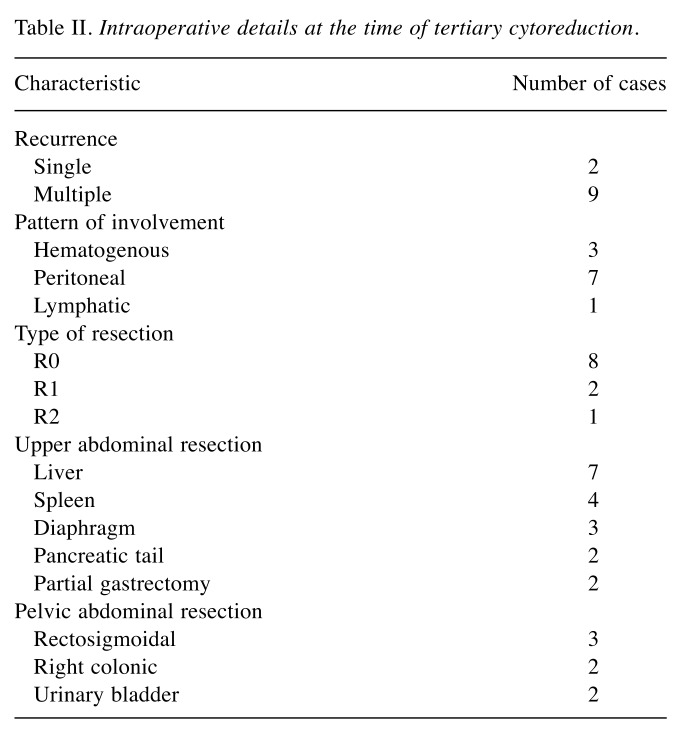
Table II. Intraoperative details at the time of tertiary cytoreduction.
As can be seen from the above data, most patients presented disseminated recurrences, only two out of the 11 cases presented isolated recurrent tumors; as for the pattern of involvement, as expected, in most cases the predominant route was peritoneal in seven cases, being followed by hematogenous in three cases and the lymphatic in one case. The patient who presented lymphatic recurrence was submitted to surgery for a large tumor mass in the splenic hilum involving both the spleen and the pancreatic tail, and was submitted to distal pancreatic resection en bloc with splenectomy. Patients presenting hematogenous lesions were diagnosed with parenchymatous liver metastases in three cases and splenic metastases in two cases (two patients presented both liver and splenic metastases). Cases presenting peritoneal recurrence were usually diagnosed with multiple peritoneal nodules involving the diaphragmatic surface, the mesenteric surface, as well as the Glissonian capsule; therefore, four cases necessitated minor liver resection for peritoneal seeding invading the adjacent liver parenchyma. One case presented a large recurrence at the level of the greater omentum invading the spleen, the greater curvature and the pancreatic tail; therefore, a complex resection consisting of splenectomy, distal pancreatectomy and partial gastrectomy was needed.
Moreover, in five cases, pelvic recurrences were also encountered; in order to maximize the debulking effect, three rectosigmoidal resections, two right colonic resections and two partial cystectomies were needed.
The median length of surgery was 240 min (range=200-320 min), while the median estimated blood loss was 400 ml (range=200-1400 ml); intraoperatively, four out of the 11 cases necessitated transfusion, the median number of packs being one. The median hospital stay was 16 days (range=7-34 days).
Postoperative complications were encountered in two cases, a single complication being related to the association of upper abdominal resection. One case submitted to a distal pancreatectomy developed a pancreatic leak, which was successfully treated in a conservative manner and was classified as grade 3 Dindo–Clavien complication. The second case developed diffuse postoperative bleeding and required emergency relaparotomy; therefore, the complication was classified as Clavien–Dindo stage IV and was not related to the upper abdominal surgery. Postoperative mortality was zero.
Discussion
Patients presenting upper abdominal involvement in the setting of advanced-stage ovarian cancer were considered for a long time as having poor tumor biology and were considered as candidates solely for systemic chemotherapy (2). However, since surgical techniques have improved, extended upper abdominal resections have been associated with good outcomes, demonstrating the effectiveness of these procedures as part of primary debulking surgery. One of the first studies which demonstrated the benefits of these procedures and which destroyed the myth of poor tumor biology was by from Eisenhauer et al. and was published in 2006 (12). The study included 262 patients submitted to surgery between 1998 and 2003 in the Memorial Sloan Kettering Cancer Center, New York, United States of America. According to the time and extent of surgery, the authors classified these patients into three groups: the first group included 57 patients presenting upper abdominal involvement submitted to surgery with curative intent; the second group included 122 patients presenting disease limited to the pelvis and submitted to standard cytoreduction; the remaining 83 cases were included in the third group and were represented by cases presenting upper abdominal involvement in which suboptimal cytoreduction was performed. The authors underlined the fact that cases submitted to suboptimal cytoreduction due to the presence of upper abdominal involvement were submitted to surgery before the year 2000, when extensive upper abdominal resection was added as part of debulking surgery. However, the authors demonstrated that progression-free and overall survival was significantly improved in cases submitted to complete debulking irrespective of the site of involvement. Therefore, patients in the second group presented a median overall survival of 84 months, significantly higher when compared to that reported for the third group (of only 38 months) and similar to that for the first group (which was not reached by 68 months). The most important prognostic factors for the long-term outcomes were the stage of disease, the preoperative level of cancer antigen-125, association with ascites and the presence of residual disease. Moreover, the authors demonstrated similar rates of postoperative complications between the two groups, underlining in this way the safety of the addition of upper abdominal resection (12).
Once the benefits of extending the field of the resection in the upper abdomen were widely demonstrated in patients presenting locally advanced ovarian cancer, attention was focused on determining if such resections were feasible and effective in recurrent disease. Such resections were successfully added in patients presenting disease relapse.
The concept of repetitive cytoreduction was initially introduced by Berek et al., this study group also being the first to demonstrate an inversely proportional relationship between the residual tumoral volume and overall survival after secondary debulking (13).
When it comes to the role of tertiary cytoreduction in relapsed ovarian cancer, the first series was reported by Leitao et al. and was conducted between 1990 and 2002, at the Memorial Sloan-Kettering Cancer Center, New York University, United States of America (14). The study included 26 patients submitted to tertiary cytoreduction after a median time from secondary cytoreduction of 25.6 months and a median treatment-free interval of 13.4 months. The authors reported median disease-specific survival of 36.3 months after tertiary cytoreduction for patients submitted to optimal cytoreduction (defined as a visible tumoral volume lower than 0.5 cm) and of only 15 months than the residual volume was higher (p=0.05). They also demonstrated that cases submitted to complete debulking (defined as no residual disease) had similar long-term outcomes when compared to those submitted to optimal debulking. The univariate analysis demonstrated that other prognostic factors for long-term survival were the time to the first recurrence (>12 months) and the treatment-free interval (>12 months). Regarding the need for extending the resection in the upper abdomen, the authors reported that three cases were submitted to hepatic resection, seven to extended lymph node dissection, one to nephrectomy and another to cholecystectomy. When it comes to postoperative complications, they were reported in 27% of cases and were classified as Clavien–Dindo grade 1, 2 or 3 complications, no reoperation or death being reported (14). Data reported in this study were further completed in the report published by the same study group in 2010. At that time, the authors reported the outcomes of 77 patients submitted to tertiary cytoreductive surgery between 1998 and 2008, the median disease-free survival between secondary and tertiary cytoreduction being of 25.7 months. On univariate analysis, the most significant prognostic factors were residual volume after tertiary cytoreduction and the treatment-free interval; however, on multivariate analysis, only the residual volume after tertiary cytoreduction remained statistically significant (15).
A more recent study which investigated the feasibility and safety of tertiary cytoreduction for relapsed ovarian cancer was a multicenter one and was published in 2017 (16). The study included 103 patients submitted to tertiary cytoreduction at 11 high-volume gynecologic oncology centers between January 2008 and December 2012. The primary endpoints of the study were to study the impact of complete debulking on overall survival and to identify the prognostic factors which might predict the feasibility of complete debulking, while the secondary endpoint was to study the value of the Arbeitsgemeinschaft Gynäkologische Onkologie (AGΟ) score on predicting the feasibility of complete cytoreduction. The median overall survival after tertiary cytoreduction was 39.5 months and was significantly influenced by the completeness of cytoreduction, the initial stage at diagnostic, the location of the recurrent tumor and by the interval between the initial diagnosας and when tertiary cytoreduction was performed. For the AGO score, the authors demonstrated that a positive score had a negative predictive value for complete cytoreduction of 43.2%. For the extent of the resectional phase in the upper abdomen, the authors reported 39 cases submitted to extended retroperitoneal dissection, nine to diaphragmatic resection, eight to splenectomy, two to cholecystectomy, two to hepatic resection, two to pancreatic tail resection, and one to partial gastrectomy. Moreover, extra-abdominal resections consisting of axillary lymph node dissection, pulmonary lobectomy and resection of brain metastases were performed in one case each. However, the early postoperative morbidity rate was 9.7%, while the postoperative mortality rate was zero, demonstrating once again the safety of tertiary cytoreduction (16).
These data underline once again the fact that the extent of upper abdominal resection, and even extra-abdominal resection, increases over time once the perioperative management of these cases improves.
In order to maximize the efficiency of this procedure and to minimize perioperative complications, certain authors went further and demonstrated the utility of minimally invasive surgery in such cases. In a study conducted by Choi et al. and published in 2014, the authors reported the cases of eight patients submitted to laparoscopic debulking in the setting of secondary cytoreduction and five cases submitted to laparoscopic debulking in the setting of tertiary cytoreduction; among these patients, upper abdominal resection consisted of liver resection in two cases, splenectomy in four, and resection of the mesentery and omentum in one case. The authors reported a single intraoperative complication and no postoperative complications, suggesting the utility of minimally invasive surgery in selected cases (17).
Conclusion
Tertiary cytoreduction seem to be feasible and safe in providing control of relapsed disease in recurrent ovarian cancer, acceptable rates of perioperative complications being reported. For the inclusion of extensive upper abdominal resections as part of tertiary cytoreduction, it seems that these resections can be safely added in order to maximize the completeness of cytoreduction. As expected, these procedures were initially rarelypart of tertiary cytoreduction, a higher number of such procedures being reported over time, once the surgical techniques and the perioperative management of such cases improved.
Conflicts of Interest
The Authors declare no conflicts of interest exist in regard to this study
Authors’ Contributions
NB: Performed surgical procedures. IB: Wrote the article. IB, SD: Performed literature review. MV: Part of the surgical team. IB: Advice about surgical procedures, finally revised the article.
Acknowledgements
This work was supported by the project entitled Multidisciplinary Consortium for Supporting the Research Skills in Diagnosing, Treating and Identifying Predictive Factors of Malignant Gynecologic Disorders, project number PN-III-P1-1.2-PCCDI2017-0833.
References
- 1.McGee J, Bookman M, Harter P, Marth C, McNeish I, Moore KN, Poveda A, Hilpert F, Hasegawa K, Bacon M, Gatsonis C, Brand A, Kridelka F, Berek J, Ottevanger N, Levy T, Silverberg S, Kim BG, Hirte H, Okamoto A, Stuart G, Ochiai K. Fifth Ovarian Cancer Consensus Conference: Individualized therapy and patient factors. Ann Oncol. 2017;28:702–710. doi: 10.1093/annonc/mdx010. PMID: 28119296. DOI: 10.1093/annonc/mdx010. [DOI] [PubMed] [Google Scholar]
- 2.Covens AL. A critique of surgical cytoreduction in advanced ovarian cancer. Gynecol Oncol. 2000;78:269–274. doi: 10.1006/gyno.2000.5926. PMID: 10985879. DOI: 10.1006/gyno.2000.5926. [DOI] [PubMed] [Google Scholar]
- 3.Le T, Krepart GV, Lotocki RJ, Heywood MS. Does debulking surgery improve survival in biologically aggressive ovarian carcinoma. Gynecol Oncol. 1997;67:208–214. doi: 10.1006/gyno.1997.4839. PMID: 9367710. DOI: 10.1006/gyno.1997.4839. [DOI] [PubMed] [Google Scholar]
- 4.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: A prospective study. Gynecol Oncol. 2003;90:390–396. doi: 10.1016/s0090-8258(03)00278-6. PMID: 12893206. DOI: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 5.Bacalbasa N, Balescu I, Vilcu M, Brasoveanu V, Tomescu D, Dima S, Suciu I, Suciu N, Bodog A, Brezean I. Distal pancreatectomy en bloc with splenectomy as part of tertiary cytoreduction for relapsed ovarian cancer. Proc 4th Congr Romanian Soc Minimal Invasive Surg in Gynecol. 2019:29–32. [Google Scholar]
- 6.Bacalbasa N, Balescu I, Vilcu M, Brezean I, Tomescu D, Dima S, Suciu I, Bodog A, Suciu N. Debulking surgery for paraaortic tumoral masses at the time of secondary cytoreduction for relapsed ovarian cancer. Proc 4th Congr Romanian Soc Minimal Invasive Surg in Gynecol. 2019:29–32. [Google Scholar]
- 7.Brasoveanu V, Bacalbasa N, Balescu I, Tomescu D, Dima S, Orban C, Vilcu M, Brezean I. Pancreatoduodenectomy as part of primary cytoreduction for advanced stage ovarian cancer. Proc 4th Congr Romanian Soc Minimal Invasive Surg in Gynecol. 2019:60–63. [Google Scholar]
- 8.Brezean I, Aldoescu S, Catrina E, Fetche N, Marin I, Pacescu E. Gallstone ileus: analysis of eight cases and review of the literature. Chirurgia. 2010;105:355–359. PMID: 20726301. [PubMed] [Google Scholar]
- 9.Bacalbasa N, Balescu I, Tanase A, Pautov M, Brezean I, Vilcu M, Brasoveanu V. Spleno-pancreatectomy en bloc with parcelar gastrectomy for splenic artery aneurysm - A case report and literature review. In Vivo. 2018;32:915–919. doi: 10.21873/invivo.112329. PMID: 29936480. DOI: 10.21873/invivo.11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacalbasa N, Balescu I, Tanase A, Brezean I, Vilcu M, Brasoveanu V. Successful resection of a non-functional paraganglioma with celiac trunk invasion followed by common hepatic artery reimplantation – a case report and literature review. In Vivo. 2018;32:911–914. doi: 10.21873/invivo.112328. PMID: 29936479. DOI:10.21873/invivo.11328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912. DOI: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, Jarnagin WR, DeMatteo RP, D’Angelica MI, Barakat RR, Chi DS. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103:1083–1090. doi: 10.1016/j.ygyno.2006.06.028. PMID: 16890277. DOI: 10.1016/j.ygyno.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Berek JS, Hacker NF, Lagasse LD, Nieberg RK, Elashoff RM. Survival of patients following secondary cytoreductive surgery in ovarian cancer. Obstet Gynecol. 1983;61:189–193. PMID: 6823360. [PubMed] [Google Scholar]
- 14.Leitao MM Jr., Kardos S, Barakat RR, Chi DS. Tertiary cytoreduction in patients with recurrent ovarian carcinoma. Gynecol Oncol. 2004;95:181–188. doi: 10.1016/j.ygyno.2004.07.033. PMID: 15385129. DOI: 10.1016/j.ygyno.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Shih KK, Chi DS, Barakat RR, Leitao MM Jr. Tertiary cytoreduction in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer: an updated series. Gynecol Oncol. 2010;117:330–335. doi: 10.1016/j.ygyno.2010.01.046. PMID: 20189234. DOI: 10.1016/j.ygyno.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Falcone F, Scambia G, Benedetti PP, Signorelli M, Cormio G, Giorda G, Bogliolo S, Marinaccio M, Ghezzi F, Rabaiotti E, Breda E, Casella G, Fanfani F, Di D V, Leone Roberti MU, Greggi S. Tertiary cytoreductive surgery in recurrent epithelial ovarian cancer: A multicentre MITO retrospective study. Gynecol Oncol. 2017;147:66–72. doi: 10.1016/j.ygyno.2017.07.008. PMID: 28716306. DOI: 10.1016/j.ygyno.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Choi JK, Paik ES, Lee YY, Lee JW, Bae DS, Kim TH. Feasibility and safety of laparoscopic secondary or tertiary cytoreduction in carefully selected patients with localized recurrent epithelial ovarian cancer. J Minimal Invas Gynecol. 2014;21(6) Supplement:S189. DOI: 10.1016/j.jmig.2014.08.614. [Google Scholar]