Abstract
Background: The C-reactive protein (CRP)-to-serum albumin ratio is associated with a poor prognosis in patients with several cancers. The purpose of this study was to clarify the relationship between the preoperative CRP/Alb ratio and overall survival of pancreatic ductal adenocarcinoma (PDAC) in patients who received radical surgery and S-1 adjuvant chemotherapy. Patients and Methods: We included 117 patients who underwent radical surgery with S-1 adjuvant chemotherapy. We constructed receiver operating characteristic curve (ROC curve) of the CRP/Alb ratio to determine the cut-off value. We analyzed the relationship among the CRP/Alb ratio, clinicopathological status, and survival. Results: The optimal cut-off value of the CRP/Alb ratio was 0.036. All patients were divided into a high-ratio group (CRP/Alb ratio ≥0.036) and low-ratio group (CRP/Alb ratio <0.036). The 5-year overall survival (OS) rates in the high- and low-ratio groups were 22.5% and 36.4%, respectively (p=0.0089). The 5-year disease-free survival (DFS) rates in the high- and low-ratio groups were 12.5% and 22.1%, respectively (p=0.0097). The univariate and multivariate analyses of the OS showed that the pathological N factor and CRP/Alb ratio were independent factors of the survival. The univariate and multivariate analyses of the RFS showed that the pathological N factor, resection margin, and CRP/Alb ratio were independent factors of the survival. Conclusion: The preoperative CRP/Alb ratio is a strong prognostic factor for PDAC patients with undergo curative resection with S-1 adjuvant chemotherapy.
Keywords: Pancreatic ductal adenocarcinoma, CRP/Alb ratio, prognostic factor
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death with a 5-year relative survival of 8% (1,2). It has been estimated that pancreatic cancer will become the second leading cause of cancer death in the United States by 2030 (3). Complete surgical resection and perioperative adjuvant treatment is the only chance for a cure. In Japan, the Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC) showed that S-1 (oral fluorouracil) adjuvant chemotherapy improved the overall survival (OS) and the disease-free survival (DFS) for completely resected pancreatic cancer patients with a 5-year OS and 3-year DFS of 44.1% and 22.6%, respectively (4).
The relationship between systemic inflammation and tumor progression has been reported in several investigations, with the involvement of the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and Glasgow Prognostic Score (GPS) suggested (5-7). Recently, the CRP/Alb ratio has been associated with poor outcomes in patients in renal cell carcinoma, gastric cancer, hepatocellular carcinoma, and systemic inflammation diseases (8-11). The C-reactive protein (CRP)-to-serum albumin ratio was first developed as a predictive system of critically ill patients (12). However, there are few reports that have investigated the prognostic and predictive value of the CRP/Alb ratio in PDAC patients preoperatively.
The purpose of this study was to clarify the relationship between the preoperative CRP/Alb ratio and the survival of PDAC patients who received radical surgery and S-1 adjuvant chemotherapy.
Patients and Methods
Patients. The consecutive 201 patients who received pancreatic surgery at Kanagawa Cancer Center from 2007 to 2016 were enrolled retrospectively. All patients were pathologically confirmed as PDAC and staged according to the UICC TNM 7th edition. The patients underwent macroscopically curative resection with pathological R0 or R1 resection. The patients who received S-1 adjuvant chemotherapy were eligible for this study. The patients who have pathologically proven of distant metastases, such as metastasis to para-aortic lymph nodes, and who did not receive adjuvant chemotherapy or received adjuvant chemotherapy with other regimens were excluded from this study.
Surgical treatment and adjuvant chemotherapy. All surgeries were performed in accordance to standardized procedures that have been described (13). After surgery, all patients received S-1 adjuvant chemotherapy. Adjuvant treatment was started within 10 weeks after curative surgery and continued for 24 weeks. The patients received 80 mg/m2/day of S-1 for 4 weeks, followed by 2 weeks of rest. If the adverse events appeared according to common terminology criteria for adverse events (CTCAE), the daily dose was reduced. Adjuvant chemotherapy was withdrawn when patients showed adverse events even after dose modification and recognized the recurrence by imaging.
Clinicopathological data. The following clinicopathological data were collected and evaluated in this study: gender, age, preoperative CA 19-9 value, complication after surgery, tumor size, lymph node metastasis, pathological stage according to TNM classification, residual tumor, and serum CRP and albumin levels. Blood samples were evaluated within 14 days before surgery and the start day of the adjuvant chemotherapy. The grade of postoperative complications was defined according to the Clavien-Dindo classification that occurred within 30 days after surgery (14).
Follow-up. All patients were followed-up at outpatient for five years after received surgical resection and adjuvant chemotherapy. Patients are regularly checked the serum CEA and CA19-9 levels with blood samples and radiological examination by computed tomography every three months until five years after surgery.
Evaluations and statistical analyses. We constructed receiver operating characteristic curve (ROC curve) to determine the appropriate cut-off value of the preoperative CRP/Alb ratio. Comparisons between the high CRP/Alb ratio and the low CRP/Alb ratio groups were compared with using χ2 test or Fisher’s exact test. The OS was defined as the period from surgery until death or the date of final observation. The Kaplan-Meier method was used to create the OS curves and compared using the log-rank test. The univariate and multivariate survival analyses were performed using Cox’s proportional hazards model. The two-sided p-values of <0.05 were defined as statistically significant. All of the statistical analyses were calculated using EZR (15). This study was approved by the IRB Committee of the Kanagawa Cancer Center.
Results
Patients. A total of 306 patients underwent surgical resection between 2007 and 2016, and 117 patients were eligible for this study. The median age was 70 years (range=44-84 years). Sixty-three patients were males, and 54 were females. Seventy-eight patients underwent pancreaticoduodenectomy, 30 distal pancreatectomy, and 9 total pancreatectomy. All patients received S-1 adjuvant chemotherapy, with 62 completing the planned therapy. Patients with obstructive jaundice and acute cholangitis were already being treated by endoscopic or percutaneous drainage. There were no patients who underwent pancreatic surgery with cholangitis.
Determination of the cut-off value of the CRP/Alb ratio. We collected data on the preoperative values (within 14 days before surgery) of CRP (mg/dl) and Alb (g/dl) by sampling the peripheral blood. We constructed a ROC curve to determine the appropriate cut-off value of the preoperative CRP/Alb ratio and determined the value as 0.036 preoperatively.
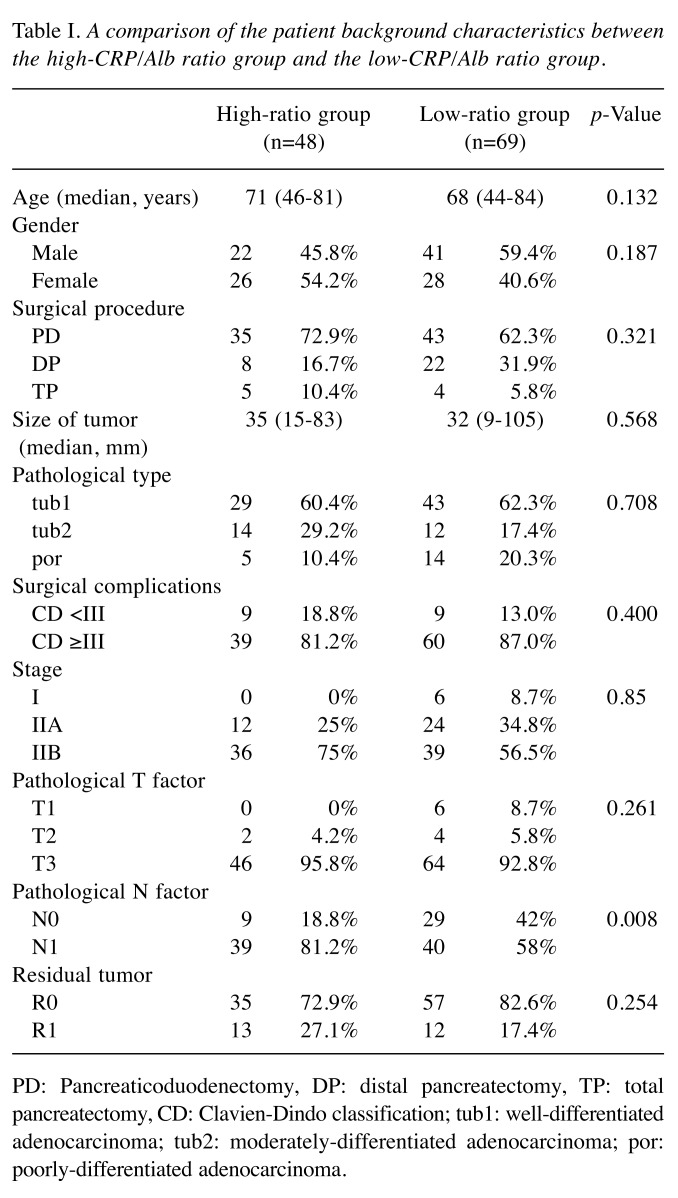
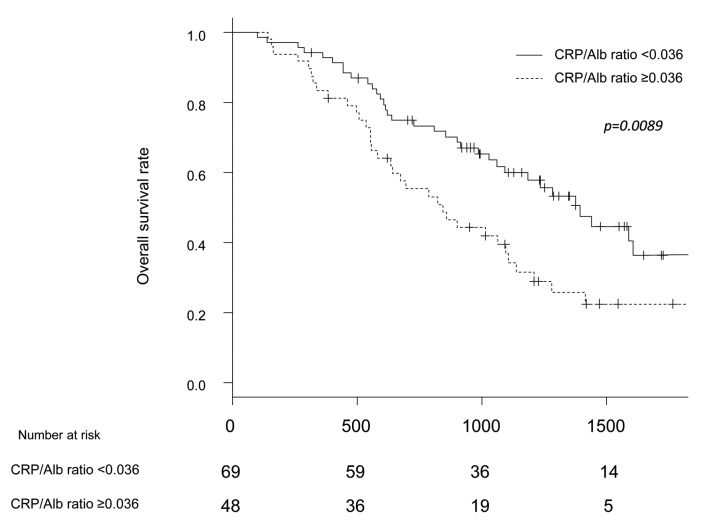
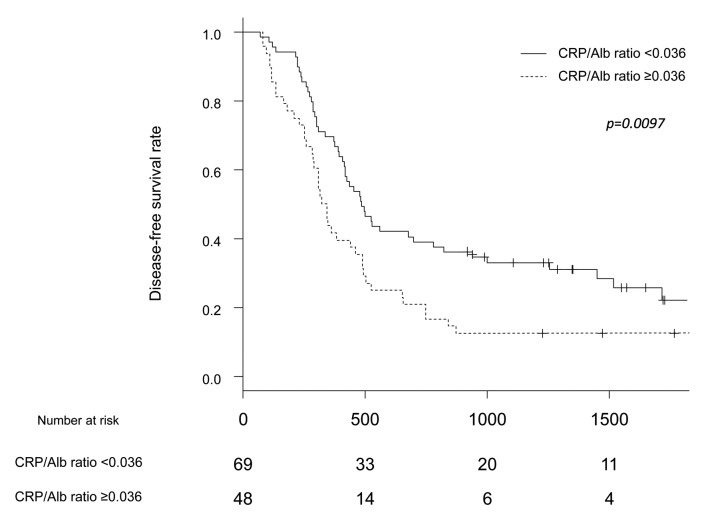
DFS and OS analyses. All patients were divided into a high-ratio group (CRP/Alb ratio ≥0.036) and low-ratio group (CRP/Alb ratio <0.036). Preoperatively, 48 patients were included in the high-ratio group, and 69 were included in the low-ratio group. The background characteristics of these two groups are shown in Table I. Only the pathological N factor differed markedly between the two groups. The 5-year OS rates in the high- and low-ratio groups were 22.5% and 36.4%, respectively (p=0.0089) (Figure 1). The 5-year DFS rates in the high- and low-ratio groups were 12.5% and 22.1%, respectively (p=0.0097) (Figure 2).
Table I. A comparison of the patient background characteristics between the high-CRP/Alb ratio group and the low-CRP/Alb ratio group.
PD: Pancreaticoduodenectomy, DP: distal pancreatectomy, TP: total pancreatectomy, CD: Clavien-Dindo classification; tub1: well-differentiated adenocarcinoma; tub2: moderately-differentiated adenocarcinoma; por: poorly-differentiated adenocarcinoma.
Figure 1. Comparison of overall survival between the high-CRP/Alb ratio group and the low-CRP/Alb ratio group.
Figure 2. Comparison of disease-free survival between the high-CRP/Alb ratio group and the low-CRP/Alb ratio group.
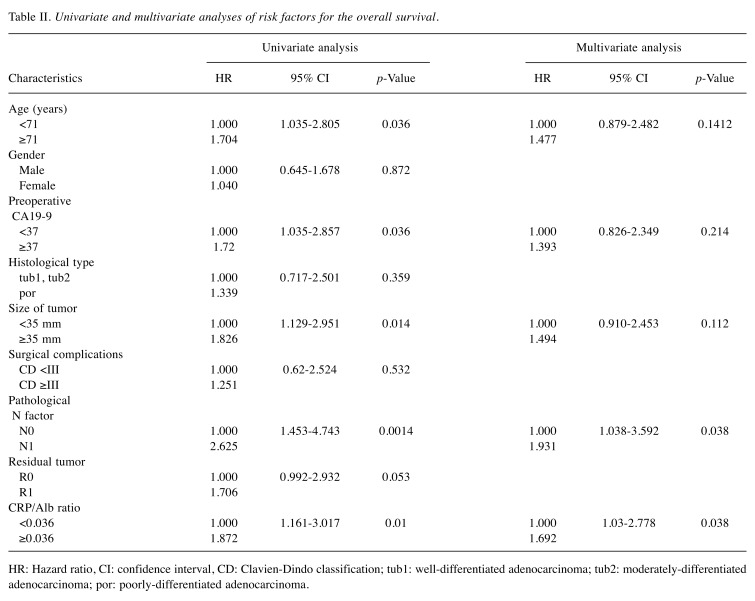
A univariate analysis of the OS showed that the age, preoperative CA19-9 value, pathological N factor, tumor size, and CRP/Alb ratio were factors affecting the survival. A multivariate analysis showed that the pathological N factor [hazard ratio (HR)=1.931; 95% confidence interval (CI)=1.038-3.592; p=0.038] and CRP/Alb ratio (HR=1.692; 95%CI=1.03-2.778; p=0.038) were independent factors affecting the survival (Table II).
Table II. Univariate and multivariate analyses of risk factors for the overall survival.
HR: Hazard ratio, CI: confidence interval, CD: Clavien-Dindo classification; tub1: well-differentiated adenocarcinoma; tub2: moderately-differentiated adenocarcinoma; por: poorly-differentiated adenocarcinoma.
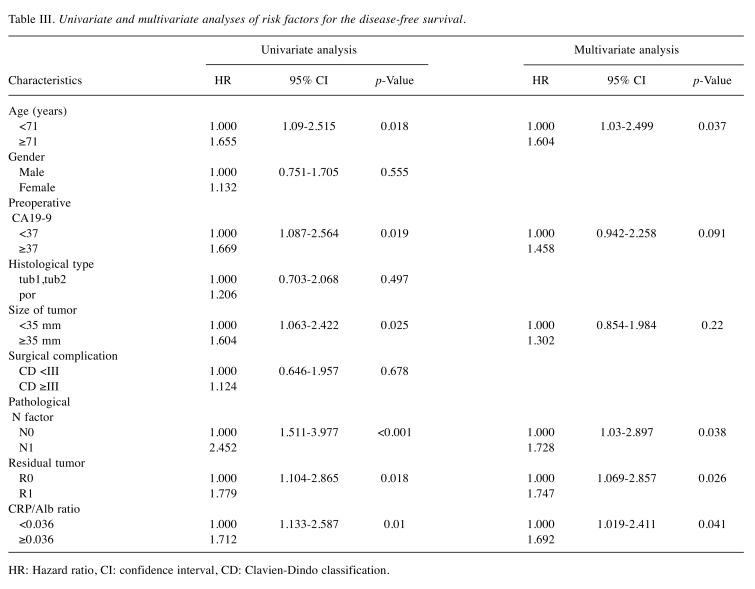
A univariate analysis for the DFS showed that the age, preoperative CA19-9 value, pathological N factor, tumor size, residual tumor, and CRP/Alb ratio were independent factors affecting recurrence. A multivariate analysis showed that the age (HR=1.604; 95%CI=1.03-2.499; p=0.037), pathological N factor (HR=1.728; 95%CI=1.03-2.897; p=0.038), residual tumor (HR=1.747; 95%CI=1.069-2.857; p=0.026), and CRP/Alb ratio (HR=1.692; 95%CI=1.019-2.411; p=0.041) were independent factors affecting the recurrence (Table III).
Table III. Univariate and multivariate analyses of risk factors for the disease-free survival.
HR: Hazard ratio, CI: confidence interval, CD: Clavien-Dindo classification.
Discussion
In the present study, we revealed the clinical significance of the preoperative CRP/Alb ratio as an independent prognostic factor for PDAC patients who received radical surgery and S-1 adjuvant chemotherapy. We determined the appropriate cut-off value of the preoperative CRP/Alb ratio to be 0.036, and statistical analyses showed that the CRP/Alb ratio was strongly related to the survival as an independent prognostic factor. This result suggests that patients with a high preoperative CRP/Alb ratio have a poorer prognosis.
The relationship between inflammation and cancer is well-documented (5,6). Inflammation plays an important role in carcinogenesis and tumor progression. Many investigators have already reported that inflammation-based prognostic scores, including the NLR, PLR, the GPS, and modified GPS, are associated with the prognosis of several types of malignancies. Recently, the CRP/Alb ratio was reported to have prognostic value in pancreatic cancer patients. However, there have been few reports evaluating the significance of the preoperative CRP/Alb ratio as a prognostic factor in PDAC patients. In a previous report, Haruki et al. reported on the significance of the preoperative CRP/Alb ratio as an independent prognostic factor in patients with pancreatic cancer (16). They analyzed 113 patients who had undergone pancreatic resection and reported the optimal cut-off level of the CRP/Alb ratio to be 0.03, which is associated with a poor prognosis after pancreatic resection with or without adjuvant chemotherapy. In another report, Wu et al. reported that the cut-off value of the CRP/Alb ratio was 0.54 in 233 advanced pancreatic cancer patients (17). They found that the CRP/Alb ratio was an independent prognostic factor in advanced PDAC patients. They also reported that the CRP/Alb ratio was a more useful marker than other inflammation-based scores by comparing the AUC values. Given the above, the CRP/Alb ratio appears to be a useful marker for identifying patients with a poor prognosis.
Our data showed that the prognosis of PDAC patients could be predicted by evaluating a small blood sample obtained preoperatively. We focused on the preoperative values, because postoperative values reflect not only cancer progression but also surgical invasion. Pancreatectomy is one of the most invasive surgical procedures, and invasive surgery can cause systemic inflammation along with postoperative complications and a poor nutritional status. For example, Arima et al. reported that the CRP/Alb ratio at postoperative day 14 was significantly associated with a shorter OS and relapse-free survival (18). The cut-off value of the CRP/Alb ratio in their study was 0.34, which is higher than that obtained in the present study. In addition, we detected a relationship between survival and postoperative complications, especially infectious complications, such as intra-abdominal abscess (19). As we mentioned above, we need to be careful when evaluating the CRP/Alb ratio postoperatively.
Several limitations associated with the present study warrant mentioning. First, the rate of pathological lymph node metastasis differed significantly between the two groups. However, while we cannot deny its effect on the survival curves, the multivariate analysis showed that the CRP/Alb ratio was an independent prognostic factor. Second, the current study was a retrospective, single-center study with a small sample size. It is very important that these results be confirmed in another cohort or in a multi-center study. Finally, our data included only patients who received upfront surgery and postoperative adjuvant chemotherapy. Whether or not the same results would have been obtained with PDAC patients who received neo-adjuvant chemotherapy is unclear.
Conclusion
The CRP/Alb ratio was found to be a useful prognostic marker in PDAC patients who underwent curative resection with S-1 adjuvant chemotherapy. The high CRP/Alb ratio is associated with poorer prognosis with the cut-off value of 0.036. This result should be confirmed by another cohort study or multi-center study.
Conflicts of Interest
The Authors declare no conflicts of interest associated with this manuscript.
Authors’ Contributions
Masaaki Murakawa: Conception of the work, collection of data, statistical analysis and writing manuscript; Yuto Kamioka, Mariko Kamiya, Yosuke Atsumi, Toru Aoyama: Contribution to collection of data; Naoto Yamamoto, Satoshi Kobayashi, Makoto Ueno, Manabu Morimoto: Contribution to advice regarding Tables, Figures and evaluation and interpretation of clinical data; Hiroshi Tamagawa, Norio Yukawa, Yasushi Rino, Munetaka Masuda and Soichiro Morinaga: Contribution to evaluation of clinical data and statistical analysis. Advice regarding Discussion.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. PMID: 25220842. DOI: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. PMID: 26742998. DOI: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. PMID: 24840647. DOI: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y, Group JS. Adjuvant chemotherapy of s-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (jaspac 01) Lancet. 2016;388(10041):248–257. doi: 10.1016/S0140-6736(16)30583-9. PMID: 27265347. DOI: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. PMID: 12490959. DOI: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. PMID: 18650914. DOI: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.McMillan DC. The systemic inflammation-based glasgow prognostic score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003. PMID: 22995477. DOI: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Tsujino T, Komura K, Hashimoto T, Muraoka R, Satake N, Matsunaga T, Tsutsumi T, Yoshikawa Y, Takai T, Minami K, Uehara H, Hirano H, Nomi H, Ibuki N, Takahara K, Inamoto T, Ohno Y, Azuma H. C-reactive protein-albumin ratio as a prognostic factor in renal cell carcinoma - a data from multiinstitutional study in japan. Urol Oncol. 2019 doi: 10.1016/j.urolonc.2019.04.002. PMID: 31053528. DOI: 10.1016/j.urolonc.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, Xu D. Preoperative c-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8(4):339–345. doi: 10.1016/j.tranon.2015.06.006. PMID: 26310380. DOI: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M. The c-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. doi: 10.1245/s10434-014-4048-0. PMID: 25190127. DOI: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 11.Qin G, Tu J, Liu L, Luo L, Wu J, Tao L, Zhang C, Geng X, Chen X, Ai X, Shen B, Pan W. Serum albumin and creactive protein/albumin ratio are useful biomarkers of crohn's disease activity. Med Sci Monit. 2016;22:4393–4400. doi: 10.12659/MSM.897460. PMID: 27848931. DOI: 10.12659/msm.897460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8(3):e59321. doi: 10.1371/journal.pone.0059321. PMID: 23555017. DOI: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakawa M, Aoyama T, Miyagi Y, Kobayashi S, Ueno M, Morimoto M, Numata M, Yamamoto N, Tamagawa H, Yukawa N, Rino Y, Masuda M, Morinaga S. The impact of sparc expression on the survival of pancreatic ductal adenocarcinoma patients after curative resection. J Cancer. 2019;10(3):627–633. doi: 10.7150/jca.28660. PMID: 30719160. DOI: 10.7150/jca.28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. PMID: 1360123. DOI: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanda Y. Investigation of the freely available easy-to-use software 'ezr' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. PMID: 3590441. DOI: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haruki K, Shiba H, Shirai Y, Horiuchi T, Iwase R, Fujiwara Y, Furukawa K, Misawa T, Yanaga K. The c-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg. 2016;40(9):2254–2260. doi: 10.1007/s00268-016-3491-4. PMID: 26956901. DOI: 10.1007/s00268-016-3491-4. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, Guo J, Guo L, Zuo Q. The c-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol. 2016;37(9):12525–12533. doi: 10.1007/s13277-016-5122-y. PMID: 27344157. DOI: 10.1007/s13277-016-5122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arima K, Yamashita YI, Hashimoto D, Nakagawa S, Umezaki N, Yamao T, Tsukamoto M, Kitano Y, Yamamura K, Miyata T, Okabe H, Ishimoto T, Imai K, Chikamoto A, Baba H. Clinical usefulness of postoperative c-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am J Surg. 2018;216(1):111–115. doi: 10.1016/j.amjsurg.2017.08.016. PMID: 28859917. DOI: 10.1016/j.amjsurg.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Aoyama T, Murakawa M, Katayama Y, Yamaoku K, Kanazawa A, Higuchi A, Shiozawa M, Morimoto M, Yoshikawa T, Yamamoto N, Rino Y, Masuda M, Morinaga S. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res. 2015;35(4):2401–2409. PMID: 25862906. [PubMed] [Google Scholar]