Abstract
Background/Aim: The number of older patients with colorectal cancer (CRC) is increasing. Stage II CRC is a heterogeneous group of cancers with different prognoses. We aimed to examine older patients in relation to clinical outcome following curative resection in stage II CRC. Patients and Methods: We analyzed data for 329 consecutive patients with stage II CRC following curative resection. Recurrence-free survival (RFS) and overall survival (OS) were compared between older patients ≥75 years of age and those <75 years. Cox proportional hazards model was used to compute hazard ratios (HRs) controlling for potential confounders. Results: In the multivariable analyses, patients ≥75 years were independently associated with shorter RFS (multivariable HR=2.56, 95% confidence interval (CI)=1.55-4.31, p<0.001) and OS (multivariable HR=4.36, 95%CI=2.08-9.97, p<0.001) in stage II CRC. Conclusion: Older patients were independently associated with shorter RFS and OS following curative resection in stage II CRC.
Keywords: Carcinoma, morbidity, mortality, geriatrics, gerontology
Colorectal cancer is the most common cancer in Japan and the third most common cancer worldwide (1,2). Surgery with complete resection represents a potentially curative treatment for most patients with stage I or II colorectal cancer (3,4). Although the current standard treatment for stage III colorectal cancer is curative surgery and adjuvant chemotherapy, the benefit of adjuvant chemotherapy in patients with stage II colorectal cancer remains controversial (5).
Stage II colorectal cancer is a heterogeneous group of cancers with differing biology (6). Large-scale studies demonstrate that patients with stage II colorectal cancer have widely different prognoses (7-9). Western clinical guidelines indicate: i) T4 stage, ii) fewer than 12 examined lymph nodes, iii) bowel perforation and obstruction, iv) lymphatic and venous invasion, and v) poorly differentiated tumors as poor prognostic factors in stage II colorectal cancer and recommend adjuvant chemotherapy for patients presenting these ‘high-risk’ features (10,11). Unfortunately, the benefit of adjuvant chemotherapy for such patients has not been confirmed yet (5,12). Hence, identifying predictive biomarkers for the selection of patients with stage II colorectal cancer who benefit from adjuvant chemotherapy are important.
Due to low birth and high longevity rates many populations around the world are aging rapidly, resulting in an increasing requirement for surgery to treat gastrointestinal cancer in older patients (13-16). Because many clinical trials for colorectal cancer have not included patients with colorectal cancer ≥75 years of age (17-19), there is a lack of evidence-based guidelines for older patients with colorectal cancer, who usually have age-related compromised organ function and host immunity (20,21). Here we tested the hypothesis that older patients may suffer worse clinical outcomes following curative resection in stage II colorectal cancer.
Patients and Methods
Patients. We retrospectively analyzed data for consecutive patients with pathological stage II colorectal cancer who underwent D3 lymph node dissection at the National Hospital Organization Kumamoto Medical Center between January 2009 and December 2016. The main inclusion criteria were as follows: i) histologically confirmed stage II colorectal adenocarcinoma following curative resection, ii) no prior chemotherapy or radiotherapy for colorectal cancer, and iii) no other active malignancy.
Recurrence-free survival (RFS) was defined as the time to recurrence or death. Overall survival (OS) was calculated from surgery to death from any cause. A single institutional pathologist diagnosed the i) depth of wall invasion, ii) status of lymph node metastasis, iii) histopathological differentiation, and iv) the degree of lymphatic and venous invasion, based on the Japanese Classification of Colorectal Carcinoma (1,22). Postoperative complications were recorded and graded as defined by the Clavien-Dindo classification system (23,24). Preoperative blood samples were obtained within two weeks before resection for colorectal cancer. The prognostic nutritional status was calculated on the basis of admission data as follows: 10× serum albumin (g/dl)+0.005× total lymphocyte count (per mm3) (25). The definition of anastomotic leakage was used as previously reported in clinical trials (26,27); peritonitis from any staple line, and pelvic abscess without radiologically proven leakage mechanism were included. Leakage was verified by i) clinical (inspection of drain contents), ii) endoscopic (flexible sigmoidoscopy), or iii) radiologic (rectal contrast study, computed tomography scan) interventions.
This study was approved by the Human Ethics Review Committee of the National Hospital Organization Kumamoto Medical Center, Kumamoto, Japan (institutional ethical committee number: 907).
Statistical analysis. All statistical analyses were conducted using JMP (version 12.2, SAS Institute, Cary, NC, USA) and all p-Values were two-sided. All statistical tests were two-sided at an α level of 0.005, considering multiple comparisons and consequent false positives (28).
The Kaplan-Meier method was used to describe the distribution of RFS and OS, and the log-rank test was performed. A Cox proportional hazards model was used to compute the hazard ratios (HRs) and the confidence intervals (95%CIs). Multivariable Cox proportional hazards regression models were used to identify independent risk factors for RFS and OS. The multivariable models included variables showing a univariable association (p<0.10) with RFS or OS.
Categorical variables are presented as proportions. Non-normally distributed variables were reported as medians with interquartile ranges. Categorical data were compared using the chi-square test or Fisher’s exact test, and non-normally distributed data were compared using the Mann-Whitney U-test.
Results
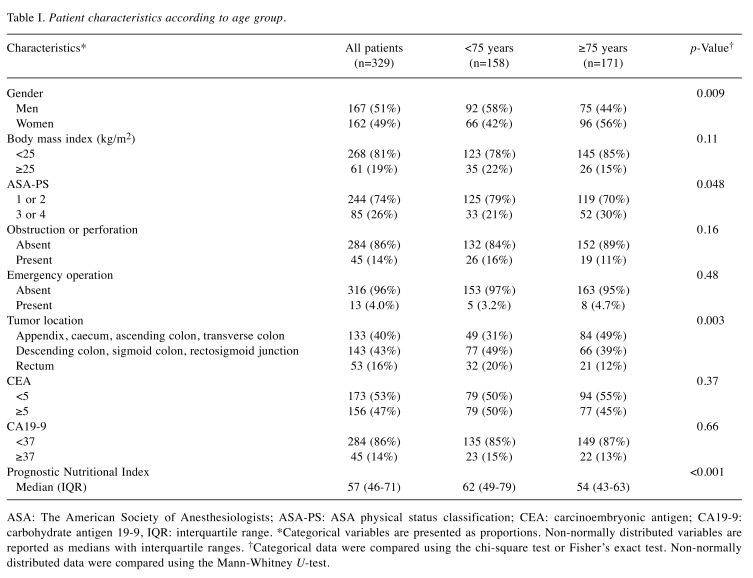
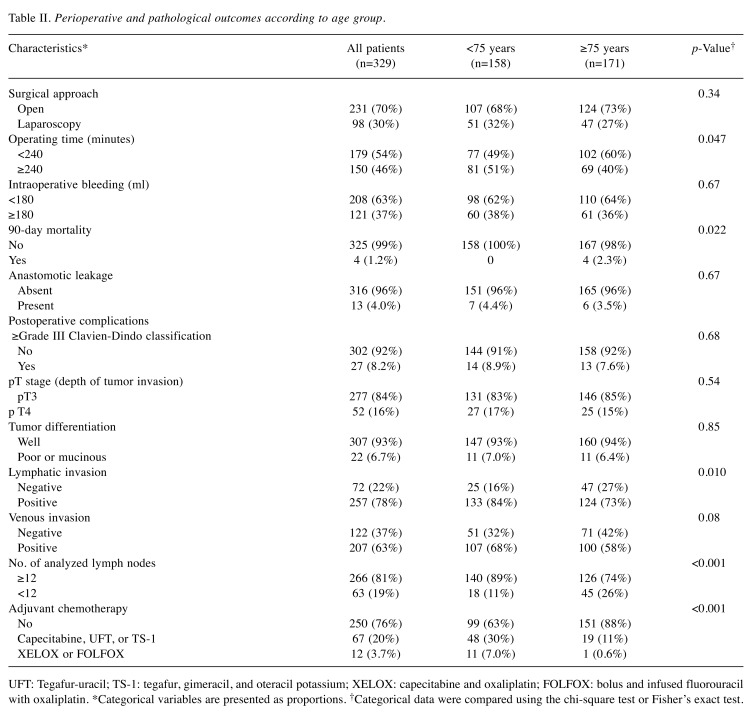
Clinicopathological features and surgical outcomes according to age group. A total of 329 patients with stage II colorectal cancer were included in this retrospective study. Of the 329 patients, 171 (52%) were ≥75 years and 158 (48%) were <75 years. Table I and Table II summarize the clinicopathological features and surgical outcomes according to age group. Advanced age in stage II colorectal cancer was associated with i) a low preoperative prognostic nutritional index, ii) right-sided colon cancer, iii) analysis of fewer than 12 lymph nodes, and iv) the absence of adjuvant chemotherapy (p<0.001, with an α level of 0.005).
Table I. Patient characteristics according to age group.
ASA: The American Society of Anesthesiologists; ASA-PS: ASA physical status classification; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9, IQR: interquartile range. *Categorical variables are presented as proportions. Non-normally distributed variables are reported as medians with interquartile ranges. †Categorical data were compared using the chi-square test or Fisher’s exact test. Non-normally distributed data were compared using the Mann-Whitney U-test.
Table II. Perioperative and pathological outcomes according to age group.
UFT: Tegafur-uracil; TS-1: tegafur, gimeracil, and oteracil potassium; XELOX: capecitabine and oxaliplatin; FOLFOX: bolus and infused fluorouracil with oxaliplatin. *Categorical variables are presented as proportions. †Categorical data were compared using the chi-square test or Fisher’s exact test.
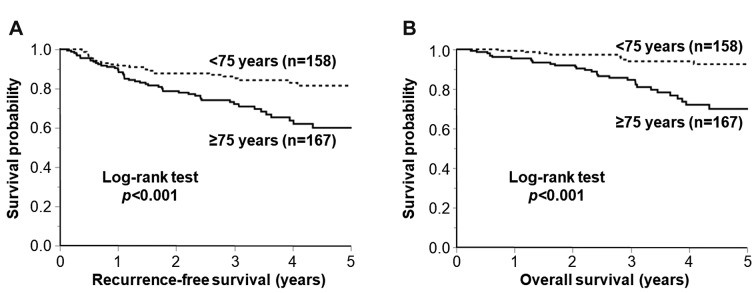
Associations of advanced age with recurrence-free and overall survival in stage II colorectal cancer. We examined the associations of advanced age with RFS and OS in stage II colorectal cancer after excluding four patients with in-hospital mortality. The median follow-up was 3.1 years (interquartile range=2.5 to 4.9 years). Using Kaplan-Meier analysis we found that older patients ≥75 years with stage II colorectal cancer were associated with shorter RFS (p<0.001 by the log-rank test) and OS (p<0.001 by the log-rank test; Figure 1).
Figure 1. Kaplan-Meier curves for recurrence-free survival (A) and overall survival (B), according to age group. The p-Value was calculated using the log-rank test (two-sided).
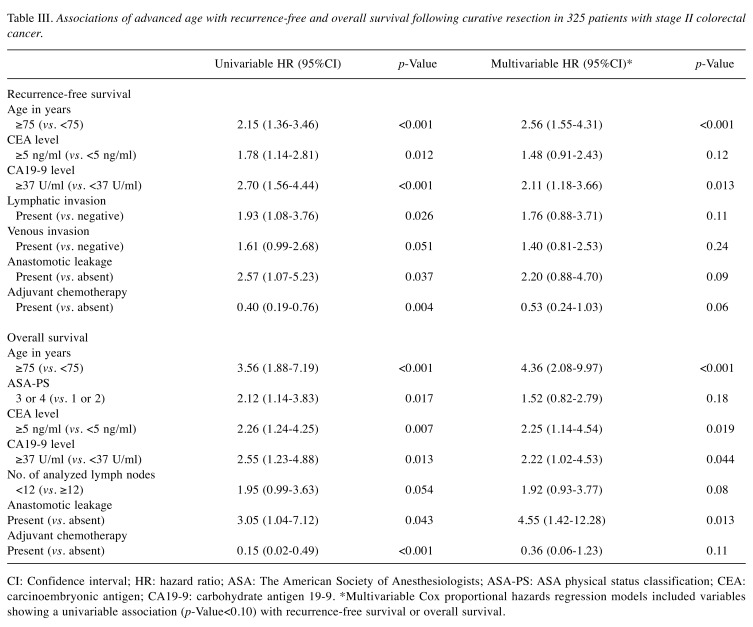
Among the high-risk features in stage II colorectal cancer that have been described in major guidelines (10,11), lymphatic invasion tended to be associated with shorter RFS in univariable analysis (p=0.026; Table III). Associations of other high-risk factors, including i) T4 stage, ii) fewer than 12 examined lymph nodes, iii) bowel perforation and obstruction, and iv) poorly differentiated tumors, with RFS or OS were not statistically significant in univariable analyses (p>0.05).
Table III. Associations of advanced age with recurrence-free and overall survival following curative resection in 325 patients with stage II colorectal cancer.
CI: Confidence interval; HR: hazard ratio; ASA: The American Society of Anesthesiologists; ASA-PS: ASA physical status classification; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9. *Multivariable Cox proportional hazards regression models included variables showing a univariable association (p-Value<0.10) with recurrence-free survival or overall survival.
Multivariable Cox regression analyses revealed that older age of patients (≥75 years) remained as a factor showing significant association with shorter RFS (p<0.001) and OS (p<0.001; Table III). Compared to patients <75 years of age, multivariable HRs (95%CI) for RFS and OS in those aged ≥75 years were 2.56 (1.55 to 4.31) and 4.36 (2.08 to 9.97), respectively.
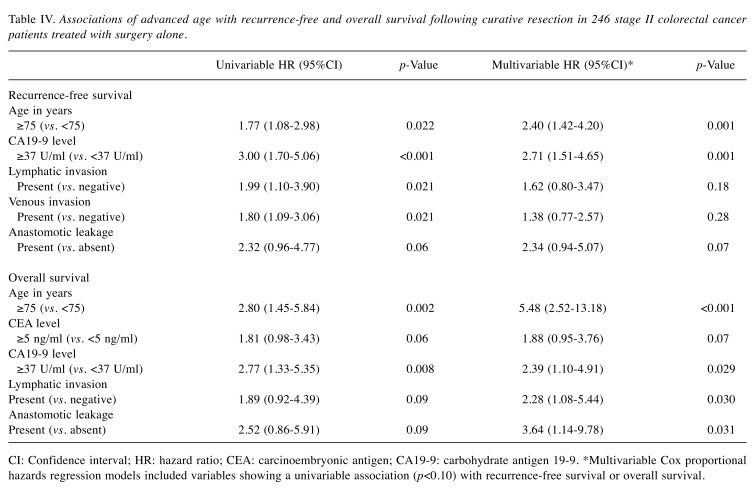
We also performed multivariable Cox regression analyses after excluding patients who received adjuvant chemotherapy and observed significant associations of age ≥75 years with shorter RFS (p=0.001) and OS (p<0.001; Table IV) in colorectal cancer patients treated with surgery alone.
Table IV. Associations of advanced age with recurrence-free and overall survival following curative resection in 246 stage II colorectal cancer patients treated with surgery alone.
CI: Confidence interval; HR: hazard ratio; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9. *Multivariable Cox proportional hazards regression models included variables showing a univariable association (p<0.10) with recurrence-free survival or overall survival.
Discussion
We conducted this study to test the hypothesis that advanced age might be associated with worse clinical outcome following curative resection in patients with stage II colorectal cancer. In the 329 stage II colorectal carcinoma cases after curative resection, we found that age ≥75 years was independently associated with a shorter RFS and OS after controlling for the high-risk features that have been described in the major guidelines. In the 246 stage II colorectal cancer patients treated with surgery alone, age ≥75 years remained as an independent risk factor showing associations with a shorter RFS and OS.
Approximately 60% of colorectal cancer cases are diagnosed in people aged ≥65years, with a median age at diagnosis of 68 years (29,30). Hence, further clinical trials are needed to establish evidence-based guidelines for older patients with colorectal cancer. Older patients have been shown to have decline in age-related immune function (31). Experimental and clinical evidence in old mice or older patients has demonstrated that dendritic cells exhibit i) impaired maturation and lower antigen uptake in the aged environment (32,33), ii) impaired T-cell expansion and differentiation, iii) impaired induction of effector molecules, such as INF-γ and granzyme B, and iv) increased production of PGE2 and IL10 (34-36). The preoperative nutritional status has been associated with the host immune response against cancers and their clinical outcome (25,37-40). In 843 colorectal cancer patients, the number of analyzed lymph nodes was associated with lymphocytic reactions against colorectal cancer, which have been associated with longer survival (41). In the current study, older patients with stage II colorectal cancer were associated with low a preoperative prognostic nutritional index and an analysis of fewer than 12 lymph nodes. Collectively, the data from our current study support the hypothesis that advanced age might represent a risk factor for worse clinical outcomes following curative resection of stage II colorectal cancer, partly due to the downregulation of the antitumor immune response. Further studies are needed to clarify the exact mechanism.
Perioperative nutrition support has been shown to reduce postoperative complications and enhance host immunity in patients with gastrointestinal cancers (42-45). A systematic review has demonstrated that older patients with colorectal cancer are less likely to receive adjuvant chemotherapy compared to younger patients (46), consistent with the current study. Enteral nutrition support has been shown to reduce the incidence of chemotherapy-related toxicities in patients with gastrointestinal cancers (47,48). Hence, perioperative nutrition support may improve prognosis in older patients with colorectal cancer.
In the current study, preoperative serum levels of CEA and CA19-9 and the incidence of anastomotic leakage were independently associated with worse RFS or OS following surgery for stage II colorectal cancer; consistent with the findings of previous studies (49-51). Identifying predictive biomarkers for the selection of patients with stage II colorectal cancer who could benefit from adjuvant chemotherapy are important. Hence, further clinical trials are needed to identify predictive biomarkers for the selection of patients with stage II colorectal cancer who could benefit from adjuvant chemotherapy.
We acknowledge that there are several limitations of our study such as, i) its retrospective nature and single-center design, and ii) the lack of data assessing tumor molecular features or immune cells in colorectal cancer tissue. Thus, further investigations are needed to examine the potential influence of aging on tumor molecular features and antitumor immunity in colorectal cancer.
A major strength of this study was that it included a relatively large number of older patients, which enabled us to assess the prognostic significance of advanced age in stage II colorectal cancer after controlling for potential confounders.
In conclusion, advanced age was associated with a shorter RFS and OS in stage II colorectal cancer following curative resection. Further clinical trials are needed to identify the robust risk factors for recurrence in stage II colorectal cancer, and to establish evidence-based guidelines for older patients with stage II colorectal cancer.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Authors’ Contributions
KM, NM, and HB contributed to the study conception and design. Data collection was performed by KM, JK, AM, SY, TM, KK, MI, TM, TK, NM, and HB. Analysis and interpretation of data were performed by KM and NM. The first draft of the manuscript was written by KM and all authors commented on previous versions of the manuscript. All Authors read and approved the final manuscript.
Acknowledgements
This study was supported by a grant from JSPS KAKENHI (grant number 17H05094). We thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Matsuda C, Ishiguro M, Teramukai S, Kajiwara Y, Fujii S, Kinugasa Y, Nakamoto Y, Kotake M, Sakamoto Y, Kurachi K, Maeda A, Komori K, Tomita N, Shimada Y, Takahashi K, Kotake K, Watanabe M, Mochizuki H, Nakagawa Y, Sugihara K, Group SS. A randomised-controlled trial of 1-year adjuvant chemotherapy with oral tegafur-uracil versus surgery alone in stage ii colon cancer: Sacura trial. Eur J Cancer. 2018;96:54–63. doi: 10.1016/j.ejca.2018.03.009. PMID: 29677641. DOI: 10.1016/j.ejca.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. PMID: 30620402. DOI: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. PMID: 27189416. DOI: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K, Japanese Society for Cancer of the Colon and Rectum Japanese society for cancer of the colon and rectum (jsccr) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1–34. doi: 10.1007/s10147-017-1101-6. PMID: 28349281. DOI: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannarkatt J, Joseph J, Kurniali PC, Al-Janadi A, Hrinczenko B. Adjuvant chemotherapy for stage ii colon cancer: A clinical dilemma. J Oncol Pract. 2017;13(4):233–241. doi: 10.1200/JOP.2016.017210. PMID: 28399381. DOI: 10.1200/JOP.2016.017210. [DOI] [PubMed] [Google Scholar]
- 6.Punt CJ, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol. 2017;14(4):235–246. doi: 10.1038/nrclinonc.2016.171. PMID: 27922044. DOI: 10.1038/nrclinonc.2016. [DOI] [PubMed] [Google Scholar]
- 7.Sato H, Maeda K, Sugihara K, Mochizuki H, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hirai T, Ohue M, Shirouzu K, Sakai Y, Watanabe T, Hirata K, Hatakeyama K. High-risk stage ii colon cancer after curative resection. J Surg Oncol. 2011;104(1):45–52. doi: 10.1002/jso.21914. PMID: 21416472. DOI: 10.1002/jso.21914. [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Andre T, Grothey A, Yothers G, Hamilton SR, Bot BM, Haller DG, Van Cutsem E, Twelves C, Benedetti JK, O’Connell MJ, Sargent DJ. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages ii and iii colon cancer between 1978 to 1995 and 1996 to 2007: Evidence of stage migration from the accent database. J Clin Oncol. 2013;31(29):3656–3663. doi: 10.1200/JCO.2013.49.4344. PMID: 23980089. DOI: 10.1200/JCO.2013.49.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino N, Hasegawa S, Hida K, Kawada K, Ganeko R, Sugihara K, Sakai Y. Nomogram for predicting recurrence in stage ii colorectal cancer. Acta Oncol. 2016;55(12):1414–1417. doi: 10.1080/0284186X.2016.1223881. PMID: 27581839. [DOI] [PubMed] [Google Scholar]
- 10.Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG. American society of clinical oncology recommendations on adjuvant chemotherapy for stage ii colon cancer. J Clin Oncol. 2004;22(16):3408–3419. doi: 10.1200/JCO.2004.05.063. PMID: 15199089. [DOI] [PubMed] [Google Scholar]
- 11.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, Arnold D, Group EGW. Early colon cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–vi72. doi: 10.1093/annonc/mdt354. PMID: 24078664. DOI: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 12.Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20(19):3999–4005. doi: 10.1200/JCO.2002.11.084. PMID: 12351597. [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi T, Haruki K, Shiba H, Sakamoto T, Saito N, Shirai Y, Iwase R, Fujiwara Y, Yanaga K. Assessment of outcome of hepatic resection for extremely elderly patients with a hepatic malignancy. Anticancer Res. 2019;39(9):5143–5148. doi: 10.21873/anticanres.13709. PMID: 31519626. DOI: 10.21873/anticanres.13709. [DOI] [PubMed] [Google Scholar]
- 14.Kuwada K, Kuroda S, Kikuchi S, Yoshida R, Nishizaki M, Kagawa S, Fujiwara T. Clinical impact of sarcopenia on gastric cancer. Anticancer Res. 2019;39(5):2241–2249. doi: 10.21873/anticanres.13340. PMID: 31092415. DOI: 10.21873/anticanres.13340. [DOI] [PubMed] [Google Scholar]
- 15.Shin SH, Park Y, Hwang DW, Song KB, Lee JH, Kwon J, Yoo C, Alshammary S, Kim SC. Prognostic value of adjuvant chemotherapy following pancreaticoduodenectomy in elderly patients with pancreatic cancer. Anticancer Res. 2019;39(2):1005–1012. doi: 10.21873/anticanres.13206. PMID: 30711988. DOI: 10.21873/anticanres.13206. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto K, Hirao M, Nishikawa K, Omori T, Yanagimoto Y, Shinno N, Sugimura K, Miyata H, Wada H, Takahashi H, Yasui M, Ohue M, Yano M, Fujitani K, Tsujinaka T. Sarcopenia is associated with impaired overall survival after gastrectomy for elderly gastric cancer. Anticancer Res. 2019;39(8):4297–4303. doi: 10.21873/anticanres.13595. PMID: 31366521. DOI: 10.21873/anticanres.13595. [DOI] [PubMed] [Google Scholar]
- 17.Itatani Y, Kawada K, Sakai Y. Treatment of elderly patients with colorectal cancer. Biomed Res Int. 2018;2018:2176056. doi: 10.1155/2018/2176056. PMID: 29713641. DOI: 10.1155/2018/2176056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kienle DL, Dietrich D, Ribi K, Wicki A, Quagliata L, Winterhalder RC, Koeberle D, Horber D, Bastian S, Kueng M, Saletti P, Helbling D, Baertschi D, Lugli A, Bernhard J, Andrieu C, von Moos R. Cetuximab monotherapy and cetuximab plus capecitabine as first-line treatment in older patients with ras- and braf wild-type metastatic colorectal cancer. Results of the multicenter phase ii trial sakk 41/10. J Geriatr Oncol. 2019;10(2):304–310. doi: 10.1016/j.jgo.2018.11.011. PMID: 30559073. DOI:10.1016/j.jgo.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Asimakopoulou N, Souglakos J, Kentepozidis N, Karampeazis A, Kotsakis A, Ziras N, Makrantonakis P, Prinarakis E, Vamvakas L, Georgoulias V, Hellenic Oncology Research Group Efficacy of panitumumab in older patients with metastatic colorectal cancer: A retrospective analysis using the database of the hellenic oncology research group (horg) J Geriatr Oncol. 2019;10(1):143–148. doi: 10.1016/j.jgo.2018.08.002. PMID: 30366852. DOI:10.1016/j.jgo.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Nikolich-Zugich J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10–19. doi: 10.1038/s41590-017-0006-x. PMID: 29242543. DOI: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- 21.Hong H, Wang Q, Li J, Liu H, Meng X, Zhang H. Aging, cancer and immunity. J Cancer. 2019;10(13):3021–3027. doi: 10.7150/jca.30723. PMID: 31281479. DOI: 10.7150/jca.30723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno H, Ishiguro M, Nakatani E, Ishikawa T, Uetake H, Matsuda C, Nakamoto Y, Kotake M, Kurachi K, Egawa T, Yasumasa K, Murata K, Ikawa O, Shinji S, Murotani K, Matsui S, Teramukai S, Tomita N, Sugihara K, Group SS. Prospective multicenter study on the prognostic and predictive impact of tumor budding in stage II colon cancer: Results from the sacura trial. J Clin Oncol. 2019;37(22):1886–1894. doi: 10.1200/JCO.18.02059. PMID: 31180819. DOI: 10.1200/JCO.18.02059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. PMID: 15273542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The clavien-dindo classification of surgical complications: Five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912. DOI: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 25.Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2018 doi: 10.1097/SLA.0000000000002985. PMID: 30308614. DOI: 10.1097/SLA.0000000000002985. [DOI] [PubMed] [Google Scholar]
- 26.Matthiessen P, Hallbook O, Rutegard J, Simert G, Sjodahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: A randomized multicenter trial. Ann Surg. 2007;246(2):207–214. doi: 10.1097/SLA.0b013e3180603024. PMID: 17667498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frasson M, Flor-Lorente B, Rodriguez JL, Granero-Castro P, Hervas D, Alvarez Rico MA, Brao MJ, Sanchez Gonzalez JM, Garcia-Granero E, Group AS. Risk factors for anastomotic leak after colon resection for cancer: Multivariate analysis and nomogram from a multicentric, prospective, national study with 3193 patients. Ann Surg. 2015;262(2):321–330. doi: 10.1097/SLA.0000000000000973. PMID: 25361221. DOI: 10.1097/SLA.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin DJ, Berger JO, Johannesson M, Nosek BA, Wagenmakers EJ, Berk R, Bollen KA, Brembs B, Brown L, Camerer C, Cesarini D, Chambers CD, Clyde M, Cook TD, De Boeck P, Dienes Z, Dreber A, Easwaran K, Efferson C, Fehr E, Fidler F, Field AP, Forster M, George EI, Gonzalez R, Goodman S, Green E, Green DP, Greenwald AG, Hadfield JD, Hedges LV, Held L, Hua Ho T, Hoijtink H, Hruschka DJ, Imai K, Imbens G, Ioannidis JPA, Jeon M, Jones JH, Kirchler M, Laibson D, List J, Little R, Lupia A, Machery E, Maxwell SE, McCarthy M, Moore DA, Morgan SL, Munafó M, Nakagawa S, Nyhan B, Parker TH, Pericchi L, Perugini M, Rouder J, Rousseau J, Savalei V, Schönbrodt FD, Sellke T, Sinclair B, Tingley D, Van Zandt T, Vazire S, Watts DJ, Winship C, Wolpert RL, Xie Y, Young C, Zinman J, Johnson VE. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6–10. doi: 10.1038/s41562-017-0189-z. PMID: 30980045. DOI: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 29.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2019 doi: 10.3322/caac.21565. PMID: 31184787. DOI: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 30.Nitsche U, Spath C, Muller TC, Maak M, Janssen KP, Wilhelm D, Kleeff J, Bader FG. Colorectal cancer surgery remains effective with rising patient age. Int J Colorectal Dis. 2014;29(8):971–979. doi: 10.1007/s00384-014-1914-y. PMID: 24924447. DOI: 10.1007/s00384-014-1914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31(2):578–585. doi: 10.1086/313947. PMID: 10987724. [DOI] [PubMed] [Google Scholar]
- 32.Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, Janssen EM. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J Immunol. 2015;195(6):2624–2632. doi: 10.4049/jimmunol.1501006. PMID: 26246142. DOI: 10.4049/jimmunol.1501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhrlaub JL, Smithey MJ, Nikolich-Zugich J. Cutting edge: The aging immune system reveals the biological impact of direct antigen presentation on cd8 t cell responses. J Immunol. 2017;199(2):403–407. doi: 10.4049/jimmunol.1700625. PMID: 28615415. DOI: 10.4049/jimmunol.1700625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castle S, Uyemura K, Wong W, Modlin R, Effros R. Evidence of enhanced type 2 immune response and impaired upregulation of a type 1 response in frail elderly nursing home residents. Mech Ageing Dev. 1997;94(1-3):7–16. doi: 10.1016/s0047-6374(96)01821-0. PMID: 9147356. [DOI] [PubMed] [Google Scholar]
- 35.Burns EA, Goodwin JS. Immunodeficiency of aging. Drugs Aging. 1997;11(5):374–397. doi: 10.2165/00002512-199711050-00005. PMID: 9359024. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Smithey MJ, Rudd BD, Nikolich-Zugich J. Age-associated alterations in cd8alpha+ dendritic cells impair cd8 t-cell expansion in response to an intracellular bacterium. Aging Cell. 2012;11(6):968–977. doi: 10.1111/j.1474-9726.2012.00867.x. PMID: 22862959. DOI: 10.1111/j.1474-9726.2012.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, Zhang Q, Li Z. Impact of the preoperative prognostic nutritional index on postoperative and survival outcomes in colorectal cancer patients who underwent primary tumor resection: A systematic review and meta-analysis. Int J Colorectal Dis. 2019;34(4):681–689. doi: 10.1007/s00384-019-03241-1. PMID: 30680451. DOI: 10.1007/s00384-019-03241-1. [DOI] [PubMed] [Google Scholar]
- 38.Nakao Y, Yamashita YI, Arima K, Miyata T, Itoyama R, Yusa T, Umezaki N, Yamao T, Nakagawa S, Okabe H, Imai K, Chikamoto A, Baba H. Clinical usefulness of perioperative c-reactive protein/albumin ratio in patients with intrahepatic cholangiocarcinoma: A retrospective single institutional study. Anticancer Res. 2019;39(5):2641–2646. doi: 10.21873/anticanres.13388. PMID: 31092463. DOI: 10.21873/anticanres.13388. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu T, Taniguchi K, Asakuma M, Tomioka A, Inoue Y, Komeda K, Hirokawa F, Uchiyama K. Lymphocyte-to-monocyte ratio and prognostic nutritional index predict poor prognosis in patients on chemotherapy for unresectable pancreatic cancer. Anticancer Res. 2019;39(4):2169–2176. doi: 10.21873/anticanres.13331. PMID: 30952764. DOI: 10.21873/anticanres.13331. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto M, Saito H, Uejima C, Tanio A, Tada Y, Matsunaga T, Sakamoto T, Honjo S, Ashida K, Fujiwara Y. Combination of serum albumin and cholinesterase levels as prognostic indicator in patients ith colorectal cancer. Anticancer Res. 2019;39(2):1085–1090. doi: 10.21873/anticanres.13217. PMID: 30711999. DOI: 10.21873/anticanres.13217. [DOI] [PubMed] [Google Scholar]
- 41.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, Dranoff G, Giovannucci EL, Fuchs CS. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and cpg island methylator phenotype. Clin Cancer Res. 2009;15(20):6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. PMID: 19825961. DOI: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805–814. doi: 10.1067/msy.2002.128350. PMID: 12464864. [DOI] [PubMed] [Google Scholar]
- 43.Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763–1770. doi: 10.1053/gast.2002.33587. PMID: 12055582. [DOI] [PubMed] [Google Scholar]
- 44.Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: The joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698–709. doi: 10.1016/j.clnu.2007.06.009. PMID: 17683831. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Liu BL, Shang B, Chen AS, Liu SQ, Sun W, Yin HZ, Yin JQ, Su Q. Nutrition support in surgical patients with colorectal cancer. World J Gastroenterol. 2011;17(13):1779–1786. doi: 10.3748/wjg.v17.i13.1779. PMID: 21483641. DOI: 10.3748/wjg.v17.i13.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surgery for colorectal cancer in elderly patients: A systematic review. Colorectal cancer collaborative group. Lancet. 2000;356(9234):968–974. PMID: 11041397. [PubMed] [Google Scholar]
- 47.Miyata H, Yano M, Yasuda T, Hamano R, Yamasaki M, Hou E, Motoori M, Shiraishi O, Tanaka K, Mori M, Doki Y. Randomized study of clinical effect of enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Clin Nutr. 2012;31(3):330–336. doi: 10.1016/j.clnu.2011.11.002. PMID: 22169459. DOI: 10.1016/j.clnu.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Miyata H, Yano M, Yasuda T, Yamasaki M, Murakami K, Makino T, Nishiki K, Sugimura K, Motoori M, Shiraishi O, Mori M, Doki Y. Randomized study of the clinical effects of omega-3 fatty acid-containing enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Nutrition. 2017;33:204–210. doi: 10.1016/j.nut.2016.07.004. PMID: 27644137. DOI: 10.1016/j.nut.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Huang SH, Tsai WS, You JF, Hung HY, Yeh CY, Hsieh PS, Chiang SF, Lai CC, Chiang JM, Tang R, Chen JS. Preoperative carcinoembryonic antigen as a poor prognostic factor in stage i-iii colorectal cancer after curative-intent resection: A propensity score matching analysis. Ann Surg Oncol. 2019;26(6):1685–1694. doi: 10.1245/s10434-019-07184-3. PMID: 30915591. DOI: 10.1245/s10434-019-07184-3. [DOI] [PubMed] [Google Scholar]
- 50.Filella X, Molina R, Grau JJ, Pique JM, Garcia-Valdecasas JC, Astudillo E, Biete A, Bordas JM, Novell A, Campo E. Prognostic value of ca 19.9 levels in colorectal cancer. Ann Surg. 1992;216(1):55–59. doi: 10.1097/00000658-199207000-00008. PMID: 1632702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmermann MS, Wellner U, Laubert T, Ellebrecht DB, Bruch HP, Keck T, Schloricke E, Benecke CR. Influence of anastomotic leak after elective colorectal cancer resection on survival and local recurrence: A propensity score analysis. Dis Colon Rectum. 2019;62(3):286–293. doi: 10.1097/DCR.0000000000001287. PMID: 30540662. DOI: 10.1097/DCR.0000000000001287. [DOI] [PubMed] [Google Scholar]