Abstract
Objective
To demonstrate the multimodal imaging and histopathology of Berger's space.
Methods
We conducted a retrospective in vivo analysis of 4 patients demonstrating Berger's space with intraocular pathological conditions, documented by slit-lamp biomicroscopic photography and, in 2 patients, also by optical coherence tomography (OCT). Additionally, we carried out a retrospective histological study of 7 enucleated eyes with retinoblastoma demonstrating Berger's space. A review of the literature was also performed.
Results
Two eyes had slit-lamp photographs. One case showed Berger's space surrounded by vitreous hemorrhage. In the other case, amyloid was trapped within Berger's space. In another 2 eyes that were pseudophakic, Berger's space was visible on anterior segment OCT. One had amyloid trapped in Berger's space that could be seen with OCT. The histological review of the 7 enucleated eyes with advanced retinoblastoma demonstrated the presence of pyknotic cells in Berger's space.
Conclusions
Berger's space is an actual space in pathological conditions and can be an important site of pathology. Additionally, to our knowledge, this is the first time that Berger's space has been documented by anterior segment OCT in a clinical setting.
Keywords: Amyloid, Berger's space, Optical coherence tomography, Vitreous
Introduction
Berger's space is described as a potential compartment between the posterior surface of the lens and the vitreous humor. The earliest reference to Berger's space was made in 1887, by Emil Berger [1]. He evaluated enucleated eyes post mortem and created meticulous anatomical illustrations of the space. Berger had help from his colleague Wieger in evaluating the fibers representing the hyalocapsular ligament [1, 2].
The contact between the posterior lens capsule and the anterior hyaloid face is maintained by a thickened circular vitreolenticular adhesion [3], known as the hyalocapsular ligament or Wieger's ligament. Under physiologic conditions, this adhesion is fairly strong. However, in patients with pathological conditions, this attachment is weakened, resulting in a retrolental space: Berger's space. The pathologic conditions associated with this phenomenon include trauma [4], pigmentary dispersion syndrome [5], vitreous hemorrhage [6], intraocular tumors, and amyloidosis. There is also a case described in the literature with the presence of a dexamethasone implant in Berger's space [7].
Surgical access to Berger's space is difficult, due to its small size and close proximity to the lens. Therefore, the authors would like to confirm the existence of Berger's space and discuss its importance based on noninvasive imaging modalities such as biomicroscopy and anterior segment optical coherence tomography (OCT). To the best of our knowledge, this is the first report of the use of OCT for documenting Berger's space in a clinical setting. Additionally, we performed a histopathological analysis of enucleated eyes for a better morphologic understanding.
Materials and Methods
A review of the English-language literature was performed using PubMed. Keywords searched included Berger's space, anterior hyaloid, lens capsule, and retinoblastoma.
This is a retrospective study of 4 patients seen at Mayo Clinic in Rochester, MN, USA, with diagnoses of amyloidosis, vitreous hemorrhage, and pseudophakia. All 4 patients underwent complete ophthalmologic examination. Biomicroscopic images were recorded using a Haag-Streit BX 900 device, and we used Spectralis HRA OCT (version 1.10; Heidelberg Engineering) in the anterior segment module, with a VAO 01083 (version 3.3) anterior chamber lens, to document Berger's space in the pseudophakic eye.
Additionally, we performed a retrospective review of 7 enucleated eyes with retinoblastoma: 5 from patients recently seen at Mayo Clinic, 1 consultation case from an outside hospital, and 1 case seen at Mayo Clinic in 1922 to which we had access to through the histopathological slides in the teaching files of one of the authors (D.R.S.). The 6 recently enucleated eyes had been fixed for a minimum of 48 h in 10% buffered formalin. Following fixation, the eyes were examined, photographed, and sectioned. The sections were then paraffin embedded and stained with hematoxylin-eosin (H&E) and periodic acid-Schiff (PAS) for subsequent microscopic examination. The histopathological evaluation was performed by an experienced ocular pathologist (D.R.S.) and a vitreoretinal surgeon (J.S.P.). Attention was focused on Berger's space, the vitreous, the posterior chamber, and the anterior hyaloid for the presence of tumor cell infiltration.
Results
Case Series
The findings on the 4 cases that demonstrated Berger's space in vivo are summarized below.
Case 1
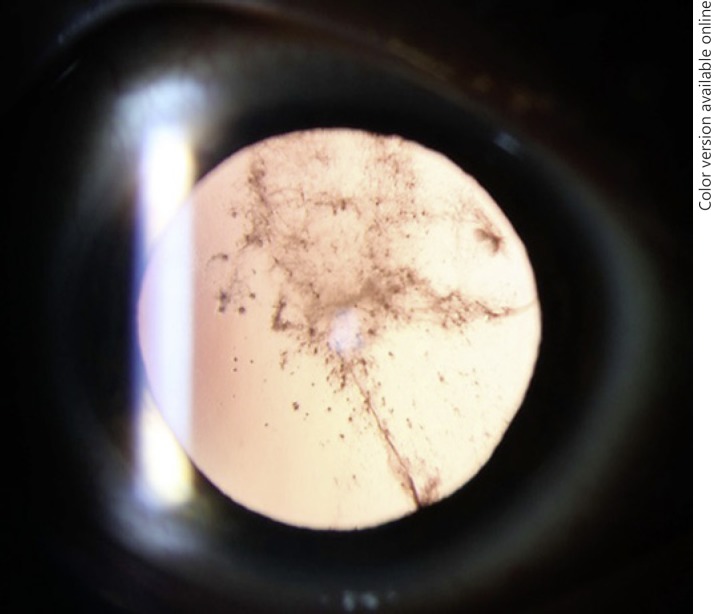
A 40-year-old male patient with a medical history of systemic transthyretin amyloidosis and an ocular history of cataract surgery and vitrectomy presented with complaint of visually significant glare. The case is described elsewhere in depth (accepted by the American Journal of Ophthalmology Case Reports). Briefly, visual acuity was 20/20 in the right eye (OD) and 20/25 in the left eye (OS). However, brightness acuity testing revealed a visual acuity of 20/200 OS. Retrocapsular opacification was noted in both eyes and the fundus examination was unremarkable. Nd:YAG (neodymium-doped yttrium aluminum garnet) laser capsulotomy was performed on both eyes, and vision improved (Fig. 1).
Fig. 1.
Amyloid material confined to Berger's space before capsulotomy.
Case 2
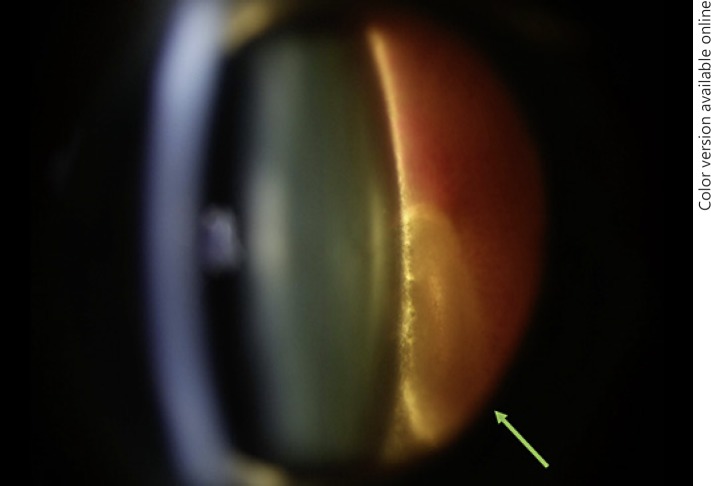
A 56-year-old female patient reported sudden decreased vision in her left eye as a result of a significant vitreous hemorrhage. Biomicroscopic examination was performed due to the presence of surrounding blood. It was possible to delineate the presence of Berger's space. It was also possible to see probable ghost cells inside Berger's space. There was no view of the fundus (Fig. 2).
Fig. 2.
Vitreous hemorrhage delineating Berger's space with blood inside inferiorly (arrow).
Case 3
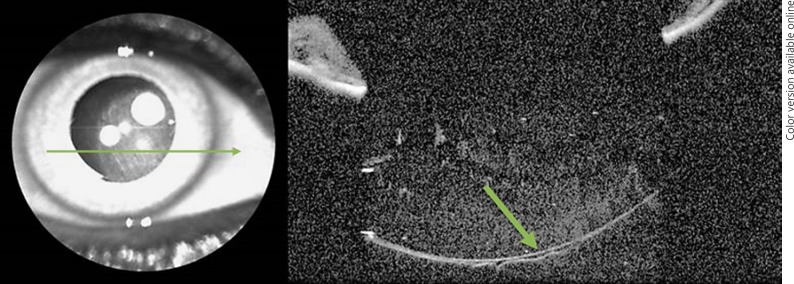
A 63-year-old pseudophakic female patient came to the clinic for routine examination. It was possible to see Berger's space on anterior segment OCT. It is possible that the absence of her natural lens combined with disturbance from cataract surgery was enough to create a visible Berger space in this patient (Fig. 3).
Fig. 3.
Berger's space in a pseudophakic patient on anterior segment OCT. The arrow shows the anterior hyaloid and Berger's space.
Case 4
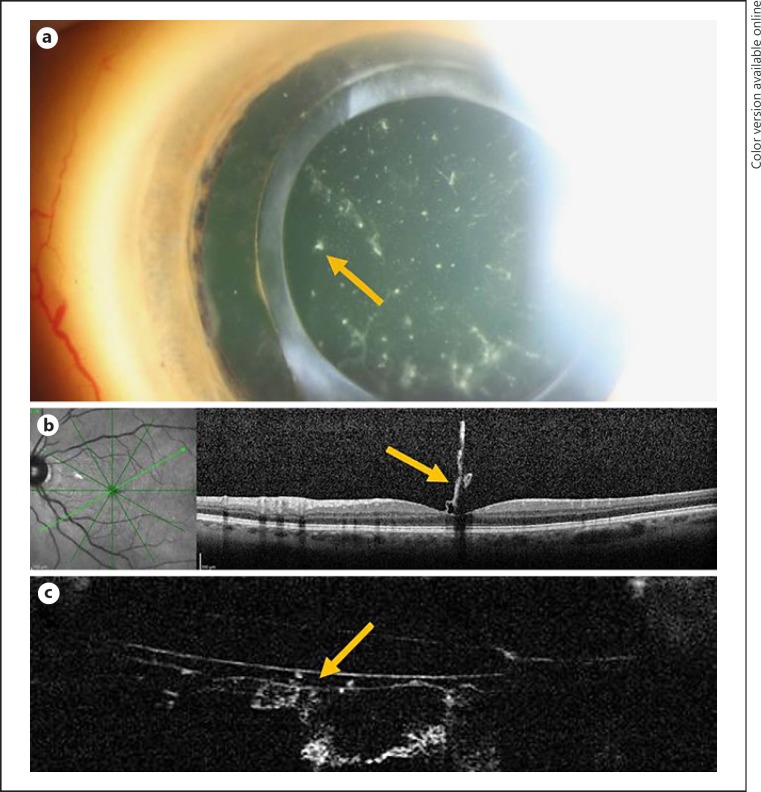
A 42-year-old female patient with a history of systemic transthyretin amyloidosis noticed blurred vision in her left eye. Her previous ocular history included cataract surgery and vitrectomy in both eyes. At the time of initial presentation, her visual acuity was 20/25 OD and 20/40 OS. Biomicroscopy revealed amyloid deposition in the retrolental space in both eyes (Fig. 4a). Fundus examination showed a macular abnormality OS compatible with amyloid deposition in the fovea. Macular OCT identified the posterior hyaloid and amyloid material attached to the fovea (Fig. 4b). Because we had been able to visualize Berger's space by anterior segment OCT in case 3, we attempted to use it here, and it showed that Berger's space was visible with deposits of amyloid material inside it as well as on the surface of the anterior hyaloid (Fig. 4c).
Fig. 4.
a Amyloid material in the retrolental space, one spot of amyloid material being indicated by the arrow. b Amyloid material attached to the fovea demonstrated by OCT. c Berger's space demonstrated by anterior segment OCT, with amyloid material inside it. The arrows identify amyloid material trapped in the anterior hyaloid and within Berger's space.
Retrospective Review of Enucleated Eyes
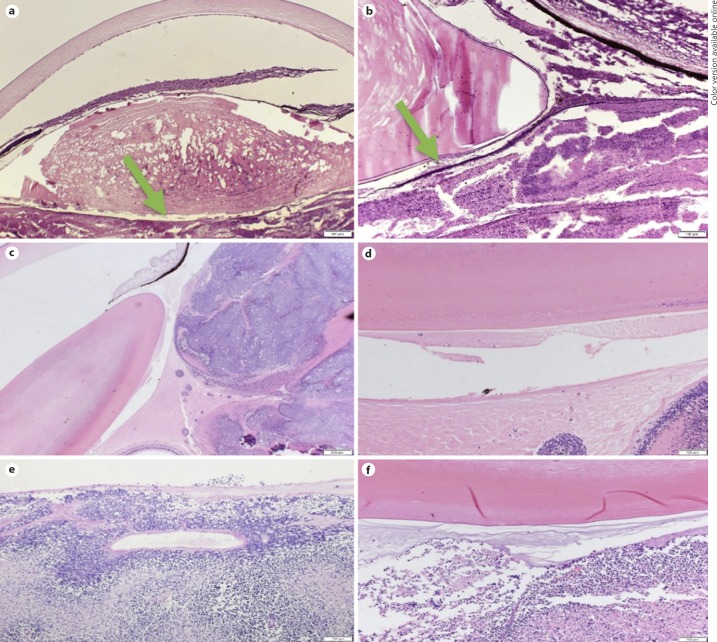
The histopathological review of 6 enucleated eyes from 6 patients with advanced retinoblastoma showed that Berger's space was well visualized in this pathological condition. On examination, there were numerous tumor cells in the vitreous. However, these tumor cells were not observed to cross the anterior hyaloid face. In Berger's space, it was possible to identify pyknotic cells and proteinaceous material.
These findings were also visualized on the slides of an enucleated eye at Mayo Clinic from 1922, from a patient with advanced retinoblastoma (Fig. 5a). Therefore, in addition to histological confirmation of the existence of Berger's space, it appears that the anterior hyaloid serves as a semipermeable membrane which does not allow the passage of tumor cells. Proteinaceous material appears to easily cross the membrane (Fig. 5b–f).
Fig. 5.
a Berger's space in a histopathological slide from a patient with advanced retinoblastoma from the year 1922. Arrow: Berger's space. H&E. ×20. b Magnified image from the same patient. The arrow shows probable proteinaceous and pyknotic cells within the space. H&E. ×40. c Lens, tumor cells, and Berger's space. PAS. ×20. d Berger's space with a pyknotic nucleus. PAS. ×100. e Retinoblastoma and probable pyknotic cells in Berger's space. PAS. ×100. f Retinoblastoma and Berger's space. PAS. ×100.
Discussion
There are a few papers describing the existence of Berger's space and its associated pathological conditions in the literature. Using Google Scholar, we found that before 1960 there were only 3 studies mentioning Berger's space. This coincides with the beginning of the use of the surgical microscope. From 1960 until 2000 there were 9 studies, and from 2000 until 2018 the number of studies mentioning Berger's space increased to 85. This signifies a growing interest in this anatomical space over the last few years.
Tolentino et al. [8], in their “Biomicroscopic Study of Vitreous Cavity in Diabetic Retinopathy,” described a visible arc between the hyalocapsular ligament and the central portion of the posterior lens surface free of blood in cases of vitreous hemorrhage, similar to our case 2. When observing optical sections, the authors described the blood deposits forming a wedge between the detached anterior hyaloid and the posterior lens capsule. Roberts et al. [9] described central retrolental pigmentation in a case of blunt trauma with pigment dispersion syndrome, and, corroborating this finding, Li et al. [6] discusses a case of traumatic hemorrhage in Berger's space in a 4-year-old child.
Most of the literature on Berger's space consists of case reports, but the importance of this anatomical location for microsurgery has increased. Tassignon and Ní Dhubhghaill [3] succeeded in imaging this space intraoperatively using anterior segment OCT following lens extraction, and Tassignon et al. [10] performed phacoemulsification with a bag-in-the-lens implant technique as opposed to the more common “lens-inside-the-capsular-bag” technique. This technique involves the use of a bag-in-the-lens ocular implant. The lens is positioned so that the posterior flange is positioned in Berger's space. A viscoelastic is used to fill the space for greater protection of the hyaloid and to physically dissect this space. The use of filtered air to pneumodissect an anatomical plane beginning at Petit's canal to completely separate the anterior hyaloid from the posterior lens capsule has also been described [11].
Our cases add further credence to Berger's space as a real space under pathological conditions, and they also show that anterior segment OCT can be used to view this space in pseudophakic patients. We believe that it is possible to capture images of Berger's space with anterior segment OCT in pseudophakic patients because the intraocular lens is a thinner interface than the natural lens. We tried to capture similar images in a young, healthy, phakic patient, but it was not possible because OCT imaging failed to reach the depth necessary to capture Berger's space.
We believe that the anterior hyaloid functions as a semipermeable membrane, protecting Berger's space from blood and tumor cells. However, it is permeable to amyloid material and pyknotic cells. It is therefore reasonable to postulate that this anatomical location could also be permeable to small microorganisms and act as a reservoir for infection. This is likely not the case, as antibiotics would also be able to pass through the hyaloid membrane, but we have to consider this possibility when thinking about retrolental abscesses, or pathologically thickened membranes in severe posterior uveitis [12].
Another condition in which Berger's space may be clinically relevant is malignant glaucoma. The pathophysiology of malignant glaucoma is multifactorial and poorly understood. One of the accepted theories of this condition is the posterior misdirection of aqueous humor into or behind the vitreous body. Because the aqueous humor cannot pass through an abnormal anterior hyaloid, the entire vitreous-lens-iris complex is displaced anteriorly [13, 14]. A known treatment for this condition is vitrectomy with anterior hyaloidectomy. This procedure can be challenging due to the close proximity of the posterior lens capsule and the anterior hyaloid face. Dissecting Berger's space would increase this distance and also separate the hyalocapsular ligament, thus making the anterior vitrectomy easier to complete. This physical dissection of Berger's space could be accomplished using viscoelastic material, hydrodissection, or pneumodissection, as previously described [13, 14, 15].
Conclusions
Berger's space is a real and clinically significant space in pathological conditions. The anterior hyaloid seems to be a semipermeable membrane protecting this space, but more research is required to further establish its anatomical and physiological importance. Changes in the anterior hyaloid and its capsular attachment may also contribute to a better understanding of the posterior segment following trauma and cataract surgery, and can help treat diseases including amyloidosis, infection, and glaucoma.
Statement of Ethics
This study received approval from the Mayo Clinic Institutional Review Board. The authors have no ethical conflicts to disclose.
Disclosure Statement
No conflicting relationship exists for V.M., M.B.N., or D.R.S. J.S.P. has stock in LAgen Laboratories, which supplies induced pluripotent stem cell-derived retinal pigment epithelial cells for in vitro studies and has no relevant financial interests.
Funding Sources
This study was funded in part by unrestricted grants from Research to Prevent Blindness, Inc., New York, NY, USA, and by grants from the Deshong and Paul Family (J.S.P.).
Author Contributions
V.M. performed the literature review, participated in all testing and analysis/interpretation, and wrote the manuscript; M.B.N. provided supervision of the research and reviewed/edited the manuscript; D.R.S. performed gross dissection as well as histopathological analysis and interpretation and reviewed/edited the manuscript; S.G. performed clinical imaging and reviewed/edited the manuscript; J.T. performed clinical imaging and reviewed/edited the manuscript; J.S.P. provided supervision of all research, testing, and analysis/interpretation and reviewed/edited the manuscript.
References
- 1.Berger E. Wiesbaden: Bergmann; 1887. Beitrage zur Anatomie des Auges in Normalem und Pathologischem Zustande. [Google Scholar]
- 2.Wieger G. Über den Canalis Petiti und ein ‘Ligamentum hyaloideocapsulare'; thesis. Strassurg. 1883 [Google Scholar]
- 3.Tassignon MJ, Ní Dhubhghaill S. Real-Time Intraoperative Optical Coherence Tomography Imaging Confirms Older Concepts About the Berger Space. Ophthalmic Res. 2016;56((4)):222–226. doi: 10.1159/000446242. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, Kim KH, Lee JE. Traumatic Dislocation of Posterior Chamber Phakic Intraocular Lens into the Berger's Space. Korean J Ophthalmol. 2016 Oct;30((5)):396–7. doi: 10.3341/kjo.2016.30.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turgut B, Türkçüoğlu P, Deniz N, Catak O. Annular and central heavy pigment deposition on the posterior lens capsule in the pigment dispersion syndrome: pigment deposition on the posterior lens capsule in the pigment dispersion syndrome. Int Ophthalmol. 2008 Dec;28((6)):441–5. doi: 10.1007/s10792-007-9158-2. [DOI] [PubMed] [Google Scholar]
- 6.Li ST, Yiu EP, Wong AH, Yeung JC, Yu LW. Management of traumatic haemorrhage in the Berger's space of a 4-year-old child. Int Ophthalmol. 2017 Aug;37((4)):1053–5. doi: 10.1007/s10792-016-0337-x. [DOI] [PubMed] [Google Scholar]
- 7.Dubrulle P, Fajnkuchen F, Qu L, Giocanti-Aurégan A. Dexamethasone implant confined in Berger's space. Springerplus. 2016 Oct;5((1)):1786. doi: 10.1186/s40064-016-3490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolentino FI, Lee PF, Schepens CL. Biomicroscopic study of vitreous cavity in diabetic retinopathy. Arch Ophthalmol. 1966 Feb;75((2)):238–46. doi: 10.1001/archopht.1966.00970050240018. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DK, Miller E, Kim LS. Pigmentation of the posterior lens capsule central to Wieger's ligament and the Scheie line: a possible indication of the pigment dispersion syndrome. Optom Vis Sci. 1995 Oct;72((10)):756–62. doi: 10.1097/00006324-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Tassignon MJ, Gobin L, Mathysen D, Van Looveren J, De Groot V. Clinical outcomes of cataract surgery after bag-in-the-lens intraocular lens implantation following ISO standard 11979-7:2006. J Cataract Refract Surg. 2011 Dec;37((12)):2120–9. doi: 10.1016/j.jcrs.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Kam YW, Funk RO, Barnard L, Ajlan RS, New endoscopic surgical approach for anterior hyaloid dissection in phakic and pseudophakic patients The journal of retinal and vitreous diseases. Retina. 2018 Jun 5; doi: 10.1097/IAE.0000000000002193. [DOI] [PubMed] [Google Scholar]
- 12.Manners RM, Canning CR. Posterior lens capsule abscess due to Propionibacterium acnes and Staphylococcus epidermidis following extracapsular cataract extraction. Bntishoumal ofOphthalmology, 1991;75:710–712. doi: 10.1136/bjo.75.12.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foreman-Larkin J, Netland PA, Salim S. Clinical Management of Malignant Glaucoma. J Ophthalmol. 2015;2015:283707. doi: 10.1155/2015/283707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003 Apr;12((2)):167–80. doi: 10.1097/00061198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda T, Sato K, Katano T, Hayashi Y. Surgically induced detachment of the anterior hyaloid membrane from the posterior lens capsule. Arch Ophthalmol. 1999 Mar;117((3)):408–9. doi: 10.1001/archopht.117.3.408. [DOI] [PubMed] [Google Scholar]