Abstract
Numerous biomarkers for somatic disorders are used in routine medical practice. Yet, despite remarkable advances in mental health research, we are not able to identify biomarkers with established clinical utility for mental disorders such as schizophrenia. While identification and characterization of biomarkers are crucial first steps in this process, their predictive diagnostic and treatment utility need to be better developed for clinical practice. The heterogeneity of psychotic disorders etiologically, pathologically and symptomatically presents both a challenge and an opportunity for the use of biomarkers in clinical practice. Simply said, a single biomarker might not exist that necessitates the search for a biomarker profile. In this review we discuss research findings in light of such an approach. We summarize some examples of emerging biomarkers in early psychosis research and delineate how these can be applied to a clinical setting to inform treatment on an individual basis fostering a personalized treatment approach.
Keywords: algorithm, biomarkers, prodrome, psychosis, schizophrenia, treatment
The term biological marker or biomarker has been defined in many different ways in the literature. For instance, the NIH Biomarkers Definitions Working Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.” In practical terms, a biomarker refers to a broad subcategory of medical signs – that is, objective indications of medical state observed from outside the patient – which can be measured accurately and reproducibly [1]. In the case of nonpsychiatric illness, such as HIV, the observation of certain clinical manifestations (Kaposi’s sarcoma) and blood tests (HIV ELISA and viral count) can provide both an accurate and reproducible measure of the presence of illness. These markers can be used in a clinical setting to not only accurately diagnose but also generate an appropriate treatment plan for patients. In the case of neuropsychiatric disorders, however, the task has been much more difficult. For instance, in schizophrenia (SCZ), no single symptom is unique to the disorder. Positive symptoms can be seen in virtually all mood and anxiety disorders; negative symptoms are common in depression; and cognitive impairments are not necessarily unique to SCZ. The prodrome of psychosis is marked by attenuated psychotic symptoms. These are symptoms that deviate from normal behavior but are not frankly psychotic, as described by Yung and colleagues [2,3]. At present, diagnosis is based largely on patient reports of symptoms and/or collateral reporting, but remains relatively devoid of objective, brain-based biological markers. Beyond diagnosis, the disorder is characterized by marked heterogeneity and impressive individual level variability. Thus, the search for biomarkers in neuropsychiatric disorders to date has taken into account the fact that psychotic disorders are neurodevelopmental disorders with genetic and environmental contributions to disease.
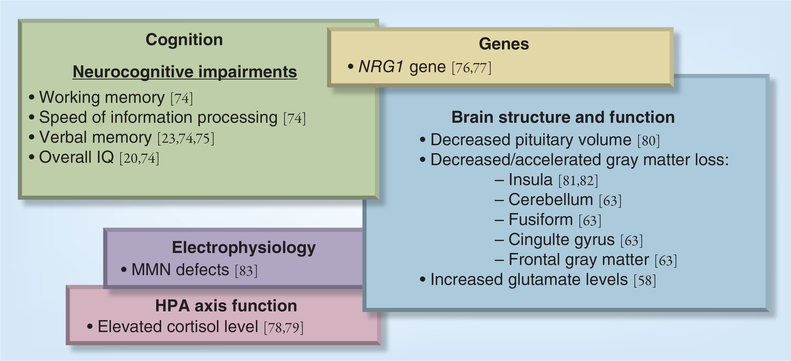
Promising research has identified candidate biomarkers in multiple domains (genetic, electrophysiological, neuroimaging, neurocognitive, inflammatory and neuroendocrine) in SCZ patients [4–7], unaffected first-degree relatives [8,9] and in the prodrome of the disorder as predictors of later psychosis (for a comprehensive review, see [1]) (Figure 1). The question that arises is how one can bridge the gap between research and practice; that is, implement and utilize this multitude of biomarkers to identify individuals at greatest risk for psychosis and individualize treatment.
Figure 1. Model of biomarkers in the prodrome predicting conversion to psychosis.
HPA: Hypothalamic–pituitary–adrenal; MMN: Mismatch negativity.
Thus far, most research in psychiatry has focused on identifying single biomarkers that are evident at the group level but are not sufficiently sophisticated to identify individual differences. Currently, research consortia such as the North American Prodromal Longitudinal Studies are in the process of developing Psychosis Risk Algorithms that combine clinical, demographic and biomarker data to inform risk and perhaps treatment. The development of a reliable algorithm that can be used clinically to inform the degree of risk and specify treatment is essential. Further research is needed to generate a biomarker-defined risk profile that can inform interventions in a more targeted and individualized fashion, matching treatment to the individual patient’s risk domain(s) of dysfunction [10–12].
The practical application of an at-risk algorithm that includes a battery of biomarkers for psychosis will in part depend on the development of reliable measures that can easily be administered in the laboratory or office, and ideally used as part of the evaluation process for each patient. Many biomarkers are linked to dysfunctional neural systems and can also be used as surrogate end points to predict and monitor clinical benefit in specific domains [13]. A number of psychosocial and pharmacologic interventions have great potential as neuroprotective, diseasemodifying or procognitive interventions in early psychosis [14], and have been shown to modify specific biomarker-defined deficits.
It is well conceivable for clinical settings to start expanding diagnosis and treatment planning by incorporating biomarkers in the evaluation process of patients in the early stages of psychosis. In the Cognitive Assessment and Risk Evaluation Program at the University of California San Diego (CA, USA), for example, patients undergo a comprehensive evaluation including clinical, functional, laboratory, neuropsychological and neuroimaging assessment – all domains that are associated with risk for psychosis. Based on the initial evaluation, a profile is generated for each individual, highlighting areas of weakness that can benefit from intervention and areas of strength that can be drawn upon. In addition, comorbid symptoms such as anxiety and depression can be immediately therapeutically addressed. Treatment of these symptoms facilitates diagnosis and at the same time decreases the illness burden for patients. Similarly, if deficits in social skills are present, these can also be addressed through social skills training. Patients can acquire more adaptive ways of communication and emotion regulation when interacting with family or healthcare providers. This, in turn, can help patients create or foster their support network, as well as potentially create a therapeutic atmosphere more conducive to success.
In terms of promising biomarkers, neurocognitive deficits are prominent across the SCZ spectrum [15–18], are known to predict functional outcomes [19,20], as well as explain 20–60% of the variance in community functioning, social problem solving and acquisition of psychosocial skills [21]. It is well documented that substantial cognitive deficits predate the onset of psychosis, and these tend to exacerbate before the onset of psychotic symptoms and may worsen after the initial episode of the illness [22]. Neurocognitive deficits across multiple domains have been documented in individuals at high clinical risk for psychosis, with more significant impairments in those individuals who later convert to psychosis [23–31]. A recent longitudinal study in individuals at risk for psychosis identified that processing speed, verbal learning and memory had highest sensitivity in discriminating between at-risk and healthy individuals, and that worse verbal memory predicted more rapid conversion [26].
Consequently, through comprehensive neuropsychological testing, individuals who demonstrate weakness in the cognitive domain can be offered cognitive training and remediation, which have shown promise in patients early in the course of illness [32–35], when intervention is likely to make the greatest impact on the developing brain. Cognitive remediation or training interventions have included restorative (e.g., computer-based approaches [36]), compensatory (e.g., strategy-based approaches [37,38]) or environmental adaptation [39]. It appears that patients with SCZ benefit most from compensatory strategy-based approaches in the context of psychiatric rehabilitation [40]. These strategies, also known as cognitive prosthetics, teach patients to use their cognitive strengths to work around their deficits within a real-world context. Improvements in memory and attention also help with concomitant treatments such as medication adherence. Although a number of cognition-enhancing medications [41,42] have been tested in psychotic patients with some evidence of success, there are no reported trials in the prodromal phase of illness. Clinical trials are needed with agents known to have a safe side-effect profile with evidence of efficacy in SCZ. Interesting candidate procognitive interventions include those that target the NMDA system (benzoate, glycine, N-acetyl-cysteine, D-cylcloserine, as well as minocycline) [41,43–45]. A double-blind, randomized study by Levkovitz and colleagues reported improvements in negative symptoms as well as executive functioning in early psychotic patients treated with minocycline versus placebo [45]. Most recently, a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor demonstrated significant improvements in a variety of symptom domains, including neurocognition, in patients with chronic SCZ, highlighting the promise for D-amino acid oxidase inhibition as a novel approach for new drug development for SCZ [43]. The potential for cognitive enhancement in the early phases is intriguing and may have even more positive and longer lasting results than those seen in SCZ.
In the not too distant future, patients may undergo electrophysiological testing in an initial evaluation. For instance, some of the electrophysiological paradigms currently under development as potential biological markers with treatment implications include mismatch negativity (MMN), P300, γ-band synchrony [46–49] and markers derived from neural targets in the mirror neuron system. In the case of MMN, duration of MMN predicts conversion to psychosis, and could therefore be used at initial testing to identify patients at the highest risk of psychotic transition. Furthermore, the fact that treatment of SCZ subjects with N-acetyl-cysteine, a glutathione precursor, increases MMN makes it an especially useful biomarker [50]. In this way, MMN could be used to identify patients at high risk for psychosis and to match them with a treatment that specifically modifies MMN. Another application of this concept utilizes μ rhythm suppression, an EEG marker derived from mirror neuron function. Mounting evidence indicates that μ rhythm suppression is impaired in patients with SCZ, and that the neural impairment is correlated with loss of social functioning. This finding, combined with early indications that μ suppression is improved with oxytocin treatment, provides a practical avenue to identify patients with neural evidence of poor social information processing and treating those patients with prosocial treatments such as oxytocin or social skills training. Similarly, in a recent review of the literature, Lewis et al. showed evidence for impaired γ synchrony in SCZ [51]. In addition, based on preclinical and clinical data, they hypothesize that compounds that impact GABA-ergic and cholinergic signaling are likely to improve γ synchrony in SCZ.
Similarly, neuroimaging methods show promise and can be utilized to facilitate diagnosis and treatment planning. Structural and functional MRI, PET and proton spectroscopy have been implemented in finding biomarkers in SCZ, as well as in the prodrome and early psychosis, all with reasonable success [52–55]. In general, differential prefrontal cortical functioning along the SCZ spectrum has been repeatedly reported [56], with recent studies showing hyperactivity in multiple brain regions in prodromal subjects, specifically the ones who later converted to psychosis [57,58]. Similarly, changes in cortical gray matter and aberrant neurochemical levels have been linked to SCZ and psychosis [56,59]. Identification of the latter two biomarkers is less invasive, more accessible and reliably assessed. MRI scans are easily administered and should become a regular assessment tool for individuals who show early signs of psychosis, not only because they may pick up rare neurological causes of psychosis (e.g., brain tumors), but because of the potential importance as a biomarker for psychosis. Although as previously mentioned we are still unable to predict conversion or identify the emergence of psychosis at an individual level using MRI scans, longitudinal MRIs at the individual level can be helpful in treatment planning. Progressive neuroanatomical changes that are greater than those seen in normal development have been repeatedly reported in SCZ [60–63]. As reviewed by Pantelis et al. [64], extant neuroimaging data provide evidence of pre- and/or peri-natal neurodevelopmental changes in SCZ that may lead to a vulnerability to postpubertal insults that contribute to the accelerated loss of gray matter and aberrant connectivity in the prefrontal regions. These, in conjunction with substance abuse, stress and hypothalamic–pituitary–adrenal axis dysregulation, may lead to neurodevelopmental abnormalities that may be neurodegenerative, involving medial temporal and orbital prefrontal regions. Thus, while disturbances of brain structure early in life may be necessary for the future emergence of SCZ [65], neurodevelopmental events during the late adolescent period may participate in psychotic symptom formation via a range of possible mechanisms including inflammation, glutamatergic or dopaminergic transmission [63,65,66]. Pharmacological agents show promise. For example, neuroprotective properties of serotonin reuptake inhibitors are documented in animal models, showing increased neurogenesis, dendritic arborization and synaptogenesis [67]. A preliminary study by Berger et al. showed a reduction in neuropathological change in the hippocampus of putatively prodromal subjects treated with low doses of lithium in comparison with untreated prodromal subjects [68].
In addition to pharmacologic interventions, there is research on nonpharmacologic treatments that indicate slowing of gray matter loss in SCZ. A recent study by Falkai and colleagues reported an increase in gray matter density after 3 months of aerobic exercise training in healthy individuals [69], while another group demonstrated hippocampal changes associated with improvements in memory performance in SCZ [70]. While the authors were unable to find exercise effects in chronic SCZ, aerobic exercise in at-risk and early psychotic patients may show results similar to healthy individuals. Thus, as part of treatment planning for individuals who present to the clinic, a moderate level exercise regimen can be suggested. Other interventions can include computer-based cognitive enhancement therapy. Eack et al. demonstrated greater perseveration of gray matter in early psychotic patients who were involved in computer-based cognitive enhancement therapy compared with those who received supportive psychotherapy over 2 years [33].
Finally, SCZ is associated with increased inflammation, including abnormal blood levels of the acute-phase reactant C-reactive protein (CRP) [71,72]. Testing for inflammatory biomarkers in the early phases of disease may be useful and inform treatment. Recent data suggest that baseline elevated plasma CRP is predictive of increased risk of developing late-onset SCZ [73]. While studies are necessary to replicate risk prediction in the early phases, assessment of CRP in the early phases may prove to be beneficial.
Conclusion
Psychiatric disorders are complex and necessitate a multidimensional approach for diagnosis and to inform treatment planning. In the SCZ spectrum, advances in research have identified promising biomarkers, although more research is needed to fortify current research findings and refine diagnostic accuracy. At the same time it would be futile for patient care not to incorporate existent knowledge into treatment planning. While caution is warranted, a more tailored treatment approach guided by comprehensive evaluation is preferable to a one-fits-all approach.
Future perspective
Further research in biomarkers, especially to aid in generating biomarker profiles addressing relevant domains of dysfunction or deficits, is exigent. Such an approach has been successfully used in the Framingham studies introducing the risk score calculator for coronary heart disease. It may be necessary to shift focus away from a one-size-fits-all approach and instead generate a profile for each patient highlighting his/her strengths or deficiencies. For research purposes it may be helpful to use stratification and investigate groups of patients with shared or similar characteristics or profiles to study the optimal management for the patients and achieve the best possible treatment outcome. For example, in clinical settings patients may test positive for neurochemical biomarkers and cognitive deficits, indicating risk for psychosis, but test negative when it comes to social functioning biomarkers. This profile suggests a different treatment approach compared with a profile that is positive for only some mild prodromal symptoms and social functioning problems. In the case of the former example, in addition to cognitive-enhancing medication, the particular patient’s treatment could be augmented by cognitive remediation therapy. On the other hand, the latter patient may benefit from social skills training and other interventions addressing social functioning, which may be sufficient to prevent exacerbation of symptoms [74].
Several robust biomarkers for conversion to psychosis have already been established [59,75–84] and many putative biomarkers show promising results but need to be further investigated. Ideally, longitudinal studies in large samples could demonstrate linkage between putative biomarkers and clinical end points. To further advance research it may be necessary to create shared datasets as well as draw on successful models outside of psychiatry, such as lessons learned from the Framingham studies or the Alzheimer’s Disease Neuroimaging Initiative [85,86].
Executive summary.
Despite remarkable advances in mental health research, there is a lack of biomarkers with clinical utility.
Promising candidates include genetic, electrophysiological, neuroimaging, neurocognitive and inflammatory biomarkers.
All these biomarkers have potential implications for treatment and personalized mental healthcare.
Research in schizophrenia thus far has focused on single biomarkers.
A shift towards a biomarker profile may be necessary to identify individuals at greatest risk for psychosis and individualize treatment.
Future directions may draw on successful models outside of psychiatry such as Framingham studies or the Alzheimer’s Disease Neuro-Imaging Initiative.
Acknowledgments
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Correll CU, Hauser M, Auther AM, Cornblatt BA. Research in people with psychosis risk syndrome: a review of the current evidence and future directions. J. Child Psychol. Psychiatry 51(4), 390–431 (2010).■■ Comprehensive discussion of the psychosis prodrome, review of the literature and future directions.
- 2.Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr. Bull 22(2), 283–303 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr. Res 67(3), 131–142 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Pillai A, Buckley PF. Reliable biomarkers and predictors of schizophrenia and its treatment. Psychiatr. Clin. North Am 35(3), 645–659 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Light GA, Swerdlow NR, Rissling AJ et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS ONE 7(7), e39434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong A, Feldcamp L. Biomarkers in schizophrenia In: Biomarkers for Psychiatric Disorders. Turck C (Ed.). Springer, USA, 23–55 (2009). [Google Scholar]
- 7.Dean B Dissecting the syndrome of schizophrenia: progress toward clinicaly useful biomarkers. Schizophr. Res. Treatment 2011, 614730 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snitz BE, MacDonald AW, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr. Bull 32(1), 179–194 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ. Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinicaly unaffected first-degree relatives and first-episode and chronic schizophrenia. Biol. Psychiatry 64(5), 385–391 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vesell ES. Twin studies in pharmacogenetics. Hum. Genet. Suppl. 1, 19–30 (1978). [DOI] [PubMed] [Google Scholar]
- 11.Foster A, Miller DD, Buckley P. Pharmacogenetics and schizophrenia. Clin. Lab. Med 30(4), 975–993 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Wilke RA, Dolan ME. Genetics and variable drug response. JAMA 306(3), 306–307 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther 69(3), 89–95 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Tandon R, Nasrallah HA, Keshavan MS. ‘Just the facts’: meandering in schizophrenia’s many forests. Schizophr. Res 128(1–3), 5–6 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Cadenhead KS, Perry W, Shafer K, Braff DL. Cognitive functions in schizotypal personality disordered subjects. Schizophr. Res 37(2), 123–132 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Cannon TD, Zorrilla LE, Shtasel D et al. Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch. Gen. Psychiatry 51(8), 651–666 (1994). [DOI] [PubMed] [Google Scholar]
- 17.Hawkins KA, Addington J, Keefe RS et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr. Res 67(2–3), 115–122 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Heinrichs R, Zakanis K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12(3), 426–445 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 153, 321–330 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophr. Bull. 25(2), 309–319 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff’? Schizophr. Bull 26(1), 119–136 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Bilder RM, Reiter G, Bates J et al. Cognitive development in schizophrenia: follow-back from the first episode. J. Clin. Exp. Neuropsychol 28(2), 270–282 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr. Res 88(1–3), 26–35 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr. Res 93(1–3), 266–277 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hambrecht M, Lammertink M, Klosterkötter J, Matuschek E, Pukrop R. Subjective and objective neuropsychological abnormalities in a psychosis prodrome clinic. Br. J. Psychiatry Suppl 43, S30–S37 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Seidman LJ, Giuliano AJ, Meyer EC et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch. Gen. Psychiatry 67(6), 578–588 (2010).■ Highlights the utility of neuropsychological biomarkers to detect risk for conversion.
- 27.Cosway R, Byrne M, Clafferty R et al. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh high risk study. Psychol. Med 30(5), 1111–1121 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Brewer WJ, Francey SM, Wood SJ et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am. J. Psychiatry 162(1), 71–78 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Whyte MC, Brett C, Harrison LK et al. Neuropsychological performance over time in people at high risk of developing schizophrenia and controls. Biol. Psychiatry 59(8), 730–739 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Pukrop R, Schultze-Lutter F, Ruhrmann S et al. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J. Clin. Exp. Neuropsychol 28(8), 1388–1407 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology 24(1), 109–120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr. Res. 94(1–3), 221–230 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Eack SM, Hogarty GE, Cho RY et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch. Gen. Psychiatry 67(7), 674–682 (2010).■■ Demonstrates the positive effects of cognitive enhancement on the brain in a well-designed longitudinal study.
- 34.Barlati S, De Peri L, Deste G, Fusar-Poli P, Vita A. Cognitive remediation in the early course of schizophrenia: a critical review. Curr. Pharm. Des 18(4), 534–541 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Breitborde NJ, Moreno FA, Mai-Dixon N et al. Multifamily group psychoeducation and cognitive remediation for first-episode psychosis: a randomized controlled trial. BMC Psychiatry 11, 9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatry 166(7), 805–811 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twamley EW, Savla GN, Zurhellen CH, Heaton RK, Jeste DV. Development and pilot testing of a novel compensatory cognitive training intervention for people with psychosis. Am. J. Psychiatr. Rehabil 11(2), 144–163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twamley EW, Burton CZ, Vella L. Compensatory cognitive training for psychosis: who benefits? Who stays in treatment? Schizophr. Bull 37(Suppl. 2), S55–S62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velligan DI, Diamond PM, Maples NJ et al. Comparing the efficacy of interventions that use environmental supports to improve outcomes in patients with schizophrenia. Schizophr. Res 102(1–3), 312–319 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry 168(5), 472–485 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Goff DC. D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr. Bull 38(5), 936–941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyaoka T Minocycline for schizophrenia: a critical review. Open J. Psychiatry 2(4), 399–406 (2012). [Google Scholar]
- 43.Lane H, Lin CH, Green MF et al. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry doi: 10.1001/jamapsychiatry.2013.2159 (2013) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow N Behavioral Neurobiology of Schizophrenia and its Treatment. Springer, USA, 4 (2010). [PubMed] [Google Scholar]
- 45.Levkovitz Y, Mendlovich S, Riwkes S et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 71(2), 138 (2010).■■ Highlights the treatment implications of minocycline in early psychosis.
- 46.Crossley NA, Constante M, Fusar-Poli P, Bramon E. Neurophysiological alterations in the prepsychotic phases. Curr. Pharm. Des 18(4), 479–485 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Rissling AJ, Light GA. Neurophysiological measures of sensory registration, stimulus discrimination, and selection in schizophrenia patients. Curr. Top. Behav. Neurosci 4, 283–309 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull 34(5), 927–943 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Stelt O, Belger A. Application of electroencephalography to the study of cognitive and brain functions in schizophrenia. Schizophr. Bull 33(4), 955–970 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavoie S, Murray MM, Deppen P et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 33(9), 2187–2199 (2008).■■ Demonstrates the utility of N-acetyl-cysteine in improving mismatch negativity, a robust biomarker of psychosis.
- 51.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of γ-aminobutyric acid and glutamate alterations. Arch. Neurol 63(10), 1372–1376 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Fu CH, Costafreda SG. Neuroimaging-based biomarkers in psychiatry: clinical opportunities of a paradigm shift. Can. J. Psychiatry 58(9), 499–508 (2013).■■ Delineates new, thoughtful approaches to using neuroimaging techniques for clinical purposes, comparing schizophrenia and Alzheimer’s disease.
- 53.Weber-Fahr W, Englisch S, Esser A et al. Altered phospholipid metabolism in schizophrenia: a phosphorus 31 nuclear magnetic resonance spectroscopy study. Psychiatry Res. doi: 10.1016/j.pscychresns.2013.06.011 (2013) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 54.Fusar-Poli P, Howes OD, Allen P et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch. Gen. Psychiatry 67(7), 683–691 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia – a systematic review and meta-analysis. Biol. Psychiatry 69(5), 495–503 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Fusar-Poli P, Perez J, Broome M et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci. Biobehav. Rev 31(4), 465–484 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Sabb FW, van Erp TG, Hardt ME et al. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr. Res 116(2–3), 173–183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen P, Seal ML, Valli I et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr. Bull 37(4), 746–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de la Fuente-Sandoval C, León-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff- Guerrero A. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int. J. Neuropsychopharmacol 16(2), 471–475 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry 60(6), 585–594 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Gur RE, Cowell P, Turetsky BI et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch. Gen. Psychiatry 55(2), 145–152 (1998). [DOI] [PubMed] [Google Scholar]
- 62.Jacobsen LK, Giedd JN, Castellanos FX et al. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am. J. Psychiatry 155(5), 678–685 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J. Psychiatr. Res 28(3), 239–265 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Pantelis C, Yucel M, Wood SJ et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr. Bull 31(3), 672–696 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44(7), 660–669 (1987). [DOI] [PubMed] [Google Scholar]
- 66.Feinberg I Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res 17(4), 319–334 (1982). [DOI] [PubMed] [Google Scholar]
- 67.Richtand NM, McNamara RK. Serotonin and dopamine interactions in psychosis prevention. Prog. Brain Res 172, 141–153 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Berger GE, Wood SJ, Ross M et al. Neuroprotective effects of low-dose lithium in individuals at ultra-high risk for psychosis. A longitudinal MRI/MRS study. Curr. Pharm. Des 18(4), 570–575 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Falkai P, Malchow B, Wobrock T et al. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur. Arch. Psychiatry Clin. Neurosci 263(6), 469–473 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Pajonk FG, Wobrock T, Gruber O et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry 67(2), 133–143 (2010).■■ Demonstrates the positive effect of nonpharmacologic intervention on the brain and emphasizes brain plasticity in schizophrenia.
- 71.Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia. Clin. Schizophr. Relat. Psychoses 1–22 (2013). [PubMed] [Google Scholar]
- 72.Dickerson F, Stallings C, Origoni A et al. C-reactive protein is elevated in schizophrenia. Schizophr. Res 143(1), 198–202 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Wium-Andersen MK, Ørsted DD, Nordestgaard BG. Elevated C-reactive protein associated with late- and very-late-onset schizophrenia in the general population: a prospective study. Schizophr. Bull doi: 10.1093/schbul/sbt120 (2013) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am. Heart J. 121(1 Pt 2), 293–298 (1991). [DOI] [PubMed] [Google Scholar]
- 75.Pukrop R, Ruhrmann S, Schultze-Lutter F et al. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr. Res 92(1–3), 116–125 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Lencz T, Smith CW, McLaughlin D et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol. Psychiatry 59(9), 863–871 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Bousman CA, Yung AR, Pantelis C et al. Effects of NRG1 and DAOA genetic variation on transition to psychosis in individuals at ultra-high risk for psychosis. Transl. Psychiatry 3, e251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kéri S, Kelemen O, Janka Z. Diagnosis and treatment of mental states at high risk for psychosis. Psychiatr. Hung 21(2), 130–137 (2006). [PubMed] [Google Scholar]
- 79.Walker EF, Trotman HD, Pearce BD et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol. Psychiatry 74(6), 410–417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J. Abnorm. Psychol 119(2), 401–408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garner B, Pariante CM, Wood SJ et al. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol. Psychiatry 58(5), 417–423 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Takahashi T, Wood SJ, Yung AR et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry 66(4), 366–376 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Borgwardt SJ, McGuire PK, Aston J et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br. J. Psychiatry Suppl 51, S69–S75 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Bodatsch M, Ruhrmann S, Wagner M et al. Prediction of psychosis by mismatch negativity. Biol. Psychiatry 69(10), 959–966 (2011).■■ The authors demonstrate the utility of mismatch negativity in prediction of psychosis in a well-designed study.
- 85.Kannel WB. Fifty years of Framingham study contributions to understanding hypertension. J. Hum. Hypertens 14(2), 83–90 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Carrillo MC, Bain LJ, Frisoni GB, Weiner MW. Worldwide Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 8(4), 337–342 (2012). [DOI] [PubMed] [Google Scholar]