Abstract
The recent discovery of a mutational variant in the CEP290 gene (CEP290: IVS50 + 9T>G), conferring recessive retinal degeneration in Abyssinian and Somali (long-haired Abyssinian) cats (rdAc) prompted a survey among 41 cat breeds (846 individuals) to assess the incidence, frequency and clinical consequence of rdAc. The rdAc allele displayed widespread distribution, observed in 16/43 (37%) breeds, exhibiting a high allele frequency (~33%) in North American and European Siamese populations. Clinical evaluations demonstrated high concordance between rdAc pathology and the CEP290 (IVS50 + 9T>G) homozygous genotype (P = 1.1E–6), with clinical disease similar to affected Abyssinians/Somalis. This retinal degeneration has not been reported in breeds other than the Abyssinian/Somali and poses a significant health risk particularly in the Siamese breed group. Alertness of the veterinary community and the present availability of commercial diagnostic testing could synergistically enable breeders to reduce the incidence of rdAc blindness in pure-bred cat populations.
Keywords: Retinal degeneration, Cat breeds, Mutation, CEP290, Photoreceptors
Introduction
Cat breeds exhibit a high incidence of hereditary disease pathologies (Pontius et al., 2007), as has been seen in other domesticated animal breed populations (Higgins and Nicholas, 2008; Taberlet et al., 2008). Small effective population sizes, the use of popular sires, line breeding and founder effects increase the likelihood of expression of rare pathogenic mutations. Over 280 pathologies with a hereditary component have been reported in the domestic cat.1 Many conditions are not seen outside of the breed or breed group in which the causative mutation occurred, such as gangliosidosis in the Korat (Baker et al., 2001; Martin et al., 2004; Muldoon et al., 1994) and Type IV glycogen storage enzyme disease in the Norwegian Forest cat (Fyfe and Kurzhals, 1998), possibly due to the constraints that registration rules may place on gene transfer between breeds. Others such as polycystic kidney disease have most likely spread into newer breeds from contributing parental breeds (Persian) (Barthez et al., 2003).
Whilst there have been reports of sporadic retinal pathologies identified in cats (Bistner et al., 1976; Glaze, 2005; Gould and Sargan, 2002; Narfström, 1999, 1983; Rah et al., 2005), hereditary retinal blindness has not generally been considered a significant health factor in pure-bred cats. With the discovery of an unusually high prevalence (45%) of hereditary rod cone degeneration in the Abyssinian cat approximately 25 years ago in Sweden, it became clear that there was increased risk for hereditary retinal dystrophies causing blindness within some cat breeds (Narfström, 1983, 1985a; Narfström and Nilsson, 1983). Hereditary blindness was observed in a group of American short-haired cats (West-Hyde and Buyukmihci, 1982) and another Abyssinian cat model was subsequently described for retinal blinding disease in the United Kingdom (Barnett and Curtis, 1985; Curtis et al., 1987). More recently, an autosomal recessive form of early onset progressive retinal atrophy has been described in Persian cats (Rah et al., 2005).
The development of critical genetic mapping resources in the cat, including comprehensive genetic maps (Davis et al., 2009; Menotti-Raymond et al., 1999, 2003, 2009; Murphy et al., 2007), the recent 1.9X whole genome sequence of the cat (Pontius et al., 2007; Pontius and O’Brien, 2007) and the generation of a pedigree segregating for rdAc (Narfström et al., in press) has enabled the identification of the causal mutation for rdAc (Menotti-Raymond et al., 2007b). A single base pair substitution in an intron of the centrosomal protein 290 gene (CEP290) (IVS50 + 9T>G) (previously referred to as the rdAc allele) results in alternative splicing of the CEP290 transcript, with subsequent introduction of a premature stop codon and truncation of the mature protein (Menotti-Raymond et al., 2007b).
Mutations in the homologous human CEP290 gene are a common cause of human blindness, including approximately 30% of patients with Leber’s congenital amaurosis (Hollander et al., 2006). Additionally, mutations in CEP290 are causative of several rare, severe, early onset syndromic diseases in humans including Joubert’s, Senior-Loken, Meckel-Gruber, and Bardet-Biedl syndromes, which cause blindness, mental retardation and kidney failure among other severe clinical signs (Baala et al., 2007; Leitch et al., 2008; Sayer et al., 2006; Valente et al., 2006). None of these additional clinical manifestations are observed in rdAc affected cats (Narfström et al., in press).
A recent survey of 21 cat breeds in the USA (n = 2/breed) (Menotti-Raymond et al., 2007b) suggested that the rdAc allele was confined to the Abyssinian/Somali (Somali cats are long-haired Abyssinians) and Abyssinian related breeds, including a single rdAc allele identified in an Ocicat. This recently generated hybrid breed has had input from both Abyssinian and Siamese cats (Helgren, 1997). Furthermore, a recent genetic survey of Abyssinian and Somali cat populations from the USA, UK, Australia and Scandinavia, identified rdAc allele frequencies of 0.07, 0.21, 0.11, 0.20, respectively (Menotti-Raymond et al., 2007b; Narfström et al., in press). In this extended survey the rdAc allele genotype was in complete concordance with the presence or absence of retinal atrophy (P = 3.2E–8), demonstrating that the rdAc genotype is highly predictive of rdAc disease progression (n = 846).
As the rdAc allele has a world-wide distribution in Abyssinian and Somali populations with a significant clinical impact on homozygous affected cats, it became imperative to determine whether the rdAc allele is present in additional related breeds. In this report 41 further breeds (n = 846 individuals) and 92 outbred (random bred) cats were genotyped at the rdAc locus, and 27 individual cats screened for evidence of retinal degeneration.
Surprisingly, the rdAc allele was detected in 34% of the cat breeds examined with relatively high frequencies in Siamese and Siamese-related breeds in both North America and Europe. Based on a recent publication on the genetic relatedness of cat breeds (Menotti-Raymond et al., 2007a), we were unable to identify genetic distinctiveness between the Siamese, Colorpoint Shorthair, Oriental Shorthair, Balinese and Javanese breeds, which we will refer to in this study as ‘Siamese related breeds’ or the ‘Siamese breed group’. In addition, the rdAc genotype was predictive of the disease phenotype, where all homozygous rdAc pure-bred cats that were examined in the study had evidence of retinal degeneration, similar to that observed in Abyssinian and Somali cats. Clearly the rdAc mutation has a global distribution in multiple breeds of cats, and this form of heritable retinal degeneration has been previously unrecognised in several popular breeds of cats.
Materials and methods
DNA and tissue samples
DNA samples representing 43 cat breeds and one outbred population were used in the study (Table 1). DNA samples of individuals representing 36 breeds were used from a previous collection (Menotti-Raymond et al., 2005). DNA from five additional breeds was obtained from a population genetic survey (Thai) (see below), the Laboratory of Genomic Diversity DNA resources (Tennessee Rex, Munchkin, Angora) and from a commercial DNA testing laboratory (see below) which included the Peterbald, a breed recently developed with Siamese influence (Fogle, 2001).
Table 1.
Frequencies observed for rdAc genotypes in 43 cat breeds and one outbred population.
| Cat breed | na | Genotype | Frequency of CEP290 risk allele | Anticipated frequency of affected individualsc | Potential breed introducing risk allele | |||
|---|---|---|---|---|---|---|---|---|
| Unrelatedb | CEP290 +/+ (homozygous unaffected) | CEP290 +/− (carrier) | CEP290 −/− (affected) | |||||
| Abyssinian/Somali (USA)d | 16 | 16 | 14 | 2 | 0 | 0.070 | 0.005 | |
| Abyssinian (UK)e | 34 | 34 | 22 | 10 | 2 | 0.206 | 0.042 | |
| Abyssinian (Australia)e | 57 | 57 | 46 | 10 | 1 | 0.105 | 0.011 | |
| Abyssinian/Somali (Scandinavia)e | 130 | 130 | 85 | 39 | 6 | 0.196 | 0.038 | |
| American curl | 10 | 10 | 9 | 1 | 0 | 0.050 | 0.003 | ? |
| American wirehair | 10 | 10 | 8 | 2 | 0 | 0.100 | 0.010 | Siamese? |
| Bengal | 18 | 18 | 16 | 2 | 0 | 0.056 | 0.003 | Siamese |
| Balinese/Javanese | 28 | 24 | 10 | 12 | 2 | 0.333 | 0.111 | Siamese |
| Colorpoint shorthair | 11 | 11 | 5 | 4 | 2 | 0.364 | 0.132 | Siamese |
| Cornish rex | 20 | 20 | 19 | 1 | 0 | 0.025 | 0.001 | Siamese |
| Munchkin | 15 | 15 | 14 | 1 | 0 | 0.033 | 0.001 | ? |
| Ocicat | 18 | 18 | 15 | 3 | 0 | 0.083 | 0.007 | Siamese/Abyssinian |
| Oriental shorthair | 46 | 25 | 11 | 11 | 3 | 0.340 | 0.116 | Siamese/Abyssinian? |
| Siamese | 91 | 49 | 28 | 16 | 5 | 0.265 | 0.070 | ? |
| Singapura | 6 | 6 | 6 | 0 | 1 | 0.167 | 0.028 | ? |
| Tonkinese | 7 | 7 | 6 | 1 | 0 | 0.071 | 0.005 | Siamese |
| American shorthair | 9 | 9 | 9 | 0 | 0 | 0.000 | 0.000 | |
| Angora | 13 | 13 | 13 | 0 | 0 | 0.000 | 0.000 | |
| Birman | 10 | 10 | 10 | 0 | 0 | 0.000 | 0.000 | |
| Bobtail | 13 | 13 | 13 | 0 | 0 | 0.000 | 0.000 | |
| Bombay | 9 | 9 | 9 | 0 | 0 | 0.000 | 0.000 | |
| British shorthair | 9 | 9 | 9 | 0 | 0 | 0.000 | 0.000 | |
| Burmese | 35 | 35 | 35 | 0 | 0 | 0.000 | 0.000 | |
| Chartreux | 10 | 10 | 10 | 0 | 0 | 0.000 | 0.000 | |
| Devon rex | 20 | 20 | 20 | 0 | 0 | 0.000 | 0.000 | |
| Egyptian Mau | 19 | 19 | 19 | 0 | 0 | 0.000 | 0.000 | |
| Exotic | 18 | 18 | 18 | 0 | 0 | 0.000 | 0.000 | |
| Havana | 8 | 8 | 8 | 0 | 0 | 0.000 | 0.000 | |
| Himalayan | 17 | 17 | 17 | 0 | 0 | 0.000 | 0.000 | |
| Korat | 7 | 7 | 7 | 0 | 0 | 0.000 | 0.000 | |
| Maine coon cat | 13 | 13 | 13 | 0 | 0 | 0.000 | 0.000 | |
| Manx | 19 | 19 | 19 | 0 | 0 | 0.000 | 0.000 | |
| Norwegian forest cat | 19 | 19 | 19 | 0 | 0 | 0.000 | 0.000 | |
| Persian | 19 | 19 | 19 | 0 | 0 | 0.000 | 0.000 | |
| Ragdoll | 8 | 8 | 8 | 0 | 0 | 0.000 | 0.000 | |
| Russian blue | 10 | 10 | 10 | 0 | 0 | 0.000 | 0.000 | |
| Scottish fold | 20 | 20 | 20 | 0 | 0 | 0.000 | 0.000 | |
| Selkirk rex | 20 | 20 | 20 | 0 | 0 | 0.000 | 0.000 | |
| Siamese (‘appleheads’) | 31 | 18 | 18 | 0 | 0 | 0.000 | 0.000 | |
| Sphynx | 20 | 20 | 20 | 0 | 0 | 0.000 | 0.000 | |
| Tennessee rex | 19 | 19 | 19 | 0 | 0 | 0.000 | 0.000 | |
| Thai | 2 | 2 | 2 | 0 | 0 | 0.000 | 0.000 | |
| Turkish angora | 14 | 14 | 14 | 0 | 0 | 0.000 | 0.000 | |
| Turkish van | 9 | 9 | 9 | 0 | 0 | 0.000 | 0.000 | |
| Outbred (USA) | 92 | 92 | 92 | 2 | 0 | 0.011 | 0.000 | |
| Total cats this study (North America) | 792 | |||||||
| European samples | ||||||||
| Balinese | 1 | 0 | 0 | 1 | 1.000 | 1.000 | ||
| Bengal | 2 | 2 | 0 | 0 | 0.000 | 0.000 | ||
| Ocicat | 3 | 1 | 2 | 0 | 0.333 | 0.111 | ||
| Oriental shorthair | 12 | 7 | 4 | 1 | 0.250 | 0.063 | ||
| Peterbald | 8 | 2 | 4 | 2 | 0.500 | 0.250 | Siamese | |
| Siamese | 28 | 18 | 9 | 1 | 0.196 | 0.039 | ||
| Total European | 54 | |||||||
| Total cats | 846 | |||||||
Numbers of individuals include cats in this study, only.
Total number of unrelated cats used for statistical analyses.
Estimates are based on expectations for populations in Hardy Weinberg equilibrium (see text).
See Menotti-Raymond et al. (2007).
DNA from 92 random bred, feral cats was extracted from discarded tissues provided by veterinary hospitals and spay clinics in Frederick and Howard County, Maryland. Additionally, DNA samples (n = 54) were obtained from a commercial testing laboratory in Europe LABOKLIN (Bad Kissingen, Germany), including 28 Siamese cats, 1 Balinese, 8 Peterbald, 3 Ocicat, 12 Oriental Short-hair, and 2 Bengal, under the condition of anonymity of the individuals and their owners.
Animals
For cats of recognised breeds, buccal swab samples were obtained, under an approved Animal Care and Use Protocol, from Siamese (n = 107), Oriental Shorthair (n = 40), Javanese (n = 4), Balinese (n = 11), and Colorpoint Shorthair (n = 3) cats from cat breeders in Maryland, Massachusetts, Pennsylvania, Virginia, Texas and Ohio. Breeders’ names were withheld under request for anonymity.
DNA extraction and genotyping of the rdAc allele
DNA was extracted from buccal or tissue samples using Qiagen QiAmp DNA Blood Midi and Mini Extraction Kits following the manufacturer’s suggested protocols. DNA was quantified using a Hoefer DNA Quant 200 Fluorometer (Amersham BioSciences). A proportion of each sample was diluted to a standard concentration of 2.5 ng/μL with sterile distilled water (Quality Biological). Genotyping of the rdAc causative single nucleotide polymorphism (SNP) (IVS50 + 9T>G) was performed as described by Menotti-Raymond et al. (2007b).
Clinical examinations
Clinical study of rdAc has been extensively characterized in the Abyssinian cat (Narfström, 1983, 1985a,b; Narfström et al., 1989, 2001, 1988; Narfström and Nilsson, 1986, 1989). To determine if the rdAc genotype correlated with the development of retinal degeneration pathology as observed in the Abyssinian, a subset of individuals (n = 27) from 8 months to 18 years of age, representing six specific breeds in the Siamese breed group (Table 2), were clinically evaluated after genotyping. Ophthalmologic examinations were performed in a masked fashion, in that the veterinary ophthalmologist (KN) examined cats without knowledge of their rdAc genotype. Pupils were dilated using short acting mydriatics (Tropicamide 1%, Alcon), and fundic examinations were performed through indirect ophthalmoscopy (Welch Allyn Distributors) in all cats included in the study.
Table 2.
Results of clinical ophthalmoscopic exams.
| Cat breeda | Age (years) | Sex | rdAc genotypeb | Clinical diagnosis | Stage of disease | Examination performed |
|---|---|---|---|---|---|---|
| Balinese | 5 | M | Affected | Affected | Stage 2 | Ophthalmoscopic |
| Colorpoint shorthair | 11 | F | Affected | Affected | Stage 3+ | Ophthalmoscopic |
| Oriental shorthair | 3 | F | Affected | Affected | Stage 3+ | Ophthalmoscopic |
| Oriental shorthair | 1 | M | Affected | Affected | Stage 2 | Ophthalmoscopic |
| Siamese | 3.5 | F | Affected | Affected | Stage 2 | Ophthalmoscopic |
| Siamese | 1.5 | M (N) | Affected | Affected | Stage 1 | Ophthalmoscopic, ERG |
| Siamese | 8 | F | Affected | Affected | Stage 4 | Ophthalmoscopic |
| Oriental shorthair | 8 months | F | Carrier | Unaffected | ||
| Oriental shorthair | 2 | F | Carrier | Unaffected | ||
| Oriental shorthair | 2 | F | Unaffected | Unaffected | ||
| Oriental shorthair | 10.5 | F | Carrier | Unaffected | ||
| Oriental shorthair | 3 | F | Carrier | Unaffected | ||
| Oriental shorthair | 1 | F | Carrier | Unaffected | ||
| Oriental shorthair | 6.5 | M | Carrier | Unaffected | ||
| Oriental shorthair | 4 | F | Carrier | Unaffected | ||
| Oriental shorthair | 1 | M | Carrier | Unaffected | ||
| Siamese | 18 | F | Carrier | Unaffected | ||
| Siamese | 1.5 | F | Carrier | Unaffected | ||
| Siamese (A) | 3.5 | F | Unaffected | Unaffected | ||
| Siamese (A) | 5 | F | Unaffected | Unaffected | ||
| Siamese (classic wedge) | 9 | F (S) | Unaffected | Unaffected | ||
| Siamese (wedge:traditional) | 18 | M (N) | Unaffected | Unaffected | ||
| Siamese | 8 | M (N) | Carrier | Unaffected | ||
| Siamese | 5.5 | M (N) | Unaffected | Unaffected | ||
| Siamese | 13 | M (N) | Unaffected | Unaffected | ||
| Siamese | 6 | M | Unaffected | Unaffected | ||
| Thaic | 1 | M | Unaffected | Unaffected | Ophthalmoscopic, ERG |
(A) refers to the rounder head style Siamese known as the ‘applehead’ – see text in results for detailed description; cats not so designated are wedge or modified wedge style head.
Affected: homozygous for the rdAc defining SNP, CEP290 −/−; Carrier; CEP290 +/−; Unaffected: CEP290 +/+.
The Thai breed is a recently recognized breed of the International Cat Association, representing recent imports from Thailand which exhibit features of the rounder-headed old-style Siamese.
Full-field flash electroretinography (ERG) was performed in a 1-year old Siamese with suspected disease (stage 1) according to the ophthalmoscopic examination and normal age-matched cat, both under medetomidine, 150 μg/kg, equivalent to 0.15 mL/kg (Domitor, Pfizer) and ketamine anaesthesia (5 mg/kg IM). A portable ERG unit was used (HMsERG, RetVetCorp.) with an automated protocol for evaluation of rod and cone function (Narfström et al., 2002; Katz et al., 2008).
Results
In a genetic survey of 846 pure-bred and 92 outbred cats, the rdAc single nucleotide polymorphism (CEP290: IVS50 + 9T>G) previously characterized in the Abyssinian and Somali breeds was detected in 14/41 breeds (34%) (Table 1). Frequencies for the rdAc allele in the cat breeds from the US ranged from 0.02 (Cornish Rex) to 0.36 (Balinese) (Table 1), although sample sizes were small for some of the breeds and may not accurately reflect the true frequencies. While the rdAc allele was also detected in two carriers out of 92 outbred cats sampled in Maryland, the frequency was extremely low (0.01). Whereas, individuals in the Siamese and Siamese-related breeds (Colorpoint Shorthair, Oriental Shorthair, Balinese, Javanese), which will be referred to in this study as the ‘Siamese breed group’, exhibited the highest allele frequencies for the rdAc mutation ranging from 0.27 to 0.36 (Table 1).
A significant part of our study focused on the Siamese breed group, as initial estimates in 36 individuals had demonstrated elevated rdAc allele frequencies (data not shown). To confirm these initial estimates within the Siamese and Siamese-related breeds, additional samples were collected from several independent US breed registries (n = 187) (Table 3) and European countries (n = 52) (Table 1). Samples obtained from 52 cats from six breeds (Balinese, Bengal, Ocicat, Oriental Shorthair, Peterbald, Siamese) from geographically separated countries, demonstrated presence of the rdAc allele in 5/6 breeds with elevated frequencies in the Siamese breed group (Table 1).
Table 3.
Frequency of rdAc genotypes in the Siamese breed group in different cat registries.
| Cat breed (registry) | n | Unrelated | Genotype | Frequency of CEP290 allele | Anticipated frequency of affected catsc | P-value | ||
|---|---|---|---|---|---|---|---|---|
| CEP290 +/+ (homozygous unaffected) | CEP290 +/− (carrier) | CEP290 −/− (affected) | ||||||
| Siamese (CFA, TICA, TCA)ab | 71 | 49 | 28 | 16 | 5 | 0.265 | 0.070 | 0.791 |
| Siamese (CFA) | 25 | 25 | 11 | 11 | 3 | 0.340 | 0.116 | 0.734 |
| Siamese (TICA) | 25 | 17 | 14 | 2 | 1 | 0.118 | 0.014 | 0.906 |
| Siamese: TCA (classic:wedge-faced)d | 21 | 7 | 3 | 3 | 1 | 0.357 | 0.128 | 0.721 |
| Siamese: TCA (‘applehead’) | 31 | 18 | 18 | 0 | 0 | 0.000 | 0.000 | |
| Oriental shorthair | 46 | 25 | 11 | 11 | 3 | 0.340 | 0.116 | 0.734 |
| Balinese/Javanese | 28 | 24 | 10 | 12 | 2 | 0.333 | 0.111 | 0.739 |
| Colorpoint shorthair | 11 | 11 | 5 | 4 | 2 | 0.364 | 0.132 | 0.716 |
| Total | 187 | |||||||
CFA: Cat Fancy Association; TICA: The International Cat Association; TCA: The Traditional Cat Association.
Numbers do not include ‘applehead’ cats.
Frequency is based on expectations of populations in Hardy Weinberg equilibrium.
The TCA ‘classic’ Siamese has a less severe wedge-shaped face and more robust body than the TICA, CFA Siamese.
Within the US additional samples were obtained from populations of Siamese cats maintained in separate registries (CFA, TICA, TCA) (Table 3). Within the TCA registry, breeders have developed two populations of Siamese cats with different conformational standards. Siamese cats which exhibit a rounder head shape and more robust body (reminiscent of the old-style Siamese conformation) are referred to as classic or ‘appleheads’, and are shown and maintained as a distinct group from those with a ‘wedge-shaped’ face. A slim body and more extreme ‘wedge-shaped’ face has become the standard for most contemporary Siamese cat registries, including the CFA and TICA. No rdAc alleles were identified in any of the 31 ‘appleheads’ examined. However, the rdAc allele was found at high frequencies in all ‘wedge-faced’ Siamese, confirming earlier estimates, regardless of registry (Table 3).
A subset of cats from the Siamese and Siamese breed group was examined to determine, if the rdAc genotype was predictive of the development of retinal degeneration, as in Abyssinian and Somali cats. Ophthalmic evaluations and functional testing using ERG showed similar changes to those which been described for the Abyssinian cat (Hyman et al., 2005; Kang Derwent et al., 2006; Vaegan and Narfström, 2005, 2008), and it was possible to stage the disease in these cats according to criteria established in these earlier studies (Narfström, 1985a,b) (Table 2). The age of onset and developmental progression of rdAc within these additional cat breeds appears to be similar to the slowly progressive retinal degeneration previously described in rdAc Abyssinians, in which affected kittens may have reduced retinal function by 8 months of age with early funduscopic changes detectable at 1–2 years and visual impairment by 5–6 years of age.
Seven individual cats homozygous for the rdAc allele, including three Siamese, one Balinese, one Colorpoint Shorthair and two Oriental Shorthair cats, were found to have clinical signs of rdAc disease (Table 2). Fig. 1 demonstrates typical funduscopic changes in an 8-year old Siamese cat. ERG recordings resulting from white light stimulation using 10 cd s/m2 in the dark adapted state (Fig. 2) performed in a 1-year old Siamese with stage 1 disease showed a reduced a-wave in comparison to an age-matched normal cat, while the b-wave in the affected animal was less reduced (43% reduction for the a-wave and 33% for the b-wave, respectively). No signs of retinal degeneration were detected in the 20 remaining cats that were either carriers of the rdAc allele or homozygous for the unaffected (wildtype) allele. Complete concordance between rdAc genotype and rdAc disease status was observed in all 27 individuals examined representing six breeds (P = 1.1E–6) (Table 2).
Fig. 1.
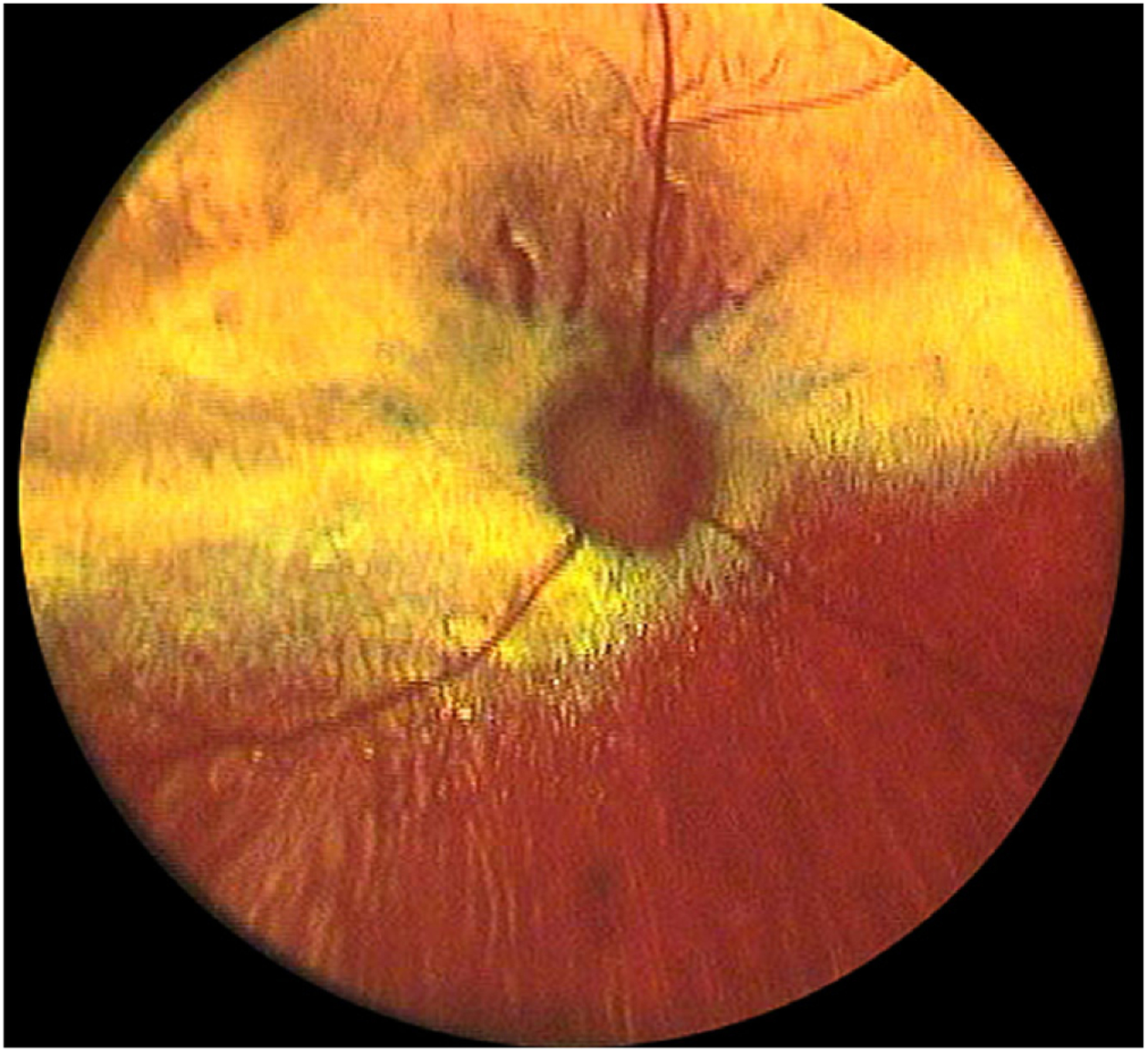
Fundus photograph of an 8-year-old Siamese cat homozygous for the rdAc mutation in an advanced stage of retinal degeneration. Note the generalized retinal color changes in this submelanotic fundus. There is greyish discoloration horizontally along the visual streak area, hyper-reflectivity in the central fundus and also in the mid-peripheral parts and a generalized vascular attenuation. Courtesy Dr. David A. Wilkie.
Fig. 2.
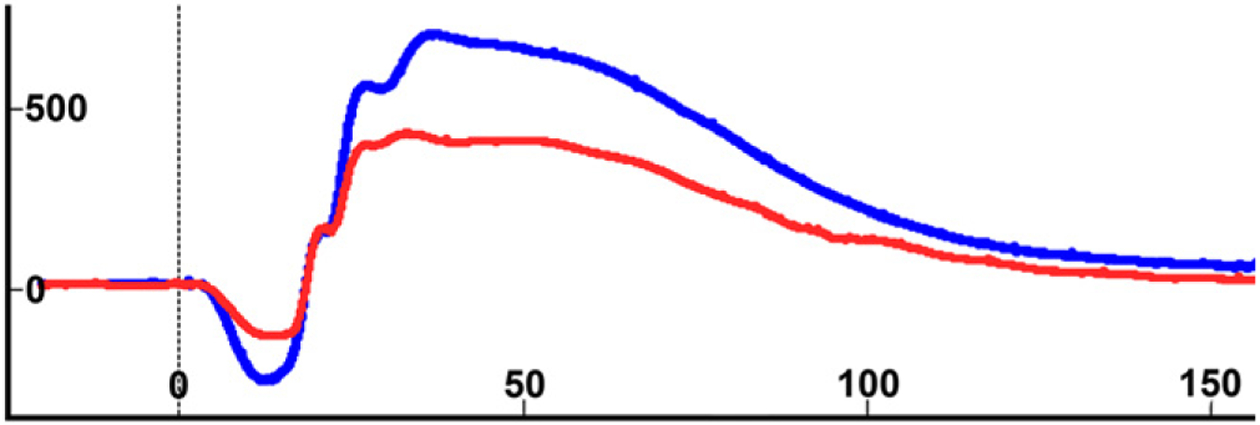
ERG tracings from an affected 1-year-old Siamese cat (red) in stage I (stage of suspected disease) and from an age-matched normal cat (blue) showing responses using scotopic high intensity white light stimulation (10 cd s/m2) and the HMsERG under similar recording conditions for both cats. Note the severely reduced a-wave and the less reduced b-wave in the affected individual in comparison to the normal cat. Amplitude calibration in microvolts and implicit time calibration in milliseconds are shown on the ordinate and on the abscissa, respectively. See also Vaegan and Narfström (2005, 2008).
Discussion
The mutational variant in the CEP290 gene (CEP290: IVS50 + 9T>G or rdAc) originally identified as the cause of heritable retinal degeneration in Abyssinian and Somali cats has a previously unrecognised world-wide distribution in additional cat breeds. In this present study the rdAc allele was detected in 14/41 breeds (34%) sampled, with a relatively high frequency found in Siamese and Siamese-related breeds (Table 1). The incidence of rdAc affected individuals in outbred populations was negligible, as would be expected for a recessively inherited pathology, with two rdAc allele carrier individuals detected from farms in adjacent counties of rural Maryland. The rdAc allele was also detected at high frequency in cats from the Siamese breed group residing in Europe, confirming that this mutation is present in geographically separated populations.
Most importantly, all 26 individual cats within the Siamese breed group that were directly examined exhibited complete concordance of rdAc genotype with affection status (P = 1.1E–6), demonstrating that rdAc genotype is an excellent predictor of a cat’s rdAc affection status. Within this small subset of affected Siamese breed group cats, progression of rdAc proceeded in an age-dependent manner, similar to that observed in Abyssinian cats (Table 2). Additional clinical studies will be necessary to confirm these observations in other breeds unrelated to Abyssinians or Siamese in which the rdAc allele was detected.
Although sample sizes were small, the rdAc allele frequency may be as high as 27–36% in the wedge-faced Siamese breed group of cats from North America (Table 1). If these allele frequencies are confirmed by increased sampling, 7–13% of the individuals in this breed group could be affected with rdAc, and would be expected to be severely visually impaired by middle age, at approximately 5–6 years of age. This estimate is based on principals of population genetic theory, specifically the Hardy–Weinberg equilibrium (HWE), which posits that alleles in a population associate randomly unless acted upon by selection (Hartl and Clark, 2006). Certain assumptions about gene flow, genetic drift and non-random mating in populations in the HWE may not apply in populations maintained by the Cat Fancy. However, the rdAc polymorphism conformed to HWE within and among each of five Siamese-related breeds (P >0.1) (Table 3).
Of interest was the absence of the rdAc allele in our sample set of ‘applehead’ Siamese which is highly suggestive that these individuals represent a separate gene pool from the wedge-faced Siamese. There is little motivation for breeders to cross between the two widely different phenotypes, resulting in relative isolation. Thus it should not be surprising that there is a significant difference in rdAc allele frequency among different registries (see Table 3). The high rdAc allele frequency in the wedge-faced individuals could result from a founder effect within the Siamese, which are bred for showing. Additionally, the rdAc risk allele could be linked to a gene involved in the distinctive wedge-shaped face phenotype. RdAc is not observed in other cat breeds with the distinctive color points of the Siamese (i.e., Birman, Himalayan, Ragdoll), though this linkage was not expected as the gene/gene variants responsible for ‘colorpoint’ and rdAc phenotypes, tyrosinase and CEP290, respectively, are located on different chromosomes (D1 and B4) (Lyons et al., 2005; Schmidt-Küntzel et al., 2005).
We demonstrate a single SNP in the CEP290 gene that exhibits high predictability for rdAc affection status in multiple breeds. Additionally, we have observed a single DNA haplotype over an approximate 500 kilobase region 5′ and 3′ of the CEP290 gene in three breeds (Abyssinian, Siamese, Oriental Shorthair) affected with rdAc (data not shown). These data support the hypothesis that the mutation causative of rdAc occurred only once, which raises the question of how rdAc become so widespread in cat breeds.
The high frequency of rdAc, first reported in Abyssinian populations in Sweden (45%) (Narfström, 1983), and the present high incidence in the Siamese breed group, suggest that the rdAc mutation originated in either the Abyssinian or Siamese breed group. Based on breeding practice within the Cat Fancy and allele frequency comparisons with other cat populations, it appears most likely that the Abyssinian was the point of origin for introduction into the Cat Fancy. The Abyssinian has anecdotally experienced little genetic input from other breeds, but has itself had input in the creation of several of the modern breeds (Lipinski et al., 2008). Although it cannot be proven, the presence of the allele in the Siamese most likely reflects outcrosses to the Abyssinian sometime after World War II, with selection bias for the more extreme phenotype contributing to the high frequency of the rdAc allele in some populations within the Siamese breed group. At the end of WWII, cat breeds in Europe were practically decimated. The long-legged Abyssinian would have been an attractive candidate to ‘contribute’ to the Siamese breed gene pool.
The absence of the allele in the applehead population study suggests introduction of the rdAc allele after the phenotypic split within the Siamese population and argues against the original imports from Siam (now Thailand) as the source for rdAc in the Siamese breed group. We have not found the mutation in other established breeds tracing their origin to Southeast Asia (including the Korat and a large sampling of Burmese), nor in more recent imports from Thailand (Thai) (Table 1), further argument in favor of a more recent introduction into the Siamese.
The majority of modern cat breeds have been developed within the last 100 years, many with genetic input from the Siamese, and some from the Abyssinian (Fogle, 2001; Helgren, 1997). Recently we conducted a study of the relatedness of 38 cat breeds (Menotti-Raymond et al., 2007a). Members of the Siamese breed group exhibited a common genetic pool for markers in the study, facilitating transfer of rdAc across these breeds. The majority of the additional rdAc affected breeds have had ‘input’ genetically at some point in the recent past from either the Siamese or Abyssinian breeds, as noted in Table 1 (Fogle, 2001; Helgren, 1997). Founder effects or the use of popular sires in the small effective population sizes of cat breeds can have dramatic impacts on allele frequencies in a relatively short period of time. Our sample numbers are small in some breeds. With a larger and wider sampling, rdAc may be identified in other breeds. Additionally, a larger sampling in affected breeds is needed in order to get a more accurate estimate of rdAc allele frequency, and perhaps to identify ‘lines’ that are especially at risk for rdAc.
The Siamese cat is one of the most popular breeds. Sporadic reports of blindness in Siamese cats have been made in the veterinary literature (Barnett, 1965; Carlile, 1981; Giuliano and van der Woerdt, 1999), but the condition has not been clinically evaluated nor has the magnitude of the problem, as suggested by the present study, been appreciated. Our data suggests that rdAc represents a significant problem in the Siamese breed group and a condition that veterinarians should be alert to in a wide range of pure-bred cats.
The fact that rdAc has gone undetected across a wide spectrum of cat breeds and appears with a relatively high frequency in the Siamese breed group of cats, is testament to the cats’ phenomenal ability to adapt to visual impairment. Pure-bred cats are often maintained in a closed environment of the home and not allowed outside. Given the slowly progressive nature of the disease, generally over several years, affected cats learn to adapt to their decreased visual function. It has been shown in previous studies that the clinical expression of the disease (i.e., visual impairment or blindness) is difficult for cat owners to recognise, since indoor cats can manoeuvre in known surroundings, using their other well developed senses (Narfström et al., in press). In addition, pupillary light reflexes (PLRs), if tested with conventional instrumentation (such as a penlight or a Finhoff transilluminator) appear reactive in affected cats until late in the disease process. However, recent studies have shown that using white and chromatic light stimulation under controlled conditions, and video monitoring, it is possible to observe subtle changes in the PLRs of affected cats already in the early stage of disease (S. Thompson et al., unpublished data).
With a concerted effort between veterinarians and breeders to diagnose and test for rdAc induced retinal degeneration, this condition can be reduced in pure-bred cat populations. In the early 1980s rdAc had an extremely high incidence of approximately ~45% in the Swedish Abyssinian population. The current incidence of individuals affected with rdAc has decreased to approximately 4% through a concerted effort among breeders reporting carrier individuals (Narfström et al., in press). Two commercial animal testing laboratories provide genotyping for the rdAc causative SNP. As rdAc is a recessive condition, testing is recommended in the breeding population, particularly in the Siamese breed group. Cooperation and alertness on the part of the cat breeder and veterinarian community could work to reduce significantly the incidence of rdAc in cat breed populations.
Conclusions
These results demonstrate that the mutational variant (CEP290: IVS50 + 9T>G), causative of rdAc, so far unreported in breeds other than the Abyssinian/Somali, displays widespread distribution among cat breeds and exhibits a high allele frequency (~33%) in both North American and European Siamese cat populations. Clinical evaluations demonstrate high concordance between rdAc pathology and the presence of a homozygous genotype for the rdAc risk allele. This retinal degeneration poses a significant health risk particularly in the Siamese breed group. Alertness of the veterinary community and the present availability of commercial diagnostic testing could synergistically enable breeders to reduce the incidence of rdAc blindness in pure-bred cat populations.
Acknowledgements
We wish to thank all of the cat breeders who donated buccal swab samples for this study. Names have been withheld under the desire for anonymity. We thank Dr. David Wilkie for the photograph used in Fig. 1. We additionally thank LABOKLIN for their contribution of 54 pure-bred cat DNA samples. We also wish to thank Donald Crouser DVM of the Boston Road Animal Hospital, Springfield, MA, USA for the contribution of his time facilitating ERG procedures.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
See: http://omia.angis.org.au.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, Munnich A, Lyonnet S, Salomon R, Encha-Razavi F, Gubler MC, Boddaert N, de Lonlay P, Johnson CA, Vekemans M, Antignac C, Attie-Bitach T, 2007. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. American Journal of Human Genetics 80, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Smith BF, Martin DR, Foureman P, 2001. Molecular diagnosis of gangliosidosis: a model of inherited diseases in pure breeds In: August JR (Ed.), Consultations in Feline Internal Medicine, vol. 4 W.B. Saunders Company, Philadelphia, pp. 615–620. [Google Scholar]
- Barnett KC, 1965. Retinal atrophy. Veterinary Record 77, 1543–1560. [PubMed] [Google Scholar]
- Barnett KC, Curtis R, 1985. Autosomal dominant progressive retinal atrophy in Abyssinian cats. Journal of Heredity 76, 168–170. [DOI] [PubMed] [Google Scholar]
- Barthez PY, Rivier P, Begon D, 2003. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. Journal of Feline Medicine and Surgery 5, 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistner SI, Aguirre G, Shively JN, 1976. Hereditary corneal dystrophy in the Manx cat: a preliminary report. Investigative Ophthalmology 15, 15–26. [PubMed] [Google Scholar]
- Carlile JL, 1981. Feline retinal atrophy. Veterinary Record 108, 311. [DOI] [PubMed] [Google Scholar]
- Curtis R, Barnett KC, Leon A, 1987. An early-onset retinal dystrophy with dominant inheritance in the Abyssinian cat. Clinical and pathological findings. Investigative Ophthalmology and Visual Science 28, 131–139. [PubMed] [Google Scholar]
- Davis BW, Raudsepp T, Pearks Wilkerson AJ, Agarwala R, Schäffer AA, Houck M, Chowdhdary BP, Murphy WJ, 2009. A high-resolution cat radiation hybrid and integrated FISH mapping resource for phylogenomic studies across Felidae. Genomics 93, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle B, 2001. The new encyclopedia of the cat. DK Publishing, Inc., New York, NY. [Google Scholar]
- Fyfe JC, Kurzhals RL, 1998. Glycogen Storage Disease Type IV in Norwegian Forest Cats: Molecular Detection of Carriers. In: First International Feline Genetic Disease Conference, University of Pennsylvania. [Google Scholar]
- Giuliano EA, van der Woerdt A, 1999. Feline retinal degeneration: clinical experience and new findings (1994–1997). Journal of the American Animal Hospital Association 35, 511–514. [DOI] [PubMed] [Google Scholar]
- Glaze MB, 2005. Congenital and hereditary ocular abnormalities in cats. Clinical Techniques in Small Animal Practice 20, 74–82. [DOI] [PubMed] [Google Scholar]
- Gould DJ, Sargan DR, 2002. Autosomal dominant retinal dystrophy (Rdy) in Abyssinian cats: exclusion of PDE6G and ROM1 and likely exclusion of Rhodopsin as candidate genes. Animal Genetics 33, 436–440. [DOI] [PubMed] [Google Scholar]
- Hartl DL, Clark AG, 2006. Principles of Population Genetics, fourth ed Sinauer Associates Inc., Sunderland, MA. [Google Scholar]
- Helgren JA, 1997. Barron’s encyclopedia of cat breeds: a complete guide to the domestic cats of North America Barron’s Educational Series, Hauppauge, NY. [Google Scholar]
- Higgins AJ, Nicholas FW, 2008. The breeding of pedigree dogs: time for strong leadership. The Veterinary Journal 178, 157–158. [DOI] [PubMed] [Google Scholar]
- Hollander AID, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KEJ, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, Hoyng CB, Born L.I.V.d., Rohrschneider K, Cremers FPM, 2006. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. American Journal of Human Genetics 79, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JA, Vaegan Lei, B., Narfström KL, 2005. Electrophysiologic differentiation of homozygous and heterozygous Abyssinian-crossbred cats with late-onset hereditary retinal degeneration. American Journal of Veterinary Research 66, 1914–1921. [DOI] [PubMed] [Google Scholar]
- Kang Derwent JJ, Padnick-Silver L, McRipley M, Giuliano E, Linsenmeier RA, Narfström K, 2006. The electroretinogram components in Abyssinian cats with hereditary retinal degeneration. Investigative Ophthalmology and Visual Science 47, 3673–3682. [DOI] [PubMed] [Google Scholar]
- Katz ML, Coates JR, Cooper JJ, O’Brien DP, Jeong M, Narfström K, 2008. Retinal pathology in a canine model of late infantile neuronal ceroid lipofuscinosis. Investigative Ophthalmology and Visual Science 49, 2686–2695. [DOI] [PubMed] [Google Scholar]
- Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, Dollfus H, Beales PL, Badano JL, Katsanis N, 2008. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nature Genetics 40, 443–448. [DOI] [PubMed] [Google Scholar]
- Lipinski MJ, Froenicke L, Baysac KC, Billings NC, Leutenegger CM, Levy AM, Longeri M, Niini T, Ozpinar H, Slater MR, Pedersen NC, Lyons LA, 2008. The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics 91, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LA, Imes DL, Rah HC, Grahn RA, 2005. Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus). Animal Genetics 36, 119–126. [DOI] [PubMed] [Google Scholar]
- Martin DR, Krum BK, Varadarajan GS, Hathcock TL, Smith BF, Baker HJ, 2004. An inversion of 25 base pairs causes feline GM2 gangliosidosis variant. Experimental Neurology 187, 30–37. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond M, David VA, Lyons LA, Schäffer AA, Tomlin JF, Hutton MK, O’Brien SJ, 1999. A genetic linkage map of microsatellites in the domestic cat (Felis catus). Genomics 57, 9–23. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond M, David VA, Pflueger SM, Lindblad-Toh K, Wade CM, O’Brien SJ, Johnson WE, 2007a. Patterns of molecular genetic variation among cat breeds. Genomics 91, 1–11. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond M, David VA, Roelke ME, Chen ZQ, Menotti KA, Sun S, Schäffer AA, Tomlin JF, Agarwala R, O’Brien SJ, Murphy WJ, 2003. Second-generation integrated genetic linkage/radiation hybrid maps of the domestic cat (Felis catus). Journal of Heredity 94, 95–106. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond M, David VA, Schäffer AA, Stephens R, Wells D, Kumar-Singh R, O’Brien SJ, Narfström K, 2007b. Mutation in CEP290 discovered for cat model of human retinal degeneration. Journal of Heredity 98, 211–220. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond M, David VA, Schäffer AA, Tomlin JF, Eizirik E, Phillip C, Wells D, Pontius JU, Hannah SS, O’Brien SJ, 2009. An autosomal genetic linkage map of the domestic cat, Felis silvestris catus. Genomics 93, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotti-Raymond MA, David VA, Wachter LL, Butler JM, O’Brien SJ, 2005. An STR forensic typing system for genetic individualization of domestic cat (Felis catus) samples. Journal of Forensic Sciences 50, 1061–1070. [PubMed] [Google Scholar]
- Muldoon LL, Neuwelt EA, Pagel MA, Weiss DL, 1994. Characterization of the molecular defect in a feline model for type II GM2-gangliosidosis (Sandhoff disease). American Journal of Pathology 144, 1109–1118. [PMC free article] [PubMed] [Google Scholar]
- Murphy WJ, Davis B, David VA, Agarwala R, Schäffer AA, Pearks-Wilkerson AJ, Neelam B, O’Brien SJ, Menotti-Raymond M, 2007. A 1.5-Mb-resolution radiation hybrid map of the cat genome and comparative analysis with the canine and human genomes. Genomics 89, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfström K, 1999. Hereditary and congenital ocular disease in the cat. Journal of Feline Medicine and Surgery 1, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfström K, 1983. Hereditary progressive retinal atrophy in the Abyssinian cat. Journal of Heredity 74, 273–276. [DOI] [PubMed] [Google Scholar]
- Narfström K, 1985a. Progressive retinal atrophy in the Abyssinian cat. Clinical characteristics. Investigative Ophthalmology and Visual Science 26, 193–200. [PubMed] [Google Scholar]
- Narfström K, 1985b. Retinal degeneration in a strain of Abyssinian cats: a hereditary, clinical, electrophysiological and morphological study, Vol. No. 208 Linköping University Medical Dissertations, Linköping. [Google Scholar]
- Narfström K, Arden GB, Nilsson SE, 1989. Retinal sensitivity in hereditary retinal degeneration in Abyssinian cats: electrophysiological similarities between man and cat. British Journal of Ophthalmology 73, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfström K, David V, Jarret O, Beatty JA, Barrs VR, Wilkie AL, O’Brien SJ, Menotti-Raymond M, in press Retinal degeneration in the Abyssinian cat (rdAc); correlation between genotype and phenotype and rdAc allele frequency in two continents Veterinary Ophthalmology. [DOI] [PubMed] [Google Scholar]
- Narfström K, Ehinger B, Bruun A, 2001. Immunohistochemical studies of cone photoreceptors and cells of the inner retina in feline rod-cone degeneration. Veterinary Ophthalmology 4, 141–145. [DOI] [PubMed] [Google Scholar]
- Narfström K, Ekesten B, Rosolen SG, Spiess BM, Percicot CL, Ofri R, 2002. Guidelines for clinical electroretinography in the dog. Documenta Ophthalmologica 105, 83–92. [DOI] [PubMed] [Google Scholar]
- Narfström K, Nilsson SE, 1986. Progressive retinal atrophy in the Abyssinian cat. Electron microscopy. Investigative Ophthalmology and Visual Science 27, 1569–1576. [PubMed] [Google Scholar]
- Narfström K, Nilsson SE, 1989. Morphological findings during retinal development and maturation in hereditary rod-cone degeneration in Abyssinian cats. Experimental Eye Research 49, 611–628. [DOI] [PubMed] [Google Scholar]
- Narfström K, Wilen M, Andersson BE, 1988. Hereditary retinal degeneration in the Abyssinian cat: developmental studies using clinical electroretinography. Documenta Ophthalmologica 69, 111–118. [DOI] [PubMed] [Google Scholar]
- Narfström LK, Nilsson SE, 1983. Progressive retinal atrophy in the Abyssinian cat: an update. Veterinary Record 112, 525–526. [DOI] [PubMed] [Google Scholar]
- Pontius JU, Mullikin JC, Smith DR, Team AS, Lindblad-Toh K, Gnerre S, Clamp M, Chang J, Stephens R, Neelam B, Volfovsky N, Schäffer AA, Agarwala R, Narfström K, Murphy W, Giger U, Roca AL, Antunes A, Menotti-Raymond M, Yuhki N, Pecon-Slattery J, Johnson WE, Bourque G, Tesler G, O’Brien SJ, 2007. Initial sequence and comparative analysis of the cat genome. Genome Research 17, 1675–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius JU, O’Brien SJ, 2007. Genome Annotation Resource Fields – GARFIELD: A Genome Browser for Felis catus. Journal of Heredity 98, 386–389. [DOI] [PubMed] [Google Scholar]
- Rah H, Maggs DJ, Blankenship TN, Narfström K, Lyons LA, 2005. Early-onset, autosomal recessive, progressive retinal atrophy in Persian cats. Investigative Ophthalmology and Visual Science 46, 1742–1747. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F, 2006. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nature Genetics 38, 674–681. [DOI] [PubMed] [Google Scholar]
- Schmidt-Küntzel A, Eizirik E, O’Brien SJ, Menotti-Raymond M, 2005. Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci. Journal of Heredity 96, 289–301. [DOI] [PubMed] [Google Scholar]
- Taberlet P, Valentini A, Rezaei HR, Naderi S, Pompanon F, Negrini R, Ajmone-Marsan P, 2008. Are cattle, sheep, and goats endangered species? Molecular Ecology 17, 275–284. [DOI] [PubMed] [Google Scholar]
- Vaegan, Narfström K, 2008. Electroretinographic diagnosis of feline hereditary rod cone degeneration is most efficient when amax to scotopic Imax is the only measure used. Documenta Ophthalmologica 117, 1–12. [DOI] [PubMed] [Google Scholar]
- Vaegan, Narfström K, 2005. A(max) is the best a-wave measure for classifying Abyssinian cat rod/cone dystrophy. Documenta Ophthalmologica 111, 33–38. [DOI] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, Fazzi E, Signorini S, Louie CM, Bellacchio E, Related Disorders Study Group, I.J., Bertini E, Dallapiccola B, Gleeson JG, 2006. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nature Genetics 38, 623–625. [DOI] [PubMed] [Google Scholar]
- West-Hyde L, Buyukmihci N, 1982. Photoreceptor degeneration in a family of cats. Journal of the American Veterinary Medical Association 181, 243–247. [PubMed] [Google Scholar]


