Abstract
Zn–air batteries are under revival. They have large theoretical energy density and potentially very low manufacturing cost compared to the existing Li-ion technology. However, their full potential has not been fulfilled due to challenges associated with air cathodes and Zn anodes. In this minireview, we present the current status and technical hurdles of Zn–air batteries and discuss the possible direction of their future improvements. We show that in contrast to tremendous efforts on the design and development of efficient cathode electrocatalysts over recent years, the pursuit of stable and cyclable Zn anodes is equally important but receives far less attention than deserved. We therefore call for a shift of future research focus from cathode electrocatalysts to Zn anodes in order to make this century old technology a truly commercial reality.
Introduction
Our ever-growing energy demand has spurred the increasing exploration and utilization of renewable energy sources and the burgeoning development of energy storage devices.1,2 Li-ion batteries are the leading energy storage solution for a variety of portable electronics and widely regarded as the most viable options for electric vehicles (EVs) and grid-scale energy storage.3–6 Despite the great commercial success, current lithium-ion technology is still suffering from insufficient energy density (limited to be <350 W h kg–1 based on the intercalation chemistry),7 relatively high cost (currently ∼$150 kW–1 h–1) and potential safety risk. As possible alternatives, aqueous metal (Zn, Fe, Mg and Al)–air batteries have attracted increased interest over recent years.8–12 Zn–air batteries are particularly promising by virtue of their large theoretical energy density (1353 W h kg–1 excluding oxygen), low cost (currently <$100 kW–1 h–1, and potentially <$10 kW–1 h–1) and inherent safety.13–15 They are arguably the only technically and economically viable solution for fast-charging EVs in the future.16
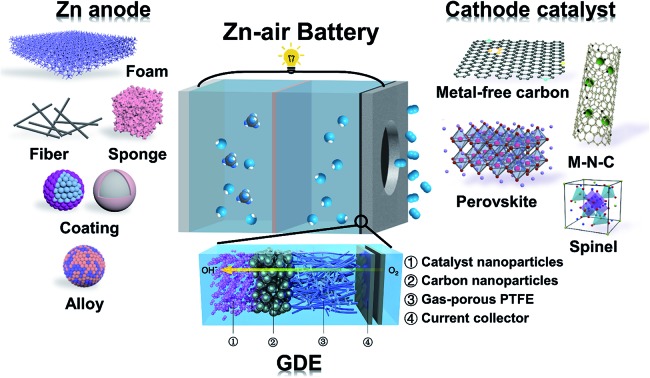
Fig. 1 schematically shows the basic configuration of a typical Zn–air battery. It consists of a porous air cathode and a Zn metal anode, separated using a membrane separator and filled with a concentrated alkaline electrolyte. During discharge, O2 from the surrounding atmosphere permeates the porous cathode and gets reduced on the electrocatalyst surface; in the meantime, the metallic Zn anode is oxidized to soluble zincate (Zn(OH)42–) ions. In the presence of a proper bifunctional oxygen electrocatalyst, the above reaction can be reversed with O2 evolving at the cathode and it can be released back to the atmosphere, and metallic Zn is plated at the anode. For many experiments in laboratory, O2 gas instead of air is directly fed to the cathode. Such batteries should be in fact called Zn–O2 batteries. We don't attempt to distinguish these two concepts here because the underlying chemistry is the same.
Fig. 1. Schematic configuration of Zn–air batteries including the GDE structure, different candidate materials for cathode electrocatalysts and different forms of Zn anode materials.
Zn–air batteries are a century old technology. The concept was first reported by Smee in 1840.17 In 1878, Maiche demonstrated functionable primary Zn–air batteries using a porous platinized carbon cathode.18 Commercial products of primary Zn–air batteries were introduced to the market in 1932.19 They typically have high energy density (200–500 W h kg–1) but very poor power output, and are widely used in medical and telecommunication applications such as hearing aids and pagers.20 Rechargeable Zn–air batteries were first commercialized by NantEnergy (formerly Fluidic Energy) in 2012, but were reported to have rather limited energy density (∼35 W h kg–1 as of 2017).21 Despite the early start, current Zn–air batteries are still far from fulfilling their full potential. This is because, on the one hand, both the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) at the cathode involve multiple proton-coupled electron transfers and are notoriously sluggish in nature, resulting in small current density and large electrode polarization; on the other hand, the corrosion and the dendrite growth of Zn metal upon recharge results in poor cyclability of most available Zn anodes.8,13,14,22 In order to address these challenges, tremendous research efforts have been recently invested on the development of efficient oxygen electrocatalysts.23–26 However, considerably less attention has been paid to the Zn side.27–29
In this minireview, we do not aim to provide a comprehensive overview on the current status of Zn–air batteries since many up-to-date accounts are already available in the literature. Instead, we plan to highlight major technical challenges that would have to be tackled with and resolved before Zn–air batteries are considered for large-scale industrial and commercial deployment. Through our discussions here, we call for a shift of research focus from cathode electrocatalysts to Zn anodes in order to expedite the future development of Zn–air batteries.
Air cathode
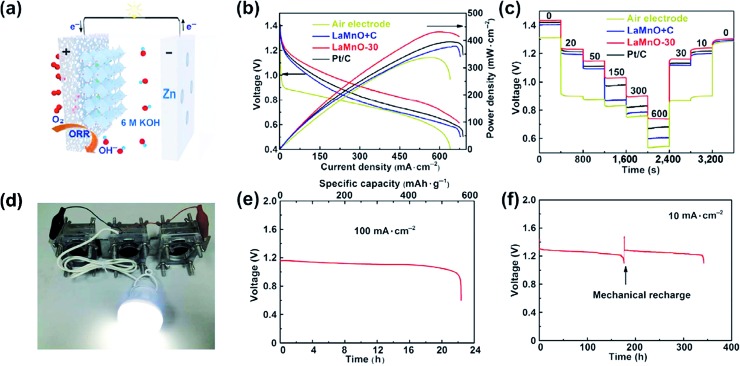
Air cathodes are conventionally the performance-limiting electrode of Zn–air batteries. They are constructed by uniformly loading appropriate electrocatalysts onto porous polytetrafluoroethylene (PTFE)-treated gas diffusion layers (GDLs). Good GDLs have balanced water repelling (hydrophobic) and water retaining (hydrophilic) properties.9,13 They allow the fast permeation of O2 gas, provide abundant gas–electrolyte–electrode triple-phase boundaries for the ORR to take place at a high rate, and can potentially sustain a large current density of up to >1 A cm–2. Electrocatalysts loaded on GDLs are the core component responsible for accelerating the ORR and OER and thereby determine the overall battery performance (e.g., power density, energy efficiency and cycle life). Pt along with its alloys is generally regarded as the benchmark material for the ORR,30 and so are IrO2 and RuO2 for the OER.31,32 Their applications in primary and rechargeable Zn–air batteries were assessed at the laboratory scale, but are unlikely to scale up any further owing to their prohibitive costs, which essentially goes against the low-cost aspect of Zn–air batteries and outweighs any potential performance gain. Conventional Zn–air batteries often use MnO2 as the cathode electrocatalyst.33 Its activity and stability, however, are not very satisfactory. This is the main reason behind the very poor power density of conventional Zn–air batteries. It is therefore very natural that recent Zn–air studies are predominantly focused on the search for better oxygen electrocatalysts. A large variety of non-precious-metal-based candidates have been explored and are now available as the necessary material basis for Zn–air battery application, ranging from metal-free carbonaceous materials (e.g., heteroatom (N, P, S, F and B)-doped carbons)34,35 and M–N–C type materials (e.g., Fe–N–C and Co–N–C, also popularly known as “single-atom catalysts”),36–41 to transition metal oxides such as spinels (e.g., Co3O4)42–44 and perovskites (e.g. La0.6Ca0.4CoO3)45,46 as well as transition metal hydroxides and sulfides.47,48 Even though they may not have intrinsic activities as high as those of Pt, one can suitably increase their areal loading and achieve an “apparent activity” comparable or even superior to that of Pt in concentrated alkaline solution. With these advances, the performances of primary Zn–air batteries now are no longer throttled by cathode electrocatalysts like before. Remarkable peak power density (up to >400 mW cm–2) has been attained at room temperature using non-precious-metal-based electrocatalysts.45,49 For instance, Chen and coworkers prepared La0.99MnO3.03/C nanocomposites via a rapid gel auto-combustion method.45 The resulting electrocatalyst exhibited an ORR activity comparable to that of Pt, and delivered a peak power density of 430 mW cm–2 and an energy density of 837 W h kgZn–1 when used as the cathode electrocatalyst for primary Zn–air batteries (Fig. 2).
Fig. 2. (a) Schematic configuration of the primary Zn–air battery; (b) polarization and power density curves of Zn–air batteries with different electrocatalysts; (c) discharge curves of Zn–air batteries with different electrocatalysts at various current densities; (d) a lamp (3 V; 1 W) powered using three series-connected Zn–air batteries; (e and f) discharge curve of the Zn–air battery with the LaMnO-30 cathode electrocatalyst at (e) 100 mA cm–2 and (f) 10 mA cm–2, the arrow in (f) indicates the point where the Zn anode and electrolyte were replaced, ref. 45, copyright© Tsinghua University Press and Springer-Verlag GmbH Germany 2017.
Zn–air batteries can be recharged either mechanically or electrically. Mechanically rechargeable Zn–air batteries are recharged by replenishing the Zn anode and electrolyte. They are replenishable primary batteries and only need ORR electrocatalysts as the cathode.13,14 The pursuit of electrically rechargeable Zn–air batteries poses more stringent requirements on the choice of cathode electrocatalysts for catalyzing the ORR during discharge and the OER during recharge. This is often realized using bifunctional oxygen electrocatalysts or the combination of different ORR and OER electrocatalysts as discussed in our previous studies.8,13 Unfortunately, due to large ORR and OER overpotentials, even rechargeable Zn–air batteries using state-of-the-art cathode electrocatalysts have a low round-trip energy efficiency of <65% under real working conditions. This represents an intrinsic drawback of rechargeable Zn–air batteries compared to conventional lithium-ion batteries (typically having an energy efficiency of 80–90%). Another challenge associated with rechargeable Zn–air batteries is the poor cycling stability of the air cathodes (and of course, the cyclability of Zn anodes as well, which would be discussed in the next section). The harsh electrochemical environment during the OER in concentrated alkaline solution is severely detrimental to the ORR-active component by corroding the carbon support and leaching transition metals.8 As a matter of fact, it remains to be seen if there is any truly cyclable air cathode under meaningful test conditions. The majority (if not all) of previous studies on rechargeable Zn–air batteries were tested with small current density (1–10 mA cm–2) and under very shallow cycling depths and adopted short charge/discharge periods from tens of seconds to a few minutes. Such experimental conditions would not effectively expose the real cycling performances of air cathodes. We recommend that in order to establish that Zn–air batteries can be electrically rechargeable in the future, cycling measurements should be carried out at a current density of at least 10–20 mA cm–2 and charge/discharge periods of >1 h (corresponding to an areal capacity of >10 mA h cm–2) for at least 10 cycles.
Zn electrode
Zn anodes are also subject to severe challenges.27–29 The parasitic reaction between Zn and the electrolyte leads to spontaneous H2 generation and electrode corrosion, lowering the active material utilization. In concentrated alkaline solution, the corrosion is further aggravated due to the lack of surface passivation on Zn anodes. Moreover, the non-uniform distribution of current density at the electrode surface inevitably triggers electrode shape change or dendrite growth upon recharge. These dendrites may penetrate the separator and reach the cathode side, leading to a short circuit and catastrophic failure of the battery. The above challenges associated with Zn anodes chronically impede the progress of Zn–air batteries and other Zn-related batteries including Zn–MnO2 and Zn–NiOOH. However, it has come to our attention that in stark contrast to the intense research efforts on cathode electrocatalysts, Zn anodes so far have attracted much less attention than deserved. Their problems are often diluted or completely ignored in previous studies by using excessive amounts of Zn metals and electrolytes.50,51 We note that the depth of discharge (DOD) in previous studies is mostly very low and even <1% for rechargeable batteries so as to maximize the impact of air cathodes. Such shallow discharges barely challenge Zn anodes. To be considered for practical applications at a large scale, ideal Zn electrodes should have high material utilization and be capable of sustaining large capacity and electrochemical reversibility under realistic conditions.
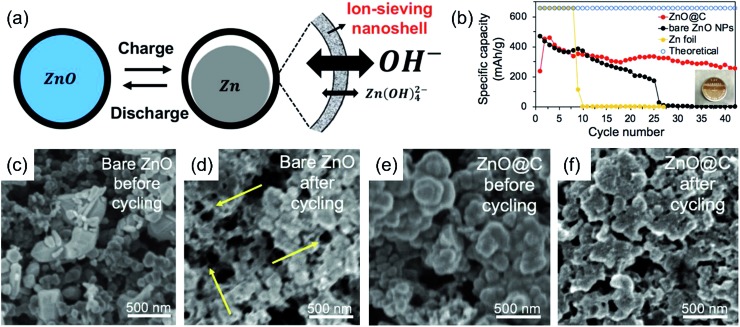
Several strategies are investigated to improve Zn anodes in the literature. High-surface-area Zn anodes are usually the preferable choice. Various forms of metallic Zn such as particles, fibers, sponges and foams have been explored as Zn anodes with enhanced electrochemical performances.52–55 However, they often come at the price of faster electrode corrosion due to the enlarged electrode–electrolyte contact areas.22 To tackle with the corrosion and dendrite problems, composition or surface modifications of Zn anodes are necessary. Studies show that Zn electrodes alloyed with certain metals (e.g., Pb, Cd, Bi, Sn and In)13,56,57 or modified with additives (e.g., silicates, surfactants and polymers)58–61 can suppress H2 evolution to different extents. The incorporation of alloying metals or additives may increase the conductivity, improve the current distribution and promote the formation of compact, thin Zn deposits. Furthermore, in order to increase the electrode reversibility, efforts have also been made to coat Zn anodes with surface trapping layers for better retention of the soluble zincate discharge product. Wrapping Zn or ZnO with thin protective layers (e.g., GO, TiNxOy and TiO2) to form core/shell structures has been pursued in the literature.28,29,62–65 For example, Liu and coworkers reported ion-sieving carbon nanoshell coated ZnO nanoparticles as the starting anode material of Zn–air batteries.65 The microporous carbon shell suppressed the dissolution of zincate ions but allowed smaller hydroxide ions to pass freely. They exhibited significantly improved performance compared to Zn foil and bare ZnO nanoparticles (Fig. 3). Despite this progress, the cycling stability of most Zn anodes, especially under deep discharge conditions, still falls short of expectations. There are few demonstrations about fully cyclable Zn anodes for >100 cycles.51
Fig. 3. (a) Schematic showing ZnO nanoparticles coated with an ion-sieving carbon nanoshell; (b) specific capacity and coulombic efficiency of bare ZnO, ZnO@C, and bulk Zn foil anodes during discharge; (c and d) SEM images of the bare ZnO anode (c) before and (d) after cycling; (e and f) SEM images of the ZnO@C anode (e) before and (f) after cycling, ref. 65, copyright© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
An alternative approach to improve the anode cyclability is to use non-alkaline electrolytes. Near-neutral aqueous electrolytes (e.g. ZnCl2–NH4Cl) were previously investigated for Zn–air batteries.66 They can slow down the corrosion of Zn metal and relieve the dendrite growth during Zn plating. However, Zn anodes are much less active in neutral electrolytes due to the surface passivation by resistive ZnO films. ORR and OER kinetics are also considerably slower in neutral pH than under alkaline conditions. Similarly, non-aqueous electrolytes (e.g. ionic liquids) are proposed and evaluated for Zn–air batteries.67,68 They are beneficial for the cyclability of the Zn electrodes, but may completely alter the reaction pathways of the ORR and OER at the air cathode (as we have learned from the case of Li–O2 batteries) and significantly reduce the reaction kinetics. Non-alkaline electrolytes are probably useful for other Zn batteries, but in our opinion, they are not very suitable for Zn–air batteries especially when high powder density is heavily demanded.
In a recent perspective, Schröder and coworkers propose seven descriptors for Zn anodes that they believe should be reported in future publications.50 These descriptors include the mass ratio of the active material and anode mixture (mAM/manode), ratio of capacity of active material and volume of electrolyte (QAM/VE), number of cycles (NC), averaged coulombic efficiency (ΦQ), average utilization of active material (XAM), average discharge capacity per mass of the anode mixture (qdis), and product of averaged discharge capacity per mass of the anode mixture and number of cycles (NC × qdis). Reporting these key descriptors would help us to rigorously assess the practical relevance of Zn anodes for battery applications and allow reliable cross-comparison of different studies. It is essential for the rapid transformation of Zn–air technology from mainly laboratory-scale science to large-scale applications. In the next ten years, we suggest that an ambiguous but achievable goal would be to develop Zn anodes with high active material utilization (>80%), capability to deeply discharge and charge (DOD > 50%) with reasonable cycle life (>500 cycles) and high coulombic efficiency (>80%). This goal would have to be achieved in practical cells instead of beaker cells with excess electrolyte.
Conclusion
In this minireview, we overviewed the current status and technical challenges of Zn–air batteries and discussed possible directions and solutions for future improvements. As a result of intense research activities over the past decade, the performance of Zn–air batteries is no longer limited by air cathodes and electrocatalysts. It is believed that a quick shift of research attention from air cathodes to Zn anodes would therefore greatly benefit this community and eventually realize the full potential of this century-old technology.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51972219) and the Priority Academic Program Development of Jiangsu Higher Education Institutions and Collaborative Innovation Center of Suzhou Nano Science and Technology.
References
- Larcher D., Tarascon J. M. Nat. Chem. 2015;7:19–29. doi: 10.1038/nchem.2085. [DOI] [PubMed] [Google Scholar]
- Chu S., Majumdar A. Nature. 2012;488:294–303. doi: 10.1038/nature11475. [DOI] [PubMed] [Google Scholar]
- Goodenough J. B., Park K. S. J. Am. Chem. Soc. 2013;135:1167–1176. doi: 10.1021/ja3091438. [DOI] [PubMed] [Google Scholar]
- Nitta N., Wu F. X., Lee J.-T., Yushin G. Mater. Today. 2015;18:252–264. [Google Scholar]
- Winter M., Barnett B., Xu K. Chem. Rev. 2018;118:11433–11456. doi: 10.1021/acs.chemrev.8b00422. [DOI] [PubMed] [Google Scholar]
- Li M., Lu J., Chen Z. W., Amine K. Adv. Mater. 2018;30:1800561. doi: 10.1002/adma.201800561. [DOI] [PubMed] [Google Scholar]
- Noorden R. V. Nature. 2014;507:26–28. [Google Scholar]
- Li Y. G., Lu J. ACS Energy Lett. 2017;2:1370–1377. [Google Scholar]
- Cai X. Y., Lai L. F., Lin J. Y., Shen Z. X. Mater. Horiz. 2017;4:945–976. [Google Scholar]
- McKerracher R. D., Leon C. P. d., Wills R. G. A., Shah A. A., Walsh F. C. ChemPlusChem. 2015;80:323–335. [Google Scholar]
- Li C.-S., Sun Y., Gebert F., Chou S.-L. Adv. Energy Mater. 2017;7:1700869. [Google Scholar]
- Ryu J., Park M., Cho J. Adv. Mater. 2019;31:1804784. doi: 10.1002/adma.201804784. [DOI] [PubMed] [Google Scholar]
- Li Y. G., Dai H. J. Chem. Soc. Rev. 2014;43:5257–5275. doi: 10.1039/c4cs00015c. [DOI] [PubMed] [Google Scholar]
- Fu J., Cano Z. P., Park M. G., Yu A., Fowler M., Chen Z. Adv. Mater. 2017;29:1604685. doi: 10.1002/adma.201604685. [DOI] [PubMed] [Google Scholar]
- Gu P., Zheng M. B., Zhao Q. X., Xiao X., Xue H. G., Pang H. J. Mater. Chem. A. 2017;5:7651–7666. [Google Scholar]
- The Zinc Air Battery and the Zinc Economy: A Virtuous Circle, http://www.meridian-int-res.com/Projects/The_Zinc_Air_Solution.pdf.
- Smee A. Philos. Mag. 1840;16:315–321. [Google Scholar]
- Maiche L., French Pat., 127069, 1878.
- Heise G. W., US Pat., 1899615, 1933.
- Linden D. and Reddy T. B., Handbooks of Batteries, McGraw-Hill, 2001. [Google Scholar]
- Batteries that breathe air, https://cen.acs.org/articles/95/i9/Batteries-breathe-air.html.
- Chen X. C., Zhou Z., Karahan H. E., Shao Q., Wei L., Chen Y. Small. 2018;14:1801929. doi: 10.1002/smll.201801929. [DOI] [PubMed] [Google Scholar]
- Pan J., Xu Y. Y., Yang H., Dong Z. H., Liu H. F., Xia B. Y. Adv. Sci. 2018;5:1700691. doi: 10.1002/advs.201700691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-F., Tang C., Zhang Q. Adv. Funct. Mater. 2018;28:1803329. [Google Scholar]
- Wang Y.-J., Fan H., Ignaszak A., Zhang L., Shao S., Wilkinson D. P., Zhang J. Chem. Eng. J. 2018;348:416–437. [Google Scholar]
- Huang Z.-F., Wang J., Peng Y., Jung C.-Y., Fisher A., Wang X. Adv. Energy Mater. 2017;7:1700544. [Google Scholar]
- Fang G. Z., Zhou J., Pan A. Q., Liang S. Q. ACS Energy Lett. 2018;3:2480–2501. [Google Scholar]
- Yi J., Liang P. C., Liu X. Y., Wu K., Liu Y. Y., Wang Y. G., Xia Y. Y., Zhang J. J. Energy Environ. Sci. 2018;11:3075–3095. [Google Scholar]
- Wu T.-H., Zhang Y., Althouse Z. D., Liu N. Mater. Today Nano. 2019;6:100032. [Google Scholar]
- Shao M., Chang Q. W., Dodelet J. P., Chenitz R. Chem. Rev. 2016;116:3594–3657. doi: 10.1021/acs.chemrev.5b00462. [DOI] [PubMed] [Google Scholar]
- Suen N. T., Hung S. F., Quan Q., Zhang N., Xu Y. J., Chen H. M. Chem. Soc. Rev. 2017;46:337–365. doi: 10.1039/c6cs00328a. [DOI] [PubMed] [Google Scholar]
- Reier T., Oezaslan M., Strasser P. ACS Catal. 2012;2:1765–1772. [Google Scholar]
- Mainar A. R., Colmenares L. C., Leonet O., Alcaide F., Iruin J. J., Weinberger S., Hacker V., Iruin E., Urdanpilleta I., Blazquez J. A. Electrochim. Acta. 2016;217:80–91. [Google Scholar]
- Lu Z. Y., Wang J., Huang S. F., Hou Y. L., Li Y. G., Zhao Y. P., Mu S. C., Zhang J. J., Zhao Y. F. Nano Energy. 2017;42:334–340. [Google Scholar]
- Wu M. G., Wang Y. Q., Wei Z. X., Wang L., Zhuo M., Zhang J. T., Han X. P., Ma J. M. J. Mater. Chem. A. 2018;6:10918–10925. [Google Scholar]
- Zeng M., Liu Y. L., Zhao F. P., Nie K. Q., Han N., Wang X. X., Huang W. J., Song X. N., Zhong J., Li Y. G. Adv. Funct. Mater. 2016;26:4397–4404. [Google Scholar]
- Fu G. T., Cui Z. M., Chen Y. F., Li Y. T., Tang Y. W., Goodenough J. B. Adv. Energy Mater. 2017;7:1601172. [Google Scholar]
- Su C.-Y., Cheng H., Li W., Liu Z.-Q., Li N., Hou Z., Bai F.-Q., Zhang H.-X., Ma T.-Y. Adv. Energy Mater. 2017;7:1602420. [Google Scholar]
- Amiinu I. S., Pu Z. H., Liu X. B., Owusu K. A., Monestel H. G. R., Boakye F. O., Zhang H. N., Mu S. C. Adv. Funct. Mater. 2017;27:1702300. [Google Scholar]
- Qiao Y. Y., Yuan P. F., Hu Y. F., Zhang J. N., Mu S. C., Zhou J. H., Li H., Xia H. C., He J., Xu Q. Adv. Mater. 2018;30:1804504. doi: 10.1002/adma.201804504. [DOI] [PubMed] [Google Scholar]
- Amiinu I. S., Liu X. B., Pu Z. H., Li W. Q., Li Q. D., Zhang J., Tang H. L., Zhang H. N., Mu S. C. Adv. Funct. Mater. 2018;28:1704638. [Google Scholar]
- Chen X., Liu B., Zhong C., Liu Z., Liu J., Ma L., Deng Y. D., Han X. P., Wu T. P., Hu W. B., Lu J. Adv. Energy Mater. 2017;7:1700779. [Google Scholar]
- Zhang Y. Q., Li M., Hua B., Wang Y., Sun Y. F., Luo J. L. Appl.Appl. Catal., BCatal., B. 2018;236:413–419. [Google Scholar]
- Liu Z. Q., Wang X. T., Ouyang T., Wang L., Zhong J. H., Ma T. Angew. Chem., Int. Ed. 2019;58:1–7. [Google Scholar]
- Yan Z. H., Sun H. M., Chen X., Fu X. R., Chen C. C., Cheng F. Y., Chen J. Nano Res. 2018;11:3282–3293. [Google Scholar]
- Bradley K., Giagloglou K., Hayden B. E., Jungius H., Vian C. Chem. Sci. 2019;10:4609–4617. doi: 10.1039/c9sc00412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Wu J. H., Liu Y. L., Cui X., Ding P., Deng J., Zha C. Y., Coy E., Li Y. G. Energy Storage Materials. 2019;16:24–30. [Google Scholar]
- Wang H. F., Tang C., Wang B., Li B. Q., Zhang Q. Adv. Mater. 2017;29:1702327. doi: 10.1002/adma.201702327. [DOI] [PubMed] [Google Scholar]
- Pei P. C., Ma Z., Wang K. L., Wang X. Z., Song M. C., Xu H. C. J. Power Sources. 2014;249:13–20. [Google Scholar]
- Stock D., Dongmo S., Janek J., Schröder D. ACS Energy Lett. 2019;4:1287–1300. [Google Scholar]
- Parker J. F., Ko J. S., Rolison D. R., Long J. W. Joule. 2018;2:2519–2527. [Google Scholar]
- Chamoun M., Hertzberg B. J., Gupta T., Davies D., Bhadra S., Tassell B. V., Erdonmez C., Steingart D. A. NPG Asia Mater. 2015;7:e178. [Google Scholar]
- Zhang X. G. J. Power Sources. 2006;163:591–597. [Google Scholar]
- Parker J. F., Nelson E. S., Wattendorf M. D., Chervin C. N., Long J. W., Rolison D. R. ACS Appl. Mater. Interfaces. 2014;6:19471–19476. doi: 10.1021/am505266c. [DOI] [PubMed] [Google Scholar]
- Joseph C. N. C., Parker F., Nelson E. S., Rolison D. R., Long J. W. Energy Environ. Sci. 2014;7:1117–1124. [Google Scholar]
- Lee C., Sathiyanarayanan K., Eom S., Yun M. S. J. Power Sources. 2006;160:1436–1441. [Google Scholar]
- Otani T., Fukunaka Y., Homma T. Electrochim. Acta. 2017;242:364–372. [Google Scholar]
- Tan Z. Y., Yang Z. H., Ni X., Chen H. Y., Wen R. J. Electrochim. Acta. 2012;85:554–559. [Google Scholar]
- Schmid M., Willert-Porada M. J. Power Sources. 2017;351:115–122. [Google Scholar]
- Gan W. G., Zhou D. B., Zhou L., Zhang Z. J., Zhao J. Electrochim. Acta. 2015;182:430–436. [Google Scholar]
- Stock D., Dongmo S., Walther F., Sann J., Janek J., Schroder D. ACS Appl. Mater. Interfaces. 2018;10:8640–8648. doi: 10.1021/acsami.7b18623. [DOI] [PubMed] [Google Scholar]
- Zhang Y. M., Wu Y. T., Ding H. R., Yan Y., Zhou Z. B., Ding Y., Liu N. Nano Energy. 2018;53:666–674. [Google Scholar]
- Zhao K. N., Wang C. X., Yu Y. H., Yan M. Y., Wei Q. L., He P., Dong Y. F., Zhang Z. Y., Wang X. D., Mai L. Q. Adv. Mater. Interfaces. 2018;5:1800848. [Google Scholar]
- Zhou Z. B., Zhang Y. M., Chen P., Wu Y. T., Yang H. C., Ding H. C., Zhang Y., Wang Z. Z., Du X., Liu N. Chem. Eng. Sci. 2019;194:142–147. [Google Scholar]
- Wu Y. T., Zhang Y. M., Ma Y., Howe J. D., Yang H. C., Chen P., Aluri S., Liu N. Adv. Energy Mater. 2018;8:1802470. [Google Scholar]
- Clark S., Mainar A. R., Iruin E., Colmenares L. C., Blázquez J. A., Tolchard J. R., Latz A., Horstmann B. J. Mater. Chem. A. 2019;7:11387–11399. [Google Scholar]
- Simons T. J., Torriero A. A. J., Howlett P. C., MacFarlane D. R., Forsyth M. Electrochem. Commun. 2012;18:119–122. [Google Scholar]
- Liu Z., Cui T., Pulletikurthi G., Lahiri A., Carstens T., Olschewski M., Endres F. Angew. Chem., Int. Ed. 2016;55:2889–2893. doi: 10.1002/anie.201509364. [DOI] [PubMed] [Google Scholar]