Abstract
Background
Type 2 diabetes mellitus (T2DM) is a chronic disorder that is characterised by insulin resistance and hyperglycaemia, which over time may give rise to vascular complications. Resveratrol is a plant‐derived nutritional supplement shown to have anti‐diabetic properties in many animal models. Less evidence is available on its safety and efficacy in the management of T2DM in humans.
Objectives
To assess the efficacy and safety of resveratrol formulations for adults with type 2 diabetes mellitus.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, PubMed, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and International Pharmaceutical Abstracts, as well as the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov. The date of the last search was December 2018 for all databases. No language restrictions were applied.
Selection criteria
All randomised controlled trials (RCTs) comparing effects of oral resveratrol (any dose or formulation, duration, or frequency of administration) with placebo, no treatment, other anti‐diabetic medications, or diet or exercise, in adults with a diagnosis of T2DM.
Data collection and analysis
Two review authors independently identified and included RCTs, assessed risk of bias, and extracted study‐level data. Study authors were contacted for any missing information or for clarification of reported data. We assessed studies for certainty of the evidence using the GRADE instrument.
Main results
We identified three RCTs with a total of 50 participants. Oral resveratrol not combined with other plant polyphenols was administered at 10 mg, 150 mg, or 1000 mg daily for a period ranging from four weeks to five weeks. The comparator intervention was placebo. Overall, all three included studies had low risk of bias. None of the three included studies reported long‐term, patient‐relevant outcomes such as all‐cause mortality, diabetes‐related complications, diabetes‐related mortality, health‐related quality of life, or socioeconomic effects. All three included studies reported that no adverse events were observed, indicating that no deaths occurred (very low‐quality evidence for adverse events, all‐cause mortality, and diabetes‐related mortality). Resveratrol versus placebo showed neutral effects for glycosylated haemoglobin A1c (HbA1c) levels (mean difference (MD) 0.1%, 95% confidence interval (CI) ‐0.02 to 0.2; P = 0.09; 2 studies; 31 participants; very low‐certainty evidence). Due to the short follow‐up period, HbA1c results have to be interpreted cautiously. Similarly, resveratrol versus placebo showed neutral effects for fasting blood glucose levels (MD 2 mg/dL, 95% CI ‐2 to 7; P = 0.29; 2 studies; 31 participants), and resveratrol versus placebo showed neutral effects for insulin resistance (MD ‐0.35, 95% CI ‐0.99 to 0.28; P = 0.27; 2 studies; 36 participants). We found eight ongoing RCTs with approximately 800 participants and two studies awaiting assessment, which, when published, could contribute to the findings of this review.
Authors' conclusions
Currently, research is insufficient for review authors to evaluate the safety and efficacy of resveratrol supplementation for treatment of adults with T2DM. The limited available research does not provide sufficient evidence to support any effect, beneficial or adverse, of four to five weeks of 10 mg to 1000 mg of resveratrol in adults with T2DM. Adequately powered RCTs reporting patient‐relevant outcomes with long‐term follow‐up periods are needed to further evaluate the efficacy and safety of resveratrol supplementation in the treatment of T2DM.
Plain language summary
Resveratrol for adults with type 2 diabetes mellitus
Review question
What are the effects of oral resveratrol supplementation compared with placebo, no treatment, anti‐diabetic medications, or diet or exercise, for the management of type 2 diabetes mellitus?
Background
Type 2 diabetes mellitus is a chronic disorder characterised by increased opposition of the cells in the body to circulating insulin in the blood, possibly leading to long‐term complications in organs such as kidneys, eyes, nerves, and heart. Resveratrol is a plant‐based nutritional supplement found mainly in grapes, peanuts, blueberries, and mulberries. Many animal studies have shown it to have anti‐diabetic properties. Few human studies have been conducted so far, and it is very important that current evidence from well‐performed studies is synthesised to inform the public and researchers.
Study characteristics
We identified three randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) with a total of 50 participants with type 2 diabetes. Among the included studies, the duration of resveratrol supplementation ranged from four to five weeks. Resveratrol as a capsule or Softgel was taken at 10 mg, 150 mg, or 1000 mg daily and was compared to placebo.
This evidence is up‐to‐date as of December 2018.
Key results
None of the included studies reported on important long‐term, patient‐relevant outcomes such as death from any cause, diabetes‐related death, diabetes‐related complications, health‐related quality of life, or impact on treatment costs. However, no side effects and no deaths were observed in these short‐term studies. No clear changes were observed for indicators of glucose management. We found eight ongoing studies with approximately 800 participants and two studies awaiting assessment, which, when published, could contribute to our findings.
Certainty of the evidence
The overall certainty of evidence from the included studies was very low, mainly because the number of participants and the number of studies reporting the outcomes were small . Also, the duration of the studies was very short.
Summary of findings
Summary of findings for the main comparison. Summary of findings for resveratrol plus oral anti‐diabetic drugs versus placebo plus oral anti‐diabetic drugs.
| Resveratrol compared with placebo for type 2 diabetes mellitus | ||||||
| Patient: adults with type 2 diabetes mellitus Setting: outpatients Intervention: resveratrol + OAD Comparison: placebo + OAD | ||||||
| Outcomes | Risk with placebo + OAD | Risk with resveratrol + OAD | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Diabetes‐related complications | Not reported | |||||
|
All‐cause mortality Follow‐up: 4 to 5 weeks |
See comment | See comment | See comment | 50 (3) |
Very lowa ⊕⊕⊝⊝ |
No adverse events were reported, indicating that no deaths occurred |
|
Diabetes‐related mortality Follow‐up: 4 to 5 weeks |
See comment | See comment | See comment | 50 (3) |
Very lowa ⊕⊕⊝⊝ |
No adverse events were reported, indicating that no deaths occurred |
| Health‐related quality of life | Not reported | |||||
|
Adverse events Follow‐up: 4 to 5 weeks |
See comment | See comment | See comment | 50 (3) |
Very lowa ⊕⊕⊝⊝ |
No adverse events were reported |
|
HbA1c (%) Follow‐up: 30 days and 5 weeks |
Mean HbA1c in the intervention group was 0.1% higher (0.02% lower to 0.2% higher) | ‐ | 31 (2) |
Very lowb ⊕⊕⊝⊝ |
Due to the short follow‐up period, HbA1c results have to be interpreted cautiously | |
| Socioeconomic effects | Not reported | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HbA1c: glycosylated haemoglobin A1c; OAD: oral anti‐diabetic drug(s). | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level because of indirectness (insufficient time frame) and by two levels because of serious imprecision (low median sample size and small number of studies) ‐ see Appendix 14. bDowngraded by one level because of indirectness (surrogate outcome and insufficient time frame) and by two levels because of serious imprecision (low median sample size and small number of studies, CI ranging between benefit and harm) ‐ see Appendix 14.
Background
Description of the condition
Type 2 diabetes mellitus (T2DM) is a chronic condition characterised by insulin resistance. There is an initial early compensatory increase in insulin levels, but at a later stage, beta‐cell failure leads to decreased insulin secretion (Martin‐Gronert 2012). More than 366 million people worldwide are diabetic, and this number is predicted to nearly double by 2030 (Moser 2012). Prevalence continues to increase at an alarming rate, especially in low‐ and middle‐income countries (Moser 2012). T2DM is the fourth leading cause of death in high‐income nations, with a two‐fold excess risk of mortality and a two‐ to four‐fold increase in the risk of cardiovascular disease (McKinlay 2000).
Macrovascular complications of T2DM include coronary artery disease, peripheral arterial disease, and stroke; microvascular complications include diabetic nephropathy and retinopathy (Hemmingsen 2013). Management of T2DM has traditionally been approached in a step‐wise manner, starting with 'lifestyle' modifications, exercise, and, if still uncontrolled, pharmacotherapy with oral anti‐diabetic drugs and insulin (Bird 2012; El‐Kaissi 2011). Although studies have shown that improved long‐term glycaemic control in people with T2DM, measured by glycosylated haemoglobin A1c (HbA1c), leads to a reduction in both microvascular and macrovascular complications (Moser 2012), a recent systematic review and meta‐analysis concluded that evidence is insufficient to show the influence of targeting intensive glycaemic control on macrovascular complications (Hemmingsen 2013). However, targeting intensive glycaemic control has been shown to possibly reduce the risk of microvascular complications (Hemmingsen 2013).
Anti‐diabetic drugs are not taken without adverse effects and complications. Hypoglycaemia is a major concern among individuals taking these agents, as they may directly cause or contribute to hypoglycaemia (Germino 2011).
Description of the intervention
Resveratrol is a natural polyphenolic anti‐oxidant synthesised by several plant species, including grapes, peanuts, mulberries, and blueberries (Burns 2002; Rimando 2004), and it is available in tablet form. Although resveratrol has therapeutic properties, its pharmacokinetic properties, reflected by its poor bioavailability due to rapid metabolisation, pose a major challenge to its use. To circumvent this issue, novel drug delivery systems to improve its stability and increase its bioavailability have been formulated (Pangeni 2014). Studies have reported the use of micro‐formulations and nano‐formulations for encapsulation of resveratrol, such as polymeric nanoparticles, liposomes, lipospheres, solid lipid nanoparticles, polymeric microspheres, cyclodextrins, calcium or zinc pectinate beads, and yeast cell carriers (Neves 2012). However, use of these novel delivery systems generally has not been attempted in humans.
Adverse effects of the intervention
A small number of studies have reported on the safety and tolerability of resveratrol in humans (Almeida 2009; Boocock 2007; Brown 2010; Chow 2010; La Porte 2010). Brown 2010 reported mild gastrointestinal adverse effects at higher doses of 2.5 g and 5 g, and recommended that safe doses should perhaps not exceed 1 g; however, no hypoglycaemia was reported. In addition, these mild adverse effects could not directly be attributed to resveratrol due to lack of a control group in the study design.
How the intervention might work
Numerous animal studies have demonstrated the beneficial effects of resveratrol in managing diabetes through various mechanisms such as preservation of beta‐cells (Hansen 2004; Palsamy 2010), improvement in the action of insulin and blood insulin concentrations (Palsamy 2009), and improvement in insulin sensitivity (Baur 2006; Lagouge 2006). Anti‐cytotoxic and anti‐oxidant effects of resveratrol have been proposed to play an important role in protecting the pancreas in diabetes. Studies have also revealed diminished levels of HbA1c in response to administration of resveratrol in diabetic rats, which is a reflection of a prolonged reduction in hyperglycaemia (Palsamy 2008; Palsamy 2010).
A few clinical studies have shown resveratrol to improve insulin sensitivity and HbA1c levels in persons with T2DM (Bhatt 2012; Brasnyo 2011). Based on these preliminary findings, it is possible that resveratrol could lead to clinical improvement in insulin sensitivity and glycaemic control, and to a decrease in diabetic complications.
Why it is important to do this review
Type 2 diabetes is a serious chronic disease that presents a huge healthcare and economic burden. A few clinical studies have been published on the efficacy of resveratrol in the management of T2DM (Bhatt 2012; Brasnyo 2011). Primary studies have indicated that resveratrol represents a potential treatment strategy for T2DM; therefore it is important to synthesise available evidence to explore its beneficial effects as well as any associated adverse effects. Previous systematic reviews have evaluated and reported the effects of resveratrol on cardio‐metabolic biomarkers in individuals with or without diabetes, and in individuals already undergoing pharmaceutical interventions for T2DM (Hausenblas 2015; Liu 2014; Zhu 2017). However, these systematic reviews did not assess the effects of resveratrol on important, clinically relevant outcomes such as mortality, diabetes‐related complications, and health‐related quality of life. Although surrogate outcomes may provide useful information, it is very important to assess clinically relevant outcomes that are directly meaningful to individuals living with diabetes, as this may help not only to decrease the burden of the disease but also to highlight potential research gaps that researchers need to address.
Objectives
To assess the efficacy and safety of resveratrol for adults with type 2 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
Adults (18 years or older) with diagnosis of T2DM. In the case of mixed studies, at least 80% of participants had to be adult persons with T2DM.
Diagnostic criteria for diabetes mellitus
To be consistent with changes over the years in the classification and diagnostic criteria for diabetes mellitus, we included studies with diagnoses established using the standard criteria valid at the time the study commenced (e.g. ADA 1999; ADA 2008; WHO 1998). If diagnostic criteria were not described, we used the study authors' definition of diabetes mellitus.
Types of interventions
We planned to investigate the following comparisons.
Intervention
Oral resveratrol (any dose, formulation, duration, or frequency of administration).
Comparator
Placebo.
Anti‐diabetic medications (oral anti‐diabetic drugs, herbal supplements, nutritional preparations, insulin).
Diet and/or exercise.
No treatment.
Concomitant interventions had to be the same in intervention and comparator groups to establish fair comparisons.
Types of outcome measures
Primary outcomes
All‐cause mortality
Diabetes‐related complications
Adverse events
Secondary outcomes
Diabetes‐related mortality
Health‐related quality of life
HbA1c
Fasting blood glucose
Insulin sensitivity
Socioeconomic effects
Method of outcome measurement
All‐cause mortality: defined as number of deaths due to any cause in the study population
Diabetes‐related complications: macrovascular complications defined as stroke, angina, myocardial infarction (heart attack), leg and foot pain; microvascular complications defined as retinopathy (vision loss or blindness), nephropathy (renal failure), cardiomyopathy (heart failure), neuropathy (diabetic foot)
Diabetes‐related mortality: defined as death from myocardial infarction, stroke, peripheral vascular disease, renal disease, hyperglycaemia, hypoglycaemia, sudden death
Adverse events: any adverse events, serious adverse events, and all other reported adverse events
HbA1c: measured as percentage or as mmol/mol
Fasting blood glucose: any measurement of fasting blood glucose
Insulin sensitivity: any measurement of homeostasis model assessment of insulin resistance (HOMA‐IR)
Health‐related quality of life: measured with any validated instrument
Socioeconomic effects: defined as the economic and social position of the individual in relation to others, as determined by education, income, and/or occupation
Timing of outcome measurement
All outcomes measured at any time after participants were randomised to intervention/comparator groups
Search methods for identification of studies
Electronic searches
We searched the following sources from the inception of each database to the specified date and placed no restrictions on the language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO) (December 2018; Issue 11 of 12).
MEDLINE (Ovid SP MEDLINE ALL 1946 to present) (12 April 2018).
Embase (Ovid SP 1974 to 3 December 2018) (12 April 2018).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO (12 April 2018).
International Pharmaceutical Abstracts (12 April 2018).
ClinicalTrials.gov (12 April 2018).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch/) (12 April 2018).
For detailed search strategies, see Appendix 1.
Searching other resources
We tried to identify other potentially eligible studies or ancillary publications by searching the reference lists of retrieved included studies, systematic reviews, meta‐analyses, and health technology assessment reports. We performed a citation search for key studies in Scopus and Web of Science. In addition, we contacted authors of included studies to obtain additional information on the retrieved studies and to establish whether we have missed further studies. We defined grey literature as records detected in ClinicalTrials.gov or on the WHO ICTRP.
Data collection and analysis
Selection of studies
Two review authors (MJ, ASM) independently screened the title, the abstract, or both, of every record we retrieved in the literature searches, to determine which studies we should assess further. We obtained the full text of all potentially relevant records. We resolved disagreements through consensus or by recourse to a third review author (AMAS). If we could not resolve a disagreement, we categorised the study as one of the Studies awaiting classification and contacted the study authors for clarification. We present an adapted PRISMA flow diagram to show the process of study selection (Liberati 2009). We listed all articles excluded after full‐text assessment in the Characteristics of excluded studies table and provided the reasons for exclusion.
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (MJ, NA) independently extracted key participant and intervention characteristics. We reported data on efficacy outcomes and adverse events using standardised data extraction sheets from the Cochrane Metabolic & Endocrine Disorders (CMED) Group. We resolved disagreements by discussion or, if required, by consultation with a third review author (AMAS) (for details, see Characteristics of included studies; Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 11; Appendix 12; Appendix 13; Appendix 14).
1. Overview of study populations.
| Study ID (study design) | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (N) | Randomised (N) | Analysed (N) | Finishing study (N) | Randomised finishing study (%) | Follow‐up |
|
Timmers 2016 (cross‐over RCT, 30‐day washout period) |
I: trans‐resveratrol | "We estimated 14 subjects were required to achieve 80% power, with an assumed treatment difference of 1.4 mg/kg fat‐free mass/min after 30 days and an assumed SD of 1.7 mg/kg fat‐free mass/min for a hyperinsulinaemic clamp. A dropout of 20% was taken into account, so 17 subjects were recruited" | 17 | 17a | 17 | 17 | 100 | 30 days |
| C: placebo | 17a | 17 | 17 | 100 | ||||
| Total: | 17 | 17 | 17 | 100 | ||||
|
Thazhath 2016 (cross‐over RCT, 5‐week washout period) |
I: resveratrol capsule | "A sample size of 14 subjects would have 80% power (at alpha = 0.05) to detect a 50% difference in the postprandial AUC for plasma total GLP‐1, which was the primary endpoint" | 16 | 14a | 14 | 14 | 100 | 5 weeks |
| C: placebo | 14a | 14 | 14 | 100 | ||||
| Total: | 14 | 14 | 14 | 100 | ||||
|
Brasnyo 2011 (parallel RCT) |
I: resveratrol capsule | — | 19 | 10 | 10 | 10 | 100 | 4 weeks |
| C: placebo | 9 | 9 | 9 | 100 | ||||
| Total: | 19 | 19 | 19 | 100 | ||||
| Grand total | All interventions | 41 | 41 | |||||
| All comparators | 40 | 40 | ||||||
| All interventions and comparators | 81 | 81 | ||||||
— denotes not reported.
aCross‐over study: each participant received both placebo and resveratrol; total number of participants was 50. AUC: area under the curve.
C: comparator, GLP‐1: glucagon‐like peptide‐1, I: intervention, RCT: randomised controlled trial.
We provided information including study identifier for potentially relevant ongoing trials in the Characteristics of ongoing studies table and in Appendix 7, 'Matrix of study endpoints (publications and trial documents)'. We tried to find the protocol for each included trial and reported in Appendix 7 primary, secondary and other outcomes in comparison with data in the publications.
We emailed all authors of included studies to ask whether they would be willing to answer questions regarding their studies. We have presented the results of this survey in Appendix 13. We thereafter sought relevant missing information on the study from the primary author(s) of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximised the information yield by collating all available data and used the most complete dataset aggregated across all known publications. We listed duplicate publications, companion documents, multiple reports of a primary study, and trial documents of included studies (such as trial registry information) as secondary references under the study identifier (ID) of the included study. Furthermore, we listed duplicate publications, companion documents, multiple reports of a study, and trial documents of excluded studies (such as trial registry information) as secondary references under the study ID of the excluded study.
Data from clinical trials registers
If data from included studies were available as study results in clinical trials registers, such as ClinicalTrials.gov or similar sources, we made full use of this information and extracted the data. If there was also a full publication of the study, we collated and critically appraised all available data. If an included study was marked as a completed study in a clinical trial register but no additional information (study results, publication, or both) was available, we added this study to the Characteristics of studies awaiting classification table.
Assessment of risk of bias in included studies
Two review authors (MJ, NA) independently assessed the risk of bias for each included study. We resolved disagreements by consensus, or by consultation with a third review author (AMAS). In cases of disagreement, we consulted the remainder of the review author team and made a judgement based on consensus. If adequate information was unavailable from the publication, study protocols, or other sources, we contacted the study authors for more detail to request missing data on 'Risk of bias' items.
We used the Cochrane 'Risk of bias' assessment tool (Higgins 2011a; Higgins 2017), and we assigned assessments of low, high, or unclear risk of bias (for details, see Appendix 2; Appendix 3). We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions according to the criteria and associated categorisations contained therein (Higgins 2017).
Summary assessment of risk of bias
We presented a 'Risk of bias' graph and a 'Risk of bias' summary figure.
We distinguished between self‐reported, investigator‐assessed, and adjudicated outcome measures.
We considered the following endpoints as self‐reported.
Adverse events.
Health‐related quality of life.
We considered the following endpoints as investigator‐assessed.
Adverse events.
All‐cause mortality.
Diabetes‐related complications.
Diabetes‐related mortality.
HbA1c.
Fasting blood glucose.
Insulin sensitivity.
Socioeconomic effects.
Risk of bias for a study across outcomes
Some 'Risk of bias' domains, such as selection bias (sequence generation and allocation sequence concealment), affect the risk of bias across all outcome measures in a study. In case of high risk of selection bias, we marked all endpoints investigated in the associated study as high risk. Otherwise, we did not perform a summary assessment of risk of bias across all outcomes for a study.
Risk of bias for an outcome within a study and across domains
We assessed the risk of bias for an outcome measure by including all entries relevant to that outcome (i.e. both study‐level entries and outcome‐specific entries). We considered low risk of bias to denote low risk of bias for all key domains, unclear risk to denote unclear risk of bias for one or more key domains, and high risk to denote high risk of bias for one or more key domains.
Risk of bias for an outcome across studies and across domains
These are the main summary assessments that we incorporated into our judgements about the certainty of evidence in the 'Summary of findings' tables. We defined outcomes as at low risk of bias when most information came from studies at low risk of bias, at unclear risk of bias when most information came from studies at low or unclear risk of bias, and at high risk of bias when a sufficient proportion of information came from studies at high risk of bias.
Measures of treatment effect
When at least two included studies were available for a comparison of a given outcome, we tried to express dichotomous data as a risk ratio (RR) or an odds ratio (OR) with 95% confidence interval (CI). For continuous outcomes measured on the same scale (e.g. fasting blood glucose in mg/dL), we estimated the intervention effect using the mean difference (MD) with 95% CI. For continuous outcomes that measured the same underlying concept (e.g. health‐related quality of life) but used different measurement scales, we planned to calculate the standardised mean difference (SMD). We planned to express time‐to‐event data as a hazard ratio (HR) with 95% CI.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over studies, cluster‐randomised trials, and multiple observations for the same outcome. If outcomes were presented at several time points, we included only data from the longest follow‐up period. For cross‐over studies, we included only data obtained before the cross‐over unless the intraclass correlation (ICC) coefficient was reported. We planned to include data from cluster‐randomised trials only if the intracluster correlation coefficient was reported (Higgins 2011b).
Dealing with missing data
If possible, we obtained missing data from the authors of included studies. We carefully evaluated important numerical data such as number screened, randomly assigned participants, and intention‐to‐treat (ITT) and as‐treated and per‐protocol populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up, withdrawals), and we critically appraised issues concerning missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report study results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1 (Deeks 2017). In view of the low power of this test, we considered the I² statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003).
Had we found heterogeneity, we would have attempted to determine possible reasons for this by examining individual study and subgroup characteristics.
Assessment of reporting biases
If we included 10 or more studies that investigated a particular outcome, we used funnel plots to assess small‐study effects. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to study size, poor methodological design (and hence bias of small studies), and publication bias (Sterne 2017). Therefore we interpreted the results carefully (Hart 2012; Sterne 2011).
Data synthesis
We planned to undertake (or display) a meta‐analysis only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure an answer that was clinically meaningful. Unless good evidence showed homogeneous effects across studies of different methodological quality, we primarily summarised low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration for the whole distribution of effects and planned to present a prediction interval (Borenstein 2017a; Borenstein 2017b; Higgins 2009). A prediction interval requires at least three studies to be calculated and specifies a predicted range for the true treatment effect in an individual study (Riley 2011). For rare events such as event rates below 1%, we planned to use the Peto odds ratio method, provided there was no substantial imbalance between intervention and comparator group sizes, and intervention effects were not exceptionally large. In addition, we performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to potentially introduce clinical heterogeneity, and we planned to carry out the following subgroup analyses with investigation of interactions (Altman 2003).
Sex (male/female).
Participants with or without comorbidities (participants with heart attack, stroke, peripheral vascular disease).
Treatment effect by co‐intervention (insulin, oral anti‐diabetic drugs).
Treatment dosage (low dose (< 250 mg/d), moderate dose (250 to 1000 mg/d), high dose (> 1000 mg/d)).
Treatment duration (short (< 6 months), medium (6 months to 2 years), long (> 2 years)).
Treatment formulation (pure resveratrol, timed release, other additives).
Different comparators (e.g. placebo, no additional treatment, oral hypoglycaemic medications, insulin).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting analysis to the following.
Published studies.
Very long (> 1 year) or large studies to establish the extent to which they dominate the results (< 100 participants vs ≥ 100 participants).
Use of the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry vs other), country.
Effect of risk of bias, as specified in the Assessment of risk of bias in included studies section.
We planned to test the robustness of results by repeating analysis using different measures of effect size (i.e. RR, OR, etc) and different statistical models (fixed‐effect and random‐effects models).
Certainty of the evidence
We presented the overall certainty of evidence for each outcome specified below, according to the GRADE approach, which took into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and to external validity (directness of results). Two review authors (MJ, AMAS) independently rated the certainty of evidence for each outcome.
We included Appendix 14 entitled 'Checklist to aid consistency and reproducibility of GRADE assessments', to help with standardisation of the 'Summary of findings' tables (Meader 2014). Alternatively, we planned to use the GRADEpro Guideline Development Tool (GDT) software and would have presented evidence profile tables as an appendix (GRADEproGDT 2015). We presented results for outcomes as described in the Types of outcome measures section. When meta‐analysis was not possible, we presented the results in a narrative format in the 'Summary of findings' table. We justified all decisions to downgrade the certainty of evidence by using footnotes, and we made comments to aid the reader's understanding of the Cochrane Review when necessary.
'Summary of findings' table
We presented a summary of evidence in Table 1. This provides key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, numbers of participants, and studies addressing each important outcome, and a rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017), using Review Manager (RevMan 5.3) table editor (RevMan 2014).
The intervention presented in the 'Summary of findings' table was oral resveratrol, and the comparator was placebo.
We reported the following outcomes, listed according to priority.
Diabetes‐related complications.
All‐cause mortality.
Diabetes‐related mortality.
Health‐related quality of life.
Adverse events.
HbA1c.
Socioeconomic effects.
Results
Description of studies
For a detailed description of studies, see the Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies sections.
Results of the search
Our comprehensive literature searches conducted until December 2018 initially identified 832 records. After de‐duplication, we included 762 abstracts for initial title/abstract screening. After title/abstract screening, we excluded 702 records that clearly were not relevant to our review question. Full texts and records were retrieved for the rest of the 60 records. After full‐text screening, we excluded 26 studies and gave the reasons for exclusion in the Excluded studies section. Ten of the 60 full‐text articles/records could not be included for reasons of incomparability (due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol ‐ Bashmakov 2014; Bhatt 2013; Bo 2018; Goh 2014; Imamura 2017; Khodabandehloo 2018; Movahed 2014; Sattarinezhad 2018; Seyyedebrahimi 2018; Javid 2017).
Thirteen studies (18 records) met the review inclusion criteria. Eight of the 13 included studies were ongoing clinical trials (CTRI/2017/04/008384; IRCT201411112394N14; IRCT201601022394N19; IRCT20171118037528N1; NCT01158417; NCT01881347; NCT02549924; SLCTR/2018/019). Two of the 13 included studies are awaiting classification (ACTRN12614000891628; Verges 2014). Finally, we included three clinical studies (Brasnyó 2011; Thazhath 2016; Timmers 2016). All three included studies were published in English. The PRISMA study flow diagram (Figure 1) depicts the study selection process.
1.
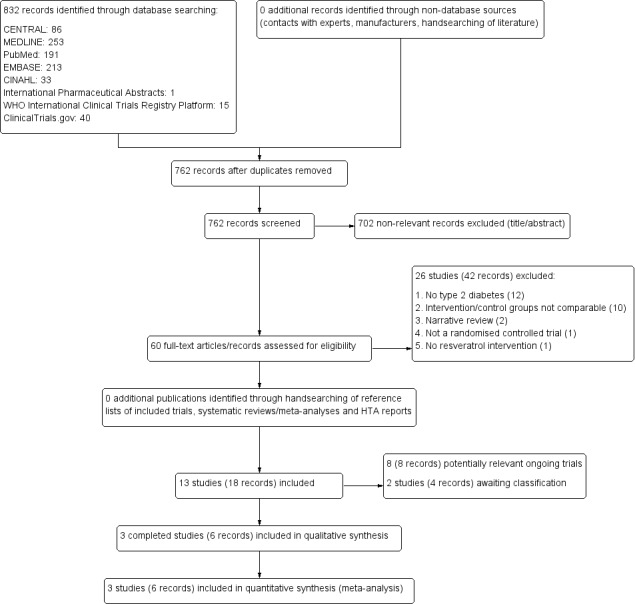
Study flow diagram.
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 9; and Table 2). The following is a succinct overview.
Source of data
Most data from included studies presented in this review were obtained from published full‐text articles. We made an attempt to contact the study authors of all included studies to obtain any unpublished data on relevant outcomes and to request clarification of methodological issues (Brasnyó 2011; Thazhath 2016; Timmers 2016). We received responses from authors of two studies (Thazhath 2016; Timmers 2016). We obtained study characteristics for ongoing clinical trials from clinical trials registers. We also made an attempt to contact authors of ongoing trials to obtain unpublished data if available. However, we did not receive a response from these authors to date.
Comparisons
We planned to include the following six different comparisons for this review.
Resveratrol vs placebo.
Resveratrol vs anti‐diabetic medications.
Resveratrol vs diet.
Resveratrol vs exercise.
Resveratrol vs diet and exercise.
Resveratrol vs no treatment.
All three included published studies compared the effect of resveratrol treatment versus placebo in participants with T2DM.
Overview of study populations
A total of 50 participants with T2DM were included in the three included studies of this review. The total number of participants randomised to the resveratrol treatment group was 41, and the total number of participants randomised to the placebo group was 40 (due to two studies with a cross‐over design, these numbers appear greater than the actual numbers of participants). The percentage of participants finishing the study in the intervention and comparator groups was 100%. Individual sample size in the included studies of this review ranged from 14 to 19 participants.
Study design
All included studies were randomised controlled studies; two had a cross‐over study design (Thazhath 2016; Timmers 2016), and one had a parallel design (Brasnyó 2011). All studies were single‐centre studies. All studies used placebo as comparison. In terms of blinding, all three studies were double‐blinded and were published between 2011 and 2016. Study duration ranged from four weeks to five weeks. One study reported a run‐in period of four weeks (Brasnyó 2011). None of the studies reported a post‐intervention follow‐up period. Also, none of the included studies was terminated before the planned study end.
Settings
The three included studies in this review were conducted in different countries: Australia (Thazhath 2016), Hungary (Brasnyó 2011), and The Netherlands (Timmers 2016). For other details, see Characteristics of included studies. Funding sources for the included studies were as follows: two studies were funded by research grants from national funding agencies (Thazhath 2016; Timmers 2016), and one study reported that investigators did not receive any funding (Brasnyó 2011).
Participants
A total of 50 participants with a diagnosis of T2DM were included in this review. All included participants were adults with a mean age ranging between 53 and 67 years. In two studies, all participants were males (Brasnyó 2011; Timmers 2016), and in one study, 27% of participants were females (Thazhath 2016). The mean duration of T2DM in study participants ranged from five years to seven years. Brasnyó 2011 did not report the duration of diabetes. Two studies included participants from high‐income countries (Thazhath 2016; Timmers 2016), and one study included participants from a middle‐income country (Brasnyó 2011). All included studies recruited participants who were white. Mean HbA1c levels at baseline ranged from 6.4% to 7.6%. Mean baseline body mass index (BMI) ranged from 27.7 kg/m² to 30.5 kg/m². One study did not report baseline BMI (Brasnyó 2011). One study reported that there were no comorbidities among study participants (Thazhath 2016), whereas another study did not report any details on comorbidities (Timmers 2016). One study reported comorbidities such as Ischaemic heart disease, peripheral arterial disease, hypercholesterolaemia, angina pectoris, diabetic neuropathy, and diabetic nephropathy (Brasnyó 2011). Among the three included studies, one study included participants who were on an anti‐diabetic medication or diet (Timmers 2016); most study participants were on oral hypoglycaemic agents (sulphonylurea derivatives and/or metformin), and some were on diet alone (6%). Another study included participants who were treated by diet alone (Thazhath 2016). In the third study, participants were treated with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blocker medication (Brasnyó 2011). The inclusion and exclusion criteria for the included studies are outlined in the Characteristics of included studies section.
Diagnosis
All participants in the included studies had T2DM. Two studies confirmed the diagnosis against WHO diagnostic criteria (Brasnyó 2011; Thazhath 2016). The third study relied on third party diagnosis of T2DM and did not report a reference to standard diagnostic criteria for T2DM (Timmers 2016).
Interventions
All included studies reported using oral resveratrol in capsule or tablet form not combined with other plant polyphenols. Two studies used trans‐resveratrol (Brasnyó 2011; Timmers 2016). The daily dose of resveratrol varied widely between the included studies: 10 mg/d (Brasnyó 2011), 150 mg/d (Timmers 2016), and 1000 mg/d (Thazhath 2016). All included studies reported use of placebo as a comparator. Placebo ingredients were reported by two studies as microcrystalline cellulose (Brasnyó 2011; Thazhath 2016). One study did not report the ingredient for the placebo (Timmers 2016). Another study reported using matching placebo (Brasnyó 2011). The duration of the intervention ranged from 4 weeks in Brasnyó 2011 and Timmers 2016 to 5 weeks in Thazhath 2016.
Outcomes
Two studies explicitly reported primary and secondary outcome measures in their publications and trial documents (Thazhath 2016; Timmers 2016). Primary outcomes reported in these studies were insulin sensitivity, plasma total glucagon‐peptide 1 (GLP‐1) concentrations, and insulin resistance/sensitivity. For one study, we were unable to find a trial protocol (Brasnyó 2011). We contacted study authors but did not receive a response. For a summary of all outcome measures reported in each of the studies included in this review, see Appendix 7.
None of the included studies reported on diabetes‐related complications, health‐related quality of life, or socioeconomic effects. Because no adverse events were observed, we concluded that there were no diabetes‐related deaths or deaths from any cause. However, due to short follow‐up, no study was powered to investigate mortality.
Excluded studies
After thorough full‐text screening, we excluded 26 studies (Bashmakov 2014; Bhatt 2013; Bo 2018; Elliott 2009; Fujitaka 2011; Goh 2014; Imamura 2017; Javid 2017; Khazaei 2014; Khodabandehloo 2018; Kjaer 2014; Mendez‐Del 2014; Movahed 2014; NCT00937222; NCT01038089; NCT01150955; NCT01375959; NCT01714102; NCT01997762; NCT02129595; NCT02216552; NCT02219906; NCT02565979; Sattarinezhad 2018; Seyyedebrahimi 2018; Tomé‐Carneiro 2012). The main reasons for exclusion were incomparability of interventions and controls because a combination of a rather unspecified mixture of oral anti‐diabetic agents was used with/without resveratrol (Bashmakov 2014; Bhatt 2013; Bo 2018; Goh 2014; Imamura 2017; Javid 2017; Khodabandehloo 2018; Movahed 2014; Sattarinezhad 2018; Seyyedebrahimi 2018), or because study participants did not have T2DM (see Characteristics of excluded studies).
Risk of bias in included studies
Overall, all three included studies were adjudicated to be at low risk of bias (Brasnyó 2011; Thazhath 2016; Timmers 2016). For details on risk of bias of the included studies, see Characteristics of included studies. For an overview of review authors' judgements about each risk of bias item for individual studies and across all studies, see Figure 2 and Figure 3.
2.
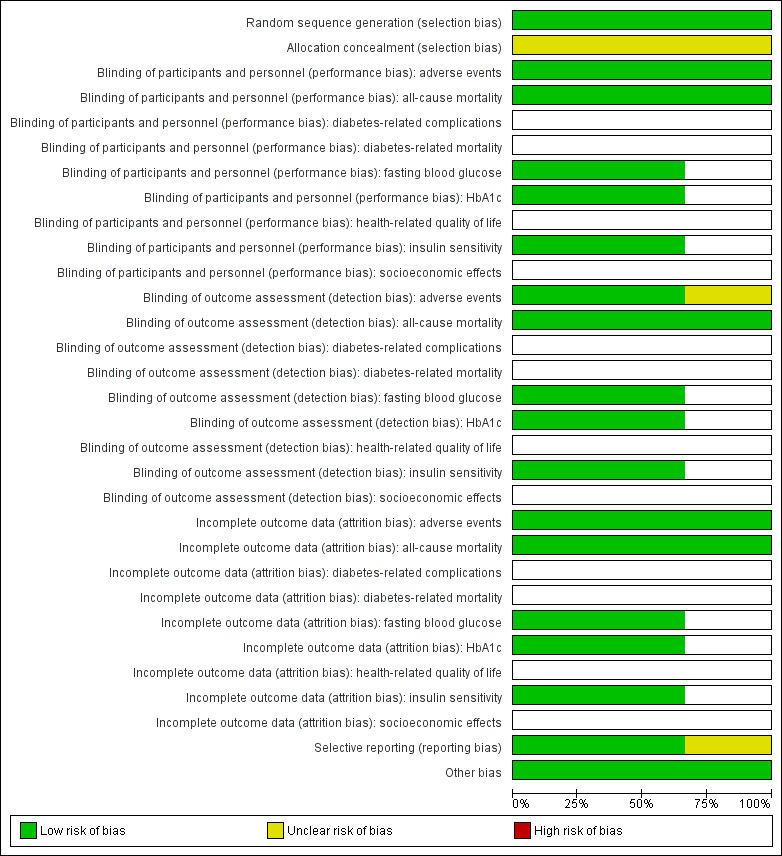
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (blank cells indicate that the particular outcome was not measured in some studies).
3.
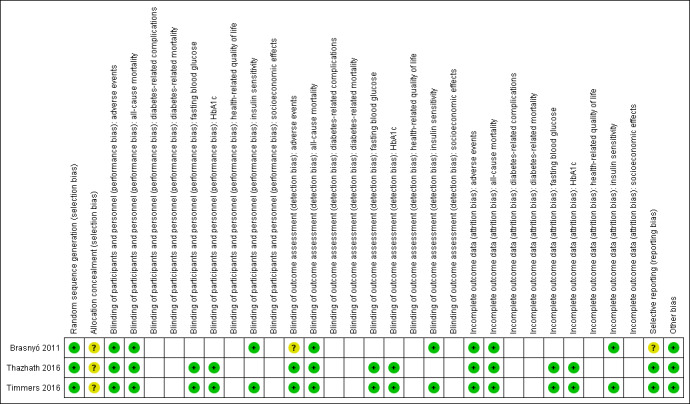
Risk of bias summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the study did not measure that particular outcome).
Allocation
With regard to random sequence generation, we judged all studies to be at low risk of bias. None of the three included studies provided information on allocation concealment; studies were assessed as having unclear risk of bias for this selection bias domain.
Blinding
With the exception of adverse events in Brasnyó 2011, we judged all other outcome to be at low risk of performance and detection bias.
Incomplete outcome data
We judged all reported outcome measures for the three included studies to be at low risk of attrition bias.
Selective reporting
Two included studies had study documents in the clinical trial registry (Thazhath 2016; Timmers 2016); outcomes reported in the trial documents matched outcomes reported in the results section of the publication and were extractable. Thus, we judged both studies to be at low risk of reporting bias. One study did not have trial documents in the clinical trial registry (Brasnyó 2011), and we assigned this study unclear risk of reporting bias.
Other potential sources of bias
Two of the included studies were of cross‐over design (Thazhath 2016; Timmers 2016). Because T2DM is a stable condition over short time periods and because an adequate washout period was reported, we attributed low risk of bias in this domain. In addition, we found no evidence for other potential sources of bias in any of the included studies.
Effects of interventions
See: Table 1
Baseline characteristics
For details of baseline characteristics, see Appendix 5 and Appendix 6.
Resveratrol versus placebo
Primary outcomes
All‐cause mortality
No study was powered to investigate all‐cause mortality (Brasnyó 2011; Timmers 2016; Thazhath 2016). However, all study authors reported that no adverse events were observed, indicating that no deaths occurred (very low‐certainty evidence).
Diabetes‐related complications
None of the included studies reported this outcome.
Adverse events
All included studies reported that no adverse events were observed (very low‐certainty evidence).
Secondary outcomes
Diabetes‐related mortality
No study was powered to investigate all‐cause mortality. However, study authors reported that no adverse events were observed, indicating that no deaths occurred (very low‐certainty evidence).
Health‐related quality of life
None of the included studies reported this outcome.
HbA1c
Two studies with a cross‐over design reported data on HbA1c for 31 participants with T2DM (Thazhath 2016; Timmers 2016). Using individual participant data provided by study authors, we controlled for intraclass correlations and then calculated mean differences between the two intervention groups using a mixed analysis of variance (ANOVA) model. Resveratrol versus placebo showed neutral effects for HbA1c levels (mean difference (MD) 0.1%, 95% confidence interval (CI) ‐0.02 to 0.2); P = 0.09; 2 studies; 31 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.
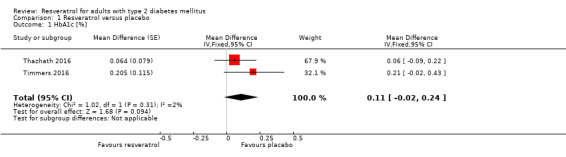
Comparison 1 Resveratrol versus placebo, Outcome 1 HbA1c [%].
Fasting blood glucose
Two cross‐over studies reported data on fasting blood glucose (FBG) levels for 31 participants with T2DM (Thazhath 2016; Timmers 2016). Using individual participant data provided by study authors, we controlled for intraclass correlations and then calculated mean differences between the two intervention groups using a mixed ANOVA model. Resveratrol versus placebo showed neutral effects for FBG levels (MD 2 mg/dL, 95% CI ‐2 to 7; P = 0.29; 2 studies; 31 participants; Analysis 1.2).
1.2. Analysis.
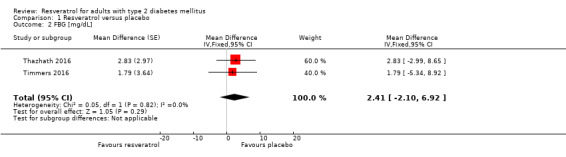
Comparison 1 Resveratrol versus placebo, Outcome 2 FBG [mg/dL].
Insulin sensitivity
Two studies reported data on insulin sensitivity as measured by HOMA‐IR for 36 participants with type 2 diabetes mellitus (Brasnyó 2011; Timmers 2016). We obtained individual participant data from study authors for one cross‐over RCT (Timmers 2016), controlled for intraclass correlations, and calculated mean differences between the two intervention groups using a mixed ANOVA model. The second study used a parallel design (Brasnyó 2011). Resveratrol versus placebo showed neutral effects for insulin resistance (MD ‐0.35, 95% CI ‐0.99 to 0.28; P = 0.27; 2 studies; 36 participants; Analysis 1.3).
1.3. Analysis.
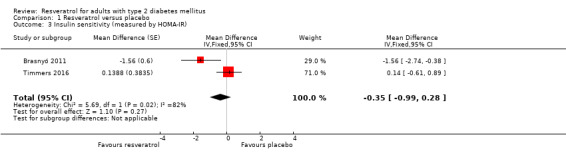
Comparison 1 Resveratrol versus placebo, Outcome 3 Insulin sensitivity (measured by HOMA‐IR).
Socioeconomic effects
None of the included studies reported this outcome.
Subgroup analyses
We did not perform subgroup analyses because we found insufficient trials to estimate effects in various subgroups.
Sensitivity analyses
We did not perform sensitivity analyses due to insufficient studies reporting our primary outcome.
Assessment of reporting bias
We did not generate funnel plots due to the limited number of included studies (N = 3).
Ongoing trials
We found eight ongoing RCTs that fit the inclusion criteria of this review (CTRI/2017/04/008384; IRCT201411112394N14; IRCT201601022394N19; IRCT20171118037528N1; NCT01158417; NCT01881347; NCT02549924; SLCTR/2018/019). Out of these eight ongoing trials, seven are of parallel design (CTRI/2017/04/008384; IRCT201411112394N14; IRCT201601022394N19; IRCT20171118037528N1; NCT01158417; NCT02549924; SLCTR/2018/019), and one is a cross‐over trial (NCT01881347). The approximate number of participants in these eight ongoing trials is 800.
Furthermore, we identified two studies that are awaiting assessment, which could possibly contribute to the findings of our systematic review (ACTRN12614000891628; Verges 2014).
Discussion
Summary of main results
In this systematic review, we sought to identify the efficacy and safety of resveratrol in the treatment of adults with type 2 diabetes mellitus (T2DM). Using a comprehensive search strategy and an independent duplicate study selection process, we identified three randomised controlled trials (RCTs) (one parallel‐design RCT and two cross‐over design RCTs) with 50 adult participants with T2DM that fit our inclusion criteria. All three studies administered different doses of oral resveratrol as their intervention, and all used placebo as a comparator. None of our patient‐relevant outcomes such as all‐cause mortality, diabetes‐related complications, diabetes‐related mortality, socioeconomic effects, and health‐related quality of life were reported by the included studies. Instead, surrogate outcomes such as glycosylated haemoglobin A1c (HbA1c), fasting blood glucose (FBG), and insulin sensitivity were reported. Results for the outcomes HbA1c, FBG, and insulin resistance showed neutral effects.
Based on current limited evidence, resveratrol seems to be well tolerated, with none of the study participants reporting adverse events. Although this systematic review offers up‐to‐date evidence on the efficacy of resveratrol for adults with T2DM, this evidence is very limited, as it is supported by only a few small RCTs reporting surrogate outcomes. Another limitation is that the follow‐up periods in these studies were very short (follow‐up periods ranged from four weeks to five weeks), and the long‐term effects of resveratrol remain unclear. Thus, evidence is currently insufficient to support the use of resveratrol supplementation in adults for treatment of T2DM.
Overall completeness and applicability of evidence
The main objective of this systematic review was to investigate the safety and efficacy of resveratrol in adults with T2DM. The three studies included in this review reported a few of our predefined primary and secondary outcome measures. Some of our patient‐important primary outcomes such as all‐cause mortality, diabetes‐related mortality, and diabetes‐related complications were not reported by any of the included studies. The one‐month measurements of HbA1c reported in the included studies were too short for this outcome to be considered reliable. Also, variation in the dose of resveratrol reported in the included studies (10 mg/d to 1000 mg/d) was too wide to allow any meaningful conclusions regarding the effective dose of resveratrol. Lack of studies reporting outcomes, use of different doses and varying duration of intervention among studies, and lack of long‐term follow‐up made comparisons among the included studies very difficult. The body of evidence on resveratrol for treatment of adults with T2DM is very limited. With limited evidence available from a few small studies, resveratrol appears to be generally safe with the potential for beneficial effects on insulin resistance in people with T2DM. But no firm conclusions can be reached. Future studies with longer follow‐up periods are needed to investigate the impact of resveratrol on T2DM.
Quality of the evidence
The certainty of evidence was adjudicated according to the GRADE approach for all key patient‐important outcomes. The seven patient‐important outcomes for which we evaluated the strength of evidence were adverse events, all‐cause mortality, diabetes‐related mortality, diabetes‐related complications, HbA1c, health‐related quality of life, and socioeconomic effects. Due to the small number of studies reporting, small sample sizes, and short‐term follow‐up, we judged all reported outcomes to provide very low‐certainty evidence. Thus we can place only low confidence in our effect estimates. Funnel plot asymmetry was not tested as included studies were too few.
Potential biases in the review process
We followed the methods listed in the protocol and made no post‐hoc decisions. We made an attempt to contact all authors of included studies for specific information regarding available outcome data. Only a few study authors responded, and this could have limited the availability and analysis of missing outcomes from these studies. Although we did a comprehensive database search for this review without limitations on language of publication, unpublished studies (English and non‐English) could have been missed, leading to the possibility of publication and language bias.
Agreements and disagreements with other studies or reviews
Three systematic reviews have reported the effects of resveratrol on individuals with T2DM (Hausenblas 2015; Liu 2014; Zhu 2017). The first systematic review published the effects of resveratrol as an adjunct treatment to pharmaceutical interventions for T2DM on cardio‐metabolic biomarkers (Hausenblas 2015). The second and the third systematic reviews published the effects of resveratrol in participants with and without T2DM on glucose control and insulin sensitivity (Liu 2014; Zhu 2017). These three systematic reviews reported positive effects of resveratrol on some but not all cardio‐metabolic markers, and they did not assess the effects of resveratrol on key patient‐important outcomes such as all‐cause mortality, diabetes‐related mortality, diabetes‐related complications, and health‐related quality of life that we have addressed in our review. Although surrogate outcomes provide useful information, it is very important to investigate patient‐important endpoints that are directly meaningful to individuals with T2DM. It is important to note that in these three systematic reviews, the review authors included a broad mixture of interventions (resveratrol plus various additional compounds), making it impossible to reliably delineate the effects of resveratrol only.
Authors' conclusions
Implications for practice.
This systematic review provides limited evidence that orally administered resveratrol in Softgel or capsule form at a dose of 10 mg/d to 1000 mg/d (not combined with other plant polyphenols) for a period of four to five weeks may be safe in individuals with type 2 diabetes mellitus (T2DM) compared to placebo, but no firm conclusions on the efficacy of resveratrol could be drawn due to a dearth of studies reporting these outcomes. Although many studies using animal models have shown promising results of resveratrol in improving insulin sensitivity, insulin secretion, and blood glucose levels, there is not enough evidence yet in the scientific literature to justify supplementation of resveratrol in humans for treatment of T2DM. Lack of scientific evidence does not necessarily reflect harm, but currently, many over‐the‐counter products containing resveratrol claim a beneficial effect of resveratrol on T2DM in humans, mainly based on findings from animal studies. Humans consume resveratrol at low doses from dietary sources such as grapes, berries, and peanuts. Recommendations for higher doses of resveratrol through supplementation in the treatment of humans with T2DM should be made only on the basis of evidence from future robust clinical studies reporting patient‐relevant outcomes.
Implications for research.
Based on the findings of our systematic review, it is clear that scientific evidence on the safety and efficacy of resveratrol in individuals with T2DM is very limited and is still in its infancy. We found a definite research gap in the literature investigating effects of resveratrol‐only formulations not combined with other plant polyphenols in adults with T2DM. To provide healthcare stake holders such as consumers, funders, and healthcare professionals access to best evidence on the use of resveratrol for T2DM, there is a great need to grow the available evidence base by conducting high‐quality clinical studies with large sample sizes and long‐term follow‐up periods that measure patient‐important endpoints. In addition, future studies must consider exploring socioeconomic effects as one of their study outcomes, as this may help reduce the healthcare burden that we currently face due to an alarming increase in the incidence of T2DM around the world. Although ongoing studies do not appear to address these issues (eight ongoing trials with a total of approximately 800 participants with follow‐up periods ranging from four weeks to 12 months), they still can provide valuable safety data.
Notes
Portions of the background and methods sections, the appendices, additional tables, and Figures 1 to 3 of this review are based on a standard template established by the CMED Group.
Acknowledgements
Search strategies were revised and were partially designed by the CMED Information Specialist (Maria‐Inti Metzendorf). We are thankful to study authors Christopher K. Rayner, Patrick Schrauwen, and Marlies de Ligt for providing us with study data. We are thankful to Nicole Askin for helping with the updated search.
The review authors and the CMED editorial base are grateful to the peer reviewer James M. Smoliga, Department of Physical Therapy, High Point University, High Point, NC, USA, for his time and comments.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
| 1. (resveratrol* or SRT 501 or SRT501 or 501‐36‐0):TI,AB,KY 2. MESH DESCRIPTOR Diabetes Mellitus, Type 2 EXPLODE ALL TREES 3. (MODY OR NIDDM OR T2DM OR T2D):TI,AB,KY 4. diabet*:TI,AB,KY 5. #2 OR #3 OR #4 6. #1 AND #5 |
| MEDLINE (Ovid SP) |
| 1. resveratrol.rn. 2. (resveratrol* or SRT 501 or SRT501 or 501‐36‐0).tw. 3. or/1‐2 4. exp Diabetes Mellitus, Type 2/ 5. (MODY or NIDDM or T2DM or T2D).tw. 6. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 7. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 8. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 9. or/4‐8 10. 3 and 9 |
| PubMed („NOT MEDLINE[sb]“: ‐ as supplied by publisher; ‐ in process; ‐ OLDMEDLINE; ‐ pubmednotMEDLINE) |
| #1 (resveratrol*[tw] OR "SRT 501"[tw] OR SRT501[tw] OR 501‐36‐0[tw]) AND (diabete*[tw] OR diabeti*[tw] OR MODY[tw] OR NIDDM[tw] OR T2DM[tw] OR T2D[tw]) #2 (medline[sb] or pmcbook) #3 #1 NOT #2 |
| Embase (Ovid SP) |
| 1. resveratrol/ 2. (resveratrol* or SRT 501 or SRT501 or 501‐36‐0).tw. 3. or/1‐2 4. non insulin dependent diabetes mellitus/ 5. (MODY or NIDDM or T2D*).tw. 6. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 7. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 8. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 9. or/4‐8 10. 3 and 9 [10:Wong 2006"sound treatment studies" filter – BS version] 11. random*.tw. or clinical trial*.mp. or exp health care quality/ 12. 10 and 11 |
| CINAHL (EBSCO Host) |
| S1. MH "resveratrol" S2. TI (resveratrol* or SRT 501 or SRT501 or 501‐36‐0) S3. AB (resveratrol* or SRT 501 or SRT501 or 501‐36‐0) S4. S1 OR S2 OR S3 S5. MH "Diabetes Mellitus, Type 2+" S6. TI (MODY OR NIDDM OR T2D*) S7. AB (MODY OR NIDDM OR T2D*) S8. TI ("non insulin* depend*" OR "noninsulin* depend*" OR noninsulin#depend* OR "non insulin#depend*") S9. AB ("non insulin* depend*" OR "noninsulin* depend*" OR noninsulin#depend* OR "non insulin#depend*") S10.TI (("typ* 2" OR "typ* II" OR typ#2 OR typ#II) N3 diabet*) S11.AB (("typ* 2" OR "typ* II" OR typ#2 OR typ#II) N3 diabet*) S12.TI (((late OR adult* OR matur* OR slow OR stabl*) N3 onset) AND diabet*) S13.AB (((late OR adult* OR matur* OR slow OR stabl*) N3 onset) AND diabet*) S14.S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15.S4 AND S14 |
| International Pharmaceutical Abstracts (Ovid) |
| 1. resveratrol.mp. 2. (resveratrol* or SRT 501 or SRT501 or 501‐36‐0).tw. 3. or/1‐2 4. (MODY or NIDDM or T2DM or T2D).tw. 5. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 6. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 7. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 8. or/4‐7 9. 3 and 8 10. (random* or placebo* or double‐blind*).mp. 11. 9 and 10 |
| ClinicalTrials.gov (Basic search) |
| (resveratrol OR "SRT 501" OR SRT501 OR "501‐36‐0") AND (diabetes OR diabetic) |
| ICTRP Search Portal (Standard search) |
| diabet* AND resveratrol* OR T2D* AND resveratrol* OR NIDDM AND resveratrol* OR MODY AND resveratrol* OR diabet* AND SRT‐501 OR T2D* AND SRT‐501 OR NIDDM AND SRT‐501 OR MODY AND SRT‐501 OR diabet* AND SRT501 OR T2D* AND SRT501 OR NIDDM AND SRT501 OR MODY AND SRT501 OR diabet* AND 501‐36‐0 OR T2D* AND 501‐36‐0 OR NIDDM AND 501‐36‐0 OR MODY AND 501‐36‐0 |
Appendix 2. 'Risk of bias' assessment
| 'Risk of bias' domains |
|
Random sequence generation (selection bias due to inadequate generation of a randomised sequence) For each included trial, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment (selection bias due to inadequate concealment of allocation before assignment) We described for each included trial the method used to conceal allocation to interventions before assignment, and we assessed whether intervention allocation could have been foreseen in advance of or during recruitment or changed after assignment.
We also evaluated trial baseline data to incorporate assessment of baseline imbalance into the 'Risk of bias' judgement for selection bias (Corbett 2014). Chance imbalances may also affect judgements on the risk of attrition bias. In the case of unadjusted analyses, we distinguished between trials that we rate as being at low risk of bias on the basis of both randomisation methods and baseline similarity, and trials that we judge as being at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We re‐classified judgements of unclear, low, or high risk of selection bias as specified in Appendix 3. Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed, or adjudicated outcome measures (see below).
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed, or adjudicated outcome measures (see below).
Incomplete outcome data (attrition bias due to quantity, nature, or handling of incomplete outcome data) For each included trial or each outcome, or both, we described the completeness of data, including attrition and exclusions from analyses. We stated whether the trial reported attrition and exclusions, and we reported the number of participants included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups). We also noted if the trial reported the reasons for attrition or exclusion, and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
Selective reporting (reporting bias due to selective outcome reporting) We assessed outcome reporting bias by integrating the results of the appendix 'Matrix of trial endpoints (publications and trial documents' (Boutron 2014; Jones 2015; Mathieu 2009), with those of the appendix 'High risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Other bias
|
Appendix 3. Selection bias decisions
| Selection bias decisions for studies that reported unadjusted analyses: comparison of results obtained using method details alone vs results obtained using method details and study baseline informationa | |||
| Reported randomisation and allocation concealment methods | 'Risk of bias' judgement using methods reporting | Information gained from study characteristics data | 'Risk of bias' using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskb |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly randomised or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Unclear risk | ||
| No baseline details | High risk | ||
| aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias compared with use of methods reporting alone. bImbalance was identified that appears likely to be due to chance. cDetails for the remaining important prognostic variables are not reported. | |||
Appendix 4. Description of interventions
| Study ID | Intervention(s) (route, frequency, total dose/d) | Comparator(s) (route, frequency, total dose/d) |
|
Timmers 2016 (cross‐over RCT) |
Trans‐resveratrol Softgel Oral, daily, 150 mg/d + oral hypoglycaemic agent(s) or diet alone |
Placebo Oral, daily, 150 mg/d + oral hypoglycaemic agent(s) or diet alone |
|
Thazhath 2016 (cross‐over RCT) |
Resveratrol capsule Oral, twice a day, 1000 mg/d |
Placebo (microcrystalline cellulose) capsule Oral, twice a day, 1000 mg/d |
|
Brasnyo 2011 (parallel RCT) |
Trans‐resveratrol capsule Oral, twice a day, 10 mg/d |
Placebo (microcrystalline cellulose) capsule Oral, twice a day, 10 mg/d |
| C: comparator, I: intervention, RCT: randomised controlled trial. | ||
Appendix 5. Baseline characteristics (I)
| Study ID | Intervention(s) and comparator(s) | Duration of intervention/follow‐up | Description of participants | Study period (year to year) | Country | Setting | Ethnic groups (%) | Duration of type 2 diabetes (mean years (SD)) |
|
Timmers 2016 (cross‐over RCT) |
I: trans‐resveratrol + oral hypoglycaemic agent(s) or diet alone | 30 days with 30‐day washout period (cross‐over study) | Adults with well‐controlled type 2 diabetes managed by diet and/or oral hypoglycaemic agents | Jun 2012 to Jun 2014 | Netherlands | Outpatients | White: 100 | 6.8 (1) |
| C: placebo + oral hypoglycaemic agent(s) or diet alone | ||||||||
|
Thazhath 2016 (cross‐over RCT) |
I: resveratrol | 5 weeks with 5‐week washout period (cross‐over study) | Adults with type 2 diabetes managed by diet alone | Sep 2013 to Jan 2015 | Australia | Outpatients | White: 100 | 5 (1) |
| C: placebo | ||||||||
|
Brasnyo 2011 (parallel RCT) |
I: trans‐resveratrol | 4 weeks | Adults with type 2 diabetes | — | Hungary | Outpatients | White: 100 | — |
| C: placebo | White: 100 | — | ||||||
| — denotes not reported. C: comparator, I: intervention, RCT: randomised controlled trial, SD: standard deviation. | ||||||||
Appendix 6. Baseline characteristics (II)
| Study ID | Intervention(s) and comparator(s) | Sex (female %) | Age (mean years (SD), or as reported) | HbA1c (mean % (SD)) | BMI (mean kg/m² (SD)) | Comedications/Cointerventions (%) | Comorbidities (%) |
|
Timmers 2016 (cross‐over RCT) |
I: trans‐resveratrol + oral hypoglycaemic agent(s) or diet alone | 0 | 64 (59.2 to 67.3)a | 6.8 (0.2) | 30.5 (0.6) | Metformin + sulphonylurea: 35
Metformin: 59 Diet only: 6 Cholesterol‐lowering medications: 65 Anti‐hypertensives: 71 |
— |
| C: placebo + oral hypoglycaemic agent(s) or diet alone | |||||||
|
Thazhath 2016 (cross‐over RCT) |
I: resveratrol | 27 | 67.5 (1.6) | 6.4 (0.2) | 27.7 (1.4) | Diet | 0 |
| C: placebo | |||||||
|
Brasnyo 2011 (parallel RCT) |
I: trans‐resveratrol | 0 | 58 (8) | 7.5 (2.2) | — | Angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker: 100 | Ischaemic heart disease: 10
Peripheral arterial disease: 10
Hypercholesterolaemia: 50
Angina pectoris: 0
Diabetic neuropathy: 0 Diabetic nephropathy: 70 |
| C: placebo | 0 | 53 (11) | 7.6 (1.8) | — | Angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker: 100 | Ischaemic heart disease:11 Peripheral arterial disease: 11 Hypercholesterolaemia: 44 Angina pectoris: 11 Diabetic neuropathy: 22 Diabetic nephropathy: 44 | |
| — denotes not reported aMedian and 95% confidence interval. BMI: body mass index, C: comparator, HbA1c: glycosylated haemoglobin A1c, I: intervention, RCT: randomised controlled trial, SD: standard deviation. | |||||||
Appendix 7. Matrix of study endpoints (publications and trial documents)
| Timmers 2016 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT01638780 Primary outcome measure: insulin sensitivity (overall, muscle and liver specific), time frame: 30 days after supplementation Secondary outcome measure(s): muscle mitochondrial oxidative capacity, intramyocellular lipid content, intrahepatic lipid content, intracardiac lipid content, heart function; time frame for all outcomes: 30 days after supplementation Other outcome measure(s): — Trial results available in trial register: no | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): insulin sensitivity Secondary outcome measure(s): intrahepatic lipid content, intramyocellular lipids, mitochondrial function (in vivo and ex vivo), blood pressure, and cardiac function Other outcome measure(s): adverse events | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): (main outcome measure) insulin sensitivity by the hyperinsulinaemic‐euglycaemic clamp technique Secondary outcome measure(s): — Other outcome measure(s): intrahepatic lipid content, intramyocellular lipid content, systolic blood pressure, ex vivo mitochondrial function | |
| Thazhath 2016 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:ACTRN12613000717752 Primary outcome measure: plasma total GLP‐1 concentrations (concentrations measured at time point = ‐5, 15, 30, 45, 60, 90, 120, 150, 180, and 240 minutes in relation to consumption of the test meal at time point = 10 minutes) Secondary outcome measure(s): gastric half‐emptying time as measured by 13C‐octanoic breath test (breath samples collected at time point = 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 minutes, and then every 15 minutes until time point = 240 minutes), blood glucose concentrations (concentrations measured at time point = ‐5, 15, 30, 45, 60, 90, 120, 150, 180, and 240 minutes in relation to consumption of the test meal at time point = 10 minutes) Other outcome measure(s): — Trial results available in trial register: no | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): postprandial AUC for plasma total GLP‐1 Secondary outcome measure(s): changes in AUC blood glucose concentrations, fasting and peak postprandial GLP‐1, blood glucose concentrations, HbA1c, gastric emptying, daily energy intake, body weight Other outcome measure(s): adverse effects | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): plasma total GLP‐1 concentrations, fasting and peak postprandial GLP‐1 and blood glucose concentrations, HbA1c, gastric emptying, daily energy intake, and body weight | |
| Brasnyo 2011 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: — Secondary outcome measure(s): —c Other outcome measure(s): insulin resistance/sensitivity, creatinine‐normalised ortho‐tyrosine level in urine samples (as a measure of oxidative stress), incretin levels and phosphorylated protein kinase B (pAkt):protein kinase B (Akt) ratio in platelets | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): insulin resistance/sensitivity (homeostasis model of assessment for insulin resistance), creatinine‐normalised ortho‐tyrosine level in urine samples (as a measure of oxidative stress), incretin levels and phosphorylated protein kinase B (pAkt):protein kinase B (Akt) ratio in platelets, homeostasis model of assessment of beta‐cell function, adverse effects | |
| — denotes not reported.
aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturers' websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary references, duplicate publications, companion documents, or multiple reports of a primary trial). cPrimary and secondary outcomes refers to verbatim specifications in publications/records. Unspecified outcome measures refers to all outcomes not described as primary or secondary outcome measures. AUC: area under the curve, EMA: European Medicines Agency, FDA: Food and Drug Administration (US), GLP‐1: glucagon‐like peptide 1, HbA1c: glycosylated haemoglobin A1c, NT: no trial document available, pAkt: phosphorylated protein kinase B. | |
Appendix 8. High risk of outcome reporting bias according to ORBIT classification
| Study ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Timmers 2016 | ND | ||||
| Thazhath 2016 | ND | ||||
| Brasnyo 2011 | ND | ||||
|
aClear that outcome was measured and analysed; study report states that outcome was analysed but reports only that result was not significant.
(Classification 'A', Table 2, Kirkham 2010)
bClear that outcome was measured and analysed; study report states that outcome was analysed but does not report results.
(Classification 'D', Table 2, Kirkham 2010)
cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results.
(Classification 'E', Table 2, Kirkham 2010)
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results.
(Classification 'G', Table 2, Kirkham 2010) ND: none detected, ORBIT: Outcome Reporting Bias In Trials. | |||||
Appendix 9. Definition of endpoint measurementa
| Study ID | All‐cause mortality | Diabetes‐related complications | Diabetes‐related mortality | HbA1c | Health‐related quality of life | FBG | Insulin sensitivity | Socioeconomic effects | Severe/serious adverse events |
| Timmers 2016 | NR | NR | NR | Glycosylated haemoglobin A1c (IO) | NR | Fasting blood glucose (IO) | "Insulin Sensitivity (HOMA‐IR) and Substrate Kinetics Assessed by Hyperinsulinemic‐Euglycemic Clamp" (IO) | NR | ND |
| Thazhath 2016 | NR | NR | NR | Glycosylated haemoglobin A1c (IO) | NR | Fasting blood glucose (IO) | NR | NR | ND |
| Brasnyo 2011 | NR | NR | NR | NR | NR | NR | HOMA‐IR: "The values of homeostasis model of assessment for insulin resistance and related to B‐cell function (HOMAIR and HOMAb, respectively) were calculated as in Nagaretani et al.and Matthews et al., respectively" (IO) | NR | ND |
|
aIn addition to the definition of endpoint measurement, description of who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement). FBG: fasting blood glucose, HbA1c: glycosylated haemoglobin A1c, HOMA‐IR: homeostasis model of assessment for insulin resistance, ND: not defined, NR: not reported. | |||||||||
Appendix 10. Adverse events (I)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Deaths (N) | Deaths (%) | Participants with at least one adverse event (N) | Participants with at least one adverse event (%) | Participants with at least one severe/serious adverse event (N) | Participants with at least one severe/serious adverse event (%) |
|
Timmers 2016 (cross‐over RCT) |
I: trans‐resveratrol + oral hypoglycaemic agent(s) or diet alone | 17 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo + oral hypoglycaemic agent(s) or diet alone | 17 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
Thazhath 2016 (cross‐over RCT) |
I: resveratrol | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 14 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
Brasnyo 2011 (parallel RCT) |
I: trans‐resveratrol | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 9 | 0 | 0 | 0 | 0 | 0 | 0 | |
| — denotes not reported. C: comparator, I: intervention, N: number of participants. | ||||||||
Appendix 11. Adverse events (II)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants discontinuing study due to an adverse event (N) | Participants discontinuing study due to an adverse event (%) | Participants with at least one hospitalisation (N) | Participants with at least one hospitalisation (%) | Participants with at least one outpatient treatment (N) | Participants with at least one outpatient treatment (%) |
| Timmers 2016 | I: trans‐resveratrol + oral hypoglycaemic agent(s) or diet alone | 17 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo + oral hypoglycaemic agent(s) or diet alone | 17 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Thazhath 2016 | I: resveratrol | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 14 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Brasnyo 2011 | I: trans‐resveratrol | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 9 | 0 | 0 | 0 | 0 | 0 | 0 | |
| — denotes not reported. C: comparator, I: intervention. | ||||||||
Appendix 12. Adverse events (III)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least one specific adverse events (N) | Participants with at least one specific adverse event (%) |
| Timmers 2016 | I: trans‐resveratrol + oral hypoglycaemic agent(s) or diet alone | 17 | 0 | 0 | 0 |
| C: placebo + oral hypoglycaemic agent(s) or diet alone | 17 | 0 | 0 | 0 | |
| Thazhath 2016 | I: resveratrol | 14 | 0 | 0 | 0 |
| C: placebo | 14 | 0 | 0 | 0 | |
| Brasnyo 2011 | I: trans‐resveratrol | 10 | 0 | 0 | 0 |
| C: placebo | 9 | 0 | 0 | 0 | |
| — denotes not reported. C: comparator, I: intervention. |
|||||
Appendix 13. Survey of study investigators providing information on included trials
| Study ID | Date study author contacted | Date study author replied | Date study author asked for additional information (short summary) | Date study author provided data (short summary) |
| ACTRN12614000891628 | 09 Jan 2019 | No reply | NA | NA |
| Brasnyo 2011 | 23 November 2015 | No reply | NA | NA |
| CTRI/2017/04/008384 | 09 Jan 2019 | No reply | NA | NA |
| IRCT20171118037528N1 | 09 Jan 2019 | No reply | NA | NA |
| IRCT201601022394N19 | 09 Jan 2019 | No reply | NA | NA |
| IRCT201411112394N14 | 09 Jan 2019 | No reply | NA | NA |
| NCT01158417 | 07 August 2019 | No reply | NA | NA |
| NCT01881347 | 07 August 2019 | No reply | NA | NA |
| NCT02549924 | 07 August 2019 | No reply | NA | NA |
| SLCTR/2018/019 | Not contacted (estimated trial completion date January 2019) | |||
| Timmers 2016 | 10 Sep 2018 | 11 Sep 2018 | See summary | 11 September 2018 (individual participant data on HbA1c, fasting insulin, and fasting blood glucose (baseline and end of study values were shared). Later in May 2019, the author contacted us and provided corrected data for fasting insulin and fasting blood glucose to calculate insulin resistance (HOMA‐IR). |
| Thazhath 2016 | 28 November 2015 | 06 December 2015 | 07 December 2015 (requested for change from baseline values for outcomes) | 07 December 2015 (participants' data on HbA1c and fasting blood glucose for both study periods were shared) |
| Verges 2014 | Not contacted (conference abstract without contact information) | |||
| HbA1c: glycosylated haemoglobin A1c, HOMA‐IR: homeostasis model of assessment for insulin resistance, NA: not applicable. | ||||
Appendix 14. Checklist to aid consistency and reproducibility of GRADE assessments
| Diabetes‐related complications | All‐cause mortality | Diabetes‐related mortality | Health‐related quality of life | Adverse events | HbA1c | Socioeconomic effects | ||
| Study limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | NR | Yes | Yes | NR | Yes | Yes | NR |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | ||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias)? | Yes | Yes | Yes | Yes | ||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias)? | Yes | Yes | Yes | Yes | ||||
| Was an objective outcome used? | Yes | Yes | Yes | Yes | ||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | Yes | ||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | ||||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | ||||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | ||||
| Inconsistencyb | Point estimates did not vary widely? | NA | NA | NA | Yes | |||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies' point estimates; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies does not overlap with those of most included studies)? | NA | NA | NA | Substantial | ||||
| Was the direction of effect consistent? | NA | NA | NA | Yes | ||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | NA | NA | NA | Low | ||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | NA | NA | NA | Not statistically significant | ||||
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Applicable | Applicable | Applicable | Applicable | |||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable) | ||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | No (↓) | ||||
| Was the outcome time frame sufficient? | Insufficient (↓) | Insufficient (↓) | Insufficient (↓) | Insufficient (↓) | ||||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | ||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | NA | NA | NA | No (↓) | |||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | Low (↓) | Low (↓) | ||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to o10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | Small (↓) | ||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | NA | NA | NA | NA | ||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | |||
| Was grey literature searched? | Yes | Yes | Yes | Yes | ||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | ||||
| Was there no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | ||||
| Was there no evidence of funnel plot asymmetry? | NA | NA | NA | NA | ||||
| Was there no discrepancy in findings between published and unpublished trials? | NA | NA | NA | NA | ||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual studies.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval, it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry, and discrepancies between published and unpublished studies. eDepends on the context of the systematic review area. (↓): key item for possible downgrading of the quality of evidence (GRADE), as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; HbA1c: glycosylated haemoglobin A1c. NA: not applicable. | ||||||||
Data and analyses
Comparison 1. Resveratrol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c [%] | 2 | Mean Difference (Fixed, 95% CI) | 0.11 [‐0.02, 0.24] | |
| 2 FBG [mg/dL] | 2 | Mean Difference (Fixed, 95% CI) | 2.41 [‐2.10, 6.92] | |
| 3 Insulin sensitivity (measured by HOMA‐IR) | 2 | Mean Difference (Fixed, 95% CI) | ‐0.35 [‐0.99, 0.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brasnyó 2011.
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria
Exclusion criteria
Diagnostic criteria: quote from publication: "A total of nineteen Caucasian male patients previously diagnosed with type 2 diabetes (according to the WHO diagnostic guidelines) were included in the study" Setting: outpatients Age group: adults Gender distribution: males Country where study was performed: Hungary |
|
| Interventions |
Intervention(s): trans‐resveratrol Comparator(s): placebo Duration of intervention: 4 weeks Duration of follow‐up: 4 weeks Run‐in period: 4 weeks Number of study centres: 1 Extension period: none |
|
| Outcomes | Reported outcome(s) in full text of publication: insulin resistance/sensitivity, creatinine normalised ortho‐tyrosine level in urine samples (as a measure of oxidative stress), incretin levels, and phosphorylated protein kinase B (pAkt):protein kinase B (Akt) ratio in platelets | |
| Study details |
Trial identifier: none Study terminated early: no |
|
| Publication details |
Language of publication: English Funding: "The present study received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors" Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To determine whether the polyphenol resveratrol improves insulin sensitivity in type 2 diabetic patients and to gain some insight into the mechanism of its action" | |
| Notes | "Resveratrol of herbal origin (with > 98% t‐resveratrol content) and the placebo (both in gelatin capsules) were obtained from Argina Nutraceuticals (previously Admarc Nutraceuticals, Fót,
Hungary) and dosed 5 mg/capsule. The identical placebo capsules contained only the carrier microcrystalline cellulose" "The general examination was followed by a 4‐week washout period before the trial began (during which all lipid‐lowering medication was ceased)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "They underwent a blinded randomisation into two groups: ten patients to receive oral resveratrol twice daily (in gelatin capsules containing 5 mg resveratrol) and nine patients to placebo" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "nineteen patients enrolled in the 4‐week long double‐blind study were randomly assigned into two groups" "The identical placebo capsules contained only the carrier microcrystalline cellulose" |
| Blinding of participants and personnel (performance bias) all‐cause mortality | Low risk |
Quote from publication: "nineteen patients enrolled in the 4‐week long double‐blind study were randomly assigned into two groups" "The identical placebo capsules contained only the carrier microcrystalline cellulose" |
| Blinding of participants and personnel (performance bias) insulin sensitivity | Low risk |
Quote from publication: "nineteen patients enrolled in the 4‐week long double‐blind study were randomly assigned into two groups" "The identical placebo capsules contained only the carrier microcrystalline cellulose" |
| Blinding of outcome assessment (detection bias) adverse events | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) all‐cause mortality | Low risk | Comment: not reported; outcome measure unlikely influenced by potential lack of blinding |
| Blinding of outcome assessment (detection bias) insulin sensitivity | Low risk | Comment: not reported; outcome measure unlikely influenced by potential lack of blinding |
| Incomplete outcome data (attrition bias) adverse events | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) all‐cause mortality | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) insulin sensitivity | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available |
| Other bias | Low risk | Comment: none detected |
Thazhath 2016.
| Methods | Study design: cross‐over randomised controlled clinical trial, phase 2 trial | |
| Participants |
Inclusion criteria
Exclusion criteria
Diagnostic criteria: World Health Organization (WHO) diagnostic criteria for diagnosis of type 2 diabetes Setting: outpatients Age group: adults Gender distribution: females and males Country where study was performed: Australia |
|
| Interventions |
Intervention(s): resveratrol Comparator(s): placebo Duration of intervention: 2 × 5 weeks intervention period with 5 weeks washout period (cross‐over study) Duration of follow‐up: 5 weeks Run‐in period: not reported Number of study centres: 1 Extension period: none |
|
| Outcomes | Reported outcome(s) in full text of publication: plasma total GLP‐1 concentrations, changes in blood glucose concentrations, fasting and peak postprandial GLP‐1 and blood glucose concentrations, HbA1c, gastric emptying, daily energy intake, body weight, adverse effects | |
| Study details |
Trial identifier:ACTRN12613000717752 Study terminated early: no |
|
| Publication details |
Language of publication: English Funding: not reported Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote: "We hypothesized that supplementation with resveratrol for 5 wk in patients with type 2 diabetes would increase both fasting and postprandial GLP‐1 concentrations and, accordingly, lower both fasting and postprandial blood glucose concentrations and slow gastric emptying" | |
| Notes | "After an initial screening visit, each patient was treated with 500 mg oral resveratrol or placebo (microcrystalline cellulose) capsules twice daily for two 5‐wk treatment periods in a double‐blind, randomized, crossover design with a 5‐wk washout period between treatments. This dose of resveratrol was chosen to approximate the human equivalent of the dose shown to enhance GLP‐1 release in rodents" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Random assignment was performed by the hospital pharmacy with established software" Quote from trials register: "computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Random assignment was performed by the hospital pharmacy with established software" Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Blinding of participants and personnel (performance bias) all‐cause mortality | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Comment: reported as a double blinded study; matching placebo given (investigator‐assessed outcome measurement) Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Blinding of participants and personnel (performance bias) fasting blood glucose | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Comment: reported as a double‐blinded study; matching placebo given (investigator‐assessed outcome measurement) Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Comment: reported as a double‐blinded study; matching placebo given (investigator‐assessed outcome measurement) Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Blinding of outcome assessment (detection bias) adverse events | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Comment: reported as a double‐blinded study; matching placebo given (investigator‐assessed outcome measurement) Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Blinding of outcome assessment (detection bias) all‐cause mortality | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Comment: reported as a double‐blinded study; matching placebo given (investigator‐assessed outcome measurement) Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Blinding of outcome assessment (detection bias) fasting blood glucose | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Comment: reported as a double‐blinded study; matching placebo given (investigator‐assessed outcome measurement) Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "in this double‐blind, randomized, crossover study of patients with type 2 diabetes" Comment: reported as a double‐blinded study; matching placebo given (investigator‐assessed outcome measurement) Quote from trials register: "Who is/are masked/blinded? The people receiving the treatment/s, the people administering the treatment/s, the people assessing the outcomes, the people analysing the results/data" |
| Incomplete outcome data (attrition bias) adverse events | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) all‐cause mortality | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) fasting blood glucose | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) HbA1c | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Low risk | Comment: trial register information available; outcomes reported in the trial register match the outcomes reported in the publication |
| Other bias | Low risk | Quote: "Carryover effects from one intervention period to the other were excluded with the use of the methods reported by Wellek et al. (24). The presence of treatment effects was analyzed with the use of period‐adjusted t tests to account for the crossover design (24)" |
Timmers 2016.
| Methods | Study design: cross‐over randomised controlled clinical trial; randomisation ratio not reported | |
| Participants |
Inclusion criteria
Exclusion criteria
Diagnostic criteria: not reported Setting: outpatients Age group: adults Gender distribution: males Country where study was performed: Netherlands |
|
| Interventions |
Intervention(s): trans‐resveratrol Softgel Comparator(s): placebo Duration of intervention: 2 × 30 days intervention period with 30 days washout period (cross‐over study) Duration of follow‐up: 30 days Run‐in period: not reported Number of study centres: 1 Extension period: none |
|
| Outcomes | Reported outcome(s) in full text of publication: insulin sensitivity, intrahepatic lipid content, intramyocellular lipids, mitochondrial function (in vivo and ex vivo), blood pressure, and cardiac function | |
| Study details |
Trial identifier:NCT01638780 Study terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding (European Foundation for the Study of Diabetes Clinical Research Grant) Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote: "to examine if 30 days of resveratrol (resVida) supplementation leads to an improvement in peripheral and hepatic insulin sensitivity in subjects with well‐controlled T2D" | |
| Notes | "In randomized order, participants underwent two experimental trials: a placebo and a resVida (150 mg/day trans‐resveratrol [99.9%]; provided by DSM Nutritional Products Ltd.) condition, with a washout period of at least 30 days" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomization was performed according to standard procedures as described in Statistical Methods by Snedecor and Cochran" Comment: probably performed correctly |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of participants and personnel (performance bias) all‐cause mortality | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of participants and personnel (performance bias) fasting blood glucose | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of participants and personnel (performance bias) insulin sensitivity | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) adverse events | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) all‐cause mortality | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) fasting blood glucose | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) insulin sensitivity | Low risk |
Quote from publication: "Subjects with well‐controlled type 2 diabetes (T2D) were treated with placebo and 150 mg/day resveratrol (resVida) in a randomized double‐blind crossover study for 30 days" Quote from trials register: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Incomplete outcome data (attrition bias) adverse events | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) all‐cause mortality | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) fasting blood glucose | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) HbA1c | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) insulin sensitivity | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available; outcomes reported in the protocol match the outcomes reported in the results section |
| Other bias | Low risk | Quote: "potential carryover effect between treatment and period was examined by unpaired t test analyses according to Pocock (29)" |
Note: Where the judgement is 'Unclear' and the description is blank, the study did not report that particular outcome.
BMI: body mass index, eGFR: estimated glomerular filtration rate, GLP‐1: glucagon‐peptide 1, HbA1c: glycosylated haemoglobin A1c, IFCC: International Federation of Clinical Chemistry, WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bashmakov 2014 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Bhatt 2013 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Bo 2018 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Elliott 2009 | Narrative review |
| Fujitaka 2011 | Study participants had metabolic syndrome. Primary investigator for that study was Dipak K Das, who was found to have falsified data and was charged with fraud (https://www.nytimes.com/2012/01/12/science/fraud‐charges‐for‐dipak‐k‐das‐a‐university‐of‐connecticut‐researcher.html) |
| Goh 2014 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Imamura 2017 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Javid 2017 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Khazaei 2014 | Narrative review |
| Khodabandehloo 2018 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Kjaer 2014 | Study participants had metabolic syndrome |
| Mendez‐Del 2014 | Study participants had metabolic syndrome |
| Movahed 2014 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| NCT00937222 | Study intervention was not resveratrol (peanuts and peanut butter intervention) |
| NCT01038089 | Not a randomised controlled trial |
| NCT01150955 | Study participants had metabolic syndrome |
| NCT01375959 | Study participants had impaired glucose tolerance |
| NCT01714102 | Study participants did not have type 2 diabetes mellitus |
| NCT01997762 | Study participants had gestational diabetes |
| NCT02129595 | Study participants did not have type 2 diabetes mellitus |
| NCT02216552 | Study participants did not have type 2 diabetes mellitus |
| NCT02219906 | Study participants had metabolic syndrome |
| NCT02565979 | Study participants had impaired glucose homeostasis |
| Sattarinezhad 2018 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Seyyedebrahimi 2018 | Study participants were not comparable due to a combination of a rather unspecified mixture of oral anti‐diabetic agents with/without resveratrol (not identical in the intervention/comparator groups) |
| Tomé‐Carneiro 2012 | Only 36% to 48% of study participants had type 2 diabetes mellitus |
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12614000891628.
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: cross‐over assignment Masking: double‐blinded (participants and study personnel) Primary purpose: treatment |
| Participants |
Condition: non‐insulin‐dependent type 2 diabetes mellitus Enrollment: 39 Inclusion criteria: adults with non‐insulin‐dependent type 2 diabetes mellitus; non‐smoking; women must be postmenopausal; dementia‐free; clinical systolic blood pressure between 130 and 160 mmHg; BMI < 40 kg/m²; computer literate; measurable ultrasound signal on both sides of the head; unlikely to change medication/supplements during the intervention Exclusion criteria: suspected dementia (3MS score < 78/100 determined at screening); smoker or currently on nicotine therapy; neurological conditions; kidney/liver disease; insulin therapy; major depression as diagnosed by a healthcare professional; visual problems including inability to distinguish the colours of red, green, blue, and yellow; illiterate; physical difficulty in both hands that will impede motor performance of the hand/arm; inability to obtain a measurable signal in the MCA; unwillingness to provide 2 blood samples at each visit; unwillingness to maintain pre‐enrolment physical activity levels and dietary habits for the duration of the study; unwillingness to fast for 4 hours; currently consuming resveratrol or other grape extract supplements |
| Interventions |
Intervention(s): resveratrol capsules (75, 150, or 300 mg once daily) Comparator(s): placebo capsules |
| Outcomes |
Primary outcome(s): use of transcranial doppler (TCD) ultrasound to determine the most efficacious dose of resveratrol to improve cerebral vasodilator responsiveness (CVR) to hypercapnia in the anterior circulation (MCA) in adults with T2DM Secondary outcome(s): clinic BP and arterial compliance (AC), CVR to hypercapnia measured with TCD ultrasound, CVR to neuropsychological tests measured with TCD ultrasound, blood sample collection and analysis, blood sample collection, analysis of plasma resveratrol concentration Other outcome(s): ‐ |
| Study details | Trial identifier:ACTRN12614000891628 |
| Publication details | Dose response evaluation of resveratrol supplementation on cerebrovascular function, mood, and cognitive performance in type 2 diabetes mellitus (T2DM) |
| Stated aim of study | Quote: "dose response evaluation of resveratrol supplementation on cerebrovascular function, mood, and cognitive performance in type 2 diabetes mellitus" |
| Notes | Two identified reports of this trial (Wong RH, et al. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutrition, Metabolism, and Cardiovascular Diseases 2016;26(5):393‐9; and Wong RH, et al. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 2016;8(7)) do not report any of our review outcomes. The other outcomes of interest were planned to be investigated but have not yet been reported |
Verges 2014.
| Methods | Randomised cross‐over double‐blinded placebo‐controlled trial |
| Participants | Patients with statin‐treated type 2 diabetes |
| Interventions | 40 mg/d long‐acting resveratrol vs placebo |
| Outcomes | Changes in weight, HbA1c; lipid changes |
| Study details | Two 3‐month periods |
| Publication details | Conference abstract |
| Stated aim of study | To study the effect of resveratrol on plasma lipids in patients with type 2 diabetes |
| Notes | This is a conference abstract with no author contact information provided to obtain study data |
3MS: Modified Mini Mental State Examination, AC: arterial compliance, BMI: body mass index, BP: blood pressure, CVR: cerebral vasodilator responsiveness, HbA1c: glycosylated haemoglobin A1c, MCA: middle cerebral artery, TCD: transcranial doppler, T2DM: type 2 diabetes mellitus.
Characteristics of ongoing studies [ordered by study ID]
CTRI/2017/04/008384.
| Trial name or title | Acronym: — |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes mellitus Enrolment: estimated 180 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol supplement (1 g once daily for 12 months) Comparator(s): placebo |
| Outcomes |
Primary outcome(s): change in blood sugar level (fasting and fed), lipid profile, and systolic blood pressure and diastolic blood pressure from baseline to end of study visit (12 months) Secondary outcome(s): change in blood sugar level (fasting and fed), lipid profile, haemoglobin A1c, and systolic and diastolic blood pressure |
| Starting date |
Trial start date: September 2014 Trial completion date: not reported |
| Contact information | Responsible party/principal investigator: Hemant Mishra, Department of Medicine, Vikas Hospital, Kalyan West, Thane, Maharashtra, India |
| Study identifier |
Trial identifier:CTRI/2017/04/008384 Trial registered retrospectively (registered on 20/04/2017) |
| Official title | A randomized, open‐label, active‐control, phase IV clinical study evaluating efficacy and safety of resveratrol as an adjuvant therapy in patients with diabetes, dyslipidemia and hypertension |
| Stated purpose of study | Quote: "a study to check whether addition of resveratrol is beneficial and safe in patients with diabetes, dyslipidemia and hypertension (who are already on standard therapy)" |
| Notes |
IRCT201411112394N14.
| Trial name or title | Acronym: — |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: parallel Masking: double‐blinded Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes mellitus Enrolment: estimated 60 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol supplement (240 mg daily for 8 weeks) Comparator(s): placebo |
| Outcomes |
Primary outcome(s): mood, cognitive performance, serum BDNF (brain‐derived neurotrophic factor) Secondary outcome(s): HbA1c, fasting blood sugar |
| Starting date |
Trial start date: June 2015 Trial completion date: not reported |
| Contact information | Responsible party/principal investigator: Shima Jazayeri, Iran University of Medical Sciences |
| Study identifier | Trial identifier:IRCT201411112394N14 |
| Official title | Effects of resveratrol on mood, cognitive function, and serum BDNF in patients with type 2 diabetes |
| Stated purpose of study | Quote: "to determine effects of resveratrol on mood, cognitive function and serum BDNF in patients with type 2 diabetes. Design: randomized double‐blind placebo‐controlled clinical trial" |
| Notes |
IRCT201601022394N19.
| Trial name or title | Acronym: — |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: parallel Masking: double‐blinded Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes mellitus Enrolment: estimated 50 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol supplement (240 mg daily with lunch for 8 weeks) Comparator(s): placebo |
| Outcomes |
Primary outcome(s): irisin, adiponectin Secondary outcome(s): fasting blood sugar (FBS), HbA1C, insulin |
| Starting date |
Trial start date: June 2015 Trial completion date: not reported |
| Contact information | Responsible party/principal investigator: Shima Jazayeri, Iran University of Medical Sciences |
| Study identifier | Trial identifier:IRCT201601022394N19 |
| Official title | Effects of resveratrol supplementation on serum irisin and adiponectin in patients with type 2 diabetes |
| Stated purpose of study | Quote: "to determine effects of resveratrol on serum irisin and adiponectin in patients with type 2 diabetes" |
| Notes |
IRCT20171118037528N1.
| Trial name or title | Acronym: — |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: parallel assignment Masking: double‐blinded (participants and administrators) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes mellitus Enrolment: estimated 72 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol (500 mg oral 2 times a day) Comparator(s): placebo capsules |
| Outcomes |
Primary outcome(s): peroxisome proliferator‐activated receptor alpha, p53 gene, p21 gene, p16 gene, soluble cluster of differentiation 163, TNF‐related weak inducer of apoptosis Secondary outcome(s): total triglycerides, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, fasting blood glucose, glycated haemoglobin, fasting insulin Other outcome(s): — |
| Starting date |
Trial start date: July 2018 Trial completion date: not reported |
| Contact information | Responsible party/principal investigator: Shima Abdollahi, Shahid Sadoughi University of Medical Science |
| Study identifier | Trial identifier:IRCT20171118037528N1 |
| Official title | Effects of resveratrol on lipid and glycemic profile indices, expression of PPARα, some factors associated with cell cycle arrest and sCD163 to sTWEAK ratio in T2DM patients |
| Stated purpose of study | Quote: "effects of resveratrol on lipid and glycemic profile indices, expression of PPARα, some factors associated with cell cycle arrest and sCD163 to sTWEAK ratio in T2DM patients" |
| Notes |
NCT01158417.
| Trial name or title | Acronym: — |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: parallel assignment Masking: single‐blind (participant) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes mellitus, obesity, and Insulin resistance Enrolment: estimated 102 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol (40 mg oral 3 times a day or 500 mg oral once daily) Comparator(s): placebo tablets |
| Outcomes |
Primary outcome(s): NF‐Kb Secondary outcome(s): GLP‐1 Other outcome(s): — |
| Starting date |
Trial start date: December 2008 Trial completion date: August 2014 (estimated) |
| Contact information | Responsible party/principal investigator: Paresh Dandona, MD, Kaleida Health |
| Study identifier | Trial identifier:NCT01158417 |
| Official title | Effect of resveratrol on insulin resistance and inflammatory mediators in obese and type 2 diabetic subjects |
| Stated purpose of study | Quote: "The main objective of this study is to investigate the effect of resveratrol on inflammatory mediators and insulin resistance at the cellular and molecular level in obese non diabetic and type 2 diabetic subjects in vivo" |
| Notes |
NCT01881347.
| Trial name or title | Acronym: — |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: cross‐over assignment Masking: double‐blinded (participants, caregiver, investigator, outcomes assessor) Primary purpose: basic science |
| Participants |
Condition: type 2 diabetes mellitus Enrolment: estimated 50 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol supplement (100 mg daily for 2 weeks followed by 300 mg daily for 2 weeks) Comparator(s): placebo |
| Outcomes |
Primary outcome(s): change from baseline in brachial artery flow‐mediated dilation Secondary outcome(s): change from baseline in fingertip peripheral arterial tonometry, change from baseline in carotid femoral pulse wave velocity, change from baseline in reactive hyperaemia Other outcome(s): change from baseline in serum glucose, change from baseline in serum insulin, change from baseline in mononuclear cell mitochondrial DNA damage, change from baseline in mononuclear cell mitochondrial mass, change from baseline in mononuclear cell mitochondrial production of reactive oxygen species, change from baseline in endothelial cell gene expression, change from baseline in endothelial cell protein expression |
| Starting date |
Trial start date: June 2013 Trial completion date: June 2016 |
| Contact information | Responsible party/principal investigator: Joseph A Vita, MD, Boston University (Contact: Monika Holbrook: monica.holbrook@bmc.org) |
| Study identifier | Trial identifier:NCT01881347 |
| Official title | Dose response evaluation of resveratrol supplementation on cerebrovascular function, mood and cognitive performance in type 2 diabetes mellitus (T2DM) |
| Stated purpose of study | Quote: "a dose response evaluation of resveratrol supplementation on cerebrovascular function, mood and cognitive performance in type 2 diabetes mellitus" |
| Notes |
NCT02549924.
| Trial name or title | Acronym: — |
| Methods |
Type of trial: safety/efficacy trial Allocation: randomised Intervention model: parallel assignment Masking: double‐blinded (participant, investigator) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes mellitus Enrolment: estimated 22 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol (500 mg 3 times daily) Comparator(s): placebo |
| Outcomes |
Primary outcome(s): area under the curve, mean amplitude of glucose excursions (MAGE) Secondary outcome(s): fasting plasma glucose, postprandial glucose, A1C, total cholesterol, triglycerides, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, very low‐density lipoprotein, alanine aminotransferase, aspartate aminotransferase, creatinine, blood pressure, body and visceral fat % Other outcome(s): — |
| Starting date |
Trial start date: September 2015 Trial completion date: February 2018 |
| Contact information | Responsible party/principal investigator: Esperanza Martínez‐Abundis, PhD, Institute of Experimental and Clinical Therapeutics (INTEC), CUCS, University of Guadalajara (esperanzamartnezabundi@yahoo.com) |
| Study identifier | Trial identifier:NCT02549924 |
| Official title | Effect of administration of resveratrol on glycemic variability in individuals with type 2 diabetes mellitus inadequately controlled with metformin |
| Stated purpose of study | Quote: "to know the effect of resveratrol on the glycemic variability [GV] in patients with T2DM who are not in control with metformin monotherapy based" |
| Notes |
SLCTR/2018/019.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: parallel assignment Masking: double‐blinded (participants and healthcare providers) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes mellitus Enrolment: estimated 275 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): resveratrol (200 mg/d) Comparator(s): placebo capsules |
| Outcomes |
Primary outcome(s): mean reduction from baseline in serum high‐sensitivity C‐reactive protein (hsCRP), mean reduction from baseline in glycosylated haemoglobin (HbA1c), and mean reduction from baseline in serum malondialdehyde (MDA), at 24 weeks Secondary outcome(s): mean change in insulin resistance as measured by HOMA‐IR, mean change in microalbuminuria, mean change in lipid profile, mean change in serum creatinine, mean change in cytokines (TNF‐alpha (tumour necrosis factor‐alpha), interleukin (IL)‐6, IL‐10, IL‐12, TGF‐ß (transforming growth factor‐beta), vascular endothelial growth factor (VEGF), intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM)), mean change in circulatory mitochondrial RNA (miRNA): miRNA‐21, miRNA‐34a, miRNA‐126, miRNA‐132, miRNA‐148, mi‐RNA 217, miRNA‐375 Other outcome(s): — |
| Starting date |
Trial start date: July 2017 Trial completion date: January 2019 (estimated) |
| Contact information | Responsible party/principal investigator: Dr. Dilshad Ahmed Khan, National University of Medical Sciences (NUMS), Islamabad, Pakistan |
| Study identifier | Trial identifier:SLCTR/2018/019 |
| Official title | Synergistic effects of delta‐tocotrienol, resveratrol and vitamin D supplementation on modulation of biochemical markers, cytokines and miRNAs in patients of type 2 diabetes mellitus |
| Stated purpose of study | Quote: "effects of delta‐tocotrienol, resveratrol and vitamin D supplementation mixture on biochemical markers in diabetic patients" |
| Notes |
— denotes not reported, ACE: angiotensin‐converting enzyme, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: body mass index, BP: blood pressure, GLP‐1: glucagon‐like peptide‐1, HbA1c: glycosylated haemoglobin A1c, HOMA‐IR: homeostasis model assessment of insulin resistance, NF‐Kb: nuclear factor kappa‐light‐chain‐enhancer of activated B cells, PPAR: peroxisome proliferator‐activated receptor, T2DM: type 2 diabetes mellitus, TSH: thyroid‐stimulating hormone.
Differences between protocol and review
The 'Method and timing of outcome measurement' section under methods has been changed. We changed the timing of outcome measurement to include any time point.
Contributions of authors
All review authors read and approved the final review draft.
Maya M Jeyaraman (MJ): protocol draft, search strategy development, acquisition of study reports, study selection, data extraction, data analysis, data interpretation, and future review updates.
Amrinder Singh Mann (AM): study selection and data extraction.
Nameer Al‐Yousif (NA): study selection and data extraction.
Vernon W Dolinsky (VD): protocol draft and expert review of content.
Rasheda Rabbani (RR): analysis of study data.
Ryan Zarychanski (RZ): protocol draft and search strategy development.
Ahmed M Abou‐Setta (AA): protocol draft, search strategy development, study selection, data interpretation, and future review updates.
Declarations of interest
MJ: none known.
AM: none known.
NA: none known.
RR: none known.
VD: none known.
AA: none known.
RZ: Ryan Zarychanski receives salary support from the Canadian Institute of Health Research (CIHR). This agency will have no role in the design or conduct of the review, including but not limited to study identification, collection, management, analysis and interpretation of data, or preparation, review, or approval of the final report.
New
References
References to studies included in this review
Brasnyó 2011 {published data only}
- Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, et al. Effect of resveratrol on insulin sensitivity, oxidative stress and Akt pathway in humans. Diabetologia 2010;53:S301. [Google Scholar]
- Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition 2011;106:383‐9. [DOI] [PubMed] [Google Scholar]
Thazhath 2016 {published data only}
- ACTRN12613000717752. The effect of resveratrol supplementation on gut hormone secretion, gastric emptying, and blood glucose responses to meals in patients with type 2 diabetes. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364511 (first received 1 June 2013).
- Thazhath SS, Wu T, Bound MJ, Checklin HL, Standfield S, Jones KL, et al. Administration of resveratrol for 5 weeks has no effect on glucagon‐like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: a randomized controlled trial. American Journal of Clinical Nutrition 2016; Vol. 103:66‐70. [DOI] [PubMed]
Timmers 2016 {published data only}
- NCT01638780. Resveratrol and type 2 diabetes. clinicaltrials.gov/show/NCT01638780 (first received 12 July 2012).
- Timmers S, Ligt M, Phielix E, Weijer T, Hansen J, Moonen‐Kornips E, et al. Resveratrol as add‐on therapy in subjects with well‐controlled type 2 diabetes: a randomized controlled trial. Diabetes Care 2016;39(12):2211‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bashmakov 2014 {published data only}
- ACTRN12610000565044. Pilot clinical trial for treatment of diabetic foot ulcers with dietary nano‐formulated phytochemicals. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335696 (first received 09 July 2010).
- Bashmakov YK, Assaad‐Khalil SH, Abou Seif M, Udumyan R, Megallaa M, Rohoma KH, et al. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinology 2014;2014:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhatt 2013 {published data only}
- Bhatt JK, Nanjan MJ. Resveratrol supplementation in patients with type 2 diabetes mellitus: a prospective, open label, randomized controlled trial. International Research Journal of Pharmacy 2013;4(8):245‐9. [Google Scholar]
- Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutrition Research (New York, N.Y.) 2012;32(7):537‐41. [DOI] [PubMed] [Google Scholar]
- CTRI/2011/05/001731. Evaluation of resveratrol as a dietary supplement in type 2 diabetes mellitus. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=2998&EncHid=&modid=&compid=%27,%272998det%27 (first received 16 April 2010).
Bo 2018 {published data only}
- Bo S, Gambino R, Ponzo V, Cioffi I, Goitre I, Evangelista A, et al. Effects of resveratrol on bone health in type 2 diabetic patients. a double‐blind randomized‐controlled trial. Nutrition and Diabetes 2018;8(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo S, Ponzo V, Ciccone G, Evangelista A, Saba F, Goitre I, et al. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. a randomized, double blind, placebo‐controlled trial. Pharmacological Research 2016;111:896‐905. [DOI] [PubMed] [Google Scholar]
- Bo S, Ponzo V, Evangelista A, Ciccone G, Goitre I, Saba F, et al. Effects of 6 months of resveratrol versus placebo on pentraxin 3 in patients with type 2 diabetes mellitus: a double‐blind randomized controlled trial. Acta Diabetologica 2017;54(5):499‐507. [DOI] [PubMed] [Google Scholar]
- Bo S, Togliatto G, Gambino R, Ponzo V, Lombardo G, Rosato R, et al. Impact of sirtuin‐1 expression on H3K56 acetylation and oxidative stress: a double‐blind randomized controlled trial with resveratrol supplementation. Acta diabetologica 2018;55(4):331‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02244879. Effects of resveratrol on inflammation in type 2 diabetic patients. clinicaltrials.gov/show/NCT02244879 (first received 19 September 2014).
Elliott 2009 {published data only}
- Elliott PJ, Walpole SM, Morelli L, Lambert PD, Lunsmann W, Westphal CH, et al. Resveratrol/SRT501. Sirtuin SIRT1 activator treatment of type 2 diabetes. Drugs of the Future 2009;34:291‐5. [Google Scholar]
Fujitaka 2011 {published data only}
- Fujitaka K, Otani H, Jo F, Jo H, Nomura E, Iwasaki M, et al. Modified resveratrol longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutrition Research (New York, N.Y.) 2011;31:842‐7. [DOI] [PubMed] [Google Scholar]
Goh 2014 {published data only}
- Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AF. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. International Journal of Sport Nutrition and Exercise Metabolism 2014;24:2‐13. [DOI] [PubMed] [Google Scholar]
- NCT01677611. Effects of resveratrol in patients with type 2 diabetes (RED). clinicaltrials.gov/show/NCT01677611 (first received 3 September 2012).
Imamura 2017 {published data only}
- Imamura H, Yamaguchi T, Nagayama D, Saiki A, Shirai K, Tatsuno I. Resveratrol ameliorates arterial stiffness assessed by cardio‐ankle vascular index in patients with type 2 diabetes mellitus. International Heart Journal 2017;58(4):577‐83. [DOI] [PubMed] [Google Scholar]
Javid 2017 {published data only}
- IRCT2015012420765N1. Impact of resveratrol supplementation on periodontal indices, antioxidant status, inflammatory markers and blood sugar in periodontal patients with type 2 diabetes mellitus. en.irct.ir/trial/18354 (first received 29 March 2015).
- Javid AZ, Hormoznejad R, Yousefimanesh HA, Zakerkish M, Haghighi‐Zadeh MH, Dehghan P, et al. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytotherapy Research 2017;31(1):108‐14. [DOI] [PubMed] [Google Scholar]
Khazaei 2014 {published data only}
- Khazaei S, Firouzei MS, Afghari P, Khazaei M. Resveratrol may improve osseointegration of dental implants in type 2 diabetes mellitus patients. Journal of Research in Medical Sciences 2014;19:81. [PMC free article] [PubMed] [Google Scholar]
Khodabandehloo 2018 {published data only}
- IRCT2015080223336N2. The effect of resveratrol on plasma and peripheral blood mononuclear cells (PBMCs) inflammatory factors of patients with type 2 diabetes. en.irct.ir/trial/19923 (first received 25 July 2015).
- Khodabandehloo H, Seyyedebrahimi S, Esfahani EN, Razi F, Meshkani R. Resveratrol supplementation decreases blood glucose without changing the circulating CD14(+)CD16(+) monocytes and inflammatory cytokines in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled study. Nutrition Research (New York, N.Y.) 2018;54:40‐51. [DOI] [PubMed] [Google Scholar]
Kjaer 2014 {published data only}
- Kjaer TN, Poulsen MM, Ornstrup MJ, Jorgensen J, Richelsen B, Pedersen SB. The metabolic and anti‐inflammatory effects of long‐term resveratrol intake; a randomised clinical trial. Diabetologia 2014;57:S306‐7. [Google Scholar]
Mendez‐Del 2014 {published data only}
- Mendez‐Del Villar M, Gonzalez‐Ortiz M, Martinez‐Abundis E, Perez‐Rubio KG, Lizarraga‐Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metabolic Syndrome and Related Disorders 2014;12:497‐501. [DOI] [PubMed] [Google Scholar]
Movahed 2014 {published data only}
- IRCT201111198129N1. Effect of resveratrol in controlling blood glucose in type 2 diabetic patients. en.irct.ir/trial/8549 (first received 19 September 2011).
- Movahed A, Nabipour I, Lieben XL, Thandapilly SJ, Yu L, Kalantarhormozi M, et al. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evidence‐based Complementary and Alternative Medicine 2013;2013:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahed A, Nabipour I, Louis XL, Joseph S, Yu L, Netticadan T. Short‐term resveratrol supplementation improves metabolic profile in type 2 diabetes. Diabetes Technology & Therapeutics 2014;16:A46‐A47. [Google Scholar]
NCT00937222 {published data only}
- NCT00937222. Effects of peanut and peanut butter consumption on blood lipids and glycemic control in adults with type 2 diabetes. clinicaltrials.gov/show/NCT00937222 (first received 10 July 2009).
NCT01038089 {published data only}
- NCT01038089. Pilot study of the effects of resveratrol on endothelial function in subjects with type 2 diabetes mellitus. clinicaltrials.gov/show/NCT01038089 (first received 23 December 2009).
NCT01150955 {published data only}
- NCT01150955. Potential beneficial effects of resveratrol. clinicaltrials.gov/show/NCT01150955 (first received 25 June 2010).
NCT01375959 {published data only}
- NCT01375959. Pilot study of resveratrol in older adults with impaired glucose tolerance. clinicaltrials.gov/show/NCT01375959 (first received 20 June 2011).
NCT01714102 {published data only}
- NCT01714102. Resveratrol and the metabolic syndrome. clinicaltrials.gov/show/NCT01714102 (first received 25 October 2012).
NCT01997762 {published data only}
- NCT01997762. Can resveratrol improve insulin sensitivity and preserve beta cell function following gestational diabetes?. clinicaltrials.gov/show/NCT01997762 (first received 28 November 2013).
NCT02129595 {published data only}
- NCT02129595. Resveratrol and first‐degree relatives of type 2 diabetic patients. clinicaltrials.gov/show/NCT02129595 (first received 2 May 2014).
NCT02216552 {published data only}
- NCT02216552. Resveratrol for the treatment of non alcoholic fatty liver disease and insulin resistance in overweight adolescents. clinicaltrials.gov/show/NCT02216552 (first received 15 August 2014).
NCT02219906 {published data only}
- NCT02219906. Resveratrol in metabolic syndrome. clinicaltrials.gov/show/NCT02219906 (first received 19 August 2014).
NCT02565979 {published data only}
- NCT02565979. Long‐term resveratrol and metabolism. clinicaltrials.gov/show/NCT02565979 (first received 1 October 2015).
Sattarinezhad 2018 {published data only}
- IRCT2016100716876N2. Resveratrol's effects in diabetic nephropathy. https://en.irct.ir/trial/15666 (first received 25 November 2016).
- NCT02704494. Resveratrol's effects in diabetic nephropathy. clinicaltrials.gov/show/NCT02704494 (first received 10 March 2016).
- Sattarinezhad A, Roozbeh J, Yeganeh BS, Omrani GR, Shams M. Resveratrol reduces albuminuria in diabetic nephropathy: a randomized double‐blind placebo‐controlled clinical trial. Diabetes and Metabolism 2018;S1262‐3636(18):30114‐9. [DOI] [PubMed] [Google Scholar]
Seyyedebrahimi 2018 {published data only}
- IRCT2015072523336N1. The effects of resveratrol on plasma and peripheral blood mononuclear cells (PBMCs) oxidative stress factors of type 2 diabetic patients. https://en.irct.ir/trial/19922 (first received 19 August 2015).
- Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. Correction to: the effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled clinical trial. Acta Diabetologica 2018 Jun 16 [Epub ahead of print]. [DOI: 10.1007/s00592-018-1160-9] [DOI] [PubMed]
- Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled clinical trial. Acta Diabetologica 2018;55(4):341‐53. [DOI] [PubMed] [Google Scholar]
Tomé‐Carneiro 2012 {published data only}
- Tomé‐Carneiro J, Gonzálvez M, Larrosa M, Yáñez‐Gascón MJ, García‐Almagro FJ, Ruiz‐Ros JA, et al. One‐year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. American Journal of Cardiology 2012;110:356‐63. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12614000891628 {published data only}
- ACTRN12614000891628. Dose response evaluation of resveratrol supplementation on cerebrovascular function, mood and cognitive performance in type 2 diabetes mellitus (T2DM). www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366701 (first received 12 August 2014).
- Wong RH, Nealon RS, Scholey A, Howe PR. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutrition, Metabolism, and Cardiovascular Diseases 2016;26(5):393‐9. [DOI] [PubMed] [Google Scholar]
- Wong RH, Raederstorff D, Howe PR. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 2016;8(7):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verges 2014 {published data only}
- Verges B, Brindisi MC, Bouillet B, Crevisy E, Buffier P, Baillot‐Rudoni S, et al. Is long‐acting resveratrol effective on the residual dyslipidemia in statin‐treated type 2 diabetic patients?. Atherosclerosis 2014;235(2014):e192. [Google Scholar]
References to ongoing studies
CTRI/2017/04/008384 {published data only}
- CTRI/2017/04/008384. A study to check whether addition of resveratrol is beneficial and safe in patients with diabetes, dyslipidemia and hypertension (who are already on standard therapy). www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=11073 (first received 20 April 2017).
IRCT201411112394N14 {published data only}
- IRCT201411112394N14. Effects of resveratrol on mood and cognition in patients with type 2 diabetes. en.irct.ir/trial/2084?revision=2084 (first received 18 August 2015).
IRCT201601022394N19 {published data only}
- IRCT201601022394N19. Effect of resveratrol on serum irisin and adiponectin in patients with type 2 diabetes. en.irct.ir/trial/2085 (first received 24 July 2016).
IRCT20171118037528N1 {published data only}
- IRCT20171118037528N1. Effect of resveratrol on vascular function. en.irct.ir/trial/27734 (first received 29 December 2017).
NCT01158417 {published data only}
- NCT01158417. Resveratrol in type2 diabetes and obesity. clinicaltrials.gov/show/NCT01158417 (first received 8 July 2010).
NCT01881347 {published data only}
- NCT01881347. Effects of resveratrol on endothelial function in type 2 diabetes mellitus. clinicaltrials.gov/show/NCT01881347 (first received 19 June 2013).
NCT02549924 {published data only}
- NCT02549924. Effect of administration of resveratrol on glycemic variability in individuals with type 2 diabetes mellitus. clinicaltrials.gov/show/NCT02549924 (first received 15 September 2015).
SLCTR/2018/019 {published data only}
- SLCTR/2018/019. Effects of delta‐tocotrienol, resveratrol and vitamin D supplementation mixture on biochemical markers in diabetic patients. slctr.lk/trials/1304 (first received 21 June 2018).
Additional references
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, the American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1999;22(Suppl 1):S5‐19. [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31(Suppl 1):S12‐54. [DOI] [PubMed] [Google Scholar]
Almeida 2009
- Almeida L, Vaz‐da‐Silva M, Falcao A, Soares E, Costa R, Loureiro AI, et al. Pharmacokinetic and safety profile of trans‐resveratrol in a rising multiple‐dose study in healthy volunteers. Molecular Nutrition & Food Research 2009;53(Suppl 1):S7‐15. [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baur 2006
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high‐calorie diet. Nature 2006;444:337‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhatt 2012
- Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutrition Research (New York, NY) 2012;32:537‐41. [DOI] [PubMed] [Google Scholar]
Bird 2012
- Bird SR, Hawley JA. Exercise and type 2 diabetes: new prescription for an old problem. Maturitas 2012;72:311‐6. [DOI] [PubMed] [Google Scholar]
Boocock 2007
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiology, Biomarkers & Prevention 2007;16:1246‐52. [DOI] [PubMed] [Google Scholar]
Borenstein 2017a
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. [DOI] [PubMed] [Google Scholar]
Borenstein 2017b
- Borenstein M. Prediction intervals. www.meta‐analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. [DOI] [PubMed] [Google Scholar]
Brasnyo 2011
- Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the akt pathway in type 2 diabetic patients. British Journal of Nutrition 2011;106:383‐9. [DOI] [PubMed] [Google Scholar]
Brown 2010
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin‐like growth factor axis. Cancer Research 2010;70:9003‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2002
- Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. Journal of Agricultural and Food Chemistry 2002;50:3337‐40. [DOI] [PubMed] [Google Scholar]
Chow 2010
- Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, et al. Resveratrol modulates drug‐ and carcinogen‐metabolizing enzymes in a healthy volunteer study. Cancer Prevention Research (Philadelphia, Pa.) 2010;3:1168‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79‐85. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
El‐Kaissi 2011
- El‐Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Current Diabetes Reviews 2011;7:392‐405. [DOI] [PubMed] [Google Scholar]
Germino 2011
- Germino FW. Noninsulin treatment of type 2 diabetes mellitus in geriatric patients: a review. Clinical Therapeutics 2011;33:1868‐82. [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Version accessed 1 October 2018. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Hansen 2004
- Hansen JB, Arkhammar PO, Bodvarsdottir TB, Wahl P. Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders. Current Medicinal Chemistry 2004;11:1595‐615. [DOI] [PubMed] [Google Scholar]
Hart 2012
- Hart B, Lundh A, Bero L. Effect of reporting bias on meta‐analyses of drug trials: reanalysis of meta‐analyses. BMJ 2012;344:d7202. [DOI: 10.1136/bmj.d7202] [DOI] [PubMed] [Google Scholar]
Hausenblas 2015
- Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus ‐ systematic review and meta‐analysis. Molecular Nutrition & Food Research 2015;59(1):147‐59. [DOI] [PubMed] [Google Scholar]
Hemmingsen 2013
- Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD008143.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; Vol. 343:d5928. [DOI] [PMC free article] [PubMed]
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.cochrane.org. [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editors(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
La Porte 2010
- Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, et al. Steady‐state pharmacokinetics and tolerability of trans‐resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clinical Pharmacokinetics 2010;49:449‐54. [DOI] [PubMed] [Google Scholar]
Lagouge 2006
- Lagouge M, Argmann C, Gerhart‐Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1alpha. Cell 2006;127:1109‐22. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014
- Liu K, Zhou R, Wang B, Mi MT. Effect of resveratrol on glucose control and insulin sensitivity: a meta‐analysis of 11 randomized controlled trials. American Journal of Clinical Nutrition 2014;99(6):1510‐9. [DOI] [PubMed] [Google Scholar]
Martin‐Gronert 2012
- Martin‐Gronert MS, Ozanne SE. Metabolic programming of insulin action and secretion. Diabetes, Obesity & Metabolism 2012;14(Suppl 3):29‐39. [DOI] [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. [DOI] [PubMed] [Google Scholar]
McKinlay 2000
- McKinlay J, Marceau L. US public health and the 21st century: diabetes mellitus. Lancet 2000;356:757‐61. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moser 2012
- Moser EG, Morris AA, Garg SK. Emerging diabetes therapies and technologies. Diabetes Research and Clinical Practice 2012;97:16‐26. [DOI] [PubMed] [Google Scholar]
Neves 2012
- Neves AR, Lucio M, Lima JL, Reis S. Resveratrol in medicinal chemistry: a critical review of its pharmacokinetics, drug‐delivery, and membrane interactions. Current Medicinal Chemistry 2012;19(11):1663‐81. [DOI] [PubMed] [Google Scholar]
Palsamy 2008
- Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin‐nicotinamide induced experimental diabetic rats. Biomedecine & Pharmacotherapie [Biomedicine & Pharmacotherapy] 2008;62:598‐605. [DOI] [PubMed] [Google Scholar]
Palsamy 2009
- Palsamy P, Subramanian S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin‐nicotinamide‐induced diabetic rats. Chemico‐biological Interactions 2009;179:356‐62. [DOI] [PubMed] [Google Scholar]
Palsamy 2010
- Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta‐cell dysfunction in streptozotocin‐nicotinamide‐induced diabetic rats. Journal of Cellular Physiology 2010;224:423‐32. [DOI] [PubMed] [Google Scholar]
Pangeni 2014
- Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S. Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert Opinion on Drug Delivery 2014;11(8):1285‐98. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Rimando 2004
- Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. Journal of Agricultural and Food Chemistry 2004;52:4713‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2017
- Zhu X, Wu C, Qiu S, Yuan X, Li L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta‐analysis. Nutrition and Metabolism 2017;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]


