Abstract
Background
Increased intracranial pressure has been shown to be strongly associated with poor neurological outcomes and mortality for patients with acute traumatic brain injury. Currently, most efforts to treat these injuries focus on controlling the intracranial pressure. Hypertonic saline is a hyperosmolar therapy that is used in traumatic brain injury to reduce intracranial pressure. The effectiveness of hypertonic saline compared with other intracranial pressure‐lowering agents in the management of acute traumatic brain injury is still debated, both in the short and the long term.
Objectives
To assess the comparative efficacy and safety of hypertonic saline versus other intracranial pressure‐lowering agents in the management of acute traumatic brain injury.
Search methods
We searched Cochrane Injuries' Specialised Register, CENTRAL, PubMed, Embase Classic+Embase, ISI Web of Science: Science Citation Index and Conference Proceedings Citation Index‐Science, as well as trials registers, on 11 December 2019. We supplemented these searches with searches of four major Chinese databases on 19 September 2018. We also checked bibliographies, and contacted trial authors to identify additional trials.
Selection criteria
We sought to identify all randomised controlled trials (RCTs) of hypertonic saline versus other intracranial pressure‐lowering agents for people with acute traumatic brain injury of any severity. We excluded cross‐over trials as incompatible with assessing long‐term outcomes.
Data collection and analysis
Two review authors independently screened search results to identify potentially eligible trials and extracted data using a standard data extraction form. Outcome measures included: mortality at end of follow‐up (all‐cause); death or disability (as measured by the Glasgow Outcome Scale (GOS)); uncontrolled intracranial pressure (defined as failure to decrease the intracranial pressure to target and/or requiring additional intervention); and adverse events e.g. rebound phenomena; pulmonary oedema; acute renal failure during treatment).
Main results
Six trials, involving data from 287 people, met the inclusion criteria. The majority of participants (91%) had a diagnosis of severe traumatic brain injury. We had concerns about particular domains of risk of bias in each trial, as physicians were not reliably blinded to allocation, two trials contained participants with conditions other than traumatic brain injury and in one trial, we had concerns about missing data for important outcomes. The original protocol was available for only one trial and other trials (where registered) were registered retrospectively.
Meta‐analysis for both the primary outcome (mortality at final follow‐up) and for 'poor outcome' as per conventionally dichotomised GOS criteria, was only possible for two trials. Synthesis of long‐term outcomes was inhibited by the fact that two trials ceased data collection within two hours of a single bolus dose of an intracranial pressure‐lowering agent and one at discharge from the intensive care unit (ICU). Only three trials collected data after participants were released from hospital, one of which did not report mortality and reported a 'poor outcome' by GOS criteria in an unconventional way. Substantial missing data in a key trial meant that in meta‐analysis we report 'best‐case' and 'worst‐case' estimates alongside available case analysis. In no scenario did we discern a clear difference between treatments for either mortality or poor neurological outcome.
Due to variation in modes of drug administration (including whether it followed or did not follow cerebrospinal fluid (CSF) drainage, as well as different follow‐up times and ways of reporting changes in intracranial pressure, as well as no uniform definition of 'uncontrolled intracranial pressure', we did not perform meta‐analysis for this outcome and report results narratively, by individual trial. Trials tended to report both treatments to be effective in reducing elevated intracranial pressure but that hypertonic saline had increased benefits, usually adding that pretreatment factors need to be considered (e.g. serum sodium and both system and brain haemodynamics). No trial provided data for our other outcomes of interest. We consider evidence quality for all outcomes to be very low, as assessed by GRADE; we downgraded all conclusions due to imprecision (small sample size), indirectness (due to choice of measurement and/or selection of participants without traumatic brain injury), and in some cases, risk of bias and inconsistency.
Only one of the included trials reported data on adverse effects; a rebound phenomenon, which was present only in the comparator group (mannitol). None of the trials reported data on pulmonary oedema or acute renal failure during treatment. On the whole, trial authors do not seem to have rigorously sought to collect data on adverse events.
Authors' conclusions
This review set out to find trials comparing hypertonic saline to a potential range of other intracranial pressure‐lowering agents, but only identified trials comparing it with mannitol or mannitol in combination with glycerol. Based on limited data, there is weak evidence to suggest that hypertonic saline is no better than mannitol in efficacy and safety in the long‐term management of acute traumatic brain injury. Future research should be comprised of large, multi‐site trials, prospectively registered, reported in accordance with current best practice. Trials should investigate issues such as the type of traumatic brain injury suffered by participants and concentration of infusion and length of time over which the infusion is given.
Plain language summary
Concentrated salt solution versus other treatments to lower pressure around the brain for people with acute traumatic brain injury
Review question
We reviewed the evidence for the effectiveness and safety of infusions (where a substance is given through a vein) of hypertonic saline (concentrated salt (sodium chloride) solution) compared with other types of infusion for lowering intracranial pressure (pressure in and around the brain) in the management of acute traumatic brain injury.
Background
Acute traumatic brain injury (sudden and severe injury to the brain, often due to accidents) is a leading cause of death and disability worldwide, especially in children and young people. Intracranial hypertension (the build‐up of high pressure within and around the brain) is common after damage to the brain. This is because the skull is a rigid compartment that contains three parts: soft brain tissue, blood, and cerebrospinal fluid. If an increase occurs in the volume of one component, such as hematomas (collections of blood) within the brain's soft tissue, the volume of one or more of the other components must decrease ‐ otherwise intracranial pressure will rise. If intracranial pressure increases beyond certain limits, there is an imbalance, and blood flow to the brain becomes dangerously low. This high intracranial pressure can cause serious effects that include brain damage and death. Hyperosmolar therapy is an important treatment for raised intracranial pressure. One kind of hyperosmolar therapy involves an infusion of concentrated (hypertonic) saline (table salt/sodium chloride) solution into the blood; other treatments including mannitol (a form of sugar) can also be used. Such treatments may lower intracranial pressure by reducing water volume inside and between brain cells.
Trial characteristics
In December 2019, the authors of this review searched for randomised trials comparing the effects and safety of hypertonic saline with other fluid infusions that are used to lower intracranial pressure in people with acute traumatic brain injury. The review authors searched a wide variety of medical databases and identified six relevant trials, with data from a total of 287 participants. The trials were all randomised controlled trials, which produce the most reliable evidence. Three trials took place in India, one each in France and Germany, and one included people from both France and Israel. Most people in the trials (91%) had traumatic brain injury. Trials compared various concentrations of hypertonic saline with either mannitol or mannitol in combination with glycerol.
Key results
Based on limited data of these six trials, there is no clear evidence to support the use of hypertonic saline infusion over mannitol infusion for people with acute traumatic brain injury. Adverse effects of the treatments were not routinely measured.
More research is needed. Future trials should be larger and better reported. Potential points for research include investigating whether there is a particular concentration of infusion, or length of time over which the infusion is given, that benefits people with raised intracranial pressure after traumatic brain injury.
Summary of findings
Summary of findings for the main comparison. Hypertonic saline compared with other intracranial pressure‐lowering agents for acute traumatic brain injury.
| Hypertonic saline compared with other intracranial pressure‐lowering agents for acute traumatic brain injury | ||||||
|
Patient or population: people with acute traumatic brain injury Settings: intensive care units Intervention: hypertonic saline (between 3% and 7.5% solution) delivered by infusions Comparison: all other intracranial pressure‐lowering agents eligible (but mannitol infusions the sole comparator within the included trials) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (RCTs) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other intracranial pressure‐lowering agents (mannitol or mannitol with glycerol) | Hypertonic saline | |||||
| Mortality (short‐term) | 2 RCTs reported mortality in the short term, prior to discharge from hospital. Meta‐analysis was not possible. 1 RCT (n = 38) reported that 3/18 participants in the HTS group and 1/20 in the mannitol group died in the first 6 days following treatment, after which no further deaths occurred in the HTS group but a further 9 deaths occurred in the mannitol group prior to discharge. At this time point, there is a slight trend favouring HTS compared to mannitol (RR 0.33, 95% 0.11 to 1.02). The other RCT (n = 32, in which only a third of participants had TBI) reported that 7/17 (41.2%) participants in the HTS group and 9/15 (60%) in the mannitol group died by the end of stay in the ICU. Here, HTS did not reduce all‐cause mortality in people with acute TBI (RR 0.69, 95% CI 0.34 to 1.39). |
70 (2 RCTs) |
⊕⊝⊝⊝ Very lowa |
Not advisable to pool data due to difference in time of assessment and in populations | ||
| Mortality at 6 months | 35.6% risk (available case) 35.6% risk (worst case for intervention, HTS) 44.4% risk (best case for intervention, HTS) |
30% risk (available case) 40% risk (worst case for intervention, HTS) 30% risk (best case for intervention, HTS) |
RR 0.84 (0.46 to 1.55) (available case) RR 1.12 (0.66 to 1.89) (worst case for intervention, HTS) RR 0.67 (0.38 to 1.18) (best case for intervention, HTS) |
85 (2 RCTs) | ⊕⊝⊝⊝ Very lowb |
Pooling done with available, best‐case and worst‐case scenarios. In no case do results show a clear difference between groups. |
|
Glasgow Outcome Scale: poor outcome at 6 months |
66.7% risk (available case) 66.7% risk (worst case for intervention, HTS) 75.6% risk (best case for intervention, HTS) |
72.5% risk (available case) 82.5% risk (worst case for intervention, HTS) 72.5% risk (best case for intervention, HTS) |
RR 1.09 (0.82 to 1.44) (available case) RR 1.24 (0.97 to 1.58) (worst case for intervention, HTS) RR 0.96 (0.74 to 1.24) (best case for intervention, HTS) |
85 (2 RCTs) | ⊕⊝⊝⊝ Very lowb |
Pooling done with available, best‐case and worst‐case scenarios from two studies which supplied sufficient data. One study on children reported GOS non‐traditionally (defining 'poor outcome' as survival with any level of disability excluding persistent vegetative state and found no clear difference between groups |
| Uncontrolled ICP during treatment |
HTS vs mannitol 1 RCT reported a difference in the magnitude of ICP reduction and found both HTS and mannitol effectively and equally reduced ICP levels with subsequent elevation of CPP and CBF, although this effect was significantly stronger and of longer duration after HTS. Another 2 RCTs reported a difference in the ratio of uncontrolled ICP between the 2 groups, and the definition of uncontrolled ICP in these 2 RCTs differed, as did the time frames for data collection. 1 of these RCTs found both interventions to be effective, but added pretreatment factors need to be considered (e.g. serum sodium and haemodynamics). The 5th RCT found HTS to be more effective for increased ICP than mannitol but cautioned the benefit in this trial might be explained by "local osmotic effects". 2 RCTs in which intervention was only given after CSF drainage had failed, reported the mean fall in ICP following a dose averaged over hundreds of episodes across 4‐6 days of treatment. Of these 2 RCTs, 1 found HTS to be superior to mannitol; the other found no clear difference between groups. HTS vs mannitol vs mannitol plus glycerol A 6th RCT comparing HTS with mannitol and also with mannitol plus glycerol reported means and ranges of change within an hour following a single dose. They reported all 3 hyperosmolar agents (HTS, mannitol and mannitol plus glycerol) as effective, but HTS was slightly superior, effecting a greater change in reducing ICP, and more quickly, than other agents, while at a lower dose. |
287 (6 RCTs) |
⊕⊝⊝⊝ Very lowb |
It is not possible to pool these data because of variations in timings and ways of reporting ICP | ||
| A rebound phenomenon during treatment | None of the RCTs reported on this outcome systematically, although it is mentioned in passing as potentially affecting those treated with mannitol in 1 RCT. | 32 (1 RCT) |
⊕⊝⊝⊝ Very lowc |
This outcome was reported in just 1/4 RCTs available. | ||
| Pulmonary oedema during treatment | No data available | This outcome was not reported. | ||||
| Acute renal failure during treatment | No data available | This outcome was not reported. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPP: cerebral perfusion pressure; CSF: cerebrospinal fluid; HTS: hypertonic saline; ICP: Intracranial pressure: ICU: intensive care unit; RCT: randomised controlled trial; RR: risk ratio; TBI: traumatic brain injury | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aWe downgraded for imprecision (low number of participants), risk of bias and indirectness (1 trial included a mixed population of whom only a third had TBI). bWe downgraded for imprecision, inconsistency and risk of bias. cWe downgraded for imprecision, indirectness and risk of bias.
Background
Description of the condition
Traumatic brain injury is a major cause of death and disability worldwide (Corrigan 2010). Intracranial hypertension secondary to traumatic brain injury is well known to have a profound influence on outcome, and severe intracranial hypertension has been associated with higher morbidity (Miller 1977). Intracranial pressure is the pressure in the cranium, and the brain has a very limited ability to compensate for haemorrhage, swelling, oedema, or mass effects due to the invariant constraints of the cranial vault (Stevens 2012). Intracranial pressure in the intact cranium is determined by the brain parenchyma tissue pressure, presence of mass lesions, cerebral blood volume, and intracranial cerebrospinal fluid volume (Greve 2009). Intracranial hypertension is defined as a sustained (longer than five minutes) elevation of ICP above 20 mmHg (Bratton 2007). Sustained intracranial hypertension indicates life‐threatening neurological emergencies that require immediate recognition and treatment to prevent irreversible injury and death. In a review of trials of the value of intracranial pressure in predicting outcomes in traumatic brain injury, the risk of death was 18.4% for participants with intracranial pressure less than 20 mmHg and 24.8% for participants with intracranial pressure between 20 mmHg and 40 mmHg but 55.6% for those with intracranial pressure greater than 40 mmHg (Treggiari 2007). Achieving a sustained reduction in elevated intracranial pressure remains a focus of neurocritical care.
Description of the intervention
Currently available medical treatments for raised intracranial pressure include hyperosmolar therapy, sedation and paralysis, hyperventilation, barbiturates, hypothermia, steroids and surgical intervention (Rangel‐Castillo 2008).
Hyperosmolar therapy is the cornerstone of pharmaceutical treatment for intracranial hypertension. The physiological basis of osmotherapy was first published by Weed and Mckibben (Weed 1919). Intravenous injection of a hypertonic solution was followed by a marked decrease in size of the brain. Since that time, mannitol, a sugar alcohol that acts as an osmotic diuretic, causing sustained hyperosmolarity by dehydration, has become the most widely used hyperosmolar solution to treat elevated intracranial pressure. Increasingly, hypertonic saline has emerged as an alternative hyperosmolar agent after several trials reported its relative superiority, especially for refractory intracranial pressure (Horn 1999; Khanna 2000; Oddo 2009).
Hypertonic saline firstly gained attention as a potentially more effective alternative to normal saline in the initial resuscitation of haemorrhagic shock (Gunnar 1986). A survival benefit was shown when used for patients with combined haemorrhagic shock with head injury. The favourable effect on survival was attributed to the hyperosmolar characteristics of hypertonic saline and the resultant reduction in intracranial pressure. Since then, more clinical trials have found that the intravenous bolus administration of hypertonic saline resulted in a sustained reduction of intracranial pressure on patients with traumatic cerebral oedema, even when elevated intracranial pressure is resistant to other intracranial pressure‐lowering agents including mannitol (Ziai 2007).
Although treatment protocols for administering hypertonic saline vary (Mortazavi 2012), retrospective trials suggest a definite intracranial pressure reduction is observed with the use of hypertonic saline independent of the dosage, the concentration or the administration strategy (Lewandowski‐Belfer 2014; Maguigan 2017; Roquilly 2011). Reported concentration and volume of hypertonic saline for clinical use range from 2% to 23.4% in concentration and 10 to 30 mL/kg in volume (Mortazavi 2012).
How the intervention might work
The intracranial pressure‐lowering mechanisms of hypertonic saline solutions are believed to be due to their effects on microcirculation and osmotic action (Ziai 2007). Hypertonic saline solutions decrease serum viscosity and hematocrit, leading to an increase in cerebral perfusion and causing cerebral arteriole vasoconstriction that reduces cerebral blood volume and intracranial pressure. Water always flows from body compartments with low osmolality to those with higher osmolality. Hypertonic saline solutions increase plasma osmolality after administration, thus promoting gradual movement of water from tissues into the circulation. As fluid moves into the vascular space and is carried away by the blood, the brain shrinks and intracranial pressure is reduced.
Why it is important to do this review
Hyperosmolar therapy is standard practice in most neurosurgical centres worldwide (Bratton 2007). The recent consensus suggest the use of mannitol or hypertonic saline solutions for reducing increased intracranial pressure in neuro‐intensive care patients (Oddo 2018). Guidelines for the management of severe pediatric brain traumatic injury recommend bolus hypertonic saline (3%) in patients with intracranial hypertension (Kochanek 2019). Intravenous infusion of mannitol has been considered by some to be the ‘gold standard’ for the treatment of increased intracranial pressure, mostly due to its long history (Marko 2012). Some clinical trials suggest that hypertonic saline solutions can reduce raised intracranial pressure (Horn 1999; Kerwin 2009; Khanna 2000; Worthley 1988), but its use can still be controversial in the field of TBI. We undertook this review to enable better understanding of the comparative efficacy and safety of hypertonic saline versus other intracranial pressure‐lowering agents for people with acute traumatic brain injury.
Objectives
To assess the comparative efficacy and safety of hypertonic saline versus other intracranial pressure‐lowering agents in the management of acute traumatic brain injury.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) with a parallel design. We excluded cross‐over trials as incompatible with assessing long‐term outcomes.
Types of participants
We included participants of any age, with clinically defined traumatic brain injury of any severity, seen in the acute setting.
Types of interventions
Any hypertonic saline in any dosage for any duration, given at any time within eight weeks following injury. Hypertonic saline had to be compared with another intracranial pressure‐lowering agent, such as mannitol, barbiturates or steroids.
We excluded any trials that used sodium lactate as an hypertonic saline, as, although it is a hyperosmolar solution, its effects cannot be attributed to a classical osmotic effect (Ichai 2009; Ichai 2013). Sodium lactate differs fundamentally from sodium chloride because lactate is a metabolisable anion; this means that even with comparable osmolarity in vitro, sodium lactate becomes two times less hypertonic than equiosmotic sodium chloride in the body.
Types of outcome measures
Primary outcomes
Death at final follow‐up (grouped by period of reporting e.g. short term (while in ICU); long term (at six months)
Secondary outcomes
'Poor outcome' (death, persistent vegetative state or severe disability) at final follow‐up (as measured by the Glasgow Outcome Scale (GOS); Jennett 1975; Teasdale 1974). The GOS score, where possible, was converted into a dichotomous outcome. A 'poor outcome' is defined above; a ‘good outcome’ includes GOS categories of moderate disability or good recovery.
Uncontrolled intracranial pressure during treatment (we define uncontrolled intracranial pressure as failure to decrease the intracranial pressure to target and/or requiring additional intervention; Burgess 2016).
A 'rebound phenomenon' during treatment (we define rebound phenomenon as intracranial pressure rising above its original level after hyperosmolar therapy. Leakage of osmotic agent into the brain parenchyma through an altered blood brain barrier and secondary reversal of osmotic gradient with subsequent increase in brain oedema is considered the major cause of rebound).
Pulmonary oedema during treatment.
Acute renal failure during treatment.
Sample size calculation
At protocol stage, we judged that 474 people are required to have a 90% chance of detecting, at a significance level of 5%, a decrease in death from 27% in the control group to 15% in the experimental group (Lu 2005).
Search methods for identification of studies
We did not restrict searches by date, language or publication status.
Electronic searches
The Cochrane Injuries' Information Specialist ran searches in December 2019 (Appendix 1); we also report earlier searches (Appendix 2). The main amendment in the updated search in February 2017 was the inclusion of a more sensitive list of terms for hypertonic saline solution and hyperosmolar therapy. Searches were back‐dated to accommodate these changes. Additional searches were run in Chinese databases (see below) in August 2018. No relevant records resulted from the latter searches, and these were not re‐run in 2019.
The Information Specialist ran searches on the following databases (all years):
Cochrane Injuries' specialised register (11 December 2019);
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 12) in the Cochrane Library;
Ovid MEDLINE databases (1946 to 11 December 2019);
Embase Classic + Embase (OvidSP) (1947 to 11 December 2019);
ISI Web of Science: Science Citation Index‐Expanded (SCI‐EXPANDED) (1970 to 11 December 2019);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 11 December 2019).
Shuang Yang Liu, from the Department of Documentation and Retrieval, Xiangya Medical College, Central South University, searched the following Chinese databases in December 2013, November 2017 and September 2018:
ChinaBiologyMedicinedisc (CBMdisc);
Wanfang Data;
China National Knowledge Infrastructure (CNKI);
VIP Database for Chinese Technical Periodicals.
The CBMdisc search strategy (Appendix 3) was adapted as necessary for each of the other databases.
Searching other resources
We also searched the following clinical trials registers (December 2019):
Clinicaltrials.gov (www.clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
We checked the reference lists of all identified relevant articles.
Data collection and analysis
Selection of studies
Two review authors (HC and ZS) independently screened the search results and discussed the trials eligible for inclusion for searches run in 2013, examining each potential title. If titles were ambiguous, we read the abstracts. We resolved disagreement about inclusion of one trial (Ichai 2009), by seeking the advice of the trial's correspondence author by email (Ichai 2013), as the trial author informed us that they had used sodium lactate to decrease the raised intracranial pressure, and that sodium lactate differs fundamentally from sodium chloride because the absence of significant modification of plasma osmolality does not support a pure osmotic effect of sodium lactate, we excluded this trial.
For the searches run after in 2017 and thereafter, JD assisted HC in screening the English language results.
Data extraction and management
Two review authors (HC and ZS; HC and JD) independently extracted and recorded the data on specially designed forms and subsequently cross‐checked the data. We collected the following data from the trial reports: trial design, participant characteristics, intervention characteristics, outcome data, and adverse effects. Participant characteristics included age, sex and traumatic brain injury severity. Intervention characteristics included concentration, dosage, timing of administration and duration of intervention. Outcome measures included uncontrolled intracranial pressure (however defined by trial authors: our own definition was "failure to decrease the intracranial pressure to target and/or requiring additional intervention", mortality and disability according to GOS score (Jennett 1975; Teasdale 1974), dichotomised in the standard way. We used Review Manager 2014 software in the completion of this review.
Assessment of risk of bias in included studies
Two review authors (HC and JD) independently assessed the risk of bias for each included trial. We evaluated six domains: sequence generation, allocation concealment, blinding (subdivided into blinding of participants, treating physicians, and outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias. We judged the risk of bias in each category as high risk, low risk or unclear risk according to guidance on use of the risk of bias tool within The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We approached the contact authors of all included trials to ask for clarification of trial methods and to request the trial protocols (where available). We intended to resolve any disagreements by consensus; however, no disagreements arose between review authors on the 'Risk of bias' judgements.
Measures of treatment effect
For dichotomous data, we present results as summary risk ratios (RRs) with 95% confidence intervals (CIs). Additionally, as described above, we determined at the protocol development stage that we would transform the GOS score into a dichotomous outcome. 'Death or disability' would mean death, persistent vegetative state and severe disability; a 'good outcome' would include moderate disability and good recovery.
Where trials presented other data as continuous data, such as 'uncontrolled intracranial pressure during treatment', we proposed to use mean differences (MDs) or standardised mean differences (SMDs), if data were measured in varying scales, with 95% CIs between the trial groups.
Unit of analysis issues
We did not include cross‐over RCTs in this review, although we identified several. For rapidly changing intracranial pressure, we determined at protocol stage that although feasible, cross‐over trials were not suitable for our review question, given that our primary outcome (death) was a long‐term one and that even in the short term, 'carry‐over' effects confound the estimates of the treatment effects in rapidly changing intracranial pressure.
Our searches did not identify any cluster‐randomised trials. For the one trial included within this review with more than two eligible arms, data were unsuitable for pooling with those from other trials. Therefore, for this review, the unit of analysis is the individual participant.
Dealing with missing data
As planned at protocol stage, we made every effort to contact trial authors to acquire missing data. We initially planned to prefer data reported according to intention‐to‐treat principles. As this was not possible in one key trial, we conducted best‐case and worst‐case analyses for the primary outcome (death at longest follow‐up) and also for GOS (in trials that provided data suitable for this purpose).
Assessment of heterogeneity
We planned at protocol stage to use the Chi2 test and the I2 statistic (Higgins 2003), to assess statistical heterogeneity and we report these values, where relevant, in Results. Heterogeneity in terms of treatment protocols, mode and timing of intervention delivery are described below, and discussed in relevant sections of the review (Deeks 2019).
Assessment of reporting biases
In the future, if meta‐analysis is feasible and if more than 10 trials are available for the primary outcome, we plan to use a funnel plot to assess publication bias. In this version of the review, we compared the trial protocols or trial registrations (where available) with the published reports, to assess the likelihood of selective outcome reporting.
Data synthesis
We planned, if data were available for the same outcome measure in more than one trial, to attempt a meta‐analysis, analysing outcomes providing dichotomous data using the RR and 95% CI using a fixed‐effect model, conducting all analyses using Review Manager 5 (Review Manager 2014). We did not anticipate continuous outcomes at protocol stage, but henceforth plan to do so utilising MD where the same scales are used, and SMD where different trials report different scales, with 95% CI.
Subgroup analysis and investigation of heterogeneity
We planned to explore the effects by subsets of participants (children and adults) and by subsets of interventions (e.g. different dosage and different duration, with or without a colloid), but data were not sufficient for such analyses for the present version of the review. We will attempt these subgroup explorations in future versions of the review if and when there are sufficient data.
Sensitivity analysis
We planned to perform a sensitivity analysis for allocation concealment (adequate vs 'unclear' or 'not done'), but there were not enough trials to enable us to do so.
Results
Description of studies
See also: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Initial searches run in December 2013 identified 2841 records. After deduplication, 2488 potentially relevant search results remained, which we (HC and ZS) screened. We excluded 2478 of these records on the basis of their title or abstract. We examined the full text of the 10 remaining reports, and thus identified three trials that met the inclusion criteria, and seven were formally excluded.
An update search (10 February 2017) retrieved 1249 new records, of which 740 proved to be internal duplicates or overlaps with previous searches, yielding 509 unique records. We identified and included one further eligible trial (Jagannatha 2016), and assessed and excluded a further 11 reports. We ran 'top‐up' searches in August 2018 and in December 2019. The 2018 searches found 265 records, 204 records when deduplicated, which we screened. We did not include any eligible trials, but nine were formally excluded at full‐text stage. The 2019 searches identified 217 records, 180 when deduplicated. Of these, we identified and included two eligible trials (Kumar 2019; Patil 2019), and formally excluded two trials. The trial identification process is outlined in Figure 1 (Moher 2009).
1.
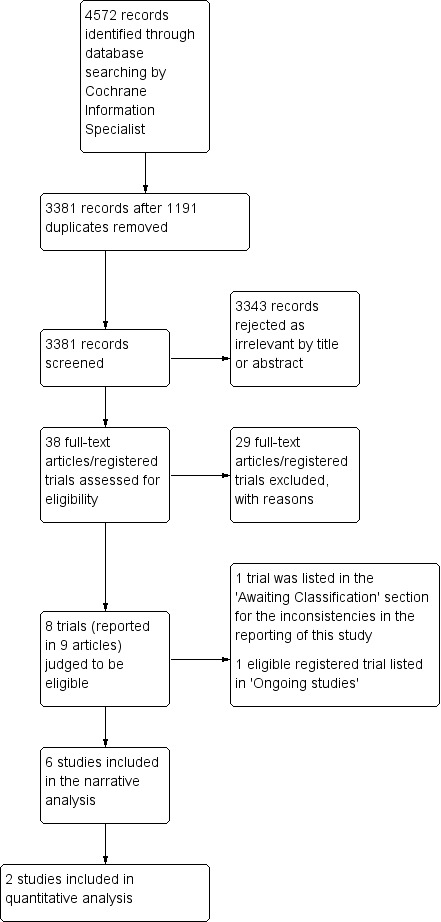
Study flow diagram
We have identified one large trial as ongoing. In March 2019, we learned of a relevant RCT soon to commence in the UK, likely to report after 2023 (Salt or Sugar 2019), details of which can be found in Characteristics of ongoing studies; a record of this was identified in register searches later in the year.
Included studies
Design
All six included trials (Cottenceau 2011; Francony 2008; Harutjunyan 2005; Jagannatha 2016; Kumar 2019; Patil 2019), were conducted as parallel, RCTs. Five involved two arms and one (Patil 2019), three arms.
Sample sizes
Sample sizes tended to be small, ranging from 20 (Francony 2008), to 120 (Patil 2019). Data from 287 participants overall are included within this review, but as described below, 9% of these participants did not have diagnoses of traumatic brain injury. At protocol stage, we had estimated that 474 people were required to have a 90% chance of detecting, as significant at the 5% level, a decrease in death from 27% in the control group to 15% in the experimental group (Lu 2005); this target was manifestly not met within this review.
Only one trial ‐ the smallest included within the review (Francony 2008, n = 20), which did not collect data beyond 120 minutes of infusion, reported undertaking a power calculation, using the following assumptions: "The study population size for the trial was calculated assuming a 40% ± 15% ICP [intracranial pressure] in the HSS group and a 20% ± 15% ICP reduction in the mannitol group, according to previous studies ....Based on the formula for a normal distribution and assuming a two‐sided type I error of .05 and a power of .80, ten patients in each of the two groups were required" (Francony 2008, p 796).
No other trial reported undertaking a sample size calculation a priori, and all other trials (with the exception of Patil 2019), describe their small sizes as limitations. The authors of Jagannatha 2016 (n = 38), and Kumar 2019 (n = 30), both explicitly reported small trial size underneath the 'limitations' sections of their papers; likewise, investigators involved in Cottenceau 2011 (n =47), comment that "although the number of included patients was significantly larger than the number of patients included in most other comparable studies, the figures remained smaller than what would be needed to draw definite conclusions, especially when differences observed between subgroups are analyzed" (Cottenceau 2011, p. 210). Authors of Harutjunyan 2005 (n =32) also mention "the small patient population of each group" as a limitation, especially in the context of the "heterogeneity in the underlying neurological illness" (p R530).
Setting
All trials took place in ICUs, usually based within university hospitals. Three trials were conducted in India (Jagannatha 2016; Kumar 2019; Patil 2019), one trial each in France (Francony 2008), and Germany (Harutjunyan 2005), whilst the sixth trial included participants from both France and Israel (Cottenceau 2011). Trials were published between 2005 and 2019; the earliest recruitment period appears to have commenced in 2002 (Francony 2008), and the latest in 2015 (Patil 2019).
Participants
Inclusion and exclusion criteria varied amongst trials. Two were restricted solely to participants with traumatic brain injury severe enough to require intracranial pressure monitoring (Cottenceau 2011; Jagannatha 2016); a third (Francony 2008), required that they were stable patients (with or without traumatic brain injury) with sustained elevated intracranial pressure of 20 mm Hg for 10 minutes, not related to procedural pain (resulting in a participant group of whom 85% (17/20) had severe traumatic brain injury and the remainder of whom had suffered strokes). The fourth included trial required that participants have severe brain damage (Glasgow Coma Scale (GCS) < 8) with cerebral oedema, resulting in a participant group of whom only 31% (10) had a diagnosis of traumatic brain injury (the remainder had subarachnoid haemorrhage (9); brain infarctions (7); intracerebral haemorrhage (4) or "other" (2) (Harutjunyan 2005)). The inclusion criteria for Patil 2019 were that participants be screened by CT "to eliminate the need for surgery, then included if they had a GCS ≤8, and had sustained elevated ICP of >20 mm Hg for more than 5 minutes". The sole paediatric trial (Kumar 2019), required that participants have severe traumatic brain injury, defined as a score of ≤8 on the Pediatric GCS, and present within 24 hours of trauma.
The one trial that focused on children under 16 years (Kumar 2019), did not report an overall mean for age, but provided ranges of ages. Participants were very young; 18 out of 30 were under five years of age, and one child was 22 months old. Within the trials largely focused on adults, minimum age criteria ranged from 15 (Jagannatha 2016), to 16 (Cottenceau 2011), to 18 years (Francony 2008; Harutjunyan 2005); the mean ages of included participants varied from around 30 (Jagannatha 2016) to 47 years (Harutjunyan 2005). In the four trials that reported on gender, male participants constituted the majority.
In terms of severity, participants ranged from means of GCS scores on admission of 7 (SD 2) to 8 (SD 2) in the two groups within the Francony 2008 trial (n = 20), to the most severely affected participants within the review, those within the Jagannatha 2016 trial (n = 38; median GCS scores = 4 in the hypertonic saline group (range 4 to 5) and 5 in the mannitol group (range 4 to 6). Authors of the two 'mixed' trials, which did not focus on traumatic brain injury, did not report the source of traumatic brain injuries (Francony 2008; Harutjunyan 2005); authors of the traumatic brain injury‐only trials (Jagannatha 2016; Cottenceau 2011; Kumar 2019; Patil 2019), did. Where reported, road traffic accidents accounted for the majority of traumatic brain injuries, followed by falls, assaults and 'other'. Three trials explicitly categorised the nature of lesions, for example, extradural haematoma, subdural haematoma, contusions, intraventricular haemorrhage, diffuse axonal injury (Cottenceau 2011; Jagannatha 2016; Kumar 2019).
Interventions
Initiation of therapy; mode of intracranial pressure assessment
All six trials provided a detailed treatment protocol showing the stages by which clinicians determined if hyperosmolar therapy was indicated. In one trial (Cottenceau 2011), hyperosmolar therapy was initiated when intracranial pressure elevation was above 15 mmHg. In Kumar 2019, the sole pediatric trial, trial authors aimed at maintaining an intracranial pressure of below 15 mmHg in children between 1 and 10 years of age and 18 mmHg in children aged 11 to 16 years of age, and treatment was only administered if intracranial pressure remained persistently above the cut‐off value for more than five minutes, in spite of cerebrospinal fluid drainage. The four other included trials (Francony 2008; Harutjunyan 2005; Jagannatha 2016; Patil 2019), initiated the trial medications if intracranial pressure exceeded the 20 mmHg threshold; Jagannatha 2016 stood out amongst the adult trials for initiating treatment only after cerebrospinal fluid drainage.
It must be noted that methods of intracranial pressure assessment differed between trials. Two trials (Harutjunyan 2005; Francony 2008), used a standard intraparenchymal intracranial pressure device (Codman Microsensor intracranial pressure Monitoring System; Codman & Shurtleff Inc, Raynham, MA, USA); one used a subdural bolt (Patil 2019), one trial did not state technology used (Cottenceau 2011), two used an intraventricular device alongside an extraventricular device allowing cerebrospinal fluid drainage (Jagannatha 2016; Kumar 2019). In these latter trials, the hyperosmolar treatment that is the subject of this review was only initiated if cerebrospinal fluid drainage failed to control intracranial pressure, compelling one set of trial authors to see hyperosmolar treatment in this context as a 'second‐tier' and not a first‐line therapy (Kumar 2019).
Concentrations, duration of therapy and comparators
Four trials compared equiosmolar doses of hypertonic saline to mannitol; a fifth, equiosmolar doses of hypertonic saline, mannitol and mannitol in combination with glycerol, the sixth trial featured hypertonic saline hydroxyethyl starch versus mannitol. Concentrations and durations of infusions varied and are presented, put in order of concentration of hypertonic saline, as follows:
one trial (n = 120) compared 3% hypertonic saline with 20% mannitol and with mannitol 10% plus 10% glycerol combination; "infused via the central venous line at a defined infusion rate, that is, 6 mL/minute or 120 drops/minute (osmolarity of mannitol, mannitol plus glycerol combination, and 3% HTS [hypertonic saline] are almost the same, ie, 1100 mOsm/L, 1049 mOsmo/L, and 1027 mOsm/L, respectively). The infusion was stopped when ICP [intracranial pressure] was reduced to <15 mm Hg, which was our treatment goal" (Patil 2019, e222). Assessment stopped after a single infusion;
-
two trials (Jagannatha 2016 (n = 38); Kumar 2019 (n = 30)) compared administration of either 3% saline or 20% mannitol in an equiosmolar dose infused as a bolus through a central venous catheter over five minutes. Both mannitol and hypertonic saline were administered as 2.5 mL/kg doses. Both trials dealt with multiple episodes of raised intracranial pressure over four to six days, following cerebrospinal fluid drainage as mentioned above;
Jagannatha 2016 administered a maximum of three doses of the same drug if the first dose of the osmotic agent failed to decrease the intracranial pressure to below 20 mmHg;
Kumar 2019 administered a maximum of two doses if the agent failed to decrease the intracranial pressure to one of two targets based on the age of the child (15 mmHg or 18 mmHg);
one trial (n = 32) compared 7.2% hypertonic saline hydroxyethyl starch (200/0.5) 6% versus 15% mannitol, infused via the central venous line using an automated infusion system at a defined infusion rate (Harutjunyan 2005). The infusion was stopped when intracranial pressure was reduced to less than 15 mmHg, defined as the treatment goal. Multiple infusions were often required; assessment stopped after discharge from ICU.
one trial (n = 20) compared a single infusion of 100 mL of 7.45% saline (osmolarity, 2548 mOsm/L; hypertonic saline group) versus 231 mL of 20% mannitol (osmolarity, 1100 mOsm/L; mannitol group) for 20 minutes of administration via the central venous catheter (Francony 2008). Assessment was performed during a study period of 120 minutes;
one trial (n = 47) compared hypertonic saline 7.5% (2 mL/kg) versus mannitol 20% (4 mL/kg) delivered intravenously for 20 minutes (Cottenceau 2011); and as long as intracranial pressure remained elevated and monitored, all participants had a daily evaluation during which a baseline assessment was followed by two additional tests performed at 30 and 120 minutes after administration of hypertonic saline or mannitol.
Outcomes
The length of follow‐up (from one hour post infusion, to six months) impacted on the range of outcomes chosen. Four of six trials assessed mortality; one at end of stay in ICU, one at discharge from hospital and two at six months. We cannot disaggregate mortality data from the overall neurological outcome (GOS categories) in two trials (Cottenceau 2011;Kumar 2019).
All trials measured intracranial pressure, although in different ways and by different means.
Neurological outcome, even when assessed by a single instrument (GOS) used within three of six trials, did not make comparison straightforward. At six months, two trials reported GOS results dichotomised in the traditional way, grouping death, persistent vegetative state and severe disability together as a 'poor' outcome (Cottenceau 2011; Jagannatha 2016), a third trial dichotomised a poor outcome as death or persistent vegetative state, versus all other outcomes (Kumar 2019). Patil 2019 was short term in nature and so measured only the GCS at baseline and one hour after a successful infusion.
No trial appeared systematically to assess adverse effects.
Each trial is described in more detail in the Characteristics of included studies table and a separate table listing data reported by each trial at baseline and within the trial is also supplied (Table 2).
1. Data collected within studies.
| Study ID | Cottenceau 2011 | Francony 2008 | Harutjunyan 2005 | Jagannatha 2016 | Kumar 2019 | Patil 2019 |
| Baseline measures | ||||||
| Demographic data (age, source of injury) | Demographic data (age, gender, weight) | Demographic data (age, gender, weight) | Demographic data (age, gender, source and type of injury, duration from injury to hospital ) | Demographic data (age, gender, source and type of injury, duration from injury to EVD insertion) | Demographic data apparently collected, but not reported. Source of injury | |
| Neurological condition data included: initial GCS score; CT scan studies analyzed using Marshall criteria considering presence of basal cisterns compression, midline shift > 5mm, and lesions > 25cm3 in volume. Lesions were categorized into two subgroups: diffuse and focal | Neurological condition data at baseline including: GCS score; 'preserved cerebral autoregulation'; 'injury to studied treatment (time in days), diagnosis (not all participants had TBI) | Neurological condition data at baseline including: initial GCS score; SAPS, 'basic illness', brain infarct or not) etc; "simplified acute physiology score" | Neurological condition data at baseline including: Initial GCS score; GCS score post‐resuscitation; median GCS score at inclusion to trial; Predominant lesion on CT scan | Neurological condition data at baseline including: Post‐resuscitation GCS; motor score; Marshal CT grade; predominant lesion in CT scan | Neurological condition data at baseline: GCS | |
| ICP data collected during trial | ||||||
| Fall in ICP (mean and SD before infusion, after 30 min, after 120 min, comparisons made using repeated measures model of ANOVA) | Fall in ICP reported as percentage decline from baseline values (mean ± SD) | Fall in ICP (mean and range before infusion, terminating infusion, after 10 min, 30 min, 60 min); percentage decline calculated | Absolute values of ICP; response to individual boluses of agents (means and SDs) | Mean reduction in ICP | Minimum and maximum ICP (mmHG) at 1 hr | |
| ICP responses also compared based on analysis of percentages of the baseline (instead of absolute values) | Time course of ICP changes | Time required to reduce ICP below 20mm Hg | Duration of monitoring | Mean duration of ICP monitoring | Maximum change in ICP in percentage | |
| Day 1: hours of ICP > 20 | Duration of ICP fall | 24 h mean ICP monitoring | Dose and time required to reduce ICP below 15mm Hg | |||
| Numbers of ICP elevations during average monitoring duration of 3.7 to 3.8 days | 24 h mean ICP monitoring | ICP tracings | ||||
| Time required to achieve ICP < 20 mmHg | Mean numbers of raised ICP episodes | |||||
| Duration of time ICP was maintained at < 20 mmHg in a given day | ||||||
| 'Effective' doses/'ineffective' doses (as regards ICP) | Mean number of doses per day; instances of refractory ICP (mean (SD)) (defined as persistently elevated ICP despite 3 consecutive doses) | |||||
| Other interventions recorded | ||||||
| Surgical intervention | Inotrope intervention/duration of inotrope | Surgery for evacuation of EDH, depressed skull fracture or contusion | ||||
| Barbiturate intervention | Ionotropes | |||||
| CSF drainage | CSF drainage | |||||
| Other data measured in ICU | ||||||
| Common measures | MAP | MAP | MAP | MAP | MAP | |
| CPP | CPP | CPP | CPP | CPP | ||
| CVP | CVP | |||||
| Heart rate | Heart rate | Heart rate | Heart rate | |||
| Serum Na | Serum Na | Serum Na | Serum Na | Serum Na | ||
| Cerebral metabolic rate of glucose (CMRGlc (mg/100 g/min)) | Blood glucose | Blood glucose | ||||
| Serum osmolality | Serum osmolality | Serum osmolality | Serum osmolarity | |||
| Fluid balance | Fluid balance | |||||
| Haemoglobin | Haemoglobin | Haemoglobin | ||||
| Hematocrit | Hematocrit | Hematocrit | ||||
| Volume of CSF drained | Volume of CSF drained | |||||
| Mean (SD) duration of ventilation | ||||||
| Measures unique to individual trials | ||||||
| Global CBF (mL/100 g/min) | Preserved cerebral autoregulation | |||||
| AVDO2, AVDGlc and AVDLct contents were calculated allowing the determination of CMRO2; CMRGlc and CMRLct | Serum chloride | SpO2 | ||||
| Blood urea nitrogen | Serum creatinine | |||||
| Shear Rate | ||||||
| Blood flow velocities/arterial blood pressure (systolic, mean and diastolic) | ||||||
| Brain tissue oxygen tension | ||||||
| Urine output (vol) | ||||||
| Arterial PH | ||||||
| PaO2 | ||||||
| Duration of stay data | Days on ICU | Duration of ICU stay/ duration of overall hospital stay | Duration of ICU stay/ duration of overall hospital stay | |||
| Mortality/ neuro outcome | In ICU, GCS score following interruption of sedative drugs. Neurological outcome at 6 months: GOS as 'good recovery', 'moderate disability', 'severe disability', 'persistent vegetative state' and 'death' | Mortality at the end of stay in ICU | In‐hospital mortality plus GOS scores/mortality at 6 months | GOS scores at 6 months (unconventionally dichotomised) | GCS at one hour after one dose | |
| AVDGlc: Arterial jugular differences for glucose; AVDLct: Arterial jugular differences for lactate; AVDO2: Arterial jugular differences for oxygen; CBF: cerebral blood flow; CMRGlc: cerebral metabolic rate of glucose; CMRLct: cerebral metabolic rate of lactate; CMRO2: cerebral metabolic rate of oxygen; CPP: cerebral perfusion pressure; CSF: cerebrospinal fluid; CVP: central venous pressure; CT: computed tomography; EVD: extraventricular drain; GCS: Glasgow Coma Scale; GOS: Glasgow Outcomes Scale; ICH: intracranial hypertension;ICP: intracranial pressure; ICU: intensive care unit; MAP: mean arterial pressure; NICU: neuro‐intensive care unit; PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; SAPS: simplified acute physiology score; SD: standard deviation; SPO2 = peripheral oxygen saturation | ||||||
Excluded studies
See also the Characteristics of excluded studies table.
29 trials are formally excluded from this review.
Studies excluded for design features
We excluded 16 trials in total due to features of their design. We excluded five trials due to their cross‐over design (Battison 2005; Bourdeaux 2011; Huang 2014; Polushin 2009; Sakellaridis 2011). Searches of the Chinese literature identified 11 trials, which had to be excluded due to unreliable data or methods (incorrect 'T' values, questionable sequence generation), which we could not clarify through contact with trial authors (Huang 2015; Jin 2018; Li 2018; Liang 2013; Liu 2018; Mei 2016; Ni 2018; Shu 2015; Zhang 2014; Zhang 2015; Zhang 2018).
Termination
We excluded four registered trials because they were terminated, largely due to difficulties in recruitment (NCT01028339; NCT01108744; NCT01111682; NCT01215019.
Miscellaneous (chiefly ineligible intervention or comparator)
We excluded nine trials for other reasons. We excluded two trials (Jiang 2018; Yang 2019), because they compared differing doses of hypertonic saline, with no eligible control; one trial (Jafari 2018), because they administered hypertonic saline to both groups, with furesimide as an added treatment; and one trial (Du 2017) because there was no defined trigger for starting hyperosmotherapy to reduce ICP, and in addition, mannitol was administrated Q8h (every 8 hours). Following email communication with the trial author (Ichai 2013), we excluded Ichai 2009 because they used sodium lactate with the aim of decreasing the raised intracranial pressure; although sodium lactate is a hyperosmolar solution, its effects cannot be attributed to a classical osmotic effect. It differs fundamentally from sodium chloride because lactate is a metabolisable anion; this means that even with comparable osmolarity in vitro, sodium lactate becomes two times less hypertonic than equiosmotic sodium chloride in the body. We excluded one trial (Hong 2017), because of ineligible population. They administered hypertonic saline but not as a result of intracranial pressure monitoring. One trial appeared to meet the inclusion criteria, but was a nontherapeutic investigation that only considered coagulation (Wang 2017). We excluded one trial (Upadhyay 2010), for multiple reasons: quasi‐randomisation; too low a proportion of participants with traumatic brain injury (only 7% traumatic brain injury). We excluded one ongoing trial (Roquilly 2017), because its comparator, 'standard care', is unlikely to include alternative intracranial pressure‐lowering agents.
One trial (Vialet 2003). awaits classification (see also Characteristics of studies awaiting classification) due to unreconciled discrepancies in numbers of participants reported within the paper.
Risk of bias in included studies
Two figures show our assessment of the risk of bias of the included trials (Figure 2; Figure 3).
2.
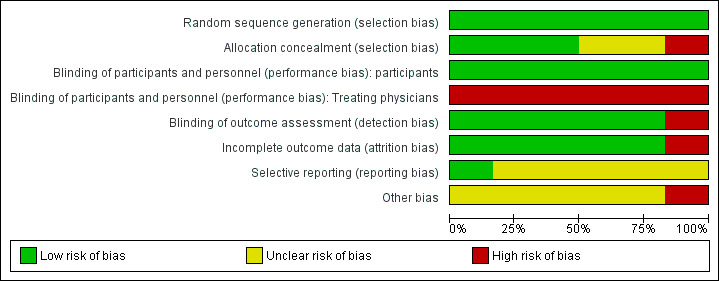
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials. Six trials are included in this review
3.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Random sequence generation
All included trials reported acceptable methods of generating randomisation sequence (either computer‐generated random‐number tables, or sealed, opaque envelopes) and so we judged them to be at low risk of bias.
Allocation concealment
Three trials (Cottenceau 2011; Francony 2008; Patil 2019), concealed allocation by using sealed and opaque envelopes and we judged them to be at low risk of bias. Harutjunyan 2005 and Kumar 2019 did not describe the method of allocation concealment, and the assessment for both is thus 'unclear'. The trial author of Jagannatha 2016 told us in a personal communication that, "we did not do allocation concealment", and so we judged it to be at high risk of bias.
Blinding
Participants
We judged all the trials to have a low overall risk of bias for blinding of participants; all of the people recruited to the trials had a brain injury severe enough to have a GCS score of 8 or lower, were sedated, and so were unaware of the treatment they were receiving.
Treating physicians
Overall, we judged the included trials to have a high risk of bias for blinding treating physicians. In Francony 2008, it was not possible to blind administration because the two treatments were of different volumes. Cottenceau 2011 explained in 2015 through correspondence that the team was well aware of the regimen at the acute phase. Harutjunyan 2005, Jagannatha 2016, Kumar 2019 and Patil 2019 did not describe blinding and it is unlikely to have been ensured.
Outcome assessors
We judged trials to have varying risk of bias in blinding outcome assessors. Cottenceau 2011 told us in a personal communication that, "as for the outcome, it was assessed in both centers by blinded medical staff during follow‐up visits or by phone calls issued by blinded personnel". Kumar 2019 reported using an assessor unaware of treatment status. In Francony 2008, Harutjunyan 2005 and Jagannatha 2016, objective outcomes such as laboratory measures or death are unlikely to have been affected by problems of blinding. We judged the above trials at low risk of bias for blinding outcome assessors. We judged risk of bias for blinding outcome assessors in Patil 2019 as high risk, as they do not mention blinding and all outcomes were assessed in the short‐term.
Incomplete outcome data
Complete outcome data were available for Francony 2008 (n = 20) and for Patil 2019 (n = 30), two trials that followed participants up for a maximum of two hours post treatment. We therefore assessed these trials as being at low risk of bias for this domain. In Harutjunyan 2005, data from eight out of 40 (20%) of the participants initially recruited were missing, but these participants were withdrawn from analysis before initiation of treatment, as their intracranial pressure never exceeded the treatment threshold of 20 mmHg. Cottenceau 2011 excluded nine out of 56 randomised participants early, either because of intracranial pressure lower than 15 mmHg (n = 7) or serum osmolarity greater than 320 mOsm/L on admission (n = 2). Data for the remaining participants were complete. As we consider the data of participants who never reached treatment threshold to be missing completely at random, we also judged these trials to be at low risk of bias. The paediatric trial Kumar 2019 reported that they assessed data on an intention‐to‐treat basis and these appear to be complete (all 30 participants analysed at all time points), so we judged this trial to be at low risk of bias for this domain.
In the sixth included trial (Jagannatha 2016), no data were missing at the point where 22 surviving participants were discharged from hospital, but we were confronted with the problem of missing data for four participants in each group at the time of six‐month follow‐up. Personal communication with trial authors confirmed that contact could not be maintained with participants or their carers by telephone (Jagannatha 2017). Data were insufficient to impute and we confined ourselves to using available data, conducting a best‐case and worst‐case scenario analysis, and commenting on the high risk of bias introduced by 35% loss to follow‐up for surviving participants leaving hospital. Given that this affects our assessment of the primary outcome for a large proportion of the sample of this trial, we therefore assess the risk of bias for this domain to be 'high'.
Selective reporting
In correspondence with the trial authors during 2014, Francony 2008 sent us a trial protocol (Payen 2002): a comparison with the completed trial revealed no suggestion of reporting bias; our assessment for bias is therefore 'low'. The assessment for all other trials is 'unclear' as either we could not identify any registration or protocol (Cottenceau 2011; Patil 2019), or trials were retrospectively registered (Harutjunyan 2005; Jagannatha 2016; Kumar 2019).
Other potential sources of bias
Two of the trials included within this review did not restrict recruitment to participants with traumatic brain injury (Francony 2008; Harutjunyan 2005), meaning that only 91% of included data come from participants meeting all our inclusion criteria.
-
In addition, as mentioned, trials within this review are small, which means that it is not surprising that half report important differences between groups at baseline.
Authors of the second largest trial (Cottenceau 2011, n = 47), note that, "Although there was a statistical trend suggestive of a better outcome in patients in the MTL [mannitol] group, similar differences found in the cerebral metabolic rate of oxygen (CMRO2) values and GCS scores on admission between the two groups probably indicated some asymmetry in the degree of severity of injury and accounted for this neurological outcome difference (Fig. 5; x2 p = 0.0662)" (Cottenceau 2011, p 2007).
Potential bias may have run in the other direction in a second trial, as trial authors noted here: "The occurrence of twice the number of subdural hematomas in the mannitol group (15 versus seven) may have introduced a bias. Subdural hematomas by virtue of being a pathologically more severe form of injury may have necessitated a higher number of hyperosmolar boluses in the mannitol group" (Jagannatha 2016, p 73).
Authors of a third trial report "the clinical values in both groups were not normally distributed [at baseline]" (Harutjunyan 2005, p R532).
Authors of Jagannatha 2016 also note that some "[other] methodological issues need to be taken into account when interpreting our results. ... Though we intended to recruit consecutive patients, some patients were excluded for logistic reasons. The GCS at inclusion of the patient into the trial was much lower in this trial compared with other trials, with a median of 5 and 4 (eye opening and motor scores) in the mannitol and HTS groups, indicating a more severe injury. Some of the patients in the trial underwent surgery, which might have conferred some benefit in terms of ICP [intracranial pressure] reduction. The patients were controlled for GCS at the time of inclusion and not the type of lesion on CT scan. .... The groups, however, were comparable with respect to the overall radiological profile and findings at surgery. Also, to our surprise, even in the operated patients, the initial reduction of ICP was followed by a progressive increase over time. Poor glycaemic control in the mannitol group may also have influenced the outcome in these patients. The number of patients in the trial was small and this limited our outcome analysis" (Jagannatha 2016) p 73.
Authors of Kumar 2019 wrote that hyperosmolar therapy was effectively "used as a second tier treatment" ‐ only after failure of an extraventricular drain in promoting cerebrospinal fluid drainage to reduce intracranial pressure. They wrote that, "EVD [extraventricular drain] as an initial treatment may dilute the effect of hyperosmolar therapy. It is not known, whether there will be any difference in intracranial pressure reduction between mannitol and hypertonic saline if any of these agents are administered as first‐line therapy. Many centers do not use EVD for ICP [intracranial pressure] monitoring. When ICP monitoring is done using parenchymal sensor, option of CSF [cerebrospinal fluid] drainage is not available, and true effect of hyperosmolar therapy can be assessed". Related to this, different trials measured intracranial pressure in different ways: Patil 2019 listed the use of a subdural bolt, and they cited their failure to assess the advantages or disadvantages of the intracranial pressure‐measuring technique as a limitation.
Effects of interventions
See: Table 1
All included trials compared hypertonic saline with mannitol, or mannitol in combination with glycerol, for the control of intracranial pressure.
Primary outcome
Mortality
Three out of six included trials reported on our primary outcome (mortality); timings varied and meta‐analysis was feasible between only two trials.
Mortality (short‐term) prior to discharge from hospital
Jagannatha 2016 (n = 38) reported that there were four deaths within the first six days of treatment, but did not specify in which group the deaths occurred. Personal contact established that during this period, three out of 18 participants in the hypertonic saline group and one out of 20 in the mannitol group died. The same trial further reported data on deaths after six days, but before participants left hospital, recording no further deaths in the hypertonic saline group but a further nine in the mannitol group. At this time point, there is a slight trend favouring hypertonic saline compared to mannitol (RR 3.33, 95% CI 0.38 to 29.25).
Investigators within Harutjunyan 2005 (n = 32), reported that seven of 17 (41.2%) people in the hypertonic saline group and nine of 15 (60%) people in the mannitol group died by the end of stay in the ICU. Within this small trial with a mixed population of whom only a third had traumatic brain injury, hypertonic saline did not reduce all‐cause mortality (RR 0.69, 95% CI 0.34 to 1.39).
Mortality at six months
We pooled data from two trials (Cottenceau 2011; Jagannatha 2016), for this outcome, but given the high loss to follow‐up in Jagannatha 2016 (8 of 22 participants who survived to leave hospital could not be contacted at six months), we have chosen to present the data three ways (per protocol, 'worst‐case' scenario for hypertonic saline and 'best‐case' scenario for hypertonic saline; Figure 4). Here we have considered the effects of all four missing participants from either group alternately surviving or dying. Available data results are as follows. Per protocol RR 0.84 (95% CI 0.46 to 1.55; I2 = 0%; 2 trials, 85 participants). 'Worst‐case' and 'best‐case' scenarios suggest the following (extreme) potential parameters of effect: worst‐case RR 1.12 (95% CI 0.66 to 1.89; I2 = 0%; 2 trials, 85 participants); best‐case RR 0.67 (95% CI 0.38 to 1.18; I2 = 50%; 2 trials, 85 participants). None of these analyses show a difference between treatments.
4.
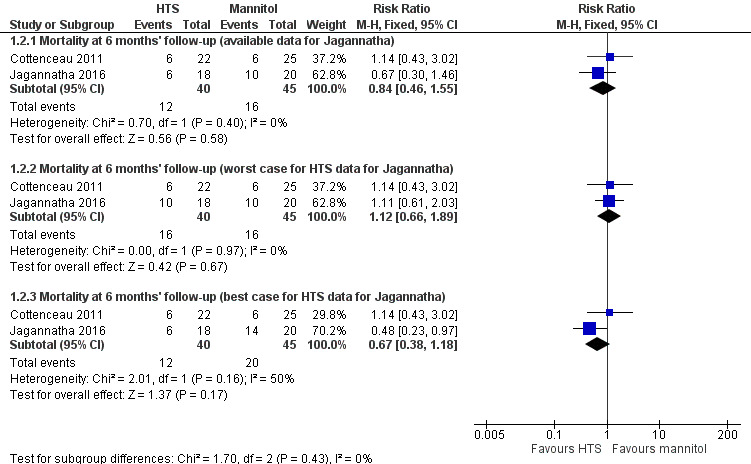
Forest plot of comparison 1. Hypertonic saline vs mannitol, outcome: 1.2 mortality: 6 months
Secondary outcome
Poor outcome (measured by the dichotomised Glasgow Outcome Scale)
At protocol stage, we planned to report this outcome in the conventional manner, that is, by converting GOS scores into a dichotomous outcome where ‘death or disability’ signifies death, persistent vegetative state and severe disability; and a ‘good outcome’ includes moderate disability and good recovery. Two trials reported data suitable for pooling for this outcome at six months (Cottenceau 2011; Jagannatha 2016), although the uncertainty due to missing data from Jagannatha 2016 referred to above remains a concern. We thus reported findings in a similar way (available data, 'best‐case' and 'worst‐case').
Within Cottenceau 2011: 17 of 22 (77.3%) people in the hypertonic saline group and 14 of 25 (56%) people in the mannitol group died or had a severe disability at the end of the follow‐up period. In Jagannatha 2016, trial authors report that, "GOS scores at 6 months, dichotomized as unfavourable (GOS 1–3) and favorable (GOS 4–5)" are regarded as "comparable (p = 0.21)" (Jagannatha 2016, p. 71). Twelve of 18 (67%) people in the hypertonic saline group and 16 of 20 (80%) people in the mannitol group died or had a severe disability at six months. These findings are difficult to interpret in the light of the missing data on mortality referred to above. Pooling produces a result in which we have low confidence due to missing data as well as acknowledged baseline imbalance in Cottenceau 2011 (RR 1.09, 95% CI 0.82 to 1.44; I2 = 67%; 2 trials, 85 participants; Analysis 1.3). We have also presented best‐ and worst‐case scenarios in Figure 5.
1.3. Analysis.
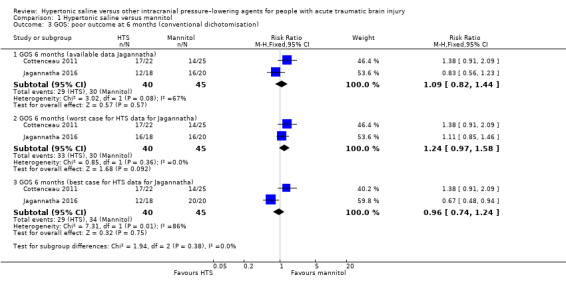
Comparison 1 Hypertonic saline versus mannitol, Outcome 3 GOS: poor outcome at 6 months (conventional dichotomisation).
5.
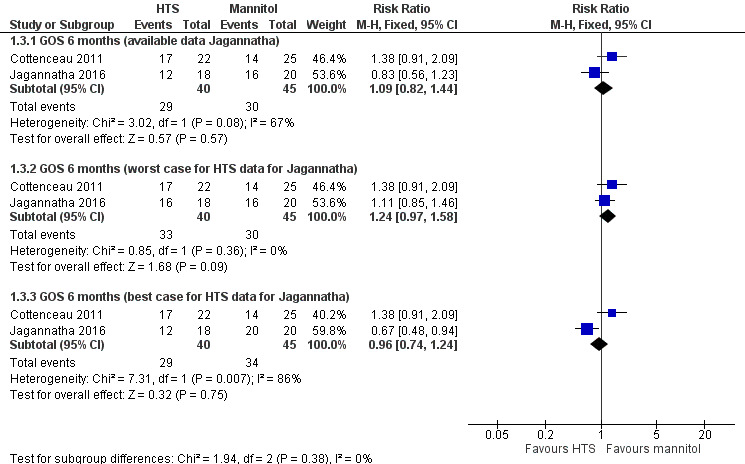
Forest plot of comparison: 1 Hypertonic saline versus mannitol, outcome: 1.3 GOS: poor outcome at 6 months (conventional dichotomisation).
Kumar 2019 included solely children aged under 16 years. They also reported GOS, but dichotomised differently, grouping all children who survived (regardless of severity of disability) against those who died or remained in a persistent vegetative state. We could not, therefore, pool findings with those above. Results suggest no clear difference between groups (RR 0.72, 95% CI 0.14 to 3.61; 1 trial, 25 participants; Analysis 1.4).
1.4. Analysis.
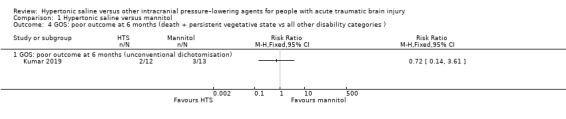
Comparison 1 Hypertonic saline versus mannitol, Outcome 4 GOS: poor outcome at 6 months (death + persistent vegetative state vs all other disability categories ).
Uncontrolled intracranial pressure
Only two trials (Francony 2008; Harutjunyan 2005) reported ‘uncontrolled ICP’ and they each had a different definition of ‘uncontrolled ICP’. In addition, the unit of analysis of Harutjunyan 2005 is 'episodes', while Francony 2008 is 'participants', so we could not incorporate them in a meta‐analysis.
All six trials reported the effect of HTS or mannitol on ICP. Three trials (Francony 2008; Jagannatha 2016; Kumar 2019) reported the mean magnitude of ICP reduction, with standard deviations. The other three trials (Cottenceau 2011; Harutjunyan 2005; Patil 2019) reported the initial ICP prior to, and immediately following, the administration by infusion of the study medication , in the form of means and ranges. We could not pool data for this outcome due to manifest heterogeneity in timings and modes of assessment, and other issues (including non‐normally distributed data, differing populations (adults and children, non‐TBI participants)). Therefore, we report results for this outcome narratively.
Cottenceau 2011: absolute ICP measurement values were reported as 12.2 ± 6.1 mmHg and 13.9 ± 7.8 mmHg in the hypertonic saline group versus 10.5 ± 6.8 mmHg and 13.6 ± 7.5 mmHg in the mannitol group after 30 minutes and 120 minutes of the infusion, respectively. The mean magnitude of intracranial pressure reduction after 30 minutes and 120 minutes of the infusion in the hypertonic saline groups were 1.70 mmHg higher (1.99 lower to 5.39 higher) and 0.30 mmHg higher (4.09 lower to 4.69 higher), respectively. Trial authors reported that, "both HTS [hypertonic saline] and MTL [mannitol] effectively and equally reduced ICP [intracranial pressure] levels with subsequent elevation of CPP [cerebral perfusion pressure] and CBF [cerebral blood flow], although this effect was significantly stronger and of longer duration after HTS ... Further, effect of HTS on ICP appeared to be more robust in patients with diffuse brain injury" (Cottenceau 2011, p 2003).
Francony 2008: intracranial pressure was reported as being reduced by 45% ± 19% of baseline values (‐14 ± 8 mmHg) and by 32% ± 12% of baseline values (‐10 ± 4 mmHg ) in the mannitol group versus 35% ± 14% (‐10 ± 5 mmHg) and by 23% ±10% (‐6 ± 3 mmHg) in the HTS group at 60 minutes and at 120 minutes after the start of the study medication infusion, respectively). Only one person from the hypertonic saline group was a low responder to osmotherapy (with a reduction in intracranial pressure of < 20% of baseline values at 60 minutes after the start of infusion). Trial authors found both interventions to be effective for this outcome but added pretreatment factors need to be considered (e.g. serum sodium and haemodynamics).
Harutjunyan 2005: a total of 53 episodes of raised intracranial pressure exceeding 20 mmHg from 15 people in the mannitol group required infusion of trial medication. For four of these episodes (7.5%), intracranial pressure was uncontrolled within an average of 8.7 (4.2 to 19.9) minutes. 57 episodes of increased intracranial pressure occurred in the 17 people in the hypertonic saline group. For two of these episodes (3.5%), intracranial pressure was uncontrolled within 6.0 (1.2 to 15.0) minutes. Trial authors in this trial concluded hypertonic saline to be more effective for increased intracranial pressure than mannitol but cautioned that the benefit in this trial might be explained by "local osmotic effects" as there had been no differences in baseline haemodynamics.
Jagannatha 2016: trial authors here report that mean fall in intracranial pressure following a dose of hyperosmolar agent was 8.9 ± 8.4 mmHg in the mannitol group and 10.1 ± 8.7 mmHg in the hypertonic saline group, based on a comparison of 488 episodes of raised intracranial pressure across six days. The mean fall in intracranial pressure following a dose of hyperosmolar agent in the hypertonic saline group was 1.20 mmHg higher (‐0.37 lower to 2.77 higher) than with mannitol. Trial authors reported that this was significant and also that the percentage time for which intracranial pressure remained below a threshold of 20 mmHg on day 6 was significantly higher for hypertonic saline than mannitol. They also reported that cerebrospinal fluid drainage was performed on 41 ± 38 occasions in the mannitol group and 45 ± 31 occasions in the HTS group (p= 0.73).
Kumar 2019: trial authors report that most episodes of raised intracranial pressure were first managed by cerebrospinal fluid drainage which was effective in "almost more than two thirds of episodes". Thereafter, if not managed and requiring administration of a hyperosmolar agent, the mean (SD) reduction in intracranial pressure was −5.67 (SD 3.9) in the hypertonic saline group; − 7.13 (SD 2.9) in the mannitol group (Kumar 2019, p 1003). Trial authors report that the difference was not statistically different.
Patil 2019: all findings in this trial relate to a single episode of raised intracranial pressure, after which assessment was carried on for a maximum of one hour (assessments were made at 10, 30 and 60 minutes). Investigators reported data in the form of means and ranges (rather than standard deviations); they then report findings in percentage improvements. All three hyperosmolar agents (hypertonic saline, mannitol and mannitol plus glycerol) were reported as effective, but hypertonic saline was slightly superior, effecting a greater change in reducing intracranial pressure, and more quickly, than other agents, while at a lower dose. The "maximum change in ICP [intracranial pressure] occurred after the bolus dose of 3% HTS [hypertonic saline]"; the "maximum decrease in ICP was produced by 3% HTS (60%), followed by the 10% mannitol plus 10% glycerol combination group (57%) and then 20% mannitol (55%). When the 3 groups were compared, 3% HTS required the lowest dose, that is, 1.4 mL/kg, followed by the 10% mannitol plus 10% glycerol combination group, that is, 1.7 mL/kg, and then the 20% mannitol group, that is, 2 mL/kg." The time required to reduce intracranial pressure below 15 mm Hg was 16 minutes (range 6 to 39 minutes) in the 3% hypertonic saline group, 23 minutes in the mannitol group (range, 10 to 70 minutes) and 19 minutes in the 10% mannitol plus 10% glycerol combination group (range, 7 to 50) minutes.
A rebound phenomenon during treatment
None of the trials reported on this outcome systematically, although it is mentioned in passing as potentially affecting those treated with mannitol in one trial (Jagannatha 2016).
Pulmonary oedema during treatment
None of the trials reported data on pulmonary oedema during treatment.
Acute renal failure during treatment
None of the trials reported data on acute renal failure during treatment.
Discussion
Summary of main results
There are six trials, involving a total of 287 people, included in this review. We assessed the overall risk of bias in most trials as unclear or high, due either to mixed population or other factors, such as the impact of incomplete outcome data.
Some pooling of data was possible for the primary outcome (mortality), as well as for the outcome of 'poor outcome' as assessed by traditional dichotomisation of GOS. We report other results narratively. Our certainty in all results is very low. Not enough people have been randomised into eligible trials to be able to give a reliable result.
Mortality
Two trials reported mortality in the short term, prior to discharge from hospital. One (n = 38) reported that three out of 18 participants in the hypertonic saline group and one out of 20 participants in the mannitol group died in the first six days following treatment, after which no further deaths occurred in the hypertonic saline group but a further nine deaths occurred in the comparator group (mannitol) prior to discharge (Jagannatha 2016). At this time point, there is no clear difference between groups (RR 0.33, 95% CI, 0.11 to 1.02). In another trial (n = 32), in which only a third of participants had traumatic brain injury), trial authors reported that seven of 17 (41.2%) people in the hypertonic saline group and 9 of 15 (60%) people in the mannitol group died by the end of stay in the ICU (Harutjunyan 2005). Here, hypertonic saline did not reduce all‐cause mortality in people with acute traumatic brain injury (RR 0.69; 95% CI 0.34 to 1.39). We were able to pool data for two trials (n = 85) for mortality, but given the high loss to follow‐up in one, we presented data in three ways (per protocol, 'worst‐case' scenario for hypertonic saline and 'best‐case' scenario for hypertonic saline and there was no difference between groups at any time point (Cottenceau 2011;Jagannatha 2016).
'Poor outcome' on the GOS
Using the method described above for mortality at six‐month follow‐up, and for the same reason, we present inconclusive results for this outcome based on pooling of two trials (n = 85). Again, there were no differences between groups; nor was there a difference in GOS reported in a smaller trial (n = 30) in which the GOS scale was dichotimised by grouping death and persistent vegetative status versus all other outcomes.
Uncontrolled intracranial pressure
For the outcome of 'uncontrolled intracranial pressure', no meta‐analysis was feasible due to no uniform definition of 'uncontrolled intracranial pressure', and heterogeneity in reporting intracranial pressure changes. Therefore we reported these and all other results by individual trial. Essentially, trial results indicated that both treatments appeared effective compared with baseline, with some additional benefits for hypertonic saline, but the data are too few to be definitive and treatment selection might be individually based on sodium level and cerebral haemodynamics.
Adverse effects
None of the trials systematically reported data on adverse effects, such as a rebound phenomenon, pulmonary oedema or acute renal failure during treatment.
Overall completeness and applicability of evidence
This review set out to compare hypertonic saline against a potential range of other therapies. However, the only trials that met the inclusion criteria involved mannitol or mannitol with glycerol as a comparator. We thus have no evidence of how hypertonic saline compares to other intracranial pressure‐lowering agents.
In addition, despite having found six trials, all were of small sample size and were powered (if powered at all) for short‐term outcomes. Heterogeneneity of modes of administration, dosages, outcome measurement and populations were so diverse as to make meta‐analysis difficult. Not all trials contained only participants with traumatic brain injury, and those from 'mixed' trials reported little about the source of participants' traumatic brain injury. Longer‐term (six‐month) follow‐up data were available from only three of the six trials. Clinical evidence is therefore insufficient at present to answer the objectives of the review.
Quality of the evidence
As stated above, sample sizes were small (287 people across six trials) and the outcome measures and methods used were heterogeneous. Data were missing for our primary outcome (mortality) as well as for 'poor outcome' on the GOS scale. Blinding of this intervention is often unfeasible, an inevitable weakness in such trials. A comment must also be made on the quality of the reporting of trials included within this review. None were reported in accordance with CONSORT standards (Moher 2010). Registration of trials (where extant) was retrospective; only one protocol was available. Results were equivocal and the quality of the evidence was low to very low, as these issues obliged us to downgrade for a variety of criteria.
Potential biases in the review process
The review is based on a thorough search of medical literature in English and Chinese, and we believe it is a complete compilation of the RCTs on this topic; however, we have made choices differently to that of other systematic review authors in the area. We have not incorporated data from one trial (Vialet 2003), used within other reviews focusing on mannitol (Wakai 2013; Wang 2015), because of unreconciled discrepancies within the paper. We have (unlike the authors of Boone 2015, Burgess 2016 and Li 2015), excluded cross‐over trials. We maintain this is appropriate for our review question and focus on long‐term outcome.
Agreements and disagreements with other studies or reviews
There are two literature reviews (Kamel 2011; Mortazavi 2012), of hypertonic saline for treating raised intracranial pressure, which included people with raised intracranial pressure from multiple aetiologies, which found that hypertonic saline was more effective than mannitol.
One recent systematic review with meta‐analysis (Rickard 2014), found that both hypertonic saline and mannitol effectively lower intracranial pressure. Authors identified a trend favouring the use of hypertonic saline solutions in people with acute traumatic brain injury. However, this meta‐analysis included three trials that our review does not: two had cross‐over designs (Battison 2005; Sakellaridis 2011). We maintain that the issue of 'carry‐over' effects in cross‐over trials confounds the estimates of the treatment effects and further, that it is impossible to assess the effect of hypertonic saline on death and neurological outcomes after traumatic brain injury in a cross‐over trial. This review also incorporated data from Ichai 2009, which we have excluded.
Another recent meta‐analysis (Burgess 2016), found no clinically important differences in mortality, neurological outcomes, and intracranial pressure reduction between hypertonic saline or mannitol in the management of severe traumatic brain injury. It included data from four trials that we have excluded (Battison 2005; Ichai 2009; Sakellaridis 2011; Vialet 2003), but fundamentally, our conclusions are similar. Authors of Schwimmbeck 2019 performed a systematic review, meta‐analysis and trial sequential analysis comparing hypertonic saline and mannitol, concluding that there were, "indications that HS [hypertonic saline] might be superior to mannitol in the treatment of TBI [traumatic brain injury]‐related raised ICP [intracranial pressure]"; however, "there are insufficient data to reach a definitive conclusion, and further trials are warranted" (Schwimmbeck 2019). Authors of Gu 2019 synthesised 12 RCTs including those excluded for reasons mentioned above. Searches did not capture Kumar 2019 or Patil 2019. Once again, authors found a benefit that was not statistically significant for intracranial pressure control with hypertonic saline as compared to mannitol, but not enough to "lend a specific recommendation to select hypertonic saline or mannitol as a first‐line [treatment]"; all other outcomes (including function and mortality) were "close".
In 2018, the European Society of Intensive Care Medicine (ESICM) reported the consensus and clinical practice recommendations on fluid therapy in neuro‐intensive care patients (Oddo 2018). ESCICM recommendations in the area of hyperosmolar fluids for the management of elevated intracranial pressure guidelines are consonant with our findings, stating, "we suggest the use of mannitol or hypertonic saline solutions for reducing increased intracranial pressure (weak recommendation)". A weak recommendation was made when votes in favour or against (a mix of strong and weak options) reached the 80% threshold.
Authors' conclusions
Implications for practice.
We identified six trials suitable for inclusion in this review; all compared a type of hypertonic saline with mannitol or mannitol combined with glycerol; all evidence was of low or very low quality. Trial authors noted that where immediate benefits of hypertonic saline are suggested, they do not translate into long‐term benefit. Therefore at present, there is not sufficient evidence to enable conclusions to be drawn about the efficacy and safety of hypertonic saline versus mannitol or other intracranial pressure‐lowering agents in the management of acute traumatic brain injury.
Implications for research.
Carefully planned, high‐quality randomised controlled trials are warranted. We are concerned that searches of trials registers for this review have revealed several terminated trials in this field, but are pleased also to have identified an ongoing trial of adequate size (Characteristics of ongoing studies). Future investigators should consider implications of such experiences. The following factors are relevant.
Due to the different definitions of 'uncontrolled intracranial pressure' within identified trials and important differences in reporting intracranial pressure changes from baseline, a widely uniform definition for raised intracranial pressure and intracranial pressure control is necessary. Recently, ESCIM have suggested using a predefined trigger for starting osmotherapy to treat elevated intracranial pressure, and they also suggest using an intracranial pressure threshold above 25 mmHg, independent of other variables, as a trigger for starting osmotherapy to reduce intracranial pressure (Oddo 2018).
Consideration should be paid to assessing optimal concentration and infusion time of any intracranial pressure‐lowering agent.
Investigators should collect and report data on types of traumatic brain injury experienced by participants (e.g. extradural haematoma, diffuse axonal injury)
To test the effect of hypertonic saline on death and neurological recovery for acute traumatic brain injury but not just on intracranial pressure control, longer‐term follow‐up than most of the published trials in this area currently provide, is essential.
Trials must be pre‐registered and transparent, and reasonably detailed protocols should be made available. Failure to do so will, in future, result in exclusion of data from this review, in accordance with Cochrane Injuries' policies (CIG 2015).
Results of trials should be reported according to CONSORT (Moher 2010).
Failure to generate a truly informative sample size is an ethical as well as a procedural problem. To combat difficulties in recruitment and attaining a necessary sample size, the possibility of multicentre trials could be considered.
Acknowledging obvious recruitment difficulties within this field, in order not to waste valuable data from persistent 'mixed population' trials, investigators could be encouraged to report outcomes disaggregated by type of neurological complaint, for example, stroke or traumatic brain injury.
What's new
| Date | Event | Description |
|---|---|---|
| 16 January 2020 | New citation required but conclusions have not changed | This review was republished to enable electronic databases to hold a copy of the final version. This review was first published on 30 December 2019 but was not electronically linked to some online databases. No changes have been made to the text of the review; republication was solely in order to overcome a technological problem. |
History
Protocol first published: Issue 2, 2014 Review first published: Issue 12, 2019
| Date | Event | Description |
|---|---|---|
| 30 December 2019 | Amended | Minor copy edits made to the table of excluded studies. |
Acknowledgements
The review authors would like to thank all staff at Cochrane Injuries. We are particularly grateful for help in drafting the English‐language search strategy at protocol stage, and for support in the completion of the review.
We also want to thank Dr Ichai, Dr Soustiel, Dr Payen and Dr Jagannatha for replying to our requests for additional information.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to Cochrane Injuries. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search Dec 2019 (English language sources)
Note. Amendments were made to update searches in 2017, including more sensitive list of terms for hypertonic saline solution and hyperosmolar therapy. The searches were back‐dated to accommodate these changes and records de‐duplicated from the 2013 search results. Thereafter, 2018 and 2019 searches used the same terms.
Cochrane Injuries Group specialised register (SR‐INJ)
#1 (((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertensi* or oedema or edema or swell*))):TI,AB,KY AND SR‐INJ:CC #2 (((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) near (infarct* or injur* or trauma* or damag* or wound* or fracture* or contusion*))):TI,AB,KY AND SR‐INJ:CC #3 ((TBI or mTBI or sTBI) ):TI,AB,KY AND SR‐INJ:CC #4 (("subarachnoid h?emorrhage" or tSAH) ):TI,AB,KY AND SR‐INJ:CC #5 ((diffuse axonal injury or diffuse axonal injuries or persistent vegetative state or glasgow outcome scale or glasgow coma scale)):TI,AB,KY AND SR‐INJ:CC #6 ((Glasgow adj3 (coma or outcome) adj3 (scale* or score*))):TI,AB,KY AND SR‐INJ:CC #7 (((midbrain or mid brain) NEXT syndrome)):TI,AB,KY AND SR‐INJ:CC #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 (((hypertonic or hyper‐tonic) AND (saline or salt or sodium chloride or NaCl))):TI,AB,KY AND SR‐INJ:CC #10 ((osmotherap* or "osmo* therap*" or "hyperosmo* therap*" or osmolar* or hyperosmolar*) ):TI,AB,KY #11 ((fluid NEXT (manage* or therap* or resuscitat*))):TI,AB,KY AND SR‐INJ:CC #12 (#9 OR #10 OR #11) #13 (#8 AND #12)
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library)
#1 ((hypertonic or hyper‐tonic) near (saline or salt or sodium chloride or NaCl)) #2 hts:ti,ab,kw #3 MeSH descriptor: [Saline Solution, Hypertonic] this term only #4 MeSH descriptor: [Hypertonic Solutions] this term only #5 MeSH descriptor: [Sodium Chloride] this term only #6 MeSH descriptor: [Fluid Therapy] this term only and with qualifier(s): [Methods ‐ MT, Standards ‐ ST] #7 (fluid and (manage* or therap* or resuscitat*)):ti #8 (osmotherap* or "osmo* therap*" or "hyperosmo* therap*" or osmolar* or hyperosmolar*) #9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) #10 MeSH descriptor: [Intracranial Pressure] this term only #11 MeSH descriptor: [Intracranial Hypertension] explode all trees #12 MeSH descriptor: [Brain Edema] explode all trees #13 ((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) near (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertensi* or oedema or edema or swell*)) #14 ICP:ab (Word variations have been searched) #15 (TBI or mTBI or sTBI) #16 MeSH descriptor: [Cerebrovascular Trauma] explode all trees #17 MeSH descriptor: [Craniocerebral Trauma] explode all trees #18 ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) near (infarct* or injur* or trauma* or damag* or wound* or fracture* or contusion*)) #19 MeSH descriptor: [Glasgow Coma Scale] explode all trees #20 MeSH descriptor: [Glasgow Outcome Scale] explode all trees #21 MeSH descriptor: [Unconsciousness] in all MeSh products #22 ("subarachnoid h?emorrhage" or tSAH) #23 (diffuse axonal injury or diffuse axonal injuries or persistent vegetative state or glasgow outcome scale or glasgow coma scale):ti,ab,kw (Word variations have been searched) #24 ((unconscious* or coma* or concuss* or postconcuss*) near (injur* or trauma* or damag* or wound* or fracture*)) #25 ((midbrain or mid brain) next syndrome) #26 (#9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25) #27 (#9 and # 26)
Ovid MEDLINE databases
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1. randomi#ed.ab,ti. 2. randomized controlled trial.pt. 3. controlled clinical trial.pt. 4. placebo.ab. 5. clinical trials as topic.sh. 6. double blind method.sh. 7. randomly.ab. 8. (RCT or at random or (random* adj (assign* or allocat* or divid* or division or number))).ti,ab,kf. 9. trial.ti. 10. or/1‐9 11. (animals not (humans and animals)).sh. 12. 10 not 11 13. Intracranial Pressure/ 14. exp Intracranial Hypertension/ 15. ((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertensi* or oedema or edema or swell*)).ti,ab,kf. 16. Brain Edema/ 17. exp Craniocerebral Trauma/ 18. Glasgow Coma Scale/ 19. Glasgow Outcome Scale/ 20. exp Unconsciousness/ 21. exp Cerebrovascular Trauma/ 22. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) adj3 (infarct* or injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti,kf. 23. (Glasgow adj3 (coma or outcome) adj3 (scale* or score*)).ab,ti,kf. 24. rancho los amigos scale.ti,ab,kf. 25. diffuse axonal injur*.ti,ab,kf. 26. ((unconscious* or coma* or concuss* or postconcuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture*)).ti,ab,kf. 27. (subarachnoid h?emorrhage or tSAH).ti,ab,kf. 28. ((midbrain or mid brain) adj syndrome).ti,ab,kf. 29. (TBI or mTBI or sTBI).ti,ab,kf. 30. or/13‐29 31. Sodium Chloride/ 32. Saline Solution, Hypertonic/ 33. Hypertonic Solutions/ad, st, tu, th [Administration & Dosage, Standards, Therapeutic Use, Therapy] 34. ((hypertonic or hyper‐tonic) adj3 (saline or salt or sodium chloride or NaCl)).ti,ab,kf,nm. 35. HTS.ab,ti,kf. 36. Fluid Therapy/ 37. (osmotherap* or osmo* therap* or hyperosmo* therap*).ti,ab,kf. 38. (hyperosmolar* or hyper osmolar*).ti,ab,kf. 39. or/31‐38 40. 12 and 30 and 39 41. remove duplicates from 40
Ovid Embase
1. randomized controlled trial/ 2. controlled clinical trial/ 3. randomi#ed.ti,ab,kw. 4. randomization/ 5. placebo.ti,ab,kw. 6. placebo/ 7. *Clinical Trial/ 8. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,kw. 9. double blind procedure/ 10. (RCT or at random or (random* adj (assign* or allocat* or divid* or division or number))).ti,ab,kw. 11. trial.ti. 12. or/1‐11 13. ((animal or nonhuman) not (human and (animal or nonhuman))).de. 14. 12 not 13 15. intracranial pressure/ 16. exp intracranial hypertension/ 17. ((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertensi* or oedema or edema or swell*)).ti,ab,kw. 18. ICP.ab. 19. exp brain hemorrhage/ 20. brain edema/ 21. brain perfusion/ 22. head injury/ 23. exp brain injury/ 24. cerebrovascular accident/ 25. exp brain injury assessment/ 26. exp unconsciousness/ 27. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) adj3 (infarct* or injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti,kw. 28. (Glasgow adj3 (coma or outcome) adj3 (scale* or score*)).ab,ti,kw. 29. rancho los amigos scale.ti,ab,kw. 30. diffuse axonal injur*.ti,ab,kw. 31. ((unconscious* or coma* or concuss* or postconcuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture*)).ti,ab,kw. 32. (subarachnoid h?emorrhage or tSAH).ti,ab,kw. 33. ((midbrain or mid brain) adj syndrome).ti,ab,kw. 34. (TBI or mTBI or sTBI).ti,ab,kw. 35. or/15‐34 36. ((hypertonic or hyper‐tonic) adj3 (saline or salt or sodium chloride or NaCl)).ti,ab,kw,rn. 37. sodium chloride/ and hypertonic solution/ 38. ((hypertonic or fluid) adj3 (therapy or resuscitat*)).ti,kw. 39. *fluid therapy/ 40. fluid resuscitation/ 41. (osmotherap* or osmo* therap* or hyperosmo* therap*).ti,ab,kw. 42. or/36‐41 43. 14 and 35 and 42 44. (rat or rats or mouse or mice or rodent* or pig or pigs or piglet* or swine or porcine or murine or sheep or lambs or rabbit or rabbits or cat or cats or feline or dog or dogs or canine).ti. 45. 43 not 44 46. remove duplicates from 45
Web of Science
Science Citation Index‐Expanded (SCI‐EXPANDED) Conference Proceedings Citation Index‐Science (CPCI‐S) TS=((randomised or randomized or randomly or “random order” or “random sequence” or “random allocation” or “randomly allocated” or “at random” or placebo or ((singl* or doubl* or trebl* or tripl*) same (blind* or mask*))) AND (((hypertonic or hyper‐tonic) same (saline or salt or sodium chloride or NaCl))) AND (((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) same (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertensi* or oedema or edema or swell*)) or ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) same (infarct* or injur* or trauma* or damag* or wound* or fracture* or contusion*)) or “diffuse axonal injur*” or “glasgow outcome scale” or “glasgow coma scale” or TBI or sTBI or "subarachnoid h?emorrhage" or tSAH)) NOT TI=(rat or rats or mouse or mice or rodent* or pig or pigs or piglet* or swine or porcine or murine or sheep or lambs or rabbit or rabbits or cat or cats or feline or dog or dogs or canine)
Appendix 2. Searches to Dec 2013 (English language sources)
Cochrane Injuries Group specialised register
((fluid management or sodium or fluid therapy* or hypertonic saline resuscitation or maintenance fluid* or "hts" or hypertonic saline infusion* or hypertonic sodium chloride solution*) AND (((haematoma* or hematoma* or haemorrhag* or hemorrhage* or bleed* or pressure) adj3 (head or cranial or cerebral or brain* or intra‐cranial or inter‐cranial)) or (diffuse axonal injury or diffuse axonal injuries or persistent vegetative state or glasgow outcome scale or Glasgow coma scale) or ((injury* or injuries or trauma or damage or damaged or wound* or fracture*OR contusion* or haematoma* or hematoma* or Haemorrhag* or hemorrhag* or bleed* or pressure) adj3 (unconscious* or coma* or concuss*)))) AND ( INREGISTER)
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library
#1fluid management:ti,ab,kw (Word variations have been searched) #2sodium:ti,ab,kw (Word variations have been searched) #3fluid therapy*:ti,ab,kw (Word variations have been searched) #4hypertonic saline resuscitation:ti,ab,kw (Word variations have been searched) #5maintenance fluid*:ti,ab,kw (Word variations have been searched) #6"hts":ti,ab,kw (Word variations have been searched) #7hypertonic saline infusion*:ti,ab,kw (Word variations have been searched) #8hypertonic sodium chloride solution*:ti,ab,kw (Word variations have been searched) #9MeSH descriptor: [Saline Solution, Hypertonic] explode all trees #10MeSH descriptor: [Hypertonic Solutions] this term only #11MeSH descriptor: [Fluid Therapy] explode all trees #12MeSH descriptor: [Sodium] explode all trees #13MeSH descriptor: [Sodium Chloride] explode all trees #14#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 #15MeSH descriptor: [Craniocerebral Trauma] explode all trees #16MeSH descriptor: [Brain Edema] explode all trees #17MeSH descriptor: [Glasgow Coma Scale] explode all trees #18MeSH descriptor: [Glasgow Outcome Scale] explode all trees #19MeSH descriptor: [Unconsciousness] explode all trees #20MeSH descriptor: [Cerebrovascular Trauma] explode all trees #21((haematoma* or hematoma* or haemorrhag* or hemorrhage* or bleed* or pressure) near/3 (head or cranial or cerebral or brain* or intra‐cranial or inter‐cranial)):ti,ab,kw (Word variations have been searched) #22(diffuse axonal injury or diffuse axonal injuries or persistent vegetative state or glasgow outcome scale or glasgow coma scale):ti,ab,kw (Word variations have been searched) #23((injury* or injuries or trauma or damage or damaged or wound* or fracture*OR contusion* or haematoma* or hematoma* or Haemorrhag* or hemorrhag* or bleed* or pressure) near/3 (unconscious* or coma* or concuss*)):ti,ab,kw (Word variations have been searched) #24#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25#14 and #24
PubMed
((((((((((fluid management[Title/Abstract]) OR sodium[Title/Abstract] OR fluid therapy*[Title/Abstract]) OR hypertonic saline resuscitation[Title/Abstract]) OR maintenance fluid*[Title/Abstract]) OR "hts"[Title/Abstract]) OR hypertonic saline infusion*[Title/Abstract]) OR hypertonic sodium chloride solution*[Title/Abstract])) OR ((((("Saline Solution, Hypertonic"[Mesh]) OR "Hypertonic Solutions"[Mesh:NoExp]) OR "Fluid Therapy"[Mesh]) OR "Sodium"[Mesh]) OR "Sodium Chloride"[Mesh]))) AND ((((((((("Comparative Study"[Publication Type]) OR "Randomized Controlled Trial"[Publication Type]) OR "Controlled Clinical Trial"[Publication Type])) OR (((((((randomized[Title/Abstract]) OR randomised[Title/Abstract]) OR placebo[Title/Abstract]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract]) OR group[Title/Abstract]))) NOT (("Animals"[Mesh]) NOT ("Animals"[Mesh] AND "Humans"[Mesh])))) AND (((((((((((((("Craniocerebral Trauma"[Mesh])) OR "Brain Edema"[Mesh]) OR "Glasgow Coma Scale"[Mesh]) OR "Glasgow Outcome Scale"[Mesh]) OR "Unconsciousness"[Mesh]) OR "Cerebrovascular Trauma"[Mesh])) OR ((((((((haematoma*[Title/Abstract]) OR hematoma*[Title/Abstract]) OR haemorrhag*[Title/Abstract]) OR hemorrhage*[Title/Abstract]) OR bleed*[Title/Abstract]) OR pressure[Title/Abstract])) AND ((((((head[Title/Abstract]) OR cranial[Title/Abstract]) OR cerebral[Title/Abstract]) OR brain*[Title/Abstract]) OR intra‐cranial[Title/Abstract]) OR inter‐cranial[Title/Abstract]))) OR (((((diffuse axonal injury[Title/Abstract]) OR diffuse axonal injuries[Title/Abstract]) OR persistent vegetative state[Title/Abstract]) OR glasgow outcome scale[Title/Abstract]) OR glasgow coma scale[Title/Abstract])) OR ((((((((((((((((injury*[Title/Abstract]) OR injuries[Title/Abstract]) OR trauma[Title/Abstract]) OR damage[Title/Abstract]) OR damaged[Title/Abstract]) OR wound*[Title/Abstract]) OR fracture*[Title/Abstract]) OR contusion*[Title/Abstract]) OR haematoma*[Title/Abstract]) OR hematoma*[Title/Abstract]) OR Haemorrhag*[Title/Abstract]) OR hemorrhag*[Title/Abstract]) OR bleed*[Title/Abstract]) OR pressure[Title/Abstract])) AND (((unconscious*[Title/Abstract]) OR coma*[Title/Abstract]) OR concuss*[Title/Abstract]))))))
Embase Classic + Embase (OvidSP)
1. fluid management.tw. 2. sodium.tw. 3. fluid therapy*.tw. 4. hypertonic saline resuscitation.tw. 5. maintenance fluid*.tw. 6. "hts".tw. 7. hypertonic saline infusion*.tw. 8. hypertonic sodium chloride solution*.tw. 9. sodium chloride/ 10. hypertonic solution/ 11. exp fluid therapy/ 12. sodium/ 13. or/1‐12 14. exp head injury/ 15. brain edema/ 16. Glasgow coma scale/ 17. Glasgow outcome scale/ 18. exp unconsciousness/ 19. exp cerebrovascular accident/ 20. ((haematoma* or hematoma* or haemorrhag* or hemorrhage* or bleed* or pressure) adj3 (head or cranial or cerebral or brain* or intra‐cranial or inter‐cranial)).ab,ti. 21. (diffuse axonal injury or diffuse axonal injuries or persistent vegetative state or glasgow outcome scale or glasgow coma scale).ab,ti. 22. ((injury* or injuries or trauma or damage or damaged or wound* or fracture*OR contusion* or haematoma* or hematoma* or Haemorrhag* or hemorrhag* or bleed* or pressure) adj3 (unconscious* or coma* or concuss*)).ab,ti. 23. or/14‐22 24. 13 and 23 25. exp Randomized Controlled Trial/ 26. exp controlled clinical trial/ 27. exp controlled study/ 28. comparative study/ 29. randomi?ed.ab,ti. 30. placebo.ab. 31. *Clinical Trial/ 32. exp major clinical study/ 33. randomly.ab. 34. (trial or study).ti. 35. 25 or 26 or 27 or 29 or 30 or 31 or 32 or 33 or 34 36. exp animal/ not (exp human/ and exp animal/) 37. 35 not 36 38. 24 and 37
ISI Web of Science: Science Citation Index‐Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index‐Science (CPCI‐S)
#18#17 AND #9 AND #6 #17#16 OR #13 OR #12 #16#15 AND #14 #15TS=(unconscious* or coma* or consuss*) #14TS=(injur* or trauma or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure) #13TS=("diffuse axonal injur*" or "persistent vegetative state" or "glasgow outcome scale" or "glasgow coma scale") #12#11 AND #10 #11TS=(head or cranial or cerebral or brain* or intra‐cranial or inter‐cranial) #10TS=(haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure) #9#8 OR #7 #8TS=("hypertonic sodium chloride solution*") #7TS=("fluid management" or sodium or "fluid therap*" or "hypertonic saline resuscitation" or "maintenance fluid*" or hts or "hypertonic saline infusion*") #6#5 AND #4 #5TS=(human*) #4#3 OR #2 OR #1 #3TS=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) #2TS=(controlled clinical trial OR controlled trial OR clinical trial OR placebo) #1TS=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial)
Appendix 3. CBMdisc search
#1 "随机对照试验"[扩展全部树]/全部副主题词
#2 "随机分配"[扩展全部树]/全部副主题词
#3 "双盲法"[扩展全部树]/全部副主题词
#4 "单盲法"[扩展全部树]/全部副主题词
#5 "临床试验"[扩展全部树]/全部副主题词
#6 "临床试验"
#7 "双盲"
#8 "单盲"
#9 "安慰剂"
#10 "盲法"
#11 "随机"
#12 "研究设计"[扩展全部树]/全部副主题词
#13 CT=对比研究
#14 "评价研究"[扩展全部树]/全部副主题词
#15 "随访研究"[扩展全部树]/全部副主题词
#16 "前瞻性研究"[扩展全部树]/全部副主题词
#17 "对照"
#18 "前瞻"
#19 "志愿者"
#20 CT=动物 AND NOT(CT=人类 AND CT=动物)
#21 #1‐#11/ OR 查找包括随机试验在内的所有临床实验文献)
#22 #21 AND NOT #20(排除动物试验后所有人体实验的临床实验文献)
#23 #12‐#19/OR(查找所有包括评价、对比、随访及前瞻性研究的文献)
#24 #23 AND NOT #20(排除动物试验后所有包括评价、对比、随访及前瞻性研究的文献)
#25 #22 OR #24(排除动物试验后所有包括随机对照试验在内的人体临床试验及评价、对比、随访及前瞻性研究的人体试验文献)
#26 "盐水, 高渗"[不加权:扩展]
#27 "高渗溶液"[不加权:不扩展]
#28 "补液疗法"[不加权:扩展]
#29 "钠"[不加权:扩展]
#30 "氯化钠"[不加权:扩展]
#31 "高渗氯化钠溶液复苏"[常用字段:智能]
#32 ("液体疗法"[常用字段:智能]) OR "补液疗法"[常用字段:智能]
#33 "hts"[常用字段:智能]
#34 "高渗盐水"[常用字段:智能]
#35 "高渗氯化钠溶液"[常用字段:智能]
#36 #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35
#37 "颅脑损伤"[不加权:扩展]
#38 "脑水肿"[不加权:扩展]
#39 "格拉斯哥昏迷量表"[不加权:扩展]
#40 "格拉斯哥预后评分"[不加权:扩展]
#41 "意识丧失"[不加权:扩展]
#42 "脑血管损伤"[不加权:扩展]
#43 #37 OR #38 OR #39 OR #40 OR #41 OR #42
#44 "脑出血"[常用字段:智能]
#45 "脑溢血"[常用字段:智能]
#46 "脑水肿"[常用字段:智能]
#47 "脑血肿"[常用字段:智能]
#48 #44 OR #45 OR #46 OR #47
#49 "弥漫性轴索损伤"[常用字段:智能]
#50 "弥漫性轴突损伤"[常用字段:智能]
#51 "植物状态"[常用字段:智能]
#52 "格拉斯哥昏迷量表"[常用字段:智能]
#53 "格拉斯哥预后评分"[常用字段:智能]
#54 #49 OR #50 OR #51 OR #52 OR #53
#55 "创伤和损伤"[不加权:扩展]
#56 "损伤"[常用字段:智能]
#57 "创伤"[常用字段:智能]
#58 "出血"[常用字段:智能]
#59 "溢血"[常用字段:智能]
#60 "血肿"[常用字段:智能]
#61 "水肿"[常用字段:智能]
#62 #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61
#63 "昏迷"[常用字段:智能]
#64 "震荡"[常用字段:智能]
#65 #63 OR #64
#66 #62 AND #65
#67 #43 OR #48 OR #54 OR #66
#68 #25 AND #36 AND #67
Data and analyses
Comparison 1. Hypertonic saline versus mannitol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality: short‐term | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mortality within 6 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Mortality at discharge from ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Mortality at discharge from hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mortality: 6 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Mortality at 6 months' follow‐up (available data for Jagannatha) | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.55] |
| 2.2 Mortality at 6 months' follow‐up (worst case for HTS data for Jagannatha) | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.66, 1.89] |
| 2.3 Mortality at 6 months' follow‐up (best case for HTS data for Jagannatha) | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.38, 1.18] |
| 3 GOS: poor outcome at 6 months (conventional dichotomisation) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 GOS 6 months (available data Jagannatha) | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.44] |
| 3.2 GOS 6 months (worst case for HTS data for Jagannatha) | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.97, 1.58] |
| 3.3 GOS 6 months (best case for HTS data for Jagannatha) | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.24] |
| 4 GOS: poor outcome at 6 months (death + persistent vegetative state vs all other disability categories ) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 GOS: poor outcome at 6 months (unconventional dichotomisation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
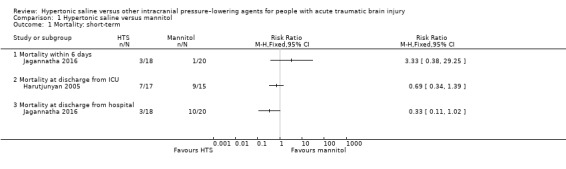
Comparison 1 Hypertonic saline versus mannitol, Outcome 1 Mortality: short‐term.
1.2. Analysis.
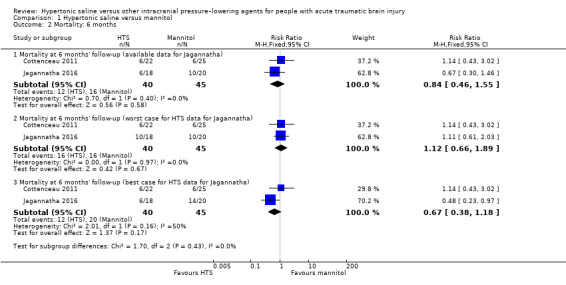
Comparison 1 Hypertonic saline versus mannitol, Outcome 2 Mortality: 6 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cottenceau 2011.
| Methods |
Design: parallel, RCT (2 sites) Setting: ICUs in university hospitals in Bordeaux, France, and in Haifa, Israel Recruitment period: June 2002‐June 2003 Maximum follow‐up: 6 months |
|
| Participants | 56 people (age > 16 years) with severe TBI (GCS ≤ 8) eligible for enrolment into the trial. Of these, 9 were excluded, either because of ICP < 15 mmHg (n = 7) or serum osmolarity > 320 mOsm/L on admission (n = 2) Inclusion criteria: participants with "TBI severe enough to justify ICP monitoring and mechanical ventilation under sedation with a Glasgow Coma Scale (GCS) score... of ≤ 8 at the time of admission" (Cottenceau 2011, p 2004). Exclusion criteria: being < 16 years of age; previous history of cerebral vascular disease; bilateral fixed dilated pupils on admission; hypovolaemic shock Baseline demographics Age: mean of 42.7 years (SD 19.9) in the HTS group; mean of 36.1 years (SD 16.8) in the MTL group Gender: (F/M): not reported Severity: median admission GCS score: 5 (range 4‐7) HTS group, 7 (range 5‐8) in the MTL group N randomized: 47 (HTS, n = 22; MTL n = 25) N for whom data analysed for ICP: this number varied as number of ICP elevations decreased after day 3. Trial authors report data for each of the 1st 3 days, until the point where values are available for < 50% of either group. The last data (day 3) are for 24 participants (HTS = 11, MTL = 13) N for whom GOS data analysed at 6 months: 6 deaths occurred in each group; these formed part of the GOS categories. Data were available for the full sample (47; HTS, n = 22; MTL, n = 25) |
|
| Interventions | Quote: "Assessment of patients was initiated prior induction of hyperosmolar therapy in presence of ICP elevation above 15 mm Hg. Although ... lower than the 20mm Hg threshold quoted in the Guidelines for the Management of Severe TBI .... this threshold was chosen in accordance with the management protocols of both participating centers and was consistent with clinical evidence showing that ICP > 15 was one of the five independent factors associated with death following TBI ....Whenever appropriate, patients received equiosmolar infusions of either..." Intervention: HTS 7.5% (2 mL/kg; n = 22), delivered IV within 20 min or Comparator: MTL 20% (4 mL/kg; n = 25) Quote: "As long as ICP remained elevated and monitored, all patients had a daily evaluation during which a baseline assessment was followed by two additional tests performed at 30 and 120 min after administration" (Cottenceau 2011, p 2004). |
|
| Outcomes | Outcomes included (in hospital):
At 6 months:
|
|
| Notes | Trial authors note their focus differs from others, i.e. "we aimed to comparatively assess the effect of HTS and MTL, not only on ICP and neurological outcome but also on indices of cerebral blood flow (CBF) and metabolism" (Cottenceau 2011 p 2004). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated random‐number table was used to assign each consecutive envelope to receive a sheet indicating either MTL or HTL group....Randomization was based on blocks of four" (Cottenceau 2011, p. 2004) |
| Allocation concealment (selection bias) | Low risk | Quote: Before the trial, "30 opaque envelopes in each hospital had been prepared and numbered sequentially....Envelopes were then sealed.... The sealed envelopes were opened sequentially throughout the study when a patient fulfilled inclusion criteria" (Cottenceau 2011, p. 2004) |
| Blinding of participants and personnel (performance bias) participants | Low risk | Participants had GCS score of < 8 and therefore they were unconscious at the time of admission. |
| Blinding of participants and personnel (performance bias) Treating physicians | High risk | Correspondence with trial author in 2015. Quote: "at the acute phase where the team was well aware of the regimen" (6 May 2015). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Correspondence with trial author in 2015. Quote: "as for the outcome, it was assessed in both centers by blinded medical staff during follow‐up visits or by phone calls issued by blinded personnel" (2 March 2015). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are reported in full for ICP for as long as a reasonable number of participants require it (day 3). |
| Selective reporting (reporting bias) | Unclear risk | Correspondence with trial author in 2015. Quote: "the protocol is not of reach after such a long time" (Soustiel 2015) |
| Other bias | Unclear risk | Authors note that "Comparative analysis of neurological outcome at 6 months did not disclose any significant difference between the two groups. Although there was a statistical trend suggestive of a better outcome in patients in the MTL group, similar differences found in the CMRO2 [cerebral metabolic rate of oxygen] values and GCS scores on admission between the two groups probably indicated some asymmetry in the degree of severity of injury and accounted for this neurological outcome difference (Fig. 5; x2 p = 0.0662)." (Cottenceau 2011, p 2007) |
Francony 2008.
| Methods |
Design: Parallel, RCT Setting: 2 ICUs at the same hospital in Grenoble, France Recruitment period: October 2002‐June 2005 Maximum follow‐up: 2‐h trial period |
|
| Participants | A total of 20 stable patients with a sustained ICP of > 20 mmHg secondary to TBI (n = 17) or stroke (n = 3) were recruited. Inclusion criteria: "Patients were included if they were aged 18 yrs and had sustained elevated ICP of 20 mm Hg for 10 mins, not related to procedural pain. They had to be mechanically ventilated and in stable conditions for 2 hrs before the trial, as defined by the following criteria: MABP of 80 mm Hg, PaO2 of 80 mm Hg, PaCO2 of 45 mm Hg, serum osmolality ranging between 280 and 320 mOsm/kg, and body temperature of 38.0°C" (Francony 2008, p 796). Exclusion criteria: "Patients were excluded if they had any of the following criteria: an imminent cranial or extracranial surgery, a previous decompressive craniectomy, a leakage or a drainage of cerebrospinal fluid, unstable respiratory and hemodynamic conditions, oliguric renal failure, hemoglobin content of 100 g/L, serum osmolality of 320 mOsm/kg, the use of mannitol or HSS in the previous 6 hrs, or a concomitant use of thiopentone." (Francony 2008, p 796). Baseline demographics Age: mean of 37 years (SD 16) in the HTS group; mean of 43 years (SD 11) in the MTL group Gender: (F/M); 2:8 in the HTS group; 1:9 in the MTL group Severity: mean GCS at baseline 8 (SD 2) HTS group, 7 (SD 2) in the MTL group N randomized: 20 (HTS = 10; MTL = 10) N for whom ICP data analysed: 20 (HTS = 10; MTL = 10) |
|
| Interventions | Prior to the intervention, if the patient "met the inclusion criteria, a static cerebral autoregulation test was performed because the response to osmotherapy may differ according to the pressure autoregulation status.... After this test, the patient was assigned ...to receive... " either: Intervention: a single equimolar infusion (255 mOsm dose) of 100 mL of 7.45% HTS in 20 min of administration via the central venous catheter Comparator: a single equimolar infusion (255 mOsm dose) of 20% MTL in 20 min of administration via the central venous catheter "No therapeutic intervention (e.g., nursing procedure, manipulation of ventilatory variables, changes in vasoactive support and in sedative drug regimens) was allowed during the experiment, except the administration of 6% hydroxyethyl starch solution ... if MABP decreased 10% from baseline" (Francony 2008, p 796). |
|
| Outcomes | During a trial period of 120 min, the following were measured:
"Clinical and biological variables (blood gases, arterial pH, serum osmolality, hemoglobin) were collected at baseline (reference time, T0) and repeated every 30 mins after the start of infusion (T30, T60, T90) until the end of the study period (T120). Blood levels of sodium, chloride, glucose, and creatinine were collected at T0 and at T120" (Francony 2008, p 796). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patient was assigned by randomization using sealed, numbered envelopes" (Francony 2008, p. 796) |
| Allocation concealment (selection bias) | Low risk | Quote: "The patient was assigned by randomization using sealed, numbered envelopes" (Francony 2008, p. 796) |
| Blinding of participants and personnel (performance bias) participants | Low risk | Participants had severe brain injury with sustained elevated ICP of > 20 mmHg and therefore had reduced cognitive function. |
| Blinding of participants and personnel (performance bias) Treating physicians | High risk | Quote: "The two treatments were of different volumes, it was not possible to blind their administration" (Francony 2008, p. 796) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were reported (the trial lasted 120 minutes). |
| Selective reporting (reporting bias) | Low risk | Following correspondence with the trial author in 2015, we obtained a copy of the trial protocol. It is clear that the 2002 protocol focused on cerebral blood flow and metabolism, which were adequately described. |
| Other bias | Unclear risk | 3/17 participants do not have TBI |
Harutjunyan 2005.
| Methods |
Design: RCT Setting: ICU setting in a university hospital in Halle, Germany Recruitment period: February 2003‐August 2004 Maximum follow‐up: end of stay in the ICU |
|
| Participants | 40 "neurosurgical patients at risk of increased ICP" were recruited; 8 did not receive medication as ICP threshold of 20 mmHg was not met; of the remaining 32, only a minority (n = 10) had TBI. Inclusion criteria:"Age >18 years, severe brain damage (Glasgow Coma Score <8) with cerebral edema – visualized by CT scan and continuous monitoring of ICP". Exclusion criteria:"elevated ICP due to space‐occupying lesions with indication for neurosurgical intervention (e.g. bleeding, hydrocephalus), severe renal failure, metabolic disorders, initial serum sodium >150 mmol/l and initial serum osmolarity >320 mosm/kg" (Harutjunyan 2005, p R531). Baseline demographics Age: mean of 47 years (SD = 16 years) across both groups Gender: 17 men, 15 women (8/9 F/M in HTS/HES Group; 7/8 in MTL group) Severity: GCS at baseline 6 ± 1.3 HTS/HES group, 5.8 ± 1.4 MTL group N randomized: 32 (HTS/HES = 17; MTL = 15). Of these, 6 in HTS/HES group had TBI and 4 in MTL group) N for whom ICP data analysed: 32 (HTS/HES = 17; MTL = 15) |
|
| Interventions | A standard treatment protocol was followed (details of which appear Harutjunyan 2005, p R531). "All patients were intubated and received pressure‐controlled mechanical ventilation .... Care was taken to keep the arterial partial oxygen pressure above 15 kPa, the hemoglobin concentration above 5.5 mmol/l and the CPP above 70 mmHg. If necessary, blood pressure was supported with vasopressor therapy. Blood glucose was adjusted to values between 6–8 mmol/l by continuous application of human insulin. Patients' core temperature was measured via the bladder, with a target temperature of 36.0–37.0°C. ....Analgosedation and continuous patient monitoring were managed ....Analgosedation at days 1–4 was performed using propofol and sufentanil or remifentanil. Thereafter, midazolam and sufentanil were administered. The standard monitoring ... An increase in ICP was treated first by deepening the sedation and analgesia by titrating the medication and adjusting to adequate ventilator settings. If ICP exceeded the 20 mmHg threshold for more than 5 min, the study medication ... was infused via the central venous line using an automated infusion system at a defined infusion rate. The infusion was stopped when ICP was reduced to <15 mmHg, defined as the treatment goal." Intervention: 7.2% HTS/HES (200/0.5) 6% by infusion Comparator: MTL 15%, by infusion "However, in the case of sustained ICP problems (ICP >15 mmHg or CPP <70 mmHg) after these measures, bolus applications of thiopentone (maximum single bolus: 5 mg/kg) were allowed. In these patients, the possibility of a space‐occupying lesion was excluded by CT scan." |
|
| Outcomes | The following were continuously measured:
At the end of stay in the ICU, the following were measured:
|
|
| Notes | Trial authors note their focus differs from others, i.e.: "The substantial difference in the design of the present and a comparable study is the fact that we did not administer a fixed total dose, but infused the study medication at a defined infusion rate until ICP decreased to <15 mmHg, the primary goal of our treatment. No clinical study has so far identified an exact dose‐effect relationship for hypertonic saline." (Harutjunyan 2005, R537) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random code for group assignment was generated by computer" (Harutjunyan 2005, p R532) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) participants | Low risk | All participants had GCS score of < 8 and therefore had reduced cognitive function at the time of admission; outcome assessment was not long‐term, so participants had no chance of learning their assignment. |
| Blinding of participants and personnel (performance bias) Treating physicians | High risk | There is no indication that the treating physicians were blind to the treatments given, as the time of treatment and doses given were different between trial groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 40 neurosurgical patients were recruited and randomised to receive either 7.2% HTS/HES 200/0.5 or MTL 15%. Only 32 participants were evaluated since in 8 participants, ICP did not exceed 20 mmHg,therefore no trial medication was administered. No relevant clinical characteristics were revealed in the 8 participants not undergoing osmotic therapy. Full data were available for the 32 eligible by reason of exceeding 20 mmHg. |
| Selective reporting (reporting bias) | Unclear risk | This trial was retrospectively registered in 2005, the year of its publication (ISRCTN62699180). Although expected outcomes are present, in the absence of a published protocol or prospective trial registration, assessment of selective outcome reporting must remain as "unclear." |
| Other bias | High risk | For the purposes of this review question, this trial's data must be considered at high risk of bias because only a minority of participants had suffered a TBI; a situation further complicated by acknowledged baseline imbalances (trial authors note that "the clinical values in both groups were not normally distributed [at baseline]" (Francony 2008, p R532) |
Jagannatha 2016.
| Methods |
Design: RCT Setting: ICU of a tertiary neurosurgical center, Bangalore, India Recruitment period: May 2008 to unknown date Maximum follow‐up: 6 months |
|
| Participants |
Inclusion criteria: participants with severe TBI; aged 15‐70 years; to be enrolled into the trial within 24 h of injury Exclusion criteria: people with GCS score of 3 and absent brainstem reflexes; pregnant women; those with spinal cord injury or multiple systemic injuries Baseline demographics Age: mean of 27 years (SD 8) in the HTS group; mean of 31 years (SD 13) in the MTL group Gender: (F/M); 1:8 in the HTS group; 1:9 in the MTL group Severity: mean admission GCS score post‐resuscitation median 4 (range 4‐5) in the HTs group and 5 (range 4‐6) in the MTL group N randomized: 38 (HTS, n = 18; MTL n = 20) N for whom data analysed at 6 days: 30 (HTS, n = 15; MTL, n = 15) had ICP/CPP data available for the main data collection period (6 days). Of the rest, 4 died before day 6 and in 4, ICP monitoring was discontinued after < 6 days as clinical status had improved. N for whom GOS and mortality data analysed at 6 months: 22 (HTS, n = 12; MTL, n = 10) |
|
| Interventions | Prior to treatment "All patients were managed in the ICU according to the Brain Trauma Foundation guidelines [2007] .... The aim of the therapy was to maintain the ICP below 20 mmHg and CPP above 50 mmHg. Any spontaneous ICP increase to >20 mmHg qualified as an ICH episode. If an ICH episode occurred despite adequacy of sedation, ventilation and head position, CSF was drained until it stopped flowing spontaneously as a first line intervention. If the ICP remained elevated (>20 mmHg for >10 minutes) in spite of CSF drainage (until the CSF egress ceased), patients received osmotic therapy" (Jagannatha 2016, p 69) as follows: Intervention: 3% HTS, in an equiosmolar dose infused as a bolus through a central venous catheter over 5 min Comparator: 20% MTL, in an equiosmolar dose infused as a bolus through a central venous catheter over 5 min Subsequently, "If the first dose of the osmotic agent failed to decrease the ICP to below 20 mmHg, a maximum of three doses of the same drug were administered. If the ICH persisted, hyperosmolar therapy was considered a failure and thiopentone, propofol, or moderate hyperventilation (PaCO2 = 30 mmHg) were instituted. As per the Brain Trauma Foundation guidelines, decompressive craniectomy was considered after exhausting general measures, CSF drainage, osmotic therapy and metabolic suppression. Hyperosmolar therapy was temporarily suspended if serum sodium increased to > 160 mmol/dL or if serum osmolality increased to >320 mosm/kg. Inotropes/vasopressors (dopamine, adrenaline and noradrenaline) were administered as and when required to maintain CPP. A CT scan of the head was repeated at 24 hours and 5 days post‐trauma, and whenever the patient suffered a neurological deterioration. The ICP catheter was left in situ for 6 days. The catheter was removed earlier if the patient started obeying commands or the ICP was maintained <20 mmHg for 24 hours." |
|
| Outcomes | For each bolus of the hyperosmolar agent administered over 6 days, the following were recorded:
The following outcomes were also assessed in hospital:
At 6 months, the following was measured:
|
|
| Notes | Trial authors note their focus differs from others, i.e.: "Overall, the literature is centered on efficacy in individual episodes of ICH than the sustenance of ICP control during the acute phase of TBI, which is more relevant to the outcome. Our study differs from the other studies in this important respect" (Jagannatha 2016, p. 71) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was recruited into the mannitol or HTS group based on a computer generated randomization chart" (p 69) (Jagannatha 2016) Appears adequate |
| Allocation concealment (selection bias) | High risk | Not described. Trial authors reported not concealing allocation (Jagannatha 2017, personal communication) |
| Blinding of participants and personnel (performance bias) participants | Low risk | Participants had severe brain injury with sustained elevated ICP of > 20 mmHg and therefore had reduced cognitive function. |
| Blinding of participants and personnel (performance bias) Treating physicians | High risk | There is no indication that the treating physicians were blind to the treatments given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For the outcome of ICP, there appear to be no data missing for reasons other than participants' not having ICP assessed due to improvement in status, or to their deaths. In the longer term however, of the 30 who left hospital, 8 were lost to follow‐up, as explained by trial authors in a personal communication (Jagannatha 2017). Data were "based on telephonic interview with the patient/ patients relative. Unfortunately due to various reasons (wrong phone number, no one to answer the ringing phone, change of number) we could not collect the data for 8 patients in total." |
| Selective reporting (reporting bias) | Unclear risk | This trial was retrospectively registered in 2016. Enrolment appears to have commenced in May 2008 (CTRI//04/006829 2016). Although expected outcomes are present, in the absence of a published protocol or prospective trial registration, assessment of selective outcome reporting must remain as "unclear." |
| Other bias | Unclear risk | Trial authors note that some "methodological issues need to be taken into account when interpreting our results. ... Though we intended to recruit consecutive patients, some patients were excluded for logistic reasons. The GCS at inclusion of the patient into the study was much lower in this study compared with other studies, with a median of 5 and 4 (eye opening and motor scores) in the mannitol and HTS groups, indicating a more severe injury. Some of the patients in the study underwent surgery, which might have conferred some benefit in terms of ICP reduction. The patients were controlled for GCS at the time of inclusion and not the type of lesion on CT scan. The occurrence of twice the number of subdural hematomas in the mannitol group (15 versus seven) may have introduced a bias. Subdural hematomas by virtue of being a pathologically more severe form of injury may have necessitated a higher number of hyperosmolar boluses in the mannitol group. The groups, however, were comparable with respect to the overall radiological profile and findings at surgery. Also, to our surprise, even in the operated patients, the initial reduction of ICP was followed by a progressive increase over time. Poor glycemic control in the mannitol group may also have influenced the outcome in these patients. The number of patients in the study was small and this limited our outcome analysis" (Jagannatha 2016) p 73. |
Kumar 2019.
| Methods |
Design: parallel, equivalence RCT (single site) Setting: ICU of a tertiary neurosurgical centre in Bangalore, India Recruitment period: January 2012‐June 2014 Maximum follow‐up: 6 months |
|
| Participants | 50 children were assessed; 30 met eligibility criteria (20 were excluded with reasons given). All children underwent initial resuscitation, evaluation and treatment for TBI as required; CT scan studies on admission were analysed using Marshall criteria (Marshall 1992) Inclusion criteria: children in age group 1‐16 years with severe TBI, defined as post‐resuscitation Pediatric GCS of ≤ 8, and presenting within 24 h of trauma Exclusion criteria: having a GCS of 3; absent brain stem reflexes; systemic injuries requiring immediate treatment; clinical evidence of significant spinal cord injuries; or presenting > 24 h after injury Baseline demographics Age: a mean for age is not reported. Trial authors report no significant difference between groups. There is a typographical error in the total number for the ranges of age groups, but the disaggregated totals add up to 30, and are reported as follows: of eligible children admitted to the trial, 14 children were aged 1‐5 years; 7 children age 6‐10; 9 children aged 11‐16 Gender: 12 girls, 18 boys; 6:14 in the HTS group; 6:16 in the MTL group Severity: post resuscitation GCS means and SDs were given by group. These were 7.4 (SD = 0.9) in the HTS group and 6.6 (SD=1.1) in the MTL group. Data on manner of injury (road accidents, falls); pupillary reaction to light; head CT findings; interval between injury and insertion of EVD, duration of monitoring, duration of ventilation, duration of ICU stay, and duration of hospital stay, were also collected N randomized: 30 (HTS, n = 14; MTL n = 16) N for whom data analysed for ICP/GOS data analysed: trial authors report no missing data; data available for the full sample (HTS, n = 14; MTL, n = 16) |
|
| Interventions | "The aim of the therapy [ICP monitor setup and treatment, protocol described] was to maintain the ICP below 15 mmHg in children between 1 and 10 years of age and 18mmHg in children age 11–16 years of age [7, 8]. When the ICP remained raised more than the cutoff value for more than 5 min in the absence of noxious stimuli like suction, positioning, etc., it qualified as an intracranial hypertensive (ICH) episode. For an ICH episode, the EVD was opened to drain CSF until it stopped flowing or up to 20 cc release of CSF whichever is first...After successful insertion of EVD and ICP monitoring, the patients were randomized to receive one of the interventional agents. ...[which]were administered if ICP remained persistently above the cutoff value for more than 5 min in spite of CSF drainage. The ICU staff informed each episode of raised ICP to one of the investigators (AK or DS), who was available at bedside before initiating treatment for reduction of ICP. The investigator personally documented ICP before initiation and after completion of treatment, and measured reduction in ICP for each dose of medication" (Kumar 2019 p 1000) Intervention: equiosmolar dose of 3% HTS (1027 mOsm/L) as a bolus of 2.5 mL/kg through the central venous line over a period of 5 min (n = 14 participants) Comparator: equiosmolar dose of 20% MTL (1098 mOsm/L) as a bolus of 0.5 g/kg (2.5 mL/kg; n = 16 participants) "If the ICP did not decrease even after two consecutive doses of the hyperosmolar agent, it was considered refractory to therapy" (Kumar 2019 p 1000) Monitoring of active treatment appears to have continued for 5‐6 days |
|
| Outcomes |
Primary outcome: mean reduction of ICP ‐ defined as "the difference between ICP value before administering hyperosmolar agent and lowest ICP value after completion of bolus for each dose. The mean reduction in ICP was obtained by summing the difference in ICP values before and after treatment divided by number of doses during the entire period of ICP monitoring" (Kumar 2019, p 1001). Secondary outcome:
Other data collected included:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was done through computer‐generated random numbers" (Kumar 2019, p 1000). Appears adequate |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) participants | Low risk | Participants had brain injury and neither they nor parents/carers were likely to be aware of treatment allocation. |
| Blinding of participants and personnel (performance bias) Treating physicians | High risk | There is no indication that the treating physicians were blind to the treatments given, and the trial is described as "single blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial authors report that "The person who assessed outcome was blinded for the interventional agent" (Kumar 2019, p 1001). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For the outcome of ICP, there appear to be no data missing. |
| Selective reporting (reporting bias) | Unclear risk | Trial authors report that this trial was registered with Clinical Trials Registry of India (REF/2015/03/008696). The date suggests retrospective registration. |
| Other bias | Unclear risk | Authors note that "The limitation of our study was small sample size. ...For adequate power of study, a large multicenter study is warranted. The present underpowered study cannot generate a good class of evidence but demonstrates the feasibility of such studies at a larger scale. The second limitation was use of hyperosmolar therapy as second tier treatment after failure of CSF drainage to reduce the ICP. The EVD, when available, is recommended prior to hyperosmolar therapy.... The EVD as an initial treatment may dilute the effect of hyperosmolar therapy. It is not known, whether there will be any difference in ICP reduction between mannitol and hypertonic saline if any of these agents are administered as first‐line therapy. Many centers do not use EVD for ICP monitoring. When ICP monitoring is done using parenchymal sensor, option of CSF drainage is not available, and true effect of hyperosmolar therapy can be assessed. The third limitation was that we did not measure time to peak effect and duration of effect, cerebral blood flow, cerebral tissue oxygen, cerebral metabolism, cerebral injury biomarkers, cerebrospinal compliance, and pressure reactivity. The multimodal monitoring is labor intensive, and is not available in our set up" (Kumar 2019, pp 1004‐5). |
Patil 2019.
| Methods |
Design: parallel, 3‐armed RCT (single site) Setting: ICU in a department of neurosurgery at a teaching hospital in Indore, Madhya Pradesh, India Recruitment period: 2015‐2017 Maximum follow‐up: 1‐h observation period following the treatment goal of reduction of ICP below 15 mmHg (after a single bolus) |
|
| Participants | A total of 120 participants with isolated severe TBI due to road traffic accidents were recruited. Inclusion criteria: "After assessing the Glasgow Coma Scale (GCS), computed tomography of the head was performed to rule out the need for immediate surgery. Patients were included if they were aged 18 years, GCS 8, and had sustained elevated ICP of >20 mm Hg for more than 5 minutes" Exclusion criteria: "... imminent cranial or extracranial surgery Previous decompressive craniectomy Leakage or drainage of cerebrospinal fluid Polytrauma Oliguria, renal failure Hemoglobin <8 g/L Serum osmolality of >320 mOsm/L. The use of mannitol or HTS in the previous 6 hours." (Patil 2019 p e222) Baseline demographics Age: mean was given as 38.42 (+/‐15.5 years). The range was 18‐75 years. Gender: (F/M); not reported Severity: mean GCS at baseline reported as 6 (range 3‐8) in the HTS group; 5 (range 3‐7) in the MTL group and 5 (range 3‐6) in the combined MTL and glycerol group, BP also reported N randomized: 120 (HTS = 40; MTL = 40; MTL plus glycerol 40) N for whom ICP data analysed: not stated, but data presumed to be complete (assessments gathered across 1 h): 120 (HTS = 40; MTL = 40; MTL plus glycerol 40) |
|
| Interventions | At first‐ "Analgesia was provided to all the patients and if required sedation also provided in irritable patients (dexmedetomidine). Vasoactive support (norepinephrine) was administered in hypotensive patients. Insulin treatment was administered to maintain glycemia at <140 mg/dL. For each patient, a set of variables was collected that included demographic characteristics data, initial GCS, and timing of studied treatment. The ICP was continuously monitored by using an intracranial bolt..." When ICP exceeded 20 mm Hg for a period of > 5 min, interventions comprised a single bolus dose of one of the following: Intervention: HTS 3% (n = 40); mean dose of 1.4 mL/kg (range 0.5‐3.3); mean dose mL application 94 (range 38‐234) or Comparator 1: MTL 20% (n =40); mean dose of 2.0 mL/kg (range 0.5‐6.3); mean dose mL application 137 (range 40‐422) Comparator 2: MTL 10% plus 10% glycerol combination (n =40); mean dose of 1.7 mL/kg (range 1.6‐4); mean dose mL application 118 (range 44‐302) All treatments were "infused via the central venous line at a defined infusion rate, that is, 6 mL/minute or 120 drops/minute (osmolarity of mannitol, mannitol plus glycerol combination, and 3% HTS are almost the same, ie, 1100 mOsm/L, 1049 mOsmo/L, and 1027 mOsm/L, respectively). The infusion was stopped when ICP was reduced to <15 mm Hg, which was our treatment goal" (Patil 2019, e222). |
|
| Outcomes |
The lack of data collection on adverse events is mentioned as a trial limitation. |
|
| Notes | This trial is not reported according to CONSORT/PRISMA. There is no flowchart; scant description of methods. Baseline data including lesion type, gender, are unreported. All data are reported in ranges (minimum‐maximum). Mortality is not specifically reported although GCS (after a single bolus dose) is reported. The paper states that recruitment took place 2015‐2017, but submission of the present paper was apparently made in 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All the patients were divided into 3 groups (40 in each group) using the sealed envelope method of physical randomization" (Patil 2019, p e223) Appears adequate although phrasing unusual |
| Allocation concealment (selection bias) | Low risk | Quote: "All the patients were divided into 3 groups (40 in each group) using the sealed envelope method of physical randomization" (.Patil 2019, p e223) Appears adequate although phrasing unusual |
| Blinding of participants and personnel (performance bias) participants | Low risk | Participants had severe brain injury with sustained elevated ICP of > 20 mmHg and therefore had reduced cognitive function and would be unaware of treatment status. |
| Blinding of participants and personnel (performance bias) Treating physicians | High risk | Agents were of different substances (or at different doses). Trial authors do not report any blinding of those administering or collecting data. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | We have some concerns about the lack of information on assessment of GCS, which was assessed alongside hematocrit, ICP, etc., to a maximum of 1 h, during a period of nearly constant assessment. It is not stated who undertook this, and as stated above it is unlikely staff were unaware of intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were reported (the post‐intervention data collection period lasted 60 min) |
| Selective reporting (reporting bias) | Unclear risk | Trial authors mention ethics approval, but not trial registration. Although most expected outcomes are present, in the absence of a published protocol or prospective trial registration, assessment of selective outcome reporting must remain as 'unclear' |
| Other bias | Unclear risk | Trial authors comment that they did not assess any complications associated with the placement of the subdural bolt used for measuring ICP. |
BP: blood pressure; CBF: cerebral blood flow; CPP: cerebral perfusion pressure; CSF: cerebrospinal fluid; CT: computerised tomography scan; EVD: external ventricular drain(age); GCS: Glasgow Coma Scale; GOS: Glasgow Outcome Scale; HES: hydroxyethyl starch; HTS: hypertonic saline; ICP: intracranial pressure; ICU: intensive care unit; IV: intravenous; MAP/MABP: mean arterial [blood] pressure; MTL: mannitol; RCT: randomised controlled trial; SD: standard deviation; SpO2: peripheral capillary oxygen saturation; TBI: traumatic brain injury
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Battison 2005 | This is a prospective, cross‐over, pilot trial. |
| Bourdeaux 2011 | Participants received doses of HTS or sodium bicarbonate in a random order, some having both. This may result in cross‐over effects between treatments. |
| Du 2017 | This trial was excluded because there was no defined trigger for starting hyperosmotherapy to reduce ICP, and mannitol was administered every eight hours. |
| Hong 2017 | This trial was excluded due to unreliable methods (HTS or other ICP‐lowering agents were administered by time but not according to ICP monitoring). |
| Huang 2014 | This is a randomised clinical trial with a cross‐over design. |
| Huang 2015 | This trial was excluded for questionable sequence generation; there was no reporting on outcomes of interest to this review. |
| Ichai 2009 | Sodium lactate was used to decrease the raised ICP. Sodium lactate differs fundamentally from sodium chloride; infusion of sodium lactate in TBI decreases the occurrence of raised ICP. The absence of significant modification of plasma osmolarity does not support a pure osmotic effect of sodium lactate. |
| Jafari 2018 | This trial was excluded because it assessed the effects of adding furosemide to 1 of 2 groups both receiving HTS. |
| Jiang 2018 | RCT. Excluded because of ineligible comparator (there were 3 arms, all of HTS, in the same concentration but delivered at different speeds (6 mL/3 mL/2 mL per h). |
| Jin 2018 | Excluded due to doubts about sequence generation and apparently unreliable data. We were unable to contact trial authors for clarification. |
| Li 2018 | Excluded due to unreliable data and methods (there is an error in the judgement of P value). |
| Liang 2013 | Excluded due to unreliable data and methods (incorrect 'T' values, questionable sequence generation). We were unable to contact trial authors for clarification. |
| Liu 2018 | Excluded due to unreliable methods (HTS or other ICP‐lowering agents were administered by time but not according to ICP monitoring). |
| Mei 2016 | Excluded due to unreliable data and methods (incorrect 'T' values, questionable sequence generation). We were unable to contact trial authors for clarification. |
| NCT01028339 | Appeared to meet inclusion criteria but according to the clinicaltrials.gov record it has been "terminated. (No patients enrolled during 2 years)". |
| NCT01108744 | This trial met inclusion criteria but according to the clinicaltrials.gov record was "...withdrawn prior to enrolment. Timeline to consent prior to intervention start was unfeasible". |
| NCT01111682 | This trial met inclusion criteria but according to the clinicaltrials.gov record was "...terminated. (A significant reduction in head injuries coupled with more frequent use of craniectomy reduced the number of potential subjects)". |
| NCT01215019 | This trial met inclusion criteria but according to the clinicaltrials.gov record was "...withdrawn prior to enrolment. (Lack of funding; no subjects enrolled)". |
| Ni 2018 | Excluded due to unreliable data and methods (e.g. no clear definition of ICP). |
| Polushin 2009 | This is a randomised clinical trial with a cross‐over design. |
| Roquilly 2017 | This planned RCT is measuring continuous hyperosomolar therapy (HTS) in participants with TBI vs standard care (which is unlikely to include other ICP‐lowering agents). |
| Sakellaridis 2011 | This is a prospective, cross‐over, pilot trial. |
| Shu 2015 | Excluded due to unreliable data and methods (incorrect 'T' values, questionable sequence generation). We were unable to contact trial authors for clarification. |
| Upadhyay 2010 | The sequence generation was described (quasi‐randomisation). The included participants were 200 children with increased ICP of various different aetiologies, of whom only 14 had TBI. Comparison of average reduction of MAP at defined intervals was performed to indirectly assess reduction in ICP. |
| Wang 2017 | This trial was non‐therapeutic in nature, measuring only coagulation. |
| Yang 2019 | This trial was excluded because it compared two different doses of HTS (7.5% HTS (4 ml/kg) and 3% HS treatment (4 ml/kg)). |
| Zhang 2014 | Excluded due to unreliable data and methods (incorrect 'T' values, questionable sequence generation). We were unable to contact trial authors for clarification. |
| Zhang 2015 | Excluded due to unreliable data and methods (incorrect 'T' values, questionable sequence generation). We were unable to contact trial authors for clarification. |
| Zhang 2018 | Excluded due to unreliable methods (HTS or other ICP‐lowering agents were administered by time, but not according to results of ICP monitoring). |
HTS: hypertonic saline; ICP: intracranial pressure; MAP: mean arterial pressure; RCT: randomised controlled trial; TBI: traumatic brain injury;
Characteristics of studies awaiting assessment [ordered by study ID]
Vialet 2003.
| Methods | Prospective, randomised trial |
| Participants | 20 consecutive patients with head trauma and persistent coma who required infusions of an osmotic agent to treat episodes of ICP resistant to well‐conducted standard modes of therapy. |
| Interventions | Participants were randomly assigned to receive isovolume infusions of either 7.5% HTS solution (n =10) or 20% MTL (n =10). |
| Outcomes |
|
| Notes | The numbers quoted in the abstract and the main results in Table 2 were not the same. Direct contact with the trial authors did not resolve this issue. |
HTS: hypertonic saline; ICP: intracranial pressure; MTL: mannitol
Characteristics of ongoing studies [ordered by study ID]
Salt or Sugar 2019.
| Trial name or title | Salt or Sugar (SOS) trial: hyperosmolar in traumatic brain injury |
| Methods | RCT. Sample size intended to be 219 per group (638 in total) |
| Participants |
Target population
Adult patients (aged > 16 years) with severe TBI and raised intracranial pressure (ICP).
Exclusion criteria
|
| Interventions | 2 mL/kg bolus of 20% MTL vs 2 mL/kg bolus of 3% HTS (or equivalent osmolar bolus) |
| Outcomes |
Primary outcome
Secondary outcomes
Health economic outcomes
|
| Starting date | 1 June 2019‐1 December 2023 |
| Contact information | Professor Gavin Perkins,University of Warwick UK G.D.Perkins@warwick.ac.uk |
| Notes | Information above came directly from a 'Trial Summary Sheet' sent to review authors in March 2019; trial was then registered here: ISRCTN16075091 (apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN16075091) |
CT: computed tomography; GOS‐E: Glasgow Outcome Scale‐Extended; HTS: hypertonic saline; ICP: intracranial pressure; ICU: intensive care unit; MTL: mannitol; RCT: randomised controlled trial; TBI: traumatic brain injury;
Differences between protocol and review
In the protocol for this review (Chen 2014), we intended to include people with acute traumatic brain injury only; however, eligible trials are so few that we were obliged to include trials with mixed populations within the final review. Two trials included people who did not have a traumatic brain injury but did have increased intracranial pressure (Francony 2008; Harutjunyan 2005). Naturally this complicates interpretation of the results; however, we did not perform any meta‐analysis including data from these trials and comment on the heterogeneity of participants where relevant.
In the Types of interventions section, we included any hypertonic saline in any dosage for any duration in the protocol, but in the review we excluded a trial that compared sodium lactate with mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain injury. Sodium lactate is a hyperosmolar solution but it differs fundamentally from sodium chloride because lactate is a metabolisable anion which means that even with comparable osmolarity in the bottle, sodium lactate becomes two times less hypertonic than equiosmotic sodium chloride. The effect of sodium lactate could not be attributed to a 'classical osmotic effect' as sodium lactate administration was not associated with any increase in plasma osmolality, contrary to the equiosmotic mannitol administration which was associated with a concomitant increase in plasma osmolality (personal communication Ichai 2009).
Under 'Methods', we had not planned to consider conducting 'best‐worst case' scenarios, but missing mortality data in a key trial made this seem a reasonable course in order to demonstrate the parameters of uncertainty for important outcomes including mortality and 'poor outcome' on the Glasgow Outcome Scale (GOS).
We have included plans for future updates to incorporate continuous data, if necessary, given the variation with which intracranial pressure values are reported.
In future updates, we may, if appropriate, use Trial Sequential Analysis (CIG 2015).
Contributions of authors
All authors contributed to the production of this review.
Writing and editing the manuscript: HC, ZS, JD
Screening titles and abstracts: HC, ZS, JD
Assessment for inclusion: HC, ZS, JD
Quality assessment: HC, ZS, JD
Data entry into Review Manager 5 and analysis: HC, JD
Resolution of disagreement (not required): ZS
Sources of support
Internal sources
-
London School of Hygiene & Tropical Medicine, UK.
J Dennis received payment from Cochrane Injuries during the completion of this review. Cochrane Injuries is based at LSHTM.
External sources
-
Natural Science Foundation of Hunan Province, China.
Grant No. 2018JJ3806, Han Chen
Declarations of interest
HC: no known conflict of interest
ZS: no known conflict of interest
JD: no known conflict of interest. JD was employed by Cochrane Injuries during her part in the development of the review.
Edited (no change to conclusions)
References
References to studies included in this review
Cottenceau 2011 {published data only}
- Cottenceau V, Masson F, Mahamid E, Petit L, Shik V, Sztark F, et al. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. Journal of Neurotrauma 2011;28:2003‐12. [DOI] [PubMed] [Google Scholar]
- Soustiel JF. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline [personal communication]. Email to: H Chen 2 March 2015.
- Soustiel JF. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline [personal communication]. Email to: H Chen 6 May 2015.
Francony 2008 {published data only}
- Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Critical Care Medicine 2008;36:795‐800. [DOI] [PubMed] [Google Scholar]
- Payen J‐F, Fauvage B, Canet C, Lavagne P, Falcon D. Comparing the effects of mannitol and of hypertonic saline solution on post traumatic intracranial hypertension: a study with direct individual benefit [Effets comparés du mannitol et du sérum salé hypertonique sur l’hypertension intracrânienne post‐traumatique: Etude avec bénéfice individuel direct.]. Unpublished document [sent to H Chen by Dr J‐F Payen May 2002].
Harutjunyan 2005 {published data only}
- Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients ‐ a randomized clinical trial [ISRCTN62699180]. Critical Care 2005;9:R530‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jagannatha 2016 {published data only}
- CTRI//04/006829. A comparative study of 3% hypertonic saline and 20% mannitol in the treatment of refractory posttraumatic intracranial hypertension. Available online (ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10708&EncHid=&modid=&compid=%27,%2710708det%27) (accessed 8 December 2017).
- Jagannatha AT. An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. Email to: J Dennis 27 December 2017. [DOI] [PubMed]
- Jagannatha AT, Kamath S, Devi I, Rao UGS. The salt versus sugar debate: Urinary sodium losses following hypertonic saline administration curtails its superior osmolar effect in comparison to mannitol in severe traumatic brain injury. Clinical Neurosurgery. 2016; Vol. 63:212.
- Jagannatha AT, Sriganesh K, Devi BI, Rao GS. Urinary sodium loss following hypertonic saline administration curtails its superior osmolar effect in comparison to mannitol in severe traumatic brain injury: a secondary analysis of a randomized controlled trial. Journal of Neuroanaesthesiology and Critical Care 2018;5(3):164‐7. [Google Scholar]
- Jagannatha AT, Sriganesh K, Devi BI, Rao SU. An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. Journal of Clinical Neuroscience 2016;27:68‐73. [DOI: 10.1016/j.jocn.2015.08.035] [DOI] [PubMed] [Google Scholar]
Kumar 2019 {published data only}
- Kumar SA, Devi BI, Reddy M, Shukla D. Comparison of equiosmolar dose of hyperosmolar agents in reducing intracranial pressure‐a randomized control study in pediatric traumatic brain injury. Child's Nervous System : ChNS : Official Journal of the International Society for Pediatric Neurosurgery 2019;35(6):999‐1005. [DOI] [PubMed] [Google Scholar]
Patil 2019 {published data only}
- Patil H, Gupta R. A comparative study of bolus dose of hypertonic saline, mannitol, and mannitol plus glycerol combination in patients with severe traumatic brain injury. World Neurosurgery 2019;125:e221‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Battison 2005 {published data only}
- Battison C, Andrews PJ, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Critical Care Medicine 2005;33:196‐202; discussion 257‐8. [DOI] [PubMed] [Google Scholar]
Bourdeaux 2011 {published data only}
- Bourdeaux CP, Brown JM. Randomized controlled trial comparing the effect of 8.4% sodium bicarbonate and 5% sodium chloride on raised intracranial pressure after traumatic brain injury. Neurocritical Care 2011;15:42‐5. [DOI] [PubMed] [Google Scholar]
Du 2017 {published data only}
- Du DY, Sun LT, Zhang WS, Li K, Xu C, Li ZF. The clinical efficacy of hypertonic saline in reducing intracranial pressure inpatients with severe traumatic brain injury. Neural Injury and Functional Reconstruction 2017;12:215–17. [Google Scholar]
Hong 2017 {published data only}
- Hong DQ, Wu XX, Hu Y. The change of intracranial pressure and NSE after hypertonic saline in the treatment of severe brain injuries. Modern Doctor of China (Chinese) 2017;55(36):15‐18. [Google Scholar]
Huang 2014 {published data only}
- Huang X, Yang L. Comparison of 20% mannitol and 15% hypertonic saline in doses of similar osmotic burden for treatment of severe traumatic brain injury with intracranial hypertension. Journal of Southern Medical University 2014;34(5):723‐6. [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang GR, Guan YJ, Zhen XH. The effects of 7.5% hypertonic saline in the severe traumatic brain injury with hemorrhagic shock. Journal of Bethune Medical Science 2015;13(14):435‐6. [Google Scholar]
Ichai 2009 {published data only}
- Ichai C. Re: Sodium Lactate versus Hypertonic Saline [personal communication]. Email to: H Chen 4 July 2013.
- Ichai C, Armando G, Orban J C, Berthier F, Rami L, Samat‐Long C, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain‐injured patients. Intensive Care Medicine 2009;35:471‐9. [DOI] [PubMed] [Google Scholar]
Jafari 2018 {published data only}
- Jafari M, Ala S, Haddadi K, Alipour A, Mojtahedzadeh M, Ehteshami S, et al. Cotreatment with furosemide and hypertonic saline decreases serum neutrophil gelatinase‐associated lipocalin (NGAL) and serum creatinine concentrations in traumatic brain injury: a randomized, single‐blind clinical trial. Iranian Journal of Pharmaceutical Research : IJPR 2018;17(3):1130‐40. [PMC free article] [PubMed] [Google Scholar]
Jiang 2018 {published data only}
- Jiang Z, Xu H, Wang M, Li Z, Su X, Li X, et al. Effect of infusion speed of 7.5% hypertonic saline on brain edema in patients with craniocerebral injury: an experimental study. Gene 2018;665:201‐7. [DOI: 10.1016/j.gene.2018.05.005] [DOI] [PubMed] [Google Scholar]
Jin 2018 {published data only}
- Jin HH, Jin Y, Mao TM, Chen Y. 3% hypertonic saline in the treatment of severe brain injuries. Zhejiang Journal of Traumatic Surgery (Chinese) 2018;23(4):659‐60. [Google Scholar]
Li 2018 {published data only}
- Li DH, Zhao XZ, Li HL, Zhang XB. Effect of hypertonic saline on intracranial pressure and cerebral edema in patients with severe craniocerebral injury. Journal of Preventive Medicine of Chinese People Liberation Army (Chinese) 2018;36(3):357‐60. [Google Scholar]
Liang 2013 {published data only}
- Liang CY. Comparison of effects of 3% hypertonic saline and 20% mannitol in raised intracranial pressure. Clinical Medicine 2013;33(4):70‐1. [Google Scholar]
Liu 2018 {published data only}
- Liu Q, Yang F. The application of hypertonic saline on the brain injuries. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine (Chinese) 2018;18(2):114‐15. [Google Scholar]
Mei 2016 {published data only}
- Mei LK, Wang L, Yu JB. Comparison of effects of hypertonic saline and mannitol in raised intracranial pressure. China Medical Engineering 2016;24(7):126‐7. [Google Scholar]
NCT01028339 {published data only}
- NCT01028339 (Direction Centrale du Service de Santé des Armées). Mannitol vs HS to treat ICHT after severe TBI : comparison on PtiO2 and microdialysis values. clinicaltrials.gov/show/NCT01028339 (accessed 1 December 2017).
NCT01108744 {published data only}
- NCT01108744. Double blind study of hypertonic saline vs mannitol in the management of increased intracranial pressure (ICP). clinicaltrials.gov/show/NCT01108744 (accessed 1 December 2017).
NCT01111682 {published data only}
- NCT01111682 (University of Cinncinati / US Dept of Defense). Hypertonic saline vs. mannitol for elevated intercranial pressure. clinicaltrials.gov/show/NCT01111682 (accessed 1 December 2017).
NCT01215019 {published data only}
- NCT01215019 (Indiana University). Osmotic therapy for treatment of intracranial hypertension for traumatic brain injury. clinicaltrials.gov/ct2/show/NCT01215019 (accessed 1 December 2017).
Ni 2018 {published data only}
- Ni XW, Chen F. Comparison of 3% hypertonic saline and 20% mannitol in the treatment of severe brain injuries. Modern Practical Medicine (Chinese) 2018;30(6):739‐41. [Google Scholar]
Polushin 2009 {published data only}
- Polushin LS, Krylov VV, Svistov DV, Belkin AA, Petrikov SS, Shchegolev AV, et al. Correction of intracranial hypertension syndrome using hyperosmolar solutions in patients with severe brain damage (multicenter randomized clinical study). Anesteziologiia I Reanimatologiia 2009;5:4‐8. [PubMed] [Google Scholar]
Roquilly 2017 {published data only}
- Roquilly A, Asehnoune K. Continuous hyperosomolar therapy for traumatic brain‐injured patients (COBI). clinicaltrials.gov/ct2/show/NCT03143751 2017.
Sakellaridis 2011 {published data only}
- Sakellaridis N, Pavlou E, Karatzas S, Chroni D, Vlachos K, Chatzopoulos K, et al. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. Clinical article. Journal of Neurosurgery 2011;114:545‐8. [DOI] [PubMed] [Google Scholar]
Shu 2015 {published data only}
- Shu Z, Xu Y, Shen XM, Qiu YF. Comparison of effects of 3% hypertonic saline and mannitol in raised intracranial pressure. Chinese Journal of Hemorheology 2015;1:67‐68, 117. [Google Scholar]
Upadhyay 2010 {published data only}
- Upadhyay P, Tripathi VN, Singh RP, Sachan D. Role of hypertonic saline and mannitol in the management of raised intracranial pressure in children: a randomized comparative study. Journal of Pediatric Neurosciences 2010;5:18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang HF, Cao HS, Zhang XH, Ge L, Bie L. The effect of hypertonic saline and mannitol on coagulation in moderate traumatic brain injury patients. American Journal of Emergency Medicine 2017;35:1404‐7. [DOI] [PubMed] [Google Scholar]
Yang 2019 {published data only}
- Yang X, Chen Y, Li J, Chen L, Ren H, Liu Y, et al. Hypertonic saline maintains coagulofibrinolytic homeostasis following moderate‐to‐severe traumatic brain injury by regulating monocyte phenotype via expression of lncRNAs. Molecular Medicine Reports 2019;19(2):1083‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y. Comparison of effects of hypertonic saline and mannitol in raised intracranial pressure. Henan Medical Research 2014;23(11):74‐5. [Google Scholar]
Zhang 2015 {published data only}
- Zhang YB. Comparison of effects of 3% hypertonic saline and 20% mannitol in severe traumatic brain injury. Contemporary Medicine 2015;21(1):66‐7. [Google Scholar]
Zhang 2018 {published data only}
- Zhang RJ, Wang XF, Luo WY, Wei YJ, Zhang HB, Chen BB, et al. Comparison of hypertonic saline and mannitol in the treatment of intracranial pressure. Chinese Journal of Neurosurgery (Chinese) 2018;34(6):632. [Google Scholar]
References to studies awaiting assessment
Vialet 2003 {published data only}
- Vialet R, Albanese J, Thomachot L, Antonini F, Bourgouin A, Alliez B, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Critical Care Medicine 2003;31:1683‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Salt or Sugar 2019 {unpublished data only}
- Salt or Sugar (SOS) trial: hyperosmolar in traumatic brain injury. Ongoing study 1 June 2019‐1 December 2023.
Additional references
Beers 2012
- Beers SR, Wisniewski SR, Garcia‐Filion P, Tian Y, Hahner T, Berger RP, et al. Validity of a pediatric version of the Glasgow Outcome Scale‐Extended. Journal of Neurotrauma 2012;29(6):1126‐39. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boone 2015
- Boone MD, Oren‐Grinberg A, Robinson TM, Chen CC, Kasper EM. Mannitol or hypertonic saline in the setting of traumatic brain injury: what have we learned?. Surgical Neurology International 2015;6:177. [DOI: 10.4103/2152-7806.170248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bratton 2007
- Bratton SL, Chestnut RM, Ghajar J, McConnell FH, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. Journal of Neurotrauma 2007;24:S55‐8. [DOI] [PubMed] [Google Scholar]
Burgess 2016
- Burgess S, Anu‐Laban RB, Slavik RS, Vu EN, Zed PJ. A systematic review of randomized controlled trials comparing hypertonic sodium solutions and mannitol for traumatic brain injury: implications for emergency department management. Annals of Pharmacotherapy 2016;50(4):291‐300. [DOI] [PubMed] [Google Scholar]
CIG 2015
- Cochrane Injuries Group. Cochrane Injuries Group Editorial Policy. injuries.cochrane.org/editorial‐policy‐2015 2015.
Corrigan 2010
- Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. Journal of Head Trauma Rehabilitation 2010;25(2):72. [DOI] [PubMed] [Google Scholar]
CTRI//04/006829 2016
- CTRI//04/006829. A comparative study of 3% hypertonic saline and 20% mannitol in the treatment of refractory posttraumatic intracranial hypertension. Available online (ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10708&EncHid=&modid=&compid=%27,%2710708det%27) (accessed 8 December 2017).
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Greve 2009
- Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mount Sinai Journal of Medicine 2009;76(2):97‐104. [DOI] [PubMed] [Google Scholar]
Gu 2019
- Gu Jiajie, Huang H, Huang Y, Sun H, Xu H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: a meta‐analysis of randomized controlled trials. Neurosurgical Review 2019;42(2):499‐509. [DOI] [PubMed] [Google Scholar]
Gunnar 1986
- Gunnar WP, Merlotti GJ, Barrett J, Jonasson O. Resuscitation from hemorrhagic shock. Alterations of the intracranial pressure after normal saline, 3% saline and dextran‐40. Annals of Surgery 1986;204:686‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editors. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Horn 1999
- Horn P, Münch E, Vajkoczy P, Herrmann P, Quintel M, Schilling L, et al. Hypertonic saline solution for control of elevated intracranial pressure in patients with exhausted response to mannitol and barbiturates. Neurological Research 1999;21(8):758‐64. [DOI] [PubMed] [Google Scholar]
Ichai 2013
- Ichai C. Re: Sodium Lactate versus Hypertonic Saline [personal communication]. Email to: H Chen 4 July 2013.
Jagannatha 2017
Jennett 1975
- Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet 1975;305(7905):480‐4. [DOI] [PubMed] [Google Scholar]
Kamel 2011
- Kamel H, Navi BB, Nakagawa K, Hemphill JC 3rd, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta‐analysis of randomized clinical trials. Critical Care Medicine 2011;39(3):554‐9. [DOI] [PubMed] [Google Scholar]
Kerwin 2009
- Kerwin AJ, Schinco MA, Tepas JJ 3rd, Renfro WH, Vitarbo EA, Muehlberger M. Hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. Journal of Trauma 2009;67(2):277‐82. [DOI] [PubMed] [Google Scholar]
Khanna 2000
- Khanna S, Davis D, Peterson B, Fisher B, Tung H, O'Quigley J, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in paediatric traumatic brain injury. Critical Care Medicine 2000;28(4):1144‐51. [DOI] [PubMed] [Google Scholar]
Kochanek 2019
- Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, et al. Guidelines for the management of pediatric severe traumatic brain injury: update of the brain trauma foundation guidelines, executive summary. Neurosurgery 2019;84:1169‐78. [DOI] [PubMed] [Google Scholar]
Lewandowski‐Belfer 2014
- Lewandowski‐Belfer JJ, Patel AV, Darracott RM, Jackson DA, Nordeen JD, Freeman WD. % hypertonic saline for refractory intracranial hypertension. Neurocritical Care 2014;20:436‐42. [DOI] [PubMed] [Google Scholar]
Li 2015
- LI M, Chen T, Chen SD, Cai J, Hu YH. Comparison of equimolar doses of mannitol and hypertonic saline for the treatment of elevated intracranial pressure after traumatic brain injury: a systematic review and meta‐analysis. Medicine 2015;94(17):e736. [Google Scholar]
Lu 2005
- Lu J, Marmarou A, Choi S, Maas A, Murray G, Steyerberg EW, Impact and Abic Study Group. Mortality from traumatic brain injury. Acta Neurochirurgica. Supplementum 2005;95:281‐5. [DOI] [PubMed] [Google Scholar]
Maguigan 2017
- Maguigan KL, Dennis BM, Hamblin SE, Guillamondegui OD. Method of hypertonic saline administration: effects on osmolality in traumatic brain injury patients. Journal of Clinical Neuroscience 2017;39:147‐50. [DOI] [PubMed] [Google Scholar]
Marko 2012
- Marko NF. Hypertonic saline, not mannitol, should be considered gold‐standard medical therapy for intracranial hypertension. Critical Care 2012;16(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 1992
- Marshall LF, Marshall SB, Klauber MR, Berkum Clark M, Eisenberg H, et al. The diagnosis of head injury requires a classification based on computed axialtomography. Journal of Neurotrauma 1992;9(Suppl 1):S287–92. [PubMed] [Google Scholar]
Miller 1977
- Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. Journal of Neurosurgery 1977;47(4):503‐16. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz K, Montori V, Gøtzsche P, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2010;340:28‐55. [Google Scholar]
Mortazavi 2012
- Mortazavi MM, Romeo AK, Deep A, Griessenauer CJ, Shoja MM, Tubbs RS, et al. Hypertonic saline for treating raised intracranial pressure: literature review with meta‐analysis. Journal of Neurosurgery 2012;116(1):210‐21. [DOI] [PubMed] [Google Scholar]
Oddo 2009
- Oddo M, Levine JM, Frangos S, Carrera E, Maloney‐Wilensky E, Pascual JL, et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. Journal of Neurology, Neurosurgery, and Psychiatry 2009;80:916‐20. [DOI] [PubMed] [Google Scholar]
Oddo 2018
- Oddo M, Poole D, Helbok R, Meyfroidt G, Stocchetti N, Bouzat P, et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Medicine 2018;44(4):449‐63. [DOI] [PubMed] [Google Scholar]
Payen 2002
- Payen J‐F, Fauvage B, Canet C, Lavagne P, Falcon D. Comparing the effects of mannitol and of hypertonic saline solution on post traumatic intracranial hypertension: a study with direct individual benefit [Effets comparés du mannitol et du sérum salé hypertonique sur l’hypertension intracrânienne post‐traumatique: Etude avec bénéfice individuel direct]. Unpublished document [sent to HC by Dr J‐F Payen] 2002 (May).
Rangel‐Castillo 2008
- Rangel‐Castillo L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurologic Clinics 2008;26(2):521‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rickard 2014
- Rickard AC, Smith JE, Newell P, Bailey A, Kehoe A, Mann C. Salt or sugar for your injured brain? A meta‐analysis of randomised controlled trials of mannitol versus hypertonic sodium solutions to manage raised intracranial pressure in traumatic brain injury. Emergency Medicine Journal 2014;31(8):679‐83. [DOI] [PubMed] [Google Scholar]
Roquilly 2011
- Roquilly A, Mahe PJ, Latte DD, Loutrel O. Continuous controlled‐infusion of hypertonic saline solution in traumatic brain‐injured patients: a 9‐year retrospective study. Critical Care 2011;15:R260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwimmbeck 2019
- Schwimmbeck F, Voellger B, Chappell D, Eberhart L. Hypertonic saline versus mannitol for traumatic brain injury: a systematic review and meta‐analysis with trial sequential analysis. Journal of Neurosurgical Anesthesiology 2019;Sept 20; epub ahead of print:no pagination. [DOI] [PubMed] [Google Scholar]
Soustiel 2015
- Soustiel JF. Re: Comparison of effects of equiosmolar doses of mannitol and hypertonic saline [personal communication]. Email to: H Chen 6 May 2015.
Stevens 2012
- Stevens RD, Huff JS, Duckworth J, Papangelou A, Weingart SD, Smith, WS. Emergency neurological life support: intracranial hypertension and herniation. Neurocritical Care 2012;17(1):60‐5. [DOI] [PubMed] [Google Scholar]
Teasdale 1974
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2(7872):81‐4. [DOI] [PubMed] [Google Scholar]
Treggiari 2007
- Treggiari MM, Schutz N, Yanez ND, Romand JA. Role of intracranial pressure values and patterns in predicting outcome of traumatic brain injury: a systematic review. Neurocritical Care 2007;6(2):104‐12. [DOI] [PubMed] [Google Scholar]
Wakai 2013
- Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001049.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015
- Wang K, Sun M, Jiang H, Cao XP, Zeng J. Mannitol cannot reduce the mortality on acute severe traumatic brain injury (TBI) patients: a meta‐analyses and systematic review. Burns & Trauma 2015;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weed 1919
- Weed LH, McKibben PS. Experimental alteration of brain bulk. American Journal of Physiology‐Legacy Content 1919;48(4):531‐58. [Google Scholar]
Worthley 1988
- Worthley LI, Cooper DJ, Jones N. Treatment of resistant intracranial hypertension with hypertonic saline: report of two cases. Journal of Neurosurgery 1988;68(3):478‐81. [DOI] [PubMed] [Google Scholar]
Ziai 2007
- Ziai WC, Toung TJ, Bhardwaj A. Hypertonic saline: first‐line therapy for cerebral edema?. Journal of the Neurological Sciences 2007;261(1‐2):157‐66. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2014
- Chen H, Song Z. Hypertonic saline versus other intracranial pressure–lowering agents for people with acute traumatic brain injury. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010904] [DOI] [PMC free article] [PubMed] [Google Scholar]


