Abstract
Background
Pressure ulcers (also known as pressure sores, decubitus ulcers or bedsores) are localised injuries to the skin or underlying tissue, or both. Pressure ulcers are a disabling consequence of immobility. Electrical stimulation (ES) is widely used for the treatment of pressure ulcers. However, it is not clear whether ES is effective.
Objectives
To determine the effects (benefits and harms) of electrical stimulation (ES) for treating pressure ulcers.
Search methods
In July 2019 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. We did not impose any restrictions with respect to language, date of publication or study setting.
Selection criteria
We included published and unpublished randomised controlled trials (RCTs) comparing ES (plus standard care) with sham/no ES (plus standard care) for treating pressure ulcers.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data, and assessed risk of bias. We assessed the certainty of evidence using GRADE.
Main results
We included 20 studies with 913 participants. The mean age of participants ranged from 26 to 83 years; 50% were male. ES was administered for a median (interquartile range (IQR)) duration of five (4 to 8) hours per week. The chronicity of the pressure ulcers was variable, ranging from a mean of four days to more than 12 months. Most of the pressure ulcers were on the sacral and coccygeal region (30%), and most were stage III (45%). Half the studies were at risk of performance and detection bias, and 25% were at risk of attrition and selective reporting bias. Overall, the GRADE assessment of the certainty of evidence for outcomes was moderate to very low. Nineteen studies were conducted in four different settings, including rehabilitation and geriatric hospitals, medical centres, a residential care centre, and a community‐based centre.
ES probably increases the proportion of pressure ulcers healed compared with no ES (risk ratio (RR) 1.99, 95% confidence interval (CI) 1.39 to 2.85; I2 = 0%; 11 studies, 501 participants (512 pressure ulcers)). We downgraded the evidence to moderate certainty due to risk of bias.
It is uncertain whether ES decreases pressure ulcer severity on a composite measure compared with no ES (mean difference (MD) ‐2.43, 95% CI ‐6.14 to 1.28; 1 study, 15 participants (15 pressure ulcers) and whether ES decreases the surface area of pressure ulcers when compared with no ES (12 studies; 494 participants (505 pressure ulcers)). Data for the surface area of pressure ulcers were not pooled because there was considerable statistical heterogeneity between studies (I2 = 96%) but the point estimates for the MD of each study ranged from ‐0.90 cm2 to 10.37 cm2. We downgraded the evidence to very low certainty due to risk of bias, inconsistency and imprecision.
It is uncertain whether ES decreases the time to complete healing of pressure ulcers compared with no ES (hazard ratio (HR) 1.06, 95% CI 0.47 to 2.41; I2 = 0%; 2 studies, 55 participants (55 pressure ulcers)). We downgraded the evidence to very low certainty due to risk of bias, indirectness and imprecision.
ES may be associated with an excess of, or difference in, adverse events (13 studies; 586 participants (602 pressure ulcers)). Data for adverse events were not pooled but the types of reported adverse events included skin redness, itchy skin, dizziness and delusions, deterioration of the pressure ulcer, limb amputation, and occasionally death. We downgraded the evidence to low certainty due to risk of selection and attrition bias and imprecision.
ES probably increases the rate of pressure ulcer healing compared with no ES (MD 4.59% per week, 95% CI 3.49 to 5.69; I2 = 25%; 12 studies, 561 participants (613 pressure ulcers)). We downgraded the evidence to moderate certainty due to risk of bias. We did not find any studies that looked at quality of life, depression, or consumers' perception of treatment effectiveness.
Authors' conclusions
ES probably increases the proportion of pressure ulcers healed and the rate of pressure ulcer healing (moderate certainty evidence), but its effect on time to complete healing is uncertain compared with no ES (very low certainty evidence). It is also uncertain whether ES decreases the surface area of pressure ulcers. The evidence to date is insufficient to support the widespread use of ES for pressure ulcers outside of research. Future research needs to focus on large‐scale trials to determine the effect of ES on all key outcomes.
Plain language summary
Is electrical stimulation effective for treating pressure ulcers?
What is the aim of this review?
The aim of this review was to find out whether electrical stimulation (ES; an electrical current applied to the skin) can help heal pressure ulcers. We collected and analysed all relevant studies (randomised controlled trials) to answer this question and found 20 relevant studies.
Key messages
ES compared with no ES probably increases the proportion of pressure ulcers healed and the rate of pressure ulcer healing (moderate certainty evidence) but its effect on time to complete healing and the surface area of pressure ulcers is uncertain (very low certainty evidence). The most commonly reported side effects of ES were reddening of the skin and discomfort. There is a need for better quality research to determine whether ES is safe and effective.
What was studied in the review?
Pressure ulcers (also known as pressure sores, bed sores or pressure injuries) are injuries to the skin and/or underlying tissue caused by sustained pressure over bony parts of the body such as the hips, heels or lower back. People with reduced mobility due to age, disability or illness are at risk of developing pressure ulcers.
ES is provided by an electrical current that can be applied to the skin in different ways. ES requires the placing of at least two small electrodes on the skin connected to a small battery‐powered device which controls the intensity of the current. ES can be delivered either as a direct or pulsed current. It causes a tingling or vibratory sensation in most people except those who cannot feel due to conditions such as spinal cord injury. We reviewed the evidence about whether ES affects the number of pressure ulcers healed, the size and severity of the pressure ulcers, the time to complete healing, and quality of life. We also wanted to find out about any side effects associated with ES.
What are the main results of the review?
This review includes the results of 20 randomised controlled trials dating from 1985 to 2018 and involving 913 participants. The average age of participants ranged from 26 to 83 years; 50% were male. Participants had their pressure ulcers for at least four days and in some cases for more than 12 months. The majority of pressure ulcers (60%) were serious and on or adjacent to the buttocks (62%). Studies were conducted in four different settings, including rehabilitation and geriatric hospitals, medical centres, a residential care centre, and a community‐based centre. ES was administered for an average of five hours per week. Studies compared ES plus usual care (e.g. wound dressing, pressure relief, regular turning, nutritional advice and supplements) to no ES (but with usual care). Eight studies out of 20 were funded by a device manufacturer with a vested interest in the results of the studies.
Eleven studies that compared ES with no ES indicated that ES probably improves the proportion of pressure ulcers healed (moderate certainty evidence based on 501 participants (512 pressure ulcers)). It is uncertain whether ES decreases pressure ulcer severity on a composite measure (based on 1 study with 15 participants (15 pressure ulcers)). The effect of ES on pressure ulcer area was not estimable because different studies showed very different results. It is uncertain whether ES decreases the surface area of pressure ulcers (very low certainty evidence based on 494 participants (505 pressure ulcers)). We cannot be certain whether ES has an effect on time to complete healing (very low certainty evidence based on 55 participants (55 pressure ulcers)). The common complications related to ES were skin redness and discomfort (low certainty evidence based on 586 participants (602 pressure ulcers)). Twelve studies also indicated that ES probably increases the rate of pressure ulcer healing (moderate certainty evidence based on 561 participants (613 pressure ulcers)). No studies reported results for quality of life or depression.
How up‐to‐date is this review?
We searched for studies that had been published up to July 2019.
Summary of findings
Summary of findings for the main comparison. Electrical stimulation (plus standard care) versus sham/no ES (plus standard care) for treating pressure ulcers.
| Electrical stimulation (plus standard care) versus sham/no ES (plus standard care) for treating pressure ulcers | ||||||
| Patient or population: people with pressure ulcers Setting: inpatients and outpatients Intervention: electrical stimulation (plus standard care) Comparison: sham/no ES (plus standard care) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of ulcers (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sham/no ES (plus standard care) | Risk with Electrical stimulation (plus standard care) | |||||
| Proportion of pressure ulcers healed (3 to 12 weeks) |
Study population | RR 1.99 (1.39 to 2.85) | 512 (11 RCTs) | ⊕⊕⊕⊝ Moderatea | ES may increase the proportion of pressure ulcers healed when compared with no ES. Absolute effect: 297 out of 1000 (from 207 more to 425 more). |
|
| 149 per 1,000 | 297 per 1,000 (207 to 425) | |||||
| Time to complete healing (3 and 8 weeks) |
Study population | HR 1.06 (0.47 to 2.41) | 55 (2 RCTs) | ⊕⊝⊝⊝ Very lowb | It is uncertain if ES decreases time to complete healing when compared with no ES. | |
| 18 per 100 | 19 per 100 (9 to 38) | |||||
| Complications/ adverse events related to pressure ulcers (3 to 12 weeks) | Adverse events included redness of the skin, itchy skin, dizziness and delusions, deterioration of the pressure ulcer, limb amputation and occasionally death. | 602 (13 RCTs) |
⊕⊕⊝⊝ Lowc | The data were not sufficiently detailed or comparable to analyse quantitatively. | ||
| Quality of life (QoL) | No studies measured quality of life | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level: once for serious risk of bias because a lot of the studies had either high or unclear risk of bias for performance bias and selective reporting. bDowngraded four levels: once for serious risk of bias because both studies had high risk of bias for two domains and one study had unclear risk of bias for another three domains; once for indirectness because the two studies were not reflective of all who are vulnerable to pressure ulcers; twice for imprecision. cDowngraded two levels: once for serious risk of bias because a lot of the studies had either high or unclear risk of bias for selection and attrition bias; once for imprecision.
Background
Description of the condition
Pressure ulcers (also known as pressure sores, pressure injuries, decubitus ulcers or bedsores) are localised injuries to the skin or underlying tissue, or both. Pressure ulcers usually occur over a bony prominence and are caused by pressure, friction or shear forces. Friction or shear forces occur when layers of the skin are forced to slide over one another or over deeper layers of tissue (NPUAP/EPUAP 2014).
Pressure ulcers are a disabling consequence of immobility. They most often occur in people with neurological conditions (e.g. people with spinal cord injuries (Rintala 2008), acute illnesses (for example, people in comas in intensive care units) (Schoonhoven 2006), or in people who are elderly and immobile (for example, older people in nursing home care) (Perneger 2002).
Pressure ulcers are a common problem (Cowan 2019). They affect up to 32% of people admitted to hospital (Kaltenthaler 2001), and 40% of people with spinal cord injuries (Zakrasek 2015). They hinder rehabilitation and have many harmful consequences. For example, they can lead to contractures (shortening of muscles, tendons, or ligaments), permanent scarring, deformities, osteomyelitis (infection of bones), loss of limbs, and sepsis (a life‐threatening response to infection) (Allman 1989; Rodriguez 1994). People with severe pressure ulcers commonly require hospitalisation. Pressure ulcers affect health‐related quality of life and participation in meaningful community activities (New 2004). In addition, they affect a person's family life, and are costly and difficult to manage (Brem 2010). Pressure ulcers can also be life‐threatening, particularly in low‐ and middle‐income countries (Hossain 2015; Zakrasek 2015).
Two key guidelines recommend using the Pressure Ulcer Classification System to classify the severity of pressure ulcers (NPUAP/EPUAP 2014). This system is based on the level of tissue injury, and classifies pressure ulcers into four 'stages' ('grades' or 'categories') with two additional unstageable categories. A stage I pressure ulcer is indicated by non‐blanching superficial red areas, and a stage IV pressure ulcer is indicated by full skin thickness injuries that can involve the underlying bone, tendon or joint capsule, and invariably requires hospitalisation and surgery (see Appendix 1 for further details of the Pressure Ulcer Classification System).
Description of the intervention
Electrical stimulation (ES) is advocated as a way of healing pressure ulcers (Bogie 2000), and is recommended in at least four clinical practice guidelines (AWMA 2012; Consortium for Spinal Cord Medicine 2014; Houghton 2013; SCIRE 2014). It is provided by an electrical current that can be applied in different ways, however, in this review we are only investigating ES that is applied on the skin. Application requires placing at least two electrodes on the skin, which are connected to a small battery‐like device. The intensity of the ES is controlled through dials or switches. The cost of an ES device ranges from USD 80 to USD 750 (Mittmann 2011).
ES for the treatment of pressure ulcers can be delivered either as a direct or pulsed current. When a direct current is used, the current flows constantly in one direction. When a pulsed current is used, each pulse is separated by a period of no flow of current. There are two types of pulsed current; monophasic and biphasic. In both types of pulsed current, the electric current is delivered in short bursts, however in monophasic, the current flows in one direction, whilst in biphasic the current flows in two directions.
There are different ways of placing the electrodes, for example, they can be placed in or around the pressure ulcer, or on other parts of the body. In addition, the parameters of the ES can be varied. This includes the frequency (low or high Hz), polarity (negative, positive or mixed), pulse type (monophasic or biphasic), duration of stimulation (per session) and amplitude (low or high mA). ES causes a comfortable tingling or vibratory sensation (except in those with neurological lesions that results in the loss of sensation) and can cause a muscle contraction.
How the intervention might work
There are many theories about how ES may help heal pressure ulcers but the veracity of these theories is unclear. Most work in this area was done in the 1980s with very little recent work directed at furthering our understanding. Researchers have suggested that ES affects all four phases of healing, that is, the inflammatory, proliferative, epithelialisation and remodelling phases. Most believe that ES increases blood flow to the affected area (Alvarez 1983; Bourguignon 1987; Cruz 1989). This may increase the flow of cells important for the inflammatory and proliferation phases (Foulds 1983; Orida 1982), or promote tissue oxygenation and reduce oedema (Sussman 2012). It may also influence the increase of epidermal growth factors and their receptors (Zhao 2002). Some have even suggested that ES has an antibacterial effect that helps reduce infection and enhance healing (Fakhri 1987). However, none of these theories have been substantiated and the effects of ES have not been reviewed systematically.
In addition, ES may indirectly help treat and prevent pressure ulcers in people with neurological disorders by its possible effects on the properties of muscles. For example, some clinicians apply ES to facilitate a contraction of the gluteal muscles of people with paralysis. The ES is used to induce muscle hypertrophy and hence better distribute pressure over the ischial tuberosities; a region that is highly vulnerable to pressure ulcers with prolonged sitting. However, there is no strong evidence to support these beliefs.
Why it is important to do this review
This review is important because pressure ulcers are very common and debilitating, and there is initial evidence to suggest that ES is therapeutic. There are a small number of non‐Cochrane reviews that claim that ES is effective for the treatment of pressure ulcers (Barnes 2014; Kawasaki 2014; Lala 2016; Liu 2014). These have prompted clinical guidelines to start recommending ES (AWMA 2012; Houghton 2013; NPUAP/EPUAP 2014), however, it is not clear whether these recommendations are justified because the reviews and studies that they are based upon have methodological limitations. It is important to know the certainty of the evidence that underpins any recommendation for ES because ES is costly, time‐consuming to administer, and inconvenient for patients. It also requires specialised equipment, training and daily application. In addition, there is the potential for harm. For example, ES can cause electric burns and it is possible that ES could hinder the healing of pressure ulcers. Therefore it is important to establish whether ES is effective, whether the potential for therapeutic effect outweighs any potential for harm, and whether the associated cost, time and inconvenience of ES are justified.
Objectives
To determine the effects (benefits and harms) of electrical stimulation (ES) for treating pressure ulcers.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs). We included studies irrespective of language of publication. We included studies that used parallel‐group designs, within‐participant designs or cross‐over designs.
Types of participants
We included participants of all ages and both genders, provided participants had at least one pressure ulcer. We excluded studies that only involved participants with other types of wounds (e.g. diabetic and venous ulcers). If a study involved participants with different types of wounds, we extracted the data for participants with pressure ulcers. If this was not possible, we only included the study if more than 75% of participants had pressure ulcers. There were no restrictions on the type or stage of the pressure ulcers, that is, we included acute or chronic pressure ulcers of any stage (including non‐open wounds classified as stage I) and due to any cause. We anticipated most pressure ulcers would be due to neurological conditions (e.g. people with spinal cord injuries) and acute illnesses (for example, people in comas in intensive care units), or due to age and immobility (e.g. older people in nursing homes). We included studies even if the causes of the pressure ulcers were not reported but it was reasonable to assume that they were due to pressure injuries (e.g. the pressure ulcers were on the sacrum).
Types of interventions
We included studies that determined the effectiveness of any type of ES for treating pressure ulcers. We included studies that compared ES (plus standard care) with sham/no ES (plus standard care).
We included ES which was administered through either direct or pulsed current. Standard care could include any of the following: wound dressing, pressure relief, regular turning, nutritional advice, and nutritional supplements.
Types of outcome measures
Our primary outcomes are mainly reflective of pressure ulcer healing. For example, proportion of pressure ulcers healed, composite measures of pressure ulcer severity, surface area of pressure ulcers and time to complete healing. This focus is justified because ES for pressure ulcers is primarily administered in an effort to promote healing.
Primary outcomes
Proportion of pressure ulcers healed; the data expressed as the number of pressure ulcers healed in each group.
Composite measures of pressure ulcers that captured different aspects of severity; this includes measures such as the Pressure Ulcer Scale for Healing (PUSH) (Gardner 2005), Sussman Wound Healing Tool (Sussman 1997) and Pressure Sore Status Tool (Bates‐Jensen 1992).
Surface area of pressure ulcers; data expressed as cm2. If not provided in the study, areas were calculated by multiplying the length and width of the pressure ulcers.
Time to complete healing; these data expressed as days to wound closure (time‐to‐event data).
Complications/adverse events; these include death, skin irritation, spasm, or pain, or the number of pressure ulcer infections.
Secondary outcomes
Rate of pressure ulcer healing expressed as percentage rate of healing per week.
Quality of life; including any validated standardised questionnaire that captured quality of life. For example, the Short Form‐36 (Forchheimer 2004) and Euro Quality of Life (Whitehurst 2012).
Depression; this includes any validated standardised outcome that captures depression e.g., the Hospital and Anxiety Depression Scale (Woolrich 2006) and Depression Anxiety Stress Scale (Üstün 2010).
Consumers' perception of treatment effectiveness; this includes any outcome that captured consumers' satisfaction, impression of treatment effectiveness or comfort with ES.
We only extracted one type of measure from a study to reflect each of the primary and secondary outcomes. If a study had more than one type of measure for any primary or secondary outcome, then we chose the measure that was most valid and reliable.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 02 July 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6) in the Cochrane Library (searched 02 July 2019);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 02 July 2019);
Ovid Embase (1974 to 02 July 2019);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 02 July 2019);
PEDro (www.pedro.org.au) (Physiotherapy Evidence Database; 1929 to 02 July 2019).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus and PEDro can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) Lefebvre 2019). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2019). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018). We did not impose any restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries to identify unpublished and ongoing studies (searched 02 July 2019) (see Appendix 2 for search terms).
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch);
ClinicalTrials.gov (www.clinicaltrials.gov);
the International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.controlled‐trials.com);
EU Clinical Trials Register (www.clinicaltrialsregister.eu);
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
Stroke Trials Registry (www.strokecenter.org/trials).
Searching other resources
To identify further published, unpublished and ongoing studies, we:
used the Cited Reference Search within Web of Science (Thomson Reuters) Science Citation Index (SCI) and Social Science Citation Index (SSCI) (searched 02 July 2019) to track relevant references (see Appendix 2 for search terms);
scanned the reference lists of all identified studies and reviews;
searched grey literature using Open Grey (www.opengrey.eu), Google Scholar (scholar.google.com), and Proquest Dissertations & Theses databases;
contacted key researchers in the area and international organisations to enquire about unpublished or ongoing studies;
contacted manufacturers of ES devices and authors regarding any published or unpublished data.
Searching reference lists of included trials and relevant reviews
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports.
Searching by contacting individuals or organisations
When necessary, we contacted authors of key papers and abstracts to request further information about their trials.
Adverse effects
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
We carried out data collection and analysis according to methods stated in the published protocol (Arora 2016), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors (MA and LAH) independently screened the titles and abstracts of the search output to identify potentially relevant studies. We retrieved full‐length articles for all potentially relevant studies and used these to identify studies that met the selection criteria. The full‐length articles were re‐examined to ensure that they met the inclusion criteria. Review authors did not screen studies in which they were involved. In such instances studies were screened by another review author who was not involved in the study. Disagreements between the two review authors (MA and LAH) were resolved by discussion and, when necessary, arbitrated by a third review author (JVG).
We compiled a table of the excluded studies and detailed the primary reason for exclusion. We created a flow diagram using the PRISMA template within Review Manager 2014 (Liberati 2009). The flowchart included the number of:
records identified by the database and other searches;
records after removal of duplicates;
records excluded after preliminary screening (i.e. of titles and abstracts);
records retrieved in full text;
records or studies excluded after assessment of the full text with brief reasons;
studies included in qualitative synthesis and quantitative syntheses (meta‐analysis).
Data extraction and management
Two review authors (MA and LAH) independently performed data extraction for all included studies. Differences between the two review authors were resolved by discussion and, when necessary, arbitrated by a third author (JVG). If data were missing from reports, we attempted to contact the study authors to obtain the missing data. We resolved discrepancies by consensus.
We extracted the data from included studies into an Excel spreadsheet designed to capture the trial information detailed below. Initially, we piloted the Excel spreadsheet to explore any issues that may arise in relation to the data extraction process. We expanded and amended the spreadsheet as necessary after the piloting process.
We extracted the maximal amount of data without duplicating results from dual publications. We extracted the following data as listed below.
Author: year of publication
Methods: study design
Participants: health condition; sample size; study setting and country; inclusion and exclusion criteria; characteristics of pressure ulcers; age; gender
Interventions: details of the experimental group (i.e. duration of ES, electrode placement, name of device and manufacturer, intensity of ES, type of current and polarity) and control group; details of co interventions
Outcomes: details of outcomes included in the review; other outcomes not included in the review; time points (i.e. when outcomes were measured)
Withdrawals and reason for withdrawals
Funding source; registry; published protocol
Assessment of risk of bias in included studies
Two review authors (MA and LAH) assessed the risk of bias in each study using the following eight methodological domains as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Random sequence generation
Allocation sequence concealment
Blinding of participants
Blinding of personnel
Blinding of outcome assessors
Incomplete outcome data
Selective outcome reporting
Other potential sources of bias
We assessed each of the eight domains for low, high, or unclear risk of bias (see Appendix 3). We rated studies as high risk of bias for incomplete outcome data if more than 15% of participants had dropped out of the study (this decision was made after the protocol was published).
We attempted to contact the study authors, wherever applicable, to clarify any ambiguities. Disagreements in judgements about the risk of bias were resolved by discussion or, when necessary, arbitrated by an independent third review author (JVG). Review authors did not extract data, or rate the risk of bias of studies in which they were involved. In such instances these tasks were performed by two authors who were not involved in conducting the study.
We used Review Manager 5 to generate two figures detailing the risk of bias (Review Manager 2014). The first figure (the 'Risk of bias' graph) was used to illustrate the judgements about the risk of bias ('low risk', 'high risk', 'unclear risk' of bias) for each study. The second figure (the 'Risk of bias' summary) was used to present the judgements about the risk of bias in a cross‐tabulation format.
Measures of treatment effect
For continuous data:
we expressed mean differences (MDs) with 95% confidence intervals (CIs) for outcomes that used the same units (this was done for surface area of pressure ulcers and rate of pressure ulcer healing);
we planned to express summary estimates as standardised MDs (SMDs) with 95% CIs for outcomes that used different units.
We converted available data, where possible, using the calculator incorporated into Review Manager 5 (e.g. when data were reported as standard errors) (Higgins 2011a; Review Manager 2014). Change scores were given preference over postintervention scores, however, we did not intend to combine postintervention scores with change scores in meta‐analyses using SMDs, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). Ultimately, this was not relevant because we did not express any results as SMDs.
Wherever possible, data measured on the same scale but with different units were converted to the same units (this was done for surface area of pressure ulcers and rate of pressure ulcer healing). In addition, we planned to express rate of pressure ulcer healing as either mm2 healed per day, cm2 healed per day or percentage healed per day. However, ultimately, we expressed these data as percentage healed per week because this was how most authors presented these data. This required converting some data that were expressed differently by an appropriate conversion constant (e.g. percentage healed per day was multiplied by 7 and percentage healed per 4 weeks was divided by 4 to obtain percentage healed per week).
We planned to convert SMD from meta‐analyses into MD to aid clinical interpretation (Deeks 2011). We intended to do this by using an outcome and its standard difference (SD) from one of the studies included in the meta‐analysis. We planned to choose an outcome that was widely used, provided the study from which it was taken had a reasonable sample size. We planned to calculate the MD by multiplying the SMD by the baseline SD from the control group of the selected study. Ultimately, this was not done because we did not express any results as SMDs.
For dichotomous data, we expressed summary estimates as:
risk ratios (RRs) with 95% CIs (this was done for proportion of pressure ulcers healed).
For time‐to‐event data, we expressed summary estimates as:
hazard ratios (HRs) with 95% CIs (this was done for time to complete healing).
Unit of analysis issues
We planned to include cross‐over studies, studies with more than one ES group, studies in which multiple observations were taken on the same individual, and studies in which more than one pressure ulcer was treated per participant. Where these types of studies were included in the review we dealt with them in the following way.
Cross‐over studies
We planned to use the first period of cross‐over studies in our analyses (Curtin 2002), rather than combined data for subsequent periods (Higgins 2011a). However, there were no cross‐over studies.
Studies with more than one ES group
Where multiple arms were reported in a single study, we included only the relevant arms. In studies where two or more different types of ES, or two or more types of electrode placements were compared with a control arm, we extracted data from each intervention arm but divided the control group by the number of intervention arms so that participants were not double‐counted. If the study data could not be analysed correctly, outcome data were extracted and presented but not analysed together.
Studies in which multiple observations were taken on the same individual
In studies with multiple observations for an individual, we extracted the data collected at the end of the intervention period for all analyses. For example, if ES was applied for six weeks and outcomes were measured at two, four, six, eight and 10 weeks, we used the data collected at six weeks (i.e. at the end of the intervention).
We planned to do subgroup analyses with data categorised as either (Schünemann 2011a):
short‐term effects of ES: data collected within four weeks of the completion of the intervention; or
long‐term effects of ES: data collected more than four weeks after the completion of the intervention.
However, ultimately this was not done because only one study measured outcomes more than four weeks after the completion of the intervention.
Studies in which more than one pressure ulcer was treated per participant
Where studies randomised at the participant level and measured outcomes at the pressure ulcer level (e.g. pressure ulcer healing), we treated the participant as the unit of analysis when the number of pressure ulcers assessed appeared to equal the number of participants (e.g. one pressure ulcer per participant).
We planned to incorporate cluster trials into the meta‐analyses, if the studies had been analysed correctly. Where a cluster trial was incorrectly analysed, we planned to record this as part of the 'Risk of bias' assessment. If possible, we planned to approximate the correct analyses based on the Cochrane Handbook for Systematic Reviews of Interventions guidance (Deeks 2011), using information on:
the number of clusters (or groups) randomised to each intervention group; or the average (mean) size of each cluster;
whether the outcome data ignored the cluster design for the total number of individuals (e.g. number or proportion of individuals with events, or means and SDs); and
an estimate of the intracluster (or intraclass) correlation coefficient (ICC) (Schünemann 2011a).
If studies randomised participants, but collected and reported outcome data on multiple pressure ulcers in some, but not all participants, we did not consider this a cluster trial per se, but rather a study that incorrectly included a mixture of individual and clustered data. We noted such studies and recorded the issue in the 'Risk of bias' assessment. We included these studies in the meta‐analysis but then conducted a sensitivity analysis to determine the effect of their inclusion.
Dealing with missing data
If data were not provided in numerical format and only provided in graphs, we planned to estimate the mean scores and SDs from the graphs. If studies did not provide a mean (SD) for continuous data, and it could not be derived, but studies did provide medians and interquartile ranges, we planned to extract medians and we planned to estimate the SD as 80% of the interquartile range.
If data were missing altogether, we contacted study authors. If authors did not respond or were unable to provide the additional data, we included whatever data were available. If insufficient data were available for analyses, we only presented descriptive data in the review.
If authors of trials provided both intention‐to‐treat and per protocol data, we planned to use the intention‐to‐treat data. We did not plan to impute missing data.
Assessment of heterogeneity
We considered conducting a meta‐analysis if there were at least two clinically homogenous studies (studies that investigated the effect of similar interventions on similar participant groups and reported similar outcomes). In such circumstances the I2 statistic was used to quantify the statistical heterogeneity and inform decisions about whether to pool data (Higgins 2003). We considered I2 values less than, or equal to 40% indicative of a low level of heterogeneity, and values that exceeded 75% indicative of a very high level of heterogeneity (Deeks 2011). We analysed data using Review Manager 5 (Review Manager 2014).
Assessment of reporting biases
We used a funnel plot to assess the possibility of small sample and reporting bias on the estimates for the effects of ES on the proportion of pressure ulcers healed (Sterne 2011).
Data synthesis
We pooled results in meta‐analyses, provided there was not excessive clinical or methodological heterogeneity, and the studies were appropriately similar in terms of type of ES, duration of pressure ulcers, type of participants, duration of treatments, and outcome assessments. Clinical and methodological heterogeneity were based on the review authors' judgement.
We presented meta‐analyses of outcome data using Review Manager 5 (Review Manager 2014). The decision to pool data in a meta‐analysis was based on the availability of outcome data and assessment of between‐trial heterogeneity. For comparisons where there was no apparent clinical heterogeneity and the I2 value was less than, or equal to 40%, we pooled data using a fixed‐effect model (Demets 1987). Where there was no apparent clinical heterogeneity and the I2 value was greater than 40%, we planned to pool data using a random‐effects model (DerSimonian 1986). However, we did not pool data where heterogeneity was very high (I2 values of 75% or above).
We presented data using forest plots, where possible, in the following ways.
For continuous outcomes (e.g. surface area of pressure ulcers), we used the inverse variance method when summary estimates were presented as MDs with 95% CIs or SMDs with 95% CIs.
For dichotomous outcomes (e.g. proportion of pressure ulcers healed), we used the inverse variance method when summary estimates were presented as RRs with 95% CIs and we planned to use the Peto method when summary estimates were presented as odds ratios (ORs) with 95% CIs.
For time‐to‐event outcomes (e.g. time to complete healing), we used the generic inverse variance method when summary estimates were presented as HRs with 95% CIs. If HRs were not reported, but time‐to‐event data were reported, we calculated the HR with 95% CI using the methods suggested by Tierney 2007. If data were provided but could not be analysed, they were included in this review but not pooled.
For all analyses we obtained pooled estimates of treatment effect by using Review Manager 5 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to explore the influence of the following variables on effect size, but these were not carried out because of an insufficient number of studies or participants.
Type of ES: (direct current versus pulsating current). We planned to explore differences in the response to the two types of ES because it is possible that different currents have different effects on healing.
Duration of pressure ulcers: (acute versus chronic i.e. less than 3 months versus more than 3 months). We planned to explore differences in the response to ES of acute and chronic pressure ulcers because acute pressure ulcers may respond better and more quickly to ES than chronic pressure ulcers.
Type of participants: (participants with spinal cord injuries versus participants without spinal cord injuries). We planned to explore differences in the response to ES of participants with spinal cord injuries versus participants without spinal cord injuries because people with spinal cord injuries have additional impairments that may influence the effectiveness of ES.
Duration of treatment effect: (short‐term treatment effect versus long‐term treatment effect i.e. effects present up to 4 weeks after the last intervention versus effects present for 4 weeks and more after the last intervention). We planned to explore differences in the duration of treatment effects because the short‐term effects of ES may differ to the long‐term effects.
Sensitivity analysis
Four sensitivity analyses were conducted to examine the robustness of the meta‐analyses to the inclusion of studies at high risk of bias from the following four domains on the 'Risk of bias' tool: the generation of the random allocation sequence, use of concealed allocation, use of blinded assessors, and dropouts (Deeks 2011). For each sensitivity analysis we excluded studies that were rated at high or unclear risk of bias. We performed additional sensitivity analyses to determine the effect of including studies with unit of analysis issues.
'Summary of findings' tables
We present the main results of the review in a 'Summary of findings’ table. This table presents key information concerning the certainty of the evidence, the magnitude of the effects of ES and the sum of available data for the main outcomes as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b). The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach (Schünemann 2011a). The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect is close to the true value for an outcome (Guyatt 2011; Higgins 2011a). The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological certainty), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a).
We present the following outcomes in the 'Summary of findings' tables.
Proportion of pressure ulcers healed.
Time to complete healing.
Complications/adverse events related to pressure ulcers.
Quality of life.
Ethics and inequalities
We addressed considerations of inequities by ensuring that we extracted data about population characteristics that are associated with health inequalities or disadvantage (Welsh 2016).
Context
We addressed contextual factors by ensuring that we extracted data about the target groups or populations (Armstrong 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search generated 370 records. Forty‐five of these were duplicates, leaving 325 potentially eligible records. We retrieved 55 full‐text articles for consideration for inclusion (Figure 1) (Liberati 2009). We excluded 28 full‐text articles, two studies are awaiting classification (Feldman 2005; Karba 1995), and five studies are ongoing (ACTRN12617001534370; ACTRN12618000345280; JPRN‐UMIN000029516; NCT03753581; NTR6450). Ultimately, 20 studies met the inclusion criteria (Adegoke 2001; Adunksy 2005; Ahmad 2008; Asbjornsen 1990; Baker 1996; Carley 1985; Feeder 1991; Franek 2011; García‐Pérez 2018; Gentzkow 1991; Griffin 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Kloth 1988; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018; Wood 1993). We contacted authors of 11 studies for additional information (Adegoke 2001; Adunksy 2005; Ahmad 2008; Baker 1996; Feeder 1991; Franek 2011; Houghton 2010; Karba 1995; Kloth 1988; Polak 2016a; Polak 2016b; missing data and/or any ambiguities), and we received replies from authors of seven studies (Adunksy 2005; Feeder 1991; Franek 2011; Karba 1995; Kloth 1988; Polak 2016a; Polak 2016b).
1.
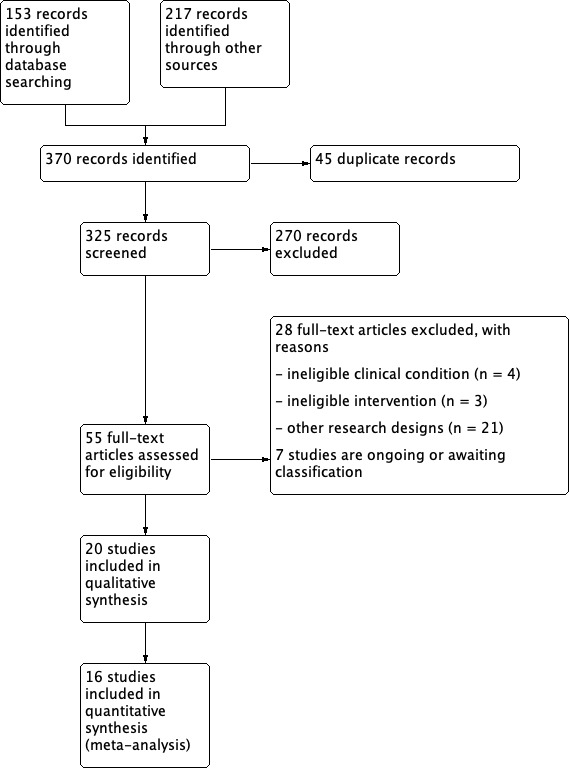
Study flow diagram.
Included studies
The details of the 20 included studies are provided in the Characteristics of included studies table.
Study design and setting
All included studies used a parallel‐group design. Twelve studies were single‐centred RCTs (Adegoke 2001; Asbjornsen 1990; Baker 1996; Carley 1985; Franek 2011; Griffin 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Kloth 1988; Polak 2017; Polak 2018), and eight were multicentred RCTs (Adunksy 2005; Ahmad 2008; Feeder 1991; García‐Pérez 2018; Gentzkow 1991; Polak 2016a; Polak 2016b; Wood 1993). Nineteen studies were conducted in four different settings, including rehabilitation and geriatric hospitals (11 studies; Adegoke 2001; Adunksy 2005; Asbjornsen 1990; Carley 1985; Franek 2011; Gentzkow 1991; Griffin 1991; Jercinovic 1994; Karba 1995; Kloth 1988; Polak 2018), medical centres (4 studies; Baker 1996; Feeder 1991; Polak 2016a; Wood 1993), a residential care centre (2 studies; Polak 2016b; Polak 2017), and a community‐based centre (2 studies; García‐Pérez 2018; Houghton 2010). The setting of one study was unknown (Ahmad 2008). Studies were conducted in nine different countries including Canada (2 studies; Gentzkow 1991; Houghton 2010), Egypt (1 study; Ahmad 2008), Israel (1 study; Adunksy 2005), Nigeria (1 study; Adegoke 2001), Norway (1 study; Asbjornsen 1990), Poland (5 studies; Franek 2011; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018), Spain (1 study; García‐Pérez 2018), Slovenia (2 studies; Jercinovic 1994; Karba 1995), and the USA (6 studies; Baker 1996; Carley 1985; Feeder 1991; Griffin 1991; Kloth 1988; Wood 1993).
Thirteen studies did not clearly state whether participants or pressure ulcers were randomised, but the number of pressure ulcers equalled the number of participants, so there was not a unit of analysis issue (Adegoke 2001; Adunksy 2005; Ahmad 2008; Asbjornsen 1990; Carley 1985; Franek 2011; García‐Pérez 2018; Houghton 2010; Karba 1995; Kloth 1988; Polak 2016a; Polak 2017; Polak 2018). One study clearly stated that participants (not pressure ulcers) were randomised and the number of participants equalled the number of pressure ulcers, so there was not a unit of analysis issue (Griffin 1991). Four studies did not clearly state whether participants or pressure ulcers were randomised and the number of pressure ulcers was greater than the number of participants. There was no accounting for non‐independence of data in the analysis, resulting in a unit of analysis issue (Baker 1996; Feeder 1991; Jercinovic 1994; Polak 2016b). One study did not clearly state whether participants or pressure ulcers were randomised, however, the authors provided the individual participant data and from this it appeared that pressure ulcers (not participants) were randomised. There was no accounting for non‐independence of data in the analysis, resulting in a unit of analysis issue (Wood 1993). One study clearly stated that pressure ulcers (not participants) were randomised. There was no accounting for non‐independence of data in the analysis, resulting in a unit of analysis issue (Gentzkow 1991).
Participants
A total of 913 participants were randomised with sample sizes ranging from seven participants in Adegoke 2001 to 80 participants in Baker 1996. The mean age of the participants in the included studies ranged from 26 years to 83 years. Overall, 50% of participants were male. The chronicity of the pressure ulcers was variable, ranging from a mean of 4 days in Adunksy 2005 to more than 12 months in Feeder 1991. In 16 studies, pressure ulcers were on the sacral and coccygeal region (30%), ischium (24%), lower extremities including heels (23%), greater trochanter of the femur (7%), and other parts of the body (4%). Four studies did not provide information about the location of the pressure ulcers (Ahmad 2008; Carley 1985; Karba 1995; Kloth 1988). Fourteen studies provided data on the severity of the pressure ulcers. In these studies, most participants had stage II (37%) or stage III (45%) pressure ulcers. Six studies did not provide information about the severity of the pressure ulcers (Asbjornsen 1990; Baker 1996; Carley 1985; Jercinovic 1994; Karba 1995; Wood 1993). Two studies had participants with wounds from different causes (Baker 1996; Feeder 1991). In both studies, more than 75% of the wounds were pressure ulcers, but neither study provided individual participant data.
Interventions
Electrical stimulation (ES) was administered from two to 20 hours per week (median 5, interquartile range 4 to 8) and for between three and 12 weeks (median 6, interquartile range 4 to 8). Four studies administered direct current (Adegoke 2001; Adunksy 2005; Carley 1985; Griffin 1991), and 16 studies administered pulsating current (Ahmad 2008; Asbjornsen 1990; Baker 1996; Feeder 1991; Franek 2011; García‐Pérez 2018; Gentzkow 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Kloth 1988; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018; Wood 1993).
All studies, but one, used two electrodes for the administration of ES (i.e. an active electrode and a dispersive electrode). Electrodes were placed in three different ways, namely:
in 13 studies, one electrode was placed over the treating pressure ulcer and the other electrode was placed on healthy skin next to the pressure ulcer (Adegoke 2001; Ahmad 2008; Carley 1985; Feeder 1991; Franek 2011; Gentzkow 1991; Griffin 1991; Houghton 2010; Kloth 1988; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018);
in five studies, both electrodes were placed on healthy skin around the pressure ulcer (Adunksy 2005; Baker 1996; Jercinovic 1994; Karba 1995; Wood 1993);
in one study, the two electrodes were placed on either side of one hand for pressure ulcers on the sacrum and heel (Asbjornsen 1990); and
in one study, the four electrodes were placed around the ulcer (García‐Pérez 2018).
Studies used different current intensities, namely:
in nine studies, the intensity was set to elicit a visible minimal motor contraction (Adegoke 2001; Ahmad 2008; Asbjornsen 1990; Baker 1996; Griffin 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Kloth 1988); and
in six studies, the intensity was set to elicit a mild tingling sensation (Franek 2011; García‐Pérez 2018; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018).
Studies used different frequencies, namely:
in five studies, frequency was set to less than or equal to 50 Hz (Adegoke 2001; Baker 1996; García‐Pérez 2018; Jercinovic 1994; Wood 1993);
in seven studies, frequency was set between 50 Hz and 100 Hz, inclusive (Asbjornsen 1990; Franek 2011; Griffin 1991; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018);
in two studies, frequency was set to more than 100 Hz (Ahmad 2008; Kloth 1988);
in three studies, frequency was changed over the course of the study (Feeder 1991; Gentzkow 1991; Houghton 2010), and one study did not report the frequency (Karba 1995); and
two studies used direct current, therefore frequency was not applicable (Adunksy 2005; Carley 1985).
Five studies did not provide information about the intensity of the current (Adunksy 2005; Carley 1985; Feeder 1991; Gentzkow 1991; Wood 1993).
Fourteen studies used a placebo or sham ES as the control (Adegoke 2001; Adunksy 2005; Ahmad 2008; Asbjornsen 1990; Baker 1996; Feeder 1991; Gentzkow 1991; Griffin 1991; Karba 1995; Kloth 1988; Polak 2016a; Polak 2017; Polak 2018; Wood 1993), and six studies had no type of ES as the control (Carley 1985; Franek 2011; García‐Pérez 2018; Houghton 2010; Jercinovic 1994; Polak 2016b). Standard nursing or wound care was provided to all groups in all studies.
Outcomes
Seventeen studies (out of 20) provided data on five of our outcomes of interest. Twelve studies measured the proportion of pressure ulcers healed, one measured pressure ulcer severity on a composite measure, 13 measured the surface area of pressure ulcers, five measured the time to complete healing, 14 measured the rate of pressure ulcer healing, and 13 reported adverse events. Ten studies did not provide useable data for one or more of our outcomes of interest (Adegoke 2001; Adunksy 2005; Asbjornsen 1990; Baker 1996; Feeder 1991; Gentzkow 1991; Griffin 1991; Houghton 2010; Kloth 1988; Polak 2017).
Registry and funding source
Two studies were prospectively registered (Polak 2017; Polak 2018), and two studies were retrospectively registered (Polak 2016a; Polak 2016b), on the Australian and New Zealand Clinical Trials Registry. Eight studies received full or partial funding from medical device companies (Adunksy 2005; Carley 1985; Gentzkow 1991; Griffin 1991; Houghton 2010; Karba 1995; Kloth 1988; Wood 1993), four studies received full or partial funding from their institutions (Polak 2016b; Polak 2017; Polak 2018; Wood 1993), four studies received funding from research grants (Baker 1996; Houghton 2010; Jercinovic 1994; Karba 1995), three studies did not receive any funding (Franek 2011; García‐Pérez 2018; Polak 2016a), and four studies did not provide information about any source of funding (Adegoke 2001; Ahmad 2008; Asbjornsen 1990; Feeder 1991).
Excluded studies
We excluded a total of 28 full‐text articles for one or more of the following reasons (see Characteristics of excluded studies).
Study design (20 studies): these studies were excluded because they were before‐and‐after intervention studies, prospective non‐randomised intervention studies, reviews, economic analyses (Allen 2004; Barczak 2001; Barron 1985; Chalker 1983; Clegg 2007; Cukjati 2001; Edsberg 2002; Gault 1976; Gentzkow 1993; Karsli 2017; Koel 2014; Lawson 2007; Lee 2007; Lippert‐Gruner 2003; Polak 2014; Recio 2012; Stefanovska 1993; Trontelj 1994; Ullah 2007; Wolcott 1969).
Population (6 studies): these studies were excluded because they were either preclinical or included participants with leg ulcers or other types of wounds (Goldman 2004; Houghton 2003; Jankovic 2008; Sugimoto 2012; Van Londen 2008; Yoshikawa 2015).
Intervention (2 studies): these studies were excluded because they assessed electromagnetic therapy or acupuncture for the treatment of pressure ulcers (Comorosan 1993; Jia 2015).
Studies awaiting classification
There are two studies awaiting classification (Feldman 2005; Karba 1997; see Characteristics of studies awaiting classification).
Ongoing studies
There are five ongoing studies (ACTRN12617001534370; ACTRN12618000345280; JPRN‐UMIN000029516; NCT03753581; NTR6450; see Characteristics of ongoing studies).
Risk of bias in included studies
We assessed all 20 included studies for risk of bias across the eight domains. The results are shown in Figure 2 and Figure 3 with judgements explained in the Characteristics of included studies table.
2.
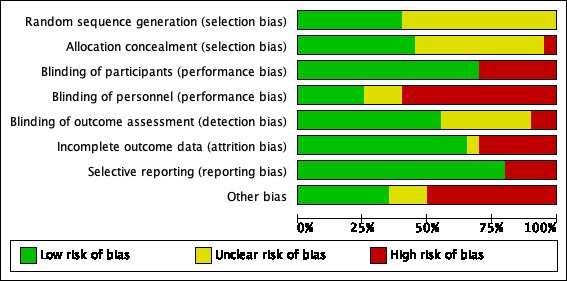
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
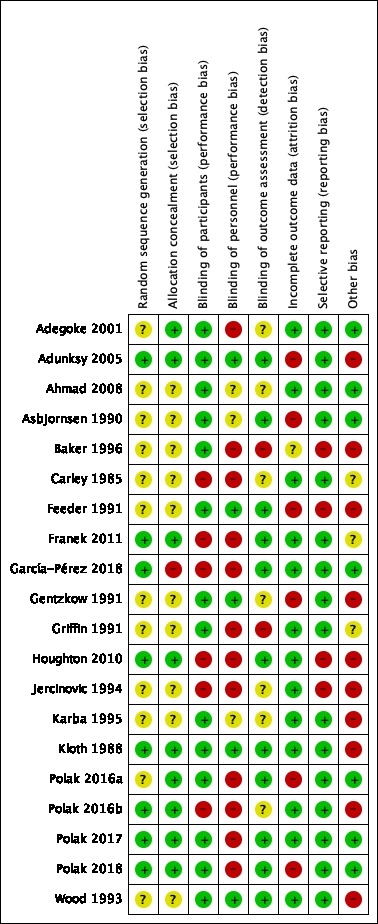
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Eight studies had a low risk of bias for this domain. These studies used computer software or coin tossing to generate their randomisation sequences (Adunksy 2005; Franek 2011; García‐Pérez 2018; Houghton 2010; Kloth 1988; Polak 2016b; Polak 2017; Polak 2018).
Twelve studies had an unclear risk of bias for this domain. These studies did not provide sufficient details to make a judgement (Adegoke 2001; Ahmad 2008; Asbjornsen 1990; Baker 1996; Carley 1985; Feeder 1991; Gentzkow 1991; Griffin 1991; Jercinovic 1994; Karba 1995; Polak 2016a; Wood 1993).
No studies had a high risk of bias for this domain.
Concealed allocation
Nine studies had a low risk of bias for this domain. These studies reported that participants' allocation to groups was either done by an independent person or with the use of opaque sealed sequentially numbered envelopes (Adegoke 2001; Adunksy 2005; Franek 2011; Houghton 2010; Kloth 1988; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018).
Ten studies had an unclear risk of bias for this domain. These studies did not provide sufficient details to make a judgement (Ahmad 2008; Asbjornsen 1990; Baker 1996; Carley 1985; Feeder 1991; Gentzkow 1991; Griffin 1991; Jercinovic 1994; Karba 1995; Wood 1993).
One study had a high risk of bias for this domain. This study stated that the study personnel who determined eligibility also prepared the randomisation sequence (García‐Pérez 2018).
Blinding
Blinding of participants
Fourteen studies had a low risk of bias for this domain. These studies provided control participants with a placebo or sham ES treatment (Adegoke 2001; Adunksy 2005; Ahmad 2008; Asbjornsen 1990; Baker 1996; Feeder 1991; Gentzkow 1991; Griffin 1991; Karba 1995; Kloth 1988; Polak 2016a; Polak 2017; Polak 2018; Wood 1993).
No studies had an unclear risk of bias for this domain.
Six studies had a high risk of bias for this domain. These studies could not blind participants because there was no placebo or sham ES treatment for control participants (Carley 1985; Franek 2011; García‐Pérez 2018; Houghton 2010; Jercinovic 1994; Polak 2016b).
Blinding of personnel
Five studies had a low risk of bias for this domain. These studies clearly reported that personnel were blinded to the treatment group or were unaware of the participants’ allocation (Adunksy 2005; Feeder 1991; Gentzkow 1991; Kloth 1988; Wood 1993).
Three studies had an unclear risk of bias for this domain. These studies did not provide sufficient details to make a judgement (Ahmad 2008; Asbjornsen 1990; Karba 1995).
Twelve studies had a high risk of bias for this domain. These studies could not blind personnel because control participants did not receive placebo or sham ES. Alternatively, these studies clearly stated that it was not possible to blind personnel even though control participants received placebo or sham ES treatment (Adegoke 2001; Baker 1996; Carley 1985; Franek 2011; García‐Pérez 2018; Griffin 1991; Houghton 2010; Jercinovic 1994; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018).
Blinding of outcome assessment
Eleven studies had a low risk of bias for this domain. These studies clearly reported blinding of outcome assessors to group allocation (Adunksy 2005; Asbjornsen 1990; Feeder 1991; Franek 2011; García‐Pérez 2018; Houghton 2010; Kloth 1988; Polak 2016a; Polak 2017; Polak 2018; Wood 1993).
Seven studies had an unclear risk of bias for this domain. These studies did not provide sufficient details to make a judgement (Adegoke 2001; Ahmad 2008; Carley 1985; Gentzkow 1991; Jercinovic 1994; Karba 1995; Polak 2016b).
Two studies had a high risk of bias for this domain. These studies clearly reported that the outcome assessors were not blinded (Baker 1996; Griffin 1991).
Incomplete outcome data
Thirteen studies had a low risk of bias for this domain. These studies had a dropout rate of less than 15% (Adegoke 2001; Ahmad 2008; Carley 1985; Franek 2011; García‐Pérez 2018; Griffin 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Kloth 1988; Polak 2016b: Polak 2017; Wood 1993).
One study had an unclear risk of bias for this domain. This study did not provide sufficient details to make a judgement (Baker 1996).
Six studies had a high risk of bias for this domain. These studies had a dropout rate of between 18% and 40% (Adunksy 2005; Asbjornsen 1990; Feeder 1991; Gentzkow 1991; Polak 2016a; Polak 2018).
Selective reporting
Sixteen studies had a low risk of bias for this domain. These studies reported data on all outcomes stated in the methods (Adegoke 2001; Adunksy 2005; Ahmad 2008; Asbjornsen 1990; Carley 1985; Franek 2011; García‐Pérez 2018; Gentzkow 1991; Griffin 1991; Karba 1995; Kloth 1988; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018; Wood 1993).
No studies had an unclear risk of bias for this domain.
Four studies had a high risk of bias for this domain. These studies either reported outcome data for only a specific group of those randomised or did not report data on all outcomes stated in the methods (Baker 1996; Feeder 1991; Houghton 2010; Jercinovic 1994).
Other potential sources of bias
Seven studies had a low risk of bias for this domain. These studies were free of other sources of potential bias (Adegoke 2001; Ahmad 2008; Asbjornsen 1990; García‐Pérez 2018; Polak 2016a; Polak 2017; Polak 2018).
Three studies had an unclear risk of bias for this domain. These studies did not provide sufficient details to make a judgement but there were sufficient reasons to believe that there may be other sources of bias (Carley 1985; Franek 2011; Griffin 1991).
Ten studies had a high risk of bias for this domain. These studies had potential bias due to some aspect of study design. This included extreme baseline imbalance or unit of analysis issues (i.e. participants with multiple pressure ulcers were recruited and data were presented at the pressure ulcer level rather than participant level) (Adunksy 2005; Baker 1996; Feeder 1991; Gentzkow 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Kloth 1988; Polak 2016b; Wood 1993). Alternatively (or in addition), these studies were sponsored by industry (Adunksy 2005; Carley 1985; Gentzkow 1991; Griffin 1991; Houghton 2010; Kloth 1988; Wood 1993).
Effects of interventions
See: Table 1
All included studies compared a type of ES (plus standard care) with sham, placebo or no ES (plus standard care). We have not attempted to distinguish between placebo and sham because the two terms are used interchangeably by authors of included studies. Standard care included any of the following: wound dressings, pressure relief, regular turning, nutritional advice, and nutritional supplements. The studies administered standard care in the same manner to both groups.
All studies included a measure of at least one of the outcomes of interest. They examined the proportion of pressure ulcers healed, pressure ulcer severity on a composite measure, surface area of pressure ulcers, time to complete healing, complication/adverse events and rate of pressure ulcer healing. One study included a composite measure of pressure ulcer severity but did not provide useable data. No study included measures reflective of three of the outcomes of interest, including quality of life, depression and consumers' perceptions of treatment effectiveness. The results of all analyses are reported below.
Electrical stimulation (plus standard care) versus sham/no ES (plus standard care)
Primary outcome: proportion of pressure ulcers healed
Twelve studies with a total of 581 participants (697 pressure ulcers) examined the proportion of pressure ulcers healed (Adunksy 2005; Asbjornsen 1990; Baker 1996; Feeder 1991; Franek 2011; Griffin 1991; Houghton 2010; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018; Wood 1993). The data in all these studies were expressed as the number of pressure ulcers healed. Eleven studies with a total of 501 participants (512 pressure ulcers) provided sufficient data for meta‐analysis (Adunksy 2005; Asbjornsen 1990; Feeder 1991; Franek 2011; Griffin 1991; Houghton 2010; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018; Wood 1993), and were pooled using a fixed‐effect model. ES probably increases the proportion of pressure ulcers healed when compared with no ES (risk ratio (RR) 1.99, 95% confidence interval (CI) 1.39 to 2.85; I2 = 0%; Analysis 1.1; Table 1). We downgraded the evidence to moderate certainty for serious risk of bias (because a lot of the studies had either high or unclear risk of bias for performance bias and selective reporting). Importantly, three of the included studies did not account for non‐independence of data, resulting in unit of analyses issues (Feeder 1991; Polak 2016b; Wood 1993). Two of these studies randomised at the participant level, but included a few participants with more than one pressure ulcer and analysed data at the pressure ulcer, not participant level, without taking into account the clustered nature of data (Feeder 1991; Polak 2016b). One of these studies randomised at the pressure ulcer level and included a few participants with more than one pressure ulcer, and analysed data at the pressure ulcer level without taking into account the non‐independence of data (Wood 1993). We performed a sensitivity analysis to determine the effect of these three studies on the overall estimate; they made little difference in the overall treatment effect (RR 1.99, 95% CI 1.39 to 2.85 versus RR 1.79, 95% CI 1.17 to 2.73) although as expected the estimate is less precise with the removal of the three studies.
1.1. Analysis.
Comparison 1 Electrical stimulation (plus standard care) versus sham/no electrical stimulation (plus standard care), Outcome 1 Proportion of pressure ulcers healed.
Primary outcome: composite measures of pressure ulcer severity
Two studies with a combined total of 51 participants (51 pressure ulcers) reported a composite measure of pressure ulcer severity (García‐Pérez 2018; Houghton 2010). The data in both these studies were expressed as a composite number. One study used the photographic wound assessment tool (Houghton 2010), but did not provide sufficient data to be included in the analyses. The other study used the Resultados Esperados de la Valoracion y Evolucion de la Cicatrizacion de las Heridas cronicas (RESVECH) Index (García‐Pérez 2018). The point estimate for the mean difference (MD) was ‐2.43 points (95% CI ‐6.14 to 1.28; Analysis 1.2).
1.2. Analysis.
Comparison 1 Electrical stimulation (plus standard care) versus sham/no electrical stimulation (plus standard care), Outcome 2 Composite measure of pressure ulcer severity.
Primary outcome: surface area of pressure ulcers
Fourteen studies with a total of 590 participants (706 pressure ulcers) examined the surface area of pressure ulcers (Adegoke 2001; Adunksy 2005; Ahmad 2008; Asbjornsen 1990; Baker 1996; Feeder 1991; Franek 2011; García‐Pérez 2018; Karba 1995; Kloth 1988; Polak 2016b; Polak 2017; Polak 2018; Wood 1993). The data in all these studies were expressed as mm2 or cm2. For the purpose of analyses we converted surface area into cm2. Twelve studies with a total of 494 participants (505 pressure ulcers) provided sufficient data (Adegoke 2001; Adunksy 2005; Ahmad 2008; Asbjornsen 1990; Feeder 1991; Franek 2011; García‐Pérez 2018; Karba 1995; Polak 2016b; Polak 2017; Polak 2018; Wood 1993). We did not pool the data because there was considerable statistical heterogeneity between studies (I2 = 96%). The source of the heterogeneity was not apparent, but is probably due to a variety of factors, including differences in the types of participants, length of intervention, duration of pressure ulcers and different risks of bias. It is uncertain whether ES decreases the surface area of pressure ulcers when compared with no ES. The MD for each included study is presented as Analysis 1.3. The point estimates for the MD of each study ranged from ‐0.90 cm2 to 10.37 cm2. We did not include this outcome in the 'Summary of findings' table but nonetheless rated it using the GRADE assessment of the certainty of evidence. We downgraded the evidence to very low certainty: downgrading once for serious risk of bias (because a lot of the studies had either high or unclear risk of bias for selection and detection bias), once for inconsistency and once for imprecision.
1.3. Analysis.
Comparison 1 Electrical stimulation (plus standard care) versus sham/no electrical stimulation (plus standard care), Outcome 3 Surface area of pressure ulcers.
Primary outcome: time to complete healing
Five studies with a total of 181 participants (184 pressure ulcers) examined time to complete healing (Adunksy 2005; Asbjornsen 1990; Feeder 1991; Griffin 1991; Polak 2017). The data in these studies were expressed as number of days to complete healing. Two studies with a total of 55 participants (55 pressure ulcers) provided sufficient data (Adunksy 2005; Griffin 1991), and were pooled using a fixed‐effect model. It is uncertain whether ES decreases the time to complete healing of pressure ulcers compared with no ES (hazard ratio (HR) 1.06, 95% CI 0.47 to 2.41; I2 = 0%; Analysis 1.4). We downgraded the evidence to very low certainty: once for serious risk of bias (because both studies had high risk of bias for 2 domains and 1 study had unclear risk of bias for another 3 domains), once for indirectness (because the 2 studies were not reflective of all who are vulnerable to pressure ulcers) and twice for imprecision (Table 1).
1.4. Analysis.
Comparison 1 Electrical stimulation (plus standard care) versus sham/no electrical stimulation (plus standard care), Outcome 4 Time to complete healing.
Primary outcome: complications/adverse events
Thirteen studies with a total of 586 participants (602 pressure ulcers) provided statements about adverse events (Adunksy 2005; Asbjornsen 1990; Carley 1985; Feeder 1991; Franek 2011; García‐Pérez 2018; Gentzkow 1991; Griffin 1991; Houghton 2010; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018). However, the data were not sufficiently detailed or comparable to analyse quantitatively. We downgraded the evidence to low certainty: once for serious risk of bias (because a lot of the studies had either high or unclear risk of bias for selection and attrition bias) and once for imprecision (Table 1). The narrative descriptions of the adverse events in these 13 studies are therefore provided below.
Adunksy 2005 reported that 25 participants withdrew. Ten participants (5 in the experimental group and 5 in the control group) withdrew for a variety of medical reasons. Another 15 participants withdrew because of other adverse events, such as limb amputation (3 participants), deterioration of the pressure ulcer (1 participant), other medical problems (pneumonia, urosepsis, ischaemic colitis, installation of a cardiac pacemaker; 8 participants), or other reasons (3 participants). In addition to the withdrawals, there were four adverse events in two participants from the experimental group (excessive granulation and local irritation to the ES).
Asbjornsen 1990 reported two adverse events (1 leg amputation in the experimental group, and 1 death in the control group).
Carley 1985 reported no complications/adverse events for participants in the experimental group. They however stated that "the control wounds would typically redevelop eschars that required repeated debridement as often as every two weeks" (p444). This was associated with pain and discomfort.
Feeder 1991 stated that 15% of participants had minor uncomfortable tingling in the wound bed (20% of participants in the experimental group and 10% of participants in the control (sham ES) group).
Franek 2011 stated that three participants had complications that were not related to the intervention, including one death (2 in the experimental group and 1 in the control group).
Gentzkow 1991 stated that 14% of participants in the experimental group and 4% of participants in the control group had occasional uncomfortable sensations in the wound bed.
Griffin 1991 stated that three participants (2 in the experimental group and 1 in the control group) withdrew from the study because of medical complications (n = 2) and need for surgical repair of the pressure ulcer (n = 1).
Houghton 2010 stated that the adverse events were minor for participants in the experiment group. For example, two participants in the experiment group had red, raised and itchy skin under one of the electrodes (lasting more than 24 hours and less than 48 hours). A third participant in the experimental group complained of dizziness and delusions. These were not attributed to the intervention. No information on adverse events was provided for participants in the control group.
García‐Pérez 2018, Polak 2016a, Polak 2016b, Polak 2017 and Polak 2018 stated that no adverse events were observed in the experimental group/s.
Secondary outcome: rate of pressure ulcer healing
Fourteen studies with a total of 657 participants (812 pressure ulcers) examined the rate of pressure ulcer healing (Baker 1996; Feeder 1991; Franek 2011; Gentzkow 1991; Griffin 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Kloth 1988; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018; Wood 1993). The data in these studies were expressed in various ways including percentage healed per day, percentage healed per week, percentage healed per four weeks, percentage healed per six weeks and percentage healed per eight weeks. For the purpose of analysis, we expressed data as percentage healed per week by dividing or multiplying the data by an appropriate conversion constant (e.g. percentage healed per day was multiplied by 7 and percentage healed per 4 weeks was divided by 4 to obtain percentage healed per week). Twelve studies with a total of 561 participants (613 pressure ulcers) provided sufficient data (Feeder 1991; Franek 2011; Gentzkow 1991; Griffin 1991; Houghton 2010; Jercinovic 1994; Karba 1995; Polak 2016a; Polak 2016b; Polak 2017; Polak 2018; Wood 1993), and we analysed these studies using a fixed‐effect model. ES probably increases the rate of pressure ulcer healing when compared with no ES (MD 4.59% per week, 95% CI 3.49% to 5.69%; I2 = 25%; Analysis 1.5). This outcome was not included in the 'Summary of findings' table but we nonetheless rated this using the GRADE assessment of the certainty of evidence. We downgraded the evidence to moderate certainty: twice for serious risk of bias (because a lot of the studies had either high or unclear risk of bias for selection bias and some studies had high risk of bias for attrition and reporting bias). Importantly, five of the included studies did not account for non‐independence of data, resulting in unit of analyses issues (Feeder 1991; Gentzkow 1991; Jercinovic 1994; Polak 2016b; Wood 1993). Three of these studies randomised at the participant level, but included a few participants with more than one pressure ulcer and analysed data at the pressure ulcer, not participant level, without taking into account the clustered nature of the data (Feeder 1991; Jercinovic 1994; Polak 2016b). Two of these studies randomised at the pressure ulcer level and included a few participants with more than one pressure ulcer, and analysed data at the pressure ulcer level, without taking into account the non‐independence of data (Gentzkow 1991; Wood 1993). We performed a sensitivity analysis to determine the effect of including these five studies. The results indicated very little difference in treatment effect with or without these five studies (MD 4.59% per week, 95% CI 3.49% to 5.69% versus MD 4.21% per week, 95% CI 3.03% to 5.40%).
1.5. Analysis.
Comparison 1 Electrical stimulation (plus standard care) versus sham/no electrical stimulation (plus standard care), Outcome 5 Rate of pressure ulcer healing.
Secondary outcome: quality of life, depression and consumers' perceptions of treatment effectiveness
No studies were found that measured quality of life, depression or consumers' perceptions of treatment effectiveness.
Subgroup analyses
We did not perform subgroup analyses to explore the influence of the variables on effect size because of an insufficient number of studies or participants.
Sensitivity analysis
We conducted sensitivity analyses to examine the effects of the randomisation process (adequate sequence generation versus inadequate sequence generation), concealed allocation (concealed allocation versus non‐concealed allocation), blinding of assessors (blinding of assessors versus no blinding of assessors) and dropouts (more than 15% dropouts versus 15% or less dropouts) on the primary outcome of proportion of pressure ulcers healed. For each analysis, we excluded between three and five studies (out of 12 studies) because of high or unclear risk. These exclusions had no effect on the RR (see Table 2). We performed additional sensitivity analyses to determine the effect of including studies with unit of analyses issues (these results are reported for each relevant outcome in the results section above).
1. Sensitivity analyses‐ bias.
| Outcomes | Pooled results | Randomisation (studies with adequate sequence generation) | Allocation (studies with concealed allocation) | Assessors (studies with blinded assessors) | Dropout rate (studies with < 15% dropouts) |
|
Proportion of pressure ulcers healed |
1.99 (1.39 to 2.85) (n = 12) |
2.12 (1.36 to 3.30) (n = 6) |
1.98 (1.35 to 2.90) (n = 7) |
1.93 (1.26 to 2.95) (n = 9) |
2.34 (1.47 to 3.71) (n = 6) |
Results are presented as RR (95% CI) n = number of studies included in analysis
Small sample bias
We assessed the possibility of small sample and reporting bias on the estimates for the effects of ES on the proportion of pressure ulcers healed using a funnel plot (see Figure 4). There is no indication of small sample or reporting bias (Sterne 2011).
4.
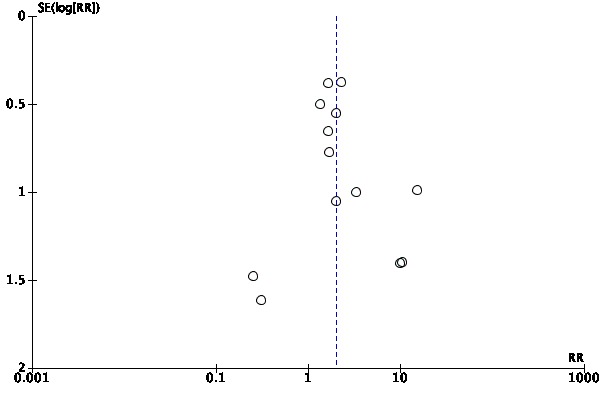
Funnel plot of comparison: 1 Electrical stimulation (plus standard care) versus sham/no electrical stimulation (plus standard care), outcome: 1.1 Proportion of pressure ulcers healed.
Discussion
Summary of main results
We identified 20 eligible studies. There is moderate certainty evidence that electrical stimulation (ES) probably increases the proportion of pressure ulcers healed compared with no ES.
Two studies examined a composite measure of pressure ulcer severity. One used a photographic wound assessment tool, but the data were not useable (Houghton 2010), and it was not possible to determine the effect of ES on this outcome.
There is uncertainty as to whether ES reduces the surface area of pressure ulcers. We could not pool the data for this outcome because there was considerable statistical heterogeneity between studies (I2 = 96%), and the certainty of the evidence for this outcome is very low.
Similarly, there is uncertainty as to whether ES decreases the time to complete healing of pressure ulcers when compared with no ES. The 95% confidence interval associated with the hazard ratio extends from 0.47 (favouring the control) to 2.41 (favouring ES), reflecting the uncertainty; the certainty of evidence for this outcome is very low.
Adverse events were poorly reported and included redness of the skin, itchy skin, dizziness and delusions, deterioration of the pressure ulcer, limb amputation, and occasionally death; the certainty of evidence for this outcome is low and it is difficult to attribute adverse events to interventions.
ES probably increases the rate of pressure ulcer healing when compared with no ES (moderate certainty evidence). We did not include this outcome in the 'Summary of findings' table, but we nonetheless rated it using the GRADE assessment of the certainty of evidence.
No studies included measures of quality of life, depression, or consumers' perceptions of treatment effectiveness.
Overall completeness and applicability of evidence
In some respects this review is reasonably complete and the findings are generalisable. For example, it included participants reflective of our target population. That is, participants were of both genders, various ages, and had a variety of conditions (e.g. spinal cord injury, frail older people) who were recruited from in‐ and outpatient settings. Most of the studies were recent and from different countries. In addition, the ES was applied in a way that is reflective of current practice. For example, the electrodes were applied over or around the pressure ulcers. There was one exception where the electrodes were placed on either side of one hand for pressure ulcers on the sacrum and heel (Asbjornsen 1990). However, this study only contributed to two meta‐analyses and its point estimates were similar to the other studies, suggesting that electrode placement may make little difference. This would seem surprising because presumably the mechanism of action for ES varies depending on electrode placement. More studies are required to explore this further.
However, in other aspects there are some limitations to the completeness of this review. For example, the included studies either did not provide useable data or did not assess some of the outcomes of interest to this review, such as composite measures for pressure ulcer severity, quality of life, depression, and consumers' perspective of treatment effectiveness. In addition, most studies included in this review only investigated the effects of ES over a relatively short period of time (between 20 days to 8 weeks). These aspects limit the generalisability of the results. The completeness of our review is also limited because we did not include children. Future reviews could consider expanding the inclusion criteria to children with pressure ulcers.
Certainty of the evidence
The certainty of evidence for the two healing outcomes rated in the 'Summary of findings' table was either moderate (proportion of pressure ulcers healed) or very low (time to complete healing). We downgraded the evidence mostly because of the serious risk of publication bias and because the included studies were at high or unclear risk of bias (Figure 2; Figure 3). We downgraded one outcome (surface area of pressure ulcers) for imprecision, even though we did not pool the results because the point estimates of the included studies were imprecise. Some of the more serious risks of bias in the included studies were failure to blind therapists (60% of studies), incomplete outcome data (30% of studies) and failure to blind assessors to outcomes (10% of studies). There were also other potential risks of bias (50% of studies). However, we included results from all studies in the main analyses regardless of their risk of bias.
The data extraction for this systematic review was challenging. This was due to poor reporting and the lack of consistency between authors in the way outcome data were expressed. This was particularly problematic for the rate of healing data with some authors expressing this as percentage change while others expressed it as percentage healed. For example, a change in a pressure ulcer size from 10 cm2 to 8 cm2 over one month was expressed by some as a reduction to 80% of original pressure ulcer size and by others as a 20% improvement. It was often difficult to ascertain how the data were expressed. In addition, the units varied between studies with some reporting rate of healing per day and others reporting rate of healing per week or per month. We dealt with this later issue by converting all different units into percentage rate of healing per week, where for example, a rate of healing of 5% reflects a 5% reduction each week with respect to the size of the pressure ulcer at the beginning of the week. In all, we would encourage readers to interpret the results of this outcome cautiously. We would strongly recommend better reporting of this outcome in future studies.
Six studies did not account for non‐independence of data, resulting in unit of analyses issues (Baker 1996; Feeder 1991; Gentzkow 1991; Jercinovic 1994; Polak 2016b; Wood 1993). Three of these studies randomised at the participant level, but included a few participants with more than one pressure ulcer and analysed data at the pressure ulcer, not participant level, without taking into account the clustered nature of the data (Feeder 1991; Jercinovic 1994; Polak 2016b). Two of these studies randomised at the pressure ulcer level and included a few participants with more than one pressure ulcer, and analysed data at the pressure ulcer level without taking into account the non‐independence of data (Gentzkow 1991; Wood 1993). We rated all of these studies as high risk on the 'other bias' domain in the 'Risk of bias' tool. Importantly, we included five of these six studies in one of the meta‐analyses, i.e. rate of pressure ulcer healing (Feeder 1991; Gentzkow 1991; Jercinovic 1994; Polak 2016b; Wood 1993), and three of these six studies were included in another meta‐analysis, i.e. proportion of pressure ulcer healed (Feeder 1991; Polak 2016b; Wood 1993). In these two meta‐analyses, the number of pressure ulcers, not the number of participants, was used to estimate the pooled effect without adjustment for the non‐independence of the data. This was done because the difference between the number of participants and number of pressure ulcers was only small and therefore unlikely to influence the results. The potential for bias in these two analyses due to this problem was captured on the GRADE risk of bias outcome. In addition, our sensitivity analyses to determine the influence of the addition of these studies on the estimates indicated that they had little effect.
Potential biases in the review process
There were two main sources of potential bias in our review process.
We attempted to contact authors for missing details but not all responded. Our failure to gather all missing data may have introduced bias.
We may have missed some potentially eligible studies. We were most likely to have missed unpublished studies, studies in languages other than English, and studies with negative results. However, there was no evidence of publication bias from the funnel plot.
Agreements and disagreements with other studies or reviews
There are five notable systematic reviews which have examined the effect of ES for the treatment of pressure ulcers (Cullum 2001; Koel 2014; Lala 2016; Reddy 2008; Vélez‐Díaz‐Pallarés 2015). We broadly agree with the interpretation of three of these reviews (Cullum 2001; Lala 2016; Vélez‐Díaz‐Pallarés 2015), despite discrepancies in data extraction between two of these reviews and our own (Lala 2016; Vélez‐Díaz‐Pallarés 2015). They, like us, conclude that there is uncertainty as to whether ES is effective. We however, do not agree with the findings of two of the five reviews (Koel 2014; Reddy 2008);
In Koel 2014, the authors examined the rate of healing, but included any type of wound, not just pressure ulcers. The pooled estimate is surprisingly similar to our pooled estimate given there are some notable discrepancies between the data extraction of the studies in common. Nonetheless, the authors made a definitive conclusion stating that: "a clear and positive recommendation is available regarding the effectiveness of ES to increase wound healing" (Koel 2014, pg. 124). We do not agree that the evidence justifies such a strong and clear conclusion.
In the second review that we disagree with (Reddy 2008), the authors examined the rate of healing of pressure ulcers. The authors of this review did not pool data because of high clinical heterogeneity. Interestingly, they concluded that: "Among the good quality RCTs examining adjunctive therapies [electric current], there were no benefits to the interventions, which included electric current (vs placebo electric current)…..." (Reddy 2008, pg. 2658). Their conclusions are in stark contrast to the conclusions of the other reviews in this area and not fully supported by our findings. Our findings indicate uncertainty, but do not indicate that ES is ineffective.
There are also some reviews that have not extracted between‐group differences and are therefore difficult to summarise (Gardner 1999; Houghton 2014; Kawasaki 2014; Lampe 1998; Polak 2014; Regan 2009; Thakral 2013). Regardless, they differ to our review by concluding that ES is effective.
In addition to systematic reviews, there are six key guidelines (AWMA 2012; Consortium for Spinal Cord Medicine 2014; Houghton 2013; NICE 2014; NPUAP/EPUAP 2014; SCIRE 2014). We agree with the recommendations from the National Institute for Health and Care Excellence (NICE 2014). They state: "Do not offer… [electrical stimulation] to adults to treat a pressure ulcer". However, they acknowledge, like we do, that this recommendation is based on very low or low GRADE evidence.
Surprisingly, most of the other guidelines state the opposite and recommend the use of ES, and all grade the certainty of evidence as high. For example, The Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury (AWMA 2012) state: "Consider using electrotherapy as an adjunct for promoting healing in pressure injuries". They rate this recommendation as level "B" according to the National Health and Medical Research Council (NHMRC) grading matrix (good evidence ‐ body of evidence can be trusted to guide practice in most situations).
In even greater contrast to our findings, The Canadian Best Practice Guidelines for the Prevention and Management of Pressure Ulcers in People With Spinal Cord Injury (Houghton 2013), emphatically state: "Use electrical stimulation combined with standard wound care interventions to promote closure of stage III or IV pressure ulcers". They rate this recommendation as "1A” according to the grading of the Registered Nurses' Association of Ontario level of evidence (evidence from meta‐analysis or systematic review of randomised controlled trials). We do not believe this rating or recommendation is justified.
The Consortium for Spinal Cord Medicine 2014 provide a similar clear directive to use ES, stating: "Use electrical stimulation (ES) to promote closure of category/stage III or IV pressure ulcers, unless contraindicated in the cases of untreated, underlying osteomyelitis or infection". They state that this recommendation is based on grade "A" according to the grading of Sackett 1989 (supported by direct scientific evidence from properly designed and implemented controlled trials, i.e. large randomised trial(s) with clear‐cut results and low risk of error).
The Spinal Cord Injury Research Evidence similarly states (SCIRE 2014): "Electrical stimulation added to standard wound management promotes healing of Stage III and IV pressure ulcers post‐SCI [spinal cord injury]". They also base their recommendation on level "1" evidence according to the grading of Sackett 1989 (supported by direct scientific evidence from properly designed and implemented controlled trials, i.e. large randomised trial(s) with clear‐cut results and low risk of error).
The results of our systematic review do not support any of these interpretations of the evidence. We conclude that the certainty of the evidence is moderate, low or very low, and not sufficient to support promoting the use of ES in the clinical setting outside of further research.
Authors' conclusions
Implications for practice.
Electrical stimulation (ES) probably increases the proportion of pressure ulcers healed, but its effect on time to complete healing is uncertain, and the certainty of evidence for all outcomes is moderate, low or very low. The evidence to date is insufficient to support the widespread use of ES for pressure ulcers other than for research purposes.
Implications for research.
We have two broad recommendations for future research in this area. One is about research design and reporting, and the other is about areas of future research.
Our recommendations about research design and reporting are as follows.
Future studies must focus on minimising bias. Importantly, they need to randomly allocate participants to groups, use concealed allocation and blind assessors. Adequate follow‐up of participants will also help minimise bias. Importantly, studies need to register their protocols and adhere to preplanned statistical analyses.
Studies must report between‐group differences for all outcomes. They also need to examine both the short‐term (i.e. within 4 weeks of the cessation of treatment) and long‐term effects of ES (i.e. more than 4 weeks after the cessation of treatment). This will aid interpretation of results and future meta‐analyses.
Studies must follow CONSORT reporting guidelines (Moher 2010). Most studies included in this review only adhered to a few items of CONSORT. Full adherence will have an important positive impact on research in this area.
Studies must pay particular attention to some key methodological issues. In addition, time to healing data need to be appropriately analysed using Kaplan‐Meier curves and hazard ratios. The area would also benefit from consensus on key outcomes and endpoints for future studies.
Future studies should ensure they have a sufficient sample size to detect clinically important differences.
Trialists should take care to avoid unit of analyses problems by randomising, reporting and analysing at the level of the participant not the pressure ulcer.
Our recommendations about areas of future research are as follows.
Studies need to determine the effect of ES on all key outcomes. There is a pressing need to include outcome measures that are important to patients. This needs to include time to complete healing, quality of life, depression and consumers' perceptions of treatments. Future studies should also include composite measures, reflecting the severity of pressure ulcers. The PUSH tool would seem particularly appropriate given it is widely recommended in various guidelines including the NPUAP (NPUAP/EPUAP 2014).
Studies need to determine whether ES has any adverse events. No study included in this review adequately looked at this issue, yet it is feasible that ES could be harmful. This in an important issue that needs attention.
Studies need to look at the long‐term effects of ES. Only one study included in this review looked at the long‐term effects of ES (Adunksy 2005), yet, the long‐term effects are perhaps of most interest and relevance to people with pressure ulcers.
Studies need to look at the cost‐effectiveness of ES for the treatment of pressure ulcers. This should be an important consideration before its widespread use.
Studies need to determine the treatment burden for people with pressure ulcers. A better understanding of this would help guide decisions about minimally worthwhile treatment effects.
Studies need to focus on the optimal therapeutic frequency, duration, and location of treatment. There is no consensus on these parameters, and they are important issues to be considered in future studies.
Studies need to explore the possible mechanism of action of ES. That is, determine how ES could heal pressure ulcers. This will guide decisions about the optimal treatment parameters and electrode placement.
Acknowledgements
The authors would like to thank peer reviewers Susan O’Meara, Anne‐Marie Bagnall, Patricia Davies, Tarek Turk and Janet Yarrow for their contributions to the protocol, and Rebecca Jessup, Pinar Avsar, Gill Norman and Sarah Rhodes for their valuable feedback on the review. Authors would also like to thank Elizabeth Royle for copy editing the protocol and Clare Dooley for copy editing the review.
Appendices
Appendix 1. NPUAP/EPUAP pressure ulcer classification
Category/stage I: non‐blanchable redness of intact skin
Intact skin with non‐blanchable redness (erythema) of a localised area usually over a bony prominence. Discolouration of the skin, warmth, oedema, hardness, or pain may also be present. Darkly pigmented skin may not have visible blanching. Category I may be difficult to detect in individuals with dark skin tones. Presence may indicate 'at risk' persons.
Category/stage II: partial thickness skin loss or blister
Partial thickness loss of dermis (skin) presenting as a shallow open ulcer with a red‐pink wound bed, without slough. May also present as an intact or open/ruptured serum‐filled or serosanguineous‐filled blister. Presents as a shiny, or dry, shallow ulcer without slough or bruising. This category should not be used to describe skin tears, tape burns, incontinence‐associated dermatitis, maceration (skin breakdown under moist conditions), or excoriation (skin loss due to scratching, abrasion or a burn).
Category/stage III: full thickness skin loss (fat visible)
Full thickness tissue loss in which subcutaneous fat may be visible but bone, tendon, or muscle are not exposed. Some slough may be present. May include undermining and tunnelling. The depth of a category III pressure ulcer varies according to its anatomical location. The bridge of the nose, ear, occiput (back part of the head or skull), and malleolus do not have (adipose) subcutaneous tissue, so in these locations category III ulcers can be shallow. In contrast, areas with significant adiposity can develop extremely deep category III pressure ulcers. Bone/tendon is not visible or directly palpable.
Category/stage IV: full thickness tissue loss (muscle/bone visible)
Full thickness tissue loss with exposed bone, tendon, or muscle. Slough or eschar (dead skin) may be present. Often includes undermining and tunnelling. The depth of a category IV pressure ulcer varies according to its anatomical location. The bridge of the nose, ear, occiput, and malleolus do not have (adipose) subcutaneous tissue and so category IV ulcers in these locations can be shallow. Category IV ulcers can extend into muscle or supporting structures (e.g. fascia, tendon, or joint capsule), or both, making osteomyelitis or osteitis likely to occur. Exposed bone/muscle is visible or directly palpable.
Additional categories of the unclassifiable wounds
Unstageable/unclassified: full thickness skin or tissue loss ‐ depth unknown
Full thickness tissue loss, in which actual depth of the ulcer is completely obscured by slough (yellow, tan, grey, green, or brown) or eschar (tan, brown, or black), or both, in the wound bed. Until enough slough or eschar, or both, are removed to expose the base of the wound, the true depth cannot be determined; but it will be either a category III or category IV ulcer. Stable (dry, adherent, intact without erythema or fluctuance (an indication of the presence of pus in a bacterial infection)) eschar on the heels serves as 'the body's natural (biological) cover' and should not be removed.
Suspected deep tissue injury
Purple or maroon localised area of discoloured intact skin or blood‐filled blister due to damage of underlying soft tissue from pressure or shear, or both. The area may be preceded by tissue that is painful, firm, mushy, boggy, and warmer or cooler than adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may evolve further and become covered by thin eschar. Evolution may be rapid, exposing additional layers of tissue even with treatment.
Source
National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel. Clinical Practice Guidelines for the Prevention and Treatment of Pressure Ulcers (NPUAP/EPUAP 2014)
Appendix 2. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Electric stimulation EXPLODE ALL AND INREGISTER
2 MESH DESCRIPTOR Electric stimulation therapy EXPLODE ALL AND INREGISTER
3 MESH DESCRIPTOR Transcutaneous electric nerve stimulation EXPLODE ALL AND INREGISTER
4 (electric* next stimula*) AND INREGISTER
5 ((electric* NEAR3 current*)) AND INREGISTER
6 ("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous electric stimulation" or "transcutaneous electrical stimulation") AND INREGISTER
7 ((direct or puls*) next current*) AND INREGISTER
8 (("ES" or "ENS" or "TENS") NEAR5 electric*) AND INREGISTER
9 ((monophasic* or biphasic*) next (pulse or current*)) AND INREGISTER
10 (("high frequency" or "low frequency") next (current*)) AND (INREGISTER)
11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 AND INREGISTER
12 MESH DESCRIPTOR Pressure ulcer EXPLODE ALL AND INREGISTER
13 (pressure NEXT (ulcer* or sore* or injur*)) AND INREGISTER
14 (decubitus NEXT (ulcer* or sore*)) AND INREGISTER
15 ((bedsore* or bed sore*)) AND INREGISTER
16 #12 OR #13 OR #14 OR #15 AND INREGISTER
17 #11 AND #16 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Electric Stimulation] explode all trees
#2 MeSH descriptor: [Electric Stimulation Therapy] explode all trees
#3 MeSH descriptor: [Transcutaneous Electric Nerve Stimulation] explode all trees
#4 electric* next stimula*:ti,ab,kw
#5 electric* near/3 current*:ti,ab,kw
#6 ("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous electric stimulation" or "transcutaneous electrical stimulation"):ti,ab,kw
#7 ((direct or puls*) next current*):ti,ab,kw
#8 (("TENS" or "ES" or "ENS") near/5 (electric*)):ti,ab,kw
#9 ((monophasic* or biphasic*) next (pulse or current*)):ti,ab,kw
#10 (("high frequency" or "low frequency") next (current*)):ti,ab,kw
#11 {or #1‐#10}
#12 MeSH descriptor: [Pressure Ulcer] explode all trees
#13 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw
#14 (decubitus next (ulcer* or sore*)):ti,ab,kw
#15 (bedsore* or bed next sore*):ti,ab,kw
#16 {or #12‐#15}
#17 {and #11, #16} in Trials
Ovid MEDLINE
1 exp Electric Stimulation/
2 exp Electric Stimulation Therapy/
3 exp Transcutaneous Electric Nerve Stimulation/
4 electric* stimula*.tw.
5 (electric* adj3 current*).tw.
6 (transcutaneous electric* nerve stimulation or transcutaneous electric* stimulation).tw.
7 ((direct or puls*) adj current*).tw.
8 (("ES" or "ENS" or "TENS") adj5 electric*).tw.
9 ((monophasic* or biphasic*) adj (pulse or current*)).tw.
10 ((high frequency or low frequency) adj current*).tw.
11 or/1‐10
12 exp Pressure Ulcer/
13 (pressure adj (ulcer* or sore* or injur*)).tw.
14 (decubitus adj (ulcer* or sore*)).tw.
15 (bedsore* or bed sore*).tw.
16 or/12‐15
17 and/11,16
18 randomized controlled trial.pt.
19 controlled clinical trial.pt.
20 randomi?ed.ab.
21 placebo.ab.
22 clinical trials as topic.sh.
23 randomly.ab.
24 trial.ti.
25 or/18‐24
26 exp animals/ not humans.sh.
27 25 not 26
28 17 and 27
Ovid Embase
1 exp electrostimulation/
2 exp electrostimulation therapy/
3 exp transcutaneous nerve stimulation/
4 electric* stimula*.tw.
5 (electric* adj3 current*).tw.
6 (transcutaneous electric* nerve stimulation or transcutaneous electric* stimulation).tw.
7 ((direct or puls*) adj current*).tw.
8 (("ES" or "ENS" or "TENS") adj5 electric*).tw.
9 ((monophasic* or biphasic*) adj (pulse or current*)).tw.
10 ((high frequency or low frequency) adj current*).tw.
11 or/1‐10
12 exp Pressure Ulcer/
13 (pressure adj (ulcer* or sore* or injur*)).tw.
14 (decubitus adj (ulcer* or sore*)).tw.
15 (bedsore* or bed sore*).tw.
16 or/12‐15
17 and/11,16
18 Randomized controlled trials/
19 Single‐Blind Method/
20 Double‐Blind Method/
21 Crossover Procedure/
22 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
23 (doubl* adj blind*).ti,ab.
24 (singl* adj blind*).ti,ab.
25 or/18‐24
26 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
27 human/ or human cell/
28 and/26‐27
29 26 not 28
30 25 not 29
31 17 and 30
EBSCO CINAHL Plus
S30 S16 AND S29
S29 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28
S28 TI allocat* random* or AB allocat* random*
S27 MH "Quantitative Studies"
S26 TI placebo* or AB placebo*
S25 MH "Placebos"
S24 TI random* allocat* or AB random* allocat*
S23 MH "Random Assignment"
S22 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S21 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S20 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S19 TI clinic* N1 trial* or AB clinic* N1 trial*
S18 PT Clinical trial
S17 MH "Clinical Trials+"
S16 S10 AND S15
S15 S11 OR S12 OR S13 OR S14
S14 TI decubitus or AB decubitus
S13 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* )
S12 TI ( pressure ulcer* or pressure sore* or pressure injur* ) or AB ( pressure ulcer* or pressure sore* or pressure injur* )
S11 (MH "Pressure Ulcer+")
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 TX high frequency current* or low frequency current*
S8 TX monophasic* pulse or monophasic* current* or biphasic* pulse or biphasic* current*)
S7 TX (("ES" or "ENS" or "TENS") N5 electric*)
S6 TX direct current* or puls* current*
S5 TX (transcutaneous electric* nerve stimulation or transcutaneous electric* stimulation)
S4 TX (electric* N3 current*)
S3 TX electric* stimula*
S2 (MH "Transcutaneous Electric Nerve Stimulation")
S1 (MH "Electric Stimulation+")
PEDro (Physiotherapy Evidence Database)
The first PEDro search combined the following terms using “OR”: [Abstract & Title field] pressure*, ulcer*, electrical*, stimulation*, spinal*, TENS*.
The second PEDro search combined the following terms using “AND”: [Therapy field] stimulation, currents [Problem field] pressure ulcers, wounds, ulcers, spinal cord injuries, neurological conditions, geriatrics.
The third PEDro search combined the following terms using “AND”: [Therapy field] stimulation, currents [Problem field] healing, promote healing, wound
World Health Organization International Clinical Trials Registry Platform
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND pressure ulcer [Title]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND pressure ulcer [Condition]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND Pressure sore [Title]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND Pressure sore [Condition]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND Pressure injury [Title]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND Pressure injury [Condition]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND decubitus [Title]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND decubitus [Condition]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND bed sore [Title]
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic [Intervention] AND bed sore [Condition]
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Pressure Ulcer
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Pressure Injury
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Pressure Ulcer Stage 1
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Pressure Ulcers Stage III
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Pressure Ulcer, Buttock
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Pressure Ulcer Not Visible
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Pressure Ulcers Stage II
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Ulcer, Pressure
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Bed Sore
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic | Decubitus Ulcer
The International Standard Randomised Controlled Trial Number (ISRCTN) registry
electrical OR electric OR voltage OR current within Condition: pressure ulcer Interventions: electrical stimulation
voltage OR current OR TENS OR pulsed OR monophasic OR biphasic within Condition: pressure ulcer Interventions: electrical stimulation
TENS OR pulsed OR monophasic OR biphasic within Condition: pressure ulcer Interventions: electrical stimulation
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic within Condition: pressure ulcer Interventions: electrical stimulation
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic within Condition: wounds Interventions: electrical stimulation
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic within Condition: bed sore Interventions: electrical stimulation
electric OR electrical OR voltage OR current OR TENS OR pulsed OR monophasic OR biphasic within Condition: decubitus Interventions: electrical stimulation
EU Clinical Trials Register
stimulation AND pressure ulcer
electric AND pressure sore
electric AND bed sore
electrical stimulation AND pressure ulcer
electric stimulation AND bed sore
electric stimulation AND decubitus
electric current AND pressure sore
electric current AND wounds
Australian New Zealand Clinical Trials Registry
Search term: electrical stimulation AND [wounds OR pressure sore OR bed sore] Allocation to intervention: Randomised
Search term: electric current AND [wounds OR pressure sore OR bed sore] Allocation to intervention: Randomised
Search term: TENS AND [wounds OR pressure sore OR bed sore] Allocation to intervention: Randomised
Search term: [Monophasic OR biphasic] AND [wounds OR pressure sore OR bed sore] Allocation to intervention: Randomised
Stroke Trials Registry
Keywords: wound healing Condition: Wounds and injuries Intervention: Electrical stimulation OR Trancutaneous Electrical Stimulation (ES)
Keywords: pressure sore OR bed sore Condition: Wounds and injuries Intervention: Electrical stimulation OR Trancutaneous Electrical Stimulation (ES)
Keywords: chronic wounds Condition: Wounds and injuries Intervention: Electrical stimulation OR Trancutaneous Electrical Stimulation (ES)
Keywords: decubitus Condition: Wounds and injuries Intervention: Electrical stimulation OR Trancutaneous Electrical Stimulation (ES)
Science Citation Index Expanded and Social Science Citation Index Expanded
# 1 TOPIC: (electrical stimulation)
# 2 TOPIC: (electrical stimulation therapy)
# 3 TOPIC: (Transcutaneous Electric Nerve Stimulation)
# 4 TOPIC: (direct current)
# 5 TOPIC: (pulsed current)
# 6 #5 OR #4 OR #3 OR #2 OR #1
# 7 TOPIC: (pressure ulcers)
# 8 TOPIC: (pressure sores)
# 9 TOPIC: (wounds)
# 10 TOPIC: (ulcers)
# 11 TOPIC: (bedsores)
# 12 #11 OR #10 OR #9 OR #8 OR #7
# 13 TOPIC: (clinical trial*)
# 14 TOPIC: (comparative stud*)
# 15 TOPIC: (evaluation stud*)
# 16 TOPIC: (controlled trial*)
# 17 TOPIC: (follow‐up stud*)
# 18 TOPIC: (prospective stud*)
# 19 TOPIC: (random*)
# 20 TOPIC: (placebo*)
# 21 TOPIC: (single blind*)
# 22 TOPIC: (double blind*)
# 23 #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13
# 24 #23 AND #12 AND #6
Appendix 3. The Cochrane tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process, such as referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example, sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes that were unsealed, non opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding of participants/personnel/outcome assessors
Low risk of bias
Explicit statement that participants/care givers/outcome assessors were blind or inclusion of any information in the trial report suggests that participants/care givers/outcome assessors were not aware of treatment allocation.
High risk of bias
Explicit statement indicates that participants/care givers/outcome assessors were not blinded to treatment allocation.
Unclear
Terms such as 'open' or 'double‐blind' are used with no further explanation, or there is no reference at all to blinding of participants/care givers/outcome assessors.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data are balanced in numbers across experimental groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across experimental groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size.
'As‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon)
High risk of bias
Any one of the following.
Not all of the study's prespecified primary outcomes have been reported.
One or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Comparability at baseline
Low risk of bias
Groups appeared to be similar at baseline for ulcer infection status, ulcer duration and wound surface area (with median values and interquartile ranges reported for duration and area); or differences were observed, but were adjusted for in the analysis.
High risk of bias
Group imbalance was observed at baseline for ulcer infection status, ulcer duration or wound surface area, and no adjustment was made.
Unclear
Information on one or more predictive variables was not provided, or the information was difficult to interpret (e.g. only mean values provided for ulcer area/duration).
7. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Electrical stimulation (plus standard care) versus sham/no electrical stimulation (plus standard care).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of pressure ulcers healed | 11 | 512 | Risk Ratio (IV, Random, 95% CI) | 1.99 [1.39, 2.85] |
| 2 Composite measure of pressure ulcer severity | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Surface area of pressure ulcers | 12 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Time to complete healing | 2 | 55 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.47, 2.41] |
| 5 Rate of pressure ulcer healing | 12 | 613 | Mean Difference (IV, Fixed, 95% CI) | 4.59 [3.49, 5.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adegoke 2001.
| Methods | Design: 4‐week single‐centred, parallel randomised controlled study | |
| Participants |
Health condition: people with spinal cord injury Sample size:
Setting, country: neurology wards at the University College Hospital (1 site), Nigeria Inclusion criteria:
Exclusion criteria: not reported Characteristics of pressure ulcer:
Mean age (SD):
Gender: not reported §It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: interrupted direct current and standard nursing care
Control: placebo interrupted direct current and standard nursing care
Standard nursing care for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Not useable data: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: week 4 (end of intervention) Other time points: baseline and week 2 *converted to cm2 for the purpose of analyses †SD not provided |
|
| Notes |
Withdrawals, (n; reason):
Funding source: no information about funding source provided Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were then randomly assigned to either group A (IDC plus nursing care) or group B (placebo IDC plus nursing care)." p195 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignment to groups was done by an individual with no knowledge of the treatment modality as a way of reducing investigator bias." p195 |
| Blinding of participants (performance bias) All outcomes | Low risk | Comment: control participants received placebo interrupted direct currents |
| Blinding of personnel (performance bias) All outcomes | High risk | Comment: not possible to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All measurements were taken by the same therapist (BKA) to ensure reliability." p196 Comment: insufficient detail reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One of the patient[s] requested to be discharged from the hospital before the end of the study hence was not regarded as being part of the study leaving three subjects each in both groups….." p196 Comment: 1/7 (14%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Low risk | Comment: appears free of other bias |
Adunksy 2005.
| Methods | Design: 8‐week multicentred, double‐blinded, placebo, randomised controlled study | |
| Participants |
Health condition: people with spinal cord injury and advanced age Sample size:
Setting, country: Geriatric and Rehabilitation Medicine Departments (11 sites), Israel Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer$:
Overall ulcer location (n): sacrum (25), trochanters (13), heels (6), buttocks (4), ischium (2), calves and ankles (13) Mean age (SD)$:
Gender$:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. $These data were taken from Table 2, p264. The data for the control and experimental groups were not correct. We presumed that the data were swapped by mistake and the numbers in column 3 and 4 were data for the control and experimental groups, respectively. |
|
| Interventions |
Total groups in this study: two Experimental: active decubitus direct current treatment and conservative treatment
Control: placebo decubitus direct current treatment and conservative treatment
Conservative treatment for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Not useable data: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: day 57 (end of intervention) *Other time points: baseline and day 147 **Other time points: baseline, day 45, day 57 and day 147 †SD not provided |
|
| Notes |
Withdrawals, (n; reason):
Funding source: supported by Lifewave Medical Device Company Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote in the published study: "Allocation of the eligible patients to the TG [treatment group] or CG [control group] was randomised in each department by using a block design of size 4, to assure a ratio of 50:50 in the two groups." p263
Comment: insufficient detail reported in the published study but the information about the random generation has been confirmed by one of the authors of the study via email (dated: 1 Feb 2017) and clinical report. Quote in clinical report: “....random number drawn by a computer software.” p18 Reference: Clinical Report‐ Dedicated Computerized Clinical system for Decubitus Direct Current Treatment (DDCT). LifeWave Ltd., Harrison Clinical Research, Medistat Ltd. March 2007 |
| Allocation concealment (selection bias) | Low risk | Quote in published study: "Allocation of the eligible patients to the TG [treatment group] or CG [control group] was randomised in each department…." p263 Comment: insufficient detail reported in the published study but the information about the allocation concealment has been confirmed by one of the authors of the study via email (dated: 1 Feb 2017) and clinical report. Quote in clinical report: “Knowledge of the randomisation list was limited to the persons responsible for creation of the randomisation list, preparation of the random code envelopes....” and “Copies of the complete randomisation list were kept or dispensed in sealed envelopes.” p18 Reference: Clinical Report‐ Dedicated Computerized Clinical system for Decubitus Direct Current Treatment (DDCT). LifeWave Ltd., Harrison Clinical Research, Medistat Ltd. March 2007 |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "All…patients were completely blinded to treatment type." p263 |
| Blinding of personnel (performance bias) All outcomes | Low risk | Quote: "All investigators….were completely blinded to treatment type." p263 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote in the published study: "All investigators and patients were completely blinded to treatment type." p263
Comment: not sure if the assessor was one of the investigators, who was blinded to participants’ allocation but the information about the assessor blinding has been confirmed by one of the authors of the study via email (dated: 1 Feb 2017) and clinical report. Quote in clinical report: “Knowledge of the randomisation list was limited to the persons responsible for creation of the randomisation list, preparation of the random code envelopes…...” p18 Reference: Clinical Report‐ Dedicated Computerized Clinical system for Decubitus Direct Current Treatment (DDCT). LifeWave Ltd., Harrison Clinical Research, Medistat Ltd. March 2007 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Thirty‐eight patients completed the trial (54% of TG and 64% of PG)." p266, and also Section 3.5 on adverse effects. Comment: 25/63 (40%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre stated outcomes were reported |
| Other bias | High risk | Comment:
|
Ahmad 2008.
| Methods | Design: 5‐week multicentred randomised controlled study | |
| Participants |
Health condition: people with chronic pressure ulcers Sample size:
Setting, country: not reported (4 sites), Egypt Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: four Experimental 1: high voltage pulsed galvanic current administered for 45 minutes plus cointervention
Experimental 2: high voltage pulsed galvanic current administered for 60 minutes plus cointervention
Experimental 3: high voltage pulsed galvanic current administered for 120 minutes plus cointervention
Control: sham high voltage pulsed galvanic current administered for 45 minutes plus cointervention and conventional wound therapy
Conventional wound therapy included a wet dressing and 4‐5 whirlpool therapy sessions per week and an intensive amount of additional care (including the maintenance of a moist wound microenvironment). Cointervention for all groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: week 5 (end of intervention) Other time points: baseline and week 3 |
|
| Notes |
Withdrawals, (n; reason):
Funding source: no information about funding source provided Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly and equally into four groups…" p124 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: control participants received "Sham HVPC". p125 |
| Blinding of personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient detail reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Each ulcer was traced three times to establish measurement reliability." p125 Comment: insufficient detail reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Low risk | Comment: appears free of other bias |
Asbjornsen 1990.
| Methods | Design: 6‐week randomised placebo controlled study | |
| Participants |
Health condition: elderly people with pressure ulcers Sample size:
Setting, country: Department of Geriatric Medicine (1 site), Norway Inclusion criteria:
Exclusion criteria: not reported Characteristics of pressure ulcer:
Mean age (range):
Gender: not reported §It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: TENS and conventional treatment
Control: placebo TENS and conventional treatment
Conventional treatment for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Not useable data: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: week 6 (end of intervention) Other time points: baseline and week 4 *converted to cm2 for the purpose of analyses **Included but not analysed because there were zero events in the treatment group; therefore, it is not possible to estimate the hazard ratio |
|
| Notes |
Withdrawals, (n; reason):
Funding source: no information about funding source provided Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Those who gave consent were randomised..." p210 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: control participants received "placebo TENS”. p213 |
| Blinding of personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient detail reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One of us (GA) who did not know the patients' allocation…..measured the ulcers." p211 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Altogether, 20 patients were recruited, but 4 of these did not participate for a minimum of 4 weeks." p210 Comment: 4/20 (20%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Low risk | Comment: appears free of other bias |
Baker 1996.
| Methods | Design: randomised controlled study | |
| Participants |
Health condition: elderly people with spinal cord injury Sample size:
Setting, country: inpatient and outpatients of a Medical Centre (1 site), USA Inclusion criteria:
Exclusion criteria: not reported Characteristics of pressure ulcer:
Mean age (SD; range):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers is greater than the number of participants. We have assumed that randomisation was at the participant level. *Participants in the control group were moved across to the experimental group but outcome data prior to switching groups are not provided. |
|
| Interventions |
Total groups in this study: four Experimental 1: asymmetric biphasic stimulation plus standard therapy
Experimental 2: symmetric biphasic stimulation plus standard therapy
Experimental 3: microcurrent stimulation plus standard therapy
Control: sham microcurrent stimulation plus standard therapy
Standard therapy for all groups
|
|
| Outcomes |
Outcomes included in this review: none Not useable data**: presented as [name of outcome in review]‐ [name of outcome in study]
Timing of outcome measures: until healed |
|
| Notes |
Withdrawals, (n; reason)**:
Funding source: supported by the National Institute on Disability Research and Rehabilitation, Department of Education, USA Trial registration or published protocol: no information about trial registration or published protocol provided **These data are not reliable as participants from the control group were reassigned to Experimental 1 or Experimental 2 after 28 days leading to duplication of data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned..." p22 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "The control group received the same stimulation procedures as the MC [microcurrent] treatment group, but special leads were used to interrupt the passage of current so the patient received no electrical stimulation" p23 Comment: control participants received sham treatment |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: "Only the therapist doing daily stimulation treatment……knew the group assignment of each subject." p23 Comment: personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Only the therapist doing …… weekly tracings knew the group assignment of each subject." p23 Comment: assessor was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: Table 4‐ 27/192 withdrew; p25 Comment: insufficient detail reported |
| Selective reporting (reporting bias) | High risk | Comment: insufficient detail reported to include outcome data in meta‐analysis |
| Other bias | High risk | Comment:
|
Carley 1985.
| Methods | Design: 5‐week randomised controlled study | |
| Participants |
Health condition: elderly people with indolent pressure ulcers Sample size:
Setting, country: Spaulding Rehabilitation Hospital (1 site), Boston, USA Inclusion criteria:
Exclusion criteria: not reported Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: LIDC plus cointervention
Control: conventional wound therapy plus cointervention
Cointervention for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Other outcomes not included in this review: (1) "area measurement" (i.e. volume) of the ulcer (expressed as cm3) Time point included in this review: week 5 (end of intervention) Other time points: baseline, week 1, week 2, week 3 and week 4 |
|
| Notes |
Withdrawals, (n; reason):
Funding source:
Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned to LIDC" p443 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: "Conventional wound therapy" p443 Comments: not possible to blind participants |
| Blinding of personnel (performance bias) All outcomes | High risk | Comments: not possible to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Area measurement of length, width, and depth to within the nearest millimetre were performed by the nursing staff and recorded without previous knowledge" p444 Comment: not clear if assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Unclear risk | Comment: trial was sponsored by third parties who were likely to have a vested interest in a positive finding |
Feeder 1991.
| Methods | Design: 4‐week randomised, double‐blinded, multicentred study | |
| Participants |
Health condition: elderly people with chronic dermal ulcers Sample size:
Setting, country: hospital and medical centres (9 sites), USA Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD; range):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers is greater than the number of participants. We have assumed that randomisation was at the participant level. |
|
| Interventions |
Total groups in this study: two Experimental: monophasic pulsed ES plus cointervention
Control: sham ES
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review] – [name of outcome in study]
Not useable data: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: week 4 Other time points: baseline, week 1, week 2, week 3 and until healed (end of intervention) *Included but not analysed because there were zero events in the treatment group; therefore, it is not possible to estimate the hazard ratio |
|
| Notes |
Withdrawals, (n; reason):
Funding source: no information about funding source provided Trial registration or published protocol: no information about trial registration or published protocol provided $Authors stated that 4 participants were excluded because they did not meet the eligibility criteria but it is not clear if these participants had been randomised |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...a randomisation list was established for each center by the central study director. Each consecutive numbered patient at each center was then randomly assigned to either a treatment group, which used an active stimulator, or a control group, which used a stimulator that had been modified to produce no output current." p642 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each consecutive numbered patient at each center was then randomly assigned to either a treatment group, which used an active stimulator, or a control group, which used a stimulator that had been modified to produce no output current." p642 Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "Neither the ….. nor the patients were aware of which type of device was used for a particular wound during the 4‐week study period." p642 Comment: control participants received sham treatment |
| Blinding of personnel (performance bias) All outcomes | Low risk | Quote: "The clinical investigators did not have access to the randomisation lists and therefore did not know whether a particular device was active or inactive." p642 Quote: "Neither the investigators nor … were aware of which type of device was used for a particular wound during the 4‐week study period." p642 Comment: it is implied that the clinical investigators (personnel) administered the treatment and therefore were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To further ensure that the clinical trials were blinded, the persons who administered the treatments were different from those who obtained the measurements." p643 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: under heading "Subject"; p641 Comment: 17/67 (25%) dropouts |
| Selective reporting (reporting bias) | High risk | Comment: only reports wound size but indicate that wound appearance was also recorded each week. This is not reported. Comment: not stated that data will be presented as percentage change area |
| Other bias | High risk | Comment:
|
Franek 2011.
| Methods | Design: 6‐week prospective randomised controlled study | |
| Participants |
Health condition: people with pressure ulcers Sample size:
Setting, country: Traumatic Surgery Hospital (1 site), Poland Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: Group A ‐ High Voltage Monophasic Stimulation (HVMS) and pharmacologic agents
Control: Group B‐ Pharmacologic agents Pharmacologic agents for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review] – [name of outcome in study]
Other outcomes not included in this review: (1) volume, (2) length, (3) width, (4) pus‐covered area, (5) granulation area, and (6) Gilman index Time point included in this review: week 6 (end of intervention) Other time points: baseline †These data were taken from Franek 2012. |
|
| Notes |
Withdrawals, (n; reason):
Funding source: insufficient detail reported in the study but an author of this study confirmed that this study did not receive any kind of financial support or funding from any source (email dated: 27 January 2017) Trial registration or published protocol: insufficient detail reported in the study but an author of this study confirmed that they did not publish a study protocol in a journal or on a trial registry (email dated: 27 January 2017) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…computer generated randomisation numbers…" p16 |
| Allocation concealment (selection bias) | Low risk | Quote: “…numbers were sealed in sequentially numbered envelopes." p16 |
| Blinding of participants (performance bias) All outcomes | High risk | Comment: not possible to blind participants |
| Blinding of personnel (performance bias) All outcomes | High risk | Comment: not possible to blind therapists |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Measurements of area (the total surface area and isolated areas covered with pus or granulation) and volume were performed in each person before…." p19 Comment: insufficient detail reported in the study but an author confirmed that the assessors were blinded (email dated: 27‐Jan‐2017) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Unclear risk | Comment: one of the authors (GDG) was the Vice President and Medical Director of Staodyn, Inc. (medical equipment company) |
García‐Pérez 2018.
| Methods | Design: 2‐month prospective randomised controlled study | |
| Participants |
Health condition: elderly people with pressure ulcers Sample size:
Setting, country: nursing homes (6 sites), Spain Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
|
|
| Interventions |
Total groups in this study: two Experimental: TENS and standard wound care
Control: standard wound care Standard wound care for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review] – [name of outcome in study]
Other outcomes not included in this review:
Time point included in this review: 2 months (end of intervention) |
|
| Notes |
Withdrawals, (n; reason):
Funding source: no funding Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The physical therapist who examined the participants for eligibility and collected baseline demographic data prepared the randomization code using computer software.” p464 |
| Allocation concealment (selection bias) | High risk | Quote: " The physical therapist who examined the participants for eligibility and collected baseline demographic data prepared the randomization code using computer software. This research assistant was not involved in the rest of the study. Treatment allocation was concealed……” p464 |
| Blinding of participants (performance bias) All outcomes | High risk | Comment: not possible to blind participants |
| Blinding of personnel (performance bias) All outcomes | High risk | Comment: not possible to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “…physical therapists who collected all outcome measures at baseline and after 20 sessions of treatment were blinded to treatment assignment.” p464 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2/17 (12%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Low risk | Comment: appears free of other bias |
Gentzkow 1991.
| Methods | Design: 4‐week double‐blind placebo multicentred randomised controlled study | |
| Participants |
Health condition: people with pressure ulcers Sample size:
Setting, country: inpatient and outpatient departments (9 sites), Canada and USA Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is clearly stated that pressure ulcers (not participants) were randomised. |
|
| Interventions |
Total groups in this study: two Experimental: STIM and conventional care
Control: shamand conventional care
Conventional care for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Not useable data: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: week 4 (end of intervention) Other time points: baseline, week 1, week 2 and week 3 *SD not provided for the postdata |
|
| Notes |
Withdrawals, (n; reason):
Funding source: this study was supported by a grant from Staodyn, Inc. Gentzkow (first author) was the Vice President and Medical Director of Staodyn, Inc. Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned…." p160 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "Sham device…..[and the]….patients were unaware of whether the device was active or sham, and all study procedures were identical for both groups." p160 Comment: control participants received sham treatment |
| Blinding of personnel (performance bias) All outcomes | Low risk | Quote: "Investigators….were unaware of whether the device was active or sham, and all study procedures were identical for both groups." p160 Comment: personnel were blinded to the treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Investigators and patients were unaware of whether the device was active or sham, and all study procedures were identical for both groups." p160 Comment: insufficient detail reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Overall, 40/49 ulcers (19 sham, 21 stim) or 81.6% were included in the analysis. These 40 ulcers were on 37 patients; three patients each had two ulcers included in the analysis." p164 Comment: 9/49 (18%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | High risk | Comment:
|
Griffin 1991.
| Methods | Design: 20‐day double‐blinded randomised controlled study | |
| Participants |
Health condition: people with spinal cord injury Sample size:
Setting, country: Spinal Cord Injury Service, Baptist Memorial Hospital Regional Rehabilitation Centre (1 site), USA Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Median age (range):
Gender:
§It is clearly stated that participants (not pressure ulcers) were randomised. |
|
| Interventions |
Total groups in this study: two Experimental: HVPC and nursing care
Control: placebo HVPC and nursing care
Nursing care for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Not useable data: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: day 20 (end of intervention) Other time points: baseline, day 5, day 10 and day 15 *SD not provided |
|
| Notes |
Withdrawals, (n; reason):
Funding source: high voltage pulsed direct current units loaned by the Chattanooga Corporation and digitizer for analysis of WSA was provided by WC Campbell foundation Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned…" abstract, p433 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "…a placebo HVPC group…" abstract Comment: control participants received placebo treatment |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: "All treatments were administered by one of three persons‐two physical therapists (JWG and JKC) and a nursing coordinator (RAM). The nursing staff and patients were kept blinded as to patient treatment group assignment" p436 Comment: personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The same person (JWG) conducted all WSA measurements." p437 and "The design of future studies might also be improved by having the person conducting the measurements blinded as to ulcer treatment." p441 Comment: JWG was not blinded, p436 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seventeen of the 20 patients completed the study. Three patients were transferred to the acute care hospital (2 patients developed medical complications, and 1 patient required surgical repair of his ulcer) and thus were eliminated from the study." p438 Comment: 3/20 (15%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Unclear risk | Comment: trial was sponsored by third parties who were likely to have a vested interest in a positive finding |
Houghton 2010.
| Methods | Design: 3‐month, single blind, parallel‐group, randomised controlled study | |
| Participants |
Health condition: people with spinal cord injury Sample size:
Setting, country: community‐based home care setting (1 site), Canada Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: HVPC plus standard wound care programme (EST + SWC)
Control: standard wound care programme Standard wound care programme for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Not useable data: presented as [name of outcome in review]‐ [name of outcome in study]
Other outcomes not included in this review:
Time point included in this review: month 3 (end of intervention) Other time points: baseline *SD not provided |
|
| Notes |
Withdrawals, (n; reason):
Funding source: the study was supported by:
Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…random number generation…" p670 |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible subjects were then assigned to receive either SWC or EST SWC using a concealed, random process that involved opening an opaque envelope prepared by an independent person with random number generation." p670 |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: "This single‐blind study was not set up in a manner that blinded subjects receiving EST." p676 Comment: not possible to blind participants |
| Blinding of personnel (performance bias) All outcomes | High risk | Comment: not possible to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both the acetate tracings and digital images were analyzed by a single assessor who was not involved in either EST or standard wound treatment and was blind to group assignment."p672 Quote: "In this way, results from several centers can be sent to a single assessor who is blind to treatment allocation." p675 Comment: assessor was blinded to all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of the subjects enrolled in the study completed the 3‐month study period" p675 Comment: no dropouts |
| Selective reporting (reporting bias) | High risk | Comment: the total number of wounds healed is not reported. Insufficient data on PWAT and no data on PSST |
| Other bias | High risk | Comment:
|
Jercinovic 1994.
| Methods | Design: 4‐week randomised parallel controlled study | |
| Participants |
Health condition: people with spinal cord injury Sample size:
Setting, country: inpatient department (1 site), Slovenia Inclusion criteria:
Exclusion criteria: not reported Characteristics of pressure ulcer:
Mean age (SD):
Gender: not reported §It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers is greater than the number of participants. We have assumed that randomisation was at the participant level. |
|
| Interventions |
Total groups in this study: two Experimental: ES‐treated group plus conventional (standard) treatment
Control: standard treatment Standard treatment for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review] – [name of outcome in study]
Other outcomes not included in this review:
Time point included in this review: week 4 (end of intervention) Other time points: baseline |
|
| Notes |
Withdrawals, (n; reason):
Funding source: this work was supported by the Ministry of Science and Technology of the Republic of Slovenia and the National Institute for Disability and Rehabilitation Research, Department of Education, Washington D.C., USA Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned…" p226 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: "Because of visible muscle contractions, it was not possible to conduct a double‐blind clinical trial." p227 Comment: not possible to blind participants |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: "Because of visible muscle contractions, it was not possible to conduct a double‐blind clinical trial." p227 Comment: not possible to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient detail reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | High risk | Quote: “To evaluate the healing process, weekly measurements of the wound area were performed. Changes in other very important ulcer measurements, such as the wound depth and the appearance of granulation, were recorded as well. Data obtained during the first four weeks of treatment were included in the data analysis for evaluation of treatment outcomes.” p227 Comment: all pre stated outcomes were not reported |
| Other bias | High risk | Comment:
|
Karba 1995.
| Methods | Design: parallel randomised controlled study | |
| Participants |
Health condition: people with spinal cord injury Sample size:
Setting, country: inpatient department (1 site), Slovenia Inclusion criteria:
Exclusion criteria: not reported Characteristics of pressure ulcer:
Age (range):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: ES group and standard care
Control: CO group
Standard care for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: until healed† Other time points: baseline *converted to cm2 for the purpose of analyses †data were captured at varying time points (between 10 and 56 days); we extracted the data for 2 weeks before participants in the control group were crossed to experimental group |
|
| Notes |
Withdrawals, (n; reason):
Funding source: this study was supported by:
Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" p671 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: control participants received "sham treatment". p671 |
| Blinding of personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient detail reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient detail reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts. All data are available in Table 2 |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | High risk | Comment:
|
Kloth 1988.
| Methods | Design: prospective randomised controlled study | |
| Participants |
Health condition: people with ulcers Sample size:
Setting, country: Marquett University Hospital (1 site), USA Inclusion criteria:
Exclusion criteria: not reported Characteristics of pressure ulcer:
Mean age (SD):
Gender: not reported §It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: treatment group and standard care
Control: control group and standard care
Standard care for both groups
|
|
| Outcomes |
Not useable data*: presented as [name of outcome in review]‐ [name of outcome in study]
Time point of outcome measures: baseline and until healed *Individual data are presented but at different time since randomisation. The data are therefore not comparable. |
|
| Notes |
Withdrawals, (n; reason):
Funding source: the equipment used in this study was provided by DynaWave Corp. Trial registration or published protocol: insufficient detail reported in the study but an author of this study confirmed that they did not publish a study protocol in a journal or on a trial registry (email dated: 31 January 2017) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..tossed a coin…" p504 |
| Allocation concealment (selection bias) | Low risk | Comment: insufficient detail reported in the study but an author confirmed that the allocation of participants was determined by drawing of random number by a person not involved in the study (email dated: 31 January 2017) |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: control participants received "sham treatment". p504 |
| Blinding of personnel (performance bias) All outcomes | Low risk | Comment: insufficient detail reported in the study but an author confirmed that the therapists delivering the intervention were blinded to group allocation (email dated: 31 January 2017) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The same physical therapist (J.A.F.) recorded surface area wound dimensions for each patient before treatment and at weekly treatment intervals." p506 Comment: insufficient detail reported in the study but an author confirmed that the assessors were blinded (email dated: 31‐Jan‐2017) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | High risk | Comment:
|
Polak 2016a.
| Methods | Design: 6‐week prospective, double‐blind, randomised controlled study | |
| Participants |
Health condition: elderly people with pressure ulcers Sample size:
Setting, country: nursing and care centres (2 sites), Poland Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers equals the number of participants. |
|
| Interventions |
Total groups in this study: two Experimental: HVMPC groupand standard wound care
Control: sham HVMPC group and standard wound care
Standard wound care programme for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Other outcomes not included in this review:
Time point included in this review: week 6 (end of intervention) Other time point: baseline |
|
| Notes |
Withdrawals, (n; reason):
Funding source: no information about funding source provided Registry or published protocol: retrospectively registered at Australian New Zealand Clinical Trials Registry (identifier ANZCTR12614000207617) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “….they were randomly allocated between the ES group (SWC plus active HVMPC) and the control group (SWC plus sham HVMPC) using a concealed process (Figure 1).” p452 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Low risk | Quote: “The allocation sequence was concealed by using sealed envelopes with consecutive numbers.” p452 |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: “All patients……were blinded.” p453 |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: “The only person engaged in the experiment who was not blinded was the principal physiotherapist, who set the devices to apply active or sham ES.” p453 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All ….as well as the person making weekly measurements of WSA and the statistician processing the data, were blinded.” p453 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 11/60 (18%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Low risk | Comment: appears free of other bias |
Polak 2016b.
| Methods | Design: 6‐week prospective, parallel‐group, randomised controlled study | |
| Participants |
Health condition: elderly people with pressure ulcers Sample size:
Setting, country: residential care centre and temporary care facilities (number of sites: not reported), Poland Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is not clearly stated whether participants or pressure ulcers were randomised but the number of pressure ulcers is greater than the number of participants. We have assumed that randomisation was at the participant level. |
|
| Interventions |
Total groups in this study: three Experimental 1: ES group (ES plusstandard wound care)
Experimental 2: ultrasound group (US plusstandard wound care)
Control group: standard wound care
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: week 6 (end of intervention) Other time point: baseline, week 4 * converted from percentage per 6 week to percentage per week |
|
| Notes |
Withdrawals, (n; reason):
Funding source: all research activities were funded by the Academy of Physical Education, Poland Registry or published protocol: retrospectively registered at Australian New Zealand Clinical Trials Registry (identifier ANZCTR12613001374752) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patient allocation to groups was performed disregarding when and who would deliver the treatment.” p746 |
| Allocation concealment (selection bias) | Low risk | Quote: “The allocation sequence was concealed by using sealed envelopes with consecutive numbers.” P746 |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: “……is that patients were not blinded and that control groups with sham ES and sham US were not created;” p753 |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: “…..the main investigator opened the envelopes one at a time in the presence of the principal physiotherapist and the particular patient was directed to the indicated group.” p746 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “WSA was determined and the statistical analysis was performed blinded.” p749 Comment: not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8/60 (13%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | High risk | Comment: the unit of randomisation was the participant but some participants had more than one pressure ulcer and data were analysed by pressure ulcers with no account for non‐independence of data |
Polak 2017.
| Methods | Design: 6‐week prospective, parallel‐group, randomised controlled study | |
| Participants |
Health condition: elderly people with chronic pressure ulcers Sample size:
Setting, country: nursing and care centre (number of sites: 3), Poland Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is clearly stated that in participant with multiple pressure ulcer, all wounds were treated but only one pressure ulcer per participant was analysed. |
|
| Interventions |
Total groups in this study: three Experimental 1: cathodal ES group plus standard wound care (CG group)
Experimental 2: cathodal and anodal ES group plus standard wound care (CAG group)
Control: placebo ES group current plus standard wound care (PG group)
Standard wound care
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Not useable data§: presented as [name of outcome in review]‐ [name of outcome in study]
Time point included in this review: week 6 (end of intervention) * converted from percentage per 6 week to percentage per week § data are not extractable and therefore not comparable |
|
| Notes |
Withdrawals, (n; reason):
Funding source: all research activities were funded by the Academy of Physical Education, Poland Registry or published protocol: prospectively registered at Australian New Zealand Clinical Trials Registry (identifier ANZCTRN12614000992606) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “….in the trial generated 4 blocks of 6 letters (combinations of A, B, and C) using computer software.” p779 |
| Allocation concealment (selection bias) | Low risk | Quote: “To conceal the allocation sequence, consecutively numbered, opaque, and sealed envelopes were used.” p779 |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: “All patients, medical personnel, and researchers were blinded.” p779 |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: “All patients….were blinded. The exception was the main investigator and principal physical therapist, who set the equipment to apply active or sham ES.” p779 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The person responsible for wound surface area measurements and statistical analysis was blinded too.” p779 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 6/63 (10%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Low risk | Comment: appears free of other bias |
Polak 2018.
| Methods | Design: 8‐week prospective, parallel‐group, randomised controlled study | |
| Participants |
Health condition: people with neurological injuries Sample size:
Setting, country: rehabilitation centre (number of sites: 1), Poland Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§It is clearly stated that in participant with multiple pressure ulcer, all wounds were treated but only one pressure ulcer per participant was analysed. |
|
| Interventions |
Total groups in this study: three Experimental 1: anodal ES group plus standard wound care (AG group)
Experimental 2: cathodal ES group plus standard wound care (CG group)
Control: placebo ES group plus standard wound care (PG group)
Control group: standard wound care
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Other outcomes not included in this review:
Time point included in this review: week 8 (end of intervention) converted from percentage per 8 weeks to percentage per week † measured at 2 weeks and 4 weeks only |
|
| Notes |
Withdrawals, (n; reason):
Funding source: all research activities were funded by the Academy of Physical Education, Katowice, Poland Registry or published protocol: prospectively registered at Australian New Zealand Clinical Trials Registry (identifier ANZCTRN12615001281583) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “……..inserted the slips into 45 computer‐generated, randomly drawn envelopes.” and “3 additional sets of 6 envelopes were prepared for each group and the randomization of patients proceeded as described.”p12 |
| Allocation concealment (selection bias) | Low risk | Quote: “Before the trial commenced, the envelopes were opened 1 at a time in the presence of a physiotherapist and the patient concerned was directed to the appropriate group.” p12 |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: “All patients……were blinded…..” p12 |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: “The exceptions were … the principal physiotherapist who set the equipment to apply active or sham ES.” p12 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “First, the research team (physicians, nurses, physiotherapists), the person in charge of measuring WSA, and the statistician were blinded as to treatment provided.” p27 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 20/60 (33%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | Low risk | Comment: appears free of other bias |
Wood 1993.
| Methods | Design: 8‐week multicentred, placebo, randomised controlled study | |
| Participants |
Health condition: people with chronic decubitus ulcers Sample size:
Setting, country: medical centre (4 sites), USA Inclusion criteria:
Exclusion criteria:
Characteristics of pressure ulcer:
Mean age (SD):
Gender:
§The number of pressure ulcers is greater than the number of participants but it is not clearly stated whether participants or pressure ulcers were randomised. However, the authors provided the individual participant data and from this it appears that pressure ulcers (not participants) were randomised. |
|
| Interventions |
Total groups in this study: two Experimental: PLIDC (treated ulcer) and standard treatment
Control: control group and standard treatment
Standard treatment for both groups
|
|
| Outcomes |
Outcomes included in this review: presented as [name of outcome in review]‐ [name of outcome in study]
Other outcomes:
Time point included in this review: week 8 (end of intervention) Other time point: baseline |
|
| Notes |
Withdrawals, (n; reason):
Funding source: this study is supported by:
Trial registration or published protocol: no information about trial registration or published protocol provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" p1000 Comment: insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail reported |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: control participants received "….sham instrument." p1000 |
| Blinding of personnel (performance bias) All outcomes | Low risk | Quote: "All treatments were given by the same investigator, at each of the four facilities, without the investigator knowing which was the active or sham instrument." p1000 Comment: personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Surface area tracings and photographs of the ulcers were controlled by persons not involved directly in the treatments to minimize bias." p1000 Comment: assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8/74 (11%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all pre stated outcomes were reported |
| Other bias | High risk | Comment:
|
ABPI: ankle‐brachial pressure index EPUAP: European Pressure Ulcers Advisory Panel ES: electrical stimulation EST: electrical stimulation therapy HVMPC: high voltage monophasic pulsed current HVPC: high voltage pulsed current IAET: International Association of Enterostomal Therapists IDC: interrupted direct current LIDC: low intensity direct current NPUAP: National Pressure Ulcers Advisory Panel PLIDC: pulsed low‐intensity direct current PSST: Pressure Sore Status Tool PWAT: Photographic Wound Assessment Tool SD: standard deviation SWC: standard wound care TENS: transcutaneous electrical nerve stimulation WSA: Wound Surface Area
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2004 | Design: not a randomised controlled trial |
| Barczak 2001 | Design: not a randomised controlled trial |
| Barron 1985 | Design: not a randomised controlled trial |
| Chalker 1983 | Design: not a randomised controlled trial |
| Clegg 2007 | Design: not a randomised controlled trial |
| Comorosan 1993 | Intervention: electromagnetic therapy |
| Cukjati 2001 | Design: not a randomised controlled trial |
| Edsberg 2002 | Design: not useable pilot data |
| Gault 1976 | Design: not a randomised controlled trial |
| Gentzkow 1993 | Design: not a randomised controlled trial |
| Goldman 2004 | Participants: venous ulcers |
| Houghton 2003 | Participants: leg ulcers |
| Jankovic 2008 | Participants: leg ulcers |
| Jia 2015 | Intervention: acupuncture |
| Karsli 2017 | Design: no control group |
| Koel 2014 | Design: not a randomised controlled trial |
| Lawson 2007 | Design: not a randomised controlled trial |
| Lee 2007 | Design: not a randomised controlled trial |
| Lippert‐Gruner 2003 | Design: not a randomised controlled trial |
| Polak 2014 | Design: not a randomised controlled trial |
| Recio 2012 | Design: not a randomised controlled trial |
| Stefanovska 1993 | Design: not a randomised controlled trial |
| Sugimoto 2012 | Intervention: in vitro |
| Trontelj 1994 | Design: not a randomised controlled trial |
| Ullah 2007 | Design: not a randomised controlled trial |
| Van Londen 2008 | Participants: no pressure ulcers |
| Wolcott 1969 | Design: not a randomised controlled trial |
| Yoshikawa 2015 | Intervention: in vitro |
Characteristics of studies awaiting assessment [ordered by study ID]
Feldman 2005.
| Methods | Randomised cross‐over trial |
| Participants | People with spinal cord injuries |
| Interventions | ES bandage (experimental group) |
| Outcomes | Rate of healing of pressure ulcers |
| Notes | This is a conference abstract; no useable data. The contact person of this abstract has responded (email dated 26 August 2016) to our query and stated they did not publish these data but only presented as an abstract. Awaiting full report |
Karba 1997.
| Methods | Double‐blind study |
| Participants | People with spinal cord injuries |
| Interventions | ES (experimental group) versus sham stimulation (control group) |
| Outcomes | Relative healing rate (percentage per day) |
| Notes | Not sure if randomised: awaiting response from the author of the study |
ES: electrical stimulation
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617001534370.
| Trial name or title | Effects of pulsed currents applied by cathode and anode in the treatment of category I pressure ulcers |
| Methods | Randomised controlled trial |
| Participants | People with pressure ulcers |
| Interventions | Monophasic pulsed current (MPC) delivered by the cathode (intervention 1) as the treatment electrode and MPC delivered by the anode as the treatment electrodes (intervention 2) |
| Outcomes | Healing progress |
| Starting date | 6 November 2017 |
| Contact information | Dr Anna Polak (a.polak@awf.katowice.pl) |
| Notes | The trial is prospectively registered |
ACTRN12618000345280.
| Trial name or title | Influence of low voltage monophasic pulsed current and low voltage biphasic pulsed current on pressure ulcer healing based on clinical treatment effects and basic research |
| Methods | Randomised controlled trial |
| Participants | People with pressure ulcers |
| Interventions | Low voltage monophasic pulsed current (intervention 1) and low voltage biphasic pulsed current (intervention 2) |
| Outcomes | Wound surface area |
| Starting date | 26 March 2018 |
| Contact information | Dr Anna Polak (a.polak@awf.katowice.pl) |
| Notes | The trial is prospectively registered |
JPRN‐UMIN000029516.
| Trial name or title | The effect of electrical stimulation on pressure injuries: double‐blind controlled cross over study |
| Methods | Cross‐over randomised controlled trial |
| Participants | People with pressure injuries |
| Interventions | Monophasic pulsed microcurrent |
| Outcomes | Wound contraction |
| Starting date | 10 October 2017 |
| Contact information | Terutaka Hiramatsu (rihaptot@seiwa‐h.org) |
| Notes |
NCT03753581.
| Trial name or title | Effectiveness of microcurrents therapy in pressure ulcers in elderly people |
| Methods | Controlled and randomised triple‐blind clinical trial |
| Participants | Elderly people with pressure ulcers |
| Interventions | Microcurrents |
| Outcomes | Wound surface area and rate of pressure ulcer healing |
| Starting date | 31 October 2018 |
| Contact information | Juan Avendaño Coy |
| Notes | https://clinicaltrials.gov/ct2/show/NCT03753581 |
NTR6450.
| Trial name or title | Wireless microcurrent stimulation: adjunctive therapy for hard‐to‐heal chronic wounds – a double‐blind, placebo controlled trial |
| Methods | Randomised controlled trial |
| Participants | People with hard to heal chronic wounds |
| Interventions | Wireless microcurrent stimulation |
| Outcomes | Wound surface area and adverse events |
| Starting date | 28 September 2015 |
| Contact information | M.C.H.A. Doomen (m.doomen@vumc.nl) |
| Notes | http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR6450 |
Differences between protocol and review
We clarified the inclusion criteria. We included studies even if the causes of the pressure ulcers were not reported but it was reasonable to assume that they were due to pressure injuries (e.g. the pressure ulcers were on the sacrum).
We initially intended to express rate of pressure ulcer healing as mm2 per day, cm2 per day and percentage per day. However, for the purposes of analysis, these data were expressed as percentage healed per week.
We clarified the distinction between acute and chronic pressure ulcers for the subgroup analysis. We classified acute and chronic pressure ulcers on the basis of the mean duration of the pressure ulcers (e.g. less than 3 months versus more than 3 months).
We examined the effect of "duration of treatment effect" within a subgroup analysis rather than a sensitivity analysis. This change did not affect the results because there were insufficient studies for either type of analysis.
We clarified our methodology for dealing with one type of unit of analysis problem. We included studies where participants were randomised but data were reported on multiple pressure ulcers for some participants in the meta‐analysis. We conducted a sensitivity analysis to determine the effect of their inclusion.
We rated studies as high risk of bias for incomplete outcome data if there were more than 15% dropouts.
Contributions of authors
Mohit Arora: conceived, designed and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; wrote to study authors/experts/companies; provided data; approved the final review prior to submission; and is a guarantor of the review.
Lisa Harvey: conceived and designed the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; contributed to writing or editing the review; advised on the review; provided data; approved the final review prior to submission; is a guarantor of the review.
Joanne Glinsky: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Lianne Nier: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Lucija Lavrencic: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Annette Kifley: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Ian Cameron: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Contributions of the editorial base
Nicky Cullum (Editor): edited the review; advised on methodology interpretation and content; approved the final review prior to submission.
Liz McInnes (Editor): edited the protocol; advised on methodology interpretation and content; approved the final protocol prior to submission. Gill Rizzello (Managing Editor): co‐ordinated the editorial process; advised on content; edited the review. Sophie Bishop and Naomi Shaw (Information Specialists); designed the search strategy, ran the searches and edited the search methods section. Tom Patterson and Ursula Gonthier (Editorial Assistants): edited the Plain Language Summary and the reference section.
Sources of support
Internal sources
John Walsh Centre for Rehabilitation Research, Sydney Medical School, Northern Clinical School, The University of Sydney, Australia.
Kolling Institute of Medical Research, Royal North Shore Hospital, Northern Sydney Local Health District, Australia.
Spinal Cord Injury Unit, Royal North Shore Hospital, Northern Sydney Local Health District, Australia.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Ian Cameron’s salary is supported by an Australian National Health and Medical Research Council Practitioner Fellowship, Australia.
Declarations of interest
Mohit Arora: none known
Lisa Harvey: I am Editor‐in‐Chief of Spinal Cord and receive an honorarium.
Joanne Glinsky: none known
Lianne Nier: none known
Lucija Lavrencic: none known
Annette Kifley: none known
Ian Cameron: none known
New
References
References to studies included in this review
Adegoke 2001 {published data only}
- Adegoke BO, Badmos KA. Acceleration of pressure ulcer healing in spinal cord injured patients using interrupted direct current. African Journal of Medicine and Medical Sciences 2001;30(3):195‐7. [PubMed] [Google Scholar]
Adunksy 2005 {published data only}
- Adunsky A, Ohry A, Group D. Decubitus direct current treatment (DDCT) of pressure ulcers: results of a randomized double‐blinded placebo controlled study. Archives of Gerontology and Geriatrics 2005;41(3):261‐9. [DOI] [PubMed] [Google Scholar]
- Ohry A, Tamir J, Hartwell SD. An interim analysis from a 62 patient randomised double‐blind study of a novel non‐invasive therapy (LifeGen) versus a placebo in stage II pressure ulcers. Wound Repair and Regeneration 2003;11(5):A39. [Abstract P.030] [Google Scholar]
Ahmad 2008 {published data only}
- Ahmad ET. High‐voltage pulsed galvanic stimulation: effect of treatment duration on healing of chronic pressure ulcers. Annals of Burns and Fire Disasters 2008;21(3):124‐8. [PMC free article] [PubMed] [Google Scholar]
Asbjornsen 1990 {published data only}
- Asbjornsen G, Hernaes B, Molvaer G. The effect of transcutaneous electrical nerve stimulation on pressure sores in geriatric patients. Journal of Clinical and Experimental Gerontology 1990;12(4):209‐14. [Google Scholar]
Baker 1996 {published data only}
- Baker LL, Rubayi S, Villar F, Demuth SK, Villar F, Demuth SK. Effect of electrical stimulation waveform on healing of ulcers in human beings with spinal cord injury. Wound Repair and Regeneration 1996;4(1):21‐8. [DOI] [PubMed] [Google Scholar]
Carley 1985 {published data only}
- Carley PJ, Wainapel SF. Electrotherapy for acceleration of wound healing: low intensity direct current. Archives of Physical Medicine & Rehabilitation 1985;66(7):443‐6. [PubMed] [Google Scholar]
Feeder 1991 {published data only}
- Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Physical Therapy 1991;71(9):639‐49. [DOI] [PubMed] [Google Scholar]
- Gentzkow GD, Pollack, SV, Kloth LC, Stubbs HA. Improved healing of pressure ulcers using Dermapulse, a new electrical stimulation device. Wounds 1991;3(5):158‐70. [Google Scholar]
- Mulder GD. Treatment of open‐skin wounds with electric stimulation. Archives of Physical Medicine and Rehabilitation 1991;72(6):375‐7. [PubMed] [Google Scholar]
Franek 2011 {published data only}
- Franek A, Kostur R, Polak A, Taradaj J, Szlachta Z, Blaszczak E, et al. Using high‐voltage electrical stimulation in the treatment of recalcitrant pressure ulcers: results of a randomized, controlled clinical study. Ostomy/Wound Management 2012;58(3):30‐44. [PubMed] [Google Scholar]
- Franek A, Kostur R, Taradaj J, Blaszczak E, Szlachta Z, Dolibog P, et al. Effect of high voltage monophasic stimulation on pressure ulcer healing: results from a randomized controlled trial. Wounds 2011;23(1):15‐23. [Google Scholar]
García‐Pérez 2018 {published data only}
- García‐Pérez S, García‐Ríos MC, Pérez‐Mármol JM, Tapia‐Haro RM, Albornoz‐Cabello M, Valenza MC, et al. Effectiveness of transcutaneous electrical nerve stimulation energy in older adults: a pilot clinical trial. Advances in Skin and Wound Care 2018;31(10):462‐9. [DOI] [PubMed] [Google Scholar]
Gentzkow 1991 {published data only}
- Gentzkow GD, Pollack VP, Kloth LC, Stubbs HA. Improved healing of pressure ulcers using Dermapulse, a new electrical stimulation device. Wounds 1991;3(5):158‐70. [Google Scholar]
Griffin 1991 {published data only}
- Griffin JW, Tooms RE, Mendius RA, Clifft JK, Zwaag RC, El‐Zeky F. Efficacy of high voltage pulsed current for healing of pressure ulcers in patients with spinal cord injury. Physical Therapy 1991;71(6):433‐42. [DOI] [PubMed] [Google Scholar]
Houghton 2010 {published data only}
- Houghton P, Koel G. RR‐PL‐2735: Electrical stimulation therapy increases closure of pressure ulcers and leg ulcers and speeds healing of diabetic foot ulcers: a meta‐analysis. Physiotherapy 2011;97(Suppl 1):eS504. [Google Scholar]
- Houghton PE, Campbell KE, Fraser C, Harris C, Keast DH, Potter PJ, et al. Electrical stimulation therapy (EST) increases healing of pressure ulcers in community dwelling people with spinal cord injury (SCI). Archives of Physical Medicine and Rehabilitation 2010;91(5):669‐77. [DOI] [PubMed] [Google Scholar]
- Houghton PE, Campbell KE, Fraser C, Harris C, Woodbury MG. Electrical stimulation therapy increases healing of pressure ulcers in community dwelling people with spinal cord injury. Third congress of the World Union of Wound Healing Societies meeting; 2008 Jun 4‐8; Toronto, Canada. 2008:OR121.
- Mittmann N, Chan BC, Craven BC, Isogai PK, Houghton P. Evaluation of the cost‐effectiveness of electrical stimulation therapy for pressure ulcers in spinal cord injury. Archives of Physical Medicine and Rehabilitation 2011;92(6):866‐72. [DOI] [PubMed] [Google Scholar]
Jercinovic 1994 {published data only}
- Jercinovic A, Karba R, Vodovnic L, Stefanovska A, Kroselj P, Turk R, et al. Low frequency pulsed current and pressure ulcer healing. IEEE Transactions on Rehabilitation Engineering 1994;2(4):225‐33. [Google Scholar]
Karba 1995 {published data only}
- Karba R, Benko H, Savrin R, Vodovnik L. Combination of occlusive dressings and electrical stimulation in pressure ulcer treatment. Medical Science Research 1995;23(10):671‐4. [Google Scholar]
Kloth 1988 {published data only}
- Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic, pulsed current. Physical Therapy 1988;68(4):503‐8. [DOI] [PubMed] [Google Scholar]
Polak 2016a {published data only}
- Polak A, Kloth LC, Blaszczak E, Taradaj J, Nawrat‐Szoltysik A, Walczak A, et al. Evaluation of the healing progress of pressure ulcers treated with cathodal high‐voltage monophasic pulsed current: results of a prospective, double‐blind, randomized clinical trial. Advances in Skin and Wound Care 2016;29(10):447‐59. [DOI] [PubMed] [Google Scholar]
Polak 2016b {published data only}
- Polak A, Taradaj J, Nawrat‐Szoltysik A, Stania M, Dolibog P, Blaszczak E, et al. Reduction of pressure ulcer size with high‐voltage pulsed current and high‐frequency ultrasound: a randomised trial. Journal of Wound Care 2016;25(12):742‐54. [DOI] [PubMed] [Google Scholar]
Polak 2017 {published data only}
- Polak A, Kloth LC, Blaszczak E, Taradaj J, Nawrat‐Szoltysik A, Ickowicz T, et al. The efficacy of pressure ulcer treatment with cathodal and cathodal‐anodal high‐voltage monophasic pulsed current: a prospective, randomized, controlled clinical trial. Physical Therapy 2017;97(8):777‐89. [DOI] [PubMed] [Google Scholar]
Polak 2018 {published data only}
- Polak A, Kucio C, Kloth LC, Paczula M, Hordynska E, lckowicz T, et al. A randomized, controlled clinical study to assess the effect of anodal and cathodal electrical stimulation on periwound skin blood flow and pressure ulcer size reduction in persons with neurological injuries. Ostomy Wound Management 2018;64(2):10‐29. [PubMed] [Google Scholar]
Wood 1993 {published data only}
- Wood JM, Evans PE, Schallreuter KU, Jacobson WE, Sufit R, Newman J, et al. A multicenter study on the use of pulsed low‐intensity direct current for healing chronic stage II and stage III decubitus ulcers. Archives of Dermatology 1993;129(8):999‐1009. [PubMed] [Google Scholar]
- Wood JM, Jacobson WF, Schallreuter KU, Evans P, Jacobson M, Suffer R, et al. Pulsed low‐intensity direct current (PLIDC) is effective in healing chronic decubitus ulcers in stages II and III. Journal of Investigative Dermatology 1992;98(4):574. [Google Scholar]
References to studies excluded from this review
Allen 2004 {published data only}
- Allen J, Houghton PE. C20A case study for electrical stimulation on a stage iii pressure ulcer. Wound Care Canada 2004;2(1):34‐6. [Google Scholar]
Barczak 2001 {published data only}
- Barczak M, Kluger P, Kluger J, Bauerle J, Puhl W. Therapeutic effectiveness of electric stimulation in paraplegic patients with pressure sores [Doctoral thesis]. Ulm (Germany): Medical School of the University of Ulm, 2001. [Google Scholar]
Barron 1985 {published data only}
- Barron JJ, Jacobson WE, Tidd G. Treatment of decubitus ulcers. A new approach. Minnesota Medicine 1985;68(2):103‐6. [PubMed] [Google Scholar]
Chalker 1983 {published data only}
- Chalker M. Healing of decubitus ulcers of patients in neuro‐kinesthetic program with the Electro‐Acuscope 80. Critical Care Update 1983;10(3):50‐2. [PubMed] [Google Scholar]
Clegg 2007 {published data only}
- Clegg JP, Guest JF. Modelling the cost‐utility of bio‐electric stimulation therapy compared to standard care in the treatment of elderly patients with chronic non‐healing wounds in the UK. Current Medical Research and Opinion 2007;23(4):871‐83. [DOI] [PubMed] [Google Scholar]
Comorosan 1993 {published data only}
- Comorosan S, Vasilco R, Arghiropol M, Paslaru L, Jieanu V, Stelea S. The effect of Diapulse therapy on the healing of decubitus ulcer. Romanian Journal of Physiology 1993;30(1‐2):41‐5. [PubMed] [Google Scholar]
Cukjati 2001 {published data only}
- Cukjati D, Robnik‐Sikonja M, Rebersek S, Kononenko I, Miklavcic D. Prognostic factors in the prediction of chronic wound healing by electrical stimulation. Medical and Biological Engineering and Computing 2001;39(5):542‐50. [DOI] [PubMed] [Google Scholar]
Edsberg 2002 {published data only}
- Edsberg LE, Brogan MS, Jaynes CD, Fries K. Topical hyperbaric oxygen and electrical stimulation: exploring potential synergy. Ostomy/Wound management 2002;48(11):42‐50. [PubMed] [Google Scholar]
Gault 1976 {published data only}
- Gault WR, Gatens PF Jr. Use of low intensity direct current in management of ischemic skin ulcers. Physical Therapy 1976;56(3):365‐9. [DOI] [PubMed] [Google Scholar]
Gentzkow 1993 {published data only}
- Gentzkow GD, Alon G, Taler GA, Eltorai IM, Montroy RE. Healing of refractory stage III and IV pressure ulcers by a new electrical stimulation device. Wounds 1993;5(3):160‐71. [Google Scholar]
Goldman 2004 {published data only}
- Goldman R, Rosen M, Brewley B, Golden M. Electrotherapy promotes healing and microcirculation of infrapopliteal ischemic wounds: a prospective pilot study. Advances in Skin and Wound Care 2004;17(6):284‐94. [DOI] [PubMed] [Google Scholar]
Houghton 2003 {published data only}
- Houghton PE, Kincaid CB, Lovell M, Campbell KE, Keast DH, Woodbury MG, et al. Effect of electrical stimulation on chronic leg ulcer size and appearance. Physical Therapy 2003;83(1):17‐28. [PubMed] [Google Scholar]
Jankovic 2008 {published data only}
- Jankovic A, Binic I. Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers. Archives of Dermatological Research 2008;300(7):377‐83. [DOI] [PubMed] [Google Scholar]
Jia 2015 {published data only}
- Jia C, Su B, Gong L, Wang W, Zhang X. Encircling needling combined with physical factor therapy for severe pressure sore. Chinese Acupuncture and Moxibustion 2015;35(11):1131‐4. [PubMed] [Google Scholar]
Karsli 2017 {published data only}
- Karsli PB, Gurcay E, Karaahmet OZ, Cakci A. High‐voltage electrical stimulation versus ultrasound in the treatment of pressure ulcers. Advances in Skin & Wound Care 2017;30(12):565‐70. [DOI] [PubMed] [Google Scholar]
Koel 2014 {published data only}
- Koel G, Houghton PE. Electrostimulation: current status, strength of evidence guidelines, and meta‐analysis. Advances in Wound Care 2014;3(2):118‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawson 2007 {published data only}
- Lawson D, Petrofsky JS. A randomized control study on the effect of biphasic electrical stimulation in a warm room on skin blood flow and healing rates in chronic wounds of patients with and without diabetes. Medical Science Monitor 2007;13(6):CR258‐63. [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee BY, Wendell K, Al‐Waili N, Butler G. Ultra‐low microcurrent therapy: a novel approach for treatment of chronic resistant wounds. Advances in Therapy 2007;24(6):1202‐9. [DOI] [PubMed] [Google Scholar]
Lippert‐Gruner 2003 {published data only}
- Lippert‐Gruner M. Gluteal neuromuscular stimulation in therapy and prophylaxis of recurrent sacral pressure ulcers. Spinal Cord 2003;41(6):365‐6. [DOI] [PubMed] [Google Scholar]
Polak 2014 {published data only}
- Polak A, Franek A, Taradaj J. High‐voltage pulsed current electrical stimulation in wound treatment. Advances in Wound Care 214;3(2):104‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Recio 2012 {published data only}
- Recio AC, Felter CE, Schneider AC, McDonald JW. High‐voltage electrical stimulation for the management of stage III and IV pressure ulcers among adults with spinal cord injury: demonstration of its utility for recalcitrant wounds below the level of injury. Journal of Spinal Cord Medicine 2012;35(1):58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stefanovska 1993 {published data only}
- Stefanovska A, Vodovnik L, Benku H, Turk R. Treatment of chronic wounds by means of electric and electromagnetic fields. Part 2: value of FES parameters for pressure sore treatment. Medical and Biological Engineering and Computing 1993;31(3):213‐20. [DOI] [PubMed] [Google Scholar]
Sugimoto 2012 {published data only}
- Sugimoto M, Maeshige N, Honda H, Yoshikawa Y, Uemura M, Yamamoto M, et al. Optimum microcurrent stimulation intensity for galvanotaxis in human fibroblasts. Journal of Wound Care 2012;21(1):5‐10. [DOI] [PubMed] [Google Scholar]
Trontelj 1994 {published data only}
- Trontelj K, Karaba R, Vodovnik L, Savrin R, Strukelj MP. Treatment of chronic wounds by low frequency pulsed electrical current. Journal of Tissue Viability 1994;4(4):105‐9. [Google Scholar]
Ullah 2007 {published data only}
- Ullah MO. A study to detect the efficacy of micro‐current electrical therapy on decubitus wound. Journal of Medical Sciences 2007;7(8):1320‐4. [Google Scholar]
Van Londen 2008 {published data only}
- Londen A, Herwegh M, Zee CH, Daffertshofer A, Smit CA, Niezen A, et al. The effect of surface electric stimulation of the gluteal muscles on the interface pressure in seated people with spinal cord injury. Archives of Physical Medicine and Rehabilitation 2008;89(9):1724‐32. [DOI] [PubMed] [Google Scholar]
Wolcott 1969 {published data only}
- Wolcott LE, Wheeler PC, Hardwicke HM, Rowley BA. Accelerated healing of skin ulcer by electrotherapy: preliminary clinical results. Southern Medical Journal 1969;62(7):795‐801. [DOI] [PubMed] [Google Scholar]
Yoshikawa 2015 {published data only}
- Yoshikawa Y, Maeshige N, Sugimoto M, Uemura M, Fujishima R, Shuntoh H. The effects of direct micro‐current stimulation on the proliferation of human dermal fibroblasts. Physiotherapy 2015;101(Suppl 1):e1555‐6. [Google Scholar]
References to studies awaiting assessment
Feldman 2005 {published data only}
- Feldman D, Andino RV, Jennings JA. Clinical evaluation of an electrical stimulation bandage (Posifect Dressing). Abstract 037. Wound Repair and Regeneration. Fifteenth Annual Meeting and Exhibition of the Wound Healing Society 2005;13(2):A13. [Google Scholar]
- Feldman D, Jennings A, Andino R. Preliminary evaluation of an electrical stimulation bandage (POSiFECT dressing). Abstract 242. Wound Repair and Regeneration 2006;14(1):A29. [Google Scholar]
Karba 1997 {published data only}
- Karba R, Semrov D, Vodovnik L, Benko H, Savrin R. DC electrical stimulation for chronic wound healing enhancement. Part 1. Clinical study and determination of electrical field distribution in the numerical wound model. Bioelectrochemistry and Bioenergetics 1997;43(2):265‐70. [Google Scholar]
References to ongoing studies
ACTRN12617001534370 {published data only}
- ACTRN12617001534370. Effects of pulsed currents applied by cathode and anode in the treatment of category I pressure ulcers. www.anzctr.org.au/ACTRN12617001534370.aspx (first registered 6 November 2017).
ACTRN12618000345280 {published data only}
- ACTRN12618000345280. Influence of low voltage monophasic pulsed current and low voltage biphasic pulsed current on pressure ulcer healing based on clinical treatment effects and basic research. www.anzctr.org.au/ACTRN12618000345280.aspx (first registered 7 March 2018).
JPRN‐UMIN000029516 {published data only}
- JPRN‐UMIN000029516. The effect of electrical stimulation on pressure injuries: double‐blind controlled cross over study. (Verification by double blind crossover comparison test). upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000033714 (first registered 13 October 2017).
NCT03753581 {published data only}
- NCT03753581. Effectiveness of microcurrents therapy in pressure ulcers in elderly people. clinicaltrials.gov/show/NCT03753581 (first registered 27 November 2018).
NTR6450 {published data only}
- NTR6450. Wireless micro current stimulation: adjunctive therapy for hard‐to‐heal chronic wounds – a double‐blind, placebo controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR6450 (first registered 15 May 2017).
Additional references
Allman 1989
- Allman RM. Epidemiology of pressure sores in different populations. Decubitus 1989;2(2):30‐3. [PubMed] [Google Scholar]
Alvarez 1983
- Alvarez OM, Mertz PM, Smerbeck RV, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. Journal of Investigative Dermatology 1983;81(2):144‐8. [DOI] [PubMed] [Google Scholar]
Armstrong 2011
- Armstrong R, Waters E, Doyle J. Chapter 21: Reviews in health promotion and public health. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
AWMA 2012
- Australian Wound Management Association (AWMA). Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury. www.awma.com.au/publications/2012_AWMA_Pan_Pacific_Abridged_Guideline.pdf (accessed 5 January 2016).
Barnes 2014
- Barnes R, Shahin Y, Gohil R, Chetter I. Electrical stimulation vs. standard care for chronic ulcer healing: a systematic review and meta‐analysis of randomised controlled trials. European Journal of Clinical Investigation 2014;44(4):429‐40. [DOI] [PubMed] [Google Scholar]
Bates‐Jensen 1992
- Bates‐Jensen BM, Vredevoe DL, & Brecht ML. Validity and reliability of the Pressure Sore Status Tool. Decubitus 1992;5(6):20‐8. [PubMed] [Google Scholar]
Bogie 2000
- Bogie KM, Reger SI, Levine SP, Sahgal V. Electrical stimulation for pressure sore prevention and wound healing. Assistive Technology 2000;12(1):50‐66. [DOI] [PubMed] [Google Scholar]
Bourguignon 1987
- Bourguignon GJ, Bourguignon LY. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB Journal 1987;1(5):398‐402. [DOI] [PubMed] [Google Scholar]
Brem 2010
- Brem H, Maggi J, Nierman D, Rolnitzky L, Bell D, Rennert R, et al. High cost of stage IV pressure ulcers. American Journal of Surgery 2010;200(4):473‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Consortium for Spinal Cord Medicine 2014
- Consortium for Spinal Cord Medicine. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health‐care professionals. Second edition. Washington DC: Paralyzed Veterans of America; 2014 August. Clinical practice guideline: pressure ulcer. Available from www.pva.org/atf/cf/%7BCA2A0FFB‐6859‐4BC1‐BC96‐6B57F57F0391%7D/CPG_Pressure%20Ulcer.pdf.
Cowan 2019
- Cowan LJ, Ahn H, Flores M, Yarrow J, Barks LS, Garvan C, et al. Pressure ulcer prevalence by level of paralysis in patients with spinal cord injury in long‐term care. Advances in Skin and Wound Care 2019;32(3):122‐30. [DOI] [PubMed] [Google Scholar]
Cruz 1989
- Cruz NI, Bayron FE, Suarez AJ. Accelerated healing of full‐thickness burns by the use of high‐voltage pulsed galvanic stimulation in the pig. Annals of Plastic Surgery 1989;23(1):49‐55. [PubMed] [Google Scholar]
Cullum 2001
- Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technology Assessment 2001;5(9):1‐221. [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Altman DG, Elbourne D. Meta‐analysis combining parallel and cross‐over clinical trials. I: continuous outcomes. Statistics in Medicine 2002;21(15):2131‐44. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Demets 1987
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Fakhri 1987
- Fakhri O, Amin MA. The effect of low‐voltage electric therapy on the healing of resistant skin burns. Journal of Burn Care and Rehabilitation 1987;8(1):15‐18. [DOI] [PubMed] [Google Scholar]
Forchheimer 2004
- Forchheimer M, McAweeney M, Tate DG. Use of the SF‐36 among persons with spinal cord injury. American Journal of Physical Medicine and Rehabilitation 2004;83(5):390‐5. [DOI] [PubMed] [Google Scholar]
Foulds 1983
- Foulds IS, Barker AT. Human skin battery potentials and their possible role in wound healing. British Journal of Dermatology 1983;109(5):515‐22. [DOI] [PubMed] [Google Scholar]
Gardner 1999
- Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta‐analysis. Wound Repair and Regeneration 1999;7(6):495‐503. [DOI] [PubMed] [Google Scholar]
Gardner 2005
- Gardner SE, Frantz RA, Bergquist S, Shin CD. A prospective study of the pressure ulcer scale for healing (PUSH). Journals of Gerontology. Series A: Biological Sciences and Medical Sciences 2005;60(1):93‐7. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hossain 2015
- Hossain MS, Rahman MA, Herbert RD, Quadir MM, Bowden JL, Harvey LA. Two‐year survival following discharge from hospital after spinal cord injury in Bangladesh. Spinal Cord 2016;54(2):132‐6. [DOI] [PubMed] [Google Scholar]
Houghton 2013
- Houghton PE, Campbell KE, CPG Panel. Canadian Best Practice Guidelines for the Prevention and Management of Pressure Ulcers in People with Spinal Cord Injury: a resource handbook for clinicians. Ontario Neurotrauma Foundation; 2013. Available from onf.org/system/attachments/168/original/Pressure_Ulcers_Best_Practice_Guideline_Final_web4.pdf.
Houghton 2014
- Houghton PE. Clinical trials involving biphasic pulsed current, microcurrent, and/or low‐intensity direct current. Advances in Wound Care 2014;3(2):166‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaltenthaler 2001
- Kaltenthaler E, Whitfield MD, Walters SJ, Akehurst RL, Paisley S. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare?. Journal of Wound Care 2001;10(1):530‐5. [DOI] [PubMed] [Google Scholar]
Kawasaki 2014
- Kawasaki L, Mushahwar VK, Ho C, Dukelow SP, Chan LL, Chan KM. The mechanisms and evidence of efficacy of electrical stimulation for healing of pressure ulcer: a systematic review. Wound Repair and Regeneration 2014;22(2):161‐73. [DOI] [PubMed] [Google Scholar]
Lala 2016
- Lala D, Spaulding SJ, Burke SM, Houghton PE. Electrical stimulation therapy for the treatment of pressure ulcers in individuals with spinal cord injury: a systematic review and meta‐analysis. International Wound Journal 2016; Vol. 13, issue 6:1214‐26. [DOI] [PMC free article] [PubMed]
Lampe 1998
- Lampe KE. Electrotherapy in tissue repair. Journal of Hand Therapy 1998;11(2):131‐9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook..
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 2009;151(4):W65‐94. [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu LQ, Moody J, Traynor M, Dyson S, Gall A. A systematic review of electrical stimulation for pressure ulcer prevention and treatment in people with spinal cord injuries. Journal of Spinal Cord Medicine 2014;37(6):703‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mittmann 2011
- Mittmann N, Chan BC, Craven BC, Isogai PK, Houghton P. Evaluation of the cost‐effectiveness of electrical stimulation therapy for pressure ulcers in spinal cord injury. Archives of Physical Medicine and Rehabilitation 2011;92(6):866‐72. [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
New 2004
- New PW, Rawicki HB, Bailey MJ. Non‐traumatic spinal cord injury rehabilitation: pressure ulcer patients, prediction, and impact. Archives of Physical Medicine and Rehabilitation 2004;85(1):87‐93. [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence (NICE). Pressure ulcers: prevention and management. NICE clinical guideline [CG179]; 2014 April. Available from www.nice.org.uk/guidance/cg179. [PubMed]
NPUAP/EPUAP 2014
- National Pressure Ulcers Advisory Panel (NPUAP) and European Pressure Ulcers Advisory Panel (EPUAP). Prevention and treatment of pressure ulcers: clinical practice guideline. www.npuap.org/resources/educational‐and‐clinical‐resources/prevention‐and‐treatment‐of‐pressure‐ulcers‐clinical‐practice‐guideline/ (accessed 5 January 2016).
Orida 1982
- Orida N, Feldman JD. Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motility 1982;2(3):243‐55. [DOI] [PubMed] [Google Scholar]
Perneger 2002
- Perneger TV, Rae AC, Gaspoz JM, Borst F, Vitek O, Heliot C. Screening for pressure ulcer risk in an acute care hospital: development of a brief bedside scale. Journal of Clinical Epidemiology 2002;55(5):498‐504. [DOI] [PubMed] [Google Scholar]
Reddy 2008
- Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300(22):2647‐62. [DOI] [PubMed] [Google Scholar]
Regan 2009
- Regan MA, Teasell RW, Wolfe DL, Keast D, Mortenson WB, Aubut JA, Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Archives of Physical Medicine and Rehabilitation 2009;90(2):213‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rintala 2008
- Rintala DH, Garber SL, Friedman JD, Holmes SA. Preventing recurrent pressure ulcers in veterans with spinal cord injury: impact of a structured education and follow‐up intervention. Archives of Physical Medicine and Rehabilitation 2008;89(8):1429‐41. [DOI] [PubMed] [Google Scholar]
Rodriguez 1994
- Rodriguez GP, Garber SL. Prospective study of pressure ulcer risk in spinal cord injury patients. Paraplegia 1994;32(3):150‐8. [DOI] [PubMed] [Google Scholar]
Sackett 1989
- Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 1989;95(2 Suppl):2s‐4s. [PubMed] [Google Scholar]
Schoonhoven 2006
- Schoonhoven L, Grobbee DE, Donders AR, Algra A, Grypdonck MH, Bousema MT, et al. Prediction of pressure ulcer development in hospitalized patients: a tool for risk assessment. Quality and Safety in Health Care 2006;15(1):65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SCIRE 2014
- Hsieh J, McIntyre A, Wolfe D, Lala D, Titus L, Campbell K, et al. Pressure ulcers following spinal cord injury. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. 2014. scireproject.com/evidence/rehabilitation‐evidence/pressure‐ulcers/ (accessed 21 February 2017).
SIGN 2018
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 02 July 2018).
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sussman 1997
- Sussman C, Swanson G. Utility of the Sussman Wound Healing Tool in predicting wound healing outcomes in physical therapy. Advances in Wound Care 1997;10(5):74‐7. [PubMed] [Google Scholar]
Sussman 2012
- Sussman C. Chapter 23: Electrical stimulation for wound healing. In: Sussman C, Bates‐Jensen B editor(s). Wound Care. 4th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2012:594‐5. [Google Scholar]
Thakral 2013
- Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabetic Foot and Ankle 2013;16(4):22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vélez‐Díaz‐Pallarés 2015
- Vélez‐Díaz‐Pallarés M, Lozano‐Montoya I, Abraha I, Cherubini A, Soiza RL, O'Mahony D, et al. Nonpharmacologic interventions to heal pressure ulcers in older patients: an overview of systematic reviews (the SENATOR‐ONTOP series). Journal of the American Medical Directors Association 2015;16(6):448‐69. [DOI] [PubMed] [Google Scholar]
Welsh 2016
- Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, et al. the PRISMA‐Equity Bellagio group. Extending the PRISMA statement to equity‐focused systematic reviews (PRISMA‐E 2012): explanation and elaboration. Journal of Clinical Epidemiology 2016;70:68‐89. [DOI] [PubMed] [Google Scholar]
Whitehurst 2012
- Whitehurst DGT, Noonan VK, Dvorak MFS, Bryan S. A review of preference‐based health‐related quality of life questionnaires in spinal cord injury research. Spinal Cord 2012;50(9):646‐54. [DOI] [PubMed] [Google Scholar]
Woolrich 2006
- Woolrich RA, Kennedy P, Tasiemski T. A preliminary psychometric evaluation of the Hospital Anxiety and Depression Scale (HADS) in 963 people living with a spinal cord injury. Psychology, Health and Medicine 2006;11(1):80‐90. [DOI] [PubMed] [Google Scholar]
Zakrasek 2015
- Zakrasek EC, Creasey G, Crew JD. Pressure ulcers in people with spinal cord injury in developing nations. Spinal Cord 2015;53(1):7‐13. [DOI] [PubMed] [Google Scholar]
Zhao 2002
- Zhao M, Pu J, Forrester JV, McCaig CD. Membrane lipids, EGF receptors, and intracellular signals colocalize and are polarized in epithelial cells moving directionally in a physiological electric field. FASEB Journal 2002;16(8):857‐9. [DOI] [PubMed] [Google Scholar]
Üstün 2010
- Üstün TB, Kostanjsek N, Chatterji S, Rehm J. Measuring health and disability: manual for WHO Disability Assessment Schedule (WHODAS 2.0). Geneva: WHO Press 2010.
References to other published versions of this review
Arora 2016
- Arora M, Harvey LA, Glinsky JV, Nier L, Lavrencic L, Kifley A, et al. Electrical stimulation for treating pressure ulcers. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012196] [DOI] [PMC free article] [PubMed] [Google Scholar]


