Abstract
Introduction:
Pulse pressure variation (PPV) has been shown to be useful to predict fluid responsiveness in patients ventilated at tidal volume (Vt) >8 mL kg−1. Nevertheless, most conditions in critical care force to use lower Vt. Thus, we sought to evaluate the operative performance of PPV when a Vt ⩽8 mL kg−1 is used during mechanical ventilation support.
Methods:
We searched PubMed and Embase databases for articles evaluating the operative performance of PPV as a predictor of fluid responsiveness in critical care and perioperative adult patients ventilated with tidal volume ⩽8 mL kg−1 without respiratory effort and arrhythmias, between January 1990 and January 2019. We included cohort and cross-sectional studies. Two authors performed an Independently selection using predefined terms of search. The fitted data of sensitivity, specificity, and area under the curve (AUC) were assessed by bivariate and hierarchical analyses.
Results:
We retrieved 19 trials with a total of 777 patients and a total of 935 fluid challenges. The fitted sensitivity of PPV to predict fluid responsiveness during mechanical ventilation at Vt ⩽8 mL kg−1 was 0.65 (95% confidence interval [CI]: 0.57-0.73), the specificity was 0.79 (95% CI: 0.73-0.84), and the AUC was 0.75. The diagnostic odds ratio was 5.5 (95% CI: 3.08-10.01, P < .001) by the random-effects model.
Conclusions:
Pulse pressure variation shows a fair operative performance as a predictor of fluid responsiveness in critical care and perioperative patients ventilated with a tidal volume ⩽8 mL kg−1 without respiratory effort and arrhythmias.
Keywords: Critical care, hemodynamic, perioperative care, pulse pressure, sepsis, tidal volume
Introduction
Intravenous fluid resuscitation is a key piece in the management of patients with circulatory shock.1 Fluid loading aims to increase cardiac output (CO) to improve the convective transport of oxygen to the tissues. Nevertheless, fluids can be harmful when excessively administered.2 Indeed, higher fluid balances have been related to adverse clinical outcomes in septic shock,3 whereby strategies to prevent fluid overload are highly desirable and represent a priority in sepsis research.4
Prediction of fluid response could potentially avoid unnecessary volume load during resuscitation of circulatory shock. Several tools can be used to predict the increase in CO after a fluid load5 and potentially, some of these might improve clinical outcomes when incorporated as a part of treatment algorithms of intravenous fluid management.6-10 Pulse pressure variation (PPV) can predict fluid responsiveness in critically ill patients,11-13 and although with some limitations, it might better predict fluid responsiveness than stroke volume and systolic pressure variations.11
Mechanical ventilation with low tidal volumes is widely recommended in patients with acute respiratory distress syndrome14 and other many circumstances in critical care.15 Nevertheless, the operative performance of PPV may be substantially reduced when mechanical ventilation is set at low tidal volumes16 or when lung compliance is severely compromised17 because, under such conditions, the effects of mechanical ventilation on the cardiac extramural and intramural pressures are limited. Besides, at higher respiratory rates (RR) and low heart to RR ratios, the usefulness of PPV could also be limited18,19
Although several meta-analyses and systematic reviews have described the operative performance of PPV as a predictor of fluid responsiveness, the particular usefulness of PPV under Vt ⩽8 mL kg−1 and high heart rate to RR ratio is controversial. Thus, we propose to perform a meta-analysis and systematic review about the performance of PPV as a predictor of fluid responsiveness in adult patients ventilated at tidal volume ⩽8 mL kg−1 without arrhythmias and active respiratory efforts in the critical care and perioperative settings.
Methodology
Protocol
This systematic review was conducted by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations.20
Study selection and inclusion criteria
We look for prospective studies assessing the operative performance of PPV as a predictor of fluid responsiveness in the critical care and perioperative adult patients ventilated at Vt ⩽8 mL kg−1 without excessive respiratory efforts and arrhythmias. Only those in which PPV was used as a predictor of fluid responsiveness and revealing data about its operative performance were finally included for analysis. Indeed, an explicit definition of fluid responsiveness and the percentage of fluid response should also be revealed to be included. Only manuscripts written in English were selected. We did not include studies including patients younger than 18 years of age or pregnant women. We also did not include case reports, studies in abstract form, or studies conducted in animals.
Search strategy and data extraction
A highly sensitive search strategy was conducted in Embase and in MEDLINE using the PubMed interface from January 1990 to January 2019. We applied no restrictions apart of language restrictions (as previously described). Data extraction and eligibility assessment were performed independently in an unblinded, standardized manner by 2 reviewers (J.I.A.S. and J.D.C.R.). We used the following terms: (“pulse pressure variation” [All Field] OR “Fluid Challenge” [All field]) AND (respiration [MeSH] OR Respiration, Artificial [MeSH] OR Respiratory Distress Syndrome, Adult [MeSH] OR Tidal volume [MeSH] OR Lung Compliance [MeSH]) filtered by full text.
Study selection and data collection process
Two authors (J.I.A.S. and J.D.C.R.) reviewed titles independently and abstracts potentially eligible. Those studies fulfilling the inclusion criteria were pooled in a list, and then, the 2 files were compared to select those to be finally included for analysis. We also search for additional studies using the bibliography of previously chosen studies. Any disagreement between the authors was resolved through discussion; if it continued, a third author reviewed the article and facilitated a consensus among all review authors.
Data items
Data extracted from each clinical trial included authors, year of publication, type of population (critical or surgical) enrolled in the trial, type of study, number of patients enrolled, device or technique used to determinate PPV; type and volume of fluid used during the fluid loading; operational definition of intravenous fluid responsiveness; percentage of positive fluid response; cut-off point of PPV used; ventilatory settings (particularly tidal volume, RR, and lung compliance); finally, data about sensitivity, specificity, and area under the curve (AUC) reported for PPV.
Quality assessment
The quality of studies was assessed by the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) using 4 domains: patient selection, index test, reference standard, and flow and time. Each area was assessed for risk of bias, classified as “low,” “high,” or “unclear.” Besides, its risk of applicability had 3 domains: patient selection, index test, and reference standard, which were assessed as “low,” “high,” or “unclear.”21
Statistical analysis
Analysis of individual studies
The data of sensitivity, specificity, diagnostic odds ratio (DOR) were calculated by a contingency table.
Analysis of summary measures
The pooled data of sensitivity, specificity, DOR were assessed by the random-effects methods. The fitted data of sensitivity, specificity, and AUC were assessed by bivariate and hierarchical analyses. The summary receiver operating characteristic (ROC) curve was evaluated by the Rutter and Gatsonis method. An area under curve receiver operating (AUROC) greater than 0.7 would mean a fair operative performance.22
The heterogeneity of trials was assessed by Cochran Q statistics; its effects were quantified using inconsistency (I2). I2 greater than 50% would mean significant heterogeneity.23
Analysis of risk of bias across studies
Asymmetry was assessed by a contour-enhanced funnel plot and by the Thompson and Sharp tests. Publication bias was fitted by the trim-and-fill method.
Additional analysis
We performed a subgroup and meta-regression analysis to assess the association between clinical setting, lung compliance, variable measured to determine fluid responsiveness, a method for indices, type of fluid, hemodynamic endpoint, outlier studies, and tidal volume used, and DOR and Log-DOR. The threshold effect was assessed by Spearman rank correlation coefficient and by the Moses-Shapiro-Littenberg method.
The data were analyzed using R version 3.4.3 with the mada and meta packages. The data are expressed as a value (95% confidence interval [CI]). P < .05 was considered statistically significant.
Results
Study selection
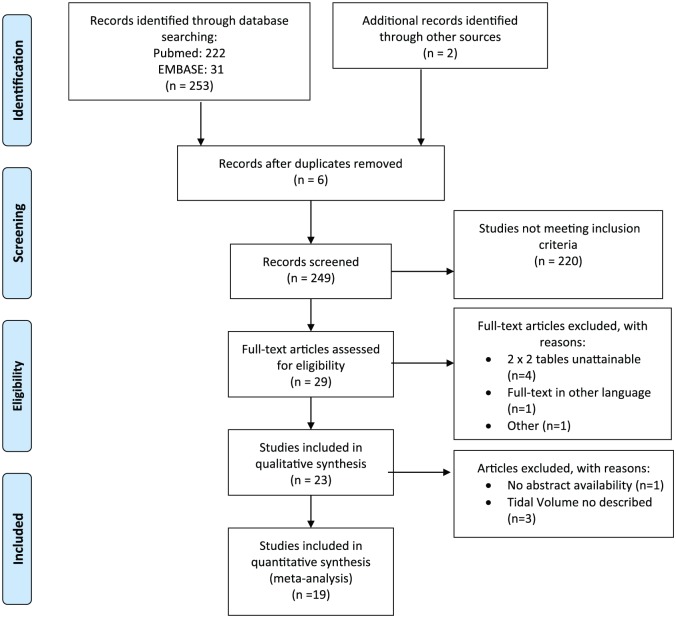
From a total of 255 studies, we finally retrieved 19 fulfilling all inclusion criteria and providing complete information about mechanical ventilation settings. The complete searching process is depicted in Figure 1.
Figure 1.
Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009).20
Study characteristics
A total of 19 studies were incorporated in the meta-analysis; these included 18 prospective cohort studies and 1 cross-sectional study for a total of 777 patients. General characteristics are summarized in Tables 1 and 2. A total of 935 fluid challenges were performed, with an average of fluid responsiveness of 51.51%. Seven studies were performed in a surgical setting, whereas 12 were conducted in the critical care setting. Eight studies used crystalloids, 8 used colloids, and 3 used both fluids during the fluid loads. Cardiac output was determined by different methods: pulmonary artery catheter (PAC) (n = 7), Pulse Contour Cardiac Output (PiCCO and PiCCO2) (n = 7), ProAQT (n = 2), PAC or PiCCO (n = 2), and Lithium Dilution Cardiac Output (LiDCO) (n = 1). A positive response to fluids was considered when CO, stroke volume index, or cardiac index increase >15% in 15 studies and >10% in 4 studies.
Table 1.
Select characteristics of included studies.
| Order | Authors | Year | Setting | Type of study | Sample size | No. of fluid challenge | Method for indices | Infusion volume | Hemodynamic end point | Tidal volume, mL kg−1 | Compliance, mL cm H2O−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | De Backer et al.16 | 2005 | Critically ill patients | Prospective cohort | 33 | 33 | PAC | 1000 mL CR or 500 mL HES 10% | Increase in CI ⩾ 15% | 6.3 (6.0-7.1) | 26.0 (23.0-33.0) |
| 2 | Huang et al.24 | 2008 | Critically ill patients | Prospective cohort | 22 | 22 | PAC | 500 mL HES | Increase in CI ⩾ 15% | 6.4 (5.7-1)7. | 26.1 (18.5-33.7) |
| 3 | Auler et al.25 | 2008 | Post cardiac surgery | Prospective cohort | 59 | 59 | PAC | 20 mL kg-1 LR | Increase in CI ⩾ 15% | 8.0 | NR |
| 4 | Vallée et al.26 | 2009 | Critically ill patients | Prospective cohort | 42 | 42 | PiCCO | 6 mL kg−1 HES | Increase in CI ⩾ 15% | 6.0 (6.2-7.3) | 27.0 (23.0-35.0) |
| 5 | Muller et al.18 | 2010 | Critically ill patients | Prospective cohort | 57 | 57 | PAC o PiCCO | 250 or 500 mL saline or HES | Increase in SVI ⩾ 15% | 6.0 (4.8-7.8) IBW | 28.0a |
| 6 | Lakhal et al.19 | 2011 | Critically ill patients | Prospective cohort? | 65 | 65 | PAC o PiCCO | 500 mL of gelatin | Increase in CO ⩾ 10% | 6.9 (5.95-7.85) PBW | 40.4 (24.6-56.2) |
| 7 | Oliveira-Costa et al.27 | 2012 | Critically ill patients | Cross-sectional observational | 37 | 37 | PAC | 1000 mL saline or LR or 500 mL HES 6% | Increase in CI ⩾ 15% | 6.5 (6.0-6.5) IBW | 34.0 (19.0-49.0) |
| 8 | Monnet et al.17 | 2012 | Critically ill patients | Prospective cohort | 28 | 28 | PiCCO2 | 500 mL saline | Increase in CI ⩾ 15% | 7.1 (6.3-7.9) PBW | 23.0 (20.0-26.0) |
| 9 | Yazigi et al.28 | 2012 | Post cardiac surgery | Prospective cohort | 60 | 60 | PAC | 7 mL kg−1 HES | Increase in SVI ⩾ 15% | 8.0 | NR |
| 10 | Cecconi et al.29 | 2012 | Post high-risk surgery | Prospective cohort | 31 | 47 | LiDCO | 250 mL colloid | Increase in SV ⩾ 15% | 8.0 IBW | NR |
| 11 | Freitas et al.30 | 2013 | Critically ill patients | Prospective cohort | 40 | 40 | PAC | 7 mL kg−1 HES | Increase in CO ⩾ 15% | 6.0 PBW | 31.0 (23.6-37.9) |
| 12 | Trepte et al.31 | 2013 | Intraoperative high-risk surgery | Prospective cohort | 24 | 72 | PiCCO2 | 300 mL HES | Increase in CI ⩾ 10% | 8.0 | NR |
| 13 | Song et al.32 | 2014 | Intraoperative—cardiac surgery | Prospective cohort | 40 | 40 | PAC | 6 mL kg−1 HES | Increase in SVI ⩾ 15% | 8.0 IBW | NR |
| 14 | Ibarra-Estrada et al.33 | 2015 | Critically ill patients | Prospective cohort | 19 | 59 | PiCCO | 7 mL kg−1 saline | Increase in SVI ⩾ 15% | 6.0 (6.0-6.3) PBW | NR |
| 15 | Liu et al.34 | 2016 | Critically ill patients | Prospective cohort | 96 | 96 | PiCCO | 500 mL saline | Increase in CO ⩾ 15% | 7.0 (6.2-7.8) mL kg−1 | 28.0 (15.9-40.1)a |
| 16 | Myatra et al.35 | 2017 | Critically ill patients | Prospective cohort | 20 | 30 | PiCCO | 7 mL kg-1 saline | Increase in CI ⩾ 15% | 6.0 (5.8-6.2) PWB | 29.0 (21.0-37.0) |
| 17 | Biais et al.36 | 2017 | Intraoperative—neurosurgery | Prospective cohort | 41 | 41 | ProAQT | 250 mL saline | Increase in SVI ⩾ 10% | 6.8 (6.3-7.3) ± IBW | 38.0 (28.0-48.0) |
| 18 | Biais et al.37 | 2017 | Intraoperative—neurosurgery | Prospective cohort | 44 | 88 | ProAQT | 250 mL saline | Increase in SVI ⩾ 10% | 6.9 (6.5-7.2) IBW | 42.2a |
| 19 | Yonis et al.38 | 2017 | Critically ill patients | Prospective cohort | 19 | 19 | PiCCO | 500 mL LR | Increase in CI ⩾ 15% | 6.0 PBW | 30.0 (23.0-39.0) |
Abbreviations: CI, cardiac index; CO, cardiac output; CR, crystalloid; HES, hydroxyethyl starch; IBW, ideal body weight; LR, Ringer’s lactate; NA, not available; NR: not reported; PAC, pulmonary artery catheter; PBW, predicted body weight; SV, stroke volume; SVI, stroke volume index; PPV, pulse pressure variation.
Values are expressed as pooled value (95% confidence interval) or median (IQR).
Calculated.
Table 2.
Diagnostic performance of pulse pressure variation for prediction of fluid responsiveness in patients with tidal volume ⩽8 mL kg−1 from included studies.
| Order | Authors | Year | tp | n1 | tn | n2 | nt | Sensitivity | Specificity | AUC | Threshold, % | Method use to measure PPV | Fluid responsiveness rate, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | De Backer et al.16 | 2005 | 7 | 18 | 10 | 15 | 33 | 0.39 (0.20-0.61) | 0.65 (0.42-0.85) | 0.71 (0.62-0.80) | PPV ⩾ 12 | Analysis of the monitored arterial tracing | 55.00a |
| 2 | Huang et al.24 | 2008 | 7 | 10 | 11 | 12 | 22 | 0.70 (0.40-0.89) | 0.92 (0.65-0.99) | 0.76 | PPV ⩾ 11.8 | Analysis of the monitored arterial tracing | 45.45 |
| 3 | Auler et al.25 | 2008 | 38 | 39 | 19 | 20 | 59 | 0.97 (0.87-1.00) | 0.95 (0.76-0.99) | 0.98 (0.97-0.99) | PPV ⩾ 12 | Analysis of the monitored arterial tracing | 66.10 |
| 4 | Vallée et al.26 | 2009 | 6 | 19 | 17 | 23 | 42 | 0.32 (0.15-0.54) | 0.74 (0.54-0.87) | 0.63 (0.45-0.81) | PPV ⩾ 15 | Analysis of the monitored arterial tracing | 46.42a |
| 5 | Muller et al.18 | 2010 | 25 | 41 | 15 | 16 | 57 | 0.61 (0.46-0.74) | 0.94 (0.72-0.99) | 0.77 (0.65-0.90) | PPV ⩾ 7 | Analysis of the monitored arterial tracing | 72.00 |
| 6 | Lakhal et al.19 | 2011 | 19 | 26 | 33 | 39 | 65 | 0.73 (0.54-0.86) | 0.85 (0.70-0.93) | 0.75 (0.62-0.85) | PPV ⩾ 5 | Analysis of the monitored arterial tracing | 40.00 |
| 7 | Oliveira-Costa et al.27 | 2012 | 9 | 17 | 19 | 20 | 37 | 0.53 (0.31-0.74) | 0.95 (0.76-0.99) | 0.74 (0.56-0.90) | PPV ⩾ 10 | Analysis of the monitored arterial tracing | 44.73 |
| 8 | Monnet et al.17 | 2012 | 14 | 15 | 4 | 13 | 28 | 0.93 (0.79-99)0. | 0.31 (0.13-58)0. | 0.69 (0.68-70)0. | PPV ⩾ 4 | Analysis with PiCCO2 | 53.57 |
| 9 | Yazigi et al.28 | 2012 | 33 | 41 | 14 | 19 | 60 | 0.80 (0.66-0.90) | 0.74 (0.51-0.88) | 0.85 | PPV >11.5 | Analysis of the monitored arterial tracing | 68.33 |
| 10 | Cecconi et al.29 | 2012 | 10 | 12 | 14 | 19 | 31 | 0.83 (0.55-0.95) | 0.74 (0.51-0.88) | 0.87 (0.76-0.99) | PPV >13 | Analysis with LiDCO | 39.00 |
| 11 | Freitas et al.30 | 2013 | 17 | 19 | 19 | 21 | 40 | 0.89 (0.69-0.97) | 0.90 (0.71-0.97) | 0.91 (0.82-1.0) | PPV ⩾ 6.5 | Analysis with computer software | 47.50 |
| 12 | Trepte et al.31 | 2013 | 25 | 41 | 25 | 31 | 72 | 0.61 (0.46-0.74) | 0.81 (0.64-0.91) | 0.70 (0.21-0.85) | PPV ⩾ 10.1 | Analysis with PiCCO2 | 57.00 |
| 13 | Song et al.32 | 2014 | 17 | 23 | 12 | 17 | 40 | 0.74 (0.54-0.87) | 0.71 (0.47-0.87) | 0.74 (0.58-0.90) | PPV ⩾ 13 | Analysis with computer software | 57.50 |
| 14 | Ibarra-Estrada et al.33 | 2015 | 15 | 30 | 23 | 29 | 59 | 0.5 (0.33-0.67) | 0.79 (0.62-0.90) | 0.63 | PPV ⩾ 14 | Analysis of the monitored arterial tracing | 50.80 |
| 15 | Liu et al.34 | 2016 | 35 | 52 | 37 | 44 | 96 | 0.67 (0.54-0.78) | 0.84 (0.71-0.92) | 0.78 (0.69-0.86) | PPV ⩾ 10 | Analysis with PiCCO | 54.16 |
| 16 | Myatra et al.35 | 2017 | 12 | 16 | 13 | 14 | 30 | 0.75 (0.51-90)0. | 0.93 (0.69-99)0. | 0.91 (0.81-0)1. | PPV ⩾ 11.5 | Analysis with computer software | 53.33 |
| 17 | Biais et al.36 | 2017 | 12 | 20 | 18 | 21 | 41 | 0.60 (0.36-0.78) | 0.86 (0.65-0.95) | 0.75 (0.60-0.90) | PPV >9 | Analysis with computer ProAQT | 48.78 |
| 18 | Biais et al.37 | 2017 | 15 | 28 | 41 | 60 | 88 | 0.54 (0.39-0.78 | 0.68 (0.56-0.79) | 0.65 (0.53-0.78) | PPV >10 | Analysis with computer Pulsioflex | 31.81 |
| 19 | Yonis et al.38 | 2017 | 3 | 9 | 8 | 10 | 19 | 0.33 (0.12-0.65) | 0.8 (0.49-0.94) | 0.49 (0.21-0.77) | PPV >10 | Analysis with computer software | 47.36 |
Abbreviations: tn, true negative; tp, true positive; PPV, pulse pressure variation.
Values are expressed as pooled value (95% confidence interval). AUC; area under curve; n1, number of patients who were positive fluid responsiveness; n2, number of patients who were negative fluid responsiveness; nt, number total of patients included.
Calculated.
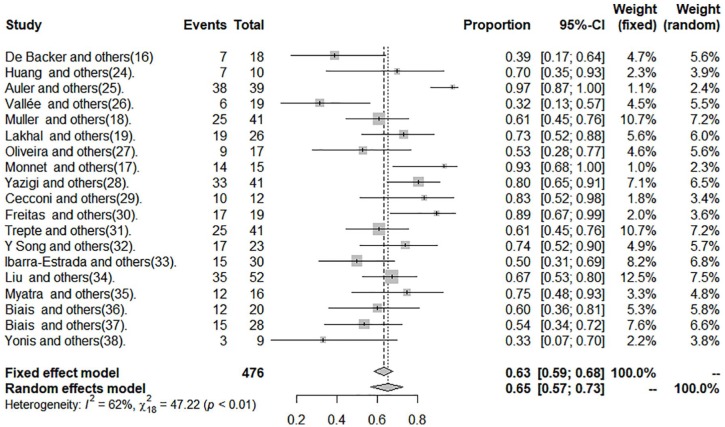
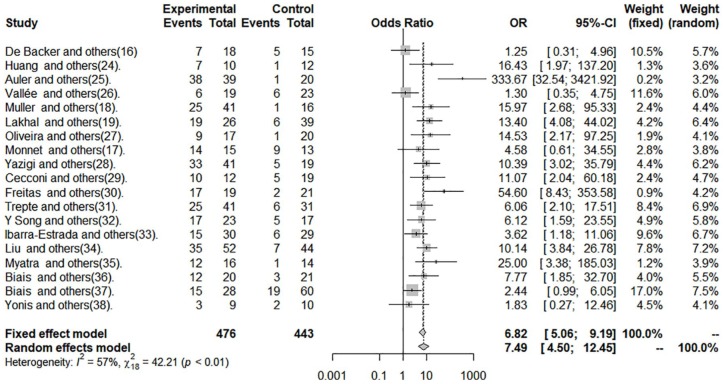
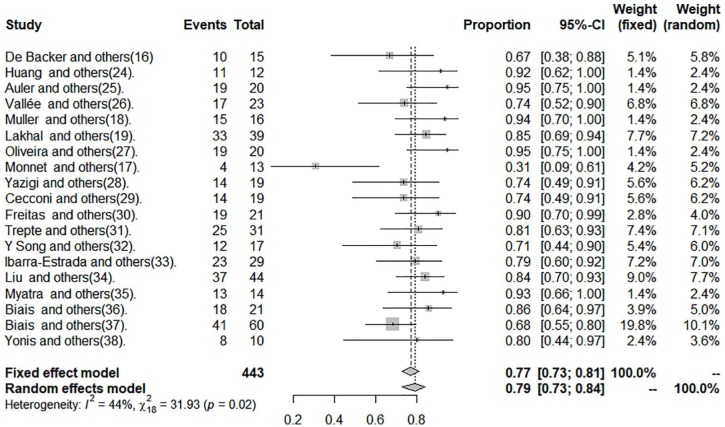
The data of sensitivity, specificity, and DOR are shown in Figures 2 to 4 and in Table 2.
Figure 2.
Sensitivity of pulse pressure variation in patients with tidal volume ⩽8 mL kg−1.
Figure 4.
Diagnostic odds ratio of pulse pressure variation in patients with tidal volume ⩽8 mL kg−1.
Figure 3.
Specificity of pulse pressure variation in patients with tidal volume ⩽8 mL kg−1.
Risk of bias within studies
The risk of bias was low in most of the included studies. Two studies were at high risk of bias in the item of patient selection and flow and timing (see additional Supplemental Table 1).
Syntheses of results
The cut-off point average of PPV was 10.28%. Pooled sensitivity was 0.65 (95% CI: 0.57-0.73) by the random-effects model. The pooled specificity was 0.79 (95% CI: 0.73-0.84) by the random-effects model. The pooled DOR was 7.49 (95% CI: 4.50-12.45) by the random-effects model. The studies revealed moderate heterogeneity (Q = 42.21, degrees of freedom [df] = 18, P = .001; I2 = 57.4, 95% CI: 28.9-74.4). Fitted sensitivity was 0.65 (95% CI: 0.57-0.73, P < .01), whereas the fitted specificity was 0.79 (95% CI: 0.73-0.84, P < .001). The AUC was 0.75 (Figure 5).
Figure 5.
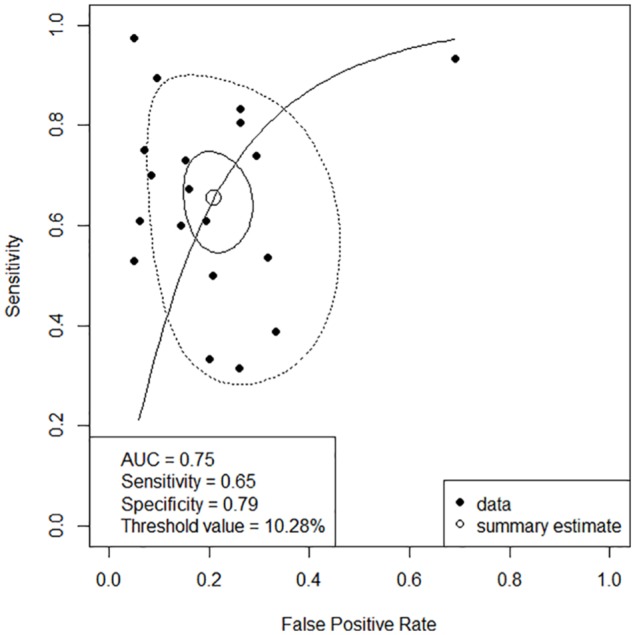
Summary ROC curve. Solid closed curve: 95% confidence region; dotted closed curve: 95% prediction region; solid line: summary ROC curve; open circle: summary estimate; close circle: study.
Risk of bias across studies
Two studies showed a specificity of 100%,24,35 and 1 study showed a sensitivity of 100%.17 Their specificity and sensitivity needed to decrease to values nearer to 0.9 to calculate their standard error and perform an asymmetry analysis. We found asymmetry in the contour-enhanced funnel plot (Figure 6), and it was statistically significant (P < .01). The asymmetry was by publication bias; we found 4 studies with P > .1 versus 15 studies with P < .1, and then the asymmetry was fitted by the trim-and-fill method. We found a fitted DOR by the random-effects model (5.5; 95% CI: 3.08-10.01, P < .001; Figure 7).
Figure 6.
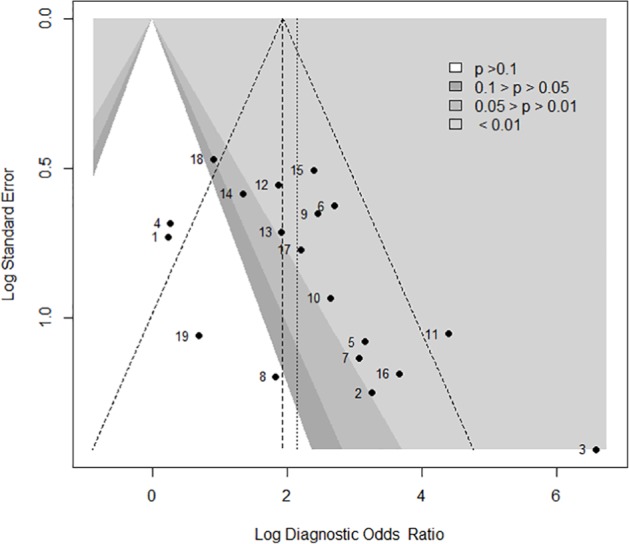
Contour enhanced funnel plot for a meta-analysis of pulse pressure variation for prediction of fluid responsiveness in patients with tidal volume ⩽8 mL kg−1. Filled circles show an estimated treatment effect (Log diagnostic odds ratio) and its precision (standard error). In addition to individual study results, the fixed-effect estimates (vertical dashed line) with 95% confidence interval limits (diagonal dashed lines) and the random-effects estimate (vertical dotted line) are shown in the figure.
Figure 7.
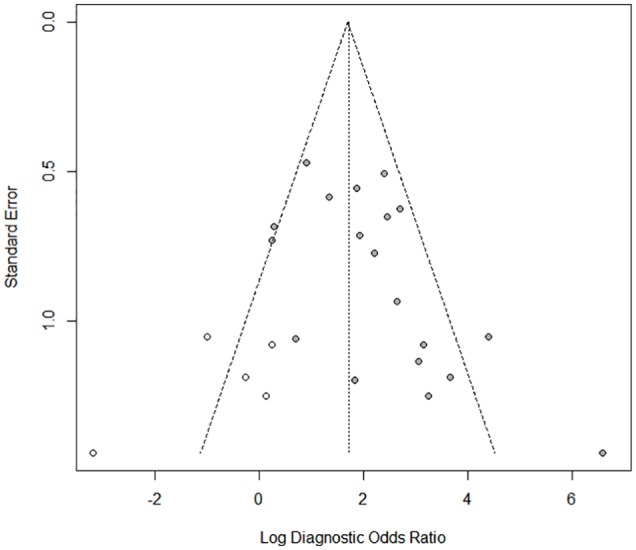
Funnel plot for meta-analysis analysis of pulse pressure variation for prediction of fluid responsiveness in patients with tidal volume ⩽8 mL kg−1 after applying the trim-and-fill method. Filled circles in the figure show trials included in the meta-analysis, whereas open circles in the figure show unpublished trials.
Additional analysis
When we performed a subgroup and meta-regression analysis, we found that lung compliance was associated with an improvement in its operative performance (DOR = 13.14 [95% CI: 6.48-26.65, P = .058 by random-effects, P = .03 by fixed-effect]); see additional Supplemental Table 2. Neither clinical setting nor tidal volume was associated with a change in its operative performance. Also, We found 2 outlier studies25,30; these were a source of heterogeneity.
We did not find a correlation between sensitivity and specificity among the studies included (ρ = 0.11, P = .63). Moreover, the slope found by the Moses-Shapiro-Littenberg method was not statistically significant (P = .465).
Discussion
This systematic review and meta-analysis suggest a fair operative performance of PPV on fluid responsiveness in patients mechanically ventilated at Vt ⩽8 mL kg−1.
Respiratory variations in stroke volume have been shown to be associated with preload dependency in mechanically ventilated patients without spontaneous breathing efforts. Pulse pressure variation could be considered, in some extent, a surrogate of stroke volume variation (SVV), and it might predict fluid responsiveness better than static indices of cardiac preload.39-41 However, SVV and PPV are generated by respiratory-induced variations, which might be limited when mechanical ventilation is provided at low tidal volumes. Thus, the reliability of PPV could be theoretically limited in mechanically ventilated patients with increased lung elastance or under conditions in which mechanical ventilation at low tidal volume is selected. Several meta-analyses assessed the operative performance of PPV as a predictor of intravenous fluid responsiveness.11-13 Nevertheless, studies incorporated in such meta-analyses included patients using a wide variety of tidal volumes. For example, Marik and collaborators included patients with Vt >7 mL kg−1, whereas other authors included mechanically ventilated patients at Vt from 4.9 to 12 mL kg−1 and even >8 mL kg−1.12,13 Conversely, we focused on studies including patients ventilated at Vt ⩽8 mL kg−1, and we also extended the search to the perioperative setting. As suggested by our results, PPV exhibits a fair operative performance in mechanically ventilated patients at such tidal volumes. Surprisingly, such operative performance was not as bad as expected, which suggests that some patients included in the studies should be highly preload dependent. Thus, although some physicians could consider the sensitivity and specificity of the PPV as low in this clinical setting, PPV can retain some capacity to predict fluid responsiveness in cases of a high preload dependence in patients ventilated with a tidal volume ⩽8 mL kg−1.
Variations in Vt influences PPV.42 De Backer et al suggested that low Vt (⩽8 mL kg−1) decreases the operative performance of PPV to predict fluid responsiveness,16 whereas other studies have shown different results.24,26,28,30 Our meta-analysis confirms a fair performance of PPV to predict fluid responsiveness when mechanical ventilation is set at Vt ⩽8 mL kg−1. However, some strategies could improve such performance, and although these are out of the scope of this meta-analysis, we can mention the adjusting of PPV by the changes in pleural pressure34 and the use of “tidal volume challenges” consisting in transitory increases in Vt to evaluate variations in PPV.35 Nevertheless, such transitory increments of Vt could be not harm-free, and there are no broad data confirming its reliability.
So, the practical question would be, “What can we do to predict fluid responsiveness in mechanically ventilated patients with tidal volumes lower than 8 mL kg−1?” One possibility would be using low cut-offs for PPV to identify responders and nonresponders such as suggested by De Backer et al.16 However, lower cut-offs might be more profoundly influenced by small errors in measurements. Another possibility might be to consider the traditional cut-off values (ie, 12%), expecting a low sensitivity but a convenient specificity.24 Unfortunately, data showed in the studies included in our meta-analysis do not provide sufficient information to conduct additional analysis using different cut-off points.
Significant limitations or advantages can result from different statistical strategies to perform meta-analyses, comparing the efficacy of diagnostic tests.43,44 We found moderate heterogeneity between studies, which decreased when we removed 2 outlier ones. Importantly, we did not find another source to clinical heterogeneity by analysis of subgroups and meta-regression; also, we did not find a threshold effect and methodological heterogeneity because, within the quality assessment of the included studies, the risk of bias was low in most of them. All of these reflect the strength of our results.
Our meta-analysis has some limitations. First, most studies included represent small and heterogeneous populations. Second, most of such studies did not evaluate the coexistence of other limitations of PPV, such as right-heart failure, intra-abdominal hypertension, increased lung elastance, or even high RRs, which might limit the conclusions. Moreover, the information retrieved from these studies did not allow exploring other sources of clinical heterogeneity potentially influencing the operative performance of PPV as a predictor of fluid responsiveness in patients mechanically ventilated at Vt lower than 8 mL kg−1. Third, the information provided in the studies included is not enough to conduct new analysis searching for different cut-off points predicting fluid responsiveness in these particular and prevailing conditions.
Future investigations should resolve some questions about the predictors of fluid intravenous responsiveness in patients under protective ventilatory strategies and limited respiratory system and lung elastance.
Conclusions
Our meta-analysis shows a fair operative performance of PPV as a predictor of intravenous fluid responsiveness in critical care and perioperative patients ventilated with a tidal volume ⩽8 mL kg−1 without respiratory effort and arrhythmias.
Supplemental Material
Supplemental material, Table_1_additional for Use of Pulse Pressure Variation as Predictor of Fluid Responsiveness in Patients Ventilated With Low Tidal Volume: A Systematic Review and Meta-Analysis by Jorge Iván Alvarado Sánchez, Juan Daniel Caicedo Ruiz, Juan José Diaztagle Fernández, Gustavo Adolfo Ospina-Tascón and Luis Eduardo Cruz Martínez in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental material, Table_2_additional for Use of Pulse Pressure Variation as Predictor of Fluid Responsiveness in Patients Ventilated With Low Tidal Volume: A Systematic Review and Meta-Analysis by Jorge Iván Alvarado Sánchez, Juan Daniel Caicedo Ruiz, Juan José Diaztagle Fernández, Gustavo Adolfo Ospina-Tascón and Luis Eduardo Cruz Martínez in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JIAS contributed to design, performed the literature review, acquisition of data, statistical analysis, interpretation of data, and wrote the manuscript. JDCR contributed to design, performed the literature review, acquisition of data, interpretation of data, and wrote the paper. JJDF and GAO-T contributed to design, analysis of data, and wrote the article. LECM contributed to design, interpretation of data, and wrote the paper.
ORCID iD: Jorge Iván Alvarado Sánchez  https://orcid.org/0000-0003-4320-3150
https://orcid.org/0000-0003-4320-3150
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Vincent J, De Backer Circulatory shock. N Engl J Med. 2013;41:580-637. [DOI] [PubMed] [Google Scholar]
- 2. Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43:68-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakr Y, Rubatto Birri PN, Kotfis K, et al. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017; 45:386-394. [DOI] [PubMed] [Google Scholar]
- 4. Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Crit Care Clin. 2018;46:1334-1356. [DOI] [PubMed] [Google Scholar]
- 5. Alvarado Sanchez JI, Amaya Zuniga WF, Monge Garcia MI. Predictors to intravenous fluid responsiveness. J Intensive Care Med. 2018;33:227-240. [DOI] [PubMed] [Google Scholar]
- 6. Simmons RS, Berdine GG, Seidenfeld JJ, et al. Fluid balance and the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:924-929. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 1992;145:990-998. [DOI] [PubMed] [Google Scholar]
- 8. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564-2575. [DOI] [PubMed] [Google Scholar]
- 9. Bednarczyk JM, Fridfinnson JA, Kumar A, et al. Incorporating dynamic assessment of fluid responsiveness into goal-directed therapy. Crit Care Med. 2017;45:1538-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cecconi M, Corredor C, Arulkumaran N, et al. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642-2647. [DOI] [PubMed] [Google Scholar]
- 12. Hong J-Q, He H-F, Chen Z-Y, et al. Comparison of stroke volume variation with pulse pressure variation as a diagnostic indicator of fluid responsiveness in mechanically ventilated critically ill patients. Saudi Med J. 2014;35:261-268. [PubMed] [Google Scholar]
- 13. Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18:650-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walkey AJ, Del Sorbo L, Hodgson CL, et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome. Ann Am Thorac Soc. 2017;14:S297-S303. [DOI] [PubMed] [Google Scholar]
- 15. Serpa Neto A, Hemmes SNT, Barbas CSV, et al. Protective versus conventional ventilation for surgery. Anesthesiology. 2015;123:66-78. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000542-201507000-00019. [DOI] [PubMed] [Google Scholar]
- 16. De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517-523. [DOI] [PubMed] [Google Scholar]
- 17. Monnet X, Bleibtreu A, Ferre A, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40:152-157. [DOI] [PubMed] [Google Scholar]
- 18. Muller L, Louart G, Bousquet P-J, et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010;36:496-503. [DOI] [PubMed] [Google Scholar]
- 19. Lakhal K, Ehrmann S, Benzekri-Lefevre D, et al. Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Crit Care. 2011;15:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. PRISMA. Transparent reporting of systematic reviews and meta-analyses. PRISMA Checklist. http://prisma-statement.org/prismastatement/Checklist.aspx.Updated 2009.
- 21. Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155: 529-536. [DOI] [PubMed] [Google Scholar]
- 22. Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29: 1043-1051. [DOI] [PubMed] [Google Scholar]
- 23. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006. [DOI] [PubMed] [Google Scholar]
- 24. Huang C-C, Fu J-Y, Hu H-C, et al. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med. 2008;36:2810-2816. [DOI] [PubMed] [Google Scholar]
- 25. Auler JO, Jr, Galas F, Hajjar L, Santos L, Carvalho T, Michard F. Online monitoring of pulse pressure variation to guide fluid therapy after cardiac surgery. Anesth Analg. 2008;106:1201-1206. [DOI] [PubMed] [Google Scholar]
- 26. Vallée F, Richard JC, Mari A, et al. Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med. 2009;35:1004-1010. [DOI] [PubMed] [Google Scholar]
- 27. Oliveira-Costa C, Friedman G, Vieira S, Fialkow L. Pulse pressure variation and prediction of fluid responsiveness in patients ventilated with low tidal volumes. Clinics (Sao Paulo). 2012;67:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yazigi A, Khoury E, Hlais S, et al. Pulse pressure variation predicts fluid responsiveness in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2012;26:387-390. [DOI] [PubMed] [Google Scholar]
- 29. Cecconi M, Monti G, Hamilton MA, et al. Efficacy of functional hemodynamic parameters power analysis in surgical patients. Minerva Anestesiol. 2012;78:527-533. [PubMed] [Google Scholar]
- 30. Freitas FGR, Bafi AT, Nascente APM, et al. Predictive value of pulse pressure variation for fluid responsiveness in septic patients using lung-protective ventilation strategies. Br J Anaesth. 2013;110:402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trepte CJC, Eichhorn V, Haas SA, et al. Comparison of an automated respiratory systolic variation test with dynamic preload indicators to predict fluid responsiveness after major surgery. Br J Anaesth. 2013;111:736-742. [DOI] [PubMed] [Google Scholar]
- 32. Song Y, Kwak YL, Song JW, Kim YJ, Shim JK. Respirophasic carotid artery peak velocity variation as a predictor of fluid responsiveness in mechanically ventilated patients with coronary artery disease. Br J Anaesth. 2014;113:61-66. [DOI] [PubMed] [Google Scholar]
- 33. Ibarra-Estrada M, Lopez-Pulgarin JA, Mijangos-Mendez JC, Diaz-Gomez JL, Aguirre-Avalos G. Respiratory variation in carotid peak systolic velocity predicts volume responsiveness in mechanically ventilated patients with septic shock: a prospective cohort study. Crit Ultrasound J. 2015;7:29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Wei L, Li G, Yu X, Li G, Li Y. Pulse pressure variation adjusted by respiratory changes in pleural pressure, rather than by tidal volume, reliably predicts fluid responsiveness in patients with acute respiratory distress syndrome. Crit Care Med. 2016;44:342-351. [DOI] [PubMed] [Google Scholar]
- 35. Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul J. The changes in pulse pressure variation or stroke volume variation after a “tidal volume challenge” reliably predict fluid responsiveness during low tidal volume ventilation. Crit Care Med. 2017;45:415-421. [DOI] [PubMed] [Google Scholar]
- 36. Biais M, Larghi M, Henriot J, de Courson H, Sesay M, Nouette-Gaulain K. End-expiratory occlusion test predicts fluid responsiveness in patients with protective ventilation in the operating room. Anesth Analg. 2017;125:1889-1895. [DOI] [PubMed] [Google Scholar]
- 37. Biais M, de Courson H, Lanchon R, et al. Mini-fluid challenge of 100 ml of crystalloid predicts fluid responsiveness in the operating room. Anesthesiology. 2017;127:450-456. [DOI] [PubMed] [Google Scholar]
- 38. Yonis H, Bitker L, Aublanc M, et al. Change in cardiac output during Trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit Care. 2017;21:295-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic. Am J Respir Crit Care Med. 2000;162:134-138. [DOI] [PubMed] [Google Scholar]
- 40. Michard F, Teboul J. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000-2008. [DOI] [PubMed] [Google Scholar]
- 41. Michard F. Changes in arterial pressure during mechanical. Anesthesiology. 2005;103:34-36. [DOI] [PubMed] [Google Scholar]
- 42. Reuter D, Bayerlein J, Goepfert MSG, et al. Influence of tidal volume on left ventricular stroke volume variation measured by pulse contour analysis in mechanically ventilated patients. Intensive Care Med. 2003;29:476-480. [DOI] [PubMed] [Google Scholar]
- 43. Gatsonis C, Paliwal P. Meta-analysis of diagnostic and screening test accuracy evaluations: methodologic primer. AJR Am J Roentgenol. 2006;187:271-281. [DOI] [PubMed] [Google Scholar]
- 44. Botella J, Huang H. Procedimientos para realizar meta-análisis de la precisión de instrumentos de clasificación binaria. Psicothema. 2012;24:133-141. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Table_1_additional for Use of Pulse Pressure Variation as Predictor of Fluid Responsiveness in Patients Ventilated With Low Tidal Volume: A Systematic Review and Meta-Analysis by Jorge Iván Alvarado Sánchez, Juan Daniel Caicedo Ruiz, Juan José Diaztagle Fernández, Gustavo Adolfo Ospina-Tascón and Luis Eduardo Cruz Martínez in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental material, Table_2_additional for Use of Pulse Pressure Variation as Predictor of Fluid Responsiveness in Patients Ventilated With Low Tidal Volume: A Systematic Review and Meta-Analysis by Jorge Iván Alvarado Sánchez, Juan Daniel Caicedo Ruiz, Juan José Diaztagle Fernández, Gustavo Adolfo Ospina-Tascón and Luis Eduardo Cruz Martínez in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine