Abstract
The identification of driver mutations in epidermal growth factor receptor, anaplastic lymphoma kinase, the BRAF and ROS1 genes and subsequent successful clinical development of kinase inhibitors not only significantly improves clinical outcomes but also facilitates the discovery of other novel driver mutations in non-small cell lung cancer. These driver mutations can be categorized into mutations in or near the kinase domain, gene amplification or fusion. In this review, BRAF V600E, EGFR and HER-2 exon 20 mutation, FGFR1–4, K-RAS, MET, neuregulin-1, NRTK, PI3K/AKT/mTOR, RET and ROS1 gene aberration and their therapeutics will be discussed.
Keywords: BRAF mutation, driver mutations, EGFR exon 20, HER-2, K-RAS, non-small cell lung cancer (NSCLC), NRG, NTRK, MET amplification, MET exon 14
Introduction
Chemotherapy was the systemic therapy option in patients with treatment-naïve and pretreated, advanced or metastatic non-small cell lung (mNSCLC) until the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), gefitinib, erlotinib and afatinib, were shown to improve overall response rate (ORR), median progression-free survival (mPFS), safety and tolerability when compared with platinum-based chemotherapy in treatment-naïve patients with mNSCLC who harboured activating EGFR mutations (deletion in exon 19 and L858R point mutation in exon 21).1–8 Afatinib and dacomitinib are second-generation, irreversible EGFR-TKIs that bind covalently to both wild-type (WT) and mutated (EGFRm+), and have shown improved mPFS when compared with gefitinib in patients who were treatment-naive, EGFRm+ mNSCLC. Furthermore, dacomitinib demonstrated an improvement in median overall survival proceeding (mOS) in the population that did not have brain metastases.9,10 Osimertinib, a third-generation EGFR-TKI, which selectively inhibits EGFR-activating and exon 20 T790M-resistant mutations, is also reported to have superior ORR, mPFS and tolerability over gefitinib or erlotinib, in the patient population with or without brain metastases.11 In a press release from August 2019, osimertinib was reported to have a clinically meaningful improvement in mOS over gefitinib. The result was presented to the European Society of Medical Oncologists in September 2019.
Anaplastic lymphoma kinase translocation (ALK) was first identified in NSCLC by Soda and colleagues,12 which led to rapid clinical development of a number of ALK inhibitors (ALKi). Crizotinib was the first ALKi to demonstrate improvement in mPFS, tolerability and mOS over chemotherapy and to receive regulatory approval in both treatment-naïve and pretreated mNSCLC with ALK translocation.13–15 To date, ceritinib,16,17 alectinib18–20 and brigatinib21 have been shown to improve ORR and mPFS when compared with either chemotherapy or crizotinib in the ALKi-naïve or pretreated settings.
In addition, the combination of trametinib and dabrafenib in both treatment-naïve and previously treated patients with mutations in BRAF V600E and crizotinib in patients with ROS1 translocation have received regulatory approval throughout the world based on encouraging phase I–II data. Details of these studies are discussed below.
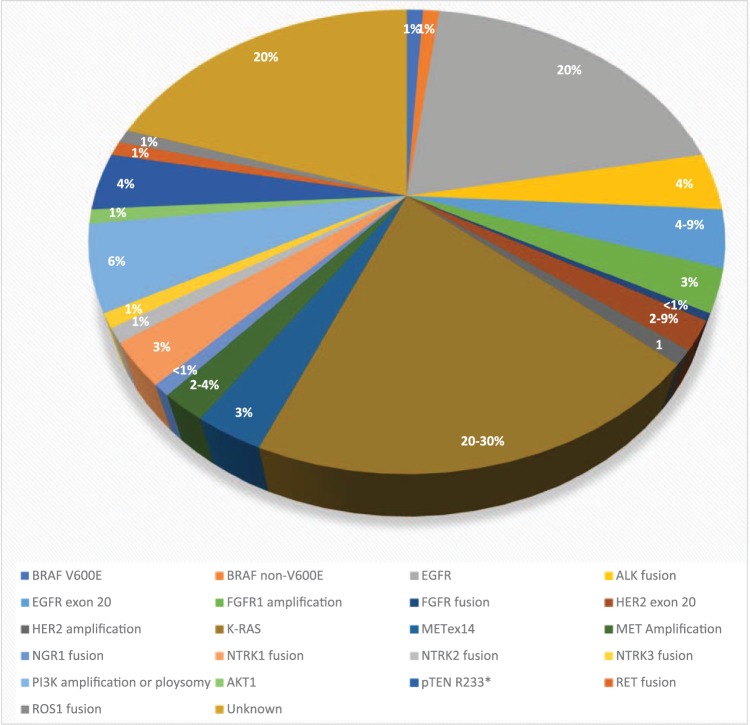
Advances in lung cancer therapeutics have led to the adaptation of comprehensive molecular profiling of known and novel driver mutations in mNSCLC, which may lead to the development of novel therapeutics that can further improve clinical outcomes.22–25 This review will provide an update on the clinical development of novel driver mutations, other than EGFR and ALK, in mNSCLC (Table 1, Figure 1).
Table 1.
Incidence, method of detection, and known secondary mutation in selected driver mutations.
| Driver mutation | Histology of NSCLC | Incidence | Method of detection | Secondary mutation |
|---|---|---|---|---|
| BRAF V600E non-V600E |
Adenocarcinoma | 1–2% 1–2% |
DNA sequencinga | Unknown |
| EGFR exon 20 | Adenocarcinoma | 4–19% | DNA sequencing | Unknown |
| FGFR FGFR1 amplification FGFR2–4 mutation gene fusion/translocation |
Squamous Adenocarcinoma Squamous Squamous Adenocarcinoma |
25% 2–3% 2–3% 0.3–3.5% 0.5% |
FISH or RNA NGSb
DNA sequencing FISH or RNA NGS or possibly IHCc |
Unknown Unknown Unknown |
| Her-2 Exon 20 Amplification overexpression |
Adenocarcinoma Adenocarcinoma Adenocarcinoma |
2–9% 1–2% Not reported |
DNA sequencing FISH or RNA NGS or possibly IHC IHC |
Unknown Loss of extracellular domain Unknown |
| K-RAS Mutation |
Adenocarcinoma | 20–30% | DNA sequencing | Unknown |
| MET METex14 mutation MET amplification |
Adenocarcinoma, particularly sarcomatoid subtype Adenocarcinoma |
3–4% 2–4% |
DNA sequencing or possibly IHC DNA sequencing, FISH or IHC |
Y1230C mutation WT KRAS amplification Unknown |
| NGR1 gene fusion/translocation |
Adenocarcinoma | 0.2–0.8% | FISH or RNA NGS, possibly IHC | Unknown |
| NTRK gene fusion/translocation mutation |
Adenocarcinoma Large cell neuroendocrine |
NTRK1: 3.5% NTRK2: 0.2–1% NTRK3: <1% NTRK 2 or 3: 10% |
FISH or RNA NGS or possibly IHC DNA sequencing |
Gatekeeper and solvent front mutation Unknown |
| PI3K pathway pTEN protein loss PI3K amplification PI3K mutation pTEN R233* mutation AKT1 |
Adenocarcinoma and squamous Adenocarcinoma and squamous Adenocarcinoma and squamous Adenocarcinoma and squamousAdenocarcinoma and squamous |
75% and 33% 6.2% 4% 4–7% 1% |
IHC FISH or RNA NGS DNA sequencing DNA sequencing DNA sequencing |
Unknown Unknown Unknown Unknown Unknown |
| RET gene fusion/translocation |
Adenocarcinoma | 1–2% | FISH or RNA NGS or possibly IHC | Gatekeeper or solvent front mutation Bypass pathway activation |
| ROS1 gene fusion/translocation |
Adenocarcinoma | 1–2% | FISH or RNA NGS or IHC | Gatekeeper or solvent front mutation Bypass pathway activation |
DNA sequencing includes direct sequencing, hot spot sequencing and DNA NGS.
RNA sequencing using NGS.
Validation study of the sensitivity and specificity of IHC relative to FISH or NGS for NTRK fusion and RET fusion are ongoing.
FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; WT, wild type.
Figure 1.
The distribution of various driver mutations in non-small cell lung cancer in Asian and White populations.
BRAF mutation
BRAF is an intracellular serine/threonine kinase that is activated by RAS, which, in turn, activates the downstream kinases, MEK and ERK (MAPK). BRAF mutation is identified in 50% of melanomas, 90% of which are of the subtype V600E.26 BRAF mutation is detected in 2–5% of mNSCLC and can be classified into V600E and non-V600E subtypes. The former occurs in 1–2% of all mNSCLC. Multiple studies on clinical characteristics of BRAF-mutated NSCLC have been reported. Not only is there no distinguishing clinical characteristics, there is also no consistent information on the benefit of chemotherapy and prognosis of BRAF mutation, except that 20–30% of patients with the V600E subtype are nonsmokers and all patients with the non-V600E subtype are heavy smokers.27–34
Joshi and colleagues demonstrated that the treatment of a V600E NSCLC cell line with vemurafenib led to G1 arrest and an increase in Bcl-2-like protein 11 (BIM), followed by apoptosis. In addition, co-administration with trametinib abrogated the upregulation of AKT activity in both V600E and non-V600E BRAF-mutant lung cancer cell lines. Dual inhibition of BRAF and MEK was also shown to prevent paradoxical reactivation of MAPK, resulting in greater antitumour activity than each single agent alone.35
Single-agent BRAF inhibition by either vemurafenib36 and dabrafenib37 demonstrated an ORR of 33–42% and mPFS of 5.5–7.3 months in previously treated BRAF-mutant NSCLC. Given the superior preclinical antitumour activity with concurrent BRAF and MEK inhibition, dabrafenib and trametinib were investigated in previously treated (n = 57)38 and untreated (n = 36),39 BRAF V600E mutation-positive mNSCLC. The respective ORRs determined by an independent review committee were 63.2% and 64%. The mPFS was 14.6 months in patients who were treatment-naïve and 9.7 months in those who were previously treated. The interim result of MyPathway study with vemurafenib in BRAF V600E and other patients who were BRAF positive, reported an ORR of 43% (n = 14) and 0% (n = 7), respectively.40 A phase II study of dabrafenib and trametinib in V600E mNSCLC is currently underway in South Korea (ClinicalTrials.gov identifier: NCT03543306).
Novel paradox-breaking BRAF inhibitors are currently in early clinical development. These inhibitors may have improved efficacy and tolerability as they bind both BRAF and CRAF monomers, homodimers and heterodimers without activating the MAPK pathway.41–44 BGB-283 is a novel inhibitor to WT ARAF, BRAF, CRAF, BRAF V600E and EGFR. The recommended phase II dose (RDII) was 40 mg daily in patients with BRAF or KRAS/NRAS-mutated solid tumours. A partial response (PR) was observed in one patient who was BRAF or MEK inhibitor-naïve, KRAS-mutated mNSCLC. Dose-limiting toxicity (DLT) was caused by thrombocytopenia. The majority of patients experienced grade 1 or 2 fatigue (68%), anorexia (48%), constipation (42%), thrombocytopenia (39%), nausea (39%), vomiting (39%), acneiform rash (39%), hand–foot syndrome (35%), hypertension (35%), and dysphonia (32%). Grade 3 and 4 toxicities were thrombocytopenia (13%), fatigue (10%) and liver dysfunction (10%).45 Janku and colleagues reported the phase I study of PLX8394 alone or in combination with cobistat, a CYP3A4 inhibitor, to increase the exposure of PLX8394, in refractory solid tumours. DLT for the combination was grade 3 elevation of aspartate transaminase (AST) and alanine transaminase (ALT). Other ⩾grade 3 treatment-related adverse events were diarrhoea (n = 1) and anorexia (n = 1). No BRAF inhibitor-related skin toxicity was reported. The combination of 900 mg of PLX8394 with 150 mg of cobistat was determined to be the RDII. PRs were reported in patients who were BRAF-inhibitor naïve, had V600E-positive gliomas or colorectal cancer. A phase II study in BRAF-mutated cancers is ongoing.46 Thus far, there are no clinical data demonstrating antitumour activity of BRAF and MEK inhibitors in patients with non-V600E mutations despite preclinical efficacy.
EGFR exon 20 insertion
EGFR exon 20 insertion occurs in 4–10% of mNSCLC47,48 and in patients with adenocarcinoma who are nonsmokers, Asian and female.49 This mutation involves the addition of 1–7 amino acids between residues 762 and 774 in the C-helix or regulatory domain of the kinase, leading to constitutive activation and dimerization of EGFR.50,51 Preclinical studies and clinical data demonstrated resistance of this heterogeneous mutation to gefitinib, erlotinib, afatinib and dacomatinib.50,52 Wu and colleagues reported a phase II trial of gefitinib in 16 evaluable patients with EGFR exon 20 mutation and only 4 patients had a response.53 In a retrospective series of over 1000 patients with EGFR mutation, only 1 out of 25 patients with exon 20 mutation responded to EGFR-TKIs.54 Naidoo and colleagues reported that the sensitivity of exon 20 mutation to EGFR-TKIs depended on the length and site of insertion. Those cases that harboured A703-Y704 insertion with FQEA amino acid residues were sensitive to EGFR-TKIs.55 A phase II study of neratinib, a second-generation, irreversible, EGFR, HER2 and HER4 inhibitor, demonstrated an ORR of 3% and mPFS of <3 months.56 In the post hoc analysis of LuxLung 2, 3 and 6 trials, response to afatinib was only observed in 8.7% of patients who harboured the exon 20 mutation.57 The phase II study of osimertinib in patients who are positive for EGFR exon 20 mutation is ongoing (ClinicalTrials.gov identifier: NCT03414814).
Poziotinib is an orally available, quinazoline, irreversible inhibitor to EGFR and HER2. Exon 20 mutation leads to steric hinderance to binding of currently available EGFR-TKIs. Based on its small size, poziotinib slips into the ATP pocket and inhibits EGFR signalling in preclinical models. Poziotinib may be less potent to exon 20 mutation with addition of single amino acid than currently available EGFR-TKIs.58 In 2018, the MD Anderson Cancer Center reported the preliminary result of a phase II study of poziotinib in 44 patients with EGFR exon 20 mutations. The ORR was 55% and the mPFS was 5.6 months. Grade 3 or higher toxicity occurred in 60% of patients, including skin rash (35%), diarrhoea (17.5%) and paronychia (9.5%). Overall, 45% of patients required dose reduction to 12 mg and 17.5% required further reduction to 8 mg. Response was reported irrespective of previous EGFR-TKI exposure.59 Another phase II study of poziotinib in treatment-naïve and previously treated EGFR exon 20 or HER-2 exon 20 mutation is ongoing (ClinicalTrials.gov identifier: NCT03318933).
TAK788 (AP32788) is another orally available, irreversible EGFR and HER-2 tyrosine kinase inhibitor with broad spectrum preclinical antitumour activity to 14 EGFR and 6 HER2 mutant variants, including 8 variants of exon 20 mutation at an IC50 < 26 nM.60 Doebele and colleagues presented the first report of the phase I study of TAK788 in advanced NSCLC. A total of 34 patients were treated at doses from 5–120 mg daily. Overall, 65% were female and 88% had two or more previous lines of systemic therapy. There were two patients that had dose-limiting pneumonitis at 80 mg and 120 mg. Common treatment-related toxicities included diarrhoea (47%), nausea (26%) and fatigue (21%). Grade 3 treatment-related toxicities were dyspnoea, anaemia, asthenia, dehydration, lung infection, pleural effusion, pneumonia and pneumonitis. All 3 PRs in the first 14 evaluable patients harboured EGFR exon 20 mutation.61 Based on the encouraging preliminary antitumour activity, a phase III study of TAK788 or platinum/pemetrexed in treatment-naïve, EGFR exon 20 mutation mNSCLC is being planned. In addition, a phase I study of tarloxotinib in patients with EGFR or HER-2 exon 20 mutation is ongoing (ClinicalTrials.gov identifier: NCT038065841).
One of the most conceivable mechanisms of resistance to the current EGFR exon 20 TKIs is secondary mutation within the kinase or solvent front domain. It is highly possible that some patients who progress on one TKI will be sensitive to another. One of the important issues to investigate is the sequential use of these agents in this population.
FGFR pathway aberrations
The fibroblast growth factor (FGF) pathway consists of four receptors, FGFR1–4 and 18 ligands, including the hormone-like FGFs: FGF15, FGF19, FGF21 and FGF23, the canonical FGFs: FGF1, FGF4, FGF7, FGF8 and FGF9, and intracellular FGF11. Binding of the ligand to the corresponding receptor leads to activation of RAS/RAF/MAPK, PI3K/AKT/mTOR, STAT and PLC γ, which in turn leads to cell growth, proliferation, differentiation, migration and survival.62,63 Dysregulation of the FGF pathway can be a result of overexpression of the ligand or receptor, gene alteration from alternative splicing, amplification, point mutation or chromosomal rearrangement and loss of downregulation or degradation of the activated FGF pathway.64 In squamous cell carcinoma of the lung, the FGFR pathway aberration includes FGFR1 amplification65–67 (25%), point mutation in FGFR2 (3%), FGFR3 (3%), FGFR4 (2%)68 and chromosomal translocation such as FGFR3-TACC3 (3.5%), BAG4-FGFR1 (<1%) and FGFR2-KIAA1967 (0.3%).63 FGFR2 or FGFR3 point mutation in either the extracellular domain or the kinase domain leads to constitutive dimerization and thus ligand-independent activation of the receptor.68 FGF pathway dysregulation is less common in nonsquamous NSCLC, which includes FGFR1 amplification in 2–3% and FGFR3-TACC3 translocation in 0.5% of cases.63 Chandrani and colleagues showed that S249C mutations in the extracellular domain and G691R mutations in the kinase domain of FGFR3 were sensitive to FGFR inhibition in preclinical models.69
In the phase I study of BGJ398, a dose-expansion cohort of 36 patients with NSCLC with FGFR1 amplification were enrolled. Only 11.1% patients achieved a PR and 50% patients had disease controlled,70 whereas there was no response reported with erdafitinib (JNJ-42756493) in this population.71 Both studies did not explicitly define the criterion for FGFR1 amplification. In two phase II studies of AZD4547, only one PR was reported in an FGFR1-amplified mNSCLC, which was defined as FGFR1:CEP8 ⩾ 2.8.72,73
The LUNG-MAP SWOG S1400D was a randomized phase II study of AZD4547 or docetaxel in previously treated, squamous cell carcinoma patients with FGFR pathway activation. A total of 43 patients with FGFR1 amplification (56%), FGFR3 S249C mutation (9%), FGFR3 amplification (7%) and FGFR3 fusion (5%) were enrolled. The median age was 66.3 years and 30% were female. Out of the 25 patients with an evaluable response treated with AZD4547, only 1 with an S249C FGFR3 mutation had an unconfirmed PR (uPR) for a duration of 1.5 months. The mPFS for patients treated with AZD4547 was 2.7 months. Grade 3 or higher treatment-related toxicity was reported in five patients, including dyspnoea, fatigue, hyponatremia, lung infection and retinopathy.74
Although the initial studies in this population are disappointing, additional studies are ongoing using different criteria for FGFR amplification. For example, the study of rogaratinib (BAY1163877, ClinicalTrials.gov identifier: NCT03762122) uses elevated FGFR1–3 mRNA levels, which may be more reflective of FGFR pathway activation. Other agents in clinical development include TAS-120 (ClinicalTrials.gov identifier: NCT02052778), pemigatinib (ClinicalTrials.gov identifier: NCT02393248) and others in malignancies with FGF/FGFR aberrations, including mNSCLC, are ongoing. It is hoped that these ongoing studies will help confirm whether FGFR gene aberration is a true driver mutation for NSCLC.
HER-2 exon 20 mutation and amplification
HER-2 exon 20 insertion or deletion occurs in 2–9% of adenocarcinomas of the lung, which accounts for 90% all HER-2 gene aberrations. The rest harbour HER-2 amplifications.75–80 Comparable with EGFR exon 20 mutation, the length of insertion or deletion is highly variable,78,79 which leads to constitutive kinase activation.58 Most of the insertion occurs between codons 775 and 781, with either duplication or insertion of four amino acid residues (YVMA) or three base pair insertions with complex insertions or substitutions at codon 775. L755S and G776V/C point mutations have also been reported.78,80
HER-2 is first identified to be a potential target for mNSCLC based on a superior ORR (83% versus 41%) and mPFS (8.5 versus 7.0 months) to trastuzumab in combination with cisplatin/gemcitabine in patients with HER-2 2+ or 3+ by immunohistochemistry (IHC).81
A phase II study of dacomitinib in mNSCLC with either HER-2 exon 20 mutation or amplification, reported an ORR of 12% and 0%, respectively.82 The two phase II studies of afatinib in patients with exon 20 mutation reported disappointing results of disease control rate (DCR) at 71% including one out of seven uPRs, and a progression-free rate at 12-weeks of 54%, respectively.83,84 In a multinational, retrospective study of afatinib, 3 out of 27 patients with HER-2 exon 20 mutations responded. Overall, two of the responders harboured YVA or VAG insertions and had received previous trastuzumab and pertuzumab.85 Heymach and colleagues reported a preliminary report of poziotinib with an ORR of 50% in 12 patients with HER-2 exon 20 mutation.59
HER-2 antibodies, including trastuzumab, ado-trastuzumab emtansine and DS-8201a have been investigated in this population. Li and colleagues reported an ORR of 44% and mPFS of 5 months with ado-trastuzumab emtansine in 18 patients with either exon 20 insertion or point mutation at the extracellular or transmembrane domain.86 Responses were independent of HER-2 mutation subtype. Another phase II study with ado-trastuzumab emtansine in patients who had HER-2 2+ or 3+ by IHC reported an ORR of 0% and 25% respectively.87 However, Hotta and colleagues reported no patients with Her-2 2+ or 3+ by IHC and only one patient with an exon 20 mutation responded to aldo-trastuzumab emtansine.88 The interim result of the MyPathway study with trastuzumab and pertuzumab, targeting HER-2 dimerization with other HER family receptors, reported an ORR of 13% and 19% in 16 patients with HER-2 amplification and 12 patients with mutated mNSCLC, respectively.40
DS-8201a is a HER-2 targeted antibody drug conjugate with a topoisomerase I payload. An update of the phase I study in HER-2 IHC 1+ or higher or mutated mNSCLC was presented at the World Conference for Lung cancer in 2018. The median age was 58 years and patients had a median of three previous regimens. A total of 18 patients were evaluable for response: 8 out of 11 patients who were HER-2 mutated and 10 out of 17 patients with either HER-2 overexpression or mutation responded. Only 3 of 12 patients experienced ⩾grade 3 toxicity. The common toxicity included grade 1 or 2 anorexia (66.7%), nausea (58.3%), alopecia (41.7%) and fatigue (41.7%).89
The EUHER2 study reported an ORR of 45.3% and mPFS of 6 months in HER-2 exon 20 mNSCLC treated with platinum-based chemotherapy. Patients treated with ado-trastuzumab emtansine or trastuzumab had an ORR of 51% and mPFS of 4.8 months. No response was observed in patients treated with TKIs. The mOS of the entire cohort was 24 months, which was comparable with that reported in prospective phase II studies.90
To date, targeting the HER-2 exon 20 mutation has been more successful with HER-2 antibodies than TKIs, except for poziotinib. The benefit to the HER-2 antibody or antibody drug conjugate confined to either patients who were HER-2 over-expressed or amplified was modest, whereas efficacy with TKIs was a disappointment. Only with further elucidation of the biology of HER-2 amplification or overexpression can we improve the therapeutic benefit of HER-2 targeting agents. Comparable with EGFR exon 20, poziotinib is active in mNSCLC with exon 20 mutations, which is probably due to its small size and flexible structure, allowing it to bind to the kinase domain, while other HER-2 TKIs are too bulky to fit into the ATP pocket.58 Tarloxotinib is currently being investigated in patients with exon 20 mutations in EGFR or HER-2 (ClinicalTrials.gov identifier: NCT03805841). Given the heterogeneous nature of exon 20 mutation, translational study is needed to understand which subgroup will not benefit from the TKIs, poziotinib or tarloxotinib, and HER-2 targeted antibodies. Resistance mechanisms have yet to be reported in NSCLC; however, it is conceivable that the loss of the extracellular domain in patients treated with HER-2 targeted antibodies can be a potential mechanism, as observed in breast cancer. Other mechanisms may include concurrent HER-2 amplification and bypass pathways, including EGFR amplification, activation or mutation.
K-RAS mutation
RAS is a guanosine triphosphatase gene superfamily, including K-RAS, H-RAS and N-RAS. It can be activated by either upstream tyrosine kinase or by point mutation at codons 12, 13, 14, and 60/61 of RAS. In turn, RAS activates the RAF–MAPK and PI3K pathways for cell proliferation, growth and anti-apoptosis.
In NSCLC, K-RAS is mutated in 20–30% of patients.22,91 Mutation at codon 12 is the most common: G12C (G→T, 43%), G12V (G→T, 18%) transversion and G12D (G→A, 11%) transition.91–93 K-RAS mutation occurs predominantly in patients who have adenocarcinoma, are non-Asian and smokers. The incidence seems to correlate with the number of cigarettes smoked, particularly for transversion.22,91 The meta-analysis by Meng and colleagues showed that early-stage K-RAS-mutated adenocarcinoma had a poorer outcome,94,95 especially in those patients with a codon 12 mutation95 or transversion.96 Therefore, K-RAS mutation is heterogeneous in biology and different therapeutic approaches will be required.
One of the most logical strategies to target K-RAS is to inhibit downstream MEK, which, in turn, inhibits ERK. The phase III study of docetaxel with or without selumetinib, an allosteric MEK1/2 inhibitor, in previously treated, advanced K-RAS mutation-positive NSCLC failed to improve both mPFS [3.9 versus 2.8 months, hazard ratio (HR) = 0.93] and mOS (8.7 versus 7.9 months, HR = 1.05) despite an improvement in mPFS in the randomized phase II study (HR = 1.14).97,98
RAS activation leads to G1/S cell cycle progression via cyclin-dependent kinase 2 and 4 (CDK2/4), which induces cyclin D1 and downregulates p27KIP. Cyclin D1 activates CDK4/6, which phosphorylates retinoblastoma protein, leading to G1/S transition.99 Preclinical studies demonstrated that K-RAS-mutant NSCLC cell lines were sensitive to CDK4/6 inhibition.100,101 Based on the synthetic lethal relationship between K-RAS and CDK4/6, inhibition of CDK4/6 alone or in combination with inhibition of the RAF/MEK/ERK pathway may be a potential therapeutic strategy.
JUNIPER was a randomized phase III study of abemaciclib 200 mg twice daily, a CDK4/6 inhibitor, or erlotinib in patients with mNSCLC with K-RAS codon 12 or 13 mutations who had no more than two previous lines of therapy, including platinum-based chemotherapy. Despite an improvement in mPFS (3.6 versus 1.9 months, HR = 0.58, p < 0.001) and ORR (8.9% versus 2.7%, p = 0.01), mOS was not improved (7.4 versus 7.8 months, HR = 0.97, p = 0.77) with abemaciclib. Molecular subgroup analysis has yet to be presented. It will be of interest if there is any difference in benefit between codon 12 and 13, transversion and transition and cyclin D overexpression or amplification status.102
Small molecular inhibitors to K-RAS G12C mutations have been shown to have significant antitumour activity in animal models. Phase I–II studies of AMG 510 (ClinicalTrials.gov identifier: NCT03600883) and MRTX849 (ClinicalTrials.gov identifier: NCT03785249) in this population have just commenced and the results are highly anticipated.
Sullivan and colleagues reported on the phase Ib study of trametinib and palbociclib in patients with solid tumours without selecting for K-RAS mutation. The RDII was once daily of 2 mg of trametinib and 75 mg of palbociclib or 1 mg of trametinib and 100 mg of palbociclib for 3 out of 4 weeks. DLTs observed at the RDII were grade 3 mucositis (1 in 7 patients) and grade 3 decrease in left ventricular ejection fraction (1 in 6 patients), respectively. Common adverse events were diarrhoea (67.9%), acneiform rash (64.3%) and fatigue (53.6%). Overall, two patients with WT BRAF or RAS melanoma and one patient with colorectal cancer and NRAS Q61K transversion responded.103 Preliminary result of the phase Ib study of palbociclib and PD0325901 (MEKi) in RAS-mutant tumours reported the maximum administered dose to be 125 mg of palbociclib daily and 8 mg of PD-0325901 twice daily. A dose-limiting pneumonitis occurred at the 100 mg palbociclib and 4 mg PD-0325901 dose level. The most frequent drug-related toxicities were leukopenia (72%), anaemia (72%), thrombocytopenia (72%), neutropenia (64%), acneiform rash (64%), diarrhoea (52%), fatigue (44%), lower extremity oedema (32%), vomiting (28%), nausea (28%), oral mucositis (24%), increased AST (20%), increased creatinine (12%), epistaxis (12%) and blurred vision (12%). One PR and five cases of stable disease (SD) for more than 6 months were observed in K-RAS mutant NSCLC. K-RAS mutation with or without concomitant p53 or CDKN2A/B loss was predictive for response.104 Other MEK and CDK4/6 inhibitor combinations are currently in early clinical development (ClinicalTrials.gov identifiers: NCT03170206 and NCT02703571). Based on the significant toxicity and modest clinical activity, these combinations are not ready for clinical use. Further optimization of the schedule and the dose of these combinations is needed to reduce toxicity and improve efficacy.
Even though K-RAS G12C-specific inhibitors may have significant clinical benefit, there is still an unmet need for other K-RAS mutation subtypes. Only through continual preclinical and translational studies of non-G12C mutants, can successful clinical strategies be developed.
MET EX14 point mutation and amplification
MET and its ligand hepatocyte growth factor play a role in cell growth and development and epithelial mesenchymal transition in cancer cells, through the activation of the downstream RAS/RAF/MAPK, Pi3K/AKT/mTOR, WNT/β-catenin and STAT pathways (Table 2).
Table 2.
Comparative toxicity and ORR of selected MET inhibitor.
| Drug | Target | All-grade common toxicity | ORR in METex14 mutation (incidence: 3–4%) | ORR in MET amplification (incidence: 2–4%) |
|---|---|---|---|---|
| Crizotinib105–107 | ALK, MET, ROS1 | Cardiac: oedema (35%), bradycardia (24%) GI: nausea (35%), vomiting (24%) CNS: visual disorder (29%) |
5 PR and 5 uPR in 15 patients | Low: 33.3% Intermediate: 14.3% High: 40% |
| Capmatinib108 | MET | Constitutional: fatigue (21%) Cardiac: peripheral oedema (49%) GI: nausea (43%), vomiting (43%), anorexia (21%) Respiratory: dyspnoea (24%) Laboratory: increase in creatinine (25%) |
Treatment naïve: 72% Pretreated: 39% |
IHC 3+: 5/17 GCN 4–6: 2/7 GCN ⩾6: 9/12 |
| Tepotinib109 | MET | Cardiac: peripheral oedema (26%) GI: diarrhoea (21%) Respiratory: pneumonitis (3%) Laboratory: increase in amylase (6%) or GGT (3%) |
43% by independent review committee | Pending |
| Savolitinib110 | MET | Constitutional: pyrexia (10%) Cardiac: peripheral oedema (36.8%) GI: nausea (41.5%), vomiting (24.4%), anorexia (14.6%) Laboratory: increase in AST/ALT (26.8%) |
54.8% | Pending |
ALT, alanine transaminase; AST, aspartate transaminase; CNS, central nervous system; GCN, gene copy number; GGT, xxxxxxx; GI, gastrointestinal; IHC, immunohistochemistry; ORR, overall response rate; PR, partial response; uPR, unconfirmed PR.
MET point mutation occurs in 3–4% of mNSCLC, with the most common being exon 14 skip mutations (METex14), which occurs in 2–3% NSCLC. Smokers, who are older than 70 years and with a sarcomatoid histology more commonly harbour this mutation. Other reports found METex14 mutation in acinar or solid subtypes of adenocarcinoma. Furthermore, stage I–IIIA METex14-positive NSCLC is more likely to recur. For those patients who had recurrence, the OS was comparable with that in ALK-positive mNSCLC.111 MET exon14 is located in the juxtamembrane domain of the receptor and is involved in degradation by ubiquitin ligase, Cbl. Thus, METex14 leads to an increase in MET expression and activity.111–115 METex14 may have concurrent MET amplification, defined as a MET:CEP7 ratio ⩾5.0 and MET overexpression.112,114,115 Both METex14 (HR = 2.156, p = 0.026) and amplification (HR = 3.444, p = 0.007) are independent negative prognostic factors in treatment-naïve mNSCLC.116
Crizotinib, an ALK, ROS1, MET inhibitor, was the first TKI to demonstrate clinical activity in METex14 mNSCLC.117 Drilon and colleagues then reported 5 PRs and 5 uPRs for crizotinib in 15 patients who were evaluable for response with METex14 mutation. The mPFS was not reached in the 2016 update.105 Responses to cabozantinib, a multikinase inhibitor to MET, VEGFR2 and RET, were reported in patients who were naïve to MET inhibitors or pretreated with crizotinib.118–120 Ou and colleagues reported that Y1230C mutations conferred resistance to crizotinib but sensitivity to other type II MET inhibitors, such as cabozantinib.119 About 4% of METex14 was found to have concurrent K-RAS G12 mutation irrespective of previous crizotinib exposure. K-RAS mutation not only led to activation of the RAS/MEK/ERK pathway, but also enhanced MET expression, leading to resistance to MET inhibition. Dual inhibition of MET with either EGR/HER-2 or MEK inhibited growth in cell lines and xenograft models more significantly than MET inhibition alone.121
The phase II study of capmatinib, a selective type I MET inhibitor, in patients with METex14 mNSCLC reported an ORR of 39%. Over 70% of patients had received 1–2 previous lines of therapy. Toxicity included peripheral oedema (49%), nausea (43%), vomiting (43%), an increase in creatinine (25%), dyspnoea (24%), anorexia (21%) and fatigue (21%), with the majority being grade 1–2.108 Preliminary results from the phase II study of tepotinib, another selective type I MET inhibitor, reported an ORR of 60% in the first 13 evaluable patients. Tepotinib was well tolerated with mostly grade 1–2 treatment-related oedema and diarrhoea. Grade 3 toxicities were increased amylase, Gamma-glutamyl transpeptidase (GGT) and interstitial pneumonia.109
An interim report of a phase II study of savolitinib in 41 mNSCLC patients with either sarcomatoid (n = 11) or other histology (n = 20) and METex14 mutation was presented at the American Association of Cancer Research Meeting in 2019. Of the 31 patients evaluable for response, the ORR was 54.8% for both cohorts and the mPFS and median duration of response (mDOR) were not reached at the time of the presentation. The treatment-related adverse events that occurred in ⩾10% patients were grade 1–2 nausea (41.5%), peripheral oedema (36.8%), elevation of AST/ALT (26.8%), vomiting (24.4%), anorexia (14.6%) and pyrexia (10%). Grade 3 toxicities observed were peripheral oedema (5%), elevation of AST/ALT (7.2%) and pyrexia (2.4%). Overall, five patients discontinued due to toxicity.110 Many other MET inhibitors, such as sitravatinib, AMG337 and tivantinib, are in clinical development for this patient population.
MET amplification is not only a primary driver mutation but also a resistance mechanism to EGFR-TKIs. In this review, only de novo MET amplification will be discussed, which occurs in 2–4% of newly diagnosed mNSCLC.122–126 MET amplification is associated with poorer survival in both resected122,127 and metastatic NSCLC.126 The definition of MET amplification remains to be clearly defined. The ORRs to crizotinib were 0%, 20% and 50% in patients with a low (⩾1.8 to ⩽2.2), intermediate (>2.2 to <5) and high (⩾5) MET:CEP7 ratio.106 The responses were more common in females and ex-smokers. Patients with high MET gene copy number (GCNs) did not respond. This study was recently updated with a change in the cutoff for intermediate and high-level amplification. The ORRs were 33.3%, 14.3% and 40% for low (⩾1.8 to ⩽2.2), intermediate (>2.2 to <4) and high (⩾4) MET amplification. The corresponding mPFS rates were 1.8, 1.9 and 6.7 months.107
Capmatinib is also being investigating in patients with MET amplification, defined as a MET:CEP7 ratio ⩾2, GCN ⩾ 4 to <6 or ⩾6 or IHC 2+ and 3+. In the preliminary report, 5 of 17 patients with IHC 3+, 2 of 7 with GCN ⩾ 4 and <6 and 9 of 12 with GCN ⩾ 6 had a PR.128 Other MET inhibitors (METi) are being investigated in the MET-amplified setting, including tepotinib (ClinicalTrials.gov identifier: NCT028664992), cabozantinib (ClinicalTrials.gov identifier: NCT03911193) and sitravatinib (ClinicalTrials.gov identifier: NCT02219711).
To date, the definition of MET amplification is still unclear. Hopefully, this will be confirmed with ongoing trials using various definitions of MET amplification. The selective MET inhibitors seem to have an improved efficacy and toxicity profile over multitargeted or type II kinase inhibitors. Mechanisms of resistance, including Y1230C kinase domain mutation and K-RAS mutation, and hence corresponding therapeutic strategies will be elucidated from ongoing trials.
Neuregulin-1 fusion
The first neuregulin-1 (NRG1) fusion with CD74 in lung adenocarcinoma was reported in 2014.129 Liu and colleagues reported NRG1 fusion in 0.2–0.8% of multiple tumour types, including cholangiocarcinoma, thyroid, ovarian, pancreas and breast cancers, NSCLC and sarcoma. In NSCLC, multiple 5′ fusion partners have been identified and the break point occurs either at exon 2 or 5 of NRG1. NRG1 fusion NSCLC is more commonly found in mucinous adenocarcinoma, females and nonsmokers with a median age of 70 years. Tp53 (50%) and pTEN loss are two common co-mutations.130,131 NRG1 is a natural ligand for HER-3, which leads to dimerization and activation of HER-2 and HER-3 followed by PI3K/AKT129,131. These findings support targeting NRG1 fusion with a pan-HER inhibitor. There are case reports of prolonged responses to anti-HER3 antibodies and afatinib.132–135 Tirunagaru and colleagues reported a novel, irreversible EGFR and HER-2 inhibitor, tarloxotinib, with potent antitumour activity in NRG fusion-positive cell lines. Responses are more durable than with afatinib.136 Trials with pan-HER or EGFR/HER-2 TKIs, such as tarloxotinib and HER2 or 3-directed antibodies will be of interest in this population.
NTRK mutation and chromosomal fusion
There are three members in the neurotrophic tyrosine kinase (NTRK) family, which are involved in the development of different neuronal tissues. In adults, NTRK1 and NTRK3 are responsible for sensory function, while NTRK2 controls cortical function. Neurotrophin-3 (NT-3) and nerve growth factor are ligands for NTRK1, NT-3–5 and brain-derived neurotrophic factor for NTRK2 and NT-3 for NTRK3. Binding of the ligands to the respective NTRK activates downstream PI3K/AKT/mTOR, RAS/RAF/MAPK and PLC-γ, leading to proliferation, growth and survival of neurons in the peripheral and central nervous system. NTRK dysregulation can result from chromosomal fusion, point mutation, overexpression and alternative splicing.137–139 To date, alternative splicing of NTRK has not been identified in NSCLC. Gene fusion of NTRK1, NTRK2 and NTRK3 occurs in 3.5%,140 0.2–1%141 and <1%142 of NSCLC, respectively. Marchetti and colleagues also reported point mutations in NTRK2 and NTRK3 in 10% of large-cell neuroendocrine carcinoma of the lung and none was documented in other NSCLC histological subtypes. All the NTRK somatic mutations are in the activation or catalytic domain, which lead to constitutive activation. Furthermore, NTRK mutations are only found in the neuroendocrine portion of large-cell neuroendocrine tumours. These findings suggest somatic NTRK2 or NTRK3 mutation may be an oncogenic driver for large-cell neuroendocrine tumour (Table 3).143
Table 3.
Comparative toxicity and ORR of selected NTRK and ROS1 inhibitors.
| Drug | Target | All-grade common toxicity | ORR in NTRK fusion (incidence for NTRK 1–3 fusion: ~4%) | ORR in ROS1 fusion (incidence: 2%) |
|---|---|---|---|---|
| Larotrectinib144–146 | NTRK1–3 | Constitutional: fatigue (36%), pyrexia (18%) Cardiac: peripheral oedema (15%) CNS: headache (16%), myalgia (16%) GI: nausea (29%), constipation (27%), diarrhoea (23%), vomiting (23%) Respiratory: cough (26%), dyspnoea (18%) Laboratory: anaemia (27%) and increase in AST/ALT (26%) |
5/7 | NA |
| Entrectinib147–150 | ALK, NTRK1-3 and ROS1 | Constitutional: fatigue (27.9%), weight gain (19.5%) Cardiac: peripheral oedema (14.1%) CNS: dizziness (25.4%), paraesthesia (18.9%), myalgia (15.2%), muscle weakness (7%) GI: dysgeusia (41.4%), constipation (23.7%), diarrhoea (22.8%), nausea (20.8%), vomiting (13.5%) Laboratory: increase in creatinine (15.2%), anaemia (12.1%) and increase in AST (10.7%) |
7/10 | 77.4% |
| Reprotrectinib151 | NTRK1-3 and ROS1 | CNS: dizziness (49%), paraesthesia (28%) GI: dysgeusia (48%), constipation (20%) |
2/7 | ROS1 naïve: 71% ROS1 pretreated: 11% |
| Ceritinib152 | ROS1 | Constitutional: fatigue (38%), fever (19%) CNS: dizziness (13%) GI: diarrheal (78%), nausea (62%), anorexia (59%), vomiting (53%), abdominal pain (41%), dyspepsia (13%) Respiratory: dyspnoea (25%), pneumonia (25%), pleural effusion (3%) Skin: pruritis (16%) |
NA | ROS1 naïve: 72% |
| Lorlatinib153 | ALK and ROS1 | Constitutional: weight gain (17%), fatigue (15%) Cardiac: Peripheral oedema (39%) CNS: peripheral neuropathy (39%), speech changes (19%), cognitive changes (17%), mood changes (15%), tinnitus (13%), visual changes (13%) GI: constipation (13%), nausea (11%) Laboratory: hypercholesterolaemia (72%), hypertriglyceridaemia (39%), increase in lipase (17%), increase in amylase (13%) and increase in AST (13%) |
NA | 50% |
ALT, alanine transaminase; AST, aspartate transaminase; CNS, central nervous system; GI, gastrointestinal; ORR, overall response rate.
Larotrectinib and entrectinib are the first NTRK inhibitors (NTRKi) in clinical development. Larotrectinib is a highly selective NRTKi. Drilon and colleagues reported the RDII to be 100 mg twice daily and an ORR of 75% by independent radiological review in the first 55 NTRK-positive solid tumours. Response was observed irrespective of age, histology, NTRK1–3 and 5′ fusion partner.144 This study was recently updated to include a total of 122 patients. The ORR was 81% with a 17% complete response (CR) across all tumour types. Preliminary intracranial antitumour responses were observed. Both the mDOR and mPFS have not been reached after a median follow up of 7.4 months.145 A total of 11 mNSCLC with NTRK fusions were enrolled. The median age was 52 years. Overall, eight patients had NTRK1 fusion and the reminder had NTRK3 fusion. In total, seven patients with mNSCLC were evaluable for response. Overall, one CR and four PRs with mDOR ⩾ 7 months were reported and no patients had primary progressive disease.146 Larotrectinib was generally well tolerated with mostly grade 1 and 2 toxicity, including fatigue (36%), dizziness (29%), nausea (29%), constipation (27%), anaemia (27%), elevation of AST/ALT (26%), cough (26%), diarrhoea (23%), vomiting (23%), pyrexia (18%), dyspnoea (18%), headache (16%), myalgia (16%) and peripheral oedema (15%). The most common grade 3 or higher toxicity was anaemia, which was reported in 10% of patients. Only 9% of patients required a dose reduction and <1% terminated treatment, secondary to toxicity.145
Entrectinib is a pan-TRK, ROS and ALK inhibitor. The combined results of the phase I studies, ALKA-372-001 and STARTRK1, determined 600 mg daily to be the RDII. Overall, three patients with NTRK fusions responded. Furthermore, five out of eight patients with NTRK fusion with brain metastases in the phase II study, STARTRK2, had a documented intracranial response.147 Demetri and colleagues reported the combined analysis of 54 patients with refractory NTRK-fusion-positive solid tumours from the ALKA-372-001, STARTRK-I and STARTRK-2 trials. The ORR was 57.4% with CR at 27.3%. Comparable with larotrectinib, responses were observed across all histology and fusion partners. The mPFS and mDOR were 11.2 and 10.4 months respectively, and the mOS was 20.9 months. The enrolled patients with NTRK fusion and mNSCLC had a median age of 62.5 years and 60% had documented brain metastases. Males and females were equally likely to harbour an NTRK fusion. The ORR was 70% (7/10) and four out of six patients had intracranial responses.148 Majority of the patients experienced grade 1 or 2 toxicity including dysgeusia (41.4%), fatigue (27.9%), dizziness (25.4%), constipation (23.7%), diarrhoea (22.8%), nausea (20.8%), weight gain (19.5%), paraesthesia (18.9%), increase in creatinine (15.2%), myalgia (15.2%), peripheral oedema (14.1%), vomiting (13.5%), arthralgia (12.4%), anaemia (12.1%), AST elevation (10.7%) and muscular weakness (7%). The most common grade 3 toxicity was weight gain (5.1%), anaemia (4.5%) and fatigue (2.8%). A total of 27% and 25% of patients required dose reduction and interruption, respectively, but only 4% of patients discontinued due to adverse events.149
Both larotrectinib and entrectinib reported on-target neurological toxicity, such as dizziness, paraesthesia and myalgia. The pathophysiology of anaemia and peripheral oedema remain to be elucidated.
Unfortunately, resistance will occur in patients treated with larotrectinib and entrectinib due to mutation in the solvent front region of the kinase (for example G595R and G623R in NTRK1 and NTRK3) or the xDFG motif (for example G667C in NTRK1), leading to steric hinderance to current NTRKi binding in the kinase domain.154 LOXO-195 and repotrectinib (TPX-0005) are currently in early clinical development to overcome these NTRK resistance mechanisms.
LOXO-195 is a low molecular weight macrocyclic inhibitor that binds to the kinase domain without steric hinderance from the bulky amino acid moiety, resulting from secondary NTRK mutation. Preliminary antitumour activity has been reported in a patient with colorectal cancer and infantile fibrosarcoma with a G595R mutation in the NTRK fusion.154 The phase I/II study is currently ongoing (ClinicalTrials.gov identifier: NCT03215511). Repotrectinib is a novel ALK/ROS1/NTRK inhibitor designed to reside within the ATP binding pocket and avoid steric hindrance from solvent front substitutions.151 Dose-limiting dizziness and dyspnoea occurred at doses ⩾160 mg daily. Other common toxicity, mostly grade 1 and 2, were dizziness (49%), dysgeusia (48%), paraesthesia (28%) and constipation (20%). A total of seven patients who were NTRK fusion-positive were evaluable for response and two patients who were NTRKi-pretreated responded. Overall, one of the responders had mammary analogue secretory carcinoma and G623E solvent front mutation in the NTRK3 fusion.155 The phase II study of reprotectinib is currently ongoing (ClinicalTrials.gov identifier: NCT03093116). Thus far, proof-of-principle responses have been observed. The ongoing studies will confirm the clinical efficacy and will help to elucidate the resistance mechanism to these second-generation NTRKi.
PIK3CA/AKT/PTEN/MTOR pathway gene aberrations
Activation of the PI3K/AKT/mTOR pathway is central to cellular proliferation, growth and apoptosis. In normal cells, following activation of PIK3CA and downstream AKT/TORC1/p70S6K, activated p70S6K exerts a negative feedback on PIK3CA. In addition, pTEN acts as a negative regulator of this pathway via inhibition of AKT. Overall, three classes of PI3K have been identified. PIK3CA is a heterodimer of a p85 regulatory subunit and one of the four isoforms of p110 catalytic subunits (α, β, γ and δ). Each p110 subunit is preferentially expressed in both normal and malignant tissue. There are also three isoforms of AKT. Malignant transformation from this pathway results from activation or mutation of upstream pathways, such as tyrosine kinase or RAS and from aberration in PI3K, AKT, pTEN or RICTOR, which leads to tumour proliferation, growth and survival.156–158
In NSCLC, activating mutations in exon 9 helical (E542K, E545A/G/K/Q) and exon 20 kinase (H1047L/R/Y) domains of PIK3CA are detected in up to 4% of patients with mNSCLC.159–161 These mutations are more common in patients with chronic obstructive pulmonary disease (10.4% versus 1.7%, p = 0.015).162 Amplification or polysomy of PIK3CA is the predominant molecular aberration in squamous cell carcinoma (33.1% versus 6.2% in adenocarcinoma).161 All in all, PIK3CA pathway aberration is more commonly found in squamous cell carcinoma, which is also prognostic (mOS of 8.5 versus 10.1 months, p < 0.0001) and predictive of higher disease burden, intracranial metastases and genomic heterogeneity.163 Activating E17K AKT1 mutations in exon 4 kinase domains occurs in 1% of all NSCLC, with the majority being squamous in histology.164–166 Loss of pTEN protein expression can result from allelic loss167,168 or promotor methylation,167,169 which occurs in up to 75% of NSCLC. Overall, 4–7% of NSCLC have activating R233* mutations in exon 7 or the C2 domain. This mutation controls the attachment of pTEN to the cell membrane, leading to activation of AKT.168,170,171 Loss of pTEN function leads to PIK3CAβ activation, which may predict sensitivity to PIK3Caβ, AKT172 or mTOR173 inhibition.
Buparlisib (BKM120), a pan-PI3K inhibitor, was tested in 30 patients with squamous and 30 patients with nonsquamous mNSCLC with PI3K/AKT/mTOR pathway activation. Approximately 50% of patients had previous chemotherapy. The progression-free rates at 12 weeks were 23.3% and 20%, respectively. The corresponding ORRs were 3.3% and 3.0% and mOS were 7.98 months and 7.20 months. PIK3CA mutation in exons 1, 5, 7, 9 and 20 were documented in 20.7% of squamous and nonsquamous NSCLC. Almost 28% of squamous and 4% of nonsquamous mNSCLC had pTEN mutations in exons 1–9. pTEN protein losses by IHC were found in 62.1% of squamous and 24% of nonsquamous mNSCLC. None of these aberrations correlated with outcome. The most common adverse events in ⩾25% of patients, regardless of relationship to study drug treatment, were nausea, vomiting, and diarrhoea (61.9%), asthenia/fatigue (49.2%), hyperglycaemia (39.7%), liver toxicity (31.7%) and hypersensitivity or rash (28.6%). Treatment-related grade 3 or 4 events were hyperglycaemia (23%), asthenia (6.7%) and fatigue (6.7%) in the squamous cohort, whereas elevation of AST/ALT (15.2%), hyperglycaemia (12%), asthenia (6%) and rash (6%) were reported in the nonsquamous cohort. Grade 3 psychiatric toxicity, described as confusion, anxiety or hallucination was reported in 10% of patients with squamous histology, whereas grade 3 depression or altered mood was reported in 6% of adenocarcinoma patients. A total of 13% patients with squamous NSCLC discontinued due to hyperglycaemia, whereas 21% of nonsquamous NSCLC discontinued due to dyspnoea, elevation in ALT or depression. The most common reason for discontinuation was toxicity in the squamous cohort as compared with disease progression in the nonsquamous cohort.174 There seemed to be some difference in the toxicity profile between the two histologies, which may be related to the fact that patients with squamous NSCLC might have more comorbidity.
Preliminary results of taselisib, a selective PI3Kα inhibitor, in the SWOG 1400B study in previously treated, squamous NSCLC that harboured PIK3CA exon 9 or exon 20 mutations reported an ORR of 4%, mPFS of 2.8 months and mOS of 5.9 months. Further enrollment was halted after this interim analysis. Overall, five patients each experienced grade 3 hyperglycaemia or diarrhoea and three had grade 3 lymphopenia.175
The initial results from the trials targeting the PI3K pathway have been disappointing from both efficacy and toxicity perspectives. Hyperglycaemia and gastrointestinal toxicity limit the dose intensity which may, in turn, limit continuous inhibition of the pathway and thus, antitumour activity. LoRusso suggested inhibition to specific PIK3CA or AKT isoforms may lead to compensatory activation of other isoforms, such as chronic lymphocytic leukaemia treated with PI3Kδ leading to activation of PI3Kα.176,177 Furthermore, inhibition of the PI3K/AKT pathway also leads to loss of AKT inhibition by p70S6K and paradoxical activation of the pathway.172,177
Until a better understanding of whether aberration in the PI3K/AKT/pTEN pathway is a driver mutation in mNSCLC and novel agents have improved toxicity profiles, successful clinical development of agents targeting this pathway is doubtful.
RET chromosomal fusion
Giant cell-derived neurotrophic factor is the ligand for RET, a tyrosine kinase receptor, and its activation leads to downstream activation of RAS/RAF/MAPK, PI3K/AKT/mTOR and PLC-γ, followed by cellular proliferation, migration and differentiation. In normal tissue, RET is responsible for renal and enteric nervous system development.178,179 RET is expressed in cells of neural crest origin, such as neurons, sympathetic and parasympathetic ganglia, testicular germ cells, urogenital tract, adrenal medulla and C cells in the thyroid (Table 4).180
Table 4.
Comparative toxicity and ORR in selected RET inhibitors.
| Drug | Target | All-grade common toxicity | ORR in RET fusion (incidence: 1–2%) |
|---|---|---|---|
| Cabozantinib181 | AXL, C-KIT, MET, RET, ROS1, TIE2 and VEGFR2 | Constitutional: fatigue (46%), weight loss (23%), hoarseness (16%) Cardiac: hypertension (19%) Cutaneous: palmar-plantar erythrodysaethesia (62%), hypopigmentation (50%), dry skin (19%), alopecia (12%) Endocrine: hypothyroidism (69%) GI: diarrhoea (61%), mucositis (46%), nausea (31%), dysgeusia (315), vomiting (23%), constipation (19%), anorexia (19%) Laboratory: increase in ALT (97%), increase in AST (74%), thrombocytopenia (46%), increase in lipase (35%), increase in amylase (27%), hypomagnesaemia (27%), hypophosphataemia (20%), increase in serum bilirubin (20%), anaemia (12%), increase in alkaline phosphatase (12%) |
28% |
| Lenvatinib182 | VEGFR1–3, FGFR1–4, PDGFRα, C-KIT and RET | Cardiac: hypertension (68%) GI: nausea (60%), anorexia (52%), diarrhoea (52%), vomiting (44%) Laboratory: proteinuria (48%) |
16% |
| RXDX105183 | RET | Constitutional: fatigue (26%) CNS: muscle spasm (13%) Cutaneous: rash (25%) GI: diarrhoea (24%), nausea (15%), anorexia (11%), vomiting (11%) Laboratory: hypophosphatemia (18%), increase in AST/ALT (14%) |
19% only in non-KIF5B-RET fusion |
| BLU-667184,185 | RET | Constitutional: fatigue (12%) Cardiac: hypertension (12%) GI: constipation (23%), diarrhoea (14%) L:aboratory: increase in AST/ALT (16%), increase in creatinine (12%) and neutrophilia (12%) |
4 PR and 1 uPR in 11 RETi naïve patients |
| LOXO-292186 | RET | CNS: headache (12%) GI: diarrhoea (21%), dry mouth (21%), constipation (20%), nausea (12%) Respiratory: cough (12%) Laboratory: hypomagnesaemia (13%) |
68% in RETi naïve patients |
ALT, alanine transaminase; AST, aspartate transaminase; CNS, central nervous system; GI, gastrointestinal; ORR, overall response rate; PR, partial response; uPR, unconfirmed PR.
RET fusion occurs in 1–2% of patients with NSCLC who are younger than 60 years, have never smoked or are minimal smokers and is equally common in men and women. Multiple 5′ fusion partners have been identified with KIF5B being the most common. Most of RET fusion mNSCLC cases have either mixed or solid subtype adenocarcinoma and 30% demonstrate signet ring morphology.187–190
Currently, the multitarget kinase inhibitors, cabozantinib, vandetanib, lenvatinib, sitravatinib, alectinib and ponatinib, and the specific RET inhibitors, RXDX-105, BLU-667, LOXO-292, BOS172738, are in clinical development.
A phase II study of cabozantinib at 60 mg daily in 25 patients with previously untreated (25%) and treated (75%) mNSCLC with RET fusion had an ORR of 28%, mPFS of 5.5 months and mOS of almost 10 months. Grade 3 treatment-related adverse events were elevation of lipase in four patients and elevation of AST/ALT or thrombocytopenia and hypophosphatemia in two patients each.181 Cabozantinib-treated patients in the Global RET Registry (GLORY) reported an ORR of 33%, mPFS of 3.6 months and mOS of 4.9 months.191
The two phase II studies of vandetanib in heavily pretreated RET fusion NSCLC reported an ORR of 18–47%, mPFS of 4.5–4.7 months and mOS of 11.6 months. The grade 3 toxicities reported were hypertension, due to vascular endothelial growth factor receptor (VEGFR) inhibition, diarrhoea, rash, dry skin, prolongation of QTc interval and elevation of AST/ALT.192,193 The GLORY registry reported an ORR of 18%, mPFS of 2.9 months and mOS at 10.2 months in 11 patients treated with vandetainib.191
Lenvatinib is a multitargeted kinase inhibitor to VEGFR1–3, FGFR1–4, PDGFRα, c-KIT and RET. It has received United States Food and Drug Administration and other regulatory agency approval for radioactive iodine-refractory, well-differentiated thyroid cancer, anti-angiogenic inhibitor pretreated renal cell carcinoma and untreated hepatocellular carcinoma. The ORR of lenvatinib at 14 mg daily in 25 patients with heavily pretreated, RET fusion-positive mNSCLC was 16% with mDOR of 16 weeks. The mPFS was 7.3 months and the mOS was not reached at the time of the report. Treatment-related adverse events occurred in 92% of patients with three being fatal. In addition, adverse event that required dose reduction, dose interruption and termination occurred in 64%, 76% and 20% patients, respectively. Common toxicities included hypertension, nausea, anorexia, diarrhoea, proteinuria and vomiting.182 The efficacy of lenvatinib may not be justified by the toxicity.
Alectinib is an ALK and RET kinase inhibitor and has been found to have activity to V804L/M gatekeeper mutations in a KIF5B-RET fusion NSCLC mouse model. Lin and colleagues reported that two out of four patients with RET fusion who had previous RET inhibitors responded to alectinib.194 A phase I/II study determined 450 mg twice daily to be the RDII. DLTs observed in 3 of 6 patients treated with 600 mg twice daily were grade 3 rash, elevation of AST, erythema multiforme, thromboembolic disease and elevation of creatine phosphokinase (CPK). No efficacy data have been presented to date. The phase II study at 450 mg twice daily is currently ongoing, which is higher than the 300 mg twice daily dose approved in Japan for ALK-positive mNSCLC.18,195 The B-FAST study is also evaluating alectinib in patients with RET fusion detected by blood-based next-generation sequencing (ClinicalTrials.gov identifier: NCT03178552).
RXDX-105 is a novel VEGFR1/2-sparing, multikinase inhibitor with antitumour activity in WT RET, KIF5B-, CCDC6- and NCOA4-RET fusion, WT or V600E mutated BRAF tumour xenografts. Drilon and colleagues reported a dose-escalating phase I study followed by dose expansion in RET fusion-positive mNSCLC, colorectal cancer, hepatocellular carcinoma, medullary thyroid cancer and other solid tumours. Only 11% of patients had no previous systemic therapy. The RDII was 275 mg daily in the fed state. A total of 39 patients who were RET fusion-positive with mNSCLC and 31 who were RET inhibitor-naïve were enrolled in the phase Ib portion. The most common fusion partners were KIF5B (65%), followed by CCDC6 (20%). The ORR in RET fusion-positive mNSCLC who were RET inhibitor-naïve was 19%. All the responses were reported in the patients who were non-KI5FB-RET-positive. A total of four DLTs were observed during the dose-escalating portion of the study: grade 3 rash at 200 mg daily, grade 3 fatigue and grade 3 diarrhoea at 275 mg and grade 3 hyperbilirubinaemia at 350 mg. Dose reduction was required in 28% of all patients enrolled with 31% at 275 mg daily. The most common adverse events that led to dose reduction were elevation of AST/ALT (9%) and cutaneous toxicity (6%). A total of 25 patients discontinued due to toxicity. The most common RXDX-105-related toxicity, included fatigue (26%), diarrhoea (24%), rash, maculopapular and nonmaculopapular, (25%), hypophosphatemia (18%), nausea (15%), elevated AST/ALT (14%), muscle spasms (13%), anorexia (11%) and vomiting (11%). Grade 3 or higher toxicities were nausea, diarrhoea, hypophosphatemia, rash and elevated AST/ALT in <10% of patients. Interestingly, three patients experienced cutaneous hypersensitivity consistent with drug rash with eosinophilia and systemic symptoms, which has also been observed in vemurafenib.183 It is postulated that the rash may be related to BRAF inhibition. It is intriguing about the discrepancy of response in xenograft models and in patients with KIF5B-RET fusion NSCLC. Further exploration of the biology of the various fusion partners of RET will help to understand this observation. Overall, the antitumour activity was comparable with other multikinase RET inhibitors, such as cabozantinib, vandetanib and lenvatinib.
BLU-667 is a next-generation highly selective RET inhibitor with preclinical activity to the most common RET fusion, KIF5B-RET and CCDC6-RET, and activating mutation, C643W, M918T and V804L/M, and WT RET at sub-nanomolar concentrations. With VEGFR2 sparing, BLU-667 is anticipated not to have toxicities such as hypertension, thrombosis and haemorrhage. Proof of concept antitumour activity from the ongoing phase I study (ClinicalTrials.gov identifier: NCT03037385) has been reported in two patients with medullary thyroid cancer with RET mutation. In addition, 4 PRs and 1 uPR were reported in 11 patients with NSCLC. Most toxicity was grade 1, including constipation (23%), elevated AST/ALT (16%), diarrhoea (14%), fatigue, elevated creatinine, neutrophilia and hypertension (12% each). Overall, three patients experienced grade 3 toxicity: elevation in ALT, hypertension and tumour lysis syndrome.184,185
LOXO-292 is another next-generation RET inhibitor with preclinical antitumour activity to various RET 5′ fusion partners, including KIF5B and CCDC6. It demonstrated better response and survival than cabozantinib and ponatinib in xenograft or orthotopic models. LIBERETTO-1 is a dose-escalation phase I study of LOXO-292 in patients with solid tumours who have failed or were not candidates for standard therapy. A total of 82 patients were enrolled at doses from 20 mg to 240 mg once or twice daily. Patients had a median of three previous lines of therapy. A total of 45% of patients had no previous RET inhibitor. The most commonly fusion partners were KIF5B (60.6%), followed by CCDC6 (26.3%). DLTs were observed in four patients at 240 mg twice daily: grade 3 diarrhoea, elevation of AST/ALT, thrombocytopenia and tumour lysis syndrome. The RDII was determined to be 160 mg twice daily. The majority of the treatment-related toxicity were grade 1, including diarrhoea (23%), fatigue (22%), dry mouth (21%), constipation (20%), hypomagnesaemia (13%), cough (12%), headache (12%) and nausea (12%). The grade 3 treatment-related toxicities reported were diarrhoea (1%) and headache (1%). A total of 68% of the 38 patients with RET fusion-positive mNSCLC enrolled had a PR. A response for ⩾6 months was reported in 92% of the responders. Primary progression was only observed in two patients. Furthermore, all four patients with measurable intracranial metastases responded. Over 90% of patients had a decrease in circulating tumour RET fusion DNA. The study is currently enrolling patients with RET fusion-positive solid tumours with ⩾1 previous standard therapy, treatment-naïve RET fusion-positive solid tumours, RET-mutated medullary thyroid carcinoma with ⩾1 previous therapy, treatment-naïve RET-mutated medullary thyroid cancer and RET-altered solid tumours without measurable lesions or with RET fusion detected in circulating DNA (ClinicalTrials.gov identifier: NCT03157128).186
To date, the antitumour activity of RET-specific inhibitors, LOXO292 and BLU-667, is encouraging with manageable toxicity. Tumour and plasma biopsy collected will help to understand the secondary resistance, which will likely include both gatekeeper and solvent mutations as well as bypass pathway activation such as EGFR and RAS.
ROS1 fusion
ROS1 is a kinase receptor that has significant structural homology to ALK. ROS1 fusion activates downstream RAS/RAF/MAPK, PI3K/AKT/mTOR and STAT-3, and leads to cell growth, proliferation and survival. Multiple 5′ fusion partners have been identified in NSCLC and other cancers, which may impact on the therapeutic benefit to ROS1 inhibitors.196 ROS1 fusion is found in 1–2% of lung adenocarcinoma and these patients are usually younger with a median age of 48.9 years, nonsmokers, female, Asian and in the advanced setting.197–199 A total of 36% of patients with ROS1 fusions presented with brain metastases, which was similar to that reported in ALK and other driver-mutation-positive mNSCLC.200 Ng and colleagues also reported a higher incidence of thromboembolic disease in patients with ROS1 (34.7%) than patients with ALK (22.3%), EGFR (13.7%) or K-RAS (18.4%) mutations.201 Overall, patients with ROS1 may have a better outcome with mOS of 3 years in those who had received only standard chemotherapy and over 5 years in those with both chemotherapy and crizotinib (Table 3).202
Crizotinib is the first TKI reported to have significant activity in ROS1 fusion mNSCLC. In this phase Ib/II study, the ORR was 72%, mPFS was 19.2 months and 1-year OS rate was 85% in the 50 patients with ROS1 fusion. A total of seven 5′ fusion partners were identified with two being novel, LIMA1 and MSN, which had no differential benefit to crizotinib.203 Unfortunately, the brain was the first site of treatment failure in patients with ROS1 treated with crizotinib at even higher incidence than crizotinib-treated ALK+ mNSCLC (47% versus 33%).200 The Korean investigators also reported an impressive ORR of 84%, mPFS of 19.3 months and mOS of 24 months for ceritinib in ROS1 inhibitor-naïve mNSCLC. Overall, five out of nine patients with documented intracranial metastases responded. Toxicity was not different from that in the patients who were ALK positive. A total of 15 patients underwent FISH, IHC and next-generation sequencing (NGS) for ROS1. A patient who was FISH+ with negative IHC and NGS progressed after 1 month, whereas three additional patients who were FISH+ who were negative by NGS or IHC had at least SD.152 Thus far, there is no clear evidence whether FISH, NGS or IHC is the superior companion diagnostic or a combination of these diagnostic tests is required.
Resistance mechanism to patients with ROS1 treated with crizotinib includes solvent front mutation,204–209 G2032R, D2033N, and gatekeeper mutation, G2026N, and L2155S,208 c-KIT mutation210 and upregulation of RAS211 or the EGFR pathway.197 Next-generation ROS1 inhibitors to the secondary mutation are in clinical development, including for example, lorlatinib, entrectinib and repotrectinib.
Lorlatinib is a selective ALK/ROS1 inhibitor that has preclinical antitumour activity in G2032R and G2026M mutations. Lorlatinib is also shown to penetrate the blood–brain barrier.206 Shaw and colleagues reported the phase I study of lorlatinib in ALK or ROS1-positive mNSCLC, with the RDII at 100 mg twice daily. Only one dose-limiting grade 2 neurocognitive change was observed. The most common toxicities were hypercholesterolaemia (72%), hypertriglyceridaemia (39%), peripheral neuropathy (39%), peripheral oedema (39%), speech changes (19%), cognitive disturbances (17%), elevation of lipase (17%), weight gain (17%), fatigue (15%), mood changes (15%), increase in amylase (13%), elevation of AST (13%), constipation (13%), tinnitus (13%), visual changes (13%) oedema (11%) and nausea (11%). Grade 3 or higher toxicities were hypercholesterolaemia, hypertriglyceridaemia, cognitive changes and elevation of amylase or AST, which each occurred in <10% of patients. A total of 12 patients with ROS1 were enrolled, out of which 7 had previous crizotinib. The preliminary ORR was 50% and the mDOR and mPFS were 12 and 7 months, respectively. Overall, 60% (3 of 5) patients had a response in their brain metastases.153
A total of 53 patients with ROS1 fusion were treated with entrectinib in the STARTRK-2, STARTRK-1 and ALKA372-001 studies, with an ORR of 77.4% and mPFS of 19 months. The mPFS in patients with or without brain metastases was 13.6 and 26.3 months, respectively. Intracranial responses to entrectinib was observed in 55% of the 23 patients with brain metastases.150
Repotrectinib has antitumour activity in G2032R and D2033N-resistant mutation ROS1 cell lines. Preliminary results of repotrectinib in 29 patients with ROS1 fusion mNSCLC reported an ORR of 11% and 71% in those with and without previous ROS1 inhibitors, respectively. A proof-of-principle response was reported in two patients with G2032R mutations and two additional patients had SD. A patient experienced an intracranial response.155 Enrolment in patients with ROS1 is ongoing (ClinicalTrials.gov identifier: NCT03093116).
In additional to the evaluation of resistance mechanism to second-generation ROS1 inhibitors, translational research to evaluate the differential benefit of various fusion partners to ROS1 inhibitors will be important. Clinical investigation with concurrent inhibition of ROS1 and bypass resistance mechanisms, such as EGFR and RAS, is warranted.212
Conclusion
Many novel driver mutations are or have been investigated in mNSCLC, with some successes and some failures. With better understanding of the biology of various subtypes of each driver mutation will help not only to choose the right inhibitor for the right patient, but also to elucidate the respective resistance mechanisms and their therapeutic strategies. These goals can only be achieved through serial tumour or plasma biopsy and multiplex molecular testing. In addition, the optimal sequence of the inhibitors for each driver mutation remains to be determined. It is not inconceivable that different molecular subtype of each driver mutation will require a different sequence of inhibitors. It is still not clear whether selective or multitargeted kinase inhibitors have superior antitumour activity; however, selective inhibitors seem to have more favourable toxicity profiles. Activation of bypass pathways represents an important resistance mechanism to driver mutation inhibitors. Further preclinical and clinical evaluation of concurrent inhibition of the bypass pathways and driver mutation is warranted. These efforts may both delay the emergence of resistance or further improve therapeutic benefits from an existing treatment strategy and overcome some secondary resistance.
Footnotes
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author declares that there is no conflict of interest.
ORCID iD: Quincy S. Chu  https://orcid.org/0000-0003-4814-3126
https://orcid.org/0000-0003-4814-3126
References
- 1. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 2. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open-label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 4. Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012; 30: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–742. [DOI] [PubMed] [Google Scholar]
- 6. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 7. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 8. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–222. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017; 18: 1454–1466. [DOI] [PubMed] [Google Scholar]
- 10. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018; 36: 2244–2250. [DOI] [PubMed] [Google Scholar]
- 11. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 12. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561–566. [DOI] [PubMed] [Google Scholar]
- 13. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385–2394. [DOI] [PubMed] [Google Scholar]
- 14. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371: 2167–2177. [DOI] [PubMed] [Google Scholar]
- 15. Solomon BJ, Kim DW, Wu YL, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018; 36: 2251–2258. [DOI] [PubMed] [Google Scholar]
- 16. Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017; 18: 874–886. [DOI] [PubMed] [Google Scholar]
- 17. Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017; 389: 917–929. [DOI] [PubMed] [Google Scholar]
- 18. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017; 390: 29–39. [DOI] [PubMed] [Google Scholar]
- 19. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377: 829–838. [DOI] [PubMed] [Google Scholar]
- 20. Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018; 29: 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 2018; 379: 2027–2039. [DOI] [PubMed] [Google Scholar]
- 22. Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol 2015; 10: 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barlesi F, Mazières J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT). Lancet 2016; 387: 1415–1426. [DOI] [PubMed] [Google Scholar]
- 24. Ku BM, Heo MH, Kim JH, et al. Molecular screening of small biopsy samples using next-generation sequencing in Korean patients with advanced non-small cell lung cancer: Korean lung cancer consortium (KLCC-13-01). J Pathol Transl Med 2018; 52: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volckmar AL, Leichsenring J, Kirchner M, et al. Combined targeted DNA and RNA sequencing of advanced NSCLC in routine molecular diagnostics: analysis of the first 3,000 Heidelberg cases. Int J Cancer 2019; 145: 649–661. [DOI] [PubMed] [Google Scholar]
- 26. Ascierto PA, Puzanov I, Agarwala SS, et al. Perspectives in melanoma: meeting report from the Melanoma bridge (30 November-2 December, 2017, Naples, Italy). J Transl Med 2018; 16: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011; 29: 2046–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011; 29: 3574–3579. [DOI] [PubMed] [Google Scholar]
- 29. Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013; 19: 4532–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of non-small cell lung carcinomas with BRAF mutations. Ann Oncol 2014; 25: 138–142. [DOI] [PubMed] [Google Scholar]
- 31. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014; 311: 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tissot C, Couraud S, Tanguy R, et al. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016; 91:23–28. [DOI] [PubMed] [Google Scholar]
- 33. Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the lung cancer mutation consortium. Cancer 2015; 121: 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baik CS, Myall NJ, Wakelee HA. Targeting. Oncologist 2017; 22: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joshi M, Rice SJ, Liu X, et al. Trametinib with or without vemurafenib in BRAF mutated non-small cell lung cancer. PLoS One 2015; 10: e0118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015; 373: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Planchard D, Kim TM, Mazières J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016; 17: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016; 17: 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF. Lancet Oncol 2017; 18: 1307–1316. [DOI] [PubMed] [Google Scholar]
- 40. Hainsworth JD, Bose R, Sweeney C, et al. Targeted therapy for non-small cell lung cancer (NSCLC) with HER2, BRAF or Hedghodge alterations: interim data from mypathway. J Clin Oncol 2017; 35: 9073. [Google Scholar]
- 41. Girotti MR, Lopes F, Preece N, et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell 2015; 27: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng SB, Henry JR, Kaufman MD, et al. Inhibition of RAF isoforms and active dimers by LY3009120 leads to anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell 2015; 28: 384–398. [DOI] [PubMed] [Google Scholar]
- 43. Yao Z, Torres NM, Tao A, et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 2015; 28: 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang C, Spevak W, Zhang Y, et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 2015; 526: 583–586. [DOI] [PubMed] [Google Scholar]
- 45. Desai J, Gan H, Barrow C, et al. A phase IB study of RAF dimer inhibitor BGB-283 in patients with B-RAF or K-RAS/N-RAS mutated solid tumors. In: AACR Annual Meeting, 1–5 April 2017; Washington, DC, Abstract CT002. [Google Scholar]
- 46. Janku F, Vaishampayan U, Khemka V, et al. Results of a phase I study of PLX8349, a next-generation BRAF inhibitor, in refractory solid tumors. In: AACR-NCI-EORTC international conference: molecular targets and cancer therapeutics, Dublin, Ireland, 13–16 November 2018, Eur J Cancer, Elsevier. Abstract B176. [Google Scholar]
- 47. Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J 2010; 277: 301–308. [DOI] [PubMed] [Google Scholar]
- 48. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013; 12: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013; 8: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013; 5: 216ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta 2010; 1804: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuza Y, Glatt KA, Jiang J, et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol Ther 2007; 6: 661–667. [DOI] [PubMed] [Google Scholar]
- 53. Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res 2008; 14: 4877–4882. [DOI] [PubMed] [Google Scholar]
- 54. Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 2011; 17: 3812–3821. [DOI] [PubMed] [Google Scholar]
- 55. Naidoo J, Sima CS, Rodriguez K, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib. Cancer 2015; 121: 3212–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010; 28: 3076–3083. [DOI] [PubMed] [Google Scholar]
- 57. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–151. [DOI] [PubMed] [Google Scholar]
- 58. Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018; 24: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heymach J, Negaro M, Robichaux J, et al. A phase II trial of poziotinib in EGFR and HER2 exon 20 mutation non-small cell lung cancer (NSCLC). In: World conference in lung cancer, Toronto, Canada, 23–26 September 2018, Abstract OA02.06. [Google Scholar]
- 60. Gonzalvez F, Zhu X, Hunag WS, et al. AP32788, a potent, selective inhibitor of EGFR and HER2 oncogenic mutants, including exon 20 insertions, in preclinical models. In: AACR 107th Annual Meeting, 16–20 April 2016, New Orleans, LA, Abstract 2644. [Google Scholar]
- 61. Doebele RC, Riely GJ, Spira AI, et al. First report of safety, PK, preliminary antitumor activity of the oral EGFR/HER2 exon 20 inhibitor TAK788 (AP32788) in non-small cell lung cancer (NSCLC). Am Soc Clin Oncol 2018; 36(Suppl. 15): Abstract 9015. [Google Scholar]
- 62. Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010; 10: 116–129. [DOI] [PubMed] [Google Scholar]
- 63. Tiseo M, Gelsomino F, Alfieri R, et al. FGFR as potential target in the treatment of squamous non small cell lung cancer. Cancer Treat Rev 2015; 41: 527–539. [DOI] [PubMed] [Google Scholar]
- 64. Ahmad I, Iwata T, Leung HY. Mechanisms of FGFR-mediated carcinogenesis. Biochim Biophys Acta 2012; 1823: 850–860. [DOI] [PubMed] [Google Scholar]
- 65. Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010; 2: 62ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dutt A, Ramos AH, Hammerman PS, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One 2011; 6: e20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim HR, Kim DJ, Kang DR, et al. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. J Clin Oncol 2013; 31: 731–737. [DOI] [PubMed] [Google Scholar]
- 68. Liao RG, Jung J, Tchaicha J, et al. Inhibitor-sensitive FGFR2 and FGFR3 mutations in lung squamous cell carcinoma. Cancer Res 2013; 73: 5195–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chandrani P, Prabhash K, Prasad R, et al. Drug-sensitive FGFR3 mutations in lung adenocarcinoma. Ann Oncol 2017; 28: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol 2017; 35: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tabernero J, Bahleda R, Dienstmann R, et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol 2015; 33: 3401–3408. [DOI] [PubMed] [Google Scholar]
- 72. Paik PK, Shen R, Berger MF, et al. A phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers. Clin Cancer Res 2017; 23: 5366–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Smyth EC, Turner NC, Peckitt C, et al. Phase II multicenter proof of concept study of AZD4547 in FGFR amplified tumours. J Clin Oncol 2015; 33(Suppl. 15): Abstract 2508. [Google Scholar]
- 74. Aggarwal C, Redman MW, Lara P, et al. Phase II study of the FGF inhibitor AZD4547 in previously treated patients with FGF pathway-activated stage IV squamous cell lung cancer (SqNSCLC): LUNG-MAP substudy SWOG 1400D. J Clin Oncol 2017; 35(Suppl. 15): Abstract 9055. [Google Scholar]
- 75. Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004; 431: 525–526. [DOI] [PubMed] [Google Scholar]
- 76. Buttitta F, Barassi F, Fresu G, et al. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int J Cancer 2006; 119: 2586–2591. [DOI] [PubMed] [Google Scholar]
- 77. Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013; 31: 1997–2003. [DOI] [PubMed] [Google Scholar]
- 78. Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012; 18: 4910–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li BT, Ross DS, Aisner DL, et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol 2016; 11: 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li C, Sun Y, Fang R, et al. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol 2012; 7: 85–89. [DOI] [PubMed] [Google Scholar]
- 81. Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004; 15: 19–27. [DOI] [PubMed] [Google Scholar]
- 82. Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 2015; 26: 1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. De Grève J, Moran T, Graas MP, et al. Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer 2015; 88: 63–69. [DOI] [PubMed] [Google Scholar]
- 84. Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC with HER2 mutations: results of the prospective, open-label phase II NICHE trial of European thoracic oncology platform (ETOP). J Thorac Oncol 2019; 14: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 85. Lai WV, Lebas L, Barnes TA, et al. Afatinib in patients with metastatic or recurrent HER2-mutant lung cancers: a retrospective international multicentre study. Eur J Cancer 2019; 109: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol 2018; 36: 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Peters S, Stahel R, Bubendorf L, et al. Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non-small cell lung cancer: efficacy, safety, and biomarkers. Clin Cancer Res 2019; 25: 64–72. [DOI] [PubMed] [Google Scholar]
- 88. Hotta K, Aoe K, Kozuki T, et al. A phase II study of trastuzumab emtansine in HER2-positive non-small cell lung cancer. J Thorac Oncol 2018; 13: 273–279. [DOI] [PubMed] [Google Scholar]
- 89. Tsurutani J, Park H, Doi T, et al. Updated results of the phase 1 study of DS-8201a in HER2-expressing or -mutated advanced non-small-cell lung cancer. In: World Conference in Lung Cancer, Toronto, Canada, 23–26 September 2018, Abstract OA02.07. [Google Scholar]
- 90. Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016; 27: 281–286. [DOI] [PubMed] [Google Scholar]
- 91. Aviel-Ronen S, Blackhall FH, Shepherd FA, et al. K-ras mutations in non-small-cell lung carcinoma: a review. Clin Lung Cancer 2006; 8: 30–38. [DOI] [PubMed] [Google Scholar]
- 92. Adderley H, Blackhall FH, Lindsay CR. KRAS-mutant non-small cell lung cancer: converging small molecules and immune checkpoint inhibition. EBioMedicine 2019; 41: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Piva S, Ganzinelli M, Garassino MC, et al. Across the universe of K-RAS mutations in non-small-cell-lung cancer. Curr Pharm Des 2014; 20: 3933–3943. [DOI] [PubMed] [Google Scholar]
- 94. Meng D, Yuan M, Li X, et al. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: a systematic review with meta-analysis. Lung Cancer 2013; 81: 1–10. [DOI] [PubMed] [Google Scholar]
- 95. Rosell R, Monzó M, Pifarré A, et al. Molecular staging of non-small cell lung cancer according to K-ras genotypes. Clin Cancer Res 1996; 2: 1083–1086. [PubMed] [Google Scholar]
- 96. Vega F, Iniesta P, Caldes T, et al. Association of K-ras codon 12 transversions with short survival in non-small cell lung cancer. Int J Oncol 1996; 9: 1307–1311. [DOI] [PubMed] [Google Scholar]
- 97. Jänne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA 2017; 317: 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013; 14: 38–47. [DOI] [PubMed] [Google Scholar]
- 99. Aktas H, Cai H, Cooper GM. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol 1997; 17: 3850–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Puyol M, Martín A, Dubus P, et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 2010; 18: 63–73. [DOI] [PubMed] [Google Scholar]
- 101. Mao CQ, Xiong MH, Liu Y, et al. Synthetic lethal therapy for KRAS-mutant non-small-cell lung carcinoma with nanoparticle-mediated CDK4 siRNA delivery. Mol Ther 2014; 22: 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Goldman JW, Mazières J, Barlesi F, et al. A randomized phase 3 study of abemaciclib versus erlotinib in previously treated patients with stage IV NSCLC with KRAS mutation: JUNIPER. J Clin Oncol 2018; 36(Suppl. 15): Abstract 9025. [Google Scholar]
- 103. Sullivan RJ, Amaria RN, Lawrence DP, et al. Phase 1b dose-escalation study trametinib (MEKi) and palbociclib (CDK4/6i) in patients with advanced solid tumors. In: AACR-NCI-EORTC international conference: molecular targets and cancer therapeutics, Boston, MA, 5 November–9 November 2015, Abstract PR06. [Google Scholar]
- 104. Shapiro GI, Hilton J, Gandi L, et al. Phase I dose-escalation study of the CDK4/6 inhibitor palbociclib in combination with the MEK inhibitor PD-0325901 in patients with RAS mutant solid tumors. Cancer Res 2017; 77(Suppl): Abstract CT046. [Google Scholar]
- 105. Drilon A, Camidge DR, Ou SH I, et al. Antitumor activity and safety of crizotinib in patients with advanced MET exon 14-aletered non-small cell lung cancer (NSCLC). In: American Society of Clinical Oncology Annual Meeting, Chicago, IL, 3–7 June 2016, Abstract 108. [Google Scholar]
- 106. Camidge DR, Ou S-H I, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). In: Annual Meeting American Society of Clinical Oncology, Chicago, IL, 30 May–3 June 2014, Abstract A8001. [Google Scholar]
- 107. Camidge DR, GA O, Clark JW, et al. Crizotinib in patients (pts) with MET-amplified non-small cell lung cancer (NSCLC): updated safety and efficacy findings from a phase 1 trial. J Clin Ocnol 2018; 36(Suppl. 15): Abstract 9062. [Google Scholar]
- 108. Wolf J, Seto T, Han J, et al. Results of the GEOMETRY mono-1 phase II study for evaluation of the MET inhibitor capmatinib (INC280) in patients with METex14 mutated advanced non-small cell lung cancer (NSCLC). In: ESMO congress, Munich, Germany, 19–23 October 2018, Abstract LBA 52. [Google Scholar]
- 109. Felip E, Horn L, Patel JD, et al. Tepotinib in patients with advanced non-small cell lung cancer (NSCLC) harboring MET exon 14-skipping mutation: phase II trial. J Clin Oncol 2018; 36(Suppl. 15): Abstract 9016. [Google Scholar]
- 110. Lu S, Fang J, Cao L, et al. Preliminary efficacy and safety results of savolitinib treating patients with pulmonary sarcomatoid carcinoma (PSC) and other types of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping mutations. In: Annual Meeting American Association Cancer Research, Atlanta, GA, 29 March–3 April 2019, Abstract CT031. [Google Scholar]
- 111. Lee GD, Lee SE, Oh DY, et al. MET exon 14 skipping mutations in lung adenocarcinoma: clinicopathologic implications and prognostic values. J Thorac Oncol 2017; 12: 1233–1246. [DOI] [PubMed] [Google Scholar]
- 112. Drilon A. MET exon 14 alterations in lung cancer: exon skipping extends half-life. Clin Cancer Res 2016; 22: 2832–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015; 5: 850–859. [DOI] [PubMed] [Google Scholar]
- 114. Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol 2016; 34: 721–730. [DOI] [PubMed] [Google Scholar]
- 115. Awad MM. Impaired c-Met receptor degradation mediated by MET exon 14 mutations in non-small-cell lung cancer. J Clin Oncol 2016; 34: 879–881. [DOI] [PubMed] [Google Scholar]
- 116. Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016; 22: 3048–3056. [DOI] [PubMed] [Google Scholar]
- 117. Caparica R, Yen CT, Coudry R, et al. Responses to crizotinib can occur in high-level MET-amplified non-small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol 2017; 12: 141–144. [DOI] [PubMed] [Google Scholar]
- 118. Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015; 5: 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ou SI, Young L, Schrock AB, et al. Emergence of preexisting MET Y1230C mutation as a resistance mechanism to crizotinib in NSCLC with MET exon 14 skipping. J Thorac Oncol 2017; 12: 137–140. [DOI] [PubMed] [Google Scholar]
- 120. Wang SXY, Zhang BM, Wakelee HA, et al. Case series of MET exon 14 skipping mutation-positive non-small-cell lung cancers with response to crizotinib and cabozantinib. Anticancer Drugs 2019; 30: 537–541. [DOI] [PubMed] [Google Scholar]
- 121. Suzawa K, Offin M, Lu D, et al. Activation of KRAS mediates resistance to targeted therapy in MET exon 14-mutant non-small cell lung cancer. Clin Cancer Res 2019; 25: 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Okuda K, Sasaki H, Yukiue H, Yano M, Fujii Y. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci 2008; 99: 2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007; 104: 20932–20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cappuzzo F, Jänne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 2009; 20: 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Onozato R, Kosaka T, Kuwano H, et al. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol 2009; 4: 5–11. [DOI] [PubMed] [Google Scholar]
- 126. Chen HJ, Mok TS, Chen ZH, et al. Clinicopathologic and molecular features of epidermal growth factor receptor T790M mutation and c-MET amplification in tyrosine kinase inhibitor-resistant Chinese non-small cell lung cancer. Pathol Oncol Res 2009; 15: 651–658. [DOI] [PubMed] [Google Scholar]
- 127. Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol 2009; 27: 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schuler MH, Berardi R, Lim WT, et al. Phase (Ph) I study of the safety and efficacy of the cMET inhibitor capmatinib (INC280) in patients (pts) with advanced cMET+ non-small cell lung cancer (NSCLC). In: Annual Meeting American Society of Clinical Oncology 2016; 3 and 5 June 2016; Chicago, IL, Abstract 9067. [Google Scholar]
- 129. Fernandez-Cuesta L, Plenker D, Osada H, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov 2014; 4: 415–422. [DOI] [PubMed] [Google Scholar]
- 130. Liu SV, Feldman RA, Borghaei H, et al. Incidecne of Neuregulin 1 (NRG1) gene fusions across tumor types. In: Annual Meeting American Society of Clinical Oncology, 1–5 June 2018, Chicago, IL, Abstract 12084. [Google Scholar]
- 131. Trombetta D, Graziano P, Scarpa A, et al. Frequent NRG1 fusions in Caucasian pulmonary mucinous adenocarcinoma predicted by phospho-Erb3 expression. Oncotarget 2018; 9: 9661–9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jones MR, Lim H, Shen Y, et al. Successful targeting of the NRG1 pathway indicates novel treatment strategy for metastatic cancer. Ann Oncol 2017; 28: 3092–3097. [DOI] [PubMed] [Google Scholar]
- 133. Gay ND, Wang Y, Beadling C, et al. Durable response to afatinib in lung adenocarcinoma harboring NRG1 gene fusions. J Thorac Oncol 2017; 12: e107–e110. [DOI] [PubMed] [Google Scholar]
- 134. Cheema PK, Doherty M, Tsao MS. A case of invasive mucinous pulmonary adenocarcinoma with a CD74-NRG1 fusion protein targeted with afatinib. J Thorac Oncol. 2017; 12: e200–e202. [DOI] [PubMed] [Google Scholar]
- 135. Drilon A, Somwar R, Mangatt BP, et al. Response to ERBB3-directed targeted therapy in NRG1-rearranged cancers. Cancer Discov 2018; 8: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tirunagaru VG, Estrada-Bernal A, Yu H, et al. Tarloxotinib exhibits potent activity in NRG1 fusion and rearranged cancers. In: Annual Meeting American Association Cancer Research, 29 March–3 April 2019, Atlanta, GA, Abstract 2202. [Google Scholar]
- 137. Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett 2001; 169: 107–114. [DOI] [PubMed] [Google Scholar]
- 138. Deinhardt K, Chao MV. Trk receptors. Handb Exp Pharmacol 2014; 220: 103–119. [DOI] [PubMed] [Google Scholar]
- 139. Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol 1994; 25: 1386–1403. [DOI] [PubMed] [Google Scholar]
- 140. Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med 2013; 19: 1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun 2014; 5: 4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Passiglia F, Caparica R, Giovannetti E, et al. The potential of neurotrophic tyrosine kinase (NTRK) inhibitors for treating lung cancer. Expert Opin Investig Drugs 2016; 25: 385–392. [DOI] [PubMed] [Google Scholar]
- 143. Marchetti A, Felicioni L, Pelosi G, et al. Frequent mutations in the neurotrophic tyrosine receptor kinase gene family in large cell neuroendocrine carcinoma of the lung. Hum Mutat 2008; 29: 609–616. [DOI] [PubMed] [Google Scholar]
- 144. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018; 378: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lassen U, Albert CM, Kummar S, et al. Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach. Ann Oncol 2018; 29(Suppl. 8): Abstract 4090. [Google Scholar]
- 146. Drilon A, Kummar S, Moreno V, et al. Activity of larotrectinib in TRK fusion lung cancer. In: European lung cancer conference, 10–13 April 2019, Ann Oncol by Oxford University Press. Geneva, Switzerland, Abstr 111O. [Google Scholar]
- 147. Drilon A, Siena S, Ou SI, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov 2017; 7: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Paz-Ares L, Doebele RC, Farago AF, et al. Entrectinib in NTRK fusion-positive non-small cell lung cancer (NSCLC): integrated analysis of patients (pts) enrolled in STARTRK-2, STARTRK-1 and ALKA-372-001. In: European lung cancer conference, 10–13 April 2019, Ann Oncol by Oxford University Press. Geneva, Switzerland, Abstr 113O. [Google Scholar]
- 149. Demetri GD, Paz-Ares L, Farago AF, et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive tumours: pooled analysis of STARTRK-2, STARTRK-1 amd ALKA-372-001. In: European lung cancer conference, 10–13 April 2018, Ann Oncol by Oxford University Press. Geneva, Switzerland, Abstr LAB17. [Google Scholar]
- 150. Doebele RC, Ahn M, Siena S, et al. Efficacy and safety of entrectinib in local advanced or metastatic ROS1 fusion positive non-small cell lung cancer (NSCLC). In: World conference on lung cancer, Toronto, Canada, 23–26 September 2018 J Thorac Oncol by Elsevier, Abstr OA02.01. [Google Scholar]
- 151. Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov 2018; 8: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 152. Lim SM, Kim HR, Lee JS, et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol 2017; 35: 2613–2618. [DOI] [PubMed] [Google Scholar]
- 153. Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase I trial. Lancet Oncol 2017; 18: 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Drilon A, Nagasubramanian R, Blake JF, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov 2017; 7: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Drilon AE, Ou S-HI, Cho BC, et al. A Phase I study of the next-generation ALK/ROS1/TRK inhibitor ropotrectinib (TPX-0005) in patients with advanced ALK/ROS1/NTRK+ cancers (TRIDENT-1). In. Annual Meeting American Society of Clinical Oncology, 1–5 June 2018, Chicago, IL. [Google Scholar]
- 156. Yuan TLand Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008; 27: 5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Solomon Band Pearson RB. Class IA phosphatidylinositol 3-kinase signaling in non-small cell lung cancer. J Thorac Oncol 2009; 4: 787–791. [DOI] [PubMed] [Google Scholar]
- 158. Dienstmann R, Rodon J, Serra V, et al. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther 2014; 13: 1021–1031. [DOI] [PubMed] [Google Scholar]
- 159. Samuels Yand Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle 2004; 3: 1221–1224. [DOI] [PubMed] [Google Scholar]
- 160. Kawano O, Sasaki H, Endo K, et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer 2006; 54: 209–215. [DOI] [PubMed] [Google Scholar]
- 161. Yamamoto H, Shigematsu H, Nomura M, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res 2008; 68: 6913–6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sawa K, Koh Y, Kawaguchi T, et al. PIK3CA mutation as a distinctive genetic feature of non-small cell lung cancer with chronic obstructive pulmonary disease: a comprehensive mutational analysis from a multi-institutional cohort. Lung Cancer 2017; 112: 96–101. [DOI] [PubMed] [Google Scholar]
- 163. Paik PK, Shen R, Won H, et al. Next-generation sequencing of stage IV squamous cell lung cancers reveals an association of PI3K aberrations and evidence of clonal heterogeneity in patients with brain metastases. Cancer Discov 2015; 5: 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Malanga D, Scrima M, De Marco C, et al. Activating E17K mutation in the gene encoding the protein kinase AKT1 in a subset of squamous cell carcinoma of the lung. Cell Cycle 2008; 7: 665–669. [DOI] [PubMed] [Google Scholar]
- 165. Do H, Solomon B, Mitchell PL, et al. Detection of the transforming AKT1 mutation E17K in non-small cell lung cancer by high resolution melting. BMC Res Notes 2008; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bleeker FE, Felicioni L, Buttitta F, et al. AKT1(E17K) in human solid tumours. Oncogene 2008; 27: 5648–5650. [DOI] [PubMed] [Google Scholar]
- 167. Marsit CJ, Zheng S, Aldape K, et al. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum Pathol 2005; 36: 768–776. [DOI] [PubMed] [Google Scholar]
- 168. Jin G, Kim MJ, Jeon HS, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer 2010; 69: 279–283. [DOI] [PubMed] [Google Scholar]
- 169. Soria JC, Lee HY, Lee JI, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res 2002; 8: 1178–1184. [PubMed] [Google Scholar]
- 170. Kohno T, Takahashi M, Manda R, et al. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer 1998; 22: 152–156. [DOI] [PubMed] [Google Scholar]
- 171. Lee SY, Kim MJ, Jin G, et al. Somatic mutations in epidermal growth factor receptor signaling pathway genes in non-small cell lung cancers. J Thorac Oncol 2010; 5: 1734–1740. [DOI] [PubMed] [Google Scholar]
- 172. Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010; 28: 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A 2001; 98: 10314–10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Vansteenkiste JF, Canon JL, De Braud F, et al. Safety and efficacy of buparlisib (BKM120) in patients with PI3K pathway-activated non-small cell lung cancer: results from the phase II BASALT-1 study. J Thorac Oncol 2015; 10: 1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wade JL, Langer CJ, Redman M, et al. A phase II study of GDC-0032 (taselisib) fpr previously treated PI3K positive patients with stage IV squamous cell lung cancer (SqNSCLC): LUNG-MAP substudy SWOG S1400B. J Clin Oncol 2017; 35(Suppl. 15): Abstract 9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Iyengar S, Clear A, Bödör C, et al. P110α-mediated constitutive PI3K signaling limits the efficacy of p110δ-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood 2013; 121: 2274–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol 2016; 34: 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Gainor JF, Shaw AT. The new kid on the block: RET in lung cancer. Cancer Discov 2013; 3: 604–606. [DOI] [PubMed] [Google Scholar]
- 179. Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 2013; 18: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Nakamura T, Ishizaka Y, Nagao M, et al. Expression of the ret proto-oncogene product in human normal and neoplastic tissues of neural crest origin. J Pathol 1994; 172: 255–260. [DOI] [PubMed] [Google Scholar]
- 181. Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016; 17: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Velcheti V, Hida T, Reckamp KL, et al. Phase 2 study of lenvatinib in patients with RET fusion-positive adenocarcinoma of the lung. Eur J Cancer 2017; 72(Suppl. 1): s178. [DOI] [PubMed] [Google Scholar]
- 183. Drilon A, Fu S, Patel MR, et al. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov 2019; 9: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov 2018; 8: 836–849. [DOI] [PubMed] [Google Scholar]
- 185. Subbiah V, Taylor M, Lin J, et al. Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase 1 study of advanced, RET-altered solid tumors. In: Annual Meeting of American Association Cancer Research, Chicago, IL, 15–18 April 2018, Abstract: CT043. [Google Scholar]
- 186. Oxynard GR, Subbiah V, Park K, et al. Clinical activity of LOXO-292, a highly selective RET inhibitor, in patients with RET fusion+ non-small cell lung cancer: an update from ASCO 2018. In: World Conference on Lung Cancer, Toronto, Canada, 23–26 September 2018, Abstract: OA12:07. Same for 23. [Google Scholar]
- 187. Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012; 30: 4352–4359. [DOI] [PubMed] [Google Scholar]
- 188. Yokota K, Sasaki H, Okuda K, et al. KIF5B/RET fusion gene in surgically-treated adenocarcinoma of the lung. Oncol Rep 2012; 28: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 189. Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012; 18: 382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012; 18: 375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol 2017; 35: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2017; 5: 42–50. [DOI] [PubMed] [Google Scholar]
- 193. Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 2017; 28: 292–297. [DOI] [PubMed] [Google Scholar]
- 194. Lin JJ, Kennedy E, Sequist LV, et al. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J Thorac Oncol 2016; 11: 2027–2032. [DOI] [PubMed] [Google Scholar]
- 195. Nosaki K, Takeuchi T, Takahara S, et al. Safety of alectinib in non-small cell lung cancer patients with RET fusion (ALL-RET): results of the dose-finding portion of a phase 1/2 study. Ann Oncol 2017; 28: Abstract 419O. [Google Scholar]
- 196. Stumpfova M, Jänne PA. Zeroing in on ROS1 rearrangements in non-small cell lung cancer. Clin Cancer Res 2012; 18: 4222–4224. [DOI] [PubMed] [Google Scholar]
- 197. Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res 2012; 18: 4570–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012; 30: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zhang S, Yan B, Zheng J, et al. Gene status and clinicopathologic characteristics of lung adenocarcinomas with mediastinal lymph node metastasis. Oncotarget 2016; 7: 63758–63766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Patil T, Smith DE, Bunn PA, et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol 2018; 13: 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ng TL, Smith DE, Mushtaq R, et al. ROS1 gene rearrangements are associated with an elevated risk of peridiagnosis thromboembolic events. J Thorac Oncol 2019; 14: 596–605. [DOI] [PubMed] [Google Scholar]
- 202. Scheffler M, Schultheis A, Teixido C, et al. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget 2015; 6: 10577–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014; 371: 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Awad MM, Engelman JA, Shaw AT. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med 2013; 369: 1173. [DOI] [PubMed] [Google Scholar]
- 205. Davare MA, Saborowski A, Eide CA, et al. Foretinib is a potent inhibitor of oncogenic ROS1 fusion proteins. Proc Natl Acad Sci U S A 2013; 110: 19519–19524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second-generation ALK inhibitors in preclinical models. Cancer Cell 2015; 28: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res 2015; 21: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Song A, Kim TM, Kim DW, et al. Molecular changes associated with acquired resistance to crizotinib in ROS1-rearranged non-small cell lung cancer. Clin Cancer Res 2015; 21: 2379–2387. [DOI] [PubMed] [Google Scholar]
- 209. Drilon A, Somwar R, Wagner JP, et al. A novel crizotinib-resistant solvent-front mutation responsive to cabozantinib therapy in a patient with ROS1-rearranged lung cancer. Clin Cancer Res 2016; 22: 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Dziadziuszko R, Le AT, Wrona A, et al. An activating KIT mutation induces crizotinib resistance in ROS1-positive lung cancer. J Thorac Oncol 2016; 11: 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cargnelutti M, Corso S, Pergolizzi M, et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget 2015; 6: 5182–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wiesweg M, Eberhardt WEE, Reis H, et al. High prevalence of concomitant oncogene mutations in prospectively identified patients with ROS1-positive metastatic lung cancer. J Thorac Oncol 2017; 12: 54–64. [DOI] [PubMed] [Google Scholar]