Short abstract
Background
Epidemiological, preclinical, and non-interventional studies link vitamin D (VD) serum levels and disease activity in multiple sclerosis (MS). It is unclear whether high-dose VD supplementation can be used as an intervention to reduce disease activity.
Objectives
The study aimed to compare the effects of every other day high- (20,400 IU) versus low-dose (400 IU) cholecalciferol supplementation on clinical and imaging markers of disease activity in patients with relapsing–remitting MS or clinically isolated syndrome.
Methods
The EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial was a multicentre randomized/stratified actively controlled explorative phase 2a pilot trial with a double-blind intervention period of 18 months, add on to interferon-β1b.
Results
Fifty-three patients were randomized, and 41 patients completed the study. Cholecalciferol supplementation was well tolerated and safe in both arms. After 18 months, clinical (relapse rates, disability progression) and radiographical (T2-weighted lesion development, contrast-enhancing lesion development, brain atrophy) did not differ between both treatment arms. Post-study power calculations suggested that the sample size was too low to prove the hypothesis.
Conclusions
The results neither support nor disprove a therapeutic benefit of high-dose VD supplementation but provide a basis for sound sample size estimations in future confirmatory studies. www.clinicaltrials.gov/NCT01440062
Keywords: Multiple sclerosis, clinical trial, vitamin D, efficacy, treatment, supplementation
Introduction
Preclinical and epidemiological studies link 25-hydroxy (OH) vitamin D, hereafter referred to as vitamin D, serum levels to disease activity of multiple sclerosis (MS). In particular, associations of higher vitamin D levels in patients with MS with favourable clinical and magnetic resonance imaging (MRI) parameters of disease activity have been reported.1–3 Based on these data, MS clinicians commonly boost vitamin D levels in patients by oral cholecalciferol or ergocalciferol supplementation.
While safety data are rather unequivocal and suggest that vitamin D supplementation up to 280,000 IU/week is fairly safe,4 prospective data from interventional treatment trials of vitamin D supplementation on clinical efficacy, doses needed, and optimal serum levels in MS are sparse, inconsistent, and in part conflicting.5,6 A phase I/II randomized controlled open-label study comparing continuous intake of up to 40,000 IU/day or 4000 IU/day for 52 weeks in 49 patients with MS showed a significant reduction of annualized relapse rate in the high-dose arm.7 In contrast, two randomized, placebo-controlled and double-blind phase II trials from Finland and Norway, applying 20,000 IU/week cholecalciferol for 48 or 96 weeks, demonstrated no effect of vitamin D on relapse rates.8,9 In the Finish study, however, patients in the treatment group had significantly fewer gadolinium enhancing (gd+) lesions and a tendency to reduced disability accumulation.8 The placebo-controlled SOLAR study10 showed no effect of 14,000 IU cholecalciferol per day on the primary endpoint ‘no evidence of disease activity’ but a significant beneficial effect on the development of new lesions on cranial MRI and a trend towards a reduction of relapse rate.
The ‘efficacy of vitamin D supplementation in multiple sclerosis’ (EVIDIMS) clinical trial was an investigator-sponsored randomized actively controlled double-blind phase II trial of vitamin D supplementation in patients with MS. The detailed protocol has been published previously.11 Here, we report the major clinical and MRI results.
Patients and methods
Study design and ethics statement
The EVIDIMS clinical trial was a multicentre, randomized and stratified, active controlled, double-blind, explorative phase II trial. Two doses of vitamin D3 (cholecalciferol) were compared: in the high-dose arm participants received 20,400 IU (1 ml cholecalciferol oil (20,000 IU) + 1 tablet (400 IU)) every other day for 18 months, while in the low-dose arm 400 IU (1 ml placebo oil (0 IU) + 1 tablet (400 IU)) were given every other day. To rule out confounding effects of variable disease-modifying treatments, all participants were required to be on stable treatment with interferon-beta-1b, started at least 3 months prior to inclusion. Interferon-beta treatment was continued throughout the study.
Patients with relapsing–remitting (RR)MS or clinically isolated syndrome (CIS) were recruited at seven German study centres (Berlin (four centres), Potsdam, Halle, Teupitz; for details see supplementary information). After a short screening period eligible patients were stratified according to gender and vitamin D level (< or ≥20 ng/ml (50 nmol/l)) and then randomized 1:1 to either high-dose or low-dose treatment as an add-on to continuous treatment with IFN β-1b. Clinical evaluations including Expanded Disability Status Scale (EDSS)12 and MS Functional Composite (MSFC)13 were performed at screening and then every 6 months. Standardized MRI was performed at screening and after 12 and 18 months using identical hardware for all patients (at the Charité study centre, Berlin). Pharmacovigilance laboratory testing was done after 4 weeks and thereafter every 3 months.
The primary endpoint was the number of new T2-weighted (T2w) hyperintense lesions on brain MRI at month 18 compared with baseline. Major secondary endpoints were relapse rate and disability progression on the clinical side, T2w lesion volume, number of gd+ lesions, and brain atrophy on the MRI side, and number of adverse events on the safety side. More detailed information on the design and conduct of the EVIDIMS trial has been published previously,11 and additional information is provided as supplementary information. The study protocol (in German) is available from the corresponding author upon qualified request.
The study was approved by the German Federal Institute for Drugs and Medical Devices (BfArM, 4037578), as well as by the local ethics committees (11/0386-ZS EK 13) and is registered at European Clinical Trials Database (EudraCT 2011-002785-20) and ClinicalTrials.gov (NCT01440062). It was conducted strictly adhering to the study protocol and to applicable national laws (Arzneimittelgesetz, 14. Novelle, 2005), the Harmonized Tripartite Guideline for Good Clinical Practice (ICH GCP), and the principles of the Declaration of Helsinki in its applicable version. All participants gave written informed consent at screening prior to any study-related procedures.
Study population
The main inclusion criteria were: male and female patients with either CIS or a definite diagnosis of MS according to the 2005 revised McDonald criteria14 and a relapsing–remitting disease course; age at randomization 18–65 years; EDSS score 0–6; continuous treatment with IFN β1b for at least 3 months; and no relapse within 30 days prior to randomization. Important exclusion criteria were: relevant hepatopathy or renal dysfunction; history of sarcoidosis, nephrolithiasis, pseudo hypoparathyroidism; vitamin D supplementation >500 IU/day within 6 months prior to randomization; pregnancy; hypercalcaemia or urine calcium/creatinine ratio >1; concomitant medication with hydrochlorothiazide, digitoxin, digoxin, phenytoin, barbiturates; inability to provide informed consent; incompatibility with MRI procedures. A comprehensive listing of inclusion and exclusion criteria is provided in the supplementary information.
Outcome measures
Clinical evaluations and safety laboratory tests
All clinical evaluations and scorings were performed by trained study personnel blinded to the patients’ treatment allocations according to standardized operating procedures. MS relapses were defined as: (re)occurrence of new or previous central nervous system dysfunction in the absence of infections or hyperthermia, duration ≥24 h, time-lag from the onset of previous relapse ≥30 days. Disability was assessed by EDSS and MSFC.12,13 EDSS rating was performed by trained (Neurostatus) and board-certified physicians otherwise not involved in the management of study participants. MSFC scoring was done by trained study personnel. All study-related safety laboratory tests were done by Bioscientia laboratories (Bioscientia Institut für Medizinische Diagnostik GmbH, Ingelheim, Germany).
MRI
Standardized MRI was performed in all participants in one study centre at Charité - Universitätsmedizin Berlin, using a Magnetom TIM TRIO 3 Tesla MRI (Siemens Healthineers, Erlangen, Germany). A sagittal 3D SPACE T2w sequence (TR 5,000 ms, TE 502 ms, TI 900 ms, flip angle 9 degree, isotropic resolution 1 mm3, GRAPPA 2) was used to obtain T2w images. In addition, we applied a 3D fluid-attenuated inversion recovery sequence (SPACE-FLAIR, TR 6,000 ms, TE 388 ms, TI 2,100) with identical spatial parameters, and a high-resolution 3-dimensional T1w sequence (MPRAGE, TR 1,900 ms, TE 3.03 ms, TI 900 ms, flip angle 9 degree, isotropic resolution 1 mm3 GRAPPA 2) with identical spatial parameters. A 3D T1w sequence (VIBE, TR 4.8 ms, TE 2.2 ms, flip angle 9 degree, isotropic resolution 1 mm3 GRAPPA 2) with identical spatial parameters was applied 5 min after injection of 0.1 mmol/kg gd-DTPA (Gadovist, Bayer, Berlin, Germany). Raw data were transferred to a Linux workstation and processed following a semi-automated procedure, including an image coregistration (FMRIB’s Linear Image Registration Tool, FMRIB Analysis Group, University of Oxford, Oxford, UK) and inhomogeneity correction routine embedded into the MedX v.3.4.3 software package (Sensor Systems Inc., Sterling, VA, USA). T2w white matter lesion load, as well as number and volume of contrast-enhancing and hypointense lesions on T1w scans were routinely measured using the MedX v.3.4.3 software package. Brain atrophy (percentage brain volume change (PBVC) and normalized brain volume (NBV)) was estimated with SIENA,15 part of FSL.16 Thalamic volumetry was performed using FIRST (FMRIB Analysis Group, University of Oxford, Oxford, UK).
Statistical analyses
Due to lack of data on the effect of vitamin D on T2w lesions, no statistical sample size calculation was performed in advance using a given error of the 1st kind and stipulated power. A sample size of 80 patients (40 in each arm) was planned, based mainly on feasibility.
Due to the explorative character of the study, statistical testing has to be understood as explorative, and data analyses were mainly descriptive for all endpoints. For univariate independent group comparisons exact Mann–Whitney U tests and exact Chi-Square tests were used. Survival data such as MS relapses were analysed by Kaplan–Meier curves with appropriate log-rank tests and multiple Cox-regressions. For time series data, a nonparametric multivariate analysis of longitudinal data (MANOVA) in a two-factorial design was applied (1st factor (independent): treatment groups, 2nd factor (dependent): study visits). When appropriate, multivariate nonparametric analysis of covariance (MANCOVA) using baseline values and additional parameters including age, gender, and disease duration as covariates was complemented. The PBVC was adjusted using baseline NBV. Missing values were replaced by multiple imputations. Statistical significance was assumed at the p = 0.05 level. Because of the explorative nature of analyses, no adjustments for multiple comparisons were performed. Both intent-to-treat (ITT) and per-protocol (PP) analyses were carried out. The ITT group comprised 53 patients. The PP definition was regular study termination and protocol-conform study drug intake. The PP group comprised 38 patients. All calculations were performed using SAS Version 9.4 [TS1M3] Copyright 2002–2012 by SAS Institute Inc., Cary, NC, USA, IBM SPSS Statistics, Version 25, Copyright 1989, 2010 SPSS Inc., an IBM Company, Chicago, IL, USA and The R Project for Statistical Computing, Version 3.0.2 (2017-04-21).
Results
Study population
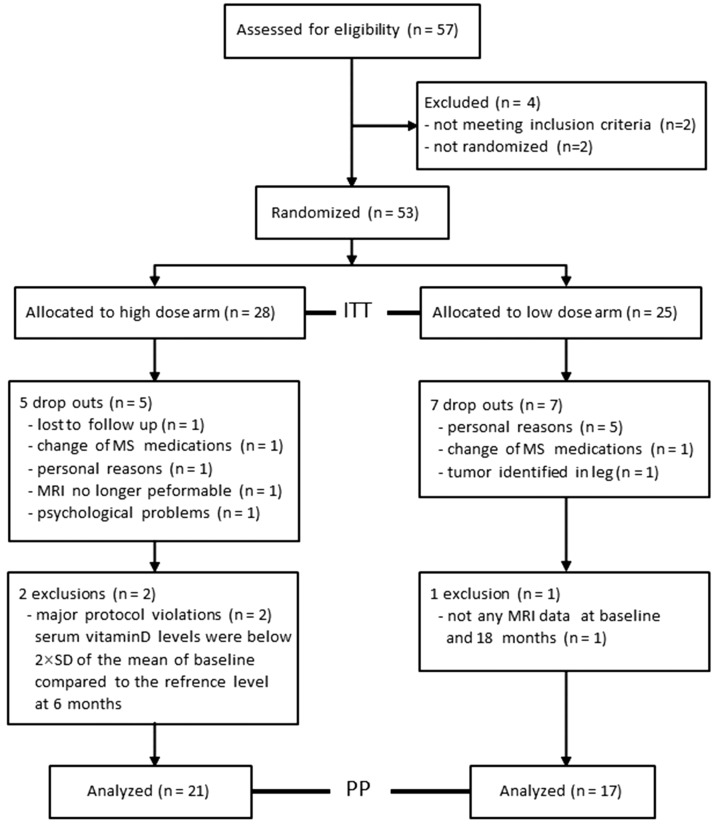
After starting recruitment, the study was terminated early. Fifty-three patients were randomized and included in the ITT analyses, 28 in the high-dose and 25 in the low-dose arm. Demographic baseline characteristics (ITT population) are shown in Table 1. T2w lesion volume was higher in the low-dose arm (p = 0.01), and thalamic volume was higher in the high-dose arm (p = 0.048). As shown in Figure 1, 41 participants completed the study. The number of dropouts did not differ between both arms. The reasons for dropping out are provided in the CONSORT flow chart (Figure 1). Two patients in the high-dose arm were excluded from analyses because of major protocol violations, which resulted in an insufficient increase of the serum vitamin D levels. In the low-dose arm, one patient was excluded because of missing MRI data at baseline and final visit (Figure 1). Thus, 38 patients were included in the PP analyses.
Table 1.
Baseline characteristics of patients (ITT).
| High dose | Low dose | p-value | |
|---|---|---|---|
| Total (n) [within group (%)] | 28 [53] | 25 [47] | >0.99a |
| Disease course (n) | |||
| RRMS | 26 | 25 | 0.49a |
| CIS | 2 | 0 | |
| Female [within group (%)] | 20 [71] | 17 [68] | >0.99a |
| Mean age at screening (years) [SE] | 41 [2.1] | 45 [1.8] | 0.26b |
| Mean disease duration onset to screening (months) [SE] | 97 [14.4] | 125 [16.8] | 0.24b |
| Mean BMI at screening [SE] | 27.2 [1.3] | 25.5 [0.9] | 0.61b |
| Median EDSS [range] | 2.0 [5.0] | 2.5 [6.0] | 0.18b |
| Mean 25OH vitamin D serum level (ng/ml) [SE] | 18.8 [1.9] | 17.8 [1.7] | 0.9b |
| Mean T2w lesion count (n) [SE] | 52.6 [6.7] | 76.1 [10.7] | 0.08b |
| Mean T2w lesion volume (ml) [SE] | 4.6 [0.9] | 10.4 [1.9] | 0.01b |
| Mean brain parenchymal fraction (ml) [SE] | 1163.1 [25.8] | 1121.3 [18.1] | 0.4b |
| Mean thalamus volume (ml) [SE] | 15.5 [0.4] | 14.4 [0.4] | 0.05b |
| Total gd+ lesions (n) | 4 | 2 | >0.99b |
BMI: body mass index; CIS: clinically isolated syndrome; EDSS: Expanded Disability Status Scale; gd+: gadolinium enhancing lesions; ITT: intention to treat; MSFC: Multiple Sclerosis Functional Composite; n: number; RRMS: relapsing remitting MS; SE: standard error.
aexact Chi-Square tests; bexact Mann–Whitney U test
Figure 1.
The CONSORT flow diagram.
Figure 1 shows the numbers of screened, randomized, and analysed patients in both treatment arms. The reasons for exclusion from randomization, drop out or exclusion from analysis are displayed.
ITT: intention to treat; PP: per protocol; n: number.
Vitamin D levels, MRI and clinical endpoints
Important explorative clinical and paraclinical outcome parameters (univariate, ITT population) are presented in Table 2. While on study medication, 25(OH)vitamin D levels increased from 19 (7–53) ng/ml at baseline to 62 (52–80) ng/ml at month 18 in the high-dose group and from 17 (4–35) to 23 (18–27) ng/ml in the low-dose group (p < 0.001).
Table 2.
Main clinical and MRI outcome parameters after 18 months and the changes from baseline (ITT).
| High dose | Low dose | p-value |
Change from baseline |
p-value | ||
|---|---|---|---|---|---|---|
| High dose | Low dose | |||||
| Mean 25OH vitamin D serum level (ng/ml) [SE] | 65.0 [5.5] | 22.3 [1.4] | <0.001c | 45.9 [5.4] | 5.9 [2.3] | <0.001c |
| Cumulative number of relapses (n) | 5 | 7 | 0.6b | |||
| Median EDSS [range] | 2.0 [3.5] | 2.0 [5.5] | 0.26a | 0 [4] | 0 [2.5] | 0.64c |
| Mean MSFC z-score change [SE] | –0.24 [0.14] | –0.44 [0.28] | 0.13a | 0.12 [0.07] | –0.09 [0.16] | 0.31c |
| Mean T2w lesion count (n) [SE] | 53.4 [7.3] | 84.1 [13.5] | 0.05a | 1.3 [0.1] | 2.1 [1.4] | 0.15c |
| Mean T2w lesion volume (ml) [SE] | 3.6 [0.5] | 11.9 [2.2] | <0.001a | 0.1 [0.1] | 0.2 [0.3] | 0.98c |
| Mean brain parenchymal fraction (ml) [SE] | 1167.7 [25.9] | 1126.6 [20.7] | 0.97a | –9.3 [3.7] | –7.3 [2.6] | 0.43c |
| Mean brain volume changes (%) [SE] | −0.61 [0.12] | −0.52 [0.10] | 0.98a | na | na | 0.84c |
| Mean thalamus volume (ml) [SE] | 15.7 [0.3] | 14.4 [0.5] | 0.048a | 0.41 [0.6] | –0.12 [0.1] | 0.95c |
| cumulative number of new gd+ lesions (V0–V6) (–) | 2 | 14 | 0.09a | na | na | na |
EDSS: Expanded Disability Status Scale; gd+: gadolinium enhancing lesions; ITT: intention to treat; MSFC: Multiple Sclerosis Functional Composite; n: number; SE: standard error; na: not applicable.
aexact Mann–Whitney U tests; bKaplan–Meier analysis, log-rank tests and multiple Cox regression; cmultivariate analysis of covariance (MANCOVA) when including baseline values, age, gender and disease duration as covariates in a longitudinal analysis.
The primary endpoint, number of new T2w hyperintense lesions on brain MRI at month 18 compared with baseline, was not different between arms, as were T2w lesion counts (p = 0.15) when including baseline, age, gender, and disease duration as covariates in a MANCOVA for longitudinal data. Similarly, T2 lesion volume at month 18 compared with baseline was not different between arms (p = 0.98). A numerical but not significant (p = 0.09) higher number of cumulative new gd+ lesions in the low-dose arm was driven by only four patients. MANCOVA with baseline as covariate showed no differences in the thalamic volume (p = 0.95), PBVC (p = 0.84), and the brain parenchymal fraction (p = 0.43) (Table 2). Disease progression measured by median EDSS (p = 0.64) and mean MSFC z-score (p = 0.31) in a MANCOVA with baseline as covariate was not different in both arms (Table 2).
Safety endpoints
The numbers and severity of adverse events did not differ between groups (p = 0.5, exact Chi-Square test). In total, 41 adverse events were recorded; of these 23 were in the high-dose and 18 in the low-dose arm. The most often recorded adverse events were respiratory infections (n = 25) and musculoskeletal complaints (n = 15), but without differences between study arms. The majority of adverse events were mild or moderate and not considered related to the study medication. No serious adverse events and no vitamin D-related toxicity or safety issues were recorded.
Discussion
Upon 18 months of continuous vitamin D intake we did not detect a different effect of high-dose versus low-dose supplementation of MS patients on important clinical and MRI parameters. We also did not record any relevant vitamin D-related adverse events. The study was carefully planned to account for heterogeneity of patients and variability of data (i.e. stratification, monocentric standardized MRI for all patients, standardization of evaluation, separation of treating and evaluating personnel). We chose an actively controlled paradigm because we expected a high incidence of vitamin D deficiency, and it was considered ethically not justifiable to deprive participants of any vitamin D supplementation. In fact, at baseline, the mean serum vitamin D levels were < 20 ng/ml in both arms (Table 1). The higher dose (corresponding to 10,200 IU per day) was chosen empirically under consideration of safety aspects and was higher than the doses given in the majority of previous trials,8,9 but was lower than the treatment arm dose (14,007 IU) in the SOLAR trial.10 The lower dose was derived from recommendations of the German Nutrition Society at the time when the study was designed, which were 200 IU per day. One might speculate that the difference between both doses was too low, or that already the low dose was immunologically active. But the observation of a rather small increase of the mean vitamin D serum level in the low-dose arm (from 18 to 22 ng/ml) in contrast to the pronounced rise in the high-dose arm argues against an insufficient difference between both doses. The rather mild disease activity in the entire cohort, which might be explained by the disease-modifying pretreatment with IFN β and the add-on treatment paradigm to an approved disease-modifying drug, may also have contributed to the negative results. But from an ethical point of view it was considered unacceptable to ‘treat’ MS patients with active disease solely by vitamin D and to withhold an approved treatment.
Finally, the rather small sample size may well account for the lack of differences in both study arms during therapy with a drug that significantly reduces relapses and MRI activity. The targeted sample size of 80 participants was mainly based on feasibility and did not result from a statistically supported sample size calculation. Nevertheless, unforeseen recruitment difficulties such as the contemporaneous approval of oral MS drugs and a highly comparative environment with a large number of recruiting clinical trials may explain why, despite a fairly long recruitment period of 45 months, only 53 patients were randomized. A substantial dropout rate further reduced the power to detect primary endpoint changes with α = 0.05 (two-sided) to 11% (PP population) and 4% (ITT population), respectively. However, these figures provide important information for future trials on vitamin D supplementation in MS. To detect a difference in the T2w lesion count with a power of 80% and a type 1 error (α) of 0.05 (two-sided) 252 participants (PP population) or 613 (ITT population) per arm would be necessary. The corresponding numbers for the hypothetical endpoint T2w lesion volume would be 3147 or 33,202 participants per arm. These power considerations suggest the in principle feasibility to demonstrate (or disprove) a disease-modifying effect of vitamin D supplementation in MS patients, at least for the endpoint T2w lesion count.
Tolerability and safety even of the high-dose were excellent in this study. The number of adverse events was similar in both arms, and adverse events recorded were not considered related to vitamin D intake. Most importantly, we did not observe hypercalcaemia. Thus, the EVIDIMS trial suggests that a mean daily intake of about 10,000 IU cholecalciferol is safe, at least under the premises of the cohort studied, i.e. in patients aged 25 to 62 without any relevant kidney dysfunction and exclusion of certain co-medications.
In conclusion, the results of the EVIDIMS study neither support nor disprove a beneficial effect of vitamin D supplementation in MS. However, the explorative trial results provide valuable information for sample size calculation and feasibility of future confirmatory trials on this topic. The data further support the safety and tolerability of a mean daily dose of 10,200 IU cholecalciferol in patients with MS.
Supplemental Material
Supplemental material, MSO903474 Supplemental material for High-dose vitamin D supplementation in multiple sclerosis – results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial by Jan Dörr Priscilla Bäcker-Koduah Klaus-Dieter Wernecke Elke Becker Frank Hoffmann Jürgen Faiss Bernd Brockmeier Olaf Hoffmann Kerstin Anvari Jens Wuerfel Sophie K Piper Judith Bellmann-Strobl Alexander U Brandt Friedemann Paul in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Contributor Information
Jan Dörr, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany; Max Delbrueck Center for Molecular Medicine and Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Experimental and Clinical Research Center, Germany.
Priscilla Bäcker-Koduah, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany; Max Delbrueck Center for Molecular Medicine and Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Experimental and Clinical Research Center, Germany.
Klaus-Dieter Wernecke, SOSTANA GmbH, Germany; Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institute of Biometry and Clinical Epidemiology, Germany.
Elke Becker, Neurologisches Facharztzentrum Berlin, Germany.
Frank Hoffmann, Department of Neurology, Krankenhaus Martha-Maria Halle-Doelau, Germany.
Jürgen Faiss, Department of Neurology, Asklepios Kliniken Teupitz and Lübben, Germany.
Bernd Brockmeier, Neurologie am Mexikoplatz, Germany.
Olaf Hoffmann, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany; Department of Neurology, Alexianer St. Josefs-Krankenhaus, Germany.
Kerstin Anvari, Neurologische Praxis Anvari, Germany.
Jens Wuerfel, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany; MIAC AG and qbig, Dep. Biomedical Engineering, University Basel, Switzerland.
Sophie K Piper, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institute of Biometry and Clinical Epidemiology, Germany; Berlin Institute of Health (BIH), Anna-Louisa-Karsch 2, Germany.
Judith Bellmann-Strobl, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany; Max Delbrueck Center for Molecular Medicine and Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Experimental and Clinical Research Center, Germany; Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Neurology, Germany.
Alexander U Brandt, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany; Department of Neurology, University of California, USA; Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Neurology, Germany.
Friedemann Paul, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany; Max Delbrueck Center for Molecular Medicine and Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Experimental and Clinical Research Center, Germany; Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Neurology, Germany.
Author Contributions
All authors approved the final version of the manuscript. JD designed the trial, drafted the study protocol, treated patients, generated data, and drafted the manuscript. PBK collected and processed data, performed statistical analyses, and drafted the manuscript. KDW was the responsible biometrician, performed the statistical analyses, and drafted the manuscript. EB, FH, JF, BB, OH and KA were principal investigators of a study site, treated patients and generated data. JW was in charge of acquisition and processing of MRI data. SKP supported the statistical analyses. JBS treated patients, generated data and coordinated as well as supervised the process of data collection. AB coordinated and supervised the process of data collection, compilation and evaluation and drafted the manuscript. FP designed the trial, drafted the study protocol, generated data, and drafted the manuscript.
Conflict of Interests
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JD received research support by Bayer and Novartis, travel support by Bayer, Novartis, Biogen, Merck Serono, and honoraria for lectures and advisory by Bayer, Novartis, Biogen, Merck Serono, Roche, Sanofi Genzyme. PBK is funded by the DFG Excellence grant to FP (DFG exc 257) and is an Einstein Junior scholar. KDW reported no conflicts of interest. EB has nothing to disclose. FH has received speaker honoraria and grant support from Alexion, Bayer Vital, Biogen, DIAMED Medizintechnik, Merck Serono, Genzyme, Grifols, Ipsen, Novartis, Roche and Teva. JF has nothing to disclose. BB reported personal fees from Biogen, Merck, Roche, and Teva. OH has received honoraria, travel and/or research grants from Bayer, Biogen, Boehringer Ingelheim, Celgene, Daiichi‐Sankyo, Merck, Novartis, Roche, Sanofi and Teva. KA has nothing to disclose. JW is CEO of MIAC AG Basel, Switzerland. He served on scientific advisory boards of Actelion, Biogen, Genzyme-Sanofi, Novartis, and Roche. He is or was supported by grants of the EU (Horizon2020), German Federal Ministries of Education and Research (BMBF) and of Economic Affairs and Energy (BMWI). SKP has nothing to disclose. JBS has received travel grants and speaking fees from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi-Aventis/Genzyme, Teva Pharmaceuticals, and Novartis. AUB is cofounder and shareholder of Motognosis and Nocturne. He is named as an inventor on several patent applications regarding MS serum biomarkers, OCT image analysis and perceptive visual computing. FP serves on the scientific advisory board for Novartis; received speaker honoraria and travel funding from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; is an academic editor for PLoS ONE; is an associate editor for Neurology® Neuroimmunology & Neuroinflammation; consulted for Sanofi Genzyme, Biogen Idec, MedImmune, Shire, and Alexion; and received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy Jackson Charitable Foundation, and National Multiple Sclerosis of the USA.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by DFG Excellence grant (DFG exc 257) and was in part funded by a limited grant by Bayer Healthcare. Bayer was not involved in the design, conduction, or analysis of the study nor in the manuscript preparation.
ORCID iDs
Jan Dörr https://orcid.org/0000-0002-5038-6496 Sophie K Piper https://orcid.org/0000-0002-0147-8992 Alexander U Brandt https://orcid.org/0000-0002-9768-014X
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: A comprehensive review. Neurol Ther 2018; 7: 59–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dörr J, Döring A, Paul F. Can we prevent or treat multiple sclerosis by individualised vitamin D supply? EPMA J 2013; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koduah P, Paul F, Dörr J-M. Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. EPMA J 2017; 8: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimball SM, Ursell MR, O’Connor Pet al. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr 2007; 86: 645–651. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin L, Clarke L, Khalilidehkordi Eet al. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J Neurol 2018; 265: 2893–2905. [DOI] [PubMed] [Google Scholar]
- 6.Shoemaker TJ, Mowry EM. A review of vitamin D supplementation as disease-modifying therapy. Mult Scler 2018; 24: 6–11. [DOI] [PubMed] [Google Scholar]
- 7.Burton JM, Kimball S, Vieth Ret al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 2010; 74: 1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soilu-Hänninen M, Aivo J, Lindström B-Met al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2012; 83: 565–571. [DOI] [PubMed] [Google Scholar]
- 9.Kampman MT, Steffensen LH, Mellgren SIet al. Effect of vitamin D3 supplementation on relapses, disease progression and measures of function in persons with multiple sclerosis: Exploratory outcomes from a double-blind randomised controlled trial. Mult Scler 2012; 18: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 10.Hupperts R, Smolders J, Vieth Ret al. Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology 2019; 93: e1906–e1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dörr J, Ohlraun S, Skarabis Het al. Efficacy of Vitamin D Supplementation in Multiple Sclerosis (EVIDIMS Trial): Study protocol for a randomized controlled trial. Trials 2012; 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 13.Cutter GR, Baier ML, Rudick RAet al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain J Neurol 1999; 122: 871–882. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Edan Get al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, Zhang Y, Jenkinson Met al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002; 17: 479–489. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Jenkinson M, Woolrich MWet al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: S208–S219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSO903474 Supplemental material for High-dose vitamin D supplementation in multiple sclerosis – results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial by Jan Dörr Priscilla Bäcker-Koduah Klaus-Dieter Wernecke Elke Becker Frank Hoffmann Jürgen Faiss Bernd Brockmeier Olaf Hoffmann Kerstin Anvari Jens Wuerfel Sophie K Piper Judith Bellmann-Strobl Alexander U Brandt Friedemann Paul in Multiple Sclerosis Journal—Experimental, Translational and Clinical