ABSTRACT
VCP (valosin containing protein) recognizes a wide variety of substrates and mediates their degradation via the ubiquitin-proteasome system and macroautophagy/autophagy. The substrate repertoire of VCP, however, is not fully understood. In our recent study, we found that Drosophila TER94/VCP mediates autophagic degradation of an Argonaute subfamily protein (AGO1), which binds microRNAs (miRNAs) and silences the expression of thousands of target genes. In the absence of TER94/VCP, miRNA-mediated gene silencing is globally impaired. Our findings reveal an unexpected connection between VCP and AGO, which may dramatically expand the biological significance of VCP.
KEYWORDS: Argonaute, gene silencing, Iruka, microRNA, ubiquitin, Ufd1–Npl4, VCP
The AGO (Argonaute) subfamily of proteins loads microRNAs (miRNAs) to form the effector RNP complex called RISC, which silences thousands of target genes. AGO uses miRNAs as guides to complementary target mRNAs and triggers their translational repression and decay. Empty AGO, but not miRNA-loaded AGO, is selectively degraded across species. For example, loss of drosha, a miRNA biogenesis factor, induces degradation of AGO1 in Drosophila. Previously, we reported that the E3 ubiquitin ligase Iru (Iruka) ubiquitinates Drosophila AGO1 in the empty state. Notably, loss of Iru results in the accumulation of dysfunctional AGO1, indicating that empty AGO degradation is a part of the quality control mechanism of AGO. However, it remained unclear how empty AGO is degraded after being ubiquitinated.
VCP/p97/Cdc48/TER94 recognizes various substrates via adaptor subunits to mediate their degradation. VCP has been best characterized for its role in directing its substrates to proteasomes, but emerging evidence shows that VCP also mediates various types of selective autophagy, including mitophagy, lysophagy, and granulophagy. In our recent study, we reported that Drosophila TER94/VCP recognizes ubiquitinated empty AGO1 via the heterodimer consisting of Ufd1-like and Npl4 and mediates its autophagic degradation [1]. Here, we summarize our findings in the recent study and provide additional insights.
Degradation of empty AGO1 by macroautophagy
How empty AGO is degraded was controversial. Previous studies reported that empty AGO is degraded by autophagy, while another study suggested that the proteasome is responsible for its degradation. In our recent study, we reported multiple lines of evidence that empty AGO1 is degraded by autophagy in Drosophila S2 cells. First, degradation of AGO1 upon the depletion of drosha is inhibited by the treatment with E-64d, pepstatin A, and leupeptin, which inhibit lysosomal protein degradation, but not by a proteasome inhibitor, bortezomib. Second, degradation of empty AGO1 is disrupted in the absence of Atg8a and Atg8b, fly orthologs of LC3, and of Atg17, the fly ortholog of RB1CC1/FIP200. In contrast, loss of Rpn2 or Rpn12, regulatory subunits of the proteasome, does not affect degradation of empty AGO1. We also found that empty AGO1 associates with ref(2)P, the fly ortholog of SQSTM1/p62, although ref(2)P is dispensable for its degradation. Together, these findings indicate that empty AGO1 is degraded by autophagy. Consistent with our finding, previous studies report that the abundance of AGO in the steady state, where AGO should exist as a mixture of empty and miRNA-loaded forms, is also regulated by autophagy.
Notably, AGO is an aggregation-prone protein and can form large (>2 MDa) complexes in cells. Thus, we envision that autophagy, which is capable of degrading aggregates, is suitable for handling AGO, compared with the proteasome, which prefers relatively small substrates. In line with this idea, AGO is degraded by the proteasome in T cells, where AGO predominantly forms small (∼100 kDa) complexes.
Identification of TER94/VCP as a mediator of empty AGO1 degradation
How is empty AGO1 directed to autophagic degradation? Given that ubiquitination of empty AGO1 triggers its degradation, we sought to identify the molecules that interact with ubiquitinated empty AGO1. To this end, we performed immunoprecipitation followed by LC-MS/MS and compared the interactomes of empty AGO1 in the presence and absence of Iru, the E3 ubiquitin ligase for empty AGO1. Our proteomic approach revealed that TER94/VCP preferentially interacts with ubiquitinated empty AGO1; the interaction between empty AGO1 and TER94/VCP is downregulated or upregulated upon the depletion or overexpression of Iru, respectively. Importantly, degradation of empty AGO1 is abrogated in the absence of TER94/VCP. Moreover, our LC-MS/MS data uncovered adaptor subunits that interact with ubiquitinated empty AGO1. Among these adaptors, we found that loss of the Ufd1-like-Npl4 heterodimer impairs the interaction between TER94/VCP and empty AGO1 and inhibits degradation of empty AGO1. Together, we concluded that TER94/VCP recognizes ubiquitinated empty AGO1 via the Ufd1-like-Npl4 heterodimer and directs its autophagic degradation (Figure 1A).
Figure 1.
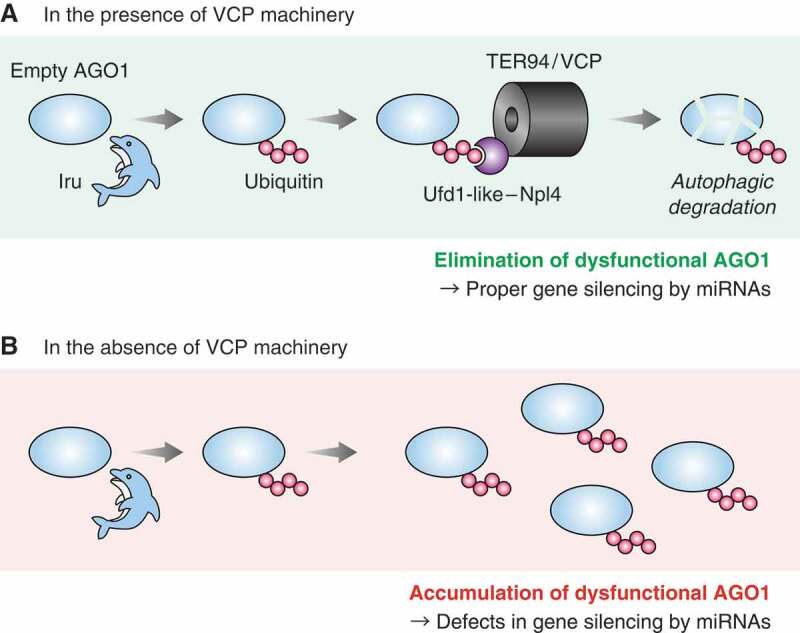
A model of autophagic degradation of AGO1 by TER94/VCP. (A) Empty AGO1 ubiquitinated by Iru is recognized by TER94/VCP via the Ufd1-like-Npl4 heterodimer. Subsequently, TER94/VCP mediates its autophagic degradation, which eliminates dysfunctional AGO1 and ensures proper gene silencing by miRNAs. (B) Loss of VCP machinery disrupts degradation of empty AGO1, which causes accumulation of dysfunctional AGO1 and dysregulation of miRNA-mediated gene silencing.
It remains unclear how TER94/VCP accomplishes autophagic degradation of empty AGO1 after recognition. It would be straightforward if TER94/VCP could link empty AGO1 and Atg8, as canonical autophagy receptors do. In our preliminary experiments, however, we did not detect the interaction between TER94/VCP and Atg8. Given that the precise roles of VCP during other selective autophagy pathways also remain elusive, future studies are needed to address this point.
Dysregulation of miRNA-mediated gene silencing in the absence of TER94/VCP
Loss of Iru causes accumulation of dysfunctional AGO1 and impairs miRNA-mediated gene silencing. Having uncovered that TER94/VCP and Ufd1-like-Npl4 mediate autophagic degradation of empty AGO1, we investigated whether these molecules are also involved in miRNA-mediated gene silencing. We performed RNA sequencing and compared transcriptomes of TER94/VCP-depleted or Ufd1-like-Npl4-depleted cells with control S2 cells. Our comprehensive analysis showed that depletion of TER94/VCP or Ufd1-like-Npl4 strongly upregulates the mRNA targets of miRNAs. Reporter assays also showed upregulation of mRNA targets upon depletion of TER94/VCP or Ufd1-like-Npl4. These data indicate that the TER94/VCP machinery is required for successful miRNA-mediated gene silencing. Previous studies reported that the autophagy pathway itself is also important for gene silencing by miRNAs. Together, these findings underscore the significance of empty AGO degradation, which contributes to quality control of AGO (Figure 1B). Notably, when we carefully compared the result of Iru-, TER94/VCP-, or Ufd1-like-Npl4-depletion, loss of TER94/VCP or Ufd1-like-Npl4 triggers much more severe defects in miRNA-mediated gene silencing than that of Iru. The difference could simply be explained by their knockdown efficiencies, but accumulation of ubiquitinated empty AGO may be more harmful than that of ubiquitin-free empty AGO. Alternatively, the TER94/VCP machinery may have an as-yet-unrecognized function to facilitate gene silencing by miRNAs beyond empty AGO degradation.
VCP is associated with multiple genetic disorders, e.g., IBMPFD, ALS, and Parkinson's disease, but it is still unclear how VCP causes such diverse pathological consequences. As we discovered that TER94/VCP is involved in miRNA-mediated gene silencing, which affects thousands of genes, the potential connection between VCP disease and the miRNA pathway should be studied in the future.
Funding Statement
This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas [“Non-coding RNA Neo-taxonomy” grant 26113007 to Y.T.] from the Ministry of Education, Culture, Sports, Science and Technology in Japan and a Grant-in-Aid for Scientific Research (S) [grant number 18H05271 to Y.T.], a Grant-in-Aid for JSPS Fellows [grant 14J10075 to H.K.], a Grant-in-Aid for Research Activity Start-up [grant 17H06616 to H.K.] from the Japan Society for the Promotion of Science, as well as by the Naito Foundation, Japan
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Kobayashi H, Shoji K, Kiyokawa K, et al. VCP machinery mediates autophagic degradation of empty argonaute. Cell Rep. 2019;28(1144–1153):e4. [DOI] [PubMed] [Google Scholar]


