ABSTRACT
Organismal aging is associated with compromised cellular function, which can be partially attributed to accumulation of cellular damage. Being the major, if not only, cellular bulk-degradation mechanism, macroautophagy (hereafter autophagy) declines with age in multiple tissues and organisms. Spermidine is an endogenous polyamine metabolite that also declines with age. It prolongs lifespan and improves tissue functions of model organisms in an autophagy-dependent manner. We report that autophagic flux is significantly reduced in B cells from old mice. Spermidine induces autophagy and improves the function of both old mouse and old human B cells. Mechanistically, spermidine post-translationally modifies (hypusinates) the translation factor EIF5A. Hypusinated EIF5A specifically regulates the synthesis of the master autophagy and lysosome transcription factor, TFEB (transcription factor EB). This pathway declines with age in both mice and humans, which may eventually lead to declining autophagy and impaired tissue functions in old individuals.
KEYWORDS: Aging, autophagy, B cells, EIF5A, hypusine, spermidine, TFEB, translation
Aging of the immune system, termed immune senescence, is characterized by altered ratios of different immune cell types and their reduced function. This causes failure of efficient clearance of pathogens and tissue damage, which directly increases the infectious disease burden in old individuals and also generally aggravates the aging process of other tissues. B cells are the only known antibody-secreting cell in the body and are key in the fight against infections and for effective vaccinations. The antibody responses against pathogens in old individuals are severely compromised, rendering them highly vulnerable to infectious diseases. Despite the long-recognized change in B cell phenotypes (by surface markers and function) that occurs during aging, the molecular mechanism underlying B cell senescence is still poorly understood. Efficient ways to improve B cell responses in the elderly are also lacking.
Using multiple LC3-II-based readouts, we confirm that autophagic flux is significantly reduced in B cells from old mice [1]. More specifically, old B cells show higher LC3-II protein levels, which are not further increased by the lysosomal inhibitor bafilomycin A1, indicating that autophagic flux is blocked at the lysosomal level. Autophagy deficiency in B cells leads to defective memory responses, mimicking the aging phenotype. Spermidine administration increases autophagic flux and improves B cell responses in old mice, but not in mice with an autophagy deficiency in B cells.
Spermidine is an essential substrate of the hypusination process, in which it donates an aminobutyl moiety to a specific lysine on EIF5A (eukaryotic translation initiation factor 5A) to form hypusine. EIF5A is a translation factor with the well-defined function of facilitating the elongation step during protein synthesis involving its hypusine residue. Hypusine is an unusual amino acid modification so far only known to exist in EIF5A. We show that depleting cellular spermidine leads to reduced hypusination of EIF5A, and that inhibiting EIF5A expression or its hypusination reduces autophagic flux in both mammalian cell lines and ex vivo activated primary B cells. To identify how EIF5A regulates autophagy, protein mass spectrometry was used. Interestingly, TFEB protein expression is repetitively found reduced upon inhibiting hypusination in several proteomic approaches, which is confirmed by western blot. Mechanistically, TFEB contains a polyproline-containing motif (… SPPPVPG …) at its N terminus, which is predicted to be a translation-stalling motif. This motif is sufficient to cause reduced translation as shown using an mCherry-GFP reporter system. Moreover, mutating this motif alone renders the expression of TFEB less dependent of hypusinated EIF5A while its function is unaltered. Therefore, EIF5A promotes TFEB synthesis at least partially via facilitating translation of hard-to-read regions. However, not all polyproline-containing proteins are downregulated upon inhibition of hypusination. The specificity of TFEB might be attributed to its long stalling motif and short protein half-life.
Spermidine levels decline with age in multiple model organisms and as confirmed by us also in human peripheral blood mononuclear cells. We find that hypusination of EIF5A, overall EIF5A protein levels, and TFEB expression are reduced in B cells from old mice, which can be fully or partially rescued by spermidine administration in vivo. A similar improvement is observed in ex vivo cultured old human B cells. Spermidine treatment induces the pathway and improves the antibody production of old human B cells in a hypusination-dependent manner. Next it would be exciting to test whether spermidine improves immune responses in the elderly in a clinical trial. Moreover, the amount of spermidine, the expression of hypusinated EIF5A and TFEB, as well as the autophagic flux in peripheral blood could be used as biomarkers to assess the biological aging status and the efficiency of other anti-aging drugs. It would also be interesting to see whether this pathway operates in other tissues.
Being a major catabolic process, autophagy can be induced by various cellular stresses. MTOR (mechanistic target of rapamycin kinase) integrates growth and nutrient signals. When conditions are favorable, MTOR phosphorylates multiple targets to promote signaling proteins that inhibit autophagy while activating anabolism and cell growth, including turning on the protein synthesis machinery. However, here we observe that activation of immune cells is associated with upregulation of both anabolic and catabolic pathways, protein synthesis and autophagy. In this case therefore translation and autophagy are induced in a coordinated fashion, and autophagy induction relies on optimal translation. It is likely that a cross-talk between catabolism and anabolism exists: Autophagy provides building blocks and energy resources to support translation, while the translation machinery maintains high levels of short-lived autophagy proteins such as TFEB (Figure 1). Based on TFEB overexpression experiments, increased expression of TFEB alone is sufficient to induce autophagy, without the need for inhibited MTOR. However, whether the induced autophagy in activated B cells is primarily driven by increased TFEB synthesis or it is more directly controlled by B-cell receptor is unclear. Moreover, although TFEB has been identified as a target of EIF5A, we postulate that more EIF5A targets may exist that regulate autophagy and other processes. In addition to this novel translational regulation of autophagy involving EIF5A, other autophagy-regulatory mechanisms that operate at the translational level are likely to be discovered.
Figure 1.
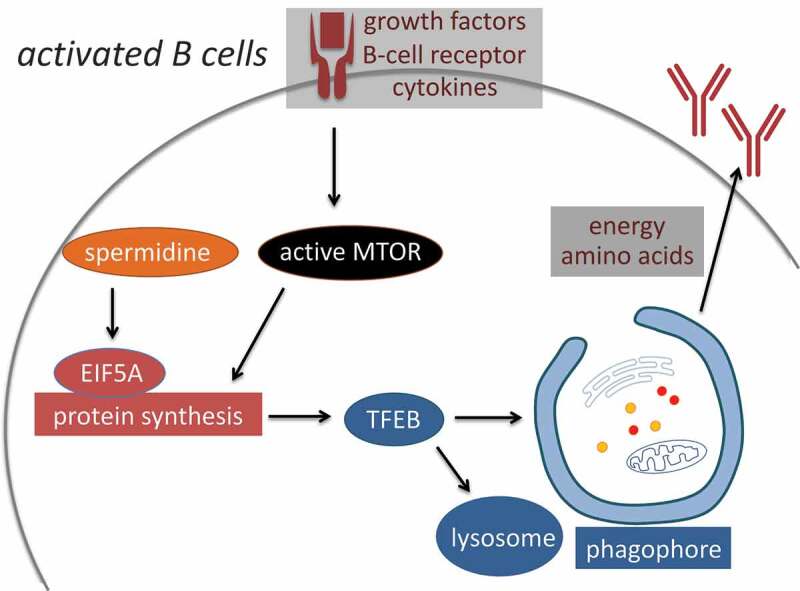
Anabolism and catabolism are upregulated in activated B cells in a coordinated manner. MTOR integrates growth and nutrient signals to promote translation and cellular growth. Despite the high MTOR activity, autophagy is also upregulated in activated B cells via MTOR-independent signaling pathways. High autophagic flux relies on efficient protein synthesis. Specifically, spermidine sustains optimal translation via hypusinating EIF5A, which is required for maintaining high TFEB protein levels. Autophagy may in turn provide nutrients and degrade damaged organelles to assist the high growth rate and antibody production of activated B cells.
Funding Statement
H.Z. is funded by the China Scholarship Council-Nuffield Department of Medicine Scholarship and the Oxford-Elysium Prize Fellowship. A.K.S. lab is funded by a Wellcome Trust Investigator Award [103830/Z/14/Z].
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Zhang H, Alsaleh G, Feltham J, et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol Cell. 2019;76:110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]


