ABSTRACT
Physiological and pathological stresses may cause swelling of the endoplasmic reticulum (ER), a biosynthetic organelle in eukaryotic cells. Upon conclusion of the stress, ER size and content return to physiological levels. The translocon component SEC62 decorates the portions of excess ER that must be cleared from cells. Our recent paper highlights the role of endosomal sorting complex required for transport (ESCRT)-III-driven micro-ER-phagy in ER remodeling during cell recovery from ER stress.
KEYWORDS: Autophagosome biogenesis, autophagy, endolysosomes, ER-centric, ER-phagy, ESCRT-III, LC3 lipidation, micro-ER-phagy, Recov-ER-phagy, unfolded protein response (UPR)
At the beginning: upon interruption of drug treatment, excess ER is delivered to acidic compartments for clearance
In the early 70s, the Swiss scientist Ewald Weibel reported that administration of the anti-epileptic drug phenobarbital causes enlargement of the ER in liver cells. He notably observed that upon interruption of phenobarbital treatment, the excess ER is delivered within acidic endolysosomal compartments (EL) for clearance. This has been the first report on a catabolic pathway, where endolysosomes regulate size, activity and homeostasis of the ER, the major biosynthetic organelle of our cells. How portions of the ER are sent to the EL for clearance re-attracted the attention of scientists only recently. These pathways are nowadays collectively defined as reticulophagy or ER-phagy, where the Greek suffix -phagy means eating or consumption.
Forty years later: SEC62 is an ER-phagy receptor controlling clearance of excess ER during recovery from ER stress
After more than 40 years, we took over the pioneering study by Weibel. In a Nature Cell Biology paper published in 2016, we subjected mammalian cells to acute exposure to cyclopiazonic acid, an inhibitor of the ER calcium pump that induces a reversible ER stress. We showed that upon interruption of cyclopiazonic acid treatment, the excess ER is delivered within EL for clearance. We named recov-ER-phagy this process that recapitulates Weibel’s observations. Recov-ER-phagy is controlled by the translocon component SEC62 that during “recovery from ER stress” engages lipidated LC3 to deliver within EL select ER subdomains containing most ER chaperones, but lacking many ERAD factors such as HERPUD1/HERP, whose return to a pre-stress level is ensured by proteasomal clearance. Our work demonstrated the “2-fold” selectivity of recov-ER-phagy: first, in contrast to starvation-induced ER-phagy (nutrient deprivation also results in -phagy of other organelles and cytoplasmic content), ER-phagy as induced on termination of acute ER stresses only clears portions of the ER. Second, only select ER subdomains enriched in chaperones and folding factors whose expression has been enhanced during the ER stress are removed from cells.
Latest work: recov-ER-phagy proceeds via ESCRT-III-driven piecemeal micro-ER-phagy
In our latest publication [1], thorough genetic and morphometric analyses of the events that characterize recov-ER-phagy eventually revealed the involvement of the LC3 lipidation machinery and dispensability of genes that are required to generate double-membrane autophagosomes. Immuno-electron microscopy supported by 3D reconstructions showed that RAB7- and LAMP1-positive endolysosomes directly engulf ER subdomains displaying SEC62 at the limiting membrane (Figure 1). This process entails inward budding of the EL membrane regulated by the ESCRT-III component CHMP4B and the accessory AAA+ ATPase VPS4A, known components of complex machineries involved in physiological and pathogen-induced membrane repair, remodeling and fission (Figure 1).
Figure 1.
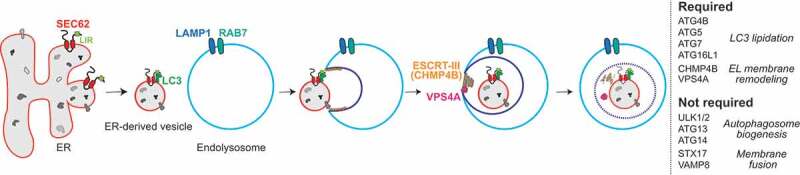
Recov-ER-phagy. Resolution of ER stress activates the LC3 binding function of the translocon component SEC62. Excess ER, in the form of ER-derived vesicles containing molecular chaperones and folding factors (but lacking degradation factors) are directly engulfed by endolysosomes. Remodeling of the ER during recovery from ER stress occurs via ESCRT-III-driven piecemeal micro-ER-phagy.
Picking up 3 of the many issues that remain to be understood
Portioning out the ER for clearance
In recov-ER-phagy and in all other types of receptor-mediated ER turnover, ER-phagy receptors (RETREG1/FAM134B, SEC62, RTN3L, CCPG1, ATL3, TEX264 in mammalian cells, Atg39 and Atg40 in yeast) delimit the ER portions that must be cleared from cells. It remains unclear how these are shed from the bulk ER because only RETREG1, RTN3L and Atg40 harbor reticulon homology domains that could facilitate these events.
Activating ER-phagy receptors
SEC62 participates in heterodimeric complexes with SEC63 that promote post-translational translocation of newly synthesized proteins in the ER. The function of SEC62 as an ER-phagy receptor is greatly enhanced during recovery from ER stress. Recov-ER-phagy is faithfully recapitulated by SEC62 overexpression or by SEC63 downregulation. This finding implies that disassembly of SEC62-SEC63 complexes is a prerequisite for ER remodeling to resume ER size and function after ER stress resolution. How cells generate orphan SEC62 to drive ER turnover, remains to be understood. Likewise, the mode of activation of other ER-phagy receptors upon nutrient deprivation or by proteasome-resistant misfolded proteins that elicit ER-to-lysosome-associated degradation (ERLAD) pathways remains to be studied.
Establishing the role of lipidated LC3
Delivery of ER portions to the EL is triggered by nutrient deprivation, recovery from ER stress, accumulation of proteasome-resistant misfolded proteins, and, no doubt, by other pleiotropic and ER-centric stimuli that remain to be elucidated. Association of ER-phagy receptors with lipidated LC3 is a common feature of all these catabolic pathways. However, in some cases this precedes capture of the ER portion by double-membrane phagophores (as in starvation-induced ER-phagy and in ERLAD of ER-derived vesicles containing procollagen). In other cases, this precedes vesicular delivery of the ER portion to EL (as in ERLAD of ER-derived vesicles containing SERPINA1 z variant/ATZ polymers) or direct engulfment of the ER portions by endolysosomes (as in recov-ER-phagy). In these latter cases, lipidated LC3 is not displayed on double-membrane phagophores or autophagosomes, but on the single membrane of the endolysosomal-target compartment or directly on the membrane of the ER-derived vesicle [1]. All in all, the outcome of the signal activating ER-phagy receptors is hard to predict, except for the fact that ER portions will eventually be destroyed within EL. To understand how they get there, well, hard work will pay off.
Funding Statement
This work was supported by the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung [310030_184827].
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Loi M, Raimondi A, Morone D, et al. ESCRT-III-driven piecemeal micro-ER-phagy remodels the ER during recovery from ER stress. Nat Commun. 2019;10:5058. [DOI] [PMC free article] [PubMed] [Google Scholar]


