Abstract
Background
Inflammation of the pulmonary parenchyma is one of the important mechanisms implicated in development of chronic lung disease (CLD) in preterm neonates.
Objectives
To evaluate the effect of alpha 1 proteinase inhibitor (a1PI) for the prevention of chronic lung disease (CLD) in preterm neonates.
Search methods
We searched the following databases: MEDLINE (1966 to February 2005), EMBASE (1980 to February 2005), CINAHL (1982 to February 2005), Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2005) and abstracts from the annual meetings of the Society of Pediatric Research, American Pediatric Society and Pediatric Academic Societies published in Pediatric Research (1991 to 2004). No language restrictions were applied.
The electronic search was updated in 2010.
Selection criteria
Randomised or quasi‐randomised trials of administration of a1PI compared to placebo or no treatment within the first week of life in preterm neonates.
Data collection and analysis
Data collection and analysis was performed in accordance with the methods of the Cochrane Neonatal Review Group.
Main results
Two eligible studies were identified. The methodological qualities of identified studies were good. One study randomised infants to either placebo or a1PI 60 mg/kg/dose for four doses while in the second study the same investigators explored the efficacy of different dose regimens of a1PI compared to placebo. There was no statistically significant difference in the risk of developing CLD at 36 weeks postmenstrual age among all randomised infants at any dose of a1PI (pooled RR 0.79, 95% CI 0.44 to 1.41) or when given 60 mg/kg/dose for four doses compared to placebo (pooled RR 0.64, 95% CI 0.35 to 1.18).
There was a trend towards reduced risk of development of oxygen dependency at 28 days postnatal age for 60 mg/kg/dose for four doses of a1PI compared to placebo. When any doses of a1PI were combined, the pooled RR was statistically significant [RR 0.80 (95% CI 0.65 to 0.98); RD ‐0.15 (95 % CI ‐0.29 to ‐0.01)].
Treatment with a1PI did not reduce the risk of CLD and/or death at 36 weeks postmenstrual age or the risk of long term neurodevelopmental abnormalities. In addition, no statistically significant difference was noted in other respiratory parameters such as duration of oxygen requirement or respiratory support.
Authors' conclusions
Prophylactic administration of a1PI did not reduce the risk of CLD at 36 weeks or long term adverse developmental outcomes in preterm neonates.
Plain language summary
Alpha‐1 proteinase inhibitor (a1PI) for preventing chronic lung disease in preterm infants
There is not enough evidence to show the long term effect of using Alpha‐1 proteinase inhibitor for chronic lung disease in premature babies. Inflammation of the lungs is one of the causes of chronic lung disease (CLD) in babies born before 37 weeks. Babies with CLD need extra oxygen and the disease can also cause serious long‐term problems. Lung damage is caused by the release of enzymes and other anti‐oxidants because babies with CLD have a low level of Alpha‐1 proteinase inhibitor (a1P1), a substance that stops lung tissue being destroyed. A medication version of AlP1 is sometimes given to protect their lungs. The review of the trials found that there is not enough evidence to show long term beneficial effects of a1P1. More research is needed.
Background
Description of the condition
Advances in perinatal and neonatal care such as use of antenatal steroids (Crowley 2001), surfactant replacement therapy (Jobe 1993) and modification in ventilatory techniques have led to improved survival of preterm neonates (Horbar 1993) and resulted in higher number of survivors with chronic lung disease (CLD) (Shaw 1993). Pulmonary sequelae after respiratory distress syndrome were identified initially by Shepard 1964; Robertson 1964 and later Northway 1967, who coined the term "bronchopulmonary dysplasia". Shennan 1988 defined CLD as oxygen requirement (with or without characteristic x‐ray findings) persisting beyond 36 weeks post menstrual age (PMA). CLD is associated with prolonged hospitalisation, increased risk of re‐hospitalisation and adverse neurodevelopmental outcomes amongst survivors.
The etiology of CLD is multi factorial. Inflammation of pulmonary parenchyma plays a major role in evolution of CLD (Ogden 1982; Rosenfeld 1984). As a result of the inflammation there is a massive influx of neutrophils in to the alveoli (Ogden 1982), liberation of pro ‐ inflammatory mediators like cytokines (Jones 1996) and release of oxygen free radicals and proteinases in the alveoli. Merritt 1983 has shown that neutrophil elastase released from neutrophils is elevated in tracheal lavage fluid of neonates who go on to develop CLD. The proteinases in the pulmonary parenchyma can digest fibrillar collagen, elastin fibers, laminin and fibronectin (Gerdes 1988; Fletcher 1990).
Description of the intervention
Strategies or interventions for the prevention of CLD include antioxidants like superoxide dismutase (Davis 1998), vitamin E (Watts 1991), vitamin A (Darlow 2001), ceruloplasmin and anti proteinases (Rosenfeld 1986). Antioxidants neutralize the initial burst of free oxygen radicals. Failure of release of antioxidants results in tissue destruction with release of proteolytic enzymes such as elastase. Alpha‐1 proteinase inhibitor (a1PI), a major inhibitor of elastase, prevents its proteolytic action on fibronectin and elastin (Rosenfeld 1984). Activity of a1PI has been evaluated in preterm and term neonates. Rosenfeld 1986 observed that a1PI activity and ceruloplasmin levels were low in all neonates, especially in less mature neonates who had higher incidence of CLD (Fletcher 1990; Evans 1972; Singer 1976). Furthermore Bruce 1981 showed that the levels of a1PI may be normal in preterm neonates but exposure to high oxygen concentration may result in inactivation of the methionine terminal of a1PI rendering it physiologically inactive.
How the intervention might work
a1PI in the form of alpha‐1 antitrypsin has been used since 1987 in adult patients with alpha‐1 antitrypsin deficiency as replacement therapy (Wewers 1987). Aerosolized alpha‐1 antitrypsin has been used in patients with cystic fibrosis who have normal alpha‐1 antitrypsin levels but excessive elastase activity (McElvaney 1991; Hubbard 1989). Koppel 1994 demonstrated that early administration of alpha‐1 antitrypsin to newborn rats exposed to hyperoxia protects them from developing the anticipated pulmonary and vascular changes and the resultant right ventricular hypertrophy. In preclinical animal experiments, Fournel 1988 demonstrated a1PI extravasation into pulmonary alveolar spaces with intravenous administration of a1PI resulting in sustained and proportionate increase in alveolar antielastase activity without abnormal clearance or significant inactivation of a1PI. No physiological or systemic adverse responses to anticipated clinical doses were observed and the toxicologic studies also failed to reveal any acute or subchronic effects. Known side effects attributable to a1PI administration are delayed fever and transient leucocytosis (Hebel 2000).
With these observations, it seems that improving the proteinase ‐ anti proteinase balance may have beneficial effects on CLD (Gadek 1981; Yoder 1991). However, Gerdes 1988 failed to show sustained clinical improvement after administration of dexamethasone which reduced bronchoalveolar lavage fluid elastase concentration.
Why it is important to do this review
The aim of this review was to assess the effectiveness of a1PI therapy when administered to ventilated preterm neonates in the first week of life in the prevention of CLD.
Objectives
The primary objective was to compare the effectiveness of a1PI versus placebo or no treatment administered to ventilated preterm neonates beginning in the first week of life for the prevention of CLD [defined as requirement for supplemental oxygen at 36 weeks PMA (Shennan 1988)].
Secondary objectives were to compare the effectiveness of a1PI versus placebo on:
1. Other indicators of CLD including: requirement for supplemental oxygen at 28 days of postnatal age; duration of requirement for supplemental oxygen; duration of assisted ventilation; requirement for systemic antiinflammatory therapy; changes in pulmonary function tests (lung compliance and airway resistance).
2. Incidence of adverse events including: mortality (expressed as early neonatal mortality < 7 days; neonatal mortality < 28 days and mortality prior to hospital discharge); growth impairment (weight, head circumference and length) at 36 weeks PMA; side effects including delayed fever, transient leucocytosis and other important side effects.
3. Long term adverse neurodevelopmental outcome (defined as presence of cerebral palsy and/or mental retardation [Bayley Scales of Infant Development‐ Mental Developmental Index < 70] and/or legal blindness [(< 20/200 bilateral visual acuity) and /or deafness (aided or < 60 dB on audiometric testing or the need for hearing aid)].
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised clinical trials comparing a1PI administered via inhalation or by systemic route (regardless of dose and duration) in preterm neonates (< 37 weeks PMA) enrolled within the first week of life.
Types of participants
Preterm neonates (< 37 weeks PMA) receiving assisted ventilation and postnatal age < one week.
Types of interventions
Alpha‐1 proteinase inhibitor (a1PI) versus placebo or no treatment
Types of outcome measures
Primary outcomes
The primary outcome measure was development of CLD [defined as requirement for supplemental oxygen at 36 weeks PMA (Shennan 1988)] amongst all randomised patients.
Secondary outcomes
Secondary outcome measures were:
1. Amongst all randomised:
a. CLD at 28 days (defined as requirement for supplemental oxygen at 28 days of age); b. death by 36 weeks PMA; c. death by 28 days postnatal age; d. death by 7 days postnatal age; e. CLD or death by 36 weeks PMA; f. CLD or death by 28 days of age postnatal age.
2. Amongst survivors:
a. CLD by 36 weeks PMA; b. CLD by 28 days postnatal age; c. duration of requirement for supplemental oxygen; d. duration of assisted ventilation; e. requirement for systemic antiinflammatory therapy; f. changes in pulmonary function tests.
3. Incidence of adverse events including:
a. mortality (expressed as early neonatal mortality < 7 days; neonatal mortality < 28 days and mortality prior to hospital discharge); b. growth impairment (weight, head circumference and length < 3rd percentile) at 36 weeks PMA; c. side effects including delayed fever, transient leucocytosis or other side effects.
4. Long term neurodevelopmental outcome (frequency of cerebral palsy and/or mental retardation, legal blindness and /or deafness)
Search methods for identification of studies
See: Cochrane Neonatal Review Group search strategy Randomized or quasi randomised controlled trials comparing a1PI versus placebo were identified from MEDLINE (1966 to February 2005) using MeSH headings: infant‐newborn, chronic lung disease, bronchopulmonary dysplasia; lung inflammation, antiinflammatory agents, alpha‐1 proteinase inhibitor, alpha‐1 antitrypsin; administration, inhalation, intravenous.
Other databases that were searched included: EMBASE (1980 to February 2005), CINAHL (1982 to February 2005), Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2005), reference lists of published trials, and abstracts from the annual meetings of the Society of Pediatric Research, American Pediatric Society and Pediatric Academic Societies published in Pediatric Research (1991 to 2005). No language restriction were applied. No dosage regimen was specified. The candidate articles were screened by the two review authors (PS, AO) to identify articles eligibility for inclusion in the review.
The search was updated in Novemberer 2010: see Appendix 1.
Data collection and analysis
Selection of studies
All published articles identified as potentially relevant by the literature search were assessed for inclusion in the review. In order to be included the trial had to meet the following criteria:
1. the study population had to be preterm neonates admitted to a neonatal intensive care unit; 2. the intervention had to be a1PI administered via inhalation or intravenous route; 3. the study had to be a randomised or quasi randomised controlled trial; 4. at least one primary or secondary outcome was reported.
Data extraction and management
Data from the primary author were obtained if published data provided inadequate information for the review. Retrieved articles were assessed and data were abstracted independently by the review authors. Discrepancies were resolved by consensus among the two review authors.
Assessment of risk of bias in included studies
Standardized methods of the Cochrane Neonatal Review Group was used to assess methodological quality of the studies.
The methodological quality of the studies were assessed using the following key criteria: allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow‐up, and blinding of outcome measurement/assessment. For each criterion, assessment was yes, no, can't tell. Two review authors separately assessed each study. Any disagreement was resolved by discussion. This information was added to the Characteristics of Included Studies Table.
For the update in 2010, the following issues were evaluated and entered into the Risk of Bias table:
1) Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated? For each included study, we categorized the method used to generate the allocation sequence as:
‐ adequate (any truly random process e.g. random number table; computer random number generator);
‐ inadequate (any non random process e.g. odd or even date of birth; hospital or clinic record number);
‐ unclear.
(2) Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
‐ adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
‐ unclear.
(3) Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
‐ adequate, inadequate or unclear for participants;
‐ adequate, inadequate or unclear for personnel;
‐ adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
‐ adequate (< 20% missing data);
‐ inadequate (≥ 20% missing data):
‐ unclear.
(5) Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
‐ adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
‐ inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
‐ unclear.
(6) Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
‐ yes; no; or unclear.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
Statistical methods included relative risk (RR), risk difference (RD), number needed to treat (NNT) and weighted mean difference (WMD) as appropriate. Ninety five percent confidence intervals were used for these estimates of treatment effects.
Dealing with missing data
Data from the primary author were obtained if published data provided inadequate information for the review.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I‐squared statistic. If we detected statistical heterogeneity, we planned to explore the possible causes (for example, differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc sub group analyses.
Data synthesis
Meta‐analysis was performed using Review Manager software (RevMan 5) supplied by the Cochrane Collaboration. For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. All meta‐analyses were done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned a priori based on route of administration (intravenous or inhalation). Statistical methods included relative risk (RR), risk difference (RD), number needed to treat (NNT) and weighted mean difference (WMD) when appropriate as recommended by the Cochrane Collaboration Neonatal Review Group. A fixed effect model was used for meta‐analysis.
Two studies contributed to this review. The analyses were performed using two different strategies: 1. comparing 60 mg/kg/dose for four doses of a1PI with placebo; 2. comparing any dose of a1PI with placebo. The decision to conduct separate analyses according to dose was made after the protocol was written.
Results
Description of studies
Two studies were included in this review, Stiskal 1998 and Dunn 2000. Dunn 2000 has been published only in abstract form. Clinical details concerning the participants, interventions and outcomes are given in the table, Characteristics of Included Studies.
Stiskal 1998 randomised patients to receive a1PI (60 mg/kg) or placebo (Human Albumin 5%, equal volume‐ 2.4 ml/kg). Infusion was administered over 5 minutes within 24 hours of birth and at four, seven and fourteen days of age. Other clinical management decisions were left to the clinical care team responsible for the patients. A total of 106 patients were enrolled (53 in each group). Recruitment was halted after 2 deaths occurred in one of the centres after a review by the safety committee the study was allowed to progress. There were four protocol violations and one family refused the participation of their neonate after the first dose had been given.
The primary outcome assessed in the Stiskal 1998 study was the incidence of CLD among the survivors (oxygen requirement at 28 postnatal days). Secondary outcomes assessed were oxygen requirements at 36 weeks PMA and the use of systemic steroids. Strict criteria were laid out to define whether patients met the criteria for steroid use (requirement for mechanical ventilation and FiO2 > 0.3 for at least 48 hours after 16 days of life) but achievement of these criteria didn't mandate the use of steroid. The decision to give steroids or not was left at the discretion of the team taking care of the infant. Other efficacy parameters monitored were age at final extubation, age off supplemental oxygenation, age off respiratory support, x‐ray changes at 28 days and evidence of right ventricular hypertrophy on electrocardiogram. The incidence of pulmonary haemorrhage, intraventricular haemorrhage, periventricular leukomalacia, necrotizing enterocolitis, retinopathy of prematurity, sepsis and PDA were recorded for both groups.
The follow‐up of the cohort was published in abstract form (Kelly 1998). Authors were contacted to confirm that the patients were the same as in the original trial. Eighty three of 94 surviving infants were assessed. Five infants were assessed at <12 months age but the rest were assessed at > 18 months of age. Patients were assessed at standard interval to >18 months of age for developmental progress. Patients were classified in 3 groups: 1. Normal 2. Mildly impaired (motor abnormality without significant functional limitation and/or mild hearing loss and/or severe myopia and/or developmental quotient > 1 < 2 SD below mean and 3. Severely impaired (motor abnormality with significant functional limitation and/or deafness and/or blindness and/or developments quotient > 2 SD below the mean).
Dunn 2000 performed an open randomised study to determine the effect of different dosing regimens of a1PI. Ventilated neonates weighing 500 ‐ 1250g were randomised to four groups.
The placebo used in this study was normal saline solution (2.4 ml/kg). Group 1. Placebo administered on days 1, 4, 7, 14 (24 patients). Group 2. a1PI at 60 mg/kg administered on days 1, 4, 7, 14 (21 patients). Group 3. a1PI at 120 mg/kg administered on days 1, 4, 7, 14 (21 patients). Group 4. a1PI at 60 mg/kg administered on days 1, 2, 3, 4, 7, 14 (23 patients). The outcomes assessed were supplemental oxygen requirement at 28 days postnatal age and 36 weeks PMA amongst survivors.
Risk of bias in included studies
For each study included in the review, assessments of methodological quality are given in the table, Characteristics of Included Studies.
The methodological details for both studies were extracted from the published information and personal contact with the authors.
In the study by Stiskal 1998 randomisation was performed centrally by the pharmacist using computer generated random table numbers. Randomization was stratified according to birth weight and whether patient was out born or inborn. Allocation was concealed in sequential opaque, sealed envelopes. Randomization was done in blocks of four. Masking of the intervention was done to ensure that investigators, parents and care takers were unaware of treatment allocation. Crossover was not allowed and contamination was not possible because study drug was not available outside the trial. Co‐interventions were possible for the use of other interventions which may be effective in reducing the incidence of chronic lung disease and were monitored. Short term outcome assessments (need for oxygen at 28 days postnatal age and 36 weeks PMA) were blinded as the assessors were not aware of treatment allocation. Roentgenograms were performed at 28 days age and reviewed by an independent radiologist.
For the long term outcomes of this group of patients as reported in Kelly 1998, personal communication with authors revealed that the outcome assessments were blinded. Outcome assessments were performed in all the major areas of infant development. Outcome data were presented amongst infants assessed at follow up.
In the study by Dunn 2000 randomisation was performed centrally by the pharmacist using computer generated random table numbers (personal communication). Allocation was not concealed. Randomization was done in blocks of four. Groups were stratified according to birth weight. Masking of the intervention was not performed as one of the groups received 120 mg/kg/dose of a1PI and it was not possible to control the volume of placebo (personal communication). Crossover was not allowed and contamination was not possible because study drug was not available outside the trial. Co‐interventions were possible for the use of other interventions which may be helpful in reducing the incidence of CLD but was monitored. Short term outcome assessments (need for oxygen at 28 days postnatal and 36 weeks PMA) were not blinded. Long‐term follow up for this study was not performed (personal communication).
Effects of interventions
PRIMARY OUTCOME:
Development of CLD at 36 weeks PMA (defined as requirement for supplemental oxygen at 36 weeks PMA) amongst all randomised patients:
There was no statistically significant difference in risk of developing CLD between treatment and placebo either with 60 mg/kg/dose for four doses [pooled RR 0.64 (95% CI 0.35, 1.18); RD ‐0.10 (95% CI ‐0.23, 0.03)] or when any doses of a1PI were combined [pooled RR 0.79 (95% CI 0.44, 1.41); RD ‐0.05 (95% CI ‐0.17, 0.06)].
SECONDARY OUTCOMES:
1. Amongst all randomised
a. CLD at 28 days (defined as requirement for supplemental oxygen at 28 days of age):
There was no statistically significant difference in risk of developing CLD between treatment and placebo with 60 mg/kg/dose for four doses [pooled RR 0.81 (95% CI 0.64, 1.01); RD ‐0.14 (95% CI ‐0.29, 0.00)]. When all doses of a1PI were combined a statistically significant reduction in risk was noted [pooled RR 0.80 (95% CI 0.65, 0.98); RD ‐0.15 (95% CI ‐0.29, ‐0.01)]. The number of patients needed to treat with a1PI to prevent the development of CLD at 28 days in one infant was seven.
b. Death by 36 weeks PMA:
No statistically significant difference were noted in the risk of death by 36 weeks between treatment and placebo either with 60 mg/kg/dose for four doses [pooled RR 1.56 (95% CI 0.58, 4.17); RD 0.04 (95% CI ‐0.05, 0.14)] or when any doses of a1PI were combined [pooled RR 1.48 (95% CI 0.59, 3.72); RD ‐0.04 (95% CI ‐0.05, 0.12)].
c. Death by 28 days
There was no statistically significant difference in risk of death by 28 days between treatment and placebo with 60 mg/kg/dose for four doses [pooled RR 1.71 (95% CI 0.56, 5.24); RD 0.04 (95% CI ‐0.04, 0.13)] or when any doses of a1PI were combined [pooled RR 1.81 (95% CI 0.58, 5.67); RD 0.04 (95% CI ‐0.03, 0.12)].
d. Death by 7 days
Similarly, there was no statistically significant difference in the risk of death before 7 days between treatment and placebo with 60 mg/kg/dose for four doses [RR 1.33 (95% CI 0.31, 5.67); RD 0.02 (95% CI ‐0.08, 0.11)].This outcome was reported by Stiskal 1998 only. The information was not available for the second study.
e. CLD or death by 36 weeks PMA
There was no statistically significant difference in risk of developing CLD or death by 36 weeks PMA between treatment and placebo either with 60 mg/kg/dose for four doses [pooled RR 0.84 (95% CI 0.53, 1.34); RD 0.06 (95% CI ‐0.20, 0.09)] or when any doses of a1PI were combined [pooled RR 0.95 (95% CI 0.61, 1.49); RD ‐0.02 (95% CI ‐0.15, 0.12)].
f. CLD or death by 28 days of age:
There was no statistically significant difference in the risk of developing CLD or death by 28 days between treatment and placebo either with 60 mg/kg/dose for four doses [pooled RR 0.65 (95% CI 0.36, 1.20); RD ‐0.10 (95% CI ‐0.24, 0.04)] or when any doses of a1PI were combined [pooled RR 0.87 (95% CI 0.73, 1.04); RD ‐0.10 (95% CI ‐0.23, 0.03)].
2. Amongst survivors:
a. CLD at 36 weeks PMA:
No statistically significant difference was noted in the risk of developing CLD at 36 weeks between treatment and placebo either with 60 mg/kg/dose for four doses [pooled RR 0.67 (95% CI 0.37, 1.24); RD ‐0.10 (95% CI ‐0.24, 0.04)] or when any doses of a1PI were combined [pooled RR 0.83 (95% CI 0.47, 1.47); RD ‐0.05 (95% CI ‐0.18, 0.08)].
b. CLD by 28 days:
There was no statistically significant difference in the risk of developing CLD at 28 days between treatment and placebo either with 60 mg/kg/dose for four doses [pooled RR 0.84 (95% CI 0.68, 1.04); RD ‐0.12 (95% CI ‐0.27, 0.02)] or when any doses of a1PI were combined [pooled RR 0.84 (95% CI 0.69, 1.02); RD ‐0.12 (95% CI ‐0.26, 0.01)].
c. Duration of requirement for supplemental oxygen:
There was no reduction in the duration of requirement of supplemental oxygen amongst the survivors [MD ‐11.2 days (95% CI ‐26.34, 3.94)]. This was reported by Stiskal 1998 only.
d. Duration of assisted ventilation:
There was no reduction in the duration of the requirement of any kind of respiratory support amongst survivors [WMD ‐5.61 days (95% CI ‐18.04, 6.82)] between treatment and placebo with 60 mg/kg/dose for four doses.
e. Requirement for systemic antiinflammatory therapy:
There was no reduction in the need for systemic anti‐inflammatory therapy amongst the survivors [RR 1.02 (95% CI 0.76, 1.38); RD 0.01 (95% CI ‐0.18, 0.20)]. This outcome was reported only by Stiskal 1998, who set strict and uniform criteria for administration of systemic anti‐inflammatory therapy.
f. Changes in pulmonary function tests: not tested in either study.
3. Incidence of adverse events including:
a. Mortality prior to hospital discharge:
There was no statistically significant difference in risk of death before discharge from the hospital with 60 mg/kg/dose for four doses [RR 1.75 (95% CI 0.54, 5.63); RD 0.06 (95% CI ‐0.06, 0.17)]. This outcome was is reported by Stiskal 1998 only.
b. Growth impairment (weight, length and head circumference below 3rd percentile) at 36 weeks PMA: not reported in either study.
c. Side effects:
No disturbance in hematologic, renal hepatic parameters was observed by Stiskal 1998.
4. Long term neurodevelopmental outcome (frequency of cerebral palsy and/or mental retardation, legal blindness and /or deafness)
There was no difference in risk of severe neurodevelopmental abnormality amongst infants assessed in either group for 60 mg/kg/dose for four doses [RR 1.29 (95% CI 0.43, 3.90); RD 0.03 (95% CI ‐0.11, 0.18)]. The long term outcome for the Dunn 2000 study is not available.
Discussion
An extensive literature search was conducted to identify trials that might qualify for this review. However, only two trials, both performed in a similar setting and by the same group of investigators, were identified. Even after combining trials appropriately the number of neonates providing data on outcomes remained very small (n = 195). As a result, the fact that there was no statistically significant difference found between groups may be due to lack of statistical power.
The quality of included studies was good suggesting that extracted data were valid. However, there were some differences in design. The first study of (Stiskal 1998) compared the drug with placebo in a blinded randomised controlled trial while the second study (Dunn 2000) compared different dose regimens with placebo in an open randomised controlled trial. Methodological rigor was strict in both instances.
There was no statistically significant difference in the development of CLD at 36 weeks PMA. The difference remained insignificant even after higher dose and increased frequency of administration. The incidence of CLD at 28 days was reduced by a1PI but it was apparent that the difference was lost at 36 weeks PMA. The other outcome measures such as duration of oxygen requirement or duration of respiratory assistance was not statistically significantly different amongst the groups irrespective of the dose of a1PI.
Long term outcomes were evaluated in the Stiskal 1998 study only and were not found to be statistically significantly different between treatment and control groups.
Authors' conclusions
Implications for practice.
The prophylactic administration of a1PI for prevention of CLD in preterm neonates may have short term benefits but to date there are no long term benefits reported. The use of a1PI cannot be recommended for routine use to prevent CLD in preterm infants.
Implications for research.
With no trend towards long term benefits of a1PI it may be argued that further placebo‐controlled trials are not warranted. However, the total number of patients enrolled to date is very small. The apparent lack of benefit may be due to a type II error.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 29 November 2010 | New search has been performed | This review updates the existing review "Alpha‐1 proteinase inhibitor (a1PI) for preventing chronic lung disease in preterm infants" published in the Cochrane Database of Systematic Reviews (Shah 2005). Updated search in October 2010 found no new trials. No changes to conclusions. |
| 15 October 2008 | Amended | Converted to new review format. |
| 15 March 2005 | New search has been performed | This review updates the existing review of "Alpha‐1 proteinase inhibitor (a1PI) for preventing chronic lung disease in preterm infants" published in The Cochrane Library, Issue 3, 2001 (Shah 2001). The updated review has no further information as no new studies were identified. |
| 23 April 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Authors would like to thank Dr. Michael Dunn, Chief, Department of Newborn and Developmental Pediatrics, Sunnybrook and Women's College Health Sciences Centre, Toronto, Canada for providing information related to both trials included in this review.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Appendices
Appendix 1. Search Strategy 2010
Medline, CCTR and Embase were searched using the following strategies.
Ovid MEDLINE(R) 1950 to August 27, 2010
Serach strategy
1 Infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, premature/ or exp Infant, Newborn, Diseases/ or pregnancy, high‐risk/ or quadruplets/ or quintuplets/ or superfetation/ or triplets/ or twins/ or twins, dizygotic/ or twins, monozygotic/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw).ti,ab. or ((intrauterine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab.
2 ("clinical trial, all" or clinical trial).pt. or clinical trials as topic/ or clinical trial, phase i.pt. or clinical trials, phase i as topic/ or clinical trial, phase iii.pt. or clinical trials, phase iii as topic/ or clinical trial, phase iv.pt. or clinical trials, phase iv as topic/ or controlled clinical trial.pt. or controlled clinical trials as topic/ or meta‐analysis.pt. or meta‐analysis as topic/ or multicenter study.pt. or multicenter studies as topic/ or randomized controlled trial.pt. or randomized controlled trials as topic/ or evaluation studies as topic/ or validation studies as topic/ or evaluation study.pt. or validation study.pt. or case‐control studies/ or retrospective studies/ or cohort studies/ or longitudinal studies/ or follow‐up studies/ or prospective studies/ or cross‐sectional studies/ or double‐blind method/ or random allocation/ or single‐blind method/ or ((singl* or doubl* or tripl* or trebl*) adj5 (blin or mask or blinded or masked)).ti,ab.
3 alpha 1 Antitrypsin\ or (alpha 1 Antiprotease \Antiprotease, alpha 1 \alpha 1‐Antiproteinase \1‐Antiproteinase, alpha \alpha 1 Antiproteinase \alpha 1‐Proteinase Inhibitor \Inhibitor, alpha 1‐Proteinase \alpha 1 Proteinase Inhibitor \Trypsin Inhibitor, alpha 1‐Antitrypsin \Trypsin Inhibitor, alpha 1 Antitrypsin \Serpin A1 \A1PI \alpha 1‐Protease Inhibitor \Inhibitor, alpha 1‐Protease \alpha 1 Protease Inhibitor).mp
4 1 and 2 and 3
5 1 and 3
EBM Reviews ‐ Cochrane Central Register of Controlled Trials <1st Quarter 2010>
Search Strategy:
1 Infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, premature/ or exp Infant, Newborn, Diseases/ or pregnancy, high‐risk/ or quadruplets/ or quintuplets/ or superfetation/ or triplets/ or twins/ or twins, dizygotic/ or twins, monozygotic/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw).ti,ab. or ((intrauterine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab.
2 alpha 1 Antitrypsin\ or (alpha 1 Antiprotease \Antiprotease, alpha 1 \alpha 1‐Antiproteinase \1‐Antiproteinase, alpha \alpha 1 Antiproteinase \alpha 1‐Proteinase Inhibitor \Inhibitor, alpha 1‐Proteinase \alpha 1 Proteinase Inhibitor \Trypsin Inhibitor, alpha 1‐Antitrypsin \Trypsin Inhibitor, alpha 1 Antitrypsin \Serpin A1 \A1PI \alpha 1‐Protease Inhibitor \Inhibitor, alpha 1‐Protease \alpha 1 Protease Inhibitor).mp
3 1 and 2
EMBASE <1980 to 2010 Week 30>
Search Strategy:
1 newborn/ or newborn period/ or low birth weight/ or extremely low birth weight/ or small for date infant/ or very low birth weight/ or Prematurity/ or exp newborn disease/ or multiple pregnancy/ or twin pregnancy/ or twins/ or dizygotic twins/ or monozygotic twins/ or human triplets/ or intrauterine growth retardation/ or small for date infant/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw or (intrautrine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab. (774681)
2 ct.fs. or clinical trial/ or controlled clinical trial/ or multicenter study/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or cohort analysis/ or double blind procedure/ or single blind procedure/ or triple blind procedure/ or meta analysis/ or randomized controlled trial/ or "systematic review"/ or case control study/ or longitudinal study/ or prospective study/ or retrospective study/ or multicenter study/ or validation study/ or (((evaluation or validation) adj2 study) or ((evaluation or validation) adj2 studies)).ti,ab. (882179)
3 alpha 1 Antitrypsin\ or (alpha 1 Antiprotease \Antiprotease, alpha 1 \alpha 1‐Antiproteinase \1‐Antiproteinase, alpha \alpha 1 Antiproteinase \alpha 1‐Proteinase Inhibitor \Inhibitor, alpha 1‐Proteinase \alpha 1 Proteinase Inhibitor \Trypsin Inhibitor, alpha 1‐Antitrypsin \Trypsin Inhibitor, alpha 1 Antitrypsin \Serpin A1 \A1PI \alpha 1‐Protease Inhibitor \Inhibitor, alpha 1‐Protease \alpha 1 Protease Inhibitor).mp
4 1 and 3
5 2 and 4
We also searched reference lists of identified trials, and abstracts from the annual meetings of the Society for Pediatric Research, American Pediatric Society and Pediatric Academic Societies published in Pediatric Research (2002‐2009). No language restrictions were applied.
Clinicaltrials.gov
Search Strategy:
(Alpha‐1 proteinase inhibitor OR a1PI) AND (neon* OR infant OR newborn)
Limited: Child (birth ‐ 17 years)
Controlled‐trials.com
Search Strategy:
(Alpha‐1 proteinase inhibitor OR a1PI) AND (neon* OR infant OR newborn)
No Limits
Data and analyses
Comparison 1. a1PI 60 mg/kg/dose for four doses versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CLD at 36 weeks amongst all randomised | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.18] |
| 2 CLD at 28 days amongst all randomised | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.01] |
| 3 Death by 36 weeks PMA amongst all randomised | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.58, 4.17] |
| 4 Death by 28 days amongst all randomized | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.56, 5.24] |
| 5 Death by 7 days amongst all randomised | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.67] |
| 6 Death or CLD by 36 weeks PMA amongst all randomised | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.34] |
| 7 Death or CLD by 28 days amongst all randomised | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| 8 CLD by 36 weeks PMA amongst survivors | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.24] |
| 9 CLD by 28 days amongst survivors | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.68, 1.04] |
| 10 Duration of requirement of supplemental oxygen amongst survivors (days) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐11.20 [‐26.34, 3.94] |
| 11 Duration of respiratory support amongst survivors (days) | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐5.61 [‐18.04, 6.82] |
| 12 Requirement for systemic anti‐inflammatory therapy amongst survivors | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.38] |
| 13 Mortality prior to hospital discharge | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.63] |
| 14 Developmental delay amongst infants assessed | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.43, 3.90] |
1.1. Analysis.
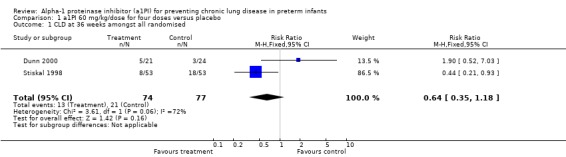
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 1 CLD at 36 weeks amongst all randomised.
1.2. Analysis.
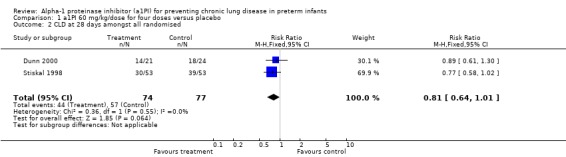
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 2 CLD at 28 days amongst all randomised.
1.3. Analysis.

Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 3 Death by 36 weeks PMA amongst all randomised.
1.4. Analysis.
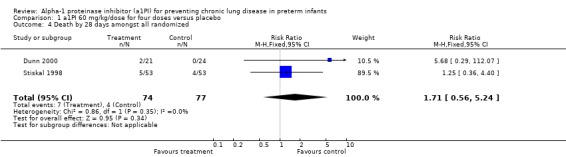
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 4 Death by 28 days amongst all randomized.
1.5. Analysis.
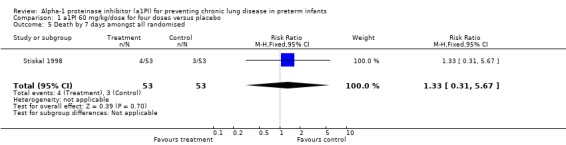
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 5 Death by 7 days amongst all randomised.
1.6. Analysis.
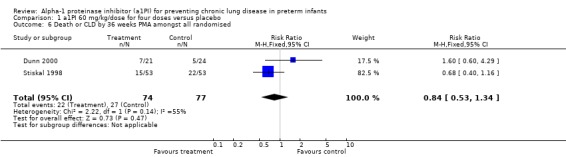
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 6 Death or CLD by 36 weeks PMA amongst all randomised.
1.7. Analysis.
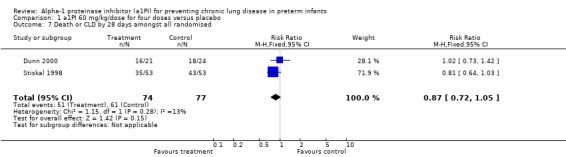
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 7 Death or CLD by 28 days amongst all randomised.
1.8. Analysis.
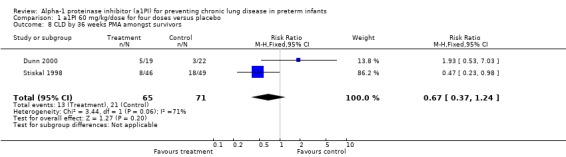
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 8 CLD by 36 weeks PMA amongst survivors.
1.9. Analysis.
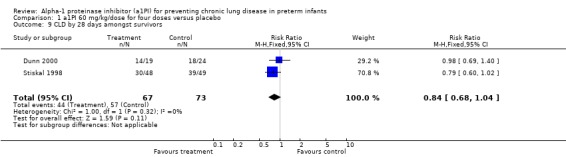
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 9 CLD by 28 days amongst survivors.
1.10. Analysis.
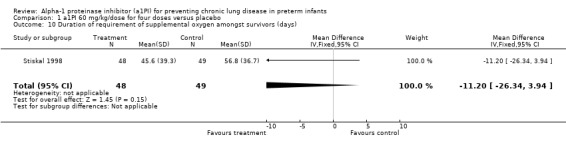
Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 10 Duration of requirement of supplemental oxygen amongst survivors (days).
1.11. Analysis.

Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 11 Duration of respiratory support amongst survivors (days).
1.12. Analysis.

Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 12 Requirement for systemic anti‐inflammatory therapy amongst survivors.
1.13. Analysis.

Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 13 Mortality prior to hospital discharge.
1.14. Analysis.

Comparison 1 a1PI 60 mg/kg/dose for four doses versus placebo, Outcome 14 Developmental delay amongst infants assessed.
Comparison 2. a1PI any dose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CLD at 36 weeks amongst all randomised | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.41] |
| 2 CLD at 28 days amongst all randomised | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 3 Death by 36 weeks PMA amongst all randomised | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.59, 3.72] |
| 4 Death by 28 days amongst all randomised | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.58, 5.67] |
| 5 Death or CLD by 36 weeks PMA amongst all randomised | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.49] |
| 6 Death or CLD by 28 days amongst all randomised | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.04] |
| 7 CLD by 36 weeks PMA amongst survivors | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.47, 1.47] |
| 8 CLD by 28 days amongst survivors | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.69, 1.02] |
2.1. Analysis.
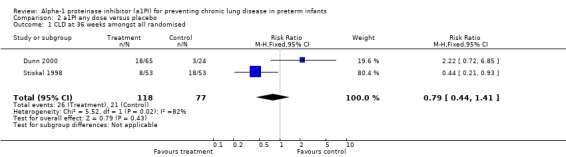
Comparison 2 a1PI any dose versus placebo, Outcome 1 CLD at 36 weeks amongst all randomised.
2.2. Analysis.

Comparison 2 a1PI any dose versus placebo, Outcome 2 CLD at 28 days amongst all randomised.
2.3. Analysis.
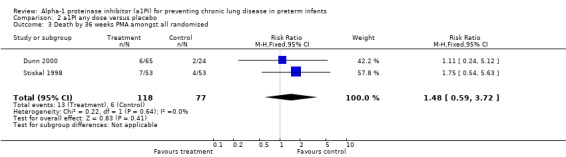
Comparison 2 a1PI any dose versus placebo, Outcome 3 Death by 36 weeks PMA amongst all randomised.
2.4. Analysis.

Comparison 2 a1PI any dose versus placebo, Outcome 4 Death by 28 days amongst all randomised.
2.5. Analysis.

Comparison 2 a1PI any dose versus placebo, Outcome 5 Death or CLD by 36 weeks PMA amongst all randomised.
2.6. Analysis.
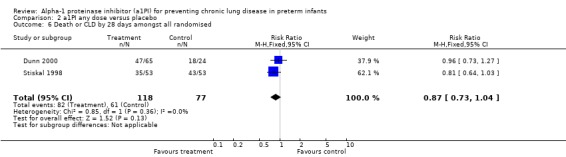
Comparison 2 a1PI any dose versus placebo, Outcome 6 Death or CLD by 28 days amongst all randomised.
2.7. Analysis.
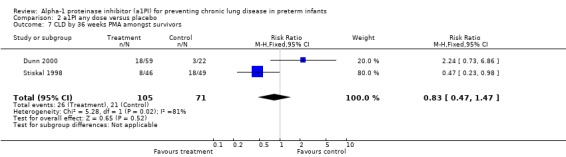
Comparison 2 a1PI any dose versus placebo, Outcome 7 CLD by 36 weeks PMA amongst survivors.
2.8. Analysis.

Comparison 2 a1PI any dose versus placebo, Outcome 8 CLD by 28 days amongst survivors.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dunn 2000.
| Methods | Masking of randomisation: Yes Masking of intervention: Yes Masking of outcome assessment: No Complete follow up: Yes for short term | |
| Participants | 89 ventilated preterm neonates between 600‐1250 grams Bw were randomised to 4 groups: Group 1: 24 neonates: mean (SD) GA 26.3 (1.6) wk and mean (SD) Bw 919.4 (142.0) grams Group 2: 21 neonates: mean (SD) GA 25.1 (1.8) wk and mean (SD) Bw 863.3 (146.9) grams Group 3: 21 neonates: mean (SD) GA 26.6 (1.9) wk and mean (SD) Bw 881.6 (184.2) grams Group 4: 23 neonates: mean (SD) GA 26.3 (1.7) wk and mean (SD) Bw 884.7 (156.4) grams | |
| Interventions | Group 1: Received placebo on days 1, 4, 7, 14 Group 2: received a1PI at 60 mg/kg/dose on days 1, 4, 7, 14 Group 3: received a1PI at 120mg/kg/dose on days 1, 4, 7, 14 Group 4: received a1PI at 60 mg/kg/dose on days 1, 2, 3, 4, 7, 14 | |
| Outcomes | Oxygen dependency at 28 days amongst survivors Oxygen dependency at 36 weeks PMA amongst survivors | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Masking of randomisation: Yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking of intervention: Yes Masking of outcome assessment: No |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: Yes for short term |
Stiskal 1998.
| Methods | Masking of randomisation: Yes Masking of intervention: Yes Masking of outcome assessment: Yes Complete follow up: No | |
| Participants | Total of 106 patients enrolled. Bw 600‐1000 requiring intubation and 1001‐1250 grams with respiratory distress syndrome were eligible for inclusion in the study Group 1: 53 neonates with mean (SD) GA 26.1 (1.7) wk and mean (SD) Bw 861 (167) grams and Group 2: 53 neonates with mean (SD) GA 26.5 (1.7) wk and mean Bw 864 (175) grams | |
| Interventions | Group 1; received 60 mg/kg/dose of a1PI over 5 minutes within first 24 hours of age and this dose was repeated on days 4, 7, 14 at standard times. Group 2: received equivalent volume of placebo (Human Albumin 5%) at the same times. | |
| Outcomes | Incidence of CLD (oxygen dependency at 28 days) in survivors Incidence of CLD (oxygen dependency at 36 weeks PMA) Death or oxygen dependence at 36 weeks PMA Need for systemic steroid administration Age at final extubation Age off all respiratory support Duration of oxygen requirement Death before discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Masking of randomisation: Yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking of intervention: Yes Masking of outcome assessment: Yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: No |
GA = Gestational age CLD = Chronic lung disease Bw = Birth weight a1PI = alpha 1 proteinase inhibitor SD = standard deviation wk = weeks
Contributions of authors
Dr. P S Shah Literature search and identification of trials Evaluation of methodological quality of trials Data collection Verification of data and entry in Revman Writing the text of review
Dr. A Ohlsson Literature search and identification of trials Evaluation of methodological quality of trials Data collection Verification of data Revision of the review
Dr. P S Shah (PS) and Dr. A Ohlsson (AO) wrote the original review and updated the review in 2004. The August 2010 search was conducted by PS and AO. The November 2010 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll). This update was reviewed and approved by PS.
Sources of support
Internal sources
Mount Sinai Hospital, Toronto, Canada.
External sources
No sources of support supplied
Declarations of interest
None.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Dunn 2000 {published data only}
- Dunn M, Stiskal J, O'Brien K, Ito S, Cox DW, Kelly E, Shennan A, et al. Alpha‐1 proteinase inhibitor (A1PI) therapy for the prevention of chronic lung disease of prematurity (CLD) ‐ a dose ranging study and meta analysis with previous randomized clinical trial (RCT). Pediatric Research 2000;47:397A. [Google Scholar]
Stiskal 1998 {published data only}
- Kelly EN, Azstalos E, O'Brien K, Stiskal J, Wylie L, Wylie G, Shennan A, Rabinovitch M, Dunn M. Neurodevelopmental outcome of babies <1250g enrolled in a randomised controlled trial (RCT) of alpha‐1‐proteinase inhibitor (a1PI) therapy. Pediatric Research 1998;43:219A. [Google Scholar]
- Stiskal JA, Dunn MS, Shennan AT, O'Brien KK, Kelly EN, Koppel RI, et al. alpha 1‐ Proteinase inhibitor therapy for the prevention of chronic lung disease of prematurity: a randomized, controlled trial. Pediatrics 1998;101:89‐94. [DOI] [PubMed] [Google Scholar]
Additional references
Bruce 1981
- Bruce MC, Boat TF, Martin RJ, Dearborn DG, Fanaroff A. Inactivation of alpha1 proteinase inhibitor in infants exposed to high concentrations of oxygen. American Review of Respiratory Disease 1981;123:166. [Google Scholar]
Crowley 2001
- Crowley P. Prophylactic corticosteroid for preterm delivery. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000065.pub2] [DOI] [Google Scholar]
Darlow 2001
- Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000501.pub2] [DOI] [Google Scholar]
Davis 1998
- Davis JM. Superoxide dismutase: a role in the prevention of chronic lung disease. Biology of the Neonate 1998;74:29‐34. [DOI] [PubMed] [Google Scholar]
Evans 1972
- Evans HE, Keller S, Mandl I. Serum trypsin inhibitory capacity and the idiopathic respiratory distress syndrome. Journal of Pediatrics 1972;81:588‐92. [DOI] [PubMed] [Google Scholar]
Fletcher 1990
- Fletcher DS, Osinga DG, Hand KM, Dellea PS, Ashe BM, Mumford RA, et al. A comparison of alpha 1‐proteinase inhibitor methoxysuccinyl‐Ala‐Ala‐Pro‐Val‐chloromethylketone and specific beta‐lactam inhibitors in an acute model of human polymorphonuclear leukocyte elastase‐induced lung hemorrhage in the hamster. American Review of Respiratory Disease 1990;141:672‐7. [DOI] [PubMed] [Google Scholar]
Fournel 1988
- Fournel MA, Newgren JO, Betancourt CM, Irwin RG. Preclinical evaluation of alpha‐1‐proteinase inhibitor. Pharmacokinetics and safety studies. American Journal of Medicine 1988;84:43‐7. [DOI] [PubMed] [Google Scholar]
Gadek 1981
- Gadek JE, Fells GA, Zimmerman RL, Rennard SI, Crystal RG. Antielastases of the human alveolar structures. Implications for the protease‐antiprotease theory of emphysema. Journal of Clinical Investigation 1981;68:889‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerdes 1988
- Gerdes JS, Harris MC, Polin RA. Effects of dexamethasone and indomethacin on elastase, alpha‐1 proteinase inhibitor, and fibronectin in bronchoalveolar lavage fluid from neonates. Journal of Pediatrics 1988;113:727‐31. [DOI] [PubMed] [Google Scholar]
Hebel 2000
- Hebel SK, Burnham TH. Drug facts and comparisons. In: Hebel SK, Burnham TH editor(s). Drug facts and comparisons. 2000. Missouri: Facts and Comparisons, 2000:691. [Google Scholar]
Horbar 1993
- Horbar JD, Wright EC, Onstad L. Decreasing mortality associated with the introduction of surfactant therapy: an observational study of neonates weighing 601 to 1300 grams at birth. Pediatrics 1993;92:191‐6. [PubMed] [Google Scholar]
Hubbard 1989
- Hubbard RC, McElvaney NG, Sellers SE, Healy JT, Czerski DB, Crystal RG. Recombinant DNA‐produced alpha 1‐antitrypsin administered by aerosol augments lower respiratory tract antineutrophil elastase defenses in individuals with alpha 1‐antitrypsin deficiency. Journal of Clinical Investigation 1989;84:1349‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 1993
- Jobe AH. Pulmonary surfactant therapy. New England Journal of Medicine 1993;328:861‐8. [DOI] [PubMed] [Google Scholar]
Jones 1996
- Jones CA, Cayabyab RG, Kwong KY, Stotts C, Wong B, Hamdan H, et al. Undetectable interleukin (IL)‐10 and persistent IL‐8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatric Research 1996;39:966‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koppel 1994
- Koppel R, Han RN, Cox D, Tanswell AK, Rabinovitch M. Alpha1‐antitrypsin protects neonatal rats from pulmonary vascular and parenchymal effects of oxygen toxicity. Pediatric Research 1994;36:763‐70. [DOI] [PubMed] [Google Scholar]
McElvaney 1991
- McElvaney NG, Hubbard RC, Birrer P, Chernick MS, Caplan DB, Frank MM, Crystal RG. Aerosol alpha1‐ antitrypsin treatment for cystic fibrosis. Lancet 1991;337:392‐4. [DOI] [PubMed] [Google Scholar]
Merritt 1983
- Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DK 3d, Gluck L. Elastase and alpha 1‐proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. Journal of Clinical Investigation 1983;72:656‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Northway 1967
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline‐membrane disease. Bronchopulmonary dysplasia. New England Journal of Medicine 1967;276:357‐68. [DOI] [PubMed] [Google Scholar]
Ogden 1982
- Ogden BE, Murphy SA, Saunders GC, Johnson JD. Lung lavage of newborns with respiratory distress syndrome (RDS): prolonged neutrophil influx is associated with bronchopulmonary dysplasia (BPD). American Review of Respiratory Disease 1982;125:194. [PubMed] [Google Scholar]
Robertson 1964
- Robertson B, Tunnell R, Rudhe U. Late stages of pulmonary hyaline membranes of the newborn. Acta Pediatrica Scandinavica 1964;53:433‐46. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1984
- Rosenfeld W, Concepcion L, Evans H, Jhaveri R, Brunot V. Alpha1 antitrypsin (AAT) activity in development of bronchopulmonary dysplasia (BPD). Pediatric Research 1984;18:403A. [Google Scholar]
Rosenfeld 1986
- Rosenfeld W, Concepcion L, Evans H, Jhaveri R, Sahdev S, Zabaleta I. Serial trypsin inhibitory capacity and ceruloplasmin levels in prematures at risk for bronchopulmonary dysplasia. American Review of Respiratory Disease 1986;134:1229‐32. [DOI] [PubMed] [Google Scholar]
Shaw 1993
- Shaw NJ, Gill AB, Weindling AM, Cooke RW. The changing incidence of chronic lung disease. Health Trends 1993;25:50‐3. [PubMed] [Google Scholar]
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82:527‐32. [PubMed] [Google Scholar]
Shepard 1964
- Shepard F, Gray J, Stahlman M. The occurrence of pulmonary fibrosis in children who had idiopathic respiratory distress syndrome. Journal of Pediatrics 1964;65:1078‐9. [Google Scholar]
Singer 1976
- Singer AD, Thibeault DW, Hobel CJ, Heiner DC. Alpha1‐antitrypsin in amniotic fluid and cord blood of preterm infants with the respiratory distress syndrome. Journal of Pediatrics 1976;88:87‐93. [DOI] [PubMed] [Google Scholar]
Watts 1991
- Watts JL, Milner R, Zipursky A, Paes B, Ling E, Gill G, Fletcher B, Rand C. Failure of supplementation with vitamin E to prevent bronchopulmonary dysplasia in infants less than 1,500 g birth weight. European Respiratory Journal 1991;4:188‐90. [PubMed] [Google Scholar]
Wewers 1987
- Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, Crystal RG. Replacement therapy for alpha 1‐antitrypsin deficiency associated with emphysema. New England Journal of Medicine 1987;316:1055‐62. [DOI] [PubMed] [Google Scholar]
Yoder 1991
- Yoder MC Jr, Chua R, Tepper R. Effect of dexamethasone on pulmonary inflammation and pulmonary function of ventilator‐ dependant infants with bronchopulmonary dysplasia. American Review of Respiratory Disease 1991;143:1044‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shah 2001
- Shah P, Ohlsson A. Alpha‐1 proteinase inhibitor (a1PI) for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2005
- Shah P, Ohlosson A. Alpha‐1 proteinase inhibitor (a1PI) for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002775] [DOI] [PMC free article] [PubMed] [Google Scholar]


