Abstract
Background
Patent ductus arteriosus (PDA) complicates the clinical course of preterm infants and increases the risk of adverse outcomes. Indomethacin has been the standard treatment to close a PDA but is associated with renal, gastrointestinal, and cerebral side effects. Ibuprofen has less effect on blood flow velocity to important organs.
Objectives
Primary objectives
To determine the effectiveness and safety of ibuprofen compared to placebo/no intervention, or other cyclo‐oxygenase inhibitor drugs in the prevention of PDA in preterm infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 10), MEDLINE via PubMed (1966 to 17 October 2018), Embase (1980 to 17 October 2018), and CINAHL; 1982 to 17 October 2018). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing ibuprofen with placebo/no intervention or other cyclo‐oxygenase inhibitor drugs to prevent PDA in preterm or low birth weight infants.
Data collection and analysis
We extracted outcomes data including presence of PDA on day three or four of life (after 72 hours of treatment), need for surgical ligation or rescue treatment with cyclo‐oxygenase inhibitors, mortality, cerebral, renal, pulmonary, and gastrointestinal complications. We performed meta‐analyses and reported treatment estimates as typical mean difference (MD), risk ratio (RR), risk difference (RD) and, if statistically significant, number needed to treat to benefit (NNTB) or to harm (NNTH), along with their 95% confidence intervals (CI). We assessed between‐study heterogeneity by the I‐squared test (I²). We used the GRADE approach to assess the quality of evidence.
Main results
In this updated analysis, we included nine trials (N = 1070 infants) comparing prophylactic ibuprofen (IV or oral) with placebo/no intervention or indomethacin. Ibuprofen (IV or oral) probably decreases the risk of PDA on day 3 or 4 (typical RR 0.39, 95% CI 0.31 to 0.48; typical RD ‐0.26, 95% CI ‐0.31 to ‐0.21; NNTB 4, 95% CI 3 to 5; 9 trials; N = 1029) (moderate‐quality evidence). In the control group, the spontaneous closure rate was 58% by day 3 to 4 of age. In addition, ibuprofen probably decreases the need for rescue treatment with cyclo‐oxygenase inhibitors (typical RR 0.17, 95% CI 0.11 to 0.26; typical RD ‐0.27, 95% CI ‐0.32 to ‐0.22; NNTB 4; 95% CI 3 to 5),and the need for surgical ductal ligation (typical RR 0.46, 95% CI 0.22 to 0.96; typical RD ‐0.03, 95% CI ‐0.05 to ‐0.00; NNTB 33, 95% CI 20 to infinity; 7 trials; N = 925) (moderate‐quality evidence). There was a possible decrease in the risk of grade 3 or 4 intraventricular haemorrhage (IVH) in infants receiving prophylactic ibuprofen (typical RR 0.67, 95% CI 0.45 to 1.00; I² = 34%; typical RD ‐0.04, 95% CI ‐0.08 to‐ 0.00; I² = 60%; 7 trials; N = 925) (moderate‐quality evidence). High quality evidence showed increased risk for oliguria (typical RR 1.45, 95% CI 1.04 to 2.02; typical RD 0.06, 95% CI 0.01 to 0.11; NNTH 17, 95% CI 9 to 100; 4 trials; N = 747). Low quality results from four studies (N = 202) showed that administering oral ibuprofen may decrease the risk of PDA (typical RR 0.47, 95% CI 0.30 to 0.74) and may increase risk of gastrointestinal bleeding (NNTH 7, 95% CI 4 to 25). No evidence of a difference was identified for mortality, any intraventricular haemorrhage (IVH), or chronic lung disease.
Authors' conclusions
This review shows that prophylactic use of ibuprofen, compared to placebo or no intervention, probably decreases the incidence of patent ductus arteriosus, the need for rescue treatment with cyclo‐oxygenase inhibitors, and for surgical ductal closure. Adverse effects associated with ibuprofen (IV or oral) included increased risks for oliguria, increase in serum creatinine levels, and increased risk of gastrointestinal haemorrhage. There was a reduced risk for intraventricular haemorrhage (grade III ‐ IV) but no evidence of a difference in mortality, chronic lung disease, necrotising enterocolitis, or time to reach full feeds. In the control group, the patent ductus arteriosus had closed spontaneously by day 3 or 4 in 58% of neonates. Prophylactic treatment exposes a large proportion of infants unnecessarily to a drug that has important side effects without conferring any important short‐term benefits. Current evidence does not support the use of ibuprofen for prevention of patent ductus arteriosus. Until long‐term follow‐up results of the trials included in this review have been published, no further trials of prophylactic ibuprofen are recommended.
A new approach to patent ductus arteriosus management is an early targeted treatment based on echocardiographic criteria within the first 72 hours of life, that have a high sensitivity for diagnosing a patent ductus arteriosus that is unlikely to close spontaneously. Such trials are currently ongoing in many parts of the world. Results of such trials will be included in updates of our "Ibuprofen for treatment of PDA" review.
Plain language summary
Ibuprofen for prevention of patent ductus arteriosus in preterm and/or low birth weight infants
Review question. Is prophylactic ibuprofen compared to placebo/no intervention or indomethacin effective and safe for prevention of PDA in preterm infants?
Background. Patent ductus arteriosus (PDA) is a common complication for very preterm (premature) or very small babies. PDA is an open vessel that channels blood from the lungs to the body. It should close after birth but sometimes remains open because of the baby's immature stage of development, and this can lead to life‐threatening complications. Indomethacin is successful in causing PDA closure but can cause serious adverse effects. Another option is the drug ibuprofen, which can be given to prevent PDA.
Study characteristics. More than 1000 infants have been enrolled in trials of ibuprofen for prevention of a PDA; most studies were of small sample size.
Key results. Prophylactic use of ibuprofen reduces the incidence of patent ductus arteriosus (PDA), the need for rescue treatment with other medications, or the need for surgical closure. Adverse effects in the ibuprofen group compared to the placebo or no interventions group included significantly increased risk of kidney complications. Risk of digestive tract bleeding was increased with ibuprofen. Risk of intraventricular haemorrhage, or bleeding into the brain (grade II to IV), of borderline significance was reduced, but researchers reported no statistically significant differences in mortality, chronic lung disease at 28 days' or 36 weeks' postmenstrual age, necrotising enterocolitis, or time to reach full feeds. In the control group, the PDA had closed spontaneously by day 3 or 4 in 58% of neonates. Preventative treatment therefore exposes a large proportion of infants unnecessarily to a drug that has important side effects without conferring any important short‐term benefit for outcomes. No long‐term follow‐up studies have been published. Current evidence does not support the use of ibuprofen for prevention of PDA. A new approach for management of PDA is an early targeted treatment based on echocardiographic (ECG), or image of the heart, criteria within the first 72 hours of life; this has high sensitivity for diagnosing a PDA that is unlikely to close spontaneously. Such trials are currently ongoing in many parts of the world.
Quality of evidence. This updated review of trials found that ibuprofen can prevent PDA but does not confer any other short‐term or long‐term benefits. The quality of evidence varied from low to high for different outcomes.
Summary of findings
Background
Description of the condition
Patent ductus arteriosus (PDA) often complicates the clinical course of preterm infants with or without respiratory distress syndrome (RDS) (Ramanathan 1997). In a large Canadian cohort (N = 3779) of very low birth weight infants (< 1500 g), the incidence of symptomatic PDA needing treatment was 28% (Lee 2000). Failure of the ductus arteriosus to constrict after birth is due to lower intrinsic tone, fewer ductal muscle fibres, and fewer subendothelial cushions in the preterm infant as compared to the term infant (Hammerman 1995). The immature ductus arteriosus has higher sensitivity to the vasodilating effects of prostaglandins and nitric oxide (Hammerman 1995). This is aggravated by haemodynamic derangements due to RDS and surfactant therapy (Hammerman 1995).
The clinical consequences of PDA are related to the degree of left‐to‐right shunting through the ductus. Despite the ability of the left ventricles in preterm infants to increase output in the face of a left‐to‐right shunt, blood flow distribution to vital organs is altered due to a drop in diastolic pressure and localised vasoconstriction (Clyman 2000). Substantial left‐to‐right shunting through the ductus may increase the risk of intraventricular haemorrhage (IVH), necrotising enterocolitis (NEC), chronic lung disease (CLD), and death (Cotton 1979).
Description of the intervention
Inhibiting prostaglandin synthesis with non‐selective blockers of both cyclo‐oxygenase 1 and 2 is effective for the non‐surgical closure of PDA (Clyman 2000). Intravenous indomethacin has become the standard pharmacological treatment for promoting closure of PDA in preterm infants; it has been used since 1976 (Friedman 1976), with reported efficacy of 66% to 80% (Gersony 1983; Lago 2002; Van Overmeire 2000).
Prophylactic use of indomethacin for prevention of PDA has been shown to reduce the incidence of a symptomatic PDA, the need for surgical ligation, and the occurrence of pulmonary haemorrhage (Couser 1996; Domanico 1994). A large trial showed that prophylactic use of indomethacin in extremely low birth weight infants reduces the frequency of PDA and severe intraventricular haemorrhage but provided no evidence of an effect on the rate of survival without neurosensory impairment at 18 months (Schmidt 2001). Similarly, a meta‐analysis of 19 eligible studies showed that prophylactic indomethacin reduces the incidence of symptomatic PDA, the need for surgical ligation, and the incidence of grade III and IV intraventricular haemorrhage in preterm infants, but without evidence of an effect on mortality or on the incidence of long‐term neurosensory impairment (Fowlie 2010).
However, the use of indomethacin may be followed by side effects such as decreased cerebral blood flow (Edwards 1990; Ohlsson 1993; Van Bel 1989), decreased cerebral blood volume and cerebral oxygen delivery (Patel 2000), oliguria or transient renal failure (Betkerur 1981; Gersony 1983; Lago 2002; Van Overmeire 2000), and necrotising enterocolitis and isolated bowel perforation or gastrointestinal haemorrhage (Gersony 1983; Grosfeld 1996). Concern regarding these complications potentially related to indomethacin use has tempered enthusiasm for its use, encouraging many researchers to seek new, safer pharmacological strategies for closure of a PDA. The only major side effect reported in the Cochrane Review by Fowlie was an increased incidence of oliguria (Fowlie 2010), but this was not associated with major renal impairment. The same review found no evidence of differences in rates of NEC, excessive clinical bleeding, or sepsis.
Other cyclo‐oxygenase inhibitors have been reported to close a PDA. In Japan, mefenamic acid is frequently used for treatment of individuals with a PDA (Ito 1994; Niopas 1994; Sakhalkar 1992).
How the intervention might work
Ibuprofen, another cyclo‐oxygenase inhibitor drug, has been used for ductal closure in animals (Coceani 1979). Preliminary experimental and clinical studies ‐ Varvarigou 1996 and Van Overmeire 1997 ‐ have shown that ibuprofen is effective in closing PDA without reducing cerebral flow (Mosca 1997; Patel 2000), and without affecting intestinal circulation (Speziale 1999), or renal circulation (Pezzati 1999). Furthermore, ibuprofen enhances cerebral blood flow autoregulation (Chemtob 1990), and it has been shown to protect neurological functions following oxidative stress in a piglet model (Chemtob 1993).
Why it is important to do this review
Trials reporting on the prophylactic use of ibuprofen in preterm neonates have been published over the years, justifying the need for a Cochrane Review. This review aims to examine the role of prophylactic use of ibuprofen for prevention of PDA in preterm infants by comparing it to no intervention, placebo, indomethacin, or other cyclo‐oxygenase inhibitors.
Objectives
Primary objectives
To determine the effectiveness and safety of ibuprofen compared to placebo or no intervention for prevention of PDA in preterm and/or low birth weight infants
To determine the effectiveness and safety of ibuprofen compared to other cyclo‐oxygenase inhibitor drugs (i.e. indomethacin, mefenamic acid) for prevention of PDA in preterm and/or low birth weight infants
Secondary objectives
To determine in subgroup analyses the effectiveness and safety of prophylactic ibuprofen for closing a PDA in relation to the following criteria
Dose of ibuprofen used
Route of administration of ibuprofen (IV or oral)
Gestational age (< 28 weeks, 28 to 32 weeks, 33 to 37 weeks) or birth weight (< 1000 g, 1000 to 1500 g, > 1500 to < 2500 g)
Method used to diagnose a PDA (only by clinical criteria or by echocardiographic criteria)
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials with or without blinding.
Types of participants
Preterm infants < 37 weeks' gestational age and low birth weight infants (< 2500 g) in their first 72 hours of life (three days).
Types of interventions
Prophylactic use of ibuprofen for prevention of PDA compared to control consisting of no intervention, placebo, other cyclo‐oxygenase inhibitor drugs (indomethacin, mefenamic acid), or rescue treatment with ibuprofen.
Types of outcome measures
Primary outcome
Presence of patent ductus arteriosus (clinically symptomatic or diagnosed by echocardiography in response to clinical suspicion or diagnosed on routine screening by echocardiography) by 72 hours after initiating treatment (three to four days of age)
Secondary outcomes
Neonatal mortality (death during first 28 days of life)
All‐cause mortality during initial hospital stay
Mortality before 36 weeks' postmenstrual age (PMA)
Infant mortality (death during first year of life)
Need for rescue treatment with cyclo‐oxygenase inhibitors for closure of PDA
Need for surgical closure of PDA
Duration of mechanical ventilation (days)
Oxygen requirement (postnatal age in days at time of last day with need for supplemental oxygen)
Chronic lung disease (CLD) (defined as oxygen requirements at 28 days' postnatal age in addition to compatible clinical and roentgenographic findings)
Chronic lung disease (CLD) (defined as oxygen requirements at 36 weeks' PMA in addition to compatible clinical and roentgenographic findings (Shennan 1988))
Chronic lung disease (CLD) (age at diagnosis not reported)
Pneumothorax
Pulmonary hypertension (PH)
Intraventricular haemorrhage (IVH) (all grades)
Intraventricular haemorrhage (IVH) (grade not stated)
Intraventricular haemorrhage (IVH) (grade III or IV) (Papile 1978)
Periventricular leukomalacia (PVL)
Necrotising enterocolitis (NEC) (any stage) (Bell 1978)
Gastrointestinal haemorrhage
Gastrointestinal perforation (defined by presence of free air in peritoneal cavity on abdominal x‐ray)
Time to full enteral feeds (postnatal age in days at time of achieving full enteral feeds)
Length of hospitalisation in days from birth to discharge home or death
Urine output after treatment (mL/kg/hr)
Oliguria (decreased urine output defined as < 1 cc/kg/hr)
Serum creatinine levels after treatment (micromol/L)
At least one episode of serum creatinine > 140 micromol/L (> 1.5 mg/dL)
At least one episode of severe hypoxaemia
Inhaled nitric oxide administration during first week of life
Retinopathy of prematurity (ROP) (according to the international classification of ROP) (ICROP 1984)
Definitive sepsis (clinical symptoms and signs of sepsis and a positive bacterial culture in a specimen obtained from normally sterile fluids or tissue obtained at autopsy)
Probable sepsis (clinical symptoms and signs of sepsis and abnormal findings on a laboratory screening test for infection)
Side effects not listed as an outcome above but reported by study authors as a side effect
Neurodevelopmental outcome (neurodevelopmental outcome assessed by a standardised and validated assessment tool and/or a child developmental specialist) at any age (outcome data grouped at 12, 18, and 24 months, if available)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialised register).
Electronic searches
We conducted a comprehensive search including Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 10), in the Cochrane Library; MEDLINE via PubMed (1966 to 17 October 2018); Embase (1980 to 17 October 2018); and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 17 October 2018), using the following search terms: terms in Appendix 1, plus database‐specific limiters for randomised controlled trials and neonates (see Appendix 1 for full search strategies for each database). We did not apply language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform; and the International Standard Randomised Controlled Trials Number (ISRCTN) Registry).
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
Data collection and analysis
Selection of studies
We applied machine learning using the Cochrane Classifier tool in the Cochrane Register of Studies (CRS) to remove reports with the least (0 to 2%) probability of being randomised controlled trials, and with the least (0 to 2%) probability of including infants in the study population.
Two review authors (AO, SS) assessed all abstracts and published full reports identified as potentially relevant by the literature search for inclusion in the review.
Data extraction and management
Each review author extracted data separately using pre‐designed data abstraction forms. The review authors compared results and resolved differences. One review author (AO) entered data into RevMan 5.3, and the other review author (SS) cross‐checked the printout against his own data abstraction forms and corrected errors by consensus. For studies identified as abstracts, some primary authors were contacted to ascertain whether a full publication is available if the full paper was not identified in an electronic database. We obtained information from the primary author if the published article provided inadequate information for the review. Review authors assessed retrieved articles and abstracted data independently.
Assessment of risk of bias in included studies
Two review authors (AO, SS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2017).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
Any disagreements were resolved by discussion or by a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
Statistical analyses followed the recommendations of Cochrane Neonatal. A weighted treatment effect was calculated using Review Manager 5 (RevMan 5). Treatment effect estimates included typical risk ratio (RR), typical risk difference (RD), number needed to treat for an additional beneficial (NNTB) or harmful (NNTH) outcome for dichotomous outcomes, and weighted mean difference (WMD) for continuous outcomes. All estimates of treatment effects were reported with 95% confidence intervals (CIs).
Unit of analysis issues
The unit of randomisation was the individual infant. We did not include cross‐over or cluster‐randomised trials, as those trial designs are unlikely for the intervention studied in this review. We identified no cross‐over or cluster‐randomised trials. An infant was considered only once even if the infant may have been randomised twice by investigators. We planned to contact study authors to obtain data resulting from the first randomisation. If we could not separate data from the first randomisation, we planned to exclude the study.
Dealing with missing data
We requested additional data from the authors of each included trial when data on important outcomes were missing or needed clarification.
Assessment of heterogeneity
We performed heterogeneity tests including the I² test to assess the appropriateness of pooling data using the following categories for heterogeneity: less than 25%, no heterogeneity; 25% to 49%, low heterogeneity; 50% to 74%, moderate heterogeneity; and 75% or greater, high heterogeneity (Higgins 2003).
Assessment of reporting biases
To ascertain the possibility of publication bias, we prepared one funnel plot for the primary outcome of 'Failure to close a PDA (after single or three doses)', which included nine studies.
Data synthesis
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
Comparison 1: ibuprofen (IV or oral) vs placebo or none. Those outcomes included presence of PDA on third or fourth day of life (after 72 hours of treatment), need for surgical closure of PDA, intraventricular haemorrhage grade III/IV, necrotising enterocolitis, GI haemorrhage, oliguria, and serum creatinine levels after treatment.
Comparison 2: ibuprofen (oral) vs placebo or none. Those outcomes included presence of PDA on third or fourth day of life (after 72 hours of treatment) and gastrointestinal haemorrhage.
Comparison 3: ibuprofen (IV) vs placebo or none. Outcomes included presence of PDA on third or fourth day of life (after 72 hours of treatment).
Comparison 4: ibuprofen (oral) vs indomethacin (oral). We did not perform quality assessments for this comparison as only one study with a total of 62 participants was included.
Two review authors (AO, SS) independently assessed the quality of evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias ‐ random sequence generation, allocation concealment, blinding of personnel, and blinding of outcome assessments), heterogeneity/consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence as one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Gestational age (< 28 weeks, 28 to 32 weeks, 33 to 36 weeks).
Birth weight (< 1000 g, 1000 to 1500 g, 1501 to 2500 g).
Method used to diagnose a PDA (by echocardiographic criteria or by clinical criteria alone).
Dosing regimen of 10 mg/kg of ibuprofen followed by 5 mg/kg of ibuprofen 24 and 48 hours later, or 0.2 mg/kg of indomethacin at 12‐hour intervals for three doses.
The prespecified subgroup analyses excluding studies that used only one dose of medication and studies that were published as abstracts only were abandoned for the previous version and for this update of the review. Only one study used a single dose, and only one abstract was identified. The results of these studies are incorporated with results of the other studies.
Oral ibuprofen had then been studied in two randomised control trials; to compare the effectiveness of oral versus IV ibuprofen, we performed two additional analyses.
Comparison: oral ibuprofen versus placebo; primary outcome ‐ "the presence of patent ductus arteriosus (diagnosed on routine screening by echocardiography) by 72 hours (three or four days) of age".
Comparison: IV ibuprofen versus placebo: primary outcome ‐ "the presence of patent ductus arteriosus (diagnosed on routine screening by echocardiography) by 72 hours (three or four days) of age".
To try to explain the heterogeneity noted in the analysis for gastrointestinal haemorrhage that included three trials ‐ one using IV ibuprofen and two using oral ibuprofen ‐ we combined In the post‐hoc analysis the two trials using oral ibuprofen.
For this update of the review in 2018, we included two additional studies using oral ibuprofen compared to no intervention and one study comparing oral ibuprofen to oral indomethacin.
Sensitivity analysis
We performed planned subgroup analyses according to the criteria listed under objectives. We performed no sensitivity analyses.
Results
Description of studies
Results of the search
The 'Study flow diagram' (Figure 1) illustrates results of the different searches and deletions and additions of identified studies. Nine studies comparing prophylactic ibuprofen with placebo or no medication or oral indomethacin qualified for inclusion in this updated review (Dani 2000; Dani 2005; De Carolis 2000; Gournay 2004; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008; Van Overmeire 2004). Inclusion of Kanmaz 2013 and Kalani 2016 increased the total number of infants enrolled in trials from 931 to 1070. All trials have been published as full‐text articles. Rubaltelli published an abstract in 1998 that reported an interim analysis of Dani 2000. The dose and duration of prophylactic ibuprofen were similar in all studies, but the age at which ibuprofen was started varied from 2 to 24 hours among studies. Four studies used oral ibuprofen(Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008), and all other studies administered ibuprofen by the IV route. PDA at 72 hours (three to four days of age) following treatment and diagnosed via echocardiographic criteria was reported as an outcome in all studies. Echocardiographic criteria of a significant PDA were similar between studies. Back‐up medical treatment with cyclo‐oxygenase inhibitors (indomethacin or ibuprofen) was permitted in the presence of significant PDA (after initial trial of ibuprofen, placebo, or no medication) in all trials (Dani 2000; Dani 2005; De Carolis 2000; Gournay 2004; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008; Van Overmeire 2004). Further details can be found in the Characteristics of included studies table. We identified one study of oral ibuprofen versus oral indomethacin (Kalani 2016).
1.
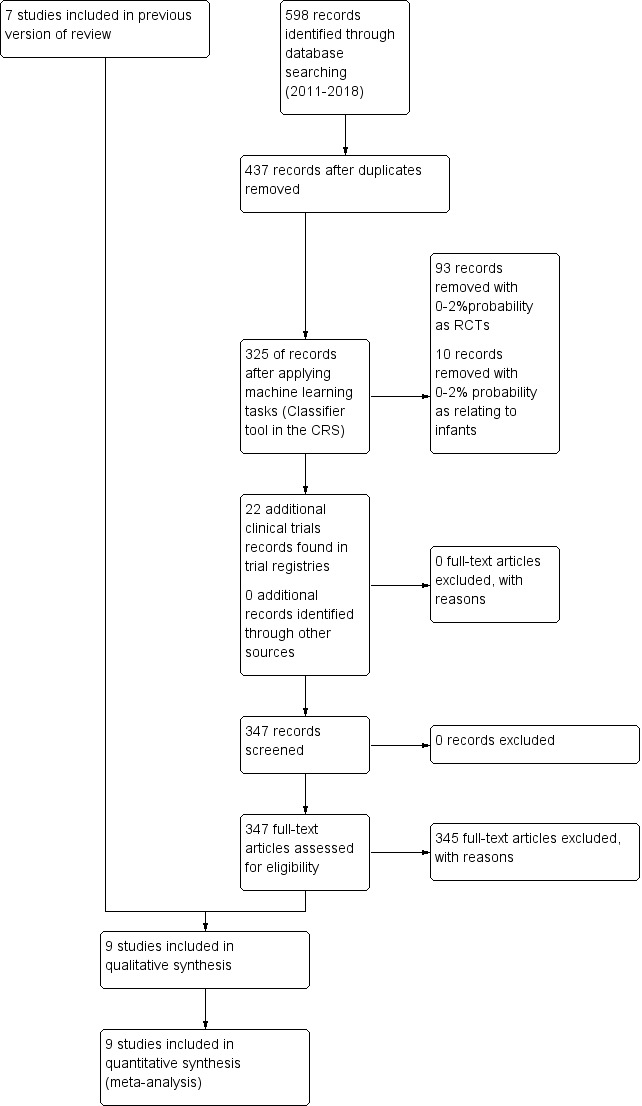
Study flow diagram.
Included studies
We included the following studies in this update of the review.
Dani 2000 ‐ This trial enrolled 80 preterm neonates with gestational age < 34 weeks with RDS requiring either continuous positive airway pressure (CPAP) with fractional inspired oxygen concentration (FiO₂) > 0.3 or mechanical ventilation (synchronised intermittent mandatory ventilation or high‐frequency ventilation). Infants were randomised to receive IV ibuprofen lysine (10 mg/kg, followed by 5 mg/kg after 24 and 48 hours) either within 24 hours of life (prophylactic) or after echocardiographic diagnosis of PDA (selective). When PDA was still present after the first course of ibuprofen, a second course was administered. Failure to respond to ibuprofen was an indication for surgical ligation. Primary outcome was incidence of significant PDA as determined by echocardiographic analysis. Echocardiographic evaluation was performed on days 3, 7, and 21 of life. Other studied variables were ventilatory support, renal function, biochemical and hematological profiles, frequency of CLD at 36 weeks' PMA, intraventricular haemorrhage, necrotising enterocolitis, ROP, and time to reach full feeds.
De Carolis 2000 ‐ The trial enrolled 50 preterm neonates with gestational age < 31 weeks. Infants were randomly assigned at two hours of age to prophylaxis or control. Two infants in each group died during the first 24 hours after birth and were not included by study authors in the final analysis. The prophylaxis group (N = 23) received IV treatment with ibuprofen lysine (10 mg/kg) followed by 5 mg/kg after 24 and 48 hours. No placebo was given to the control group (N = 23). In the presence of a significant PDA at completion of the ibuprofen cycle, treatment with indomethacin (three times 0.2 mg/kg at 12‐hourly intervals, administered by IV infusion over 20 minutes) was carried out. The same treatment was administered to infants in the control group who had a significant PDA on day 3 of life. Failure to respond to medical treatment was an indication for surgical treatment. Primary outcome was incidence of significant PDA as determined by echocardiographic analysis. Echocardiographic evaluation was performed immediately after birth, on day 3 of life, and whenever there was a clinical suspicion of PDA. Other studied variables were ventilatory support, renal function, biochemical and haematological profiles, need for surgical ligation for PDA, frequency of CLD at 28 days, IVH, NEC, ROP, and time to reach full feeds.
Gournay 2004 ‐ This multi‐centre trial enrolled 135 infants at < 28 weeks' gestational age and < 6 hours' postnatal age. Infants were randomly assigned to prophylactic ibuprofen or placebo, both of which were given as three successive doses 24 hours apart. The initial dose of ibuprofen was 10 mg/kg, and the two following doses were 5 mg/kg, infused IV over 20 minutes. The primary outcome was need for surgical ligation. Other outcomes included mortality, PDA on day 3 by echocardiogram, need for back‐up treatment with indomethacin, PVL, grade III or IV IVH, NEC, intestinal perforation, duration of mechanical ventilation, CLD at 36 weeks' PMA, renal function, and actuarial curve of survival during the study period. Occurrence of pulmonary hypertension within one hour of administration of ibuprofen was reported in three infants at < 27 weeks and < 1000 g. The trial was stopped prematurely after enrolment of 135 infants due to this adverse effect.
Van Overmeire 2004 ‐ In this multi‐centre trial, 415 preterm infants at < 31 weeks' gestation were randomised to receive either three doses of IV ibuprofen lysine (10 mg/kg followed by 5 mg/kg after 24 and 48 hours interval) or saline (1 mL/kg as initial dose, 0.5 mL/kg as subsequent doses). The initial dose of medication was given within six hours after birth, and subsequent doses were given at 24 and 48 hours after the initial dose. A total of 205 infants received ibuprofen (10 mg/mL) and 210 received saline. Cerebral and cardiac ultrasound were performed before and after treatment. The trial was conducted double‐blind. Perinatal characteristics and possible side effects were registered. The primary outcome variable was IVH grade III or IV. Secondary outcomes included echocardiographically confirmed PDA after day 3 of life and the need for its pharmacological rescue treatment or surgical ligation, occurrence of renal dysfunction measured by urine production, NEC, and death.
Dani 2005 ‐ This multi‐centre study enrolled 155 infants at < 28 weeks' gestational age and at < 6 hours' postnatal age in seven tertiary neonatal care units in Italy. Infants were assigned randomly to treatment or control using sealed envelopes. Envelopes were prepared centrally and were distributed to the different units. Infants in the prophylactic ibuprofen group received three doses of ibuprofen lysine (Arfen, Lisapharma, Erba, Italy; 10 mg/kg within 6 hours after birth, followed by 5 mg/kg after 24 and 48 hours). Infants in the control group received indistinguishable placebo. The medications were infused continuously IV over 15 minutes. The primary outcome was IVH (grade II to IV) at seven days of life. Other outcomes included IVH at days' 15 and 30 after birth and at 40 weeks PMA, PVL at 40 weeks PMA, PDA on day 3 (defined as echocardiographic evidence of a haemodynamically significant PDA), mortality, CLD at 36 weeks' PMA, NEC, sepsis (confirmed with positive blood culture), urine output after treatment, oliguria, increased serum creatinine levels after treatment, length of hospital stay, and ROP.
Sangtawesin 2006 ‐ This single‐centre trial enrolled 42 infants of 28 to 32 weeks' gestational age and birth weight ≤ 1500 g and < 24 hours' postnatal age. Infants were randomly assigned to the ibuprofen or control group by block randomisation. The prophylaxis group received ibuprofen suspension (Junifen, Boots Company, Thailand) at a dosage of 10 mg/kg via an orogastric tube, followed by 0.5 mL of distilled water. The first dose was given within the first 24 hours of life. The second and third doses were given within 24 and 48 hours after the first dose, respectively. Patients in the control group were given three doses of an orange starch suspension as placebo that looked like ibuprofen. The primary outcome was presence of a PDA (defined as echocardiographic evidence of a haemodynamically significant PDA) on day 3 of treatment. Additional outcomes included neonatal mortality, duration of mechanical ventilation, pulmonary hypertension, NEC, gastrointestinal haemorrhage, time to full enteral feeds, ROP (grades not stated), length of hospital stay, CLD (age at diagnosis not stated), days of supplemental oxygen therapy, days of mechanical ventilation, IVH (grades not stated), need for rescue treatment with indomethacin or ibuprofen, surgical closure of the PDA, and PH.
Sangtawesin 2008 ‐ This single‐centre trial enrolled 62 infants with birth weight < 1500 g and postnatal age < 24 hours. The infants were known to have a PDA diagnosed by echocardiography on entry into the trial. Infants were randomly assigned to three doses of oral ibuprofen suspension (Junifen, Boots Company, Thailand) at a dosage of 10 mg/kg for the first dose within 24 hours of life and 5 mg/kg for the second and third doses after 24 and 48 hours. The drug was given via an orogastric tube, followed by 0.5 mL of distilled water. Infants in the control group received three doses of orange starch suspension as placebo administered by the same method and time schedule as oral ibuprofen suspension in the study group. The external appearance of placebo was like ibuprofen suspension and could not be differentiated by naked eyes. The medical personnel who took care of patients were blind to group assignment. The primary outcome was closure of PDA (defined as lack of echocardiographic evidence of a haemodynamically significant PDA) on day 3 of treatment. Other outcomes included persistent pulmonary hypertension of the newborn (PPHN), bronchopulmonary dysplasia (BPD), days of assisted ventilation, days on supplemental oxygen, serum blood urea nitrogen (BUN) on day 3, serum creatinine on day 3, days to start feeding, days to reach full feeds, gastrointestinal bleeding, NEC ≥ stage 2, ROP (total and stage 1 and stage 2), IVH (grade I and grades I to III), length of hospital stay (days), and mortality during the study period (28 days).
Kanmaz 2013 (new inclusion) ‐ This single‐centre trial enrolled 46 infants (23 infants in each group) with PMA < 28 weeks and/or birth weight < 1000 g. Infants were randomly assigned to oral ibuprofen 10 mg/kg within 12 to 24 hours after birth followed by 5 mg/kg at 24 and 48 hours. The control group received standard care. The primary outcome was presence of PDA on day 4 of life. Secondary outcomes included BPD at 36 weeks, IVH (> grade II), NEC, duration of hospitalisation, mortality, GI bleeding, spontaneous intestinal perforation, and acute kidney failure.
Kalani 2016 (new inclusion) ‐ This single‐centre trial enrolled 93 preterm neonates (31 infants in each group) in their first 6 to 12 hours of life. Inclusion criteria were preterm birth (< 32 weeks) and birth weight < 1500 g. Infants were randomly assigned to the oral ibuprofen group (10 mg/kg, 5 mg/kg, and 5 mg/kg of oral ibuprofen every 24 hours during three consecutive days); to the oral indomethacin group (0.2 mg/kg oral indomethacin daily for 3 days); or to the control group, which received only standard care. The primary outcome was IVH (grade I to IV) by seven days of age. Secondary outcomes included presence of PDA, NEC, GI bleeding, and days in hospital.
Excluded studies
We did not include Varvarigou 1996 as it was not a randomised controlled trial.
Risk of bias in included studies
For details, see Risk of bias table and Risk of bias graph (Figure 2) and Risk of bias summary (Figure 3).
2.
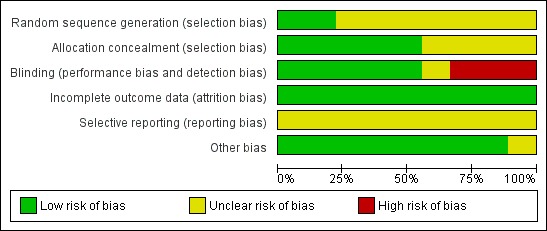
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
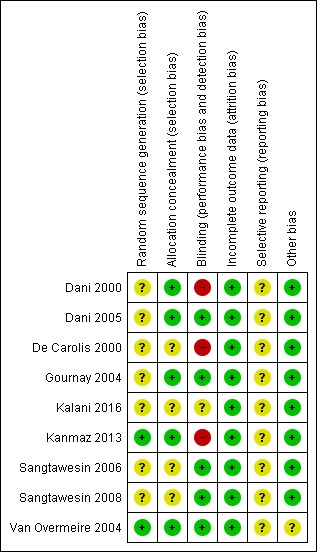
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation (selection bias) was low in two studies and unclear in seven studies. Allocation concealment (selection bias) was low in five studies and unclear in four studies.
Blinding
Blinding (performance bias and detection bias) was low in five studies, unclear in one study, and high in three studies.
Incomplete outcome data
Risk of incomplete outcome data (attrition bias) was low in all nine studies.
Selective reporting
Selective reporting (reporting bias) was unclear in all nine studies as we did not have access to the study protocols.
Other potential sources of bias
This risk was unclear in one study and low in the other eight studies.
Effects of interventions
See: Table 1; Table 2; Table 3
for the main comparison.
| Ibuprofen (IV or oral) compared with placebo or no intervention for prevention of PDA | ||||||
|
Patient or population: preterm or low birth weight infants with risk of having PDA on day 3 to 4 of life Settings: NICU Intervention: ibuprofen (IV or oral) Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Ibuprofen (IV or oral) | |||||
| Presence of PDA on third to fourth day of life (after 72 hours of treatment) | High‐risk population | RR 0.39 (0.31 to 0.48) | 1029 (9 studies) |
⊕⊕⊕⊝ moderate | Design (risk of bias): risk of bias for random sequence generation was low in 2 studies and unclear in 7 studies; risk of bias for allocation concealment was low in 5 studies and unclear in 4 studies; risk of bias regarding performance bias and detection bias was low in 5 studies, unclear in 1 study, and high in 3 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: Heterogeneity was low for RR (I² = 20%; none) and for RD (I² = 28%; low) Directness of the evidence: studies were conducted in the target population Precision of estimates: more than 1000 infants have been enrolled in the studies to date, and confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: the funnel plot for this outcome included 9 trials and was symmetrical |
|
| 424 per 1000 | 166 per 1000 (132 to 204) | |||||
|
Need for surgical closure of PDA (during hospital stay) |
High‐risk population | RR 0.46 (0.22 to 0.96) | 925 (7 studies) | ⊕⊕⊕⊝ moderate | Design (risk of bias): risk of bias for random sequence generation was low in 2 studies and unclear in 5 studies; risk of bias for allocation concealment was low in 5 studies and unclear in 2 studies; risk of bias regarding performance bias and detection bias was low in 4 studies and high in 3 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: there was no heterogeneity for RR (0%) and heterogeneity for RD was low (I² = 27%) Directness of the evidence: studies were conducted in the target population Precision of estimates: the outcome has been reported in 925 infants in the studies to date, and confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: we did not perform a funnel plot for this outcome as only 7 trials were included in the analyses |
|
| 43 per 1000 | 20 per 1000 (9 to 41) | |||||
|
IVH grade III to IV (during hospital stay) |
High‐risk population |
RR 0.67 (0.45 to 1.00) |
925 (7 studies) | ⊕⊕⊕⊝ moderate | Design (risk of bias): risk of bias for random sequence generation was low in 2 studies and unclear in 5 studies; risk of bias for allocation concealment was low in 5 studies and unclear in 2 studies; risk of bias regarding performance bias and detection bias was low in 3 studies, unclear in 1 study, and high in 3 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: heterogeneity was low for RR (I² = 34%; low) and for RD (I² = 60%; moderate) Directness of the evidence: studies were conducted in the target population Precision of estimates: 925 infants have been enrolled in the studies to date, and confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: we did not perform a funnel plot for this outcome as only 7 trials were included in the analyses |
|
| 114 per 1000 | 76 per 1000 (51 to 114) | |||||
|
NEC (during hospital stay) |
High‐risk population | RR 0.96 (0.61 to 1.50) | 1028 (9 studies) | ⊕⊕⊕⊝ moderate | Design (risk of bias): risk of bias for random sequence generation was low in 2 studies and unclear in 7 studies; risk of bias for allocation concealment was low in 5 studies and unclear in 4 studies; risk of bias regarding performance bias and detection bias was low in 5 studies, unclear in 1 study, and high in 3 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: heterogeneity was low for RR (I² = 31%) and for RD (I² = 33%) Directness of the evidence: studies were conducted in the target population Precision of estimates: the outcome of NEC has been reported in 1028 infants to date, and confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: the funnel plot for this outcome included 9 trials and was symmetrical |
|
| 64 per 1000 | 61 per 1000 (39 to 96) | |||||
|
Gastrointestinal haemorrhage (during hospital stay) |
High‐risk population | RR 2.05 (1.19 to 3.51) | 282 (5 studies) | ⊕⊕⊝⊝ low | Design (risk of bias): risk of bias for random sequence generation was low in 1 study and unclear in 4 studies; risk of bias for allocation concealment was low in 2 studies and unclear in 3 studies; risk of bias regarding performance bias and detection bias was low in 2 studies, unclear in 1 study, and high in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: there was no heterogeneity for RR (I² = 0%) but heterogeneity was moderate for RD (I² = 70%) Directness of the evidence: studies were conducted in the target population Precision of estimates: the outcome of gastrointestinal haemorrhage has been reported in 282 infants to date, and confidence intervals around the point estimates for RR and RD were wide. We downgraded the quality of the evidence by 1 step Presence of publication bias: we did not perform a funnel plot for this outcome as only 5 trials were included in the analyses |
|
| 94 per 1000 | 192 per 1000 (111 to 328) | |||||
|
Oliguria (during ibuprofen treatment) |
High‐risk population | RR 1.45 (1.04 to 2.02) | 747 (4 studies) | ⊕⊕⊕⊕ high | Design (risk of bias): risk of bias for random sequence generation was low in 2 studies and unclear in 2 studies; risk of bias for allocation concealment was low in all 4 studies; risk of bias regarding performance bias and detection bias was low in 3 studies and high in 1 study Heterogeneity/consistency: across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: the outcome of oliguria has been reported in 747 infants to date, and confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: we did not perform a funnel plot for this outcome as only 4 trials were included in the analyses |
|
| 130 per 1000 | 188 per 1000 (135 to 263) | |||||
|
Serum creatinine levels (mg/dL) (after treatment) Normal values for male and female newborns (17.7 to 88.4 µmol/L) (0.23 to 1.16 mg/dL) |
Mean serum creatinine level after treatment (mg/dL) in the intervention groups was 0.9 (mg/dL) higher (range 0.05 higher to 0.13 higher) |
800 (6 studies) | ⊕⊕⊝⊝ low | Design (risk of bias): risk of bias for random sequence generation was low in 2 studies and unclear in 4 studies; risk of bias for allocation concealment was low in 4 studies and unclear in 2 studies; risk of bias regarding performance bias and detection bias was low in 4 studies and high in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: heterogeneity was moderate for RR (I² = 56%) and for RD (I² = 56%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: the outcome serum creatinine levels after treatment has been reported in 800 infants to date, and confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: we did not perform a funnel plot for this outcome as only 6 trials were included in the analyses |
||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IV: intravenous; IVH: intraventricular haemorrhage; NEC: necrotising enterocolitis; NICU: neonatal intensive care unit; PDA: patent ductus arteriosus; RD: risk difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
2.
| Ibuprofen (oral) compared with placebo or no intervention for prevention of PDA | |||||||
|
Patient or population: preterm or low birth weight infants with risk of having PDA on day 3 to 4 of life Settings: NICU Intervention: ibuprofen (oral) Comparison: placebo or no intervention |
|||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Placebo or no intervention | Ibuprofen (oral) | ||||||
|
Presence of PDA on day 3 or 4 of life (after 72 hours of treatment) |
High‐risk population | RR 0.47 (0.30 to 0.74) | 202 (4 studies) | ⊕⊕⊝⊝ low | Design (risk of bias): risk of bias for random sequence generation was low in 1 study and unclear in 3 studies; risk of bias for allocation concealment was low in 1 study and unclear in 3 studies; risk of bias regarding performance bias and detection bias was low in 2 studies, unclear in 1 study, and high in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: there was no heterogeneity for RR nor for RD (I² = 0% for both) Directness of the evidence: studies were conducted in the target population Precision of estimates: 202 infants have been enrolled in the studies to date, and confidence intervals around the point estimates for RR and RD were wide. We downgraded the quality of the evidence by 1 step Presence of publication bias: we did not perform a funnel plot for this outcome as only 4 trials were included in the analyses |
||
| 414 per 1000 | 195 per 1000 (124 to 306) | ||||||
|
Gastrointestinal haemorrhage (during hospital stay) |
High‐risk population | RR 2.01 (1.17 to 3.48) | 202 (4 studies) | ⊕⊕⊝⊝ low | Design (risk of bias): risk of bias for random sequence generation was low in 1 study and unclear in 3 studies; risk of bias for allocation concealment was low in 1 study and unclear in 3 studies; risk of bias regarding performance bias and detection bias was low in 2 studies, unclear in 1 study, and high in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: there was no heterogeneity for RR (I² = 0%) but moderate heterogeneity for RD (I² = 72%) Directness of the evidence: studies were conducted in the target population Precision of estimates: 202 infants have been enrolled in the studies to date, and confidence intervals around the point estimates for RR and RD were wide. We downgraded the quality of the evidence by 1 step Presence of publication bias: we did not perform a funnel plot for this outcome as only 4 trials were included in the analyses |
||
| 131 per 1000 | 264 per 1000 (154 to 457) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NICU: neonatal intensive care unit; PDA: patent ductus arteriosus; RD: risk difference; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
3.
| Ibuprofen (IV) compared with placebo or no intervention for prevention of PDA | ||||||
|
Patient or population: preterm or low birth weight infants with risk of having PDA on day 3 to 4 of life Settings: NICU Intervention: ibuprofen (IV) Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Ibuprofen (IV) | |||||
| Presence of PDA on third day of life (72 hours of treatment) | High‐risk population | 0.37 (0.29 to 0.47) | 827 (5 studies) |
⊕⊕⊕⊕ high | Design (risk of bias): risk of bias for random sequence generation was low in 1 study and unclear in 4 studies; risk of bias for allocation concealment was low in 4 studies and unclear in 2 study; risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies Heterogeneity/consistency: across studies: heterogeneity was low for RR (I² = 34%) but moderate for RD (I² = 53%) Directness of the evidence: studies were conducted in the target population Precision of estimates: 827 infants have been enrolled in the studies to date, and confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: we did not perform a funnel plot for this outcome as only 5 trials were included in the analyses |
|
| 427 per 1000 | 158 per 1000 (124 to 201) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IV: intravenous; NICU: neonatal intensive care unit; PDA: patent ductus arteriosus; RD: risk difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
Ibuprofen (IV or oral) vs placebo or none (Comparison 1)
Primary outcome
Presence of patent ductus arteriosus (diagnosed on routine screening by echocardiography) by 72 hours of treatment (three to four days of age) (Outcome 1.1)
See Figure 4.
4.
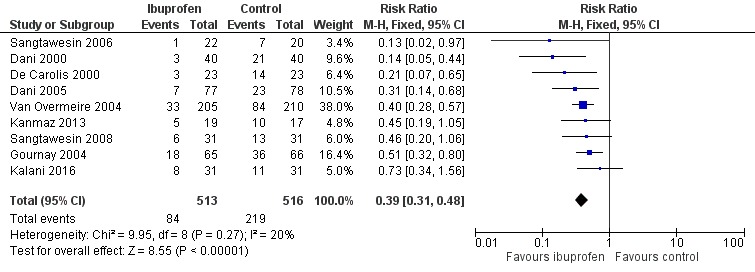
Forest plot of comparison: 1 Ibuprofen vs placebo or none, outcome: 1.1 Presence of PDA on third day of life (72 hours of age).
This was reported in all nine trials (N = 1029) (Dani 2000; Dani 2005; De Carolis 2000; Gournay 2004; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008; Van Overmeire 2004). All trials except three noted a statistically significant decrease in the incidence of PDA on day three in the group receiving prophylactic ibuprofen (Kalani 2016; Kanmaz 2013; Sangtawesin 2008). The meta‐analysis showed a statistically significant decrease in the incidence of PDA on day 3 in the prophylactic ibuprofen group as compared to the placebo group. Typical estimates were risk ratio (RR) 0.39 (95% confidence interval (CI) 0.31 to 0.48); risk difference (RD) ‐0.26 (95% CI ‐0.31 to ‐0.21); and number needed to treat for an additional beneficial outcome (NNTB) 4 (95% CI 3 to 5). There was no important between‐study heterogeneity for this outcome (I² = 20% (none) for RR and I² = 28% (low) for RD). A funnel plot was quite symmetrical around the typical point estimate for RR (Figure 5). The quality of the evidence was moderate according to GRADE.
5.
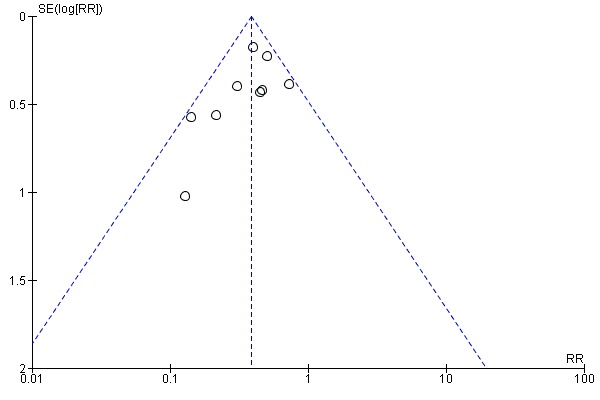
Funnel plot of comparison: 1 Ibuprofen (IV or oral) vs placebo or none, outcome: 1.1 Presence of PDA on third or fourth day of life.
In subgroup analyses including three studies (Dani 2005; Kanmaz 2013; Van Overmeire 2004) for the gestational age group ≤ 28 weeks (N = 456), the RR was 0.41 (95% CI 0.30 to 0.57); RD ‐0.24 (95% CI ‐0.32 to ‐0.16); and NNTB 4 (95% CI 3 to 6) (Outcome 1.30). For the gestational age group 29 to 30 weeks (N = 150), the RR was 0.29 (95% CI 0.13 to 0.64); RD ‐0.23 (95% CI ‐0.35 to ‐0.10); and NNTB 4 (95% CI 3 to 10) (Outcome 1.31). For the birth weight group ≤ 1000 g (N = 232), the RR was 0.39 (95% CI 0.25 to 0.59); RD ‐0.29 (95% CI ‐0.40 to ‐0.17); and NNTB 4 (95% CI 3 to 6)(Outcome 1.32). For the birth weight group 1001 to 1500 g (N = 247), the RR was 0.54 (95% CI 0.34 to 0.83); RD ‐0.16 (95% CI ‐0.27 to ‐0.05): and NNTB 6 (95% CI 4 to 20) (Outcome 1.33). There was no important heterogeneity for these secondary outcomes (RR I² = 0%; I² = 0%; or not applicable when only one trial was included). All secondary analyses were statistically significant in favour of ibuprofen versus placebo or none.
Secondary outcomes
Neonatal mortality (death during first 28 days of life) (Outcome 1.2)
Mortality at < 28 days was reported in six trials (N = 342) (Dani 2000; De Carolis 2000; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008). There was no statistically significant difference in mortality between groups in either trial. The meta‐analysis showed no statistically significant difference in mortality between the two groups. Typical estimates were RR 0.93 (95% CI 0.50 to 1.74) and RD ‐0.01 (95% CI ‐0.07 to 0.05). There was no important heterogeneity for this outcome (I² = 0% for both RR and RD).
All‐cause mortality during initial hospital stay (Outcome 1.3)
This was reported in four trials (N = 700) (Dani 2000; Dani 2005; De Carolis 2000; Van Overmeire 2004). None of the trials found a significant difference in mortality between groups. The meta‐analysis showed no statistically significant difference in the incidence of mortality. Typical estimates were RR 0.90 (95% CI 0.62 to 1.30) and RD ‐0.01 (95% CI ‐0.06 to 0.03). There was no important heterogeneity for this outcome (I² = 0% for both RR and RD).
Mortality before 36 weeks' PMA (Outcome 1.4)
This was reported in one trial (N = 131) (Gournay 2004). The RR was 0.96 (95% CI 0.56 to 1.66), and the RD was ‐0.01 (95% CI ‐0.17 to 0.14), neither of which was statistically significant. Tests for heterogeneity are not applicable.
Infant mortality (death during first year of life)
This outcome was not reported by any of the trial authors.
Need for rescue medical treatment with cyclo‐oxygenase inhibitors for closure of PDA (Outcome 1.5)
This outcome was reported in six trials (N = 776) (Dani 2000; De Carolis 2000; Gournay 2004; Sangtawesin 2006; Sangtawesin 2008; Van Overmeire 2004), and all studies found a statistically significantly reduced need for rescue medical treatment in the prophylaxis group based on RD. Dani 2000 used ibuprofen for rescue treatment, and De Carolis 2000 used indomethacin for rescue treatment. Van Overmeire 2004 used either indomethacin or ibuprofen. Gournay 2004 initiated rescue treatment with ibuprofen and if this failed used indomethacin. Sangtawesin 2006 used indomethacin and/or ibuprofen. Sangtawesin 2008 used indomethacin as rescue treatment. The meta‐analysis showed decreased need for rescue medical treatment in the group receiving prophylactic ibuprofen. Typical estimates were RR 0.17 (95% CI 0.11 to 0.26); RD ‐0.27 (95% CI ‐0.32 to ‐0.22); and NNTB 4 (95% CI 3 to 5). There was important between‐study heterogeneity for this outcome for RD (I² = 88% (high)) but not for RR (I² = 45% (low)).
Need for surgical closure of PDA (Outcome 1.6)
This outcome was reported in seven trials (N = 925) (Dani 2000; Dani 2005; De Carolis 2000; Gournay 2004; Kanmaz 2013; Sangtawesin 2008; Van Overmeire 2004). One trial found a significant difference between groups (Gournay 2004). The meta‐analysis showed a statistically significant decrease in the need for surgical ligation between the two groups. Typical estimates from the meta‐analysis were RR 0.46 (95% CI 0.22 to 0.96); RD ‐0.03 (95% CI ‐0.05 to ‐0.00);.and NNTB 33 (95% CI 20 to infinity). There was no important heterogeneity for this outcome (RR: I² = 0% (none); RD: I² = 27% (low)) (Figure 6). The quality of the evidence was moderate according to GRADE.
6.
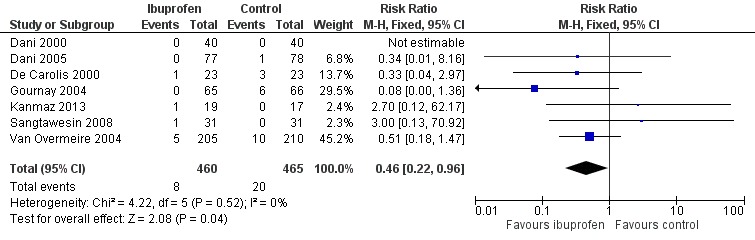
Forest plot of comparison: 1 Ibuprofen (IV or oral) vs placebo or none, outcome: 1.6 Need for surgical closure of PDA.
Duration of mechanical ventilation (days) (Outcome 1.7)
Duration of mechanical ventilation was reported in five trials (N = 470) (Dani 2000; Dani 2005; Gournay 2004; Sangtawesin 2006; Sangtawesin 2008), and there was no statistically significant difference between groups in any of the trials. The typical weighted mean difference (WMD) was 1 day (95% CI ‐2 to 4). There was no important heterogeneity for this outcome (I² = 0%) (none).
Days requiring supplemental oxygen (Outcome 1.8)
Three studies reported on this outcome (N = 259) (Dani 2005, Sangtawesin 2006; Sangtawesin 2008), and all trials found no significant differences between groups. The typical WMD was ‐0.18 days (95% CI ‐4.98 to 4.61). There was no important heterogeneity for this outcome (I² = 33%) (low). Van Overmeire (N = 415) reported (in medians and interquartile ranges) on days on supplemental oxygen (Van Overmeire 2004). Results for the ibuprofen group were 25 (6 to 52) days, and for the placebo group 24 (6 to 44) days (P = 0.36).
Chronic lung disease among survivors (defined as oxygen requirements at 28 days' postnatal age in addition to compatible clinical and roentgenographic findings) (Outcome 1.9)
This outcome was reported in one trial (N = 41) (De Carolis 2000). There was no statistically significant difference in the incidence of CLD between groups. Estimates were RR 0.88 (95% CI 0.32 to 2.42) and RD ‐0.04 (95% CI ‐0.31 to 0.24). Tests for heterogeneity were not applicable.
Chronic lung disease (defined as oxygen requirements at 36 weeks' PMA in addition to compatible clinical and roentgenographic findings) (Outcome 1.10)
Chronic lung disease at 36 weeks' PMA was reported in five trials (N = 817) (Dani 2000; Dani 2005; Gournay 2004; Kanmaz 2013; Van Overmeire 2004). There was no statistically significant difference between groups in either of the individual trials. Typical estimates were RR 1.06 (95% CI 0.89 to 1.26) and RD 0.02 (95% CI ‐0.04 to 0.08). There was no important heterogeneity for this outcome (I² = 0%) (none) for both RR and RD.
Chronic lung disease (age at diagnosis not stated) (Outcome 1.11)
Chronic lung disease (age at diagnosis not stated) was reported in two trials (Sangtawesin 2006; Sangtawesin 2008) (N = 99). There was no statistically significant difference between groups in either of the two trials. Typical estimates were RR 0.94 (95% CI 0.51 to 1.72) and RD 0.02 (95% CI ‐0.19 to 0.15). There was important heterogeneity for this outcome (RR: I² = 79% (high); RD: I² = 84% (high)).
Pneumothorax
No trial reported on this outcome.
Pulmonary hypertension (Outcome 1.12)
Pulmonary hypertension was reported in four trials (N = 390) (Dani 2005; Gournay 2004; Sangtawesin 2006; Sangtawesin 2008). In Gournay 2004, three infants in the ibuprofen group (N = 65) developed PH within one hour of administration of the drug, which was responsive to inhaled nitric oxide, as compared to none of the infants in the placebo group (N = 66). In the other three studies, no cases of PH developed. The typical RR was 7.11 (95% CI 0.37 to 135), and the RD was 0.02 (95% CI ‐0.01 to 0.04). Tests for heterogeneity were not applicable for RR; for RD, I² = 0% (low).
Intraventricular haemorrhage (all grades) (Outcome 1.13)
IVH (all grades) was reported in six trials (N = 901) (Dani 2000; Dani 2005; Gournay 2004; Kalani 2016; Sangtawesin 2008; Van Overmeire 2004). The typical RR was 0.96 (95% CI 0.78 to 1.17), and the RD was ‐0.01 (95% CI ‐0.07 to 0.05). There was no important heterogeneity for this outcome (for both RR: I² = 0% (none); for RD: I² = 41% (low)).
Intraventricular haemorrhage (grades not stated) (Outcome 1.14)
This outcome was reported in one study (N = 40) (Sangtawesin 2006). The RR was 0.45 (95% CI 0.09 to 2.20). The RD was ‐0.12 (95% CI ‐0.34 to 0.11). Tests for heterogeneity were not applicable.
Intraventricular haemorrhage (Grade III or IV) (Outcome 1.15)
IVH grade III or IV (according to Papile 1978) was reported in seven trials (N = 925) (Dani 2000; Dani 2005; De Carolis 2000; Gournay 2004; Kalani 2016; Kanmaz 2013; Van Overmeire 2004). There was no significant difference in the incidence of IVH between groups in any of the trials. The meta‐analysis showed borderline statistically significant differences in the incidence of grade III or IV IVH between the two groups. Typical estimates from the meta‐analysis were RR 0.67 (95% CI 0.45 to 1.00) (P = 0.05) and RD ‐0.04 (95% CI ‐0.08 to‐ 0.00) (P = 0.05). There was some between‐study heterogeneity for this outcome for RR (I² = 34%) (low) and RD (I² = 60%) (moderate) The quality of the evidence was moderate according to GRADE.
Periventricular leukomalacia (PVL) (Outcome 1.16)
PVL was reported in four trials (N = 747) (Dani 2005; De Carolis 2000; Gournay 2004; Van Overmeire 2004), and there was no statistically significant difference in the incidence of PVL between groups in the individual trials. Typical estimates were RR 1.19 (95% CI 0.64 to 2.18) and RD 0.01 (95% CI ‐0.02 to 0.04). There was no heterogeneity for this outcome (I² = 0% (none) for both RR and RD).
Necrotising enterocolitis (NEC) (any stage) (Bell 1978) (Outcome 1.17)
This was reported in all nine trials (N = 1028) (Dani 2000; Dani 2005; De Carolis 2000; Gournay 2004; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008; Van Overmeire 2004), and one trial found a significant difference in the incidence of NEC between groups (RD 0.12 (95% CI 0.02 to 0.23) (Gournay 2004). The meta‐analysis showed no statistically significant difference in the incidence of NEC. Typical estimates were RR 0.96 (95% CI 0.61 to 1.50) and RD ‐0.00 (95% CI ‐0.03 to 0.03). There was low between‐study heterogeneity for this outcome (RR: I² = 31% (low); RD: I² = 33% (low)). The quality of the evidence was moderate according to GRADE.
Gastrointestinal haemorrhage (Outcome 1.18)
This outcome was reported in five trials (N = 282) (Dani 2000; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008); there was no statistically significant difference between groups in the individual trials. Typical estimates were RR 2.05 (95% CI 1.19 to 3.51) and RD 0.11 (95% CI 0.03 to 0.18) and were statistically significantly increased for the ibuprofen group. Tests for heterogeneity showed no important heterogeneity for this outcome for RR (I² = 0% (none)) but for RD, I² = 70% (moderate). The quality of the evidence was low according to GRADE.
Gastrointestinal perforation (defined by presence of free air in peritoneal cavity on an abdominal x‐ray) (Outcome 1.19)
This outcome was reported in two trials (N = 167) (Gournay 2004; Kanmaz 2013), and there was no statistically significant difference between groups. The risk ratio was 4.88 (95% CI 0.87 to 27.36), and RD was 0.07 (95% CI 0.00 to 0.14). There was no heterogeneity for this outcome (I² = 0% for both RR and RD).
Time to reach full enteral feeds (days) (Outcome 1.20)
This was reported in three trials (N = 184) (Dani 2000; Sangtawesin 2006; Sangtawesin 2008), and there was no statistically significant difference between groups in the individual studies. The typical estimate was mean difference 0.5 days (95% CI ‐3 to 4). There was no statistically significant heterogeneity for this outcome (I² = 0% (none)).
Length of hospital stay (total length of hospitalisation from birth to discharge home or death in days) (Outcome 1.21)
This was reported in six trials (N = 447) (Dani 2000; Dani 2005; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008), and there was no statistically significant difference between groups. The typical WMD estimate was 1 day (95% CI ‐3 to 6). There was no important heterogeneity for this outcome (I²= 25% (low)).
Urine output after treatment (mL/kg/hr on day 3) (Outcome 1.22)
Urine output after treatment was reported in three trials (N = 650) (Dani 2000; Dani 2005; Van Overmeire 2004), and there was no statistically significant difference between groups in the individual trials. The typical WMD was ‐0.05 mL/kg/hr (95% CI ‐0.26 to 0.15). There was no important heterogeneity for this outcome (I² = 0% (none)). De Carolis 2000 reported urine output on day 3 as median (range) in the ibuprofen group 3.3 (1.3 to 4.6) mL/kg/hr, and in the control group 2.3 (1.1 to 4.9) mL/kg/hr.
Oliguria (urine output < 1 cc/kg/hr) (Outcome 1.23)
Three studies (N = 332) reported on this outcome (Dani 2005; Gournay 2004; Kanmaz 2013). The RR was 1.33 (95% CI 0.78 to 2.26), and the RD was 0.04 (95% CI ‐0.03 to 0.11) (Outcome 1.23.2). One study (N = 415) reported on oliguria defined as < 0.5 mL/kg/hr (Van Overmeire 2004) (Outcome 1.23.1). The significant RR was 1.54 (95% CI 1.01 to 2.34), and the significant RD was 0.08 (95% CI 0.00 to 0.15) (P = 0.04). Combining the four studies (N = 747) (Outcome 1.23) revealed that the typical RR was significantly increased at 1.45 (95% CI 1.04, 2.02) and the typical RD was significant at 0.06 (95% CI 0.01 to 0.11); the number needed to treat for an additional harmful outcome (NNTH) was 17 (95% CI 9 to 100). There was no statistically significant heterogeneity for the meta‐analysis of the four studies for RR and RD (I² = 0% (low)). The quality of the evidence was high according to GRADE.
Serum creatinine levels (mg/dL) after treatment (Outcome 1.24)
Serum creatinine levels after treatment were reported in six trials (N = 800) (Dani 2000; Dani 2005; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008; Van Overmeire 2004). The meta‐analysis showed a statistically significant increase in serum creatinine levels on day 3 in the group receiving ibuprofen as compared to the group receiving placebo. The typical estimate was WMD 0.09 mg/dL (95% CI 0.05 to 0.13). There was between‐study heterogeneity (I² = 56% (moderate)). De Carolis 2000 reported serum creatinine levels on day 3 as median (range) in the ibuprofen group 1.3 (0.8 to 1.7) mg/dL and in the control group 1.2 (0.8 to 1.5) mg/dL . The quality of the evidence was low according to GRADE.
At least one episode of serum creatinine > 140 micromol/L (1.6 mg/dL) (Outcome 1.25)
Gournay 2004 and Dani 2005 reported on "at least one episode of serum creatinine > 140 micromol/L (1.6 mg/dL)". The typical RR was 3.70 (95% CI 1.05 to 12.98), and the typical RD was 0.06 (95% CI 0.01 to 0.11); the NNTH was 17 (95% CI 9 to 100) (2 trials; N = 285). There was important heterogeneity for this outcome (RR: I² = 36% (low); RD: I² = 73% (moderate)).
At least one episode of severe hypoxaemia (Outcome 1.26)
One trial reported on this outcome (N = 131) (Gournay 2004). The RR was 1.69 (95% CI 0.80 to 3.59) and the RD was 0.09 (95% CI ‐0.04 to 0.23). Tests for heterogeneity were not applicable.
Inhaled nitric oxide use during first week of life (Outcome 1.27)
This outcome was reported in one study (N = 131) (Gournay 2004). The RR was 1.89 (95% CI 0.80 to 4.42), and the RD 0.09 (95% CI ‐0.03 to 0.22) (neither reached statistical significance). Tests for heterogeneity were not applicable.
Retinopathy of prematurity (ROP) (according to the international classification of ROP) (ICROP 1984) (Outcome 1.28)
ROP was reported in five trials (N = 369) (Dani 2000; Dani 2005; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008), and there was no statistically significant difference between groups. Estimates were typical RR 1.01 (95% CI 0.73 to 1.38) and RD 0.00 (95% CI ‐0.09 to 0.09). There was no heterogeneity for this outcome (I² = 0% (none) for both RR and RD).
Definite sepsis (clinical symptoms and signs of sepsis and a positive bacterial culture in a specimen obtained from normally sterile fluids or tissue obtained at autopsy) (Outcome 1.29)
The incidence of sepsis was reported in two trials (N = 201) (Dani 2005; De Carolis 2000), and there was a statistically significant difference between groups. Estimates were typical RR 2.70 (95% CI 1.10 to 6.59), RD 0.10 (95% CI 0.02 to 0.19), and NNTH 10 (95% CI 5 to 50). Tests for heterogeneity showed the following: RR: I² = 0% (none); RD: I² = 68% (moderate).
Probable sepsis (clinical symptoms and signs of sepsis and abnormal findings on a laboratory screening test for infection)
This outcome was not reported.
Neurodevelopmental outcome (neurodevelopmental outcome assessed by a standardised and validated assessment tool and/or a child developmental specialist) at any age (outcome data will be grouped at 12, 18, and 24 months if available)
No data were available for long‐term neurodevelopmental outcomes.
Subgroup analyses specified a priori could not be performed for the following reasons.
Dose of ibuprofen used was similar in all studies.
Echocardiographic criteria were used to diagnose PDA in all studies.
Demographic and outcome data were available separately for the different birth weight or gestational age (GA) categories in the Dani 2005 study and in the Van Overmeire 2004 study but did not completely correspond to our preset cutoff points. However, as they were close, we included them in subgroup analyses under the outcome "Presence of patent ductus arteriosus (clinically symptomatic or diagnosed by echocardiography in response to clinical suspicion or diagnosed on routine screening by echocardiography) by 72 hours (three days) of age" (see above).
Ibuprofen (oral) vs placebo or none (Comparison 2)
Primary outcome
Presence of patent ductus arteriosus (diagnosed on routine screening by echocardiography by 72 hours of treatment; three to four days of age) (Outcome 2.1)
This outcome was reported in four trials (N = 202) (Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008). One of the four individual trials showed a reduction in the presence of a PDA by 72 hours of age for both RR and RD (Sangtawesin 2006). The typical RR showed a significant reduction at RR 0.47 (95% CI 0.30 to 0.74); the typical RD was ‐0.22 (95% CI ‐0.34 to ‐0.10) and NNTB was 5 (95% CI 3 to 10). There was no important heterogeneity for this outcome (I² = 0% (none) for both RR and RD). The quality of the evidence was low according to GRADE.
Secondary outcome
Gastrointestinal haemorrhage (Outcome 2.2)
This outcome was reported in four trials (N = 202) (Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008). The typical RR was 2.01 (95% CI 1.17 to 3.48), and the typical RD was 0.14 (95% CI 0.04 to 0.24). NNTH was 7 (95% CI 4 to 25). Both RR and RD were statistically significantly increased with a higher occurrence of GI bleeds in the oral ibuprofen group. There was no important heterogeneity for this outcome (RR: I² = 0% (none); RD: I² = 72% (moderate)). The quality of the evidence was low according to GRADE.
Ibuprofen (IV) vs placebo or none (Comparison 3)
Presence of patent ductus arteriosus (diagnosed on routine screening by echocardiography) by 72 hours of treatment; (three to four days of age) (Outcome 3.1)
This outcome was reported in five trials (N = 827) (Dani 2000; Dani 2005; De Carolis 2000; Gournay 2004; Van Overmeire 2004). All trials showed a significant reduction in this outcome for the IV ibuprofen group compared to the control group. The typical RR was 0.37 (95% CI 0.29 to 0.47), and the typical RD was ‐0.27 (95% CI ‐0.33 to ‐0.21); NNTB was 4 (95% CI 3 to 5). There was some heterogeneity for this outcome for RR (I² = 34% (low)) and for RD (I² = 53% (moderate)). The quality of the evidence was high according to GRADE.
Ibuprofen (oral) vs indomethacin (oral) (Comparison 4)
This comparison was studied in one trial (N = 62) (Kalani 2016); therefore tests for heterogeneity were not applicable.
Primary outcome
Presence of PDA (age not stated) (Outcome 4.1)
There was no significant difference in this outcome for the oral ibuprofen group compared to the oral indomethacin group. The RR was 1.00 (95% CI 0.43 to 2.33) and the RD was 0.00 (95% CI ‐0.22 to 0.22).
Secondary outcomes
Neonatal mortality (at < 28 days) (Outcome 4.2)
There was no significant difference in this outcome for the oral ibuprofen group compared to the oral indomethacin group. The RR was 2.00 (95% CI 0.19 to 20.93) and the RD was 0.03 (95% CI ‐0.07 to 0.14).
IVH (all grades) (Outcome 4.3)
There was no significant difference in this outcome for the oral ibuprofen group compared to the oral indomethacin group. The RR was 0.11 (95%CI 0.01 to 1.98) and the RD was ‐0.13 (95% CI ‐0.26 to ‐0.00) (P = 0.05).
IVH (grades III/IV)) (Outcome 4.4)
There was no significant difference in this outcome for the oral ibuprofen group compared to the oral indomethacin group. The RR was 0.20 (95% CI 0.01 to 4.00) and the RD was ‐0.06 (95% ‐0.17 to 0.04).
NEC (Outcome 4.5)
There was no significant difference in this outcome for the oral ibuprofen group compared to the oral indomethacin group. The RR was 1.00 (95% CI 0.22 to 4.58) and the RD was 0.00 (‐0.15 to 0.15).
GI bleeding (Outcome 4.6)
There was no significant difference in this outcome for the oral ibuprofen group compared to the oral indomethacin group. The RR was 0.25 (95% CI 0.03 to 2.11) and the RD was ‐0.10 (95% CI ‐0.23 to 0.04).
Hospitalisation (days) (Outcome 4.7)
The length of hospitalisation was significantly longer in the oral ibuprofen group compared to the oral indomethacin group. The mean difference was 10.20 days (95% CI 1.24 to 19.16 days).
We were not able to identify any randomised controlled trials for the use of mefenamic acid for prevention of PDA, nor were we able to identify any trials using membrane‐bound (prostaglandin E synthase) inhibitors for prevention or treatment of PDA.
Discussion
Summary of main results
For this update, nine studies (reporting on 1070 infants) compared prophylactic ibuprofen (intravenous (IV) or oral) with placebo/no intervention or indomethacin. We included four comparisons: (1) ibuprofen (IV or oral) compared with placebo or none; (2) ibuprofen (oral) compared with placebo or none; (3) ibuprofen (IV) compared with placebo or none; and (4) ibuprofen (oral) compared with indomethacin (oral). To avoid repetition, we included in this section the GRADE scores for the Quality of the evidence for comparisons and outcomes that we have included in Table 1.
Ibuprofen (IV or oral) compared with placebo or none was significantly more effective in reducing the presence of patent ductus arteriosus (PDA) by 72 hours of treatment (moderate‐quality evidence). A funnel plot for the primary outcome "Presence of PDA on the third day of life (72 hours of treatment)" was symmetrical around the typical point estimate for risk ratio (RR), suggesting that there was no major publication bias. Similar results were found in subgroup analyses for infants ≤ 28 weeks' postmenstrual age (PMA) at birth; for infants 29 to 30 weeks' PMA at birth; for infants ≤ 1000 G at birth; and for infants 1001 to 1500 G at birth. The spontaneous closure rate of the PDA was 58% in the control group. There was no significant effect on neonatal mortality. The need for rescue medical treatment with cyclo‐oxygenase inhibitors was significantly reduced, as was the need for surgical closure of PDA (moderate‐quality evidence). The occurrence of intraventricular haemorrhage (IVH) (grade II to IV) was reduced with borderline significance (moderate‐quality evidence). The incidence of necrotising enterocolitis was not significantly changed by ibuprofen (moderate‐quality evidence). Gastrointestinal haemorrhage occurred significantly more often in the ibuprofen group (low‐quality evidence). In that analysis (Analysis 1.18), only one trial used IV ibuprofen with no increase in risk of gastrointestinal haemorrhage (Dani 2000). The incidence of gastrointestinal perforation was not significantly affected. Risk of oliguria was significantly increased in the ibuprofen group (high‐quality evidence). Serum creatinine levels after treatment were significantly increased with ibuprofen administration (low‐quality evidence). Increased risk of sepsis was noted in the ibuprofen group in the meta‐analysis of two studies (N = 201). The outcomes of chronic lung disease and retinopathy of prematurity were not significantly different between groups.
1.18. Analysis.
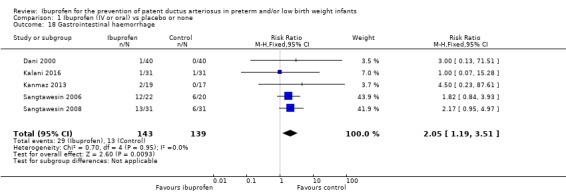
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 18 Gastrointestinal haemorrhage.
Ibuprofen (oral) compared with placebo or none was significantly more effective in reducing the presence of PDA by 72 hours of treatment (low‐quality evidence). The incidence of gastrointestinal haemorrhage was significantly increased (low‐quality evidence).
Ibuprofen (IV) compared with placebo or none was significantly more effective in reducing the presence of PDA by 72 hours of treatment (high‐quality evidence).
For the comparison ibuprofen (oral) versus indomethacin (oral), only one study has been published, and results were reported in 62 infants. There were no significant results apart from a significant increase in length of hospitalisation for the ibuprofen group.
Overall completeness and applicability of evidence
This update of our review, including two additional studies reporting on an additional 139 infants for a total of 1070 infants, confirms the findings of the previous versions of this review, published in 2003, 2006, 2009, and 2011. Several of the estimates of effect size have become more precise due to the increase in sample size. There is now stronger evidence that oral ibuprofen increases the risk of gastrointestinal haemorrhage, and that use of ibuprofen affects kidney function in a negative way. The reduction in intraventricular haemorrhage (grade III or IV) reached values of borderline significance.
This review confirmed that prophylactic ibuprofen is effective in reducing the incidence of PDA on day 3 to 4 after birth, reducing the need for rescue treatment with cyclo‐oxygenase inhibitors, and reducing the need for surgical ligation of a PDA. It has been shown that precision and accuracy of clinical and radiological signs in preterm infants at risk of PDA are poor (Davis 1995). It is therefore reassuring that the diagnosis of a PDA on day 3 or 4 (72 hours after initiation of treatment) was made using echocardiography in all studies.
Quality of the evidence
Study quality varied. Random sequence generation (selection bias) was low in two studies and unclear in seven studies. Allocation concealment (selection bias) was low in five studies and unclear in four studies; blinding (performance bias and detection bias) was low in five studies, unclear in one study, and high in three studies; risk of incomplete outcome data (attrition bias) was low in all nine studies; selective reporting (reporting bias) was unclear in all nine studies, as we did not have access to the study protocols; and other potential sources of bias was unclear in one study and low in the other eight studies. Our GRADE assessments are shown in Table 1Table 2 and Table 3, and are included in the summary of main results above.
In the main analysis "Ibuprofen (IV or oral)", there was moderate to high between‐study heterogeneity for risk ratio (RR) for the outcomes chronic lung disease (CLD) (age at diagnosis not stated) and serum creatinine levels after treatment, but not for any other outcomes. In the same comparison, there was moderate to high between‐study heterogeneity for risk difference (RD) for the outcomes need for rescue medical treatment with cyclo‐oxygenase inhibitors, CLD (age at diagnosis not stated), intraventricular haemorrhage (grade III or IV), gastrointestinal haemorrhage, serum creatinine levels after treatment, at least one episode of serum creatinine > 140 micromol/L (> 1.5 mg/dL), and sepsis.
In the second analysis, "Ibuprofen oral vs placebo or none", four trials are now included, and study authors reported on a total of 202 infants; the estimate for presence of PDA on day 3 to 4 of life was similar to the results in Comparison 1, and there was no heterogeneity for RR nor for RD. The four trials showed significantly increased risk of gastrointestinal haemorrhage with no heterogeneity for RR but moderate heterogeneity for RD.
In the 2011 update of the review, there was a statistically significantly increased risk of proven sepsis between ibuprofen and placebo groups. This outcome was reported only in two trials (N = 201), resulting in wide confidence intervals around the point estimates; no new trial reported on this outcome for the current update.
Evidence is lacking for the comparison ibuprofen (oral) versus indomethacin (oral), as only 62 infants have been enrolled in one trial.
Potential biases in the review process
We are not aware of any biases in the review process.
Agreements and disagreements with other studies or reviews
In a network meta‐analysis of indomethacin versus ibuprofen versus placebo for PDA in preterm infants, Jones and co‐workers reported an approximately 30% greater risk of CLD with IV ibuprofen given at > 24 hours of life compared to indomethacin or placebo (Jones 2011). We did not identify an increased risk of CLD with ibuprofen in this updated review nor in the review of ibuprofen for treatment of a PDA (Ohlsson 2018). Their search strategy ended in August 2008, and the review included fewer studies than were included in our two ibuprofen reviews for Cochrane.
In a previous review, we included one trial that reported the occurrence of pulmonary hypertension within one hour of administration of ibuprofen to three infants (< 27 weeks and < 1000 g) (Gournay 2004). The trial was stopped early after enrolment of 135 infants due to this adverse effect. The authors postulated that this could be due to early administration of ibuprofen (< 6 hours of age) preventing the normal fall in pulmonary vascular resistance, acidification of their ibuprofen solution (buffered with tromethamine) causing precipitation and microembolism in the lungs, or a specific effect of ibuprofen. This adverse effect was not reported in the two trials ‐ Dani 2005 and Sangtawesin 2006 ‐ included in our 2007 updated review (Shah 2007), nor in the update in 2011 (Ohlsson 2011), which included one more trial (Sangtawesin 2008), nor in trials using ibuprofen for treatment of PDA (Lago 2002; Mosca 2002; Van Overmeire 2000). Gournay 2004 concluded that prophylactic ibuprofen should not be preferred to early curative ibuprofen.
In this update, there was a significantly increased risk of gastrointestinal bleeding with ibuprofen. Five studies reported on this outcome (Dani 2000; Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008), and four studies used oral ibuprofen (Kalani 2016; Kanmaz 2013; Sangtawesin 2006; Sangtawesin 2008). The incidence of gastrointestinal bleeding was much higher in three of the four studies that used oral ibuprofen, and it may be that gastrointestinal bleeding is specific to the oral formulation of the drug.
In the current review, we found a statistically significant increase in the need for rescue treatment with cyclo‐oxygenase inhibitors (indomethacin or ibuprofen) in the placebo group as compared to the prophylactic ibuprofen group. This is an expected event and reflects common clinical practice in neonatal intensive care units for management of a symptomatic PDA. In Dani 2000, infants were randomised to prophylactic ibuprofen or rescue. In this respect, the trial was different from the rest of the trials, which randomised infants to prophylactic ibuprofen or placebo. However, the back‐up management protocol in five other studies ‐ De Carolis 2000,Gournay 2004,Van Overmeire 2004,Sangtawesin 2006, and Sangtawesin 2008 ‐ and the rescue protocol in Dani 2000 were similar in that each was based on detection of a significant PDA by echocardiography performed at regular predetermined intervals. Hence, for practical purposes, we considered the rescue ibuprofen group in Dani 2000 to be comparable to the placebo group in other studies as far as management of a PDA is concerned.
We identified one trial comparing prophylactic ibuprofen with prophylactic indomethacin (Kalani 2016). It is of note that ibuprofen did not significantly reduce IVH (grade III or IV) in that study (Kalani 2016). Prophylactic indomethacin has been shown to reduce need for surgical ligation of PDA and grade III and IV IVH (Fowlie 2010). That review found no significant effect on long‐term neurodevelopmental outcomes. It is presently unknown whether preventing IVH with the use of indomethacin is preferable to preventing ischaemia with the use of ibuprofen.
In the present update of our review, prophylactic ibuprofen was effective in reducing the incidence of PDA, the need for rescue treatment with cyclo‐oxygenase inhibitors, and the need for surgical ligation, but did not confer any substantial clinical advantages in the short term. Ibuprofen prophylaxis has a negative effect on kidney function with increased risk for oliguria and an increase in serum creatinine. A new finding in this review was an increased risk of gastrointestinal bleeding with oral ibuprofen. As 58% of the infants in the control group had closed the duct by three days of life, a large proportion of neonates would be exposed to ibuprofen unnecessarily if used as prophylaxis. No long‐term neurodevelopmental follow‐up studies are yet available.
One previous non‐systematic review with fewer included studies reported that ibuprofen lysine when administered as prophylaxis did not prevent IVH nor provide any neurodevelopmental benefits (Pai 2008). A recent narrative review concluded: "Prophylactic and early targeted treatments have the potential of closing the PDA before it is symptomatic. The prophylactic treatment, once popular, has now become controversial as it unnecessarily exposes a large proportion of preterm babies to the side effects of treatment who would have closed the PDA spontaneously. Persistence of PDA is, however, associated with increase in morbidity and mortality. Early targeted treatment seems promising as it allows selection of babies who are less likely to close the PDA spontaneously and it has the benefits of a prophylactic approach. This approach is currently being tested in large randomised trials awaiting results" (Wyllie 2018). Early targeted treatment is based on echocardiographic criteria within the first 72 hours of life that have a high sensitivity for diagnosing a PDA that is unlikely to close spontaneously ((Wyllie 2018). Such an approach might therefore substantially reduce the number of infants who in our review were exposed to unnecessary treatment, as the PDA closed in 58% of the infants in control groups. Trials are currently being conducted in France, the UK, Holland, and Australia. It is important to note most trials have neurodevelopmental outcomes at two years of age incorporated in the trial design (Wyllie 2018), which has not been reported in any of the preventive trials of ibuprofen in our review. These trials and others are included under 'ongoing trials' in our "ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants" (Ohlsson 2018). Results of those trials will be reported in a separate section of that review when it is updated.
Coceani 2005 proposed that "... an mPGES (membrane bound prostaglandin E synthase) inhibitor, once developed for therapeutic use, could become the agent of choice for PDA treatment, particularly in those instances in which prematurity is complicated by infectious or inflammatory conditions" (Coceani 2005). Di Micco 2018 reported the discovery of new potent molecular entities able to inhibit mPGES‐1. Five of the predicted compounds were shown to potently inhibit the mPGES‐1 enzyme, without affecting COX enzymes activities (Di Micco 2018). We were not able to identify any trials in neonates.
Authors' conclusions
Implications for practice.
Prophylactic use of ibuprofen reduces the incidence of patent ductus arteriosus, the need for rescue treatment with cyclo‐oxygenase inhibitors or surgical closure. Adverse effects in the ibuprofen (IV or oral) group compared to the placebo or no interventions group included significantly increased risk for oliguria and increased serum creatinine levels in the ibuprofen group. There was a significantly increased risk of gastrointestinal haemorrhage with oral ibuprofen. There was a reduced risk for intraventricular haemorrhage (grade II to IV) of borderline statistical significance but no statistically significant differences in mortality, CLD at 28 days' or 36 weeks' postmenstrual age, necrotising enterocolitis, or time to reach full feeds. The prophylactic use of ibuprofen has been associated with severe pulmonary hypertension in one of the trials included in this review but did not occur in subsequent trials included in the current review. In the control group, the patent ductus arteriosus had closed spontaneously by day 3 or 4 in 58% of neonates. Prophylactic treatment therefore exposes a large proportion of infants unnecessarily to a drug that has important side effects without conferring any important short‐term benefit on outcomes. Current evidence does not support the use of ibuprofen for prevention of PDA.
Implications for research.
Until long‐term follow‐up results are published from the trials included in this review, no further trials of prophylactic ibuprofen are recommended. Early targeted treatment seems promising, as it allows selection of neonates who by 72 hours of age are less likely to close the PDA spontaneously, and it has the benefits of retaining an element of a prophylactic approach. This intervention is currently being tested in large randomised trials awaiting results and are included as ongoing trials in our review on "Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants".
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
| 27 January 2020 | New citation required but conclusions have not changed | Contact author changed, and contact details updated. |
History
Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 12 November 2018 | New search has been performed | This updates the review titled "Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants," which was published in the Cochrane Library, Issue 7, 2011 (Ohlsson 2011) |
| 12 November 2018 | New citation required and conclusions have changed | Two new studies have been added in this update, and the review conclusions have been changed |
| 2 April 2011 | New search has been performed | This updates the review titled "Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants," which was published in the Cochrane Library, Issue 3, 2009 (new reference) |
| 2 April 2011 | New citation required and conclusions have changed | The updated search identified 1 additional trial for inclusion in the review. The review now includes 2 trials of prophylactic ibuprofen administered orally and 5 trials with IV administration Oral administration of ibuprofen appears as effective as IV administration. Risk of upper gastrointestinal bleed may be higher with orally administered ibuprofen |
| 28 February 2009 | New search has been performed | This updates the review titled "Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants," which was published in the Cochrane Library, Issue 1, 2006 (Shah 2006) The updated search identified 2 additional trials for inclusion in this review No changes were made to the conclusion of this review |
| 22 September 2005 | New citation required and conclusions have changed | Substantive amendments were made |
Acknowledgements
We are thankful to Dr. Dani, Dr. Kanmaz, and Dr. Rubaltelli for providing additional information about their trials.
We appreciate the help of Caitlin O'Connell, Assistant Managing Editor Cochrane Neonatal, in conducting the literature and trials registries searches.
Appendices
Appendix 1. Cochrane Neonatal standard search strategy
PubMed: ((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies*[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infan*[TIAB] OR neonat*[TIAB]) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase:
#1 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*).mp
#2 exp infant
#3 (#1 OR #2)
#4 (human not animal) .mp
#5 (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp
#6 (#3 and #4 and #5)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) CENTRAL: infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU
Appendix 2. 'Risk of bias' tool
'Risk of bias' tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
· low risk (any truly random process e.g. random number table; computer random number generator);
· high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
· unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
· low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
· high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
· unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
· low risk, high risk, or unclear risk for participants; and
· low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
· low risk for outcome assessors;
· high risk for outcome assessors; or
· unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
· low risk (< 20% missing data);
· high risk (≥ 20% missing data); or
· unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
· low risk (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
· high risk (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported); or
· unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
· low risk;
· high risk; or
· unclear risk.
If needed, we explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Ibuprofen (IV or oral) vs placebo or none.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Presence of PDA on third or fourth day of life | 9 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.31, 0.48] |
| 2 Neonatal mortality (at < 28 days of life) | 6 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.50, 1.74] |
| 3 All‐cause mortality during hospital stay | 4 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.62, 1.30] |
| 4 Mortality before 36 weeks' PMA | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.66] |
| 5 Need for rescue medical treatment with cyclo‐oxygenase inhibitors | 6 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.11, 0.26] |
| 6 Need for surgical closure of PDA | 7 | 925 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.22, 0.96] |
| 7 Duration of mechanical ventilation (days) | 5 | 470 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐1.99, 4.03] |
| 8 Days requiring supplemental oxygen | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐4.98, 4.61] |
| 9 CLD at 28 days of life among survivors | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.32, 2.42] |
| 10 CLD at 36 weeks' corrected GA | 5 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.26] |
| 11 CLD (age at diagnosis not stated) | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.51, 1.72] |
| 12 Pulmonary hypertension | 4 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.11 [0.37, 134.91] |
| 13 IVH all grades | 6 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.78, 1.17] |
| 14 IVH (grades not stated) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.20] |
| 15 IVH grade III or IV | 7 | 925 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 1.00] |
| 16 PVL | 4 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.64, 2.18] |
| 17 NEC | 9 | 1028 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.50] |
| 18 Gastrointestinal haemorrhage | 5 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.19, 3.51] |
| 19 Gastrointestinal perforation | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [0.87, 27.36] |
| 20 Time to full enteral feeds (days) | 3 | 184 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐2.99, 3.94] |
| 21 Length of hospital stay (days) | 6 | 447 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐3.07, 5.67] |
| 22 Urine output after treatment (mL/kg/hr) | 3 | 650 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.26, 0.15] |
| 23 Oliguria | 4 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.04, 2.02] |
| 23.1 Oliguria < 0.5 mL/kg/hr | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.01, 2.34] |
| 23.2 Oliguria < 1.0 mL/kg/hr | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.78, 2.26] |
| 24 Serum creatinine levels after treatment (mg/dL) | 6 | 800 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.05, 0.13] |
| 25 At least one episode of serum creatinine > 140 micromol/L (> 1.5 mg/dL) | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.70 [1.05, 12.98] |
| 26 At least one episode of severe hypoxaemia | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.80, 3.59] |
| 27 Nitric oxide use during first week of life | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.80, 4.42] |
| 28 ROP | 5 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.38] |
| 29 Sepsis | 2 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.10, 6.59] |
| 30 Presence of PDA on third day of life in infants ≤ 28 weeks' gestation at birth | 3 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.30, 0.57] |
| 31 Presence of PDA on third day of life in infants 29 to 30 weeks' gestation at birth | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.64] |
| 32 Presence of PDA on third day of life in infants ≤ 1000 g | 2 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.25, 0.59] |
| 33 Presence of a PDA on third day of life in infants 1001 to 1500 g | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.34, 0.83] |
1.1. Analysis.
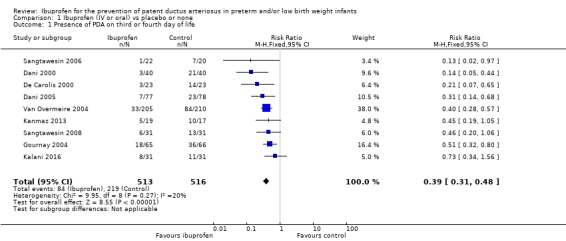
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 1 Presence of PDA on third or fourth day of life.
1.2. Analysis.
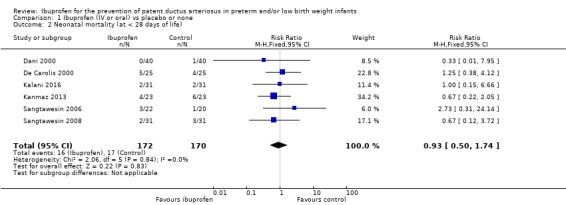
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 2 Neonatal mortality (at < 28 days of life).
1.3. Analysis.
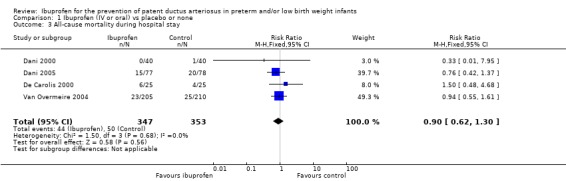
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 3 All‐cause mortality during hospital stay.
1.4. Analysis.
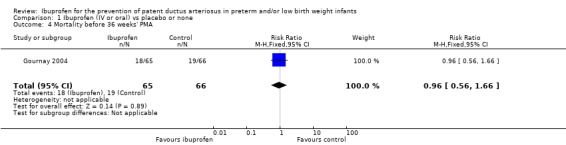
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 4 Mortality before 36 weeks' PMA.
1.5. Analysis.
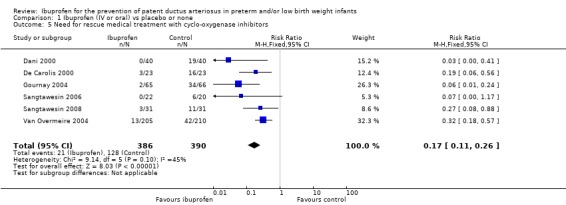
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 5 Need for rescue medical treatment with cyclo‐oxygenase inhibitors.
1.6. Analysis.
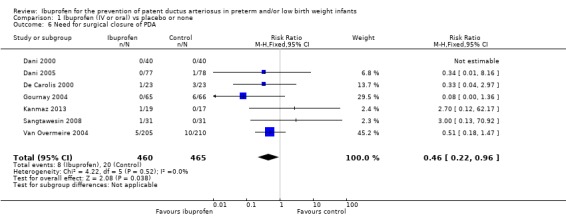
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 6 Need for surgical closure of PDA.
1.7. Analysis.
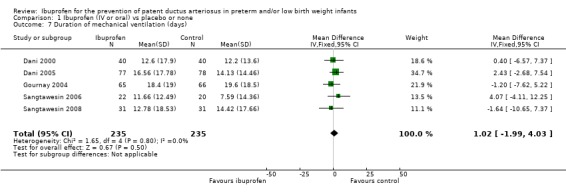
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 7 Duration of mechanical ventilation (days).
1.8. Analysis.
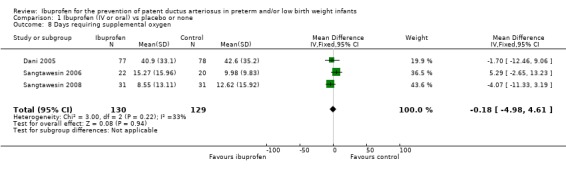
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 8 Days requiring supplemental oxygen.
1.9. Analysis.
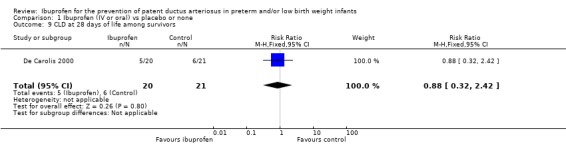
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 9 CLD at 28 days of life among survivors.
1.10. Analysis.
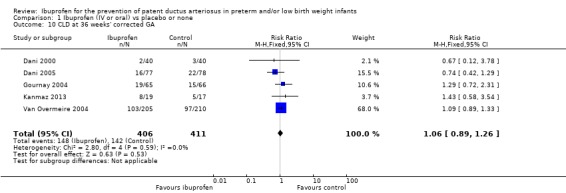
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 10 CLD at 36 weeks' corrected GA.
1.11. Analysis.
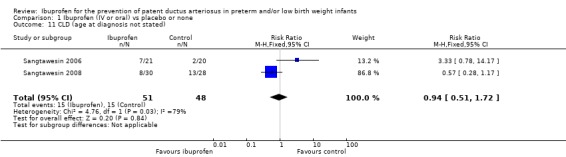
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 11 CLD (age at diagnosis not stated).
1.12. Analysis.
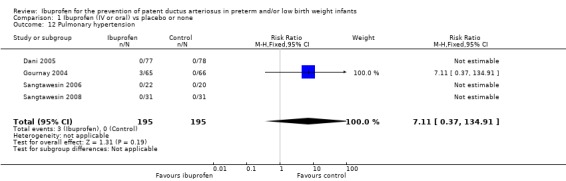
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 12 Pulmonary hypertension.
1.13. Analysis.
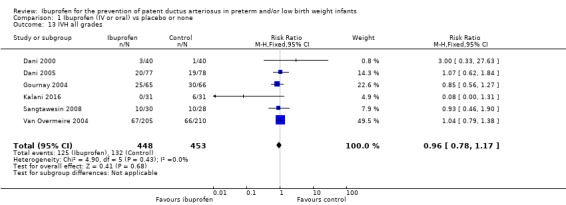
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 13 IVH all grades.
1.14. Analysis.
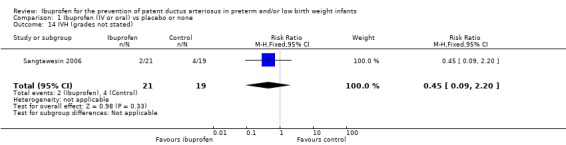
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 14 IVH (grades not stated).
1.15. Analysis.
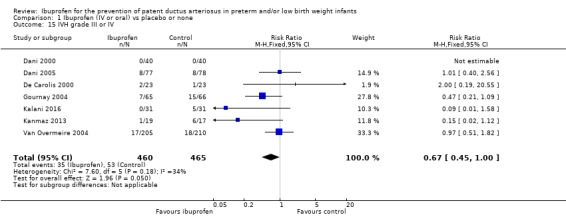
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 15 IVH grade III or IV.
1.16. Analysis.
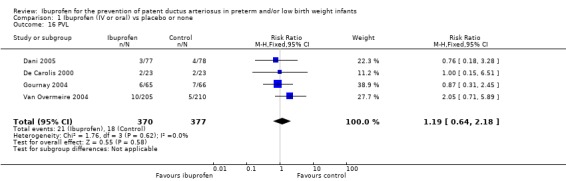
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 16 PVL.
1.17. Analysis.
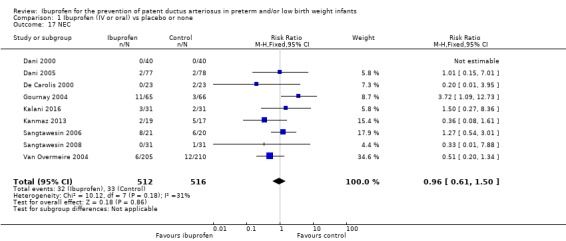
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 17 NEC.
1.19. Analysis.
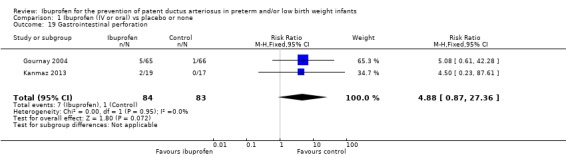
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 19 Gastrointestinal perforation.
1.20. Analysis.
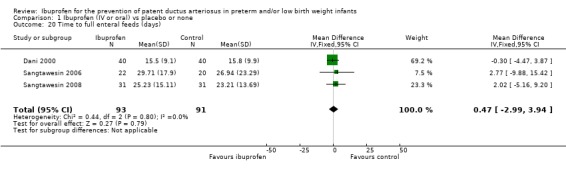
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 20 Time to full enteral feeds (days).
1.21. Analysis.
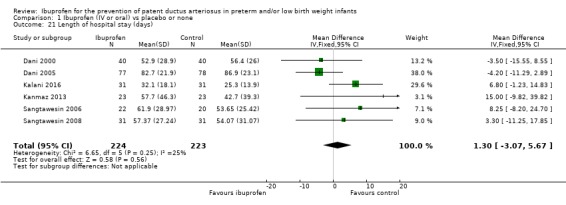
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 21 Length of hospital stay (days).
1.22. Analysis.
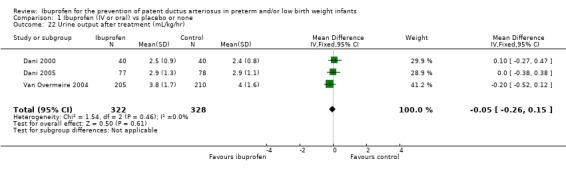
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 22 Urine output after treatment (mL/kg/hr).
1.23. Analysis.
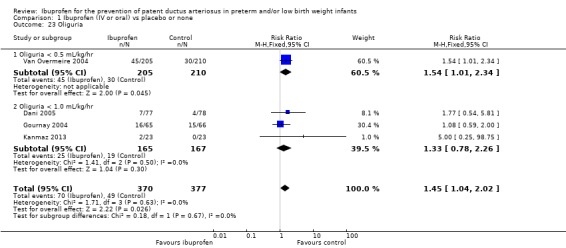
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 23 Oliguria.
1.24. Analysis.
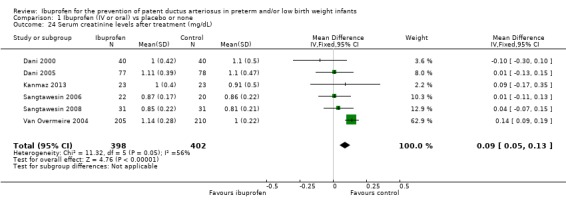
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 24 Serum creatinine levels after treatment (mg/dL).
1.25. Analysis.
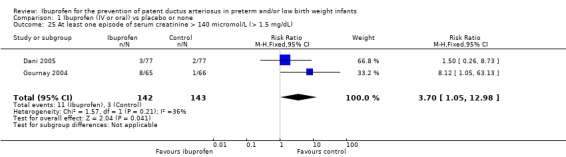
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 25 At least one episode of serum creatinine > 140 micromol/L (> 1.5 mg/dL).
1.26. Analysis.
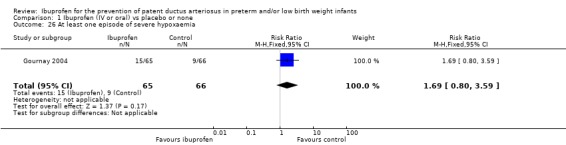
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 26 At least one episode of severe hypoxaemia.
1.27. Analysis.
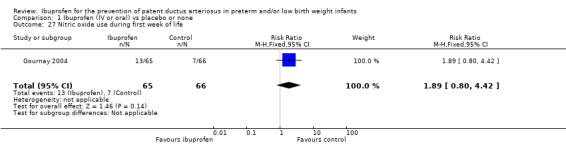
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 27 Nitric oxide use during first week of life.
1.28. Analysis.
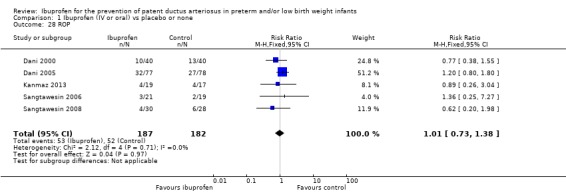
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 28 ROP.
1.29. Analysis.
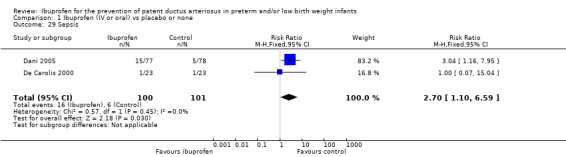
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 29 Sepsis.
1.30. Analysis.
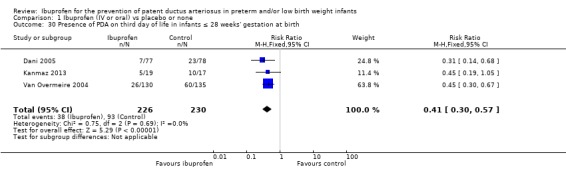
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 30 Presence of PDA on third day of life in infants ≤ 28 weeks' gestation at birth.
1.31. Analysis.
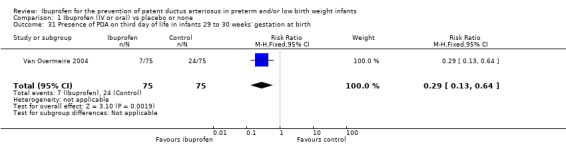
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 31 Presence of PDA on third day of life in infants 29 to 30 weeks' gestation at birth.
1.32. Analysis.
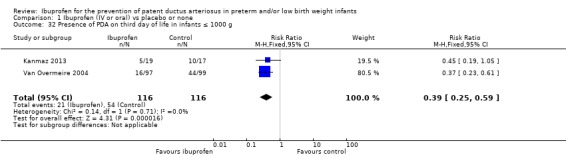
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 32 Presence of PDA on third day of life in infants ≤ 1000 g.
1.33. Analysis.
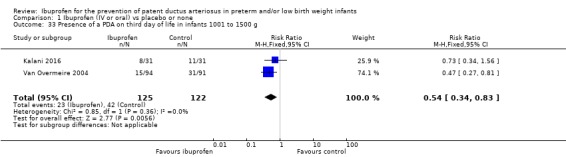
Comparison 1 Ibuprofen (IV or oral) vs placebo or none, Outcome 33 Presence of a PDA on third day of life in infants 1001 to 1500 g.
Comparison 2. Ibuprofen (oral) vs placebo or none.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Presence of PDA on day 3 or 4 of life | 4 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.30, 0.74] |
| 2 Gastrointestinal haemorrhage | 4 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.17, 3.48] |
2.1. Analysis.
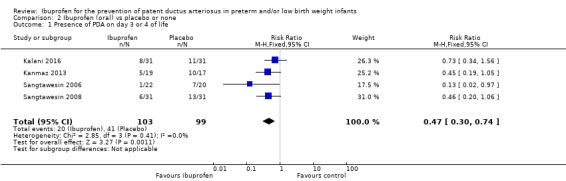
Comparison 2 Ibuprofen (oral) vs placebo or none, Outcome 1 Presence of PDA on day 3 or 4 of life.
2.2. Analysis.
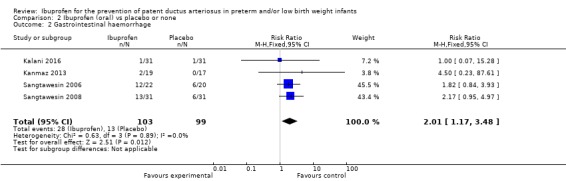
Comparison 2 Ibuprofen (oral) vs placebo or none, Outcome 2 Gastrointestinal haemorrhage.
Comparison 3. Ibuprofen (IV) vs placebo or none.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Presence of PDA on third day of life (72 hours of treatment) | 5 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.29, 0.47] |
3.1. Analysis.
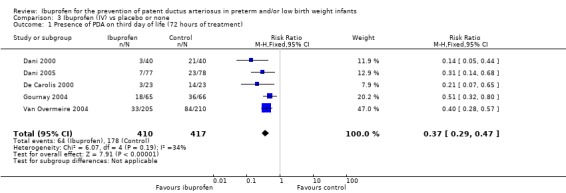
Comparison 3 Ibuprofen (IV) vs placebo or none, Outcome 1 Presence of PDA on third day of life (72 hours of treatment).
Comparison 4. Ibuprofen (oral) vs indomethacin (oral).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Presence of PDA on third day of life (72 hours of treatment) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.43, 2.33] |
| 2 Neonatal mortality (at < 28 days) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.93] |
| 3 IVH all grades | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.98] |
| 4 IVH (grade III or IV) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.00] |
| 5 NEC | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.58] |
| 6 GI bleeding | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.11] |
| 7 Hospitalisation (days) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 10.20 [1.24, 19.16] |
4.1. Analysis.
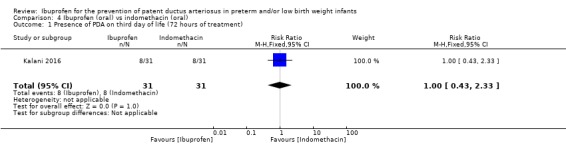
Comparison 4 Ibuprofen (oral) vs indomethacin (oral), Outcome 1 Presence of PDA on third day of life (72 hours of treatment).
4.2. Analysis.
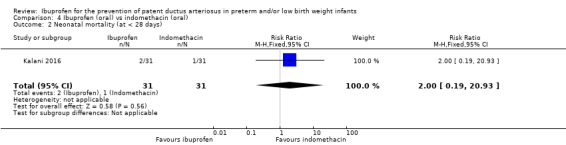
Comparison 4 Ibuprofen (oral) vs indomethacin (oral), Outcome 2 Neonatal mortality (at < 28 days).
4.3. Analysis.
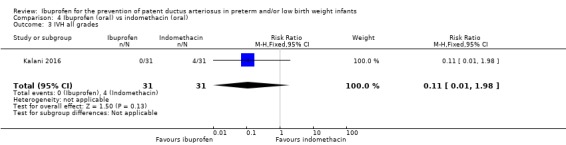
Comparison 4 Ibuprofen (oral) vs indomethacin (oral), Outcome 3 IVH all grades.
4.4. Analysis.
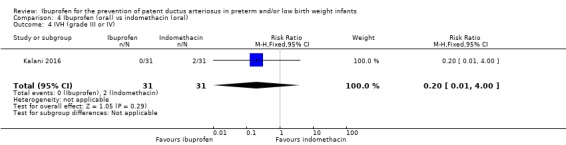
Comparison 4 Ibuprofen (oral) vs indomethacin (oral), Outcome 4 IVH (grade III or IV).
4.5. Analysis.
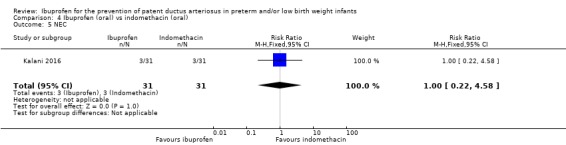
Comparison 4 Ibuprofen (oral) vs indomethacin (oral), Outcome 5 NEC.
4.6. Analysis.
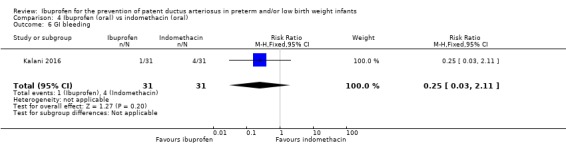
Comparison 4 Ibuprofen (oral) vs indomethacin (oral), Outcome 6 GI bleeding.
4.7. Analysis.
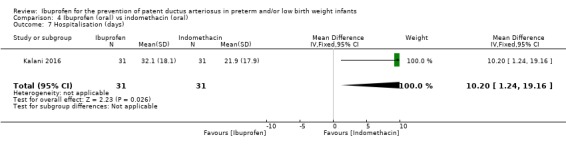
Comparison 4 Ibuprofen (oral) vs indomethacin (oral), Outcome 7 Hospitalisation (days).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dani 2000.
| Methods | Two‐centre randomised controlled trial without use of a placebo I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no | |
| Participants | Study period: February 1995 to January 1996; 2 centres, Italy Inclusion criteria: 1. GA < 34 weeks 2. Treatment with nasal continuous positive airway pressure with FiO2 > 0.3 or with synchronised mechanical ventilation or high‐frequency ventilation because of RDS 3. Platelet count ≥ 75,000/cm, serum creatinine ≤ 1.5 mg/dL, absence of clinical manifestation of abnormal clotting function 4. Absence of grade III or IV IVH before randomisation Enrolled within first 24 hours after birth Demographic data: Values presented as mean ± SD or as appropriate Prophylactic ibuprofen group: N = 40 Postmenstrual age (weeks): 29.2 ± 2.4 Birth weight (g): 1231 ± 445 Rescue ibuprofen group: N = 40 Gestational age (weeks): 29.6 ± 5.6 Birth weight (g): 1226 ± 505 |
|
| Interventions | Group A (prophylactic ibuprofen group; N = 40) received IV ibuprofen lysine (Arfen, Lisapharma, Italy) 10 mg/kg within first 24 hours of life, followed by 5 mg/kg after 24 and 48 hours Group B (rescue ibuprofen group; N = 40) received the same pharmacological treatment after echocardiographic diagnosis of PDA When significant PDA was still present after the first course of ibuprofen, a second course was administered. Failure to respond to ibuprofen was an indication for surgical ligation | |
| Outcomes | Echocardiographic diagnosis (Toshiba, Sonolayer SSH 140A with 7.5 MHz transducer) of PDA on days 3, 7, and 21 of life
Diagnosis of significant PDA was made by echocardiographic demonstration of a ductal left‐to‐right shunt, with left atrial‐to‐aortic root ratio > 1.3 or ductal size > 1.5 mm Further endpoints were severity of RDS, CLD at 36 weeks' CGA, IVH, ROP, NEC, need for surgical ligation of PDA, mortality, length of hospital stay, time to reach full feeds, renal function, biochemical and haematological profile, and any significant adverse effects |
|
| Notes | Patients enrolled in this study are the same as in the abstract of Rubaltelli 1998. This information was provided by Dr. Dani and Dr. Rubaltelli | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by the sealed envelope technique into 2 groups. Group A (prophylactic ibuprofen group; N = 40) received IV ibuprofen lysine (Arfen, Lisapharma, Italy) 10 mg/kg within first 24 hours of life, followed by 5 mg/kg after 24 and 48 hours. Group B (rescue ibuprofen group; N = 40) received the same pharmacological treatment after echocardiographic diagnosis of PDA |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used. For details, see above, under "Allocation concealment". Healthcare providers and outcome assessors were not blinded to group assignment; thus high risk of performance and detection bias existed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered in a trials registry, and we could not ascertain if there were deviations from the original protocol in the final publication |
| Other bias | Low risk | Appears free of other bias |
Dani 2005.
| Methods | Study period not stated Multi‐centre randomised double‐blind trial with use of placebo I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ yes III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ yes |
|
| Participants | 155 infants < 28 weeks' gestational age and postnatal age < 6 hours at 7 tertiary neonatal care units in Italy Prophylactic ibuprofen group: N = 77 Postmenstrual age (weeks): 25.3 ± 1.2 Birth weight (g): 832 ± 215 Placebo group: N = 78 Postmenstrual age (weeks): 25.9 ± 1.1 Birth weight (g): 812 ± 209 |
|
| Interventions | Infants were assigned randomly to treatment or control group via sealed envelopes. Envelopes were prepared centrally and were distributed to different units. Infants in the prophylactic ibuprofen group received 3 doses of ibuprofen lysine (Arfen, Lisapharma, Erba, Italy) 10 mg/kg within 6 hours after birth, followed by 5 mg/kg after 24 and 48 hours. Infants in the control group received indistinguishable placebo. The medications were infused continuously IV over 15 minutes | |
| Outcomes | The primary outcome was IVH (grade 2 to 4) at 7 days of life. Other outcomes included IVH at days' 15 and 30 and at 40 weeks' postconceptual age, PDA on day 3 (defined as echocardiographic evidence of a haemodynamically significant PDA), BPD at 36 weeks' postconceptional age, NEC, sepsis (confirmed with positive blood culture), and ROP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Infants were assigned randomly to treatment or control group via sealed envelopes. Envelopes were prepared centrally and were distributed to different units |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Infants in the control group received indistinguishable placebo. Low risk for performance and detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered in a trials registry, and we could not ascertain if there were deviations from the original protocol in the final publication |
| Other bias | Low risk | Appears free of other bias |
De Carolis 2000.
| Methods | Single‐centre randomised controlled trial without use of placebo I. Blinding of randomisation ‐ cannot be discerned II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ yes for the primary outcome | |
| Participants | Fifty infants < 2 hours of age and with GA < 31 weeks. Two infants in each group died within 24 hours after birth and were not considered in the final analysis Single‐centre study, Italy; 1 April 1996 to 30 July 1997 Assignment was performed within 2 hours after birth Demographic data: values presented as mean ± SD or as a number (percentage) Prophylactic ibuprofen group: N = 25 Gestational age (weeks): 28.1 ± 1.1 Birth weight (g): 934 ± 288 Control group: N = 25 Gestational age (weeks): 28.0 ± 1.9 Birth weight (g): 993 ± 308 |
|
| Interventions | 25 neonates received 10 mg ibuprofen lysine/kg IV over 20 minutes within 2 hours of life, and 5 mg/kg of ibuprofen lysine at 24 and 48 hours of life
25 neonates received no placebo/control treatment. Two neonates in each group died within 24 hours of life and were not considered in the final evaluation In the presence of significant PDA at completion of ibuprofen cycle, treatment with indomethacin (3 times 0.2 mg/kg at 12‐hourly intervals) administered by IV infusion over 20 minutes was carried out. The same treatment was administered to control neonates having significant PDA on the third day of life. Failure to respond to medical treatment was an indication for surgical ligation |
|
| Outcomes | PDA at 72 hours of age, need for treatment with indomethacin after 72 hours, surgical ligation, time to full oral feeds, mortality to 28 days of age, CLD at 28 days of age among survivors, sepsis. In addition, many outcomes during the first 3 days of life were reported as median and range and not as mean and SD Echocardiographic evaluation (Esaote Biomedica SPR 8000 ultrasound imaging system, using 5 MHz probe incorporating pulsed and colour‐flow doppler) was performed by the same investigator who was blinded to the treatment schedule. Neonates were studied immediately after birth, on day 3 of life, and then whenever clinical suspicion of PDA occurred. PDA was defined as symptomatic in the presence of heart murmur, bounding pulses, hyperactive precordium, decrease in diastolic arterial pressure, tachypnoea, increasing FiO2, or ventilatory requirements. Diagnosis of PDA was always confirmed by colour doppler echocardiography, and PDA was considered haemodynamically significant when the left atrial‐to‐aortic root ratio was > 1.3 |
|
| Notes | "Randomization was carried out at birth by random permuted blocks for both prophylaxis and control groups, envisaging 25 neonates in each" No further information is provided regarding randomisation and the allocation process Of the 50 randomised infants, 2 in each group died in the first 24 hours following birth and were not considered in the final analyses by study authors but were included by review authors in the analyses reported in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was carried out at birth by random permuted blocks for both prophylaxis and control groups |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was given to the control group. Risk for performance bias was high. Echocardioraphic evaluation was performed by an investigator who was blinded to the treatment schedule. Risk of detection bias for diagnosis of PDA by echocardiography was low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two neonates in each group died within 24 hours of life and were not considered in the final evaluation |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered in a trials registry, and we could not ascertain if there were deviations from the original protocol in the final publication |
| Other bias | Low risk | Appears free of other bias |
Gournay 2004.
| Methods | Randomised double‐blinded controlled trial I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ yes III. Complete follow‐up ‐ no (see notes) IV. Blinding of outcome measurement(s): yes | |
| Participants | Study period: March 2001 to December 2001
Multi‐centre trial including 11 NICUs in France
Inclusion criteria:
1. GA < 28 weeks
2. Postnatal age < 6 hours
Exclusion criteria:
1. Congenital malformations
2. Shock or right‐to‐left ductal shunt evidenced by differential cyanosis
3. Cerebral complications
4. Bleeding disorders Demographic data: values presented as mean ± SD Prophylactic ibuprofen group: N = 65 GA (weeks): 26.3 ± 0.9 BW (g): 844 ± 181 Placebo group: N = 66 GA (weeks): 26.0 ± 0.9 BW (g): 851 ± 164 |
|
| Interventions | One hundred thirty‐five infants were enrolled in the trial; 131 were randomised to receive either ibuprofen (N = 65) or placebo (N = 66) Both ibuprofen and placebo were given as 3 doses, 24 hours apart, with the first dose given within the first 6 hours of life. The initial dose of ibuprofen was 10 mg/kg, and the 2 following doses were 5 mg/kg, infused IV continuously over 20 minutes | |
| Outcomes | Decreased need for surgical ligation based on presence of significant PDA on echocardiogram Mortality PDA on day 3 by echocardiogram Need for back‐up treatment with indomethacin PVL Grade III or IV IVH NEC Intestinal perforation Duration of mechanical ventilation BPD at 36 weeks' corrected GA Renal function Actuarial curve of survival during the study period | |
| Notes | 135 infants were included
However, 4 were not randomly assigned because of errors in study drug allocation (3 mistakenly received open‐label ibuprofen prepared for the curative part of the study during their prophylactic course, and one 10‐day‐old with diagnosis of PDA was mistakenly given 2 doses of the randomised test drug (placebo) instead of curative ibuprofen). Per‐protocol analyses were performed on 131 infants. No participants were lost to follow‐up
The trial was closed earlier than planned after 3 episodes of refractory hypoxaemia with pulmonary hypertension happened after the first prophylactic injection at 3 different centres. The Agence Francaise du Medicament was notified and requested blinding of treatment received in these 3 cases. Treatment was ibuprofen in all 3 cases, and recruitment was closed on 14 December 2001 The study was supported by the industry (Orphan Europe, Paris, France) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo (saline) used. Low risk of performance and detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 135 infants were included However, 4 were not randomly assigned because of errors in study drug allocation (3 mistakenly received open‐label ibuprofen prepared for the curative part of the study during their prophylactic course, and one 10‐day‐old with diagnosis of PDA was mistakenly given 2 doses of the randomised test drug (placebo) instead of curative ibuprofen. Per‐protocol analyses were performed on 131 infants. No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered in a trials registry, and we could not ascertain if there were deviations from the original protocol in the final publication |
| Other bias | Low risk | Appears free of other bias |
Kalani 2016.
| Methods | Single‐centre RCT conducted at the NICU of Akbar‐Abadi Hospital (affiliated with Iran University of Medical Sciences, Theran, Iran) from 2013 to 2014 | |
| Participants | 93 preterm (< 32 weeks' PMA), BW < 1500 g infants, 6 to 12 hours old | |
| Interventions | One group received oral ibuprofen 10, 5, 5 mg/kg every 24 hours (N = 31); the second group received oral indomethacin 0.2 mL/kg daily for 3 days (N = 31), and the third group received only standard of care (N = 31) | |
| Outcomes | IVH, PDA, NEC, GI bleeding, mortality, hospitalisation (days). Oliguria and renal dysfunction were not defined, but no cases were reported | |
| Notes | In the abstract and under "Participants", it is stated that 96 preterm neonates entered the study, but under "Results", it is stated that 93 neonates, 31 in each group, entered the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot judge whether there were any deviations from the protocol |
| Other bias | Low risk | Appears free of other bias |
Kanmaz 2013.
| Methods | Single‐centre RCT conducted at the NICU of Zekai Tahir Burak Maternity Teaching Hosppital, Ankara, Turkey, between July 2011 and November 2011 | |
| Participants | 46 preterm infants with PMA < 28 weeks and/or BW < 1000 g were enrolled in the study 12 to 24 hours after birth | |
| Interventions | Oral ibuprofen 10 mg/kg within 12 to 24 hours after birth, followed by 5 mg/kg at 24 and 48 hours (N = 23). Control group received no treatment (N = 23) | |
| Outcomes | Presence of PDA on day 4 of life; BPD (age not stated), IVH (> grade II), NEC, duration of hospitalisation (median and IQR), mortality, GI bleeding, spontaneous intestinal perforation, acute kidney failure (defined as abnormal increase in urea, creatinine, and cystine C levels), and oliguria (defined as urine output < 1 mL/kg body weight) | |
| Notes | Computer‐based programme was used for random sequence generation as per email communication from the first author, as was clarifying information regarding acute renal failure and oliguria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was performed by computer |
| Allocation concealment (selection bias) | Low risk | Infants were randomised to the intervention (prophylactic oral ibuprofen administration) group or to the control group via sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Control group received no treatment, so caregivers must have known to which group each infant belonged. PDA assessments were done by echocardiogram by a cardiologist who was blinded to groups (low risk). Other outcomes were assessed by staff who knew to which groups the infants belonged (high risk) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mortality reported for all randomised infants. 4 infants in the ibuprofen group and 6 infants in the control group died. Apart from mortality, outcomes are reported among survivors |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot judge whether there were any deviations from the protocol |
| Other bias | Low risk | Appears free of other bias |
Sangtawesin 2006.
| Methods | I. Blinding of randomisation ‐ yes; infants were randomly assigned to ibuprofen or control group by block randomisation II. Blinding of intervention ‐ yes III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ yes | |
| Participants | Study period: July 2003 to April 2004 This single‐centre trial conducted in Thailand enrolled 42 infants of 28 to 32 weeks' gestational age and birth weight ≤ 1500 g and postnatal age < 24 hours Prophylactic ibuprofen group: N = 22 Postmenstrual age (weeks): 30.64 ± 1.76 Birth weight (g): 1279.64 ± 80.33 Control group: N = 20 Postmenstrual age (weeks): 30.20 ± 2.14 Birth weight (g): 1214.50 ± 217.52 |
|
| Interventions | The prophylaxis group received ibuprofen suspension (Junifen, Boots Company, Thailand) at a dosage of 10 mg/kg via an orogastric tube, followed by 0.5 mL of distilled water. The first dose was given within the first 24 hours of life. The second and third doses were given within 24 and 48 hours after the first dose, respectively. Participants in the control group were given 3 doses of an orange starch suspension as placebo that looked like ibuprofen | |
| Outcomes | The primary outcome was presence of a PDA (defined as echocardiographic evidence of a haemodynamically significant PDA) on day 3 of treatment Additional outcomes included neonatal mortality, duration of mechanical ventilation, pulmonary hypertension, NEC, gastrointestinal haemorrhage, time to full enteral feeds, ROP (grades not stated), length of hospital stay, BPD (age at diagnosis not stated), days of supplemental oxygen therapy, days of mechanical ventilation, IVH (grades not stated), need for rescue treatment with indomethacin or ibuprofen, and PPHN |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly assigned into the present study and control group by block randomisation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants in the control group were given 3 doses of an orange starch suspension as placebo that looked like ibuprofen. Medical personnel who took care of the patients were blind to group assignment. Risk for performance and detection bias was low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered in a trials registry, and we could not ascertain if there were deviations from the original protocol in the final publication |
| Other bias | Low risk | Appears free of other bias |
Sangtawesin 2008.
| Methods | I. Blinding of randomisation ‐ cannot tell; infants were randomly assigned to ibuprofen or control by block randomisation II. Blinding of intervention ‐ yes; medical personnel who took care of patients were blind to group assignment III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ yes | |
| Participants | Study period: October 2005 to October 2006 This single‐centre trial conducted in Thailand enrolled 62 infants with birth weight < 1500 g and postnatal age < 24 hours. The infants were known to have a PDA diagnosed by echocardiography on entry into the trial Prophylactic ibuprofen group: N = 31 Postmenstrual age (weeks): 29.32 ± 1.94 Birth weight (g): 1156.90 ± 263.6 Placebo group: N = 31 Postmenstrual age (weeks): 29.29 ± 2.16 Birth weight (g): 1162.90 ± 261.0 |
|
| Interventions | The prophylaxis group received 3 doses of oral ibuprofen suspension (Junifen, Boots Company, Thailand) at a dosage of 10 mg/kg for the first dose within 24 hours of life, and 5 mg/kg for the second and third doses after 24 and 48 hours. The drug was given via an orogastric tube, followed by 0.5 mL of distilled water. Infants in the control group received 3 doses of orange starch suspension as placebo administered by the same method and time schedule as oral ibuprofen suspension in the study group. The external appearance of placebo was like ibuprofen suspension and could not be differentiated by naked eyes | |
| Outcomes | The primary outcome was closure of PDA (defined as lack of echocardiographic evidence of a haemodynamically significant PDA) on day 3 of treatment Other outcomes included PPHN, BPD, days of assisted ventilation, days on supplemental oxygen, serum BUN on day 3, serum creatinine on day 3, days to start feeding, days to reach full feeds, gastrointestinal bleeding, NEC ≥ stage 2, ROP (total and stage 1 and stage 2), IVH (grade I and grades I to III), length of hospital stay (days), and mortality during the study period (28 days) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Infants in the control group received 3 doses of orange starch suspension as placebo administered by the same method and time schedule as oral ibuprofen suspension in the study group. The external appearance of placebo was like ibuprofen suspension and could not be differentiated by naked eyes. Risk for performance and detection bias was low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered in a trials registry, and we could not ascertain if there were deviations from the original protocol in the final publication |
| Other bias | Low risk | Appears free of other bias |
Van Overmeire 2004.
| Methods | Seven‐centre randomised double blinded controlled trial I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ yes III. Blinding of outcome measurement(s) ‐ yes IV. Complete follow‐up ‐ yes | |
| Participants | Study period: 1 February 1999 to 30 September 2001
7 centres; Belgium
Inclusion criteria: gestational age 24 to 30 weeks
Exclusion criteria: major congenital malformation or chromosomal anomaly, intraventricular haemorrhage higher than grade I already detected during baseline cranial ultrasonography, Apgar score at 5 minutes < 5, signs of congenital infection or life‐threatening septicaemia, uncontrolled hypotension, contraindications to administration of ibuprofen Demographic data: values presented as mean ± SD or as number (percentage) Prophylaxis group: N = 205 Gestational age (weeks): 28.1 ± 1.7 Birth weight (g): 1048 ± 315 Placebo group: N = 210 Gestational age (weeks): 28.1 ± 1.6 Birth weight (g): 1065 ± 324 |
|
| Interventions | 205 infants received ibuprofen‐lysine and 210 infants received placebo (saline). First dose of medication was given within 6 hours of birth, and second and third doses were given at 24 hours and 48 hours after the first dose. The dose of IV ibuprofen used was 10 mg/kg for the first dose and 5 mg/kg for subsequent doses. The dose of saline was 1 mL/kg for first dose and 0.5 mL/kg for subsequent doses | |
| Outcomes | The primary outcome variable was IVH grade III or IV Secondary outcomes included echocardiographically confirmed PDA after day 3 of life and the need for its pharmacological rescue treatment or surgical ligation, occurrence of renal dysfunction measured by urine production, NEC, and death |
|
| Notes | The study was published as an abstract when 358 infants had been enrolled. There is no mention of this interim analysis in the final publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done independently by the chief pharmacist in a 1‐to‐1 ratio between ibuprofen and placebo |
| Allocation concealment (selection bias) | Low risk | Identical looking ibuprofen‐lysine and normal saline as placebo provided by the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Attending and consulting physicians, nurses, study collaborators, and parents were unaware of treatment allocation. Risk for performance and detection bias was low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered in a trials registry, and we could not ascertain if there were deviations from the original protocol in the final publication |
| Other bias | Unclear risk | The study was published as an abstract when 358 infants had been enrolled. There is no mention of this interim analysis in the final publication |
Abbreviations: BPD: bronchopulmonary dysplasia. BUN: blood urea nitrogen. BW: birth weight. CGA: corrected gestational age. CLD: chronic lung disease. FiO2: fraction of inspired oxygen. GA: gestational age. GI: gastrointestinal. IQR: interquartile ratio. IV: intravenous(ly). IVH: intraventricular haemorrhage. NEC: necrotising enterocolitis. NICU: neonatal intensive care unit. PDA: patent ductus arteriosus. PMA: postmenstrual age. PPHN: persistent pulmonary hypertension of the newborn. PVL: periventricular leukomalacia. RCT: randomised controlled trial. RDS: respiratory distress syndrome. ROP: retinopathy of prematurity. SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Varvarigou 1996 | Not a randomised controlled study |
Differences between protocol and review
In two secondary analyses conducted for this update, we report on the effectiveness of oral ibuprofen versus placebo and of IV ibuprofen versus placebo in reducing the incidence of PDA on the third day of life (after 73 hours of treatment).
Contributions of authors
Ohlsson A ‐ contributed to all stages of the original review and conducted the updates in July 2005 and February 2009.
Shah S ‐ contributed to all stages of the protocol and the original review.
Ohlson A and Shah S contributed to all stages of the update in 2011 update and the update of the review in 2018.
Sources of support
Internal sources
Department of Paediatrics, Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
AO has no interests to declare.
SS has no interests to declare.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Dani 2000 {published data only}
- Dani C, Bertini G, Reali MF, Murru P, Fabris C, Vangi V, et al. Prophylaxis of patent ductus arteriosus with ibuprofen in preterm infants. Acta Paediatrica 2000;89(11):1369‐74. [PUBMED: 11106052] [DOI] [PubMed] [Google Scholar]
- Rubaltelli FF, Bertini G, Reali MF, Vangi V, Dani C. Does early closure of PDA with ibuprofen reduce the severity of RDS in premature infants?. Pediatric Research 1998;43:296. [DOI: 10.1203/00006450-199804001-01758] [DOI] [Google Scholar]
Dani 2005 {published data only}
- Dani C, Bertini G, Pezati M, Poggi C, Guerrini P, Martano C, et al. Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics 2005;115(6):1529‐35. [DOI: 10.1542/peds.2004-1178; PUBMED: 15930213] [DOI] [PubMed] [Google Scholar]
De Carolis 2000 {published data only}
- Carolis MP, Romagnoli C, Polimeni V, Piersigilli F, Zecca E, Papacci P, et al. Prophylactic ibuprofen therapy of patent ductus arteriosus in preterm infants. European Journal of Pediatrics 2000;159(5):364‐8. [PUBMED: 10834523] [DOI] [PubMed] [Google Scholar]
Gournay 2004 {published data only}
- Gournay V, Roze JC, Daoud P, Cambonie G, Hascoet JM, Chamboux C, et al. Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double‐blind, placebo‐controlled trial. Lancet 2004;364(9449):1939‐44. [DOI: 10.1016/S0140-6736(04)17476-X; PUBMED: 15567009] [DOI] [PubMed] [Google Scholar]
- Gournay V, Savagner C, Thirez G, Kuster A, Roze JC. Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet 2002;359(9449):1486‐8. [DOI: 10.1016/S0140-6736(04)17476-X; PUBMED: 15567009] [DOI] [PubMed] [Google Scholar]
Kalani 2016 {published data only}
- Kalani M, Shariat M, Khalesi N, Farahani Z, Ahmadi S. A comparison of early ibuprofen and indomethacin administration to prevent intraventricular hemorrhage among preterm infants. Acta Medica Iranica 2016;54(12):788‐92. [PUBMED: 28120591] [PubMed] [Google Scholar]
Kanmaz 2013 {published data only}
- Kanmaz G, Erdeve O, Canpolat FE, Oğuz SS, Uras N, Altug N, et al. Serum ibuprofen levels of extremely preterm infants treated prophylactically with oral ibuprofen to prevent patent ductus arteriosus. European Journal of Clinical Pharmacology 2013;69(5):1075‐81. [DOI: 10.1007/s00228-012-1438-8; PUBMED: 23128963] [DOI] [PubMed] [Google Scholar]
Sangtawesin 2006 {published data only}
- Sangtawesin V, Sangtawesin C, Raksasinborisut C, Sathirakul K, Kanjanapattanakul W, Khorana M, et al. Oral ibuprofen prophylaxis for symptomatic patent ductus arteriosus of prematurity. Journal of the Medical Association of Thailand 2006;89(3):314‐20. [PUBMED: 16696414] [PubMed] [Google Scholar]
Sangtawesin 2008 {published data only}
- Sangtawesin C, Sangtawesin V, Lertsutthiwong W, Kanjanapattanakul W, Khorana M, Ayudhaya JK. Prophylaxis of symptomatic patent ductus arteriosus with oral ibuprofen in very low birth weight infants. Journal of the Medical Association of Thailand 2008;91:S28‐34. [PUBMED: 19255990] [PubMed] [Google Scholar]
Van Overmeire 2004 {published data only}
- Naulaers G, Delanghe G, Allegaert K, Debeer A, Cossey V, Vanhole C, et al. Ibuprofen and cerebral oxygenation and circulation. Archives of Disease in Childhood Fetal Neonatal Edition 2005;90(1):F75‐6. [DOI: 10.1136/adc.2004.058347; PUBMED: 15613583] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeire B, Allegaert K, Casaer A, Debauche C, Decaluwe W, Jespers A, et al. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2004;364(9449):1945‐9. [DOI: 10.1016/S0140-6736(04)17477-1; PUBMED: 15567010] [DOI] [PubMed] [Google Scholar]
- Overmeire B, Casaer A, Allegaert K, Debauche C, Decaluwe W, Jespers A. Multicenter ibuprofen prophylaxis study (MIPS) in preterm infants: preliminary data. Pediatric Research 2002;52:825. [Google Scholar]
References to studies excluded from this review
Varvarigou 1996 {published data only}
- Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A, Aranda JV. Early ibuprofen administration to prevent ductus arteriosus in premature newborn infants. Journal of the American Medical Association 1996;275(7):539‐44. [PUBMED: 8606475] [PubMed] [Google Scholar]
Additional references
Bell 1978
- Bell MJ, Ternberg KL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotising enterocolitis: therapeutic decisions based on clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Betkerur 1981
- Betkerur MV, Yeh TF, Miller K, Glasser RJ, Pildes RS. Indomethacin and its effect on renal function and urinary kallikrein excretion in premature infants with patent ductus arteriosus. Pediatrics 1981;68(1):99‐102. [PUBMED: 6909683] [PubMed] [Google Scholar]
Chemtob 1990
- Chemtob S, Beharry K, Barna T, Varma DR, Aranda JV. Prostanoids determine the range of cerebral blood flow autoregulation of newborn piglets. Stroke 1990;21(5):777‐84. [PUBMED: 2339458] [DOI] [PubMed] [Google Scholar]
Chemtob 1993
- Chemtob S, Roy MS, Abran D, Fernandez H, Varma DR. Prevention of post asphyxial increase in lipid peroxides and retinal function and deterioration in the newborn pig by inhibition of cyclooxygenase activity and free radical generation. Pediatric Research 1993;33(4 Pt 1):336‐40. [DOI: 10.1203/00006450-199304000-00006; PUBMED: 8479812] [DOI] [PubMed] [Google Scholar]
Clyman 2000
- Clyman RI. Ibuprofen and patent ductus arteriosus. New England Journal of Medicine 2000;343(10):728‐30. [DOI: 10.1056/NEJM200009073431009; PUBMED: 10974138] [DOI] [PubMed] [Google Scholar]
Coceani 1979
- Coceani F, White E, Bodach E, Olley PM. Age dependent changes in the response of lamb ductus arteriosus to oxygen and ibuprofen. Canadian Journal of Physiology and Pharmacology 1979;57(8):825‐31. [PUBMED: 497895] [DOI] [PubMed] [Google Scholar]
Coceani 2005
- Coceani F, Barogi S, Brizzi F, Ackerley C, Seidlitz E, Kelsey L, et al. Cyclooxygenase isoenzymes and patency of ductus arteriosus. Prostaglandins, Leukotrienes and Essential Fatty Acids 2005;72(2):71‐7. [DOI: 10.1016/j.plefa.2004.10.004; PUBMED: 15626588] [DOI] [PubMed] [Google Scholar]
Cotton 1979
- Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. Journal of Pediatrics 1978;2(3):467‐73. [PUBMED: 632994] [DOI] [PubMed] [Google Scholar]
Couser 1996
- Couser RJ, Ferrara TB, Wright GB, Cabalka AK, Schilling CG, Hoekstra RE, et al. Prophylactic Indomethacin therapy in the first twenty four hours of life for the prevention of patent ductus arteriosus in preterm infants treated prophylactically with surfactant in the delivery room. Journal of Pediatrics 1996;128(5 Pt 1):631‐7. [PUBMED: 8627434] [DOI] [PubMed] [Google Scholar]
Davis 1995
- Davis P, Turner‐Gomes S, Cunningham K, Way C, Roberts R, Schmidt B. Precision and accuracy of clinical and radiological signs in premature infants at risk of patent ductus arteriosus. Archives of Pediatric and Adolescent Medicine 1995;149(10):1136‐41. [PUBMED: 7550818] [DOI] [PubMed] [Google Scholar]
Di Micco 2018
- Micco S, Terracciano S, Cantone V, Fischer K, Koeberle A, Foglia A, et al. Discovery of new potent molecular entities able to inhibit mPGES‐1. European Journal of Medicinal Chemistry 2018;143:1419‐27. [DOI: 10.1016/j.ejmech.2017.10.039; PUBMED: 29133047] [DOI] [PubMed] [Google Scholar]
Domanico 1994
- Domanico RS, Waldman JD, Lester LA, McPhillips HA, Catrambone JE, Covert RF. Prophylactic indomethacin reduces the incidence of pulmonary hemorrhage and patent ductus arteriosus in surfactant treated < 1250g. Pediatric Research 1994;35:331A. [Google Scholar]
Edwards 1990
- Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delply DT, et al. Effects of indomethacin on cerebral hemodynamics in very preterm infants. Lancet 1990;335(8704):1491‐5. [PUBMED: 1972434] [DOI] [PubMed] [Google Scholar]
Fowlie 2010
- Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 1976
- Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacological closure of patent ductus arteriosus in the premature infant. New England Journal of Medicine 1976;295(10):526‐9. [DOI: 10.1056/NEJM197609022951003frie; PUBMED: 820994] [DOI] [PubMed] [Google Scholar]
Gersony 1983
- Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of Indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. Journal of Pediatrics 1983;102(6):895‐906. [PUBMED: 6343572] [DOI] [PubMed] [Google Scholar]
Grosfeld 1996
- Grosfeld JL, Chaedt M, Molinari F, Engle W, Engum SA, West KW, et al. Increased risk of necrotizing enterocolitis in premature infants with patent ductus arteriosus treated with indomethacin. Annals of Surgery 1996;224(3):350‐7. [PUBMED: 8813263] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammerman 1995
- Hammerman C. Patent ductus arteriosus: clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clinics in Perinatology 1995;22(2):457‐79. [PUBMED: 7671547] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
ICROP 1984
- Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [PUBMED: 6547831] [DOI] [PubMed] [Google Scholar]
Ito 1994
- Ito K, Niida Y, Sato J, Owada E, Ito K, Umetsu M. Pharmacokinetics of mefenamic acid in preterm infants with patent ductus arteriosus. Acta Paediatrica Japonica 1994;36(4):387‐91. [PUBMED: 7942001] [DOI] [PubMed] [Google Scholar]
Jones 2011
- Jones LJ, Craven PD, Attia J, Thakkinstian A, Wright I. Network meta‐analysis of indomethacin versus placebo for PDA in preterm infants. Archives of Disease in Childhood Neonatal Edition 2011;96(1):F45‐52. [DOI: 10.1136/adc.2009.168682; PUBMED: 20876595] [DOI] [PubMed] [Google Scholar]
Lago 2002
- Lago P, Bettiol T, Salvadori S, Pitassi I, Vianello A, Chiandetti L, et al. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomised controlled trial. European Journal of Pediatrics 2002;161(4):202‐7. [PUBMED: 12014386] [DOI] [PubMed] [Google Scholar]
Lee 2000
- Lee Sk, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU network: 1996‐1997. Pediatrics 2000;106(5):1070‐9. [PUBMED: 11061777] [DOI] [PubMed] [Google Scholar]
Mosca 1997
- Mosca F, Bray M, Lattnazio M, Fumagalli M, Toscetto C. Comparative evaluation of the effect of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1997;131(4):549‐54. [PUBMED: 9386657] [DOI] [PubMed] [Google Scholar]
Mosca 2002
- Mosca F, Bray M, Stucchi I, Fumagalli M. Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet 2002;9338:1023‐4. [DOI] [PubMed] [Google Scholar]
Niopas 1994
- Niopas I, Mamzoridi K. Determination of indomethacin and mefenamic acid in plasma performance liquid chromatography. Journal of Chromatography. Biomedical Applications 1994;656(2):447‐50. [PUBMED: 7987501] [DOI] [PubMed] [Google Scholar]
Ohlsson 1993
- Ohlsson A, Bottu J, Govan J, Ryan ML, Fong K, Myhr T. The effect of indomethacin on cerebral blood flow velocities in very low birth weight neonates with patent ductus arteriosus. Developmental Pharmacology and Therapeutics 1993;20(1‐2):100‐6. [PUBMED: 7924757] [DOI] [PubMed] [Google Scholar]
Ohlsson 2018
- Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD003481.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pai 2008
- Pai VB, Sakadjian A, Puthoff TD. Ibuprofen lysine for the prevention and treatment of patent ductus arteriosus. Pharmacotherapy 2008;28(9):1162‐82. [DOI: 10.1592/phco.28.9.1162; PUBMED: 18752387] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birthweights less than 1,500 grams. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Patel 2000
- Patel J, Roberts I, Azzopardi D, Hamilton P, Edwards AD. Randomised double blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatric Research 2000;47(1):36‐42. [PUBMED: 10625080] [DOI] [PubMed] [Google Scholar]
Pezzati 1999
- Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1999;135(6):733‐8. [PUBMED: 10586177] [DOI] [PubMed] [Google Scholar]
Ramanathan 1997
- Ramanathan R, Siassi B, Gallagher R, DeLemos RA. Outcome of very low birth weight infants < 1500 g enrolled in the national database network: are there any trends in neonatology?. Pediatric Research 1997;41:171A. [Google Scholar]
Sakhalkar 1992
- Sakhalkar VS, Merchant RH. Therapy of patent ductus arteriosus in preterms with mefenamic acid and indomethacin. Indian Pediatrics 1992;29(3):313‐8. [PUBMED: 1612672] [PubMed] [Google Scholar]
Schmidt 2001
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts R, Saigal S, et al. Long‐term effects of indomethacin prophylaxis in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344(26):1966‐72. [DOI: 10.1056/NEJM200106283442602; PUBMED: 11430325] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PUBMED: 3174313] [PubMed] [Google Scholar]
Speziale 1999
- Speziale MV, Allen RG, Henderson CR, Barrington KJ, Finer NN. Effects of ibuprofen and indomethacin on the regional circulation in newborn piglets. Biology of the Neonate 1999;76(4):242‐52. [DOI: 10.1159/000014165; PUBMED: 10473899] [DOI] [PubMed] [Google Scholar]
Van Bel 1989
- Bel F, Bor M, Stijnen T, Baan J, Ruys JH. Cerebral blood flow velocity changes in preterm infants after a single dose of indomethacin: duration of its effect. Pediatrics 1989;84(5):802‐7. [PUBMED: 2677960] [PubMed] [Google Scholar]
Van Overmeire 1997
- Overmeire B, Follens I, Hartmann S, Creten WL, Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;76(3):F179‐84. [PUBMED: 9175948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Overmeire 2000
- Overmeire B, Smets K, Lecoutere D, Broek H, Weyler J, Groote K, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. New England Journal of Medicine 2000;334:674‐81. [DOI] [PubMed] [Google Scholar]
Wyllie 2018
- Wyllie JP, Gupta S. Prophylactic and early targeted treatment of patent ductus arteriosus. Seminars in Fetal and Neonatal Medicine 2018;23(4):250–4. [DOI: 10.1016/j.siny.2018.03.005; PUBMED: 29571706] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ohlsson 2009
- Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohlsson 2011
- Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD004213.pub3] [DOI] [PubMed] [Google Scholar]
Review Manager 5 (RevMan 5) [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shah 2003
- Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004213] [DOI] [PubMed] [Google Scholar]
Shah 2007
- Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI] [PubMed] [Google Scholar]


