Abstract
Background
Approximately 20% of people with cirrhosis develop ascites. Several different treatments are available; including, among others, paracentesis plus fluid replacement, transjugular intrahepatic portosystemic shunts, aldosterone antagonists, and loop diuretics. However, there is uncertainty surrounding their relative efficacy.
Objectives
To compare the benefits and harms of different treatments for ascites in people with decompensated liver cirrhosis through a network meta‐analysis and to generate rankings of the different treatments for ascites according to their safety and efficacy.
Search methods
We searched CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and trials registers until May 2019 to identify randomised clinical trials in people with cirrhosis and ascites.
Selection criteria
We included only randomised clinical trials (irrespective of language, blinding, or status) in adults with cirrhosis and ascites. We excluded randomised clinical trials in which participants had previously undergone liver transplantation.
Data collection and analysis
We performed a network meta‐analysis with OpenBUGS using Bayesian methods and calculated the odds ratio, rate ratio, and hazard ratio (HR) with 95% credible intervals (CrI) based on an available‐case analysis, according to National Institute of Health and Care Excellence Decision Support Unit guidance.
Main results
We included a total of 49 randomised clinical trials (3521 participants) in the review. Forty‐two trials (2870 participants) were included in one or more outcomes in the review. The trials that provided the information included people with cirrhosis due to varied aetiologies, without other features of decompensation, having mainly grade 3 (severe), recurrent, or refractory ascites. The follow‐up in the trials ranged from 0.1 to 84 months. All the trials were at high risk of bias, and the overall certainty of evidence was low or very low.
Approximately 36.8% of participants who received paracentesis plus fluid replacement (reference group, the current standard treatment) died within 11 months. There was no evidence of differences in mortality, adverse events, or liver transplantation in people receiving different interventions compared to paracentesis plus fluid replacement (very low‐certainty evidence). Resolution of ascites at maximal follow‐up was higher with transjugular intrahepatic portosystemic shunt (HR 9.44; 95% CrI 1.93 to 62.68) and adding aldosterone antagonists to paracentesis plus fluid replacement (HR 30.63; 95% CrI 5.06 to 692.98) compared to paracentesis plus fluid replacement (very low‐certainty evidence). Aldosterone antagonists plus loop diuretics had a higher rate of other decompensation events such as hepatic encephalopathy, hepatorenal syndrome, and variceal bleeding compared to paracentesis plus fluid replacement (rate ratio 2.04; 95% CrI 1.37 to 3.10) (very low‐certainty evidence).
None of the trials using paracentesis plus fluid replacement reported health‐related quality of life or symptomatic recovery from ascites.
Funding: the source of funding for four trials were industries which would benefit from the results of the study; 24 trials received no additional funding or were funded by neutral organisations; and the source of funding for the remaining 21 trials was unclear.
Authors' conclusions
Based on very low‐certainty evidence, there is considerable uncertainty about whether interventions for ascites in people with decompensated liver cirrhosis decrease mortality, adverse events, or liver transplantation compared to paracentesis plus fluid replacement in people with decompensated liver cirrhosis and ascites. Based on very low‐certainty evidence, transjugular intrahepatic portosystemic shunt and adding aldosterone antagonists to paracentesis plus fluid replacement may increase the resolution of ascites compared to paracentesis plus fluid replacement. Based on very low‐certainty evidence, aldosterone antagonists plus loop diuretics may increase the decompensation rate compared to paracentesis plus fluid replacement.
Plain language summary
Treatments for ascites in people with advanced liver disease
What is the aim of this Cochrane review? To find out the best available treatment for ascites (abnormal build‐up of fluid in the tummy) in people with advanced liver disease (liver cirrhosis, or late‐stage scarring of the liver with complications). People with cirrhosis and ascites are at significant risk of death. Therefore, it is important to treat such people, but the benefits and harms of different treatments available are currently unclear. The authors of this review collected and analysed all relevant research studies with the aim of finding what the best treatment is. They found 49 randomised controlled trials (studies where participants are randomly assigned to one of two treatment groups). During analysis of data, authors used standard Cochrane methods, which allow comparison of only two treatments at a time. Authors also used advanced techniques that allow comparison of multiple treatments simultaneously (usually referred as 'network (or indirect) meta‐analysis').
Date of literature search May 2019
Key messages None of the studies were conducted without flaws, and because of this, there is very high uncertainty in the findings. Approximately one in three trial participants with cirrhosis and ascites who received the standard treatment of drainage of fluid (paracentesis) plus fluid replacement died within 11 months of treatment. The funding source for the research was unclear in 21 studies; commercial organisations funded four studies. There were no concerns regarding the source of funding for the remaining 24 trials.
What was studied in the review? This review looked at adults of any sex, age, and ethnic origin, with advanced liver disease due to various causes and ascites. Participants were given different treatments for ascites. The authors excluded studies in people who had previously had liver transplantation. The average age of participants, when reported, ranged from 43 to 64 years. The treatments used in the trials included paracentesis plus fluid replacement (currently considered the standard treatment), different classes of diuretics (drugs which increase the passing of urine), and transjugular intrahepatic portosystemic shunt (an artificial channel that connects the different blood vessels that carry oxygen‐depleted blood (venous system)) within the liver to reduce the pressure built‐up in the portal venous system, one of the two venous systems draining the liver. The review authors wanted to gather and analyse data on death (percentage dead at maximal follow‐up), quality of life, serious and non‐serious adverse events, time to liver transplantation, resolution of ascites, and development of other complications of advanced liver disease. What were the main results of the review? The 49 studies included a small number of participants (3521 participants). Study data were sparse. Forty‐two studies with 2870 participants provided data for analyses. The follow‐up of the trial participants ranged from less than a week to seven years. The review shows that there is low‐ or very low‐certainty evidence for the following:
‐ Approximately one in three people with cirrhosis and ascites who received the standard treatment of drainage of fluid (paracentesis) plus fluid replacement died within 11 months. ‐ None of the interventions decrease percentage of deaths, number of complications, and liver transplantation compared to paracentesis plus fluid replacement. ‐ Transjugular intrahepatic portosystemic shunt may be nine times more effective in resolution of ascites compared to paracentesis plus fluid replacement. ‐ Adding aldosterone antagonists (a class of diuretics) may be 30 times more effective in resolution of ascites compared to paracentesis plus fluid replacement. ‐ Using aldosterone antagonists plus loop diuretics (another class of diuretics) as a substitute for paracentesis plus fluid replacement may double the development of other liver complications of cirrhosis. ‐ None of the trials that compared other treatments to paracentesis plus fluid replacement reported health‐related quality of life or symptomatic recovery from ascites. ‐ Future well designed trials are needed to find out the best treatment for people with cirrhosis and ascites.
Summary of findings
Background
Description of the condition
Liver cirrhosis
The liver is a complex organ with multiple functions including carbohydrate metabolism, fat metabolism, protein metabolism, drug metabolism, synthetic functions, storage functions, digestive functions, excretory functions, and immunological functions (Read 1972). Liver cirrhosis is a liver disease in which the normal microcirculation, the gross vascular anatomy, and the hepatic architecture have been variably destroyed and altered with fibrous septa surrounding regenerated or regenerating parenchymal nodules (Tsochatzis 2014; NCBI 2018a). The major causes of liver cirrhosis include excessive alcohol consumption, viral hepatitis, non‐alcohol‐related fatty liver disease, autoimmune liver disease, and metabolic liver disease (Williams 2014; Ratib 2015; Setiawan 2016). The global prevalence of liver cirrhosis is difficult to estimate as most estimates correspond to chronic liver disease (which includes liver fibrosis and liver cirrhosis). In studies from the USA, the prevalence of chronic liver disease varies between 0.3% to 2.1% (Scaglione 2015; Setiawan 2016); in the UK, the prevalence was 0.1% in one study (Fleming 2008). In 2010, liver cirrhosis was responsible for an estimated 2% of all global deaths, equivalent to one million deaths (Mokdad 2014). There is an increasing trend of cirrhosis‐related deaths in some countries, such as the UK, while there is a decreasing trend in other countries, such as France (Mokdad 2014; Williams 2014). The major cause of complications and deaths in people with liver cirrhosis is due to the development of clinically significant portal hypertension (hepatic venous pressure gradient at least 10 mmHg) (De Franchis 2015). Some of the clinical features of decompensation include jaundice, coagulopathy, ascites, variceal bleeding, hepatic encephalopathy, and renal failure (De Franchis 2015; McPherson 2016; EASL 2018). Decompensated cirrhosis is the most common indication for liver transplantation (Merion 2010; Adam 2012).
Ascites
Ascites is accumulation of free fluid in the abdomen (peritoneal cavity) (NCBI 2018b), and is a feature of liver decompensation (Tsochatzis 2017). Approximately 20% of people with cirrhosis have ascites (D'Amico 2014). Approximately 1% to 4% of people with cirrhosis develop ascites each year (D'Amico 2006; D'Amico 2014). Ascites is the first sign of liver decompensation in about a third of people with compensated liver cirrhosis (D'Amico 2014). Ascites can be graded as grade 1 ascites, which is mild ascites only detectable by ultrasound examination; grade 2 or moderate ascites which is manifested by moderate symmetrical distension of the abdomen; and grade 3 ascites which is large or gross ascites with marked abdominal distension (Arroyo 1996; Moore 2003). Grade 3 ascites is also called 'tense' ascites (Arroyo 1996). Ascites that is refractory to medical treatment is called 'refractory' ascites (Arroyo 1996; Moore 2003). Table 3 provides detailed criteria for the definition of refractory ascites (Moore 2003).
1. Revised 'International Ascites Club' criteria for refractory ascites.
| 1. Treatment duration: patients must be on intensive diuretic therapy (spironolactone 400 mg/day and furosemide 160 mg/day) for at least 1 week and on a salt‐restricted diet of less than 90 mmol or 5.2 g of salt/day |
| 2. Lack of response: mean weight loss of less than 0.8 kg over 4 days and urinary sodium output less than the sodium intake |
| 3. Early ascites recurrence: reappearance of grade 2 or 3 ascites within 4 weeks of initial mobilisation |
4. Diuretic‐induced complications:
|
From: Moore 2003.
In people with cirrhosis, the onset of ascites and treatment of ascites result in a decrease in health‐related quality of life (Kim 2006; Les 2010; Orr 2014). Resolution of ascites may result in improvement in health‐related quality of life in people with ascites (Orr 2014). The one‐year mortality in people with liver cirrhosis and ascites is 20%, which increases to 57% in those with ascites and variceal bleeding (D'Amico 2006). Management of ascites and its complications involve significant resources. One study reported that people with liver cirrhosis and ascites required on average one hospital admission per month and a 10‐day stay in hospital per month (Fagan 2014).
Pathophysiology of ascites
The exact mechanism by which ascites develops in people with liver cirrhosis is unknown. Portal hypertension causes arterial vasodilatation of the splanchnic circulation (dilation of the blood vessels supplying the digestive organs in the abdomen such as liver, pancreas, and intestines) (Ginès 2009; Moore 2013). This activates the renin–angiotensin system (Ginès 2009; Moore 2013), leading to fluid retention (Moore 2013). In addition, the vessel wall permeability is increased due to the pathological increase in vascular endothelial growth factor (VEGF) (Colle 2008), and the oncotic pressure is decreased due to decreased albumin synthesis by the diseased liver leading to leaky splanchnic blood vessels in people with portal hypertension (Moore 2013). This results in fluid accumulation in the peritoneal cavity, that is, ascites (Moore 2013).
Description of the intervention
Although people with cirrhosis and grade 2 ascites, grade 3 ascites, and refractory ascites should be considered for liver transplantation (EASL 2010; Runyon 2013; EASL 2016; EASL 2018), cirrhotic ascites alone without other features of end‐stage liver disease, such as jaundice, variceal bleeding, spontaneous bacterial peritonitis, or hepatorenal syndrome, are usually treated using less invasive methods than liver transplantation (EASL 2010). According to the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) guidelines, grade 1 ascites does not require any specific treatment; grade 2 requires salt‐restricted diet and diuretics; and grade 3 requires large volume paracentesis (removal of several litres of ascitic fluid) along with salt‐restricted diet and diuretics (EASL 2010; Runyon 2013; EASL 2018).
In people with diuretic‐refractory ascites, paracentesis and transjugular intrahepatic portosystemic shunt (TIPS) are the main treatments according to EASL and AASLD guidelines (EASL 2010; Runyon 2013; EASL 2018). In addition, AASLD guidelines suggests that midodrine (a vasoconstrictor) should be considered in people with refractory ascites (Runyon 2013), while midodrine is not recommended by EASL guidelines (EASL 2018).
The role of vasoconstrictors, spontaneous ultrafiltration and reinfusion (filter the removed ascitic fluid and reinfuse the proteins), and low‐flow ascites fluid pump (automatically diverts ascitic fluid to the urinary bladder, from where it is excreted in urine) in the treatment of people with ascites is unclear and neither EASL nor AASLD guidelines recommend their routine use (EASL 2010; Runyon 2013). Surgical portosystemic shunts are currently recommended only in people with refractory ascites unsuitable for TIPS, repeated paracentesis, or liver transplantation (Runyon 2013).
How the intervention might work
Diuretics increase fluid excretion, thereby decreasing the fluid accumulation: fluid accumulation is one of the mechanisms of developing ascites, and decreasing fluid accumulation can lead to resolution of ascites. Systemic vasoconstrictor drugs decrease the splanchnic vasodilation which is another mechanism of developing ascites.
Paracentesis involves removing the ascitic fluid. Removal of up to 5 litres of fluid in one session of paracentesis is unlikely to cause circulatory shock (EASL 2010; Runyon 2013), but removal of more than this volume can lead to circulatory shock. Various methods to try to overcome this are to administer albumin, colloids such as hydroxyethyl starch, vasoconstrictors such as midodrine, or reinfusing the proteins from the ascitic fluid into systemic circulation (Bruno 1992; Altman 1998; Appenrodt 2008). However, the benefits of plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis is questionable (Simonetti 2019).
TIPS procedures and other surgical forms of portosystemic shunt are aimed at decreasing portal venous pressure, the major cause of ascites in people with liver cirrhosis.
Why it is important to do this review
It is important to provide optimal treatment to people with ascites to improve their survival and health‐related quality of life. Several different treatments are available, but their relative efficacy and optimal combinations are not known. One Cochrane Review on TIPS versus paracentesis for people with cirrhosis with refractory ascites was available at the start of this project (Saab 2006); however, to date, there have not been any network meta‐analyses on the topic. Network meta‐analysis allows for a combination of direct and indirect evidence and the ranking of different interventions for different outcomes (Salanti 2011; Salanti 2012). With this systematic review and network meta‐analysis, we provide the best level of evidence for the benefits and harms of different treatments for ascites in people with decompensated liver cirrhosis. We have also presented results from direct comparisons whenever possible, as well as performing the network meta‐analysis.
Objectives
To compare the benefits and harms of different treatments for ascites in people with decompensated liver cirrhosis through a network meta‐analysis and to generate rankings of the different treatments for ascites according to their safety and efficacy.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials (including cross‐over, cluster‐randomised clinical trials) for this network meta‐analysis irrespective of language, publication status, or date of publication. We excluded studies of other designs because of the risk of bias in such studies. Inclusion of indirect observational evidence could weaken our network meta‐analysis, but this could also be viewed as a strength for assessing rare adverse events. It is well‐established that exclusion of non‐randomised studies increases the focus on potential benefits and reduces the focus on the risks of serious adverse events and those of any adverse events. However, we did not include these studies because of the findings of this review, i.e. there is considerable uncertainty about the benefits of the different treatments for ascites.
Types of participants
We included randomised clinical trials with adult trial participants (18 years old and above) undergoing treatment for ascites with decompensated liver cirrhosis. We excluded randomised clinical trials in which participants had previously undergone liver transplantation.
Types of interventions
We included any of the following treatments for comparison with one another, either alone or in combination.
Diuretics (different classes of diuretics based on their mechanism of action will be treated as separate interventions, for example, loop diuretics such as furosemide, torsemide; aldosterone antagonists such as spironolactone or potassium canrenoate);
Large volume paracentesis (removal of ascitic fluid) with different fluids to prevent circulatory dysfunction (for example, albumin, hydroxyethyl starch, etc.) ('paracentesis plus fluid replacement');
Spontaneous ultrafiltration and reinfusion (filtering the removed ascitic fluid and reinfusing the proteins);
Low‐flow ascites fluid pump (automatic diversion of ascitic fluid to the urinary bladder, from where it is excreted in urine);
Systemic vasoconstrictor (for example, terlipressin, midodrine);
TIPS procedure (decrease in portal hypertension);
Other forms of portosystemic shunt (decrease in portal hypertension);
No active intervention (no ascites‐related intervention or placebo).
We considered 'paracentesis plus fluid replacement' as the reference group. Each of the above categories was considered as a 'treatment node'; the only exception was the diuretics, where we considered different classes of diuretics as different treatment nodes. We considered variations in drugs within the same class of diuretics, doses of drugs, frequency and duration of interventions as the same treatment node. We treated each different combination of the categories as different treatment nodes.
We excluded trials that evaluated co‐interventions such as fluid restriction, restricted‐salt diet, or drugs such as vasopressin‐antagonists which are used as supplements to diuretics to overcome their adverse effects such as hyponatraemia. However, we included trials in which such co‐interventions were administered equally in both trial arms.
We evaluated the plausibility of the network meta‐analysis transitivity assumption by looking at the inclusion and exclusion criteria in the studies. The transitivity assumption means that participants included in the different trials with different treatments (in this case, ascites) can be considered to be a part of a multi‐arm randomised clinical trial and could potentially have been randomised to any of the interventions (Salanti 2012). In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions. This necessitates that information on potential effect‐modifiers such as grade of ascites (grade 2 ascites, grade 3 ascites, or refractory ascites) are the same across trials. We performed separate meta‐analysis for each of these different types of ascites, when possible, to ensure that the concerns about the transitivity assumption were minimised.
Types of outcome measures
Primary outcomes
All‐cause mortality at maximal follow‐up, i.e. the outcome measured at the last time when the participant was followed up (time‐to‐death).
Health‐related quality of life using a validated scale such as the EQ‐5D or 36‐Item Short Form Health Survey (SF‐36) at maximal follow‐up (EuroQol 2018; Optum 2018).
-
Serious adverse events (during or within six months after cessation of the intervention). We defined a serious adverse event as any event that would increase mortality; is life‐threatening; requires hospitalisation; results in persistent or significant disability; is a congenital anomaly/birth defect; or any important medical event that might jeopardise the person or require intervention to prevent it (ICH‐GCP 1997). However, none of the trial authors defined serious adverse events. Therefore, we used the list provided by trial authors for serious adverse events (as indicated in the protocol).
proportion of people with one or more serious adverse events;
number of serious adverse events per participant.
Secondary outcomes
-
Any adverse events (during or within six months after cessation of the intervention): We defined an adverse event as any untoward medical occurrence not necessarily having a causal relationship with the intervention but resulting in a dose reduction or discontinuation of the intervention (any time after commencement of the intervention) (ICH‐GCP 1997). However, none of the trial authors defined 'adverse event'. Therefore, we used the list provided by trial authors for adverse events (as indicated in the protocol).
proportion of people with one or more adverse events;
number of any adverse events per participant.
Time‐to‐liver transplantation (maximal follow‐up).
-
Time‐to‐resolution of ascites (however defined by authors at maximal follow‐up):
symptomatic recovery;
resolution as per ultrasound.
Number of decompensation episodes (maximal follow‐up).
Exploratory outcomes
Length of hospital stay (all hospital admissions until maximal follow‐up).
Number of days of lost work (in people who work) (maximal follow‐up).
Treatment costs (including the cost of the treatment and any resulting complications).
We chose the outcomes of this review based on their importance to patients in a survey related to research priorities for people with liver diseases (Gurusamy 2019), based on feedback of the patient and public representative of this project, and based on an online survey about the outcomes promoted through the Cochrane Consumer Network.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE Ovid, Embase Ovid, and Science Citation Index Expanded (Web of Science) from inception to date of search for randomised clinical trials comparing two or more of the above interventions without applying any language restrictions (Royle 2003). We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched clinicaltrials.gov, and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) which searches various trial registers, including ISRCTN and ClinicalTrials.gov. We also searched the European Medical Agency (EMA) (www.ema.europa.eu/ema/) and USA Food and Drug Administration (FDA) (www.fda.gov) registries for randomised clinical trials. We provided the search strategies along with the date of search in Appendix 1.
Searching other resources
We searched the references of the identified trials and the existing Cochrane Reviews on ascites in liver cirrhosis to identify additional trials for inclusion.
Data collection and analysis
Selection of studies
Two review authors (KG and AB, DR, LP, or MP) independently identified trials for inclusion by screening the titles and abstracts of articles identified by the literature search, and sought full‐text articles of any references identified by at least one review author for potential inclusion. We selected trials for inclusion based on the full‐text articles. We listed the references that we excluded and the reasons for their exclusion in the Characteristics of excluded studies table. We also listed any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up. We resolved any discrepancies through discussion. We illustrated the study selection process in a PRISMA diagram.
Data extraction and management
Two review authors (KG and AB, DR, LP, or MP) independently extracted the following data onto a pre‐piloted Microsoft Excel‐based data extraction form (after translation of non‐English articles).
-
Outcome data (for each outcome and for each intervention group, whenever applicable):
number of participants randomised;
number of participants included for the analysis;
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events and the mean follow‐up period for count outcomes, and number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
natural logarithm of the hazard ratio and its standard error if this was reported rather than the number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
definition of outcomes or scale used, if appropriate.
-
Data on potential effect modifiers:
participant characteristics such as age, sex, grade of ascites, whether refractory or recurrent ascites, the aetiology for cirrhosis, and the interval between diagnosis of ascites and treatment;
details of the intervention and control (including dose, frequency, and duration);
length of follow‐up;
information related to 'Risk of bias' assessment (please see below).
-
Other data:
year and language of publication;
country in which the participants were recruited;
year(s) in which the trial was conducted;
inclusion and exclusion criteria.
We collected outcomes at maximum follow‐up, but also at short‐term (up to three months) and medium‐term (from three months to five years) if this was available.
We attempted to contact the trial authors in the case of unclear or missing information. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess the risk of bias in the included trials. Specifically, we assessed sources of bias as defined below (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Savović 2018).
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random or only quasi‐randomised. We excluded such quasi‐randomised studies.
Allocation concealment
Low risk of bias: the allocation sequence was described as unknown to the investigators. Hence, the participants' allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit, an onsite locked computer, identical‐looking numbered sealed opaque envelopes, drug bottles or containers prepared by an independent pharmacist, or an independent investigator.
Unclear risk of bias: it was unclear if the allocation was hidden or if the block size was relatively small and fixed so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants. We excluded such quasi‐randomised studies.
Blinding of participants and personnel
Low risk of bias: blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken; or rarely no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; or rarely no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, adverse events, and time to resolution of ascites. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If we obtained the trial protocol from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, we did not consider those outcomes to be reliable.
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected, outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
Other bias
Low risk of bias: the trial appeared to be free of other components that could put it at risk of bias (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping).
Uncertain risk of bias: the trial may or may not have been free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. baseline differences, early stopping).
We considered a trial to be at low risk of bias if we assessed the trial to be at low risk of bias across all listed bias risk domains. Otherwise, we considered trials to be at high risk of bias. At the outcome level, we classified an outcome to be at low risk of bias if the allocation sequence generation, allocation concealment, blinding of participants, healthcare professionals, and outcome assessors, incomplete outcome data, and selective outcome reporting (at the outcome level) were at low risk of bias for objective and subjective outcomes (Savović 2018).
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we calculated the odds ratio (OR) with 95% credible interval (CrI) (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. health‐related quality of life reported on the same scale), we calculated the mean difference (MD) with 95% Crl. We planned to use standardised mean difference (SMD) values with 95% Crl for health‐related quality of life if included trials used different scales. If we calculated the SMD, we planned to convert it to a common scale, for example, EQ‐5D or SF‐36 (using the standard deviation of the common scale) for the purpose of interpretation. For count outcomes (e.g. number of serious adverse events or number of any adverse events), we calculated the rate ratio (RaR) with 95% Crl. This assumes that the events are independent of each other, i.e. if a person has had an event, they are not at an increased risk of further outcomes, which is the assumption in Poisson likelihood. For time‐to‐event data (e.g. all‐cause mortality at maximal follow‐up), we calculated hazard ratios (HRs) with 95% Crl.
Relative ranking
We estimated the ranking probabilities for all interventions of being at each possible rank for each intervention for each outcome when NMA (network meta‐analysis) was performed. We obtained the surface under the cumulative ranking curve (SUCRA) (cumulative probability), rankogram, and relative ranking table with CrI for the ranking probabilities for each outcome when NMA was performed (Salanti 2011; Chaimani 2013).
Unit of analysis issues
The unit of analysis was the participant undergoing treatment for ascites according to the intervention group to which the participant was randomly assigned.
Cluster‐randomised clinical trials
If we identified any cluster‐randomised clinical trials, we planned to include cluster‐randomised clinical trials, provided that the effect estimate adjusted for cluster correlation was available or if there was sufficient information available to calculate the design effect (which would allow us to take clustering into account). We also planned to assess additional domains of risk of bias for cluster‐randomised trials according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over randomised clinical trials
If we identified any cross‐over randomised clinical trials, we planned to include only the outcomes after the period of the first intervention because the included treatments could have residual effects.
Trials with multiple intervention groups
We collected data for all trial intervention groups that met the inclusion criteria. The codes that we used for analysis accounted for the correlation between the effect sizes from studies with more than two groups.
Dealing with missing data
We performed an intention‐to‐treat analysis, whenever possible (Newell 1992); otherwise, we used the data available to us. When intention‐to‐treat analysis was not used and the data were not missing at random (for example, treatment was withdrawn due to adverse events or duration of treatment was shortened because of lack of response and such participants were excluded from analysis), this could lead to biased results; therefore, we conducted best‐worst case scenario analysis (assuming a good outcome in the intervention group and bad outcome in the control group) and worst‐best case scenario analysis (assuming a bad outcome in the intervention group and good outcome in the control group) as sensitivity analyses, whenever possible, for binary and time‐to‐event outcomes, where binomial likelihood was used.
For continuous outcomes, we imputed the standard deviation from P values, according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we used the median for meta‐analysis when the mean was not available; otherwise, we planned to simply provide a median and interquartile range of the difference in medians. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation can decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We also planned to assess the presence of clinical heterogeneity by comparing effect estimates (please see Subgroup analysis and investigation of heterogeneity) in trial reports of different drug dosages, different grades of ascites (grade 2 or grade 3), refractory or recurrent ascites, different aetiologies for cirrhosis (for example, alcohol‐related liver disease, viral liver diseases, autoimmune liver disease), and based on the co‐interventions (for example, both groups receive prophylactic antibiotics to decrease the risk of subacute bacterial peritonitis). Different study designs and risk of bias can contribute to methodological heterogeneity.
We assessed statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study standard deviation (tau2 and comparing this with values reported in a study of the distribution of between‐study heterogeneity estimates) (Turner 2012), and by calculating the NMA‐specific I2 statistic (Jackson 2014) using Stata/SE 15.1. When possible, we explored substantial clinical, methodological, or statistical heterogeneity and addressed the heterogeneity in subgroup analysis (see 'Subgroup analysis and investigation of heterogeneity').
Assessment of transitivity across treatment comparisons
We assessed the transitivity assumption by comparing the distribution of the potential effect modifiers (clinical: grade of ascites (grade 2 versus grade 3) and whether refractory or recurrent ascites; and methodological: risk of bias, year of randomisation, duration of follow‐up) across the different pairwise comparisons.
Assessment of reporting biases
For the network meta‐analysis, we planned to perform a comparison‐adjusted funnel plot. However, to interpret a comparison‐adjusted funnel plot, it is necessary to rank the studies in a meaningful way as asymmetry may be due to small sample sizes in newer studies (comparing newer treatments with older treatments) or higher risk of bias in older studies (Chaimani 2012). As there was no meaningful way in which to rank these studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time), we judged the reporting bias by the completeness of the search (Chaimani 2012). We also considered lack of reporting of outcomes as a form of reporting bias.
Data synthesis
Methods for indirect and mixed comparisons
We conducted network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. When two or more interventions were combined, we considered this as a separate intervention ('node'). Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We obtained a network plot to ensure that the trials were connected by interventions using Stata/SE 15.1 (Chaimani 2013). We excluded any trials that were not connected to the network from the network meta‐analysis, and we reported only the direct pairwise meta‐analysis for such comparisons. We summarised the population and methodological characteristics of the trials included in the network meta‐analysis in a table based on pairwise comparisons. We conducted a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3, according to guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2016). We modelled the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and the reference group ('basic parameters') using appropriate likelihood functions and links (Lu 2006). We used binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link (a semiparametric model which excludes censored individuals from the denominator of ‘at risk’ individuals at the point when they are censored) for time‐to‐event outcomes, and normal likelihood and identity link for continuous outcomes. We used 'paracentesis plus fluid replacement' as the reference group across the networks, as this was the commonest intervention compared in the trials. We performed a fixed‐effect model and random‐effects model for the network meta‐analysis. We reported both models for comparison with the reference group in a forest plot when the results were different between the models. For each pairwise comparison in a table, we reported the fixed‐effect model if the two models reported similar results; otherwise, we reported the more conservative model, i.e. usually using the random‐effects model in the absence of ‘small‐study’ bias.
We used a hierarchical Bayesian model using three different sets of initial values to start the simulation‐based parameter estimation to assist with the assessment of convergence, employing codes provided by NICE DSU (Dias 2016). We used a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors) centred at no effect. For the random‐effects model, we used a prior distributed uniformly (limits: 0 to 5) for the between‐trial standard deviation parameter and assumed this variability would be the same across treatment comparisons (Dias 2016). We used a 'burn‐in' of 30,000 simulations, checked for convergence (of effect estimates and between‐study heterogeneity) visually (i.e. whether the values in different chains mixed very well by visualisation), and ran the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we increased the number of simulations for the 'burn‐in' and used the 'thin' and 'over relax' functions to decrease the autocorrelation. If we still did not obtain convergence, we used alternate initial values and priors employing methods suggested by Van Valkenhoef 2012. We estimated the probability that each intervention ranked at each of the possible positions using the NICE DSU codes (Dias 2016).
Assessment of inconsistency
We assessed inconsistency (statistical evidence of the violation of the transitivity assumption) by fitting both an inconsistency model and a consistency model. We used inconsistency models employed in the NICE DSU manual, as we used a common between‐study standard deviation (Dias 2014). In addition, we used design‐by‐treatment full interaction model and inconsistency factor (IF) plots to assess inconsistency (Higgins 2012; Chaimani 2013), when applicable. We used Stata/SE 15.1 to create IF plots. In the presence of inconsistency, we assessed whether the inconsistency was due to clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the Subgroup analysis and investigation of heterogeneity or limited network meta‐analysis to a more compatible subset of trials, when possible.
Direct comparison
We performed the direct comparisons using the same codes and the same technical details.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups and investigated heterogeneity and inconsistency using meta‐regression with the help of the codes provided in NICE DSU guidance (Dias 2012a), if we included a sufficient number of trials (when there were at least two trials in at least two of the subgroups). We planned to use the following trial‐level covariates for meta‐regression.
Trials at low risk of bias (risk of bias in all domains were low) compared to trials at high risk of bias (risk of bias was unclear or high in at least one of the domains).
The grade of ascites (grade 2 or grade 3 or refractory/recurrent ascites).
The aetiology for cirrhosis (for example, alcohol‐related liver disease, viral liver diseases, autoimmune liver disease).
The interval between the diagnosis of ascites and the start of treatment.
The co‐interventions (for example, both groups received prophylactic antibiotics to decrease the risk of subacute bacterial peritonitis).
The period of follow‐up (short‐term: up to three months, medium‐term: more than three months to five years, long‐term: more than five years).
The definition used by authors for serious adverse events and any adverse event (ICH‐GCP 1997 compared to other definitions).
We calculated a single common interaction term which assumes that each relative treatment effect compared to a common comparator treatment (i.e. paracentesis plus fluid replacement) is impacted in the same way by the covariate in question, when applicable (Dias 2012a). If the 95% Crl of the interaction term did not overlap zero, we considered this statistically significant heterogeneity or inconsistency (depending upon the factor being used as covariate).
Sensitivity analysis
If there were post‐randomisation dropouts, we reanalysed the results using the best‐worst case scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible. We also performed a sensitivity analysis excluding the trials in which mean or standard deviation, or both, were imputed, and we used the median standard deviation in the trials to impute missing standard deviations.
Presentation of results
We followed the PRISMA‐NMA statement while reporting (Hutton 2015). We presented the effect estimates with 95% CrI for each pairwise comparison calculated from the direct comparisons and network meta‐analysis. We originally planned to present the cumulative probability of the treatment ranks (i.e. the probability that the intervention was within the top two, the probability that the intervention was within the top three, etc) but we did not present these because of the sparse data which can lead to misinterpretation of results due to large uncertainty in the rankings (the CrI was 0 to 1 for all the ranks) in graphs (SUCRA) (Salanti 2011). We plotted the probability that each intervention was best, second best, third best, etc. for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b), but we did not present these because of the sparse data which can lead to misinterpretation of results due to large uncertainty in the rankings (the CrI was 0 to 1 for all the ranks). We uploaded all the raw data and the codes used for analysis in the European Organization for Nuclear Research open source database (Zenodo): the link is: http://doi.org/10.5281/zenodo.3531818.
Grading of evidence
We presented 'Summary of findings' tables for all the primary and secondary outcomes (see Primary outcomes; Secondary outcomes). We followed the approach suggested by Yepes‐Nunez and colleagues (Yepes‐Nunez 2019). First, we calculated the direct and indirect effect estimates (when possible) and 95% Crl using the node‐splitting approach (Dias 2010), that is, calculating the direct estimate for each comparison by including only trials in which there was direct comparison of interventions and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of interventions (and ensuring a connected network). Next, we rated the quality of direct and indirect effect estimates using GRADE methodology which takes into account the risk of bias, inconsistency (heterogeneity), directness of evidence (including incoherence, the term used in GRADE methodology for inconsistency in network meta‐analysis), imprecision, and publication bias (Guyatt 2011). We then presented the relative and absolute estimates of the meta‐analysis with the best certainty of evidence (Yepes‐Nunez 2019). We also presented the 'Summary of findings' tables in a second format presenting all the outcomes for selected interventions (Yepes‐Nunez 2019): we selected the four interventions (aldosterone antagonists plus loop diuretics, paracentesis plus systemic vasoconstrictors, aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement, and transjugular intrahepatic portosystemic shunt) which were compared in the most trials (Table 3).
Recommendations for future research
We provided recommendations for future research in the population, intervention, control, outcomes, period of follow‐up, and study design, based on the uncertainties that we identified from the existing research.
Results
Description of studies
Results of the search
We identified 4877 references through electronic searches of CENTRAL (n = 1095), MEDLINE Ovid (n = 2093), Embase Ovid (n = 875), Science Citation Index expanded (n = 779), ClinicalTrials.gov (n = 35), and WHO Trials register (n = 0). After removing duplicate references, there were 3890 references. We excluded 3713 clearly irrelevant references through reading titles and abstracts. We identified no additional references by reference searching and by searching the EMA and FDA. We retrieved a total of 177‐full text references for further assessment in detail. We excluded 97 references (78 studies) for the reasons stated in the Characteristics of excluded studies. There were six ongoing trials (seven references) without interim data (Characteristics of ongoing studies). Thus, we included a total of 49 trials described in 73 references (Characteristics of included studies). The reference flow is shown in Figure 2.
2.
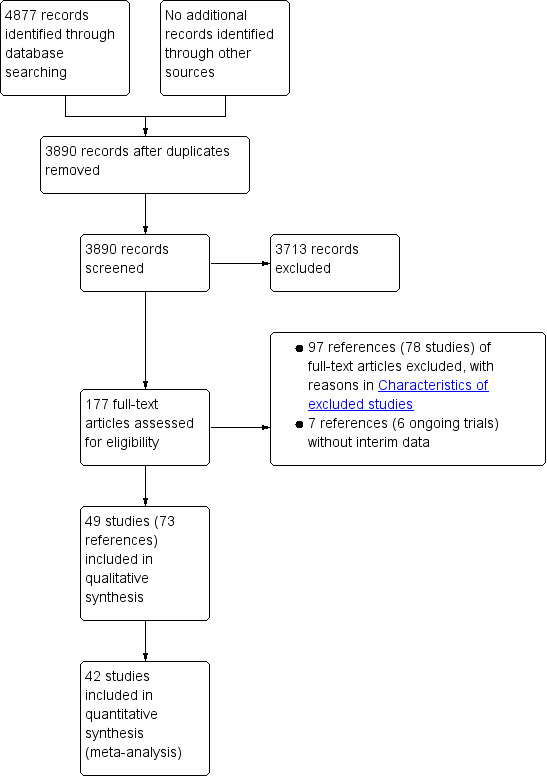
Study flow diagram.
Included studies
Forty‐nine trials were included (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Mchutchison 1989; Stanley 1989b; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Chang 1997; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Lata 2007; Appenrodt 2008; Singh 2008; Licata 2009; Narahara 2011; Raza 2011; Al Sebaey 2012; Amin 2012; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). A total of 3521 participants were randomised to different interventions. The number of participants within each trial ranged from 20 to 440. A total of 2870 participants from 42 trials were included in one or more outcomes (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Lata 2007; Singh 2008; Licata 2009; Narahara 2011; Raza 2011; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). The mean or median age in the trials ranged from 43 to 64 years in the trials that reported this information (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Lebrec 1996; Chang 1997; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Lata 2007; Appenrodt 2008; Singh 2008; Licata 2009; Narahara 2011; Raza 2011; Al Sebaey 2012; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). The proportion of females ranged from 0.0% to 47.6% in the trials that reported this information (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Lebrec 1996; Chang 1997; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Lata 2007; Appenrodt 2008; Singh 2008; Licata 2009; Narahara 2011; Raza 2011; Al Sebaey 2012; Bari 2012; Singh 2012a; Singh 2013; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). The follow‐up period in the trials ranged from 0.1 to 84 months in the trials that reported this information. Twenty‐eight trials had short‐term follow‐up (Gregory 1977; Fogel 1981; Descos 1983; Mchutchison 1989; Strauss 1991; Acharya 1992; Bruno 1992; Hagege 1992; Ljubici 1994; Schaub 1995; Chang 1997; Fernandez‐Esparrach 1997; Mehta 1998; Moreau 2002; Singh 2006a; Singh 2006b; Lata 2007; Appenrodt 2008; Singh 2008; Licata 2009; Raza 2011; Al Sebaey 2012; Amin 2012; Singh 2013; Ali 2014; Hamdy 2014; Tuttolomondo 2016; Rai 2017); 19 trials had medium‐term follow‐up (Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Sola 1994; Ginès 1995; Lebrec 1996; Graziotto 1997; Gentilini 1999a; Rossle 2000; Ginès 2002; Sanyal 2003; Salerno 2004; Narahara 2011; Bari 2012; Singh 2012a; Bureau 2017c; Caraceni 2018; Sola 2018); only two trials had long‐term follow‐up (Stanley 1989b; Romanelli 2006).
Twenty‐five trials reported the proportion of participants who had ascites grade 2: in 23 trials, none of the participants had ascites grade 2; these trials included only participants with grade 3 (Descos 1983; Gines 1987; Salerno 1987; Chesta 1990; Acharya 1992; Bruno 1992; Ljubici 1994; Sola 1994; Chang 1997; Fernandez‐Esparrach 1997; Graziotto 1997; Rossle 2000; Moreau 2002; Singh 2006a; Singh 2006b; Lata 2007; Appenrodt 2008; Singh 2008; Al Sebaey 2012; Amin 2012; Ali 2014; Hamdy 2014; Bureau 2017c); in the remaining two trials, the proportion of participants who had ascites grade 2 ranged from 65.0% to 83.1% (Romanelli 2006; Caraceni 2018). Twenty trials reported the proportion of participants who had refractory or recurrent ascites: in 19 trials, all the participants had refractory or recurrent ascites (Ginès 1991; Strauss 1991; Bruno 1992; Ginès 1995; Lebrec 1996; Rossle 2000; Ginès 2002; Sanyal 2003; Salerno 2004; Licata 2009; Narahara 2011; Raza 2011; Bari 2012; Singh 2012a; Singh 2013; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017); in the remaining trial, the proportion of participants who had refractory or recurrent ascites was 85.0% (Acharya 1992). Forty‐one trials reported the proportion of participants who had alcohol‐related cirrhosis: in two trials, none of the participants had alcohol‐related cirrhosis (Chang 1997; Raza 2011); in four trials, all the participants had alcohol‐related cirrhosis (Gregory 1977; Stanley 1989b; Ljubici 1994; Schaub 1995); in the remaining 35 trials, the proportion of participants who had alcohol‐related cirrhosis ranged from 2.0% to 90.6% (Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Hagege 1992; Sola 1994; Ginès 1995; Lebrec 1996; Fernandez‐Esparrach 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Lata 2007; Appenrodt 2008; Singh 2008; Licata 2009; Narahara 2011; Bari 2012; Singh 2012a; Singh 2013; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). Thirty‐three trials reported the proportion of participants who had viral‐related cirrhosis: in four trials, none of the participants had viral‐related cirrhosis (Gregory 1977; Stanley 1989b; Chesta 1990; Ljubici 1994); in one trial, all the participants had viral‐related cirrhosis (Chang 1997); in the remaining 28 trials, the proportion of participants who had viral‐related cirrhosis ranged from 5.6% to 95.0% (Gines 1987; Salerno 1987; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Ginès 1995; Schaub 1995; Lebrec 1996; Gentilini 1999a; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Appenrodt 2008; Singh 2008; Licata 2009; Narahara 2011; Raza 2011; Bari 2012; Singh 2012a; Singh 2013; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). Twenty‐two trials reported the proportion of participants who had autoimmune disease‐related cirrhosis: in 17 trials, none of the participants had autoimmune disease‐related cirrhosis (Gregory 1977; Salerno 1987; Ginès 1991; Ljubici 1994; Ginès 1995; Lebrec 1996; Chang 1997; Gentilini 1999a; Moreau 2002; Romanelli 2006; Singh 2006b; Appenrodt 2008; Licata 2009; Raza 2011; Singh 2013; Tuttolomondo 2016; Rai 2017); in the remaining five trials, the proportion of participants who had autoimmune disease‐related cirrhosis ranged from 2.5% to 12.0% (Chesta 1990; Singh 2006a; Singh 2008; Bari 2012; Singh 2012a). Only two trials reported whether the participants received antibiotic prophylaxis for spontaneous bacterial peritonitis (Ginès 2002; Caraceni 2018). In one trial, all participants received antibiotic prophylaxis (Ginès 2002); in the other trial, 19.3% of participants received antibiotic prophylaxis, but the reason for only a proportion of participants receiving antibiotic prophylaxis was not stated (Caraceni 2018). In 38 trials, patients with active other decompensation events such as active gastrointestinal bleeding, hepatorenal syndrome, or grade III or grade IV hepatic encephalopathy were excluded (Descos 1983; Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Lebrec 1996; Chang 1997; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Singh 2008; Narahara 2011; Raza 2011; Al Sebaey 2012; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Hamdy 2014; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). In the remaining 11 trials, it was not clear whether patients with active other decompensation events were included (Gregory 1977; Fogel 1981; Mchutchison 1989; Stanley 1989b; Schaub 1995; Rossle 2000; Lata 2007; Appenrodt 2008; Licata 2009; Amin 2012; Tuttolomondo 2016). The interval between diagnosis and treatment was not reported in any of the trials.
A total of 21 interventions were compared in these trials. Forty‐two trials (2870 participants) reported one or more outcomes for this review (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Lata 2007; Singh 2008; Licata 2009; Narahara 2011; Raza 2011; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). The important characteristics, potential effect modifiers, and follow‐up in each trial is reported in Table 4. Overall, there does not seem to be any systematic differences between the comparisons.
2. Characteristics of included studies and potential effect modifiers.
| This table is too wide to be displayed in RevMan. This table can be found at: https://doi.org/10.5281/zenodo.3604600. |
Funding: the source of funding for four trials was industries who would benefit from the results of the study (Stanley 1989b; Fernandez‐Esparrach 1997; Caraceni 2018; Sola 2018); 24 trials received no additional funding or were funded by neutral organisations with no vested interests in the results of the study (Descos 1983; Gines 1987; Ginès 1991; Sola 1994; Ginès 1995; Chang 1997; Gentilini 1999a; Ginès 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Appenrodt 2008; Singh 2008; Licata 2009; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017); the source of funding for the remaining 21 trials was unclear (Gregory 1977; Fogel 1981; Salerno 1987; Mchutchison 1989; Chesta 1990; Strauss 1991; Acharya 1992; Bruno 1992; Hagege 1992; Ljubici 1994; Schaub 1995; Lebrec 1996; Graziotto 1997; Mehta 1998; Rossle 2000; Moreau 2002; Lata 2007; Narahara 2011; Raza 2011; Al Sebaey 2012; Amin 2012).
Excluded studies
The reasons for exclusion is provided in the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias is summarised in Figure 3, Figure 4, and in Table 5. All the trials were at unclear or high risk of bias in at least one of the domains and were considered to be at high risk of bias overall.
3.
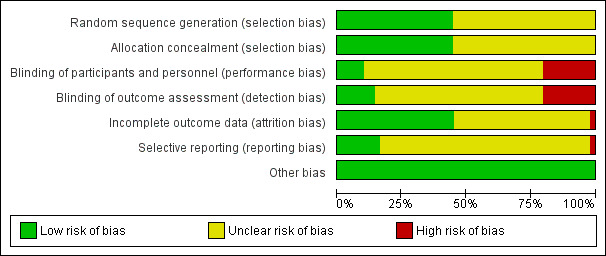
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
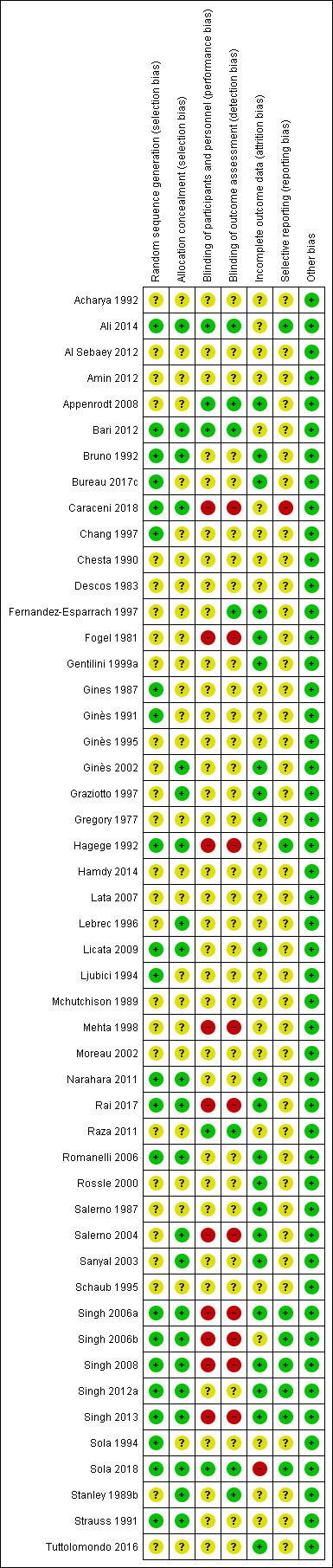
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3. Risk of bias.
| Study name | Sequence generation | Allocation concealment | Blinding of patients and healthcare providers | Blinding of outcome assessors | Missing outcome bias | Selective outcome reporting | Overall risk of bias |
| Chang 1997 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Chesta 1990 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Gines 1987 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Hagege 1992 | Low | Low | High | High | Unclear | Low | High |
| Salerno 1987 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Schaub 1995 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Al Sebaey 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Appenrodt 2008 | Unclear | Unclear | Low | Low | Low | Unclear | High |
| Bari 2012 | Low | Low | Low | Low | Unclear | Unclear | High |
| Hamdy 2014 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Lata 2007 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Moreau 2002 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Singh 2006a | Low | Low | High | High | Low | Low | High |
| Singh 2006b | Low | Low | High | High | Unclear | Low | High |
| Singh 2008 | Low | Low | High | High | Low | Low | High |
| Ljubici 1994 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Sola 1994 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Strauss 1991 | Low | Low | Unclear | Unclear | Unclear | Unclear | High |
| Bureau 2017c | Low | Unclear | Unclear | Unclear | Low | Unclear | High |
| Ginès 2002 | Unclear | Low | Unclear | Unclear | Low | Unclear | High |
| Lebrec 1996 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | High |
| Narahara 2011 | Low | Low | Unclear | Unclear | Low | Unclear | High |
| Rossle 2000 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Salerno 2004 | Unclear | Low | High | High | Low | Unclear | High |
| Sanyal 2003 | Unclear | Low | Unclear | Unclear | Low | Unclear | High |
| Gregory 1977 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Tuttolomondo 2016 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Fogel 1981 | Unclear | Unclear | High | High | Low | Unclear | High |
| Licata 2009 | Low | Low | Unclear | Unclear | Low | Unclear | High |
| Bruno 1992 | Low | Low | Unclear | Unclear | Low | Unclear | High |
| Graziotto 1997 | Unclear | Low | Unclear | Unclear | Low | Unclear | High |
| Mehta 1998 | Unclear | Unclear | High | High | Unclear | Unclear | High |
| Gentilini 1999a | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Romanelli 2006 | Low | Low | Unclear | Unclear | Low | Unclear | High |
| Caraceni 2018 | Low | Low | High | High | Unclear | High | High |
| Ginès 1991 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Ginès 1995 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Singh 2012a | Low | Low | Unclear | Unclear | Low | Low | High |
| Singh 2013 | Low | Low | High | High | Low | Low | High |
| Fernandez‐Esparrach 1997 | Unclear | Unclear | Unclear | Low | Low | Unclear | High |
| Acharya 1992 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Ali 2014 | Low | Low | Low | Low | Unclear | Low | High |
| Amin 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Descos 1983 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Rai 2017 | Low | Low | High | High | Low | Unclear | High |
| Singh 2013 | Low | Low | High | High | Low | Low | High |
| Singh 2013 | Low | Low | High | High | Low | Low | High |
| Singh 2013 | Low | Low | High | High | Low | Low | High |
| Singh 2013 | Low | Low | High | High | Low | Low | High |
| Singh 2013 | Low | Low | High | High | Low | Low | High |
| Raza 2011 | Unclear | Unclear | Low | Low | Unclear | Unclear | High |
| Stanley 1989b | Unclear | Low | Unclear | Unclear | Low | Unclear | High |
| Sola 2018 | Low | Low | Low | Low | High | Low | High |
| Mchutchison 1989 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
Allocation
With regards to sequence generation, twenty‐two trials were at low risk of bias (Gines 1987; Ginès 1991; Strauss 1991; Bruno 1992; Hagege 1992; Ljubici 1994; Sola 1994; Chang 1997; Romanelli 2006; Singh 2006a; Singh 2006b; Singh 2008; Licata 2009; Narahara 2011; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018); the remaining 27 trials, which did not provide sufficient information, were at unclear risk of bias (Gregory 1977; Fogel 1981; Descos 1983; Salerno 1987; Mchutchison 1989; Stanley 1989b; Chesta 1990; Acharya 1992; Ginès 1995; Schaub 1995; Lebrec 1996; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Lata 2007; Appenrodt 2008; Raza 2011; Al Sebaey 2012; Amin 2012; Hamdy 2014; Tuttolomondo 2016).
With regards to allocation concealment, twenty‐two trials were at low risk of bias (Stanley 1989b; Strauss 1991; Bruno 1992; Hagege 1992; Lebrec 1996; Graziotto 1997; Ginès 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Singh 2008; Licata 2009; Narahara 2011; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Rai 2017; Caraceni 2018; Sola 2018); the remaining 27 trials, which did not provide sufficient information, were at unclear risk of bias (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Mchutchison 1989; Chesta 1990; Ginès 1991; Acharya 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Chang 1997; Fernandez‐Esparrach 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Moreau 2002; Lata 2007; Appenrodt 2008; Raza 2011; Al Sebaey 2012; Amin 2012; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c).
Blinding
With regards to the blinding of patients and healthcare providers, five trials were at low risk of bias (Appenrodt 2008; Raza 2011; Bari 2012; Ali 2014; Sola 2018); 34 trials, which did not provide sufficient information, were at unclear risk of bias (Gregory 1977; Descos 1983; Gines 1987; Salerno 1987; Mchutchison 1989; Stanley 1989b; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Chang 1997; Fernandez‐Esparrach 1997; Graziotto 1997; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Romanelli 2006; Lata 2007; Licata 2009; Narahara 2011; Al Sebaey 2012; Amin 2012; Singh 2012a; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c); the remaining 10 trials were at high risk of bias (Fogel 1981; Hagege 1992; Mehta 1998; Salerno 2004; Singh 2006a; Singh 2006b; Singh 2008; Singh 2013; Rai 2017; Caraceni 2018).
With regards to blinding of outcome assessors, six trials were at low risk of bias (Fernandez‐Esparrach 1997; Appenrodt 2008; Raza 2011; Bari 2012; Ali 2014; Sola 2018); 33 trials, which did not provide sufficient information, were at unclear risk of bias (Gregory 1977; Descos 1983; Gines 1987; Salerno 1987; Mchutchison 1989; Stanley 1989b; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Chang 1997; Graziotto 1997; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Romanelli 2006; Lata 2007; Licata 2009; Narahara 2011; Al Sebaey 2012; Amin 2012; Singh 2012a; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c); the remaining 10 trials were at high risk of bias (Fogel 1981; Hagege 1992; Mehta 1998; Salerno 2004; Singh 2006a; Singh 2006b; Singh 2008; Singh 2013; Rai 2017; Caraceni 2018).
Incomplete outcome data
With regards to incomplete data, twenty‐three trials were at low risk of bias (Gregory 1977; Fogel 1981; Salerno 1987; Stanley 1989b; Bruno 1992; Fernandez‐Esparrach 1997; Graziotto 1997; Gentilini 1999a; Rossle 2000; Ginès 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Appenrodt 2008; Singh 2008; Licata 2009; Narahara 2011; Singh 2012a; Singh 2013; Tuttolomondo 2016; Bureau 2017c; Rai 2017); 25 trials were at unclear risk of bias (Descos 1983; Gines 1987; Mchutchison 1989; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Chang 1997; Mehta 1998; Moreau 2002; Singh 2006b; Lata 2007; Raza 2011; Al Sebaey 2012; Amin 2012; Bari 2012; Ali 2014; Hamdy 2014; Caraceni 2018), because it was not clear whether there were post‐randomisation dropouts or whether the post‐randomisation dropouts were related to the outcomes (if there were post‐randomisation dropouts); the remaining trial was at high risk of bias (Sola 2018), as the post‐randomisation dropouts were probably related to the intervention and the outcomes.
Selective reporting
Eight trials were at low risk of selective outcome reporting bias (Hagege 1992; Singh 2006a; Singh 2006b; Singh 2008; Singh 2012a; Singh 2013; Ali 2014; Sola 2018), as the important clinical outcomes expected to be reported in such trials were reported; 40 trials were at unclear risk of selective outcome reporting bias (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Mchutchison 1989; Stanley 1989b; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Bruno 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Chang 1997; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Lata 2007; Appenrodt 2008; Licata 2009; Narahara 2011; Raza 2011; Al Sebaey 2012; Amin 2012; Bari 2012; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017), as a protocol published prior to recruitment was not available; the remaining trial was at high risk of selective outcome reporting bias (Caraceni 2018), as adverse events were clearly collected, but not reported adequately.
Other potential sources of bias
No other bias was noted in the trials.
Effects of interventions
for the main comparison.
| Treatment for ascites in people with decompensated liver cirrhosis | ||||||||
| Patient or population: people with liver cirrhosis and ascites Settings: secondary or tertiary care Intervention: various interventions Comparison: paracentesis plus fluid replacement Follow‐up period: 0.1 to 84 months Network geometry plots:Figure 1 | ||||||||
| Outcomes | Aldosterone antagonists plus loop diuretics | Paracentesis plus systemic vasoconstrictors | Aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement | Transjugular intrahepatic portosystemic shunt | ||||
| Mortality at maximal follow‐up | ||||||||
| Paracentesis plus fluid replacement 368 per 1000 (36.8%) | HR 1.05 (0.70 to 1.69) Network estimate | 18 more per 1000 (109 fewer to 253 more) | HR 1.64 (0.46 to 6.32) Network estimate | 235 more per 1000 (200 fewer to 632 more) | HR 1.24 (0.62 to 2.59) Network estimate | 88 more per 1000 (141 fewer to 587 more) | HR 0.84 (0.60 to 1.18) Network estimate | 59 fewer per 1000 (148 fewer to 65 more) |
| Very low1,2,3 | Very low1,2,3 | Very low1,2,3 | Very low1,2,3 | |||||
| Based on 211 participants (4 RCTs) | Based on 165 participants (5 RCTs) | No direct RCT | Based on 452 participants (7 RCTs) | |||||
| Serious adverse events (number of events) | ||||||||
| Paracentesis plus fluid replacement 0 per 1000 (0 per 100 participants) | Rate ratio 1.30 (0.27 to 6.99) Direct estimate | Not estimable | ‐ | ‐ |
Not estimable (10 serious adverse events in 35 participants) |
|||
| Very low1,2,3 | Very low1,2,3 | |||||||
| Based on 41 participants (1 RCT) | Based on 70 participants (1 RCT) | |||||||
| Any adverse events (number of people) | ||||||||
| Paracentesis plus fluid replacement 100 per 1000 (10%) | OR 3.54 (0.43 to 27.41) Network estimate | 182 more per 1000 (54 fewer to 653 more) | OR 1.63 (0.30 to 11.66) Network estimate | 53 more per 1000 (68 fewer to 464 more) | ‐ | ‐ | ||
| Very low1,2,3 | Very low1,2,3 | |||||||
| Based on 84 participants (2 RCTs) | Based on 145 participants (4 RCTs) | |||||||
| Any adverse events (number of events) | ||||||||
| Paracentesis plus fluid replacement 118 per 1000 (11.8 per 100 participants) | Rate ratio 4.12 (0.87 to 34.02) Network estimate | 367 more per 1000 (15 fewer to 3885 more) | Rate ratio 1.37 (0.36 to 5.82) Network estimate | 43 more per 1000 (76 fewer to 567 more) | ‐ | ‐ | ||
| Very low1,2,3 | Very low1,2,3 | |||||||
| Based on 31 participants (1 RCT) | Based on 25 participants (1 RCT) | |||||||
| Liver transplantation at maximal follow‐up | ||||||||
| Paracentesis plus fluid replacement 121 per 1000 (12.1%) | ‐ | HR 1.08 (0.11 to 10.35) Network estimate | 10 more per 1000 (108 fewer to 879 more) | ‐ | HR 0.87 (0.52 to 1.44) Network estimate | 15 fewer per 1000 (58 fewer to 54 more) | ||
| Very low1,2,3 | Very low1,2,3 | |||||||
| Based on 145 participants (4 RCTs) | Based on 427 participants (6 RCT) | |||||||
| Resolution of ascites at maximal follow‐up (by ultrasound) | ||||||||
| Paracentesis plus fluid replacement 158 per 1000 (15.8%) | HR 1.10 (0.12 to 10.74) Network estimate | 16 more per 1000 (140 fewer to 842 more) | ‐ | HR 1.17 (0.01 to 98.79) Network estimate | 27 more per 1000 (156 fewer to 842 more) | HR 9.44 (1.93 to 62.68) Network estimate | 842 more per 1000 (147 more to 842 more) | |
| Very low1,2,3,4 | Very low1,2,3,4 | Very low1,2,4 | ||||||
| Based on 125 participants (3 RCTs) | No direct RCT | Based on 392 participants (6 RCTs) | ||||||
| Other features of decompensation at maximal follow‐up | ||||||||
| Paracentesis plus fluid replacement 439 per 1000 (43.9 per 100 participants) | Rate ratio 2.04 (1.37 to 3.10) Network estimate | 458 more per 1000 (164 more to 922 more) | Rate ratio 0.76 (0.14 to 3.61) Network estimate | 107 fewer per 1000 (377 fewer to 1144 more) | Rate ratio 1.04 (0.56 to 1.93) Network estimate | 16 more per 1000 (195 fewer to 409 more) | Rate ratio 1.17 (0.92 to 1.49) Network estimate | 76 more per 1000 (33 fewer to 217 more) |
| Very low1,2,4 | Very low1,2,3,4 | Very low1,2,3,4 | Very low1,2,3,4 | |||||
| Based on 242 participants (4 RCTs) | Based on 114 participants (3 RCTs) | No direct RCT | Based on 452 participants (7 RCTs) | |||||
| *Ranking was not provided because of the considerable uncertainty in the ranking. CrI: Credible interval; OR: Odds Ratio; HR: Hazard Ratio. | ||||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||||
1Downgraded one level for risk of bias because the trial(s) included in the analysis was/were at high risk of bias 2Downgraded one level for imprecision because the sample size was small 3Downgraded one level for imprecision because the credible intervals were wide (included clinical benefit and harms) 4Downgraded one level for inconsistency because there was evidence of statistical heterogeneity
2.
| Treatment for ascites in people with decompensated liver cirrhosis | |||||
| Patient or population: people with liver cirrhosis and ascites Settings: secondary or tertiary care Intervention: various interventions Comparison: paracentesis plus fluid replacement Follow‐up period: 0.1 to 84 months Network geometry plots:Figure 1 | |||||
| Interventions | Relative effect (95% CrI) | Anticipated absolute effect* (95% CrI) | Quality of evidence | ||
| Paracentesis plus fluid replacement | Various interventions | Difference | |||
| Mortality at maximal follow‐up Total studies: 32 Total participants: 2448 | |||||
| Paracentesis plus fluid replacement | Reference | ||||
| Aldosterone antagonists plus loop diuretics (4 RCTs; 211 participants) | HR 1.05 (0.70 to 1.69) Network estimate | 368 per 1000 | 387 per 1000 (260 to 621) | 18 more per 1000 (109 fewer to 253 more) | Very low1,2,3 |
| Paracentesis plus systemic vasoconstrictors (5 RCTs; 165 participants) | HR 1.64 (0.46 to 6.32) Network estimate | 368 per 1000 | 604 per 1000 (168 to 1000) | 235 more per 1000 (200 fewer to 632 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement (No direct RCT) | HR 1.24 (0.62 to 2.59) Network estimate | 368 per 1000 | 457 per 1000 (227 to 955) | 88 more per 1000 (141 fewer to 587 more) | Very low1,2,3 |
| Transjugular intrahepatic portosystemic shunt (7 RCTs; 452 participants) | HR 0.84 (0.60 to 1.18) Network estimate | 368 per 1000 | 309 per 1000 (221 to 433) | 59 fewer per 1000 (148 fewer to 65 more) | Very low1,2,3 |
| No active treatment (No direct RCT) | HR 1.66 (0.46 to 6.99) Network estimate | 368 per 1000 | 611 per 1000 (170 to 1000) | 243 more per 1000 (199 fewer to 632 more) | Very low1,2,3 |
| Loop diuretics (No direct RCT) | HR 0.71 (0.23 to 2.16) Network estimate | 368 per 1000 | 263 per 1000 (84 to 797) | 105 fewer per 1000 (284 fewer to 429 more) | Very low1,2,3 |
| Paracentesis plus reinfusion (1 RCT; 24 participants) | HR 0.77 (0.23 to 2.68) Network estimate | 368 per 1000 | 284 per 1000 (84 to 987) | 84 fewer per 1000 (285 fewer to 619 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus albumin (No direct RCT) | HR 1.06 (0.57 to 2.16) Network estimate | 368 per 1000 | 392 per 1000 (209 to 795) | 23 more per 1000 (159 fewer to 427 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus peritoneovenous shunt (No direct RCT) | HR 0.97 (0.40 to 2.43) Network estimate | 368 per 1000 | 358 per 1000 (148 to 894) | 10 fewer per 1000 (221 fewer to 526 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (No direct RCT) | HR 0.42 (0.15 to 1.22) Network estimate | 368 per 1000 | 153 per 1000 (55 to 450) | 215 fewer per 1000 (313 fewer to 82 more) | Very low1,2,3 |
| Aldosterone antagonists (No direct RCT) | HR 1.92 (0.24 to 20.64) Network estimate | 368 per 1000 | 708 per 1000 (90 to 1000) | 340 more per 1000 (278 fewer to 632 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus paracentesis plus fluid replacement (No direct RCT) | HR 1.11 (0.02 to 39.77) Network estimate | 368 per 1000 | 408 per 1000 (9 to 1000) | 40 more per 1000 (360 fewer to 632 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (No direct RCT) | HR 0.61 (0.02 to 9.17) Network estimate | 368 per 1000 | 226 per 1000 (9 to 1000) | 142 fewer per 1000 (360 fewer to 632 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus systemic vasodilator (No direct RCT) | HR 0.62 (0.03 to 9.10) Network estimate | 368 per 1000 | 228 per 1000 (12 to 1000) | 140 fewer per 1000 (357 fewer to 632 more) | Very low1,2,3 |
| Systemic vasoconstrictors plus albumin (No direct RCT) | HR 2.62 (0.41 to 19.28) Network estimate | 368 per 1000 | 965 per 1000 (151 to 1000) | 596 more per 1000 (218 fewer to 632 more) | Very low1,2,3 |
| Serious adverse events (number of people) | None of the trials with paracentesis plus fluid replacement as an intervention reported this outcome | ||||
| Serious adverse events (number of events) Total studies: 1 Total participants: 41 | |||||
| Paracentesis plus fluid replacement | Reference | ||||
| Aldosterone antagonists plus loop diuretics (1 RCT; 41 participants) | Rate ratio 1.30 (0.27 to 6.99) Direct estimate | 0 per 1000 | Not estimable | Very low1,2,3 | |
|
Transjugular intrahepatic portosystemic shunt (1 RCT; 70 participants) |
Not estimable (10 serious adverse events in 35 participants) |
0 per 1000 | Not estimable | Very low1,2,3 | |
| Health‐related quality of life | None of the trials with paracentesis plus fluid replacement as an intervention reported this outcome | ||||
| Any adverse events (number of people) Total studies: 6 Total participants: 229 | |||||
| Paracentesis plus fluid replacement | Reference | ||||
| Paracentesis plus systemic vasoconstrictors (4 RCTs; 145 participants) | OR 1.63 (0.30 to 11.66) Network estimate | 100 per 1000 | 153 per 1000 (32 to 564) | 53 more per 1000 (68 fewer to 464 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics (2 RCT; 84 participants) | OR 3.54 (0.43 to 27.41) Network estimate | 100 per 1000 | 282 per 1000 (46 to 753) | 182 more per 1000 (54 fewer to 653 more) | Very low1,2,3 |
| Any adverse events (number of events) Total studies: 3 Total participants: 116 | |||||
| Paracentesis plus fluid replacement | Reference | ||||
| Aldosterone antagonists plus loop diuretics (1 RCT; 31 participants) | Rate ratio 4.12 (0.87 to 34.02) Network estimate | 118 per 1000 | 485 per 1000 (103 to 4003) | 367 more per 1000 (15 fewer to 3885 more) | Very low1,2,3 |
| Paracentesis plus systemic vasoconstrictors (1 RCT; 25 participants) | Rate ratio 1.37 (0.36 to 5.82) Network estimate | 118 per 1000 | 161 per 1000 (42 to 685) | 43 more per 1000 (76 fewer to 567 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (No direct RCT) | Rate ratio 3.30 (0.38 to 38.51) Network estimate | 118 per 1000 | 388 per 1000 (45 to 4531) | 271 more per 1000 (73 fewer to 4413 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (No direct RCT) | Rate ratio 4.25 (0.53 to 46.99) Network estimate | 118 per 1000 | 501 per 1000 (62 to 5529) | 383 more per 1000 (55 fewer to 5411 more) | Very low1,2,3 |
| Aldosterone antagonists plus loop diuretics plus systemic vasodilator (No direct RCT) | Rate ratio 2.41 (0.24 to 29.67) Network estimate | 118 per 1000 | 284 per 1000 (28 to 3490) | 166 more per 1000 (89 fewer to 3372 more) | Very low1,2,3 |
| Liver transplantation at maximal follow‐up Total studies: 11 Total participants: 596 | |||||
| Paracentesis plus fluid replacement | Reference | ||||
| Paracentesis plus systemic vasoconstrictors (4 RCTs; 145 participants) | HR 1.08 (0.11 to 10.35) Network estimate | 121 per 1000 | 131 per 1000 (14 to 1000) | 10 more per 1000 (108 fewer to 879 more) | Very low1,2,3 |
| Transjugular intrahepatic portosystemic shunt (6 RCTs; 427 participants) | HR 0.87 (0.52 to 1.44) Network estimate | 121 per 1000 | 106 per 1000 (63 to 175) | 15 fewer per 1000 (58 fewer to 54 more) | Very low1,2,3 |
| Paracentesis plus reinfusion (1 RCT; 24 participants) | HR 2.56 (0.20 to 90.92) Network estimate | 121 per 1000 | 310 per 1000 (25 to 1000) | 189 more per 1000 (97 fewer to 879 more) | Very low1,2,3 |
| Symptomatic resolution of ascites at maximal follow‐up | None of the trials reported this outcome | ||||
| Resolution of ascites at maximal follow‐up (by ultrasound) Total studies: 17 Total participants: 1007 | |||||
| Paracentesis plus fluid replacement | Reference | ||||
| Aldosterone antagonists plus loop diuretics (3 RCTs; 125 participants) | HR 1.10 (0.12 to 10.74) Network estimate | 158 per 1000 | 174 per 1000 (18 to 1000) | 16 more per 1000 (140 fewer to 842 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement (No direct RCT) | HR 1.17 (0.01 to 98.79) Network estimate | 158 per 1000 | 185 per 1000 (2 to 1000) | 27 more per 1000 (156 fewer to 842 more) | Very low1,2,3,4 |
| Transjugular intrahepatic portosystemic shunt (6 RCTs; 392 participants) | HR 9.44 (1.93 to 62.68) Network estimate | 158 per 1000 | 1000 per 1000 (305 to 1000) | 842 more per 1000 (147 more to 842 more) | Very low1,2,4 |
| No active treatment (No direct RCT) | HR 0.16 (0.00 to 17.37) Network estimate | 158 per 1000 | 26 per 1000 (0 to 1000) | 132 fewer per 1000 (158 fewer to 842 more) | Very low1,2,3,4 |
| Loop diuretics (No direct RCT) | HR 2.26 (0.01 to 846.41) Network estimate | 158 per 1000 | 357 per 1000 (1 to 1000) | 199 more per 1000 (157 fewer to 842 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus albumin (No direct RCT) | HR 3.28 (0.09 to 118.39) Network estimate | 158 per 1000 | 517 per 1000 (15 to 1000) | 360 more per 1000 (143 fewer to 842 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (No direct RCT) | HR 8.81 (0.06 to 1908.36) Network estimate | 158 per 1000 | 1000 per 1000 (10 to 1000) | 842 more per 1000 (148 fewer to 842 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus paracentesis plus fluid replacement (1 RCT; 36 participants) | HR 30.63 (5.06 to 692.98) Direct estimate | 158 per 1000 | 1000 per 1000 (799 to 1000) | 842 more per 1000 (641 more to 842 more) | Low1,2 |
| Other features of decompensation at maximal follow‐up Total studies: 25 Total participants: 1756 | |||||
| Paracentesis plus fluid replacement | Reference | ||||
| Aldosterone antagonists plus loop diuretics (4 RCTs; 242 participants) | Rate ratio 2.04 (1.37 to 3.10) Network estimate | 439 per 1000 | 896 per 1000 (602 to 1360) | 458 more per 1000 (164 more to 922 more) | Very low1,2,4 |
| Paracentesis plus systemic vasoconstrictors (3 RCTs; 114 participants) | Rate ratio 0.76 (0.14 to 3.61) Network estimate | 439 per 1000 | 332 per 1000 (62 to 1582) | 107 fewer per 1000 (377 fewer to 1144 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement (No direct RCT) | Rate ratio 1.04 (0.56 to 1.93) Network estimate | 439 per 1000 | 455 per 1000 (244 to 848) | 16 more per 1000 (195 fewer to 409 more) | Very low1,2,3,4 |
| Transjugular intrahepatic portosystemic shunt (7 RCTs; 452 participants) | Rate ratio 1.17 (0.92 to 1.49) Network estimate | 439 per 1000 | 515 per 1000 (405 to 655) | 76 more per 1000 (33 fewer to 217 more) | Very low1,2,3,4 |
| No active treatment (No direct RCT) | Rate ratio 3.34 (0.85 to 13.94) Network estimate | 439 per 1000 | 1466 per 1000 (374 to 6115) | 1028 more per 1000 (64 fewer to 5677 more) | Very low1,2,3,4 |
| Loop diuretics (No direct RCT) | Rate ratio 0.95 (0.40 to 2.23) Network estimate | 439 per 1000 | 418 per 1000 (176 to 977) | 21 fewer per 1000 (262 fewer to 538 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus albumin (No direct RCT) | Rate ratio 1.56 (0.84 to 2.87) Network estimate | 439 per 1000 | 682 per 1000 (369 to 1260) | 244 more per 1000 (69 fewer to 821 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus peritoneovenous shunt (No direct RCT) | Rate ratio 0.84 (0.41 to 1.70) Network estimate | 439 per 1000 | 369 per 1000 (180 to 747) | 70 fewer per 1000 (258 fewer to 308 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (No direct RCT) | Rate ratio 0.53 (0.02 to 4.98) Network estimate | 439 per 1000 | 233 per 1000 (7 to 2185) | 205 fewer per 1000 (431 fewer to 1747 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (No direct RCT) | Rate ratio 0.53 (0.02 to 4.99) Network estimate | 439 per 1000 | 233 per 1000 (8 to 2190) | 206 fewer per 1000 (431 fewer to 1751 more) | Very low1,2,3,4 |
| Aldosterone antagonists plus loop diuretics plus systemic vasodilator (No direct RCT) | Rate ratio 0.53 (0.02 to 5.11) Network estimate | 439 per 1000 | 231 per 1000 (7 to 2241) | 208 fewer per 1000 (431 fewer to 1802 more) | Very low1,2,3,4 |
| Systemic vasoconstrictors plus albumin (No direct RCT) | Rate ratio 3.90 (0.96 to 16.98) Network estimate | 439 per 1000 | 1712 per 1000 (422 to 7447) | 1274 more per 1000 (16 fewer to 7009 more) | Very low1,2,3,4 |
| *Anticipated absolute effect. Anticipated absolute effect compares two risks by calculating the difference between the risks of the intervention group with the weighted median risk of the control group. **Ranking is not provided because of the considerable uncertainty in the ranking. CrI: Credible interval; OR: Odds Ratio; HR: Hazard Ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for risk of bias because the trial(s) included in the analysis was/were at high risk of bias 2Downgraded one level for imprecision because the sample size was small 3Downgraded one level for imprecision because the credible intervals were wide (includes clinical benefit and harms) 4Downgraded one level for inconsistency because there was evidence of statistical heterogeneity
The network plots (where relevant) are available in Figure 1. The inconsistency factor plots (where relevant) are available in Figure 5. The differences in the fixed‐effect versus random‐effects model, where relevant, are available in Figure 6. The model fit is available in Table 6. The effect estimates are available in Table 7. A formal subgroup analysis was not possible for grade of ascites because the trials that provided this information included only grade 3 ascites or included a mixture of grade 2 and grade 3 ascites, i.e. there were no trials that included grade 2 ascites only. However, there was evidence of inconsistency in some outcomes when all studies were synthesised.
1.
A high resolution version of this image can be found at: https://doi.org/10.5281/zenodo.3604788..
The network plots showing the outcomes for which network meta‐analysis was performed. The size of the node (circle) provides a measure of the number of trials in which the particular Intervention was included as one of the intervention groups. The thickness of the line provides a measure of the number of direct comparisons between two nodes (Interventions). A higher resolution image of this picture is available at: http://doi.org/10.5281/zenodo.3531818.
Abbreviations
Alb = Albumin AldoAnt = Aldosterone antagonists Fluid = Fluid replacement LoopD = Loop diuretics No active treatment = No active treatment OsmoD = Osmotic diuretics Paracen = Paracentesis PVShunt = Peritoneovenous shunt Reinf = Reinfusion Vasocons = Systemic vasoconstrictors Vasodil = Systemic vasodilator ThiazD = Thiazide diuretics TIPS = Transjugular intrahepatic portosystemic shunt
5.
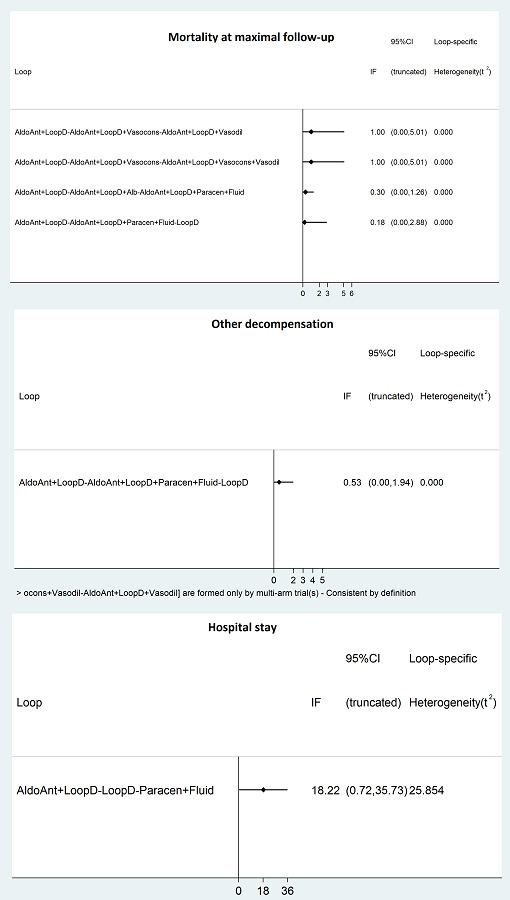
Inconsistency factor plots showing the inconsistency factors for the outcomes with direct and indirect evidence available for one or more comparisons. There was no evidence of inconsistency except for hospital stay. A higher resolution image of this picture is available at: http://doi.org/10.5281/zenodo.3531818.
Abbreviations:
Alb = Albumin AldoAnt = Aldosterone antagonists Fluid = Fluid replacement LoopD = Loop diuretics No active treatment = No active treatment OsmoD = Osmotic diuretics Paracen = Paracentesis PVShunt = Peritoneovenous shunt Reinf = Reinfusion Vasocons = Systemic vasoconstrictors Vasodil = Systemic vasodilator ThiazD = Thiazide diuretics TIPS = Transjugular intrahepatic portosystemic shunt
6.
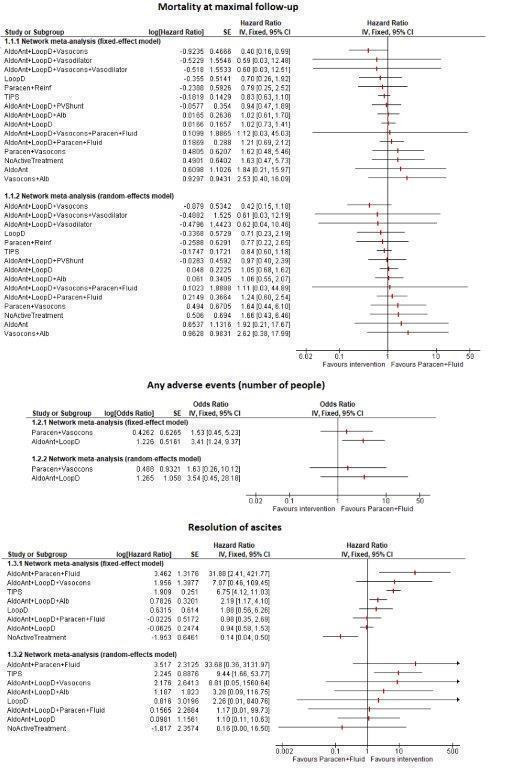
Forest plots showing the outcomes for which the random‐effects model were different from the fixed‐effect model. The more conservative random‐effects model was used. In this figure, mortality at maximal follow‐up, any adverse events (number of people), and resolution of ascites are shown. Figure 7 shows the remaining outcomes (other decompensation events and length of hospital stay), the other outcomes in which the fixed‐effect and random‐effects model were different. A higher resolution image of this picture is available at: http://doi.org/10.5281/zenodo.3531818.
Abbreviations:
Alb = Albumin AldoAnt = Aldosterone antagonists Fluid = Fluid replacement LoopD = Loop diuretics No active treatment = No active treatment OsmoD = Osmotic diuretics Paracen = Paracentesis PVShunt = Peritoneovenous shunt Reinf = Reinfusion Vasocons = Systemic vasoconstrictors Vasodil = Systemic vasodilator ThiazD = Thiazide diuretics TIPS = Transjugular intrahepatic portosystemic shunt
4. Model fit.
| Mortality at maximal follow‐up | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 255.1 | 253.7 | 255.3 |
| DIC | 297.9 | 299.2 | 303.1 |
| pD | 42.8 | 45.5 | 47.75 |
| Any adverse events (number of people) | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 38.89 | 39.75 | ‐ |
| DIC | 46.75 | 48.72 | ‐ |
| pD | 7.862 | 8.96 | ‐ |
| Liver transplantation at maximal follow‐up | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 54.83 | 54.1 | ‐ |
| DIC | 64.51 | 65.98 | ‐ |
| pD | 9.684 | 11.88 | ‐ |
| Resolution of ascites at maximal follow‐up (by ultrasound) | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 182.7 | 135 | ‐ |
| DIC | 206.4 | 165.3 | ‐ |
| pD | 23.73 | 30.24 | ‐ |
| Other features of decompensation at maximal follow‐up | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 258 | 258.1 | 253 |
| DIC | 293.9 | 294 | 294.7 |
| pD | 35.89 | 35.92 | 41.71 |
| Length of hospital stay (days) (all admissions until maximal follow‐up) | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 152.7 | 122.8 | 122.7 |
| DIC | 173.7 | 147.2 | 147.3 |
| pD | 20.95 | 24.39 | 24.56 |
| Treatment costs | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 4961 | 34.09 | ‐ |
| DIC | 4963 | 38.08 | ‐ |
| pD | 2.023 | 3.998 | ‐ |
Dbar = posterior mean of deviance
DIC = deviance information criteria
pD = effective number of parameters or leverage
5. Effect estimates (network meta‐analysis).
| This table is too wide to be displayed in RevMan. This table can be found at: https://doi.org/10.5281/zenodo.3604602 |
The table provides the effect estimates of each pairwise comparison for the different outcomes. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Statistically significant results are shown in italics.
7.
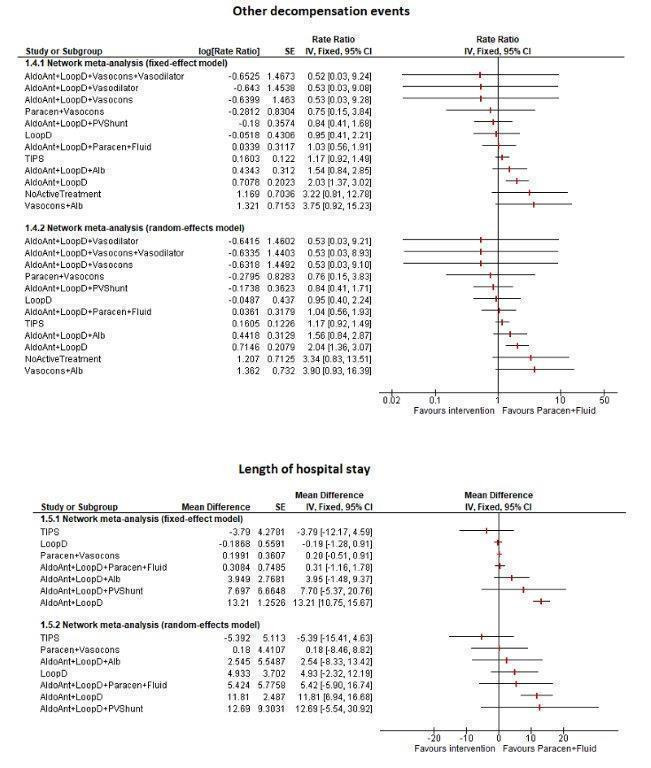
Forest plots showing the outcomes for which the random‐effects model were different from the fixed‐effect model. The more conservative random‐effects model was used. In this figure, other decompensation events and length of hospital stay are shown. Figure 6 shows the remaining outcomes (mortality at maximal follow‐up, any adverse events (number of people), and resolution of ascites), the other outcomes in which the fixed‐effect and random‐effects model were different. A higher resolution image of this picture is available at: http://doi.org/10.5281/zenodo.3531818.
Abbreviations:
Alb = Albumin AldoAnt = Aldosterone antagonists Fluid = Fluid replacement LoopD = Loop diuretics No active treatment = No active treatment OsmoD = Osmotic diuretics Paracen = Paracentesis PVShunt = Peritoneovenous shunt Reinf = Reinfusion Vasocons = Systemic vasoconstrictors Vasodil = Systemic vasodilator ThiazD = Thiazide diuretics TIPS = Transjugular intrahepatic portosystemic shunt
The 95% credible intervals of the probability ranks were wide and included 0 and 1 in most comparisons for all the primary and secondary outcomes. This was probably because of the sparse data from small trials. Therefore, we did not present the ranking probabilities (in a table), rankograms, and SUCRA plots as we considered that presenting this information would be unhelpful and potentially misleading and would ignore the differences in systematic errors in the trials.
The certainty of evidence was low or very low for all the comparisons. This was because most of the trials included in the comparison were at unclear or high risk of bias for at least one risk of bias domain at the outcome level (downgraded one level) and the sample size was small (downgraded one level). This resulted in low‐certainty evidence. In comparisons where the wide credible intervals overlapped significant clinical effect and no effect, we downgraded one more level for imprecision (downgraded one level). There was also evidence of heterogeneity (called inconsistency in the GRADE system; not to be confused with inconsistency in direct and indirect estimates in the context of network meta‐analysis) for resolution of ascites and other decompensation events (downgraded one level)
Mortality at maximal follow‐up
Thirty‐four trials (2548 participants) reported mortality at maximal follow‐up (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Acharya 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Lebrec 1996; Graziotto 1997; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Singh 2008; Licata 2009; Narahara 2011; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). A total of 18 treatments were compared in these trials. Two trials were not connected to the network because they were the only trials for the comparisons and had zero events in one of the intervention groups (Acharya 1992; Ali 2014), and thus were excluded from the analysis. The network had 16 connected treatments (32 trials; 2448 participants). There was no evidence of inconsistency according to model fit, inconsistency factor, and the 'between‐design' variance 0.16 (95% CrI 0.00 to 10.02). The random‐effects model was used because it was more conservative, even though the model fit was similar to the fixed‐effect model. The 'between‐study variance' was 0.02 (95% CrI 0.00 to 0.27).
There was no evidence of differences between interventions in any of the direct comparisons or in the comparisons included in the network meta‐analysis (i.e. there was no statistically significant difference in any of the comparisons) (Table 7) (very low‐certainty evidence; Table 2). The sensitivity analysis indicated that the different scenarios (best‐best and worst‐worst scenarios) for imputing missing data showed different interpretation of results; therefore, the results have to be interpreted with caution.
There was also no evidence of differences between the comparisons not included in the network meta‐analysis.
Aldosterone antagonists plus paracentesis plus fluid replacement (0/20; 0%) versus aldosterone antagonists plus loop diuretics (1/20; 5%) (1 trial; 40 participants; very low‐certainty evidence);
Systemic vasoconstrictors (1/30; 3.3%) versus no active intervention (0/30; 0%) (1 trial; 60 participants; very low‐certainty evidence).
Serious adverse events
Fourteen trials (761 participants) reported serious adverse events (with respect to number of people) (Acharya 1992; Hagege 1992; Ljubici 1994; Singh 2006a; Singh 2006b; Lata 2007; Singh 2008; Narahara 2011; Raza 2011; Singh 2012a; Singh 2013; Ali 2014; Rai 2017; Sola 2018). A total of 14 treatments were compared in these trials. Ten trials were not connected to the network because they had zero events in both intervention groups (Hagege 1992; Singh 2006a; Singh 2006b; Singh 2008; Narahara 2011; Raza 2011; Singh 2012a; Singh 2013; Ali 2014; Rai 2017); two trials were not connected to the network because they were the only trials for the comparison and had zero events in one of the intervention groups (Ljubici 1994; Lata 2007); the remaining trials had no common treatments, and therefore were not connected (Acharya 1992; Sola 2018). Only two treatments were compared in each of the remaining trials (Acharya 1992; Sola 2018). Therefore, random‐effects, network meta‐analysis, checking for inconsistency, or subgroup analyses were not applicable.
There was no evidence of differences in any of the direct comparisons for which it was possible to calculate the effect estimates (i.e. there was no statistically significant difference in any of the comparisons).
Aldosterone antagonists plus paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics: OR 0.68 (95% CrI 0.11 to 3.83; 1 trial; 40 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasodilator versus aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator: OR 0.84 (95% CrI 0.46 to 1.55; 1 trial; 173 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement (0/10; 0%) versus aldosterone antagonists plus loop diuretics (1/11; 9.1%) (1 trial; 21 participants; very low‐certainty evidence);
Paracentesis plus systemic vasoconstrictors (0/84; 0%) versus paracentesis plus fluid replacement (1/85; 1.2%) (4 trials; 169 participants; very low‐certainty evidence).
There was no change in the results by using the best‐worst and worst‐best scenarios for imputing missing data. Two trials (111 participants) reported serious adverse events (with respect to number of events) (Salerno 1987; Ginès 2002). A total of three treatments were compared in these trials. One trial was not connected to the network because it was the only trial for the comparison and had zero events in one of the intervention groups (Ginès 2002). Only two treatments were compared in the remaining trial (Salerno 1987; 41 participants). Therefore, random‐effects, network meta‐analysis, checking for inconsistency, or subgroup analyses were not applicable.
There was no evidence of differences in the only direct comparison for which it was possible to calculate the effect estimates (i.e. there was no statistically significant difference): aldosterone antagonists plus loop diuretics versus paracentesis plus fluid replacement: rate ratio: 1.30 (95% CrI 0.27 to 7.16; 1 trial; 41 participants; very low‐certainty evidence; Table 2). In the remaining comparison, transjugular intrahepatic portosystemic shunt versus paracentesis plus fluid replacement, there were 10 serious advents in 35 participants receiving transjugular intrahepatic portosystemic shunt (28.6 serious adverse events per 100 participants) compared to no serious adverse events in 35 participants receiving paracentesis plus fluid replacement (1 trial; 70 participants; very low‐certainty evidence).
Health‐related quality of life
One trial (431 participants) reported health‐related quality of life (EQ‐5D) (Caraceni 2018). For EQ‐5D, a higher score indicates better health‐related quality of life. A total of two treatments were compared in this trial. Since only one trial reported the outcome, random‐effects, network meta‐analysis, checking for inconsistency, or subgroup analyses were not applicable. Aldosterone antagonists plus loop diuretics plus albumin had better health‐related quality of life than aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement: MD 0.06 (95% CrI 0.03 to 0.09; 1 trial; 431 participants; low‐certainty evidence). The standard deviation was reported in the trial: therefore, sensitivity analysis of excluding trials in which standard deviations were imputed was not applicable.
Any adverse events
Eight trials (462 participants) reported any adverse events (with respect to number of people) (Chesta 1990; Hagege 1992; Singh 2006a; Singh 2006b; Singh 2008; Narahara 2011; Bari 2012; Sola 2018). A total of six treatments were compared in these trials. Two trials were not connected to the network because they were the only trials for the comparisons and had zero events in one of the intervention groups (Narahara 2011) or had unconnected treatments (Sola 2018). The network had three connected treatments (6 trials; 229 participants). There were no triangular or quadrangular loops; therefore, inconsistency was not checked. The random‐effects model was used because it was more conservative, even though the model fit was similar to the fixed‐effect model. The 'between‐study variance' was 0.37 (95% CrI 0.00 to 10.82).
There was no evidence of differences in any of the direct comparisons or network meta‐analysis (i.e. there was no statistically significant difference in any of the comparisons included in the network meta‐analysis) (Table 7) (very low‐certainty evidence; Table 2). There was no change in the results by using the best‐best and worst‐worst scenarios for imputing missing data.
The results of the remaining two comparisons which could not be included in the network meta‐analysis are as follows.
10 participants among 30 participants (10/30; 33.3%) receiving transjugular intrahepatic portosystemic shunt compared to no participant of 30 participants (0/30; 05) receiving paracentesis plus fluid replacement developed 'any adverse events' (1 trial; 60 participants; very low‐certainty evidence).
There was no evidence of differences between systemic vasoconstrictors plus albumin versus no active intervention OR 0.45 (95% CrI 0.05 to 2.61; 1 trial; 173 participants; very low‐certainty evidence).
Five trials (314 participants) reported any adverse events (number of events) (Chesta 1990; Bari 2012; Singh 2013; Rai 2017; Sola 2018). A total of 10 treatments were compared in these trials. Two trials were not connected to the network because of unconnected treatments (Rai 2017;Sola 2018). The network had six connected treatments (3 trials; 116 participants). There was no evidence of inconsistency according to model fit and the 'between‐design' variance 0.17 (95% CrI 0.00 to 3.49). The inconsistency factor plot could not be obtained since there was only one trial for the closed loops and heterogeneity could not be calculated. The fixed‐effect model was used because there was only one trial for each of the comparisons.
The following direct comparisons were statistically significant (both comparisons not included in the network meta‐analysis because of unconnected treatments):
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement: rate ratio 0.07 (95% CrI 0.00 to 0.47; 1 trial; 25 participants; low‐certainty evidence);
Systemic vasoconstrictors plus albumin versus no active treatment: rate ratio 1.17 (95% CrI 1.03 to 1.33; 1 trial; 173 participants; low‐certainty evidence).
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) or in the network meta‐analysis (Table 7) (very low‐certainty evidence; Table 2). The sensitivity analysis indicated that the different scenarios (best‐worst and worst‐best scenarios) for imputing missing data showed different interpretation of results; therefore, the results have to be interpreted with caution.
Liver transplantation at maximal follow‐up
Nineteen trials (1568 participants) reported liver transplantation at maximal follow‐up (Fogel 1981; Hagege 1992; Graziotto 1997; Rossle 2000; Ginès 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Singh 2008; Narahara 2011; Bari 2012; Singh 2012a; Singh 2013; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018). A total of 14 treatments were compared in these trials. Five trials were not connected to the network because they had zero events in both intervention groups (Fogel 1981; Hagege 1992; Singh 2012a; Singh 2013; Rai 2017); three trials were not connected to the network because of unconnected treatments (Romanelli 2006; Caraceni 2018; Sola 2018). The network had four connected treatments (11 trials; 596 participants). There were no triangular or quadrangular loops; therefore, inconsistency was not checked. The fixed‐effect model was used because it had equivalent results and model fit to the random‐effects model.
There was no evidence of differences in any of the direct comparisons or network meta‐analysis (i.e. there was no statistically significant difference in any of the comparisons) (Table 7) (very low‐certainty evidence; Table 2). There was no change in the results by using the best‐worst and worst‐best scenarios for imputing missing data.
The effect estimates in the comparisons with unconnected treatments were as follows.
Aldosterone antagonists plus loop diuretics plus albumin versus aldosterone antagonists plus loop diuretics: HR 0.22 (95% CrI 0.01 to 1.99; 1 trial; 100 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus albumin versus aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement: HR 1.03 (95% CrI 0.54 to 2.00; 1 trial; 431 participants; very low‐certainty evidence);
Systemic vasoconstrictors plus albumin versus no active intervention: HR 1.44 (95% CrI 0.96 to 2.15; 1 trial; 173 participants; very low‐certainty evidence).
The number of people who underwent liver transplantation in the trials with zero events are as follows.
Aldosterone antagonists plus loop diuretics (0/27; 0%) versus paracentesis plus fluid replacement (0/26; 0%) (1 trial; 53 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics (0/61; 0%) versus loop diuretics (0/29; 0%) (1 trial; 90 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (0/35; 0%) versus aldosterone antagonists plus loop diuretics (0/35; 0%) (2 trials; 70 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus paracentesis plus fluid replacement (0/13; 0%) versus aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement (0/12; 0%) (1 trial; 25 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence);
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics plus systemic vasodilator (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence).
Resolution of ascites at maximal follow‐up
None of the trials reported symptomatic resolution of ascites (for example, resolution of shortness of breath) at maximal follow‐up. Twenty trials (1217 participants) reported resolution of ascites (by ultrasound) at maximal follow‐up (Gregory 1977; Descos 1983; Salerno 1987; Chesta 1990; Strauss 1991; Hagege 1992; Lebrec 1996; Fernandez‐Esparrach 1997; Graziotto 1997; Gentilini 1999a; Ginès 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Licata 2009; Narahara 2011; Singh 2012a; Singh 2013; Bureau 2017c; Rai 2017). A total of 14 treatments were compared in these trials. Two trials were not connected to the network because they were the only trials for the comparison and had zero events in one of the intervention groups (Graziotto 1997; Rai 2017) and another trial was not connected because of unconnected treatments. One more trial had four arms with zero events in all four arms (Singh 2013). One comparison could be included in the network meta‐analysis as there were some events in the remaining trials of the same comparison, but the other comparisons could not be included (Singh 2013). The network had nine connected treatments (17 trials; 1007 participants). There were no triangular or quadrangular loops; therefore, inconsistency was not checked. The random‐effects model was used because it was more conservative and had better model fit. The 'between‐study variance' was 2.60 (95% CrI 0.68 to 12.29).
The following direct comparisons which could be estimated were in favour of:
Transjugular intrahepatic portosystemic shunt versus paracentesis plus fluid replacement: HR 8.37 (95% CrI 1.97 to 62.68; 6 trials; 392 participants; very low‐certainty evidence);
Aldosterone antagonists plus paracentesis plus fluid replacement versus paracentesis plus fluid replacement alone: HR 30.63 (95% CrI 5.06 to 692.98; 1 trial; 36 participants; low‐certainty evidence);
No active treatment versus aldosterone antagonists plus loop diuretics: HR 0.15 (95% CrI 0.04 to 0.43 ; 1 trial; 43 participants; low‐certainty evidence) (i.e. aldosterone antagonists plus loop diuretics versus no active treatment: HR 6.67 (95% CrI 2.33 to 25));
Loop diuretics versus aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement: HR 1.90 (95% CrI 1.03 to 3.76 ; 1 trial; 84 participants; low‐certainty evidence).
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) (Table 7) (very low‐certainty evidence). In the network meta‐analysis, the following comparisons were statistically significant:
Transjugular intrahepatic portosystemic shunt versus paracentesis plus fluid replacement: HR 9.44 (95% CrI 1.93 to 62.68) (similar effect as in direct comparison; very low‐certainty evidence)
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (Table 7) (very low‐certainty evidence; Table 2). There was no change in the results by using the best‐worst and worst‐best scenarios for imputing missing data.
The effect estimates in the comparisons with unconnected treatments were as follows.
Aldosterone antagonists versus paracentesis plus reinfusion: HR 1.11 (95% CrI 0.69 to 1.79; 1 trial; 131 participants; very low‐certainty evidence)
The number of people who had resolution of ascites in the trials with zero events are as follows.
Paracentesis plus reinfusion (2/12; 16.7%) versus paracentesis plus fluid replacement (0/12; 0%) (1 trial; 24 participants; very low‐certainty evidence)
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence)
Aldosterone antagonists plus loop diuretics plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence)
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus paracentesis plus fluid replacement (5/13; 38.5%) versus aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement (0/12; 0%) (1 trial; 25 participants; very low‐certainty evidence)
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence)
Aldosterone antagonists plus loop diuretics plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence)
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus systemic vasodilator (0/15; 0%) versus aldosterone antagonists plus loop diuretics plus systemic vasodilator (0/15; 0%) (1 trial; 30 participants; very low‐certainty evidence)
Other features of decompensation at maximal follow‐up
Twenty‐seven trials (1821 participants) reported other features of decompensation at maximal follow‐up (Gregory 1977; Fogel 1981; Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Hagege 1992; Sola 1994; Ginès 1995; Lebrec 1996; Gentilini 1999a; Rossle 2000; Ginès 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Lata 2007; Singh 2008; Licata 2009; Narahara 2011; Bari 2012; Singh 2013; Bureau 2017c; Rai 2017; Sola 2018). A total of 15 treatments were compared in these trials. Two trials were not connected to the network because they were the only trials for the comparisons and had zero events in one of the intervention groups (Acharya 1992; Rai 2017). The network had 13 connected treatments (25 trials; 1756 participants). There was no evidence of inconsistency according to the inconsistency factor plot or model fit. We could not obtain convergence for the design‐by‐treatment analysis. The random‐effects model was used because it was more conservative and had a large between‐study variance of 6.25 (95% CrI 0.02 to 23.78), even though the model fit was similar to the fixed‐effect model.
The following direct comparisons were in favour of:
Aldosterone antagonists plus loop diuretics versus paracentesis plus fluid replacement: rate ratio 2.04 (95% CrI 1.39 to 3.08; 4 trials; 242 participants; very low‐certainty evidence) (i.e. paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics: rate ratio 0.49 (95% CrI 0.72 to 0.32))
Aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics: rate ratio 0.48 (95% CrI 0.29 to 0.77 ; 2 trials; 102 participants; low‐certainty evidence)
Aldosterone antagonists plus paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors: 7/12 (0.6 other decompensation events per participant) versus 0/13 (no decompensation events per participant) (1 trial; 15 participants).
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) (Table 7) (very low‐certainty). In the network meta‐analysis, the following comparisons were in favour of:
Aldosterone antagonists plus loop diuretics versus paracentesis plus fluid replacement: rate ratio 2.04 (95% CrI 1.37 to 3.10) (i.e. paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics: rate ratio 0.49 (95% CrI 0.73 to 0.32))
Aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics: rate ratio 0.51 (95% CrI 0.32 to 0.80)
Transjugular intrahepatic portosystemic shunt versus aldosterone antagonists plus loop diuretics: rate ratio 0.57 (95% CrI 0.35 to 0.92)
Loop diuretics versus aldosterone antagonists plus loop diuretics: rate ratio 0.47 (95% CrI 0.22 to 0.96)
Aldosterone antagonists plus loop diuretics plus peritoneovenous shunt versus aldosterone antagonists plus loop diuretics: rate ratio 0.41 (95% CrI 0.23 to 0.73)
Systemic vasoconstrictors plus albumin versus aldosterone antagonists plus loop diuretics plus peritoneovenous shunt: rate ratio 4.65 (95% CrI 1.06 to 20.84) i..e. aldosterone antagonists plus loop diuretics plus peritoneovenous shunt versus systemic vasoconstrictors plus albumin: rate ratio: 0.22 (95% CrI 0.05 to 0.94).
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (Table 7) (very low‐certainty evidence; Table 2).
The number of decompensation events in the trials with zero events are as follows.
Aldosterone antagonists plus paracentesis plus fluid replacement (0/20; 0 events) versus aldosterone antagonists plus loop diuretics (3/20; 15 events per 100 participants) (1 trial; 40 participants; very low‐certainty evidence)
Aldosterone antagonists plus loop diuretics plus systemic vasoconstrictors plus paracentesis plus fluid replacement (0/13; 0 events) versus aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement (7/12; 58.3 events per 100 participants) (1 trial; 25 participants; very low‐certainty evidence).
Length of hospital stay (days)
Fifteen trials (1086 participants) reported length of hospital stay (days) (all admissions until maximal follow‐up) (Fogel 1981; Descos 1983; Gines 1987; Chesta 1990; Ginès 1991; Hagege 1992; Ginès 1995; Schaub 1995; Gentilini 1999a; Rossle 2000; Moreau 2002; Salerno 2004; Licata 2009; Tuttolomondo 2016; Bureau 2017c). A total of 10 treatments were compared in these trials. One trial was not connected to the network because it had treatments unconnected to network (Descos 1983). The network had eight connected treatments. There was evidence of inconsistency according to the 'between‐design' variance 11.04 (95% CrI 0.05 to 24.30) and inconsistency factor, but not by model fit; therefore, there is uncertainty in the validity of NMA results: direct comparisons are more reliable. The random‐effects model was used because it had better model fit. The 'between‐study variance' was 20.10 (95% CrI 8.86 to 24.79).
The following direct comparisons were in favour of:
Aldosterone antagonists plus loop diuretics versus paracentesis plus fluid replacement: MD 14.00 days (95% CrI 9.19 to 18.52; 4 trials; 218 participants; low‐certainty evidence), i.e. paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics versus paracentesis (MD ‐14.00 days (95% CrI ‐18.52 to ‐9.19)
Aldosterone antagonists plus loop diuretics plus albumin versus aldosterone antagonists plus loop diuretics: MD ‐9.28 days (95% CrI ‐14.11 to ‐4.40; 1 trial; 126 participants; low‐certainty evidence).
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) (Table 7). In the network meta‐analysis, the following comparisons were in favour of:
Aldosterone antagonists plus loop diuretics versus paracentesis plus fluid replacement: MD 11.81 days (95% CrI 6.92 to 16.67; low‐certainty evidence), i.e. paracentesis plus fluid replacement versus aldosterone antagonists plus loop diuretics versus paracentesis (MD ‐11.81 days (95% CrI ‐16.67 to ‐6.92)
Paracentesis plus systemic vasoconstrictors versus aldosterone antagonists plus loop diuretics: MD ‐11.60 days (95% CrI ‐21.67 to ‐1.68; low‐certainty evidence)
Transjugular intrahepatic portosystemic shunt versus aldosterone antagonists plus loop diuretics: MD ‐17.25 days (95% CrI ‐28.47 to ‐6.17; low‐certainty evidence).
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (Table 7). There was no imputation of mean or standard deviation in the trials. Therefore, sensitivity analysis excluding trials in which mean or standard deviation were to be imputed was not applicable.
Work days lost
None of the trials reported work days lost.
Treatment costs
Four trials (150 participants) reported treatment costs (Mehta 1998; Singh 2006a; Singh 2008; Hamdy 2014). We used an international exchange rate based on purchasing power parities (PPP) to convert cost estimates to USA dollars (USD), and we used the gross domestic product (GDP) deflators (or implicit price deflators for GDP) to convert cost estimates to 2018 USD using PPP conversion rates and GDP deflator values available from the International Monetary Fund in the World Economic Outlook Database (www.imf.org/external/data.htm (accessed in July 2019)).
A total of three treatments were compared in the four trials. All the trials were connected to the network (after imputation of standard deviation for one trial). There were no triangular or quadrangular loops; therefore, inconsistency was not checked. The random‐effects model was used because of the model fit and because the random‐effects model was the more conservative model. The 'between‐study variance' was 2,458,624 (95% CrI 265,431 to 64,689,849). Given the extremely high between‐study variance, we have presented the results in a table, without meta‐analysing the results.
Treatment costs for paracentesis plus systemic vasoconstrictors was lower than that for paracentesis plus fluid replacement in all the three trials that reported this information (Table 8). For the other comparison, paracentesis plus reinfusion versus paracentesis plus fluid replacement, the standard deviation was not reported; therefore, it was not clear whether there were differences in treatment costs between the two interventions.
6. Treatment costs (tabular results without meta‐analysis).
| Study name | Comparison | Mean in intervention group | Standard deviation in intervention group | Number of participants in intervention group | Mean in control group | Standard deviation in control group | Number of participants in control group | Mean difference and 95% confidence intervals (according to Review Manager formula) |
| Hamdy 2014 | Paracentesis plus systemic vasoconstrictors versus paracentesis plus fluid replacement | 10.5 USD | 0.1 USD | 25 | 856.1 USD | 119.6 USD | 25 | ‐845.60 (95% CI ‐892.48 to ‐798.72) |
| Singh 2006a | Paracentesis plus systemic vasoconstrictors versus paracentesis plus fluid replacement | 1629.0 USD | 76.7 USD | 20 | 3368.0 USD | 82.5 USD | 20 | ‐1739.00 (95% CI ‐1788.37 to ‐1689.63) |
| Singh 2008 | Paracentesis plus systemic vasoconstrictors versus paracentesis plus fluid replacement | 29.4 USD | 2.7 USD | 20 | 105.7 USD | 25.4 USD | 20 | ‐76.30 (95% CI ‐87.49 to ‐65.11) |
| Mehta 1998 | Paracentesis plus reinfusion versus paracentesis plus fluid replacement | 295 USD | not reported | 10 | 440 USD | Not reported | 10 | ‐ 105; no information to calculate the 95% confidence intervals |
Abbreviations:
USD = United States Dollar CI = confidence intervals
Subgroup analysis
Data were sufficient to perform only the following subgroup analysis: duration of follow‐up (short‐term, medium‐term, and long‐term). There were no subgroup differences for any of the outcomes where there were at least two different subgroups represented in the analyses.
There were insufficient data for the remaining subgroup analyses or only one subgroup was represented in the analyses. Although a formal test for subgroup differences was not relevant for grade of ascites, as the trials included either only ascites 3 or a mixture of ascites 2 and ascites 3 (or did not provide information on the grade of ascites), we have presented the subgroup estimates of grade 3 ascites only in Table 9, when possible. Similarly, we have presented the results for recurrent and refractory ascites only in Table 10, when possible. Some comparisons became statistically nonsignificant, as could be expected when fewer than 50% trials were included for the analysis, but there were no major differences that would have resulted in alterations in the overall interpretation of the results.
7. Effect estimates (Subgroup: grade 3 ascites only).
| This table is too wide to be displayed in RevMan. This table can be found at: https://doi.org/10.5281/zenodo.3604780. |
The table provides the network meta‐analysis effect estimates for the subgroup of grade 3 ascites only of each pairwise comparison for the different outcomes. To identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B.
Statistically significant results are shown in italics.
Abbreviations:
HR = hazard ratio; OR = odds ratio
8. Effect estimates (Subgroup: refractory or recurrent ascites only).
| This table is too wide to be displayed in RevMan. This table can be found at: https://doi.org/10.5281/zenodo.3604784. |
The table provides the network meta‐analysis effect estimates for the subgroup of refractory or recurrent ascites only of each pairwise comparison for the different outcomes. To identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B.
Statistically significant results are shown in italics.
Abbreviations:
HR = hazard ratio OR = odds ratio
Sensitivity analysis
All sensitivity analyses were presented under the outcome.
Assessment of reporting biases
Since there was no meaningful way in which to rank these studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time), we were unable to perform the comparison‐adjusted funnel plot. However, important outcomes such as all‐cause mortality and adverse events were not reported in some trials indicating the possibility of reporting biases.
Discussion
Summary of main results
We performed a systematic review and network meta‐analysis of all the treatments available for ascites in people with decompensated liver cirrhosis. A total of 49 trials, including a total of 3521 participants, were included in this review. A total of 21 interventions were compared in these trials. A total of 42 trials including 2870 participants were included for one or more outcomes of this review (Gregory 1977; Fogel 1981; Descos 1983; Gines 1987; Salerno 1987; Chesta 1990; Ginès 1991; Strauss 1991; Acharya 1992; Hagege 1992; Ljubici 1994; Sola 1994; Ginès 1995; Schaub 1995; Lebrec 1996; Fernandez‐Esparrach 1997; Graziotto 1997; Mehta 1998; Gentilini 1999a; Rossle 2000; Ginès 2002; Moreau 2002; Sanyal 2003; Salerno 2004; Romanelli 2006; Singh 2006a; Singh 2006b; Lata 2007; Singh 2008; Licata 2009; Narahara 2011; Raza 2011; Bari 2012; Singh 2012a; Singh 2013; Ali 2014; Hamdy 2014; Tuttolomondo 2016; Bureau 2017c; Rai 2017; Caraceni 2018; Sola 2018).
Overall, 36.8% of the trial participants who received the standard treatment of paracentesis plus fluid replacement died during the follow‐up period ranging from one week to 11 months. There was no evidence of differences in mortality or serious adverse events in any of the direct comparisons or network meta‐analysis. However, the credible intervals were wide, and clinically important differences in mortality or serious adverse events cannot be ruled out.
The health‐related quality of life was reported in only one trial comparing aldosterone antagonists plus loop diuretics plus albumin versus aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement. The mean difference was 0.06. The minimum clinically important difference for EQ‐5D in people with cirrhosis is not known. In other conditions, a difference of 0.04 to 0.20 is clinically important (Asher 2018; Sims 2018; Hoehle 2019; Kato 2019). Therefore, it is not clear whether the difference of 0.06 with aldosterone antagonists plus loop diuretics plus albumin and aldosterone antagonists plus loop diuretics plus paracentesis plus fluid replacement is clinically important. It should also be pointed out that there is no information on whether this difference was reproducible, as this was the only trial for this comparison. Therefore, there is considerable uncertainty about the difference between the groups.
There were differences between the different groups in 'any' adverse events, but none of the comparisons in which there were differences could be considered as 'standard of care'; therefore the implications of these findings are not clinically relevant. The resolution of ascites was greater with transjugular intrahepatic portosystemic shunt versus paracentesis plus fluid replacement. While the resolution of ascites was greater by adding aldosterone antagonists to paracentesis plus fluid replacement, this was based on a single small trial of high risk of bias (sample size: 36 participants), indicating that there is high uncertainty about this issue.
The number of other decompensation events and the length of hospital stay were more with aldosterone antagonists plus loop diuretics versus paracentesis plus fluid replacement. In the network meta‐analysis, a number of other treatments including transjugular intrahepatic portosystemic shunt had fewer other decompensation events and shorter length of hospital stay than aldosterone antagonists plus loop diuretics without paracentesis. Therefore, aldosterone antagonists plus loop diuretics without paracentesis seems to be the worst among the common treatments compared in this review.
Treatment costs with paracentesis plus systemic vasoconstrictors was lower than that for paracentesis plus fluid replacement in all three trials that reported this information, although the between study variance was extremely high and meta‐analysis was not performed. Furthermore, in the presence of considerable uncertainty in benefits and harms of different treatments, treatment costs alone cannot determine whether one intervention is better than another.
The weighted median mortality in the paracentesis plus fluid replacement group was 36.8% in 11 months. The sample size required to detect a relative risk reduction of 20% in the experimental group, with type I error of 5%, and type II error of 20%, is 1282 participants. Although approximately 20% of people with liver cirrhosis develop ascites, the majority may be grade 2 and may be amenable to treatment with diuretics. However, a significant proportion may be grade 3, refractory, or recurrent. There is paucity of information on the incidence or prevalence of grade 3 refractory or recurrent ascites. One small study in Tunisia estimated that about 20% of all hospital admissions in people with cirrhosis was due to refractory ascites (Ennaifer 2014). If even 5% of hospital admissions due to liver cirrhosis relate to grade 3 refractory or recurrent ascites in UK, a trial like the stipulated one, is very much feasible.
There were approximately 44 other decompensation events per 100 participants in the paracentesis plus fluid replacement group. In addition to causing death, decompensation usually results in hospital admissions and significant costs to the health service. Therefore, 'any decompensation event' is another possible primary outcome. Assuming that the variance was equal to the mean in an ordinary Poisson distribution commonly used to analyse recurrent events (that happen independently, although this is a questionable assumption), for a 20% relative risk reduction in the experimental group, with type I error of 5%, and type II error of 20%, the sample size required in a trial using any decompensation event is 786 participants.
In terms of the interventions to be compared in future trials, paracentesis plus fluid replacement was the commonest intervention in this review. So, it should be considered as one of the interventions in future trials. Aldosterone antagonists plus loop diuretics instead of paracentesis plus fluid replacement appears to increase the other decompensation events and length of hospital stay (and paracentesis or TIPS may be required in people who do not respond to diuretics), although this is based on trials at high risk of bias. However, adding diuretics to paracentesis plus fluid replacement is one of the options for intervention (particularly, because this is currently the recommended treatment by AASLD and EASL, although there is no evidence to consider this superior to paracentesis plus fluid replacement alone); transjugular intrahepatic portosystemic shunt may be another option. Such shunts may be effective in preventing variceal rebleeding (Qi 2016), but they may increase hepatic encephalopathy (Saab 2006; Zhou 2019). Therefore, the impact of decompensation events on quality of life and ability to perform daily activities, social activities, and work should be evaluated as part of future trials.
Overall completeness and applicability of evidence
There did not seem to be any restrictions based on the etiology or the presence of other features of decompensation in the trials that provided this information. Therefore, the results of the study are applicable in people with cirrhosis resulting from varied aetiologies having ascites. However, it appears that the trials included mainly people with grade 3, refractory, or recurrent ascites. Therefore, the findings of this review are applicable only to such people. There is currently no information on which diuretic is better for people with cirrhosis and grade 2 ascites which is not refractory or recurrent. Therefore, feasible randomised clinical trials which look at the potential effects of different diuretics are necessary. The incidence of grade 3, recurrent, or refractory ascites may be a suitable outcome for such a trial.
Furthermore, 38 trials excluded participants with active other decompensation features such as active variceal bleeding, hepatorenal syndrome, and grade III or grade IV hepatic encephalopathy, while the remaining 11 trials did not report whether they included any participants with active other decompensation features. Therefore, the results of the review are only applicable to people without active other decompensation events. Accordingly, more evidence on ascites treatment seems to be needed in populations with ascites and other signs of decompensation.
Quality of the evidence
The overall certainty (quality) of evidence was very low. One of the main reasons for the very low certainty of evidence was the unclear or high risk of bias in all the trials. It is possible to perform trials at low risk of bias in certain comparisons: randomisation can be performed using standard methods, for example, web‐based central randomisation; an intention‐to‐treat analysis can be performed; and a protocol should be published prior to recruitment. However, blinding of healthcare providers and participants may not be possible if TIPS is used as one of the interventions. It is possible to achieve blinding with careful planning for other comparisons, for example by using placebos for diuretics if the trial was about adding diuretics to paracentesis plus fluid replacement. Outcome assessor blinding can be achieved for all comparisons.
Another major reason for the very low certainty of evidence was imprecision: the trials had small sample sizes and the credible intervals overlapped clinically significant benefits and clinically significant harms for most comparisons. Therefore, future trials should be adequately powered with sample sizes as described above.
We used clinical outcomes; therefore, there is no issue of indirectness due to outcomes. There was no suggestion that the potential effect modifiers were systematically different across comparisons (i.e. there was no concern about the transitivity assumption). While there was evidence of inconsistency according to the model fit, inconsistency in factor plots, and between‐design variance, an analysis of a subset of participants with grade 3 ascites (when possible) did not result in major differences in the interpretation of findings. Similarly, an analysis of a subset of participants with refractory or recurrent ascites (when possible) did not result in major differences in the interpretation of findings. However, one cannot rule out inconsistency ('incoherence' according to GRADE terminology).
There was no meaningful way to rank these studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time); we have completed a thorough search for studies on effectiveness. However, different sets of trials were included for different outcomes: only 30% to 70% of the trials reported mortality, serious adverse events, liver transplantation, resolution of ascites, and other decompensation events, even though these outcomes would have been routinely measured in trials of this nature. This may suggest reporting bias for these outcomes.
Potential biases in the review process
We selected a range of databases to search without using any language restrictions and conducted the network meta‐analysis according to NICE DSU guidance. In addition, we have analysed using the fixed‐effects model and random‐effects model and assessed and reported inconsistency whenever possible. These are the strengths of the review process.
We have excluded studies that compared variations in duration or dose in the different interventions. Hence, this review does not provide information on whether one variation is better than another. Another major limitation of this review was the paucity of data: the trials were small. This paucity of data decreases the confidence in the results.
All of the network meta‐analyses included only sparse data from trials, most of which were at high risk of bias. However, the potential effect modifiers in the trials that reported them were broadly similar across comparisons. The results of direct comparisons and indirect comparisons were similar for the most outcomes where we could assess this. Therefore, the concern about the transitivity assumption was low. However, this cannot be ruled out.
We included only randomised clinical trials, which were known to focus mostly on benefits and did not collect and report harms in a detailed manner. A significant effort was required to identify nonrandomised studies that reported on harm. It was also challenging to assess the risk of bias in those studies. If future randomised clinical trials are powered on mortality or other decompensation events, a systematic review on adverse events from observational studies will likely be unnecessary.
Agreements and disagreements with other studies or reviews
This is the first network meta‐analysis on the topic. There have been several systematic reviews and direct comparisons of different interventions for treating people with cirrhosis and ascites.
Guo and colleagues assessed the role of midodrine in people with cirrhosis and ascites (Guo 2016). They did not find any benefits of midodrine in terms of clinical outcomes despite improving surrogate outcomes such as response rates and plasma renin activity (Guo 2016). They also found that midodrine could be potentially harmful when used as a substitute for fluid replacement after paracentesis (Guo 2016). We did not find any evidence of benefit or harms of systemic vasoconstrictors in people with ascites and cirrhosis. This may be because of the different methods used for meta‐analysis: we have considered that the co‐interventions such as the diuretics or vasodilators used could influence the effect of systemic vasoconstrictors and treated these as different 'nodes' in the network meta‐analysis, while Guo and colleagues combined the trials despite differences in diuretics or vasodilators used. The method used for meta‐analysis (Bayesian versus frequentist method) could be an additional reason for the difference.
Simonetti and colleagues assessed the role of different fluids after paracentesis and found no evidence of difference in outcomes between different fluids used after paracentesis including reinfusion of ascitic fluid (Simonetti 2019). While we are unable to comment on different fluids after paracentesis since we did not explore this, we agree that there was no evidence of differences between paracentesis plus reinfusion and paracentesis plus fluid replacement.
Saab and colleagues found that TIPS was more effective in the resolution of ascites than paracentesis and fluid replacement, but found that the incidence of hepatic encephalopathy was increased (Saab 2006). However, they did not find evidence of differences in other decompensation events. Our network meta‐analysis also demonstrated that TIPS may be more effective in the resolution of ascites than paracentesis plus fluid replacement. We did not analyse the individual decompensation events separately. Therefore, we are unable to comment on whether hepatic encephalopathy was increased with TIPS compared to paracentesis plus fluid replacement.
Authors' conclusions
Implications for practice.
Based on very low‐certainty evidence, there is considerable uncertainty about whether other interventions decrease mortality, adverse events, or liver transplantation compared to paracentesis plus fluid replacement in people with decompensated liver cirrhosis and ascites. Based on very low‐certainty evidence, transjugular intrahepatic portosystemic shunt and adding aldosterone antagonists to paracentesis plus fluid replacement may increase the resolution of ascites compared to paracentesis plus fluid replacement. Based on very low‐certainty evidence, aldosterone antagonists plus loop diuretics may increase the decompensation rate compared to paracentesis plus fluid replacement.
Implications for research.
Further well‐designed randomised clinical trials are necessary. Some aspects of the design of the randomised clinical trials are as follows.
Study design: parallel, randomised clinical trial
Participants: people with liver cirrhosis and grade 3 or diuretic‐refractory ascites
Interventions/control: transjugular intrahepatic portosystemic shunt versus diuretics plus paracentesis plus fluid replacement versus paracentesis plus fluid replacement.
Outcomes:
Primary outcome: medium‐term mortality (one‐year all‐cause mortality)
Secondary outcomes: health‐related quality of life, decompensation events, adverse events, resolution of ascites, and resource utilisation measures including length of hospital stay, costs
Minimum length of follow‐up: one year
Sample size:
For a simple two‐arm parallel randomised clinical trial, the sample size required to detect or reject a relative risk reduction of 20% in the experimental group from the control group proportion of 36.8% mortality, with type I error of 5%, and type II error of 20%, 1282 participants are required.
Other aspects:
Trials need to be conducted and reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement (Chan 2013) and CONSORT statement (Schulz 2010).
Notes
We based the Methods section of this protocol on a standard Cochrane Hepato‐Biliary Group template incorporating advice by the Complex Reviews Support Unit for a network meta‐analysis protocol (Best 2018).
Acknowledgements
We acknowledge the help and support of the Cochrane Hepato‐Biliary Group. We also thank the copy‐editors, Cochrane Central Editorial Unit, and the following peer referees who provided comments to improve the protocol and the review.
Peer reviewers (protocol): Michael Volk, USA; Yogesh Puri, UK Peer reviewers (review): Nadim Mahmud, USA; Christiana Kartsonaki, UK Contact Editor: Christian Gluud, Denmark Sign‐off Editor: Christian Gluud, Denmark
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
This project was funded by the National Institute for Health Research (NIHR) Systematic Reviews Programme (project number 16/114/17) and was supported by the Complex Reviews Support Unit, also funded by the National Institute for Health Research (project number 14/178/29).
Department of Health Disclaimer
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the 16/114/17 or 14/178/29 Programmes, the NIHR, the NHS, or the Department of Health.
Danish State and the Copenhagen Trial Unit Disclaimer
The views and opinions expressed in this Cochrane Review are those of the review authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | Issue 5, 2019 | #1 MeSH descriptor: [Ascites] this term only #2 ascites #3 #1 or #2 #4 MeSH descriptor: [Liver Cirrhosis] explode all trees #5 ((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)) #6 #4 or #5 #7 #3 and #6 |
| MEDLINE Ovid | January 1947 to May 2019 | 1. ascites/ 2. ascites.ti,ab. 3. 1 or 2 4. exp Liver Cirrhosis/ 5. ((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)).ti,ab. 6. 4 or 5 7. 3 and 6 8. randomized controlled trial.pt. 9. controlled clinical trial.pt. 10. randomized.ab. 11. placebo.ab. 12. drug therapy.fs. 13. randomly.ab. 14. trial.ab. 15. groups.ab. 16. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. exp animals/ not humans.sh. 18. 16 not 17 19. 7 and 18 |
| Embase Ovid | January 1974 to May 2019 | 1. exp ascites/ 2. ascites.ti,ab. 3. 1 or 2 4. exp liver cirrhosis/ 5. ((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)).ti,ab. 6. 4 or 5 7. 3 and 6 8. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 9. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 10. 8 or 9 11. 7 and 10 |
| Science Citation Index Expanded (Web of Science) | January 1945 to May 2019 | #1 TS=(ascites)
#2 TS=((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)) #3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #4 #1 AND #2 AND #3 |
| ClinicalTrials.gov | May 2019 | cirrhosis | Interventional Studies | Ascites | Phase 2, 3, 4 |
| World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx) | May 2019 | ascites |
| European Medical Agency (www.ema.europa.eu/ema/) and USA Food and Drug Administration (www.fda.gov) | May 2019 | ascites; cirrhosis; random |
Appendix 2. Data
This table is too wide to be displayed in RevMan. This table can be found at: https://doi.org/10.5281/zenodo.3604801.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acharya 1992.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 1988‐1989
Number randomised: 40
Post‐randomisation dropouts: not stated
Revised sample size: 40
Average age (years): 43
Females: 10 (25.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 40 (100.0%)
Refractory or recurrent ascites: 34 (85.0%)
Alcohol‐related cirrhosis: 11 (27.5%)
Viral‐related cirrhosis: 19 (47.5%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 10 (25.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion 1. Other features of decompensation 2. Cardiac, renal, or respiratory diseases 3. Hyponatraemia |
|
| Interventions | Group 1: Aldosterone antagonists plus paracentesis plus fluid replacement (n = 20) Further details: Spironolactone 100 mg/day after resolution of ascites + large volume paracentesis 5 litres daily and supported by dextran (30% to 50% of ascitic fluid removed) Group 2: Aldosterone antagonists plus loop diuretics (n = 20) Further details: Spironolactone 200 mg/day + furosemide 40 mg/day doubled after third day for 15 days (route not stated) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), other features of decompensation at maximal follow‐up Follow‐up (months): 0.5 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Al Sebaey 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Egypt
Period of recruitment: not stated
Number randomised: 125
Post‐randomisation dropouts: not stated
Revised sample size: 125
Average age (years): 50
Females: 56 (44.8%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 125 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion 1. Hypertension 2. Cardiac or respiratory disease 3. Other features of decompensation |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 50) Further details: Large volume paracentesis (details not available) + terlipressin 1 mg at onset of LVP, 8 hours, and 16 hours or midodrine 5 to 10 mg orally TDS for 3 days) Group 2: Paracentesis plus fluid replacement (n = 75) Further details: Large volume paracentesis (no further details) + HES or low dose albumin or high dose albumin | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Ali 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Egypt
Period of recruitment: 2012
Number randomised: 66
Post‐randomisation dropouts: 6 (9.1%)
Revised sample size: 60
Reasons for post‐randomisation dropouts: lost to follow‐up
Average age (years): 57
Females: not stated
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 60 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion 1. Acute or chronic renal failure 2. Hypertension 3. Heart diseases 4. Other features of decompensation 5. Hepatocellular carcinoma (HCC) 6. Portal vein thrombosis |
|
| Interventions | Group 1: Systemic vasoconstrictors (n = 30) Further details: Midodrine (dose not clear, but probably 2.5 mg TDS) for 2 weeks Group 2: No active treatment (n = 30) Further details: placebo | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people) Follow‐up (months): 0.5 | |
| Notes | Source of funding (quote): "No funding (author replies)" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation procedures were automated, using centrally‐allocated computer‐generated random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation procedures were automated, using centrally‐allocated computer‐generated random numbers". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a double‐blind, placebo‐controlled, randomized trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a double‐blind, placebo‐controlled, randomized trial" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there was an equal number of dropouts in the two groups as they were lost‐to‐follow‐up, but not clear if these were related to outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported or obtained by email. |
| Other bias | Low risk | Comment: no other bias noted |
Amin 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Egypt
Period of recruitment: 2009‐2011
Number randomised: 60
Post‐randomisation dropouts: not stated
Revised sample size: 60
Average age (years): not stated
Females: not stated
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 60 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Systemic vasoconstrictors (n = 30) Further details: Midodrine 2.5 mg TDS for 2 weeks Group 2: No active treatment (n = 30) Further details: placebo | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: a placebo was used, but the groups blinded were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: a placebo was used, but the groups blinded were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Appenrodt 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Period of recruitment: 2004‐2006
Number randomised: 24
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 24
Average age (years): 56
Females: 8 (33.3%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 24 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 19 (79.2%)
Viral‐related cirrhosis: 3 (12.5%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 2 (8.3%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion 1. Renal failure 2. Thrombocytopaenia |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 11) Further details: Total paracentesis performed under local anaesthesia and aseptic conditions + midodrine 12.5 mg post‐paracentesis TDS for 2 days Group 2: Paracentesis plus fluid replacement (n = 13) Further details: Total paracentesis performed under local anaesthesia and aseptic conditions + albumin (8 g/L of removed ascites) was infused immediately after the end of paracentesis | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Source of funding (quote): "Authors’ declaration of personal and funding interests: The authors do not have anything to declare". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the patient nor the physician was aware of the treatment arm". Comment: placebo was used to achieve this. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the patient nor the physician was aware of the treatment arm". Comment: placebo was used to achieve this. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Bari 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: 2003‐2010
Number randomised: 27
Post‐randomisation dropouts: 2 (7.4%)
Revised sample size: 25
Reasons for post‐randomisation dropouts: had spontaneous bacterial peritonitis on LVP and did not receive the drug
Average age (years): 58
Females: 3 (12.0%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 25 (100.0%)
Alcohol‐related cirrhosis: 13 (52.0%)
Viral‐related cirrhosis: 7 (28.0%)
Autoimmune disease‐related cirrhosis: 3 (12.0%)
Other causes for cirrhosis: 2 (8.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion 1.Other features of decompensation 2. Cardiac failure |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 12) Further details: Large volume paracentesis (details not available) + midodrine 10 mg oral TDS + long‐acting octreotide 20 mg/month for 6 months Group 2: Paracentesis plus fluid replacement (n = 13) Further details: Large volume paracentesis (details not available) + albumin 8 g/L of ascites removed once | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), any adverse events (number of events), liver transplantation at maximal follow‐up, other features of decompensation at maximal follow‐up Follow‐up (months): 10 | |
| Notes | Source of funding (quote): "Supported by a VA merit review grant and National Institutes of Health grants K‐24 DK02727 and P‐30DK 034989" Trial name/trial registry number: NCT00108355 Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random treatment allocation codes were generated at an independent biostatistical center by the study statistician using SAS version 8.2 (SAS Institute Inc, Cary, NC)". |
| Allocation concealment (selection bias) | Low risk | Quote: "A list with allocation codes was sent to the pharmacy that assigned the participants to interventions based on allocation codes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were 2 post‐randomisation dropouts because of SBP, but the diagnosis would have been made only after the intervention was administered and may or may not be related to the intervention. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Bruno 1992.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: not stated
Number randomised: 35
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 35
Average age (years): 54
Females: 13 (37.1%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 35 (100.0%)
Refractory or recurrent ascites: 35 (100.0%)
Alcohol‐related cirrhosis: 20 (57.1%)
Viral‐related cirrhosis: 7 (20.0%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Hepatocellular carcinoma |
|
| Interventions | Group 1: Paracentesis plus reinfusion (n = 17) Further details: Large volume paracentesis + polyamide fibre haemofilter (FH 88, Gambro) Group 2: Paracentesis plus fluid replacement (n = 18) Further details: Large volume paracentesis + albumin 4 to 6 g/litre of ascites removed | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned by the sealed envelope method on the basis of a computer generated list". |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly assigned by the sealed envelope method on the basis of a computer generated list". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Bureau 2017c.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 2005‐2012
Number randomised: 62
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 62
Average age (years): 57
Females: 18 (29.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 62 (100.0%)
Refractory or recurrent ascites: 62 (100.0%)
Alcohol‐related cirrhosis: 54 (87.1%)
Viral‐related cirrhosis: 4 (6.5%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Severe liver failure, other features of decompensation, or expected liver transplantation in the next months 2. Hepatocellular carcinoma 3. Cardiac failure |
|
| Interventions | Group 1: Transjugular intrahepatic portosystemic shunt (n = 29) Further details: Transjugular intrahepatic portosystemic shunt 10 mm covered stent, dilated to 8 mm to 10 mm Group 2: Paracentesis plus fluid replacement (n = 33) Further details: Large volume paracentesis + albumin 4 to 6 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 12 | |
| Notes | Source of funding (quote): "This work was funded by the French Ministry of Health, by a grant from the Délégation Régionale à la Recherche Clinique des Hôpitaux de Toulouse, and supported by the Gore company". Trial name/trial registry number: NCT00222014 Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was generated online by computer". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Caraceni 2018.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 2011‐2015
Number randomised: 440
Post‐randomisation dropouts: 9 (2.0%)
Revised sample size: 431
Reasons for post‐randomisation dropouts: withdrew consent, wrong inclusion
Average age (years): 61
Females: 135 (31.3%)
Ascites grade 2: 358 (83.1%)
Ascites grade 3: 73 (16.9%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 142 (32.9%)
Viral‐related cirrhosis: 206 (47.8%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 56 (13.0%) Prophylactic antibiotics for subacute bacterial peritonitis: 83 (19.3%) Exclusion criteria 1. Hepatic portosystemic shunt (transjugular intrahepatic portosystemic shunt) 2. Active hepatocellular carcinoma 3. Liver transplantation 4. Ongoing alcohol abuse 5. Extrahepatic organ failure 6. Albumin use for the treatment of ascites in the month preceding enrolment |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + albumin (n = 218) Further details: antialdosteronic drug (no further details) ≥ 200 mg/day + furosemide ≥25 mg/day + human albumin 20% 40 gm twice weekly for 2 weeks and then weekly for 18 months Group 2: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 213) Further details: antialdosteronic drug (no further details) ≥ 200 mg/day + furosemide ≥ 25 mg/day for 18 months | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, quality of life (maximal follow‐up) Follow‐up (months): 18 | |
| Notes | Source of funding (quote): "The trial was funded by the competitive peer‐reviewed grant FARM6P824B from the Italian Medicine Agency". Trial name/trial registry number: 2008–000625–19 and NCT01288794 Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "At the statistical data centre….blinded assignment sequence" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither patients, nor investigators, nor statisticians were masked to treatment assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Neither patients, nor investigators, nor statisticians were masked to treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts, but it was not clear whether they were related to the intervention or the outcome. |
| Selective reporting (reporting bias) | High risk | Comment: outcomes such as adverse events were collected but not reported adequately. |
| Other bias | Low risk | Comment: no other bias noted |
Chang 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: Taiwan, China
Period of recruitment: not stated
Number randomised: 26
Post‐randomisation dropouts: not stated
Revised sample size: 26
Average age (years): 59
Females: 0 (0.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 26 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 0 (0.0%)
Viral‐related cirrhosis: 26 (100.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Cardiac or respiratory disorders 2. Other features of decompensated cirrhosis |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics (n = 13) Further details: Spironolactone 100 to 400 mg/day and furosemide 80 to 240 mg/day oral for 4 to 9 days Group 2: Paracentesis plus fluid replacement (n = 13) Further details: Large volume paracentesis + albumin 6 to 8 g/litre of ascites removed | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Source of funding (quote): "This work was supported by a grant from the National Science Council of the Republic of China (NSC842331‐B075‐005)". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomly allocated to two groups (random number table)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: two patients were excluded from the analysis it was not clear whether this was pre‐randomisation or post‐randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Chesta 1990.
| Methods | Randomised clinical trial | |
| Participants | Country: Chile
Period of recruitment: 1988‐1999
Number randomised: 31
Post‐randomisation dropouts: not stated
Revised sample size: 31
Average age (years): 55
Females: not stated
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 31 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 22 (71.0%)
Viral‐related cirrhosis: 0 (0.0%)
Autoimmune disease‐related cirrhosis: 3 (9.7%)
Other causes for cirrhosis: 6 (19.4%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics (n = 14) Further details: Spironolactone 100 mg/day and if no response within a week, furosemide 40 to 80 mg/day + spironolactone 200 mg oral ‐ duration not stated (until hospital discharge) Group 2: Paracentesis plus fluid replacement (n = 17) Further details: Large volume paracentesis + albumin 4 to 6 g/litre of ascites removed (initial patients over 4 to 5 days; later patients in a single session) | |
| Outcomes | Outcomes reported: any adverse events (number of people), any adverse events (number of events), resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 18 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Descos 1983.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: not stated
Number randomised: 131
Post‐randomisation dropouts: not stated
Revised sample size: 131
Average age (years): 57
Females: 46 (35.1%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 131 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation |
|
| Interventions | Group 1: Aldosterone antagonists (n = 72) Further details: Spironolactone 100 mg to 200 mg/day for 4 weeks Group 2: Paracentesis plus reinfusion (n = 59) Further details: Large volume paracentesis with reinfusion or concentrated ascites or unconcentrated ascites | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 1.2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: ENTAC Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Fernandez‐Esparrach 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: not stated
Number randomised: 36
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 36
Average age (years): 57
Females: 9 (25.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 36 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 24 (66.7%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or kidney disease |
|
| Interventions | Group 1: Aldosterone antagonists plus paracentesis plus fluid replacement (n = 19) Further details: Spironolactone 75 mg TDS for 4 weeks + total paracentesis with IV albumin infusion (8 g per litre of ascitic fluid removed) Group 2: Paracentesis plus fluid replacement (n = 17) Further details: total paracentesis with IV albumin infusion (8 g per litre of ascitic fluid removed) | |
| Outcomes | Outcomes reported: resolution of ascites at maximal follow‐up (by ultrasound) Follow‐up (months): 1 | |
| Notes | Source of funding (quote): "This study was supported by a grant from SEARLE." Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Physicians participating in the study did not know the treatment assigned to each patient". Comment: a placebo was used, but it was not clear whether the participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Physicians participating in the study did not know the treatment assigned to each patient". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Fogel 1981.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: not stated
Number randomised: 90
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 90
Average age (years): 52
Females: 17 (18.9%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 6 (6.7%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Loop diuretics (n = 29) Further details: Furosemide 40 mg to 400 mg/day for 6 weeks orally Group 2: Aldosterone antagonists plus loop diuretics (n = 61) Further details: Sequential or combination of spironolactone 100 mg/day + furosemide 40 mg to 120 mg/day oral for 6 weeks | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 1.5 | |
| Notes | Source of funding (quote): "Sponsored in part by a research grant (#76273) from the John A Hartford Foundation" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Gentilini 1999a.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 1993‐1996
Number randomised: 126
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 126
Average age (years): 62
Females: 59 (46.8%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 16 (12.7%)
Viral‐related cirrhosis: 104 (82.5%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or kidney disease 3. Hepatocellular carcinoma |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + albumin (n = 63) Further details: Albumin 12.5 mg/day IV weekly + potassium canreonate 200 mg to 400 mg/day and furosemide 40 mg to 160 mg/day for 3 years Group 2: Aldosterone antagonists plus loop diuretics (n = 63) Further details: Potassium canreonate 200 mg to 400 mg/day and furosemide 40 mg to 160 mg/day for 3 years | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 20 | |
| Notes | Source of funding (quote): "Supported by grants from the Consiglio Nazionale delle Ricerche, Rome, Ministero Italiano dell’universita e della Ricerca Scientilica e Tecnologica (Progetto Nazionale Epatiti Virali e Cirrosi Epatica), Rome, and the Italian Liver Foundation, Florence, Italy" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Gines 1987.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: 1983‐1985
Number randomised: 117
Post‐randomisation dropouts: not stated
Revised sample size: 117
Average age (years): 57
Females: 40 (34.2%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 117 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 83 (70.9%)
Viral‐related cirrhosis: 7 (6.0%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 27 (23.1%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Hepatocellular carcinoma |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics (n = 59) Further details: Spironolactone 200 to 400 mg/day and furosemide 40 to 240 mg/day oral duration not stated, probably until follow‐up Group 2: Paracentesis plus fluid replacement (n = 58) Further details: Repeated paracentesis removing 4 to 6 litres per day + 40 g albumin after each paracentesis | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 11 | |
| Notes | Source of funding (quote): "This work was supported by grants from Comision Asesora de investigacion Cientffico y Tecnica (CAICYT 2643‐83 and 2114‐81) and from Fondo de Investigaciones Sanitarias do la Seguridad Social (FISS, 82‐410)". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomly allocated (random number table)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Ginès 1991.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: not stated
Number randomised: 89
Post‐randomisation dropouts: not stated
Revised sample size: 89
Average age (years): 56
Females: 25 (28.1%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 89 (100.0%)
Alcohol‐related cirrhosis: 65 (73.0%)
Viral‐related cirrhosis: 5 (5.6%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 19 (21.3%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + peritoneovenous shunt (n = 48) Further details: Le Veen shunt + spironolactone 200 mg/day + furosemide 80 mg/day Group 2: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 41) Further details: Repeated paracentesis removing 4 to 6 litres per day + 200 mL of 20% albumin for each paracentesis + spironolactone 200 mg/day + furosemide 80 mg/day | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 14 | |
| Notes | Source of funding (quote): "Supported by a grant (2018/84) from the Fondo de Investigaciones Sanitarias de la Seguridad Social and by the Fundació Catalana per a l'Estudi de les Malalties del Fetge" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients from each hospital were then randomly assigned to two groups with use of a random‐number table". Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Ginès 1995.
| Methods | Randomised clinical trial | |
| Participants | Country: Multiple
Period of recruitment: not stated
Number randomised: 81
Post‐randomisation dropouts: not stated
Revised sample size: 81
Average age (years): 61
Females: 36 (44.4%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 81 (100.0%)
Alcohol‐related cirrhosis: 41 (50.6%)
Viral‐related cirrhosis: 32 (39.5%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 8 (9.9%) Prophylactic antibiotics for subacute bacterial peritonitis: stated only for surgical group Exclusion criteria 1. Other features of decompensation |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + peritoneovenous shunt (n = 39) Further details: Le Veen shunt + spironolactone 200 mg/day + furosemide 80 mg/day Group 2: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 42) Further details: Repeated paracentesis removing 4 to 6 litres per day + 200 mL of 20% albumin for each paracentesis + spironolactone 200 mg/day + furosemide 80 mg/day | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 10 | |
| Notes | Source of funding (quote): "Supported by grants from Fondo de Investigaciones Sanitarias de la Seguridad Social (FISS 93/0610) and Direccion General de Investigacion Cientifica y Tecnica (DGICYT PM 91‐0216). A. Gin,s and J. Sal5 were granted by FISS (91/5549 and 93/0610, respectively)". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Ginès 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Multiple
Period of recruitment: 1996‐2000
Number randomised: 70
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 70
Average age (years): 58
Females: 20 (28.6%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 70 (100.0%)
Alcohol‐related cirrhosis: 39 (55.7%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or kidney disease 3. Hepatocellular carcinoma |
|
| Interventions | Group 1: Transjugular intrahepatic portosystemic shunt (n = 35) Further details: Transjugular intrahepatic portosystemic shunt, dilated to 8 mm to 10 mm Group 2: Paracentesis plus fluid replacement (n = 35) Further details: total paracentesis + albumin 8 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of events), liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 10 | |
| Notes | Source of funding (quote): "Supported by grants from the Fondo de Investigacio´n Sanitaria (Spain) (FIS 97/2073 and 00/0616) and the Veterans Administration Merit Review and NIH‐1K24‐DK 02727 (USA)" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization (sealed opaque envelopes) was centralized". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Graziotto 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: Multiple
Period of recruitment: 1990‐1992
Number randomised: 24
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 24
Average age (years): 57
Females: 7 (29.2%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 24 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation |
|
| Interventions | Group 1: Paracentesis plus reinfusion (n = 12) Further details: Large volume paracentesis + apheresis and reinfusion of concentrated ascites (Albusave BT 902 or Hemofilter Pan 15) Group 2: Paracentesis plus fluid replacement (n = 12) Further details: Large volume paracentesis + albumin 6 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound) Follow‐up (months): 20 | |
| Notes | Source of funding (quote): "We would like to thank Mr. Libero Barbieri and Dr. Leonardo Bigi from Dideco Co., Mirandola, Modena, Italy, for their expert technical assistance". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "using a closed envelope system" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Gregory 1977.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: not stated
Number randomised: 43
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 43
Average age (years): 48
Females: 9 (20.9%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 43 (100.0%)
Viral‐related cirrhosis: 0 (0.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: No active treatment (n = 21) Further details: placebo Group 2: Aldosterone antagonists plus loop diuretics (n = 22) Further details: Spironolactone 100 to 400 mg/day and furosemide 40 mg/day oral and then increased in incremental steps of 40 mg (maximum dose not stated), duration not stated ‐ probably until follow‐up | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: placebo was used but no information on blinding was provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: placebo was used but no information on blinding was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Hagege 1992.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: not stated
Number randomised: 53
Post‐randomisation dropouts: not stated
Revised sample size: 53
Average age (years): 56
Females: 16 (30.2%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 48 (90.6%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Heptocellular carcinoma |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics (n = 27) Further details: Spironolactone 225 to 300 mg/day and furosemide 40 mg to 80/day oral , duration not stated ‐ probably until follow‐up Group 2: Paracentesis plus fluid replacement (n = 26) Further details: paracentesis up to 4 litres/day + albumin 10 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of people), liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 3 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The treatment was designated by random draw". |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment was designated by random draw". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "there were patients excluded after randomization (author replies)". Comment: there were post‐randomisation dropouts; it was not clear whether they were related to intervention or outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported or obtained by email. |
| Other bias | Low risk | Comment: no other bias noted |
Hamdy 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Egypt
Period of recruitment: 2010‐2012
Number randomised: 50
Post‐randomisation dropouts: not stated
Revised sample size: 50
Average age (years): 57
Females: 12 (24.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 50 (100.0%)
Refractory or recurrent ascites: 50 (100.0%)
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or respiratory disease |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 25) Further details: Large volume paracentesis + midodrine 12.5 mg TDS for 3 days Group 2: Paracentesis plus fluid replacement (n = 25) Further details: Large volume paracentesis + albumin 8 g/litre of ascites removed | |
| Outcomes | Outcomes reported: treatment costs Follow‐up (months): 0.25 | |
| Notes | Source of funding (quote): "The authors declare that they have nothing to disclose". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Lata 2007.
| Methods | Randomised clinical trial | |
| Participants | Country: Czech Republic
Period of recruitment: 2002‐2004
Number randomised: 49
Post‐randomisation dropouts: not stated
Revised sample size: 49
Average age (years): 57
Females: 15 (30.6%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 49 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 29 (59.2%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Ischaemic heart disease |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 24) Further details: Large volume paracentesis + terlipressin 1 mg every 4 hours for 2 days Group 2: Paracentesis plus fluid replacement (n = 25) Further details: Large volume paracentesis + albumin 8 g/litre of ascites removed | |
| Outcomes | Outcomes reported: serious adverse events (number of people), other features of decompensation at maximal follow‐up Follow‐up (months): 0.25 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Lebrec 1996.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1992‐1994
Number randomised: 25
Post‐randomisation dropouts: not stated
Revised sample size: 25
Average age (years): 51
Females: 7 (28.0%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 25 (100.0%)
Alcohol‐related cirrhosis: 20 (80.0%)
Viral‐related cirrhosis: 5 (20.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Heart disease 3. Hepatocellular carcinoma |
|
| Interventions | Group 1: Transjugular intrahepatic portosystemic shunt (n = 13) Further details: Transjugular intrahepatic portosystemic shunt (performed after paracentesis), expanded to a diameter of 10 mm Group 2: Paracentesis plus fluid replacement (n = 12) Further details: Large volume paracentesis + albumin, no further details | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 12 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Licata 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 2002‐2007
Number randomised: 84
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 84
Average age (years): 64
Females: 31 (36.9%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 84 (100.0%)
Alcohol‐related cirrhosis: 13 (15.5%)
Viral‐related cirrhosis: 70 (83.3%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 1 (1.2%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Heart failure 3. Hepatocellular carcinoma |
|
| Interventions | Group 1: Loop diuretics (n = 60) Further details: High dose furosemide 250 mg to 1000 mg BD until 3 days before discharge along with hypertonic saline infusion Group 2: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 24) Further details: Repeated paracentesis removing 4 to 6 litres per day + albumin 5 to 8 g/litre removed + spironolactone 400 mg/day + furosemide up to 160 mg/day until 3 days before discharge | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 0.3 | |
| Notes | Source of funding (quote): "Declaration of personal and funding interests: None" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned by the use of sequentially numbered boxes (prepared before starting the study by a computerized, non‐alternating sequence)". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned by the use of sequentially numbered boxes (prepared before starting the study by a computerized, non‐alternating sequence)". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Ljubici 1994.
| Methods | Randomised clinical trial | |
| Participants | Country: Croatia
Period of recruitment: 1990‐1992
Number randomised: 21
Post‐randomisation dropouts: not stated
Revised sample size: 21
Average age (years): 56
Females: 10 (47.6%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 21 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 21 (100.0%)
Viral‐related cirrhosis: 0 (0.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 10) Further details: Large volume paracentesis removing 5 to 6 litres + albumin 6 to 8 g/litre removed + spironolactone 200 mg/day + furosemide up to 40 to 80 mg/day stepped treatment duration not reported probably for the follow‐up period Group 2: Aldosterone antagonists plus loop diuretics (n = 11) Further details: Spironolactone 200 mg/day + furosemide up to 40 to 80 mg/day stepped treatment duration not reported probably for the follow‐up period | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people) Follow‐up (months): 3 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On the third day of admission, patients were randomly allocated to two groups (random number table)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: some patients were excluded, but it was not clear whether they were excluded pre‐randomisation or post‐randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Mchutchison 1989.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: not stated
Number randomised: 21
Post‐randomisation dropouts: not stated
Revised sample size: 21
Average age (years): not stated
Females: not stated
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Thiazide diuretics (n = 11) Further details: hydrochlorothiazide 50 mg oral daily for 3 days Group 2: Loop diuretics (n = 10) Further details: furosemide 240 mg oral daily for 3 days Additional details: The intervention and control numbers were not reported. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Mehta 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: not stated
Number randomised: 20
Post‐randomisation dropouts: not stated
Revised sample size: 20
Average age (years): 52
Females: 4 (20.0%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 13 (65.0%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Malignancy 3. Pregnancy |
|
| Interventions | Group 1: Paracentesis plus reinfusion (n = 10) Further details: Large volume paracentesis + haemodialysis and reinfusion Group 2: Paracentesis plus fluid replacement (n = 10) Further details: Large volume paracentesis + polymerised gelatin haemaccel 150 mL/litre of ascites removed | |
| Outcomes | Outcomes reported: treatment costs Follow‐up (months): 3 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Moreau 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1998‐2000
Number randomised: 24
Post‐randomisation dropouts: 4 (16.7%)
Revised sample size: 20
Reasons for post‐randomisation dropouts: did not receive paracentesis (3) and had high plasma renin level (1)
Average age (years): 54
Females: 4 (20.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 20 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 17 (85.0%)
Viral‐related cirrhosis: 3 (15.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or respiratory disease |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 10) Further details: Total paracentesis + terlipressin 1 mg three doses at paracentesis, 8 hours, and 16 hours Group 2: Paracentesis plus fluid replacement (n = 10) Further details: Total paracentesis + albumin (8 g/L of removed ascites) was infused immediately after the end of paracentesis. | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 3 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts. at least one of them could be related to the treatment, but it was not clear whether this would have affected the treatment effect. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Narahara 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Period of recruitment: 2000‐2007
Number randomised: 60
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 60
Average age (years): 60
Females: 16 (26.7%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 60 (100.0%)
Alcohol‐related cirrhosis: 21 (35.0%)
Viral‐related cirrhosis: 33 (55.0%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or respiratory disease 3. Malignancy |
|
| Interventions | Group 1: Transjugular intrahepatic portosystemic shunt (n = 30) Further details: Transjugular intrahepatic portosystemic shunt (expandable stent) ‐ initially dilated to 6 to 8 mm and further dilated to 8 mm to 10 mm depending upon portoSystemic pressure gradient Group 2: Paracentesis plus fluid replacement (n = 30) Further details: Large volume paracentesis + albumin 6 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of people), liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 20 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by selecting an opaque sealed envelope that was numbered according to a random number table". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by selecting an opaque sealed envelope that was numbered according to a random number table". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Rai 2017.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 2013‐2015
Number randomised: 25
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 25
Average age (years): 48
Females: 6 (24.0%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 25 (100.0%)
Alcohol‐related cirrhosis: 16 (64.0%)
Viral‐related cirrhosis: 6 (24.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 3 (12.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac disease 3. Hepatocellular carcinoma |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + systemic vasoconstrictors + paracentesis plus fluid replacement (n = 13) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day + midodrine 7.5 mg TDS for 3 months + large volume paracentesis + albumin 8 g/litre of ascites removed Group 2: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 12) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day + large volume paracentesis + albumin 8 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of events), liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 3 | |
| Notes | Source of funding (quote): "Funding information: None" Trial name/trial registry number: NCT02173288 Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was computer‐generated and the allocation was concealed in opaque sealed envelopes". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization sequence was computer‐generated and the allocation was concealed in opaque sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients and investigators were not blinded to the treatment assignments". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Patients and investigators were not blinded to the treatment assignments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Raza 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: Pakistan
Period of recruitment: 2009‐2010
Number randomised: 60
Post‐randomisation dropouts: not stated
Revised sample size: 60
Average age (years): 51
Females: 27 (45.0%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 60 (100.0%)
Alcohol‐related cirrhosis: 0 (0.0%)
Viral‐related cirrhosis: 57 (95.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 3 (5.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Hepatocellular carcinoma |
|
| Interventions | Group 1: Osmotic diuretics (n = 30) Further details: Mannitol 30 g IV ‐ number of doses and duration not clear, but appeared to be a single dose Group 2: No active treatment (n = 30) Further details: placebo | |
| Outcomes | Outcomes reported: serious adverse events (number of people) Follow‐up (months): 0.2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "It was a double‐blind trial, so that neither the patient nor the observer knew whether drug or placebo was given". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "It was a double‐blind trial, so that neither the patient nor the observer knew whether drug or placebo was given". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Romanelli 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 1993‐2003
Number randomised: 100
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 100
Average age (years): 63
Females: 38 (38.0%)
Ascites grade 2: 65 (65.0%)
Ascites grade 3: 35 (35.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 2 (2.0%)
Viral‐related cirrhosis: 79 (79.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 19 (19.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + albumin (n = 54) Further details: Albumin 25 mg/day IV weekly for first year and after that, the same once every 2 weeks + spironolactone 100 mg to 400 mg/day and furosemide 25 mg to 150 mg/day for duration of follow‐up Group 2: Aldosterone antagonists plus loop diuretics (n = 46) Further details: Spironolactone 100 mg to 400 mg/day and furosemide 25 mg to 150 mg/day for duration of follow‐up | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 84 | |
| Notes | Source of funding (quote): "Supported by grants from the Italian Ministry of Education, University and Research and the University of Florence, Italy" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation schedule was generated using a computed random number generation system". |
| Allocation concealment (selection bias) | Low risk | Quote: "using sealed envelopes containing the treatment assignments" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Rossle 2000.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Period of recruitment: 1993‐1997
Number randomised: 60
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 60
Average age (years): 60
Females: 18 (30.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 60 (100.0%)
Refractory or recurrent ascites: 60 (100.0%)
Alcohol‐related cirrhosis: 47 (78.3%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Advanced cancer |
|
| Interventions | Group 1: Transjugular intrahepatic portosystemic shunt (n = 29) Further details: Transjugular intrahepatic portosystemic shunt (expandable stent: Palmaz–Schatz stent or a self‐expandable nitinol stent (Memotherm)) Group 2: Paracentesis plus fluid replacement (n = 31) Further details: Large volume paracentesis + albumin 8 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 44.5 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Salerno 1987.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 1985‐1986
Number randomised: 41
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 41
Average age (years): 55
Females: 9 (22.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 41 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 20 (48.8%)
Viral‐related cirrhosis: 7 (17.1%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 9 (22.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cancer |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics (n = 21) Further details: Spironolactone 200 to 400 mg/day and furosemide 50 mg/day oral added as necessary, duration not stated ‐ probably until follow‐up Group 2: Paracentesis plus fluid replacement (n = 20) Further details: paracentesis up to 4 litres/day + albumin 20 g to 60 g depending on ascites removed each day | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of events), resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 4 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Salerno 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 1996‐2002
Number randomised: 66
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 66
Average age (years): 59
Females: 17 (25.8%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 66 (100.0%)
Alcohol‐related cirrhosis: 28 (42.4%)
Viral‐related cirrhosis: 31 (47.0%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cancer |
|
| Interventions | Group 1: Transjugular intrahepatic portosystemic shunt (n = 33) Further details: Transjugular intrahepatic portosystemic shunt dilated to obtain a portal pressure gradient of 12 mmHg Group 2: Paracentesis plus fluid replacement (n = 33) Further details: Large volume paracentesis + albumin 8 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 18 | |
| Notes | Source of funding (quote): "Supported by grants of the Ministero dell’Universita` Italiana and of the Ospedale Maggiore Policlinico Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) of Milan" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization (sealed opaque envelopes)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Unblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Sanyal 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: 1997‐2000
Number randomised: 109
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 109
Average age (years): 54
Females: 37 (33.9%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 109 (100.0%)
Alcohol‐related cirrhosis: 65 (59.6%)
Viral‐related cirrhosis: 27 (24.8%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Conditions likely to limit life expectance to < 1 year 2. Acute renal failure or renal diseases 3. Cardiac failure |
|
| Interventions | Group 1: Transjugular intrahepatic portosystemic shunt (n = 52) Further details: Transjugular intrahepatic portosystemic shunt (no further details) was performed after large volume paracentesis. Group 2: Paracentesis plus fluid replacement (n = 57) Further details: Large volume paracentesis + albumin 6 to 8 g/litre of ascites removed | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 12 | |
| Notes | Source of funding (quote): "Supported by grant RO1 DK 51523 from the National Institutes of Health (to A.J.S.) and MO1‐RR‐00065" Trial name/trial registry number: NASTRA Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were then randomized centrally to either the medical therapy arm (defined by restriction of sodium, treatment with diuretics, and repeated TP as needed) or the transjugular intrahepatic portosystemic shunt arm". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Schaub 1995.
| Methods | Randomised clinical trial | |
| Participants | Country: Switzerland
Period of recruitment: not stated
Number randomised: 20
Post‐randomisation dropouts: 3 (15.0%)
Revised sample size: 17
Reasons for post‐randomisation dropouts: not stated
Average age (years): not stated
Females: not stated
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 18 (105.9%)
Viral‐related cirrhosis: 2 (11.8%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics (n = 9) Further details: Spironolactone 200 mg/day + furosemide 40 mg/day, duration not stated Group 2: Paracentesis plus fluid replacement (n = 8) Further details: Large volume paracentesis over 2 days + albumin 60 g for each puncture | |
| Outcomes | Outcomes reported: length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 0.75 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts but the reasons were not reported; therefore, it was difficult to judge whether this would have led to biased treatment effect. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Singh 2006a.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 2004‐2005
Number randomised: 40
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 40
Average age (years): 48
Females: 8 (20.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 40 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 26 (65.0%)
Viral‐related cirrhosis: 6 (15.0%)
Autoimmune disease‐related cirrhosis: 1 (2.5%)
Other causes for cirrhosis: 7 (17.5%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or respiratory disease |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 20) Further details: Total paracentesis + noradrenaline 0.5 mg/hr titrated to maintain mean arterial pressure about 10 mmHg above baseline for 72 hours Group 2: Paracentesis plus fluid replacement (n = 20) Further details: Total paracentesis + albumin (8 g/L of removed ascites) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of people), liver transplantation at maximal follow‐up, treatment costs Follow‐up (months): 0.25 | |
| Notes | Source of funding (quote): "No major funding. If patients were unable to purchase medications, we assisted them and they were provided medications (author replies)". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated (author replies)" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed envelopes (author replies)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported or obtained by email. |
| Other bias | Low risk | Comment: no other bias noted |
Singh 2006b.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 2002‐2003
Number randomised: 43
Post‐randomisation dropouts: 3 (7.0%)
Revised sample size: 40
Reasons for post‐randomisation dropouts: GI bleed, abdominal tuberculosis
Average age (years): 47
Females: 4 (10.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 40 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 28 (70.0%)
Viral‐related cirrhosis: 8 (20.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Prophylactic antibiotics for subacute bacterial peritonitis: not stated
Other causes for cirrhosis: 4 (10.0%) Exclusion criteria 1. Other features of decompensation 2. Cardiac or respiratory disease |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 20) Further details: Total paracentesis + terlipressin 1 mg at 0, 8, and 16 hours of paracentesis Group 2: Paracentesis plus fluid replacement (n = 20) Further details: Total paracentesis + albumin (8 g/L of removed ascites) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of people), liver transplantation at maximal follow‐up Follow‐up (months): 0.25 | |
| Notes | Source of funding (quote): "No major funding. If patients were unable to purchase medications, we assisted them and they were provided medications (author replies)". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated (author replies)" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed envelopes (author replies)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts, but it was not clear whether these could be related to the intervention or outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported or obtained by email. |
| Other bias | Low risk | Comment: no other bias noted |
Singh 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 2005‐2006
Number randomised: 40
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 40
Average age (years): 47
Females: 5 (12.5%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 40 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 26 (65.0%)
Viral‐related cirrhosis: 9 (22.5%)
Autoimmune disease‐related cirrhosis: 1 (2.5%)
Other causes for cirrhosis: 4 (10.0%) prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or respiratory disease |
|
| Interventions | Group 1: Paracentesis plus systemic vasoconstrictors (n = 20) Further details: Total paracentesis + midodrine 5 to 10 mg TDS for 72 hours Group 2: Paracentesis plus fluid replacement (n = 20) Further details: Total paracentesis + albumin (8 g/L of removed ascites) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of people), liver transplantation at maximal follow‐up, other features of decompensation at maximal follow‐up, treatment costs Follow‐up (months): 2 | |
| Notes | Source of funding (quote): "Financial support: None" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated (author replies)" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed envelopes (author replies)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "No blinding (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported or obtained by email. |
| Other bias | Low risk | Comment: no other bias noted |
Singh 2012a.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 2007‐2009
Number randomised: 40
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 40
Average age (years): 47
Females: 3 (7.5%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 40 (100.0%)
Alcohol‐related cirrhosis: 29 (72.5%)
Viral‐related cirrhosis: 10 (25.0%)
Autoimmune disease‐related cirrhosis: 1 (2.5%)
Other causes for cirrhosis: 0 (0.0%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation Years of recruitment: 2007‐2009 |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + systemic vasoconstrictors (n = 20) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day + midodrine 7.5 mg TDS oral for a mean of 2 months Group 2: Aldosterone antagonists plus loop diuretics (n = 20) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day, duration not stated | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound) Follow‐up (months): 6 | |
| Notes | Source of funding (quote): "The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer made randomization code" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed envelopes (author replies)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients and investigators were blinded to the treatment assignments". Comment: it was not clear how they were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients and investigators were blinded to the treatment assignments". Comment: it was not clear how they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported or obtained by email. |
| Other bias | Low risk | Comment: no other bias noted |
Singh 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 2010‐2011
Number randomised: 60
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 60
Average age (years): 53
Females: 4 (6.7%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 60 (100.0%)
Alcohol‐related cirrhosis: 49 (81.7%)
Viral‐related cirrhosis: 7 (11.7%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 5 (8.3%) Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + systemic vasoconstrictors + systemic vasodilator (n = 15) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day + midodrine 7.5 mg TDS oral + clonidine 0.1 mg BD or both until endpoints were reached ‐ probably 1 month Group 2: Aldosterone antagonists plus loop diuretics + systemic vasoconstrictors (n = 15) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day + midodrine 7.5 mg TDS oral until endpoints were reached ‐ probably 1 month Group 3: Aldosterone antagonists plus loop diuretics + systemic vasodilator (n = 15) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day + clonidine 0.1 mg BD until endpoints were reached ‐ probably 1 month Group 4: Aldosterone antagonists plus loop diuretics (n = 15) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 160 mg/day duration until endpoints were reached ‐ probably 1 month | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of events), liver transplantation at maximal follow‐up, resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 1 | |
| Notes | Source of funding (quote): "Financial support: None" Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer made the randomization code with 60 envelopes, with 15 patients in each group". |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer made the randomization code with 60 envelopes, with 15 patients in each group". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients and investigators were not blinded to the treatment assignments". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Patients and investigators were not blinded to the treatment assignments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported or obtained by email. |
| Other bias | Low risk | Comment: no other bias noted |
Sola 1994.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: not stated
Number randomised: 80
Post‐randomisation dropouts: 9 (11.3%)
Revised sample size: 71
Reasons for post‐randomisation dropouts: cross‐over or lost to follow‐up
Average age (years): 59
Females: 22 (31.0%)
Ascites grade 2: 0 (0.0%)
Ascites grade 3: 71 (100.0%)
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 63 (88.7%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 38) Further details: Total paracentesis + dextran‐40 (8 g/L of removed ascites) + spironolactone 100 to 400 mg/day + furosemide 40 to 240 mg/day; duration not reported, probably end of follow‐up Group 2: Aldosterone antagonists plus loop diuretics (n = 33) Further details: Spironolactone 100 to 400 mg/day + furosemide 40 to 240 mg/day; duration not reported, probably end of follow‐up | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, other features of decompensation at maximal follow‐up Follow‐up (months): 13 | |
| Notes | Source of funding (quote): "This work was supported by a grant from the Institut Municipal d'Investigaci6 Medica (IMIM) IM 876413601." Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts, but it is not clear whether this could have led to biased treatment effects. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Sola 2018.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: 2008‐2015
Number randomised: 196
Post‐randomisation dropouts: 23 (11.7%)
Revised sample size: 173
Reasons for post‐randomisation dropouts: liver transplantation, death, incorrect randomisation, withdrawal of consent
Average age (years): 55
Females: 36 (20.8%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 72 (41.6%)
Viral‐related cirrhosis: 80 (46.2%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: both Other inclusion criteria 1. Patients awaiting liver transplantation |
|
| Interventions | Group 1: Systemic vasoconstrictors + albumin (n = 87) Further details: Midodrine 5 mg TDS orally (increased up to 30 mg daily) + albumin IV 40 mg every 15 days for 1 year Group 2: No active treatment (n = 86) Further details: placebo | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of people), any adverse events (number of events), liver transplantation at maximal follow‐up, other features of decompensation at maximal follow‐up Follow‐up (months): 11 | |
| Notes | Source of funding (quote): "No economic support was provided by the companies, except that Grifols S.A. (Spain) gave a donation to support the transport costs incurred by patients participating in the study". Trial name/trial registry number: NCT00839358 Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated…" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed at the CTU of the Hospital Clínic of Barcelona". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All investigators and patients were blinded to treatment assignment..placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All investigators and patients were blinded to treatment assignment..placebo". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts, which were probably related to intervention and outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: a pre‐published protocol was not available, but the important outcomes were reported. |
| Other bias | Low risk | Comment: no other bias noted |
Stanley 1989b.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: not stated
Number randomised: 299
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 299
Average age (years): not stated
Females: not stated
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: not stated
Alcohol‐related cirrhosis: 299 (100.0%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated |
|
| Interventions | Group 1: Peritoneovenous shunt (n = 146) Further details: Le Veen shunt Group 2: Aldosterone antagonists plus loop diuretics (n = 153) Further details: spironolactone 100 to 400 mg/day + furosemide 40 to 320 mg/day | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Source of funding (quote): "We are indebted to Becton Dickinson (Rutherford, NJ.) for its donation of the LeVeen shunts". Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient who consented to participate was assigned to a treatment group by telephone by the Cooperative Studies Program Coordinating Center after his eligibility had been verified". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Strauss 1991.
| Methods | Randomised clinical trial | |
| Participants | Country: Brazil
Period of recruitment: 1995‐1990
Number randomised: 33
Post‐randomisation dropouts: 2 (6.1%)
Revised sample size: 31
Reasons for post‐randomisation dropouts: death before starting treatment and diagnosed with HRS
Average age (years): 52
Females: 7 (22.6%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 31 (100.0%)
Alcohol‐related cirrhosis: 19 (61.3%)
Viral‐related cirrhosis: 6 (19.4%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Cardiac or renal disease |
|
| Interventions | Group 1: Aldosterone antagonists plus loop diuretics + paracentesis plus fluid replacement (n = 16) Further details: Repeated paracentesis + albumin (20 g paracentesis) + spironolactone 150 to 300 mg/day + furosemide 40 to 80 mg/day; duration not reported, probably end of follow‐up Group 2: Aldosterone antagonists plus loop diuretics (n = 15) Further details: Spironolactone 150 to 300 mg/day + furosemide 40 to 80 mg/day; duration not reported, probably end of follow‐up | |
| Outcomes | Outcomes reported: resolution of ascites at maximal follow‐up (by ultrasound), other features of decompensation at maximal follow‐up Follow‐up (months): 0.5 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "statistical table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "system of sealed envelopes, numbered successively, after the selection of each patient for the study, the doctor made the opening of the next envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts, but it was not clear whether this could be related to intervention or could have affected the treatment effect. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
Tuttolomondo 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 2013‐2015
Number randomised: 59
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 59
Average age (years): 64
Females: 23 (39.0%)
Ascites grade 2: not stated
Ascites grade 3: not stated
Refractory or recurrent ascites: 59 (100.0%)
Alcohol‐related cirrhosis: 11 (18.6%)
Viral‐related cirrhosis: 48 (81.4%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: not stated Prophylactic antibiotics for subacute bacterial peritonitis: not stated Exclusion criteria 1. Other features of decompensation 2. Heart failure 3. Hepatocellular carcinoma |
|
| Interventions | Group 1: Loop diuretics (n = 31) Further details: High dose furosemide 125 mg to 250 mg BD until 3 days before discharge along with hypertonic saline infusion Group 2: Paracentesis plus fluid replacement (n = 28) Further details: Repeated paracentesis removing 4 to 6 litres per day + albumin 5 to 8 g/litre removed | |
| Outcomes | Outcomes reported: length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 0.3 | |
| Notes | Source of funding (quote): "Author(s) received no specific funding for this work". Trial name/trial registry number: NCT02821377 Attempts were made to contact the authors in November 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: pre‐published protocol was not available. |
| Other bias | Low risk | Comment: no other bias noted |
BD: twice daily HCC: hepatocellular carcinoma HES: hydroxy‐ethyl start HRS: hepatorenal syndrome IV: intravenous LVP: large volume paracentesis SBP: spontaneous bacterial peritonitis TDS: thrice daily TP: therapeutic paracentesis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Khalek 2010b | Comparison of variations in treatment |
| Altman 1998 | Comparison of variations in treatment |
| Angeli 1994 | Comparison of variations in treatment |
| Antillon 1993 | Not a randomised clinical trial |
| Applefeld 1994 | In this cross‐over randomised clinical trial, the cross‐over took place after 12 days of treatment with just 2 days between the cross‐over; this short duration of treatment and cross‐over period is insufficient time to determine the objectives of this review. |
| Arrigoni 1988 | Comparison of variations in treatment |
| Bories 1986 | The diuretics used in the control group was variable ‐ these may or may not have been used; therefore it is not possible to define the control group. |
| Bostan 2019 | Not a randomised clinical trial |
| Boyer 1983 | Not a randomised clinical trial |
| Brater 2001 | Not a randomised clinical trial |
| Bureau 2017a | There were several differences between the intervention and control group: the antibiotic prophylaxis was given only to intervention group, the diuretics were stopped in the intervention but not in the control group and this was variable; therefore, it was not possible to define the control group. |
| Cadranel 1992a | The unit of randomisation was the procedure (i.e. each patient underwent both procedures). |
| Cadranel 1992b | Not a randomised clinical trial |
| Castagnolo 1977 | Not a randomised clinical trial |
| Chang 1998 | Comparison of variations in treatment |
| Eknoyan 1970 | Not a randomised clinical trial |
| Fassio 1992 | Comparison of variations in treatment |
| Fuller 1977 | In this cross‐over randomised clinical trial, the cross‐over took place after 6 days of treatment with just 6 days between the cross‐over; this short duration of treatment and cross‐over period was insufficient time to determine the objectives of this review. |
| Gadano 1997 | Atrial natriuretic peptide was given for sodium retention and not for treatment of ascites. |
| Garcia‐Compean 1993 | Large volume paracentesis without fluid replacement or systemic vasoconstrictors was not one of the treatments included in this review. |
| Garcia‐Compean 2002 | Comparison of variations in treatment |
| Gentilini 1989b | Comparison of variations in treatment |
| Gerbes 1990a | Comparison of variations in treatment |
| Gines 1996 | Large volume paracentesis without fluid replacement or systemic vasoconstrictors was not one of the treatments included in this review. |
| Ginès 1988 | Comparison of variations in treatment |
| Giostra 2000 | No details of the diuretic regimen were available; so, it was not entirely clear if the drugs in the diuretic regimen were similar in the two groups. |
| He 2012 | No details of the diuretic regimen were available; so, it was not entirely clear if the drugs in the diuretic regimen were similar in the two groups. |
| Heinrich 1983 | Not clear whether this was a randomised clinical trial. |
| Hernandez 1995 | Comparison of variations in treatment |
| Inoue 1969 | Not a randomised clinical trial |
| Kalambokis 2007 | This trial included non‐ascites people and the randomisation was not stratified by presence of ascites or separate data was not available in those with ascites. |
| Kalambokis 2008 | Not a randomised clinical trial |
| Knauf 1994 | In this short‐term study, the patients who did not respond to one treatment crossed over to the other treatment. Therefore, this design will not answer our objectives. |
| Krag 2007b | The diuretics used in the intervention and control group was variable ‐ these may or may not have been used; therefore it was not possible to define the intervention or control group. |
| Krag 2008 | The diuretics used in the intervention and control group was variable ‐ these may or may not have been used; therefore it was not possible to define the intervention or control group. |
| Kurt 2011 | Not a randomised clinical trial |
| Laffi 1992 | Not an intervention included in the systematic review |
| Laffi 2003 | Not a randomised clinical trial |
| Lai 1991 | In this short‐term study, the patients who did not respond to one treatment crossed over to the other treatment. Therefore, this design will not answer our objectives. |
| Leodolter 1972 | Not a randomised clinical trial |
| Lieberman 1965 | Not a randomised clinical trial |
| Lowenthal 1973 | Not a randomised clinical trial |
| Luca 1995 | Not an intervention included in the systematic review |
| Marra 1979 | Quasi‐randomised study (allocation by order of entry to ward) |
| Merino 1967 | Not a randomised clinical trial |
| Misra 2010 | In this cross‐over RCT, the cross‐over took place after 8 hours of treatment with just 24 hours between the cross‐over. |
| Moreau 2006 | Comparison of variations in treatment |
| Nakamura 2014 | Not an intervention included in the systematic review |
| Narahara 2009 | The diuretic regimen was different in the two groups. |
| Perez 1983 | After 5 days, if there was no response, patients crossed‐over; this short duration of treatment and cross‐over period was insufficient time to determine the objectives of this review. |
| Perkins 2006 | Not a randomised clinical trial |
| Planas 1990 | Comparison of variations in treatment |
| Ring‐Larsen 1988 | The control group of diuretics was not defined; therefore it was not possible to assess whether the control group was defined. |
| Roseau 2000 | Not a randomised clinical trial |
| Runyon 1989 | The unit of randomisation was the procedures (i.e. some patients underwent both treatments). |
| Sadikali 1973 | Not a randomised clinical trial |
| Salerno 1991 | Comparison of variations in treatment |
| Salerno 1997 | Comparison of variations in treatment |
| Santos 2003 | After about 2 weeks, if there was no response, patients crossed over. |
| Sarin 1988 | Included patients without cirrhosis |
| Sarti 1984 | Not a randomised clinical trial |
| Schmukler 1968 | Not a randomised clinical trial |
| Shafei 1967 | Not a randomised clinical trial |
| Sohn 2017 | Included only patients who developed acute kidney failure following treatment |
| Sola‐Vera 2003 | Comparison of variations in treatment |
| Stanley 1989a | Not a randomised clinical trial |
| Steigmann 1966 | Not a randomised clinical trial |
| Tempini 1984 | Comparison of variations in treatment |
| Thompson 1977 | In this cross‐over trial, the wash‐out period was only 3 days; this will not answer the objectives of this systematic review. |
| Tsai 1996 | Not a comparison of interest |
| Vizzutti 2001 | In the control group, the diuretic regimen was variable. |
| Wapnick 1979 | Appeared to be a quasi‐randomised study or a nonrandomised study: authors stated "Patients in the medical group were matched with those in the surgical group according to the subgroups listed in Table I and sequentially according to the date of entry into the study". |
| Yakar 2016 | Although authors called this a randomised study, the authors also stated that the patients were studied retrospectively. An adequate method of randomisation was also not reported. |
| Yamada 1970 | Not a randomised clinical trial |
| Yosry 2019 | Quasi‐randomised study (allocation by alternate assignment) |
| Zaak 2001 | Not a randomised clinical trial |
| Zhang 2013 | Not clear if all participants had cirrhosis |
| Zhao 2000 | Comparison of variations in treatment |
Characteristics of ongoing studies [ordered by study ID]
EUCTR 2018.
| Trial name or title | None |
| Methods | Randomised clinical trial |
| Participants | Ascites and cirrhosis |
| Interventions | Albumin versus placebo |
| Outcomes | 1. Transplant‐free survival 2. Overall survival 3. Non‐resolution of ascites |
| Starting date | 25 April 2018 |
| Contact information | Instituto Grifols S.A. (IGregulatory.affairs@grifols.com) |
| Notes | EudraCT Number: 2016‐001789‐28 |
Macken 2018.
| Trial name or title | None |
| Methods | Randomised clinical trial |
| Participants | Refractory ascites and cirrhosis |
| Interventions | Tunnelled peritoneal catheter versus paracentesis plus fluid replacement |
| Outcomes | 1. Overall survival 2. Adverse events 3. Health resource utilisation and quality of life |
| Starting date | 1 October 2015 |
| Contact information | Lucia Macken (lucia.macken@bsuh.nhs.uk) |
| Notes | ISRCTN30697116 |
NCT 03172273.
| Trial name or title | PETRA |
| Methods | Randomised clinical trial |
| Participants | Diuretic resistant ascites and cirrhosis |
| Interventions | Tunnelled peritoneal catheter versus paracentesis plus fluid replacement |
| Outcomes | 1. Non‐resolution of ascites 2. Adverse events |
| Starting date | 20 January 2017 |
| Contact information | Gastro Unit, Medical Division, University Hospital Hvidovre, Hvidovre, Denmark, 2650 |
| Notes | NCT03027635; Study terminated |
NCT03027635.
| Trial name or title | None |
| Methods | Randomised clinical trial |
| Participants | Recurrant and refractory ascites and cirrhosis |
| Interventions | Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin |
| Outcomes | 1. Transplant‐free survival 2. Hepatic decompensation 3. Quality of life 4. Adverse events |
| Starting date | 29 June 2017 |
| Contact information | Guohong Han (hangh@fmmu.edu.cn) |
| Notes | NCT03172273 |
NCT03202524.
| Trial name or title | None |
| Methods | Randomised clinical trial |
| Participants | Grade 3 or refractory ascites and cirrhosis |
| Interventions | Paracentesis plus fresh frozen plasma plus albumin versus paracentesis plus albumin |
| Outcomes | 1. Incidence of post‐paracentesis circulatory dysfunction |
| Starting date | December 2017 |
| Contact information | Montefiore Medical Center |
| Notes | NCT03202524; Study withdrawn |
NCT03451292.
| Trial name or title | ARIAPUMP |
| Methods | Randomised clinical trial |
| Participants | Refractory ascites and cirrhosis |
| Interventions | Alfapump versus paracentesis plus albumin |
| Outcomes | 1. Treatment costs 2. Non‐resolution of ascites 3. Hepatic decompensation 4. Adverse events |
| Starting date | 17 July 2018 |
| Contact information | Sandra David‐Tchouda (sdavidtchouda@chu‐grenoble.fr) |
| Notes | NCT03506893 |
Differences between protocol and review
We used the 'paracentesis plus fluid replacement' as the reference group (from 'no active intervention'), as 'paracentesis plus fluid replacement' was the commonest intervention compared in the trials.
We did not perform Trial Sequential Analysis (TSA) because the risk of false positive results with Bayesian meta‐analysis is probably less or at least equivalent to TSA.
We used the latest guidance from the GRADE Working group (Yepes‐Nunez 2019) rather than the previous guidance (Puhan 2014) for presenting the 'Summary of Findings' table.
The trials did not report the proportion of people with other episodes of decompensation but reported the number of episodes of decompensation. Therefore, we treated this as a count outcome and used the Poisson likelihood to calculate the rate ratio.
In the absence of a protocol published prior to the start of the study, we classified the risk of bias as low for selective reporting bias only when mortality, adverse events, and resolution from ascites were reported, as we anticipated these outcomes to be routinely measured in clinical trials of this nature.
We used 30,000 iterations (instead of 10,000 iterations) as a minimum for burn‐in of the simulation sampler used to estimate quantities in the statistical models to ensure convergence of the simulation sampler.
We did not present some information such as ranking probability tables, rankograms, and surface area under the curve (SUCRA plots) because of the concern about the misinterpretation of the results. We have highlighted this clearly within the text of the review along with the reasons for not presenting them.
Contributions of authors
Protocol
Conceiving the protocol: KG Designing the protocol: KG Co‐ordinating the protocol: KG Designing search strategies: KG Writing the protocol: KG Providing general advice on the protocol: ET Securing funding for the protocol: KG Performing previous work that was the foundation of the current study: not applicable
Review
Co‐ordinating the review: KG Study selection: KG, AB, LP, MP, DR Data extraction: KG, AB, LP, MP, DR Writing the review: KG and AB Providing advice on the review: SF, AJS, NH, EJM, MC, DT, CSP, BRD, ET Securing funding for the review: KG
Sources of support
Internal sources
-
University College London, UK.
Writing equipment, software, etc
External sources
-
National Institute for Health Research (NIHR), UK.
Payment for writing reviews, writing equipment, software
Declarations of interest
None known for any of the authors
New
References
References to studies included in this review
Acharya 1992 {published data only}
- Acharya SK, Balwinder S, Padhee AK, Nijhawan S, Tandon BN. Large volume paracentesis and intravenous dextran to treat tense ascites. Journal of Clinical Gastroenterology 1992;14(1):31‐5. [DOI] [PubMed] [Google Scholar]
- Acharya SK, Joshi YK, Balwinder S, Padhee AK, Tandon BN. Large volume paracentesis and intravenous low molecular weight dextran to treat tense ascites in cirrhosis of the liver. Journal of Gastroenterology and Hepatology 1991;6(2):199. [Google Scholar]
Ali 2014 {published data only}
- Ali A, Farid S, Amin M, Kassem M, Al‐Garem N. Clinical study on the therapeutic role of midodrine in non azotemic cirrhotic patients with tense ascites: a double‐blind, placebo‐controlled, randomized trial. Hepato‐Gastroenterology 2014;61(135):1915‐24. [PubMed] [Google Scholar]
Al Sebaey 2012 {published data only}
- Al Sebaey A, Abdel‐Razek W, Bassuni A, Rewisha EA, Khalil M, Waked I. Alternatives to albumin for prevention of paracentesis induced circulatory dysfunction (PICD) in cirrhosis. Hepatology (Baltimore, Md) 2012;56:915a. [Google Scholar]
Amin 2012 {published data only}
- Amin MA, Elgarem N, Farid SF, Kassem M, Amin A. Clinical study on the therapeutic role of midodrine in non azotemic cirrhotic patients with tense ascites. Journal of Hepatology 2012;56:S239. [PubMed] [Google Scholar]
Appenrodt 2008 {published data only}
- Appenrodt B, Wolf A, Gruenhage F, Trebicka J, Schepke M, Sauerbruch T. Midodrine versus albumin in the prevention of paracentesis induced circulatory dysfunction in patients with cirrhosis and tense ascites? A randomized study. Journal of Hepatology 2007;46(Suppl 1):S90. [Google Scholar]
- Appenrodt B, Wolf A, Grunhage F, Trebicka J, Schepke M, Rabe C, et al. Prevention of paracentesis‐induced circulatory dysfunction: midodrine vs albumin. A randomized pilot study. Liver International 2008;28(7):1019‐25. [DOI] [PubMed] [Google Scholar]
Bari 2012 {published data only}
- Bari K, Minano C, Shea M, Inayat IB, Hashem HJ, Gilles H, et al. The combination of octreotide and midodrine is not superior to albumin in preventing recurrence of ascites after large‐volume paracentesis. Clinical Gastroenterology and Hepatology 2012;10(10):1169‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruno 1992 {published data only}
- Bruno S, Borzio M, Romagnoni M, Battezzati PM, Rossi S, Chiesa A, et al. Comparison of spontaneous ascites filtration and reinfusion with total paracentesis with intravenous albumin infusion in cirrhotic patients with tense ascites. BMJ (Clinical Research Ed) 1992;304(6843):1655‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Borzio M, Romagnoni M, Chiesa A, Rossi S, Pavesi S, et al. Spontaneous ascites filtration and reinfusion (SAFR) vs total paracentesis (TP) with IV albumin infusion in cirrhotic patients with tense ascites. Hepatology (Baltimore, Md) 1990;12(4 pt 2):855. [Google Scholar]
Bureau 2017c {published data only}
- Bureau C, Thabut D, Oberti F, Dharancy S, Cabarrou P, Carbonell N, et al. TIPS with PTFE‐covered stent improves liver transplant free survival in patients with cirrhosis and recurrent ascites: results of a multicentre randomized trial. Hepatology (Baltimore, Md) 2015;62:347a. [Google Scholar]
- Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant‐free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152(1):157‐63, Erratum in: Gastroenterology 2017; 153(3):870. [DOI] [PubMed] [Google Scholar]
Caraceni 2018 {published data only}
- Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long‐term albumin administration improves survival in patients with decompensated cirrhosis: final results of the Answer study. Journal of Hepatology 2017;66(1 Suppl 1):S93. [Google Scholar]
- Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long‐term albumin administration in decompensated cirrhosis (Answer): an open‐label randomised trial. Lancet 2018;391(10138):2417‐29. [DOI] [PubMed] [Google Scholar]
- Zaccherini G, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long‐term albumin administration in patients with cirrhosis and uncomplicated ascites: the use of serum albumin concentration for personalizing treatment. Digestive and Liver Disease 2019;51(Suppl 1):E9. [Google Scholar]
Chang 1997 {published data only}
- Chang SC, Chang HI, Chen FJ, Shiao GM, Wang SS, Lee SD. Therapeutic effects of diuretics and paracentesis on lung function in patients with non‐alcoholic cirrhosis and tense ascites. Journal of Hepatology 1997;26(4):833‐8. [DOI] [PubMed] [Google Scholar]
Chesta 1990 {published data only}
- Chesta J, Brahm J, Latorre R, Gidi J, Hurtado C, Antezana C, et al. Paracentesis combined with albumin infusion in the treatment of tense ascites in cirrhotic patients. Revista Medica de Chile 1990;118(8):874‐80. [PubMed] [Google Scholar]
Descos 1983 {published data only}
- Descos L, Gauthier A, Levy VG, Michel H, Quinton A, Rueff B, et al. Comparison of six treatments of ascites in patients with liver cirrhosis. A clinical trial. Hepato‐Gastroenterology 1983;30(1):15‐20. [PubMed] [Google Scholar]
Fernandez‐Esparrach 1997 {published data only}
- Fernandez‐Esparrach G, Arroyo V. Double‐blind placebo‐controlled study of spironolactone in the prevention of ascites recurrence after paracentesis in cirrhosis. Journal of Hepatology 1996;25(Suppl 1):S95. [Google Scholar]
- Fernandez‐Esparrach G, Guevara M, Sort P, Pardo A, Jimenez W, Gines P, et al. Diuretic requirements after therapeutic paracentesis in non‐azotemic patients with cirrhosis. A randomized double‐blind trial of spironolactone versus placebo. Journal of Hepatology 1997;26(3):614‐20. [DOI] [PubMed] [Google Scholar]
Fogel 1981 {published data only}
- Fogel MR, Sawhney VK, Neal EA, Miller RG, Knauer CM, Gregory PB. Diuresis in the ascitic patient: a randomized controlled trial of three regimens. Journal of Clinical Gastroenterology 1981;3(Suppl 1):73‐80. [DOI] [PubMed] [Google Scholar]
Gentilini 1999a {published data only}
- Gentilini P, Casini‐Raggi V, Fiore G, Romanelli RG, Buzzelli G, Pinzani M, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. Journal of Hepatology 1999;30(4):639‐45. [DOI] [PubMed] [Google Scholar]
Gines 1987 {published data only}
- Gines P, Arroyo V, Quintero E. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology 1987;93(2):234‐41. [DOI] [PubMed] [Google Scholar]
- Quintero E, Arroyo V, Bory F. Paracentesis versus diuretics in the treatment of cirrhotics with tense ascites. Lancet 1985;1(8429):611‐2. [DOI] [PubMed] [Google Scholar]
Ginès 1991 {published data only}
- Ginès P, Arroyo V, Vargas V, Planas R, Casafont F, Panés J, et al. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. New England Journal of Medicine 1991;325(12):829‐35. [DOI] [PubMed] [Google Scholar]
Ginès 1995 {published data only}
- Ginès A, Planas R, Angeli P, Guarner C, Salerno F, Ginès P, et al. Treatment of patients with cirrhosis and refractory ascites using Leveen shunt with titanium tip: comparison with therapeutic paracentesis. Hepatology (Baltimore, Md) 1995;22(1):124‐31. [DOI] [PubMed] [Google Scholar]
- Ginès A, Planas R, Angeli P, Guarner C, Salerno F, Vargas V, et al. Controlled trial of total paracentesis plus IV albumin vs Leveen shunt (LVS) with titanium tip in cirrhotic patients with refractory ascites. Hepatology (Baltimore, Md) 1994;19(4):671. [Google Scholar]
- Rodríguez N, Armengol M, Oller B, Planas R, Ginés A, Angeli P, et al. Results from a randomized multicenter study comparing Leveen shunt with titanium tip versus paracentesis with intravenous albumin infusion for the treatment of patients with cirrhosis and refractory ascites. Cirurgia Espanola 1993;54(Suppl 1):141‐2. [Google Scholar]
Ginès 2002 {published data only}
- Ginès P, Uriz J, Calahorra B, Garcia‐Tsao G, Kamath PS, Arbol LR, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology 2002;123(6):1839‐47. [DOI] [PubMed] [Google Scholar]
- Uriz J, Ginés P. Randomized, multicenter, comparative study between TIPS and paracentesis with albumin in cirrhosis with refractory ascites. Journal of Hepatology 2001;34(1):10. [Google Scholar]
Graziotto 1997 {published data only}
- Graziotto A, Rossaro L, Inturri P, Salvagnini M. Reinfusion of concentrated ascitic fluid versus total paracentesis. A randomized prospective trial. Digestive Diseases and Sciences 1997;42(8):1708‐14. [DOI] [PubMed] [Google Scholar]
Gregory 1977 {published data only}
- Gregory PB, Broekelschen PH, Hill MD, Lipton AB, Knauer CM, Egger M, et al. Complications of diuresis in the alcoholic patient with ascites: a controlled trial. Gastroenterology 1977;73(3):534‐8. [PubMed] [Google Scholar]
Hagege 1992 {published data only}
- Hagege H, Ink O, Ducreux M, Pelletier G, Buffet C, Etienne JP. Treatment of ascites in patients with cirrhosis: results of a randomized study comparing diuretics with paracentesis compensated by albumin. Gastroenterologie Clinique et Biologique 1992;16(10):751‐5. [PubMed] [Google Scholar]
Hamdy 2014 {published data only}
- Hamdy H, Baz AA, Hassan A, Hassanin O. Comparison of midodrine and albumin in the prevention of paracentesis‐induced circulatory dysfunction in cirrhotic patients: a randomized pilot study. Journal of Clinical Gastroenterology 2014;48(2):184‐8. [DOI] [PubMed] [Google Scholar]
Lata 2007 {published data only}
- Lata J, Marecek Z, Fejfar T, Zdenek P, Bruha R, Safka V, et al. The efficacy of terlipressin in comparison with albumin in the prevention of circulatory changes after the paracentesis of tense ascites ‐ a randomized multicentric study. Hepato‐Gastroenterology 2007;54(79):1930‐3. [PubMed] [Google Scholar]
Lebrec 1996 {published data only}
- Lebrec D, Giuily N, Hadengue A, Vilgrain V, Moreau R, Poynard T, et al. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. Journal of Hepatology 1996;25(2):135‐44. [DOI] [PubMed] [Google Scholar]
Licata 2009 {published data only}
- Licata G, Tuttolomondo A, Licata A, Parrinello G, Raimondo D, Sciacca R, et al. Clinical trial: high‐dose furosemide plus small‐volume hypertonic saline solutions vs. repeated paracentesis as treatment of refractory ascites. Alimentary Pharmacology & Therapeutics 2009;30(3):227‐35. [DOI] [PubMed] [Google Scholar]
Ljubici 1994 {published data only}
- Ljubici N, Bili A, Kopjar B. Diuretics vs. paracentesis followed by diuretics in cirrhosis: effect on ascites opsonic activity and immunoglobulin and complement concentrations. Hepatology (Baltimore, Md) 1994;19(2):346‐53. [DOI] [PubMed] [Google Scholar]
Mchutchison 1989 {published data only}
- McHutchison JG, Pinto PC, Reynolds TB. Hydrocholorthiazide as a third diuretic in cirrhosis with refractory ascites. Hepatology (Baltimore, Md) 1989;10(4):719. [Google Scholar]
Mehta 1998 {published data only}
- Mehta NN, Abraham P, Ashar VJ, Shikare SS, Savant SS, Karnad DR, et al. Ascitic fluid filtration and intravenous infusion versus total‐volume paracentesis with infusion of plasma expander in cirrhosis with tense or intractable ascites. Indian Journal of Gastroenterology 1998;17(3):93‐6. [PubMed] [Google Scholar]
Moreau 2002 {published data only}
- Moreau R, Asselah T, Condat B, Kerguenec C, Pessione F, Bernard B, et al. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut 2002;50(1):90‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R, Asselah TB, Kerguenec C, Pessione F, Bernard B, Poynard T, et al. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomized study. Hepatology (Baltimore, Md) 2000;32(4 pt 2):311a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narahara 2011 {published data only}
- Narahara Y, Kanazawa H, Fukuda T, Matsushita Y, Harimoto H, Kidokoro H, et al. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. Journal of Gastroenterology 2011;46(1):78‐85. [DOI] [PubMed] [Google Scholar]
Rai 2017 {published data only}
- Rai N, Singh B, Singh A, Vijayvergiya R, Sharma N, Bhalla A, et al. Midodrine and tolvaptan in patients with cirrhosis and refractory or recurrent ascites: a randomised pilot study. Liver International 2017;37(3):406‐14. [DOI] [PubMed] [Google Scholar]
- Singh V, Rai N, Singh B, Vijayvergiya R, Sharma N, Bhalla A. Midodrine and tolvaptan in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. Hepatology (Baltimore, Md) 2015;62:345a. [DOI] [PubMed] [Google Scholar]
Raza 2011 {published data only}
- Raza MA, Qureshi UF, Humayoun MA, Waseem T, Akram J. Effect of intravenous mannitol in mobilization of resistant cirrhotic ascites. European Journal of Gastroenterology and Hepatology 2011;23(2):184‐8. [DOI] [PubMed] [Google Scholar]
Romanelli 2006 {published data only}
- Romanelli RG, Villa G, Barletta G, Vizzutti F, Lanini F, Arena U, et al. Long‐term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World Journal of Gastroenterology 2006;12(9):1403‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossle 2000 {published data only}
- Rossle M, Ochs A, Gulberg V, Siegerstetter V, Holl J, Deibert P, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. New England Journal of Medicine 2000;342(23):1701‐7. [DOI] [PubMed] [Google Scholar]
Salerno 1987 {published data only}
- Salerno F, Badalamenti S, Incerti P. Repeated paracentesis and i.v. albumin infusion to treat 'tense' ascites in cirrhotic patients. A safe alternative therapy. Journal of Hepatology 1987;5(1):102‐8. [DOI] [PubMed] [Google Scholar]
Salerno 2004 {published data only}
- Salerno F, Merli M, Cazzaniga M, Riggio O, Valeriano V, Nicolini A. Randomized controlled study of TIPS vs paracentesis with albumin in cirrhosis with refractory ascites. Hepatology (Baltimore, Md) 2002;36(4 pt 2):318a. [DOI] [PubMed] [Google Scholar]
- Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology (Baltimore, Md) 2004;40(3):629‐35. [DOI] [PubMed] [Google Scholar]
Sanyal 2003 {published data only}
- Campbell NS, Brensinger CM, Sanyal AJ, Gennings C, Wong F, Kowdley KV, et al. Quality of life in refractory ascites: transjugular intrahepatic portal‐systemic shunting versus medical therapy. Hepatology (Baltimore, Md) 2005;42(3):635‐40. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, et al. The North American study for the treatment of refractory ascites. Gastroenterology 2003;124(3):634‐41. [DOI] [PubMed] [Google Scholar]
Schaub 1995 {published data only}
- Schaub N, Stieger M, Kummer H. High volume paracentesis with albumin substitution for treatment of ascites in liver cirrhosis. Schweizerische Medizinische Wochenschrift 1995;125(Suppl 69):3. [Google Scholar]
Singh 2006a {published data only}
- Singh V, Kumar B, Nain CK, Singh B, Sharma N, Bhalla A, et al. Noradrenaline and albumin in paracentesis‐induced circulatory dysfunction in cirrhosis: a randomized pilot study. Journal of Internal Medicine 2006;260(1):62‐8. [DOI] [PubMed] [Google Scholar]
Singh 2006b {published data only}
- Singh V, Kumar R, Nain CK, Singh B, Sharma AK. Terlipressin versus albumin in paracentesis‐induced circulatory dysfunction in cirrhosis: a randomized study. Journal of Gastroenterology and Hepatology 2006;21(1 pt 2):303‐7. [DOI] [PubMed] [Google Scholar]
Singh 2008 {published data only}
- Singh V, Dheerendra PC, Singh B, Nain CK, Chawla D, Sharma N. Midodrine versus albumin in the prevention of paracentesis induced circulatory dysfunction in cirrhotics: a randomized pilot study. Hepatology (Baltimore, Md) 2007;46(4 Suppl 1):563a. [DOI] [PubMed] [Google Scholar]
- Singh V, Dheerendra PC, Singh B, Nain CK, Chawla D, Sharma N, et al. Midodrine versus albumin in the prevention of paracentesis‐induced circulatory dysfunction in cirrhotics: a randomized pilot study. American Journal of Gastroenterology 2008;103(6):1399‐405. [DOI] [PubMed] [Google Scholar]
Singh 2012a {published data only}
- Singh V, Dhungana SP, Singh B, Vijayverghia R, Nain CK, Sharma N, et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. Journal of Hepatology 2012;56(2):348‐54. [DOI] [PubMed] [Google Scholar]
Singh 2013 {published data only}
- Dhungana SP, Sharma N, Vijayverghia R, Nain CK, Singh B, Bhalla A. Role of long‐term midodrine in patients with cirrhosis and refractory or recurrent ascites: a pilot study. Hepatology (Baltimore, Md) 2009;50(4 Suppl):453a. [DOI] [PubMed] [Google Scholar]
- Singh V, Singh A, Singh B, Vijayverghia R, Sharma N, Ghai A, et al. Midodrine and clonidine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. Journal of Hepatology 2012;56:S270. [DOI] [PubMed] [Google Scholar]
- Singh V, Singh A, Singh B, Vijayvergiya R, Sharma N, Ghai A, et al. Midodrine and clonidine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. American Journal of Gastroenterology 2013;108(4):560‐7. [DOI] [PubMed] [Google Scholar]
Sola 1994 {published data only}
- Sola R, Andreu M, Vila MC, Coll S, Gana J, Ledesma S. Complications associated with the treatment of ascites in cirrhotic patients with therapeutic paracentesis versus diuretics: a prospective randomized study. Journal of Hepatology 1993;16(Suppl 1):S6. [Google Scholar]
- Sola R, Vila MC, Andreu M, Oliver MI, Coll S, Gana J, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. Journal of Hepatology 1994;20(2):282‐8. [DOI] [PubMed] [Google Scholar]
Sola 2018 {published data only}
- Sola E, Simon Talero M, Martin‐Llahi M, Castellote J, Nazar A, Sole C, et al. Effects of long‐term administration of midodrine and albumin in the prevention of complications in patients with cirrhosis on waiting list for liver transplantation. A randomized, multicenter, double‐blind, placebo‐controlled trial (Macht study). Journal of Hepatology 2015;62:S848. [DOI] [PubMed] [Google Scholar]
- Sola E, Sole C, Simon‐Talero M, Martin‐Llahi M, Castellote J, Garcia‐Martinez R, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo‐controlled trial. Journal of Hepatology 2018;69(6):1250‐9. [DOI] [PubMed] [Google Scholar]
- Sola E, Sole C, Simon‐Talero M, Martin‐Llahi M, Castellote J, Garcia‐Martinez R, et al. Midodrine and albumin for prevention of complications of cirrhosis in patients in the waiting list for liver transplantation. A randomized, multicenter, double‐blind, placebo‐controlled trial. Journal of Hepatology 2017;66(1 Suppl 1):S11. [DOI] [PubMed] [Google Scholar]
Stanley 1989b {published data only}
- Stanley MM. Peritoneovenous shunting vs medical treatment of alcoholic cirrhotic ascites. Hepatology (Baltimore, Md) 1985;5(5):980. [Google Scholar]
- Stanley MM, Ochi S, Lee KK, Nemchausky BA, Greenlee HB, Allen JI, et al. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative study on treatment of alcoholic cirrhosis with ascites. New England Journal of Medicine 1989;321(24):1632‐8. [DOI] [PubMed] [Google Scholar]
Strauss 1991 {published data only}
- Strauss E, Moreira E, Caly WR, Honain N, Sa MFG. Paracentesis during diuretic treatment of ascites due to hepatic cirrhosis: results of a randomized trial. Hepatology (Baltimore, Md) 1990;12(4 pt 2):993. [Google Scholar]
- Strauss E, Moreira E, Caly WR, Honain NZ, Mfgde S. Paracentesis during diuretic treatment of ascites due to hepatic cirrhosis: results of randomized clinical assay. Gastroenterologia Endoscopia Digestiva 1991;10(2):53‐8. [Google Scholar]
Tuttolomondo 2016 {published data only}
- Tuttolomondo A, Raimondo D, Bellia C, Clemente G, Pecoraro R, Maida C, et al. Immune‐inflammatory and metabolic effects of high dose furosemide plus hypertonic saline solution (HSS) treatment in cirrhotic subjects with refractory ascites. PlOS One 2016;11(12):e0165443. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Khalek 2010b {published data only}
- Abdel‐Khalek EE, Arif S. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients with liver cirrhosis and tense ascites after large‐volume paracentesis. Journal of Hepatology 2010;52:S327. [Google Scholar]
- Abdel‐Khalek EE, Arif SE. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients with liver cirrhosis and tense ascites following large volume paracentesis. Arab Journal of Gastroenterology 2010;11(1):24‐9. [Google Scholar]
Altman 1998 {published data only}
- Altman C, Bernard B, Roulot D, Vitte RL, Ink O. Randomized comparative multicenter study of hydroxyethyl starch versus albumin as a plasma expander in cirrhotic patients with tense ascites treated with paracentesis. European Journal of Gastroenterology and Hepatology 1998;10(1):5‐10. [DOI] [PubMed] [Google Scholar]
Angeli 1994 {published data only}
- Angeli P, Dalla Pria M, Bei E, Albino G, Caregaro L, Merkel C, et al. Randomized clinical study of the efficacy of amiloride and potassium canrenoate in nonazotemic cirrhotic patients with ascites. Hepatology (Baltimore, Md) 1994;19(1):72‐9. [PubMed] [Google Scholar]
Antillon 1993 {published data only}
- Antillon MR, Runyon BA. Extracorporeal ultrafiltration and intravenous reinfusion of ascitic fluid vs. large‐volume paracentesis: a randomized controlled trial. Hepatology (Baltimore, Md) 1993;17(3):520‐2. [DOI] [PubMed] [Google Scholar]
Applefeld 1994 {published data only}
- Applefeld JJ, Kasmer RJ, Hak LJ, Dukes GE, Wermeling DP, McClain CJ. A dose‐response study of orally administered torsemide in patients with ascites due to cirrhosis. Alimentary Pharmacology & Therapeutics 1994;8(4):397‐402. [DOI] [PubMed] [Google Scholar]
Arrigoni 1988 {published data only}
- Arrigoni A, Andriulli A, Gindro T, Verme G. The use of diuretics in preventing ascites recurrence. British Journal of Clinical Practice 1988;42(3):116‐20. [PubMed] [Google Scholar]
Bories 1986 {published data only}
- Bories P, Garcia Compean D, Michel H, Bourel M, Capron JP, Gauthier A, et al. The treatment of refractory ascites by the Leveen shunt. A multi‐centre controlled trial (57 patients). Journal of Hepatology 1986;3(2):212‐8. [DOI] [PubMed] [Google Scholar]
Bostan 2019 {published data only}
- Bostan F, Cekin AH. Long‐term albumin administration in decompensated cirrhosis. Turkish Journal of Gastroenterology 2019;30(4):385‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyer 1983 {published data only}
- Boyer TD, Warnock DG. Use of diuretics in the treatment of cirrhotic ascites. Gastroenterology 1983;84(5 pt 1):1051‐5. [PubMed] [Google Scholar]
Brater 2001 {published data only}
- Brater DC, Chalasani N, Gorski JC, Horlander JC Sr, Craven R, Hoen H, et al. Effect of albumin‐furosemide mixtures on response to furosemide in cirrhotic patients with ascites. Transactions of the American Clinical and Climatological Association 2001;112:108‐15; discussion 16. [PMC free article] [PubMed] [Google Scholar]
Bureau 2017a {published data only}
- Adebayo D, Bureau C, Rieu MC, Valla D, Elkrief L, Peck‐Radosavljevic M, et al. Alfapump system versus large volume paracentesis in the treatment of refractory ascites; results from a multicenter randomised controlled study (RCT). Journal of Hepatology 2016;64(2 Suppl):S185. [Google Scholar]
- Adebayo D, Bureau C, Valla D, Peck‐Radosavljevic M, McCune A, Vargas V, et al. Alfapump system versus large volume paracentesis in the treatment of refractory ascites. A multicenter randomised controlled study. Journal of Hepatology 2015;62:S849‐S50. [DOI] [PubMed] [Google Scholar]
- Adebayo D, Mohammed A, Arora S, Chiara F, Trepte C, Whitaker S, et al. A randomized controlled trial comparing the alfapumpa© with paracentesis in patients with refractory ascites: clinical and pathophysiological effects on cardiac, haemodynamic, inflammatory, renal and nutritional parameters. Journal of Hepatology 2015;62:S374. [Google Scholar]
- Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, Peck‐Radosavljevic M, et al. Alfapump system vs. large volume paracentesis for refractory ascites: a multicenter randomized controlled study. Journal of Hepatology 2017;67(5):940‐9. [DOI] [PubMed] [Google Scholar]
- Engelmann C, Brosteanu O, Tenckhoff H, Babatz J, Lammert F, Manns M, et al. Alfapump system versus transjugular intrahepatic portosystemic shunt and paracentesis in the treatment of ascites. A multicentre randomised controlled study (Agua‐trial). Journal of Hepatology 2015;62:S846‐S7. [Google Scholar]
- Stepanova M, Nader F, Bureau C, Adebayo D, Elkrief L, Valla D, et al. Patients with refractory ascites treated with alfapump system have better health‐related quality of life as compared to those treated with large volume paracentesis: the results of a multicenter randomized controlled study. Quality of Life Research 2018;27(6):1513‐20. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Nader F, Hunt S, Bureau C, Inhaber N, et al. Patients with refractory ascites treated with alfapump system (AP) have better health‐related quality of life (HRQL) as compared to those treated with large volume paracentesis (LVP): results of a multicenter randomized controlled study. Hepatology (Baltimore, Md) 2016;64(1 Suppl 1):1033a. [Google Scholar]
Cadranel 1992a {published data only}
- Cadranel JF, Gargot D, Grippon P, Lunel F, Bernard B, Valla D, et al. Spontaneous dialytic ultrafiltration with intraperitoneal reinfusion of the concentrate versus large paracentesis in cirrhotic patients with intractable ascites: a randomized study. International Journal of Artificial Organs 1992;15(7):432‐5. [PubMed] [Google Scholar]
Cadranel 1992b {published data only}
- Cadranel JF, Grippon P, Gargot D, Lunel F, Bernard B, Valla D, et al. Simultaneous dialytic ultrafiltration and intraperitoneal reinfusion in cirrhotic patients with intractable ascites. Preliminary results of a comparative study versus large paracentesis. Journal of Hepatology 1992;15(1‐2):271‐2. [DOI] [PubMed] [Google Scholar]
Castagnolo 1977 {published data only}
- Castagnolo B, Colibus V, Napolitano L, Aliperta A, Caldarelli G, Abignente F. Clinical evaluation of the use of diuretics in the ascitic phase of hepatic cirrhosis. Clinica Terapeutica 1977;83(6):625‐9. [PubMed] [Google Scholar]
Chang 1998 {published data only}
- Chang R, Chang YW, Lee JI, Kim BH, Kim HJ, Dong SH. The efficacy of hydroxyethyl starch as plasma expander in cirrhotic patients with tense ascites treated with large volume paracentesis. Korean Journal of Gastroenterology 1998;31(5):651‐60. [Google Scholar]
Eknoyan 1970 {published data only}
- Eknoyan G, Martinez‐Maldonado M, Yium JJ, Suki WN. Combined ascitic‐fluid and furosemide infusion in the management of ascites. New England Journal of Medicine 1970;282(13):713‐7. [DOI] [PubMed] [Google Scholar]
Fassio 1992 {published data only}
- Fassio E, Terg R, Landeira G, Abecasis R, Salemne M, Podesta A, et al. Paracentesis with dextran 70 vs. paracentesis with albumin in cirrhosis with tense ascites. Results of a randomized study. Journal of Hepatology 1992;14(2‐3):310‐6. [DOI] [PubMed] [Google Scholar]
Fuller 1977 {published data only}
- Fuller RK, Khambatta PB, Gobezie GC. An optimal diuretic regimen for cirrhotic ascites. A controlled trial evaluating safety and efficacy of spironolactone and furosemide. JAMA 1977;237(10):972‐5. [PubMed] [Google Scholar]
Gadano 1997 {published data only}
- Gadano A, Moreau R, Vachiery F, Soupison T, Yang S, Cailmail S, et al. Natriuretic response to the combination of atrial natriuretic peptide and terlipressin in patients with cirrhosis and refractory ascites. Journal of Hepatology 1997;26(6):1229‐34. [DOI] [PubMed] [Google Scholar]
Garcia‐Compean 1993 {published data only}
- Garcia‐Compean D, Villarreal JZ, Cuevas HB, Cantu DAG, Estrella M, Tamez EG, et al. Total therapeutic paracentesis (TTP) with and without intravenous albumin in the treatment of cirrhotic tense ascites: a randomized controlled trial. Liver 1993;13(5):233‐8. [DOI] [PubMed] [Google Scholar]
Garcia‐Compean 2002 {published data only}
- Garcia‐Compean D, Blanc P, Larrey D, Daures JP, Hirtz J, Mendoza E, et al. Treatment of cirrhotic tense ascites with dextran‐40 versus albumin associated with large volume paracentesis: a randomized controlled trial. Annals of Hepatology 2002;1(1):29‐35. [PubMed] [Google Scholar]
Gentilini 1989b {published data only}
- Gentilini P, Meacci E, Laffi G, Buzzelli GP, Marra F, Cotrozzi G. Comparison of torasemide (T), a new loop diuretic, and furosemide (F) in the treatment of cirrhotic patients with ascites. Italian Journal of Gastroenterology 1989;21(2):31. [Google Scholar]
Gerbes 1990a {published data only}
- Gerbes AL, Berthau U, Falkner CH, Paumgartner G. Effects of torasemide, a new loop diuretic vs furosemide in patients with cirrhosis and ascites: a randomized double‐blind cross‐over study. Hepatology (Baltimore, Md) 1990;12(4 pt 2):991. [Google Scholar]
- Gerbes AL, Bertheau U, Falkner C, Jüngst D, Paumgartner G. Advantages of torasemide vs. furosemide in patients with liver cirrhosis and ascites. A randomized double‐blind cross‐over study. Gastroenterology 1991;100(5 pt 2):A746. [Google Scholar]
- Gerbes AL, Bertheau U, Falkner C, Jüngst D, Paumgartner G. Pharmacodynamic advantages of torasemide, a new loop diuretic, against furosemide in patients with cirrhosis of the liver and ascites. Results of a randomised, double‐blind study with cross‐over design. Zeitschrift für Gastroenterologie 1990;28(12):693‐4. [Google Scholar]
Ginès 1988 {published data only}
- Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology 1988;94(6):1493‐502. [DOI] [PubMed] [Google Scholar]
Gines 1996 {published data only}
- Gines A, Fernandez‐Esparrach G, Monescillo A, Vila C, Domenech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology 1996;111(4):1002‐10. [DOI] [PubMed] [Google Scholar]
Giostra 2000 {published data only}
- Giostra E, Spahr L, Nadereh AP, Martin PY, Ferraille E, Hadengue A. Effects of the vasoconstrictor terlipressin on renal function in patients with cirrhosis and ascites: a double‐blind randomized controlled trial. Hepatology (Baltimore, Md) 2000;32(4 pt 2):523a. [Google Scholar]
He 2012 {published data only}
- He HR, Li M, Wei DP, Zhu JJ, Yang JB, Feng XH, et al. Therapeutic schemes for refractory ascites of advanced schistosomiasis: a clinical control study. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2012;24(5):570‐2. [PubMed] [Google Scholar]
Heinrich 1983 {published data only}
- Heinrich F, Loew D, Dycka J. Repeated‐dose, double‐blind comparative trial of muzolimine‐spironolactone, furosemide‐spironolactone, and placebo‐spironolactone. Clinical Nephrology 1983;19(Suppl 1):S69‐75. [Google Scholar]
Hernandez 1995 {published data only}
- Hernandez Perez RE, Aguilar Ramirez JR, Hernandez Lopez JM, Gomez Maganda y Silva TG. Massive paracentesis and administration of dextran 70 vs albumin in cirrhotic patients with tense ascites. Revista de Gastroenterología de México 1995;60(1):22‐6. [PubMed] [Google Scholar]
Inoue 1969 {published data only}
- Inoue J, Mori Y, Kawamura T. Refractory hepatic cirrhosis and the ineffectiveness of diuretics in ascites and edema. Naika Internal Medicine 1969;24(5):834‐9. [PubMed] [Google Scholar]
Kalambokis 2007 {published data only}
- Kalambokis G, Fotopoulos A, Economou M, Pappas K, Tsianos EV. Effects of a 7‐day treatment with midodrine in non‐azotemic cirrhotic patients with and without ascites. Journal of Hepatology 2007;46(2):213‐21. [DOI] [PubMed] [Google Scholar]
Kalambokis 2008 {published data only}
- Kalambokis GN, Tsianos EV. Vasoconstrictor therapy for patients with cirrhosis with ascites but without hepatorenal syndrome. Hepatology (Baltimore, Md) 2008;48(2):686. [DOI] [PubMed] [Google Scholar]
Knauf 1994 {published data only}
- Knauf H, Mutschler E. Liver cirrhosis with ascites: pathogenesis of resistance to diuretics and long‐term efficacy and safety of torasemide. Cardiology 1994;84(Suppl 2):87‐98. [DOI] [PubMed] [Google Scholar]
- Knauf H, Wenk E, Scholmerich J, Goerg KJ, Gerok W, Leser HG, et al. Prediction of diuretic mobilization of cirrhotic ascites by pretreatment fractional sodium excretion. Klinische Wochenschrift 1990;68(11):545‐51. [DOI] [PubMed] [Google Scholar]
Krag 2007b {published data only}
- Krag A, Møller S, Henriksen JH, Holstein‐Rathlou N, Larsen FS, Bendtsen F. Terlipressin improves renal function and induces natriuresis in patients with cirrhosis and ascites without hepatorenal syndrome. Hepatology (Baltimore, Md) 2007;46(4 Suppl 1):566a. [DOI] [PubMed] [Google Scholar]
- Krag A, Møller S, Henriksen JH, Holstein‐Rathlou NH, Larsen FS, Bendtsen F. Terlipressin improves renal function in patients with cirrhosis and ascites without hepatorenal syndrome. Hepatology (Baltimore, Md) 2007;46(6):1863‐71. [DOI] [PubMed] [Google Scholar]
Krag 2008 {published data only}
- Krag A, Bendtsen F, Pedersen EB, Holstein‐Rathlou NH, Møller S. Effects of terlipressin on the aquaretic system: evidence of antidiuretic effects. American Journal of Physiology ‐ Renal Physiology 2008;295(5):f1295‐300. [DOI] [PubMed] [Google Scholar]
- Krag A, Møller S, Henriksen JH, Bendtsen F. Effects of a single dose of terlipressin on transcutaneous oxygen pressures. Scandinavian Journal of Gastroenterology 2010;45(7‐8):953‐8. [DOI] [PubMed] [Google Scholar]
Kurt 2011 {published data only}
- Kurt M. Deleterious effects of beta‐blockers on survival in patients with cirrhosis and refractory ascites. Hepatology (Baltimore, Md) 2011;53(4):1411‐2. [DOI] [PubMed] [Google Scholar]
Laffi 1992 {published data only}
- Laffi G, Marra F, Carloni V, Azzena G, Feo ML, Pinzani M, et al. Thromboxane‐receptor blockade increases water diuresis in cirrhotic patients with ascites. Gastroenterology 1992;103(3):1017‐21. [DOI] [PubMed] [Google Scholar]
Laffi 2003 {published data only}
- Laffi G, Gentilini P, Romanelli RG, Villa G. Is the use of albumin of value in the treatment of ascites in cirrhosis? The case in favour. Digestive and Liver Disease 2003;35(9):660‐3. [DOI] [PubMed] [Google Scholar]
Lai 1991 {published data only}
- Lai KN, Li P, Law E, Swaminathan R, Nicholls MG. Large‐volume paracentesis versus dialytic ultrafiltration in the treatment of cirrhotic ascites. Quarterly Journal of Medicine 1991;78(285):33‐41. [PubMed] [Google Scholar]
Leodolter 1972 {published data only}
- Leodolter I, Wenzl M. Operative treatment of ascites in liver cirrhosis, resistant to internal medical treatment. Munchener Medizinische Wochenschrift 1972;114(17):797‐800. [PubMed] [Google Scholar]
Lieberman 1965 {published data only}
- Lieberman FL, Reynolds TB. The use of ethacrynic acid in patients with cirrhosis and ascites. Gastroenterology 1965;49(5):531‐8. [PubMed] [Google Scholar]
Lowenthal 1973 {published data only}
- Lowenthal DT, Shear L. Use of a new diuretic agent (metolazone) in patients with edema and ascites. Archives of Internal Medicine 1973;132(1):38‐41. [PubMed] [Google Scholar]
Luca 1995 {published data only}
- Luca A, Garciapagan JC, Bosch J, Feu F, Jimenez W, Ginés A, et al. Beneficial‐effects of intravenous albumin infusion on the hemodynamic and humoral changes after total paracentesis. Hepatology (Baltimore, Md) 1995;22(3):753‐8. [PubMed] [Google Scholar]
Marra 1979 {published data only}
- Marra N, Micheli E, Spagnolo G. Controlled clinical study of the effect of combined administration of ethacrynic acid and spironolactone in ascitic liver cirrhosis. Clinica Terapeutica 1979;90(2):143‐63. [PubMed] [Google Scholar]
Merino 1967 {published data only}
- Merino I, Bardi A, Generini G, Rendic Y, Plaza de los Reyes M. The effects of the drug M.K. 870 on the diuresis, salidiuresis and plasma electrolyte concentration in liver cirrhosis patients with ascites. Revista Medica de Chile 1967;95(6):295‐9. [PubMed] [Google Scholar]
Misra 2010 {published data only}
- Misra VL, Vuppalanchi R, Jones D, Hamman M, Kwo PY, Kahi C, et al. The effects of midodrine on the natriuretic response to furosemide in cirrhotics with ascites. Alimentary Pharmacology & Therapeutics 2010;32(8):1044‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreau 2006 {published data only}
- Moreau R, Valla DC, Durand‐Zaleski I, Bronowicki JP, Durand F, Chaput JC, et al. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trial. Liver International 2006;26(1):46‐54. [DOI] [PubMed] [Google Scholar]
- Moreau R, Valla DC, Durand‐Zaleski I, Golly D, Tellier Z. Multicenter, randomized, controlled, double‐blind trial comparing albumin with polygeline for intravascular fluid loading in patients with cirrhosis and ascites. Hepatology (Baltimore, Md) 2003;38(4 Suppl 1):420a‐1a. [Google Scholar]
- Moreau R, Valla DC, Golly D, Tellier Z. Multicenter, randomized, double‐blind trial comparing albumin and polygeline for intravascular fluid loading in patients with cirrhosis and tense ascites treated with paracentesis. Liver International 2004;24(Suppl 4):7. [Google Scholar]
Nakamura 2014 {published data only}
- Nakamura T, Sata M, Suzuki K, Moriwaki H, Fukui H, Fujiyama S, et al. Open‐labeled randomized controlled trial to compare diuretic therapy with recombinant human serum albumin and diuretic therapy for therapeutic treatment of ascites in patients with advanced liver cirrhosis: an exploratory trial. Hepatology Research 2014;44(5):502‐14. [DOI] [PubMed] [Google Scholar]
Narahara 2009 {published data only}
- Narahara Y, Kanazawa H, Taki Y, Kimura Y, Atsukawa M, Katakura T, et al. Effects of terlipressin on systemic, hepatic and renal hemodynamics in patients with cirrhosis. Journal of Gastroenterology and Hepatology 2009;24(11):1791‐7. [DOI] [PubMed] [Google Scholar]
Perez 1983 {published data only}
- Perez Ayuso RM, Arroya V, Planas R, Gaya J, Bory F, Rimola A. Randomized comparative study of the efficacy of furosemide in relation to spironolactone in non‐azotemic cirrhosis with ascites relationship between the diuretic response and the activity of the renin‐aldosterone system. Folha Medica 1984;88(4):205‐11. [PubMed] [Google Scholar]
- Perez Ayuso RM, Arroyo V, Planas R. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin‐aldosterone system. Gastroenterology 1983;84(5 i):961‐8. [PubMed] [Google Scholar]
- Perez Ayuso RM, Bory F, Planas R, Gaya J, Arroyo V, Rivera F, et al. Controlled study on the efficacy of furosemide versus spironolactone in non‐azotemic cirrhosis with ascites, relationship between the diuretic response and the degree of activation on the renin‐aldosterone system. Liver 1982;2(3 pt 2):303. [PubMed] [Google Scholar]
Perkins 2006 {published data only}
- Perkins JD. Distal splenorenal shunt vs. TIPS: a rare randomized trial of a common therapy. Liver Transplantation 2006;12(11):1717‐8. [Google Scholar]
Planas 1990 {published data only}
- Planas R, Ginès P, Arroyo V, Llach J, Panés J, Vargas V, et al. Dextran‐70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology 1990;99(6):1736‐44. [DOI] [PubMed] [Google Scholar]
Ring‐Larsen 1988 {published data only}
- Ring‐Larsen H, Siemssen O, Krintel JJ, Stadager C, Henriksen JH. Denver shunt in the treatment of refractory ascites in cirrhosis a randomized controlled trial. Journal of Hepatology 1989;9(Suppl 1):S77. [Google Scholar]
- Ring‐Larsen H, Siemssen O, Krintel JJ, Stadager C, Henriksen JH. Diuretic resistant ascites in cirrhosis treated with peritoneo‐venous shunt a randomized controlled trial. Hepatology (Baltimore, Md) 1988;8(5):1353. [Google Scholar]
Roseau 2000 {published data only}
- Roseau E. Cirrhotic ascites: puncture versus transjugular intrahepatic portocaval shunt. Presse Medicale 2000;29(33):1820. [Google Scholar]
Runyon 1989 {published data only}
- Runyon BA, Antillon MR, Montano AA. Effect of diuresis versus therapeutic paracentesis on ascitic fluid opsonic activity and serum complement. Gastroenterology 1989;97(1):158‐62. [DOI] [PubMed] [Google Scholar]
Sadikali 1973 {published data only}
- Sadikali F. Spironolactone and frusemide in cirrhosis with ascites. British Journal of Clinical Practice 1973;27(6):222‐4. [PubMed] [Google Scholar]
Salerno 1991 {published data only}
- Salerno F, Badalamenti S, Lorenzano E, Moser P, Incerti P. Randomized comparative study of hemaccel vs. albumin infusion after total paracentesis in cirrhotic patients with refractory ascites. Hepatology (Baltimore, Md) 1991;13(4):707‐13. [PubMed] [Google Scholar]
Salerno 1997 {published data only}
- Salerno F, Lorenzano E, Boscani PF. Efficacy and safety of torasemide in nonazotemic cirrhotic patients with ascites: an open multicenter study. Advances in Therapy 1997;14(6):323‐37. [Google Scholar]
Santos 2003 {published data only}
- Santos J, Planas R, Pardo A, Durandez R, Cabre E, Morillas RM, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. Journal of Hepatology 2003;39(2):187‐92. [DOI] [PubMed] [Google Scholar]
- Santos J, Planas R, Pardo A, Granada ML, Jiménez JA, Durández R, et al. Spironolactone (S) alone or with furosemide (F) in the treatment of ascites in nonazotemic cirrhosis (abstract). Journal of Hepatology 2000;32(2):62. [DOI] [PubMed] [Google Scholar]
Sarin 1988 {published data only}
- Sarin SK, Sachdev G, Mishra SP, Sundaram KR, Shrivastwa A, Talukdar V, et al. Bumetanide, spironolactone and a combination of the two, in the treatment of ascites due to liver disease. A prospective, controlled, randomized trial. Digestion 1988;41(2):101‐7. [DOI] [PubMed] [Google Scholar]
Sarti 1984 {published data only}
- Sarti F, Milandri GL, Piemontese A, Macchia S, Simoni S, Dal Monte PR. Use of canrenoate potassium given orally in ascitogenic cirrhosis. Minerva Dietologica e Gastroenterologica 1984;30(3):273‐80. [PubMed] [Google Scholar]
Schmukler 1968 {published data only}
- Schmukler B, Bisio S, Iovine E, Cervini BA. New diuretic with anti‐kaliuretic effect in the treatment of cirrhotic ascitis: Mk 870(amyloid hydrochlorate). Clinico‐humoral study. Prensa Medica Argentina 1968;55(30):1442‐6. [PubMed] [Google Scholar]
Shafei 1967 {published data only}
- Shafei AZ, Habib YA, El‐Khangry HA, Abou‐Khair SA, Abaza HH, Nibal AH. Studies on furosemide: an effective oral saludiuretic for bilharzial ascites. Journal of Tropical Medicine and Hygiene 1967;70(12):286‐92. [PubMed] [Google Scholar]
Sohn 2017 {published data only}
- Sohn JH, Cho YK, Kim JH, Jung YK, Um SH, Ahn SB, et al. A prospective, randomized, multicenter and phase 3b study to evaluate the efficacy of terlipressin and albumin in cirrhotic patients with ascites and acute kidney injury in the range of serum creatinine between 1.5 mg and 2.5 mg/dl: interim analysis. Journal of Hepatology 2017;66(1 Suppl 1):S11. [Google Scholar]
Sola‐Vera 2003 {published data only}
- Sola‐Vera J, Minana J, Ricart E, Kolle L, Gómez C, Murugó M, et al. Albumin versus saline infusion in cirrhotic patients with tense ascites treated with total paracentesis. A randomized study. Hepatology (Baltimore, Md) 1997;26(4 pt 2):285a. [Google Scholar]
- Sola‐Vera J, Minana J, Ricart E, Planella M, Gonzalez B, Torras X, et al. Randomized trial comparing albumin and saline in the prevention of paracentesis‐induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology (Baltimore, Md) 2003;37(5):1147‐53. [DOI] [PubMed] [Google Scholar]
Stanley 1989a {published data only}
- Stanley MM. Randomized clinical trials of treatment of ascites in alcoholic cirrhotics medical treatment versus peritoneovenous shunting. American Society for Artificial Internal Organs Transactions 1989;35(2):174‐6. [DOI] [PubMed] [Google Scholar]
Steigmann 1966 {published data only}
- Steigmann F, Oz R, Will P, Dubin A. Furosemide therapy in intractable ascites. American Journal of the Medical Sciences 1966;252(4):436‐41. [DOI] [PubMed] [Google Scholar]
Tempini 1984 {published data only}
- Tempini S, Bellati G, Ideo G. Spironolactone and k‐canrenoate effect in decompensated liver cirrhosis: double blind study. Minerva Dietologica e Gastroenterologica 1984;30(3):255‐61. [PubMed] [Google Scholar]
Thompson 1977 {published data only}
- Thompson EJ, Torres E, Grosberg SJ, Martinez‐Maldonado M. Effect of triamterene on potassium excretion in cirrhotic patients receiving furosemide. Clinical Pharmacology and Therapeutics 1977;21(4):392‐4. [DOI] [PubMed] [Google Scholar]
Tsai 1996 {published data only}
- Tsai YT, Lin HC, Lee FY, Hou MC, Wang SS, Lee SD. Effects of captopril on renal functions, renal and portal hemodynamics in patients with cirrhosis. Proceedings of the National Science Council, Republic of China ‐ Part B, Life Sciences 1996;20(2):44‐50. [PubMed] [Google Scholar]
Vizzutti 2001 {published data only}
- Vizzutti F, Romanelli RG, Casini Raggi V, Montalto P, Pastacaldi S, Pinzani M, et al. Diuretic and natriuretic effects of long‐term albumin infusion in patients with cirrhosis and ascites: a randomised controlled study. Journal of Hepatology 2001;34(1):17. [Google Scholar]
Wapnick 1979 {published data only}
- Wapnick S, Grosberg SJ, Evans MI. Randomized prospective matched pair study comparing peritoneovenous shunt and conventional therapy in massive ascites. British Journal of Surgery 1979;66(9):667‐70. [DOI] [PubMed] [Google Scholar]
Yakar 2016 {published data only}
- Yakar T, Demir M, Dogan O, Parlakgumus A, Ozer B, Serin E. High dose oral furosemide with salt ingestion in the treatment of refractory ascites of liver cirrhosis. Clinical and Investigative Medicine 2016;39(6):S52‐60. [PubMed] [Google Scholar]
Yamada 1970 {published data only}
- Yamada S, Reynolds TB. Amiloride (mk‐870), a new antikaluretic diuretic. Comparison to other antikaluretic diuretics in patients with liver disease and ascites. Gastroenterology 1970;59(6):833‐41. [PubMed] [Google Scholar]
Yosry 2019 {published data only}
- Yosry A, Soliman ZA, Eletreby R, Hamza I, Ismail A, Elkady MA. Oral midodrine is comparable to albumin infusion in cirrhotic patients with refractory ascites undergoing large‐volume paracentesis: results of a pilot study. European Journal of Gastroenterology and Hepatology 2019;31(3):345‐51. [DOI] [PubMed] [Google Scholar]
Zaak 2001 {published data only}
- Zaak D, Paquet KJ, Kuhn R. Prospective study comparing human albumin vs. reinfusion of ultrafiltrate‐ascitic fluid after total paracentesis in cirrhotic patients with tense ascites. Zeitschrift für Gastroenterologie 2001;39(1):5‐10. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang HX, Li J, Cheng C. Abdominal indwelling central venous catheter in treatment of liver fibrosis and intractable ascites. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2013;25(4):427, 30. [PubMed] [Google Scholar]
Zhao 2000 {published data only}
- Zhao J, Yuan C, Wang D, Li X. Mannitolum infusion on cirrhotic patients with tense ascites treated by paracentesis. Chinese Medical Journal 2000;113(1):27‐30. [PubMed] [Google Scholar]
References to ongoing studies
EUCTR 2018 {published data only}
- EUCTR ES. Long‐term albumin administration in cirrhotic patients with ascites. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number: 2016‐001789‐28 (first received 25 April 2018).
- NCT03506893. Medical‐economic evaluation of the care of refractory ascites by implantation of alfapump® device in cirrhotic patients. clinicaltrialsgov/show/nct03506893 (first received 24 April 2018).
Macken 2018 {published data only}
- Macken L, Mason L, Evans C, Gage H, Jordan J, Austin M, et al. Palliative long‐term abdominal drains versus repeated drainage in individuals with untreatable ascites due to advanced cirrhosis: study protocol for a feasibility randomised controlled trial. Trials 2018;19(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03027635 {published data only}
- NCT03027635. Peritoneal catheter versus repeated paracentesis for ascites in cirrhosis. clinicaltrials.gov/ct2/show/NCT03027635 (first received 23 January 2017).
NCT 03172273 {published data only}
- NCT03172273. Early use of TIPS with polytetrafluoroethylene (PTFE) covered stents in cirrhotic patients with refractory ascites. clinicaltrialsgov/show/nct03172273 (first received 1 June 2017).
NCT03202524 {published data only}
- NCT03202524. Fresh frozen plasma as a substitute for albumin in patients receiving a large volume paracentesis. clinicaltrialsgov/show/nct03202524 (first received 28 June 2017).
NCT03451292 {published data only}
- NCT03451292. Effects of long‐term administration of human albumin in subjects with decompensated cirrhosis and ascites. clinicaltrialsgov/show/nct03451292 (first received 1 March 2018).
Additional references
Adam 2012
- Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). Journal of Hepatology 2012;57(3):675‐88. [DOI] [PubMed] [Google Scholar]
Arroyo 1996
- Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. International Ascites Club. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology (Baltimore, Md) 1996;23(1):164‐76. [DOI] [PubMed] [Google Scholar]
Asher 2018
- Asher AL, Kerezoudis P, Mummaneni PV, Bisson EF, Glassman SD, Foley KT, et al. Defining the minimum clinically important difference for grade i degenerative lumbar spondylolisthesis: insights from the quality outcomes database. Neurosurgical Focus 2018;44(1):E2. [DOI] [PubMed] [Google Scholar]
Best 2018
- Best LMJ, Freeman S, Sutton AJ, Hawkins N, Tsochatzis E, Gurusamy KS. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD013103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PlOS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colle 2008
- Colle I, Geerts AM, Steenkiste C, Vlierberghe H. Hemodynamic changes in splanchnic blood vessels in portal hypertension. Anatomical Record 2008;291(6):699‐713. [DOI] [PubMed] [Google Scholar]
D'Amico 2006
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. [DOI] [PubMed] [Google Scholar]
D'Amico 2014
- D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25‐year inception cohort study of 494 patients. Alimentary Pharmacology & Therapeutics 2014;39(10):1180‐93. [DOI] [PubMed] [Google Scholar]
De Franchis 2015
- Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63(3):743‐52. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU technical support document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). nicedsu.org.uk/wp‐content/uploads/2016/03/TSD3‐Heterogeneity.final‐report.08.05.12.pdf (accessed 17 July 2018).
Dias 2012b
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). scharr.dept.shef.ac.uk/nicedsu/wp‐content/uploads/sites/7/2016/03/TSD1‐Introduction.final_.08.05.12.pdf (accessed 17 July 2018).
Dias 2014
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). nicedsu.org.uk/wp‐content/uploads/2016/03/TSD4‐Inconsistency.final_.15April2014.pdf (accessed 17 July 2018). [PubMed]
Dias 2016
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials, August 2011 (last updated September 2016). www.ncbi.nlm.nih.gov/pubmedhealth/PMH0088912/pdf/PubMedHealth_PMH0088912.pdf (accessed 17 July 2018). [PubMed]
EASL 2010
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of Hepatology 2010;53(3):397‐417. [DOI] [PubMed] [Google Scholar]
EASL 2016
- European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. Journal of Hepatology 2016;64(2):433‐85. [DOI] [PubMed] [Google Scholar]
EASL 2018
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology 2018;69(2):406‐60. [DOI: 10.1016/j.jhep.2018.03.024] [DOI] [PubMed] [Google Scholar]
Ennaifer 2014
- Ennaifer R, Elleuch N, Romdhane H, Hefaiedh R, Cheikh M, Chaabouni S, et al. Refractory ascites in cirrhosis: prevalence and predictive factors. Journal of Liver 2014;3(4):162. [Google Scholar]
EuroQol 2018
- EuroQol. EQ‐5D Instruments | About EQ‐5D, 2018. euroqol.org/eq‐5d‐instruments/ (accessed 17 July 2018).
Fagan 2014
- Fagan KJ, Zhao EY, Horsfall LU, Ruffin BJ, Kruger MS, McPhail SM, et al. Burden of decompensated cirrhosis and ascites on hospital services in a tertiary care facility: time for change?. Internal Medicine Journal 2014;44(9):865‐72. [DOI] [PubMed] [Google Scholar]
Fleming 2008
- Fleming KM, Aithal GP, Solaymani‐Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992‐2001: a general population‐based study. Journal of Hepatology 2008;49(5):732‐8. [DOI] [PubMed] [Google Scholar]
Ginès 2009
- Ginès P, Schrier RW. Renal failure in cirrhosis. New England Journal of Medicine 2009;361(13):1279‐90. [DOI] [PubMed] [Google Scholar]
Guo 2016
- Guo TT, Yang Y, Song Y, Ren Y, Liu ZX, Cheng G. Effects of midodrine in patients with ascites due to cirrhosis: systematic review and meta‐analysis. Journal of Digestive Diseases 2016;17(1):11‐9. [DOI] [PubMed] [Google Scholar]
Gurusamy 2019
- Gurusamy K, Walmsley M, Davidson BR, Frier C, Fuller B, Madden A, et al. Top research priorities in liver and gallbladder disorders in the UK. BMJ Open 2019;9(3):e025045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoehle 2019
- Hoehle LP, Phillips KM, Speth MM, Caradonna DS, Gray ST, Sedaghat AR. Responsiveness and minimal clinically important difference for the EQ‐5D in chronic rhinosinusitis. Rhinology 2019;57(2):110‐6. [DOI] [PubMed] [Google Scholar]
Hutton 2015
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Annals of Internal Medicine 2015;162(11):777‐84. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jackson 2014
- Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design‐by‐treatment interaction model for network meta‐analysis with random inconsistency effects. Statistics in Medicine 2014;33(21):3639‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2019
- Kato S, Oshima Y, Matsubayashi Y, Taniguchi Y, Tanaka S, Takeshita K. Minimum clinically important difference in outcome scores among patients undergoing cervical laminoplasty. European Spine Journal 2019;28(5):1234‐41. [DOI] [PubMed] [Google Scholar]
Kim 2006
- Kim SH, Oh EG, Lee WH. Symptom experience, psychological distress, and quality of life in Korean patients with liver cirrhosis: a cross‐sectional survey. International Journal of Nursing Studies 2006;43(8):1047‐56. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Les 2010
- Les I, Doval E, Flavià M, Jacas C, Cárdenas G, Esteban R, et al. Quality of life in cirrhosis is related to potentially treatable factors. European Journal of Gastroenterology and Hepatology 2010;22(2):221‐7. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
McPherson 2016
- McPherson S, Lucey MR, Moriarty KJ. Decompensated alcohol related liver disease: acute management. BMJ 2016;352:i124. [DOI] [PubMed] [Google Scholar]
Merion 2010
- Merion RM. Current status and future of liver transplantation. Seminars in Liver Disease 2010;30(4):411‐21. [DOI] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Mokdad 2014
- Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Medicine 2014;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2003
- Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology (Baltimore, Md) 2003;38(1):258‐66. [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore CM, Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis and management. World Journal of Hepatology 2013;5(5):251‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCBI 2018a
- National Center for Biotechnology Information. Liver cirrhosis. www.ncbi.nlm.nih.gov/mesh/68008103 (accessed on 17 July 2018).
NCBI 2018b
- National Center for Biotechnology Information. Ascites. www.ncbi.nlm.nih.gov/mesh/68001201 (accessed on 17 July 2018).
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Optum 2018
- Optum. Patient‐reported outcomes | What we do | SF Health Surveys | SF‐36v2 Health Survey, 2018. campaign.optum.com/optum‐outcomes/what‐we‐do/health‐surveys/sf‐36v2‐health‐survey.html (accessed on 14 April 2018).
Orr 2014
- Orr JG, Homer T, Ternent L, Newton J, McNeil CJ, Hudson M, et al. Health related quality of life in people with advanced chronic liver disease. Journal of Hepatology 2014;61(5):1158‐65. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Qi 2016
- Qi X, Tian Y, Zhang W, Zhao H, Han G, Guo X. Covered tips for secondary prophylaxis of variceal bleeding in liver cirrhosis: a systematic review and meta‐analysis of randomized controlled trials. Medicine 2016;95(50):E5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratib 2015
- Ratib S, Fleming KM, Crooks CJ, Walker AJ, West J. Causes of death in people with liver cirrhosis in England compared with the general population: a population‐based cohort study. American Journal of Gastroenterology 2015;110(8):1149‐58. [DOI] [PubMed] [Google Scholar]
Read 1972
- Read AE. Clinical physiology of the liver. British Journal of Anaesthesia 1972;44(9):910‐7. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Runyon 2013
- Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology (Baltimore, Md) 2013;57(4):1651‐3. [DOI] [PubMed] [Google Scholar]
Saab 2006
- Saab S, Nieto JM, Lewis SK, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004889.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, et al. Association between risk‐of‐bias assessments and results of randomized trials in Cochrane Reviews: the ROBES Meta‐Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scaglione 2015
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the United States: a population‐based study. Journal of Clinical Gastroenterology 2015;49(8):690‐6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Setiawan 2016
- Setiawan VW, Stram DO, Porcel J, Lu SC, Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology (Baltimore, Md) 2016;64(6):1969‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severini 1993
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40. [Google Scholar]
Simonetti 2019
- Simonetti RG, Perricone G, Nikolova D, Bjelakovic G, Gluud C. Plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis. Cochrane Database of Systematic Reviews 2019, Issue 6. [DOI: 10.1002/14651858.CD004039.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sims 2018
- Sims AL, Parsons N, Achten J, Griffin XL, Costa ML, Reed MR, et al. A randomized controlled trial comparing the Thompson hemiarthroplasty with the Exeter polished tapered stem and Unitrax modular head in the treatment of displaced intracapsular fractures of the hip: the WHiTE 3: HEMI Trial. Bone and Joint Journal 2018;100‐B(3):352‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata/SE 15.1 [Computer program]
- StataCorp LLC. Stata/SE. Version 15.1. Texas, USA: StataCorp LLC, 2017.
Tsochatzis 2014
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383(9930):1749‐61. [DOI] [PubMed] [Google Scholar]
Tsochatzis 2017
- Tsochatzis EA, Gerbes AL. Diagnosis and treatment of ascites. Journal of Hepatology 2017;67(1):184‐5. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. [DOI] [PubMed] [Google Scholar]
Williams 2014
- Williams R, Aspinall R, Bellis M, Camps‐Walsh G, Cramp M, Dhawan A, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384(9958):1953‐97. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yepes‐Nunez 2019
- Yepes‐Nunez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta‐analysis. Journal of Clinical Epidemiology 2019;115:1‐13. [DOI] [PubMed] [Google Scholar]
Zhou 2019
- Zhou GP, Sun LY, Wei L, Qu W, Zeng ZG, Liu Y, et al. Comparision between portosystemic shunts and endoscopic therapy for prevention of variceal re‐bleeding: a systematic review and meta‐analysis. Chinese Medical Journal 2019;132(9):1087‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2018
- Gurusamy KS, Tsochatzis E. Treatment for ascites in people with decompensated liver cirrhosis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD013123] [DOI] [Google Scholar]


